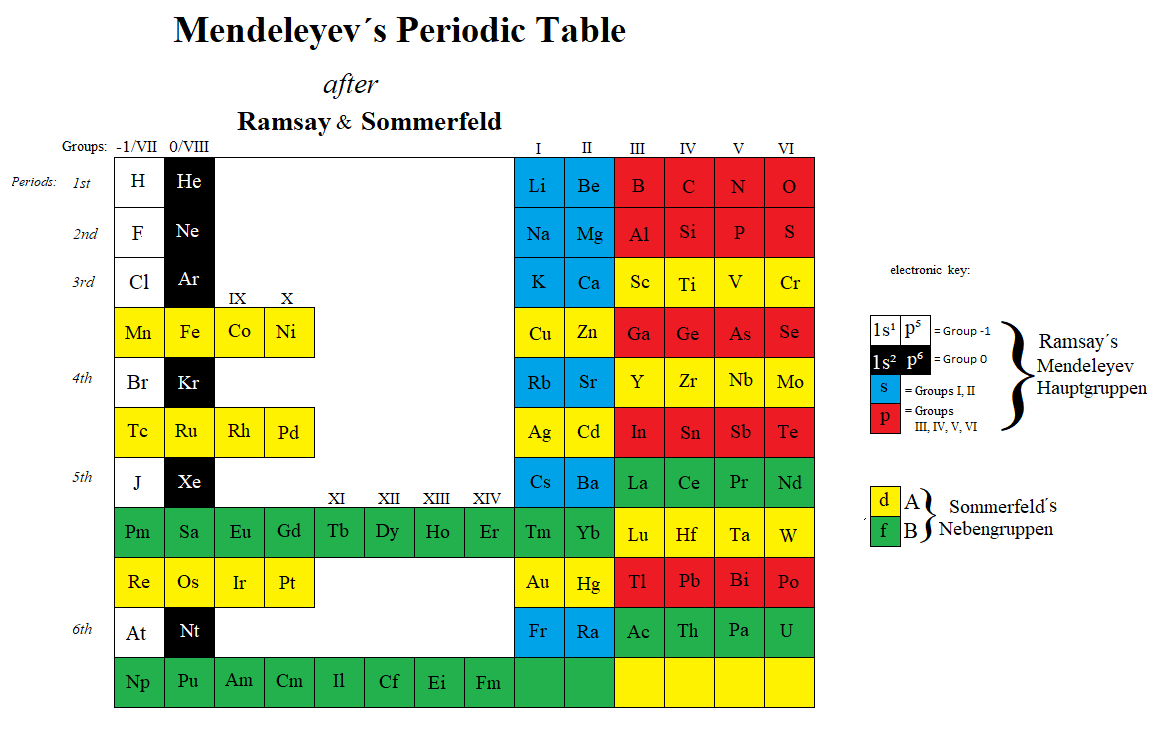
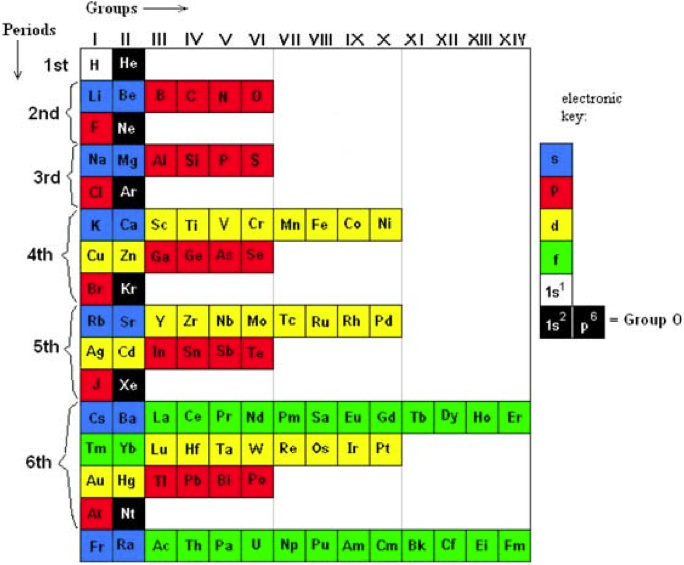
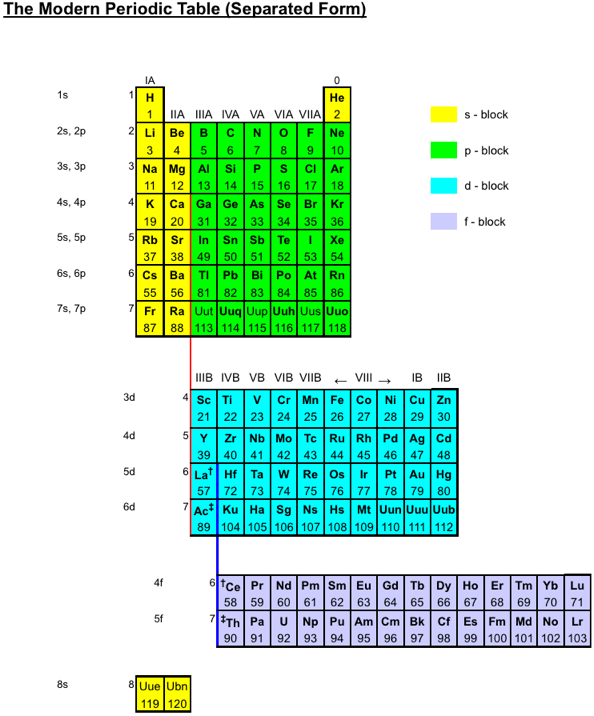
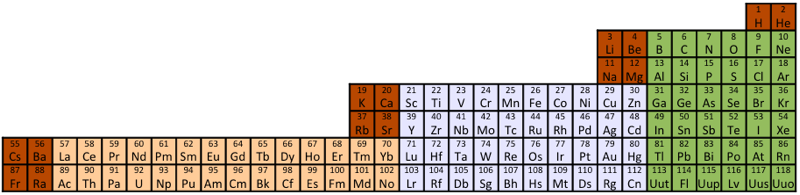
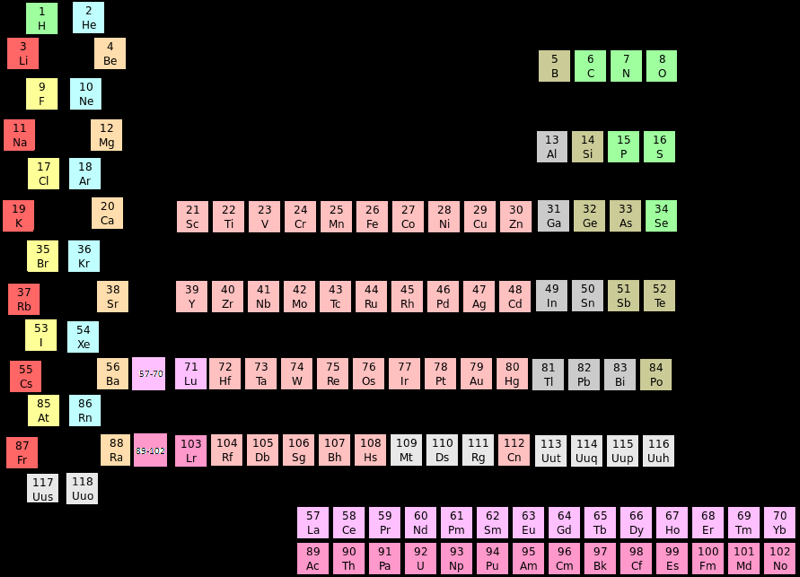
The INTERNET Database of Periodic Tables
Periodic Table formulations since 2010, by date:
2010
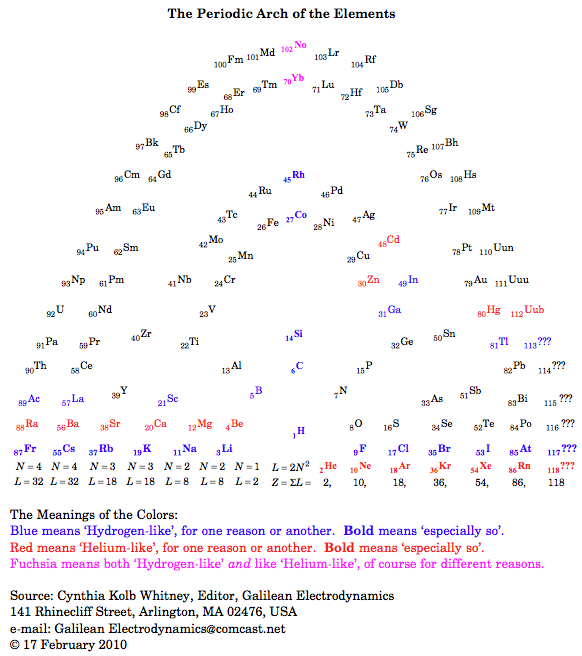
Periodic Arch of The Elements
Cynthia K. Whitney of Galilean Electrodynamics writes: "In his paper Explaining the periodic table, and the role of chemical triad, Eric Scerri mentioned the existence of at least four different candidate places for Hydrogen: Group 1 (alkali metals - Lithium, etc.), Group 17 (halogens - Fluorine, etc.), Group 14 (Carbon, etc.), or off the Periodic Table entirely, because it is so odd! The four-fold multiplicity (and maybe more) of candidate places for Hydrogen triggered in me the following thought: the excessive multiplicity of candidate places may have to do with the rectangular nature of the Periodic Tables under consideration there." Read more in this pdf file.
2010
3-D Strange Periodic Table
As Lewis Page of The Register puts it: "Top flight international reverse-alchemy boffins say they have managed to transmute gold into an entirely new form of 'negatively strange' antihypernucleic antimatter...", here.
The effect is to add a third dimension of quark strangeness to the periodic table. Read the abstract by the STAR Collaboration.
2010
Marks & Marks: Newlands Revisited
Marks & Marks – The Marks bros. – published "A periodic table explicitly for chemists rather than physicists. It is derived from Newlands’ columns. It solves many problems such as the positions of hydrogen, helium, beryllium, zinc and the lanthanoids but all within a succinct format." email here
2010
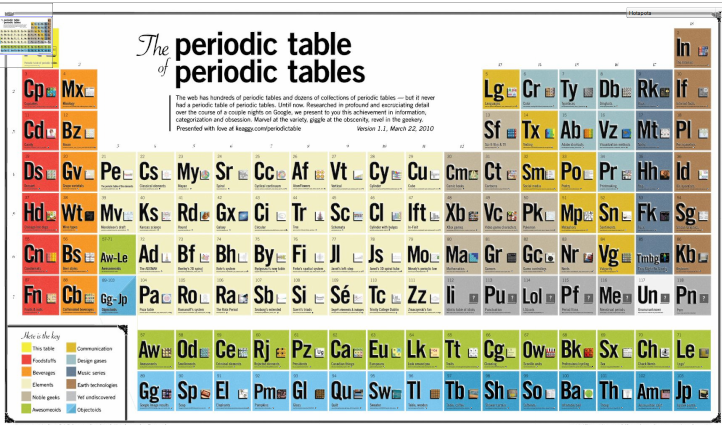
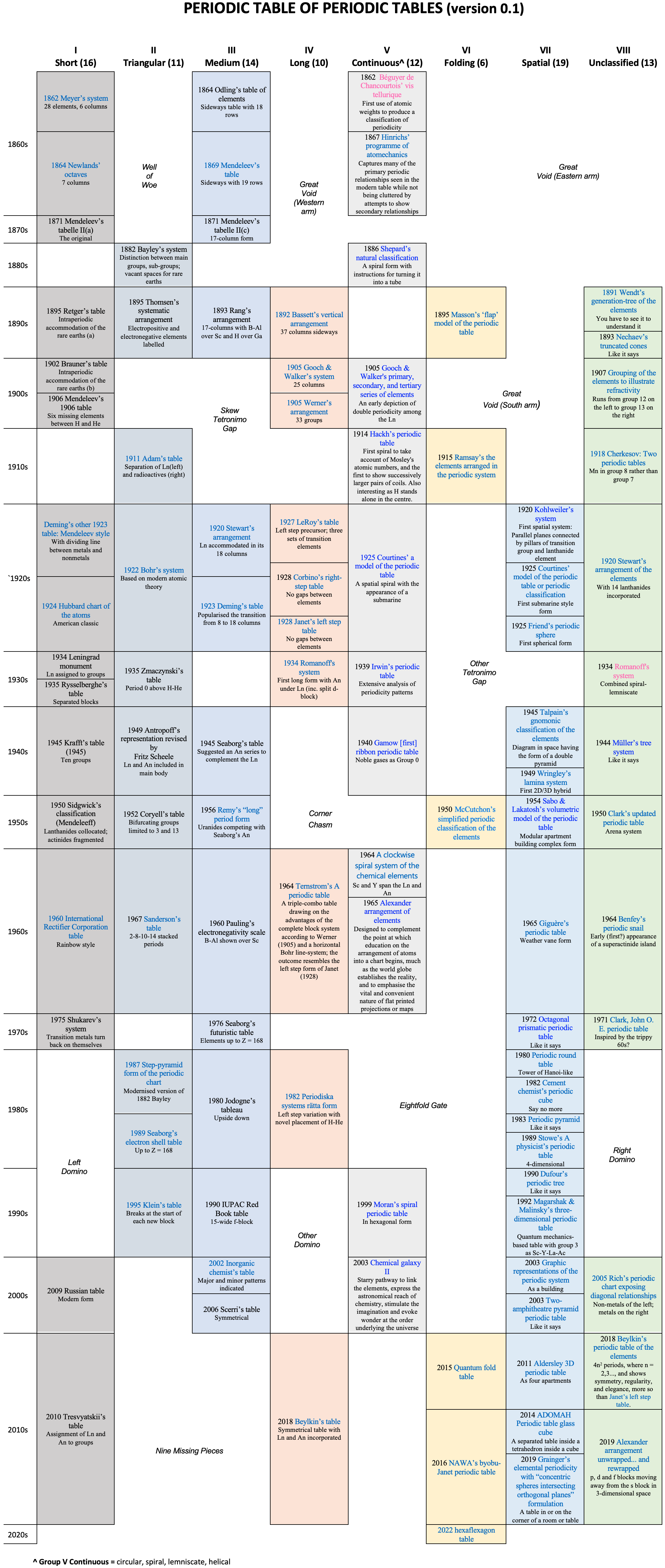
Periodic Table of Periodic Tables
Keaggy, of www.keaggy.com, has put together a rather cool 'Periodic Table of Periodic Tables', clearly using this web site as one of the major resources:
2010
Tai Chi Periodic Table
Joyous Wong, ![]() , a student at the Hebei Normal University, China presents a periodic table based on the Chinese cultural background of Tai Chi:
, a student at the Hebei Normal University, China presents a periodic table based on the Chinese cultural background of Tai Chi:
 |
 |
2010
Recipe For A Human Shirt
By Sean Fallon and available from Fashionably Geek, A Recipe For Humans Shirt:
2010
Jovanovic's 2D Periodic Table
Jovanovic's 2D Periodic Table is based on the atomic number Z and the electron configuration of the elements. There is a full explanatory pdf file on the website:
2010
Fahimi Formulations
Peyman Fahimi has posted some periodic table formulations to www.img98.com, these can be found here, here, here, here & here:
The two most interesting are are shown below:
2010
Vajra Periodic Table
The Vajra Periodic Table, which can be found at APM Periodic Tables, lays out according to electron orbitals and thus gives insights into the electron structure surrounding the nucleus. The nucleus organizes with different rules and thus a different periodic table is needed to visualize the nuclear bindings:
2010
Pauling Spheron Periodic Table
The Pauling Spheron Periodic Table, can be found at APM Periodic Tables.
Linus Pauling was a brilliant physicist who tended to think outside the mainstream. One of his many contributions to science was his spheron model for the nucleus. The word "spheron" does not mean the nucleus is spherical (although it may be), it refers to Pauling's idea that clusters might form in the nucleus. For example, a nucleus may contain a stable helium nucleus within a larger uranium nucleus. Thus, when uranium decays, it releases a helium atom. Other elements, such as oxygen, may also cluster within larger elements. This makes sense since certain atoms like helium and oxygen are more strongly bound than other elements:
2010
Bing Periodic Table
Microsoft's Bing search engine has a rather extensive way of finding element data & information that avoids any formal PT representation:
2010
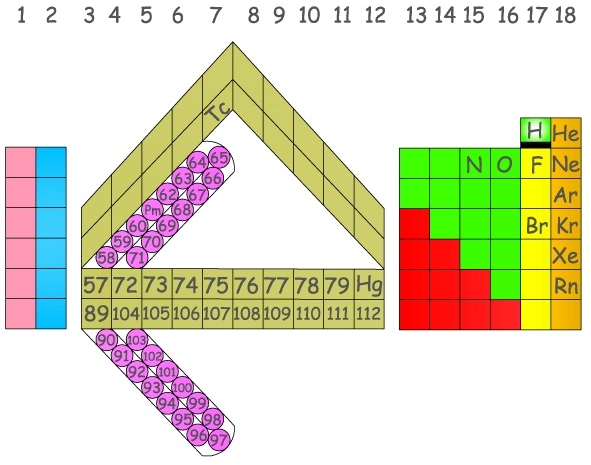
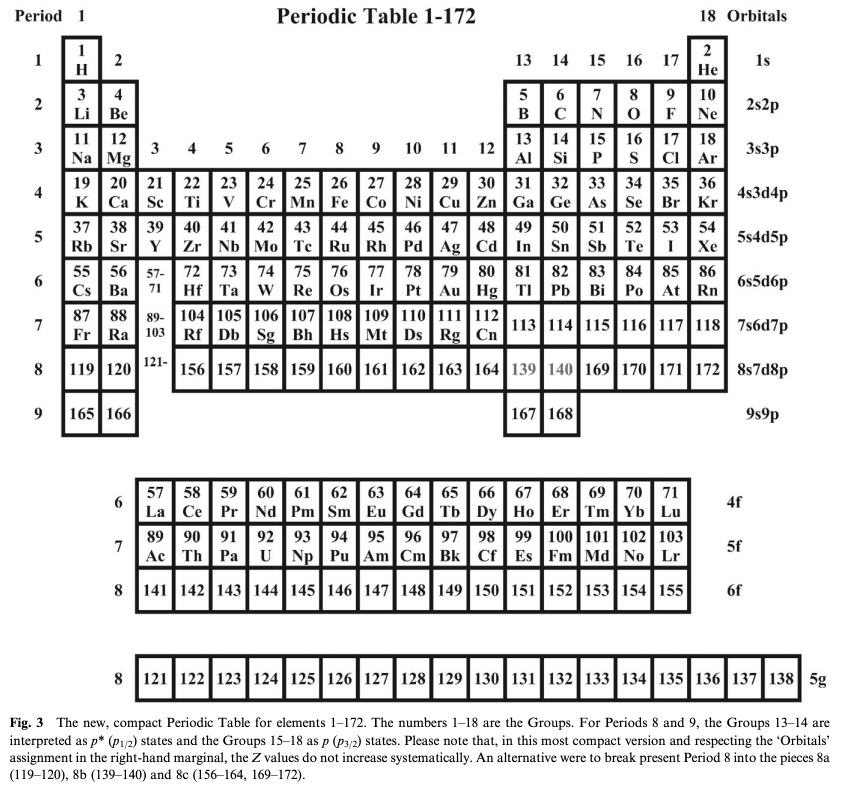
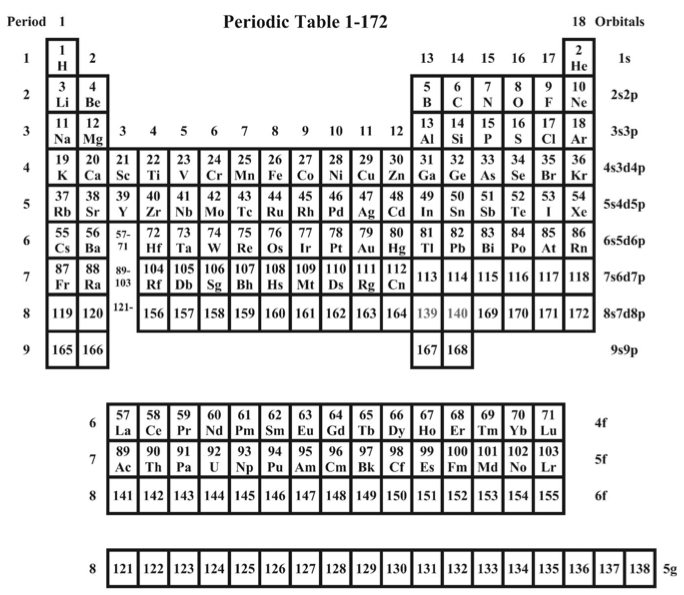
Pyykkö's Extended Elements
From an RSC new page: Pekka Pyykkö at the University of Helsinki has used a highly accurate computational model to predict electronic structures and therefore the periodic table positions of elements up to proton number 172 - far beyond the limit of elements that scientists can currently synthesise.
From the paper, A suggested periodic table up to Z = 172, based on Dirac-Fock calculations on atoms and ions:
2010
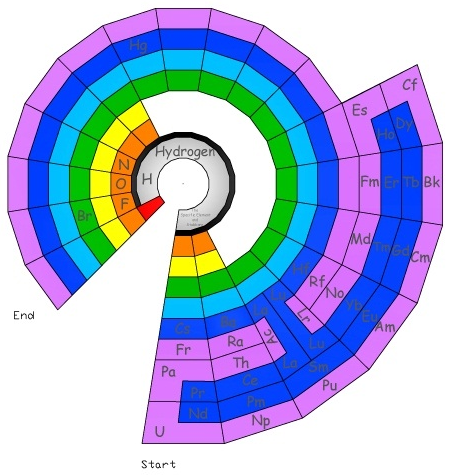
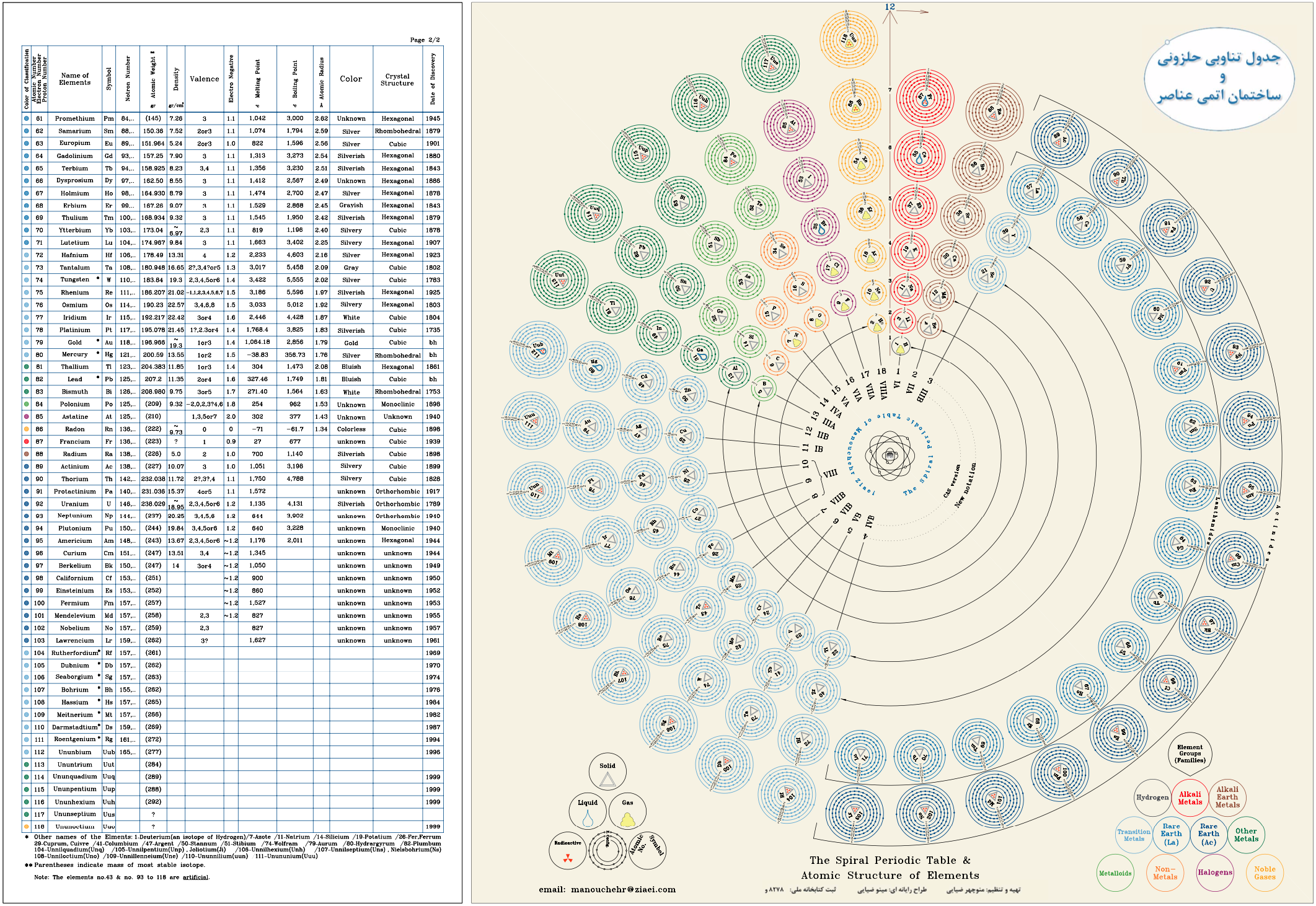
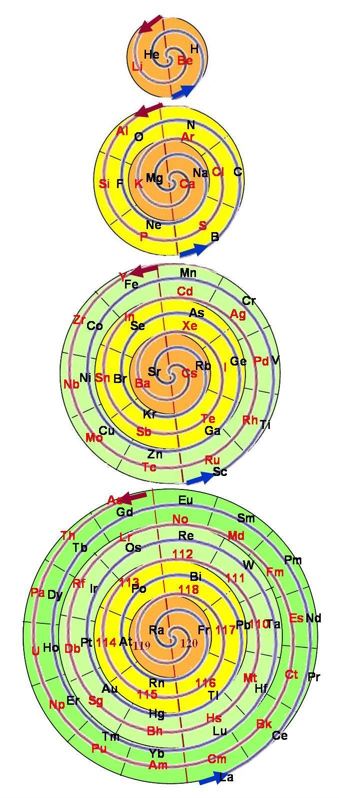
Harrison Spiral Periodic Table
This spiral, inspired by Stewart's Chemical Galaxy, is based on the modern periodic table with the elements strictly arranged in the increasing order of their atomic number and in accordance with their electron configurations.
The spiral separates the elements into the eight dominant 'A' groups of normal elements, and the eight corresponding 'B' subgroups of transitional and inner transitional elements, which have been incorporated as the inner spiral. The organisation of the elements closely follows H.G. Deming's 1923 Periodic Table where A B numeration was first utilized to correspond the characteristic oxides of the 'B' groups to those of the 'A' groups. The result of this design places Group VIII, the triads Fe, Co, Ni, etc. as a subgroup of Group 0 (or 18 Helium Group) which conflicts with some modern periodic tables, though broadly agrees with Deming's original proposal (VIIIA and VIIIB).
Hydrogen, which generally cannot be considered as part of any group, has been placed with the Fluorine group VII which appears its natural place in the spiral. Common names have been used where practicable to make the table more educational and reader-friendly. Element symbols have been included in the expanded poster of this table.
Look at a larger PDF.
2010
Spiral of Atoms and Their Periodic Table
Page 8 of my website (in Russian) shows The Spiral of Atoms and Their Periodic Table, which depicts a spiral disk of atoms with a periodic table of their relative masses.
This information clarifies the options published in the editions of my book The Axiomatics of Nature (2007-2009). Mark Adelman Samuilovich (Mark S. Eidelman)
2010
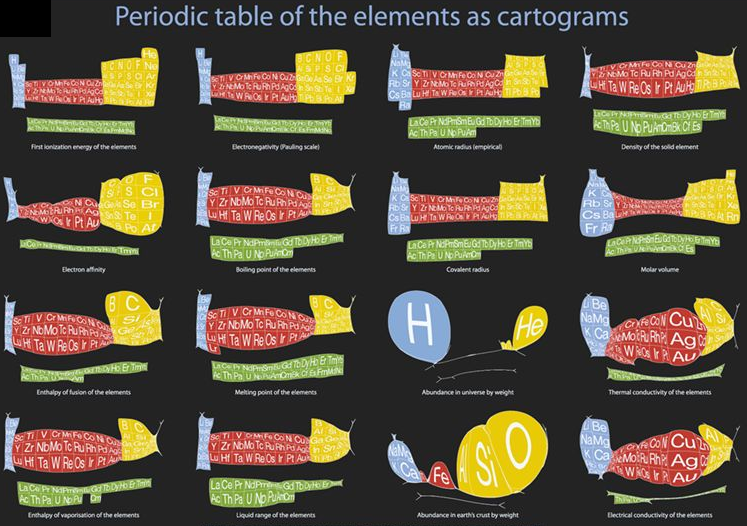
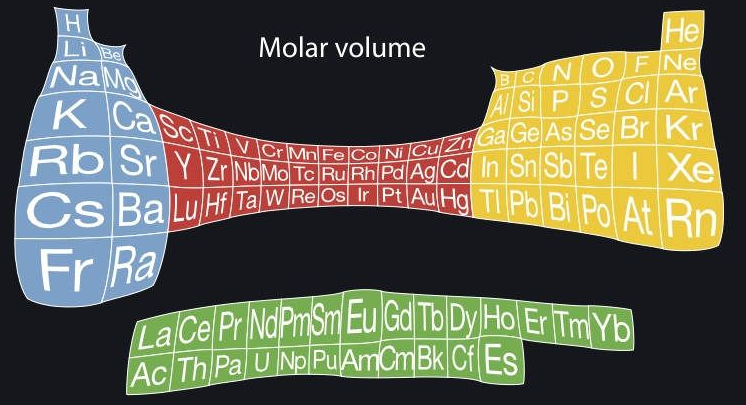
Cartogram Periodic Tables
Webelements have produced a poster with various atomic & elemental properties represented in cartographic form. From the Webelements shop:
"Periodic table cartograms are periodic table grids distorted using a computer algorithm so that the areas of the element squares are in proportion to a periodic table property. This is the first poster to show periodic properties plotted in this way".
2010
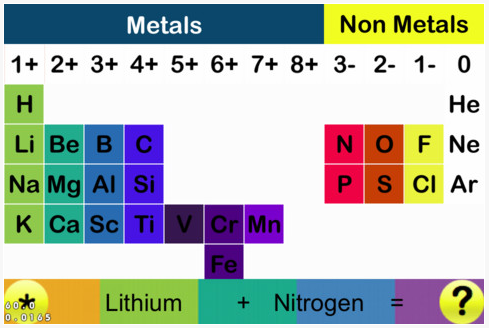
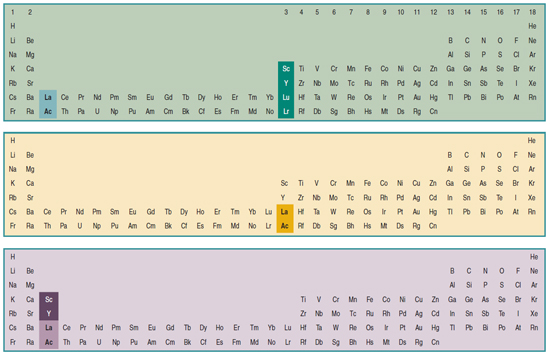
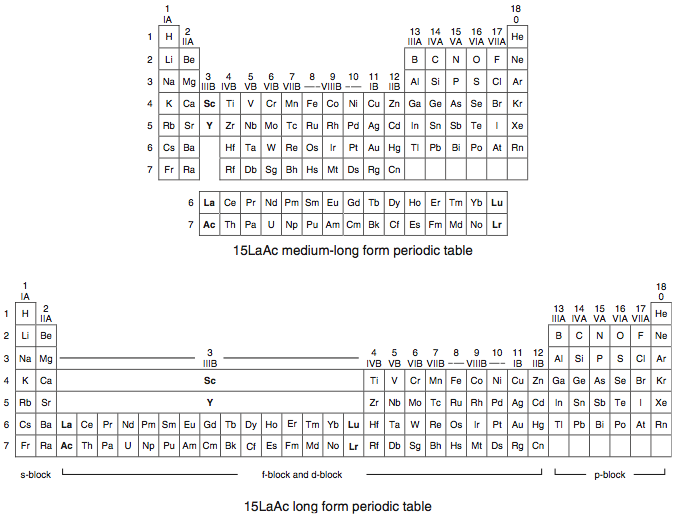
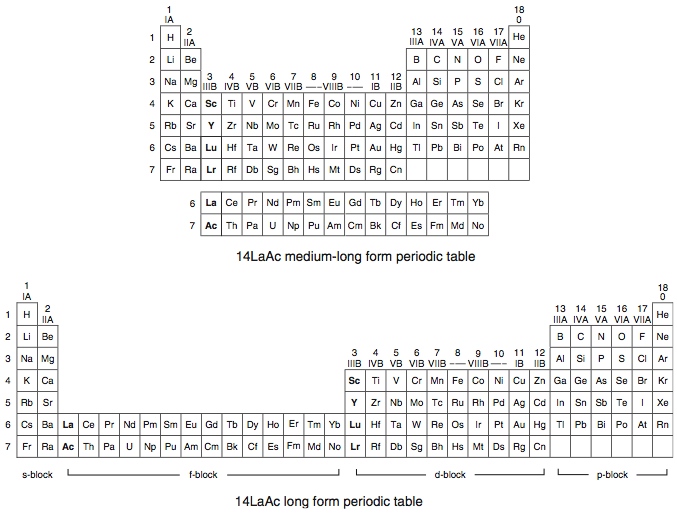
Scandium Group and The Periodic Table: Sc, Y, Lu, Lr or Sc, Y, La, Ac?
Pieter Thyssen and Koen Binnemans discuss (CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis) the confusion surrounding the members of and the positioning of the scandium group. There are three forms commonly used.
A medium-long form and long form depiction of the 15LaAc periodic table. As should be clear from the long form periodic table, an intermingling occurs between the f-block and d-block:
A medium-long form and long form depiction of the 14CeTh periodic table. The d-block has been torn apart in the long form, due to the insertion of the f-block:
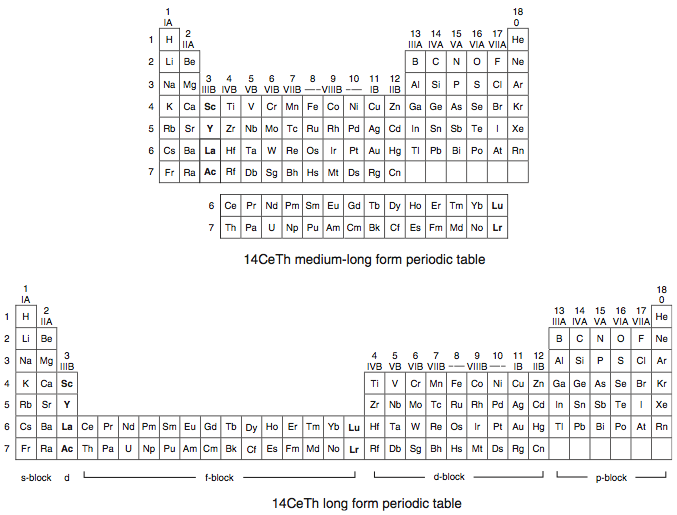
A medium-long form and long form depiction of the 14LaAc periodic table. The 14LaAc periodic table is in perfect agreement with the Madelung rule:
A recent graphic posted by Eric Scerri:
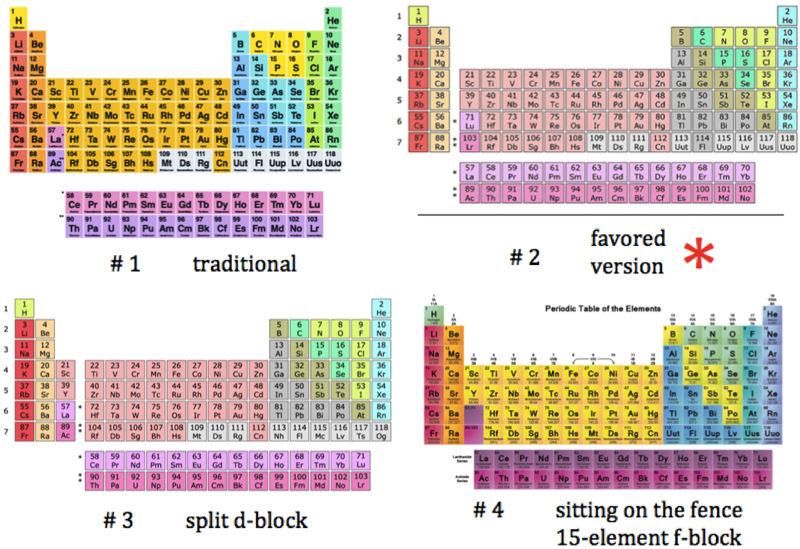
2010
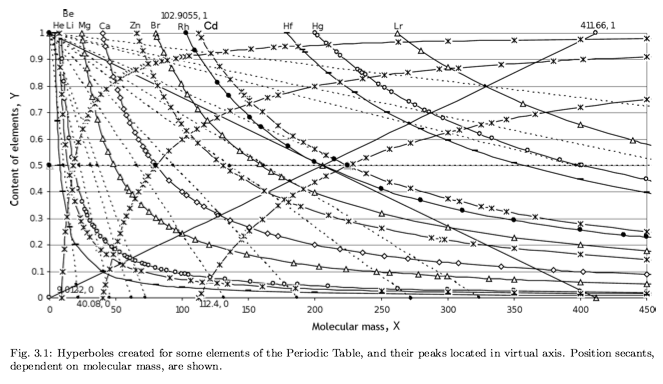
Upper Limit in Mendeleev's Periodic Table - Element No.155
This book (PDF), by Albert Khazan, represents a result of many-year theoretical research, which manifested hyperbolic law in Mendeleev's Periodic Table.
According to [Khazan's] law, an upper limit (heaviest element) exists in Mendeleev's Table, whose atomic mass is 411.66 and No.155. It is shown that the heaviest element No.155 can be a reference point in nuclear reactions. Due to symmetry of the hyperbolic law, the necessity of the Table of Anti-Elements, consisting of anti-substance, has been predicted. This manifests that the found hyperbolic law is universal, and the Periodic Table is common for elements and anti-elements.
2010
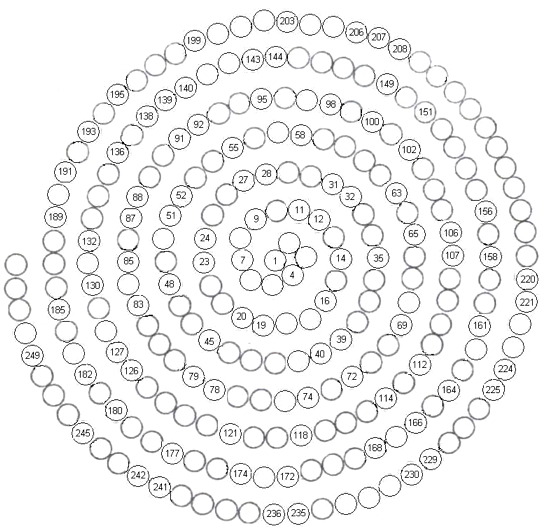
World's Smallest Periodic Table
The World's Smallest Periodic Table:
2010
Discovery of Tennessine
Ts ![]()
Tennessine, atomic number 117, has a mass of 292 au.
Synthetic radioactive element.
Tennessine was first observed in 2010 by Y. Oganessian et al.
2010
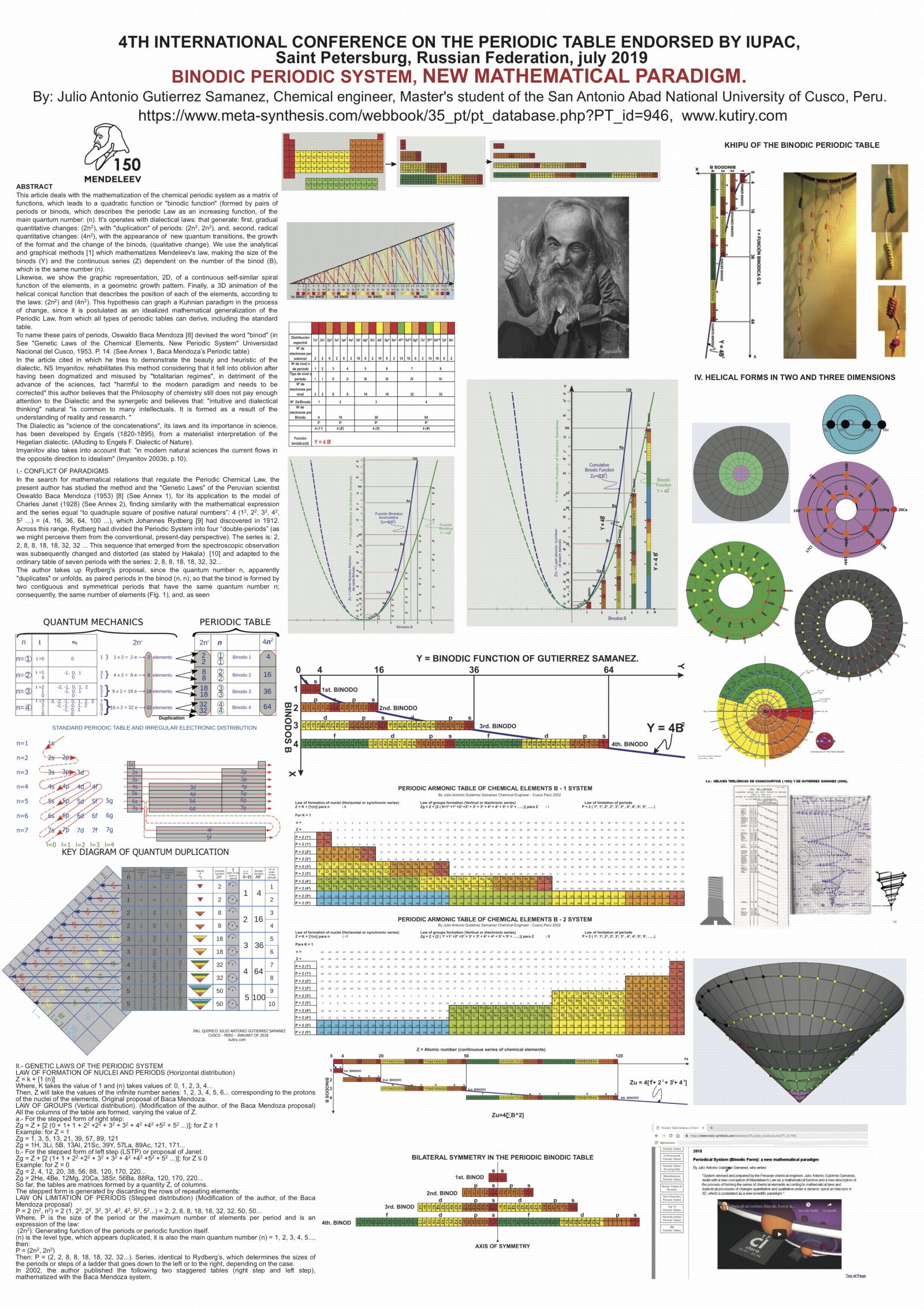
Khipu or Quipu Periodic Table
The Khipu or Quipu or Talking Knot Periodic Table, developed by Julio Antonio Gutierrez Samanez.
Google translated from the Spanish pdf file:
"As a result of bringing together each pair of periods in a single function or binod, the author has found a new regular on the subject, which has been defined as a new quantum number, since the number of orders or regulations binod growth elements in the table, under the appearance of pairs of new types of quantum structures or periods whose organization responds to a simple mathematical function: a parable of the type Y = 4 X ^ 2 - In this case report: a) That the strings correspond to pairs of periods or binod and knots are double for items with orbital s (in red), six nodes for p in orange, 10 yellow d knots and 14 knots for green f . b) That in each binod or rope, appear regularly in pairing mode or dual, new quantum or orbital structures, such as moving from within the orbital previous binod.":
2010
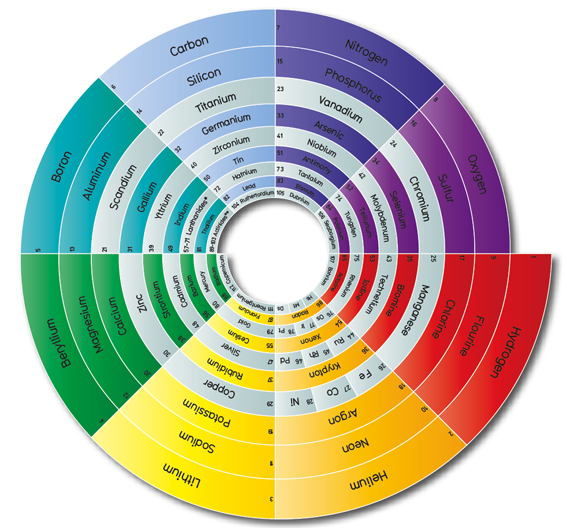
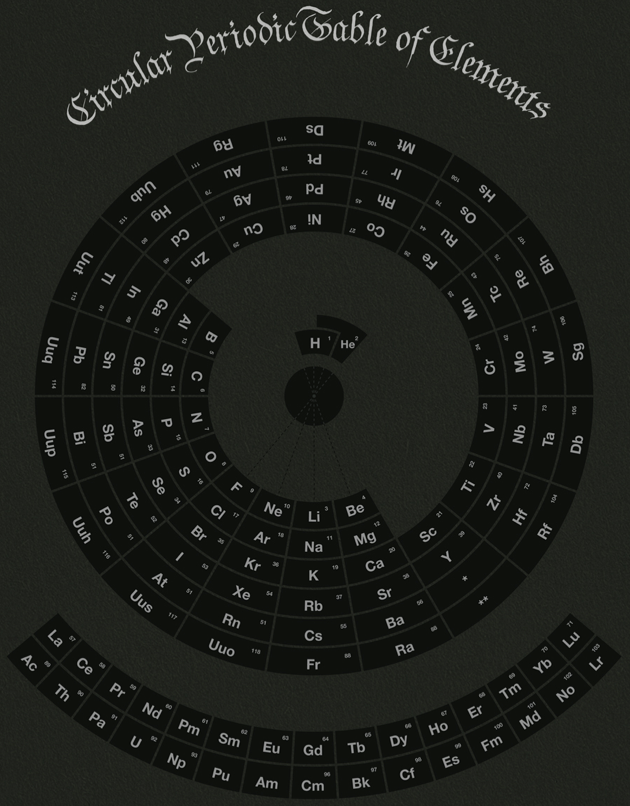
Circular Periodic Table of Elements
Michael Paukner's circular periodic table is one alternative to the standard periodic table of the elements:
2010
Newlands Revisited – Poster
At the beginning of last year (Meyers, 2009), a IUPAC editorial offered "something old, something new, something borrowed and something blue".
Marks and Marks 2010 (M&M) preserves the old subgroups (Newlands' columns) that were a feature of all short forms, although M&M would then have been described as a 'medium form' (14 groups) in contrast to Mendeleyev's 'short form' (8 groups) or Werner's 'long form' (32 groups). M&M naturally continues the grouping of the lanthanoids/actinoids whose initial four groups were also included in 'short form' tables.
The logic of the arrangement of the s-elements is a new feature. It recognizes the chemical subgroups of hydrogen, viz. the alkali metals and the halogens, and of helium, viz. the alkaline earth metals and the inert gases. It is interesting to note that subgroups differ chemically from each other inversely as the azimuth, i.e. Li:F > Ca:Zn > La:Lu.
The whole idea is, of course, borrowed from Newlands. The group numbers are borrowed from valency but also from electronic structure in that the number of s, p, d, or f subgroups corresponds to the Pauli maximum for each. Finally, the mnemonic reflects that most elementary introduction to chemistry: alkalis turn Litmus blue.
From this start, the p-bloc is red, the transition elements yellow and the "rare earth" elements green, as argued in the M&M paper. The numbering of groups I - XIV is unambiguous, it is less than IUPAC's arbitrary 18 groups, it preserves subgroups and satisfactorily accommodates hydrogen and the lanthanoids/actinoids.
As required by Leigh (2009), this table is clear, simple and brief.
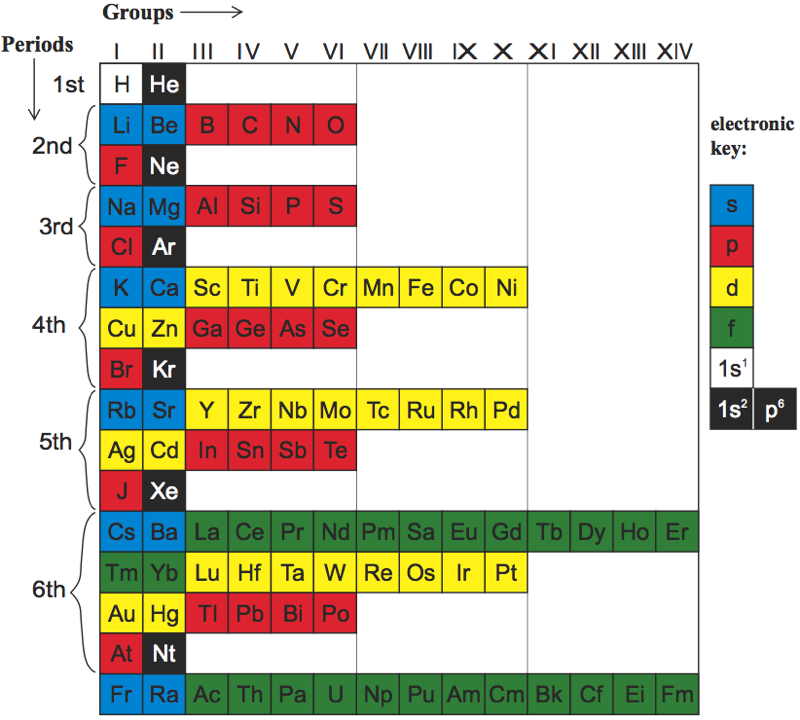
GJ Leigh "Periodic Tables and IUPAC" Chemistry International 2009, 31: 4-6. EG Marks & JA Marks "Newlands Revisited: a periodic table for chemists" Foundations of Chemistry 2010, 12: 85-93. F Meyers "From the Editor" Chemistry International 2009, 31:1-2.
2010
Schwarz & Rich's Periodic Table
W. H. Eugen Schwarz & Ronald L. Rich, Theoretical Basis and Correct Explanation of the Periodic System: Review and Update, J. Chem. Educ. 2010, 87, 4, 435-443. DOI: https://doi.org/10.1021/ed800124m
Periodic table, representing some aspects of the periodic system of chemical elements (mainly to support the discussions in [the attached] article, perhaps not for the classroom):
- Element symbol and element number Z = 0 – 118
- Period number n
- Group number G, related to the number of valence electrons
- Typical electronic valence configuration of the bound atoms ("neighbour configurations" such as s1p3 instead of s2p2 may be more important for some atoms in the group)
- Characteristic valence orbitals of highest angular momentum
- Chemical group name
- (Elements that do not belong to the group are put in parentheses.)
- The dashed lines indicate alternative or controversial group assignments, they are not meant to represent the authors' views.
Note that the richness of chemistry sometimes prevents clear-cut classifications and assignments.
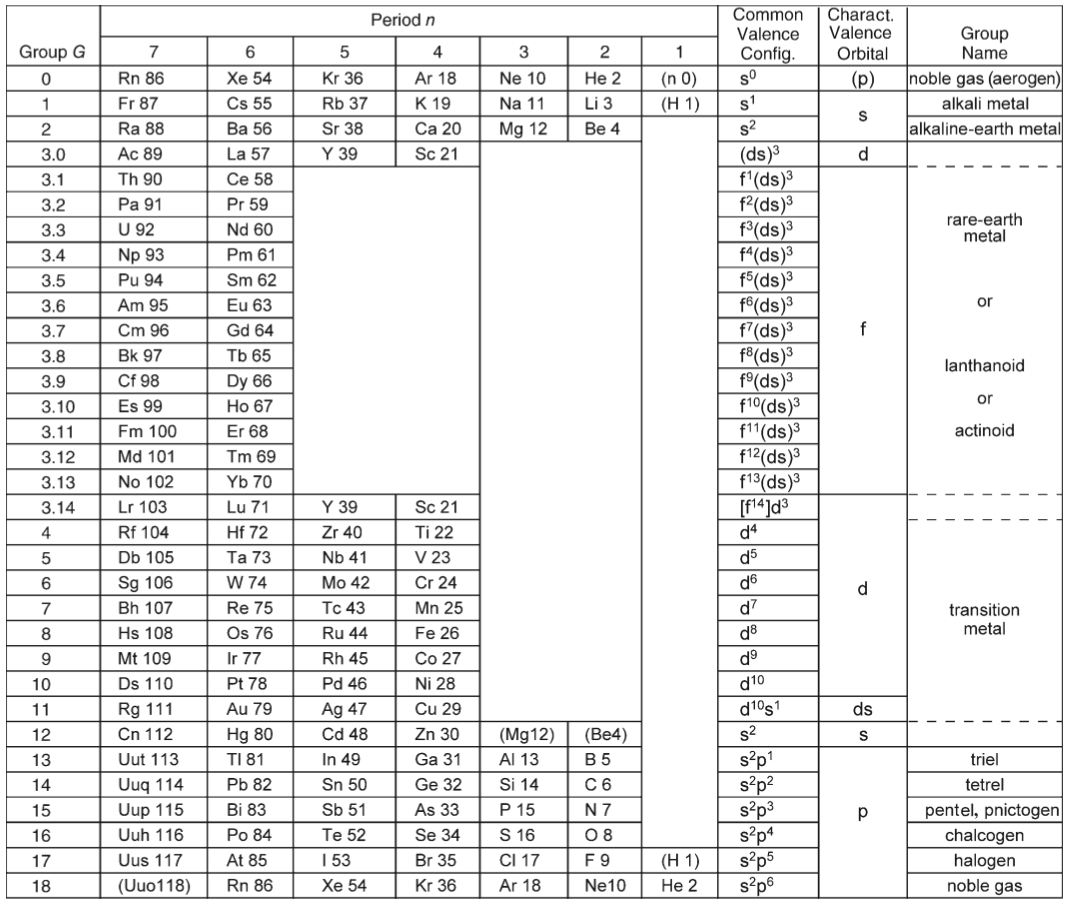
2010
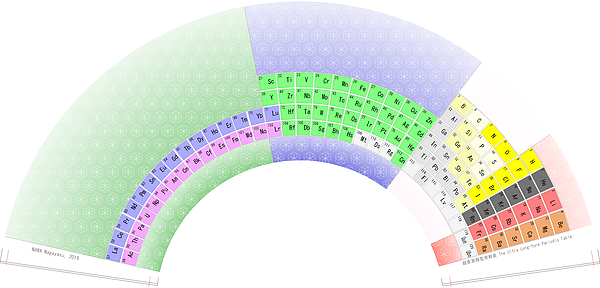
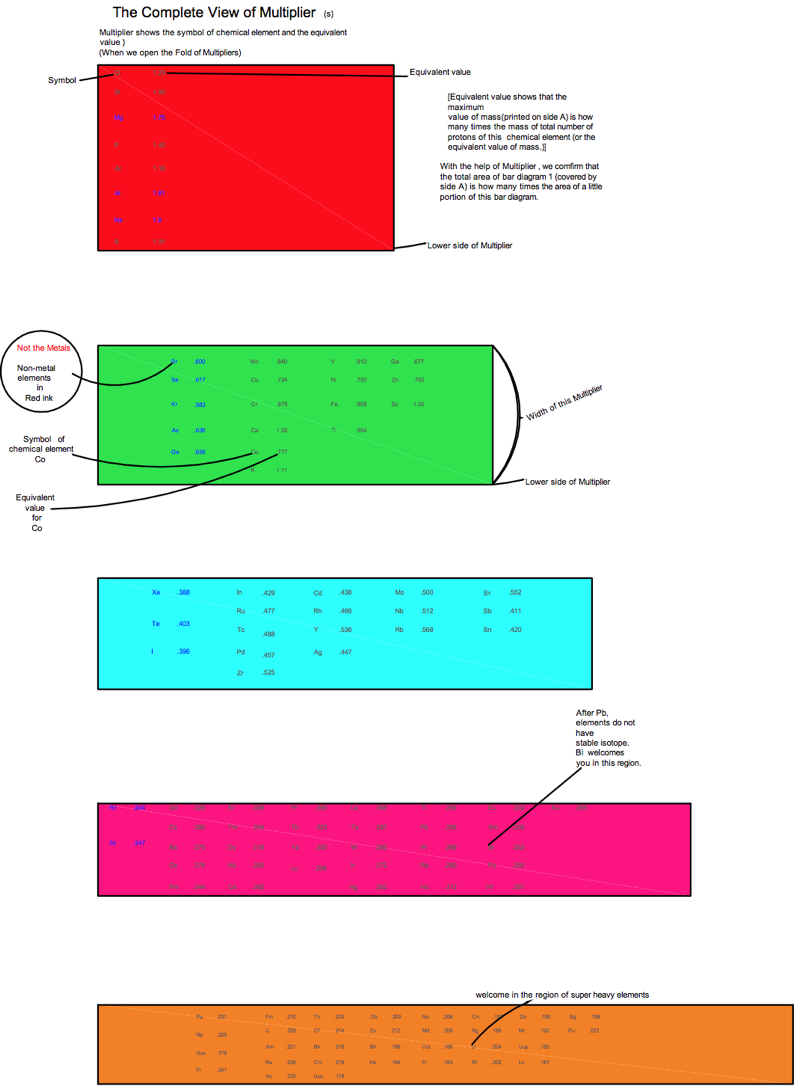
Harrington Projection for The 270 AMU Structure
From Bill Harrington, Founder/CTO of Rainforest Reactor Research and Temporal Dynamics Laboratory, comes a Harrington Projection for The 270 AMU Structure :
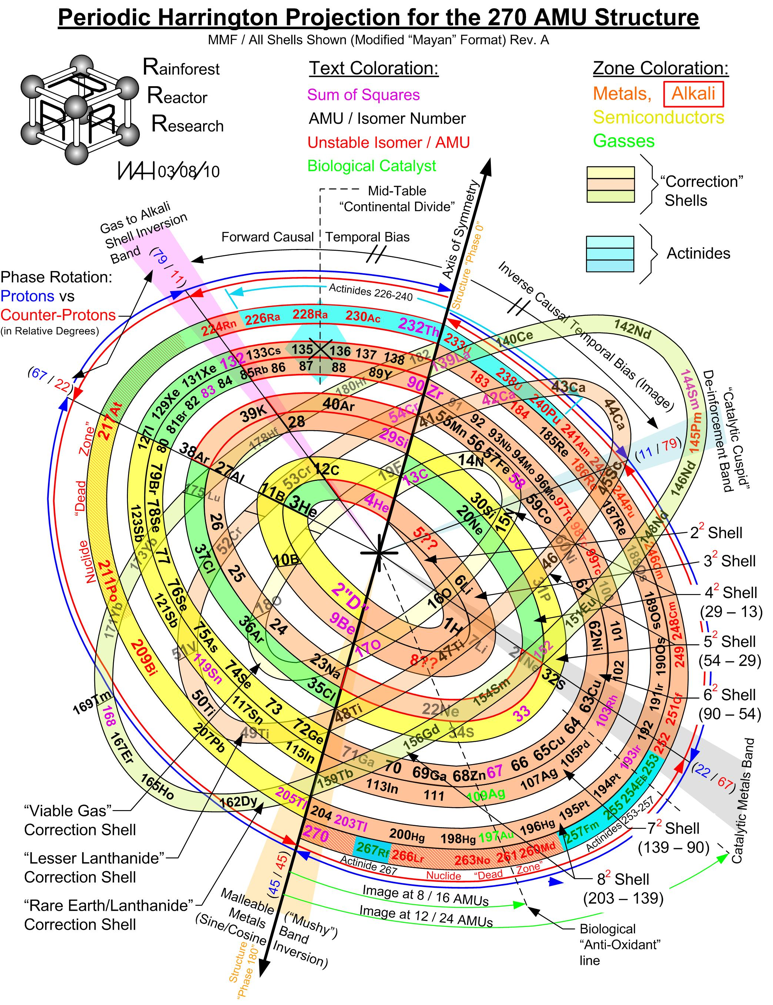
2010
Neutronic Schema of the Elements
The Neutronic Schema of the Elements, with LATIN NOTATION by Families and Groups, by Earth/matriX, Science Today, 11" x 17" laminated, color, shows each element of the periodic table with its notation in Latin letters instead of their historically accidental names and symbols:
2011
Weise's Tetrahedron
Dmitry Weise shows how it is possible to go from the Janet [left-step] periodic table formulation, to a tetrahedral formulation.
Dmitry writes:
"Three-dimensional table of the periodic law can be constructed in the form of a tetrahedron having an inner order. A comparison of the tetrahedron shells and the table of elements shows, that one tetrahedron shell corresponds to 4 periods of the 2D table."
Jess Tauber adds:
"The spheres here also aren't labeled, but I explain how they get labeled in the text accompanying the pic. Each such period (except for s-only, which are obviously simpler) we have a 'switchback' configuration. Like a road going up a mountain back and forth to minimize verticality, or a parachute folded into a pack. There are 8 different ways to do this (4 basic types in 2 chirally opposite mappings). And the original Weise-style non-continuous tetrahedron is just another way to organize half tetrahedra."
2011
Tresvyatskii's Periodic Table
Powder Metallurgy and Metal Ceramics, Vol. 49, Nos. 9-10, 2011:
The paper published below represents Tresvyatskii's fundamental study. It establishes the interrelation between the ionization potential and place of an element in the periodic table. Oxides with a certain composition may form only when an element is ionized to the needed degree. Hence, the ionization potential of elements is an important parameter that governs the formation of an oxide. In this regard, the dependence of the ionization potential on the place of an element in the periodic table is of paramount importance. The role of the ionization potential in the hightemperature chemistry of oxide compounds, which underlies modern oxide materials science, is especially significant. The paper is published in Tresvyatskii's original version.
René Vernon adds:
A depiction of the short-form table, showing some clever thinking:
- The reversal in atomic number order of Np to Am
- The return of the curides
- The placement of the Ln and the curides alongside the main table
- The assignment of the Ln and An to groups
- Triple periodicity among the Ln and heavy An
2011
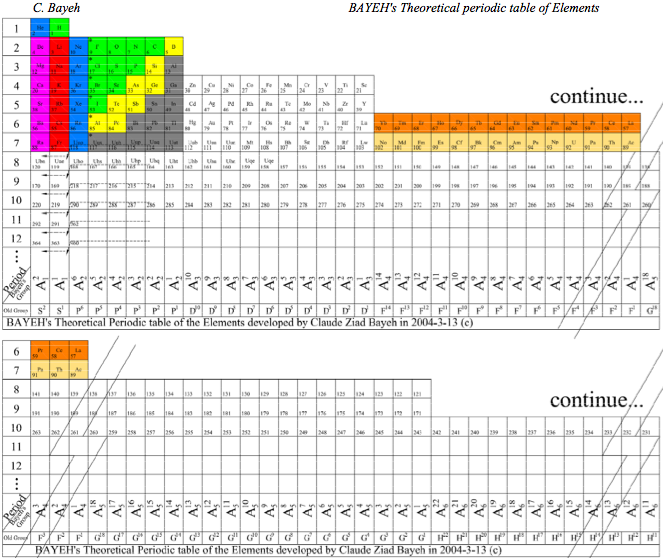
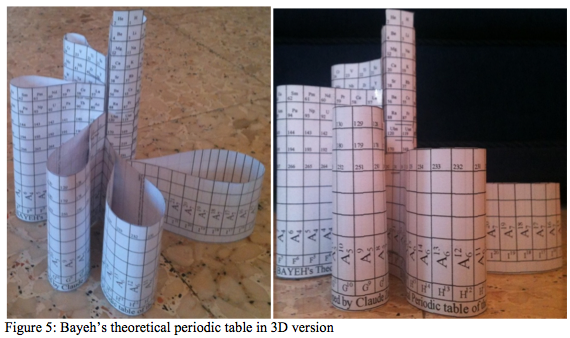
Bayeh's Theoretical Periodic Table of Elements
"The modern periodic table is based on quantum numbers and blocks, many problems faced the scientists and researchers when arranging the elements in the traditional and modern periodic tables as placing some elements in the incorrect place as (He) Helium, (La) Lanthanide and many others elements..." read the full pdf article here:
2011
Bayeh's Theoretical 3D Periodic Tables
From Bayeh Claude: "I have designed these periodic tables as developments of Bayeh's Theoretical Periodic Table, but I have introduced new shapes and 3D versions":
- Crocodile Periodic Table
- Ship Periodic Table
- Snake Periodic Table
- Spiral Periodic Table
- Spiral rectangular Periodic Table
- Spiral triangular Periodic Table
2011
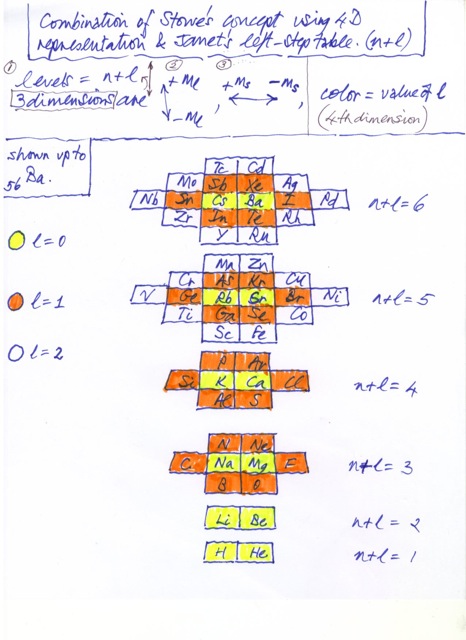
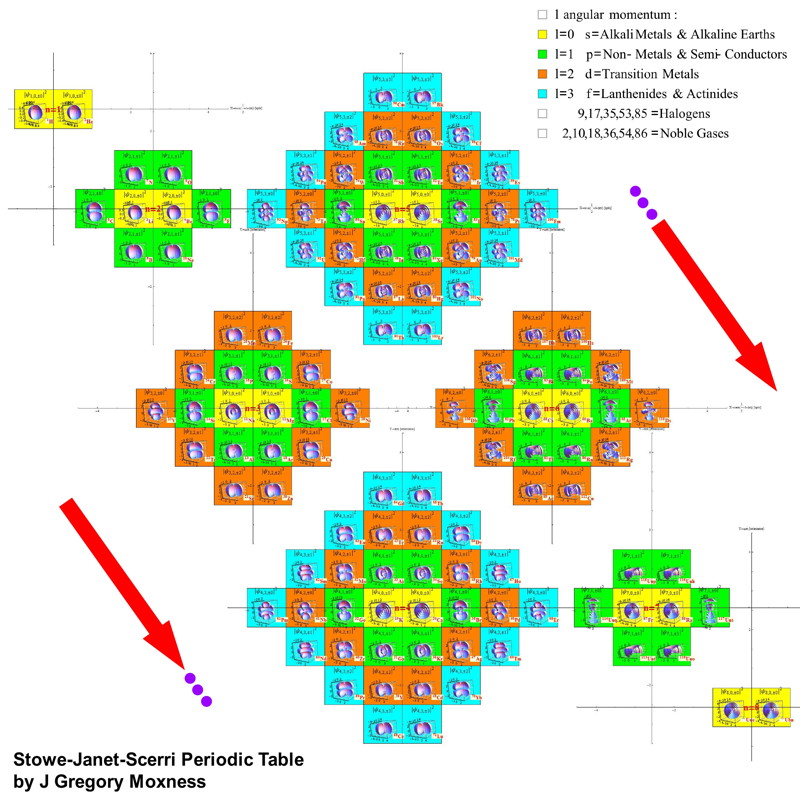
Stowe-Janet-Scerri Periodic Table
Eric Scerri made contact, writing: "Following the discussions on Periodic Table debate on the Chemistry Views website here, and as a result of recent turns, I have developed a new periodic table which I believe combines virtues of the Stowe table and also the Janet left-step table. I propose the name Stowe-Janet-Scerri Periodic Table. The explanation is posted on the Chemistry Views debate pages.
2011
Aldersley 3D Periodic Table
A Three Dimensional Periodic Table by Michael F. Aldersley, U.S. Patent 7,938,646 2B
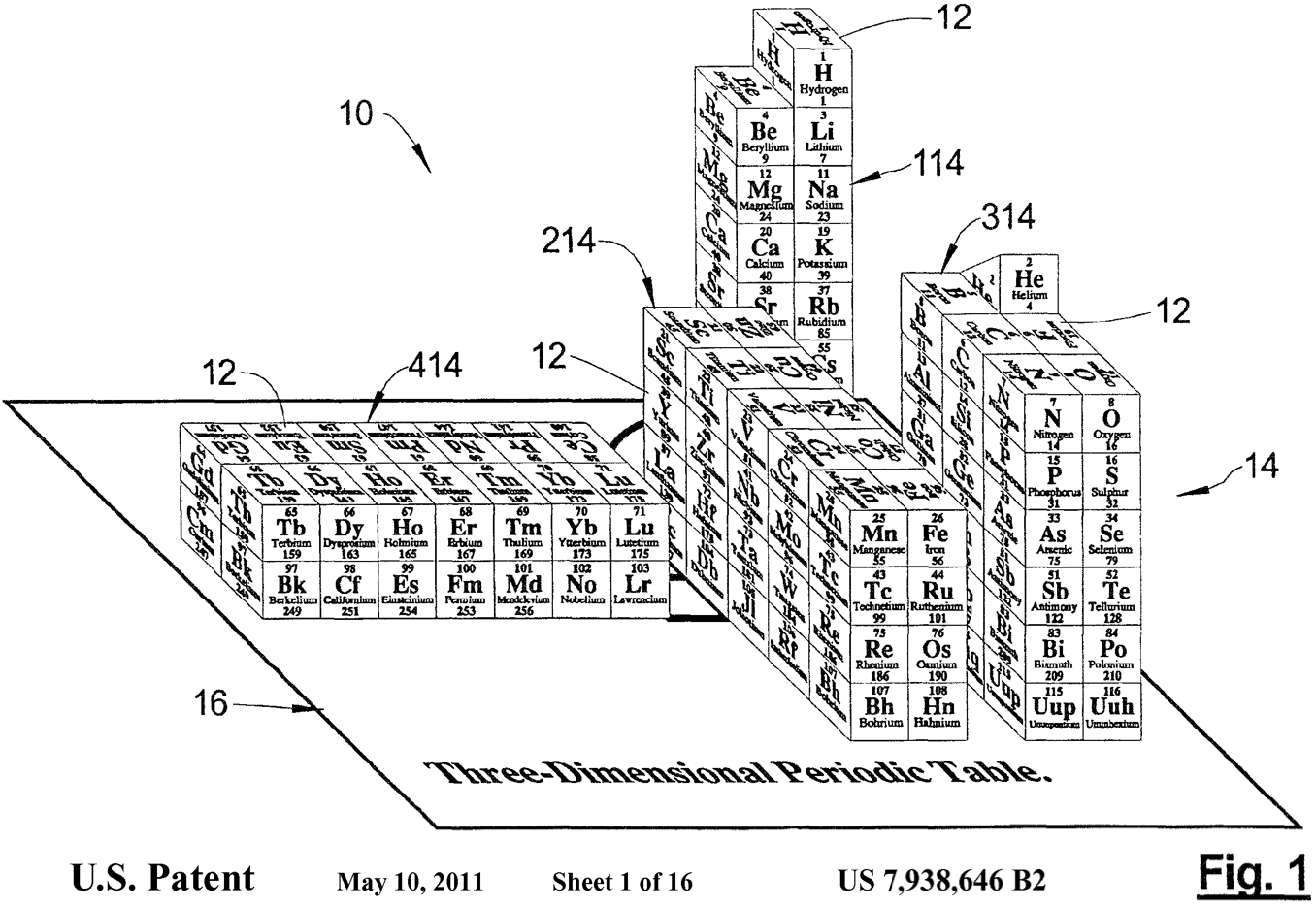
2011
Piazzalunga's Circular Periodic Table
"My name is Marco Piazzalunga, I'm from Bergamo, Italy and i'm 12 years old. I am very interested about chemistry and about your website dedicated to the periodic tables of elements. I've made one graphic version of the periodic table based on a "round" model and i would like to know your opinion about it. I'm sending you the file attached. I hope you enjoy it":
2011
Pacholek's Multipipe 3D Periodic Table
"I've recently invented a new type of periodic table. My table is 3-dimensional and is similar to the ADOMAH Periodic Table, but it's also very different from the ADOMAH Tetrahedron. Its main advantage is being fully geometric in the plane spanned by n, l and n+l quantum numbers."
Take a look at the Picasa images here and here:

