Periodic Table |
 |
 |
 |
 |
 |
 |
 |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables referencing the text string "René", listed by date:
| Year: 1946 | PT id = 1088 |
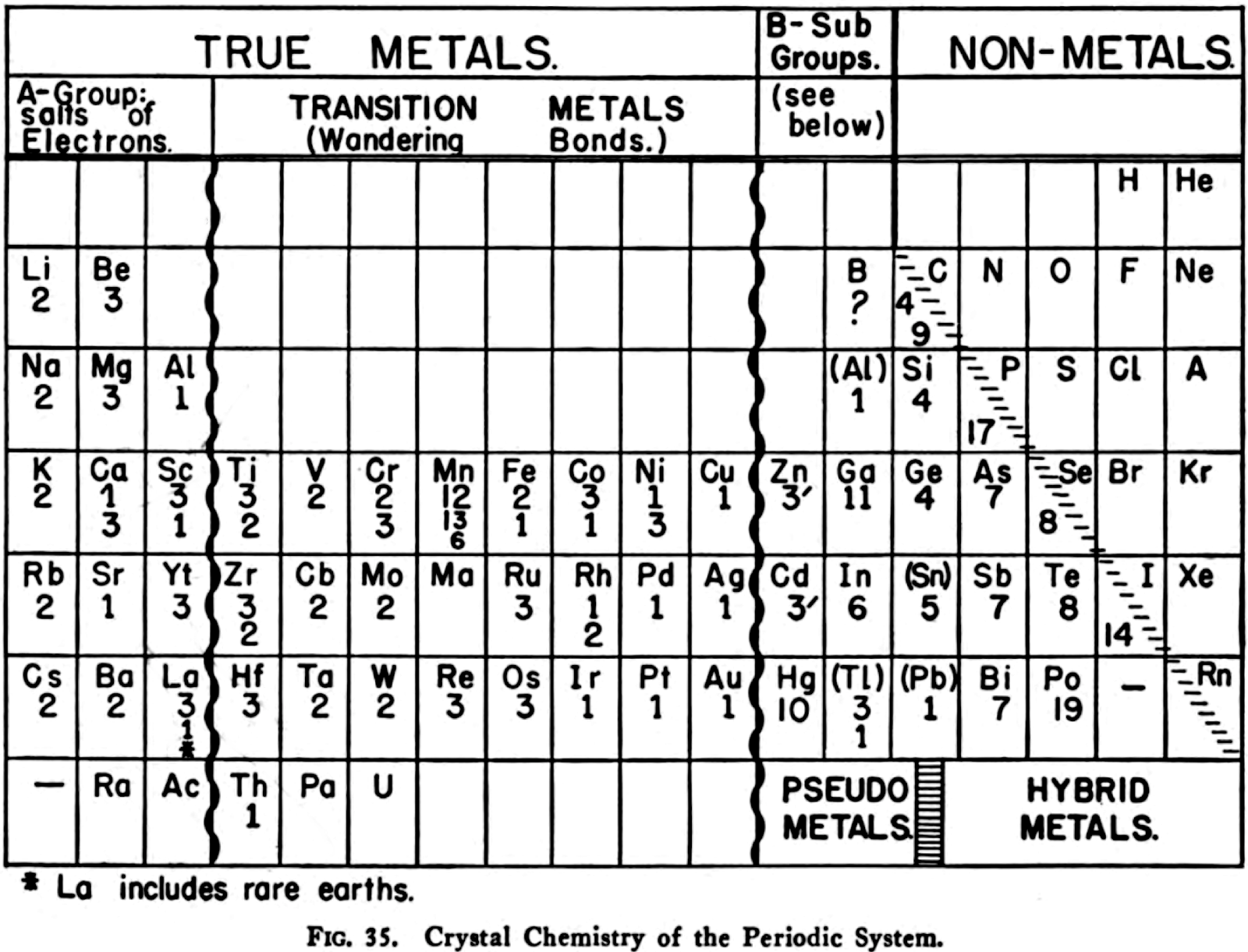
Harrington's Crystal Chemistry of the Periodic System
R.H. Harrington, The Modern Metallurgy of Alloys, John Wiley & Sons, New York, p. 143 (1946)
René Vernon writes:
- Dias JR 2004, "The periodic table set as a unifying concept in going from benzenoid hydrocarbons to fullerene carbons", in DH Rouvray & RB King (eds.), The periodic table: into the 21st century, Institute of Physics Publishing, Philadelphia, pp. 371–396 (375)
- Fernelius WC 1982, "Hafnium," J. Chem. Educ. vol. 59, no. 3, p. 242
- Greenwood NN & Earnshaw A 2002, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, Oxford, p. 1148
- Habashi F 2010, "Metals: typical and less typical, transition and inner transition", Foundations of Chemistry, vol. 12, pp. 31–39
- Lee JD 1996, Concise inorganic chemistry, 5th ed., Blackwell Science, Oxford, p. 753
- Kornilov II 1965, "Recent developments in metal chemistry", Russian Chemical Reviews, vol. 34, no. 1, p. 33
- Küpfer YJ 1954, "Rhodium uses in plating", Microtecnic, Agifa S.A., p. 294 Niedenzu K & Dawson JW 1965, Boron-nitrogen compounds, Springer, Berlin, preface
- Oshe RW (ed.) 1985, "Handbook of thermodynamic and transport properties of alkali metals", Blackwell Scientific, Oxford, p. 987
- Paine et al. 2005, "Recent developments in boron-phosphorus ring and cage chemistry", in Modern aspects of main group chemistry, M Lattman et al. (eds.), ACS Symposium Series, American Chemical Society, Washington DC, p. 163
- Rayner-Canham G 2020, The periodic table: Past, present, and future, World Scientific, Singapore
- H and P are almost on top of one another
- The proximity of Be to the post-transition metals, and its relative scarcity in the crust
- The metalloids, with their intermediate values of electronegativity, go down the middle. At the same time they span nearly the full range of abundance.
- B-Ga-Sc-Y-La are in a row
- N falls along the halogen line
- The abundance of O and Si, which we see in the form of silica
- F is more abundant in the crust than 85 percent of metals
- Al is the most abundant metal. Al and Fe are in the same vicinity: "Curiously, the chemistry of aluminium also resembles that of the iron(III) ion... These similarities may be ascribed to the same 3+ charge and near-identical ion radii (and hence charge density)." (Rayner-Canham 2020, p. 191)
