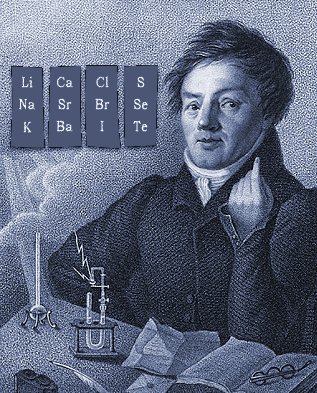The INTERNET Database of Periodic Tables
Periodic Table formulations from the years before 1900, by date:
9000 BCE
Discovery of Copper
Cu
Copper, atomic number 29, has a mass of 63.546 au.
Copper had its earliest use in about 9000 BCE, and the oldest sample dates from 6000 BCE. It was discovered by Middle East workers and the earliest sample is from Anatolia.
7000 BCE
Discovery of Lead
Pb
Lead, atomic number 82, has a mass of 207.2 au.
Lead had its earliest use in about 7000 BCE, and the oldest sample dates from 3800 BCE. It was discovered by Africa and the earliest sample is from Abydos, Egypt.
6000 BCE
Discovery of Gold
Au
Gold, atomic number 79, has a mass of 196.967 au.
Gold had its earliest use in about 6000 BCE, and the oldest sample dates from 4400 BCE. It was discovered by Bulgaria and the earliest sample is from Varna Necropolis.
5000 BCE
Discovery of Iron
Fe
Iron, atomic number 26, has a mass of 55.845 au.
Iron had its earliest use in about 5000 BCE, and the oldest sample dates from 4000 BCE from Egypt.
5000 BCE
Discovery of Silver
Ag
Silver, atomic number 47, has a mass of 107.868 au.
Silver had its earliest use in about 5000 BCE, and the oldest sample dates from 4000 BCE, and is from Asia Minor.
3750 BCE
Discovery of Carbon
C
Carbon, atomic number 6, has a mass of 12.011 au.
Carbon has many allotropes, including: graphite, diamond, graphene, C60, single wall nanotubes, etc.
Carbon had its earliest use in about 3750 BCE. It was discovered by Egyptians and Sumerians.
3500 BCE
Discovery of Tin
Sn
Tin, atomic number 50, has a mass of 118.71 au.
Tin + copper gives bronze, and so the Bronze Age.
Tin had its earliest use in about 3500 BCE, and the oldest sample dates from 2000 BCE. It is unknown who discovered the element.
2000 BCE
Discovery of Sulfur (Sulphur)
S
Sulfur, atomic number 16, has a mass of 32.068 au.
Sulfur is a pale yellow, odourless, brittle solid.
Sulfur had its earliest use in about 2000 BCE. It was discovered by Chinese/Indians.
2000 BCE
Discovery of Mercury
Hg
Mercury, atomic number 80, has a mass of 200.592 au.
Mercury had its earliest use in about 2000 BCE, and the oldest sample dates from 1500 BCE. It was discovered by Chinese/Indians and the earliest sample is from Egypt.
1000 BCE
Discovery of Zinc
Zn
Zinc, atomic number 30, has a mass of 65.38 au.
Zinc had its earliest use in about 1000 BCE, and the oldest sample dates from 1000 BCE. It was discovered by Indian metallurgists and the earliest sample is from the Indian subcontinent.
800 BCE
Discovery of Antimony
Sb
Antimony, atomic number 51, has a mass of 121.76 au.
Antimony had its earliest use in about 800 BCE.
450 BCE
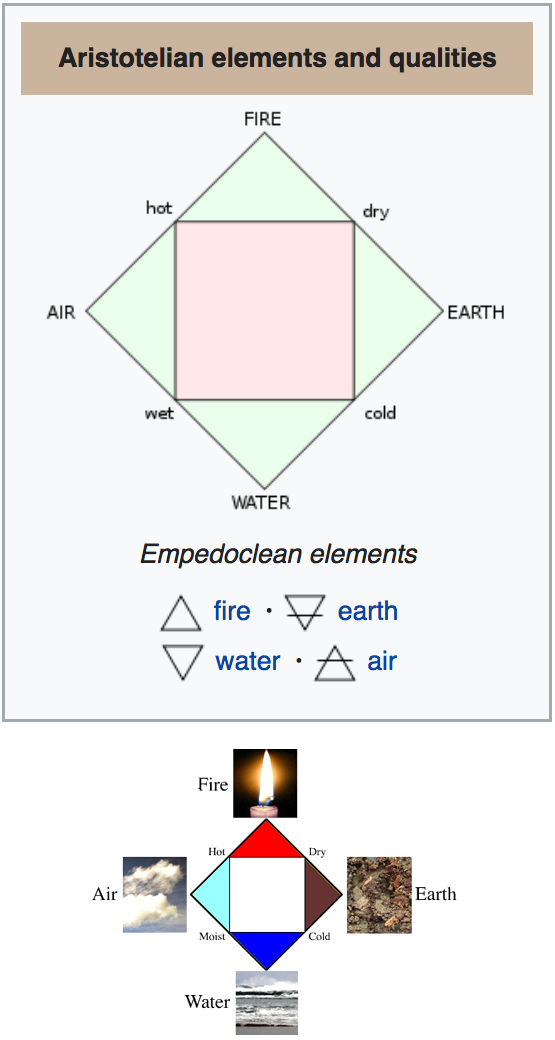
Classical Elements: Earth, Water, Air & Fire
The Greek Classical Elements — Earth, Water, Air, Fire [& Aether] — date from 450 BC or so, and persisted throughout the Middle Ages and into the Renaissance, deeply influencing European thought and culture.
A Greek text Kore Kosmou ("Virgin of the World" - associated with the Egyptian god Thoth - names the four elements fire, water, air, and earth:
And Isis answer made: Of living things, my son, some are made friends with fire, and some with water, some with air, and some with earth, and some with two or three of these, and some with all. And, on the contrary, again some are made enemies of fire, and some of water, some of earth, and some of air, and some of two of them, and some of three, and some of all. For instance, son, the locust and all flies flee fire; the eagle and the hawk and all high-flying birds flee water; fish, air and earth; the snake avoids the open air. Whereas snakes and all creeping things love earth; all swimming things love water; winged things, air, of which they are the citizens; while those that fly still higher love the fire and have the habitat near it. Not that some of the animals as well do not love fire; for instance salamanders, for they even have their homes in it. It is because one or another of the elements doth form their bodies' outer envelope. Each soul, accordingly, while it is in its body is weighted and constricted by these four.
The four elements were used by Hippocrates in describing the human body with an association with the four humours:
- yellow bile (fire)
- black bile (earth)
- blood (air)
- phlegm (water)
Plato characterizes the elements from a list created by the Sicilian philosopher Empedocles called these the four "roots." Plato seems to have been the first to use the term element:
300 BCE
Discovery of Arsenic
As
Arsenic, atomic number 33, has a mass of 74.922 au.
Arsenic had its earliest use in about 300 BCE.
1000
Elements Known in the Year 1000
Elements known in the year 1000, taken from this Wikipedia page:
1520
Tria Prima of Alchemy
Paracelsus identified three primes, the tria prima, of alchemy which are related to the Law of the Triangle, in which two components come together to produce the third. Philosophically speaking, Mercury is the Mind; Salt is the Will & Wisdom; and Sulphur is Love.
The three are components or principles of the Philosopher's Stone, and they work potently to transmute any base metal or character into golden perfection. Without these principles, the coveted Stone is ineffectual in its capacity to change vibratory rates.
- Sulfur: The fluid connecting the High and the Low. Sulfur was used to denote the expansive force, evaporation, and dissolution.
- Mercury: The omnipresent spirit of life. Mercury was believed to transcend the liquid and solid states. The belief carried over into other areas, as mercury was thought to transcend life/death and heaven/earth.
- Salt: Base matter. Salt represented the contractive force, condensation, and crystallization.
1617
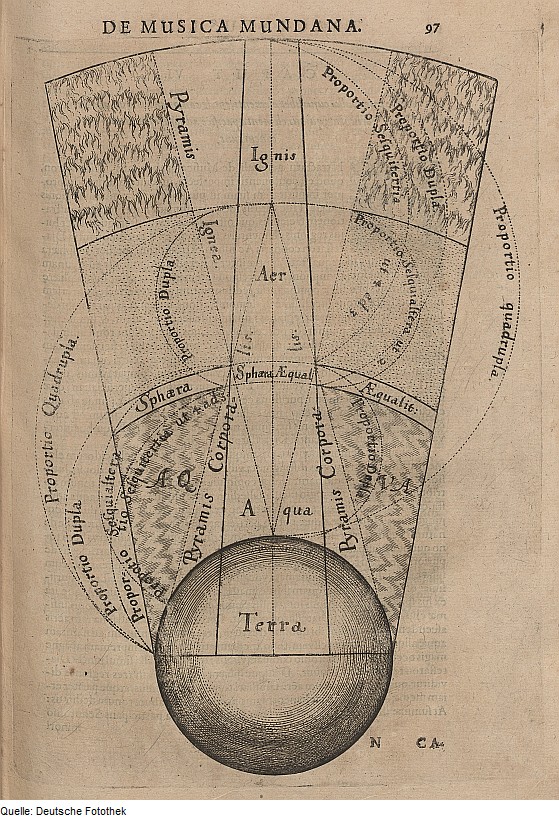
Elemental Spheres of Terra (earth), Aqua (water), Aer (air) & Ignis (fire)
From the German Photo Library Theosophie & Alchemie, a segment of the macrocosm showing the elemental spheres of terra (earth), aqua (water), aer (air), and ignis (fire), by Robert Fludd:
1624
Ripley Scroll
The Ripley Scroll, an illustrated alchemical manuscript, in English and Latin, on vellum, England [perhaps Manchester?] 1624. This item was sold by Christie's in 2017.
There are 23 known versions of the Ripley Scroll (or "Ripley Scrowle").
George Ripley (c. 1415-1490) was one of England's most famous alchemists. His alchemical writings attracted attention not only when they were published in the fifteenth century, but also later in the sixteenth and seventeenth centuries. His writings were studied by noted figures such as the alchemist John Dee, Robert Boyle (who is considered to be the first modern chemist), and even Isaac Newton.
There is a copy/version of the Ripley Scroll at the British Library.

1669
Discovery of Phosphorus
P
Phosphorus, atomic number 15, has a mass of 30.974 au.
Phosphorus exists in several allotropic forms including: white, red and black.
Phosphorus was first isolated in 1669 by H. Brand.
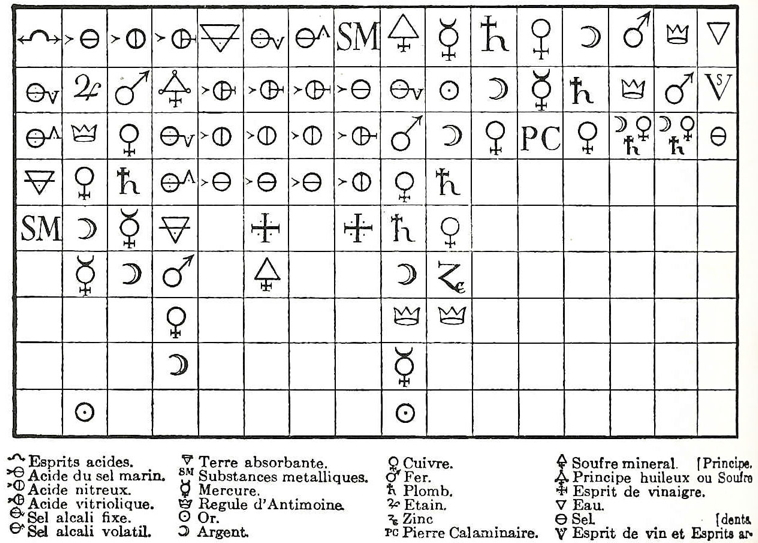
1671
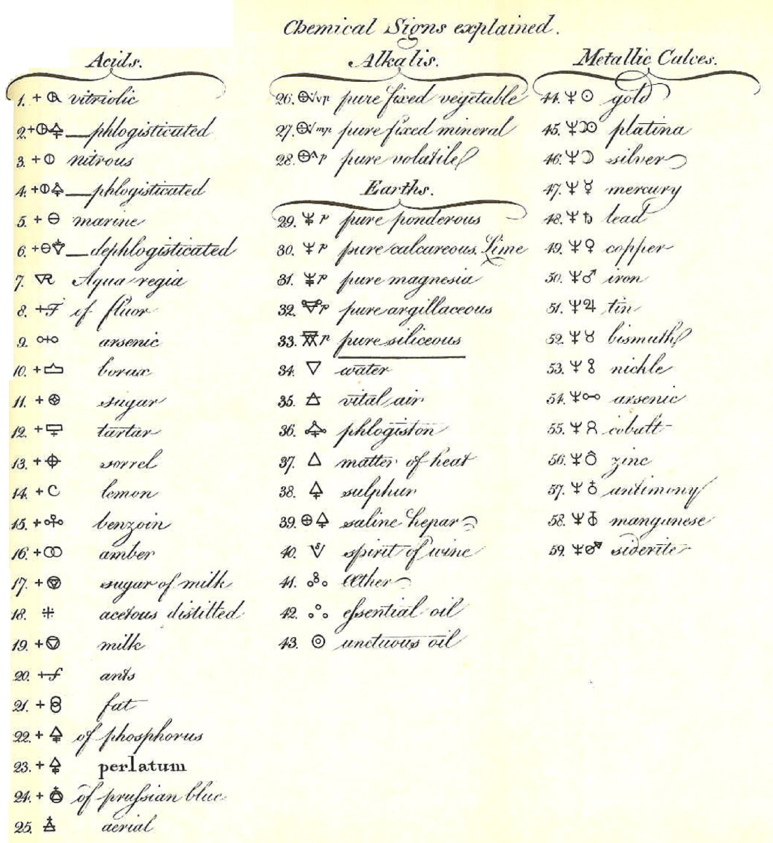
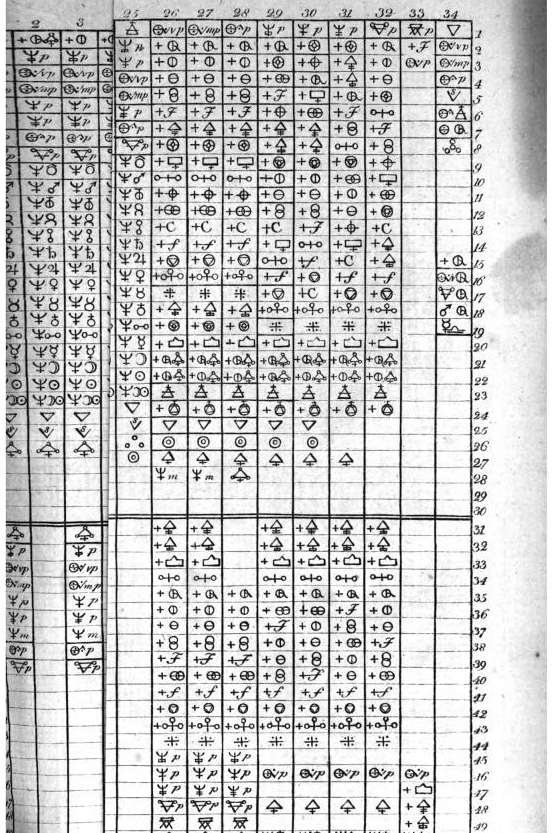
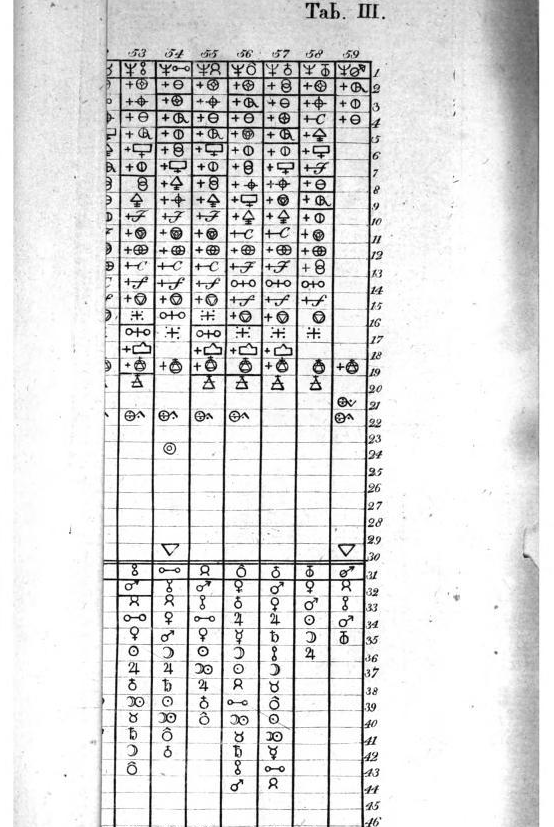
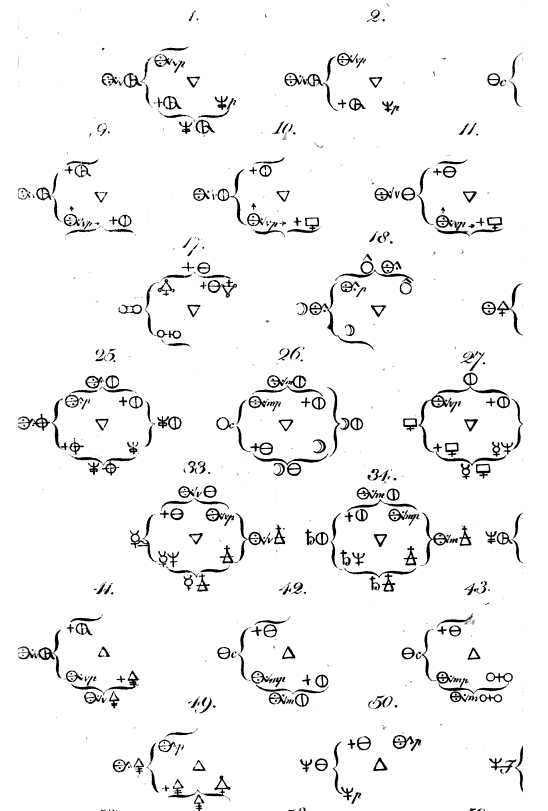
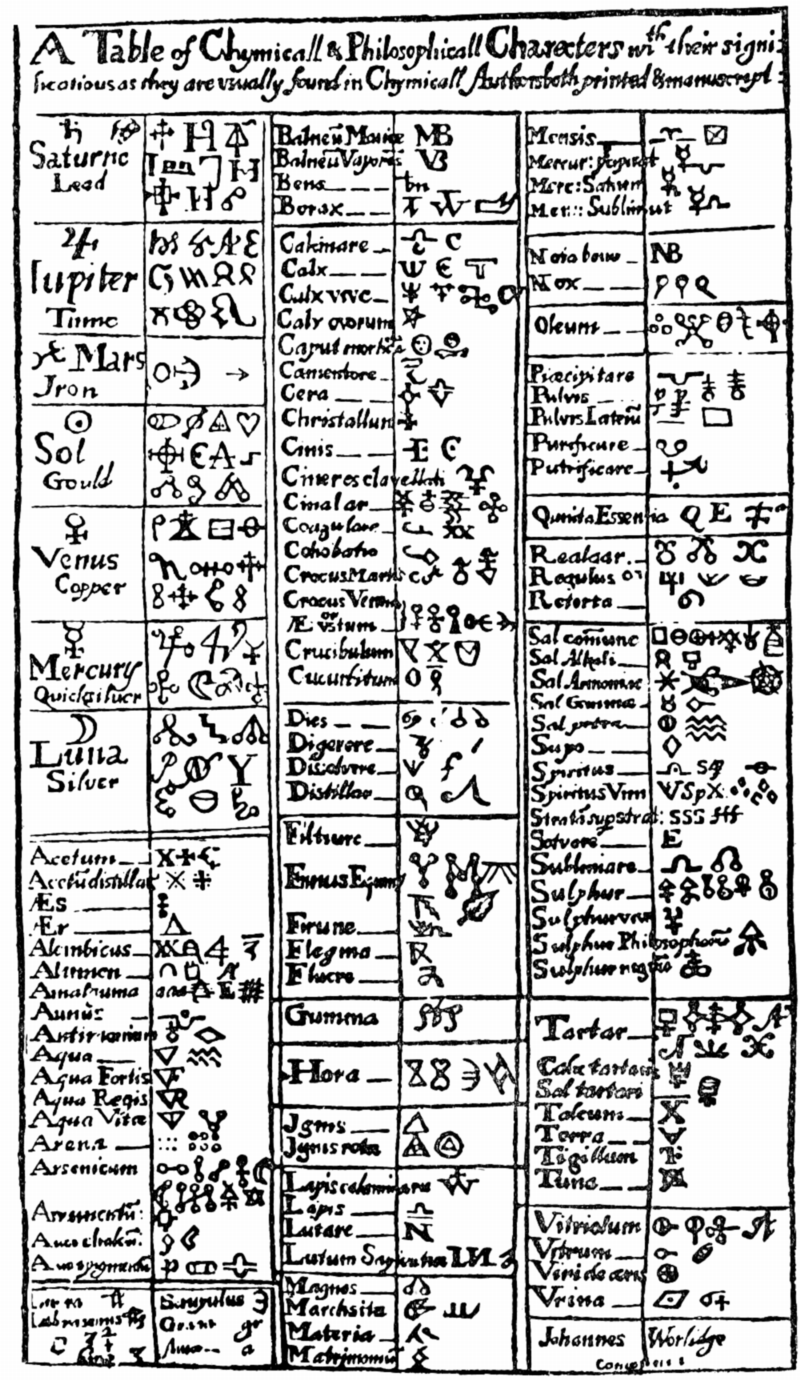
Valentinus' Table of Chymicall & Philosophicall Charecters
A table of alchemical symbols from Basilius Valentinus' (Basil Valentine) The Last Will and Testament:
1682
Digby's A Choice Collection of Rare Secrets
1687
Alchemical Emblem Showing the Four Classical Elements
From the German Photo Library Theosophie & Alchemie, a seventeenth century alchemical emblem showing the four classical elements (air, fire, earth & water) in the corners of the image, alongside the tria prima on the central triangle:
1690
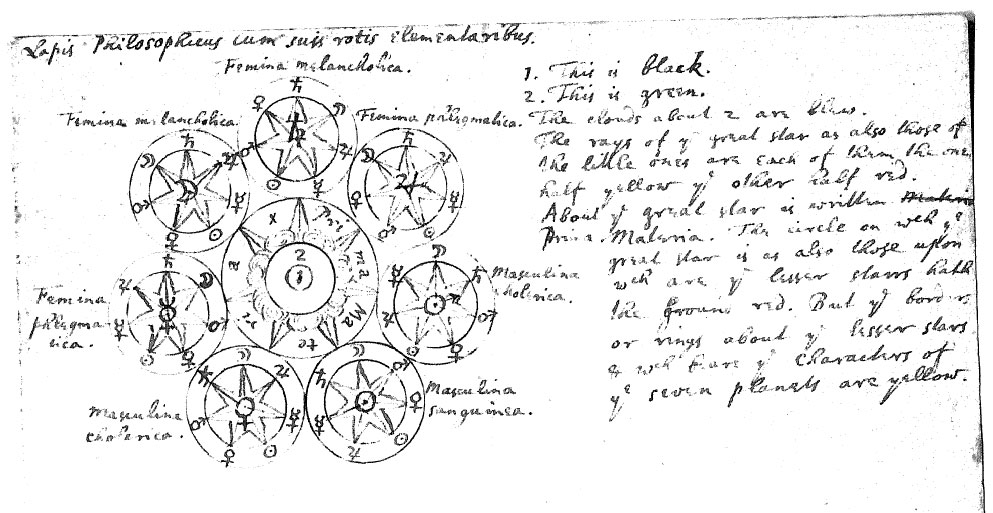
Newton's Lapis Philosophicus cum suis rotis elementaribus
In 1936 a collection of Newton's papers, amazingly regarded as of "no scientific value" when offered to Cambridge university some fifty years earlier, was purchased at Sotheby's by the respected economist and Newton scholar John Maynard Keynes. Originally left in a stack by Newton when he left his post as the director of the London mint in 1696, these documents had somehow fortuitously escaped the burning of Newton's personal writings arranged after his death, and were discovered two centuries later. Included was a handwritten manuscript entitled Lapis Philosophicus cum suis rotis elementaribus: "The philosophical stone elements with its wheels", Google Translate.
Notice how the design below also features in the Ripley Scroll, formulated in the mid-1400s. Newton is known to be influenced by this work:
1700
Elements Known in the Year 1700
Elements known in the year 1700, taken from this Wikipedia page:
1718
Geoffroy's Affinity Table
From Wikipedia, Étienne François Geoffroy's 1718 Affinity Table.
At the head of the column is a substance with which all the substances below can combine.
1735
Discovery of Cobalt
Co
Cobalt, atomic number 27, has a mass of 58.933 au.
Cobalt was first isolated in 1735 by G. Brandt.
1748
Discovery of Platinum
Pt
Platinum, atomic number 78, has a mass of 195.084 au.
Platinum was first isolated in 1748 by A. de Ulloa, although it had been used by pre-Colombian Americans.
1751
Discovery of Nickel
Ni
Nickel, atomic number 28, has a mass of 58.693 au.
Nickel was first isolated in 1751 by F. Cronstedt.
1753
Discovery of Bismuth
Bi
Bismuth, atomic number 83, has a mass of 208.98 au.
Bismuth was first isolated in 1753 by C.F. Geoffroy.
1766
Discovery of Hydrogen
H
Hydrogen, atomic number 1, has a mass of 1.008 au.
Hydrogen is the lightest element and by far the most abundant element in the universe: it makes up about about 90% of the universe by weight. Under standard conditions, hydrogen exists as a diatomic molecular gas, H2.
Hydrogen was first isolated and identified as an element in 1766 by H. Cavendish, although it was first made in 1500 by Paracelsus.
1771
Discovery of Oxygen
O
Oxygen, atomic number 8, has a mass of 15.999 au.
Oxygen exists as a diatomic molecular gas, O2; in this form it makes up about 20% of the atmosphere.
Oxygen was first isolated in 1771 by W. Scheele.
1772
Discovery of Nitrogen
N
Nitrogen, atomic number 7, has a mass of 14.007 au.
Nitrogen exists as a diatomic molecular gas, N2, and in this form it makes up about 78% of the atmosphere by volume. The element seemed so inert that Lavoisier named it azote, meaning "without life".
Nitrogen was first isolated in 1772 by D. Rutherford.
1774
Discovery of Chlorine
Cl
Chlorine, atomic number 17, has a mass of 35.452 au.
Chlorine exists as a green diatomic molecular gas, Cl2.
Chlorine was first isolated in 1774 by W. Scheele.
1774
Discovery of Manganese
Mn
Manganese, atomic number 25, has a mass of 54.938 au.
Manganese was first observed or predicted in 1774 by W. Scheele and first isolated in 1774 by G. Gahn.
1775
Bergman's Dissertation on Elective Affinities
Alchemical symbols in Torbern Bergman's 1775 Dissertation on Elective Affinities, which was translated from Latin to English in 1783 from Google Books:
1778
Diderot's Alchemical Chart of Affinities