2011
Alper's Quantum Table of The Elements
Ben Alper's Quantum Table of The Elements is a simplified periodic table which shows the elements are ordered by the energy level of their sub shells and by the number of electrons in their outer shell. Such a layout is both representative of the structure of atoms and has utility since it is easy to use.
2011
Makeyev's Periodic Table
By Alexander Makeyev – integrated interdisciplinary researcher, inventor, poet – a long pdf document (1093 pages in Russian, here) that contains a new formulation:
2011
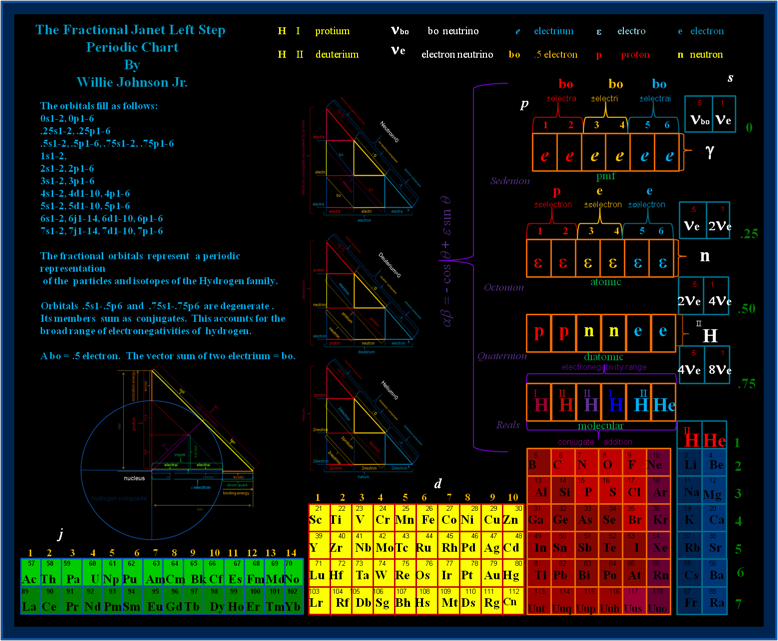
Fractional Janet Left-Step Periodic Chart
On Willie Johnson Jr.'s website – Gyroscopic Force Theory – can be found the Fractional Janet Left-Step Periodic Chart:
2011
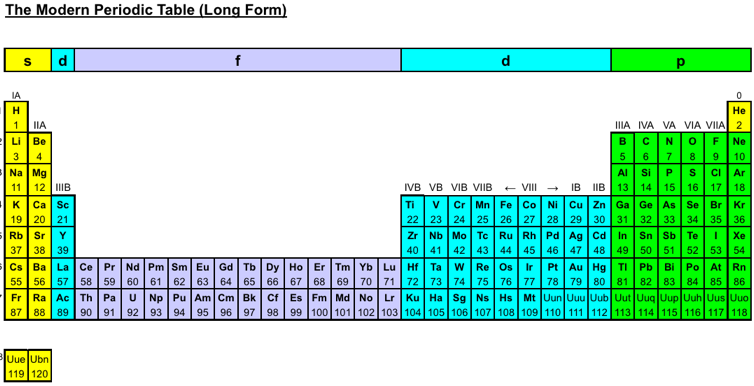
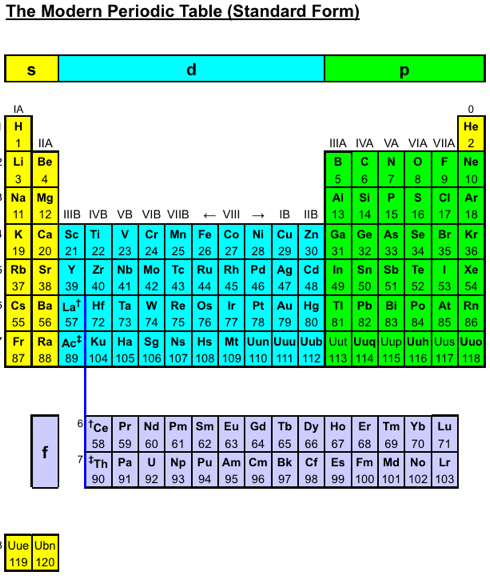
Wikipedia Long Form Periodic Table
Wikipedia has now adopted a now adopted a long form periodic table to link between the chemical elements. Scroll to the bottom of this page:
2011
Alashvili Rotating Spherical Periodikal Tabel
A nice rotating, spherical (3-D) periodic table by Tornike Alashvili, from Georgia, which can be viewed here as a .swf image:
2011
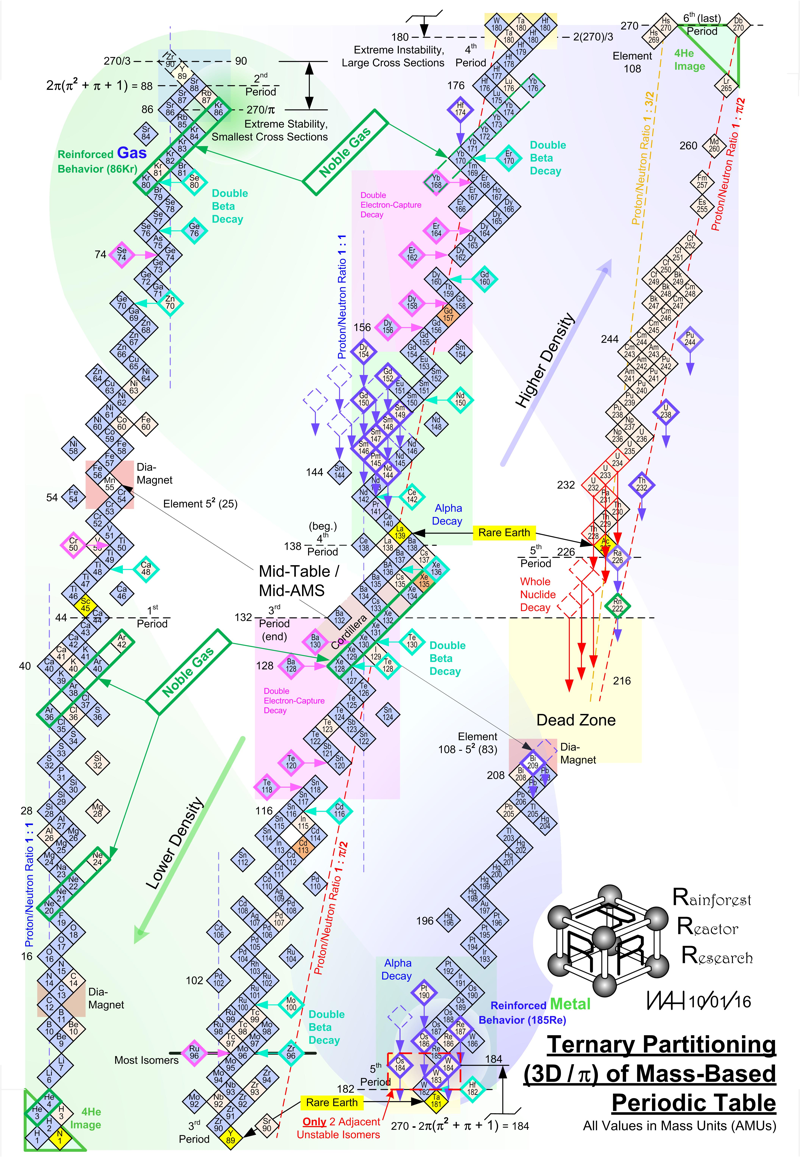
Normal vs Correction Shell "Pi Paradox" for 1-270 AMUs
From Bill Harrington, Founder/CTO of Rainforest Reactor Research and Temporal Dynamics Laboratory, comes a Normal vs Correction Shell "Pi Paradox" for 1-270 AMUs:
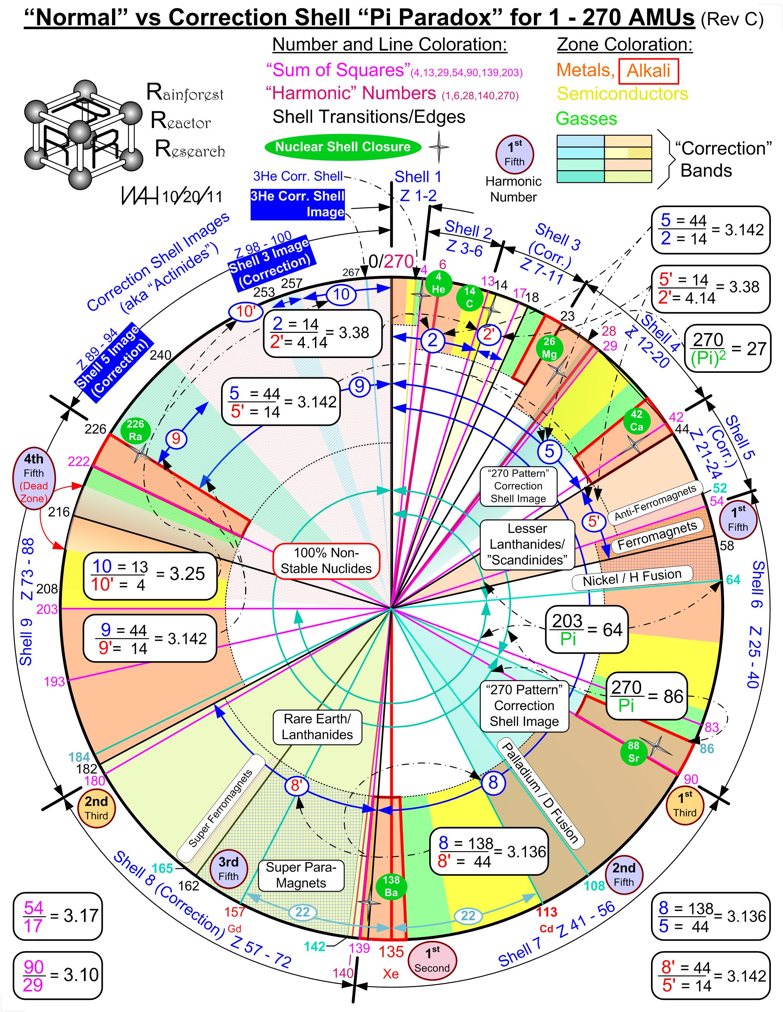
2011
Elements Known in the Year 2011
Elements known in the year 2011, taken from this Wikipedia page... all the elements to 118 are now known:
2011
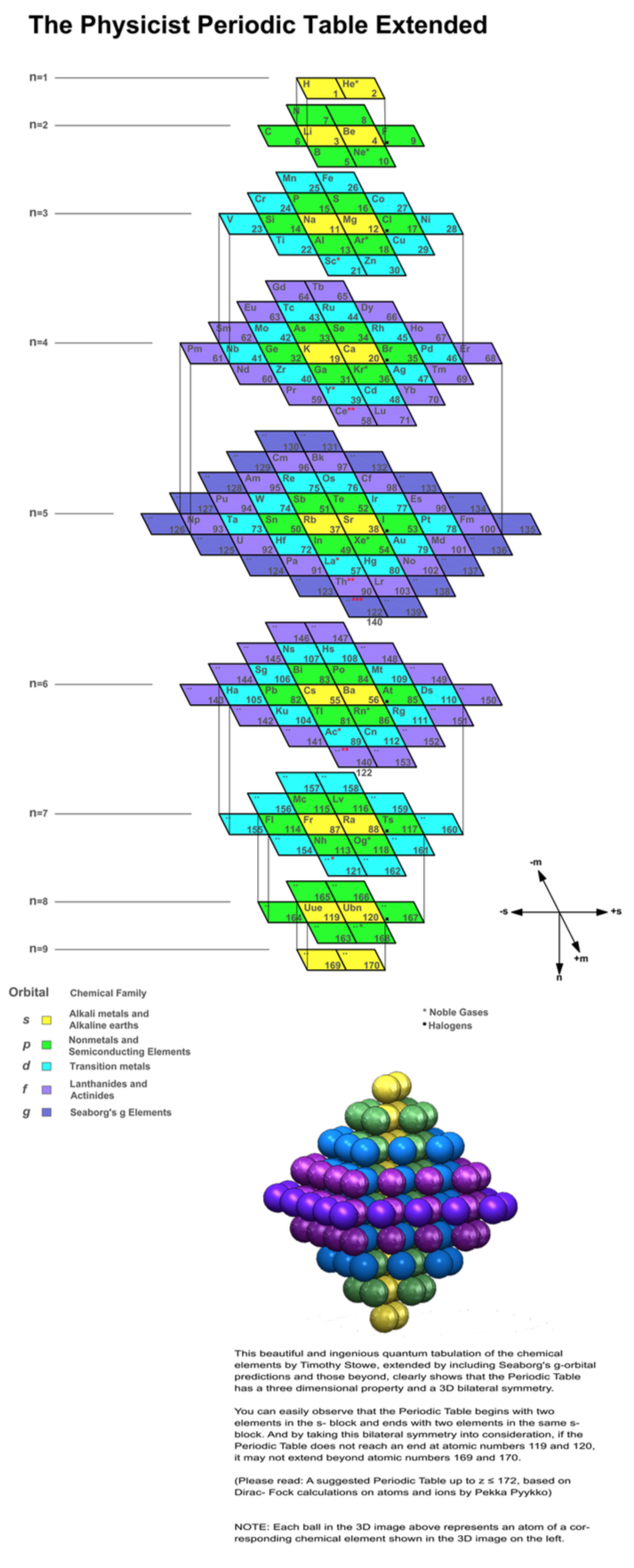
Suggested Periodic Table Up To Z ≤ 172, Based on Dirac–Fock Calculations
A suggested periodic table up to Z ≤ 172, based on Dirac-Fock calculations on atoms and ions
Pekka Pyykkö
Phys. Chem. Chem. Phys., 2011,13, 161-168
DOI: 10.1039/C0CP01575J
Extended Average Level (EAL) Dirac–Fock calculations on atoms and ions agree with earlier work in that a rough shell-filling order for the elements.
[This new] Periodic Table develops further that of Fricke, Greiner and Waber [Theor. Chim. Acta 1971, 21, 235] by formally assigning the elements 121–164 to (nlj) slots on the basis of the electron configurations of their ions. Simple estimates are made for likely maximum oxidation states, i, of these elements M in their MXi compounds:
2012
Compact Mendeleev-Moseley-Seaborg Periodic Table (CMMSPT)
A Compact Mendeleev-Moseley-Seaborg Periodic Table (CMMSPT).
This table can be found by two different ways:
- Via MMSPT - All terms of the MMSPT are shifted to the right side without spaces.
- Via Janet Periodic Table - The first row of the Janet PT is deleted. - We remove 2 from all others 118 terms.
These 2 transformations lead to the same table, with 7 rows and 32 columns. Blocks p (green), d (light grey), and f (light orange) are preserved.
The 14 terms of the s block (dark orange/red) are splited in "cascads".
This table can be seen in the A173592 sequence in the On-line Encyclopedia of Integer Sequences (OEIS). Row differences are 8, 8, 18, 18, 32, 32.
2012
Srivaths–Labarca Periodic Table
This is an improved version of the Zigzag Periodic Table (2012). In this new arrangement the main criteria proposed to settle the placement of the elements hydrogen and helium has been taken into account: electronic configurations, the number of electrons needed to fill the outer-shell, chemical behavior, and triads of atomic number.
This is a new categorial criterion recently proposed by Eric Scerri, according to which hydrogen and helium form part of the triads H(1), F(9), Cl(17) and He(2), Ne(10), Ar(18), respectively. Thus, hydrogen preserves its place between alkali metals and halogen while helium is now in between noble gases and alkaline earth elements.
This periodic table allows visualizing easily the relationships of hydrogen and of helium with the different criteria, avoiding drawing lines to see them in contrast to other similar periodic systems.
Akash Srivaths, Chennai, India
Martín Labarca, CONICET & National University of Quilmes, Argentina
2012
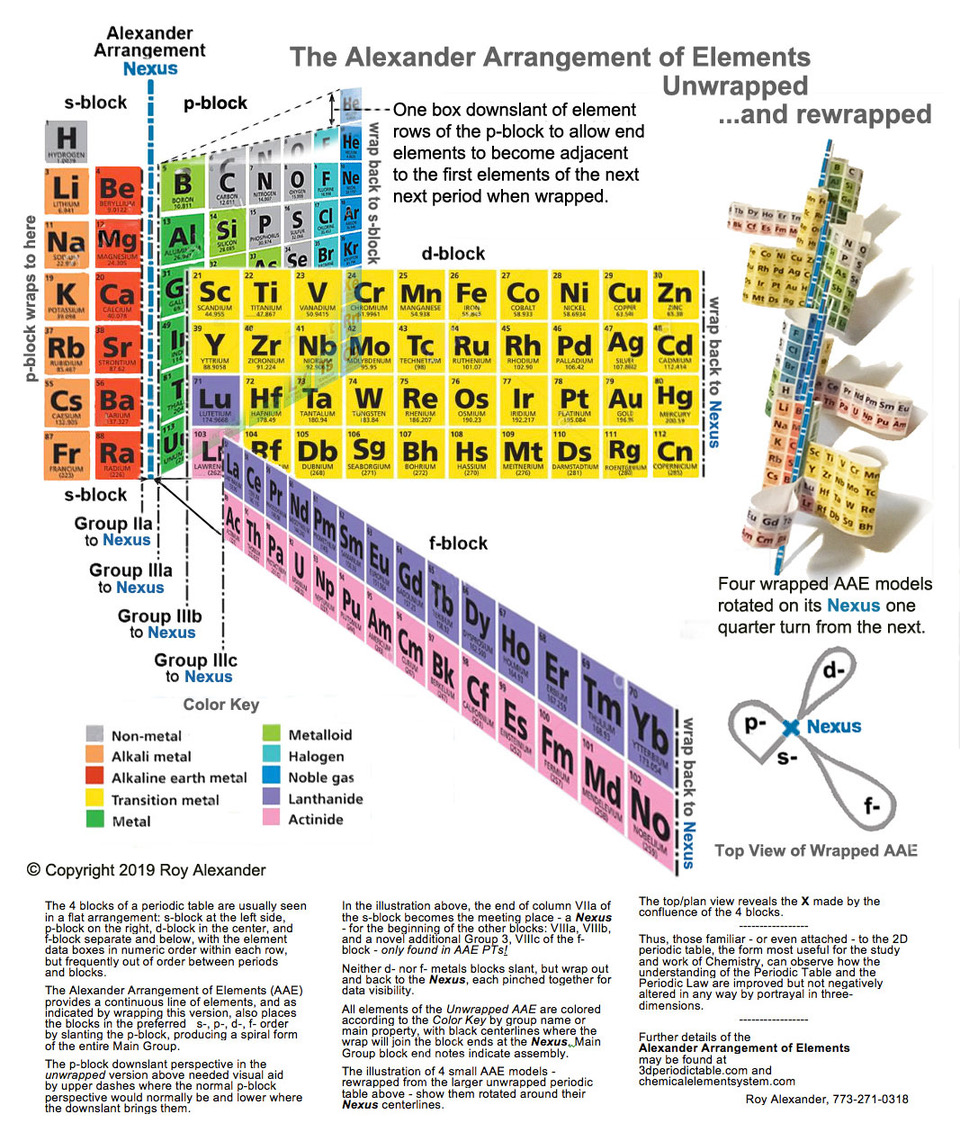
Alexander Arrangement of Elements, 3D Illustrated
The design of the 2012 Alexander Arrangement of Elements (AAE) follows the principles of a three-dimensional model developed by Roy Alexander in 1965: a printed representation of element information based on strict adherence to the Periodic Law, with every element data box physically and visually contiguous and continuous within the sequence of atomic numbers in generally accepted element property related columns - "...the periodic table the way it's supposed to be".
This is made possible by wrapping, folding, and joining the printed material and employing the patented p-block downslant of the element data boxes to allow the end element of a period to be adjacent to the first element of the next period.

Several unique features separate it from the previous four versions of the AAE
- The visual effect mirrors the look of Theodore Gray's series of posters, books, element cards and periodictable.com website and apps for the Apple iPod and iPad.
- Each element box is dominated by a Theodore Gray element photograph, with the element name, letter symbol, and atomic number relatively large, often overlapping the photo.
- The period numbers (below, right) are printed at the interface of the end/beginning of the periods, folded 90 degrees on the model, and the blocks and columns (old & new numbers), are identified below the data boxes - and in the case of the Actinoids, above.

- The element blocks connect at a central nexus (below, center), with the d- and f-blocks leaving, looping, and returning there, thus allowing the shorter period gaps above to be closed. For best visibility of the element data, these loops pinch together near the intersection. The p-block bends in a half-circle to join the s-block at the corner described above, with a patented 'downslant' where the element boxes gracefully sweep down a full box height (above) within this block to allow elimination of the "carriage return" effect: each period ending on the row above the next.

- The extended Hydrogen data box, a characteristic of all Alexander Arrangements, is more extended in this model, reaching for the multiple positions of the H box that are still under discussion among experts. The extra-extended Hydrogen box, illustrated by a composite image of a hydrogen cloud in space, (above, right) loops over the s- and p-blocks. Starting up from behind the corner of Helium & Lithium, inside the half-helical tube to loop over Helium, attach above Lithium, Beryllium and then Carbon as the loop descends (joining the ascending portion) over the data boxes of the s- & p-blocks, terminating in contact with Fluorine, Neon, and corner-on to Neon.
- The model size is the same as the previous Display Version of the AAE, but has fewer element data boxes, due to there being no photos of the lab created elements and for simplification of the educational application - introduction to property periodicity and organization of element data - the elements with atomic numbers over 94 are not included (see addendum).
- Where the f-block begins and ends, between Barium and Lutetium, the f-block is held perpendicular to the only flat segment of the element display by a pair of triangular braces, which also create the flat area, aligning the s-block with the 'pinch' of the d-block. This is particularly apparent from the bottom, when the model is supported from above. (see below)

Designed by Roy Alexander, a science museum exhibit and teaching aid designer, the Adobe Illustrator art for the model was started by Ann Grafelman, and continued by Roy from mid 2011 through November of 2012.
Photos were provided by Theodore Gray, and Element Collection funded the printing and die cutting performed by Strine Printing in York, Pennsylvania. The model kit was first offered at Theo's PeriodicTable.com, then at Roy's AllPeriodicTables.com and the new 3dPeriodicTable.com, which site is dedicated to the 3D Forever Periodic Table only, with add-ons, application suggestions, and descriptions and commentary of all sorts.
Assembly instructions and step photos, as well as a number or completed model color photographs are included with the kit. These were developed with prototype models, and while functional, have been upgraded and accompanied by an assembly video at AlexanderArrangementOfElements.com/3D
Addendum:
Text relating to the abbreviation of the ever increasing number of elements is explained at two places on the 3D AAE illustrated periodic table model kit. One will remain with the model and one is removed at the time of assembly.
That which remains runs under the Actinoids and the d-block elements, where the lab created elements might ordinarily be expected to be found, says:
The lab created elements ordinarily found in this part of a periodic table are not to be found in nature, there can be no photographs of them, so nothing needs to be added to this element photo periodic table - ever - so it will never be obsolete, a Forever Periodic Table.
That which is removed says:
Naturally-occurring elements have been numbered variously, generally between 80 and 96, all for cogent scientific reasons.
For easier teaching and learning, we have included on this periodic table only the 92 elements actually currently existing on Earth and in the remainder of the Universe, and adding Technetium and Promethium, which, although they may have no stable forms, serve to fill what would otherwise be gaps in the sequence.
Not added for practical and educational reasons are 'elements' consisting only of pages and pages of computer data from smashing atoms in particle accelerators. Another reason is that there can be no photographs of them to show, and as a result, your arrangement is complete and never be obsolete - your Forever Periodic Table.
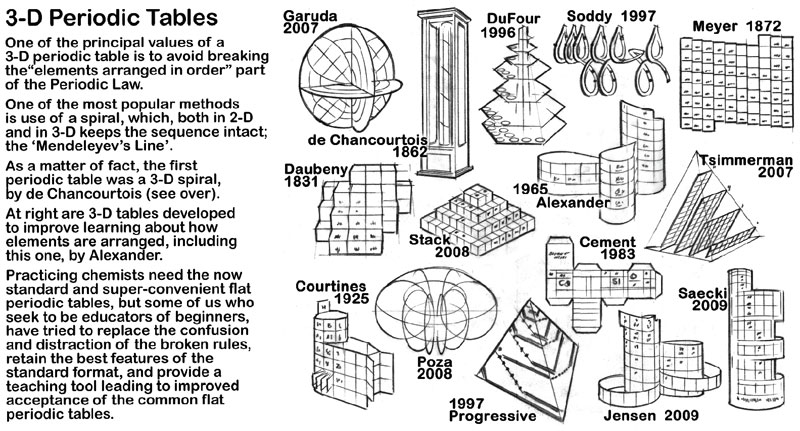
Included with the art of the periodic table on the die cut substrate that makes up the model is some background information about the the history of three dimensional periodic tables.
The first of these is about the discoverer of the concept of arranging the elements in periods suggested by the properties of the elements, de Chancourtois.

The second 3D periodic table information piece (on the rear of the de Chancourtois removable card) are sketches of a number of the 3D periodic tables found on the Chemogenesis website.
2012
Vortic Periodic Table in Marquetry
From Dr David Robson:
"My vortic periodic table created in marquetry may be of interest. I have always thought of vortic energies and with retirement time, I used my Marquetry Hobby to so create. Despite the inevitable Black Hole centre I have included the Higgs Boson there as a tribute to its discovery and potential as a window to elsewhere."
2012
Makeyev's Verticle Form Periodic Table
A new version of the periodic table of elements on the vertical table form. Alexander K. Makeyev, a member of the Moscow Society of Naturalists, section of planetonautics; freelance interdisciplinary researcher and inventor, knowall@list.ru.
1. Makeyev A.K. Normal and pathological anatomy and physiology of the human person and society. Fundamental knowledge about the qualities of the human person, human society and the software company, produces and acts of people, based on the universal algorithm of holographic structure and function at all levels and forms of matter. / / Scientific and Technical Library. July 25, 2012. 364 p, here
2. Makeyev A.K. Particles of electrostatic and magnetic fields in the system of matter photons move faster than a photon moves himself. / / The scientific debate: Proceedings IV International Correspondence scientific conference. Part I. (20 August 2012) - Moscow:. "International Centre for Science and Education", 2012. 142., S. 47-65. ISBN 978-5-905945-37-3 UDC 08. BBK 94. H 34, here:
2012
Wheelshaped Table of Elements
From Facebook, a Wheelshaped table of elements.
Please note the symmetry of this representation.
As a result, it is possible that element 118 is the very last one in the periodic table. We have the sequence:
2 x 14 (blue)
4 x 10 (brown)
6 x 6 (violet)
8 x 2 (green)
and, logically, neither first nor last factor can be 0 or -2 (they differ in two columns above respectively by 2 and 4).
On the other hand, the coherence of the structure requires the existence of two additional elements at the beginning!
2012
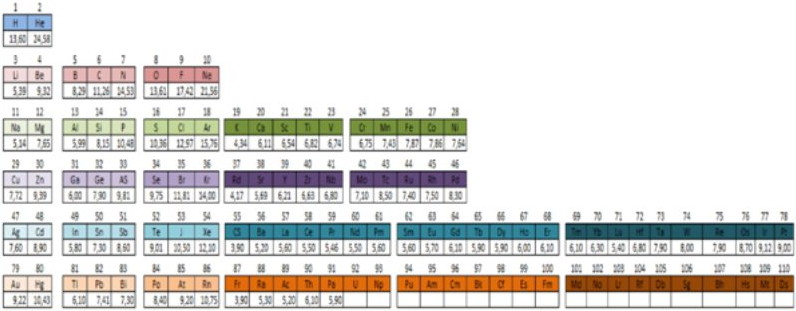
Bettermann Periodic Table
In the course of my enquiries regarding the peridoc table of the elements your comprehensive and interesting collection of the varying configuration of the elements caught my eye. Responding to a growing interest, I worked through all models but couldn't find any configuration which agrees with mine.
Find my configuration for the elements in the figure below. Please open the attachment in which you find an explanatory statement for the illustrated principle, pdf file here. It bases upon the Moseleysches diagrams and the work of Eugenie Lisitzin from the thirties of the last century.
Heiner Bettermann
2012
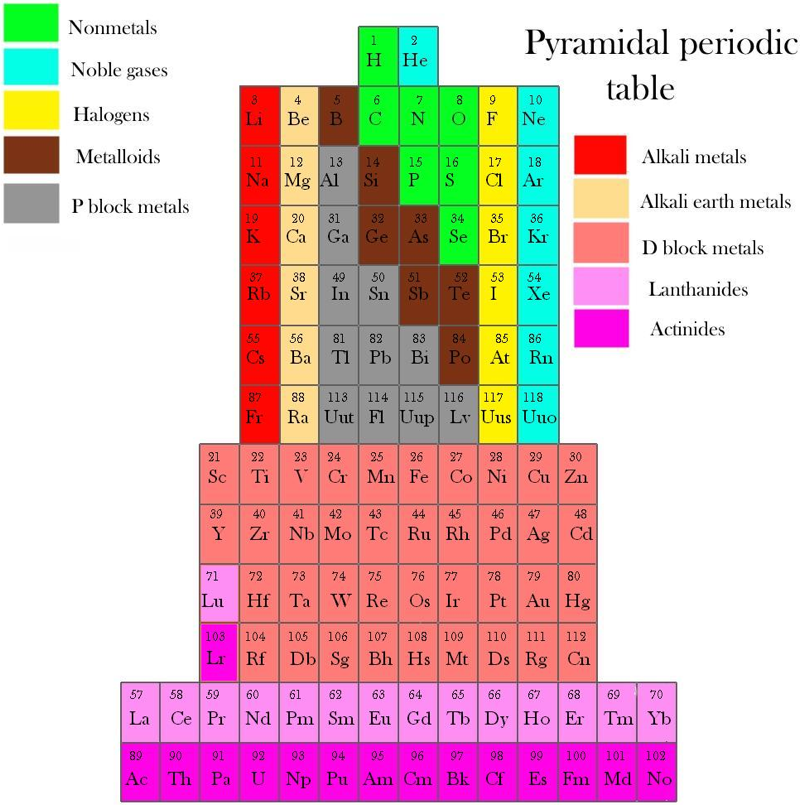
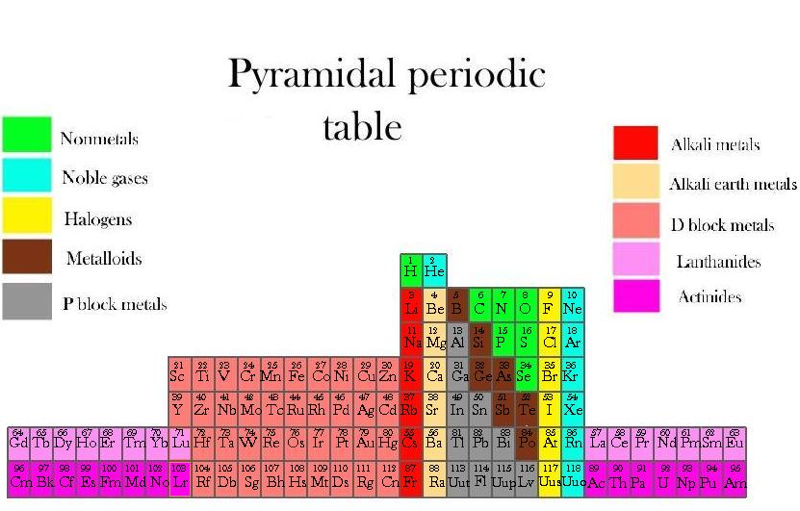
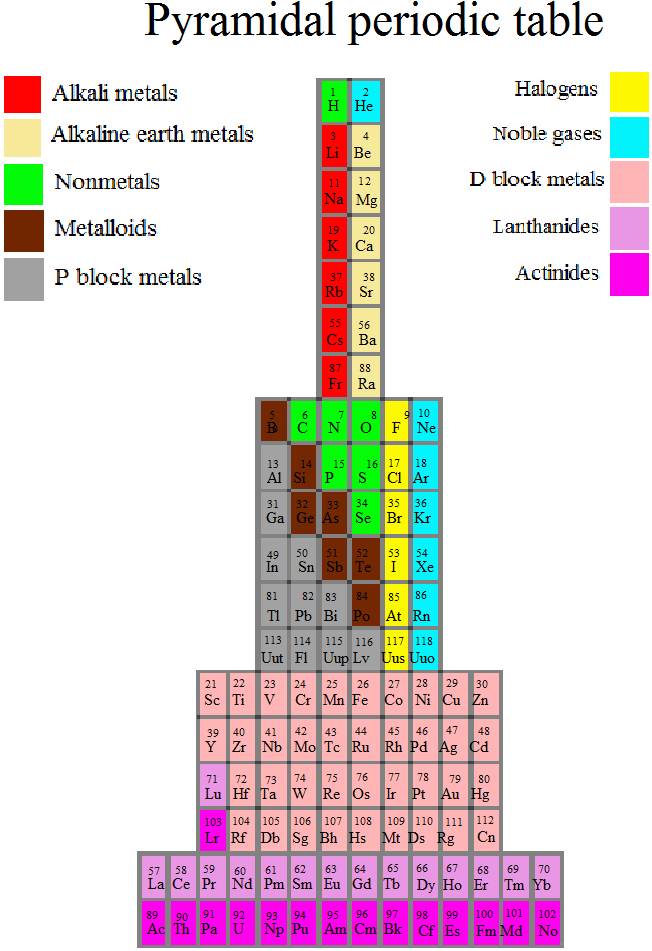
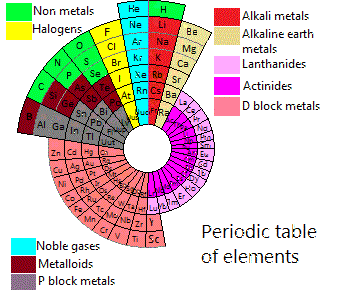
Piazzalunga's Pyramidal Periodic Table Formulations
Three Pyramidal Periodic Table Formulations, and a Spiral, from Marco Piazzalunga:
2012
Eric Scerri Lecture, Dedicated to Fernando Dufour
Dr. Eric Scerri from the Chemistry Department at UCLA giving a distinguished invited lecture at the Oscar Peterson auditorium of Concordia University, in Montreal. The topic is the history and iconic nature of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2012
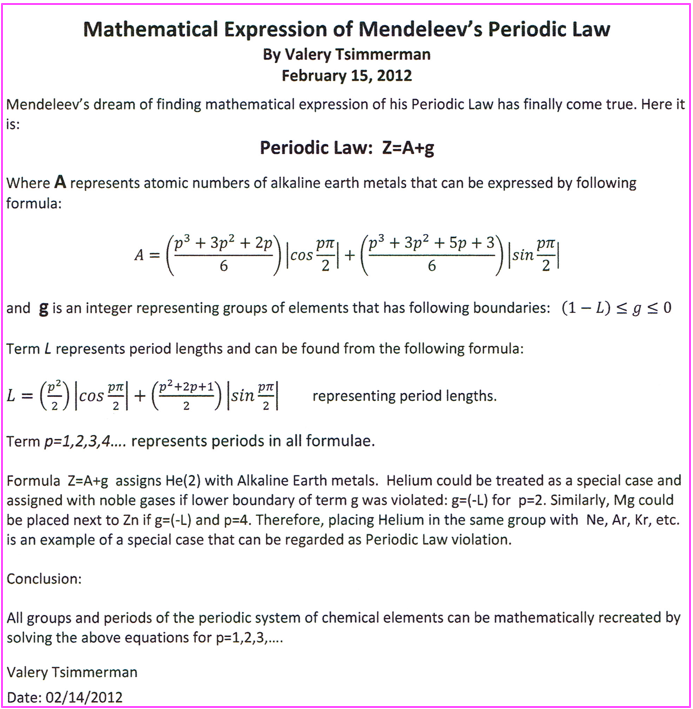
Mathematical Expression of Mendeleev's Periodic Law
Valery Tsimmerman, of the ADOMAH Tetrahedron periodic table formulation and the Perfect Periodic Table website, presents a Mathematical Expression of Mendeleev's Periodic Law:
2012
Zigzag Periodic Table
In this periodic table we can see that the elements are arranged in a different way. Hydrogen is placed in between (and above) fluorine and lithium. This is because there is an issue on the placement of hydrogen as it has the properties of both alkali metals and halogens.
How to read the Zigzag periodic table
For periods (1), (2B), (3B) etc. read from right to left.
For periods (2A), (3A), (4A) etc. read from left to rightThe arrows will guide you through the periodic table:
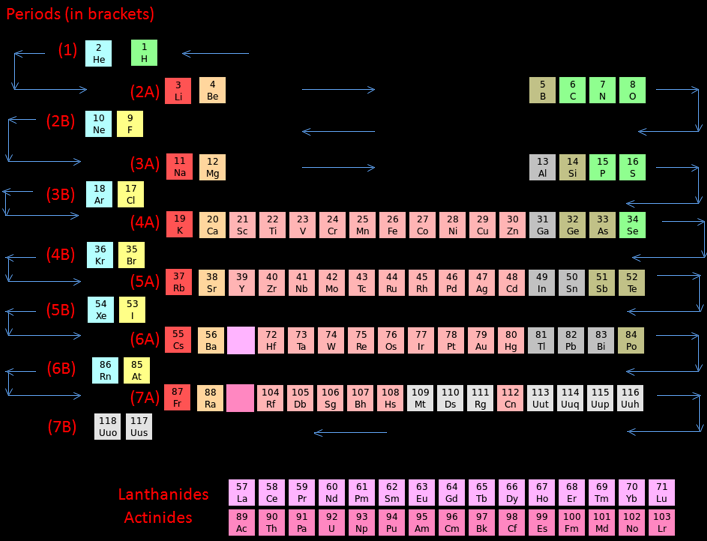
- Periodic Trends Atomic Radius- When moving opposite to the zigzag line in a particular period, the atomic radius of the elements increase.
- Metallic Character- Metallic Character decreases when moving along the zigzag line in a particular period.
- Ionization Energy- When moving along the zigzag line in a particular period, the ionization energy increases. Electron Affinity- Electron Affinity increases when moving along the zigzag line in a particular period.
By Akash Srivaths, High School Student, Chennai, India
2012
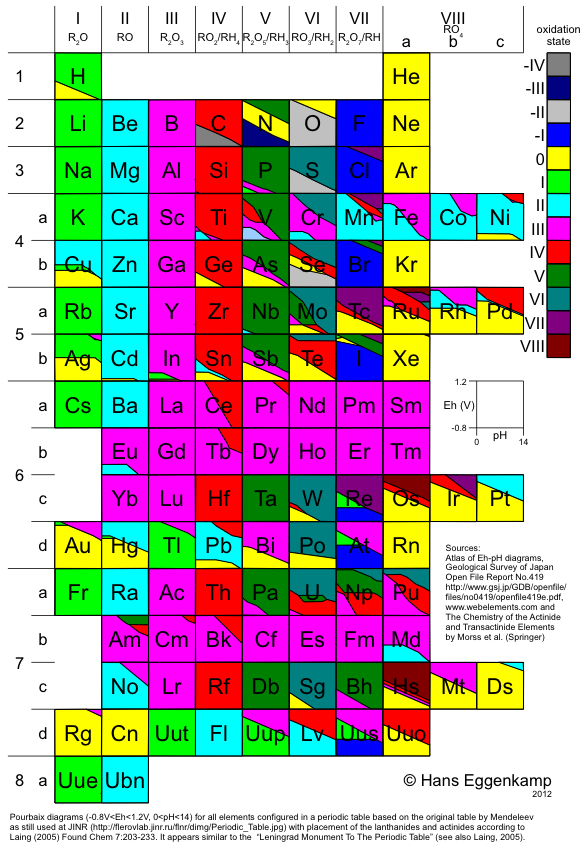
Eggenkamp's Periodic Table
Hans EggenkampI presents a periodic table based upon the table by Mendeleev, in combination with the lanthanides and actinides as suggested by Laing. A simplified Pourbaix (Eh-pH) diagram is shown for each element, colored according to the oxidation stage showing the systematics in the Periodic Table:
2012
JR's Chemistry Set
For the iPhone and iPad, JR's Chemistry Set makes chemistry interesting and fun to learn. Based upon the innovative Rota Period, it is a handy and powerful reference tool for chemistry enthusiasts and practitioners at all ages and all levels.
2012
Magnetic Periodic Table
By Particle Zoo, sellers of Higgs Boson and Anticharm Quark soft toys, comes a magnetic periodic table which you can arrange into any formulation you like!
2012
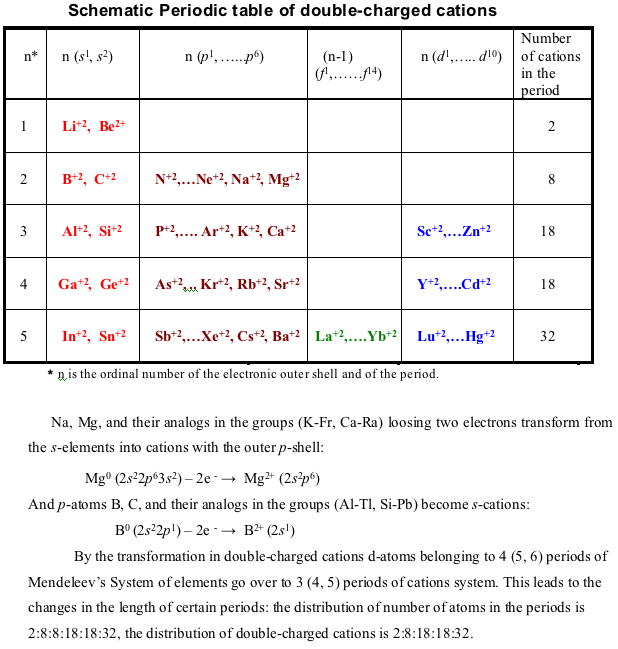
Schematic Periodic Table of Double-Charged Cations
N. S. Imyanitov / The Periodic Law. Formulations, Equations, Graphic Representations, Russian Journal of Inorganic Chemistry, Vol. 56 (14), 2183 - 2200, 2011 (In English), DOI: 10.1134/S0036023611140038
2012
Extended Periodic Table - Alternative
From Rasko Jovanovic's World of Mathematics, an Extended Periodic Table - Alternative:

2012
Three Different Long-Form, or 32-Column, Periodic Tables
From an article by Eric Scerri in the IUPAC magazine, Chemistry International, in which three different long-form, or 32-column, periodic tables with differences highlighted.
- Top: Version with group 3 consisting of Sc, Y, Lu, and Lr.
- Middle: Version with group 3 consisting of Sc, Y, La, Ac. The sequence of increasing atomic number is anomalous with this assignment of elements to group 3, e.g., Lu (71), La (57), Hf (72).
- Bottom: Third option for incorporating the f-block elements into a long-form table. This version adheres to increasing order of atomic number from left to right in all periods, while grouping together Sc, Y, La and Ac but at the expense of breaking-up the d-block into two highly uneven portions :
2012
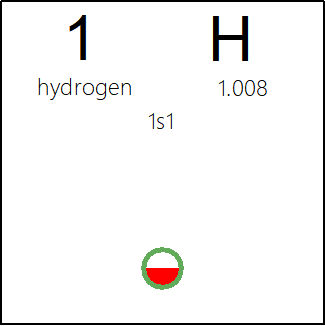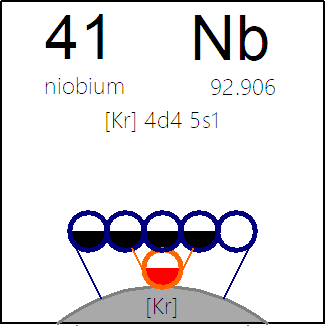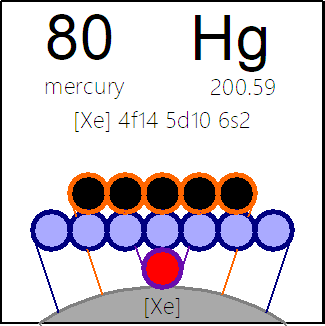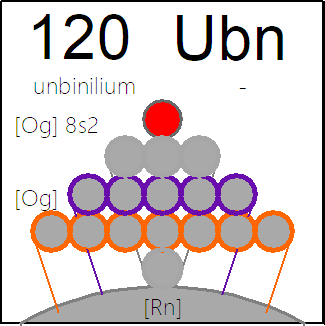
Rihani's 120 Element Periodic Table Formulations
Jeries Rihani writes: "Assuming the periodic table may reach an end at atomic number 120, I wish to draw your attention to the following three variations for the periodic table that I have on my web site Symmetry Of The Periodic Table which I think might be of interest, here, here & here":
2012
Ato Circular Periodic Table by Ramanpreet Singh Jandu
Ramanpreet Singh Jandu writes:
"The present invention relates to a device for the understanding of the periodic table and the structure of the atom together in a better way.
The device consists of seven concentric circular disks rotatable about their centre, wherein the size of the disks increases from the centre to the end like that in the structure of the atom.
Each disk is marked so as to form the sub-blocks and each disk in itself represents the periods of the periodic table.
The disks are divided into sub-blocks and labeled with elements at back side as well.
Thus the Ato Circular Periodic Table, as the name suggests, is the combination of atomic structure of the atom and that of the periodic classification of the elements."
Read more in the pdf file which describes the new formulation in detail.
2013
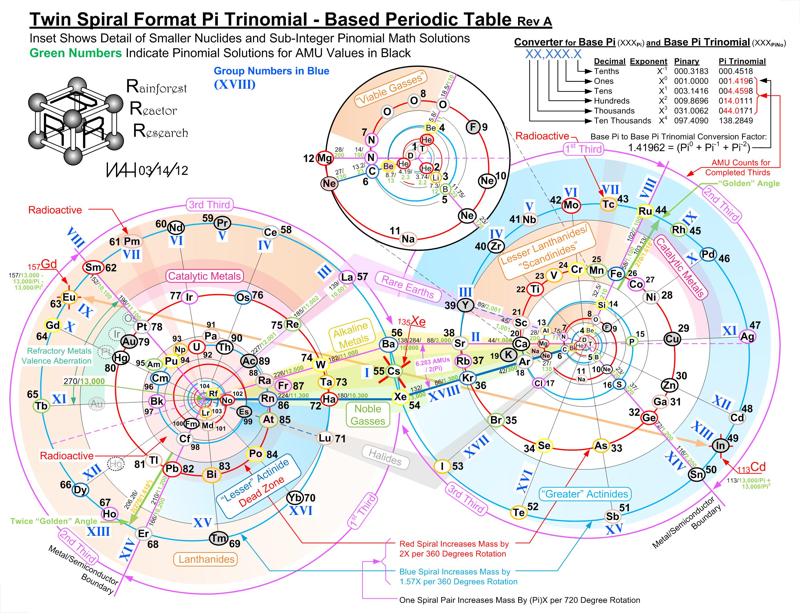
Twin Spiral Pi Trinomial - Based Periodic Table
A Twin Spiral Pi Trinomial - Based Periodic Table by Bill Harrington, Founder/CTO of Rainforest Reactor Research and Temporal Dynamics Laboratory. For full size, click the image:
2013
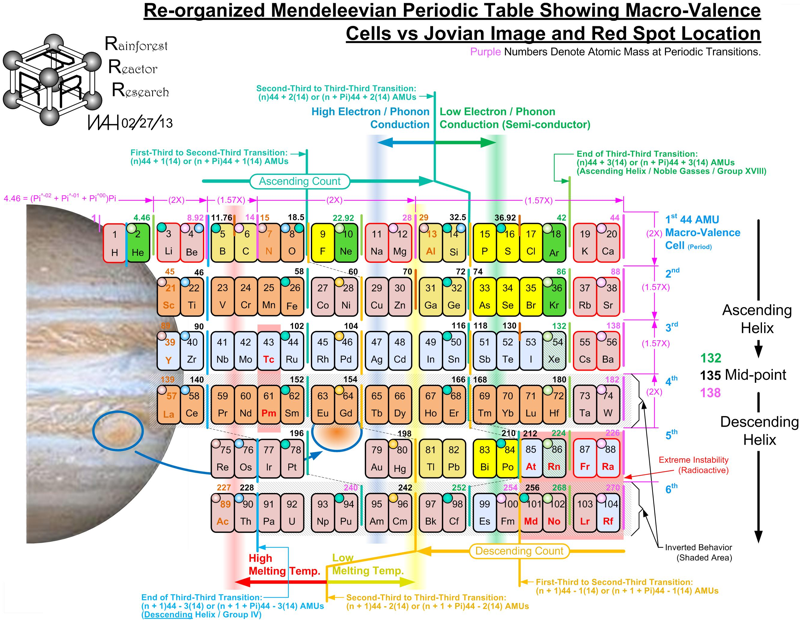
Macro-Valence Cells vs Jovian Image and Red Spot Location Periodic Table
A Macro-Valence Cells vs Jovian Image and Red Spot Location Periodic Table by Bill Harrington, Founder/CTO of Rainforest Reactor Research and Temporal Dynamics Laboratory. For full size, click the image:
2013
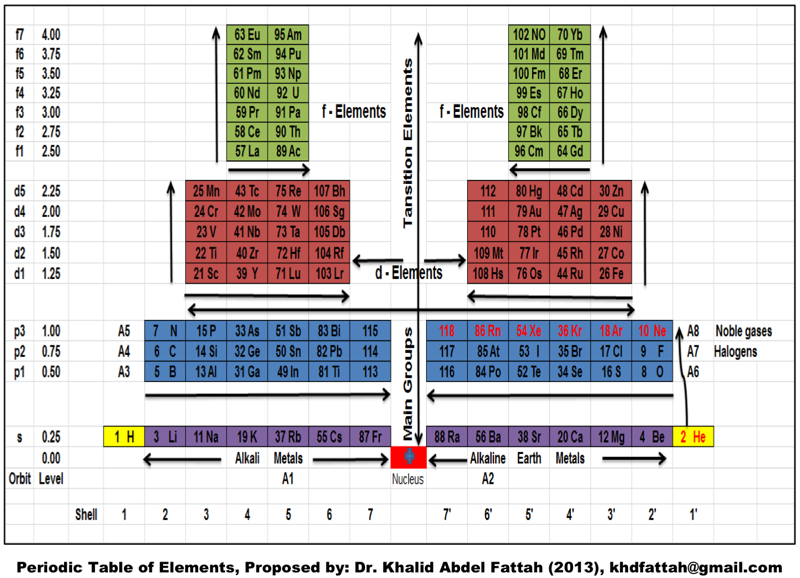
Fattah's Periodic Table
A new vertical periodic table by Dr. Khalid A. FATTAH, Faculty of Eng., Karary University, Khartoum, Sudan. For full size, click the image:
2013
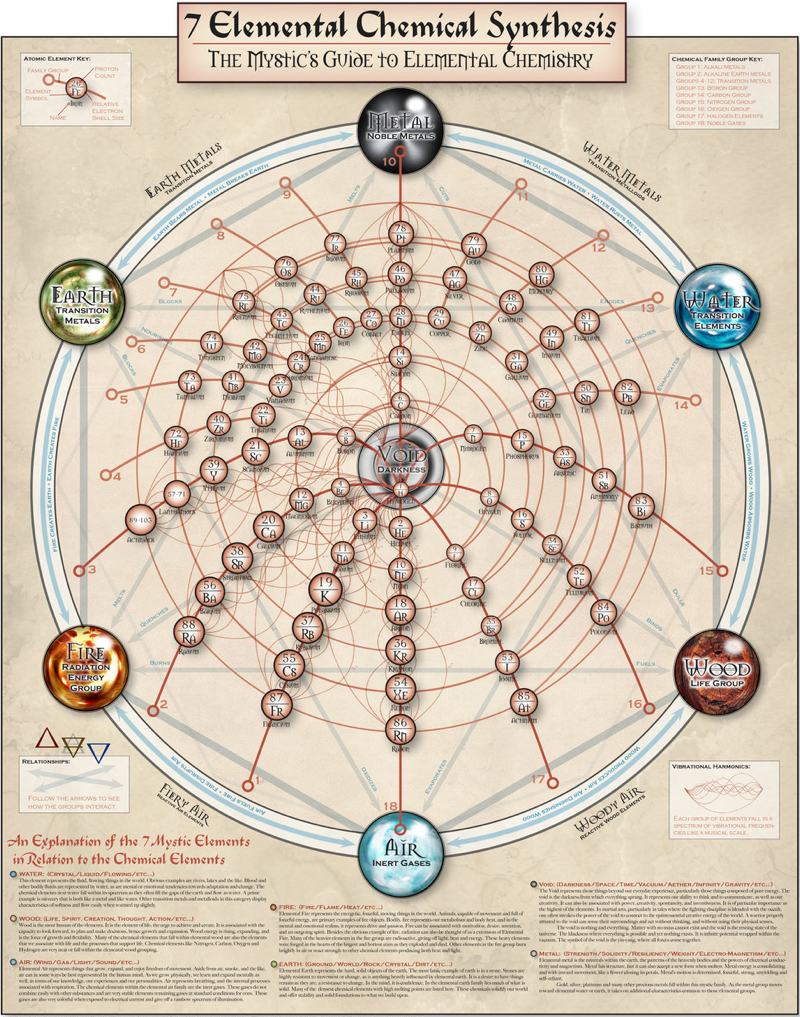
7 Elemental Chemical Synthesis
The Mystics Guide to Elemental Chemistry, by bzylman at deviantart:
[A] poster is designed to geek out the chemist and the mystic alike. It is a variation on the periodic table of chemical elements that have been rearranged into a circular structure based upon their proton count and chemical family, augmented with the concept of the 7 mystic elements of earth, air, fire, water, metal, wood and void. It was very interesting to work upon once I hit the correct organization of elements that they lined up almost perfectly.
2013
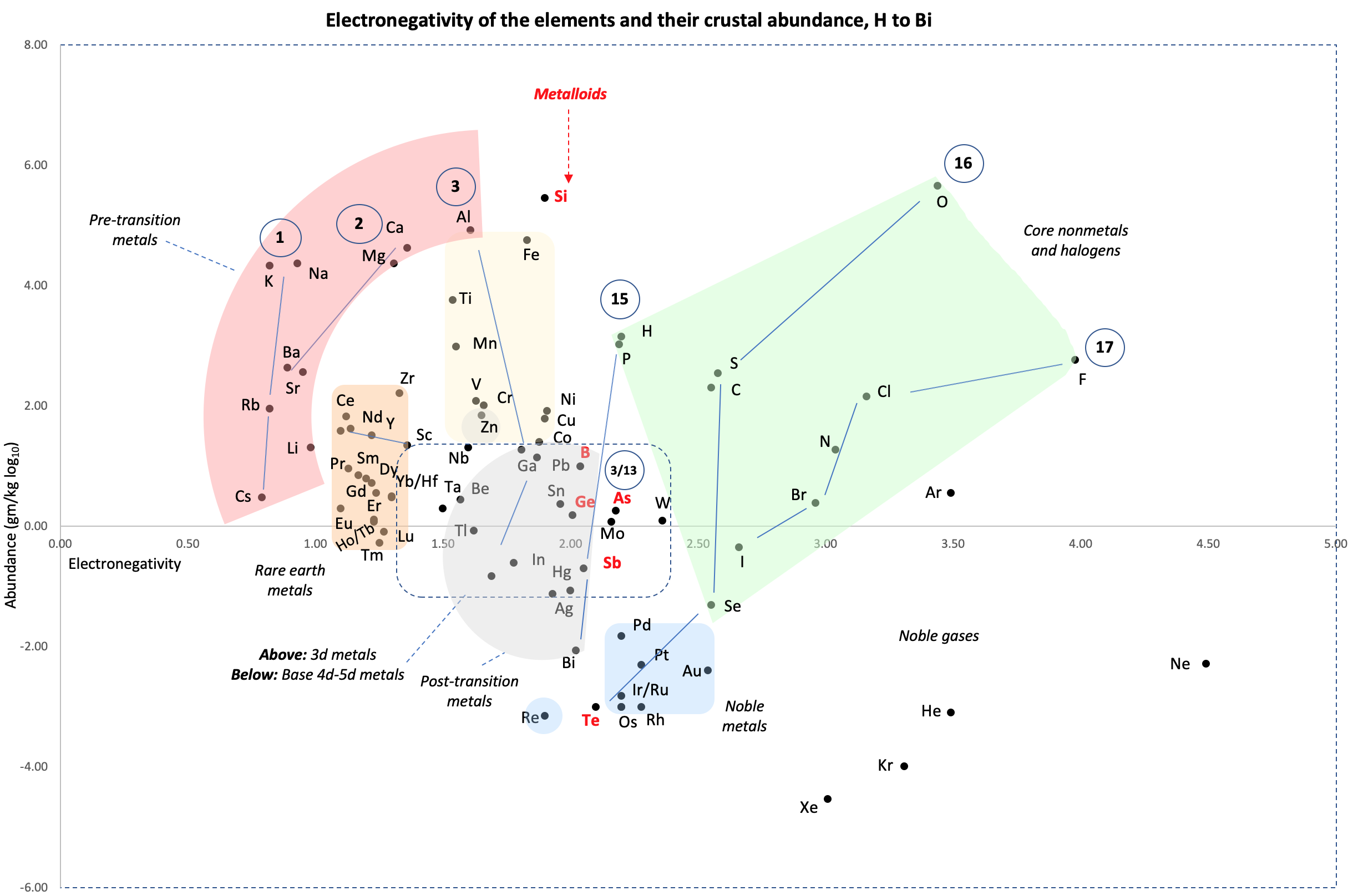
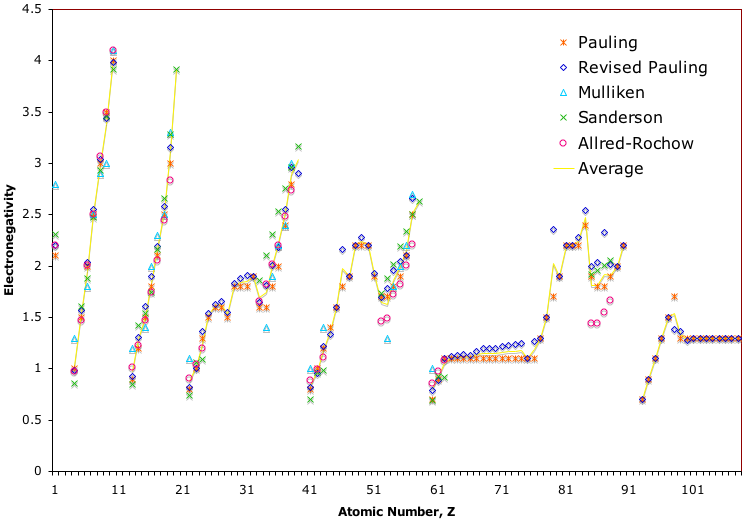
Electronegativity Chart (Leach)
From Mark R Leach's paper, Concerning electronegativity as a basic elemental property and why the periodic table is usually represented in its medium form, Journal & PDF.
Due to the importance of Pauling's electronegativity scale, as published in The Nature of The Chemical Bond (1960), where electronegativity ranges from Cs 0.7 to F 4.0, all the other electronegativity scales are routinely normalised with respect to Pauling's range.
When the Pauling, Revised Pauling, Mulliken, Sanderson and Allred-Rochow electronegativity scales are plotted together against atomic number, Z, the similarity of the data can be observed. The solid line shows the averaged data:
2013
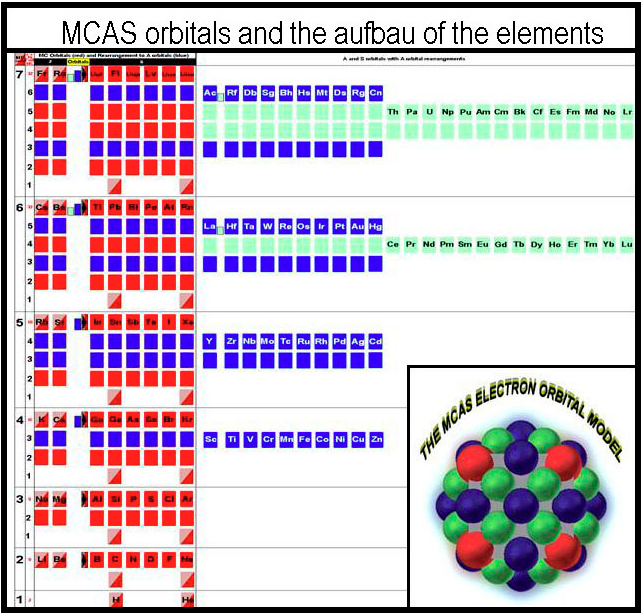
MCAS Electron Orbital Filling
From Joel M Williams:
"While the periodic table arrangement is usefully interpreted in columns of similar behavior, it is erroneous to imply that the underlying orbitals are all the same for all the elements in the columns of a block. Sub-orbital information has been excluded! From the standpoint of chemistry, the rule of eight would have provided better imagery on which to build an orbital system than was Bohr's orb turned-sphere. A sphere is useless from a chemical standpoint. Hybridization should not have to occur to explain the simplest of molecules. Simplicity would have the electrons occupying orbital spaces that are similar in shape. Only three orbital types are actually needed to describe the electron packing of the elements. Octahedral, square-planar, and pyramidal coordination complexes of the transition elements follow logically without the need to hybridize. This brief paper describes a rational packing of electrons around a nucleus that ends up mimicking the familiar periodic table when compressed to similar behavior."
Modeling the MCAS Way describes this concept of "building blocks" and can be found here.
2013
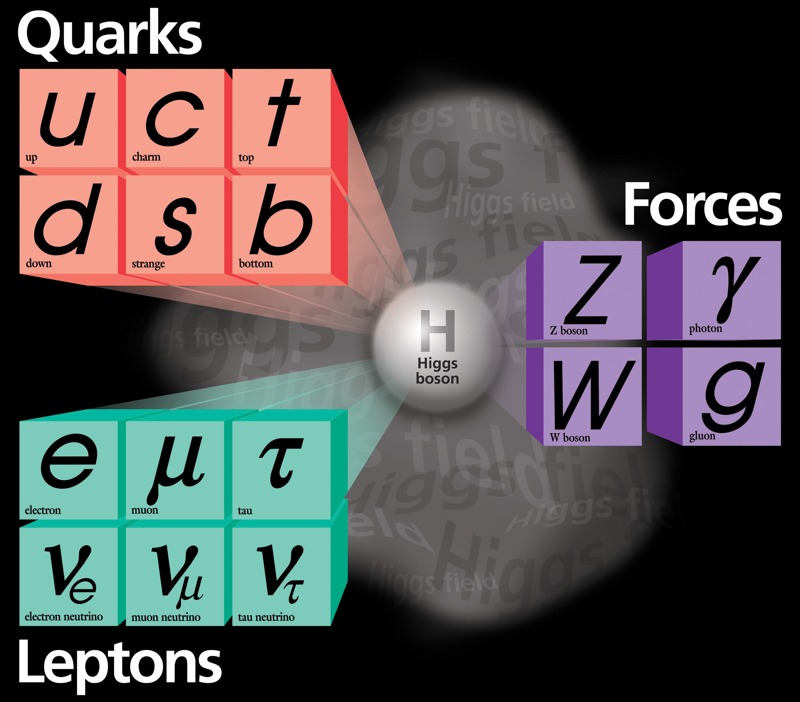
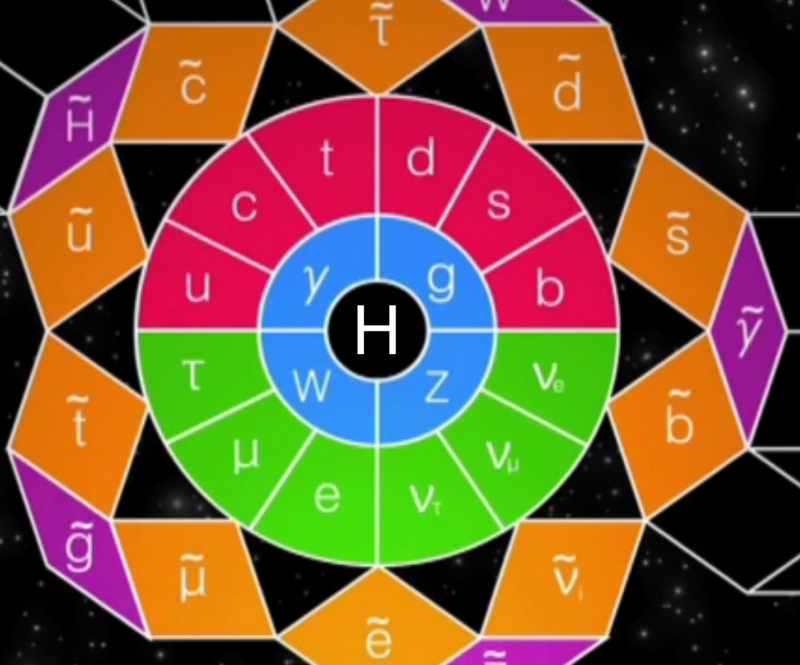
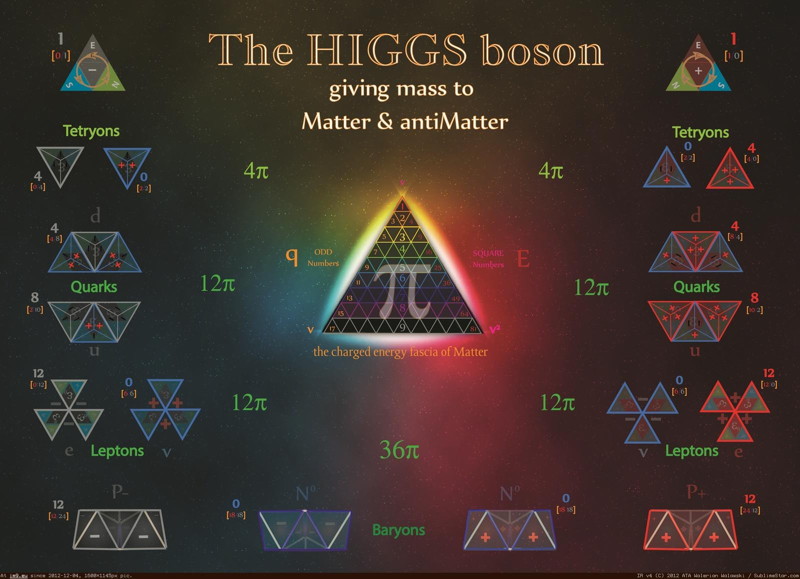
Higgs Boson and Fundamental Particle/Force Periodic Tables
The Higgs boson sits at the heart of the Standard Model of particle physics, and so is at the centre of periodic table type representations of quarks, leptons and forces.
Three representations by the UCR Today, a video interview with Particle Fever editor Walter Murch: "The Higgs boson is kind of a MacGuffin" and from im9.eu:
2013
Fattah's Extended Periodic Table
A new vertical periodic table (Extended) by Dr. Khalid A. FATTAH, Faculty of Eng., Karary University, Khartoum, Sudan:
2013
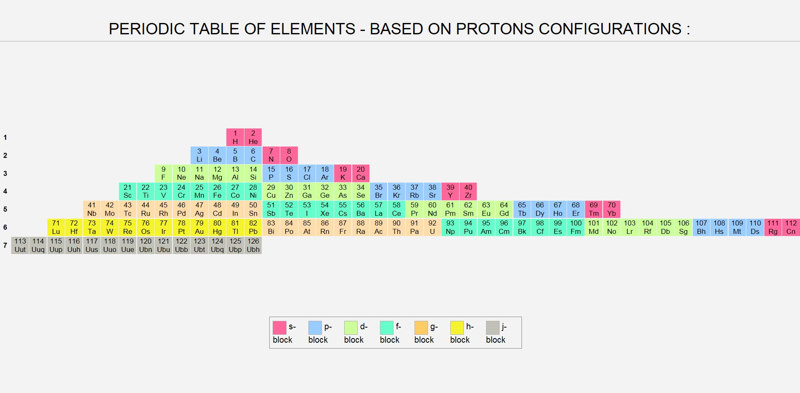
Proton Configuration Periodic Table
A Periodic Table of Proton Configurations by Radoslav Rasko Jovanovic:
2013
4D Stowe-Janet-Scerri Periodic Table
By Jgmoxness who writes:
"I've replaced the standard periodic table in the 7th "Chemistry Pane" of my E8 visualizer with a 2D/3D/4D Stowe-Janet-Scerri version of the Periodic Table.
"Interestingly, it has 120 elements, which is the number of vertices in the 600 Cell or the positive half of the 240 E8 roots. It is integrated into VisibLie_E8 so clicking on an element adds that particular atomic number's E8 group vertex number to the 3rd E8 visualizer pane.
"The code is a revision and extension of Enrique Zeleny's Wolfram Demonstration":
2013
Shapes Periodic Table
By ScienceIsGolden.com comes the Periodic Table of Shapes. The site is worth clicking around, as there is a lot of good PT stuff to find:
2013
3D Left Step Periodic Table
By Masahiko Suenaga, Kyushu University, Japan a 3D Left Step Periodic Table.
"Inspired by the work of Dr. Tsimmerman and Dr. Samanez, I have created a new 3D Left Step Periodic Table, which resembles to Mt. Fuji, recently registered as a World Heritage site. For more information, please visit my website":
2013
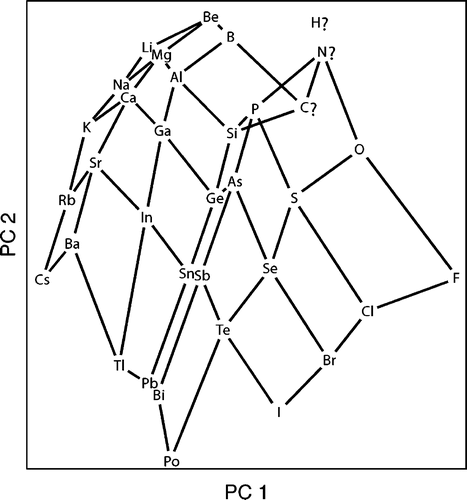
From Periodic Properties to a Periodic Table Arrangement
A paper in J.Chem. Ed.: From Periodic Properties to a Periodic Table Arrangement
Emili Besalú, Departament de Química i Institut de Química Computacíonal i Catàlisis, Universitat de Girona, C/Maria Aurèlia Capmany, 69, 17071 Girona, Catalonia, Spain.
J. Chem. Educ., 2013, 90 (8), pp 1009-1013 DOI: 10.1021/ed3004534 Publication Date (Web)
"A periodic table is constructed from the consideration of periodic properties and the application of the principal components analysis technique. This procedure is useful for objects classification and data reduction and has been used in the field of chemistry for many applications, such as lanthanides, molecules, or conformers classification. From the information given, the whole procedure can be reproduced by any interested reader having a basic background in statistics and with the help of the supplementary material provided. Intermediate calculations are instructive because they quantify several concepts the students know only at a qualitative level. The final scores representation reveals an unexpected periodic table presenting some interesting features and points for discussion."
2013
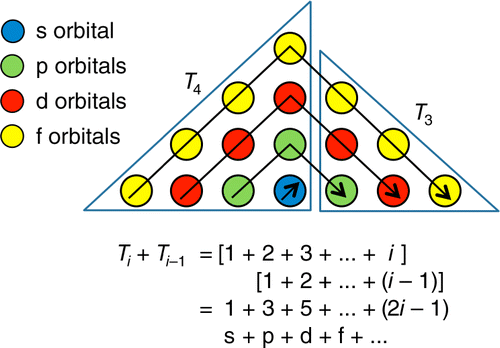
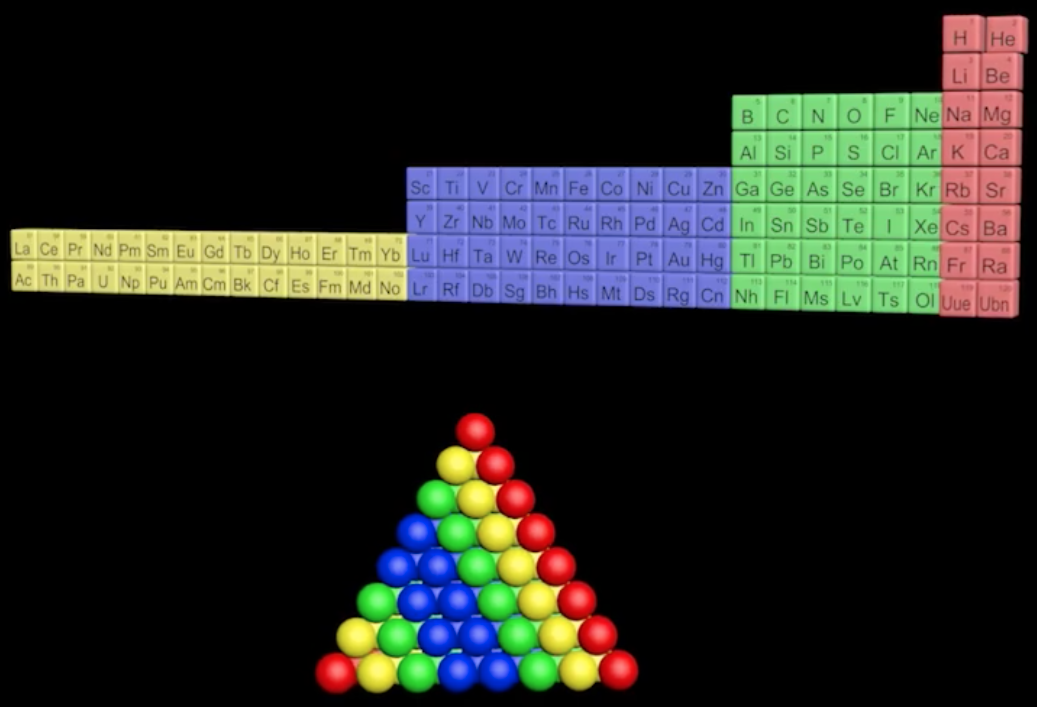
Periodic Pyramid
A Periodic Pyramid by Jennifer N. Hennigan and W. Tandy Grubbs * Department of Chemistry, Stetson University, DeLand, Florida 32723, United States
J. Chem. Educ., 2013, 90 (8), pp 1003-1008 DOI: 10.1021/ed3007567 Publication Date (Web): June 21, 2013
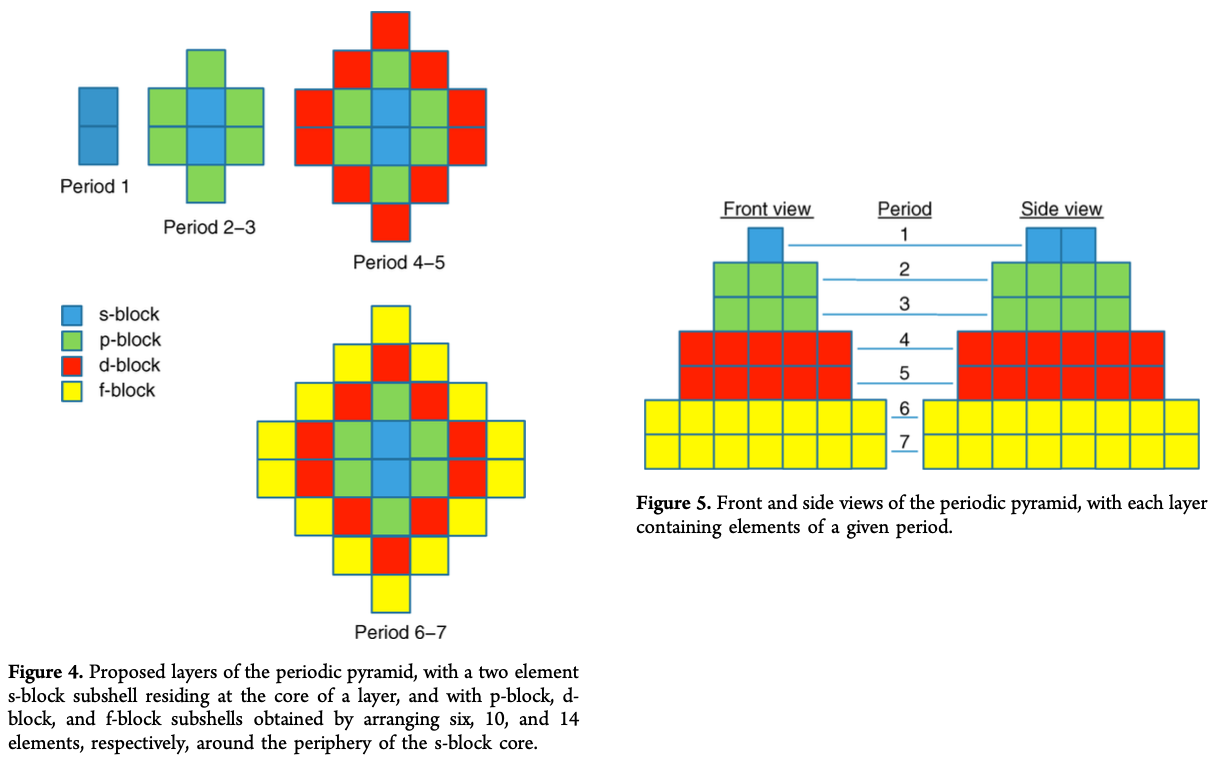
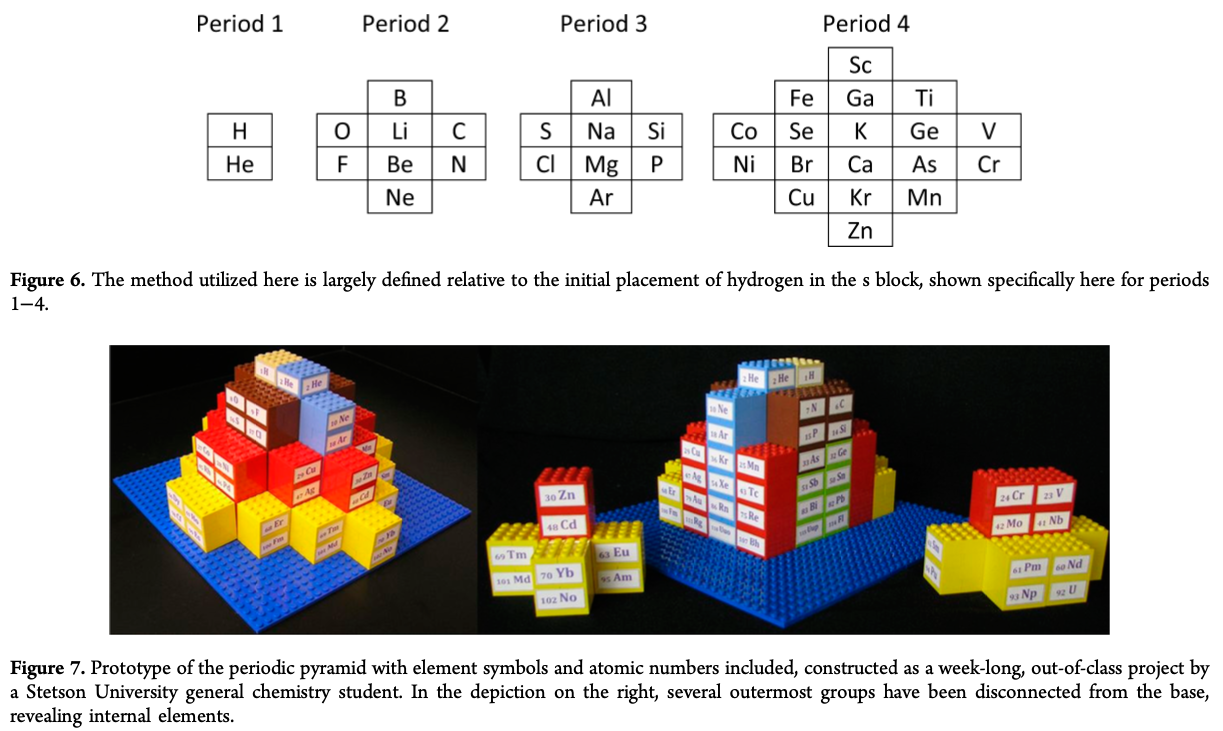
The chemical elements present in the modern periodic table are arranged in terms of atomic numbers and chemical periodicity. Periodicity arises from quantum mechanical limitations on how many electrons can occupy various shells and subshells of an atom. The shell model of the atom predicts that a maximum of 2, 8, 18, and 32 electrons can occupy the shells identified by the principle quantum numbers n = 1, 2, 3, and 4, respectively. The numbers 2, 8, 18, and 32 are shown in this work to be related to the triangular numbers from mathematical number theory. The relationship to the triangular numbers, in turn, suggests an alternate method for arranging elements in terms of periodicity. The resulting three-dimensional "periodic pyramid" is highly symmetric in shape. Just as is true in the modern periodic table, each layer of the periodic pyramid can be separated into shell and subshell contributions. Examining the pyramid's structure is arguably a pedagogically useful activity for college-level introductory or physical chemistry students, as it provides an opportunity to further ponder the shell model of the atom and the origins of periodicity. The connections to number theory are used to show that the outermost subshell of a given shell contains (2n - 1) orbitals.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2013
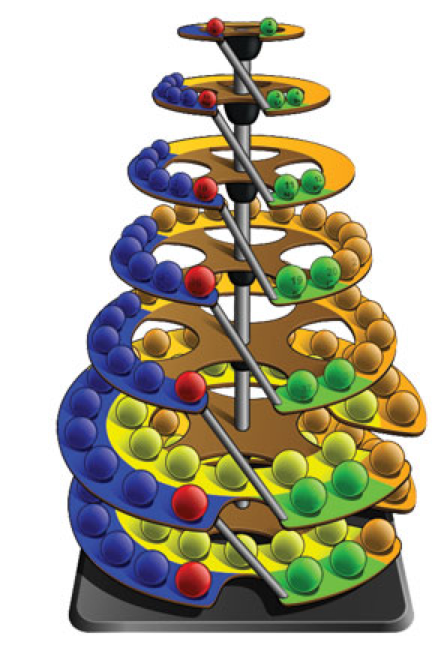
Bernard Periodic Spiral
The Bernard Periodic Spiral of the Elements (BPSE), depicts a novel rendition of the Periodic Table that replaces the flat rectangular format with a continuous unidirectional spiral that maintains all the properties of Group and Period formation.
Comparisons may be made with similar models spanning the last three decades of the 20th century (Alexander, 1971; Mazurs, 1974; & Kaufman, 1999).
In the chart form, this new rendition is referred to as the Elliptical Periodic Chart of the Elements. In the three-dimensional form, the model resembles a Christmas tree in shape with the 7 Periods represented as circular platforms situated at various levels with the elements placed appropriately at the outer edges of each of these platforms as a Period builds up. The elements may be represented as spherical objects or flat discs with radii proportionate to atomic radii (or reasonable approximations). Color schemes accentuate the four different Blocks of elements: the s-Block (green), the p-Block (blue, with the exception that the last Group is red signifying the end of a Period), d-Block (orange), and the f-Block (yellow). The grey section, called the Group-Period Interchange, is where the end of a particular Period connects to the beginning of the next Period, and, at the same time, transitions from Group 18 to Group 1.
Watch the video here:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2013
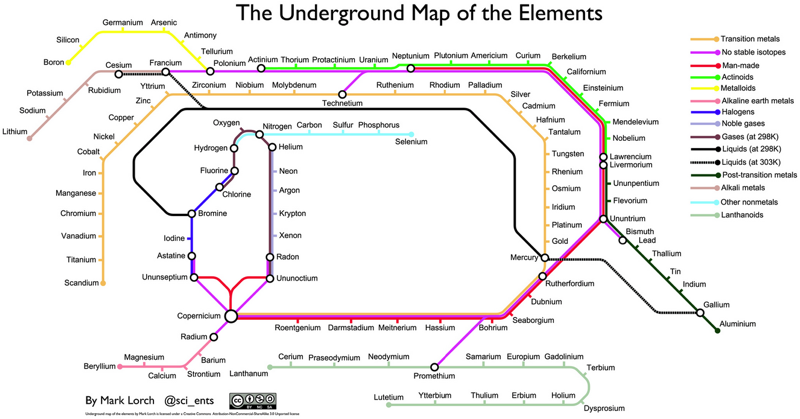
Underground Map of the Elements
By Dr Mark Lorch of the University of Hull, an Underground Map of the Elements.
From here: "My son loves trains. So I came up with a train related twist to an inspection of the periodic table. We sat and cut up a copy of the table and then rearranged each element as a 'station' on an underground rail system. Each line represents a characteristic shared by the elements on that line":
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2013
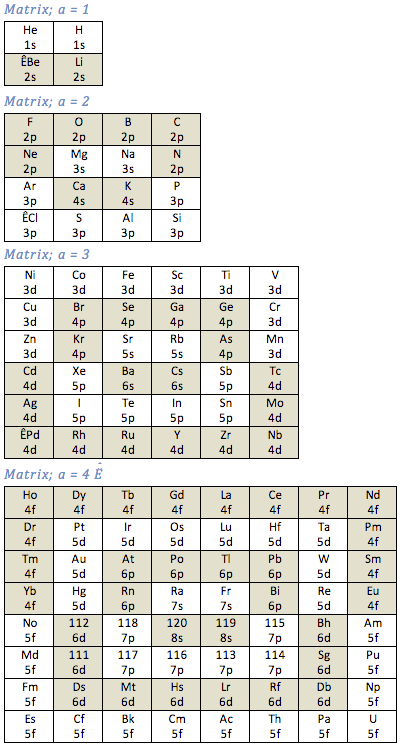
Matrix Series Periodic Table
By Richard Kingstone, a Matrix Series Periodic Table. Read more here.
The Janet Periodic Table (aka Left Step Table) may be re-arranged as a series of square matrices. Each element is represented as a cell and is identified by the atomic number (Z), shown as the upper number of each cell.
The quantum numbers (n, l, mL, mS) determine the location of an element within the table. The quantum pair (n, l ) are the lower numbers in each cell. Four matrices are required, each matrix is identified by a 'matrix number' (a) as shown below;:
2013
Music Notes of Periodic Table
By Claude Bayeh, a Musical Notes formulation:
2013
Averaged Ionisation Potential Periodic Table
By Leland Allen, a representation of the periodic table with the third dimension of energy derived from the averaged ionisation potentials of the s and p electrons. (Allen suggested that this was a direct measure of electronegativity). From J. Am. Chem. Soc. 1989, 111, 9004:
2013
Model Wooden Periodic Table
From here, and translated from Spanish:
Among the events commemorating the 75th anniversary of the creation of the School of Treball, the author of this site, B. Navarro, along with J. Semis and J. Gràcia have built a model wooden periodic table.
The table has been divided into 5 areas: representative elements, noble gases, transition elements, rare earths and finally the groups I and II of alkali and alkaline earth together. Each of these areas of the table is made with a different type of wood. The block transition elements is made with oak, ash noble gases, representative elements in cherry, sapele the rare earth and alkali/alcalinoterros beechwood.
The central idea of the model is that each element is represented by a cube of 3 cm edge so that you can see on all sides, from left to right or right to left without losing the order of increasing atomic number or the relative position of the elements:
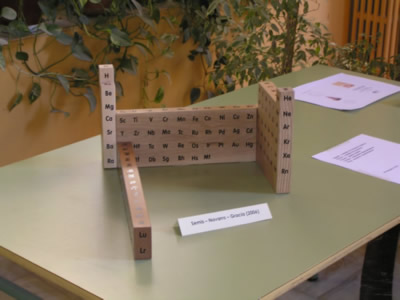
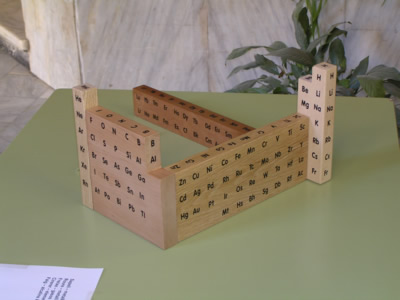
2013
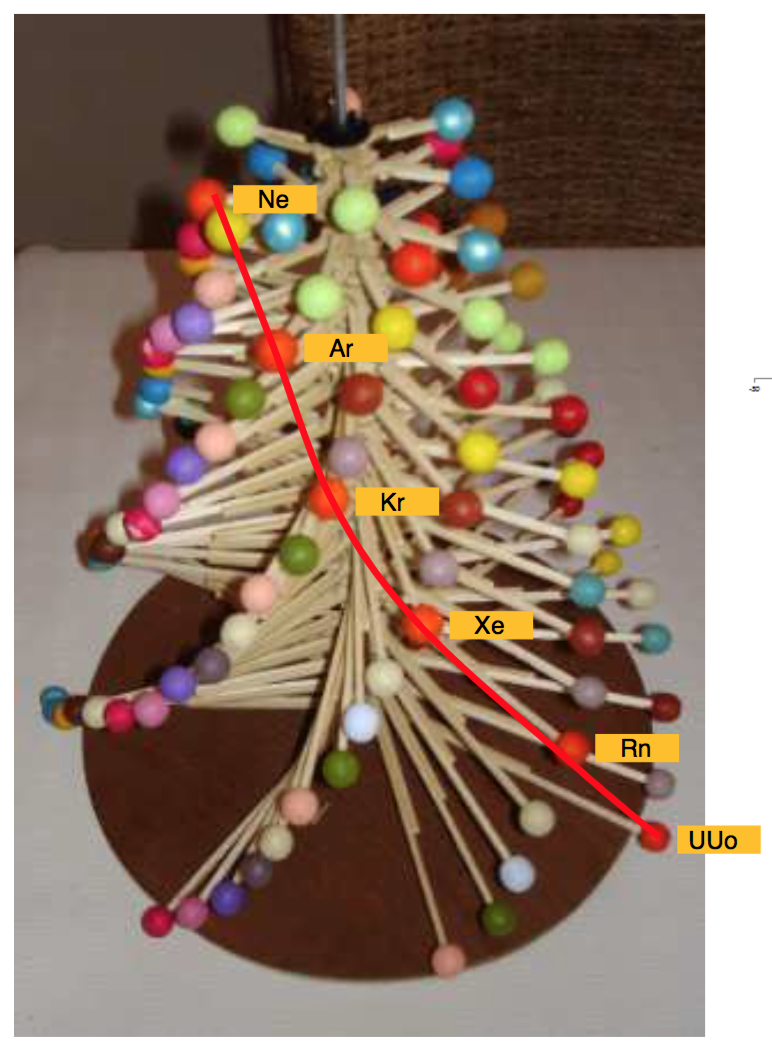
Muradjan's Mathematical Structure of The Periodic Table
From the website periodictablemathstructure:
Abstract:
The Periodic Table with a new double numerical structure, presented here is attempt to find table form which will in some new way represent the periodicity and symmetry of the Elements, with the Periodic System as base. Also this tetrahedral laminar table structure maybe will became a base for developing a new shell structure of atomic nucleus. This new rearrangement of the chemical element is based on mathematical formula which result is simple, length of the periods:
2013
Atomic Periodic Town
Three related formulations by Baha Tangour (Tangour Bahoueddine), the Atomic Town and two Boomerang periodic tables.
Baha says: "The propositions are different representation of a 3D dimensions that depend on three properties (spectral term multiplicity, lone-pairs and period number)":
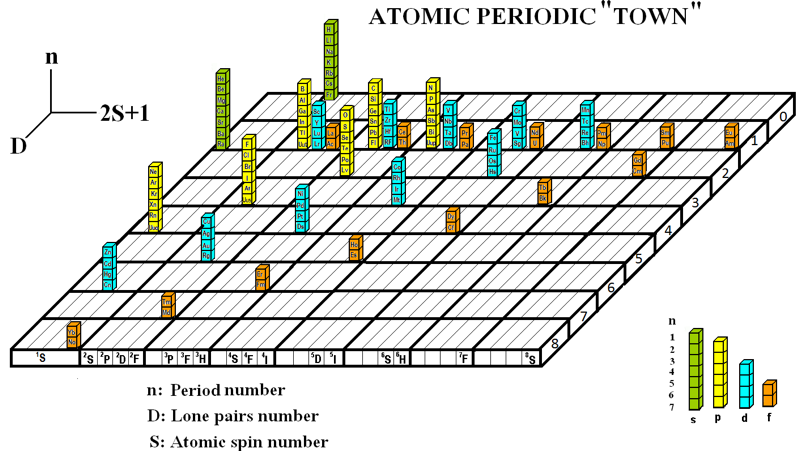
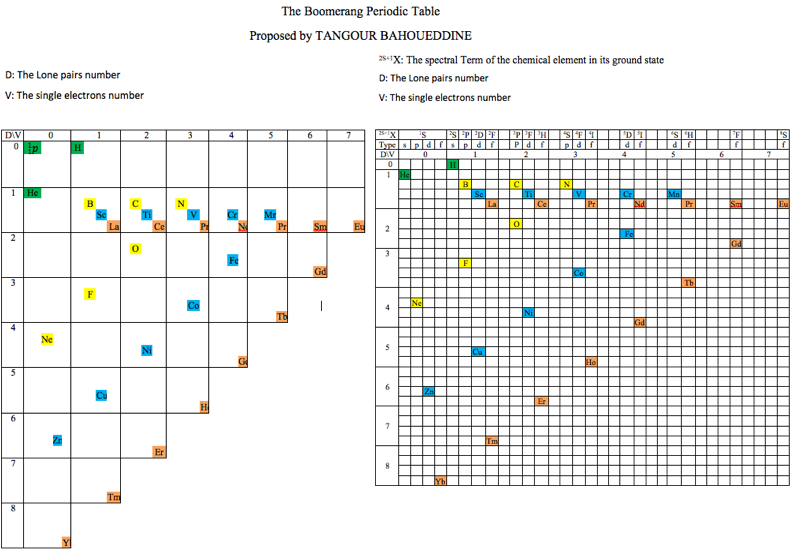
2013
Periodic Pyramid
The Periodic Pyramid by Jennifer N. Hennigan and W. Tandy Grubbs, J. Chem. Educ. 2013, 90, 8, 1003-1008, https://doi.org/10.1021/ed3007567.
"The chemical elements present in the modern periodic table are arranged in terms of atomic numbers and chemical periodicity. Periodicity arises from quantum mechanical limitations on how many electrons can occupy various shells and subshells of an atom. The shell model of the atom predicts that a maximum of 2, 8, 18, and 32 electrons can occupy the shells identified by the principle quantum numbers n = 1, 2, 3, and 4, respectively.
The numbers 2, 8, 18, and 32 are shown in this work to be related to the triangular numbers from mathematical number theory. The relationship to the triangular numbers, in turn, suggests an alternate method for arranging elements in terms of periodicity. The resulting three-dimensional 'periodic pyramid' is highly symmetric in shape. Just as is true in the modern periodic table, each layer of the periodic pyramid can be separated into shell and subshell contributions. Examining the pyramid's structure is arguably a pedagogically useful activity for college-level introductory or physical chemistry students, as it provides an opportunity to further ponder the shell model of the atom and the origins of periodicity. The connections to number theory are used to show that the outermost subshell of a given shell contains (2n – 1) orbitals."
2013
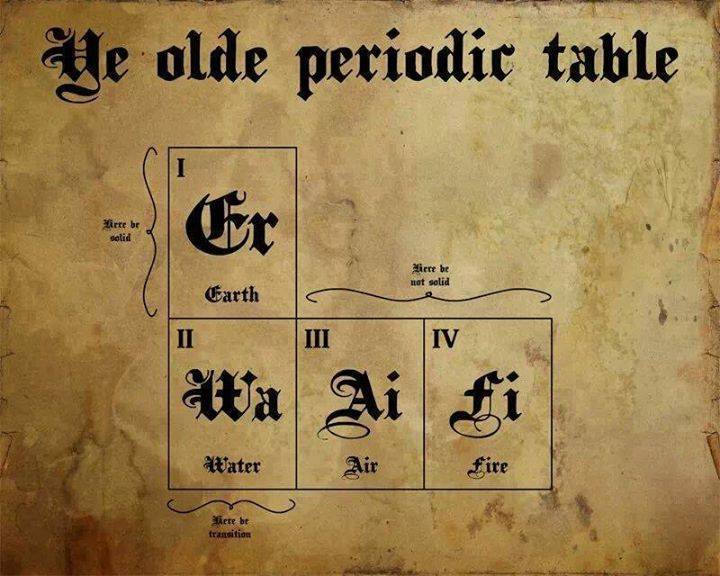
Ye Olde Periodic Table
From the Serious Severity blog, a spoof of this formulation:
Thanks to Marcus Lynch for the tip!
2013
Labarca & Zambon's Formulation
Labarca & Zambon's new representation of the periodic system.
A reconceptualization of the element concept as a basis for a new representation of the periodic system, Martín Labarca and Alfio Zambon, Educ. Quím., 24(1), 63-70, 2013.
"The aim of this paper is to propose a new conceptualization of the term 'element' as the basis for a new representation of the periodic system. For this purpose we begin by recalling the dual sense of the concept of element. Next, we develop the 'limits isotopes' argument which is the basis of the new periodic chart. This task leads us both to reconceptualize the notion of element and to characterize the term 'basic substance'. In turn, the argument is used to face the epistemological problem with hydrogen and helium in the periodic table. Finally, the Döbereiner's triads are used to calculate atomic masses in three periodic charts: the medium-long-form, the modified 'left-step' proposed by Scerri, and the proposed in this work. Evaluation results allows us to stand out the fruitful predictive power of our periodic system.
"The 46 blocks of elements are ordered vertically by the increasing number of neutrons of the lighter isotope (primary criterion) and, horizontally, by the increasing atomic number (secondary criterion). The subscript represents the value of L - Z and the superscript the value of Z."
Thanks to Eric Scerri for the tip! See the website EricScerri.com and Eric's Twitter Feed.
2014
Lado's Periodic Table Series Analysis
By Solomon F Lado, a mathematical analysis.
Abstract (full paper):
"There is a periodic table, at least in terms of atomic number and electronic configuration, for every positive integer, c >= 2, with a capacity of 20c2 – 21c + 1 elements, where c is the construction index, and c equals one plus the maximum number of electrons per orbital of an atom. The c-construction index and the c-construction-index formulae are unique to this report. The maximum number of potential orbitals per sub-shell of an atom = 3n-1 where n is the index of the s, p, d, and f sub-shells":
2014
Jodogne's Janet New Color Periodic Table
By J.C. Jodogne, "a Janet type slightly modified to enhance shells, periods and to make determination of ground state configuration (orbitals) very easily to build". Click here to get the full size pdf.
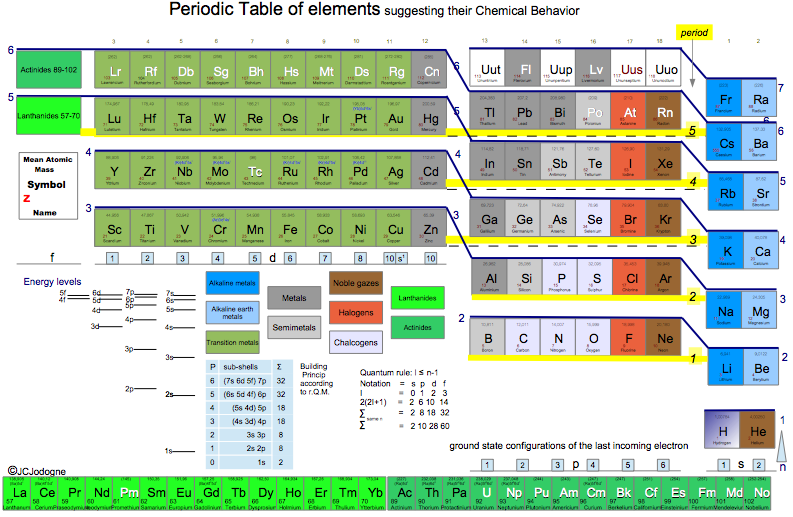
2014
Jodogne's New Color Table
By J.C. Jodogne, "a medium type with the above features but with Z continuity and a general aspect very similar to the usual presentation". Click here to get the full size pdf.
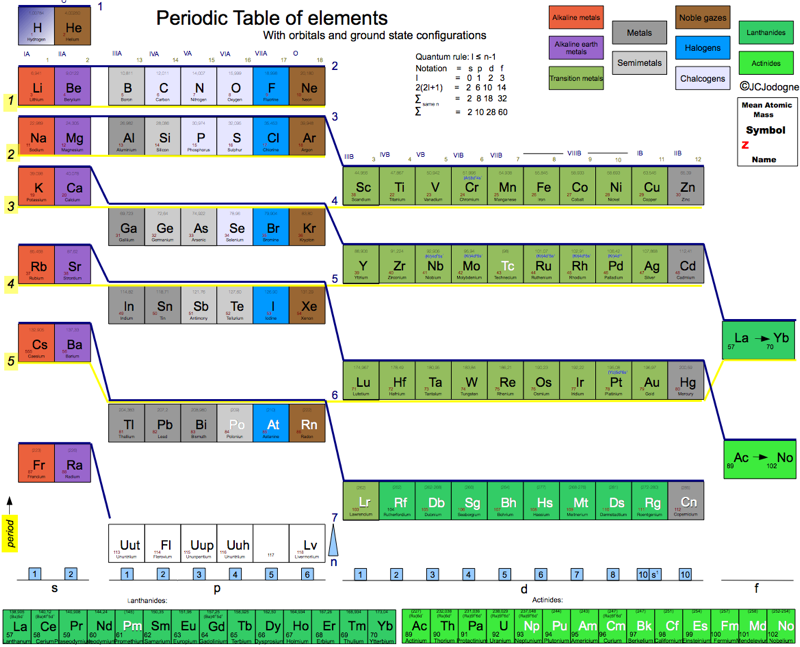
2014
ADOMAH Periodic Table Glass Cube
Valery Tsimmerman, of the ADOMAH Periodic Table and the ADOMAH Tetrahedron, has now used these ideas to produce a beautiful glass cube:

This amazing object is available for sale from Grand Illusions:
A Note by Philip Stewart stewart.phi@gmail.com
The cube represents 120 chemical elements etched into a cube of Optical Crystal glass. The s, p, d, and f blocks of the Janet periodic table form four rectangles, which are slices of a regular tetrahedron, parallel with two of its edges and with two faces of the circumscribed cube. All four quantum numbers are made visible in this arrangement. You can see a 2-D version on the Perfect Periodic Table website, click on the "skyscraper" version on the right to see the tetrahedron, and go to Regular Tetrahedron at foot of page for details.
The regular tetrahedron is the only form in which slices are rectangles of different shape and identical perimeter. When each orbital is represented by a square of unit edge, the rectangles representing the blocks all have the same perimeter, which is twice the length of the edges of the tetrahedron (which are of course √2 times the edges of the cube): 18 units = 2(values of n + values of ml).
Block |
values of n |
values of ml |
s |
8 |
1 |
p |
6 |
3 |
d |
4 |
5 |
f |
2 |
7 |
Valery Tsimmerman, orahct@gmail.com, creator of the design, has written to me as follows:
"I just had some thoughts about the Perimeter Rule that is at the basis of the tetrahedral arrangement. Dimensions of the blocks are dictated by number of values of ml and number of values of n. We know that n governs quantization of energy. Recently I learned that quantization of the possible orientations of L with respect to an external magnetic field is often referred to as space quantization. (Serway, Jewett: Physics for Scientists and Engineers. 6th edition. p.1369).
"That is, ml stands for space quantization. Therefore, the Perimeter Rule reflects a direct relationship between energy and space. I think that this could have some significance. The beautiful thing about the Universe is that each type of symmetry is related to some conservation law. Symmetry in time is related to energy. Therefore, n is related to time also, so, in the Perimeter Rule we have relationship between time and space on quantum numerical level. The interesting thing is that ml can be positive and negative, while n can only be positive. Similarly, things can move in space in positive and negative directions, but time has only one direction. There is no negative time, just as there are no negative values of quantum number n."
Adomah is a variant of Adamah, Hebrew for 'dust of the earth', from which Adam was made (Genesis 2:7).
2014
Gutierrez Samanez's Binodic Form of the Periodic Table (Video)
2014
Cross Periodic Table
By Claude Ziad Bayeh, a Cross Periodic Table:
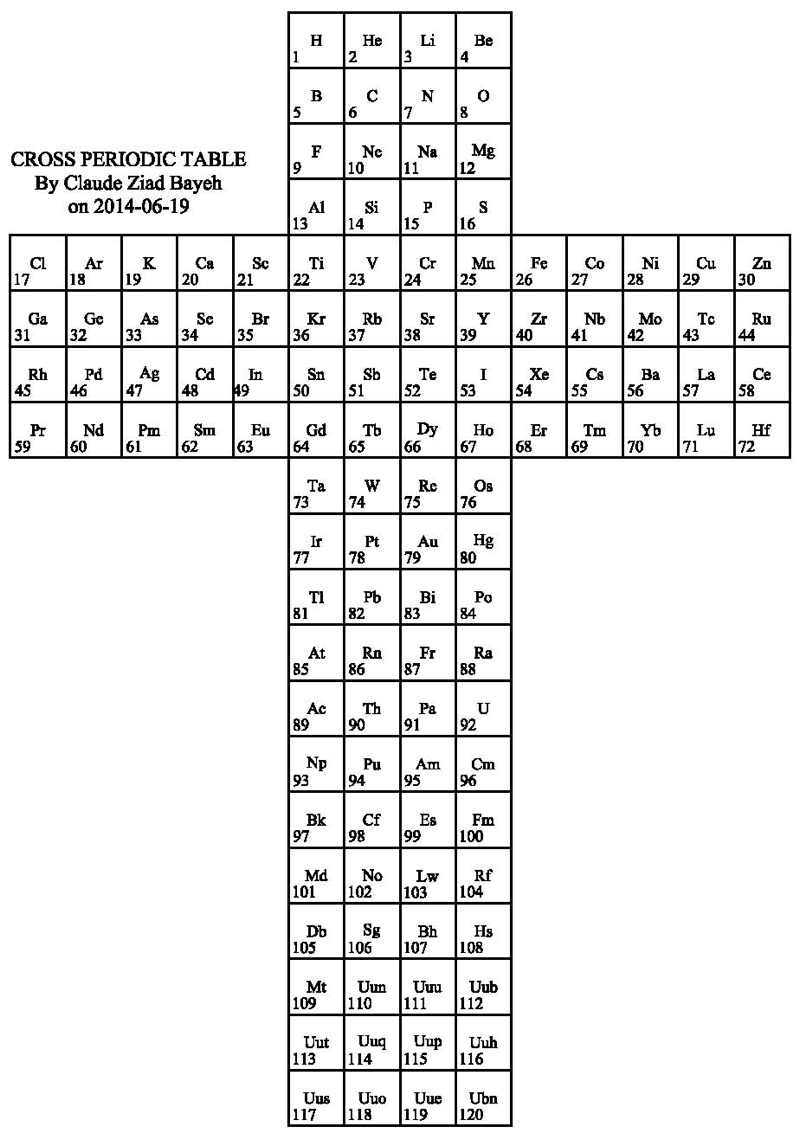
2014
Zambon's Periodic Table Based on Triads
Alfio Zambon – Universidad Nacional de la Patagonia
1. Introduction
In the last decades, the notion of triad has been recovered by Eric Scerri, who suggested it as a possible categorical criterion to represent chemical periodicity. In particular, he reformulates the notion of triad in terms of atomic number instead of atomic weights and, in this way, the value of the intermediate term of the triad is the exact average of the values of the two extremes. The author notes that the attempt of finding new triads is very important since this relation is based exclusively on the atomic number, that is, the only feature of the elements considered as basic substances. In this work, I will follow Scerri's general proposal.
Read the full paper here (pdf):
2014
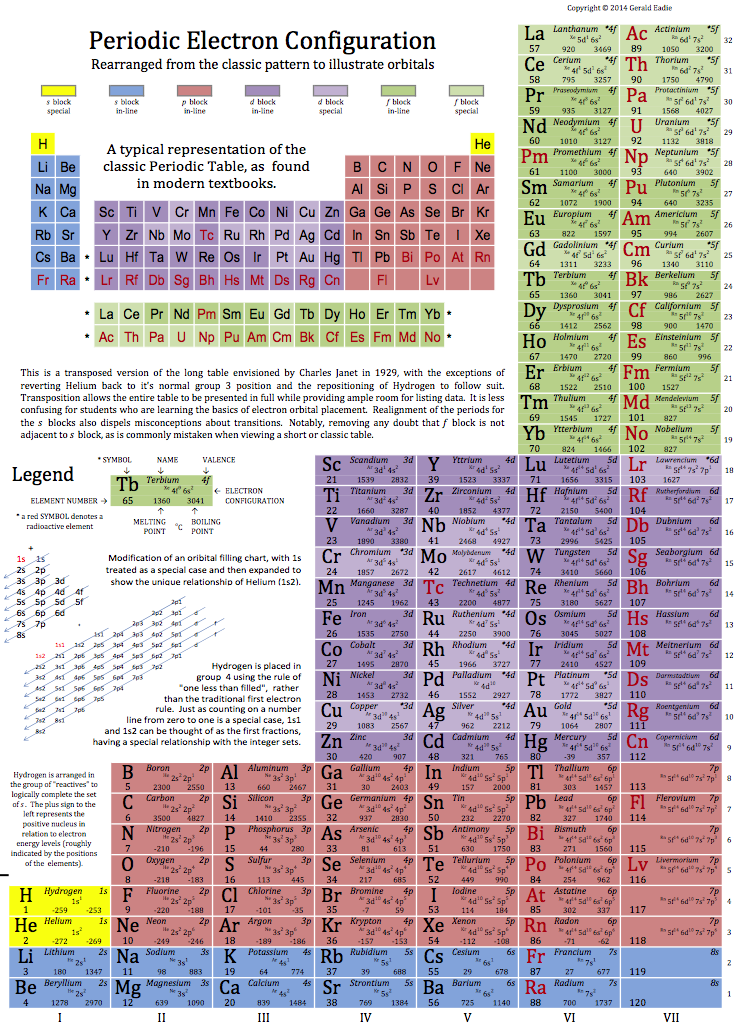
Eadie's Periodic Electron Configuration
By Gerald Eadie, a Periodic Electronic Configuration:
2014
Chandra's Polar Plot Periodic Table
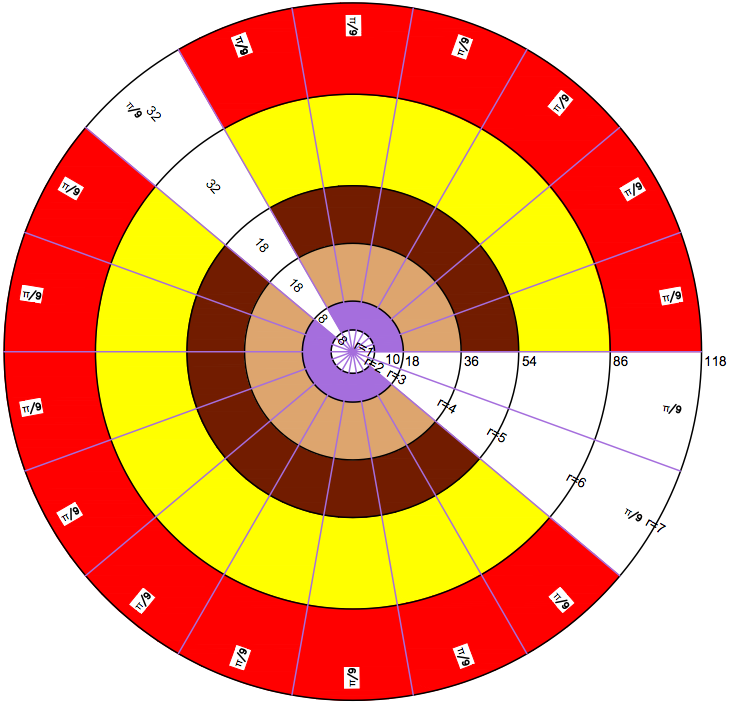
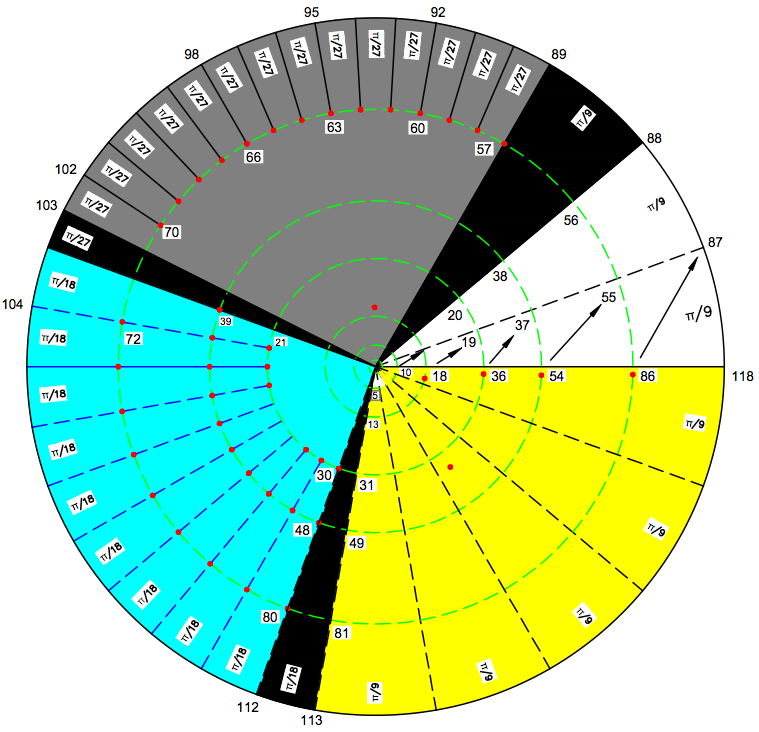
MONOGRAPH ON ATOMS, BY Dr. N. Naveen Chandra, 543 Bellamy Road North Scarborough, On, M1H1G5, 416 439 6630, chandraalex@hotmail.com >© N.Naveen Chandra, 2014.
Abstract
A new way of graphical representation of atoms is developed and presented here. Atoms are recognized as functions of two variables A(r,Θ), where r =2,10,18,36,54,86,118 (given arbitrarily r=1,2,3,4,5,6,7) represents period and Θ representing group, is actually the angle between the groups. A mathematical solution is obtained for Θ having three distinct values of (π /9) radians, (π/18) radians and (π/27) radians which define three super groups satisfying the equation 15(π/27) +10(π/18) +8 (π /9) =2π. 15 groups of two Atoms with a transition zone of (π/27) radians is nominally called Grey Super Group (GSG). 10 groups of which 9 have four Atoms and 1 has two Atoms, also including a transition zone of (π/18) radians, is nominally called Blue Super Group (BSG). 8 groups of which 7 have 6 Atoms and one has 7 Atoms, including a transition zone of (π/9) radians is called Yellow Super Group (YSG). The group with 7 atoms is the so called reference group of Atoms 2, 10, 18,36,54,86 and 118. The GSG has 30 Atoms, the BSG has 38 Atoms and the YSG has 49 Atoms. The Atom 1 is at the centre of the Hub and does not belong to any group or period and has coordinates of (0, 0). Atom 1 having no neutrons is unique.
2014
Metallic Character Table
"I would like to submit you an hexagonal periodic table. It's structured in different rings. The elements are ordered on their metallic characters so in the inner rings there are noble gases and nonmetals while in the outer rings there are alkali and alkaline earth metals. I based the order on the typical metallic characteristics: low ionization energy, electron affinity, etc... "
Marco Piazzalunga <marco.piazzalunga@live.com>

2014
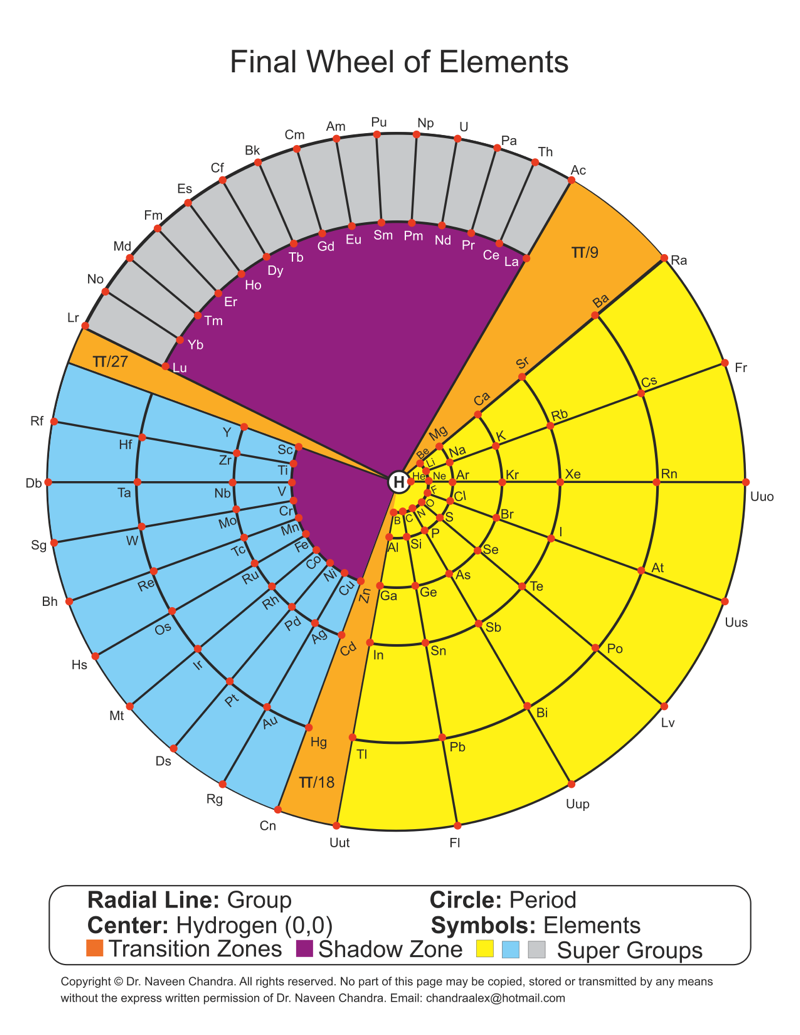
Janet Rajeuni
By Philip Stewart:
Janet Rejuvenated, with acknowledgement to Mazurs and to Valery Tsimmerman for the idea of using one square per orbital and of shifting the blocks so that each row represents one value of n, the principal quantum number.
The main objection people make to Janet is that He is placed at the head of the alkaline earth metals although it behaves as a noble gas. The essential answer is that electronic structure explains behaviour and not vice versa; like Ne (and unlike Ar, Kr, Xe and Rn), He has a complete shell. Similarly H, like C, is half way between a full and an empty shell, unlike the alkali metals and the halogens. I suggest a new argument: nobody finds it strange that the p block has a row of non-metals at its head (and that half its members are non-metals), so why not the s block?
2014
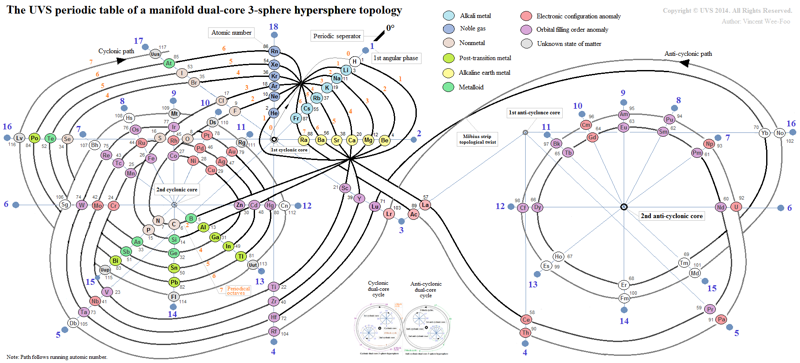
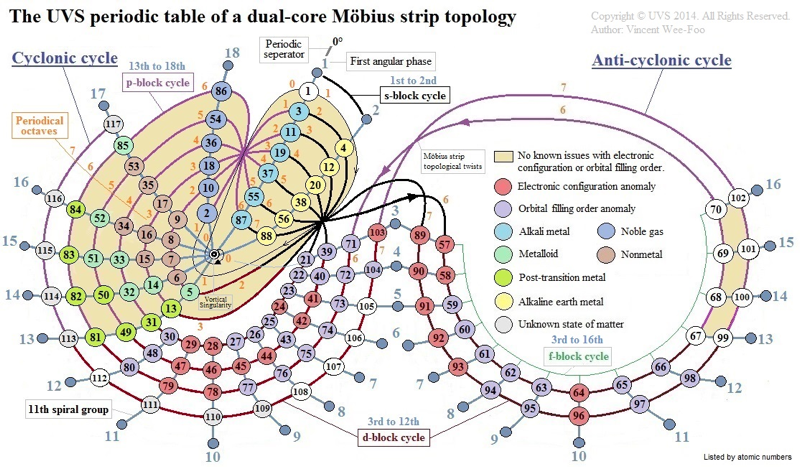
UVS Periodic Tables
From the Universal Vortical Singularity (UVS) website, two related formunations from the nucleosynthesis in the universe section, one showing a "manifold dual-core 3-sphere hypersphere topology", and the other showing a "dual-core Möbius strip topology":
2014
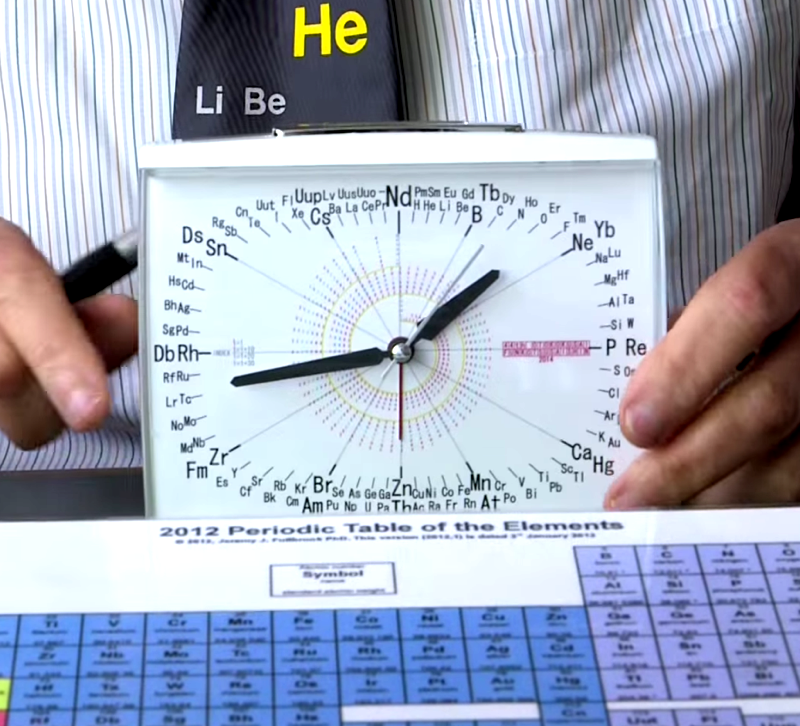
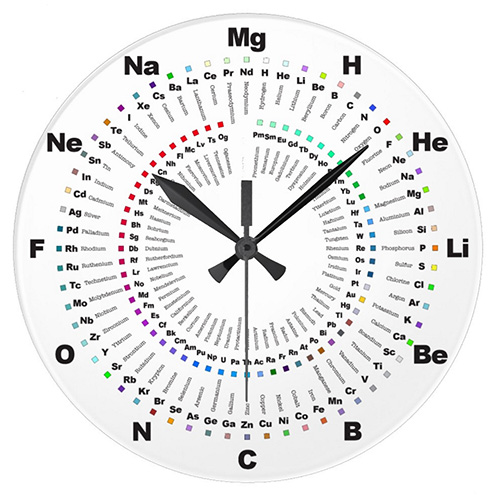
Clock Periodic Table
Prof. Martyn Poliakoff of the University of Nottingham, and star of the Periodic Videos YouTube Channel, explains how he was given a periodic table clock by a Japanese School teacher... which he likes very much:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
2014
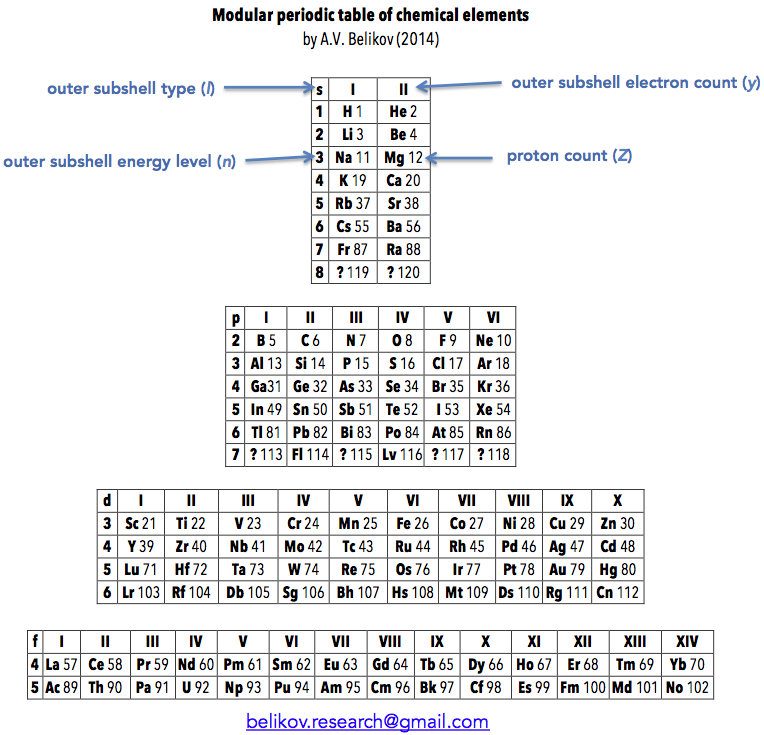
Belikov's Modular Periodic Table of Chemical Elements
"I call this version of the Modular Periodic Table of Chemical Elements. I got the idea for it some time between 2005 and 2007, during the chemistry course at my university, in attempt to rationalize the clumsy common version I was being taught. I showed it to my chemistry teacher, but he didn't seem to be impressed much, so it went into the drawer. Recently I decided to resurrect it and publish somewhere. So I had a look on the web and found your excellent database, with hundreds of versions. After the first shock, I realized that only few are actually similar to my version. These are well known Janet's table and ADOMAH table. So, it appeared to me that the idea to group elements strictly according to filling of their atomic shells is not new. However, the way I have done it is slightly different from the mentioned tables. For example, s,p,d and f blocks of elements are completely autonomous and can be placed wherever desired (hence the name 'Modular'). This reflects the notion that there is little in common in chemical behavior between the elements in different blocks. Also, outer subshell type, energy level and electron count are clearly labeled, so that these parameters can be quickly determined for each element.
"Overall, I think that this version of periodic table allows easier understanding and transition from IUPAC table and could be implemented in school and university textbooks."
Aleksey Belikov
2014
Jodogne's Janet New Colour Periodic Table
"This Periodic Table(click here for larger version) incorporates the Real Aufbau of Professor Pyykkö based on relativistic Quantum Mechanics, with Z continuity horizontally and vertically, by means of taking into account the ground level - energy increase upward - of the last incoming electron (the lower side of the element case is the level guide mark). A large yellow line indicates period. A gradual color for H induces a manifold chemical behavior."
J.C.Jodogne
2014
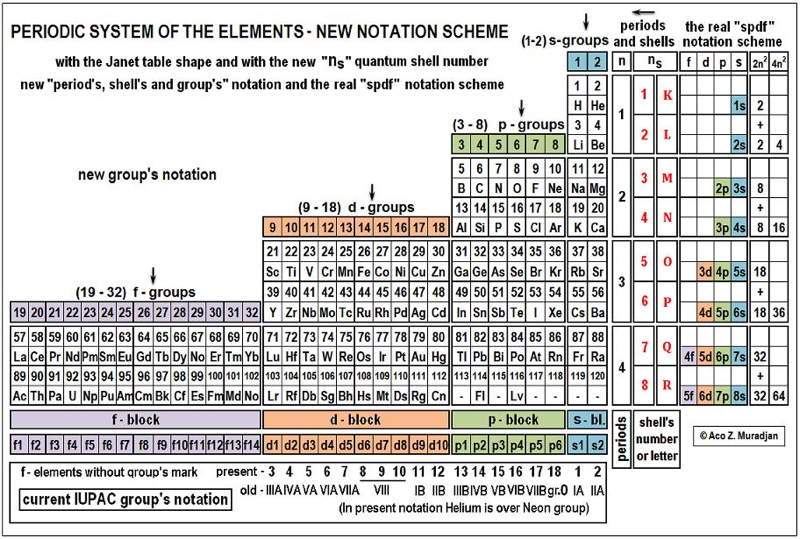
Aco Muradjan's New Notation Scheme
Aco writes:
On 08.11.2014 I added an article to the General Science Journal:
"Necessity of urgent revising and changing the present IUPAC notation scheme in the Periodic Table"
The current and present modern notation scheme for the groups in the Periodic Table exist from 1985, proposed from the IUPAC Commission on the Nomenclature of Inorganic Chemistry, as recommendation. This proposal was also verified in 2005.
Because the IUPAC Commission encourages further discussions, improvements and proposals on this subject I made this new article which article investigates the possibilities for the new notation scheme in the Periodic Table. Links:
This article has picture of the Periodic Table with new notation scheme:
2014
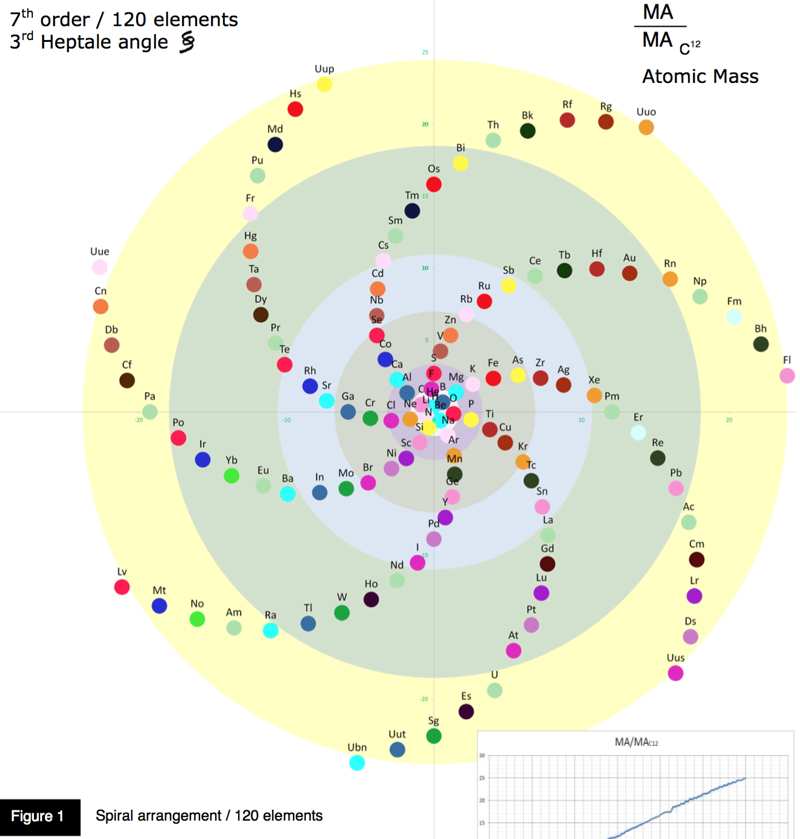
Arrangement of Elements 7th Order & Element Sequences
An exploration of some mathematics underlying the periodic table, read the PDF here, by Olivier Joseph.
Oliver says:
"May I propose you the following pattern, as the result of a personal study concerning the arrangement of the Elements, including sequences. Based on some hypothesis and as depicted in the enclosed illustrations, the elements are positioned according to a spiral function of atomic number and atomic mass, representation in 2D in a spiral form pattern, or in 3D conical helix model.
"The elements are numbered and placed consecutively along this spiral according to a specific angle, appropriately established between each element, forming a seven arm spiral pattern. With such an angle, specifically defined, a link is established between the various elements of a same group (corresponding to chemical elements with similar properties) and different layers. These latter becoming distributed among each arm of the spiral in a notable arranged way."
2014
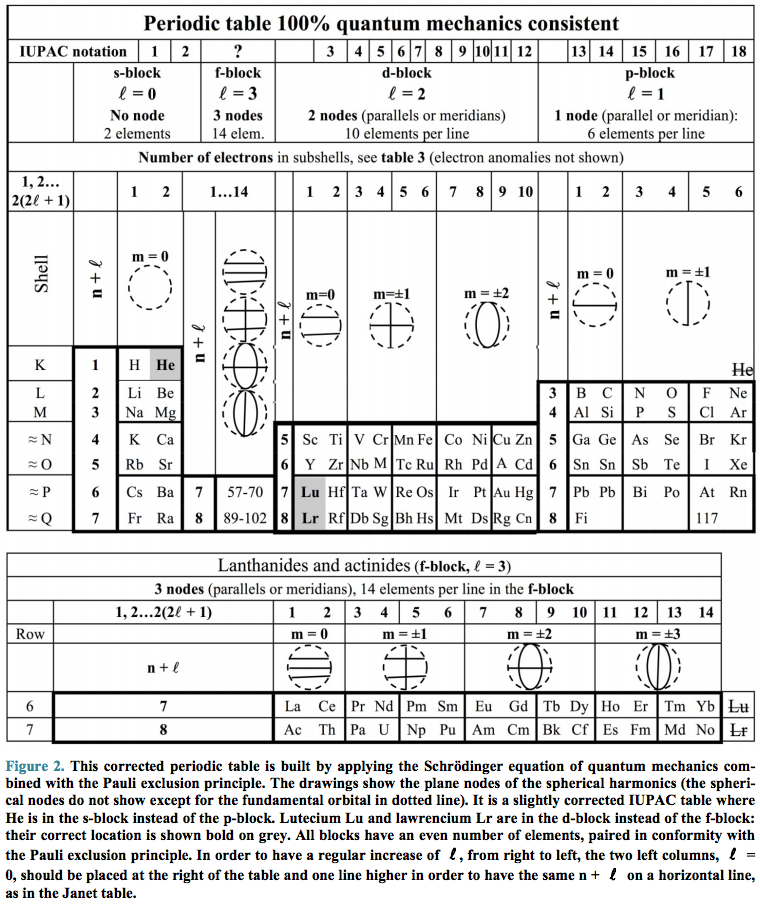
Schaeffer's IUPAC Periodic Table Quantum Mechanics Consistent
IUPAC Periodic Table Quantum Mechanics Consistent, Bernard Schaeffer, Journal of Modern Physics, Vol. 5, No. 3, February 24, 2014
DOI: 10.4236/jmp.2014.53020
Abstract: Most periodic tables of the chemical elements are between 96% and 100% in accord with quantum mechanics. Three elements only do not fit correctly into the official tables, in disagreement with the spherical harmonics and the Pauli exclusion principle. Helium, belonging to the s-block, should be placed beside hydrogen in the s-block instead of the p-block. Lutetium and lawrencium belonging to the d-block of the transition metals should not be in the f-block of the lanthanides or the actinoids. With these slight modifications, the IUPAC table becomes quantum mechanics consistent.
2015
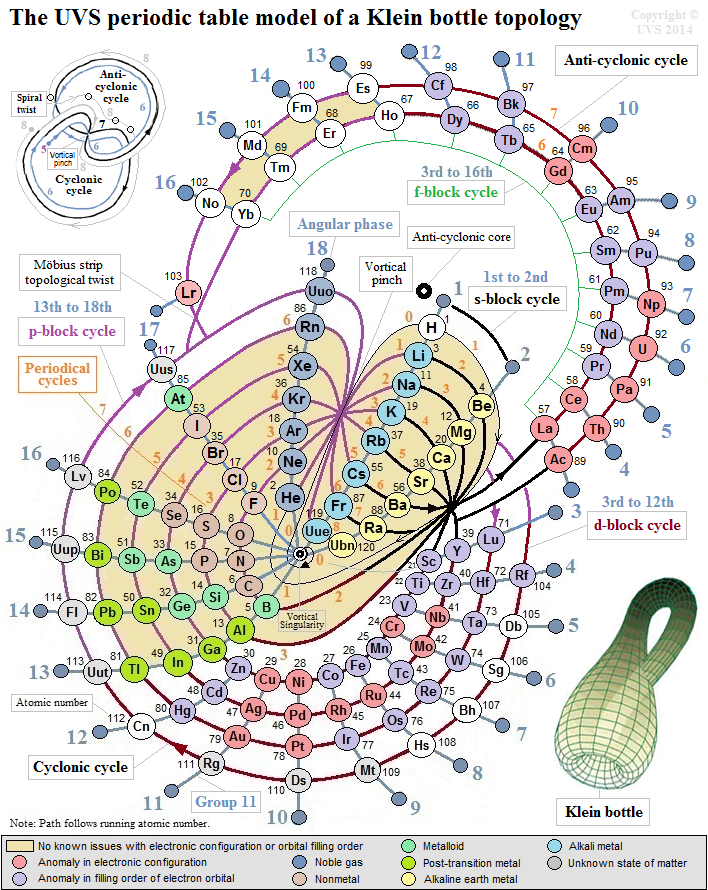
UVS Periodic Table Model of a Klein Bottle Topology
This configuration can topologically suggest the g-block cycle in the 8th period for extended periodic table.
In the Klein bottle topology as illustrated, it is plausible that after the s-block cycle in the 8th periodical cycle, the topological path continues to spiral around the outer f-block cycle to harmonically form 14 elements.
And then subjected to the spiral Möbius strip topological twist, it could resonate to form 4 more elements in the anti-cyclonic path around 17th, 18th, 1st, and 2nd angular phases of the anti-cyclonic core; this would render the 18 elemental positions for the hypothetical g-block cycle in the entire half-integral anti-cyclonic cycle of the Klein bottle topology.
Hypothetically, the topological path then moves into the cyclonic cycle, and harmonically forms its d-block and p-block cycles with 16 elemental positions to complete the 8th periodical cycle with a total of 36 elements.
2015
Quantum Fold Periodic Table
A Multi-Form Periodic Table, by keytochemistry.com, with a visual key to electronic configurations:
2015
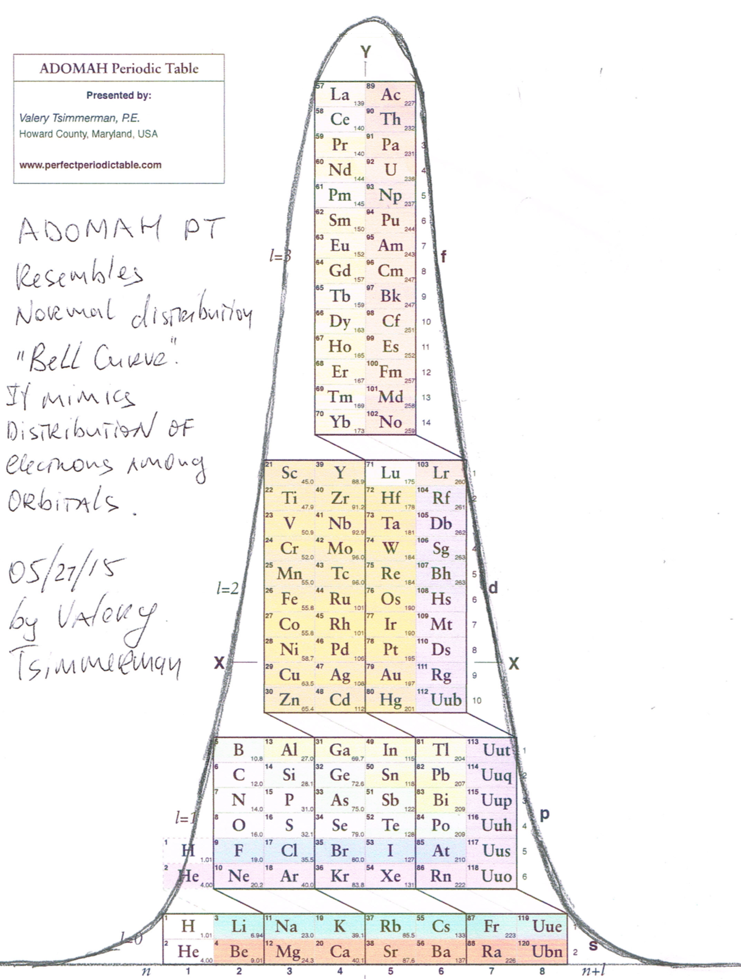
ADOMAH Periodic Table and Normal Distribution
Valery Tsimmerman writes:
The ADOMAH, from here, resembles the normal distribution or "Bell Curve". It also mimics the distribution of electrons in orbitals:
2015
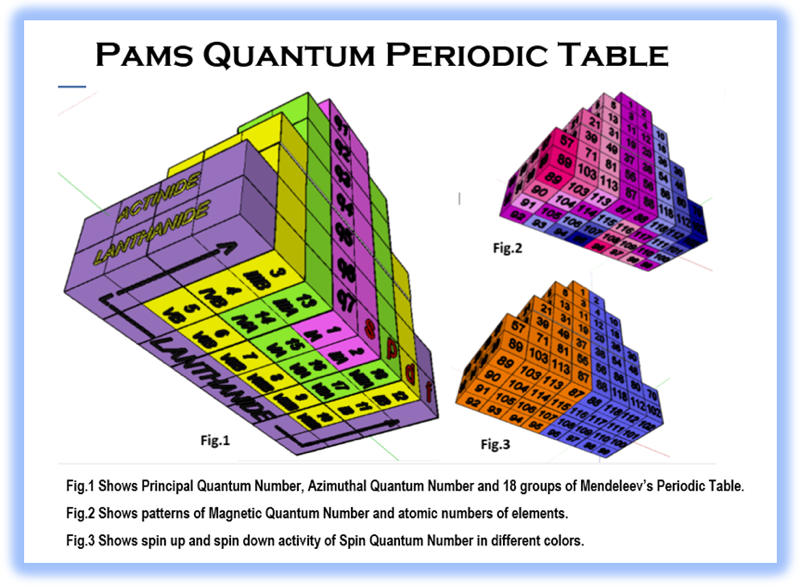
Pams Quantum Periodic Table
By Dr. N. D. Raju, the Pams Quantum Periodic Table. Read the full paper discussing the logic of the new formulation.
2015
Heart Periodic Table
A Heart Periodic Table by Claude Bayeh:
2016
Mystery of Matter: Three Videos
From Alpha-Omega, three videos about the discovery of the Periodic Table.
The Mystery of Matter: Search for the Elements is an exciting series about one of the great adventures in the history of science: the long and continuing quest to understand what the world is made of. Three episodes tell the story of seven of history's most important scientists as they seek to identify, understand and organize the basic building blocks of matter.
The Mystery of Matter: Search for the Elements shows us not only what these scientific explorers discovered but also how, using actors to reveal the creative process through the scientists' own words and conveying their landmark discoveries through re-enactments shot with replicas of their original lab equipment.
Knitting these strands together is host Michael Emerson, a two-time Emmy Award-winning actor.
Meet Joseph Priestley and Antoine Lavoisier, whose discovery of oxygen led to the modern science of chemistry, and Humphry Davy, who made electricity a powerful new tool in the search for elements.
Watch Dmitri Mendeleev invent the Periodic Table, and see Marie Curie's groundbreaking research on radioactivity crack open a window into the atom.
The Mystery of Matter: Search for the Elements brings the history of science to life for today's television audience.:
2016
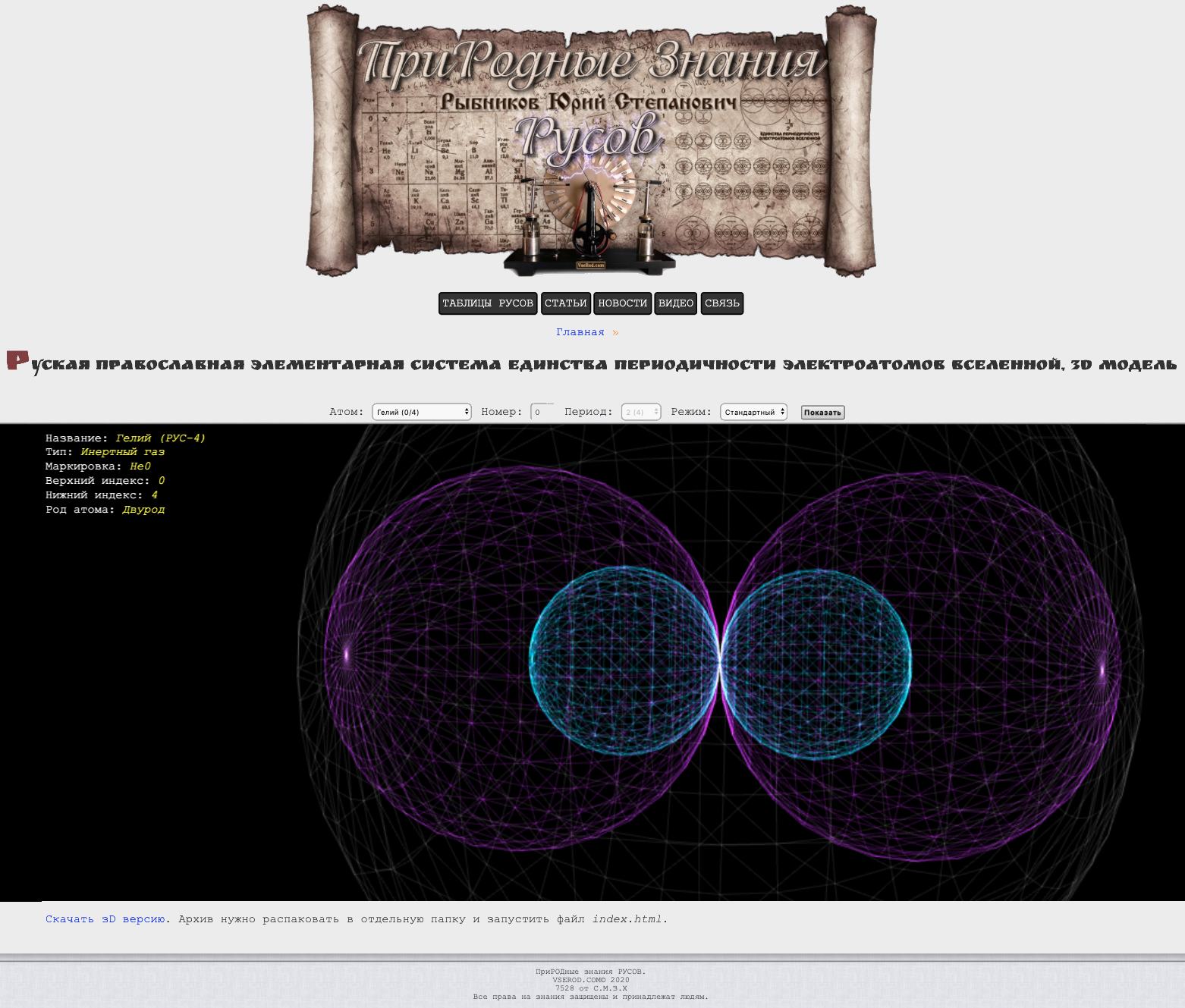
Russian Orthodox Elementary System of Unity of the Periodicity of the Electroatoms of the Universe
By Bence Szalai: Russian Orthodox Elementary System of Unity of the Periodicity of the Electroatoms of the Universe
See the 2D version here and the 3d vesion here (in Ukrainian)
2016
Genoma de la Materia
By Julio Antonio Gutiérrez Samanez, Genome of Matter:
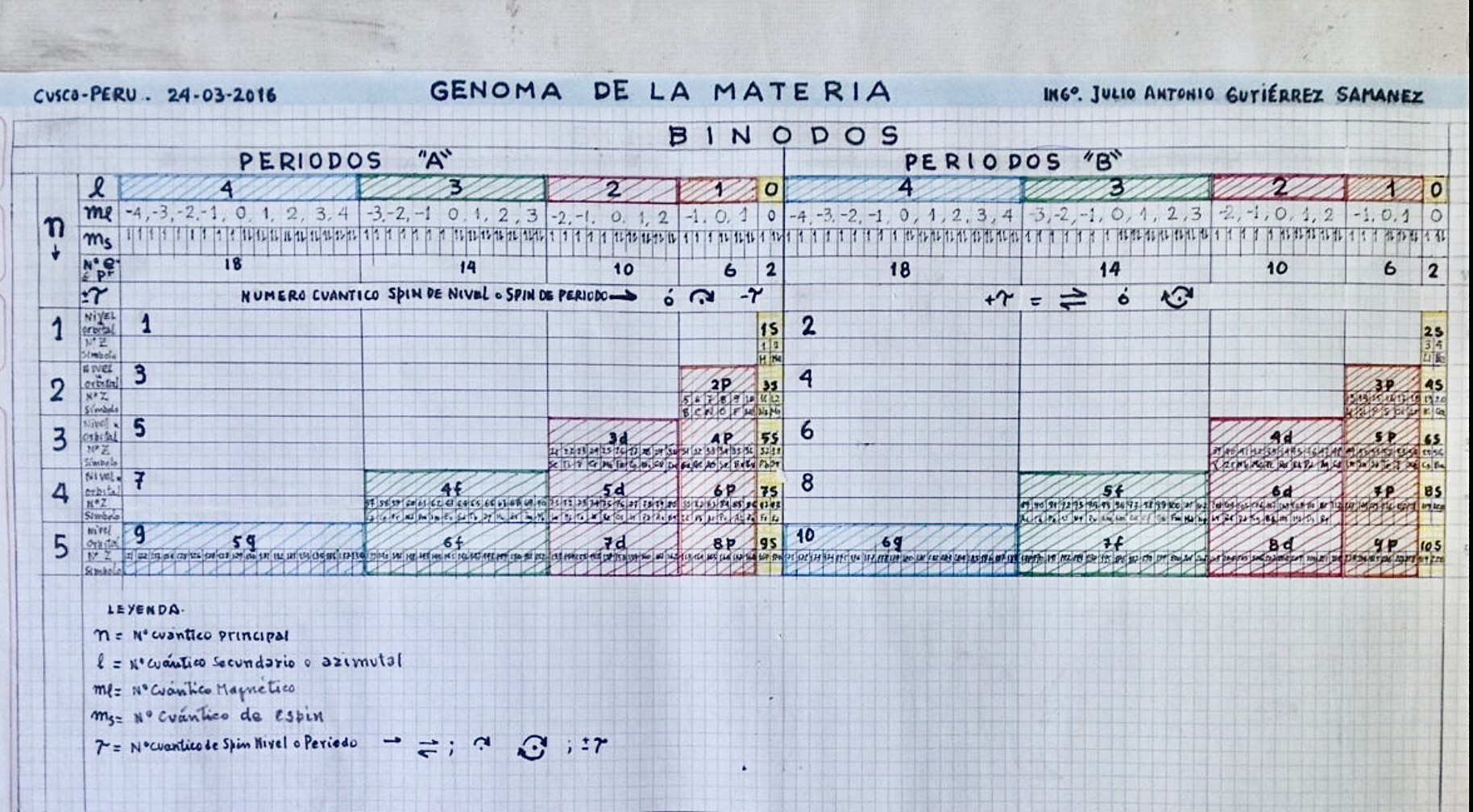
2016
Valentine Periodic Table
A Valentine Periodic Table by Claude Bayeh:

2016
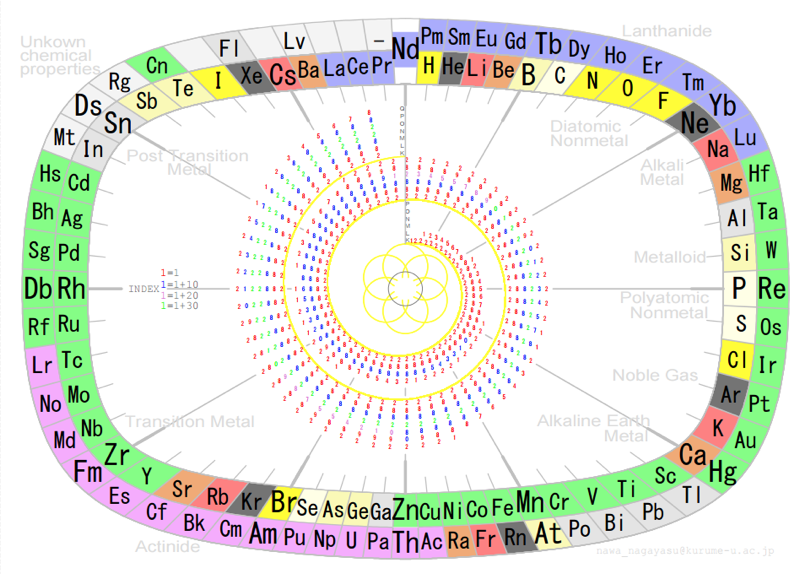
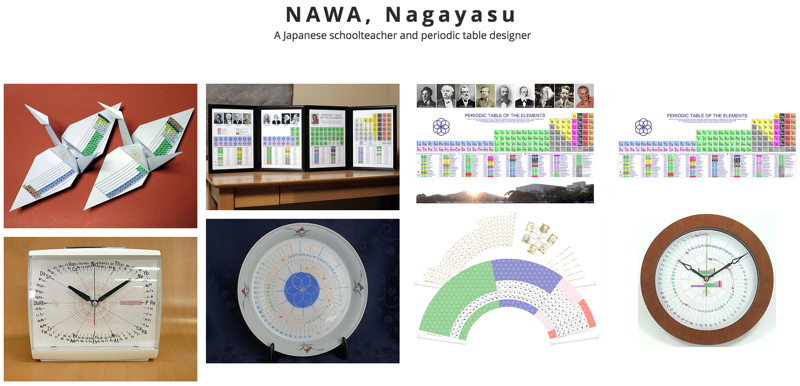
NAWA's byobu-Janet Periodic Table
NAWA, Nagayasu: A Japanese schoolteacher and periodic table designer presents a Janet form periodic table in the traditional Japanese "byobu" style:
2016
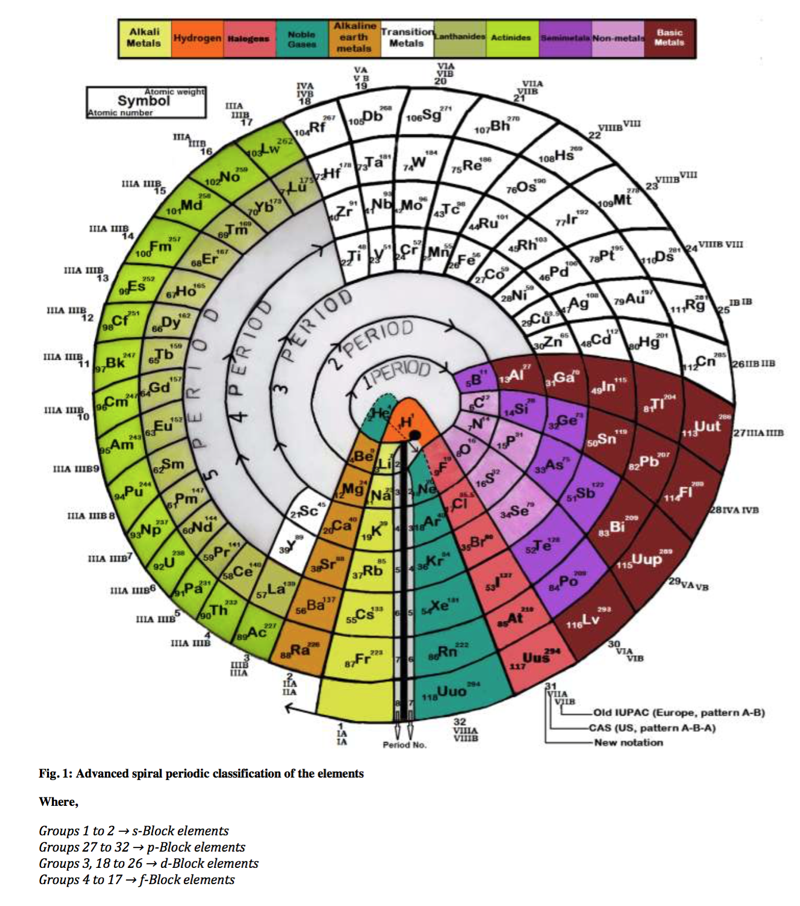
Advanced Spiral Periodic Classification of the Elements
By Imran Ali, Mohd. Suhail and Al Arsh Basheer an Advanced spiral periodic classification of the elements. Read the paper here.
2016
Triadic Networks
From orijikan.com: a great summary paper by Dr. Eric Scerri on the role of triads in evolution of the periodic table and a paper by Dr. Alfio Zambon inspired this work. Here is my contribution: the Triadic Networks (TN), which is a general mathematical design, and the Triadic Elemental Networks (TEN), that apply that design to chemical elements. For a full discussion, read the pdf here.
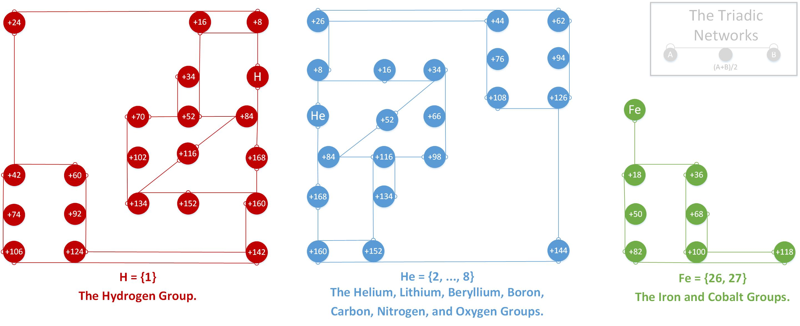
2016
Clock Face Periodic Table
In 2014 Prof. Martyn Poliakoff – of YouTube fame – showed us a working Periodic Table clock, here.
The designer of the clock, Nagayasu (a Japannese school teacher), has now provided a fuller periodic table based on the same design:
2016
Sensu or Fan Periodic Table
By NAWA, Nagayasu — A Japanese schoolteacher and periodic table designer — a "Sensu" or fan periodic table:
2016
KAS Periodic Table
The KAS periodic table reproduces and depicts the nuclear properties of chemical elements. This periodic table depicts not only the trends of nuclear properties, but also reproduces their numerical values that remain very close to the experimental values (difference less than 4%).
The Segre Chart is based on the number of protons, Z, and the number of neutrons, N. It is like a library of nuclei and shows the recorded data only. The Segre Chart can not work when the number of neutrons is not given. But KAS Periodic Table works when the number of neutrons is not given.It does not require the number of neutrons to produce the results.This is a simple chart based on the number of protons of chemical element. We identify the following properties of elements:-
- Location that remains near the Neutron Dripline of element.
- Location that remains very close to stable or long-lived isotopes of the element. Location that remains near the Proton Dripline of element.
- In the case of superheavy elements, we identify which Compound Nuclei are involved in the Hot Fusion reaction and which Compound Nuclei are involved in the Cold Fusion reaction.
- We see the r-process path and assess the r-process abundance.
- The pattern of abundance of chemical elements.
- We identify which elements are the product of exothermal fusion.
- We identify the location of isotope on the basis of two-neutron separation energy.
- Nuclear binding energy trend. Beta decay trend.
- We see the Straight Line of Nuclear Stability.
- Empirical Law discovered.
- Periodicity in the nuclear properties.
- We can compare the nuclear properties of an element with the nuclear properties of almost all the chemical elements.
Read more here, here and here.
2016
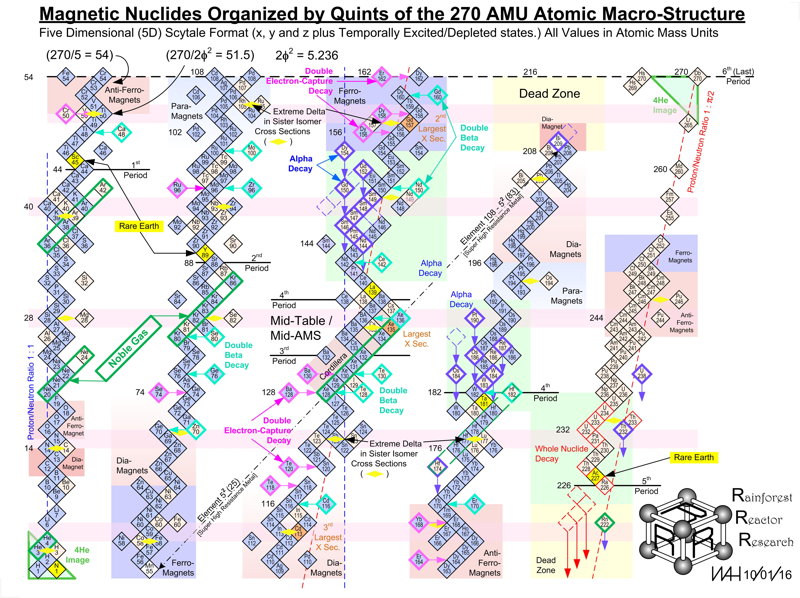
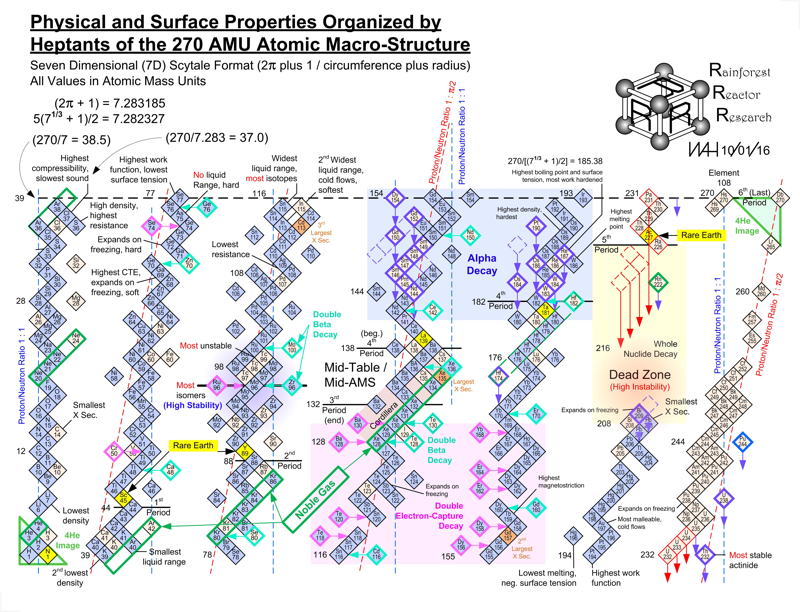
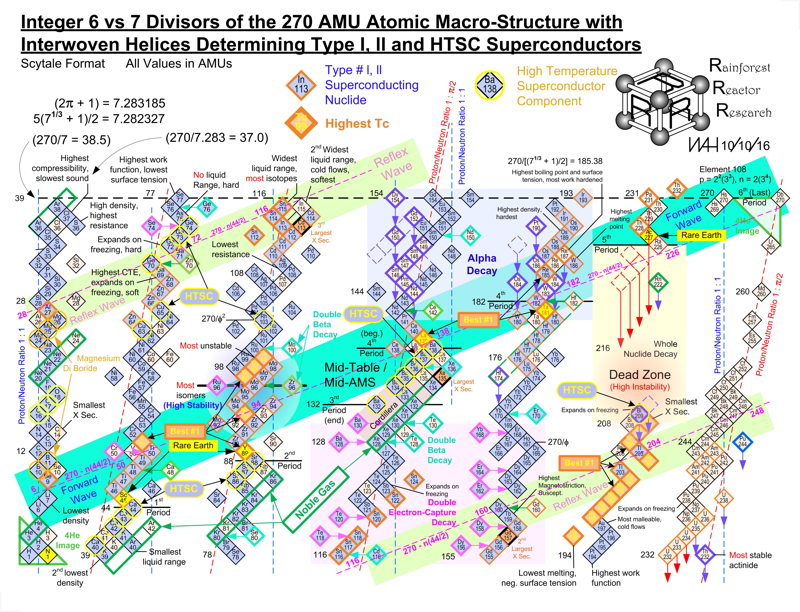
Harrington Periodic Tables
So we start this effort tabula rasa (without preconceived ideas).
1) All atoms have a default "common denominator" structure at 270 mass units, irrespective of the element under discussion. Therefore, no elements seen as wisps and glints past this point are of consequence. Ergo, the bizarre stability of Dubnium 270.
2) This common structure is divided up by the exact same divisors as are the electron orbitals - i.e. the prime numbers of 2, 3, 5, and 7.
3) Pi as a divisor produces its own, unique and dominating organizational patterns.
4) Each of these sets of plotted nuclide "boxes" use identical formats, but are arranged in vertical columns based on the set of 270 AMUs being divided by these prime numbers. So the 5D Table is 270/5 or 54 AMUs per vertical column/"tower".
5) Each system reinforces unique elemental parameters. The system based on 3/Pi, and its second "harmonic" at 6/2Pi reflects physical properties. The 2Pi configuration almost exactly emulates the "conventional" / Mendeleevian element-based table, except the periods are based upon mass not element count, and these periods do not organize in rows of 18 elements, but rather rows of 44 mass units. The organization/configuration of this default structure is: Pi(Pi^2 + Pi + 1) = 44 This is the primary physical default structure of the periodic table and spectrum of elements, as projected in 3D space, and as perceptible to humans.
6) 5D determines everything with magnetic properties. This disproves every single theory that attributes electron shell behavior as determining magnetic parameters. Clearly here we see that the nucleus is "calling the shots", with electron orbitals conforming as driven. The various red and blue shaded boxes are found at extremes of top and bottom.
7) The system of 7D determines most of all physical parameters of surface and molecular behavior. Here we see surface tension, density, softness and hardness, malleability, boiling and melting points and a few other behaviors. This system of correlation is fully unknown to conventional theory. Notice how superlative parameters bunch at the top and bottom of this configuration.
8) When this system of 270 mass units is divided by 12, for 22 mass units per period, the periodic cycle rate precisely correlates with known Type 1 and 2 elemental superconductors. The physical correlations between periodic repetition at 22 mass units, the 270 count system, and superconductors is also completely novel and not compatible to conventional BCS theory. The correlation between this 22 count system and the three largest cross section nuclides known to man (113Cd, 157Gd and 135Xe) is also completely heretical, however mathematically symmetrical and perfect it may actually be organized.
9) The center portion of this common 270 count structure is named the "Cordillera", for the habit of multiple parallel mountain ridges sharing a common alignment. This area is profoundly affected by Pi-based organizations. The very center at 135Xe indicates that the overall table should terminate at element 108 Hassium at 270 mass units. This has a Proton/Neutron ratio of 3:2. This actual nuclide has very poor stability, unlike Dubnium 105 with 270 mass counts. This nuclide has a ratio of precisely 1:Pi/2, indicating the entire table describes a spectrum of mass organizational states spanning the integer ratio of 1:1 (Deuterium) to 3:2, then on through to 1:Pi/2. Current accepted atomic theories concerning "Islands of Stability" are ridiculous.
WAH
Click on the image to see the full size version
2016
Lindsay's Periodic Table
From Geoffrey Lindsay:
"I put together a table of elements that may be useful for teaching 101 chem from the point of view of valence electrons and the energy sublevels of the valence orbitals".
Click image to see a larger version.
2016
Complete Periodic Table Chemistry Clock: H to Og
From MrEorganization: "I've created a new periodic table clock for my son, a chemistry undergrad at Whitman College, a holiday present and a celebration of the official new names."
- Hours are H to Mg Minutes H to Nd, then add 60 to the inner ring for Pm to Og.
- Colors match the ones used in the Jmol chemical structure viewer.
- I printed it though Zazzle, so anyone who wants one, can purchase it.
2016
Instructables 3D Periodic Table
From Makendo on the Instructables website:
The first periodic table was developed in 1862 by a French geologist called Alexandre-Émile Béguyer de Chancourtois. He plotted the elements on a cylinder with a circumference of 16 units, and noted the resulting helix placed elements with similar properties in line with each other. But his idea - which he called the "Telluric Spiral" (see here), because the element tellurium was near the middle - never caught on, perhaps because it was published in a geology journal unread by chemists, and because de Chancourtois failed to include the diagram and described the helix as a square circle triangle.
Mendeleev got all the glory, and it is his 1869 version (dramatically updated, but still recognizable) that nearly everyone uses today.
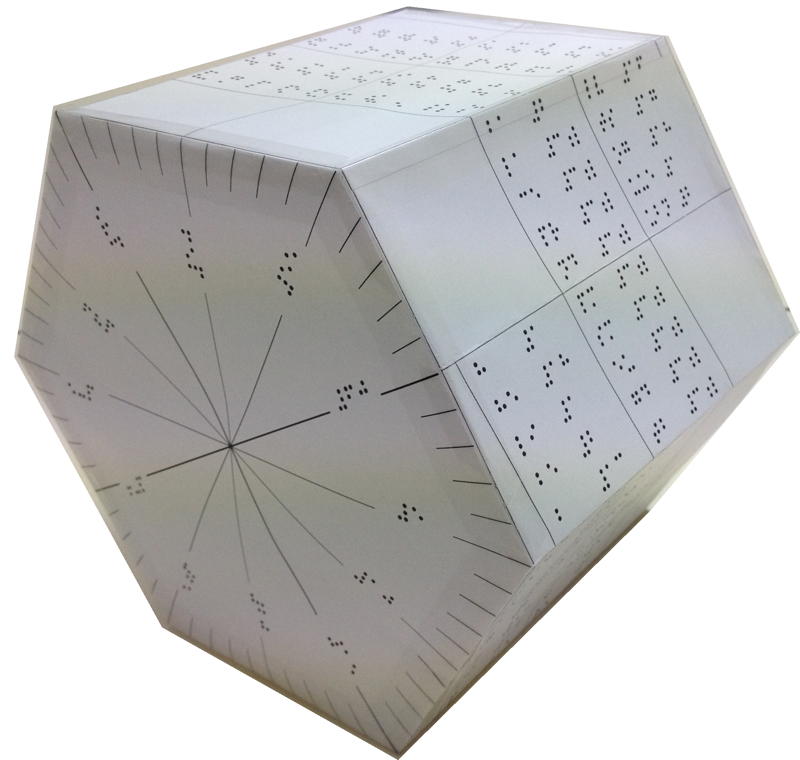
This instructable [project] documents my efforts to reimagine a 3D periodic table of the elements, using modern making methods. It's based on the structure of a chiral nanotube, and is made from a 3D printed lattice, laser cut acrylic, a lazy susan bearing, 118 sample vials and a cylindrical lamp.
2017
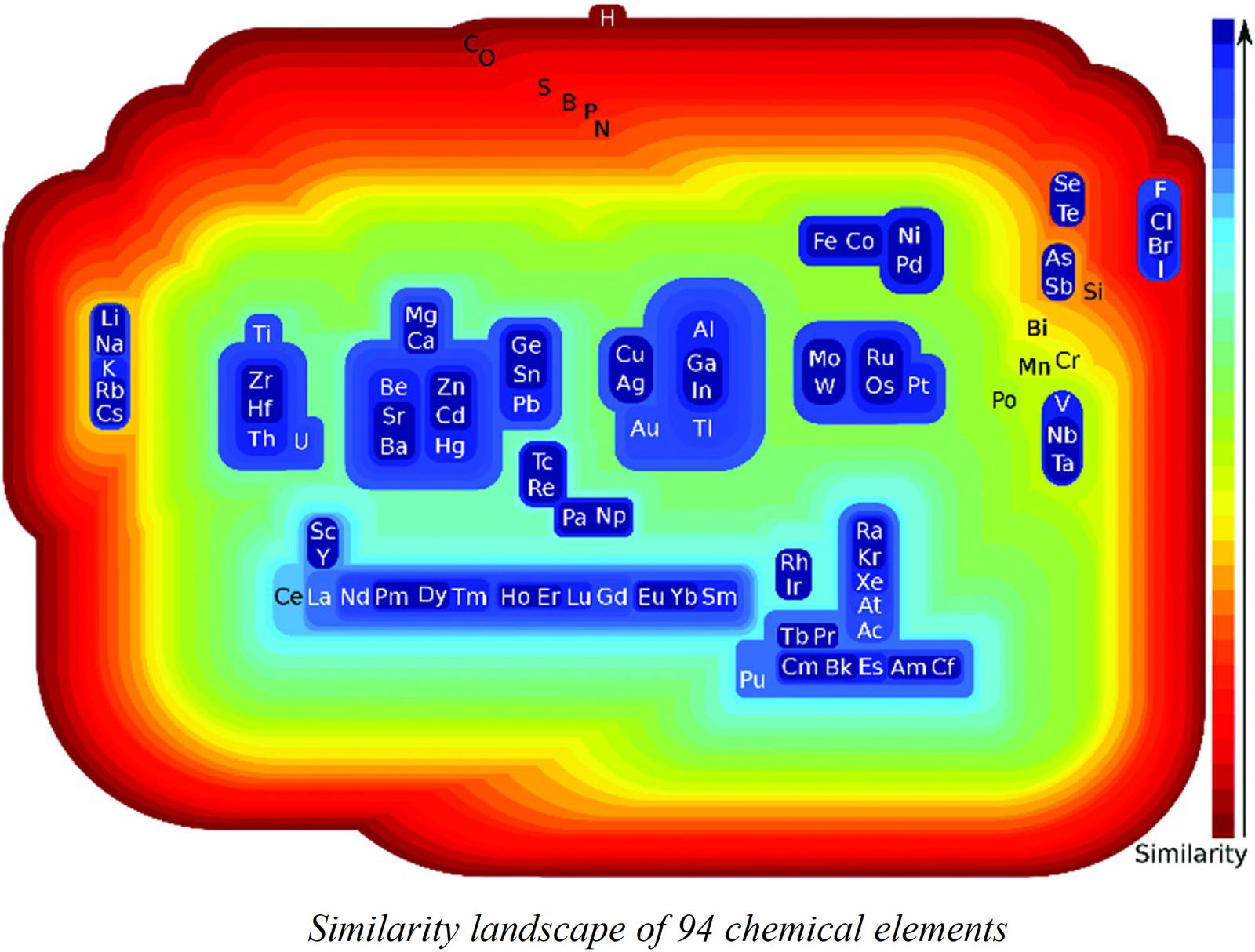
Restrepo's Similarity Landscape
Building Classes of Similar Chemical Elements from Binary Compounds and Their Stoichiometries by Guillermo Restrepo, Chapter 5 from: Elements Old and New: Discoveries, Developments, Challenges, and Environmental Implications p 95-110.
From the abstract:
Similarity is one of the key concepts of the periodic table, which was historically addressed by assessing the resemblance of chemical elements through that of their compounds. A contemporary approach to the similarity among elements is through quantum chemistry, based on the resemblance of the electronic properties of the atoms involved. In spite of having two approaches, the historical one has been almost abandoned and the quantum chemical oversimplified to free atoms, which are of little interest for chemistry. Here we show that a mathematical and computational historical approach yields well-known chemical similarities of chemical elements when studied through binary compounds and their stoichiometries; these similarities are also in agreement with quantum chemistry results for bound atoms. The results come from the analysis of 4,700 binary compounds of 94 chemical elements through the definition of neighbourhoods for every element that were contrasted producing similarity classes. The method detected classes of elements with different patterns on the periodic table, e.g. vertical similarities as in the alkali metals, horizontal ones as in the 4th-row platinum metals and mixed similarities as in the actinoids with some transition metals. We anticipate the methodology here presented to be a starting point for more temporal and even more detailed studies of the periodic table.
Thanks to René for the tip!
2017
Elements Known and now Named in the Year 2017
Elements in the year 2017, now the elements 113 – 118 have been named: Nihonium (113, Nh), Flerovium (114, Fl), Moscovium (115, Mc), Livermorium (116, Lv), Tennessine (117, Ts) & Oganesson (118, Og) have been named.
Taken from this Wikipedia page:
2017
Kurushkin's Spiral Periodic Table
Mikhail Kurushkin has a way of constructing the standard long form periodic table from the Janet Left-Step formulation.
Mikhail writes in his J.Chem.Educ paper DOI: 10.1021/acs.jchemed.7b00242; J. Chem. Educ. 2017, 94, 976?979
"Addition of another s-block to the left of the left-step periodic table [enables it] to be rolled into a spiral so that the left and right s-blocks are merged together and the number of elements is exactly 118. The resulting periodic table is called the "spiral" periodic table, which is the fundamental representation of periodicity":
2017
Alternative Periodic Table
From Useful Charts:
You'll notice that this periodic table looks quite a bit different from the one you're used to. The traditional periodic table is designed to emphasize the concept of valence, which is important for knowing which elements can easily combine with others to form compounds. In contrast, the periodic table below is designed to simply emphasize the way in which atoms are "built" (specifically, how electrons group together into shells and subshells).
It's based on a design proposed by Edward Mazurs in the 1960s. Like the traditional table, this alternative version can be used to find an elements name, number, atomic weight, state of matter, period, group, and block. However, it also contains detailed information on electron configurations and the different types of electron subshells.
2017
New Rendering of ADOMAH Periodic Table
From Valery Tsimmerman, of the PerfectPeriodicTable.com and the ADOMAH Periodic Table:
"I received email from Dr. Marcus Wolf who is a chemist, working on renewable energy and electrochemical storage in Germany, near Nuremberg. He also lectures at Georg Simon Ohm, Technische Hochschule Nürnberg. Attached to his email was new version of ADOMAH Periodic Table that he created. In this new rendering he is using Jensen's Valence Manifold (VM)."
This is what Dr. Marcus Wolf wrote:
"The first one to come up with the idea of using a valence manifold VM = [e + v] as a label for the groups, was Will B. Jensen. He derived it from the very early attempts of Richard Abegg, who, at around 1904, brought up the hypothesis of 'main- and counter-valences', derived from the observable behavior of elements and their compounds in electrochemical experiments. Eric Scerri is citing Jensen in his latest book, in the chapter about Richard Abegg. But Jensen's proper article from 1983 or so is far more detailed and in his later publications he then introduces the valence manifold concept. Last weekend I accidentally observed another consistency between the G-values and their ordering and the valence electron counts, e. If you fix the e value of the starting group in a given l-block as e(initial), you could generate every G-number of a given group by adding the valence vacancy count, v, to it:
G = e(initial) + v.
"That is another hint for the consistency of the VM labelling concept."

2017
NAWA Periodic Tables
Nagayasu Nawa - "A Japanese school teacher and periodic table designer" - has a home page showing all his designs:
2017
Stowe's A Physicist's Periodic Table UPDATED
Stowe's 'A Physicist's Periodic Table' was published in 1989, and is a famous & well respected formulation of the periodic table.
Since 1989 quite a number of elements have been discovered and Jeries A. Rihani has produced an updated and extended version. Click here to see the full size .pdf version:
2017
Clock Prism Periodic Table, Braille Version
From the prolific Nagayasu Nawa, a Braille version of the Clock Prism periodic table:
2017
Stewart's Chemosphere
P J Stewart, a good friend of the periodic table database, has mapped a PT onto a sphere.
PJS writes: "It is Janet Rajeuni 2014 wrapped round a sphere, going back to Mazurs 1965, and Tsimmerman 2006. Arabic numerals indicate shells (values of principal quantum number); Roman numerals indicate periods."
2017
Moran's Periodic Spiral (Updated)
Jeff Moran has updated his 1999 Periodic Spiral.
Click here for a larger version.
Jeff says: I offer the attached spiral formulation as a way of expressing the relationships of the f and d blocs to group 3:
- La and Ac are assigned to the Ln and An series, respectively
- The f block series is within, though apart from, the d block
- The group 3-ish relationship of Ln and An to Sc (and, by extension, to Y) is implied
- The group 3 status of Lu and Lr is explicit
2018
First Ionisation Energy to the Standard Form Periodic Table
There is debate amongst the cognoscenti about the 'best' representation of the periodic table, and how this 'best' formulation can be explained by [rationalized by] quantum mechanics (QM).
Many feel that the Janet PT formulation, the 'Left Step', is the ideal QM PT, but this formulation does not show periodicity very well, and there are issues with the placement of H, He, Be which spill over into questions about their placement in the standard form PT (the periodic table used in classrooms and textbooks around the world).
However, it is possible to get to the conventional standard form PT directly from the first ionisation energy data, where the 1st ionisation energy is the energy required to convert a gas phase atom (M) into its gas phase positive ion plus electron.
M(g) → M+(g) + e–
The process involves:
- taking the 1st ionisation data plot for the elements H to Xe (Z = 1 to 36)
- rotate 90° clockwise and stretch
- move the atoms horizontally into columns
Note that a similar logic can be applied to atomic radius and electronegativity data.
However, there are issues about the measurement of atomic radius, because atoms are 'soft at their edges', and gas phase atomic radius is not precisely defined. And, electronegativity is a derived parameter.
By Mark Leach
2018
Stowe-Janet-Scerri Periodic Table (Extended)
Stowe's A Physicist's Periodic Table was published in 1989, and is a famous & well respected formulation of the periodic table.
Since 1989 quite a number of elements have been discovered and Jeries A. Rihani has produced an updated and extended version in 2017. This has been further updated, below. Click for the full size .pdf version:
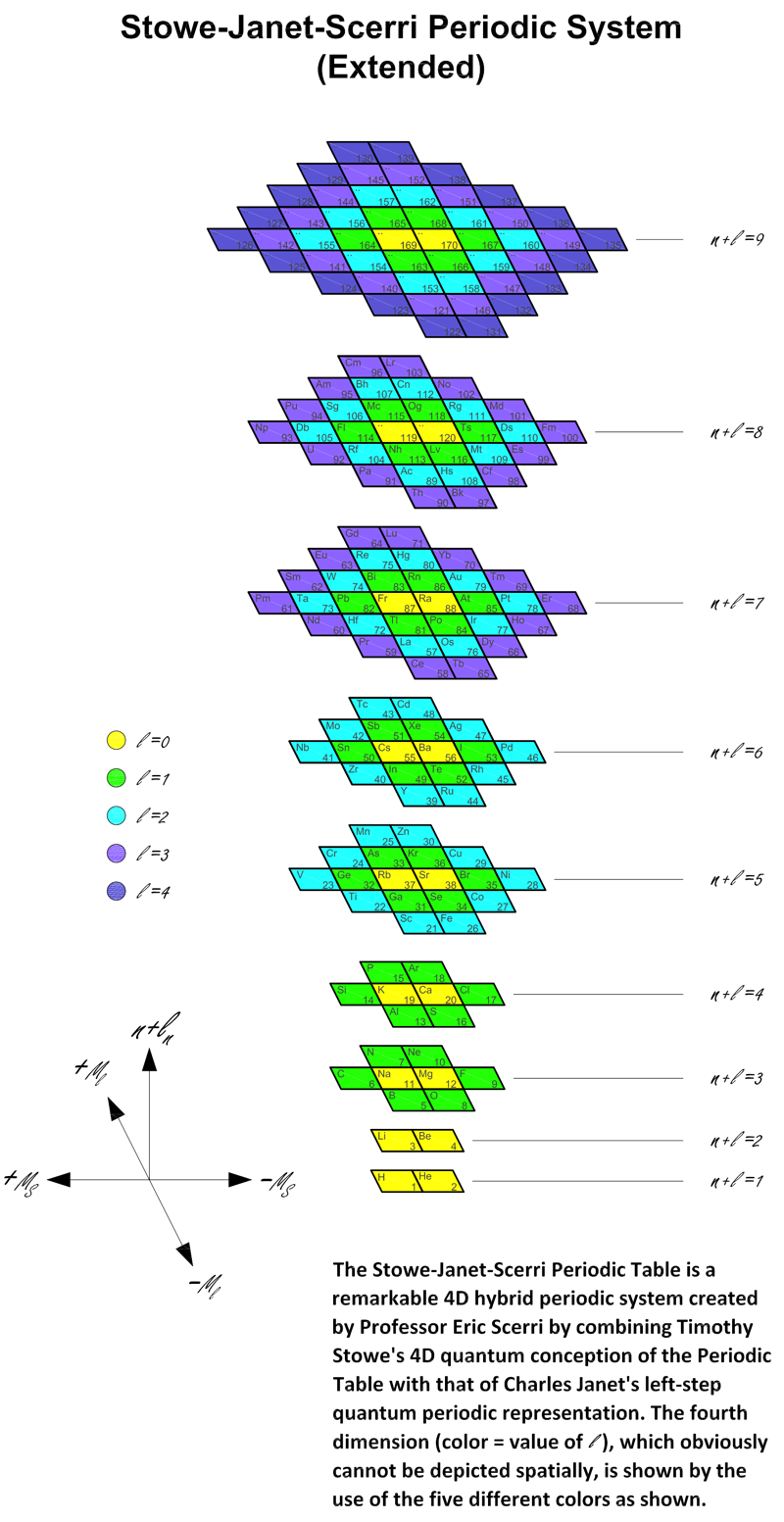
2018
Chemical Galaxy III
Updated from Philip Stewart's Chemical Galaxy II, version III shows all the recently discovered elements, including: 117 (Ts) and 118 (Og).
Click here for the full size .PDF version (which gives more data):

2018
Hoyau's Periodic Table Formulations
From Davy Hoyau in France, a selection of periodic table formulations. Davy writes:
"I can explain how i came up to this solution, to better represent graphically the mathematics of the nature. The great advantage of that solution is to make visible how important are the subrings, and not the rings, of the electronics layers. That's why we can call this representation "the table of subrings".
"They seem to be responsible of element chemistry. However, most of time the atom is represented with the full layer, of 2, 8, 18, 32 available positions for determined quanta of energy. But the most important thing is to see how strange is the table when we only have to count the additional electrons needed to finish to fill the layers, following this suit ; 2, 6, 10, 14 (because 2+6=8, 8+10=18, and 18+14=32).
"The traditional representation make easier to follow, vertically, the type of element. For example, follow the column of copper, silver, gold & roentgenium. They have the most conductivity elements of their subrings.
"Also we can follow the column of the noble gases. And that is a surprise to find a noble gas at the only first subring of the first ring, but at all the second subrings of all other rings. That let imagine an extremely innovative way to fill the free locations of energy, not simple by adding marbles on a sequence of positions, but more like a type of musical chair game (i cant be more precise actually). That show how are possibles the "errors" of filling that this 3D representation can't show, if you watch attentively the known data on the filled layers. For example, vanadium 23 is 2-8-11-2, and chrome 24 is 2-8-13-1."
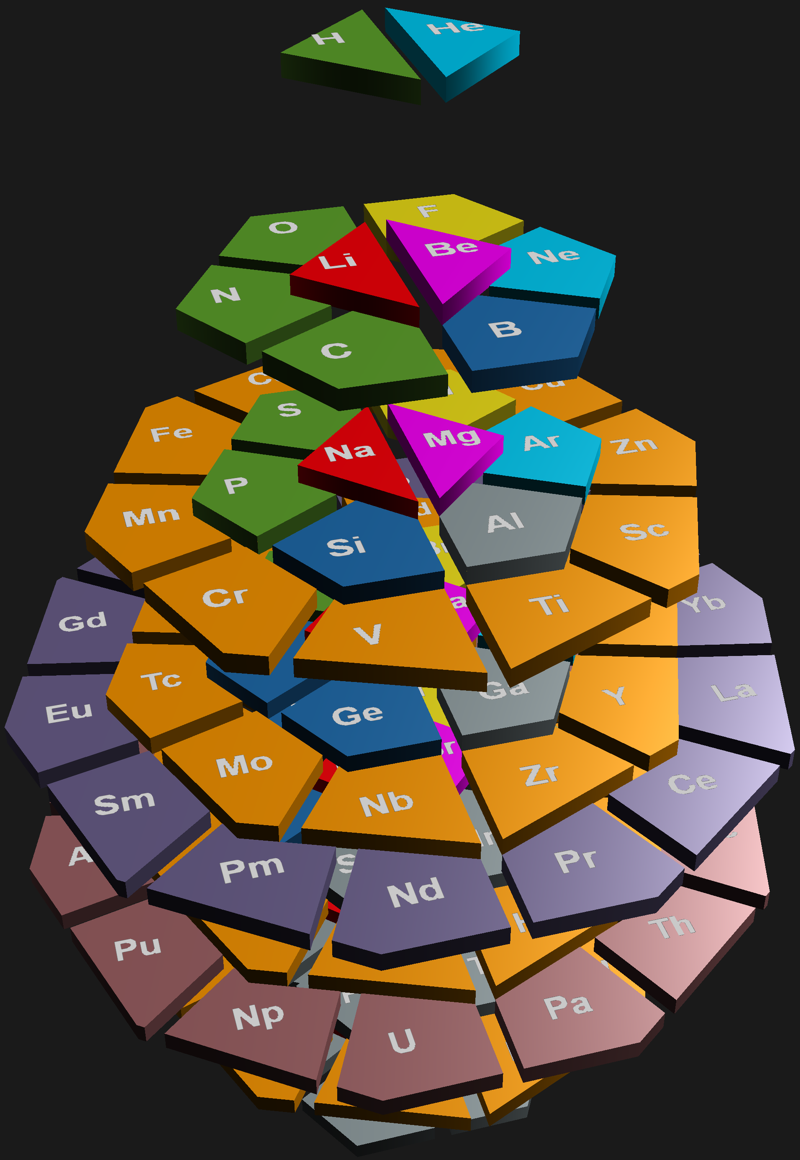
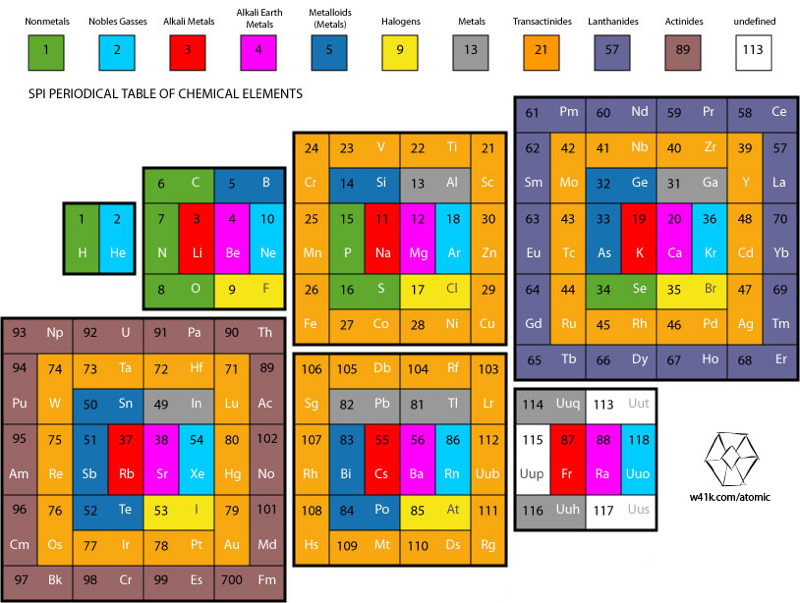
Davy has also provided some links to his ideas on the web:
- http://1nfo.net/plug/spitable
- http://1nfo.net/plug/spitablesvg
- http://1nfo.net/plug/spiline
- http://tlex.fr/frame/scene/17
- http://tlex.fr/frame/scene/17
2018
Race to Invent the Periodic Table
From PBS Digital Studios, a short-but-fast-moving video about the development of the periodic table during the 19th century, and a discussion about gallium:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
2018
Nawa–Scerri Octagonal Periodic System
A spiral periodic table formulation by Nawa, called the Nawa–Scerri Octagonal Periodic System.
Click here for a larger version:
2018
Nawa's 3-D Octagonal Pillar
A 3-D octagonal pillar periodic table model by Nawa, "acccording to Scerri's reverse engineering [of] Mendeleev's 8-column table":
2018
Scerri's Reverse Engineered Version of Mendeleev's Eight Column Table
Eric Scerri has updated – reverse engineered – the classic Mendeleev Table, here, here & here:
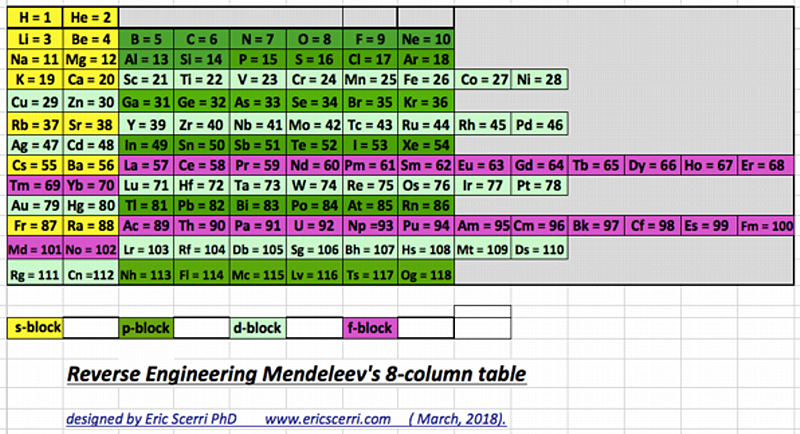
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
2018
Telluric Remix
Philip Stewart writes:
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version.
The printable version is available (click here for the full size version) to make your own:
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
2018
Sistema Periódico Binodico
By Julio Antonio Gutiérrez Samanez, who writes:
"Sistema Periódico Binodico. Nuevo Paradigma Matematizado. I have followed the work of the wise Mendeleev, of Emil de Chancourtois, of Charles Janet; inspired by the work of my countryman Dr. Oswaldo Baca Mendoza. It is in Spanish but soon I will have the English version."
2018
Puddenphatt & Monagham Periodic Table
Jeries Rihani's version of R. J. Puddenphatt and P. K. Monaghan, published in1989, but is not an exact copy. The differences are as follows:
- It adds color: YELLOW for the s-block, GREEN for the p-block, BLUE for the d-block and PURPLE for the f-block.
- It avoids being congested since it excludes the electronic configurations of the elements.
- It is updated and includes the atomic numbers 119 and 120.
- It shows that it is symmetrical around the vertical axis.
- The f-block, like all the other blocks, ends with even atomic numbers.
Puddephatt and Monaghan say "their table is after Philips and Williams":
Ref, Phillips CSG & Williams RJP 1965, Inorganic Chemistry, I: Principles and Non-metals, Clarendon Press, Oxford, p. 40.
2018
Periodical System (Binodic Form): a new mathematical paradigm
By Julio Antonio Gutiérrez Samanez, who writes:
"System devised and prepared by the Peruvian chemical engineer, Julio Antonio Gutiérrez Samanez, deals with a new conception of Mendeleev's Law as a mathematical function and a new description of the process of forming the series of chemical elements according to mathematical laws and dialectical processes of changes quantitative and qualitative under a dynamic spiral architecture in 3D, which is postulated as a new scientific paradigm."
2018
Beylkin's Periodic Table of The Elements
René Vernon writes: Beylkin's Periodic Table of The Elements has 4n2 periods, where n = 2,3..., and shows symmetry, regularity, and elegance, more so than Janet's left step table.
Beylkin (an applied mathematician) writes:
"Let us take a continuous strip of paper and, on one side of the strip, write all the elements in the order of their atomic numbers. We then form a spiral with the strip such that the two most chemically distinct groups, the group of halogens (in which we include hydrogen) and the group of noble gases, are properly aligned. By flattening the strip on a plane and folding it in the middle, we obtain the new periodic table..."
Other features:
- H is over F, which is a smoother fit in terms of physicochemical trends down the group
- He is over Ne, which is a smoother fit etc
- group 3 has lanthanum in it
- the modern relationships Ti-Zr-Hf, V-Nb-Ta, Cr-Mo-W, and Mn-Tc-Re can still be traced
- the lanthanides and actinides are integrated into the main body of the table
- 15 lanthanides and 15 actinides(!)
- the old school arrangement of B-Al-Sc-Y-La can still be traced, as can the less smooth alternative B-Al-Sc-Y-Lu
- the 1s "block" starts at H; the s block proper at Li; p at B; d at Sc; f at Ce
There are four new(ish) groups: Ti-Zr-Ce-Th, V-Nb-Pr-Pa, Cr-Mo-Nd-U and Mn-Tc-Pm-Np. For the actinide elements of these groups, the resemblance of the earlier actinides to their lighter transition metal congeners is well known. For the lanthanide elements, Johansson et al. (2014) wrote a nice article about Ce and its cross-road position. For Pr, Nd, and Pm, all of these are known in multiple oxidations states (+2, +3, +4 excl. Pm, and +5 for Pr only), just as the transitions metals are so known.
- Beylkin G 2018, The periodic table of the elements with 4n2 n = 2,3... periods, https://arxiv.org/pdf/1901.02337.pdf
- Eric 2006, https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=20
- Johansson, B., Luo, W., Li, S. et al. 2014, Cerium; crystal structure and position in the periodic table. Sci Rep 4, 6398. https://doi.org/10.1038/srep06398
- Gregory Beylkin: https://en.wikipedia.org/wiki/Gregory_Beylkin
2018
Ziaei's Circular Periodic Table
Minoo Ziaei writes:
"My father, Manouchehr Ziaei, has an interesting design of the periodic table, which I helped him draw using AutoCAD. He is very keen in introducing [this formulation] to potential interested viewers, he was recommended to visit [the Chemogenesis Database of Periodic Tables] website."
Click image to enlarge:
2018
Nawa's V.E.T. Periodic Table & Hourglass
Nagayasu Nawa, the prolific designer of periodic tables, here and here, has come up with an orbital filling periodic table and a corresponding hourglass animation. Nawa writes:
"I have turned the v.e.c. PT into the GIF animation that I call the electron hourglass, 1 second for each element. It takes 120 seconds from 1H to 120 Ubn. I have coloured orbital with colour derived from each shell's name, such as:
- K kiwi
- L lapis lazuli
- M mauve
- N navy
- O orange
- P purple
- Q quick silver"
Click image to enlarge.
2018
Janet's Left-Step with Ground Level Microstates
By Valery Tsimmerman, who writes:
Janet's LST with ground level microstate information and total spin graph shown for each group of elements. The top line represents number of electrons in open sub-shells (with exception of six anomalous elements). Information shows physical (spectroscopic) basis of the groups.
The zigzag line on top is a graphic representation of Hund's rule showing the total inherent spins of atoms and the total spin of Cu is 1/2, same as for Ag and Au. When it comes to ground level atomic microstates and Hund's rule Cu is not anomalous (2S1/2), despite its anomalous electron configuration.
The diagram represents Hund's Rule that states that "the lowest energy atomic state is the one that maximizes the total spin quantum number for the electrons in the open subshell" (Wikipedia). Y-axis is the total spin and x-axis is number of electrons in open shells (with exception of six anomalous elements).
First, I would like to make couple of general comments. When discussing periodicity, they typically talk about chemical properties and electron configurations/differentiating electrons, etc, but those are not specific enough. For each electron configuration there are multiple microstates. For example, for single electron configuration of carbon there are over 30 microstates and only one of them corresponds to ground level. So, microstates express combined physical/spectroscopic properties of whole atoms and, the most important, combined properties of electrons located in open subshells.
Now, look at ground level term symbols in each group. I see amazing consistency, especially in the main groups. It tells me that groups are not only chemical, but physical!
Looking at periods one can see that all periods in s, p & d blocks begin with elements that have multiplicity M=2 and end with M=1. This is also true for f-block if it starts with La and Ac and ends with Yb and No. This puts Lu and Lr firmly in group 3. Placing La and Ac in group three ruins spectroscopic consistency.
Click here image to enlarge the PT below.
2018
ADOMAH Periodic Table Formulation with NIST Data
By Valery Tsimmerman, who writes:
I would like to share with you another variant of my ADOMAH periodic table formulation that holds additional spectroscopic information.
Click here image to enlarge the PT below.
2018
Elements in Six Dimensions
The Elements in Six Dimensions, by Gerald Eadie, arranged by volume periods of nuclide mass averages:
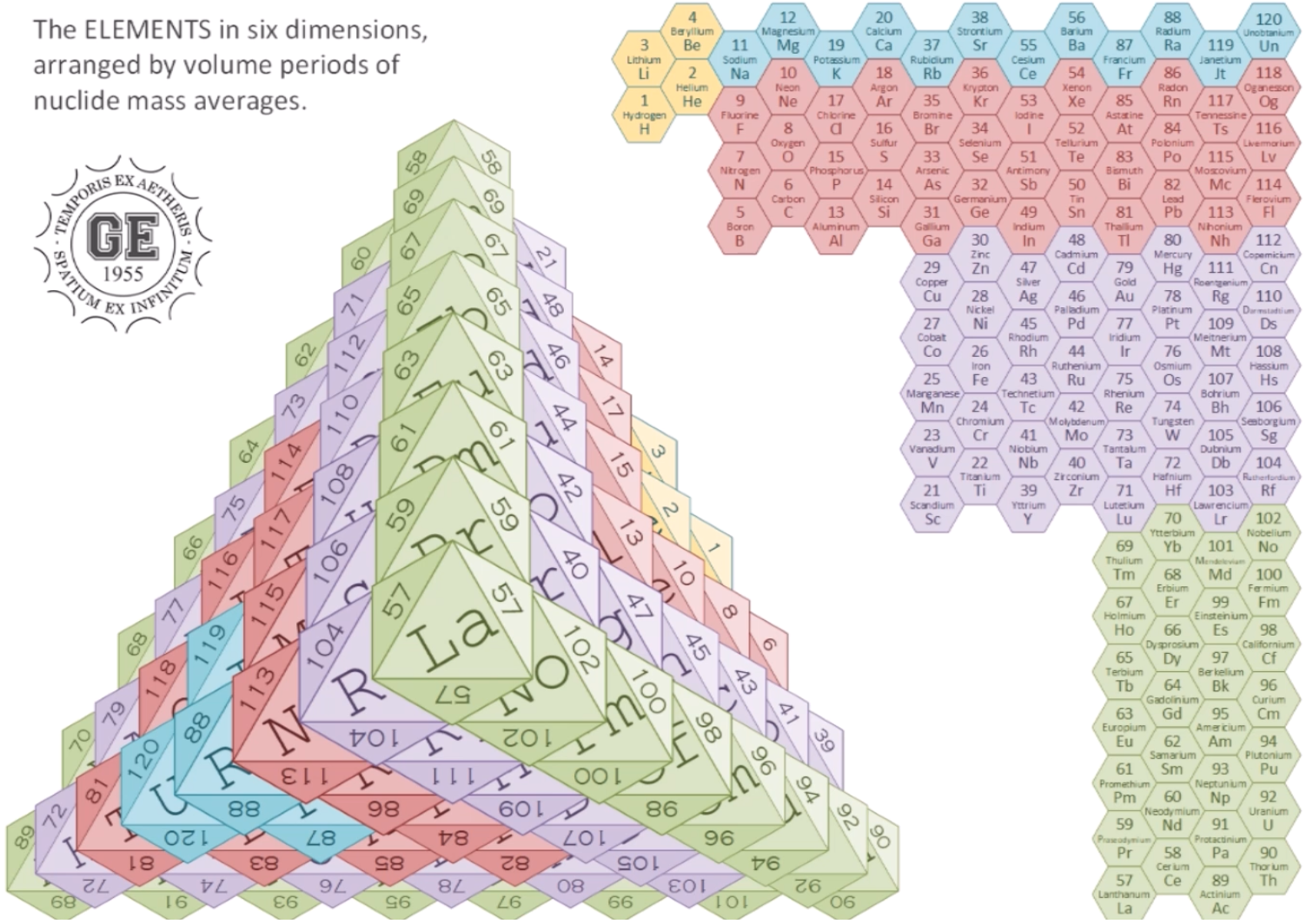
2018
Simpson's 4-Dimensional Version of the ADOMAH Periodic Table
Doug Simpson writes:
"Valery Tsimmerman's ADOMAH table and website got me started as a periodic table hobbyist. The attached photos show what I've been up to. Valery's observation that n, l, & m conspire to generate a half-filled tetrahedral lattice inspired me to create a 4D periodic table using all four quantum numbers as coordinates."