- The abundance of Ar compared to the rest of the noble gases. Apparently this is influenced by the radioactive decay of potassium-40 in Earth's core, which is considered one of the main sources of heat powering the geodynamo that generates Earth's magnetic field. It has been suggested that a large amount of Ar may be present in the core, as the compound ArNi with an L11 Laves structure (similar to an intermetallic phase, and related to a cubic close packed lattice). ArNi is stabilised by notable electron transfer from Ni to Ar, changing their electron configurations toward 3d7 and 4s1. (Adeleke et al. 2019)
- Ti, a light yet strong metal, is about 2,500 times as abundant as Sn, a weak heavy metal
- Zn is an outlaw post-transition metal
- The most active 4d-5d transition metals (Zr, Hf) occupy a boundary overlap with the rare earth metals
- Ag, which has a largely main-group chemistry, is located in the PTM region. It is about 20 times as abundant as the noble meals
- Re is an outlaw noble metal
- Adeleke AA, Kunz M, Greenberg E, Prakapenka VB, Yao Y, Stavrou R 2019, A high-pressure compound of argon and nickel: Noble gas in the Earth's core?, ACS Earth and Space Chemistry, vol. 3 no. 11, pp. 2517-2544, https://pubs.acs.org/doi/10.1021/acsearthspacechem.9b00212
- Rayner-Canham G, 2020, The periodic table: Past, present, future, World Scientific, Singapore
- Metals with lower EN, i.e. < 1.7, or active nonmetals with higher EN, tend to be concentrated in silicate or oxide phases that are more easily found in the crust due to their lower density, and hence have higher abundances.
- Metals with moderate EN 1.7 to 2.1, say the later transition metals and post-transition metals, tend to form sulfide liquid phases; are less easily found in the crust due to their relatively higher densities; and are less abundant by about two orders of magnitude compared to the metals found in silicate or oxide phases.
- Metals with EN > 2.2, i.e. the noble metals, have an affinity for a metallic liquid phase, and are depleted in the crust since they generally sank to the core and hence have very low abundances. They are about two orders of magnitude less abundant than the sulfide metals.
- Cox PA 1997, The elements: Their origin, abundance and distribution;
- Gill R 2014, Chemical fundamentals of geology and environmental geoscience;
- White WA 2020, Geochemistry
- For the nonmetals, the relative average abundance proportions are about 5: 700: 250: 1 for, respectively, the metalloids; the core nonmetals H, C, N, P, S, and Se; the halogen nonmetals; and the noble gases. Si and O were left out as outliers, in terms of their massive abundances.