1781
Discovery of Molybdenum
Mo
Molybdenum, atomic number 42, has a mass of 95.95 au.
Molybdenum was first observed or predicted in 1778 by W. Scheele and first isolated in 1781 by J. Hjelm.
1782
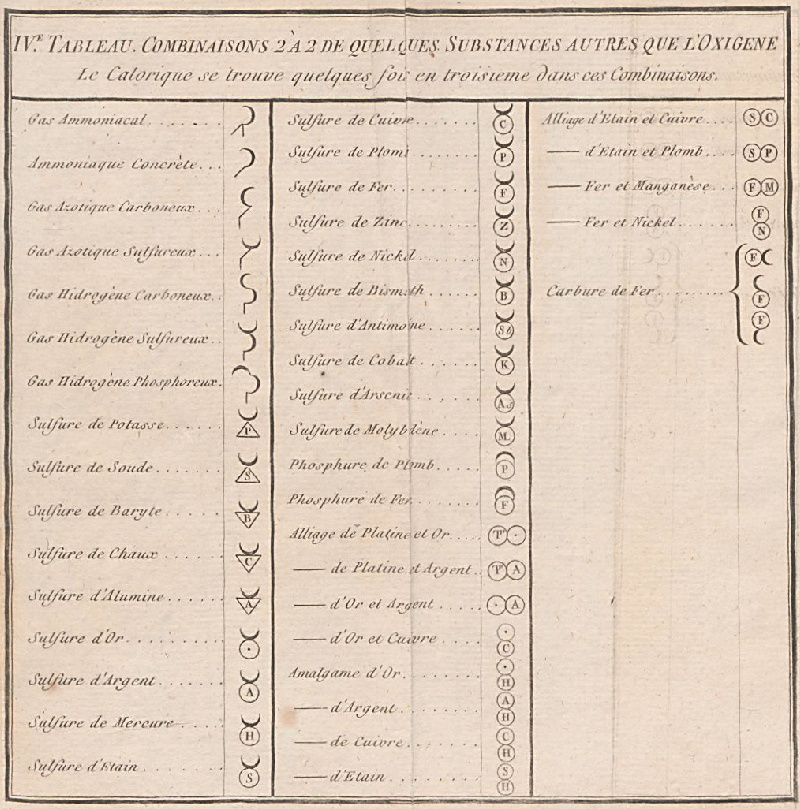
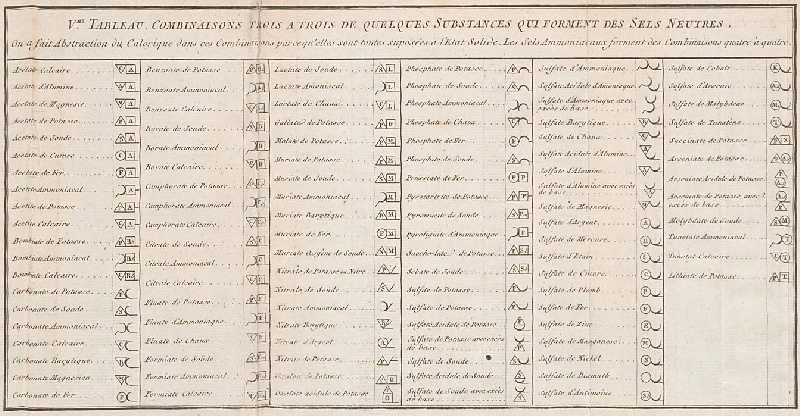
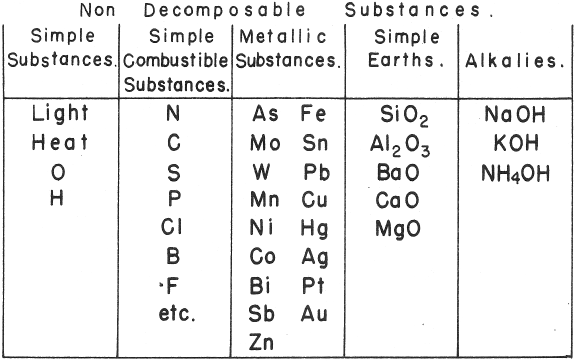
de Morveau's Table of Chemically Simple Substances
de Morveau's table of chemically simple substances (updated with modern representations by Mazurs):
1782
Discovery of Tellurium
Te
Tellurium, atomic number 52, has a mass of 127.6 au.
Tellurium caused great difficulty to the chemists who first tried to develop a periodic table, because it has an atomic weight greater than iodine (126.9). Mendeleev prioritised chemical properties over the anomalous atomic weight data, and correctly classified Te along with O, S, & Se. It was only when nuclear structure and the importance of atomic number was recognised, around 1918, that the issue was explained.
Tellurium was first isolated in 1782 by F.-J.M. von Reichenstein.
1783
Discovery of Tungsten
W
Tungsten, atomic number 74, has a mass of 183.84 au.
Tungsten was first observed or predicted in 1781 by W. Scheele and first isolated in 1783 by J. and F. Elhuyar.
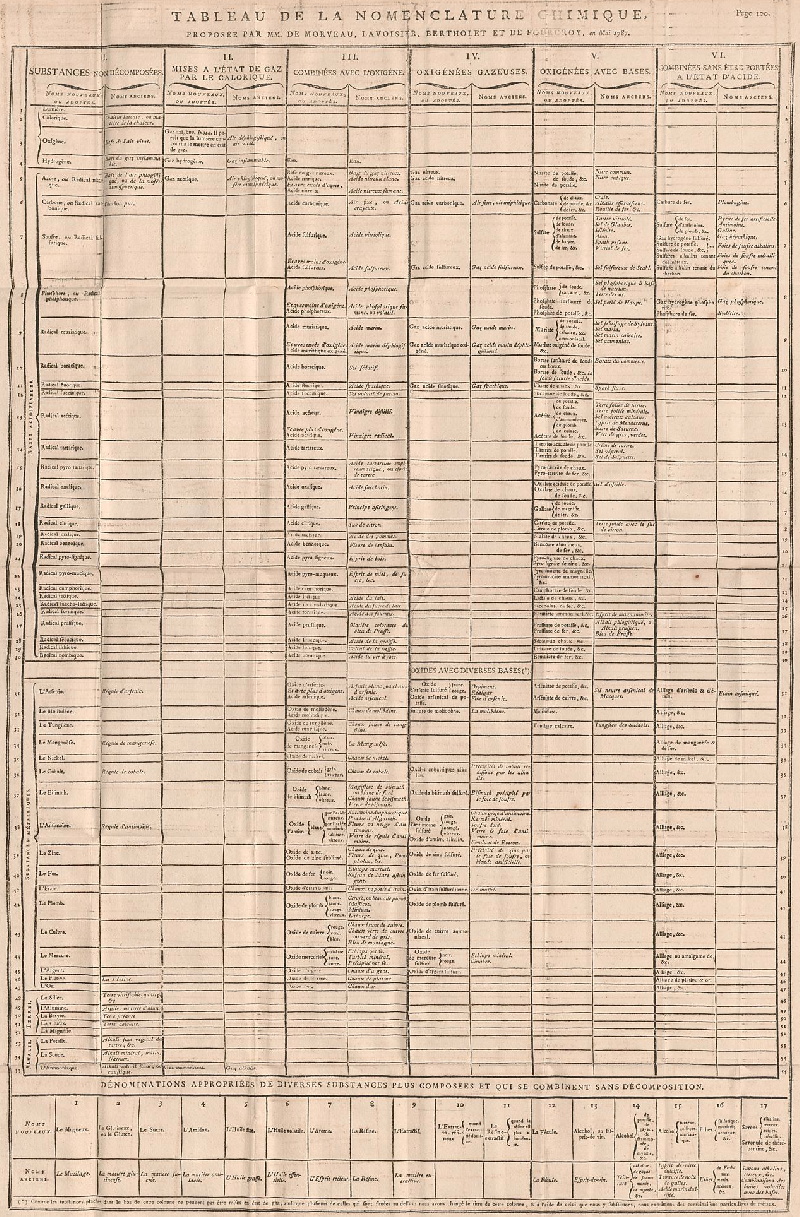
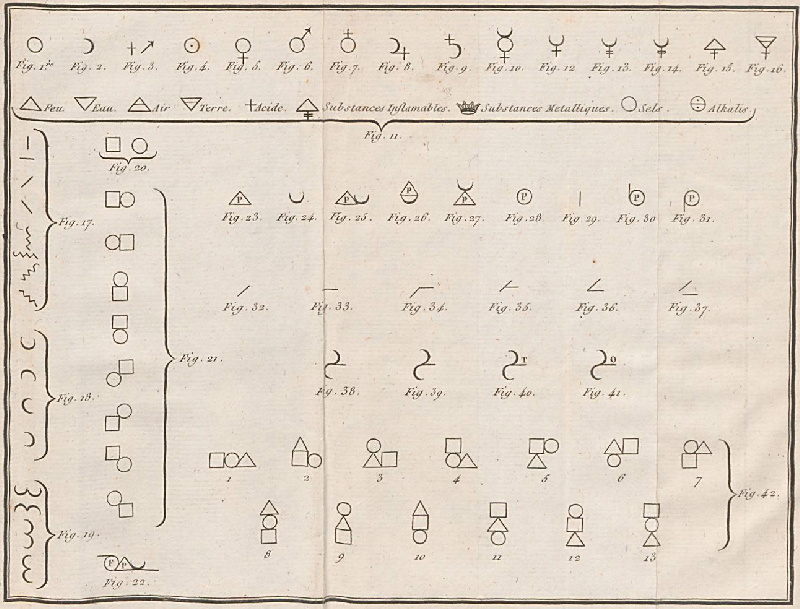
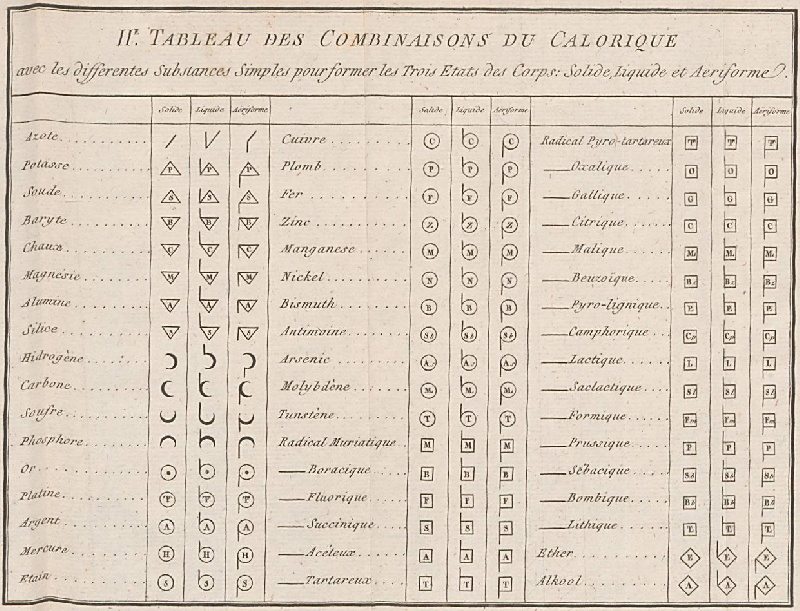
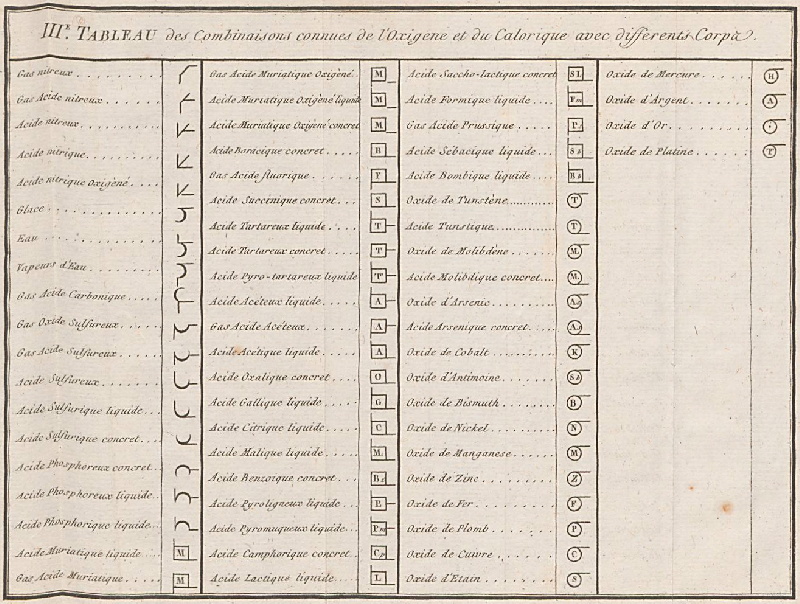
1787
Méthode de Nomeclature Chimique
By Louis Bernard Guyton de Morveau (1737-1816), Antoine Laurent Lavoisier (1743-1794) , Claude-Louis Berthollet (1748-1822) & Antoine-François de Fourcroy (1755-1809) a book: Méthode de Nomeclature Chimique.
The complete scanned book is available. (Click the 'page view' button, or here.)
The book lists the several hundred chemicals known at the time, including chemical elements, and it discusses the nomenclature (naming). Although not a periodic table as such, the information contained in this book was state of the art for 1787.
Click on an image below to enlarge.
1789
Antoine Lavoisier
Antoine Lavoisier produced a list chemical substances, that included the 23 known elements. He also refined the concept as before this time, metals - with the exception of mercury - were not considered to be elements. Wikipedia.
A list of 33 simple substances compiled by Lavoisier, from Traité Élémentaire de Chimie, Cuchet, Paris, 1789, p. 192:

From Peter van der Krogt's Elementymology & Elements Multidict web site:
| Lavoisier's Table of Simple Substances (1789) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
1789
Discovery of Zirconium
Zr
Zirconium, atomic number 40, has a mass of 91.224 au.
Zirconium was first observed or predicted in 1789 by H. Klaproth and first isolated in 1824 by J. Berzelius.
1789
Discovery of Uranium
U ![]()
Uranium, atomic number 92, has a mass of 238.029 au.
Radioactive element with a very long half-life.
Uranium was first observed or predicted in 1789 by H. Klaproth and first isolated in 1841 by E.-M. Péligot.
1791
Discovery of Titanium
Ti
Titanium, atomic number 22, has a mass of 47.867 au.
Titanium was first observed or predicted in 1791 by W. Gregor and first isolated in 1825 by J. Berzelius.
1794
Discovery of Yttrium
Y
Yttrium, atomic number 39, has a mass of 88.906 au.
Yttrium was first observed or predicted in 1794 by J. Gadolin and first isolated in 1842 by G. Mosander.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
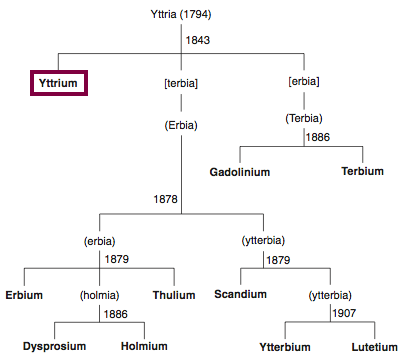
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1798
Discovery of Beryllium
Be
Beryllium, atomic number 4, has a mass of 9.012 au.
Beryllium is a metal with a high melting point. At ordinary temperatures it resists oxidation in air. Beryllium compounds are very toxic.
Beryllium was first observed or predicted in 1798 by N. Vauquelin and first isolated in 1828 by F. Wöhler and A. Bussy.
1798
Discovery of Chromium
Cr
Chromium, atomic number 24, has a mass of 51.996 au.
Chromium was first observed or predicted in 1797 by N. Vauquelin and first isolated in 1798 by N. Vauquelin.
1800
Elements Known in the Year 1800
Elements known in the year 1800, taken from this Wikipedia page:
1801
Discovery of Niobium
Nb
Niobium, atomic number 41, has a mass of 92.906 au.
Niobium was first observed or predicted in 1801 by C. Hatchett and first isolated in 1864 by W. Blomstrand.
1802
Discovery of Tantalum
Ta
Tantalum, atomic number 73, has a mass of 180.948 au.
Tantalum was first isolated in 1802 by G. Ekeberg.
1803
Dalton's Postulates About The Elements
Around the year 1803 in Manchester, John Dalton gave a series of lectures in which he presented his postulates:
- Elements are made of tiny particles called atoms.
- The atoms of a given element are different from those of any other element, and the atoms of different elements can be distinguished from one another by their respective relative atomic weigh/mass.
- All atoms of a given element are identical.
- Atoms of one element can combine with atoms of other elements to form chemical compounds, and a given compound always has the same relative numbers of types of atoms.
- Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process, and a chemical reaction simply changes the way atoms are grouped together.
From a very early notebook from around this time:

1803
Discovery of Palladium
Pd
Palladium, atomic number 46, has a mass of 106.42 au.
Palladium was first isolated in 1803 by H. Wollaston.
1803
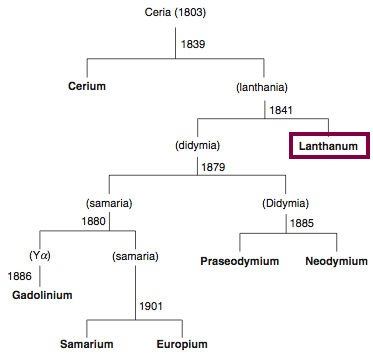
Discovery of Cerium
Ce
Cerium, atomic number 58, has a mass of 140.116 au.
Cerium was first observed or predicted in 1803 by H. Klaproth, J. Berzelius, and W. Hisinger and first isolated in 1838 by G. Mosander.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
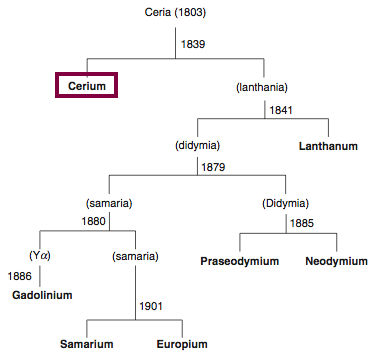
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1803
Discovery of Osmium
Os
Osmium, atomic number 76, has a mass of 190.23 au.
Osmium was first isolated in 1803 by S. Tennant.
1803
Discovery of Iridium
Ir
Iridium, atomic number 77, has a mass of 192.217 au.
Iridium was first isolated in 1803 by S. Tennant.
1804
Discovery of Rhodium
Rh
Rhodium, atomic number 45, has a mass of 102.906 au.
Rhodium was first isolated in 1804 by H. Wollaston.
1807
Discovery of Sodium
Na
Sodium, atomic number 11, has a mass of 22.99 au.
Sodium is a Group 1 element, and these are often referred to as the "alkali metals".
Sodium was first isolated in 1807 by H. Davy.
1807
Discovery of Potassium
K
Potassium, atomic number 19, has a mass of 39.098 au.
Potassium is a Group 1 element, and these are often referred to as the "alkali metals".
Potassium was first isolated in 1807 by H. Davy.
1808
Dalton's Elements
Two pages from John Dalton's A New System of Chemical Philosophy in which he proposed his version of atomic theory based on scientific experimentation (see the scanned book, page 219):
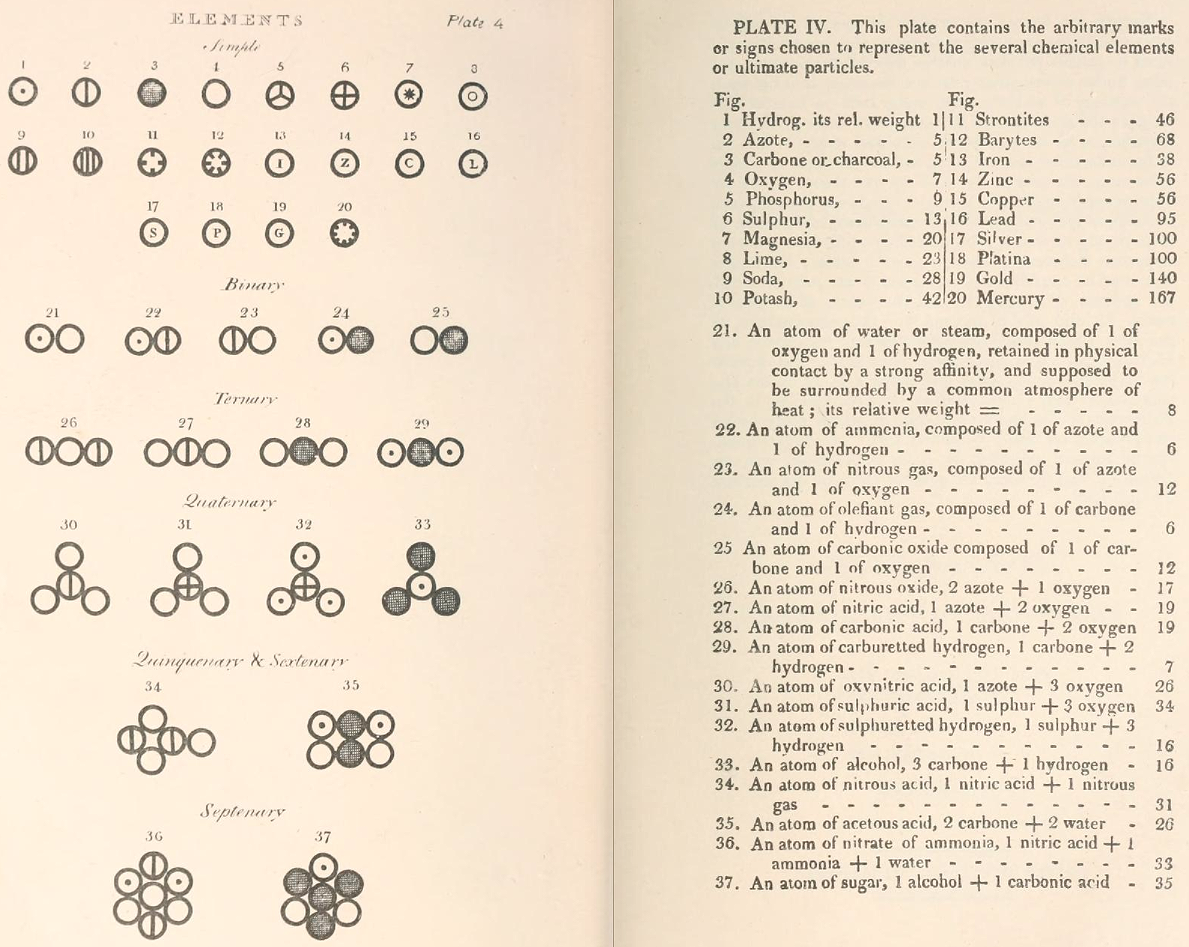
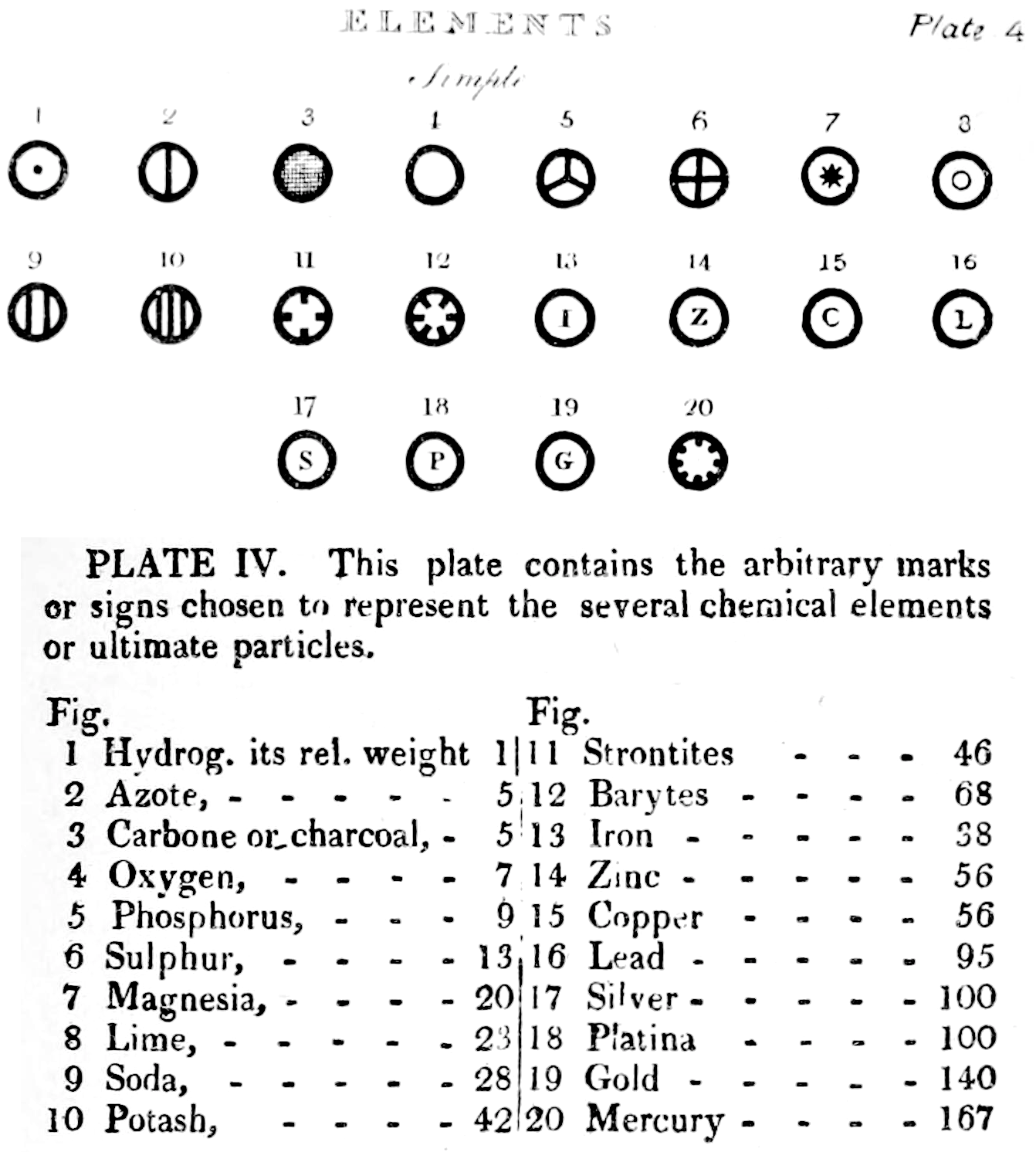
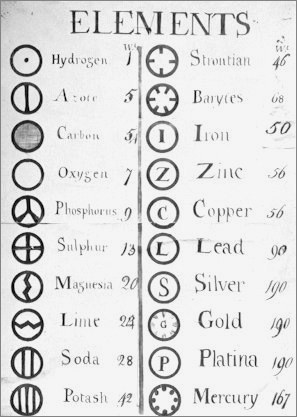
| Name | Modern Symbol | Dalton's Data | Modern Values | % error |
| Hydrog. | H | 1 | 1 | 0% |
| Azote | N | 5 | 14 | -180% |
| Carbone | C | 5 | 12 | -140% |
| Oxygen | O | 7 | 16 | -129% |
| Phosphorus | P | 9 | 31 | -244% |
| Sulphur | S | 13 | 32.1 | -147% |
| Magnesia | Mg | 20 | 24.3 | -22% |
| Lime | Ca | 24 | 40.1 | -67% |
| Soda | Na | 28 | 23 | 18% |
| Potash | K | 42 | 39.1 | 7% |
| Strontites | Sr | 46 | 87.6 | -90% |
| Barytes | Ba | 68 | 137.3 | -102% |
| Iron | Fe | 50 | 55.8 | -12% |
| Zinc | Zn | 56 | 65.4 | -17% |
| Copper | Cu | 56 | 63.5 | -13% |
| Lead | Pb | 90 | 200.6 | -123% |
| Silver | Ag | 190 | 107.9 | 43% |
| Gold | Au | 190 | 197 | -4% |
| Platina | Pt | 190 | 195.1 | -3% |
| Mercury | Hg | 167 | 200.6 | -20% |
- Dalton states that he is considering "chemical elements or ultimate particles"
- Dalton assigns hydrogen as having a relative weight of 1.
- Note the seemingly huge % errors in the atomic weights, compared with modern values.
- These errors occurred because while Dalton had deduced that atoms combine in fixed (stoichiometric) ratios in compounds, he not always know what the ratios were. Thus there were two unknowns: the atomic weights (masses) and the stoichiometric ratios.
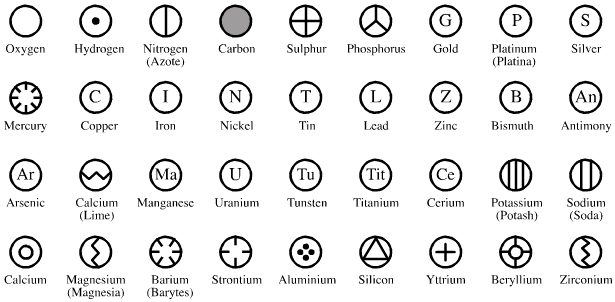
By Mark Leach
1808
Discovery of Boron
B
Boron, atomic number 5, has a mass of 10.814 au.
Boron has properties that are borderline between metal and non-metal (semimetallic).
Boron was first observed or predicted in 1808 by L. Gay-Lussac and L.J. Thénard and first isolated in 1808 by H. Davy.
1808
Discovery of Magnesium
Mg
Magnesium, atomic number 12, has a mass of 24.306 au.
Magnesium is a Group 2 element, and these are called: "alkaline earth metals".
Magnesium was first observed or predicted in 1755 by J. Black and first isolated in 1808 by H. Davy.
1808
Discovery of Calcium
Ca
Calcium, atomic number 20, has a mass of 40.078 au.
Calcium is a Group 2 element, and these are called: "alkaline earth metals".
Calcium was first isolated in 1808 by H. Davy.
1808
Discovery of Strontium
Sr
Strontium, atomic number 38, has a mass of 87.62 au.
Strontium is a Group 2 element, and these are called: "alkaline earth metals".
Strontium was first observed or predicted in 1787 by W. Cruikshank and first isolated in 1808 by H. Davy.
1808
Discovery of Barium
Ba
Barium, atomic number 56, has a mass of 137.327 au.
Barium is a Group 2 element, and these are called: "alkaline earth metals".
Barium was first observed or predicted in 1772 by W. Scheele and first isolated in 1808 by H. Davy.
1811
Discovery of Iodine
I
Iodine, atomic number 53, has a mass of 126.904 au.
Iodine exists as a black diatomic molecular solid, I2.
Iodine was first isolated in 1811 by B. Courtois.
1813
Wollaston's Slide Rule of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S. – or from here – has a diagram for a slide rule of chemical equivalents:
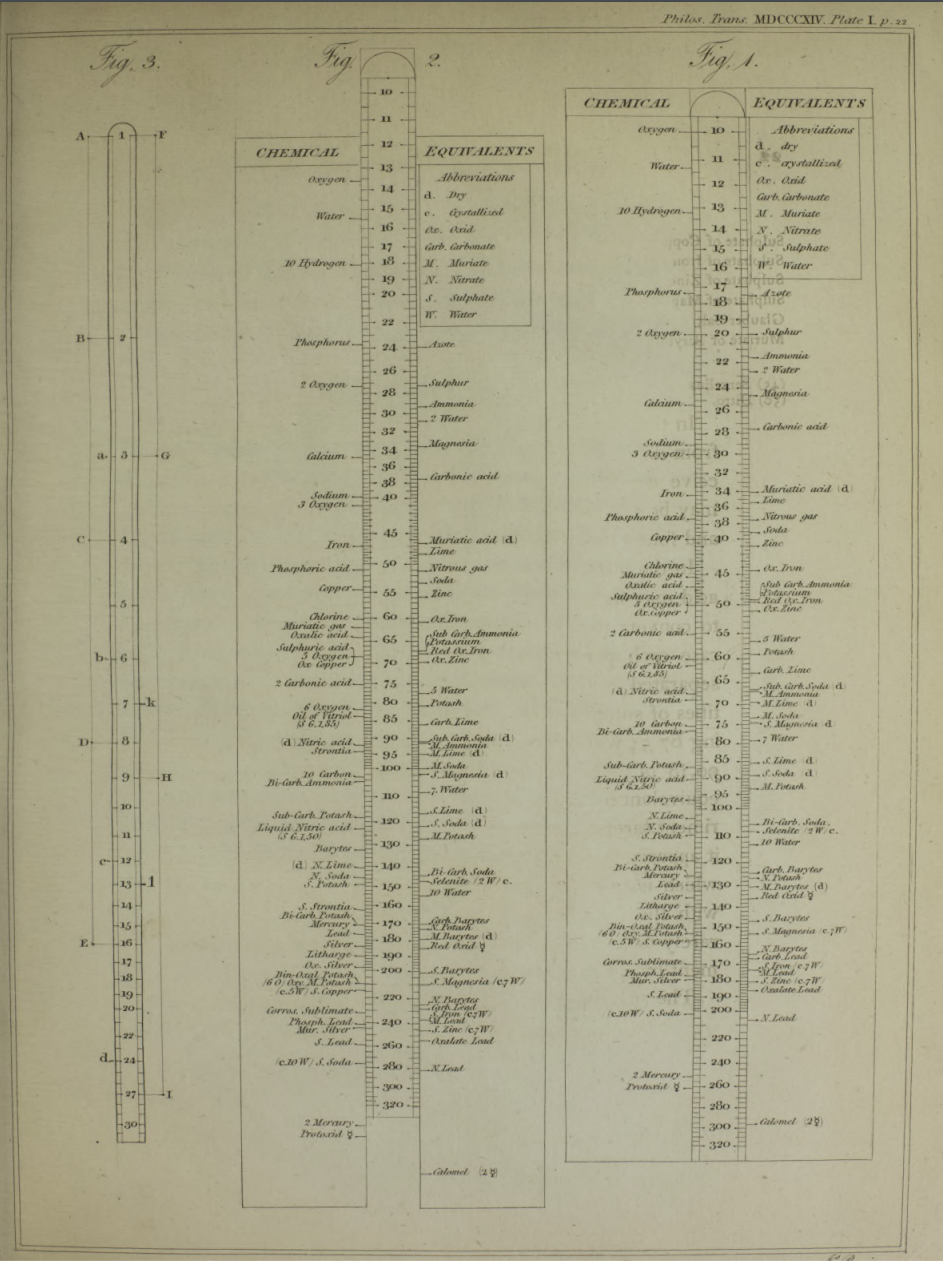
Wollaston writes:
"In order to shew more clearly the use of this scale, the Plate [diagram of the chemical slide rule] exhibits two different situations of the slider, in one of which oxygen is 10 [oxygen is defined as having an atomic weight/mass of 10.00], and other bodies are in their due proportion to it, so that carbonic acid being 27,54, and lime 35,46, carbonate of lime is placed at 63.
"In the second figure, the slider is represented drawn upwards till 100 corresponds to muriate of soda [sodium chloride, NaCl]; and accordingly the scale then shews how much of each substance contained in the table is equivalent to 100 of common salt. It shews, with regard to the different views of the analysis of this salt, that it contains 46,6 dry muriatic acid [hydrogen chloride], and 53,4 of soda, or 39,8 sodium, and 13,6 oxygen; or if viewed as chlorid of sodium, that it contains 60,2 chlorine, and 39,8 sodium."
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
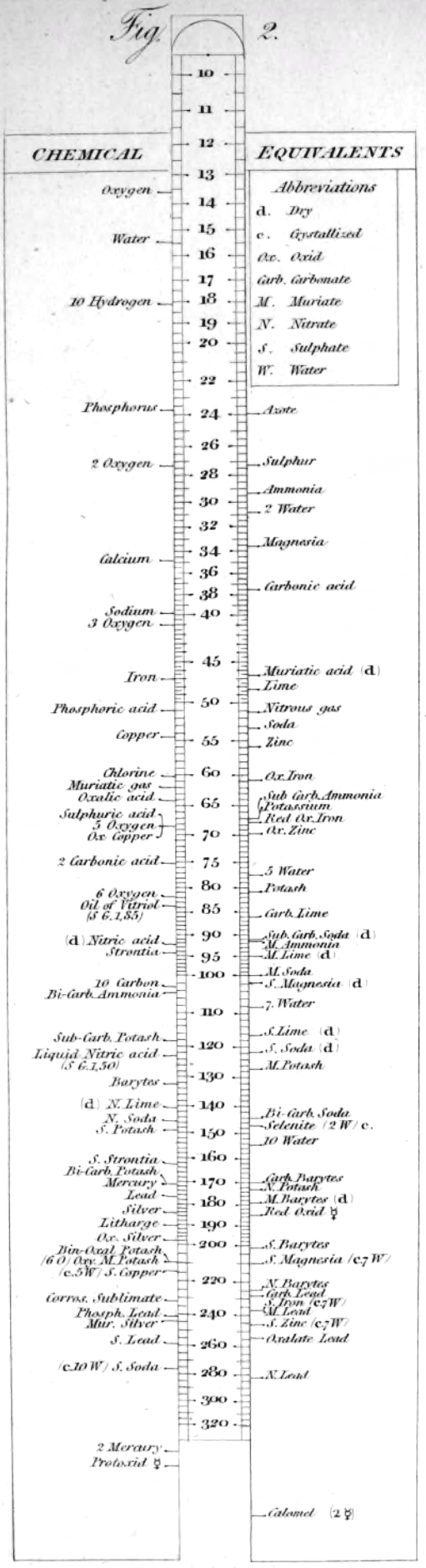
1813
Wollaston's Synoptic Scale of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S., or from here.
It is apparent that chemistry the years 1810 to 1850 was largely concerned with discovering the whole number stoichiometric ratios of atoms in chemical compounds.
Wollaston writes in the text above:
"It is impossible in several instances, where only two combinations of the same ingredients are known, to discover which of the compounds is to be regarded as consisting of a pair of single atoms, and since the decision of these questions is purely theoretical, and by no means necessary to the formation of a table adapted to most practical purposes, I have not been desirous of warping my numbers according to an atomic theory, but have endeavored to make practical convenience my sole guide, and have considered the doctrine of simple multiples, on which that of atoms is founded, merely as a valuable assistant in determining, by simple division, the amount of those quantities that are liable to such definite deviations from the original law of Richter."
"Mr. Dalton in his atomic views of chemical combination appears not to have taken much pains to ascertain the actual prevalence of that law of multiple proportions by which the atomic theory is best supported [however] it is in fact to Mr. Dalton that we are indebted for the first correct observation of such an instance of a simple multiple in the union of nitrous gas with oxygen."
"[I have] computed a series of supposed atoms, I [have] assumed oxygen as the decimal unit of my scale [ie. oxygen = 10], in order to facilitate the estimation of those numerous combinations which it forms with other bodies. Though the present table of Equivalents, I have taken care to make oxygen equally prominent on account of the important part it performs in determining the affinities of bodies by the different proportions in which it is united to them.."
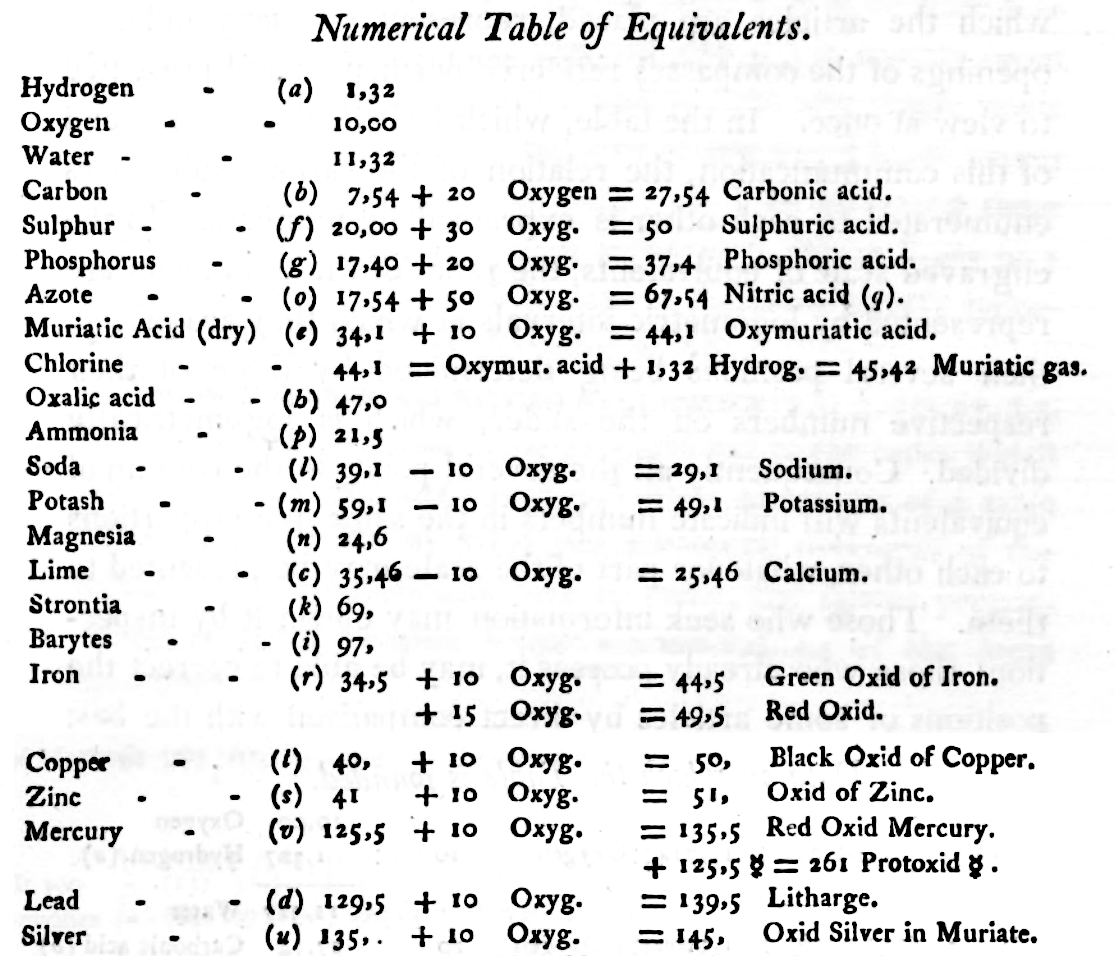
Mark Leach writes:
"When Wollaston's equivalent weights are converted from O = 10.00 to the modern value of O = 15.999, the atomic weight values can be seen to be astonishingly accurate.
"However, the language of the article is quite difficult as the meaning of many of the terms is unclear (to me, at least). For example, in modern usage adding 'ia' to a metal implies the oxide: 'magnesia' is magnesium oxide, MgO. I am not clear if this historical usage is consistent. 'Azote' is nitrogen and 'muriatic acid (dry)' is hydrogen chloride gas. I have only analyses/re-calculated the elements and a couple of common/obvious compounds:"
| Wollaston's data | Scaled to O = 15.999 | Modern Values | % error | |
| H (as H2) | 1.32 | 2.112 | 2.016 | 5% |
| O | 10.00 | 15.999 | 15.999 | ref. value |
| H2O | 11.32 | 18.111 | 18.015 | 1% |
| C | 7.74 | 12.383 | 12.011 | 3% |
| S | 20.00 | 31.998 | 32.060 | 0% |
| P | 17.40 | 27.838 | 30.974 | -11% |
| N (as N2) | 17.54 | 28.062 | 28.014 | 0% |
| Cl (as Cl2) | 44.10 | 70.556 | 70.900 | 0% |
| Fe | 34.50 | 55.197 | 55.845 | -1% |
| Cu | 40.00 | 63.996 | 63.546 | 1% |
| Zn | 41.00 | 65.596 | 65.380 | 0% |
| Hg | 125.50 | 200.787 | 200.590 | 0% |
| Pb | 129.50 | 207.187 | 207.980 | 0% |
| Ag | 135.00 | 215.987 | 107.870 | 50% |
- The elements hydrogen, nitrogen (azote) and chlorine have clearly been measured as the diatomic molecules, even if this was unknown to Wollaston in 1813.
- Phosphorus is out by 11%... [fair enough].
- Only silver is clearly wrong, but it is out by 50% so it looks like a simple stoichiometry error: Perhaps the oxide was assumed to be AgO was instead of the correct Ag2O.
Interestingly, Wollaston's analysis is far better than Daubeny's 1831 data seen in Oxford.
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
1814
Wollaston's Physical Slide Rule of Chemical Equivalents
From the Science Museum in the UK collection, the Wollaston slide rule of chemical equivalents:
"Three sliding scales of chemical equivalents, all with same manuscripts marks, published by W Cary, devised by W H Wollaston, a leading chemist and natural philosopher during the early 19th century.
"Positioning the slider with the weight of the substance set against it will show you the weights of other substances which will react with it. This fundamental ordering based on measurement paved the way for the periodic table of the elements"
Wollaston uses a decimal scale in which oxygen is defined as having an atomic weight (relative atomic mass) of 10.00 rather than the modern value of 15.999.
Read more here and here, and an entry concerning chemical slide rules:

Mark Leach writes:
"I have edited the image above, setting the scale to zero:"

1817
Discovery of Lithium
Li
Lithium, atomic number 3, has a mass of 6.968 au.
Lithium is a reactive metal, of low density: it is the least dense metal.
Lithium was first observed or predicted in 1817 by A. Arfwedson and first isolated in 1821 by W. T. Brande.
1817
Discovery of Selenium
Se
Selenium, atomic number 34, has a mass of 78.971 au.
Selenium was first isolated in 1817 by J. Berzelius and G. Gahn.
1817
Discovery of Cadmium
Cd
Cadmium, atomic number 48, has a mass of 112.414 au.
Cadmium was first isolated in 1817 by S. L Hermann, F. Stromeyer and J.C.H. Roloff.
1824
Discovery of Silicon
Si
Silicon, atomic number 14, has a mass of 28.085 au.
Silicon makes up 25.7% of the earth's crust, and after oxygen is the second most abundant element.
Silicon was first isolated in 1823 by J. Berzelius.
1825
Discovery of Aluminium (Aluminum)
Al
Aluminium (aluminum), atomic number 13, has a mass of 26.982 au.
Aluminum is a silvery-white metal.
Aluminium was first isolated in 1825 by H.C.Ørsted.
1825
Discovery of Bromine
Br
Bromine, atomic number 35, has a mass of 79.904 au.
Bromine exists as an orange diatomic molecular liquid, Br2.
Bromine was first isolated in 1825 by J. Balard and C. Löwig.
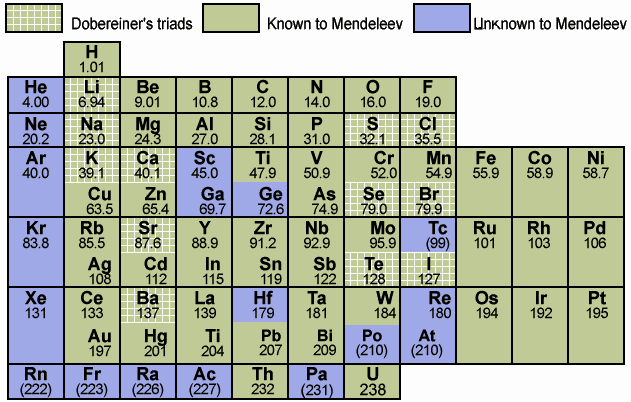
1829
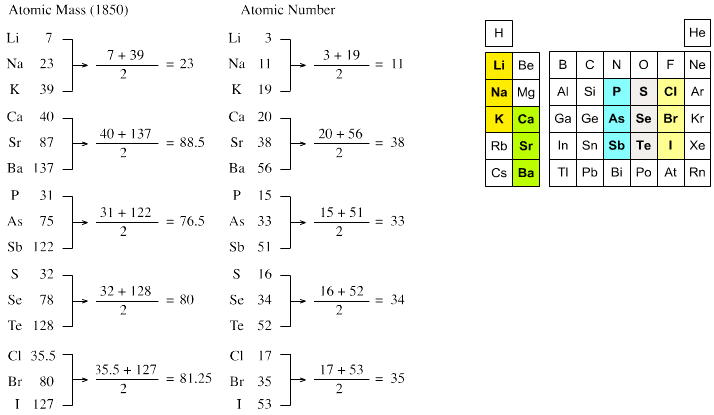
Döbereiner's Triads
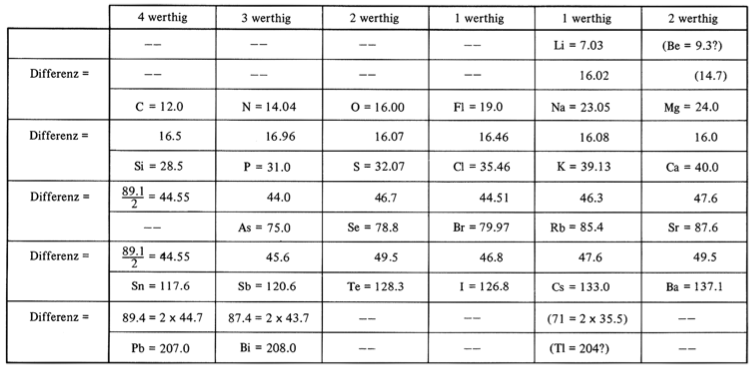
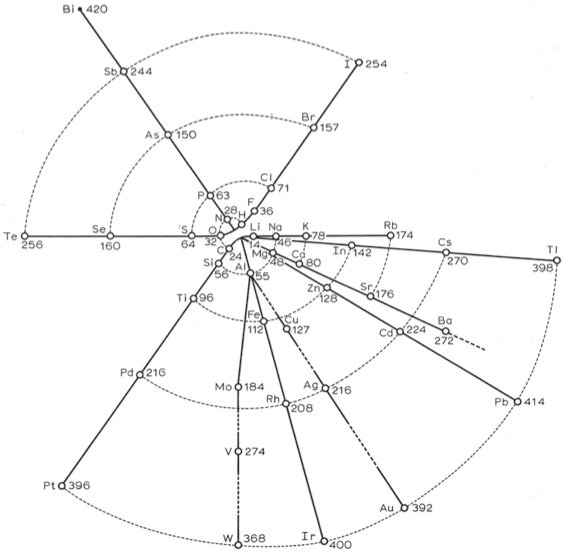
Johann Döbereiner found triads: a sequence of three similar elements, where the middle element has a mass equal to the average of the least and most massive.
A brief biography can be found on the Nature website.
Döbereiner writes in An Attempt to Group Elementary Substances according to Their Analogies (in English)
From Poggendorf's Annalen der Physik und Chemie 15, 301-7 (1829) (in German) [from Henry M. Leicester & Herbert S. Klickstein, eds., A Source Book in Chemistry, 1400-1900 (Cambridge, MA: Harvard, 1952)]:
"The work of Berzelius on the determination of the atomic weights of bromine and iodine has interested me greatly, since it has established the idea, which I expressed earlier in my lectures, that perhaps the atomic weight of bromine might be the arithmetical mean of the atomic weights of chlorine and iodine. This mean is (35.470+126.470)/2 = 80.470. This number is not much greater than that found by Berzelius (78.383); however, it comes so close that it may almost be hoped that the difference will vanish entirely after repeated careful and exact determinations of the atomic weights of these three salt-forming elements. This idea was the motive for an attempt which I made twelve years ago to group substances by their analogies."
[Note: L&K noticed an error in the above math: (35.47 + 126.47)/2 = 80.97 not 80.47. Whoops...]

The diagram below uses mid-nineteenth century atomic mass information rather than modern data. If atomic numbers (Z) are used (a property unknown in 1850), the triads are exact:
1829
Discovery of Thorium
Th ![]()
Thorium, atomic number 90, has a mass of 232.038 au.
Radioactive element with a very long half-life.
Thorium was first observed or predicted in 1829 by J. Berzelius and first isolated in 1914 by D. Lely, Jr. and L. Hamburger.
1830
Discovery of Vanadium
V
Vanadium, atomic number 23, has a mass of 50.942 au.
Vanadium was first observed or predicted in 1801 by M. del Río and first isolated in 1830 by N.G.Sefström.
1831
Daubeny's Teaching Display Board & Wooden Cubes of Atomic Weights
The Museum of the History of Science, Oxford, has a display of Charles Daubeny's teaching materials, including a black painted wooden board with "SYMBOLS OF SIMPLE BODIES": showing symbols, atomic weights and names of elements in two columns, and a small pile of cubes with element symbols.
Charles Daubeny and Chemistry at the Old Ashmolean
Charles Daubeny (1795-1867) was appointed Aldrichian Professor of Chemistry at Oxford in 1822. In 1847 he moved from the original laboratory in this basement [in the museum] to a new one built at his own expense at the Botanic Garden. His apparatus went with him and was preserved there. Daubeny actively campaigned for the teaching of science in Oxford and held several professorships in addition to chemistry. He also conducted research on subjects such as photosynthesis.
From the HSM Database (Inventory no. 17504):
DAUBENY'S LIST OF ATOMIC WEIGHTS Wooden panel, black with white lettering, listing in two columns the symbols and names of twenty elements. This lecture board is identical to the table in the third edition (1831) of E. Turner, 'Elements of Chemistry', apart from the atomic weight for bromine. Daubeny wrote a useful 'Introduction to the Atomic Theory' (published in three versions: 1831, 1840, and 1850), the first edition of which also quotes Turner's table. Probably contemporary with this lecture board are the wooden cubes with the symbols for certain elements.
The period from 1810 to 1860 was crucial in the development of the periodic table. Most of the main group and transition elements had been discovered, but their atomic weights and stoichiometries (combining ratios) had not been fully deduced. Oxygen was assumed to have a weight of 6, and consequently carbon is assumed to have a mass of 6.
Daubeny's element symbols and weights – along with the modern mass data – are tabulated:
| Symbol | Daubeny's Weight | Modern Mass Data | % error | Stoichiometry Error |
| H | 1 | 1 | 0% | |
| C | 6 | 12 | -100% | factor of 2 |
| O | 8 | 16 | -100% | factor of 2 |
| Si | 8 | 28.1 | -251% | factor of 5 (?) |
| Al | 10 | 27 | -170% | factor of 3 |
| Mg | 12 | 24.3 | -103% | factor of 2 |
| N | 14 | 14 | 0% | |
| S | 16 | 32.1 | -101% | factor of 2 |
| P | 16 | 31 | -94% | factor of 2 |
| Fl | 19 | 19 | 0% | |
| Ca | 20 | 40.1 | -101% | factor of 2 |
| Na | 24 | 23 | 4% | |
| Fe | 28 | 55.8 | -99% | factor of 2 |
| Cl | 36 | 35.5 | 1% | |
| K | 40 | 39.1 | 2% | |
| Cu | 64 | 63.5 | 1% | |
| B | 80 | 79.9 | 0% | |
| Pb | 104 | 207 | -99% | factor of 2 |
| I | 124 | 127 | -2% | |
| Hg | 200 | 200.6 | 0% |
While quite a number of weights are close to the modern values, many are way out. However, the error is usually a stiotoimetric factor error.
From the HSM Database (Inventory no. 33732): SET OF WOODEN CUBES ILLUSTRATING ATOMIC WEIGHTS
Forty-two wooden cubes numbered 1-42, painted black with symbols for certain elements, compounds or radicals painted in white on the faces, together with the corresponding atomic, molecular or radical weights. The face markings appear in various combinations:
| H | C | P | Na | Ca° | S | N | K | Fe | K | Na° | Cy | K° |
| 1 | 6 | 16 | 24 | 28 | 16 | 14 | 40 | 28 | 48 | 32 | 26 | 48 |
A typical cube (no. 3) may be represented by the following figure. They present something of an enigma as their faces do not form an obvious pattern. The numbers indicate that there were 42 cubes. In style they are similar to the figures on the panel of atomic weights.
The cubes are listed in Daubeny's 1861 catalogue, p. 11 as: "Wooden cubes for illustrating atomic weight". [See D. R. Oldroyd, The Chemical Lectures at Oxford (1822-1854) of Charles Daubeny, M.D., F.R.S. Notes and Records of the Royal Society, vol. 33 (1979), pp. 217-259.]
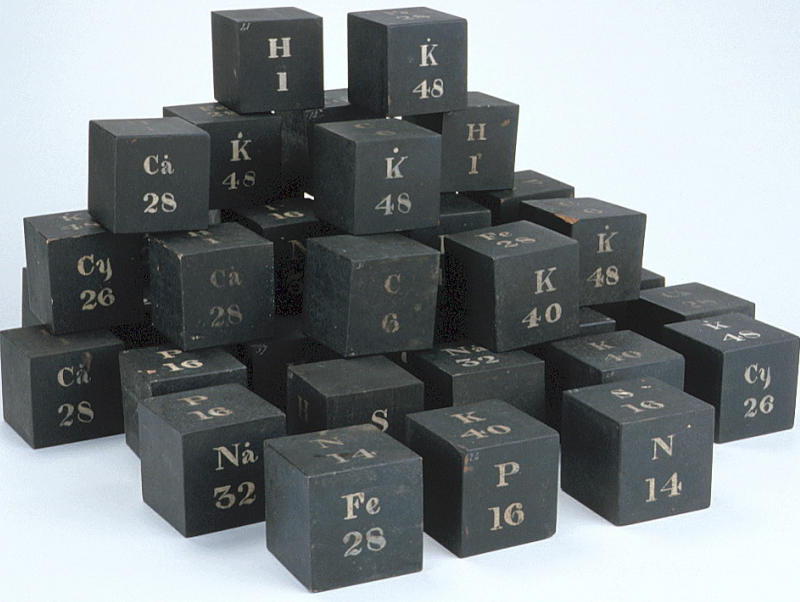
This display was spotted by Eric Scerri who was visiting the museum with Mark Leach in 2010.
There is a virtual tour on the museum, and the above display is in the basement.
1836
Berzelius' Electronegativity Table
Berzelius' electronegativity table of 1836.
The most electronegative element (oxygen or Sauerstoff) is listed at the top left and the least electronegative (potassium or Kalium) lower right. The line between hydrogen (Wasserstoff) and gold seperates the predomently electronegative elements from the electropositive elements. Page 17 and ref. 32 from Bill Jensen's Electronegativity from Avogadro to Pauling Part I: Origins of the Electronegativity Concept, J. Chem. Educ., 73, 11-20 (1996):

1838
Discovery of Lanthanum
La
Lanthanum, atomic number 57, has a mass of 138.905 au.
Lanthanum was first observed or predicted in 1838 by G. Mosander and first isolated in 1841 by G. Mosander.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1842
Discovery of Terbium
Tb
Terbium, atomic number 65, has a mass of 158.925 au.
Terbium was first observed or predicted in 1842 by G. Mosander and first isolated in 1886 by J.C.G. de Marignac.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
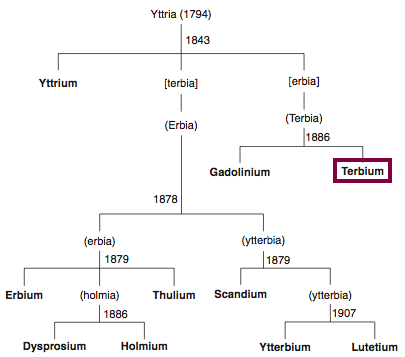
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1842
Discovery of Erbium
Er
Erbium, atomic number 68, has a mass of 167.259 au.
Erbium was first observed or predicted in 1842 by G. Mosander and first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
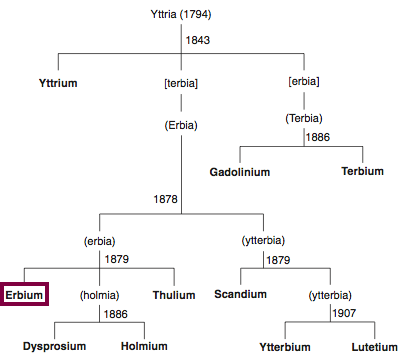
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1843
Gmelin's System
L. Gmelin, Handbuch der chemie, 4th ed., Heidelberg, 1843, vol. 1, p. 457 (Many thanks to Carmen Giunta for the ref. update & link.)
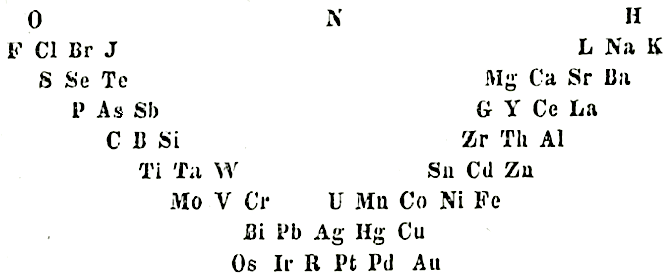
The early and important Gmelin formulation redrawn by Mark Leach with modern element symbols:
- J → I
- G → Be
- L → Li
- R → Rh
1844
Discovery of Ruthenium
Ru
Ruthenium, atomic number 44, has a mass of 101.07 au.
Ruthenium was first isolated in 1844 by K. Claus.
1850
Elements Known in the Year 1850
Elements known in the year 1850, taken from this Wikipedia page:
1858
Cannizzaro's Letter
Letter of Professor Stanislao Cannizzaro to Professor S. De Luca: Sketch of a Course of Chemical Philosophy given in the Royal University of Genoa, Il Nuovo Cimento, vol. vii. (1858), pp. 321-366.

Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about, and link to, Cannizzaro's Letter. See a list of other classic chemistry papers.
"I believe that the progress of science made in these last years has confirmed the hypothesis of Avogadro, of Ampère, and of Dumas on the similar constitution of substances in the gaseous state; that is, that equal volumes of these substances, whether simple or compound, contain an equal number of molecules: not however an equal number of atoms, since the molecules of the different substances, or those of the same substance in its different states, may contain a different number of atoms, whether of the same or of diverse nature."
From the Science History of Science Institute:
"In 1858 Cannizzaro outlined a course in theoretical chemistry for students at the University of Genoa,where he had to teach without benefit of a laboratory. He used the hypothesis of a fellow Italian, Amedeo Avogadro, who had died just two years earlier, as a pathway out of the confusion rampant among chemists about atomic weights and the fundamental structure of chemical compounds."
Mark Leach writes:
"Before a periodic table of the chemical elements – which orders the elements by atomic weight and then groups them by property – could be developed it was necessary to know the atomic weight values. However, to deduce the atomic weights was a problem as it was necessary to know the ratios of how the elements combined, the stoichiometry.
"Tables of atomic weight data by Dalton (1808), Wollaston (1813) and Daubeny (1831) show progress, but the 1858 Cannizzaro letter was the first where the atomic weight data is more or less both complete and accurate.
"I have extracted the element atomic weight data from the paper, and given the % error with respect to modern atomic weight/mass data. Only titanium is significantly out! It is clear that Cannizzaro knew that hydrogen, nitrogen, oxygen, chlorine, bromine & iodine existed as diatomic molecules."
| Element | Symbol | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H | 1 | 1.008 | -0.8% |
| Boron | B | 11 | 10.81 | 1.7% |
| Carbon | C | 12 | 12.011 | -0.1% |
| Nitrogen | N | 14 | 14.007 | 0.0% |
| Oxygen | O | 16 | 15.999 | 0.0% |
| Sodium | Na | 23 | 22.99 | 0.0% |
| Magnesium | Mg | 24 | 24.305 | -1.3% |
| Aluminium | Al | 27 | 26.982 | 0.1% |
| Silicon | Si | 28 | 28.085 | -0.3% |
| Sulphur | S | 32 | 32.06 | -0.2% |
| Phosphorus | P | 32 | 30.974 | 3.2% |
| Chlorine | Cl | 35.5 | 35.45 | 0.1% |
| Potassium | K | 39 | 39.098 | -0.3% |
| Calcium | Ca | 40 | 40.078 | -0.2% |
| Chromium | Cr | 53 | 51.996 | 1.9% |
| Manganese | Mn | 55 | 54.938 | 0.1% |
| Iron | Fe | 56 | 55.845 | 0.3% |
| Titanium | Ti | 56 | 47.867 | 14.5% |
| Copper | Cu | 63 | 63.546 | -0.9% |
| Zinc | Zn | 66 | 65.38 | 0.9% |
| Arsenic | As | 75 | 74.922 | 0.1% |
| Bromine | Br | 80 | 79.904 | 0.1% |
| Zirconium | Zr | 89 | 91.224 | -2.5% |
| Silver | Ag | 108 | 107.87 | 0.1% |
| Tin | Sn | 117.6 | 118.71 | -0.9% |
| Iodine | I | 127 | 126.9 | 0.1% |
| Platinum | Pt | 197 | 195.08 | 1.0% |
| Mercury | Hg | 200 | 200.59 | -0.3% |
| Lead | Pb | 207 | 207.2 | -0.1% |
| Diatomic Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H2 | 2 | 2.016 | -0.8% |
| Oxygen | O2 | 32 | 31.998 | 0.0% |
| Sulphur | S2 | 64 | 64.12 | -0.2% |
| Chlorine | Cl2 | 71 | 70.9 | 0.1% |
| Bromine | Br2 | 160 | 159.808 | 0.1% |
| Iodine | I2 | 254 | 253.8 | 0.1% |
| Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Water | H2O | 18 | 18.015 | -0.1% |
| Hydrochloric Acid | HCl | 36.5 | 36.458 | 0.1% |
| Methane | CH4 | 16 | 16.043 | -0.3% |
| Hydrogen sulphide | H2S | 34 | 34.076 | -0.2% |
| Diethyl ether | CH3CH2OCH2CH3 | 74 | 74.123 | -0.2% |
| Carbon disulphide | CS2 | 76 | 76.131 | -0.2% |
| Chloroethane | CH3CH2Cl | 64.5 | 64.512 | 0.0% |
1860
Discovery of Cesium
Cs
Cesium (or caesium), atomic number 55, has a mass of 132.905 au.
Cesium is a Group 1 element, and these are often referred to as the "alkali metals".
Cesium was first observed or predicted in 1860 by R. Bunsen and R. Kirchhoff and first isolated in 1882 by C. Setterberg.
1860
Karlsruhe Congress
The Karlsruhe Congress of 1860 was called so that European chemists could discuss a number of issues, including atomic weights.

From Wikipedia (lightly edited):
"The Karlsruhe meeting ended with no firm agreement on the vexing problem of atomic and molecular weights. However, on the meeting's last day reprints of Stanislao Cannizzaro's 1858 paper on atomic weights were distributed. Cannizzaro's efforts exerted an almost immediate influence on the delegates.
"Lothar Meyer later wrote that on reading Cannizzaro's paper: 'The scales seemed to fall from my eyes.'
"An important long-term result of the Karlsruhe Congress was the adoption of the now-familiar atomic weights. Prior to the Karlsruhe meeting, and going back to Dalton's work in 1803, several systems of atomic weights were in use.
"Following the Karlsruhe meeting, values of about 1 for hydrogen, 12 for carbon, 16 for oxygen, and so forth were adopted. This was based on a recognition that certain common gaseous elements, such as hydrogen, nitrogen, oxygen and chlorine were composed of diatomic molecules and not individual atoms: H2, N2, O2, Cl2, etc."
Once enough elements had been discovered, and their atomic weights correctly deduced, the time was ripe to develop versions of the periodic table systems. These came 'thick & fast' after the Karlsruhe Congress.
Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about the important Karlsruhe Congress.
1861
Discovery of Rubidium
Rb
Rubidium, atomic number 37, has a mass of 85.468 au.
Rubidium is a Group 1 element, and these are often referred to as the "alkali metals".
Rubidium was first observed, but not isolated in pure form, in 1861 by R. Bunsen and G. R. Kirchhoff.
1861
Discovery of Thallium
Tl
Thallium, atomic number 81, has a mass of 204.384 au.
Thallium was first observed or predicted in 1861 by W. Crookes and first isolated in 1862 by C.-A. Lamy.
1862
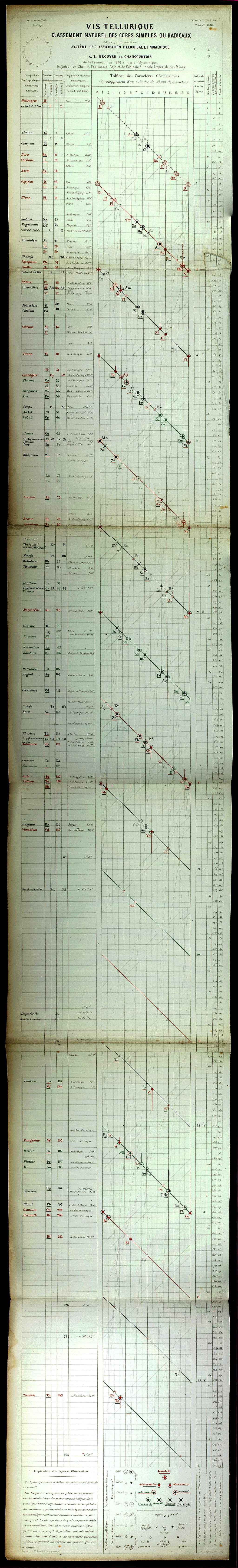
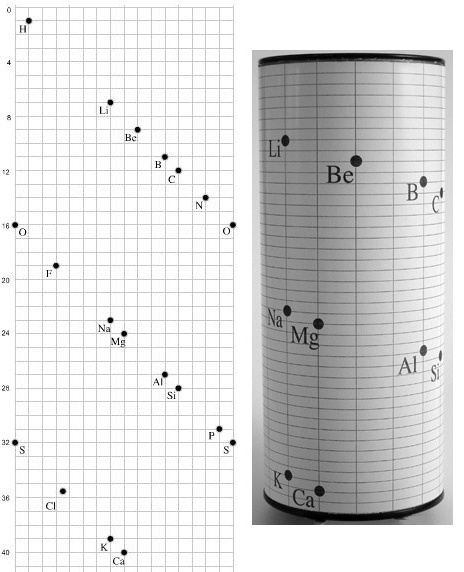
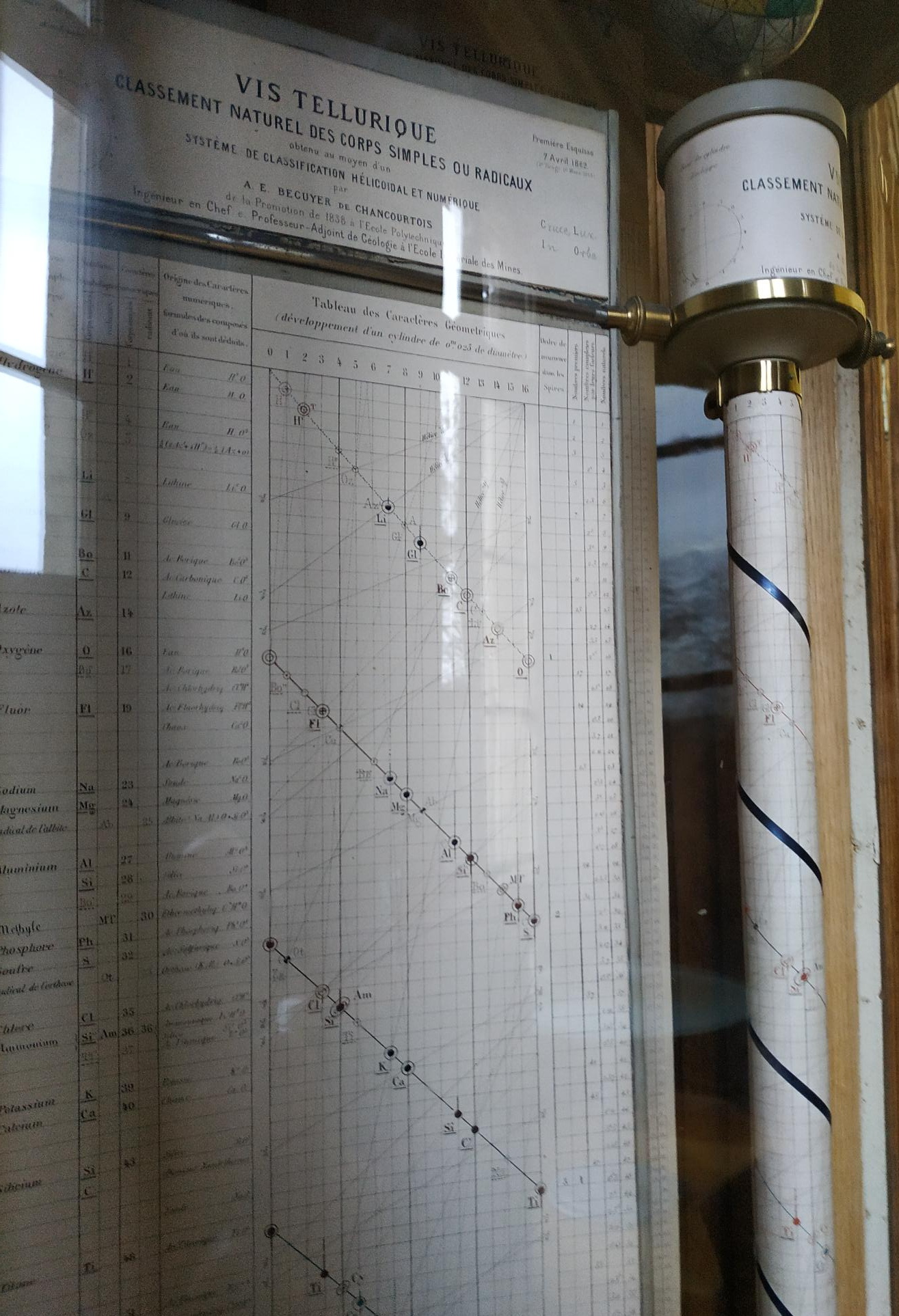
Béguyer de Chancourtois' Vis Tellurique
The French geologist , Alexandre-Émile Béguyer de Chancourtois was the first person to make use of atomic weights to produce a classification of periodicity. He drew the elements as a continuous spiral around a metal cylinder divided into 16 parts. The atomic weight of oxygen was taken as 16 and was used as the standard against which all the other elements were compared. Tellurium was situated at the centre, prompting vis tellurique, or telluric screw.
Many thanks to Peter Wothers – and courtesy of the Master and Fellows of St Catharine's College, Cambridge – comes a high quality image of the original 1862 formulation. Click here, or on the image to enlarge:
Watch Peter Wothers 'unravel' and show Prof. Martyn Poliakoff this first periodic table at 17min 50sec into the YouTube video below:
Some more information:
Chancourtois' original formulation includes elements in their correct places, selected compounds and some elements in more than one place. The helix was an important advance in that it introduced the concept of periodicity, but it was flawed.
It has been suggested that Chancourtois called his formulation a telluric helix because tellurium is found in the middle. However, most elements are found as there their 'earths' – tellus, telluris – or oxides, which for a mineralogist would have been highly significant.
The formulation was rediscovered in the 1889 (P. J. Hartog, "A First Foreshadowing of the Periodic Law" Nature 41, 186-8 (1889)), and since then it has appeared most often in a simplified form that emphasizes the virtues and eliminates its flaws. [Thanks to CG for this info.]
See also:
- Dutch Wikipedia
- ScienceWorld
- Science & Society Picture Library
- Roy Alexander's All Periodic Tables site
A three dimensional models of the telluric helix:
There are representations of the 1862 formulation at the School of Mines at ParisTech:

1862
Meyer's Periodic System
In his book, The Periodic Table: A Very Short Introduction, Eric Scerri writes how Lothar Meyer devised a partial periodic tables consisting of 28 elements arranged in order of increasing atomic weight in which the elements were grouped into vertical columns according to their chemical valences:
1863
Discovery of Indium
In
Indium, atomic number 49, has a mass of 114.818 au.
Indium was first observed or predicted in 1863 by F. Reich and T. Richter and first isolated in 1867 by T. Richter.
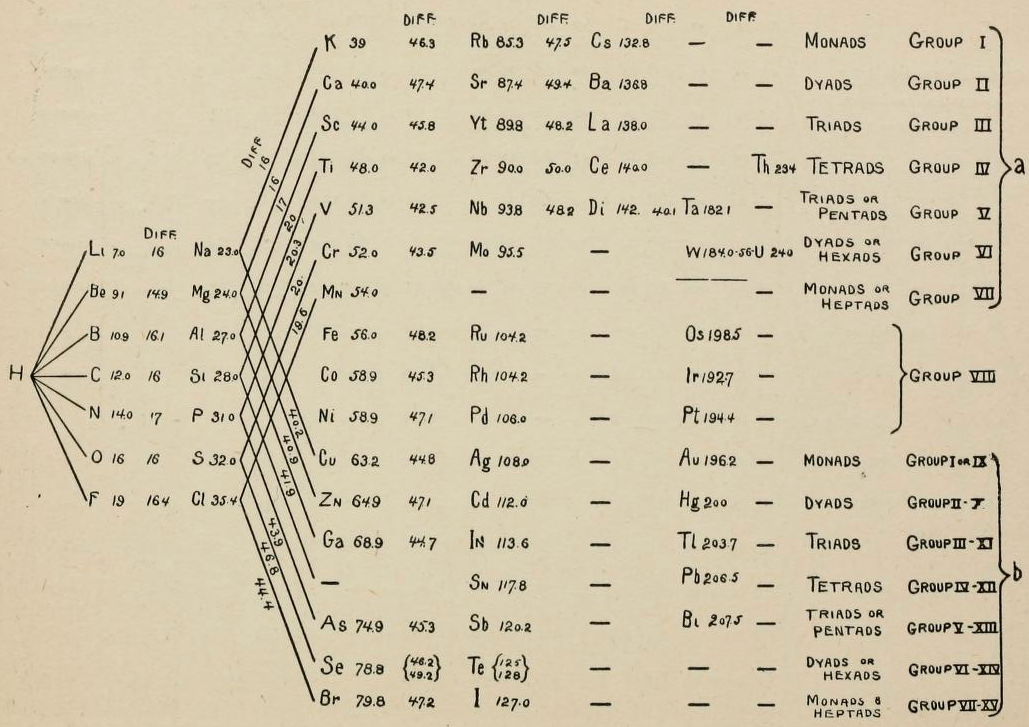
1864
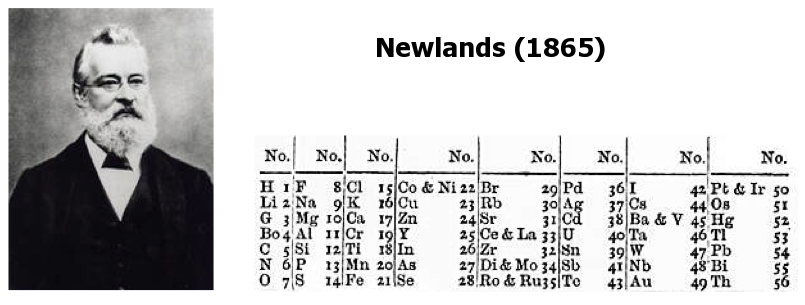
Newlands' Octaves
One of the first attempts at a periodic table that arranged the known elements by atomic weight and chemical property, was by John Newlands and is known as "Newlands Octaves".
Newland noticed that if he broke up his list of elements into groups of seven – starting a new row with the eighth element – the first element in each of those groups had similar chemistry.
Note: In the tables below, Newlands Octaves go downwards: H to O, F to S, Cl to Fe, etc.
|
H 1
|
F 8
|
Cl 15
|
Co & Ni 22
|
Br 29
|
Pd 36
|
I 42
|
Pt & Ir 50
|
|
Li 2
|
Na 9
|
K 16
|
Cu 23
|
Rb 30
|
Ag 37
|
Cs 44
|
Os 51
|
|
G 3
|
Mg 10
|
Ca 17
|
Zn 24
|
Sr 31
|
Cd 38
|
Ba & V 45
|
Hg 52
|
|
Bo 4
|
Al 11
|
Cr 19
|
Y 25
|
Ce & La 33
|
U 40
|
Ta 46
|
Tl 53
|
|
C 5
|
Si 12
|
Ti 18
|
In 26
|
Zr 32
|
Sn 39
|
W 47
|
Pb 54
|
|
N 6
|
P 13
|
Mn 20
|
As 27
|
Di & Mo 34
|
Sb 41
|
Nb 48
|
Bi 55
|
|
O 7
|
S 14
|
Fe 21
|
Se 28
|
Ro & Ru 35
|
Te 43
|
Au 49
|
Th 56
|
- Seeing the word octave applied to this table may lead one to think that Newlands recognised periods of eight elements with repeating properties, as we do with the modern periodic table, for example: Li Be B C N O F Ne.
- However, each sequence of Newlands' octaves contain only seven elements. Count the elements in the columns! In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not yet been discovered.
- To Newlands, H to F & F to Cl are octaves of eight elements, the eighth element repeating the properties of the first.
There are seven notes in a musical octave: A B C D E F G, after which you start again with A'; similarly for Newlands, seven elements H Li G Bo C N O, then the 8th is F and you start again. [Note that Newlands treated H as a halogen.] More here.
A B C D E F G A
Philip Stewart's musical representation:
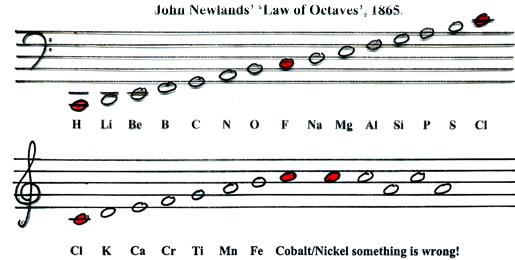
- To Newlands, H to F is an octave of eight elements.
- Today we say Li to Ne & Na to Ar are periods of eight elements, and that that Li and Na are in different periods. Indeed, the Li to Na series consists of nine elements.
- In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not been discovered.
Read more about Newland's Octaves, including a commentary on the original papers in Carmen Giunta's Elements and Atoms: Case Studies in the Development of Chemistry.
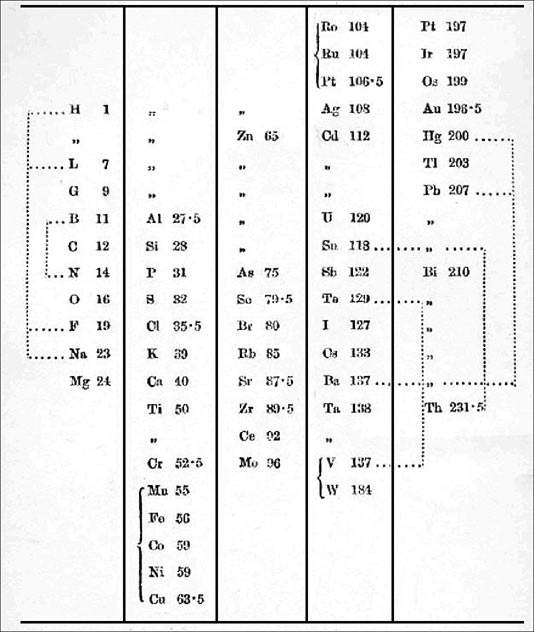
1864
Odling's Table of Elements
William Odling's table from: Q. J. Sci., 1864, 1, 642:
1864
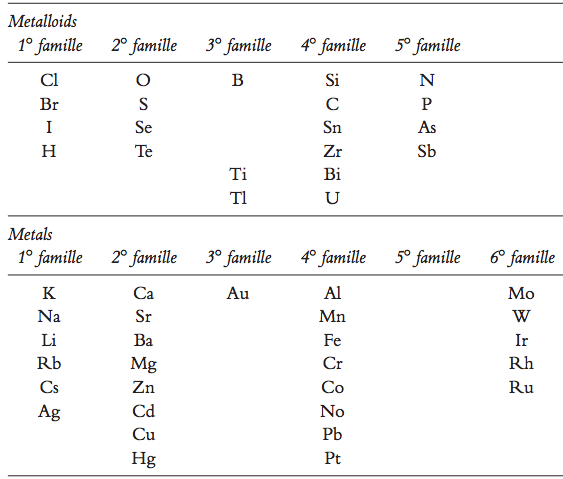
Naquet's Families of Elements
According to Naquet’s 1864 textbook, Principes de Chimie, F. Savy, Paris, (updated by Eric Scerri):
1866
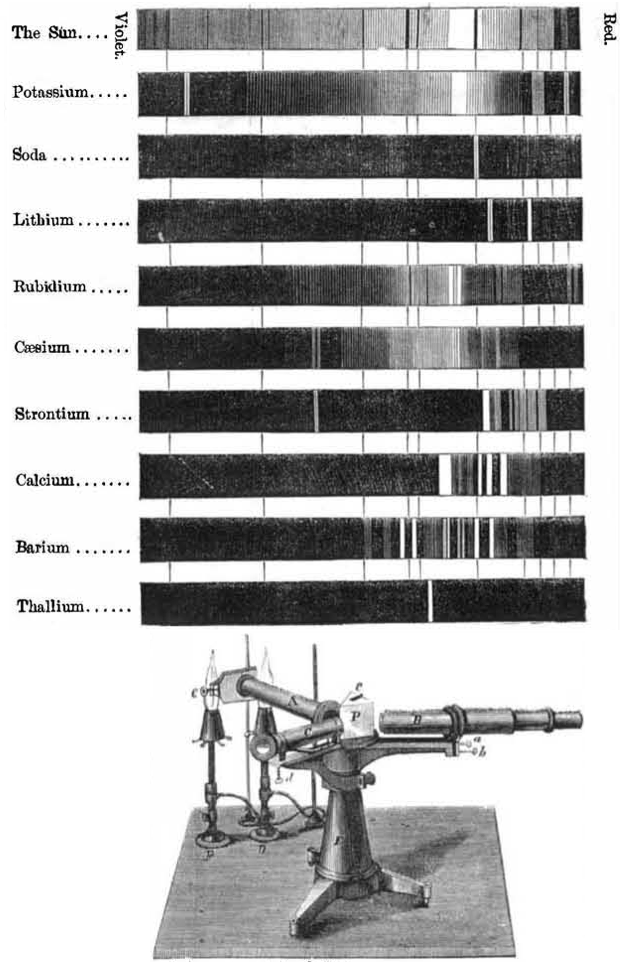
Spectroscope Revelations
From Scientific American in 1866, an article by Henry Draper concerning "The Spectroscope and Its Revelations".
At the time there was no understanding how the spectra were generated but it was recognised that every element produced a unique spectrum. Over 35,000 stars are catalogued/identified by their "HD" [Henry Draper] numbers:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.Also, thanks to Prof. Emeritus Robert J. Lancashire of The University of the West Indies for corrections & additional information.
1867
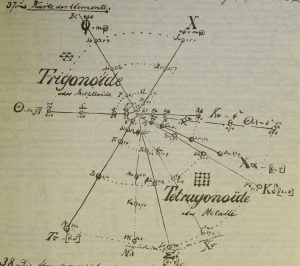
Hinrichs' Programme of Atomechanics
Gustavus Detlef Hinrichs' spiral "Programme of Atomechanics". Programm der Atomechanik oder die Chemie eine Mechanik de Pantome, Augustus Hageboek, Iowa City, IA (1867).
Hinrichs' system is based on the relationship of what he called: "pantogens, with its atoms called panatoms, which explains the numerical relations of atomic weights and gives a simple classification of the elements."
This classification system culminated in 1867 in his spiral periodic table, which better clarified the groupings of elements. Hinrichs' classification, while distinctly different from the other periodic tables of this period, "seems to capture many of the primary periodicity relationships seen in the modern periodic table... it is not cluttered by attempts to show secondary kinship relationships." (Scerri)
1868
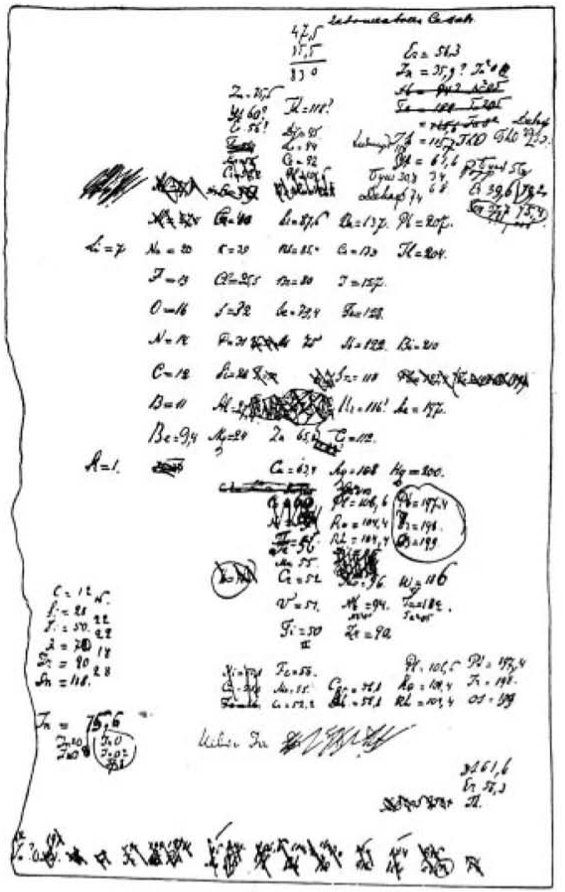
Handwritten draft of the first version of Mendeleev's Periodic Table
From of Bill Jensen, Curator of the Oesper Collection at the University of Cincinnati:
1868
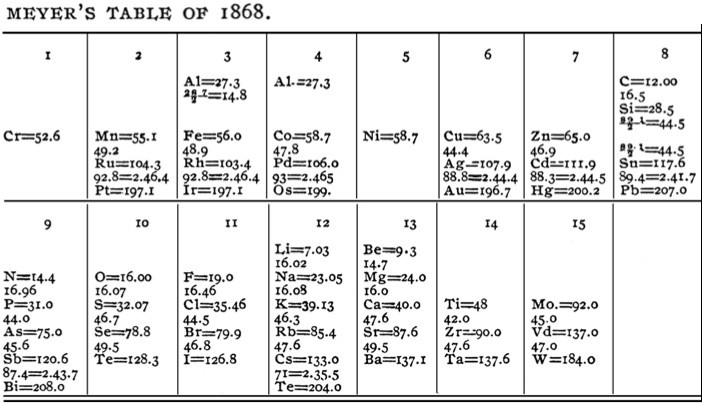
Meyer's "Lost" Table of 1868
In his book, The Periodic Table: A Very Short Introduction, Eric Scerri writes how Lothar Meyer produced an expanded periodic system for his1868 textbook which contained 53 elements. Unfortunately, the table was misplaced by the publisher and was not appear until after his death in 1895:
1869
Mendeleev's Tabelle I
Mendeleev [also spelled Mendeleyev in English] recounted in his diary:
"I saw in a dream a table where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper."