2018
Kurushkin's 32-Column Periodic Table & Left-step Periodic Table United
Dr Mikhail V. Kurushkin, 32-column Periodic Table & Left-step Periodic Table United: https://bernalinstitute.com/events/bernal-seminar-by-dr-mikhail-v-kurushkin-itmo-universityrussia/
ABSTRACT
The pursuit of optimal representation of the Periodic Table has been a central topic of interest for chemists, physicists, philosophers and historians of science for decades (Leigh, 2009; Scerri, 2009). Should the Periodic Table of Chemical Elements first and foremost serve the needs of chemists as implied by its name? Or should it start from considerations of before quantum mechanics and thus be more appealing to physicists (Scerri, 2010, 2012b)? Is there a representation which overcomes this problem? The Periodic Table is from a fundamental point of view a graphic representation of periodicity as a phenomenon of nature. While the 32-column Periodic Table, popularized by Glenn T. Seaborg, is considered by chemists the most scientifically correct representation (Scerri, 2012a), physicists apparently prefer the Left-step Periodic Table above all (Scerri, 2005; Stewart, 2010). Alternatively, it is suggested that a rigorously fundamental representation of periodicity could only take the form of a spiral as, evidently, the abrupt periods of 2-D Periodic Tables contradict the gradual increase of atomic number, and the spiral representation reconciles this debate (Imyanitov, 2016). An optimal representation is eagerly sought after both for the needs of philosophy of chemistry and chemical education as their never-ending dialogue secures a thorough methodology of chemistry. The aim of the present work is to show that the 32-column Periodic Table and the Left-step Periodic Table can co-exist in mutual tolerance in a form of what Philip Stewart has already called Kurushkin’s Periodic Table (Kurushkin, 2017), Figure 1 below.
René Vernon writes:
"Kurushkin reminds us that the Janet left step table (with Sc-Y-Lu-Lr, and He over Be), and the version of the table with the s-elements on the right (also with Sc-Y-Lu-Lr, and He over Be) are interchangeable.
"For an earlier paywall version which includes a short video see:
Kurushkin M 2018, Building the periodic table based on the atomic structure, Journal of Chemical Education, vol. 94, no. 7, pp. 976–979, https://pubs.acs.org/doi/10.1021/acs.jchemed.7b00242
"Kurushkin’s interchangeable approach extends to tables with group 3 as either Sc-Y-La-Ac or Sc-Y-Lu-Lr. See Vernon's Yin Yang of The Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1252"
2018
IUPAC Periodic Table of The Elements
The 1 Dec 2018 IUPAC (International Union of Pure and Applied Chemistry) Periodic Table of The Elements. For updates to this table click here.
By virtue of its work in relation with the chemical elements, IUPAC can dispense a periodic table that is up-to-date. IUPAC involvement covers various aspects of the table and data that it unveils, and several reports and recommendations, some quite recent, attest of that input. In particular, IUPAC is directly involved in the following:
- establishing the criteria for a new element discovery
- defining the structure of a temporary name and symbol
- assessing claims resulting in the validation and assignation of an element discovery
- coordinating the naming of a new element, involving the research laboratory and allowing for public comments
- setting up precise rules for how to name a new element
- defining Group 1-18 and collective names
- determining which elements belong to Group 3
- regularly reviewing standard atomic weights
2018
Short Form of Mendeleev’s Periodic Table of Chemical Elements
Andriiko, A.A., Lunk, HJ. The short form of Mendeleev’s Periodic Table of Chemical Elements: toolbox for learning the basics of inorganic chemistry. A contribution to celebrate 150 years of the Periodic Table in 2019. ChemTexts 4, 4 (2018). https://doi.org/10.1007/s40828-018-0059-y
2019
5 Periodic Tables We Don't Use (And One We Do)
SciShow says:
"From Mendeleev's original design to physicist-favorite "left-step" rendition, the periodic table of elements has gone through many iterations since it was first used to organize elements 150 years ago - each with its own useful insights into the patterns of the elements":
2019
Papers of Mendeleev, Odlings, Newlands & Chancourtois from the 1860s
Peter Wothers from the University of Cambridge with Sir Martyn Poliakoff, of the University of Nottingham discuss the discovery/development of the periodic table in the 1860s with the original publications.
Links to some of the formulations discussed in the video:
- Mendeleeve Table I
- Mendeleeve Table II
- Odlings' Formulation
- Newlands Octaves
- Chancourtois' Teluric Helix
2019
Chemical Bonds, Periodic Table of
The Max Planck Society (M-P-G, Max-Planck-Gesellschaft) has an article about the hidden structure of the periodic system.
Guillermo Restrepo, MPI for Mathematics in the Sciences:
"A periodic table of chemical bonds: Each of the 94 circles with chemical element symbols represents the bond that the respective element forms with an organic residue. The bonds are ordered according to how strongly they are polarized. Where there is a direct arrow connection, the order is clear: Bonds of hydrogen, for example, are more polarized than bonds of boron, phosphorus, and palladium. The same applies to rubidium in comparison to caesium, which has particularly low polarized bonds and is therefore at the bottom of the new periodic table. If there is no direct arrow between two elements, they may still be comparable – if there is a chain of arrows between them. For example, the bonds of oxygen are more polarized than the bonds of bromine. Bonds represented by the same colour have the same binding behaviour and belong to one of the 44 classes.":

Thanks to René for the tip!
2019
Slightly Different Periodic Table
The Max Planck Society (M-P-G, Max-Planck-Gesellschaft) has an article about the hidden structure of the periodic system.
Guillermo Restrepo, MPI for Mathematics in the Sciences:
"A slightly different periodic table: The table of chemical elements, which goes back to Dmitri Mendeleev and Lothar Meyer, is just one example of how objects – in this case the chemical elements – can be organized in such a system. The researchers from Leipzig illustrate the general structure of a periodic table with this example: The black dots represent the objects ordered by the green arrows. Using a suitable criterion, the objects can be classified into groups (dashed lines) in which the red arrows create a sub-order":
Thanks to René for the tip!
2019
Béguyer de Chancourtois' Vis Tellurique: A Better View
The content of Béguyer de Chancourtois' Vis Tellurique decanted into a flat table.
The flattened version – prepared by Conal Boyce – shows important aspects that cannot be 'read' from the helix itself.
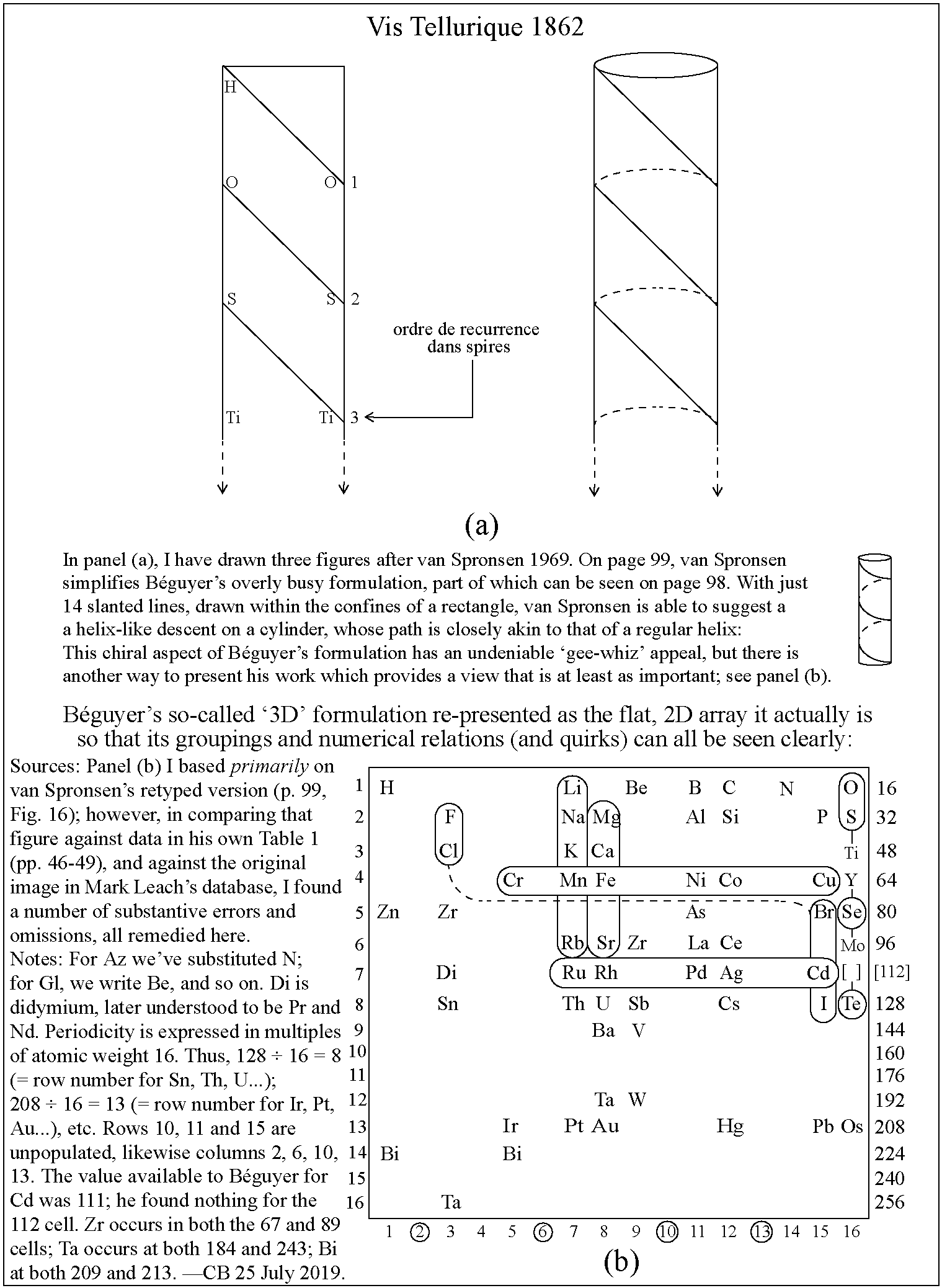
2019
Frog Periodic Table
One of the frogs from Stockport's (UK) Giant Leap Frog Art Trail. This frog is Chemit.

Thanks to Helen P for the tip!
2019
Ossmi LH & Chasib's Periodic Table
Al Ossmi LH & Chasib K F, A New Method of Elements Arrangement to Reattach the F–Block Elements of Lanthanides and Actinides in the IUPAC's Periodic Table of Elements, Med & Analy Chem Int J, 3, 4,2019, pp 1–18. DOI 10.23880/macij-16000148
The new 3-dimantional layout of the periodic table graphically shows the main body parts of the table and also these vertical and horizontal coordinators of Periods, Groups, and Nadas.
2019
Group 3 of The Periodic Table
There are several ways in which the 'common/modern medium form' periodic table are shown with respect to the Group 3 elements and how the f-block is shown. Indeed, there is even some dispute about which elements constitute Group 3. There are three general approaches to showing Group 3:
- Sc, Y, La, Ac
- Sc, Y than a gap for the lanthanides & a gap for the actinides
- Sc, Y, Lu, Lr
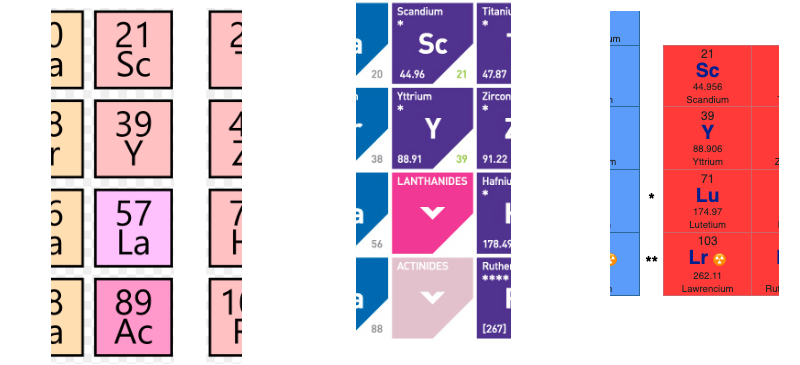
(See Scerri's take and Thyssen's view on this matter.)
So, which one of the three options is 'better'?
The general feeling amongst the knowledgeable is that leaving a gap is not an option, so it comes down to:
Sc, Y, La, Ac vs. Sc, Y, Lu, Lr
René Vernon has looked as the properties of the potential Group 3 elements, including: densities, 1st ionisation energies, ionic radii, 3rd ionisation energies, melting points & electron affinity:
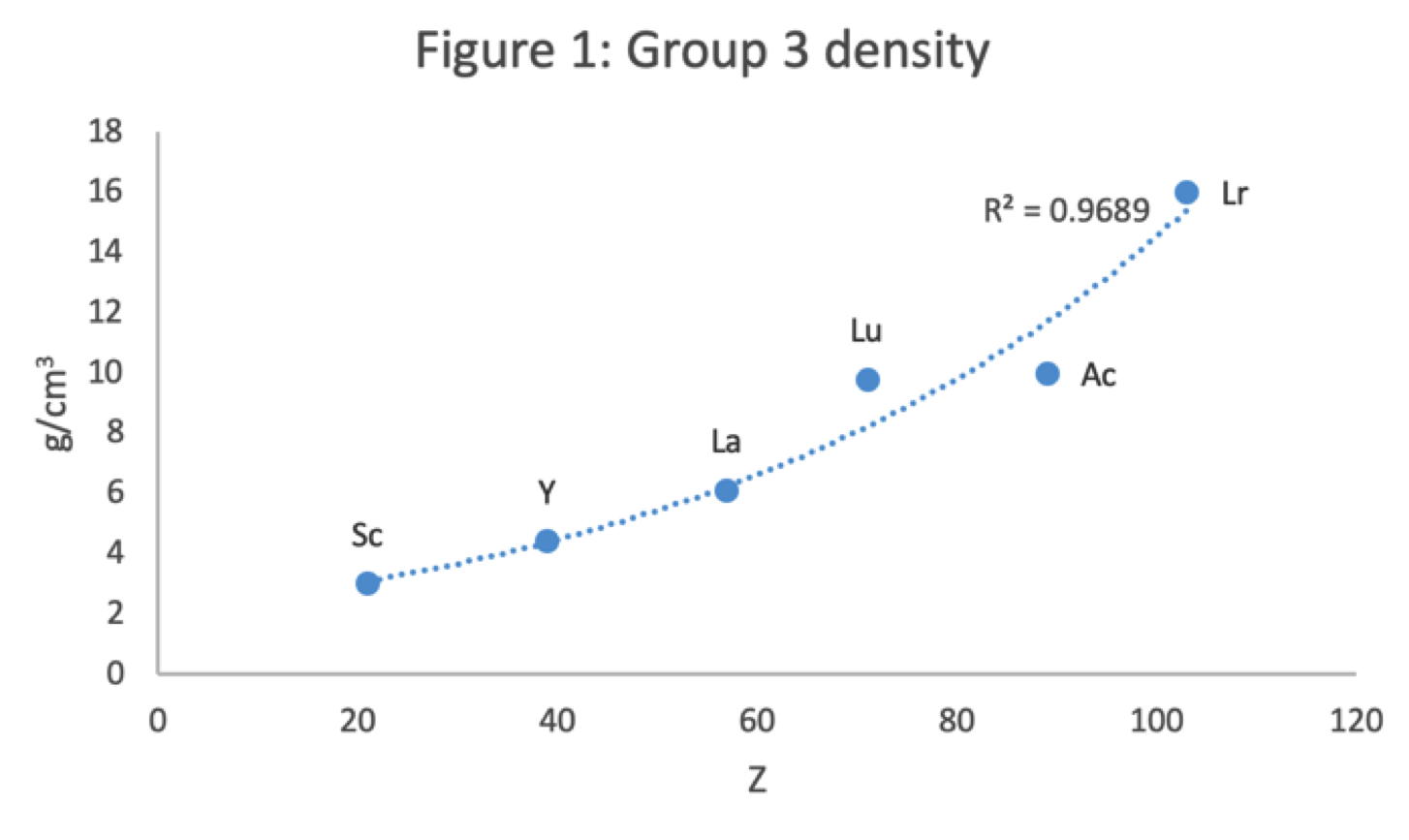
Figure 1 shows that a Z plot of the density values for Sc, Y, La, Lu Ac and Lr follows a smooth trendline.
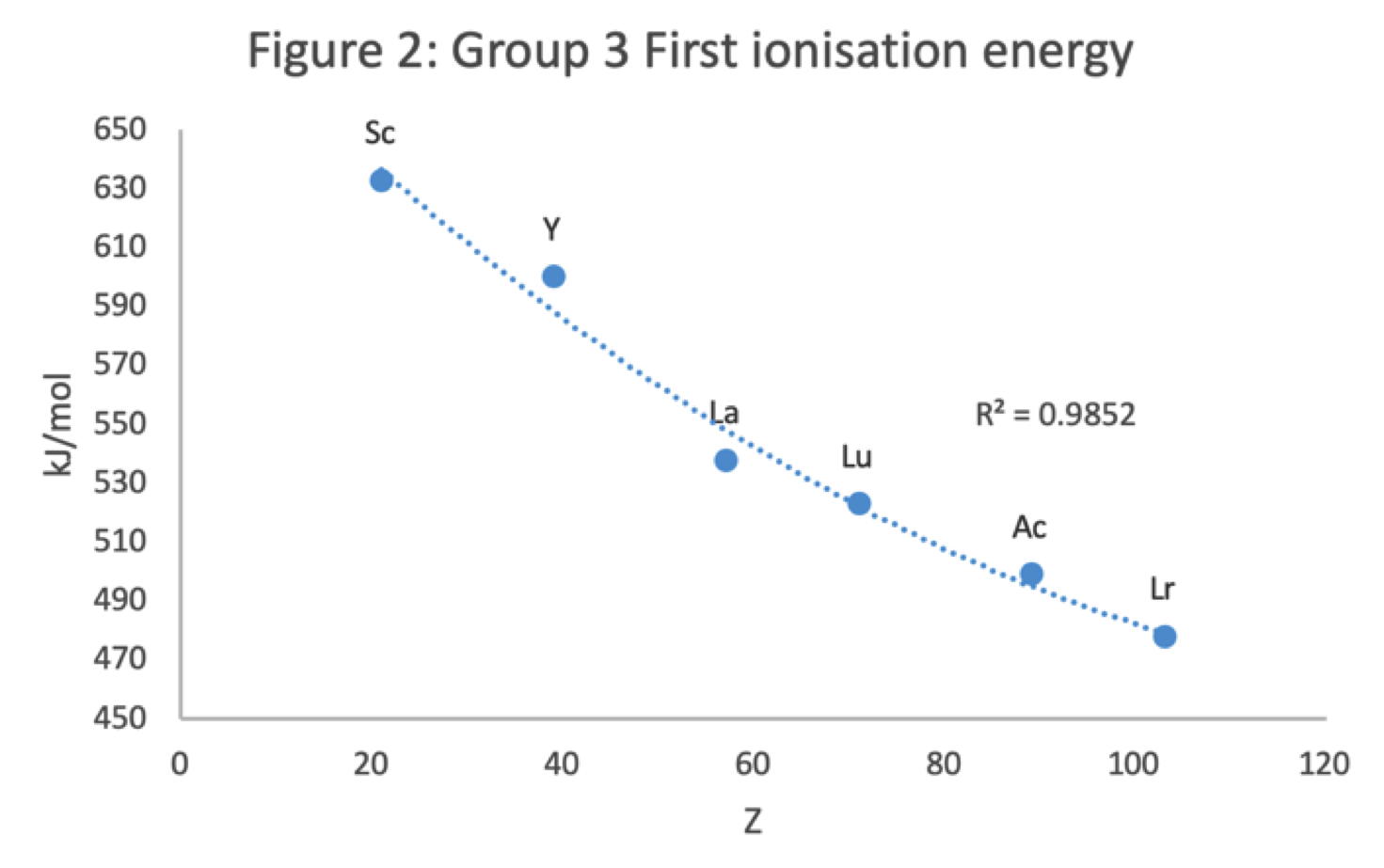
Figure 2 shows that a Z plot of the first ionization energy values follows a smooth trendline.
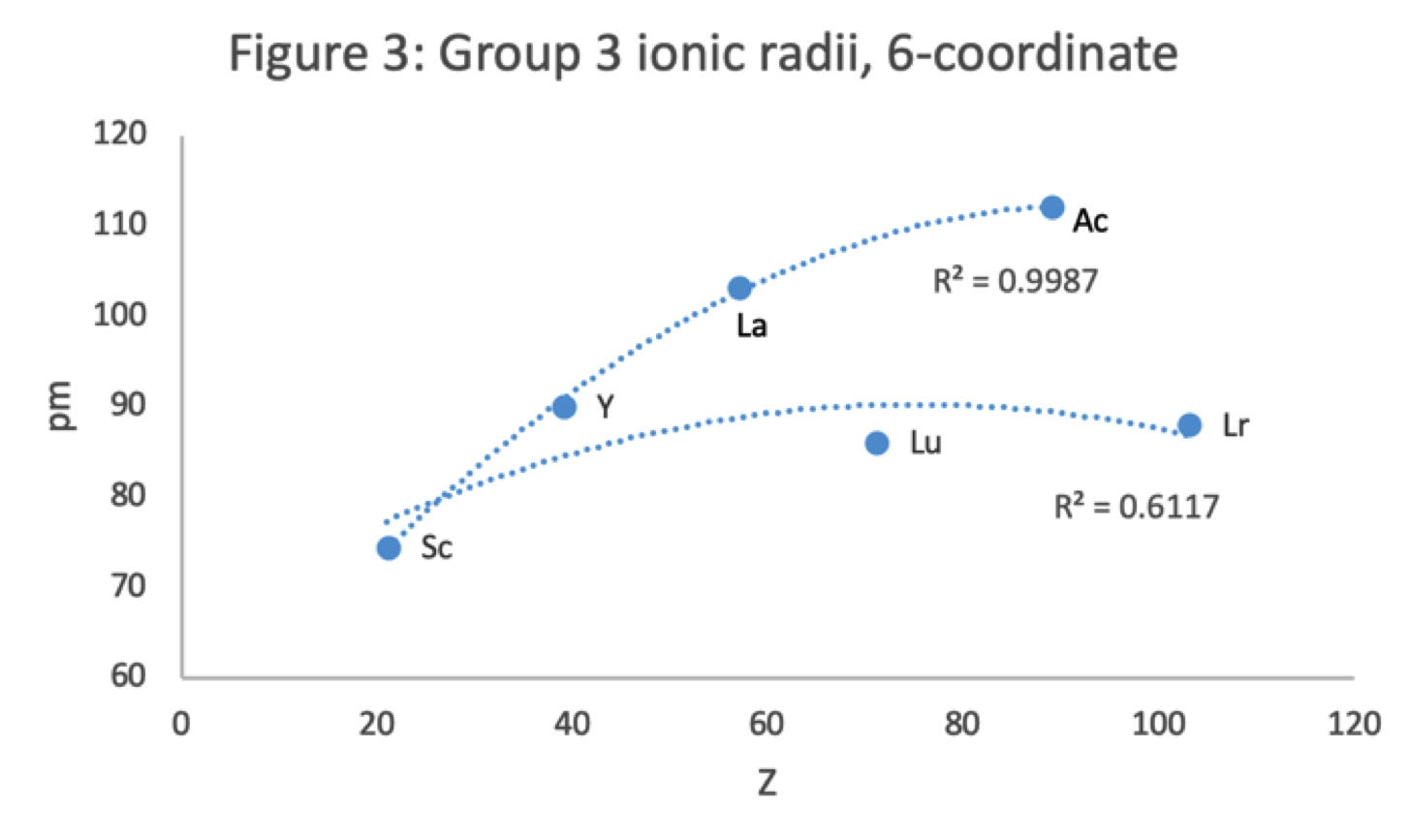
Figure 3 shows that a Z plot of the 6-coordinate ionic radii for the subject elements bifurcates after Y into an -La-Ac tranche (R2 = 0.99) and a -Lu-Lr branch (0.61). The trendline for -La-Ac is smoother.
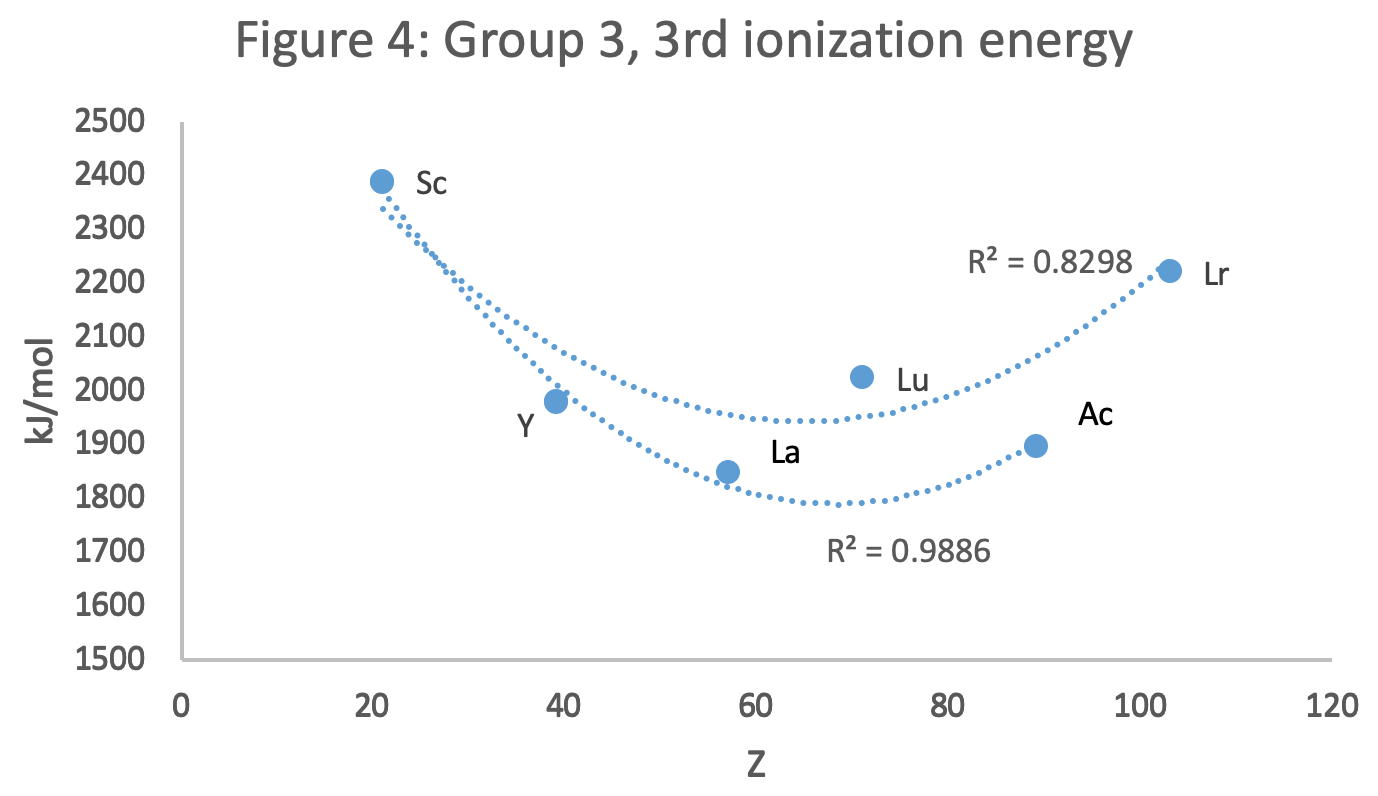
Figure 4 shows a Z plot of 3rd ionisation energy values bifurcating after Y into a -Lu-Lr tranche (R2 = 0.83) and a -La-Ac branch (0.98). The trendline for -La-Ac is smoother.
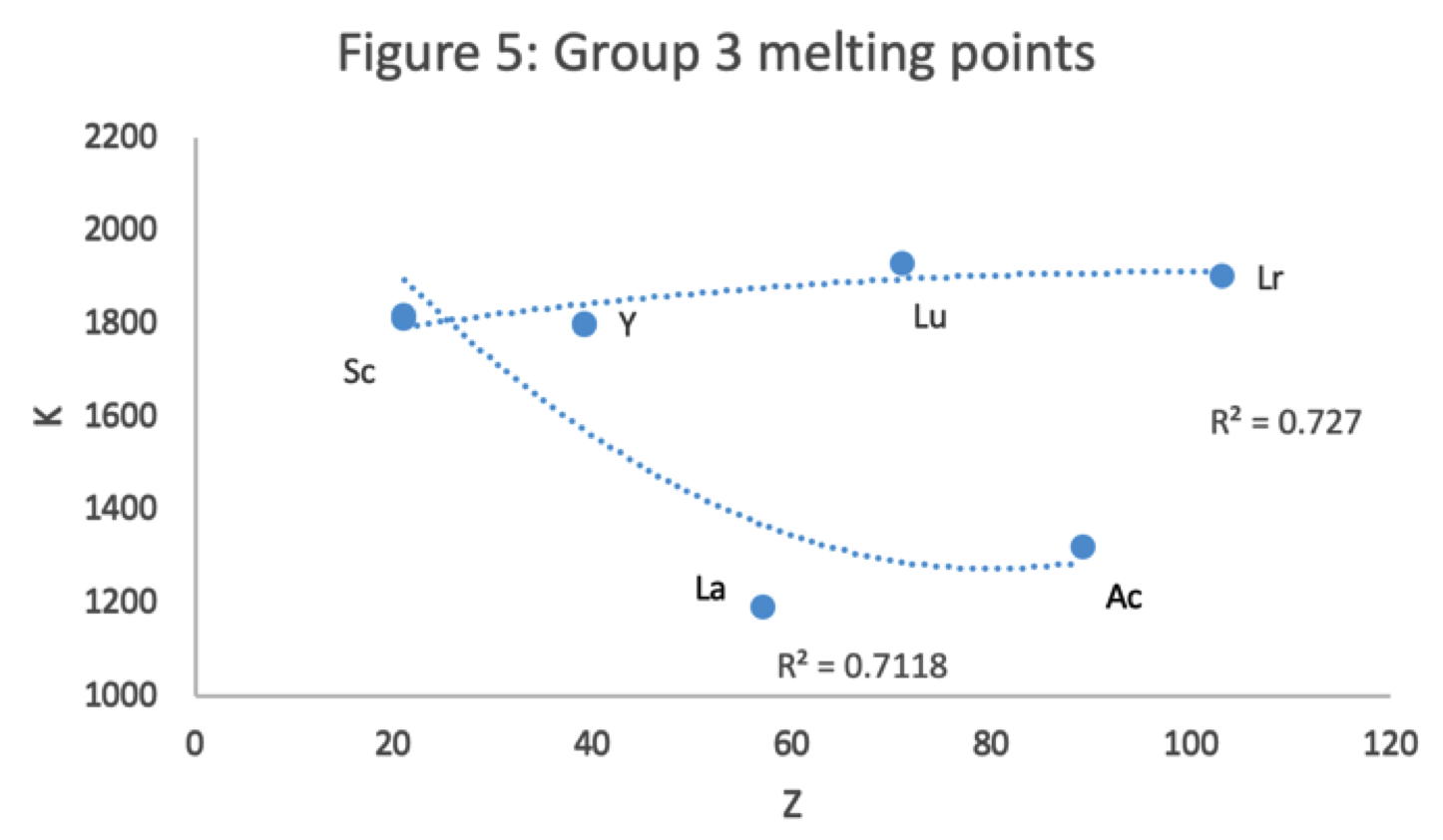
Figure 5 shows that a Z plot of the melting points bifurcates after Y into an -Lu-Lr (R2 = 0.72) tranche and a -La-Ac (0.71) branch. While the fit values for the two options are comparable, -Lu-Lr is preferred since Y and La show a greater departure from trend.
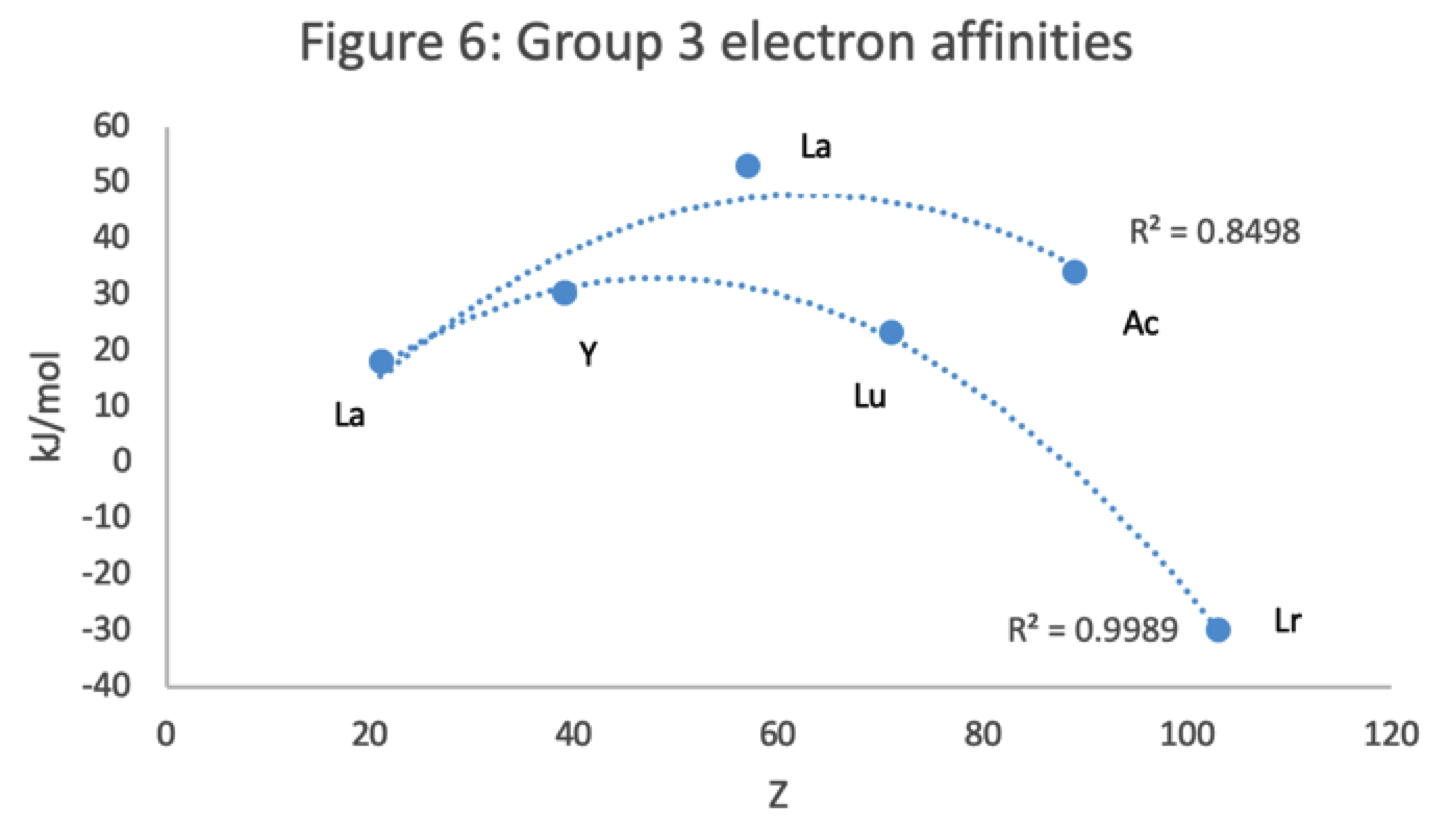
Figure 6 has a Z plot of electron affinity values bifurcating after Y into an -La-Ac tranche (R2 = 0.85) and a -Lu-Lr branch (0.99).[iii] The trendline supports Lu-Lr. The trend-lines by themselves are inconclusive: two show no difference; two support -La-Ac; two support -Lu-Lr.
Upon reviewing the data, René's comment is that: "The net result is that the two options seem inseparable" and he proposes that IUPAC adopt the following periodic table numbering system:
Professor Sir Martyn Poliakoff's [of the Periodic Videos YouTube channel & Nottiningham University] take on this matter:
2019
Janet Rejuvenated: Stewart-Tsimmerman-Nawa
An updated version of Philip Stewart's Janet Rejuvenated by Valery Tsimmerman redrawn by Nawa.
2019
Chavhan's Third Generation Periodic Table of the Elements
Randhir Bhavial Chavhan's Third Generation Periodic Table of the Elements poster, as presented 4th International Conference on Periodic Table at St. Petersburg, Russia.
Click here, or on the image, to enlarge:
2019
Meyer's NYT Graphic
A nice graphic by Alex Eben Meyer in the New York Times accompanying an article about the periodic table and some of Sir Martyn Poliakoff ideas.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Where Mendeleev Was Wrong
A paper by Gábor Lente, Where Mendeleev was wrong: predicted elements that have never been found, from ChemTexts https://doi.org/10.1007/s40828-019-0092-5.
As is well known, Mendeleev sucessfully predicted the existance of several elements, but he was not always correct.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Samanez's Binodic Periodic System, New Mathematical Paradigm Poster
Julio Antonio Gutierrez Samanez (Master's student in chemical engineering at the San Antonio Abad National University of Cusco, Peru) presented a poster at the 4th International Conference on the Periodic Table arranged by IUPAC, Saint Petersburg, Russian Federation, July 2019. See the high resolution .PDF file.
More here and at kutiry.com
2019
Mendeleev 150
Mendeleev 150 is the 4th International Conference on the Periodic Table. The event welcomed nearly 300 guests from over 30 countries and has become one of the key events of IUPAC's International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
International Year of the Periodic Table with Eric Scerri
A YouTube video about IUPAC's International Year of the Periodic Table:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Scott Van Note Periodic Table Sculpture
On the Saatchi Art website, a 3D periodic table Sculpture by Scott Van Note.
Sculpture: Metal (Bronze). Ten made for the local ASM international chapter.
Loops and changes of direction show electron shell filling. S,P,D,F with S just a change of direction. Continuous spiral from top to bottom. New loops introduce as the electron shell would. Does not show the out-of-order shell filling.
Keywords: periodic, science, sculpture, functional, nerd
Thanks to Roy Alexander for the tip!
2019
C&EN No Agreement
From C&EN: The periodic table is an icon. But chemists still can't agree on how to arrange it:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Alexander Arrangement Unwrapped... and Rewrapped
In mid-2019 Roy Alexander – of the Alexander Arrangement – produced an intriguing new formulation in sketch form that shows the p, d & f blocks moving away from the s block in three dimensional space:
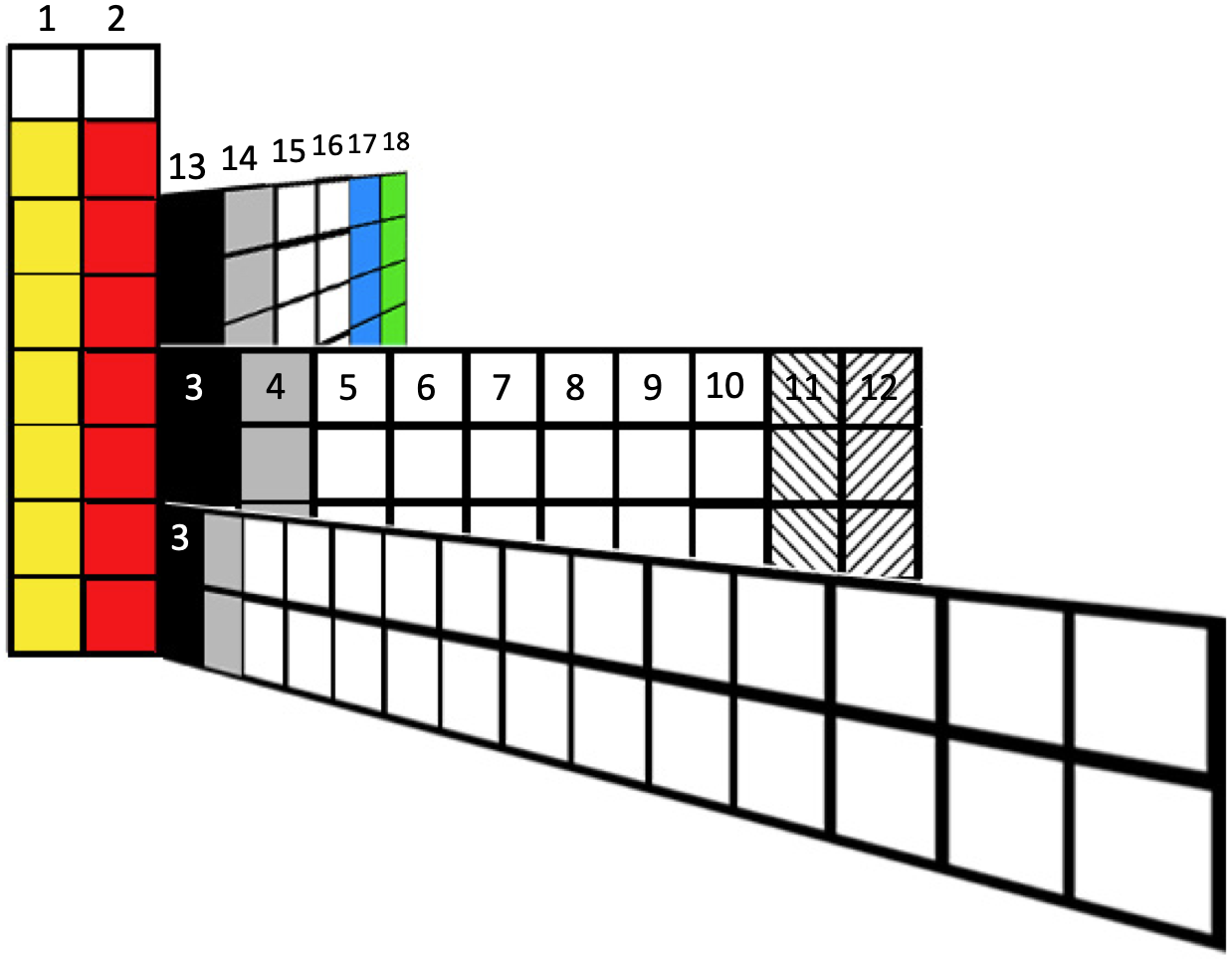
Roy has now expanded this into a full blown new formulation. (Click image to enlarge):
2019
Weise's Tetrahedral Periodic Table
A Facebook video by Dmitry Weise showing how the conventional periodic table can be morphed into a tetrahedral formulation via the Janet Left Step:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Cylindrical Periodic Table of Elements
Two YouTube videos by Takehiko Ishiguro (original & updated): Cylindrical Periodic Table of Elements.
Three types of the cylindrical periodic table of elements are demonstrated with a rotating table. Comments on them are given at the end of the video (in English).
2019
Physical Origin of Chemical Periodicities in the System of Elements
From de Gruyter: Physical origin of chemical periodicities in the system of elements, Chang-Su Cao, Han-Shi Hu, Jun Li* and W. H. Eugen Schwarz*, Pure Appl. Chem. 2019; 91(12).
Published Online: 2019-11-30 | DOI: https://doi.org/10.1515/pac-2019-0901 (open access)
Abstract:
The Periodic Law, one of the great discoveries in human history, is magnificent in the art of chemistry. Different arrangements of chemical elements in differently shaped Periodic Tables serve for different purposes. "Can this Periodic Table be derived from quantum chemistry or physics?" can only be answered positively, if the internal structure of the Periodic Table is explicitly connected to facts and data from chemistry.
Quantum chemical rationalization of such a Periodic Tables is achieved by explaining the details of energies and radii of atomic core and valence orbitals in the leading electron configurations of chemically bonded atoms. The coarse horizontal pseudo-periodicity in seven rows of 2, 8, 8, 18, 18, 32, 32 members is triggered by the low energy of and large gap above the 1s and nsp valence shells (2 ≤ n ≤ 6 !). The pseudo-periodicity, in particular the wavy variation of the elemental properties in the four longer rows, is due to the different behaviors of the s and p vs. d and f pairs of atomic valence shells along the ordered array of elements. The so-called secondary or vertical periodicity is related to pseudo-periodic changes of the atomic core shells.
The Periodic Law of the naturally given System of Elements describes the trends of the many chemical properties displayed inside the Chemical Periodic Tables. While the general physical laws of quantum mechanics form a simple network, their application to the unlimited field of chemical materials under ambient 'human' conditions results in a complex and somewhat accidental structure inside the Table that fits to some more or less symmetric outer shape. Periodic Tables designed after some creative concept for the overall appearance are of interest in non-chemical fields of wisdom and art.
2019
Global Periodic Table
Brian Gregory of keytochemicstry.com presents a Global Periodic Table.
The figure below shows the subshell strings aligned in columns based on the total pool of valence electrons as described above. This is the global periodic table. Each column constitutes a global group. Each term in column 1 launches a global period. The global periodic table is a purely mathematical matrix that assigns precise row and column coordinates to all positive integers but makes no predictions as to the order in which subshells are filled. Read more here.
2019
Colburn's 2019 Periodic Table of The Elements
Justin Lee Colburn writes:
"What is unique to my Periodic Table is the fact that any Elements Electron Spin can be identified for Orbital Diagrams using a technique I have called 'Element Shifting'.
"Elements with an Up spin Valence Electron are shifted up and Elements with a down spin are shifted down. Also, the Hund's rule Exceptions are Highlighted in the Transition Metals so their orbital diagrams can also be identified easily.
"In addition, an accurate numbering system can be applied to all the elements with Helium placed in Group 2 instead of Group 18. I believe that quantitative data should take priority when giving elements their position, but this system is meant to be dynamic rather than static. In my Periodic Table System, (1-8) corresponds to Valence Electrons in the s and p orbitals and then the 9-18 and 19-32 corresponds to core electron in d and f orbitals .
I believe that it is important to begin by showing students the first 20 Elements FIRST because they all add Outer Valence Electrons which makes the Periodic Table logic easy to follow. Also explaining that Hydrogen and Helium are anomalies with more than one logical position, can really help clear up confusion for new students.
"After element 20, the Transition Elements such as scandium 21 begin adding core electrons in the d orbital the current standard (1-18) numbering system does not reflect this. One of the reasons why I prefer keeping the s and p block elements on the outside of the table and the f and d block elements on the inside is because of how they add electrons to their orbitals.
"I have been developing a curriculum based on this system that I believe will help students learn and understand the logic and trends of The Periodic Table more efficiently than the standard. Rather than memorizing Element information, Students will truly be able to follow the logic based on the location of the Elements, simple counting and using the numbering system."
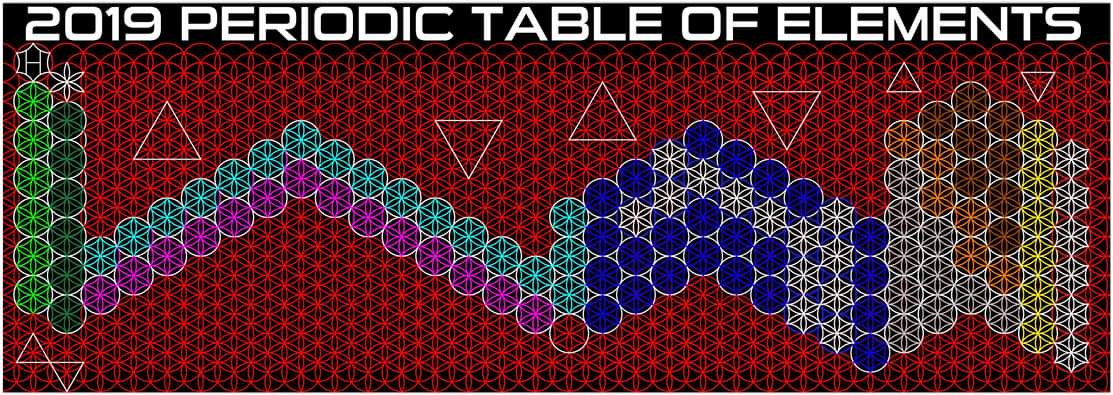
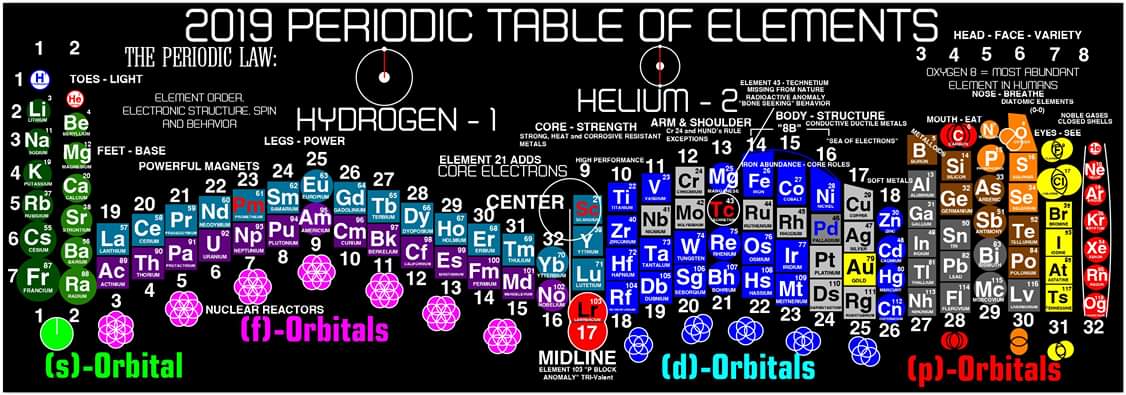
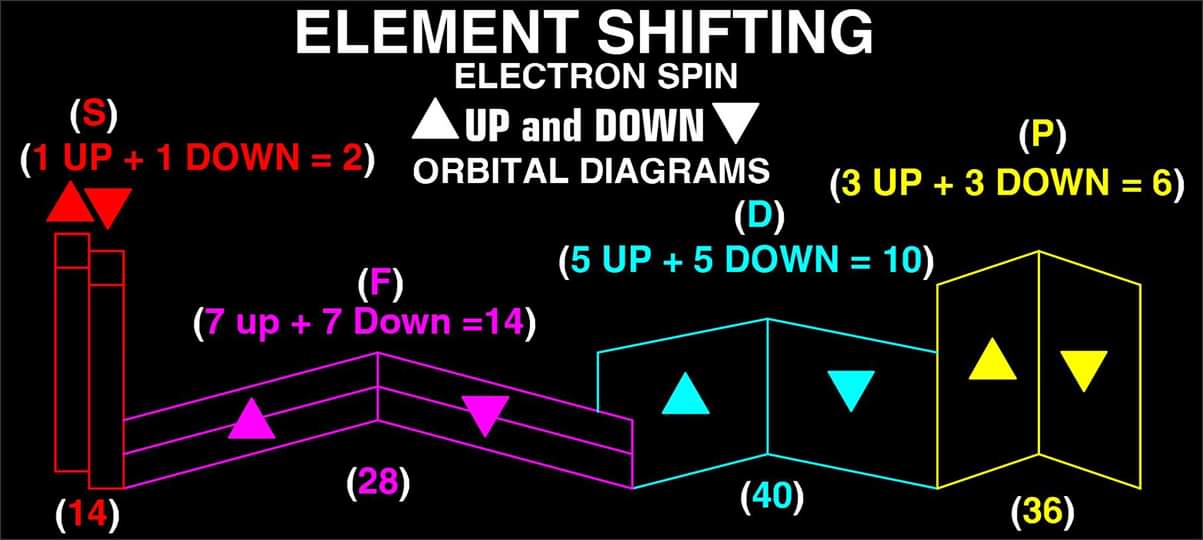
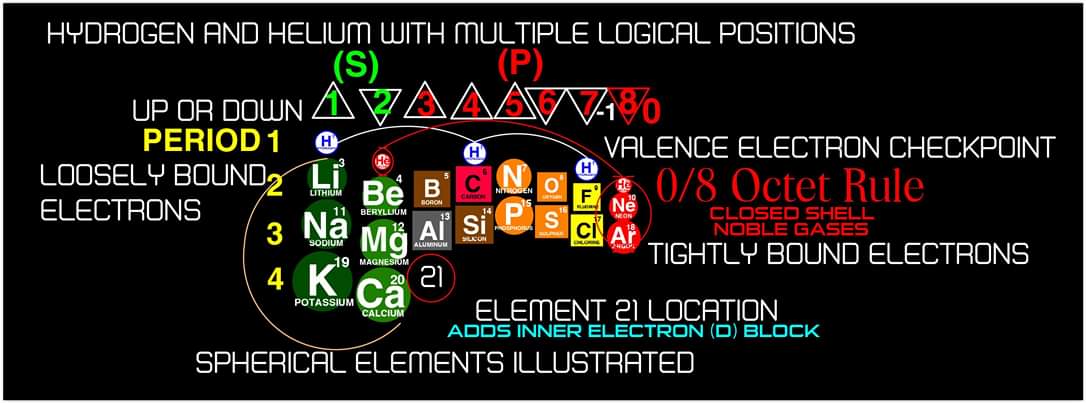
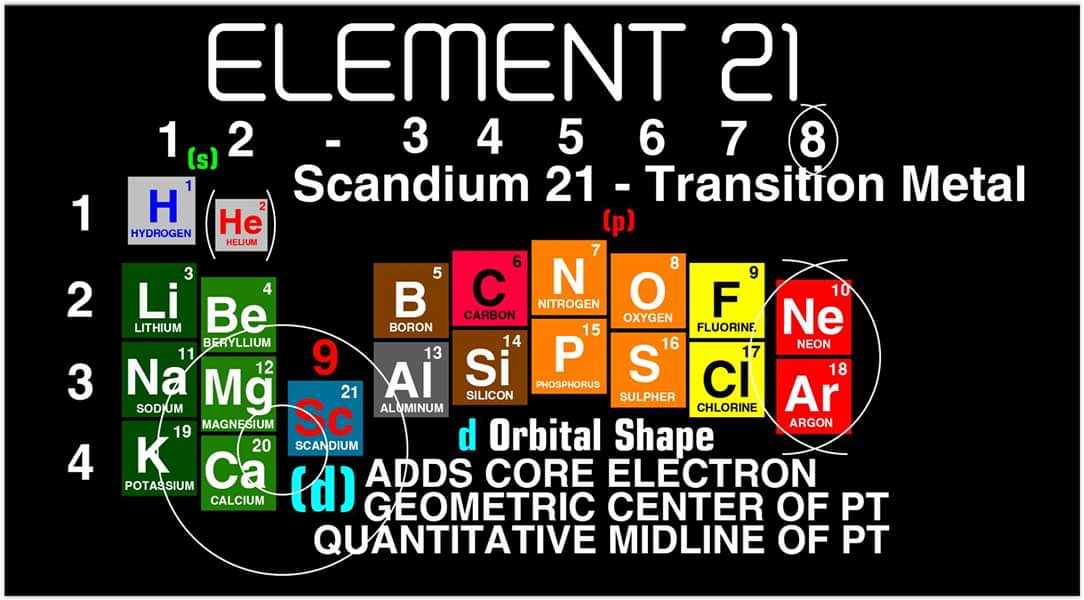
2019
International Year of the Periodic Table with Eric Scerri
Interview with leading expert on the periodic table and UCLA professor, Eric Scerri, to celebrate the International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Elements & Anti-Elements (Atom-to-Adam)
"The underlying order of the Atoms and asking the question of Intelligent Design has inspired my work. I have developed a new and improved Periodic Table of Elements that restores the Lanthanides and Actinides in their proper positions while also applying a complete and accurate numbering system. (1-8) numbering in s and p blocks correspond to Valence Electrons with (9-18) and (19-32) corresponding to core Electrons in the d and f block Elements.
"I have also inverted the Periodic Table of Elements which reflects the "missing" Anti Matter of our Universe. Unique to my Periodic Table of Elements is the ability to easily identify any Elements Electron Spin for Orbital Diagrams by shifting Elements up or down. Lastly I have highlighted some of the AUFBAU Exceptions or Electron Configuration anomalies in the transition metals. Interestingly, standing the Periodic Tables upright resembles the image of human beings, hence the title of my project, from Atom to Adam."
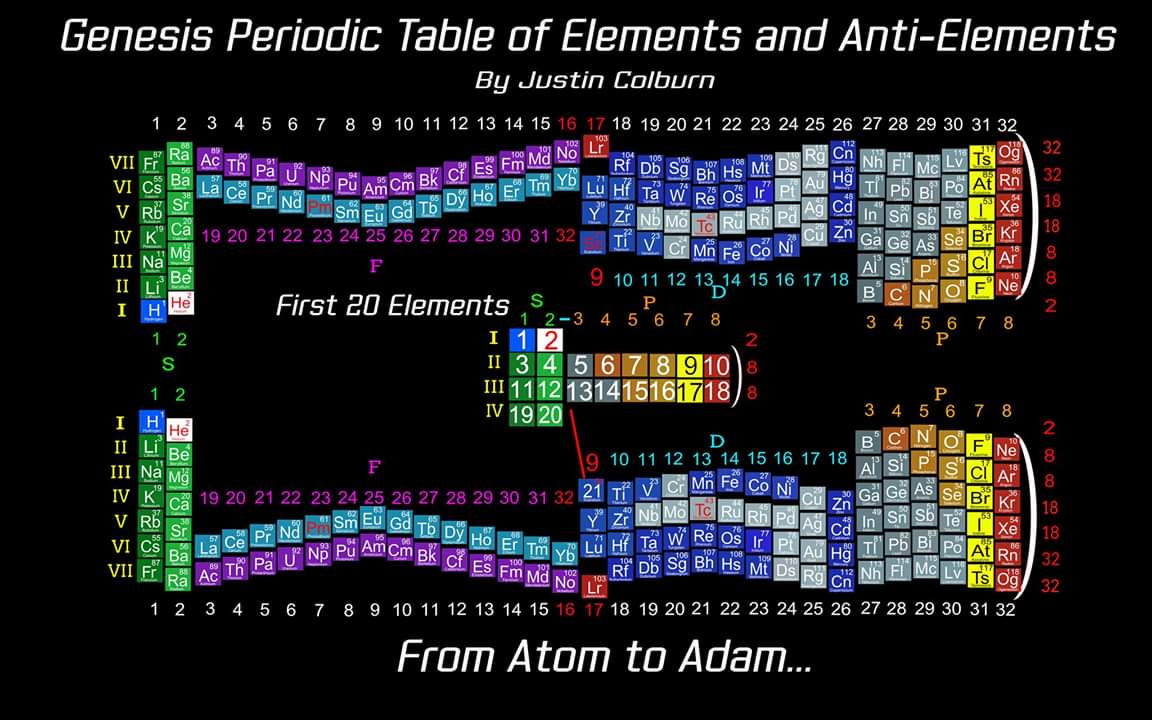
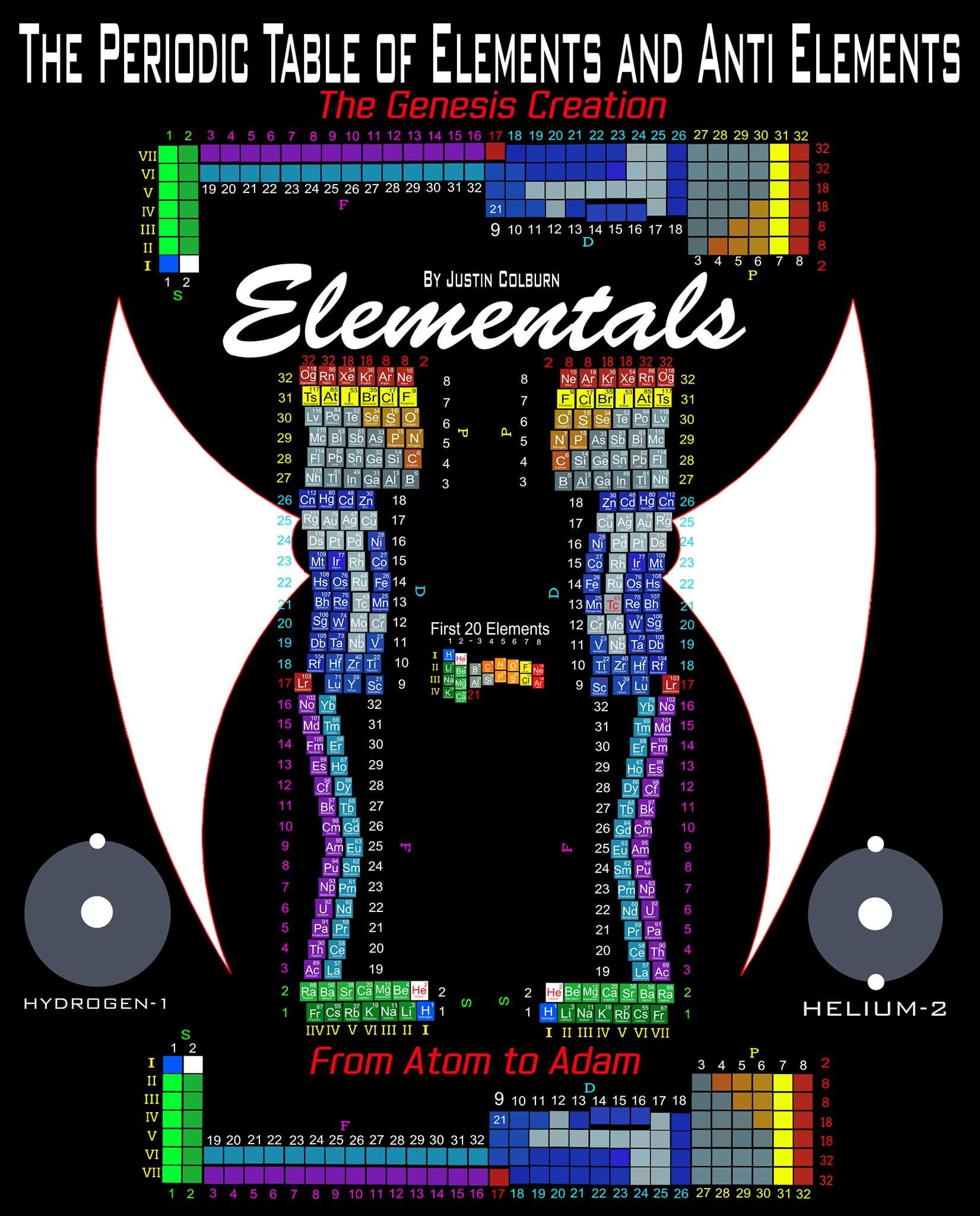
2019
Tasset's Version of Schaltenbrand
Tasset's version of Schaltenbrand's Helical Periodic Table
Harry F. Tasset writes:
"I am very happy to be able to submit a periodic table that represents the culmination of many years of study. Much of my creative inspiration came from my father Everett and my two chemist brothers, Carl and Emmett. Also, I must mention Isaac Asimov.
"I have considered the gravity of this release and believe this is for the betterment of mankind. It is a moot point at this stage to regret the mistakes made in the 1930's when the Lanthanoids and Actinoids were separated, like the middle of a tree magically suspended to its side. Suffice it to say that the table was meant to be a complete whole."
Click the image to enlarge
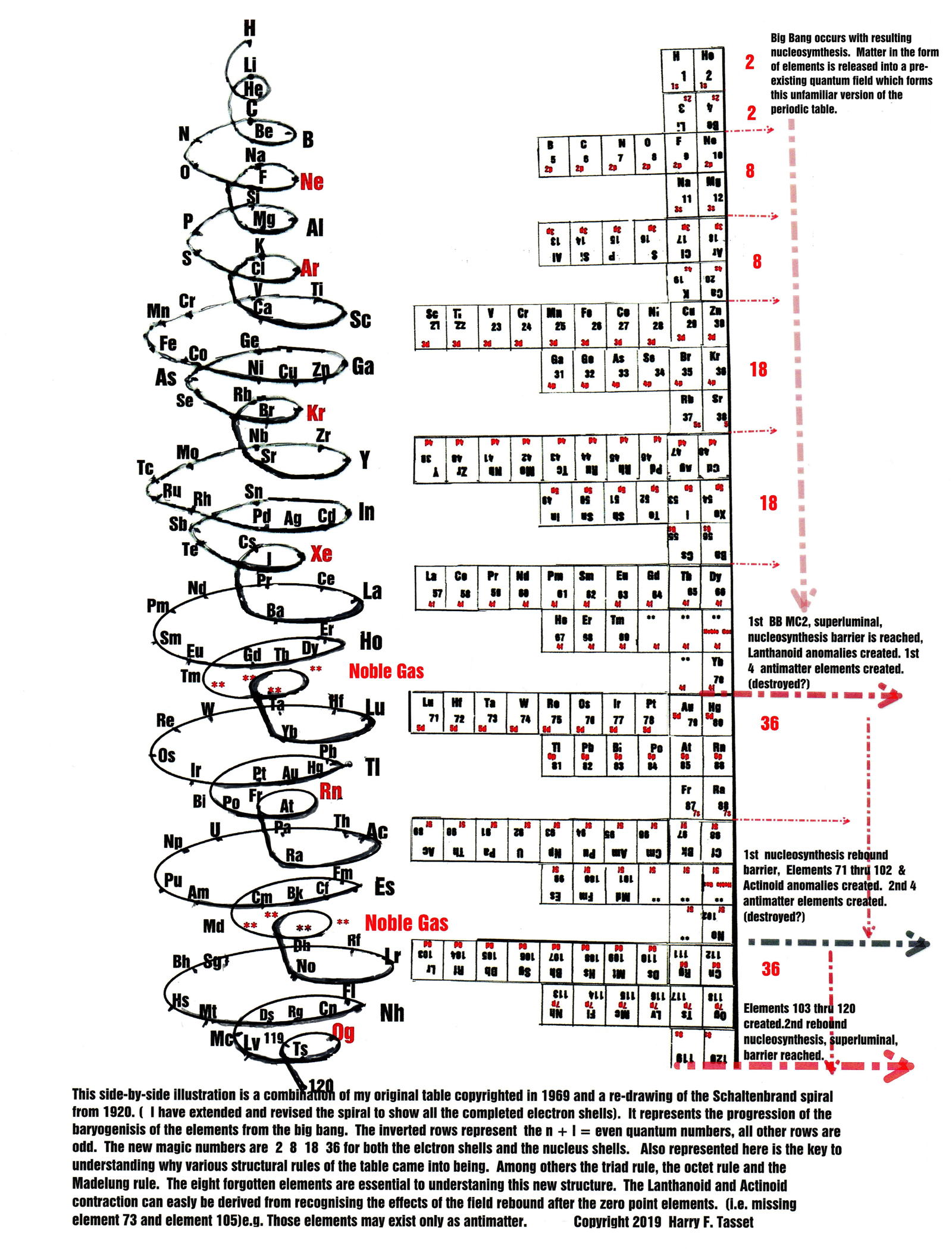
2019
Vernon's Oxidation Number Periodic Table
René Vernon's periodic table showing oxidation number trends.
René writes:
- This table is based on two tables by Fernelius (1986), one of which is a portrait version of Bohr's 1922 table, and the second of which is a conventional table highlighting oxidation number trends in groups 4 to 10, and most of the p-block. Ref. Fernelius WC , 'Some reflections on the periodic table and its use', Journal of Chemical Education, vol. 63, no. 3, pp. 263–266 (1986).
- In my table, the transition metals are in groups 4 to 11. As per Mark's Leach's email: "...It's the 'incomplete d-subshell' that gives rise to properties such as: variable oxidation state, catalytic behaviour, d-orbital splitting and [thus] coloured ions/compounds."
- I've included oxidation number details for some elements. I've tried a different way of showing the composition of a bifurcated group 3, which is more in keeping with Bohr's table.
- The horizontal bar of the "T" is for Sc → Ti; the downward bar is for Y → Lu-Lr.
- Thus, Group 3 as Sc-Y-Lu-Lr could be called group 3T.
- La-Ac and Lu-Lr are duplicated in a greyed-out style to make it clearer where the lanthanides and actinides fit into the main body of the table.
- The inner transition metals are clearly delineated. Analogously to the transition metals they're all capable of forming ions with incomplete f sub-shells.
- The early actinides resemble the transition metals.