- Thus, metalloids aside, the abundance of the nonmetals tends to fall with increasing EN. I don't know what's going on with the metalloids.
- The chart may prompt some further appreciative enquiry:
- In the case of exceptions to the initial three generalisations why do these occur?
- Why is Li so rare, compared to the other alkali metals?
- Why is Si good at forming a planetary crust?
- Why do the metalloids span such a wide range of abundances?
- If H is supposed to make up ca. 74% of the universe why does it have the same abundance in the Earth's crust as P?
- In what form is H found in Earth's crust—water, hydroxides?
- If H is supposed to make up ~ 74% of the universe why does it have the same abundance in the Earth's crust as P?
- Are there any chemical similarities between H and P, given both have some metalloidal character? The have virtually identically electron affinities. H is sometimes positioned above B due to chemical similarities. It then forms a diagonal relationship with C, which in turn has a diagonal relationship with P, which has a diagonal relationship with Se e.g. P reacts with Se to form a large number of compounds characterised by structural analogies derived from the white phosphorus P4 tetrahedron.
- The rare earth metals are relatively rare, having an average abundance of 1% that of the 3d metals. That being so, why is their rareness sometimes questioned? Why does the crustal abundance of the REM plummet by two orders of magnitude towards the end of the lanthanides?
"The numbers below each element symbol refer to the crystal: 1 = FCC, 9 = graphite structure, 11 = orthorhombic, etc. Extra numbers are for structures at higher temperatures.
"The wriggly lines between groups 3 and 4, and 11 and 12 refer to a gradation between the classes involved. Wikipedia calls these linking or bridging groups
"Harrington's class names are novel. [Who would have thought of the elements of groups 1 to 3 as being called the "salts of electrons"?] Then again, "in view of the extensive role that electrons play as anions" Dye (2015) asked: "where should electrons be placed in the periodic table?" (Note: In 1946 Achimof tried answering this, with an electron as element -1 above H and a neutron as element 0 above He.)
"Aluminium appears in group 3 and group 13 since, according to Harrington, it has the crystalline structure of a true metal. This is not quite true since its crystalline structure shows some evidence of directional bonding.
"For the transition metals as "wandering bonds", Harrington writes that the metallic bond is spatially undirected and that it may operate between any given atom and an indefinite number of neighbours" (p. 145). Since A-metals are better called, in his mind, "salts of electrons" [and B-metals show signs of significant directional bonding] the transition metals are therefore called by him as wandering bonds. This becomes confusing, however, given d electrons in partially filed d-orbitals of transition metals form covalent bonds with one another.
"Counting boron as a pseudo metals looks strange.
"Germanium is counted as a metal: "...the electrical conductivit[y]... [is] sufficiently high to show that the outer electrons are very loosely held and the linkage must be partly metallic in character." (p. 148). In fact the electrical conductivity of high purity germanium, which is a semiconductor, is around 10–2S.cm–1. Compare this with antimony, at 3.1 x 104S.cm–1
"Tin has brackets around it to show its "renegade" status, "with its white form behaving largely as would a True Metal, whereas its grey form is more non-metallic than metallic." White tin actually has an irregularly coordinated structure associated with incompletely ionised atoms.
"Thallium and lead have brackets around them since their crystalline structures are supposedly like those of true metals. This is not quite right. While both metals have close-packed structures they each have abnormally large inter-atomic distances that have been attributed to partial ionisation of their atoms.
"The B-subgroup metals are divided into pseudo metals and hybrid metals. The pseudo metals (groups 11 and 12) behave more like true metals than non-metals. The hybrid metals As, Sb, Bi, Te, Po, At – which other authors would call metalloids – partake about equally the properties of both. According to Harrington, the pseudo metals can be considered related to the hybrid metals through the carbon column.
"The location of the dividing line between metals and nonmetals, running as it does through carbon to radon is peculiar. The line is usually shown running through boron to astatine."
| Year: 2010 | PT id = 326 |
Brand Evolution Terms
By Kolbrener, a Periodic Table of Brand Evolution Terms:
| Year: 2017 | PT id = 741 |
New Rendering of ADOMAH Periodic Table
From Valery Tsimmerman, of the PerfectPeriodicTable.com and the ADOMAH Periodic Table:
"I received email from Dr. Marcus Wolf who is a chemist, working on renewable energy and electrochemical storage in Germany, near Nuremberg. He also lectures at Georg Simon Ohm, Technische Hochschule Nürnberg. Attached to his email was new version of ADOMAH Periodic Table that he created. In this new rendering he is using Jensen's Valence Manifold (VM)."