Thanks to Marcus Lynch for the tip!
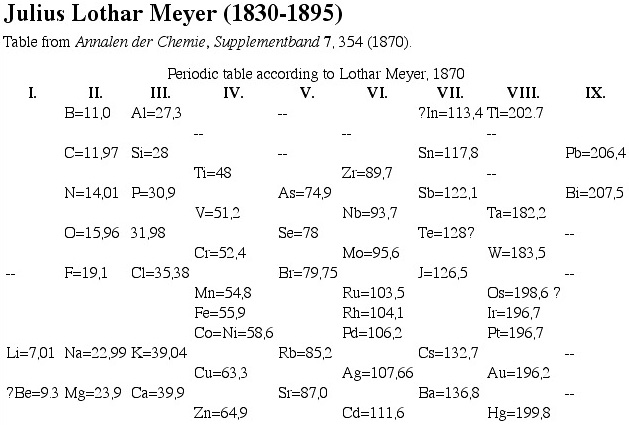
1870
Meyer's Periodic Table. This is rather similar to the Mendeleev attempt at the same time.
1870
Baumhauer's Spiral
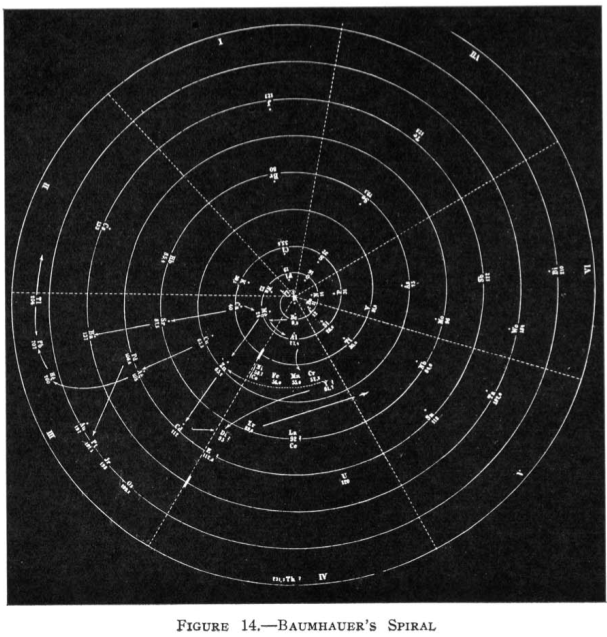
From Quam & Quam's 1934 review paper.pdf
1870
Baker's Electronegativity Table
Baker's electronegativity table of 1870 differs from Berzelius' listing of 1836 only by the addition of the newly discovered elements. Page 280 and ref. 5 from Bill Jensen's: Electronegativity from Avogadro to Pauling Part II: Late Nineteenth- and Early Twentieth-Century Developments, J. Chem. Educ., 80, 279-287 (2003):

1871
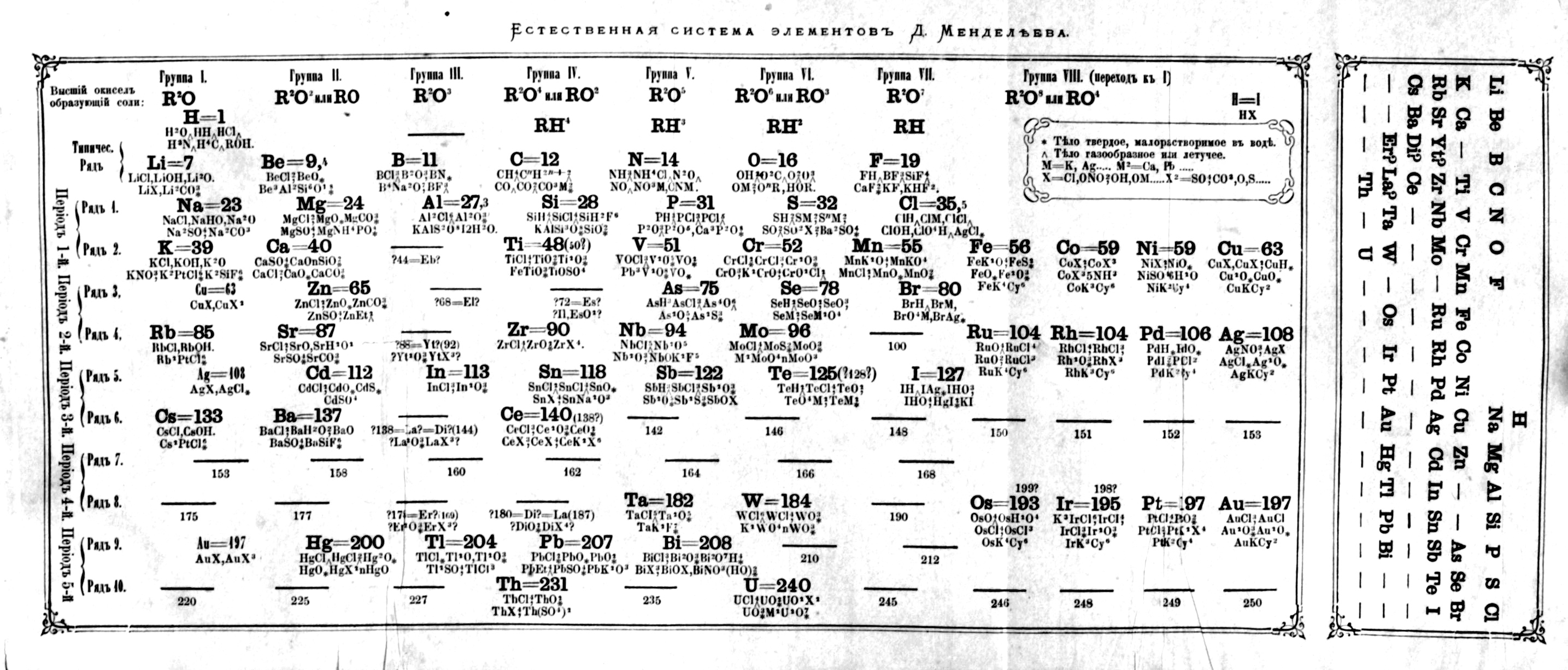
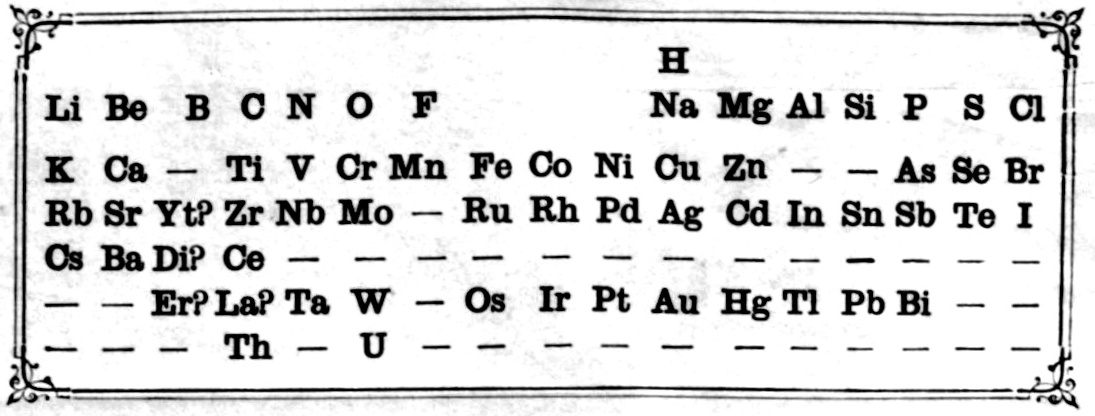
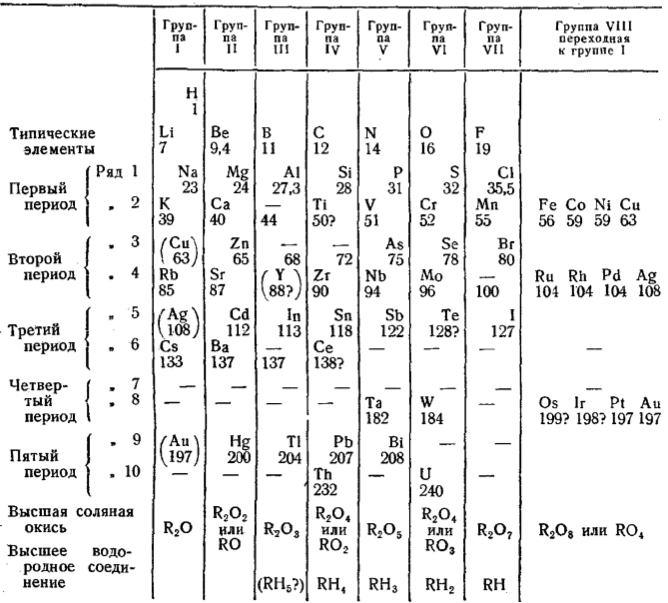
Mendeleev's Tabelle II
Some versions of Mendeleev's Tabelle II of 1871.
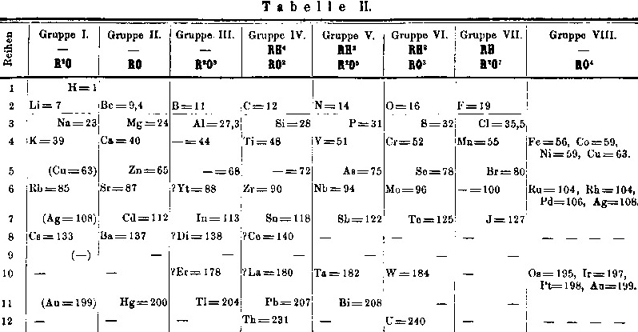
From the second volume of Mendeleev's textbook (click to enlarge):
Notice that on the right hand side, there is an additional formulation:
The two formulations above are discussed by Peter Wothers from the University of Cambridge with Sir Martyn Poliakoff, of the University of Nottingham, at 2:30 into the video below:
Mendeleev's Tabelle II can be shown in semi-modern form with the 'missing' group 18 rare gases and the f-block elements:
An alternative version of Mendeleev's Tabelle II:
1871
Mendeleev's Predicted Elements
In large part, the success of the Mendeleev's analysis can be attributed to the gaps which he predicted would contain undiscovered elements with predictable properties. Mendeleev named these unknown elements using the terms eka, dvi & tri (1, 2 & 3 from the ancient Indian language of Sanskrit).
Mendeleev predictions include:
- Eka-boron (scandium)
- Eka-aluminium (gallium)
- Eka-manganese (technetium)
- Eka-silicon (germanium)
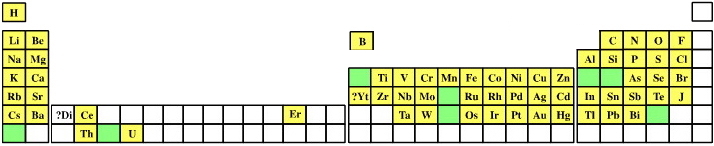
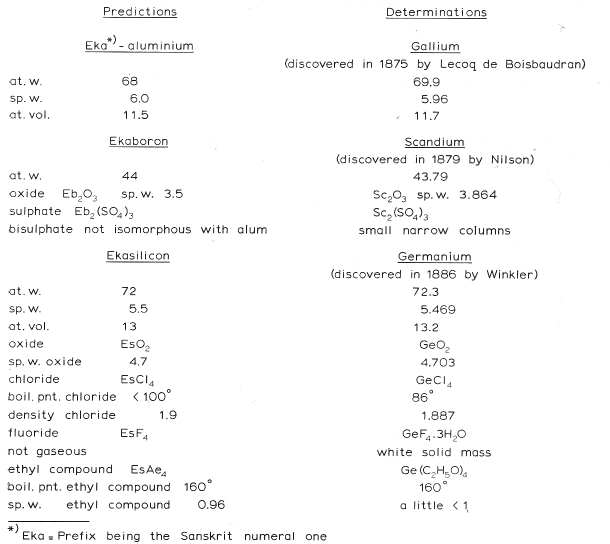
Image from van Spronsen
1871
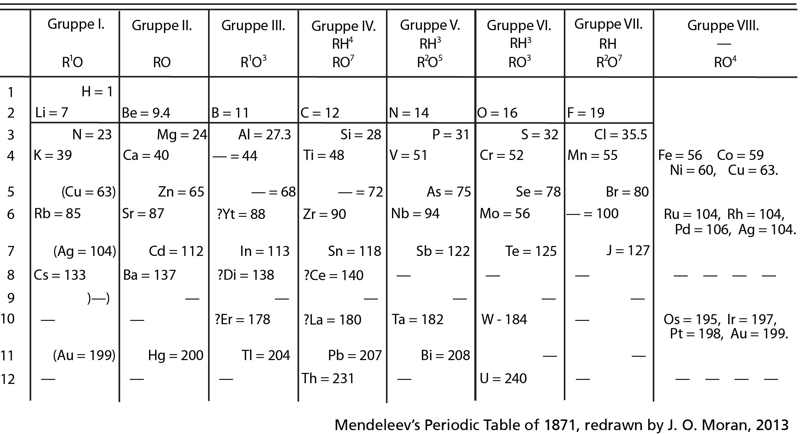
Mendeleev's Periodic Table of 1871, redrawn by J.O. Moran, 2013
Mendeleev's Periodic Table of 1871, redrawn by J.O. Moran, 2013, click here to see full size:
1872
Mendeléeff's Vertical Table (Q&Q's Spelling)
Quam & Quam's review paper states:
"Mendeléeff's first table was a vertical arrangement in which the elements could be read in order of increasing atomic weight from the top down and in successive series from left to right. The fist column consisted of H and Li; the second, of Be to Na; the third started with Mg.
"An improved form of this table, shown below, appeared in 1872: H occupied the first position separately. The vertical series in order were Li to F, Na to Cl, K to Br, etc., causing the halogens to appear in the same bottom series, while H, Li, Na, Cu, Ag, and Au constituted a midway series, and K, Rb, and Cs formed the topmost series."
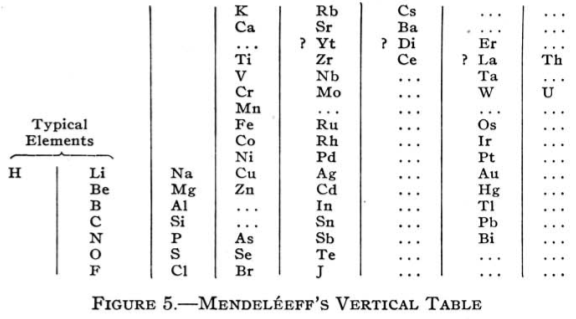
From Quam & Quam's 1934 review paper.pdf
1872
Meyer's Spiral System
Meyer's Spiral System of 1872 (from van Spronsen):