2019
IUPAC Periodic Table Challenge: Nobelium Contest
IUPAC Periodic Table Challenge: Nobelium Contest. DOI: https://doi.org/10.1515/ci-2020-0204
After a first round based on the knowledge of the participant of the chemical elements, in this new phase of the Challenge, participants were invited to share their passion and creativity about chemistry. The entries were supposed to highlight the role of the Periodic Table in a creative manner. We received a variety of submissions including videos, poems, songs, and paintings. Entries were categorized into science, art, and outreach or community activities related to the science education and the Periodic Table. We were genuinely surprised and inspired by the dozens of pieces of art that we were receiving from all around the world. A jury as well as popular votes casted for each entry decided who were to receive the special limited edition IUPAC Periodic Tables signed by the Nobel laureates. That was one of the most difficult tasks of this activity, as the quality, originality, and diversity of the entries were truly amazing.
The artist is Swaprabha Chattopadhyay, a high school student from India.
- https://iupac.org/100/pt-challenge-entry/page/3/
- https://iupac.org/100/pt-challenge-entry/adorned-with-elements/
- https://iupac.org/100/summer-winners-of-the-nobelium-contest-announced/
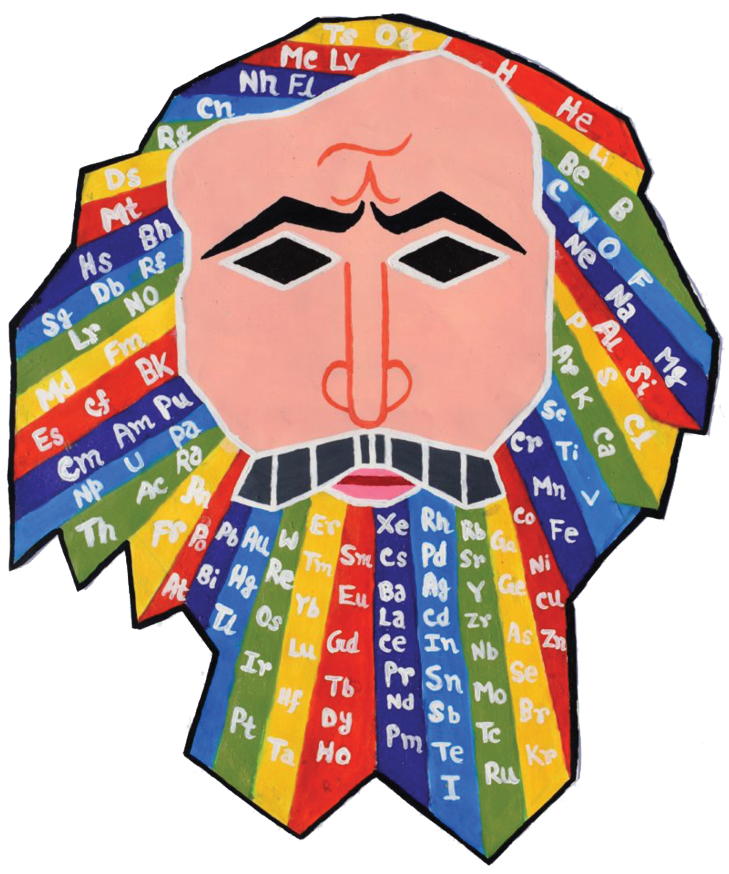
2019
Leach's Empirical Periodic Table
The common/conventional/standard 'medium form' periodic table is based on the 1945 Seaborg formulation, and it is interesting to explore where this formulation – and its 1939 predecessor – come from. (Interestingly, the Werner formulation of 1905 is not cited as a source and there are no other similar formulations in the (this) Periodic Table Database.)
However, it is possible to get to the common/conventional/standard periodic table directly from two readily available data-sets: (1) first ionisation energy of the gas phase atoms, and (2) atomic radius.
The procedure involved plotting the data, rotating 90°, squeezing vertically and smoothing. The points need a little tidying up, and then they can be mapped directly onto the Seaborg formulation periodic table.
The only element which does no obviously 'line-up' with the periodic table is hydrogen, but many modern periodic tables have H floating as it is not obvious if it should be considered to be a Group 1 alkali metal or a Group 17 halogen.
Note:
There are advantages and disadvantages to each data set. The 1st ionisation energy data from NIST is known with up to seven significant figures of precision, but the data jumps about at times due to the presence of the s & p-orbitals, which appears to make the data a little noisy. (Actually, this 'noise' is embedded information about the electronic structure of the atoms.) The atomic radius gives smoother data, but as gas phase atoms do not have hard edges calculated (Clementi 1967) rather than experimental values, must be used.
2019
UCLA Periodic Table (Proposed)
Eric Scerri writes:
During an office hour here at UCLA with a couple of students – Annelise Gazale & Chidinma Onyeonwu – we came up with a 'new periodic table'.
The basis of it is related to a point you frequently make against the Left Step formulation, namely that it messes up trends in atomic radius etc.
So how about this: Traditionally on the right side of the table elements become less reactive as we move down, but on the right side of the table elements become more reactive as we move down. Consequently, the noble gases are anomalous in the way they usually sit since they become more reactive as we move down the table.
Ergo: Move the noble gases to the left edge of the table. (Yes, this has been done before of course but not for this reason.)
Thanks to Eric Scerri for the tip! See the website EricScerri.com and Eric's Twitter Feed.
2019
Möbius-Escher Periodic Table
A comment article in Nature by Prof. Eric Scerri about quantum mechanics and the periodic table:
"Can quantum ideas explain chemistry's greatest icon? Simplistic assumptions about the periodic table lead us astray.
"Such has been the scientific and cultural impact of Dmitri Mendeleev's periodic table of the elements that many people assume it is essentially complete. [But] in its 150th year, can researchers simply raise a toast to the table's many dividends, and occasionally incorporate another heavy synthetic element?
"No – this invaluable compilation is still not settled. The placements of certain elements, even hydrogen and helium, are debated."
The article is accompanied by a fantastic illustration by Señor Salme with ideas from the Möbius strip and M.C. Escher:
2019
ElementBook Braille version of the AAE
From Roy Alexander of the AAE (Alexander Arrangement of the Elements):
"My [first] contribution to celebrate this Year of the Periodic Table is to reach out to folks who have yet to see what everyone's talking about, so they can get the feel of it: a 3D periodic table in Braille."
For the premise for this PT, here.
For your own model (with Braille) of the ElementBook: print*, cut out each part at the outside blue lines, etc.
The ElementBook Braille version of the AAE is in the beta-test phase for the rest of the year, so if any of you know of an aspiring chemist who would be willing to use it while learning (and keep me informed of that experience all year) send them to www.chemicalelementsystem.com/braille/

2019
Kid's Periodic Table
From Cognitive Classroom, a Kid's 'cut-down' Periodic Table:
2019
Döbereiner Revisited
Gordon Marks has developed a "Döbereiner Revisited" periodic table. Read all about it here.
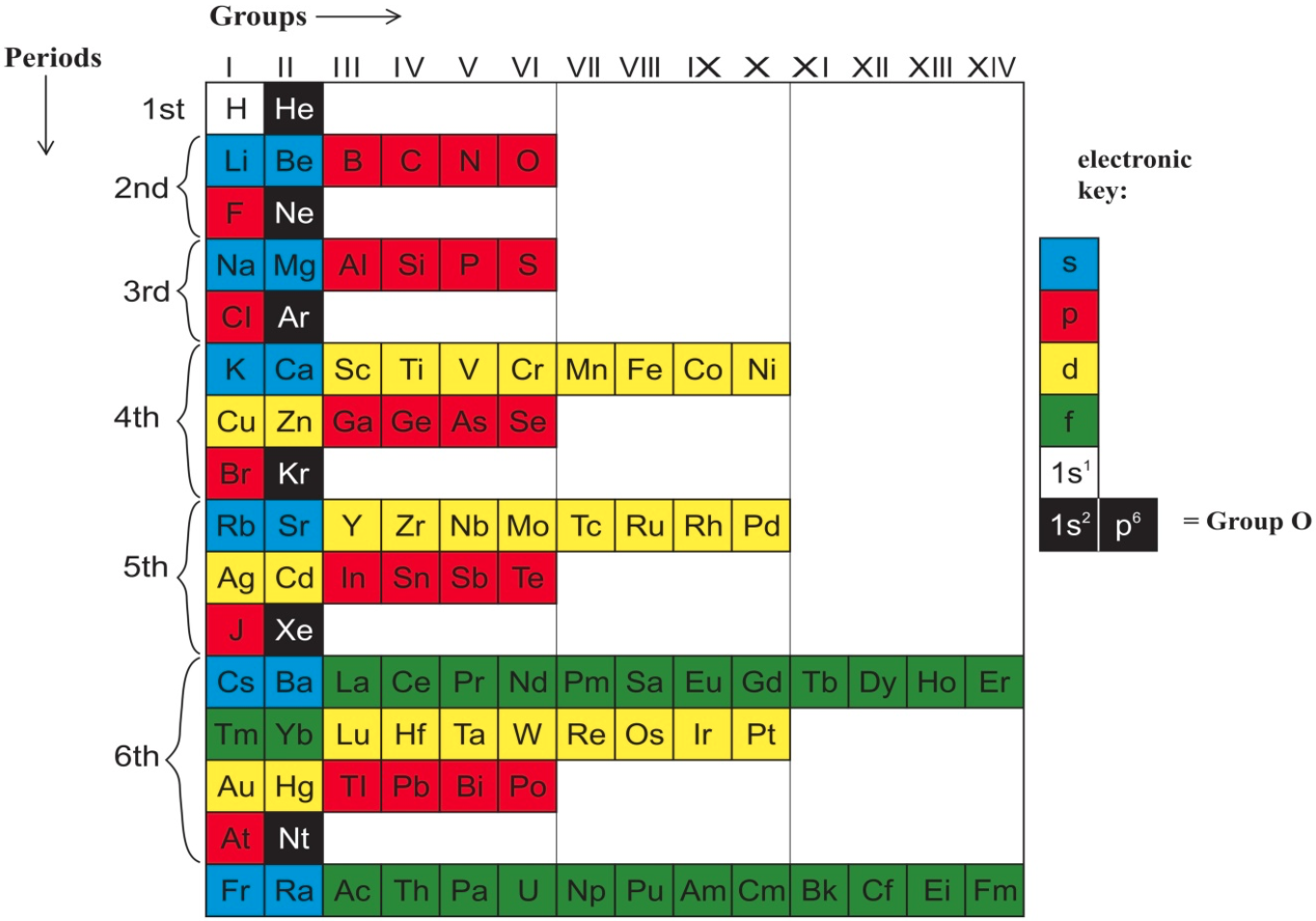
2019
Archetypes of Periodic Law
Archetypes of Periodic Law by Dmitry Weise, read more on the website.
One of the creators of quantum mechanics Wolfgang Ernst Pauli wrote in his work The Influence of Archetypal Ideas on the Scientific Theories of Kepler (1948):
"The process of understanding nature as well as the happiness that man feels in understanding – that is, in the conscious realization or new knowledge – seems thus to be based on a correspondence, a 'matching' of inner images pre-existent in the human psyche with external objects and their behavior. This interpretation of scientific knowledge, of course, goes back to Plato and is, as we shall see, advocated very clearly by Kepler. These primary images, which the soul can perceive with the aid of an innate 'instinct', are called by Kepler archetypal. Their agreement with the 'primordial images' or archetypes introduced into modern psychology by C. G. Jung and functioning as 'instincts of imagination' is very extensive. A true spiritual descendant of the Pythagoreans, he attached the utmost importance to geometric claiming that its theorems 'have been in the spirit of God since eternity'. His basic principle was: 'Geometria est archetypus pulchritudinis mundi' (Geometry is the archetype of the beauty of the world)."
Dmitry writes:
"The key archetype, in our opinion, is the concept of the square and its gnomon. This is due to the well-known fact that the electron filled shell contains 2n2 electrons, and the number of electrons on the subshell is twice the odd number; the gnomon of the square. Triangle, tetrahedron, square pyramid, octahedron, pyramid-like figures composed of square layers are also considered. The methodical concept for these constructions is the figurate numbers, actively studied by the Pythagoreans. The tables of the periodic law built on the motifs of ancient folk and modern ornaments take a special place. They include not only geometric archetypes, but also magic-symbolic, cultural and religious archetypes of the collective unconscious. Note that the periodic law table, built on the basis of the Native American ornament, surpasses the modern Mendeleev table in the parameter reflecting quantum numbers in its structure."
Note the final photograph below shows Prof. Martyn Poliakoff of The University on Nottingham and Periodic Videos:

2019
Telluric Remix in Colour
Philip Stewart writes (this is the same text that accompanies the 2018 B/W version):
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version (pdf).
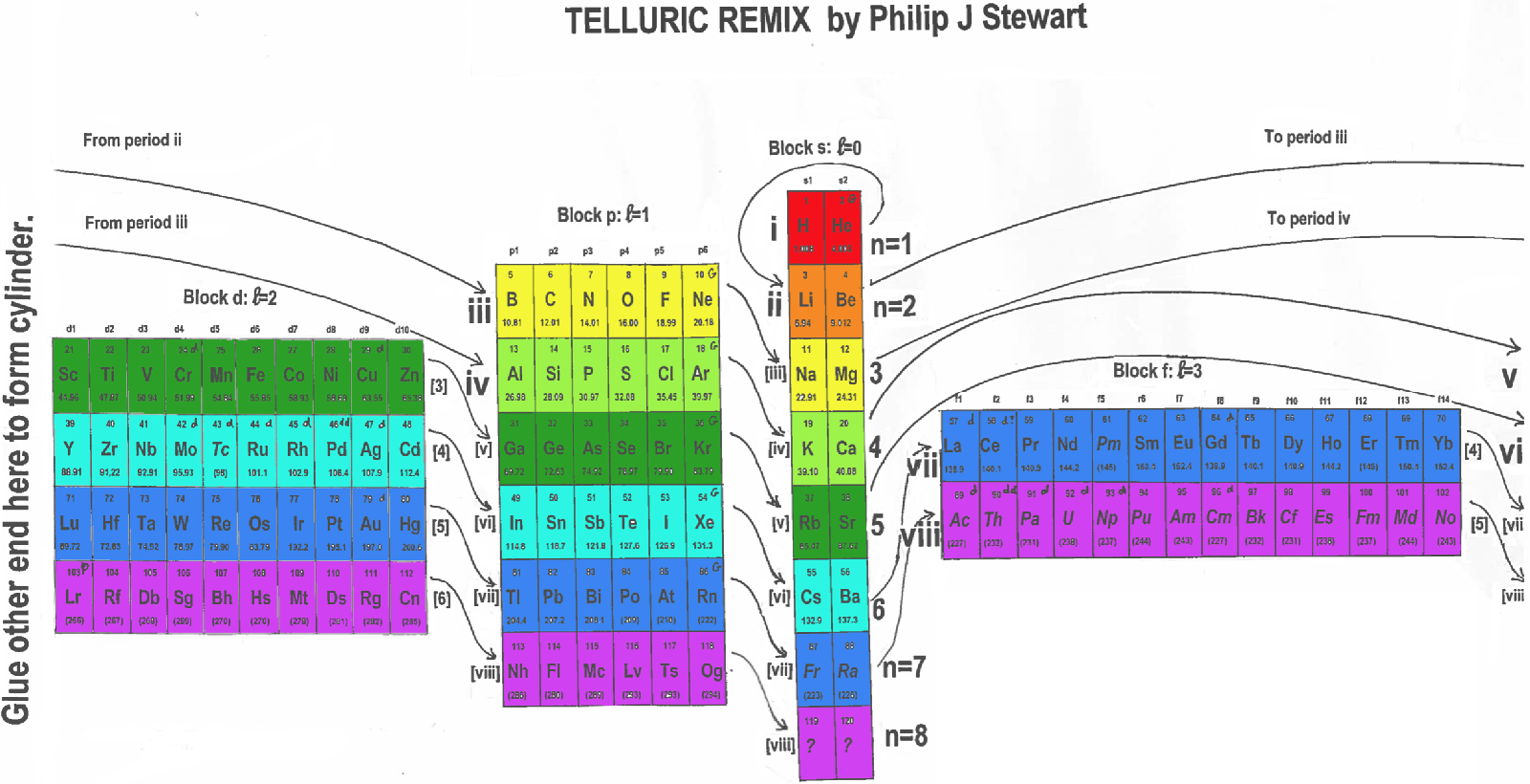
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
2019
Periodic Table of the Elements Coloring Book
Periodic Table of the Elements Coloring Book
Project managing and chemistry overseen by Yann Brouillette (Faculty, Chemistry, Dawson College). Element representations and cover by Dawson College Illustration & Design students (2nd year)* overseen by Meinert Hansen (Faculty, Illustration & Design, Dawson College).
Thanks to René for the tip!
2019
Grainger's Elemental Periodicity with "Concentric Spheres Intersecting Orthogonal Planes" Formulation
From Tony Grainger, an Elemental Periodicity formulation with concentric spheres intersecting orthogonal planes.
Tony writes:
"I hand sketched this periodic table about a decade ago and placed it on my cubicle window at UTAS, with minimal comments from work mates. It bears some similarity to other formulations in the database, especially when cut along the left axis and laid flat. The concept of all elements of a period being aligned along orthogonal planes cutting a sphere was inherent in the original sketch. When I began using SVG about five years ago I realised I could draw this as a real projection of the 3D model. It was on the back burner, until I found the original sketch during a tidy up."
There are two images of this 3D formulation: an "inside_corner_below/outside_corner_above" (top image) and an "outside_corner_below/inside_corner_above" lower image.
- The "inside corner below" is like looking at the junction of a floor and two walls in the corner of a room.
- The "outside corner above" is like looking up at the underside of an overhanging corner of a building.
- The "outside corner below" is like looking down on the corner of a large box.
- The "inside corner above" is like looking at the junction of walls and a ceiling in a room.
2019
Schaltenbrand's Helical Gathering of the Elements
From the RSC Website:
"A glistering, shining spiral made of silver, gold, platinum, palladium and a diamond forms the show-stopping apex of the tribute from the University of Cambridge's St Catharine's college to the International Year of the Periodic Table.
"Commissioned to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Poliakoff's Inverted Periodic Table
From Nature Chemistry. Martyn Poliakoff et al write:
"The inverted periodic table is obtained by rotating the conventional one by 180° about a horizontal axis.
"a, The lighter elements are now at the bottom and the filling of the electron shells occurs upwards. Just like the traditional representation, many properties (for example, atomic number) increase across the table as one proceeds from left to right, but in the inverted version, the same properties now increase as one moves from the bottom to the top, which is the way that most graphs are plotted. Also like the conventional table, the lanthanides and actinides still sit uncomfortably in an isolated block.
"b, In principle, this could be overcome by inverting the 'long form' of the table but, like the conventional long form, it is probably too elongated to be very useful to most chemists."
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2019
Moran's Periodic Spiral (2019)
Jeff Moran has been working on his Periodic Spiral for more than twenty years. Here is the latest iteration, click to enlarge:
2019
NAWA's Version of Moran's Periodic Spiral
Periodic table designer Nagayasu Nawa has put his spin on Moran's Periodic Spiral:
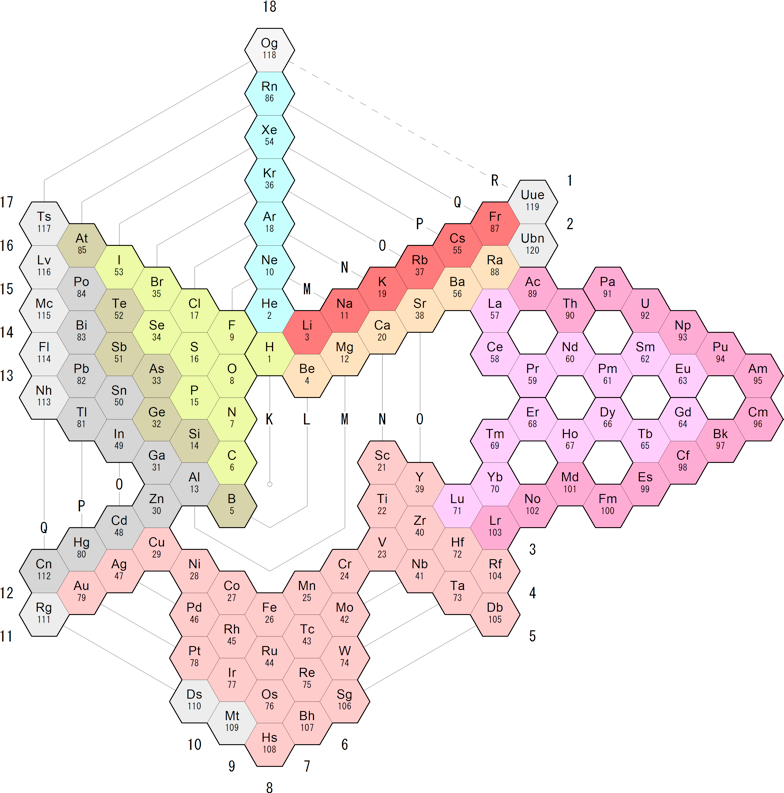
2019
Stewart's Quantahedron Formulation
From Philip Stewart, here & here, comes a three dimensional Quantahedron Formulation.
Philip writes:
"The Quantahedron is based on Tsimmerman's Adomah cube, realised in transparent plastic, in the usual order in which Z values are read, printed on separable blocks so that it can be assembled."
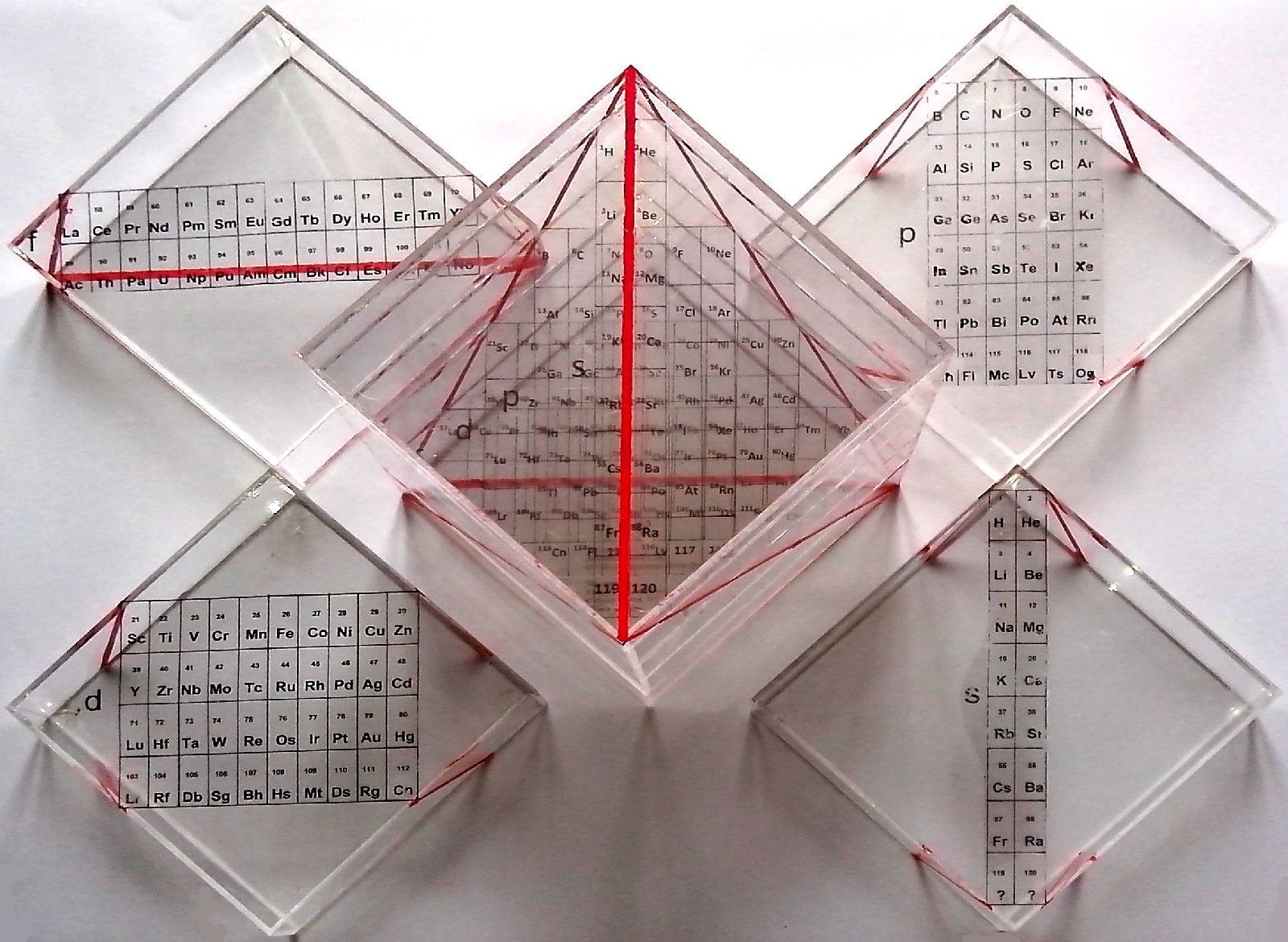
2020
Nuclear Periodic Table
A nuclear periodic table by Kouichi Hagino & Yoshiteru Maeno from Kyoto University published in Foundations of Chemistry here & here (open access).
"Elements with proton magic-number nuclei are arranged on the right-most column, just like the noble-gas elements in the familiar atomic periodic table.
"The periodic properties of the nuclei, such as their stability and deformation from spherical shape, are illustrated in the table. Interestingly, there is a fortuitous resemblance in the alignments of the elements: a set of the elements with the magic number nuclei 50(Sn), 82(Pb) and Fl(114) also appears as the group 14 elements in the atomic periodic table. Thanks to this coincidence, there are similarities in the alignments beyond 41(Nb) (e.g., Nb-Ta-Db or La-Ac in the same columns) in both the nuclear and atomic periodic tables of the elements.
"Related documents can be found: http://www.ss.scphys.kyoto-u.ac.jp/elementouch/index.html
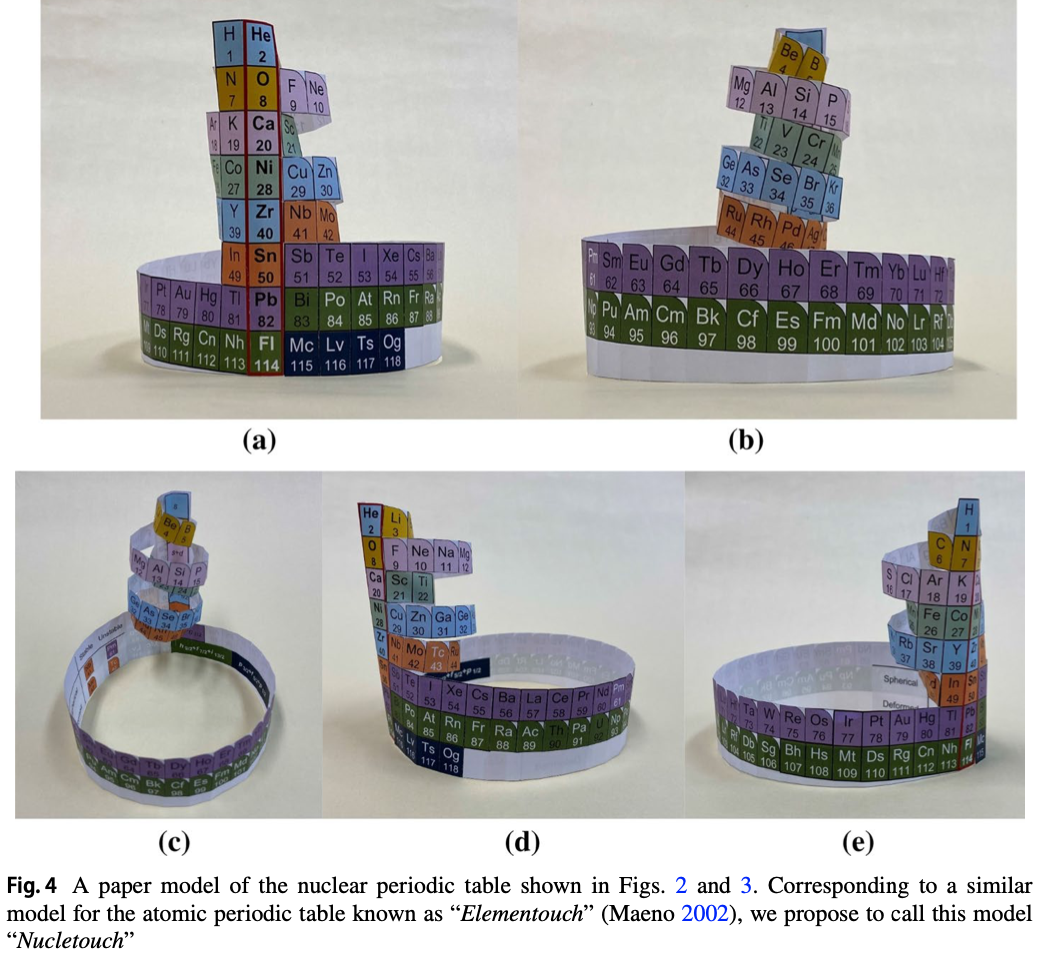
2020
Gierałtowski's Periodic Rotation Table
Sent by Tomasz Gierałtowski from Poland. There is no information, but Tomasz has provided construction diagrams for each period. Click the links to see these:
- Period 1
- Period 2
- Period 3
- Period 4
- Period 5
- Period 6a
- Period 6b
- Period 7a
- Period 7b
- Periodic Rotation Table
2020
Nawa Version of Maeno's Nuclear Periodic Table
Nagayasu Nawa - "A Japanese school teacher and periodic table designer" - has developed two versons of the Hagino-Maeno Nuclear Periodic Table.
Nawa writes:
"I have made two Nuclear PTs based on Hagino-Maeno (2020). I have tried to express the Nuclear PT visually by using symbols such as '〇','◇','☓' or small '〇' or '●' in a binary way so that people with colour blindness could understand it. And the other have been with the ' QUAD electronic data."
Click either of the images below to enlarge:
2020
Vernon's Periodic Table showing the Idealized Solid-State Electron Configurations of the Elements
René Vernon writes:
"I've attached a periodic table showing the solid-state electron configurations of the elements. Among other things, it provides a first order explanation as to why elements such as Ln (etc.) like the +3 oxidation state.
"The table includes two versions of the f-block, the first starting with La-Ac; the second with Ce-Th. The table with the first f-block version has 24 anomalies [with respect to Madelung's rule]; the table with the second f-block version has 10 anomalies.
"In the case of the Sc-Y-La-Ac form, I wonder if such a solid-state table is more relevant these days than a table based on gas phase configurations, which has about 20 anomalous configurations.
"Partly we use gas phase configurations since, as Eric Scerri mentioned to me elsewhere, configurations were first obtained (~100 years ago?) from spectroscopy, and this field primarily deals with gas phase atoms. That said, are gas phase configurations still so relevant these days – for this purpose – given the importance of solid-state physics?
"I've never been able to find a periodic table of solid-state electron configurations. Perhaps that has something to do with it? Then again, surely I'm not the first person to have drawn one of these?"
Click image below to enlarge:
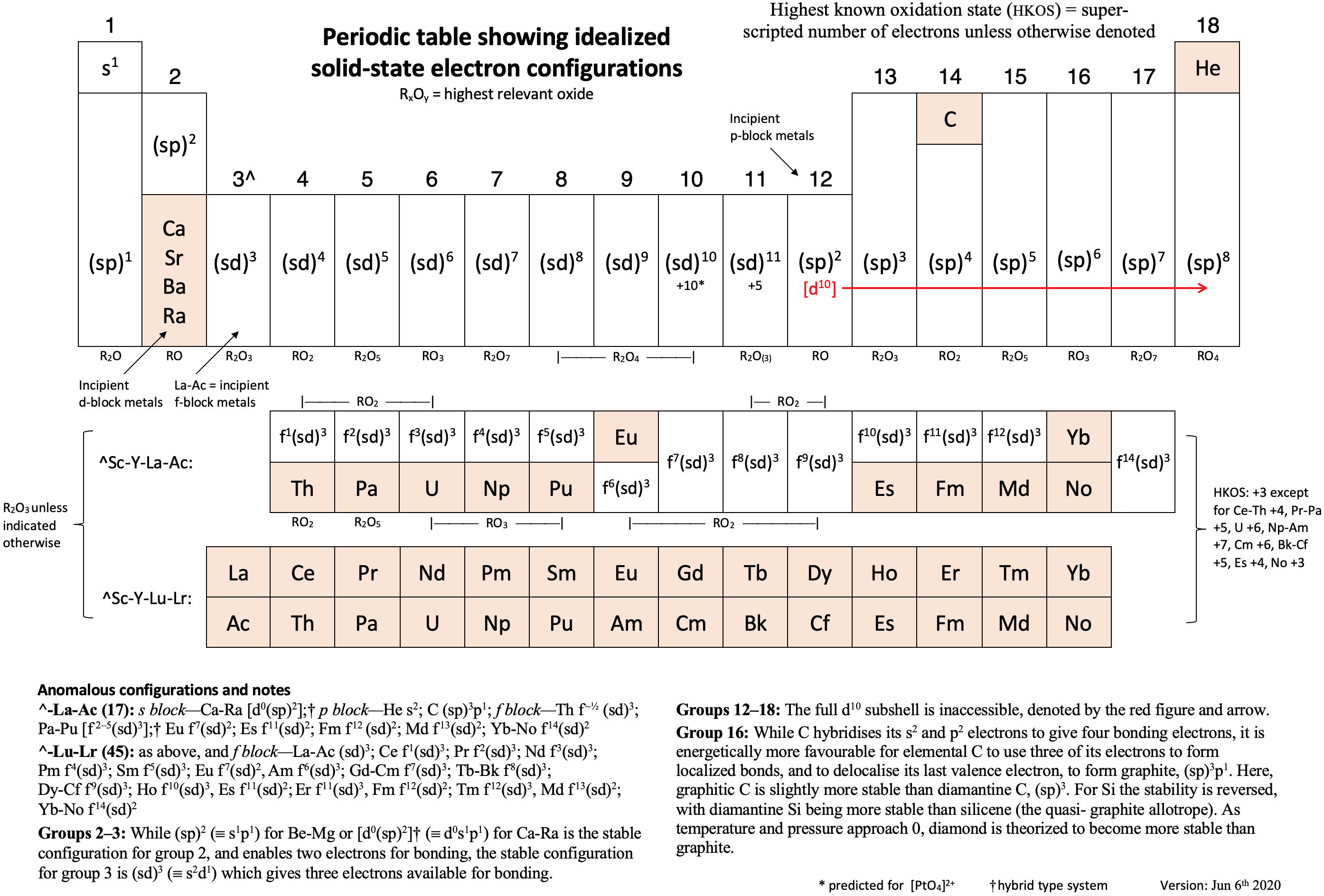
2020
Correlation of Electron Affinity (F) with Elemental Orbital Radii (rorb)
From Jour. Fac. Sci., Hokkaido Univ., Ser. IV. vol. 22, no. 2, Aug., 1987, pp. 357-385, The Connection Between the Properties of Elements and Compounds; Mineralogical-Crystallochemical Classification of Elements by Alexander A. Godovikov & Yu Hariya and expanded by René Vernon who writes.
René Vernon writes:
I was delighted to read about two properties that account for nearly everything seen in the periodic table.
Two properties
While researching double periodicity, I happened upon an obscure article, which simply correlates electron affinity with orbital radius, and in so doing reproduces the broad contours of the periodic table. Having never thought much about the value or significance of EA, and its absence of easily discernible trends, I was suitably astonished. The authors left out the Ln and An and stopped at Bi. They were sitting on a gold mine but provided no further analysis.Development
I added the data up to Lr, updated the EA values, and have redrawn their graph. It is a thing of beauty and wonderment in its simplest sufficient complexity and its return on investment. I've appended 39 observations, covering all 103 elements.
Observations
- Very good correspondence with natural categories
- Largely linear trends seen along main groups; two switchbacks seen in group 13; also falloffs (6p sub-shell) seen in groups 14-17
- First row anomalies seen for Li (in amphoteric territory), Be (ditto), C (misaligned), N (in noble gas territory), O (misaligned), F (ditto) and He (ditto)
- For group 13, the whole group is anomalous, no doubt due to the scandide contraction impacting Ga and the double whammy of the lanthanide and 5d contraction impacting Tl
- Nitrogen was called a noble gas before the discovery of the real noble gases and appropriately enough falls into that territory
- Rn is metallic enough to show cationic behaviour and falls just outside of noble gas territory
- F and O are the most corrosive of the corrosive nonmetals
- The rest of the corrosive nonmetals (Cl, Br and I) are nicely distributed, across the border from F
- The rest of the simple and complex anions, funnily enough, comprise the intermediate nonmetals
- The metalloids are nicely aligned; Ge falls a little outside of the metalloid line, being still occasionally referred to as a metal; Sb, being the most metallic of the metalloids falls outside the border; At is inside; Po is just outside
- Pd is located among the nonmetals due to its absence of 5s electrons; see here
- The proximity of H to Pd is astonishing given the latter's capacity to adsorb the former
- The post-transition metals (PTM) form an "archipelago of amphoterism" bounded by transition metals: Ni and C to the west; Fe and Re to the south; V, Tc and W to the east; noble metals to the north
- Curiously, Zn, Cd, and Hg are collocated with Be, and distant from the PTM and the TM proper (aside from Mn)
- Zn is shown as amphoteric, which it is. Cd is shown as cationic but is not too far away from amphoteric territory; it does show amphoterism, reluctantly; Hg is shown as amphoteric which is the case, weakly, for HgO, as is the congener sulfide HgS, which forms anionic thiomercurates (such as Na2HgS2 and BaHgS3) in strongly basic solutions
- The ostensibly noble metals are nicely delineated; Ag is anomalous given its greater reactivity; Cu, as a coinage metal, is a little further away
- The proximity of Au and Pt to the halogen line is remarkable given the former's capacity to form monovalent anions
- The ferromagnetic metals (Fe-Co-Ni) form a nice line
- The TM from groups 4-12 form switchback patterns e.g. Ti-Zr and the switchback to Hf
- The refractory metals, Nb, Ta, Mo, W and Re are in a wedge formation
- Tc is the central element of the periodic table in terms of mean radius and EA values; V is close, Cr is a little further away
- Ti is just inside the basic cation line; while Ti(IV) is amphoteric, Ti3+ is ionic
- Sc-Y-La shows a main group pattern up to La, when there is a switchback to Ac
- Sc-Y-Lu-Lr shows a TM switch back pattern
- La, and to lesser extent Ce are rather separated from the rest of the Ln, consistent with Restrepo and here.
- Sc and Lu are close to the amphoteric territory and are both in fact, weakly amphoteric
- The post-cerium Ln and An (but for Th) all fall within basic cation territory
- EA values for the An are estimates and need to be treated with due caution
- The light actinides (Th to Cm) occupy a tight locus, with the exception of Th, where the 5f collapse is thought to occur, and Pu, which sits on the border of 5f delocalisation and localisation
- While the light actinides U to Cm are shown as being cationic they are all known in amphoteric forms
- The heavy actinides, Bk to Lr, are widely dispersed
- All the Ln, bar Tm, are located within close proximity of the light An locus; Tm is the least abundant stable Ln
- The gap between La and Ce, and rest of the Ln is consistent with Restrepo's findings and here
- Nobelium in this edition of the chart falls off the bottom, having a radius 1.58 (cf Es) and an EA of -2.33
- There is an extraordinary alignment between He and the Group 2 metals
- Magnesium is on the cationic-amphoteric boundary; some of its compounds show appreciable covalent character
- Li, being the least basic of the alkali metals, is located just outside the alkalic zone; Li compounds are known for their covalent properties
- The reversal of the positions of Fr and Cs is consistent with Cs being the most electronegative metal
- A similar, weaker pattern is seen with Ba and Ra.
Conclusion
So there it is, just two properties account for nearly everything.
Click images below to enlarge:
2020
Vernon's Constellation of Electronegativity
René Vernon has created a "Constellation of Electronegativity" by plotting Electronegativity against Elemental Orbital Radii (rorb)
Observations on the EN plot:
- The results are similar to the orbital radii x EA plot, although not quite as clear, including being more crowded
- Very good correspondence with natural categories
- Largely linear trends seen along groups 1-2, 17 and 15-18 (Ne-Rn)
- First row anomaly seen for He (or maybe not since it lines up with the rest of group 2)
- For group 13, the whole group is anomalous
- For group 14 , the whole group is anomalous no doubt due to the scandide contraction impacting Ge and the double whammy of the lanthanide and 5d contraction impacting Pb
- F and O are the most corrosive of the corrosive nonmetals
- The rest of the corrosive nonmetals (Cl, Br and I) are nicely aligned with F
- The intermediate nonmetals (IM) occupy a trapezium
- Iodine almost falls into the IM trapezium
- The metalloids occupy a diamond, along with Hg; Po is just inside; At a little outside
- Rn is metallic enough to show cationic behaviour and falls into the metalloid diamond
- Pd is located among the nonmetals
- The proximity of H to Pd is again (coincidentally?) curious given the latter's capacity to adsorb the former
- The post-transition metals occupy a narrow strip overlapping the base of the refractory metal parallelogram
- Curiously, Zn, Cd, and Hg (a bit stand-off-ish) are collocated with Be, and relatively distant from the PTM and the TM proper
- The ostensibly noble metals occupy an oval; curiously, W is found here; Ag is anomalous given its greater reactivity; Cu, as a coinage metal, is a little further away
- Au and Pt are nearest to the halogen line
- The ferromagnetic metals (Fe-Co-Ni) are colocated
- The refractory metals, Nb, Ta, Mo, W and Re are in a parallelogram, along with Cr and V; Tc is included here too
- Indium is the central element of the periodic table in terms of mean orbital radius and EN; Tc is next as per the EA chart
- The reversal of He compared to the rest of the NG reflects #24
- All of the Ln and An fall into an oval of basicity, bar Lr
- The reversal of the positions of Fr and Cs is consistent with Cs being the most electronegative metal
- A similar, weaker pattern is seen with Ba and Ra.
2020
Jodogne's Periodic Table of The Elements
Dr.Ir.Jodogne Jean Claude writes:
"I have the pleasure to send to you my paper on the PT which appears in Chimie Nouvelle 133 of the Soc.Royale de Chimie. However for the moment it is in French. The paper contains and explains the ultimate evolution of my preceding PT but it is the most scientifically based. Pedagogically, I believe it is interesting and easy. As you will see it keeps most of the chemical usual properties of the traditional one."
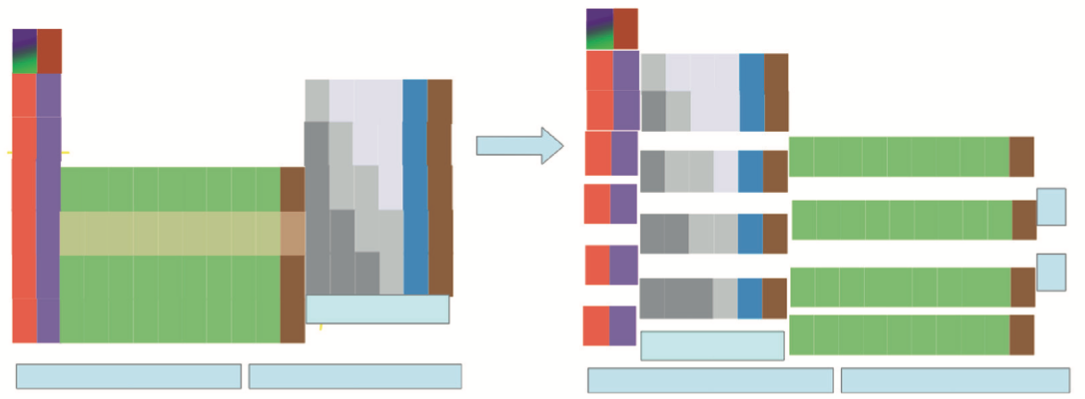
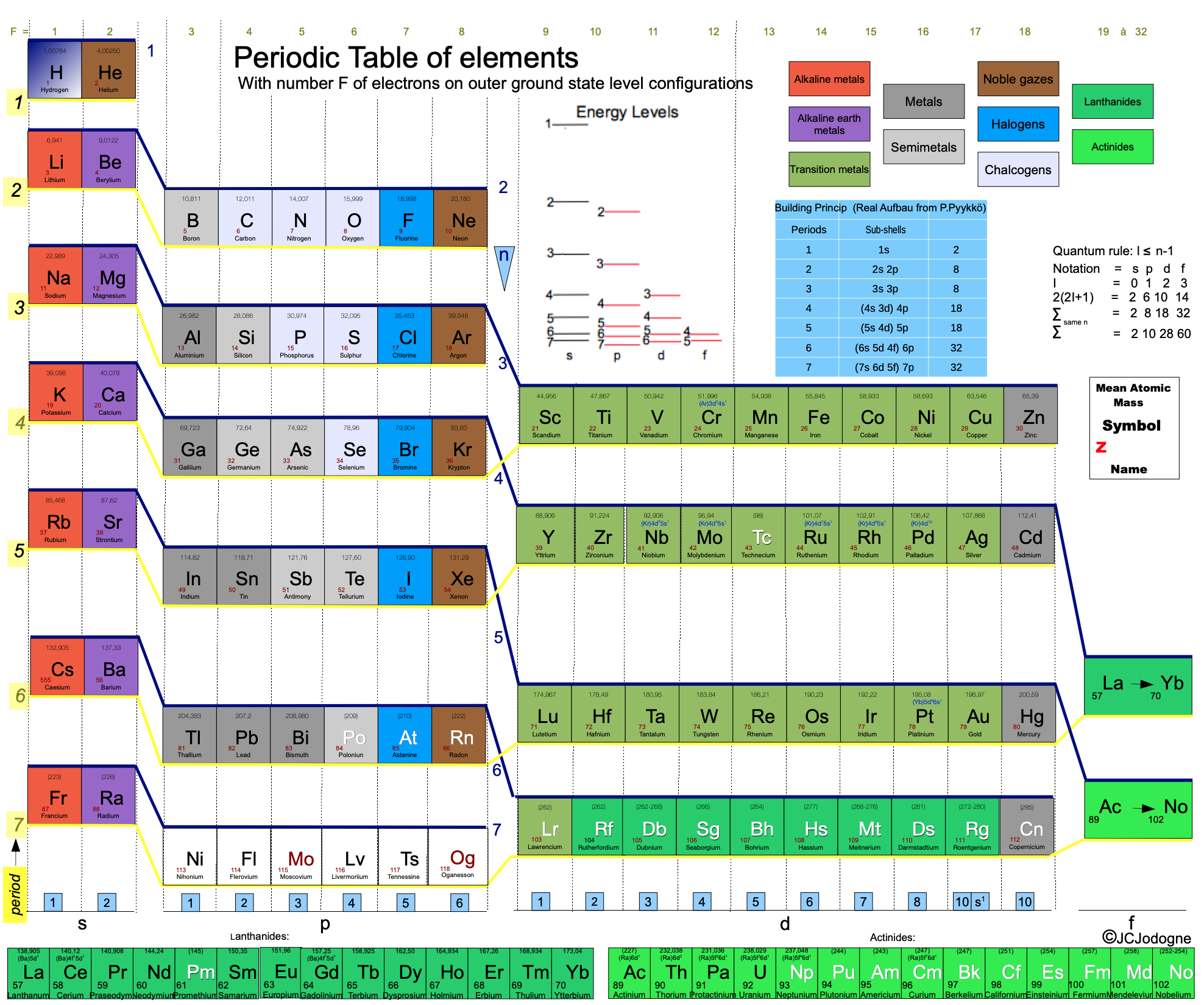
2020
artlebedev's 100,000 Permutation Periodic Table of The Elements
Moscow-based design company Art. Lebedev Studio have released a new Periodic Table which can be adapted for any task.
- Since 1869, Mendeleev's periodic law has been widely regarded as one of the most ground-breaking advances in our understanding of the laws of nature. Used around the world in classes, lecture halls, and laboratories, the periodic table helps us to understand the elements that make up our world – and the relationships between them.
- Despite this, people have never been able to agree on which information the perfect table should include. What may be useful in a professional context, for example, would be unbearably complex for a student. On the other hand, showing each element's characteristics in full would make the table almost impossible to navigate. This has always resulted in an awkward compromise between simple and detailed.
- Art. Lebedev Studio made an adaptable table which lets users compare values, reveal patterns, and make their own discoveries. If a student only needs to see the element symbols, they can simply omit the other parameters. If someone wants to find out which country discovered the largest number of elements, they can include the flags of each nation's achievements (spoiler: it's the UK with 24).
- As well as liberating scientists from the limitations of fixed tables, the Studio also focused on improving the table's appearance. Designers came up with a clean, readable typeface which makes each element almost feel like a standalone design. They also made it highly adaptable, allowing users complete control over everything from nomenclature to background and cell colours.
- With over 100 000 permutations, users are sure to find the right table for them – whether they are a lab technician, lecturer, or student.
2020
Periodic Ziggurat of The Elements
By René Vernon, the Periodic Ziggurat of the Elements. Click to enlarge:
2020
Scerri's Periodic Table of Books About The Periodic Table & The Chemical Elements
From Eric Scerri, a periodic table of books about the periodic table & the chemical elements... many by Eric Scerri himself.
Eric Scerri, UCLA, Department of Chemistry & Biochemistry. See the website EricScerri.com and Eric's Twitter Feed.
There is no particular connection between each of the elements and the book associated with it in the table, with the exception of: H, He, N, Ti, V, Nb, Ag, La, Au, Ac, U, Pu & Og.
The following is a list of references for each of the 118 books featured on Periodic Table of Books About The Periodic Table & The Chemical Elements. Books published in languages other than English are. They include the Catalan, Croatian, French, German, Italian, Norwegian & Spanish languages:
| 1 | H | J. Ridgen, Hydrogen, the Essential Element, Harvard University Press, Cambridge, MA, 2002. |
| 2 | He | W.M. Sears Jr., Helium, The Disappearing Element, Springer, Berlin, 2015. |
| 3 | Li | K. Lew, The Alkali Metals, Rosen Central, New York, 2009. |
| 4 | Be | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish) |
| 5 | B | E.R. Scerri. The Periodic Table, Its Story and Its Significance, 2nd edition, Oxford University Press, New York, 2020. |
| 6 | C | U. Lagerkvist, The Periodic Table and a Missed Nobel Prize, World Scientific, Singapore, 2012. |
| 7 | N | W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869–1905, Dover, Mineola, NY, 2005. |
| 8 | O | M. Kaji, H. Kragh, G. Pallo, (eds.), Early Responses to the Periodic System, Oxford University, Press, New York, 2015. |
| 9 | F | E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974. |
| 10 | Ne | T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009. |
| 11 | Na | N.C. Norman, Periodicity and the s- and p-Block Elements, Oxford University Press, Oxford, 2007. |
| 12 | Mg | M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, 2nd edition, Basic Books, New York, 2019. |
| 13 | Al | S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010. |
| 14 | Si | P.A. Cox, The Elements, Oxford University Press, Oxford, 1989. |
| 15 | P | J. Emsley, The 13th Element: The Sordid Tale of Murder, Fire, and Phosphorus, Wiley, New York, 2002. |
| 16 | S | P. Parsons, G. Dixon, The Periodic Table: A Field Guide to the Elements, Qurcus, London, 2014. |
| 17 | Cl | P. Levi, The Periodic Table, Schocken, New York, 1995. |
| 18 | Ar | B.D. Wiker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003. |
| 19 | K | H. Alderesey-Williams, Periodic Tales, Viking Press, 2011. |
| 20 | Ca | P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999. |
| 21 | Sc | D. Scott, Around the World in 18 Elements, Royal Society of Chemistry, London, 2015. |
| 22 | Ti | E. W. Collings, Gerhard Welsch, Materials Properties Handbook: Titanium Alloys, ASM International, Geauga County, Ohio, 1994. |
| 23 | V | D. Rehder, Bioinorganic Vanadium Chemistry, Wiley-Blackwell, Weinheim, 2008. |
| 24 | Cr | K. Chapman, Superheavy, Bloomsbury Sigma, New York, 2019. |
| 25 | Mn | E.R. Scerri, E. Ghibaudi (eds.), What is an Element? Oxford University Press, New York, 2020. |
| 26 | Fe | M. Soon Lee, Elemental Haiku, Ten Speed Press, New York, 2019. |
| 27 | Co | J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001. |
| 28 | Ni | T. James, Elemental, Robinson, London, 2018. |
| 29 | Cu | E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007. |
| 30 | Zn | H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998. |
| 31 | Ga | P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004. |
| 32 | Ge | I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966. |
| 33 | As | J. Browne, Seven Elements that Changed the World, Weidenfeld and Nicholson, London, 2013. |
| 34 | Se | N. Raos, Bezbroj Lica Periodnog Sustava Elemenata, Technical Museum of Zagreb, Croatia, 2010. (Croatian) |
| 35 | Br | P. Strathern, The Knowledge, The Periodic Table, Quadrille Publishing, London, 2015. |
| 36 | Kr | A. Ede, The Chemical Element, Greenwood Press, Westport, CT, 2006. |
| 37 | Rb | A. Stwertka, The Elements, Oxford University Press, Oxford, 1998. |
| 38 | Sr | E.R. Scerri, A Tale of Seven Elements, Oxford University Press, New York, 2013. |
| 39 | Y | H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007. |
| 40 | Zr | M. Fontani, M. Costa, M.V. Orna (eds.), The Lost Elements, Oxford University Press, New York, 2015. |
| 41 | Nb | M. Seegers, T. Peeters (eds.), Niobium: Chemical Properties, Applications and Environmental Effects, Nova Science Publishers, New York, 2013. |
| 42 | Mo | E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, Imperial College Press, London and Singapore, 2009. |
| 43 | Tc | A. Dingle, The Periodic Table, Elements with Style, Kingfisher, Richmond, B.C. Canada, 2007. |
| 44 | Ru | G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904. (German) |
| 45 | Rh | I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Publications, Norfolk, UK, 1997. |
| 46 | Pd | P. Davern, The Periodic Table of Poems, No Starch Press, San Francisco, 2020. |
| 47 | Ag | C. Fenau, Non-ferrous metals from Ag to Zn, Unicore, Brussells, 2002. |
| 48 | Cd | J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969. |
| 49 | In | M. Tweed, Essential Elements, Walker and Company, New York, 2003. |
| 50 | Sn | M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960. |
| 51 | Sb | P. Wothers, Antimony Gold Jupiter's Wolf, Oxford University Press, Oxford, 2019. |
| 52 | Te | W. Zhu, Chemical Elements in Life, World Scientific Press, Singapore, 2020. |
| 53 | I | O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001. |
| 54 | Xe | E.R. Scerri, (ed.), 30-Second Elements, Icon Books, London, 2013. |
| 55 | Cs | M. Jacob (ed.), It's Elemental: The Periodic Table, Celebrating 80th Anniversary, Chemical & Engineering News, American Chemical Society, Washington D.C., 2003. |
| 56 | Ba | J. Marshall, Discovery of the Elements, Pearson Custom Publishing, New York,1998. |
| 57 | La | K. Veronense, Rare, Prometheus Books, Amherst, New York, 2015. |
| 58 | Ce | N. Holt, The Periodic Table of Football, Ebury Publishing, London, 2016. |
| 59 | Pr | S. Alvarez, C. Mans, 150 Ans de Taules Périodiques a la Universitat de Barcelona, Edicions de la Universitat de Barcelona, Barcelona, 2019. (Catalan) |
| 60 | Nd | L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988. (Spanish) |
| 61 | Pm | P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002. |
| 62 | Sm | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish). |
| 63 | Eu | A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909. |
| 64 | Gd | M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992. |
| 65 | Tb | M. Eesa, The cosmic history of the elements: A brief journey through the creation of the chemical elements and the history of the periodic table, Createspace Independent Publishing Platform, 2012. |
| 66 | Dy | P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002. (French). |
| 67 | Ho | F. Habashi, The Periodic Table & Mendeleev, Laval University Press, Quebec, 2017. |
| 68 | Er | W.J. Nuttall, R. Clarke, B. Glowacki, The Future of Helium as a Natural Resource, Routledge, London, 2014. |
| 69 | Tm | R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010. (Spanish). |
| 70 | Yb | P.R. Polo, El Profeta del Orden Quimico, Mendeleiev, Nivola, Spain, 2008. (Spanish). |
| 71 | Lu | E.R. Scerri, A Very Short Introduction to the Periodic Table, 2nd edition, Oxford University Press, Oxford, 2019. |
| 72 | Hf | D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006. |
| 73 | Ta | P. Thyssen, A. Ceulemans, Shattered Symmetry, Oxford University Press, New York, 2017. |
| 74 | W | P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995. |
| 75 | Re | D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964. |
| 76 | Os | E. Lassner, W.-D. Schubert, Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, Springer, Berlin, 1999. |
| 77 | Ir | J.C.A. Boeyens, D.C. Levendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008. |
| 78 | Pt | R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989. |
| 79 | Au | R.J. Puddephatt, The Chemistry of Gold, Elsevier, Amsterdam, 1978. |
| 80 | Hg | D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004. |
| 81 | Tl | R.E. Krebs, The History and Use of Our Earth's Chemical Elements, Greenwood Publishing Group, Santa Barbara, CA, 2006. |
| 82 | Pb | E. Torgsen, Genier, sjarlataner og 50 bøtter med urin - Historien om det periodiske system, Spartacus, 2018. (Norwegian). |
| 83 | Bi | K. Buchanan, D. Roller, Memorize the Periodic Table, Memory Worldwide Pty Limited, 2013. |
| 84 | Po | D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003. |
| 85 | At | T. Jackson, The Elements, Shelter Harbor Press, New York, 2012. |
| 86 | Rn | R.J.P. Williams, J.J.R. Frausto da Silva, The Natural Selection of the Chemical Elements: The Environment and Life's Chemistry, Clarendon Press, Oxford, 1997. |
| 87 | Fr | G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900. |
| 88 | Ra | L. Van Gorp, Elements, Compass Point Books, Manakato, MN, 2008. |
| 89 | Ac | G.T. Seaborg, J.J. Katz, L.R. Morss, Chemistry of the Actinide Elements, Springer, Berlin, 1986. |
| 90 | Th | G. Münzenberg, Superheavy Elements - Searching for the End of the Periodic Table, Manipal Universal Press, India, 2018. |
| 91 | Pa | A. Castillejos Salazar, La Tabla Periòdica: Abecedario de la Quimica, Universidad Autonoma de Mexico, D.F. Mexico, 2005. (Spanish). |
| 92 | U | T. Zoellner, Uranium, Penguin Books, London, 2009. |
| 93 | Np | J. Barrett, Atomic Structure and Periodicity, Royal Society of Chemistry, London, 2002. |
| 94 | Pu | J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007. |
| 95 | Am | S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002. |
| 96 | Cm | H.M. Davis, The Chemical Elements, Ballantine Books, New York, 1961. |
| 97 | Bk | P.González Duarte, Les Mils Cares de la Taula Periòdica, Universitat Autonoma de Barcelona, Bellaterra Barcelona, 2005 (Catalan). |
| 98 | Cf | R. Rich, Periodic Correlations, Benjamin, New York, 1965. |
| 99 | Es | E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930. (German). |
| 100 | Fm | P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982. |
| 101 | Md | G. Villani, Mendeleev, La Tavola Periodica Degli Elementi, Grandangolo, Milan, 2016. (Italian). |
| 102 | No | J. Russell, Elementary: The Periodic Table Explained, Michael O'Mara, London, 2020. |
| 103 | Lr | P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004. |
| 104 | Rf | R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972. |
| 105 | Db | L. Ohrström, The Last Alchemist in Paris, Oxford University Press, New York, 2013. |
| 106 | Sg | N.N. Greenwood, E. Earnshaw, Chemistry of the Elements, 2nd edition, Elsevier, Amsterdam, 1997. |
| 107 | Bh | R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997. (French) |
| 108 | Hs | Science Foundation Course Team, The Periodic Table and Chemical Bonding, The Open University, Milton Keynes, 1971. |
| 109 | Mt | W.W. Schulz, J. Navratil, Transplutonium Elements, American Chemical Society, Washington D.C., 1981. |
| 110 | Ds | I. Nechaev, Chemical Elements, Lindsay Drummond, 1946. |
| 111 | Rg | F. Hund, Linienspektren und Periodisches System Der Elemente, Springer, Berlin, 1927. |
| 112 | Cn | F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896. |
| 113 | Nh | O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953 (Spanish). |
| 114 | Fl | B. Yorifuji, Wonderful Life with the Elements, No Starch Press, San Francisco, 2012. |
| 115 | Mc | D.I. Mendeléeff, The Principles of Chemistry, American Home Library, New York, 1902. |
| 116 | Lv | A. Lima-de-Faria, Periodic Tables Unifying Living Organisms at the Molecular Level: The Predictive Power of the Law of Periodicity, World Scientific Press, Singapore, 2018. |
| 117 | Ts | H.B. Gray, J.D. Simon, W.C. Trogler, Braving the Elements, University Science Books, Sausalito, CA, 1995. |
| 118 | Og | E.R. Scerri, G. Restrepo, Mendeleev to Oganesson, Oxford University Press, New York, 2018. |
2020
Scerri's Periodic Table of Books About The Periodic Table & The Chemical Elements by ERS
From Eric Scerri, a periodic table of books about the periodic table & the chemical elements... by Eric Scerri, including translations.
Eric Scerri, UCLA, Department of Chemistry & Biochemistry. See the website EricScerri.com and Eric's Twitter Feed.
2020
Spiral Electron Spin Periodic Table
The Spiral Electron Spin Periodic Table, By Justine Colburn, who also developed the Genesis formulation.
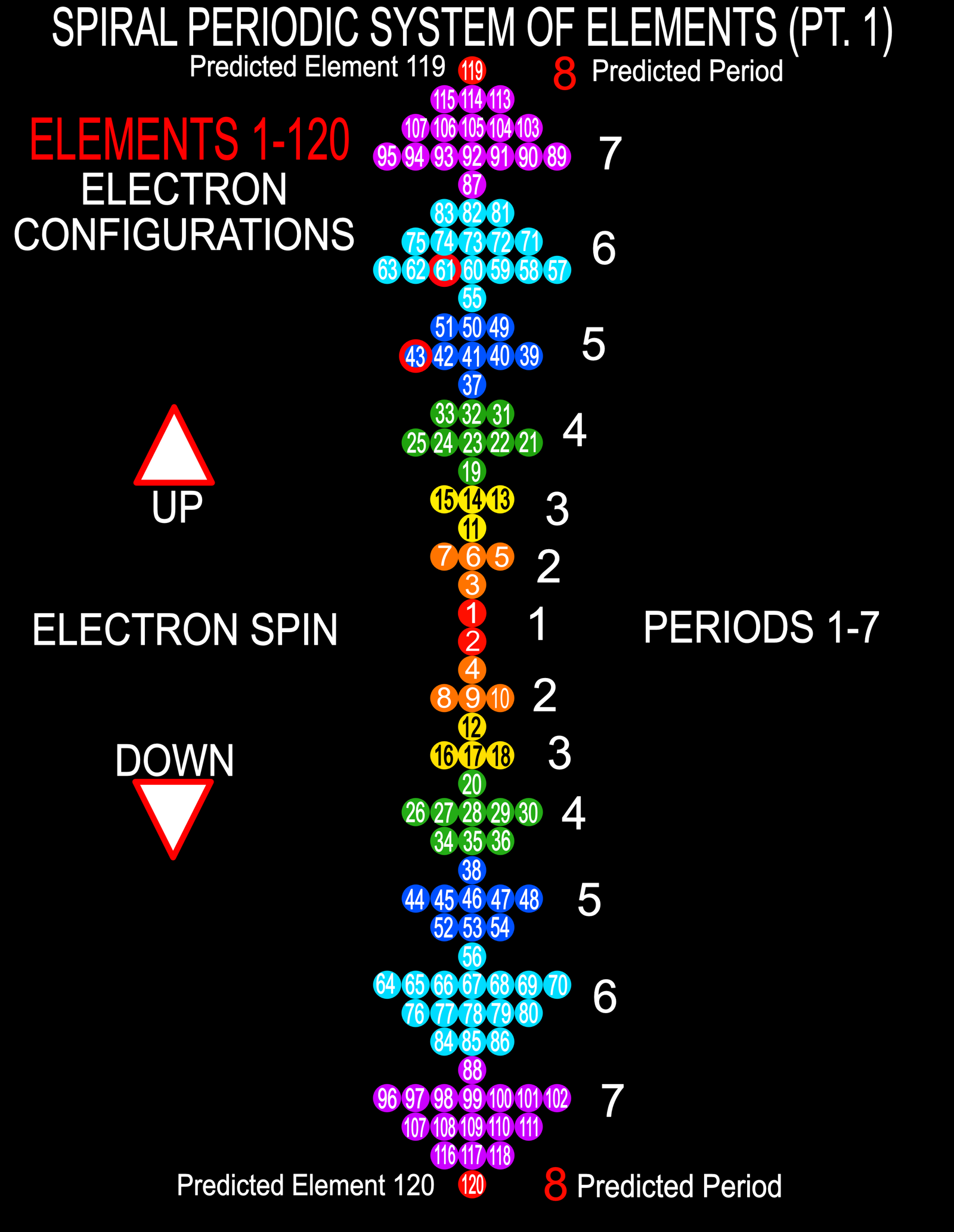
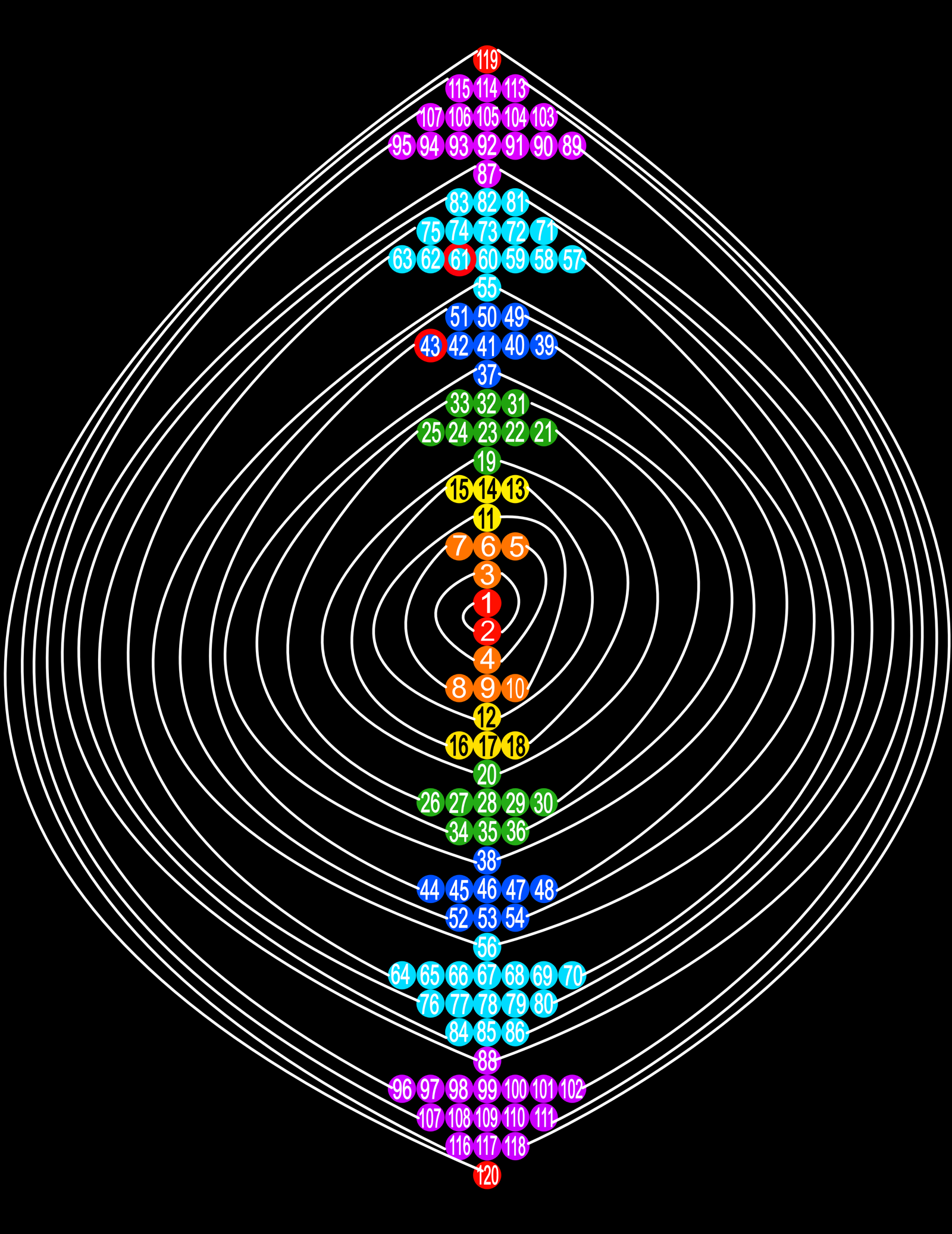
2020
Lehikoinen's Circular Clock Form
Otto Lehikoinen writes:
"A circular form separating 1s orbital to the center, set it on a wall clock as there are 48 elements of main periods, thus can be used as markers for half hours. Group 4 is centered on noon and group 7 starts the afternoon, to get anions and cations with the same but opposite charge to be beside each other. Thus the noble gases are centered on midnight which is easily remembered by neon (and other noble gas) lights. The minute hand hits the 40 d-block elements giving an accuracy of 1.5 minutes and seconds could be read from lanthanides and actinides."
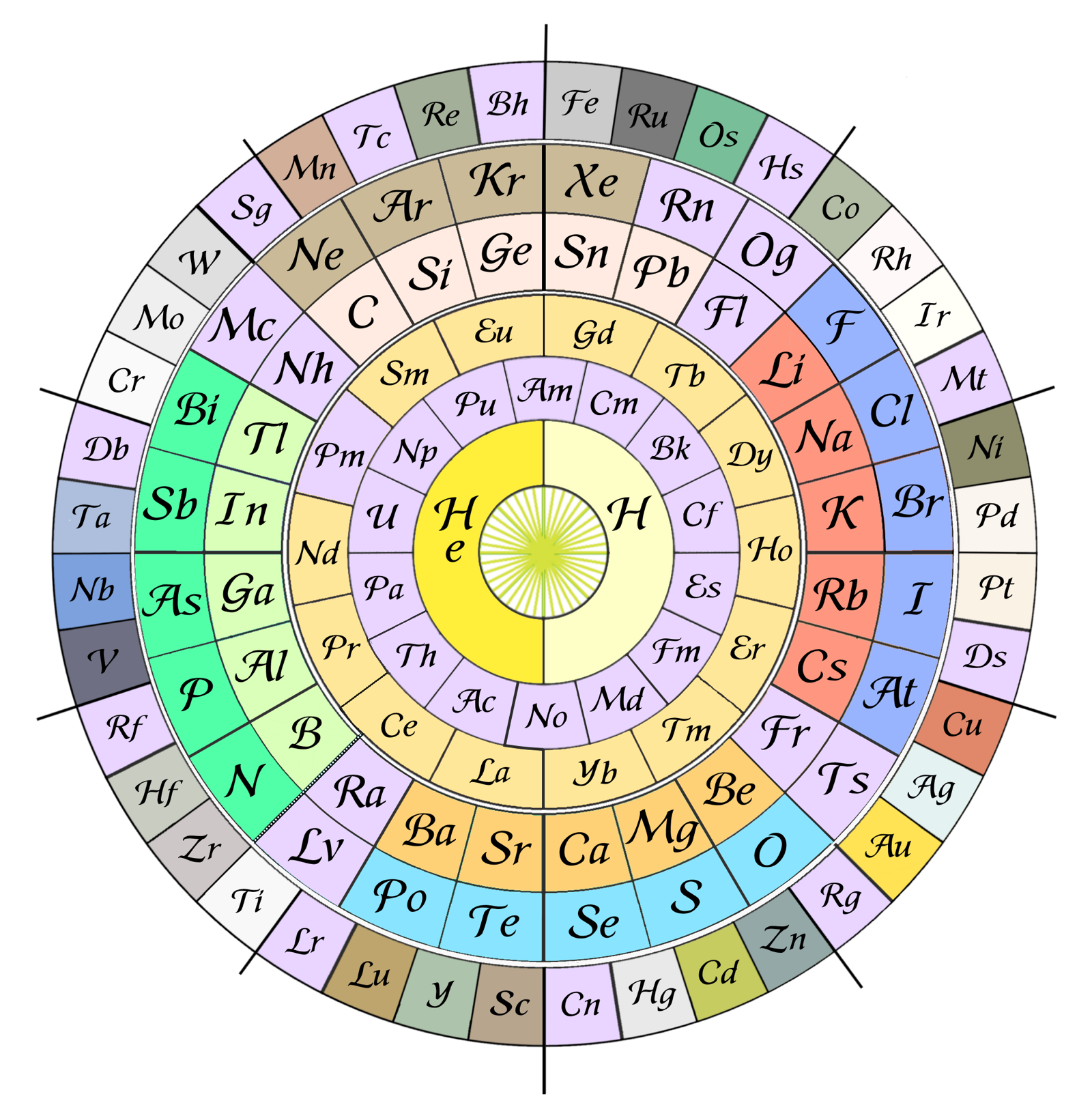