This is what Dr. Marcus Wolf wrote:
"The first one to come up with the idea of using a valence manifold VM = [e + v] as a label for the groups, was Will B. Jensen. He derived it from the very early attempts of Richard Abegg, who, at around 1904, brought up the hypothesis of 'main- and counter-valences', derived from the observable behavior of elements and their compounds in electrochemical experiments. Eric Scerri is citing Jensen in his latest book, in the chapter about Richard Abegg. But Jensen's proper article from 1983 or so is far more detailed and in his later publications he then introduces the valence manifold concept. Last weekend I accidentally observed another consistency between the G-values and their ordering and the valence electron counts, e. If you fix the e value of the starting group in a given l-block as e(initial), you could generate every G-number of a given group by adding the valence vacancy count, v, to it:
G = e(initial) + v.
"That is another hint for the consistency of the VM labelling concept."

| Year: 2020 | PT id = 1104 |
FReNeTic
FReNeTiC is the multi-Award winning 'Frenzied word game of the Elements' where players race against the clock to form as many words as possible using the Element Symbols of The Periodic Table.
In this fast and furious word game players score points equivalent to the atomic numbers of each tile used to create the word, for example Ba Na Na = Banana = 78 points.
The first player to score 1000 points wins!
Everyone plays all the time, quick set up and easy-to-follow rules with FRaNTiC FUN AcTiON! (And no, you don't need to know the Periodic Table or be a GeNiUS to play).

Thanks to Marcus for the tip!
| Year: 2020 | PT id = 1165 |
Vernon's (Partially Disordered) 15 Column Periodic Table
A formulation by René Vernon, who writes:
"Here is a 15-column table which is a hybrid of a Mendeleev 8-column table and an 18-column standard table. The key relocations are the p-block nonmetals to the far left; and the coinage and post-transition metals under their s and early d-block counterparts.
"Taking a leaf out of Mendeleev's playbook, I ignored atomic number order when this seemed appropriate. It's refreshing to see the traditional horizontal gaps between blocks disappear. (DIM did not like these.)
"Since Dias (2004, see references below) reckoned a periodic table is a partially ordered set forming a two-dimensional array, I believe I now have a partially ordered table that is partially disordered twice over.