2020
Vernon's Periodic Treehouse
René Vernon's Periodic Treehouse of the Elements, fearuring the World's longest dividing line between metals and nonmetals.
René writes:
I can't remember what started me off on this one. It may have been Mendeleev's line, as shown on the cover of Bent's 2006 book, New ideas in chemistry from fresh energy for the periodic law.
There are a few things that look somewhat arbitrary, so I may revisit these:
- Ce is known at +4, Pr is known as +5, and I recall seeing some speculation about the possibility of Nd +6. (Pm +7 may be overreach.)
- Tl is lined up under Au even though Tl prefers +1. That said Au is not adverse to +1.
- I stopped at Hs since the limits of SHE chemistry just about runs out there.
- The dividing line between metals and nonmetals is 73 element box sides long.
2020
Workshop on Teaching 3d-4s Orbitals Presented by Dr. Eric Scerri
Dr. Eric Scerri, UCLA Department of Chemistry & Biochemistry, discusses many of the issues concerning the periodic table: the aufbau principle, Madelung's rule, the electronic and anomalous electronic structures of the transition elements, the Sc2+ ion, the Janet Left Step, Group 3: Sc, Y, Lu, Lr vs. Sc, Y, La, Ac, atomic spectroscopy, etc.
Many of the topics that concern those of us interested in the periodic table are discussed.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2020
Vernon's (Partially Disordered) 15 Column Periodic Table
A formulation by René Vernon, who writes:
"Here is a 15-column table which is a hybrid of a Mendeleev 8-column table and an 18-column standard table. The key relocations are the p-block nonmetals to the far left; and the coinage and post-transition metals under their s and early d-block counterparts.
"Taking a leaf out of Mendeleev's playbook, I ignored atomic number order when this seemed appropriate. It's refreshing to see the traditional horizontal gaps between blocks disappear. (DIM did not like these.)
"Since Dias (2004, see references below) reckoned a periodic table is a partially ordered set forming a two-dimensional array, I believe I now have a partially ordered table that is partially disordered twice over.
"The table has some curious relationships. Equally, some relationships seen in the standard form are absent. The Group 2, 3, and aluminium dilemmas disappear. This confirms my impression that such dilemmas have no intrinsic meaning. Rather, their appearance or non-appearance is context dependent."
Notes & references below.
Groups 1 to 4 have either a C or F suffix where C (nonmetal) is after the importance of carbon to our existence; and F (metal) is for the importance of iron to civilisation.
Groups 1C and 1F present the greatest contrast in nonmetallic and metallic behaviour.
Coactive Nonmetals: They are capable of forming septenary heterogeneous compounds such as C20H26N4O10PSSe.
Group 2C: Helium is shaded as a noble gas. "Heliox" is a breathing gas mixture of helium and oxygen used in saturation diving, and as a medical treatment for patients with difficulty breathing.
Group 3C: Boron over nitrogen looks odd. Yet one boron atom and one nitrogen atom have the same number of electrons between them as two adjacent carbon atoms. The combination of nitrogen and boron has some unusual features that are hard to match in any other pair of elements (Niedenzu & Dawson 1965).
Boron and phosphorus form a range of ring and cage compounds having novel structural and electronic properties (Paine et al. 2005).
Metalloids. I treat them here as nonmetals given their chemistry is predominately that of chemically weak nonmetals.
Metals: The labels electropositive; transition; and electronegative are adapted from Kornilov (2008).
Group 1F: Monovalent thallium salts resemble those of silver and the alkali metals.
An alloy of cesium (73.71%), potassium (22.14%) and sodium (4.14%) has a melting point of –78.2°C (–108.76°F) (Oshe 1985).
Silver, copper, and gold, as well as being the coinage metals, are borderline post-transition metals.
Group 2F: Beryllium and magnesium are not in fact alkaline earths. Beryllium is amphoteric rather than alkaline; magnesium was isolated in impure form from its oxides, unlike the true alkaline earths. The old ambiguity over whether beryllium and magnesium should go over calcium or zinc has gone.
Nobelium is here since +2 is its preferred oxidation state, unlike other actinoids.
Group 3F: Aluminium is here in light of its similarity to scandium (Habishi 2010).
InGaAsP is a semiconducting alloy of gallium arsenide and indium phosphide, used in lasers and photonics.
There is no Group 3 "issue" since lanthanum, actinium, lutetium and lawrencium are in the same family.
Gold and aluminium form an interesting set of intermetallic compounds known as Au5Al2 (white plague) and AuAl2 (purple plague). Blue gold is an alloy of gold and either gallium or indium.
Lanthanoids: The oxidation state analogies with the transition metals stop after praseodymium. That is why the rest of lanthanoids are footnoted in dash-dot boxes.
Actinoids: The resemblance to their transition metal analogues falters after uranium, and peters out after plutonium.
Group 4F: It's funny to see titanium—the lightweight super-metal—in the same group as lead, the traditional "heavy" metal.
This is the first group impacted by the lanthanoid contraction (cerium through lutetium) which results in the atomic radius of hafnium being almost the same as that of zirconium. Hence "the twins".
The chemistry of titanium is significantly different from that of zirconium and hafnium (Fernelius 1982).
Lead zirconate titanate Pb[ZrxTi1–x]O3 (0 ≤ x ≤ 1) is one of the most commonly used piezo ceramics.
Group 5: Bismuth vanadate BiVO4 is a bright yellow solid widely used as a visible light photo-catalyst and dye.
Steel Friends: The name is reference to their use in steel alloys. They have isoelectronic soluble oxidizing tetroxoanions, plus a stable +3 oxidation state. (Rayner-Canham 2020).
Ferromagnetic Metals: The horizontal similarities among this triad of elements (as is the case among the PGM hexad) are greater than anywhere in the periodic table except among the lanthanides (Lee 1996). The +2 aqueous ion is a major component of their simple chemistry (Rayner-Canham 2020).
Group 8: "Rubiferous metals" (classical Latin rubēre to be red; -fer producing) is from the reddish-brown colour of rust; the most prevalent ruthenium precursor being ruthenium trichloride, a red solid that is poorly defined chemically but versatile synthetically; and the red osmates [OsO4(OH)4]?2 formed upon reaction by osmium tetroxide with a base.
Group 9: "Weather metals" comes from the use of cobalt chloride as a humidity indicator in weather instruments; rhodium plating used to "protect other more vulnerable metals against weather exposure as well as against concentrated acids or acids fumes" (Küpfer 1954); and the "rainbow" etymology of iridium.
Group 10: "Catalytic metals" is after a passage in Greenwood and Earnshaw, "They are... readily obtained in finely divided forms which are catalytically very active." (2002). Of course, many transition metals have catalytic properties. That said, if you asked me about transition metal catalysts, palladium and platinum would be the first to come to mind. Group 10 appear to be particularly catalytic.
References:
- Dias JR 2004, "The periodic table set as a unifying concept in going from benzenoid hydrocarbons to fullerene carbons", in DH Rouvray & RB King (eds.), The periodic table: into the 21st century, Institute of Physics Publishing, Philadelphia, pp. 371–396 (375)
- Fernelius WC 1982, "Hafnium," J. Chem. Educ. vol. 59, no. 3, p. 242
- Greenwood NN & Earnshaw A 2002, Chemistry of the elements, 2nd ed., Butterworth-Heinemann, Oxford, p. 1148
- Habashi F 2010, "Metals: typical and less typical, transition and inner transition", Foundations of Chemistry, vol. 12, pp. 31–39
- Lee JD 1996, Concise inorganic chemistry, 5th ed., Blackwell Science, Oxford, p. 753
- Kornilov II 1965, "Recent developments in metal chemistry", Russian Chemical Reviews, vol. 34, no. 1, p. 33
- Küpfer YJ 1954, "Rhodium uses in plating", Microtecnic, Agifa S.A., p. 294 Niedenzu K & Dawson JW 1965, Boron-nitrogen compounds, Springer, Berlin, preface
- Oshe RW (ed.) 1985, "Handbook of thermodynamic and transport properties of alkali metals", Blackwell Scientific, Oxford, p. 987
- Paine et al. 2005, "Recent developments in boron-phosphorus ring and cage chemistry", in Modern aspects of main group chemistry, M Lattman et al. (eds.), ACS Symposium Series, American Chemical Society, Washington DC, p. 163
- Rayner-Canham G 2020, The periodic table: Past, present, and future, World Scientific, Singapore
2020
Shukarev's Periodic System (redrawn by Vernon)
Shukarev SA 1975, "On the image of the periodic system with the use of fifth move of late a-elements", Collection of Scientific and Methodological Articles on Chemistry. M.: Higher School, no 4, pp 3-12 (in Russian). Redrawn and commented upon by René Vernon:
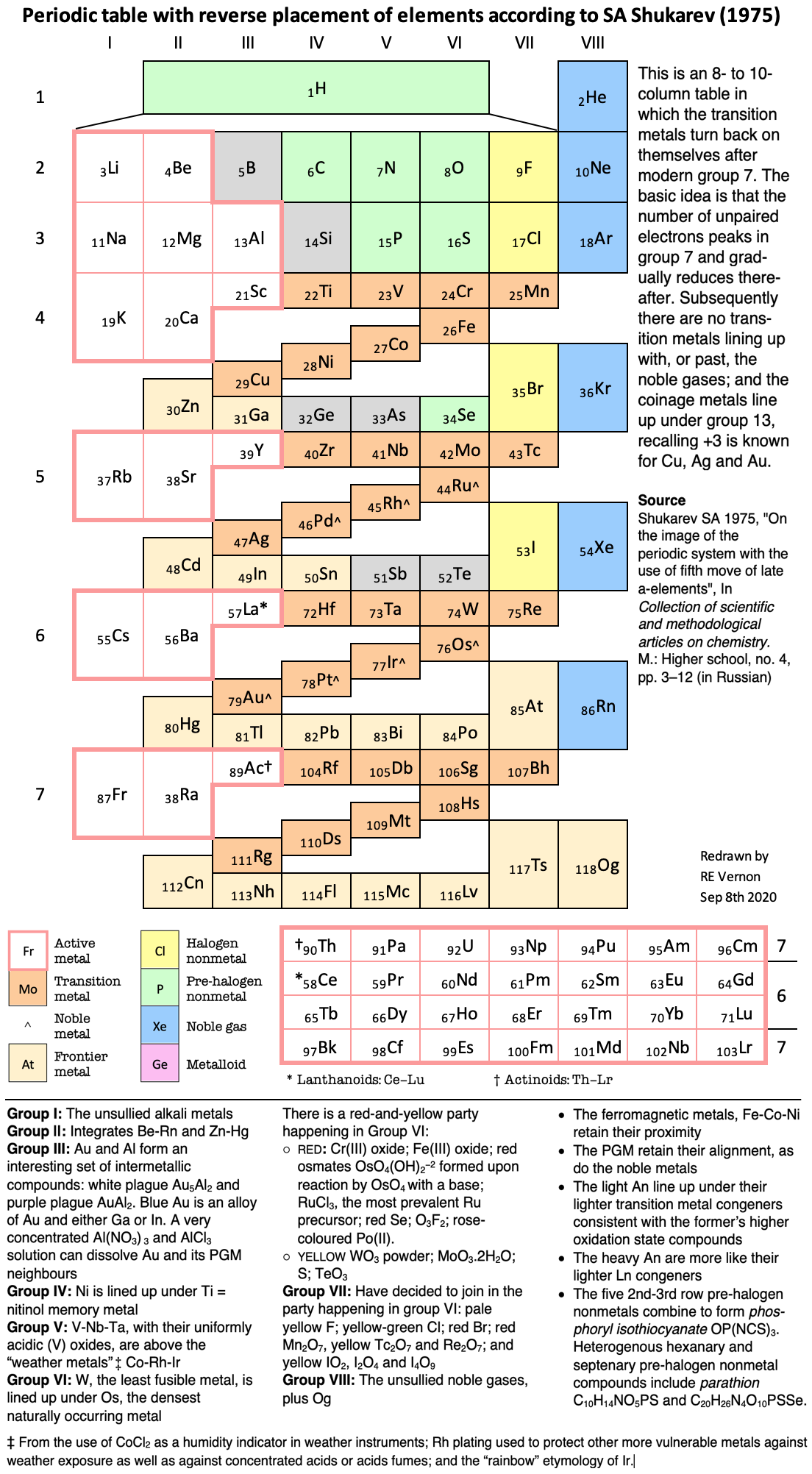
2020
Allahyari & Oganov: Mendeleev Numbers & Organising Chemical Space
This formulation may not look like a periodic table, but look again.
Zahed Allahyari & Artem R. Oganov, Nonempirical Definition of the Mendeleev Numbers: Organizing the Chemical Space, J. Phys. Chem. C 2020, 124, 43, 23867–23878, https://doi.org/10.1021/acs.jpcc.0c07857. A preprint version of the paper is available on the arxiv preprint server.
Abstract:
Organizing a chemical space so that elements with similar properties would take neighboring places in a sequence can help to predict new materials. In this paper, we propose a universal method of generating such a one-dimensional sequence of elements, i.e. at arbitrary pressure, which could be used to create a well-structured chemical space of materials and facilitate the prediction of new materials.
This work clarifies the physical meaning of Mendeleev numbers, which was earlier tabulated by Pettifor. We compare our proposed sequence of elements with alternative sequences formed by different Mendeleev numbers using the data for hardness, magnetization, enthalpy of formation, and atomization energy. For an unbiased evaluation of the MNs, we compare clustering rates obtained with each system of MNs.
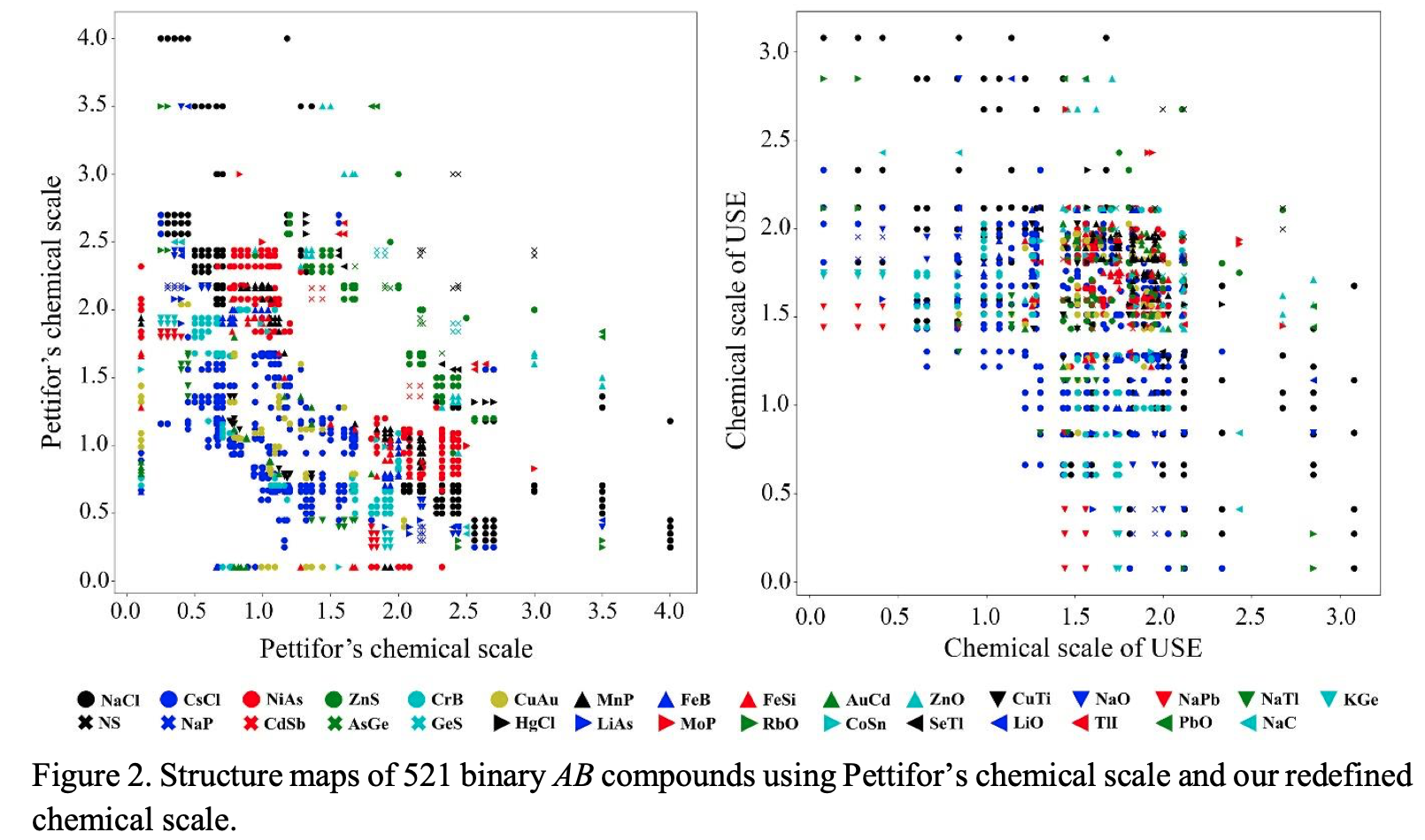
2020
Zig-Zag Line, Periodic Table
Periodic Table showing the (regular) zig-zag line by René Vernon who writes:
"It is curious that the full extent of the line has never been properly mapped (to my knowledge).
"Elements on the downside of the line generally display increasing metallic behaviour; elements on the topside generally display increasing nonmetallic behaviour.
"When you see the line you will usually see only about a quarter of it. The line actually runs all the way across the periodic table, as shown, for a total of 44 element box sides.
"Interpretations vary as to where the line runs. None of these is better than any other of them, provided the interpretation is explained to you. The thick black line (at least in the p-block) is the most common version. The metalloids tend to lie to either side of it.
"Polonium and astatine are shown here as post-transition metals although either or both of them are sometimes shown as metalloids (or, in the case of astatine, as a halogen). Polonium conducts electricity like a metal and forms a cation in aqueous solution. In 2013, astatine was predicted to be a centred cubic-metal Condensed Astatine: Monatomic and Metallic This prediction has been cited 35 times, with no dissenters. Astatine also forms a cation in aqueous solution. Oganesson is shown as having (as yet) unknown properties.
"The dashed lines show some alternative paths for the zigzag line.
"The lower one treats the metalloids as nonmetals since metalloid chemistry is predominately nonmetallic. The lower line and the upper line are sometimes shown together used when the metalloids are treated as neither metals nor nonmetals."
And in Janet Left-Step form:
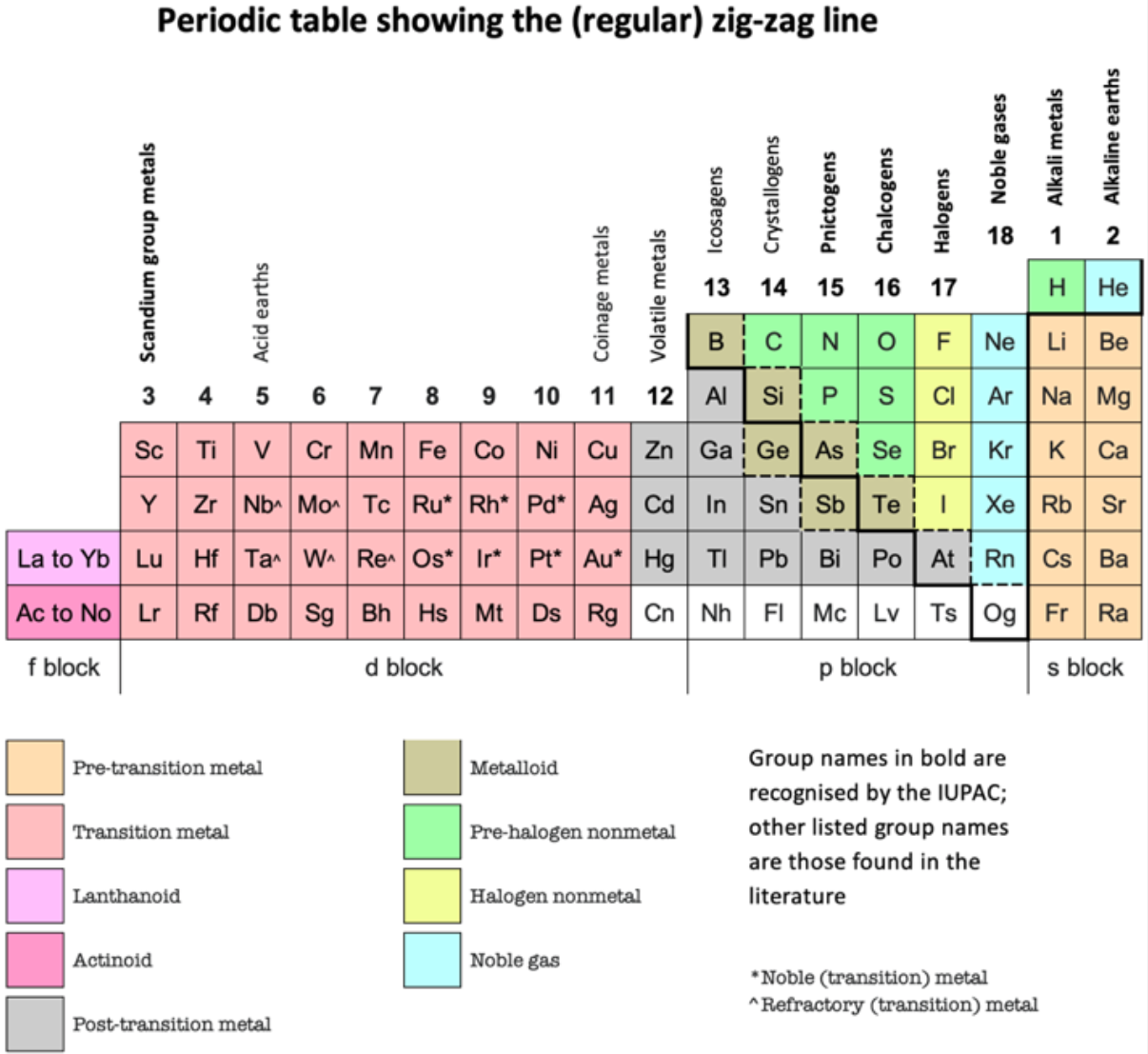
2020
16 Dividing Lines Within The Periodic Table
René Vernon points out that there are 16 dividing lines within the periodic table.
A-Z Dividing Lines:
48-crash line: Named after the dramatic reduction in physical metallic character after group 11, Cd being Z = 48. Group 12 show few transition metal attributes and behave predominantly like post-transition metals.
Big bang line: H makes up about 73% of the visible universe.
Corrosive line: O, F, Cl = most corrosive nonmetals.
d-Block fault line: Group 3 show little d-block behaviour; group 4 is the first in which characteristic d-block behaviour occurs.
Deming line: Demarcates the metalloids from the pre-halogen nonmetals. The "reactive" nonmetals to the right of the metalloids each have a sub-metallic appearance (C, O, Se, I).
Edge of the world line: No guesses for this one.
Klemm line: Klemm, in 1929, was the first to note the double periodicity of the lanthanides (Ce to Lu). Lockyer line: After the discoverer of He, the first element not found on Earth.
Ørsted line: After the magnetic effects believed to be responsible for Mn having a crystalline structure analogous to white P; Tc: First radioactive metal; Re: Last of the refractory metals; "most radioactive" of the naturally occurring elements with stable isotopes. Fe: First of the ferromagnetic metals; Ru: First noble metal; Os: Densest of naturally occurring metals. The number of unpaired d electrons peaks in group 7 and reduces thereafter.
Platypus line: Tl shows similarities to Rb, Ag, Hg, Pb.
Poor metal line: Most metals (80%) have a packing factor (PF)3 68%. Ga: Has a crystalline structure analogous to that of iodine. BCN 1+6.* PF 39.1%. Melts in your hand. In: Partly distorted structure due to incompletely ionised atoms. BCN 4+8. PE 68.6%. Oxides in preferred +3 state are weakly amphoteric; forms anionic indates in strongly basic solutions. Tendency to form covalent compounds is one of the more important properties influencing its electro-chemical behaviour. Sn: Irregularly coordinated structure associated with incompletely ionised atoms. BCN 4+2. PF 53.5%. Oxides in preferred +2 state are amphoteric; forms stannites in strongly basic solutions. Grey Sn is electronically a zero band gap semimetal, although it behaves like a semiconductor. Diamond structure. BCN 4. PF 34.0%. Pb: Close-packed, but abnormally large inter-atomic distance due to partial ionisation of Pb atoms. BCN 12. PF 74%. Oxide in preferred +2 state is amphoteric; forms anionic plumbates in strongly basic solutions. Bi: Electronic structure of a semimetal. Open-packed structure (3+3) with bonding intermediate between metallic and covalent. PF 44.6%. Trioxide is predominantly basic but will act as a weak acid in warm, very concentrated KOH. Can be fused with KOH in air, resulting in a brown mass of potassium bismuthate.
Seaborg line: No f electrons in gas phase La, Ac and Th atoms.
Triple line: N = gas; S = solid; Br = liquid.
Zigzag lobby: H needs no intro. Li: Many salts have a high degree of covalency. Small size frequently confers special properties on its compounds and for this reason is sometimes termed 'anomalous'. E.g. miscible with Na only above 380° immiscible with molten K, Rb, Cs, whereas all other pairs of AM are miscible with each other in all proportions. Be: Has a covalent component to its otherwise predominately metallic structure = low ductility. Lowest known Poisson's ratio of elemental metals. Amphoteric; predominately covalent chemistry atypical of group 2. Some aspects of its chemical properties are more like those of a metalloid.
Zigzag line: Eponymous metal-nonmetal dividing line.
Zintl line: Hypothetical boundary highlighting tendency for group 13 metals to form phases with a various stoichiometries, in contrast to group 14+ that tend to form salts with polymeric anions.
* BCN = bulk coordination number
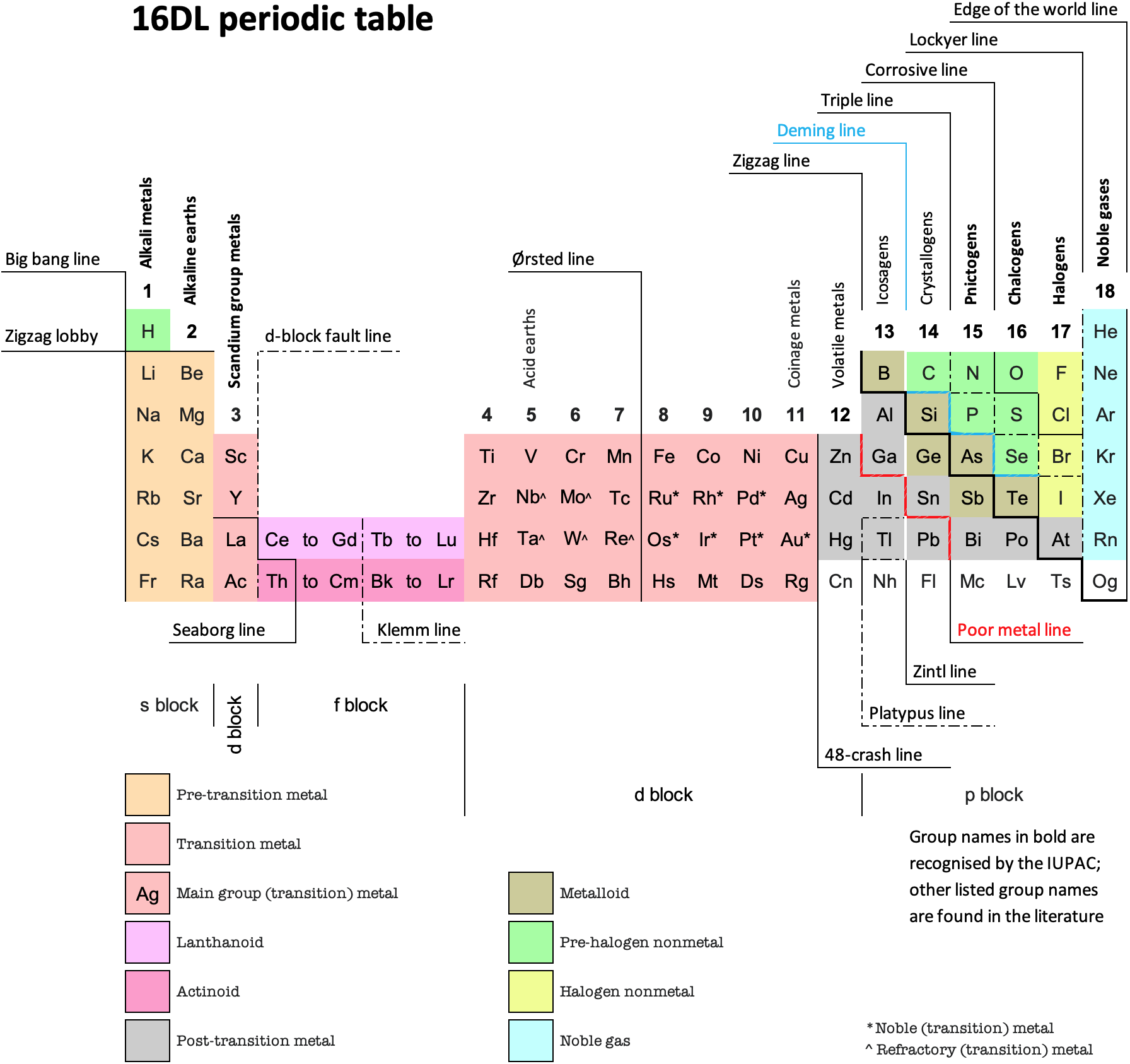
2020
Split s-, p- & d-Block Periodic Table
René Vernon presents a periodic table formulation with split s-, p- & d-blocks.
- The traditional form of periodic table is a hybrid of an electronic and a chemistry based table.
- An electronic or physics-based table would show (a) He over Be; and (b) group 3 as Sc-Y-Lu-Lr; and (c) group 13 as B-Al-Ga-In-Tl
- A chemistry-based table would show (d) He over Ne; and (e) B-Al over Sc-Y-La-Ac.
- What we have instead is a hybrid table with 1(c) and 2(d). It is not as symmetric or tidy as the pure Lu form; neither is it as irregular as the form with three split blocks.
The details: Group 3 as B-Al-Ga-In-Tl
Al over Sc has some history, which seems to have been forgotten.
Here are some other tables with B-Al-Sc-Y-La:
- Rang (1893)
- Gooch & Walker (1905)
- Cuthbertson & Metcalfe (1907)
- Baur (1911)
- Rydberg (1913)
- Black & Conant (1920)
- Lewis (1923)
- Hubbard (1924)
- Deming's table (1925), which popularised the medium-long form
- Antropoff (1926)
- LeRoy's table (1927)
- Irwin (1939)
- Seaborg (1945), with B left in group 13
- Yost & Russell (1946)
- Coryell (1952)
- Pauling's table (1960)
- Habishi's Metallurgist's Periodic Table (1992), Habishi leaves B in group 13
What was it that these luminaries knew about B-Al-Sc-Y-La-Ac that is deemed to be no longer relevant, and why is that the case?
Deming (1947, Fundamental Chemistry, 2nd ed. p. 617) located Al with the pre-transition metals in groups 1?2. Cox (2004, Inorganic Chemistry, 2nd ed. p. 185) refers to the pre-transition metals as those in groups 1 and 2, and Al. Here's that 2019 periodic table (by me), recording oxidation number trends, further suggesting B and Al are better placed over Sc.
In this vein, Rayner-Canham (2020, The periodic table: Past, present, and future, pp. 178–181) writes:
"It was Rang in 1893 who seems to have been the first, on the basis of chemical similarity, to place boron and aluminum in Group 3.
"Such an assignment seems to have been forgotten until more recent times. Greenwood and Earnshaw have discussed the way in which aluminum can be considered as belonging to Group 3 as much as to Group 13 particularly in its physical properties. Habashi has suggested that there are so many similarities between aluminum and scandium that aluminum's place in the Periodic Table should actually be shifted to Group 3.
"In terms of the electron configuration of the tripositive ions, one would indeed expect that Al3+ (electron configuration, [Ne]) would resemble Sc3+ (electron configuration, [Ar]) more than Ga3+ (electron configuration, [Ar]3d10). Also of note, the standard reduction potential for aluminum fits better with those of the Group 3 elements than the Group 13 elements (Table 9.2) – as does its melting point.
"In terms of their comparative solution behavior, aluminum resembles both scandium(III) and gallium(III). For each ion, the free hydrated cation exists only in acidic solution. On addition of hydroxide ion to the respective cation, the hydroxides are produced as gelatinous precipitates. Each of the hydroxides redissolve in excess base to give an anionic hydroxo-complex, [M(OH)4]–... There does seem to be a triangular relationship between these three elements. However, aluminum does more closely resemble scandium rather than gallium in its chemistry. If hydrogen sulfide is bubbled through a solution of the respective cation, scandium ion gives a precipitate of scandium hydroxide, and aluminum ion gives a corresponding precipitate of aluminum hydroxide. By contrast, gallium ion gives a precipitate of gallium(III) sulfide. Also, scandium and aluminum both form carbides, while gallium does not."
To answer my own question as to why group 3 as B-Al-Sc-Y-La-Ac has been forgotten.
I suspect what happened is that it was historically known that group 3 was better represented as B-Al-Sc-Y-La-[Ac]. Then, with the advent and rise of modern electronic structure theory, B-Al- got moved to the p-block because, after all, they were p-block elements, never mind the damned chemistry. And La stayed in the d-block since it was the first element to show 5d electron, and 4f did not show until Ce. And Lu stayed where it was since even thought it was learnt that the f shell become full at Yb, rather than Lu, nothing changed about the chemistry of Lu. Nowadays, this has all been forgotten.
The modern periodic table is a chemistry-physics hybrid.
Lu in group 3 demands He over Be. La in group 3 demands B-Al over Sc. Neither option gets up. The more important consideration is to teach the history and have students and chemists appreciate both perspectives.
2020
Rainbow Periodic Table in ADOMAH Cube
From the prolific Nagayasu Nawa, a version of his Rainbow Periodic Table inside Valery Tsimmerman's glass cube:
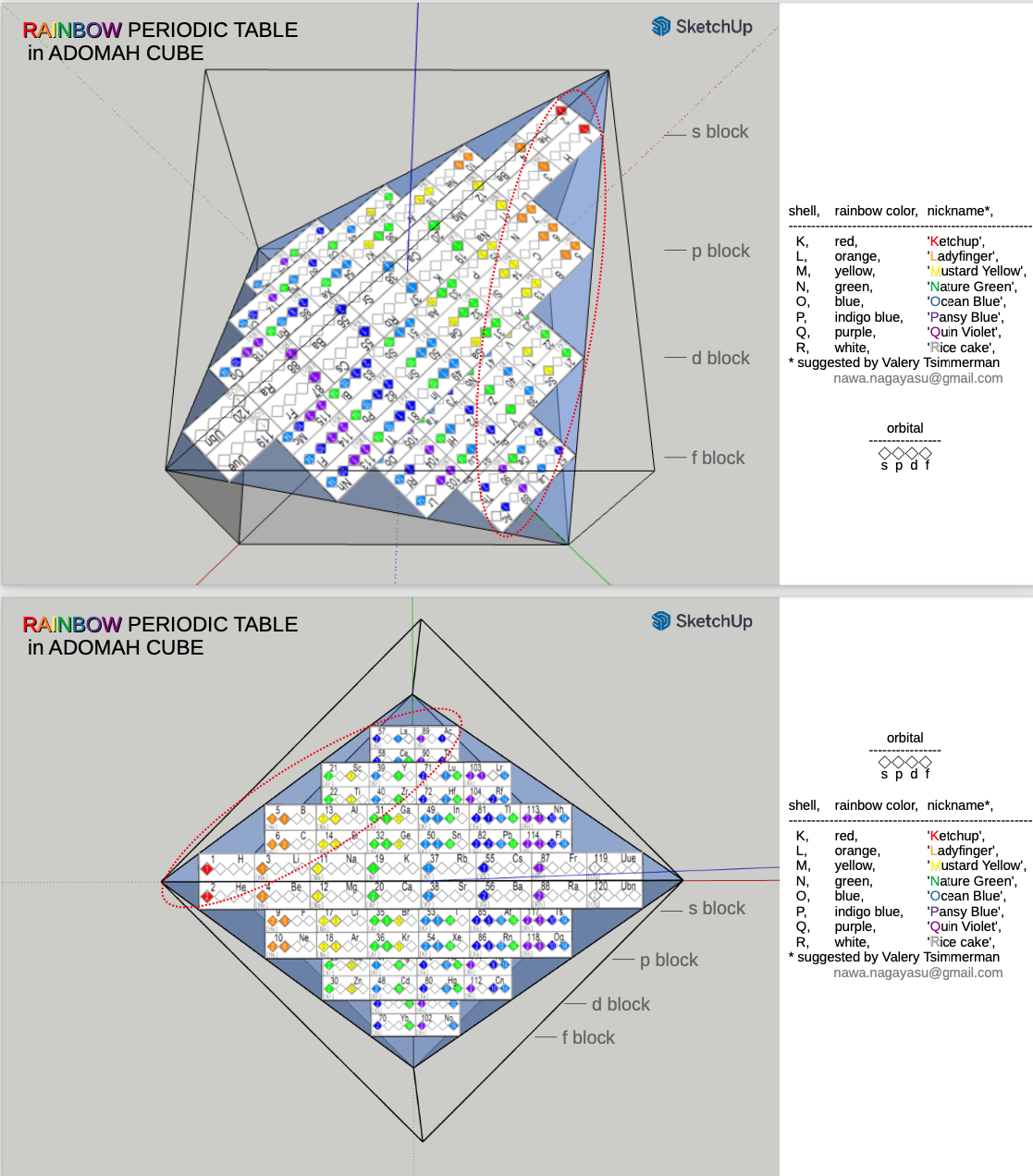
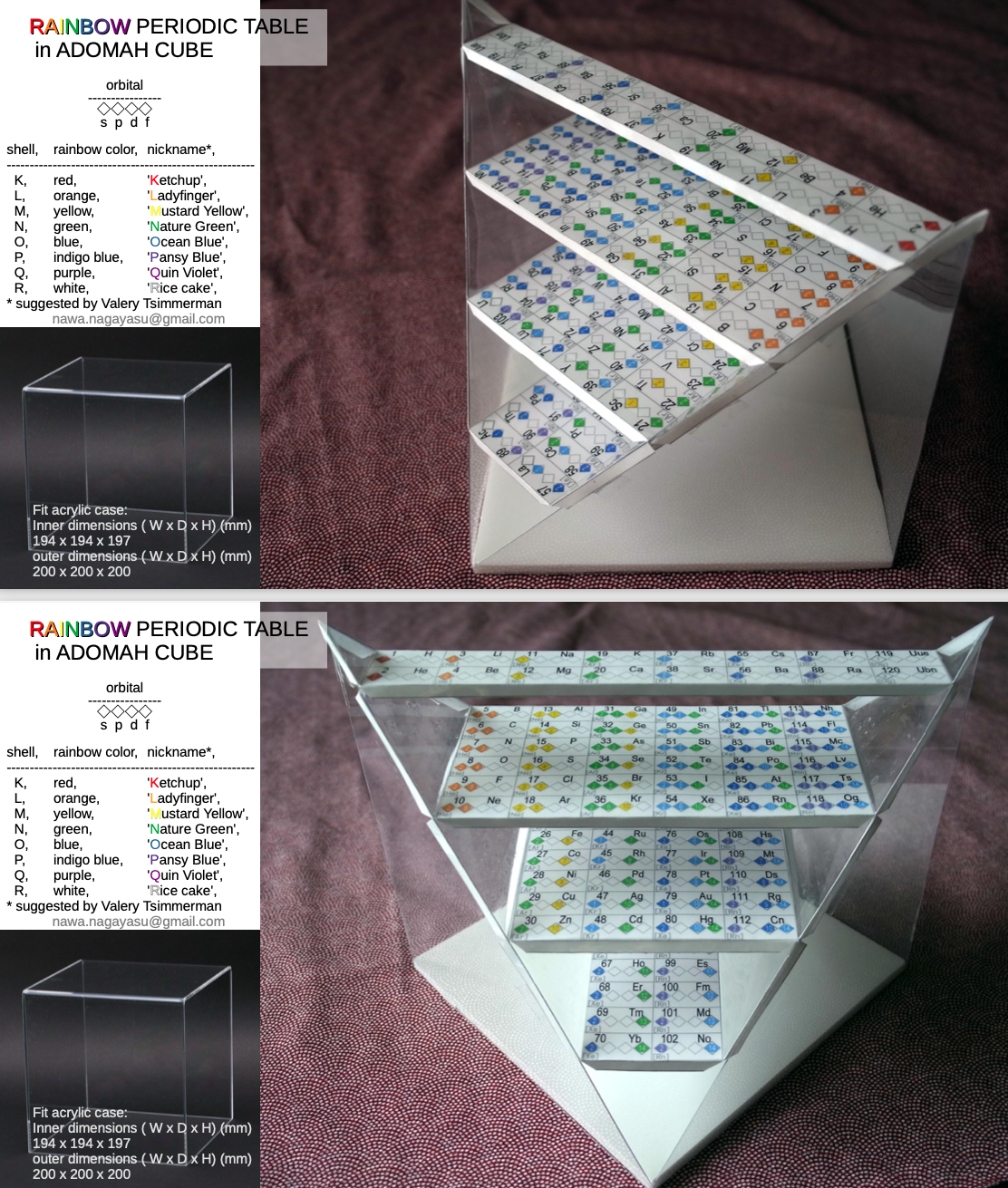
2021
Understanding Periodic and Non-periodic Chemistry in Periodic Tables
Cao C, Vernon RE, Schwarz WHE and Li J (2021). Front. Chem. 8:813. https://doi.org/10.3389/fchem.2020.00813
Abstract:
The chemical elements are the "conserved principles" or "kernels" of chemistry that are retained when substances are altered. Comprehensive overviews of the chemistry of the elements and their compounds are needed in chemical science. To this end, a graphical display of the chemical properties of the elements, in the form of a Periodic Table, is the helpful tool. Such tables have been designed with the aim of either classifying real chemical substances or emphasizing formal and aesthetic concepts. Simplified, artistic, or economic tables are relevant to educational and cultural fields, while practicing chemists profit more from "chemical tables of chemical elements."
Such tables should incorporate four aspects:
(i) typical valence electron configurations of bonded atoms in chemical compounds (instead of the common but chemically atypical ground states of free atoms in physical vacuum);
(ii) at least three basic chemical properties (valence number, size, and energy of the valence shells), their joint variation across the elements showing principal and secondary periodicity;
(iii) elements in which the (sp)8, (d)10, and (f)14 valence shells become closed and inert under ambient chemical conditions, thereby determining the "fix-points" of chemical periodicity;
(iv) peculiar elements at the top and at the bottom of the Periodic Table.
While it is essential that Periodic Tables display important trends in element chemistry we need to keep our eyes open for unexpected chemical behavior in ambient, near ambient, or unusual conditions. The combination of experimental data and theoretical insight supports a more nuanced understanding of complex periodic trends and non-periodic phenomena.
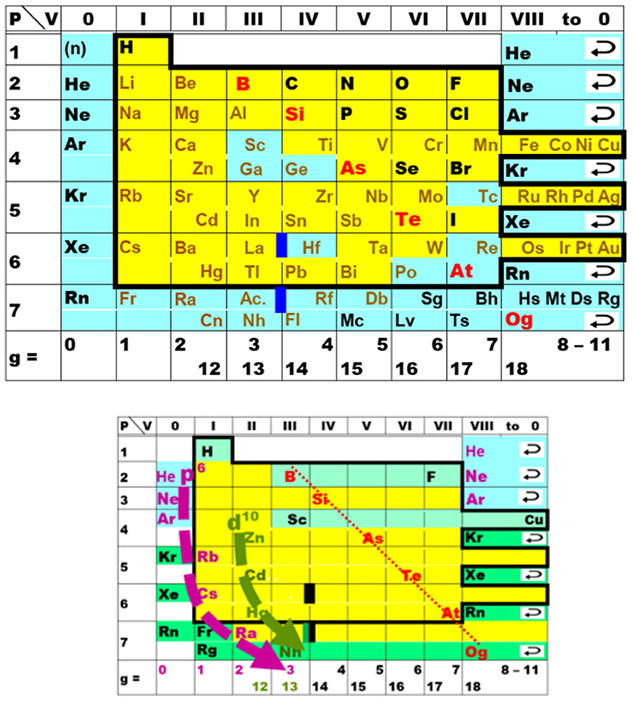
Thanks to René Vernon for the tip.
2021
Crustal Abundance vs. Electronegativity
A chart by René Vernon of Elemental Abundance (g/kg log10) vs. Electronegativity, H to Bi.
René writes:
Below is a remarkable XY chart where x = electronegativity and y = crustal abundance (log10). It stops at the end of the s-process, at Bi. The abundance figures are from the CRC Hanbook of Physics and Chemistry (2016-2017).
I say remarkable as I had little idea what the chart would end up looking like when I started plotting the values.
As well as its coloured regions, I've marked out track lines for six of the main groups and one for group 3.
Observations
The rose-coloured arc on the left encompasses the pre-transition metals i.e. the alkali and alkaline earth metals and aluminium, followed by, in the orange rectangle, the rare earth metals. Opposite these regions, along the southern boundary of the green paddock, are the halogens.
In the pale yellow field sheltered by the pre-transition metals and the REM, are the 3d transition metals and, in the white corral, are 4d and 5d base transition metals. Opposite these regions, in the green paddock, are the core nonmetals H, C, N, O, P and S, with Se as an outlier.
Following in the grey blob are the post-transtion or poor metals, immediately adjacent to the bulk of the metalloids or poor nonmetals.
Finally, in the light blue patch, the noble metals are complemented by the noble gases frolicking in the open.
Abundance tends to decrease with increasing Z. Notable exceptions are Li, B, N and Si.
Curiosities
- H and P are almost on top of one another
- The proximity of Be to the post-transition metals, and its relative scarcity in the crust
- The metalloids, with their intermediate values of electronegativity, go down the middle. At the same time they span nearly the full range of abundance.
- B-Ga-Sc-Y-La are in a row
- N falls along the halogen line
- The abundance of O and Si, which we see in the form of silica
- F is more abundant in the crust than 85 percent of metals
- Al is the most abundant metal. Al and Fe are in the same vicinity: "Curiously, the chemistry of aluminium also resembles that of the iron(III) ion... These similarities may be ascribed to the same 3+ charge and near-identical ion radii (and hence charge density)." (Rayner-Canham 2020, p. 191)
- The abundance of Ar compared to the rest of the noble gases. Apparently this is influenced by the radioactive decay of potassium-40 in Earth's core, which is considered one of the main sources of heat powering the geodynamo that generates Earth's magnetic field. It has been suggested that a large amount of Ar may be present in the core, as the compound ArNi with an L11 Laves structure (similar to an intermetallic phase, and related to a cubic close packed lattice). ArNi is stabilised by notable electron transfer from Ni to Ar, changing their electron configurations toward 3d7 and 4s1. (Adeleke et al. 2019)
- Ti, a light yet strong metal, is about 2,500 times as abundant as Sn, a weak heavy metal
- Zn is an outlaw post-transition metal
- The most active 4d-5d transition metals (Zr, Hf) occupy a boundary overlap with the rare earth metals
- Ag, which has a largely main-group chemistry, is located in the PTM region. It is about 20 times as abundant as the noble meals
- Re is an outlaw noble metal
Comment
I was intrigued by the article referring to Ni and Ar, and the suggestion of Ar becoming somewhat anionic, albeit in extreme conditions (140 GPa, 1500 K)
References
- Adeleke AA, Kunz M, Greenberg E, Prakapenka VB, Yao Y, Stavrou R 2019, A high-pressure compound of argon and nickel: Noble gas in the Earth's core?, ACS Earth and Space Chemistry, vol. 3 no. 11, pp. 2517-2544, https://pubs.acs.org/doi/10.1021/acsearthspacechem.9b00212
- Rayner-Canham G, 2020, The periodic table: Past, present, future, World Scientific, Singapore
Correlations
I wasn't looking for these but they at least exist as follows:
- Metals with lower EN, i.e. < 1.7, or active nonmetals with higher EN, tend to be concentrated in silicate or oxide phases that are more easily found in the crust due to their lower density, and hence have higher abundances.
- Metals with moderate EN 1.7 to 2.1, say the later transition metals and post-transition metals, tend to form sulfide liquid phases; are less easily found in the crust due to their relatively higher densities; and are less abundant by about two orders of magnitude compared to the metals found in silicate or oxide phases.
- Metals with EN > 2.2, i.e. the noble metals, have an affinity for a metallic liquid phase, and are depleted in the crust since they generally sank to the core and hence have very low abundances. They are about two orders of magnitude less abundant than the sulfide metals.
My references are:
- Cox PA 1997, The elements: Their origin, abundance and distribution;
- Gill R 2014, Chemical fundamentals of geology and environmental geoscience;
- White WA 2020, Geochemistry
Thus the abundance of the metals in the crust tends to fall with increasing EN.
- For the nonmetals, the relative average abundance proportions are about 5: 700: 250: 1 for, respectively, the metalloids; the core nonmetals H, C, N, P, S, and Se; the halogen nonmetals; and the noble gases. Si and O were left out as outliers, in terms of their massive abundances.
- Thus, metalloids aside, the abundance of the nonmetals tends to fall with increasing EN. I don't know what's going on with the metalloids.
- The chart may prompt some further appreciative enquiry:
- In the case of exceptions to the initial three generalisations why do these occur?
- Why is Li so rare, compared to the other alkali metals?
- Why is Si good at forming a planetary crust?
An answer from L. Bruce Railsback, creator of the Earth Scientist's Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=142:
"I think I can answer one of the questions. 'Why is Si good at forming a planetary crust?' – because it's so bad at staying in the core. Silicon isn't sufficiently metallic to stay in the core. Even in the mantle and crust, it doesn't go into non-metal solids well: in cooling magmas, it's only a lesser member of the early-forming minerals (e.g., Mg2SiO4, forsterite, where it's outnumbered two to one). The mineral only of Si as a cation, SiO2 (quartz), is the LAST mineral to form as a magma cools, in essence the residuum of mineral-forming processes. At least some this thinking is at Bowen's Reaction Series and Igneous Rocks at http://railsback.org/FundamentalsIndex.html#Bowen"
- Why do the metalloids span such a wide range of abundances?
- If H is supposed to make up ca. 74% of the universe why does it have the same abundance in the Earth's crust as P?
- In what form is H found in Earth's crust—water, hydroxides?
- If H is supposed to make up ~ 74% of the universe why does it have the same abundance in the Earth's crust as P?
- Are there any chemical similarities between H and P, given both have some metalloidal character? The have virtually identically electron affinities. H is sometimes positioned above B due to chemical similarities. It then forms a diagonal relationship with C, which in turn has a diagonal relationship with P, which has a diagonal relationship with Se e.g. P reacts with Se to form a large number of compounds characterised by structural analogies derived from the white phosphorus P4 tetrahedron.
- The rare earth metals are relatively rare, having an average abundance of 1% that of the 3d metals. That being so, why is their rareness sometimes questioned? Why does the crustal abundance of the REM plummet by two orders of magnitude towards the end of the lanthanides?
Which Electronegativity Scale?
The wide variety of methods for deriving electronegativities tend to give results similar to one another.
2021
van Spronsen's Periodic Table: Update
René Vernon writes:
I'd never before realised how clever van Spronsen's 1969 Periodic Table is. It seems to be the ultimate logical electronic version, informed by the actual filling sequence in the gas phase atoms, rather than the idealised sequence.
So, H-He are over Li-Be.
Group 3 is Sc-Y-La-Ac since that is where the d-shell starts filling. In the rest of the d-block, there are (4+1) x d5 and (4+2) x d10.
The f-block starts with Ce, as that is where the f-shell starts filling. Notice the high degree of regularity with the 4 x f7 and the 4 x f14, and how Th is treated i.e. as 5f0.
After DIM's 8-column form, I believe the periodic family tree now looks like this:
Three split-blocks
1a. He over Ne; B-Al over Sc-Y-La-Ac = old school form
1b. H-He over F-Ne; ditto = e.g. Soddy 1914?, Kipp 1942?Two split blocks
2a. He over Ne; group 3 as Sc-Y-La-Ac = popular formOne split-block
3a. He over Ne; group 3 as Sc-Y-Lu-Lr = Lu form 3b. He over Be; group 3 as Sc-Y-La-La = forgotten van Spronsen formNo split blocks
4. He over Be = Janet equivalent
2021
Helix vs. Screw
Julio Antonio Gutierrez Samanez writes:
Until today, when they write about the work of Chancourtois and his telluric helix wound in a cylinder, still no one alludes to this other telluric helix wound in a cone or screw, the idea is the same: a rope that winds a geometric solid.
The first was devised in 1862, the other in 2008 (146 years later). But, there is a big epistemological difference. In the first, the elementary series presented was: 8, 8, 8, 8, 8 ..., etc., in the second it is: 2, 2, 8, 8, 18, 18, 32, 32. Furthermore, the division of conical radii is regulated by the function 2 (n ^ 2) = 2, 8, 18, 32...
Each binode has two spirals or two periods with the same number of elements, which correspond to the function 4 (n ^ 2). I don't think it is a discovery, it is just the conclusion of the contributions of Rydberg, Janet, and, of course, Chancourtois.
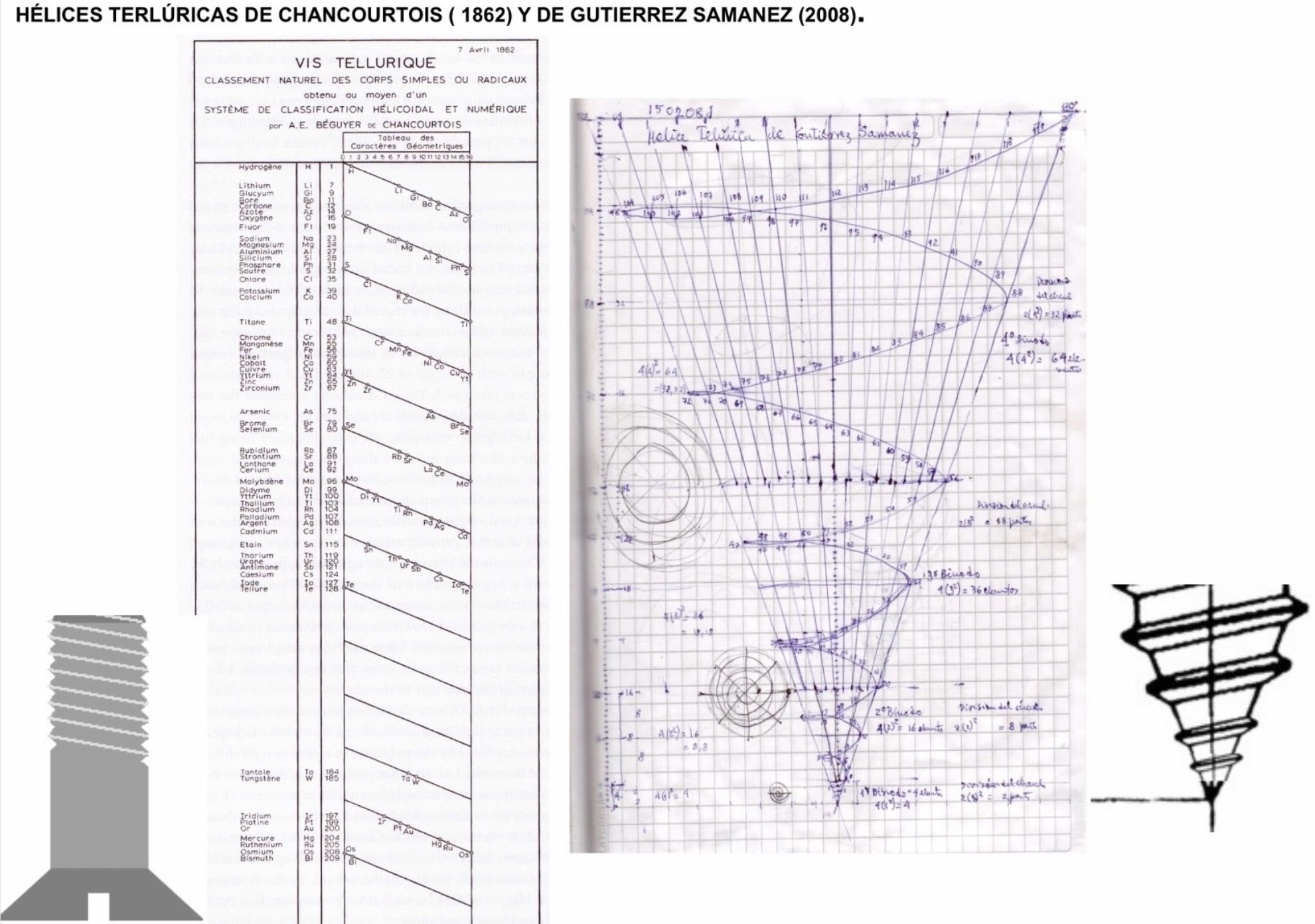
2021
Aufbau Periodic Table
An Aufbau Periodic table designed by Steven Muov at http://murov.info/aufbaupt.htm
Steven writes:
"Science has aptly been described as a search for order in the Universe. It follows that chemistry is a search for order in matter. While the search will always be a work in progress, great strides towards the finding of order in matter resulted in 1869 when Dimitri Mendeleev stood on the shoulders of many others and published his periodic table. The table has since been modified and improved but still has a remarkable resemblance to the original Mendeleev table. Excellent compilations of many alternate periodic tables have been published that use novel and intriguing approaches (e.g., circles, spirals and 3d, but the contemporary versions of the Mendeleev table are the charts found on the walls of thousands of lecture rooms around the world. The periodic table deserves recognition as one of the milestones of science along with contributions from other sciences including but not limited to: physics by Newton and Einstein, biology by Darwin, Rosalind Franklin, Watson and Crick, astronomy by Copernicus and Galileo and geology by Wegener..."
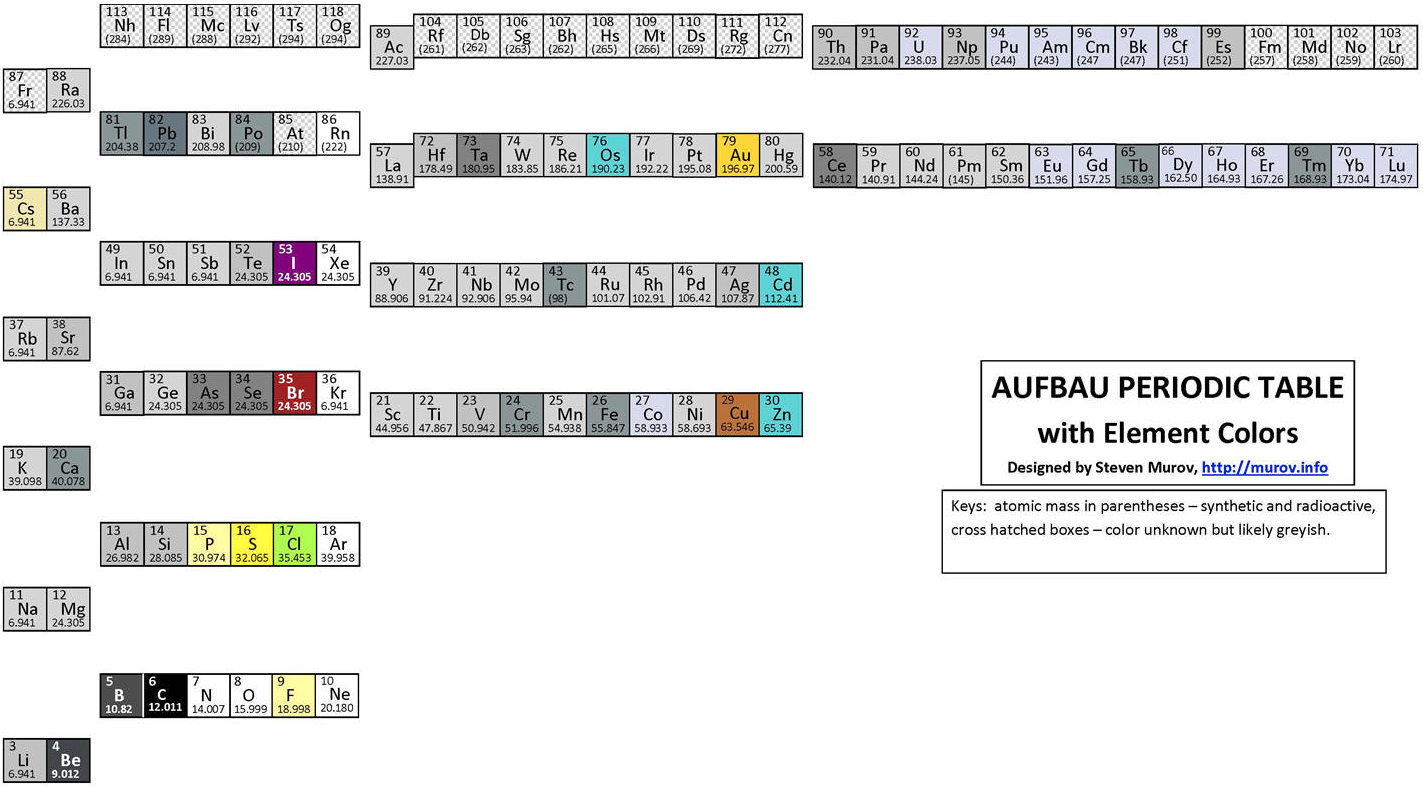
2021
Provisional Report on Discussions on Group 3 of The Periodic Table
Provisional Report on Discussions on Group 3 of The Periodic Table by Eric Scerri, De Gruyter | 2021
DOI: https://doi.org/10.1515/ci-2021-0115
The following article is intended as a brief progress report from the group that has been tasked with mak-ing recommendations to IUPAC about the constitu-tion of group 3 of the periodic table (https://iupac.org/project/2015-039-2-200). It is also intended as a call for feedback or suggestions from members of IUPAC and other readers.