"The table has some curious relationships. Equally, some relationships seen in the standard form are absent. The Group 2, 3, and aluminium dilemmas disappear. This confirms my impression that such dilemmas have no intrinsic meaning. Rather, their appearance or non-appearance is context dependent."
Notes & references below.
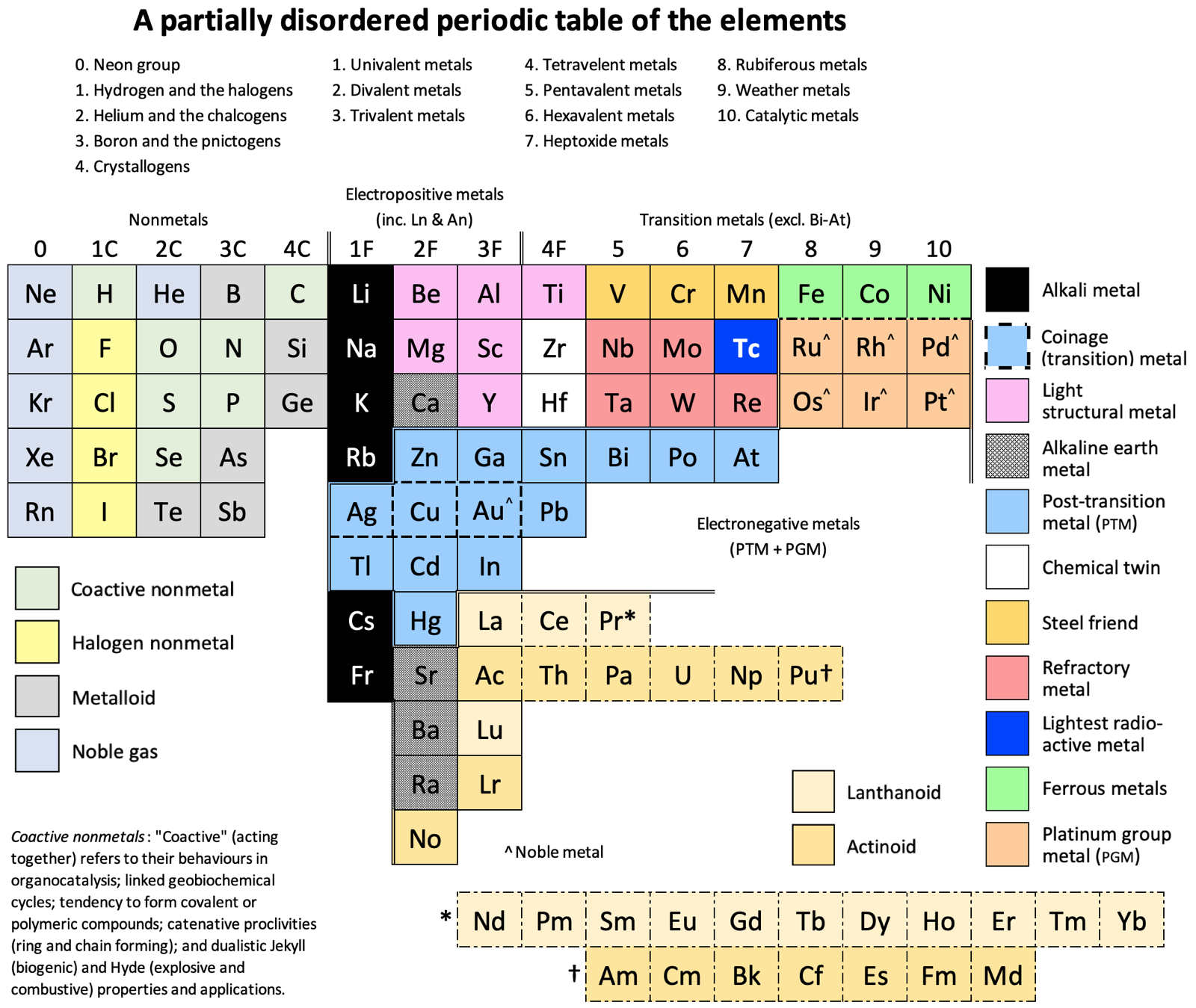
Groups 1 to 4 have either a C or F suffix where C (nonmetal) is after the importance of carbon to our existence; and F (metal) is for the importance of iron to civilisation.
Groups 1C and 1F present the greatest contrast in nonmetallic and metallic behaviour.
Coactive Nonmetals: They are capable of forming septenary heterogeneous compounds such as C20H26N4O10PSSe.
Group 2C: Helium is shaded as a noble gas. "Heliox" is a breathing gas mixture of helium and oxygen used in saturation diving, and as a medical treatment for patients with difficulty breathing.
Group 3C: Boron over nitrogen looks odd. Yet one boron atom and one nitrogen atom have the same number of electrons between them as two adjacent carbon atoms. The combination of nitrogen and boron has some unusual features that are hard to match in any other pair of elements (Niedenzu & Dawson 1965).
Boron and phosphorus form a range of ring and cage compounds having novel structural and electronic properties (Paine et al. 2005).
Metalloids. I treat them here as nonmetals given their chemistry is predominately that of chemically weak nonmetals.
Metals: The labels electropositive; transition; and electronegative are adapted from Kornilov (2008).
Group 1F: Monovalent thallium salts resemble those of silver and the alkali metals.
An alloy of cesium (73.71%), potassium (22.14%) and sodium (4.14%) has a melting point of –78.2°C (–108.76°F) (Oshe 1985).
Silver, copper, and gold, as well as being the coinage metals, are borderline post-transition metals.
Group 2F: Beryllium and magnesium are not in fact alkaline earths. Beryllium is amphoteric rather than alkaline; magnesium was isolated in impure form from its oxides, unlike the true alkaline earths. The old ambiguity over whether beryllium and magnesium should go over calcium or zinc has gone.
Nobelium is here since +2 is its preferred oxidation state, unlike other actinoids.
Group 3F: Aluminium is here in light of its similarity to scandium (Habishi 2010).
InGaAsP is a semiconducting alloy of gallium arsenide and indium phosphide, used in lasers and photonics.
There is no Group 3 "issue" since lanthanum, actinium, lutetium and lawrencium are in the same family.