2021
Nawa's Multi Periodic Table
Nagayasu Nawa - "A Japanese school teacher and periodic table designer" - has developed a "Multi" Periodic Table with three formulations: long-form, upsidedown long-form & circular with era of discovery, electronic structure and abundance data.
Click here to download the .pdf file.
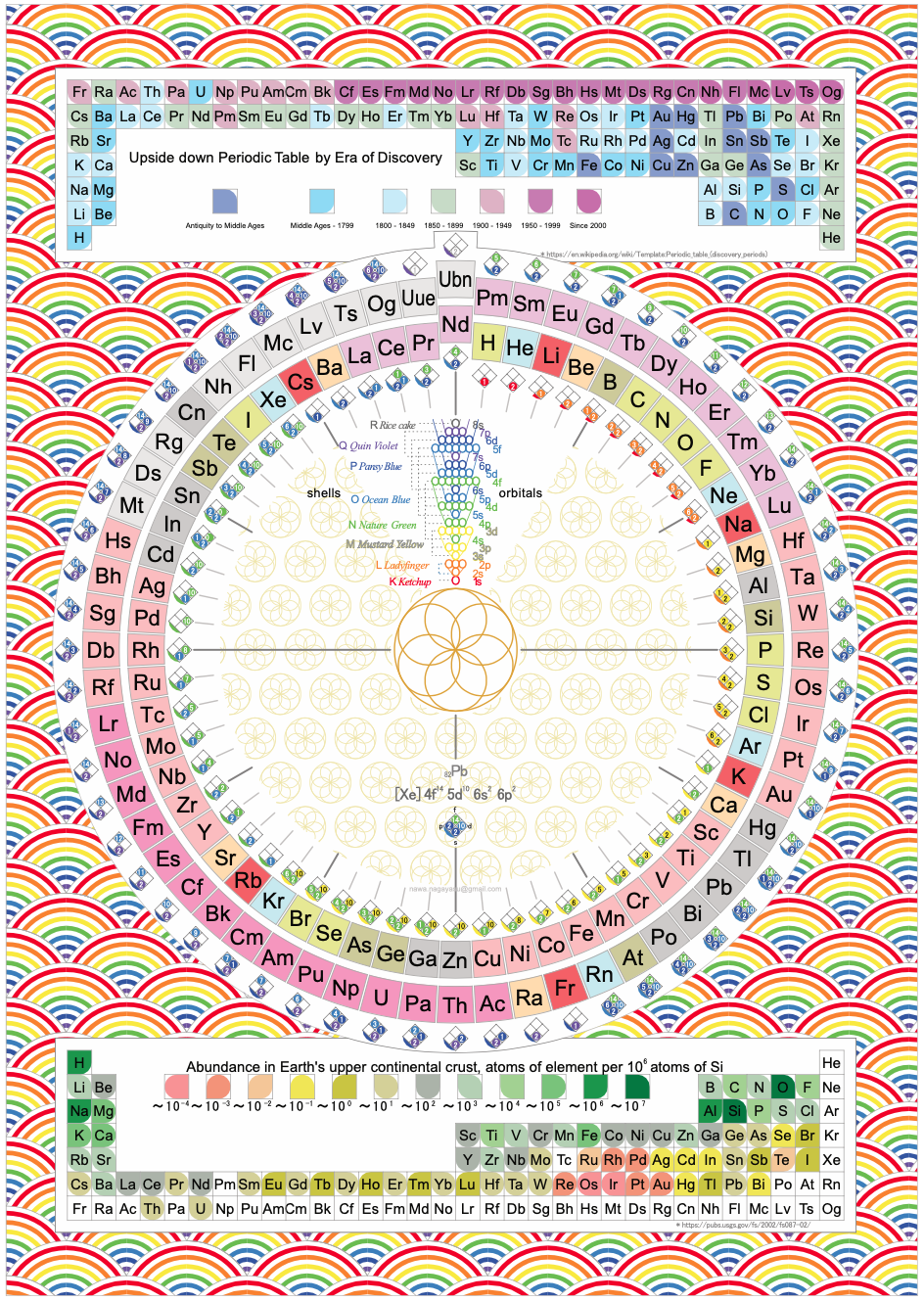
2021
Vernon's CSF Left-Step Periodic Table.
René Vernon's CSF Left-Step Periodic Table.
"I was prompted to switch to He-Be and [to develop a Janet type] left-step periodic table. I suggest it remediates concerns about H and He, and Lu in group 3.
Pros
- There is symmetry in this version.
- The physiochemical relationship of He to Ne is retained.
Cons
- There is a loss of physiochemical regularity in placing He over Be. Even if helium can be enticed to become chemically active, it will still be very much better located in group 18.
- While the d, p, and s blocks start with the appearance of the relevant electron, there is a loss of consistency with La at the start of the f-block. This is confusing to students since there is no such inconsistency in the La form.
- In terms of predominant differentiating electrons in each block, this form is less consistent than an La table.
- There is one less form of "element block-type" symmetry, than in the La form.
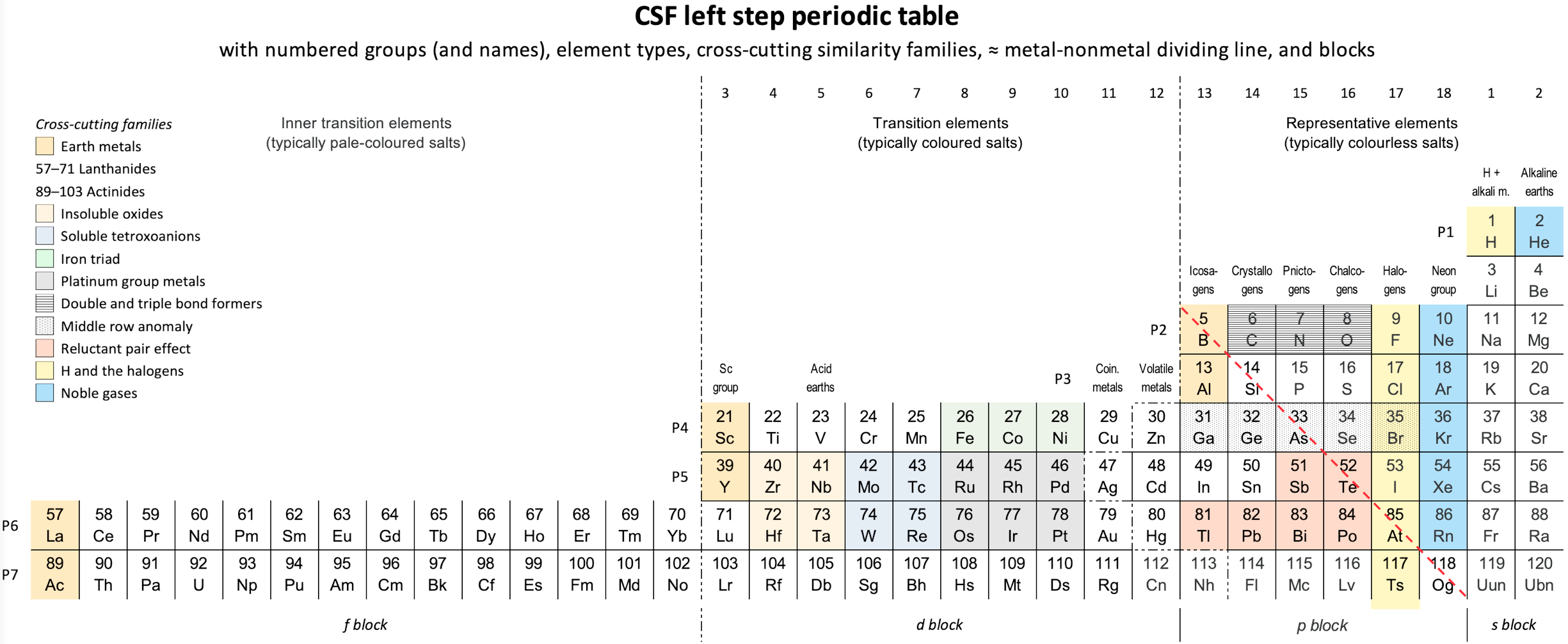
2021
Cubical-Stair Periodic Table
Sarthak Gupta's Cubical-Stair Periodic Table (Into a Whole New Dimension):
"Looking at the Modern periodic Table, somethings always bug you. The huge gap between the s and p-block when they should be side by side. The whole f-block floating around in air when it should be there in period 6 and 7. So why not experiment with shapes and structures and come up with something more space efficient?
"The cubical Periodic table paves the way taking the periodic table into a whole new dimension. Yes! from the 118 squares, we are going to transition into 67 cubes stacked onto each other like stairs."
The Cubical-Stair Periodic Table Explained:
- The table is made up of 67 cubes stacked onto each other, having three sides exposed(top, left and right)
- The top faces contain s and p-block elements
- The left side faces contain d block and right side faces contain f block
- There are two different Major Groups: A and B
- Major Group A is divided into 14 minor groups (from -1 to 12) and Major group B is divided into 8 minor groups (from 3 to 12)
- Major Group A applies to s, p & f-block. Major Group B is exclusively for d-block.
- To go down a group we follow the arrow and descend the stair in the given direction
Advantage over the Modern PT
- Although being 3 dimensional, it can be easily represented in 2 dimensions in the form of trisected hexagons
- All the elements of the same period lie in the same line (unlike MPT where f-block elements had to be depicted separately due to lack of space).
- Viewing the table from 3 different directions makes only one or two blocks visible:
1. From top: s & p-block
2. From left: d-block
3. From right: f-block - This helps in diffrentiating between the blocks easily
- The disturbing gap between s and p-block of traditional periodic table is not simply there. All advantages of Modern Periodic Table remain conserved.

2021
Vernon's Eight-Fold Way Periodic Table
René Vernon suggests that the chemical elements can be grouped into eight classes: four metallic (Active, Transition, Post-Transition and Noble) and four non-metallic (Halogen, Biogen, Metalloid and Noble gas):
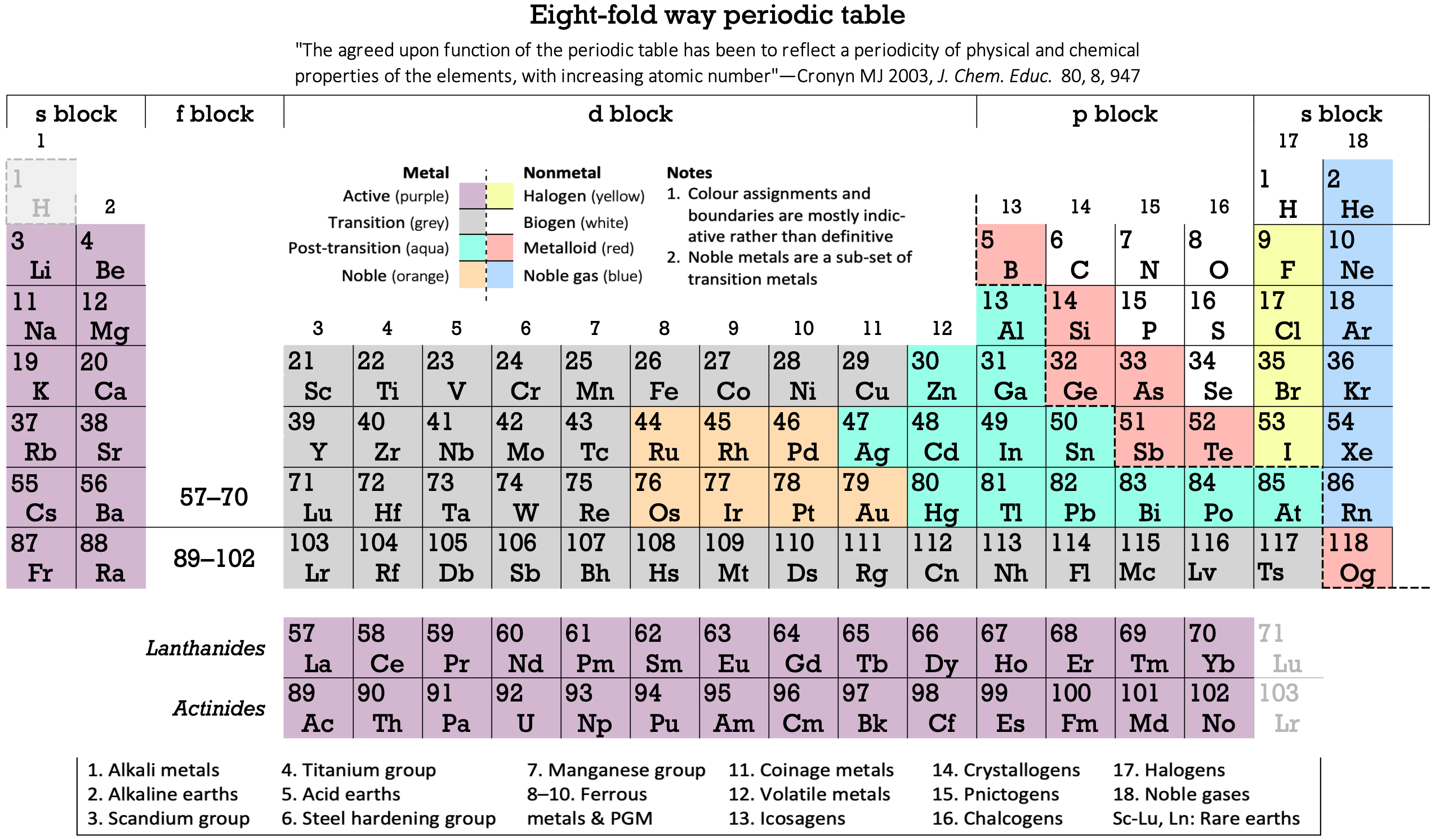
2021
Term & Spin State Periodic Table
A Tern & Spin State periodic table by Gnanamani Simiyon who writes:
"We tried to arrange the elements based on the ground state term and spin state. I attached picture of the periodic table drawn. For example, I notice that alkali metals and coinage metals grouped up indicating some relationship between the groups. Similarly with respect to alkaline Earth metals and Zinc group. We are unable to further understand other groupings based on the ground state term and spin state."
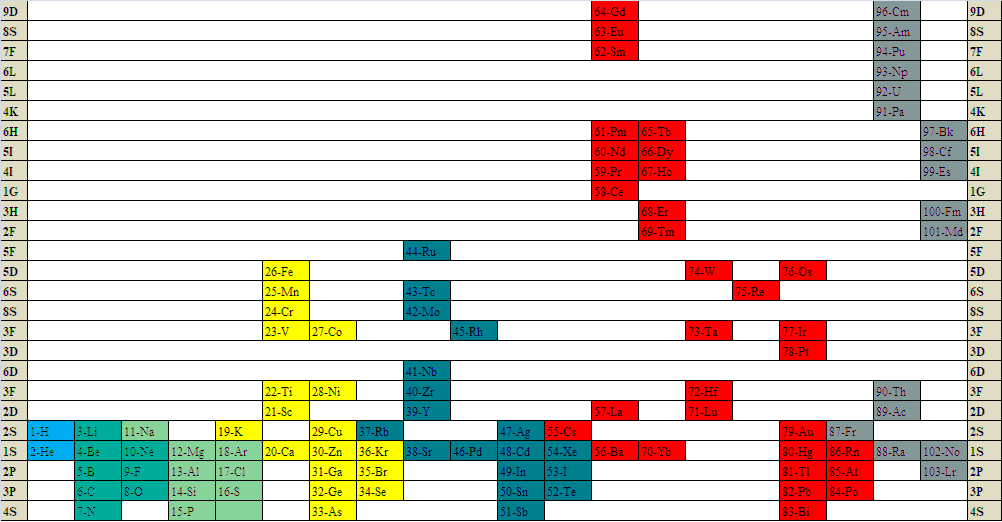
2021
Mendeleyev Revisited
An Open Access paper: Marks, E.G., Marks, J.A. Mendeleyev revisited. Found Chem 23, 215-223 (2021).
https://doi.org/10.1007/s10698-021-09398-4
"Despite the periodic table having been discovered by chemists half a century before the discovery of electronic structure, modern designs are invariably based on physicists' definition of periods. This table is a chemists' table, reverting to the phenomenal periods that led to the table's discovery. In doing so, the position of hydrogen is clarified."
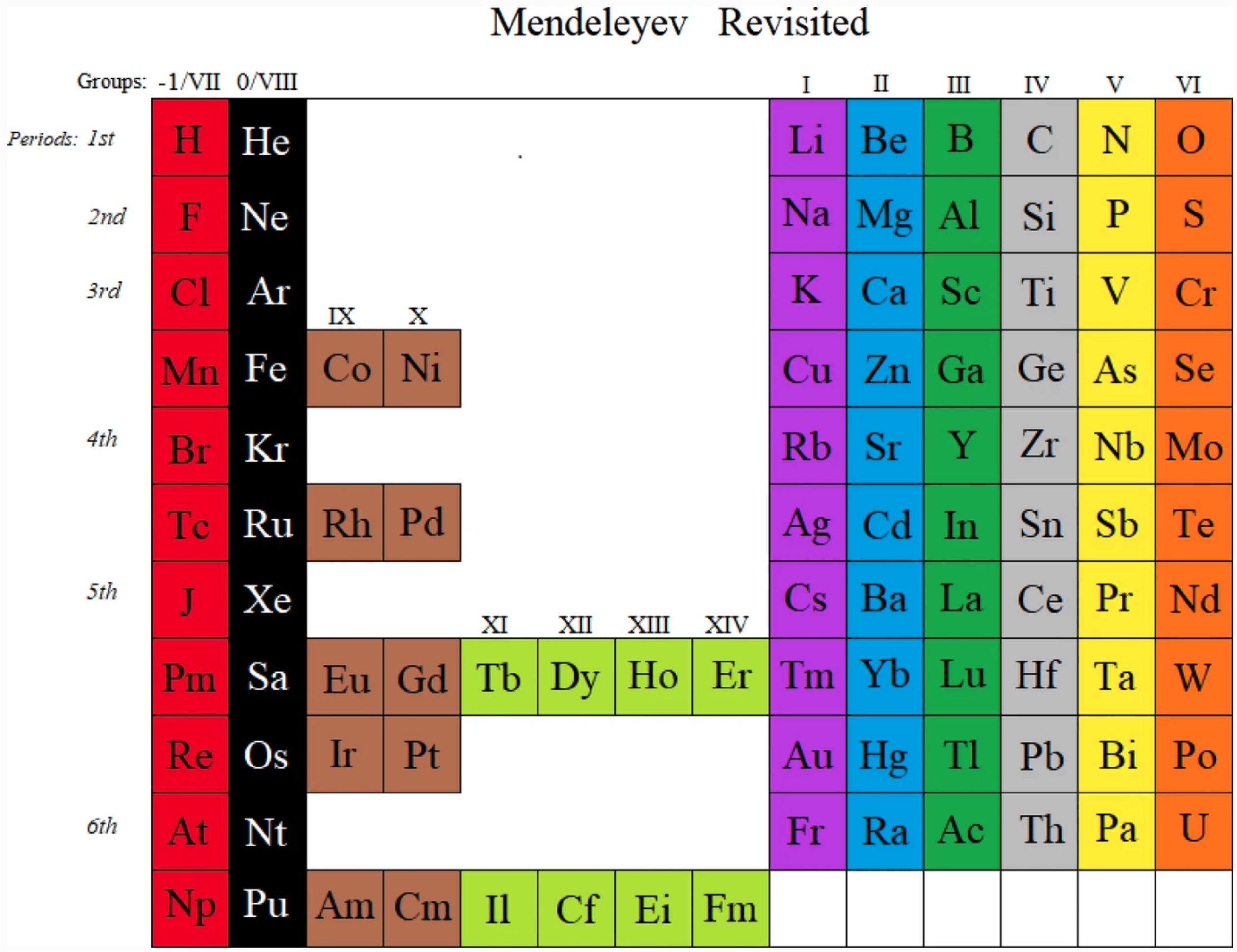
2021
History [of the] Elements and Periodic Table
From the Royal Society of Chemistry (RSC) an interactive Elements and Perioid Table History web page:

Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
2021
Mendeleyev-Sommerfeld IUPAC Periodic Table
From John Marks' updated Mendeleyev-Sommerfeld IUPAC Periodic Table.
John writes:
This is an adaptation of Fig. 4 [from https://link.springer.com/article/10.1007/s10698-021-09398-4] to match IUPAC's 18-column table. The yellow (transition metals) are Sommerfeld's 'A'-subgroups and the green (rare earths) are Sommerfeld's 'B'-subgroups.
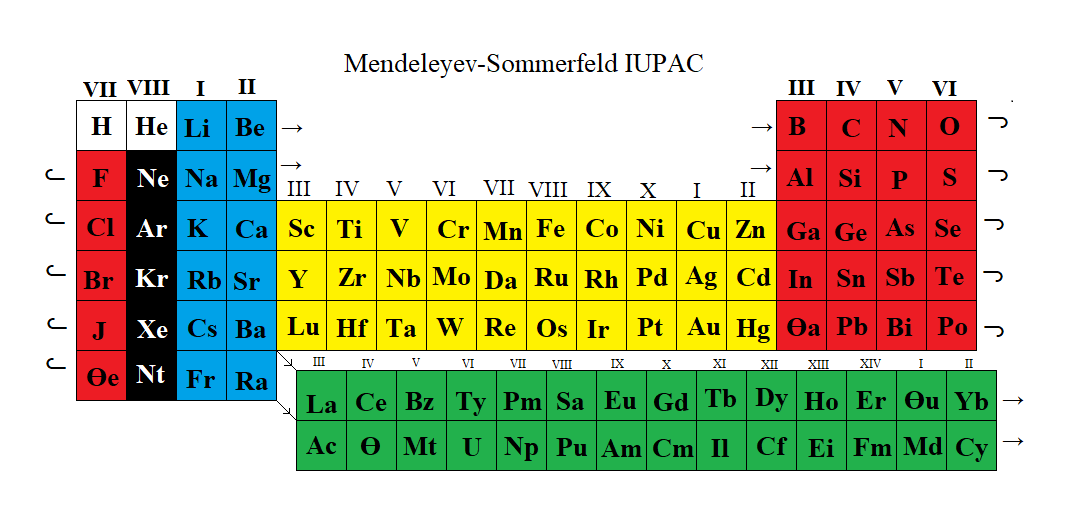
2021
Discoid Periodic Table of The Elements
Statement: "The orbital periodicity of the elements are the periodic function of their atomic number." By Muzzammil Qureshi.
Muzzammil writes:
"Years before Mendeleev's publications, there was plenty of experimentation with alternative layouts for the elements. Even after the table got its permanent right-angle flip, folks suggested some weird and wonderful twists.
"One of them are Circular in shapes. Discoid means circular in shape, and there is a great reason for choosing such a shape. The term "Periodicity" itself means "To occur in intervals", and if you walk around in a circle, you will find that you will return to the point from where you started at. Similarly, if the elements are also arranged in such way, then we shall experience more periodicity in the elements than before..."
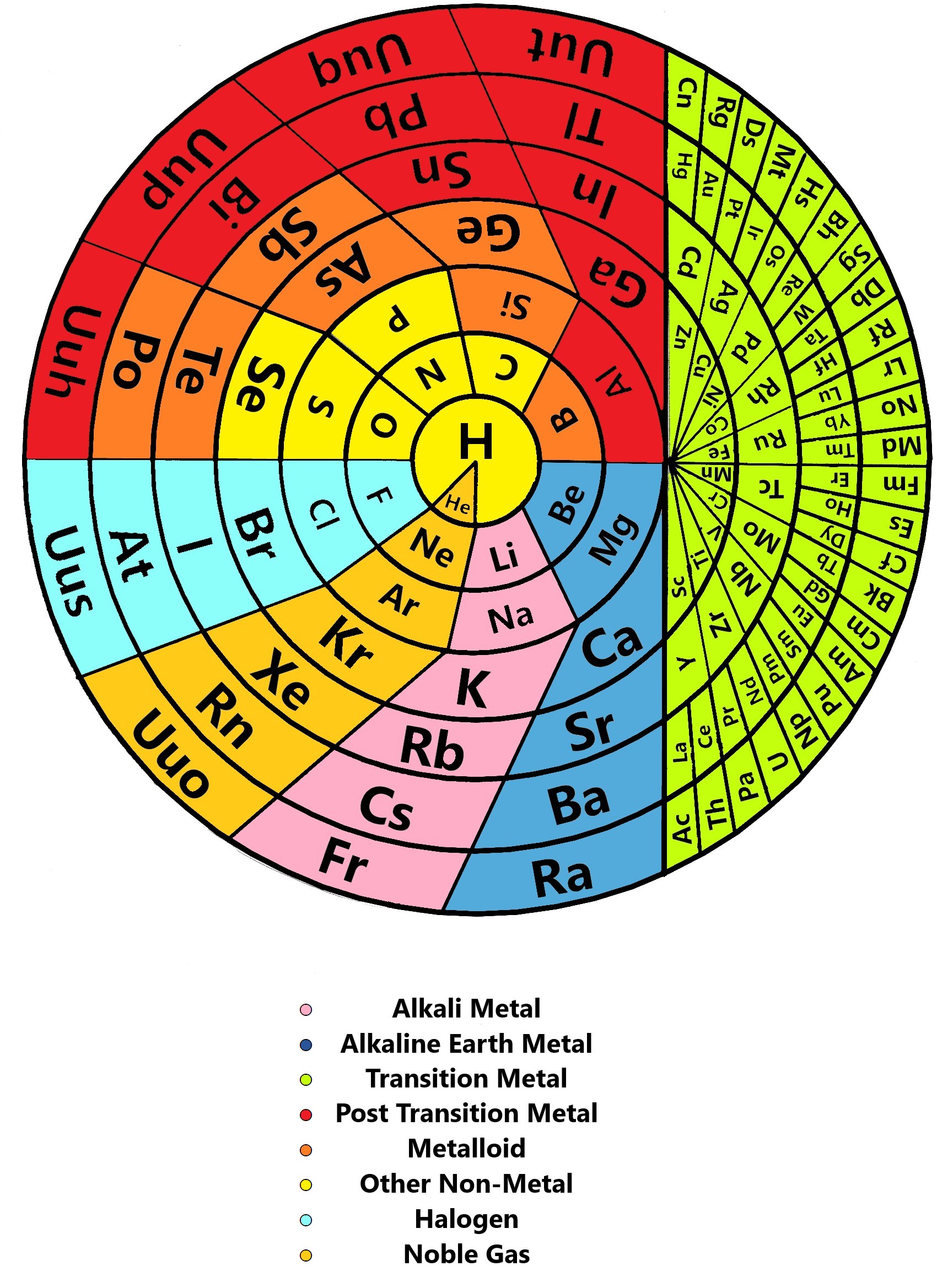
2021
Rolled-up Version of Benfey's Periodic System
Rolled-up Version of Benfey's Periodic System by Julio Antonio Gutiérrez Samanez. More on the YouTube video here.
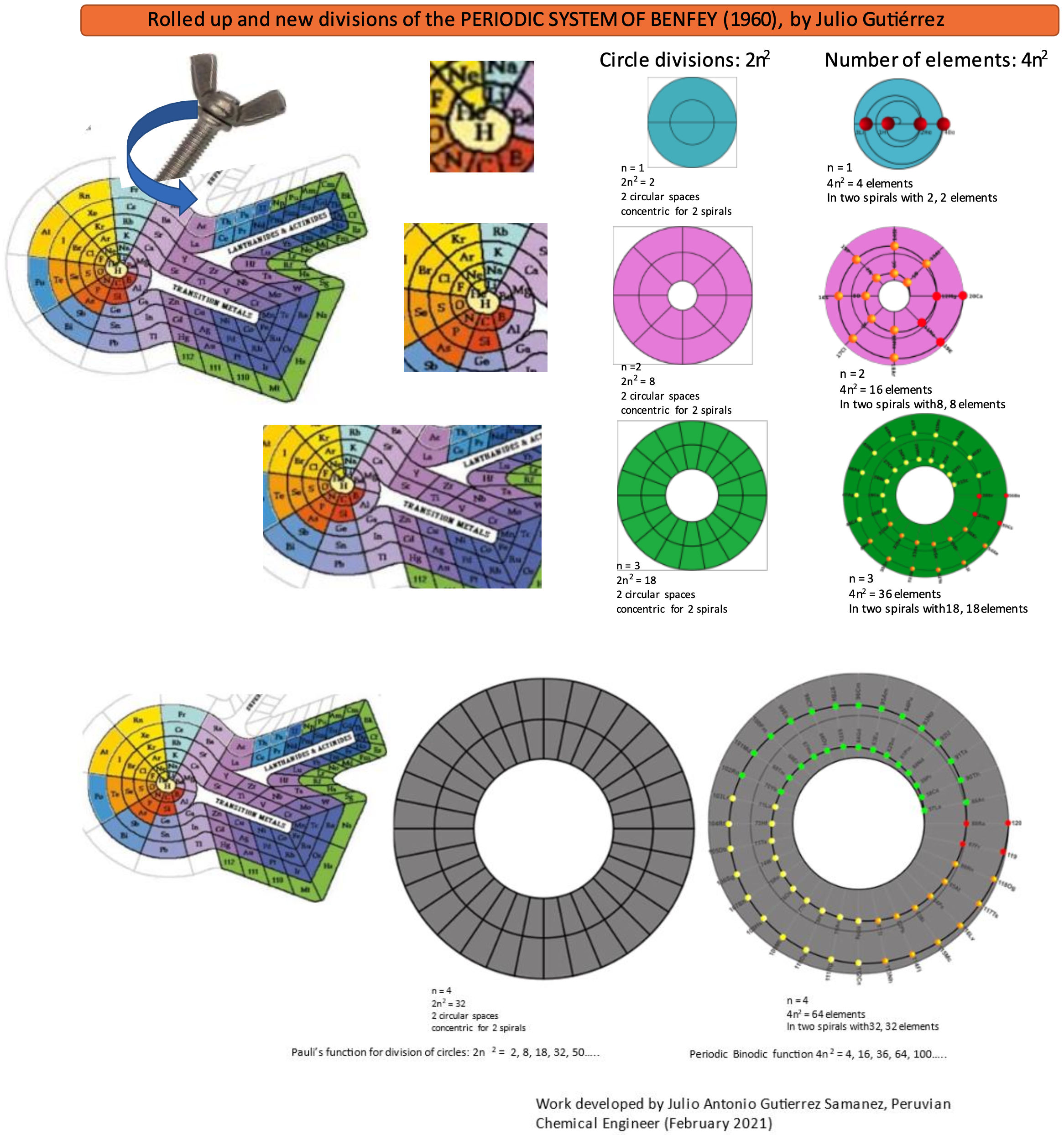
2021
Quantum Periodic Table
Shriya Tiwari & D. K. Awasthi, Quantum Periodic Table, wjpmr, 2021, 7(4), 124-130
The authors write:
"In [the] quantum periodic table, The elements are arranged according to the order of electron-shell filling, by classifying the energy levels of the atoms in the order they are filled, to create a layout based on electronic configuration. The classification of the elements is done purely on the basis clarified above, without giving any weight age to the atomic numbers. With the advent of electronic configurations and quantum mechanics, many attempts have been tried in this periodic table to unlock all the problems related with the placement of elements, which have been remained as the topic of debate by generations of chemists."
2021
Hutcheon Right Step Periodic Table
From Scott Hutcheon's Linkedin Website:
Abstract:
"Built from first principles, the RSPT is the only Periodic Table (so far) that reflects the periodicity of radioactive elements (natural and artificial) including Tc-Np and Cf/Es, the periodicity of liquids and gases (at standard and other conditions), depicts the 5 and 8 mass roadblocks, and finally clarifies the positions of the initial propagating elements H, He, and Li-Be/B in accordance with their cosmic and stellar evolutionary origins.
"Name inspired by the Janet Left Step Periodic Table (LSPT).
"Bonus Easter Egg: SPOCH BONSe, pronounced Spock Bones, is a new mnemonic device to remember the updated elements considered most essential for human life. Also considered POSCH SeNOB and SNOBS ePOCH."
2021
The Periodic Table: Is it Perfect, is it Fractured or is it Broken?
A video from Mark Leach, who writes:
The periodic table is an icon of science. Indeed, all chemical matter is made from periodic table stuff. The periodic table of the elements is often presented as being:
- With 118 elements the periodic table is now complete
- The periodic table is perfectly described (fully explained) by the application of four quantum numbers with the and some simple rules
- Chemical structure & reactivity can be deduced from the periodicity of the Groups & Periods
However, the chemistry of the chemical elements is actually a little more complicated than this. So, where & why does the predictability 'break'?
2022
Hutcheon's Animated Formulation(s) of The Periodic Table
A very cool animated periodic table that shows the PT morphing between various formulations. Read more on Scott's Linkedin Page.
2022
Kudan's Left-Step Periodic Table
From Pavel Kudan, a specialist in mass-spectrometry and identification of compounds., who suggests a variant of the left-step Periodic table:
1. Energy barriers directly shown to express correctly the Periodic Law
2. 4 periods (8 half periods)
3. 21 groups (1a-8a, 2b-8b, 3c-8c)
4. f-elements classified by groups, not families
5. La/Ac and Lu/Lr both in 3rd group
6. Cu/Ag/Au/Rg in group 8b together with Ni/Pd/Pt/Ds
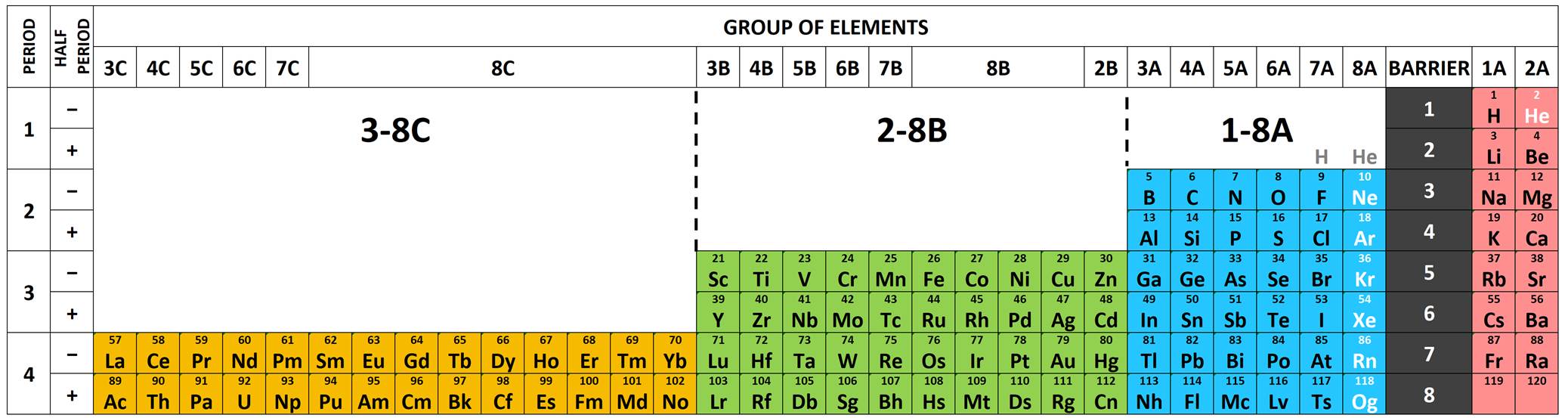
2022
Kudan's Left-Step Periodic Table (Short Form)
From Pavel Kudan, a specialist in mass-spectrometry and identification of compounds., who suggests a short-form variant of his left-step Periodic table:
1. Energy barriers directly shown to express correctly the Periodic Law
2. 4 periods (8 half periods)
3. 21 groups (1a-8a, 2b-8b, 3c-8c)
4. F-elements classified by groups, not families
5. La/Ac and Lu/Lr both in 3rd group
6. Cu/Ag/Au/Rg in group 8b together with Ni/Pd/Pt/Ds
7. Reminders for H to group 7a, He to group 8a, Cu/Ag/Au/Rg to group 1b
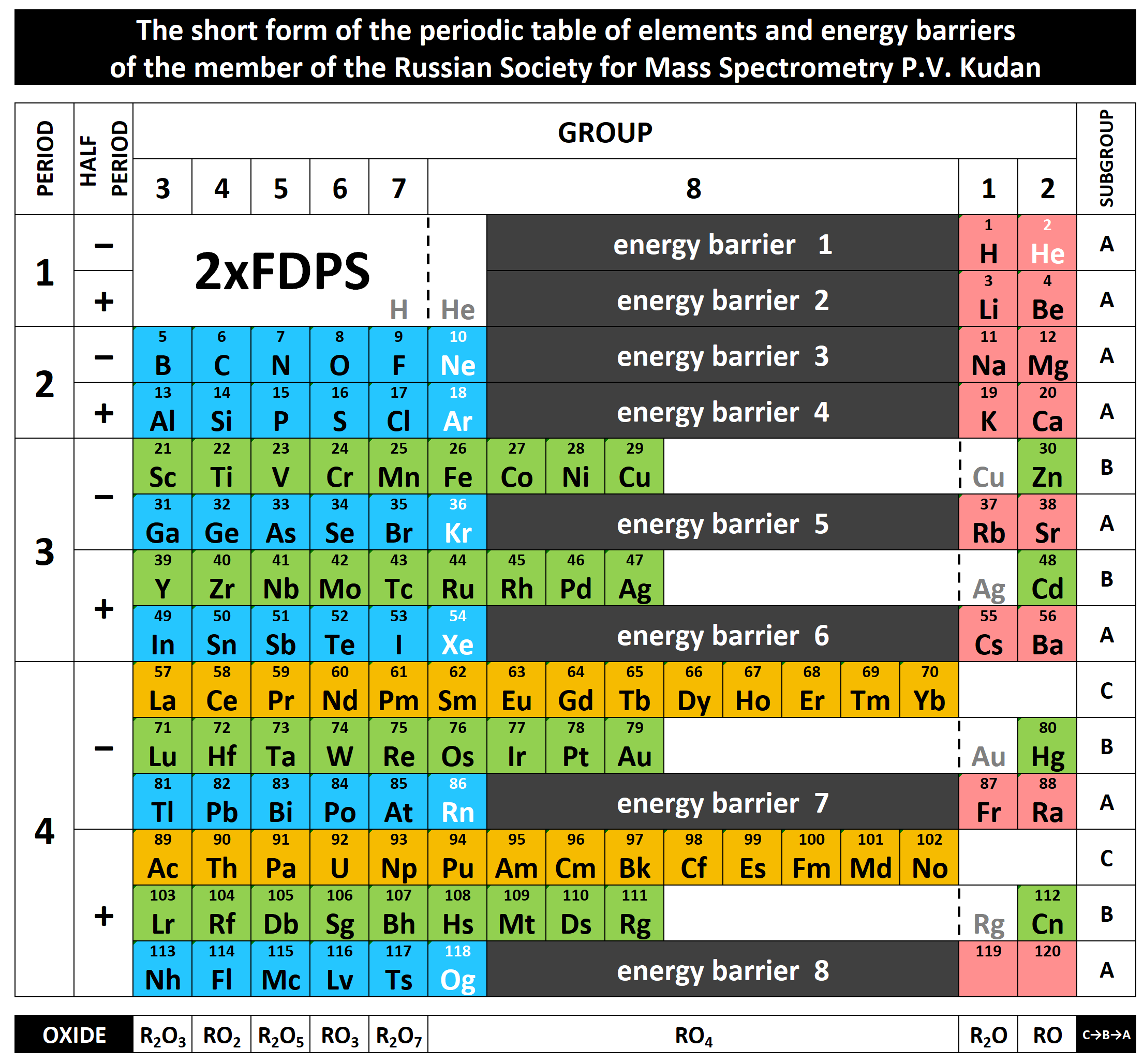
2022
Kaleidocycle of the Periodic Table
Pablo Cassinello provides a "Three-dimensional figure to improve the didactics of the Periodic Table", a Kaleidocycle of the Periodic Table.
There is a full article about this dynamic, three dimensional formulation in Pablo's blog in Chem Ed Xchange, including instructions on how to make the object.
Pablo writes:
"A kaleidocycle has four different faces, each one made of a juxtaposition of rhombuses. By turning it you can easily choose one of the four faces. On these, four different pictures can be displayed. In this three-dimensional figure of the periodic table are the elements organized in four blocks according to their final electronic structure. It is intended that students with this playful figure actively participate in classes by rotating their kaleidocycle looking for the groups or elements that are being studied. The entire periodic table fits in one palm of their hands. It is also a didactic device because students only focus their attention on one block or group of elements from the entire Periodic Table. It can be a more entertaining, motivating and exciting way of learning about the subject of the Periodic Table."
Thanks to René for the tip!
2022
Kudan's Periodic Law (Helix Form)
From Pavel V. Kudan, a specialist in mass-spectrometry and identification of compounds, who suggests a helical variant of the Periodic table. The graphical representation illustrates the Periodic Law and the following features of the Kudan’s periodic system:
- Four element periods (1, 2, 3, 4)
- Two element half periods (-,+)
- Twenty one element subgroups octet rule compatible (1a-8a, 2b-8b, 3c-8c)
- Small energy barriers between S and F, F and D, D and P elements (gray segments)
- Large energy barriers between P and S elements (black segments)
- Large energy barrier between He and Li (black segments)
(See also Kudan’s Left-Step Periodic Table)
2022
Which Element is the Best?
The That Chemist YouTube channel asks "Which Element is the Best? Elements 1-20 & Elements 21-40"
That Chemist is a synthetic organic chemist and his bias is in that direction although he gives a variety of examples:
2022
Tassitus' Periodic Table
By Harry Tassitus who wites:
"Here is my narrative; After 50 years of study I have released this periodic table which is a synthesis of the work of Dr Isaac Asimov, Dr Ida Noddack, and Edward g. Mazurs, the latter of which I found on the Internet Database of Periodic Tables web site."
2022
Makeyev's Relativity Matrix of the Elements of Matter (MOEM)
By Alexander K. Makeyev, a member of the Moscow Society of Naturalists, multidisciplinary researcher and inventor; the author of Theory Relativity of Reality: The Relativity Matrix of the Elements of Matter (MOEM)
"Periodic table of elements vertical form. The periods of atomic levels of dense matter of matter correctly end at the element of the group of alkaline earth metals. Symbols of timelessness-non-existence / time-being are displayed in front of hydrogen; loose matter of vacuum and electrostatics and magnetism of ether; dense matter of neutronium (neutron nuclei of atoms and neutron stars)."
Literature:
- Makeev A.K. Julius Lothar Meyer was the first to build a periodic system of elements // European applied sciences, No. 4 2013, (April) volume 2. - pp. 49-61. ISSN 2195-2183.
- A.K. Makeev. Self-reproduction of matter // Materials of the international scientific-practical conference: "Prospects for the Development of Modern Science" – Jerusalem, Israel: Regional Academy of Management, 2016. – 535 p. P. 213-220. UDC 001.18 BC 72 P 93 ISBN 978-601-267-398-2 https://drive.google.com/file/d/0B_W2hkSE3iXram5DX1FoX3NsLVE/view?resourcekey=0-OFX6dMXY-xOGnOLDyxFvLQ
- A.K. Makeev. The essence of time. // Prose.ru http://proza.ru/2015/11/18/796
- A.K. Makeev. What is the natural ending of periods? // Prose.ru https://proza.ru/2019/09/28/115
- A.K. Makeev. Is hydrogen a dielectric gas or an alkali metal? // Prose.ru https://proza.ru/2016/09/28/1721
- A.K. Makeev. Do vacuum and ether participate in gravity? // Prose.ru https://proza.ru/2019/04/16/1893
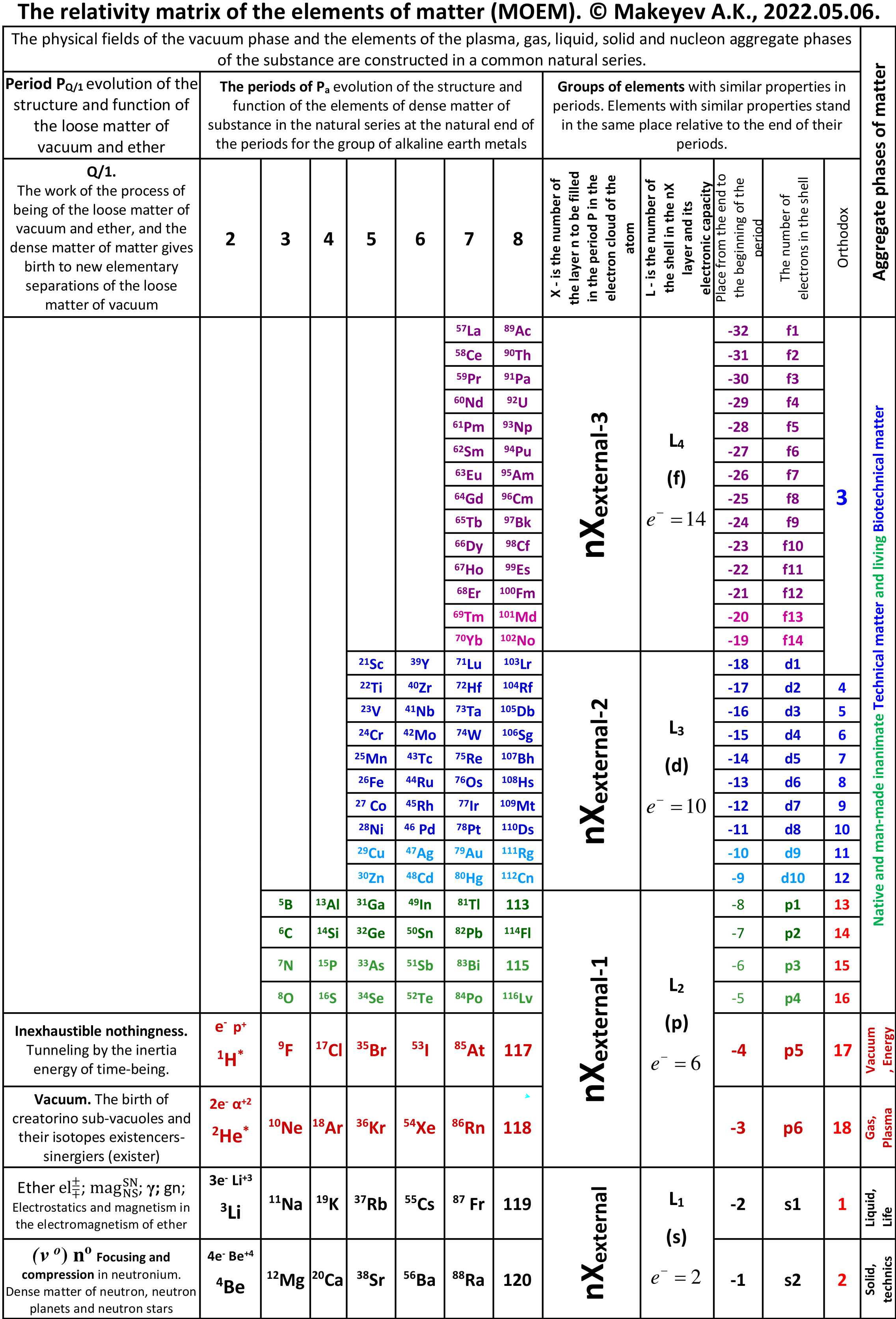
2022
Vernon's Yin Yang of The Periodic Table
René Vernon writes:
"I was prompted to [develop] this item after reading Eric Scerri's open access article: In Praise of Triads. The nub of Eric’s article is to argue for the LST on the grounds of triad regularity, first-row-anomaly regularity, and consistency with QM.
"It occurs to me that efforts to introduce more regularity to the PT invariably introduce new irregularities elsewhere. For example, as far as triad regularity goes, the left step table (LST) with He over Be introduces its own anomaly in that no element in period 1 (H, He) is part of a triad whereas this is not the case for all periods thereafter. In contrast, all periods of the traditional table have at least one element that is part of a triad.
"As far as QM goes, this tells us that there is a theoretical regularity to the PT. This regularity can be used to inform e.g. the LST, ADOMAH or Julio’s binodes. But such depictions do not reflect the factual relationships we see amongst the chemistry of the elements as well as is the case for the conventional form. A most obvious example is that the LST, while being more consistent with QM, disrupts the bottom-left to top-right trend in metallic to nonmetallic character seen in conventional tables.
(I must caveat that I'm referring to the chemistry of the elements in conditions regularly occurring on Earth. For example, it has been reported that under sufficiently high pressures the elements change their EN and electron configurations. If so, this suggests a need for a different table at high pressure.)
At this point, the chemistry educators enter the picture. They move the s-block to left. Helium is relocated over Ne on the basis of its nobility. (This could change if a few compounds of He were to be synthesized). Somewhat similarly, La was discovered well before Lu, so it ended up under Y, and most folks see no good reason to replace La with Lu. Sure, in the 32-column form, the result is a split d-block but the infrequency with which the 32-column form appears is such that most people are not bothered. The result is the conventional table.
Philosophically, while the n+l based LST might represent the most general form of table, the conventional table appears to currently represent the most pragmatic derivation for chemists and chemistry educators.
Since the PT is classification rather than theory, and there will thus always be hard cases at the boundaries, there will invariably be minor variations in the depiction of the conventional table with respect to e.g. the placement of H, the composition of group 3, or the length of the f block.
And there will always be tables such as MR that focus on particular perspectives of relationships among the the elements.
I’ve tried to sketch what's going in the attached image:"
2022
Gorodkov's Periodic System of Periodic Systems
Mikhail Gorodkov writes:
"I hope you'll find attached version of Periodic System not only funny but also informative!"
2022
99 Elements Sorted by Density & Electronegativity
René Vernon writes:
"A little while I ago I noticed that a scatter plot of EN (revised Pauling) and density values of the elements resulted in a nice distribution, as per the table below.
"According to Hein and Arena (2013) nonmetals have low densities and relatively high EN values; the table bears this out. Nonmetallic elements occupy the top left quadrant, where densities are low and EN values are relatively high. The other three quadrants are occupied by metals. Of course, some authors further divide the elements into metals, metalloids, and nonmetals although Odberg argues that anything not a metal is, on categorisation grounds, a nonmetal.
Note 25 says:
(a) Weighable amounts of the extremely radioactive elements At (element 85), Fr (87), and elements with an atomic number higher than Es (99), have not been prepared.
(b) The density values used for At and Fr are theoretical estimates.
(c) Bjerrum (1936) classified "heavy metals" as those metals with densities above 7 g/cm^3.
(d) Vernon (2013) specified a minimum electronegativity of 1.9 for the metalloids.
- Bjerrum, N (1936). Bjerrum’s Inorganic Chemistry. London: Heinemann
- Hein, M; Arena, S (2013). Foundations of College Chemistry. Hoboken: John Wiley & Sons. pp. 226, G-6. ISBN 978-1-118-29823-7.
- Oderberg DS 2007, Real Essentialism, Routledge, New York, ISBN 978-1-134-34885-5
- Vernon R 2013, "Which elements are metalloids?", Journal of Chemical Education, vol. 90, no. 12, 1703?1707, doi:10.1021/ed3008457
2022
Tutti Frutti Periodic Table
René Vernon who writes:
"As a potentially powerful teaching and learning instrument: a Tutti Frutti periodic table. I feel younger folk [will] be delighted by it. The overlay of electron configurations and blocks was designed by a colleague of mine."
2022
Deming's 1923 Periodic Table, Updated by Vernon
René Vernon writes:
"Here is the 21st century version of Deming's 1923 formulation. Lacking elegance perhaps, but that’s messy chemistry for you. 25 columns wide rather than 18 or 32. Split s, p and d blocks. The connecting lines are based on four sources:
- Deming
- The literature since his time, as shown
- The expected behaviour of the super-heavy elements
- The smoothness of Z vs physiochemical property trendlines going down groups, for up to 40 physiochemical properties
"I used [the term] frontier metals to refer to the post-transition metals, since the latter term has never applied well to Al. The frontier adjective comes from a line by Russell and Lee in which they refer to Bi and Po occupying frontier territory on the PT, adjacent to the nonmetals (2006, p. 419).
"As far as metalloids are concerned, Dingle nicely summarized their status: "With ‘no-doubt' metals on the far left of the table, and no-doubt non-metals on the far right…the gap between the two extremes is bridged first by the poor (post-transition) metals, and then by the metalloids— which, perhaps by the same token, might collectively be renamed the 'poor non-metals'." (2017, p. 101)
Ref: Dingle 2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton Russell AM & Lee KL 2005, Structure-Property Relations in Nonferrous Metals, John Wiley & Sons, Hoboken, NJ
2022
777 Periodic Wedding Cake
René Vernon's version of Courtine’s flat projection (1926) of his periodic tower, the 777 Periodic Wedding Cake:
2022
Periodic Table of Periodic Tables
René Vernon has collected the PT formulations in this PT database and classifed them as:
Short, Triangular, Medium, Long, Continious, Folding, Spatial & Unclassified
and tabulated them by date. The 'working document' is currently a word file.
2022
Ramsay-Sommerfeld Periodic Table
John Marks has updated Scerri's 2006 Triad formulation. Marks writes:
"[This is] an expansion of Scerri´s 2006 triad formulation, but brings the lanthanides & actinides in from the cold. It is lso modelled on the way IUPAC constructed its 18-group PT (but with traditional group numbering), that is to say, the total of 22 groups is simply IUPAC's 18 plus the four extra f-groups. I am not sure if this counts as a "medium" or a "long" form.
"If this PT needs a name, it would be the Ramsay-Sommerfeld PT. It has Ramsay´s 1915 arrangement for H (copied by Eric Scerri in 2006) and Sommerfeld's "greater" and "lesser" periods (1916) in green and yellow respectively. It solves the 'exile' of the f-blocks without making it too unwieldy (22 instead of 32 groups). The yellow (transition series) are Sommerfeld's 'A' subgroups and the green (rare earth series) are Sommerfeld's 'B' subgroups."
2023
Marks' Version of Mendeleyev's 1869 Formulation
John Marks, who provided the graphic, writes:
"I went back to Mendeleyev´s 1869 original and drew this (below) which demonstrates the Sommerfeldsche aufspaltung as occurring after completed s-subshells. No-one disputes the chemical phenomena of the octets formed by He/Ne, Li/Na, Be/Mg, B/Al, C/Si, N/P and O/S nor that H/F occurs at the beginning of these octets, however "irregular" H may appear.
"Chemical periodicity is clearly based on periods arising from sp3 hybridization and the aufspaltung appears to occur between the s and the p3. This gives rise to the positions of Sommerfeld's "Long" (with the d-elements) and "Very Long" (with the f-elements) periods."
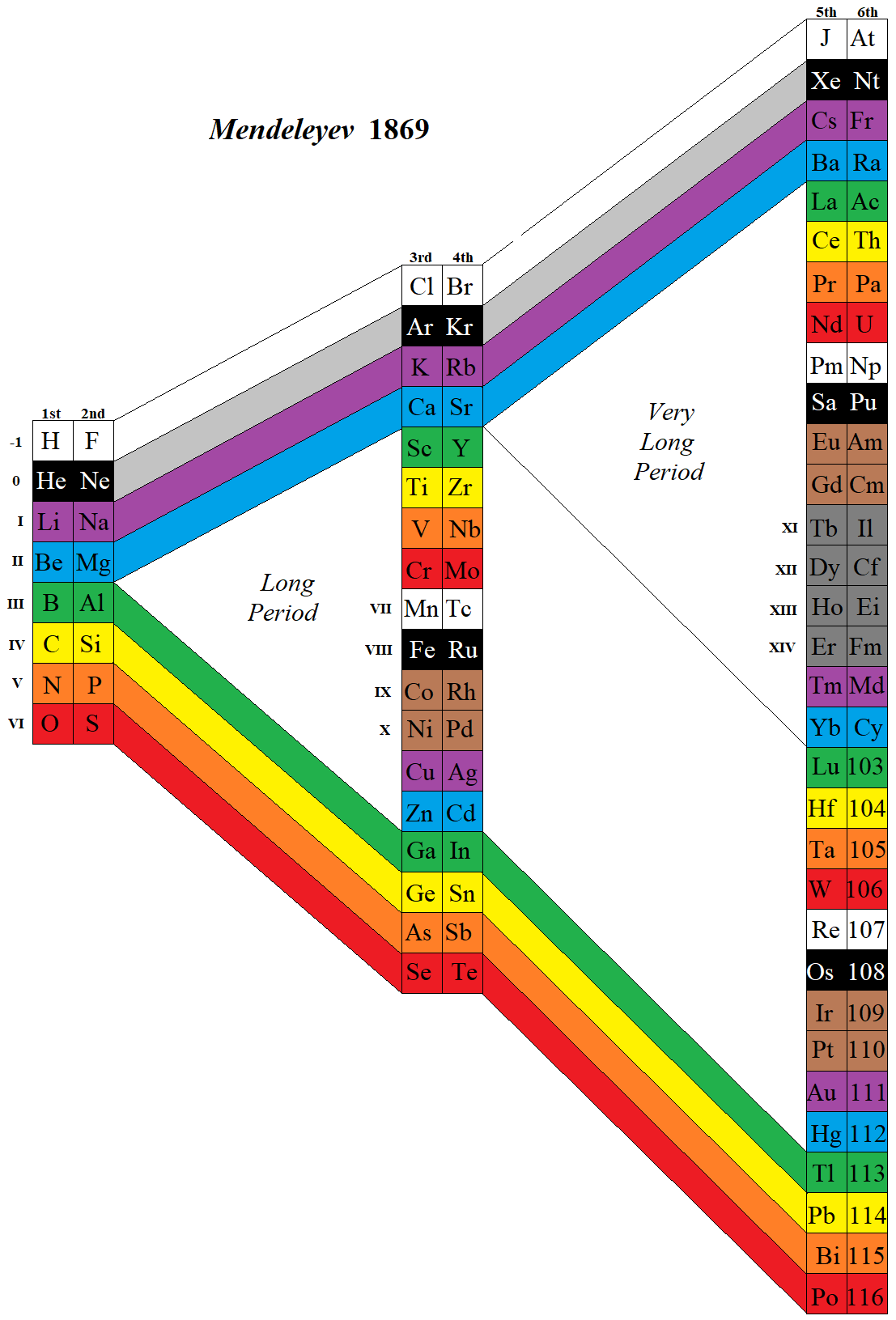
2023
Element Names: The Etymology of The Periodic Table
An excellent video by RobWords about the names of the chemical elements and how they came about:
2023
Kudan's Periodic System
From Pavel V. Kudan, a specialist in mass-spectrometry and identification of compounds, who suggests new periodic table based on new formulation of the periodic law.
See also:
Click the image to enlarge.
2023
Six Stages of The Convergence of The Periodic System
Bran, A.M., Stadler, P.F., Jost, J. et al. The six stages of the convergence of the periodic system to its final structure. Commun Chem 6, 87 (2023). https://doi.org/10.1038/s42004-023-00883-9
Abstract (abridged):
"We show, by analysing the space between 1800 and 2021, that the system has converged towards its current stable structure through six stages, respectively characterised by the finding of elements (1800–1826), the emergence of the core structure of the system (1826–1860), its organic chemistry bias (1860–1900) and its further stabilisation (1900–1948), World War 2 new chemistry (1948–1980) and the system final stabilisation (1980–)."
Periodic tables representative of each period in history. Families of similar elements (sets sharing colour) shown in each table summarise the patterns and do not necessarily imply continuity nor simultaneity of the families throughout the period:
2023
Semicircular Hybrid Chart of the Nuclides
Nawa Nagayasu has produced a new version of the Segrè Chart of the Nuclides.
Nawa writes:
"The chart has the number of neutrons on the [curved] horizontal axis and the number of protons (atomic number) on the vertical axis. I used the IAEA colour coding [scheme]. JAEA's half-life ranks are indicated by simple numbers, not rounded frames.
"In order to fit the whole chart into a semicircle, the axis representing the number of neutrons was made a spiral-like curve. For clarity, the number of neutrons is shown in the middle of each curve."
Yuri Oganessian has commented:
"Nawa Nagayasu is an original and talented designer. After all, it is not easy to work with 118 elements, but now also with isotopes, of which there are more than 3000. The fan design looks attractive and this is very important. This will make people, especially school age, guess the numbers that are written there. So they will gradually delve into the content of the Table, a truly brilliant creation."
2023
Holistic View of Metals & Nonmetals: Exploded View
From Organising the metals and nonmetals: An update by René Vernon from the chemrxiv preprint server.
Rene writes:
Abstract: This paper updates my 2020 article, Organising the metals and nonmetals in which I advocated for parsing the periodic table into four kinds of metals and four of nonmetals. This framework is retained and updated, and augmented with some additional chemistry-related and philosophical observations.
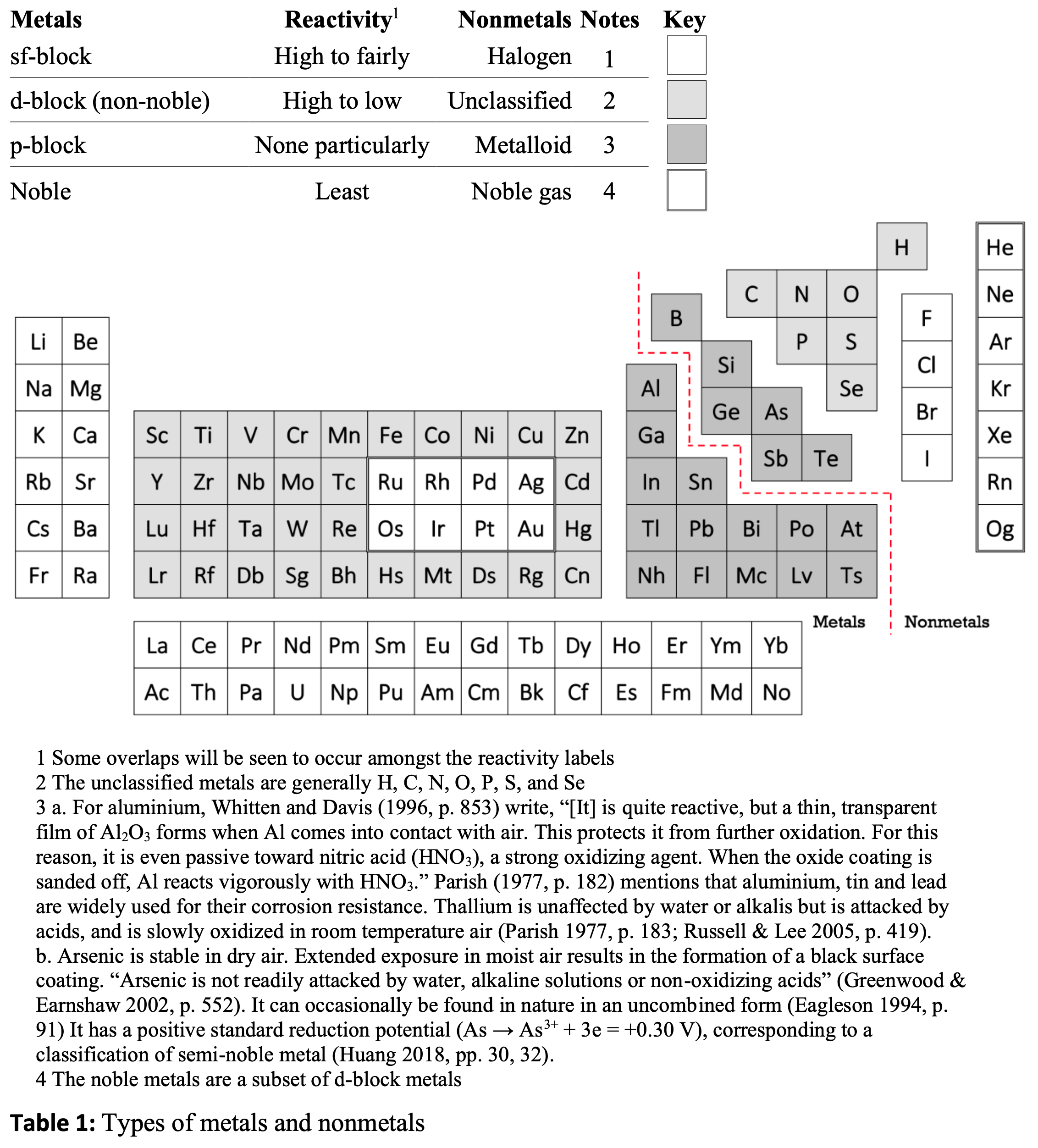
2023
Chemdex: Valence & Oxidation Number Trends
From Mark Winter's review paper Chemdex: quantification and distributions of valence numbers, oxidation numbers, coordination numbers, electron numbers, and covalent bond classes for the elements Dalton Trans., 2024,53, 493-511 https://doi.org/10.1039/D3DT03738J.
The images below show the Valence number (VN) and oxidation number (ON) proportions as percentages for the elements; and Periodic tables displaying valence number proportions (%). (There are few data for Pm and no data for Fr and elements beyond Es.)
The position of H and the group numbers are addressed in the paper.
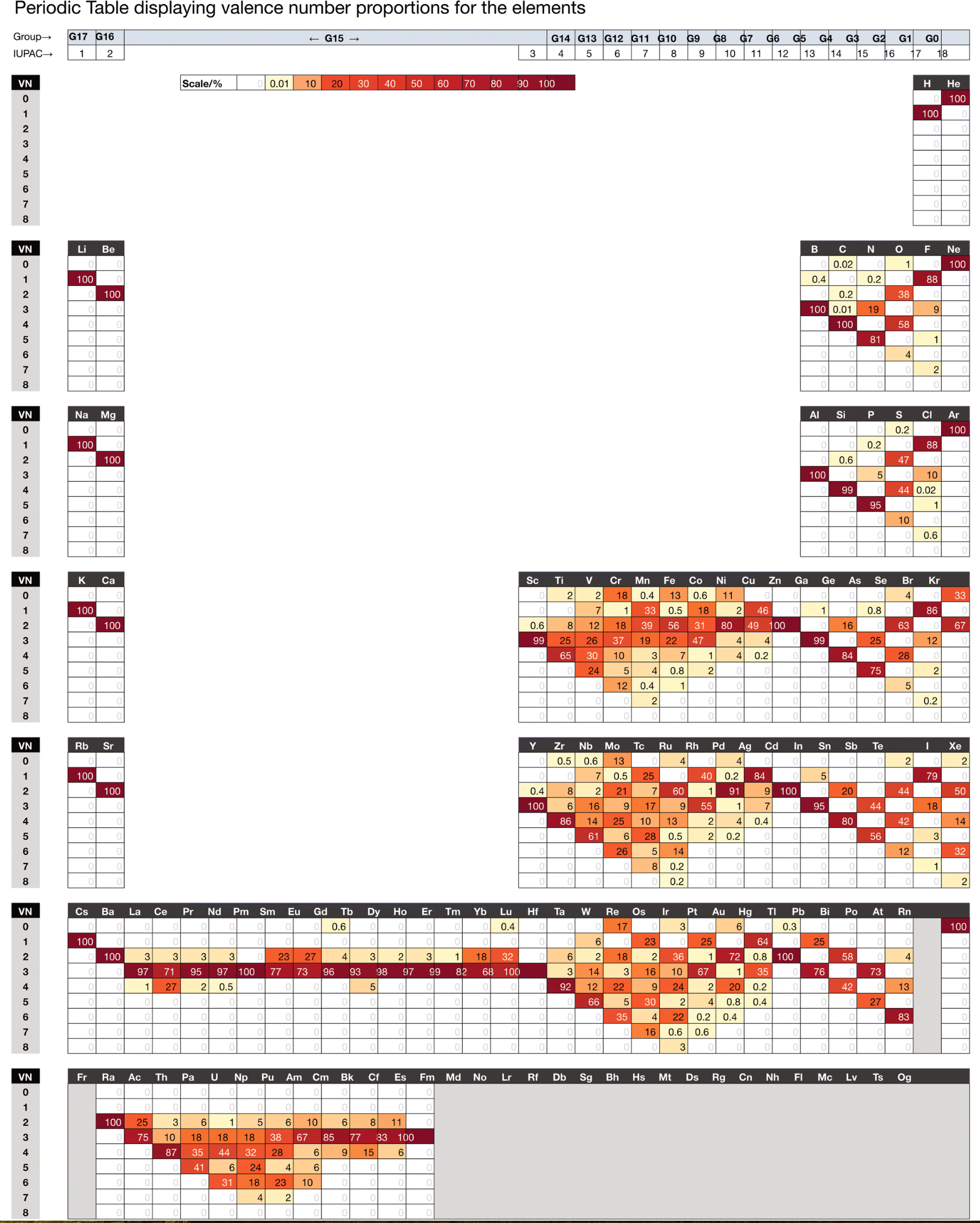
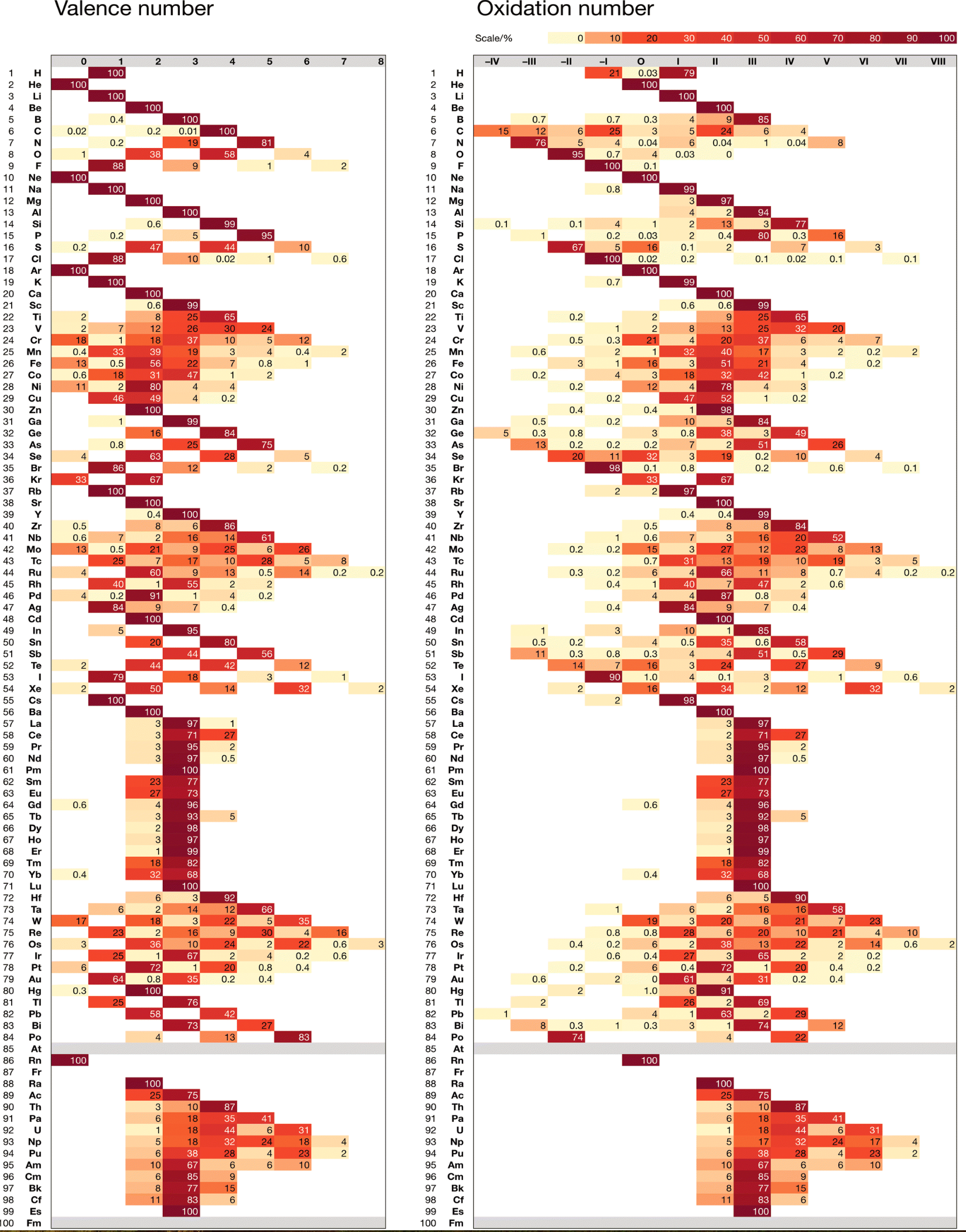
2023
DALL·E Pop Art Periodic Table
I asked DALL·E to generate a: "periodic table as pop art", and the AI produced this:

2023
Mendeleyev’s Periodic Table after Ramsay & Sommerfeld
John Marks, who provided the graphic, writes:
"This is the Ramsay-Sommerfeld PT and would seem to be the definitive PT, at least historically.
"Ramsay, a chemist, completed Mendeleyev's PT with the discovery of the inert gases in the 1890s and the position of hydrogen with the halogens by 1915.
"Sommerfeld, a physicist, generalized Bohr's atom in 1916 to yield the s-, p-, d- and f- electronic subshells that determine the layout of physicists' PTs, in particular their first "very short" period comprising H and He. Sommerfeld also explained the 'long periods', viz. the transition series ('A' subgroups) and the 'very long periods', viz. the rare earth series ('B' subgroups).
"In this chemistry/physics hybrid periodic table, the physicist Sommerfeld's first ('very short') "period" is subsumed under the chemist Ramsay's first two groups (-1 and 0) which are distinguished by colour: group -1 is white = 1s1p5, viz. H & the halogens; group 0 is black = 1s2p6, viz. He & the inert gases.
"Einstein's demonstration of atomic reality in 1905 (phenomena verified by Perrin in 1908) established the basic units of the paradigm of chemistry. Rutherford and Bohr (both physicists) went inside the atom, into the paradigm of physics.
"The PT thus straddles the borderland of the two paradigms of physics and chemistry and this has contributed significantly to the long debates on the form of the PT."