Gold and aluminium form an interesting set of intermetallic compounds known as Au5Al2 (white plague) and AuAl2 (purple plague). Blue gold is an alloy of gold and either gallium or indium.
Lanthanoids: The oxidation state analogies with the transition metals stop after praseodymium. That is why the rest of lanthanoids are footnoted in dash-dot boxes.
Actinoids: The resemblance to their transition metal analogues falters after uranium, and peters out after plutonium.
Group 4F: It's funny to see titanium—the lightweight super-metal—in the same group as lead, the traditional "heavy" metal.
This is the first group impacted by the lanthanoid contraction (cerium through lutetium) which results in the atomic radius of hafnium being almost the same as that of zirconium. Hence "the twins".
The chemistry of titanium is significantly different from that of zirconium and hafnium (Fernelius 1982).
Lead zirconate titanate Pb[ZrxTi1–x]O3 (0 ≤ x ≤ 1) is one of the most commonly used piezo ceramics.
Group 5: Bismuth vanadate BiVO4 is a bright yellow solid widely used as a visible light photo-catalyst and dye.
Steel Friends: The name is reference to their use in steel alloys. They have isoelectronic soluble oxidizing tetroxoanions, plus a stable +3 oxidation state. (Rayner-Canham 2020).
Ferromagnetic Metals: The horizontal similarities among this triad of elements (as is the case among the PGM hexad) are greater than anywhere in the periodic table except among the lanthanides (Lee 1996). The +2 aqueous ion is a major component of their simple chemistry (Rayner-Canham 2020).
Group 8: "Rubiferous metals" (classical Latin rubēre to be red; -fer producing) is from the reddish-brown colour of rust; the most prevalent ruthenium precursor being ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically; and the red osmates [OsO4(OH)4]?2 formed upon reaction by osmium tetroxide with a base.
Group 9: "Weather metals" comes from the use of cobalt chloride as a humidity indicator in weather instruments; rhodium plating used to "protect other more vulnerable metals against weather exposure as well as against concentrated acids or acids fumes" (Küpfer 1954); and the "rainbow" etymology of iridium.
Group 10: "Catalytic metals" is after a passage in Greenwood and Earnshaw, "They are... readily obtained in finely divided forms which are catalytically very active." (2002). Of course, many transition metals have catalytic properties. That said, if you asked me about transition metal catalysts, palladium and platinum would be the first to come to mind. Group 10 appear to be particularly catalytic.
References:
| Year: 2021 | PT id = 1183 |
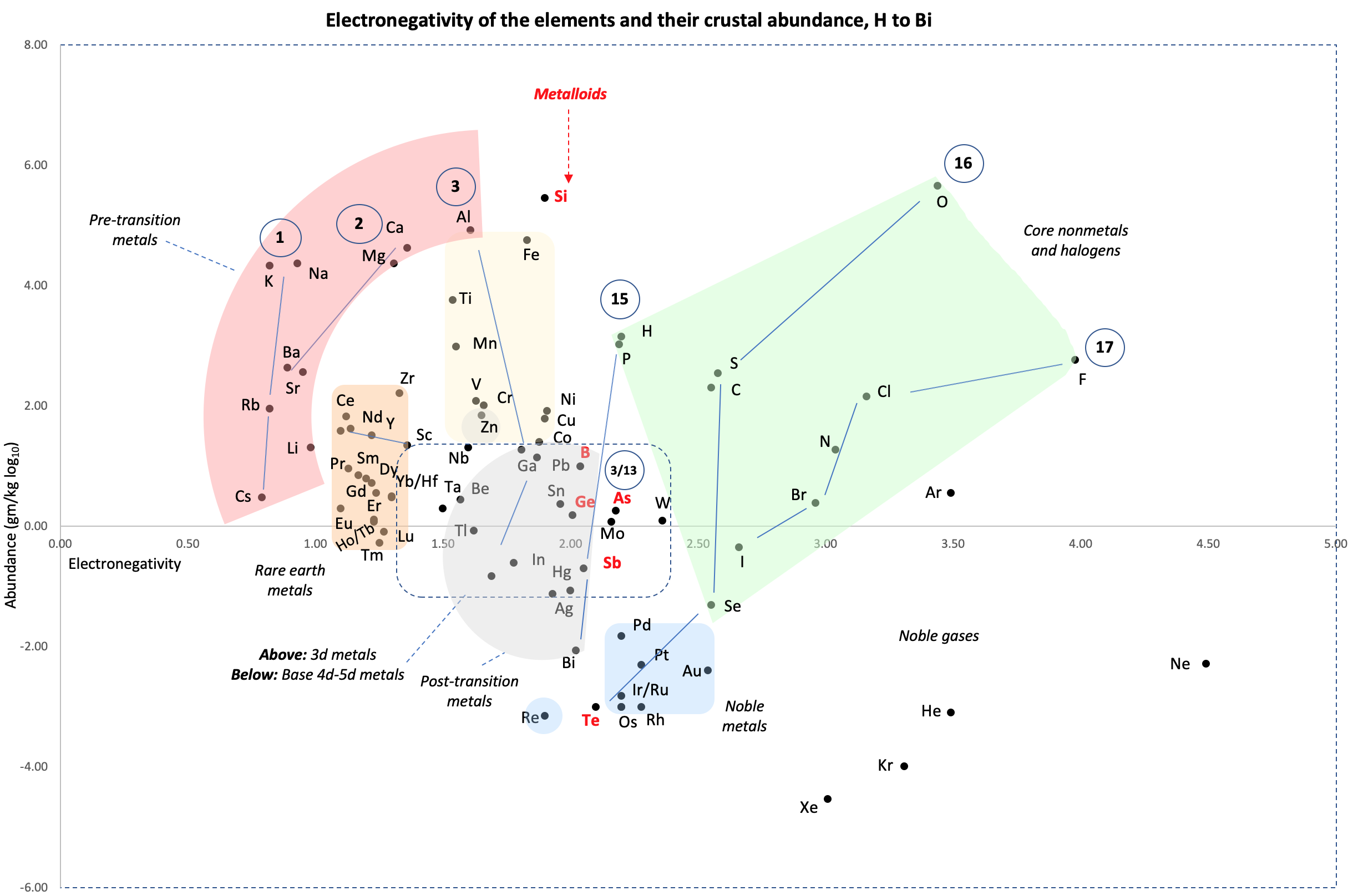
Crustal Abundance vs. Electronegativity
A chart by René Vernon of Elemental Abundance (g/kg log10) vs. Electronegativity, H to Bi.
René writes:
Below is a remarkable XY chart where x = electronegativity and y = crustal abundance (log10). It stops at the end of the s-process, at Bi. The abundance figures are from the CRC Hanbook of Physics and Chemistry (2016-2017).
I say remarkable as I had little idea what the chart would end up looking like when I started plotting the values.
As well as its coloured regions, I've marked out track lines for six of the main groups and one for group 3.
Observations
The rose-coloured arc on the left encompasses the pre-transition metals i.e. the alkali and alkaline earth metals and aluminium, followed by, in the orange rectangle, the rare earth metals. Opposite these regions, along the southern boundary of the green paddock, are the halogens.
In the pale yellow field sheltered by the pre-transition metals and the REM, are the 3d transition metals and, in the white corral, are 4d and 5d base transition metals. Opposite these regions, in the green paddock, are the core nonmetals H, C, N, O, P and S, with Se as an outlier.
Following in the grey blob are the post-transtion or poor metals, immediately adjacent to the bulk of the metalloids or poor nonmetals.
Finally, in the light blue patch, the noble metals are complemented by the noble gases frolicking in the open.
Abundance tends to decrease with increasing Z. Notable exceptions are Li, B, N and Si.
Curiosities
Comment
I was intrigued by the article referring to Ni and Ar, and the suggestion of Ar becoming somewhat anionic, albeit in extreme conditions (140 GPa, 1500 K)
References
Correlations
I wasn't looking for these but they at least exist as follows:
My references are:
Thus the abundance of the metals in the crust tends to fall with increasing EN.
An answer from L. Bruce Railsback, creator of the Earth Scientist's Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=142:
"I think I can answer one of the questions. 'Why is Si good at forming a planetary crust?' – because it's so bad at staying in the core. Silicon isn't sufficiently metallic to stay in the core. Even in the mantle and crust, it doesn't go into non-metal solids well: in cooling magmas, it's only a lesser member of the early-forming minerals (e.g., Mg2SiO4, forsterite, where it's outnumbered two to one). The mineral only of Si as a cation, SiO2 (quartz), is the LAST mineral to form as a magma cools, in essence the residuum of mineral-forming processes. At least some this thinking is at Bowen's Reaction Series and Igneous Rocks at http://railsback.org/FundamentalsIndex.html#Bowen"
Which Electronegativity Scale?
The wide variety of methods for deriving electronegativities tend to give results similar to one another.
| Year: 2023 | PT id = 1288 |
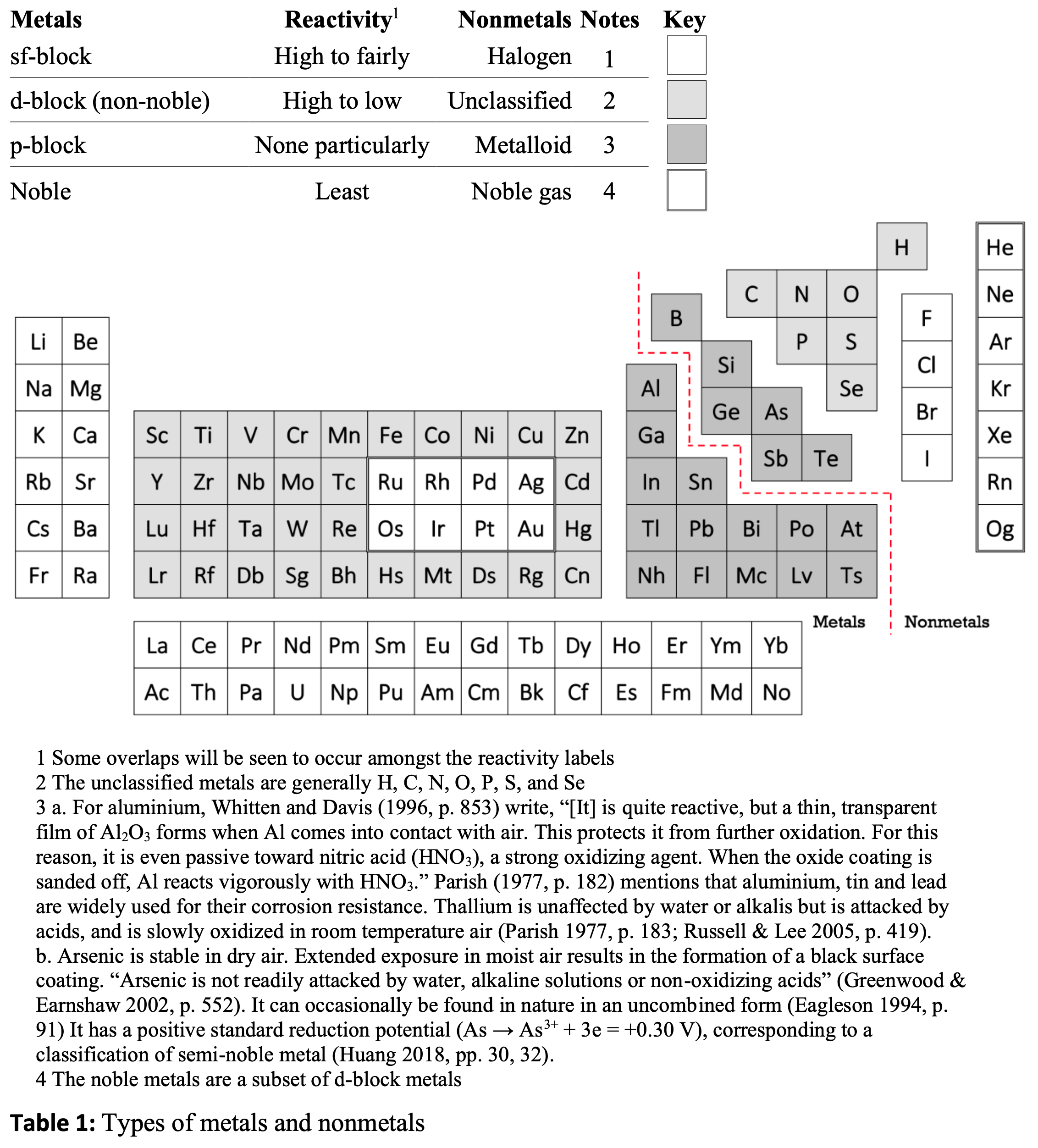
Holistic View of Metals & Nonmetals: Exploded View
From Organising the metals and nonmetals: An update by René Vernon from the chemrxiv preprint server.
Rene writes:
Abstract: This paper updates my 2020 article, Organising the metals and nonmetals in which I advocated for parsing the periodic table into four kinds of metals and four of nonmetals. This framework is retained and updated, and augmented with some additional chemistry-related and philosophical observations.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.