1875
Discovery of Gallium
Ga
Gallium, atomic number 31, has a mass of 69.723 au.
Gallium was first isolated in 1875 by P. E. L. de Boisbaudran.
1875
Gibbes' Synoptical Periodic Table
From page 127 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

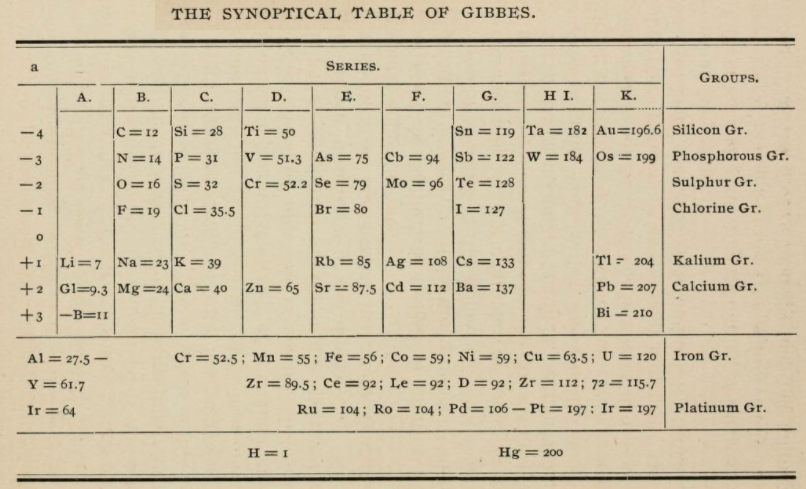
Thanks to René for the tip!
1875
Concentric Ring Arrangement of Wiik
From page 133 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

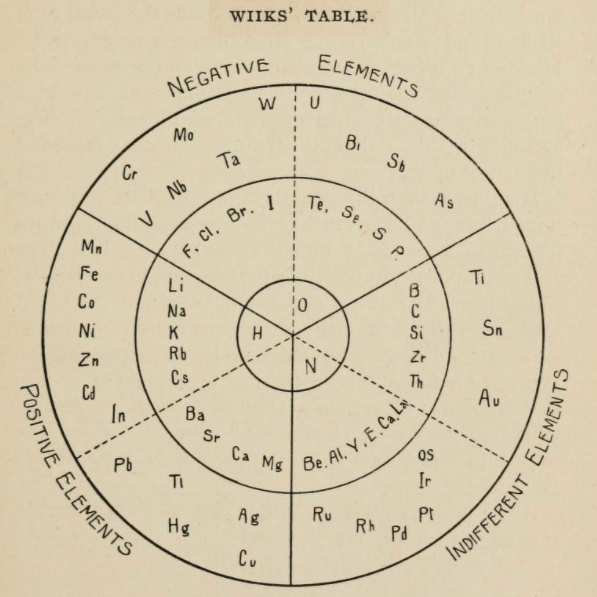
Thanks to René for the tip!
1878
Discovery of Ytterbium
Yb
Ytterbium, atomic number 70, has a mass of 173.054 au.
Ytterbium was first observed or predicted in 1878 by J.C.G. de Marignac and first isolated in 1906 by C. A. von Welsbach.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
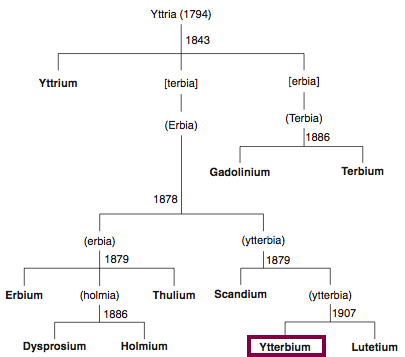
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1878
Waechter's Numerical Regularities
From page 136 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:
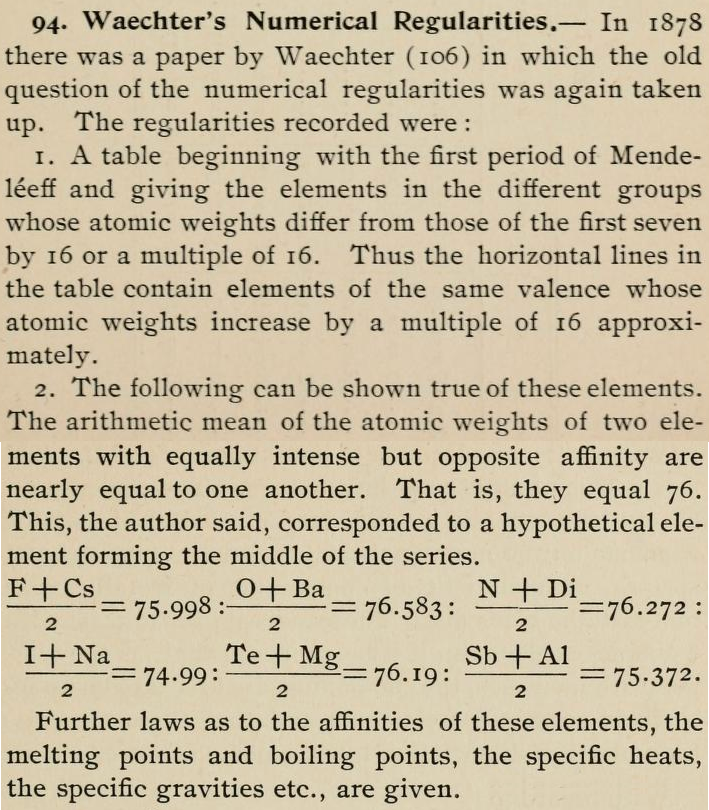
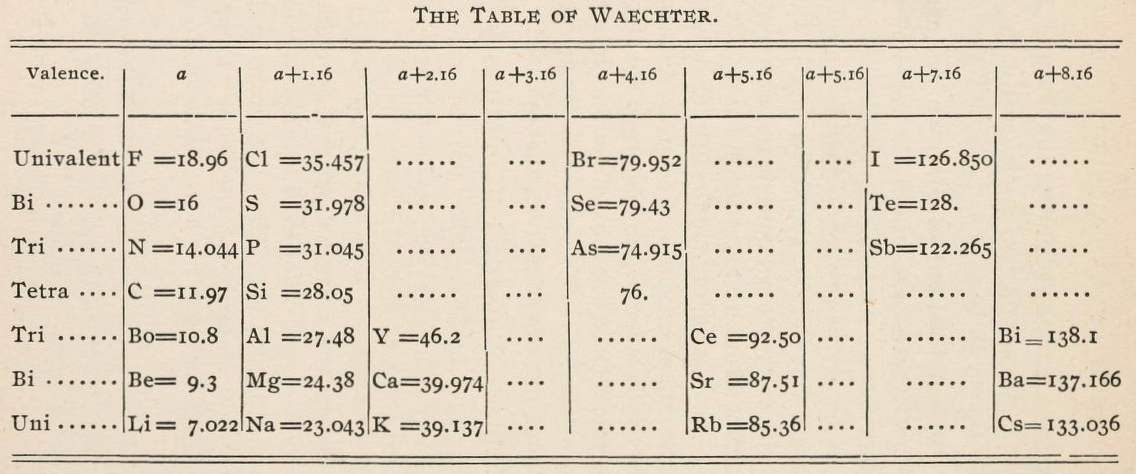
Thanks to René for the tip!
1879
Discovery of Scandium
Sc
Scandium, atomic number 21, has a mass of 44.956 au.
Scandium was first isolated in 1879 by F. Nilson.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
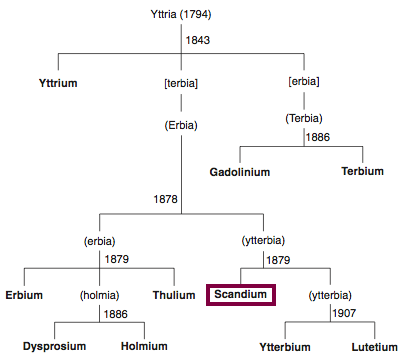
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1879
Discovery of Samarium
Sm
Samarium, atomic number 62, has a mass of 150.36 au.
Samarium was first isolated in 1879 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
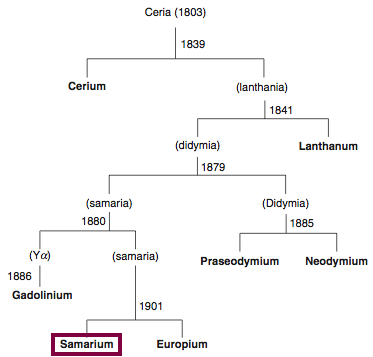
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1879
Discovery of Holmium
Ho
Holmium, atomic number 67, has a mass of 164.93 au.
Holmium was first observed or predicted in 1878 by J.-L. Soret and first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
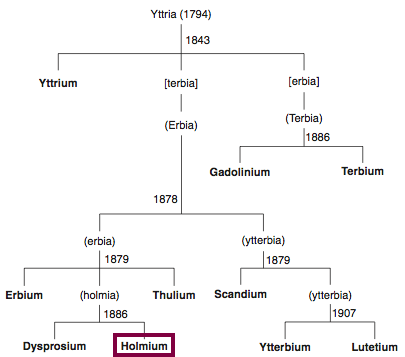
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1879
Discovery of Thulium
Tm
Thulium, atomic number 69, has a mass of 168.934 au.
Thulium was first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
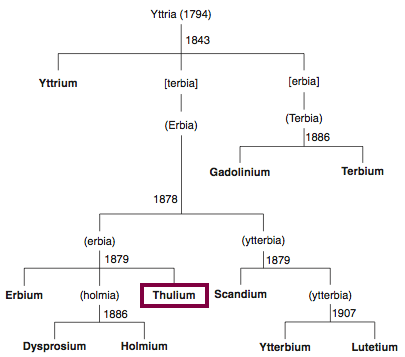
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1880
Discovery of Gadolinium
Gd
Gadolinium, atomic number 64, has a mass of 157.25 au.
Gadolinium was first observed or predicted in 1880 by J. C. G. de Marignac and first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
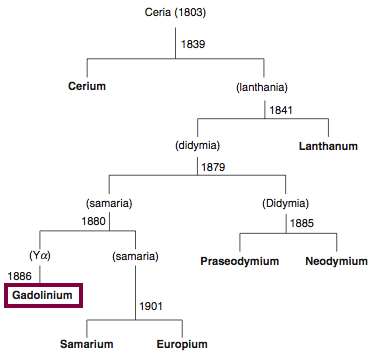
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
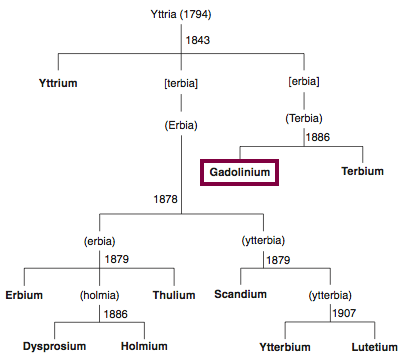
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1880
Periodische Gesetzmässigkeit der Elemente nach Mendelejeff
A lecture theatre sized periodic table, titled Periodische Gesetzmässigkeit der Elemente nach Mendelejeff, found at St Andrew's University, published and printed in Austria and dating from about from about 1880. Read more about this in The Guardian.

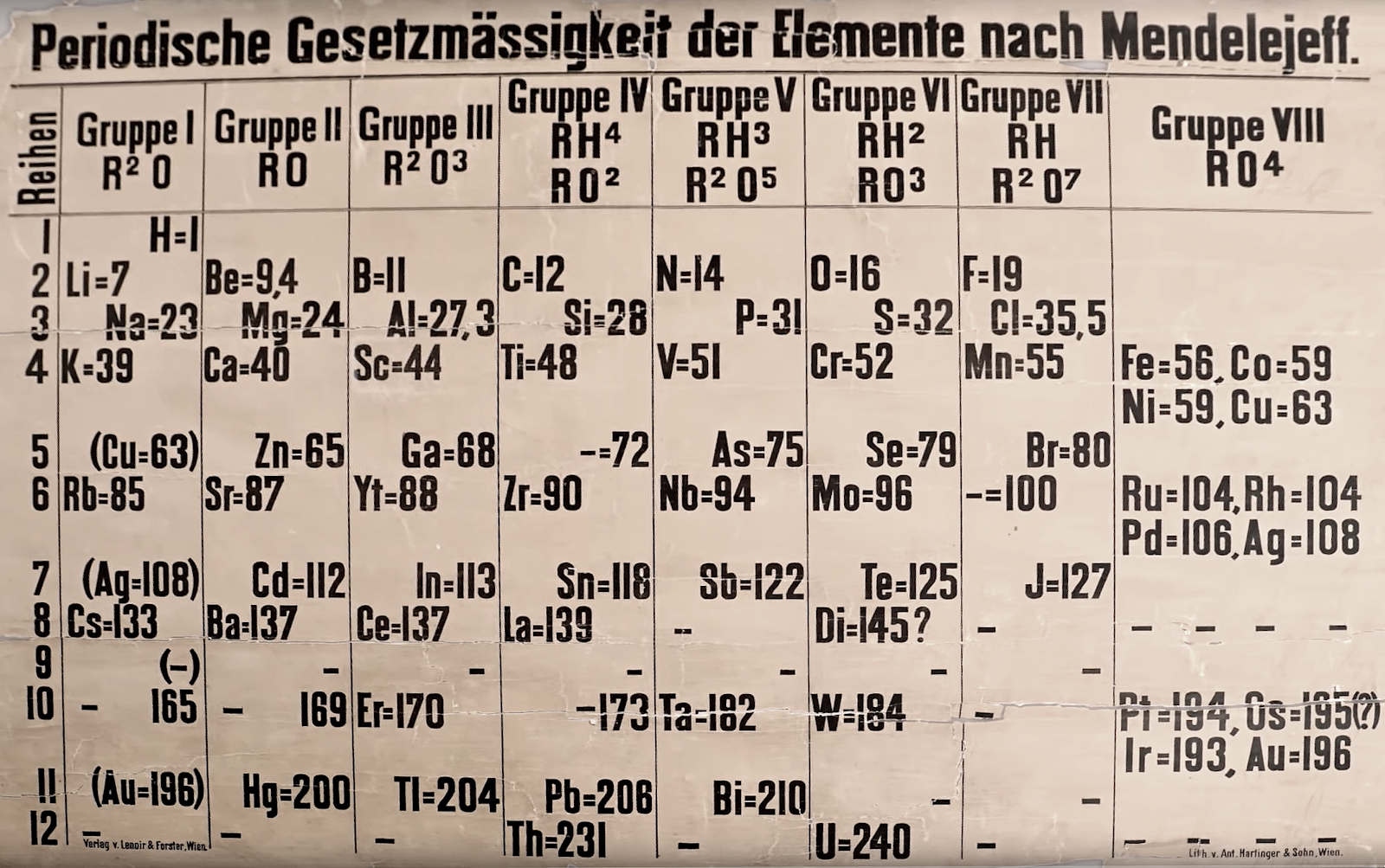
Two YouTube videos about this PT:
1881
Spring's Diagram
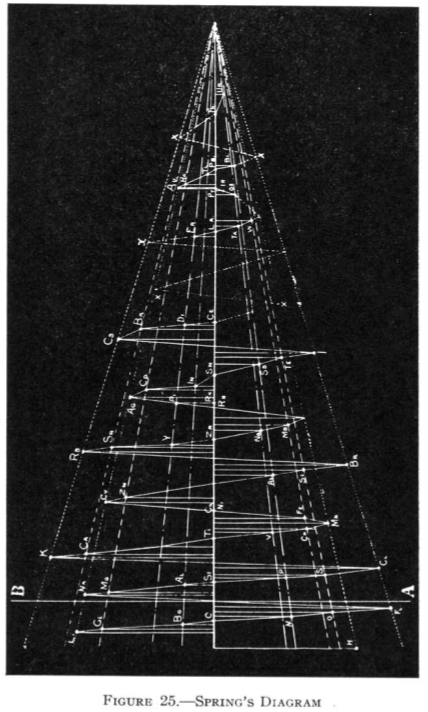
From Quam & Quam's 1934 review paper.pdf
1882
Bayley's Periodic System
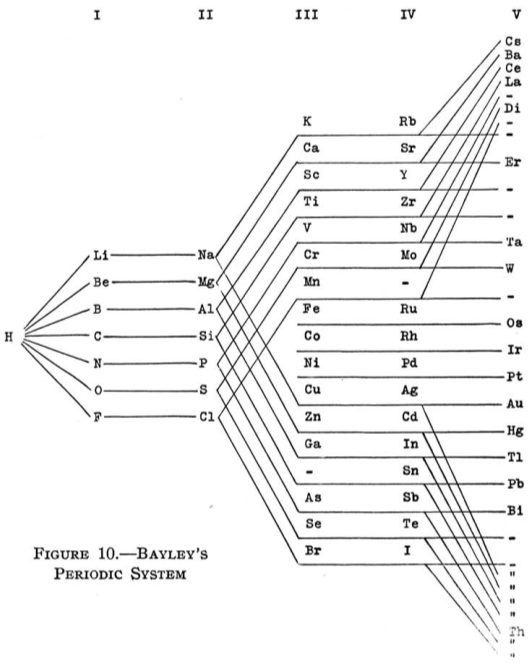
From Quam & Quam's 1934 review paper.pdf
1882
Brauner's Periodic Table
Brauner's periodic table of 1882 with a homologous accommodation of the rare-earth elements, from Chemische Berichte, 15, 1882, p. 15-121:
1882
Bayley's Attempt
From page 158 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes about Bayley:

Thanks to René for the tip!
1885
Discovery of Praseodymium
Pr
Praseodymium, atomic number 59, has a mass of 140.908 au.
Praseodymium was first isolated in 1885 by Carl Auer von Welsbach.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
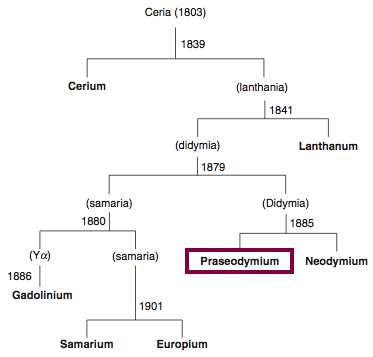
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1885
Discovery of Neodymium
Nd
Neodymium, atomic number 60, has a mass of 144.242 au.
Neodymium was first isolated in 1885 by Carl Auer von Welsbach.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
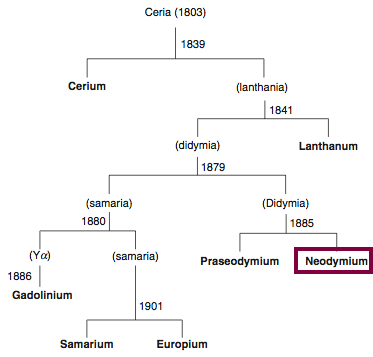
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1885
Carnelley & The Periodic Law
From page 172 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

Thanks to René for the tip!
1885
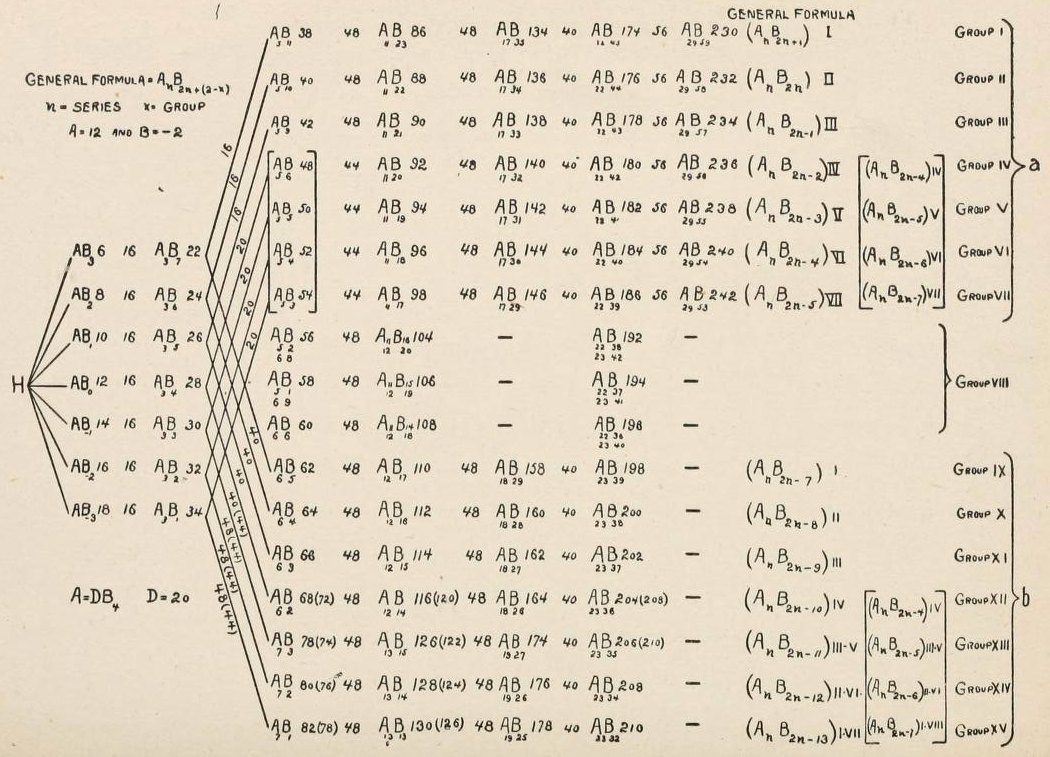
Klieber's Cosmochemical Periodic Table
Klieber's qualitative synthesis of the general composition of celestial objects in the form of a plane periodic system following atomic numbers. His diagram is probably one of the earliest versions of a "cosmochemical periodic table". (The diagram below is clearly redrawn as it has a very modern style.)
I.A. Kleiber, Zh. Russ. Fiziko-Khim. Obshch (St. Petersburg) 1885, 17, 147-171.
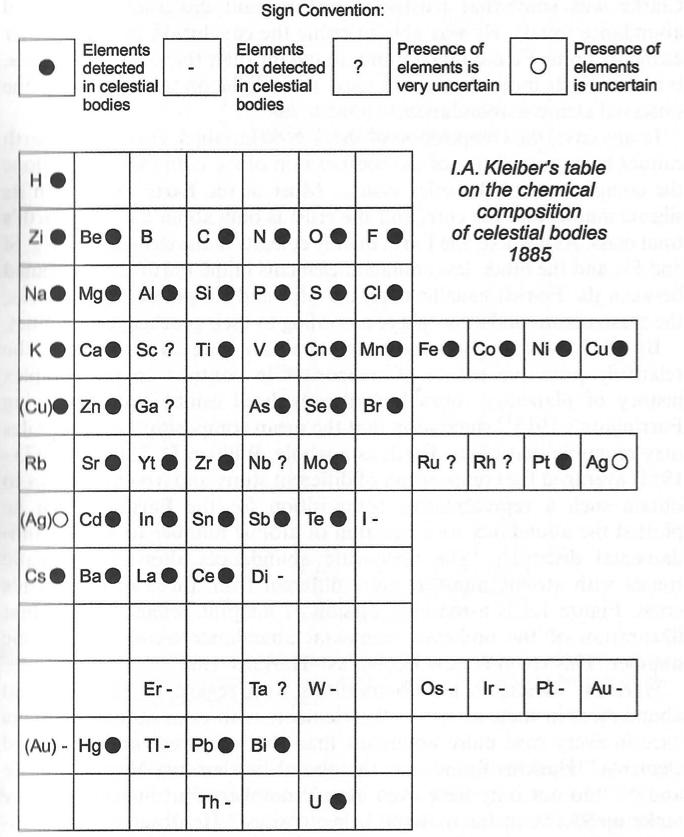
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
1885
von Richter's Periodic System of the Elements
From page 244 of A Text-book of Inorganic Chemistry by Victor von Richter, Published by Blakiston (US ed. in English, 1885). The full text (scanned) is available from archive.org. The first edition was published in 1874 in German. von Richter was was from the Baltic region, in the the Russian empire at the time.
von Richter's work is almost certainly the first chemistry textbook based on the periodic system. Many (indeed most) modern Inorganic Chemistry texts follow this format, but NOT the Chemogenesis web book!
von Richter, writes:

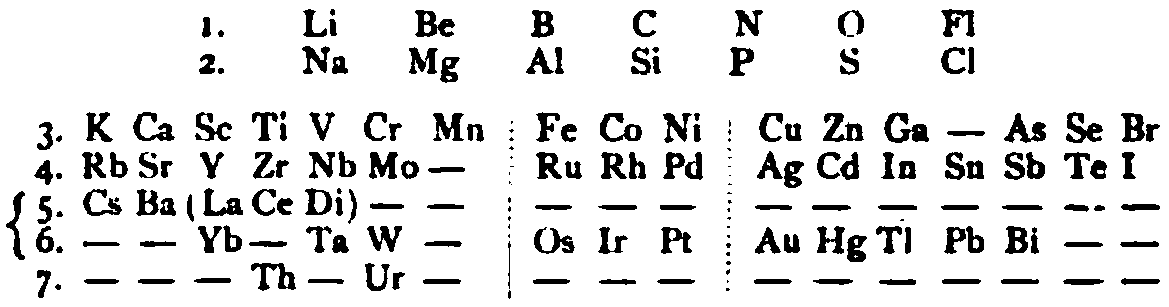

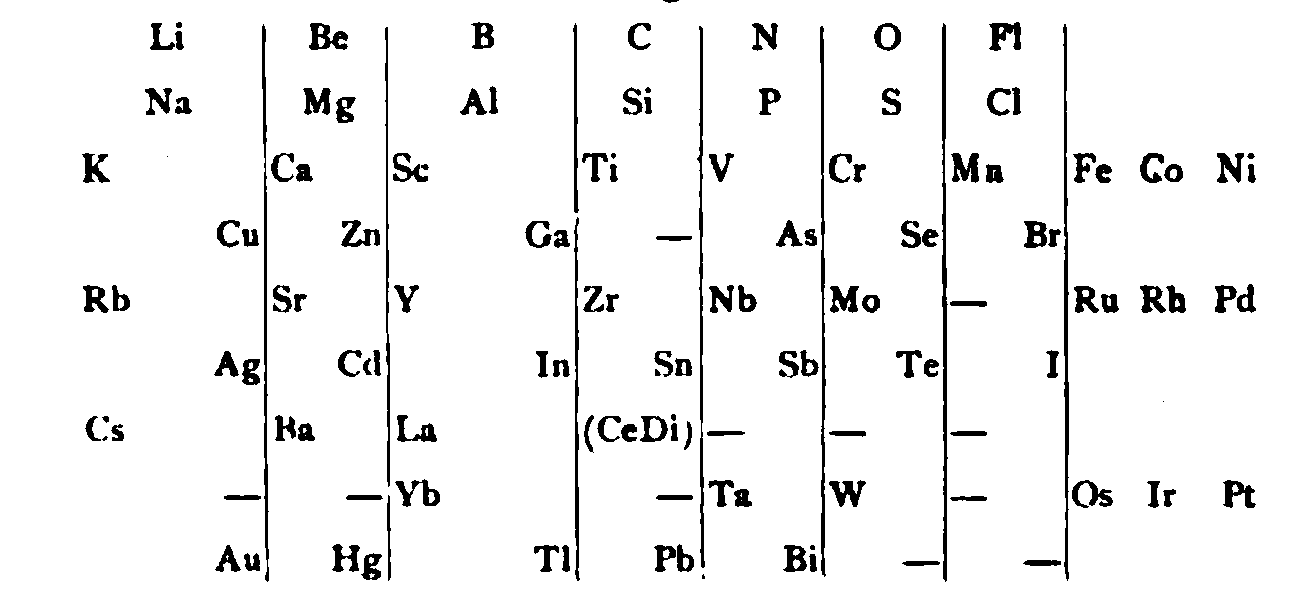
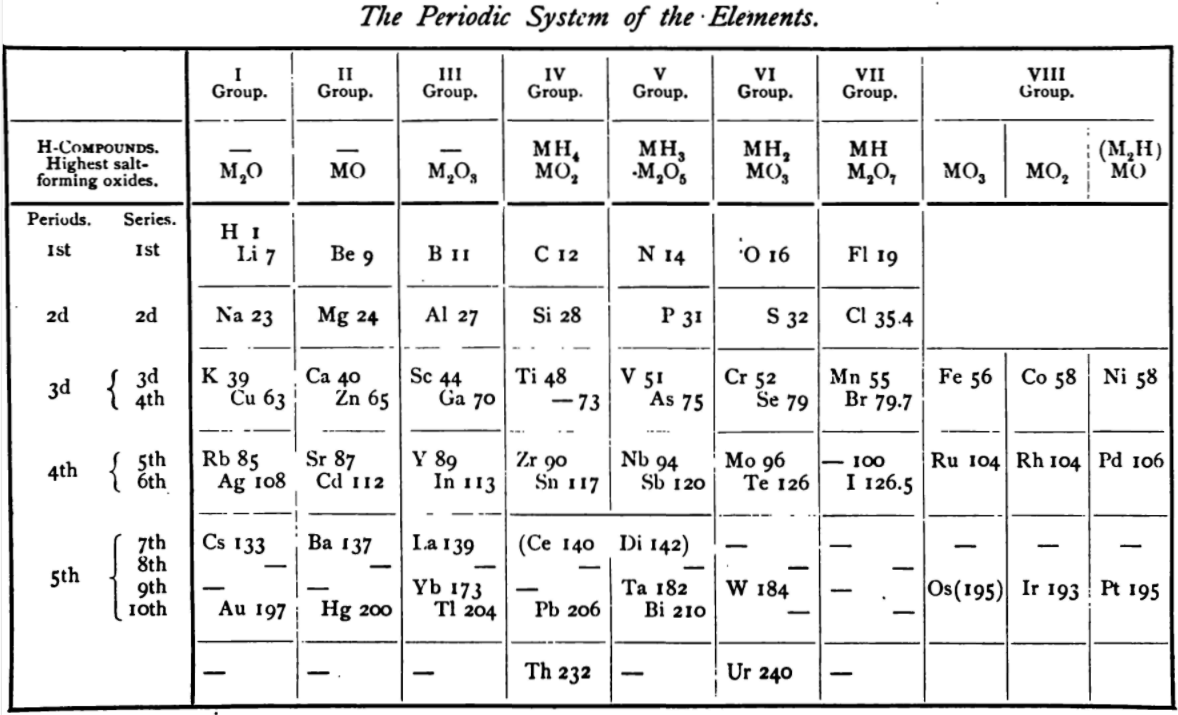
Thanks to René for the tip!
1886
Crookes' Periodic Table
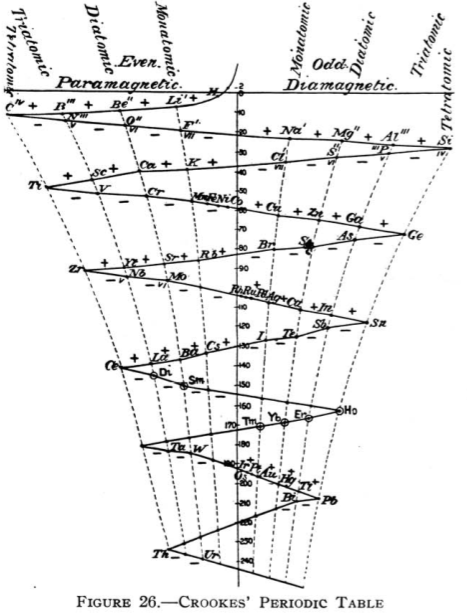
From Quam & Quam's 1934 review paper.pdf
1886
Discovery of Fluorine
F
Fluorine, atomic number 9, has a mass of 18.998 au.
Fluorine exists as a pale yellow diatomic molecular gas, F2. It is the most electronegative and reactive of all elements: it which reacts with practically all organic and inorganic substances.
Fluorine was first observed or predicted in 1810 by A.-M. Ampére and first isolated in 1886 by H. Moissan.
1886
Discovery of Germanium
Ge
Germanium, atomic number 32, has a mass of 72.63 au.
Germanium was first isolated in 1886 by C. A. Winkler.
1886
Discovery of Dysprosium
Dy
Dysprosium, atomic number 66, has a mass of 162.5 au.
Dysprosium was first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
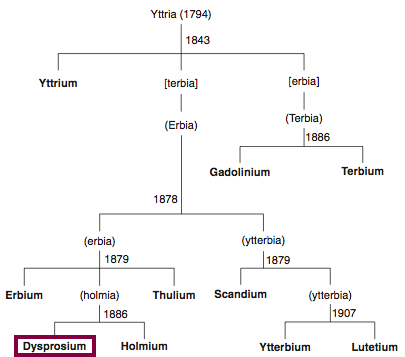
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
1886
Shepard's Natural Classification
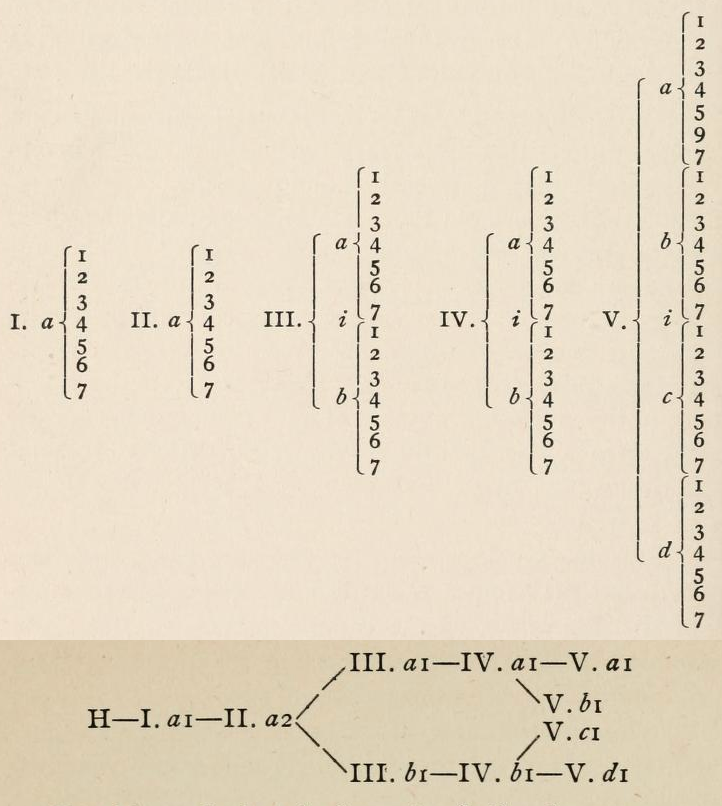
Shepard's Natural Classification of the Elements, a spiral formulation with instructions for turning it into a three-dimensional table. From: Elements of Inorganic Chemistry, Descriptive and Qualitative (pp221), by J. H. Shepard, (1886), Boston MA, pub. D. C. Heath
René Vernon writes:
Note the instructions along the side, to turn the table into a tube (spiral form) and the 19 spaces from La to eka-Ce. Here, Yb needs to be moved back one column into group II, so as to leave room for Lu under La. Then eka-Ce becomes Hf. This results in La + 15 lanthanoids.
The accompanying text says:
"Elements of most distinct basic character are found towards the left; non-metals predominate in the upper and middle parts of Groups V., VI., and VII. ; while the lower part of the table is marked by the more indifferent elements.
"A double spiral will be traced beyond Si (beginning with P and V respectively) and distinguished by heavy-face and light-face type.
"The harmony of nature here exhibited is most impressive. Is it possible that the so-called elements are really compounds? Did the various 'elements' of the earth and sun once exist as hydrogen, when our solar system was a nebula? And will modern chemists ever revive the famed problem of the alchemists, and seek to turn the base metals into gold? Far more precious than gold is the search for truth; and the more we learn of science, the broader becomes our conception of what we know in part, and the deeper should be our reverence for the infinite thought of the Creator."
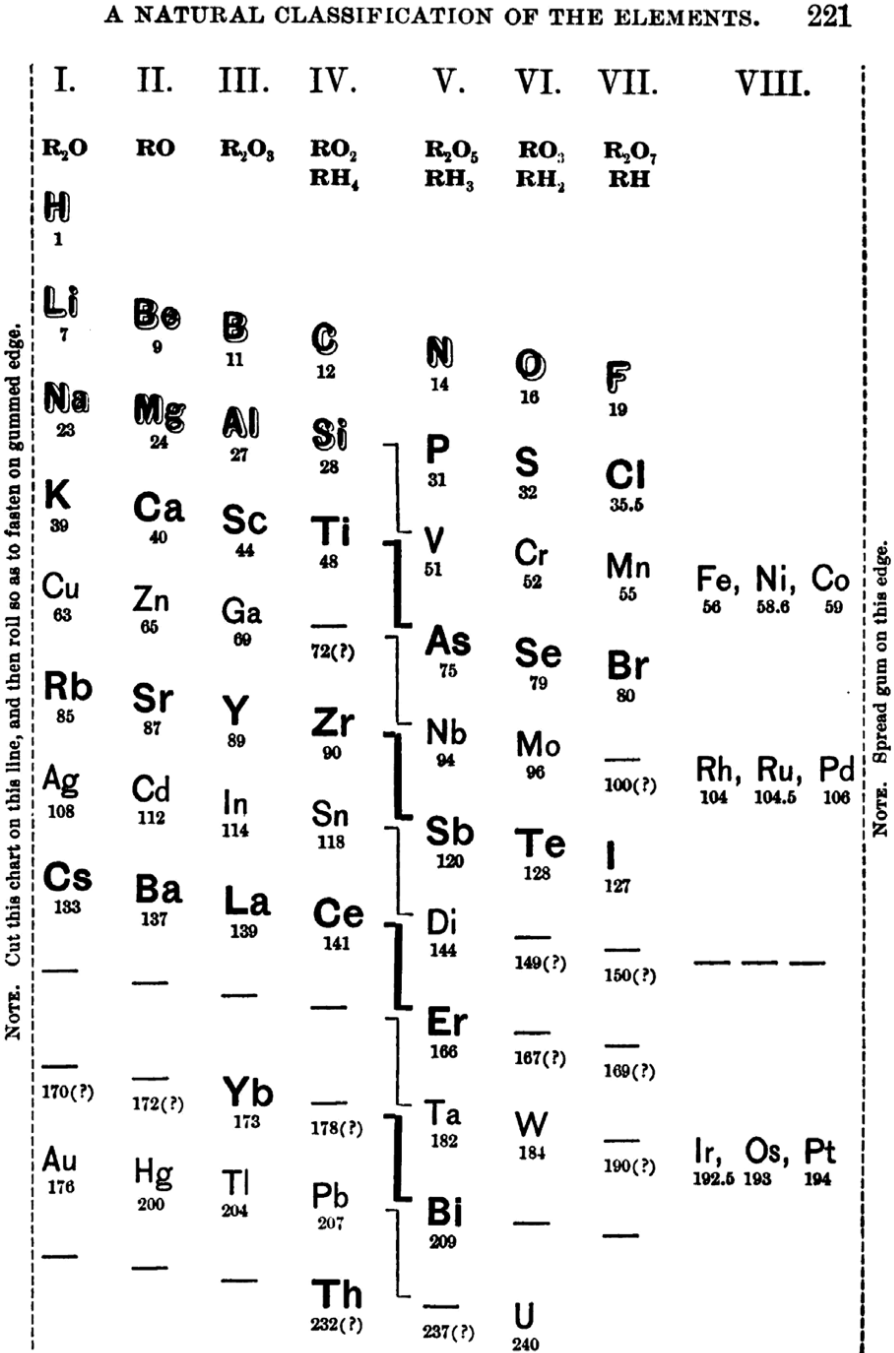
1886
Reynolds' Method of Illustrating the Periodic Law
Reynolds, J. E. (1886). Note on a method of illustrating the periodic law. Chemical News, 54, 1–4
Ann E. Robinson comments:
"Reynolds published a zig-zag version he created, noting it had been 'used in my lecture-room for some years in order to illustrate the periodic character of the relation between the atomic weights and properties of the chemical elements.'
>"Rather than a tabular form, Reynolds's form was a curve, best visualised as a string with seven knots tied in it at regular intervals, providing a 'vibrating system'. Although Reynolds's diagram is presented only as a two-dimensional picture, in a note he remarks that a friend suggested that the installation of 'a vibrating metallic chain, suspended from the ceiling and attached to the floor, would afford a more complete picture.'":
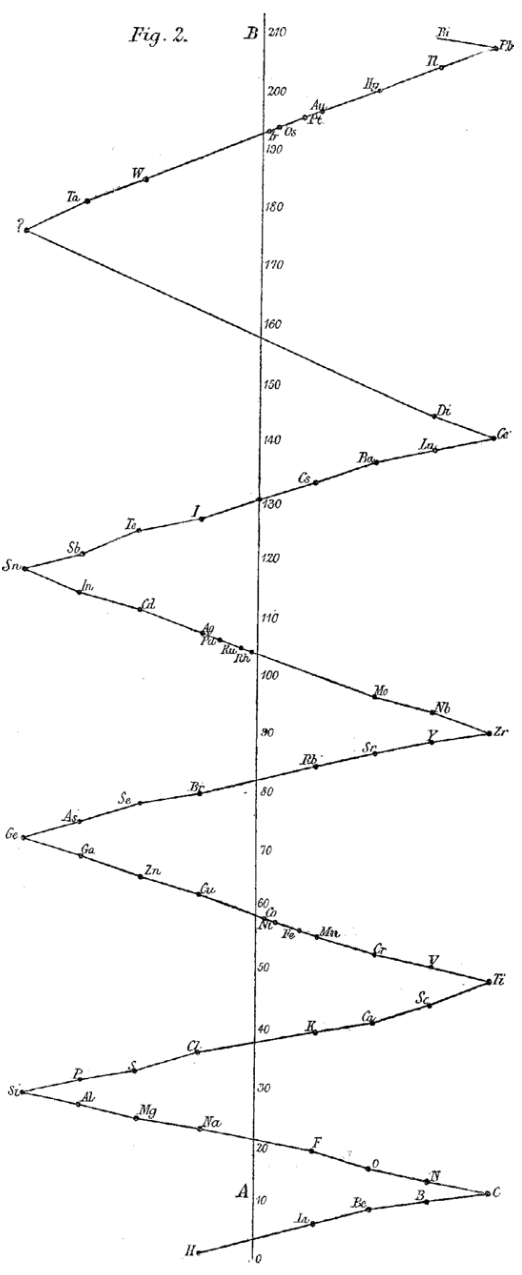
1887
Flavitzky's Arrangement
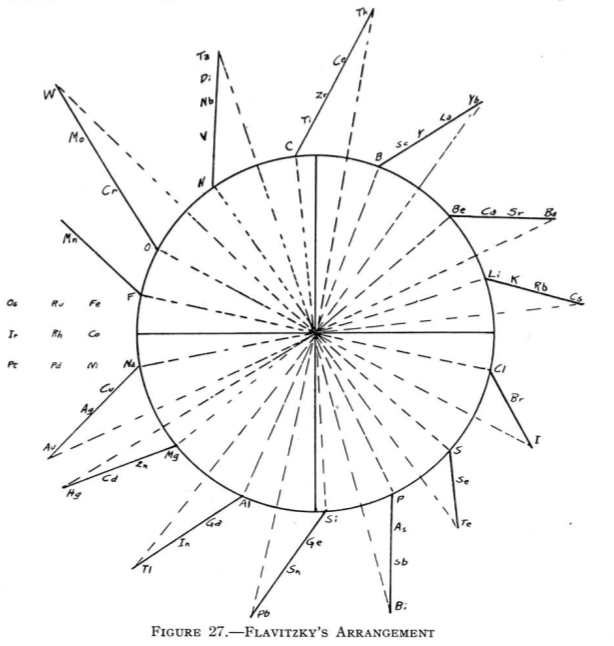
From Quam & Quam's 1934 review paper.pdf
1888
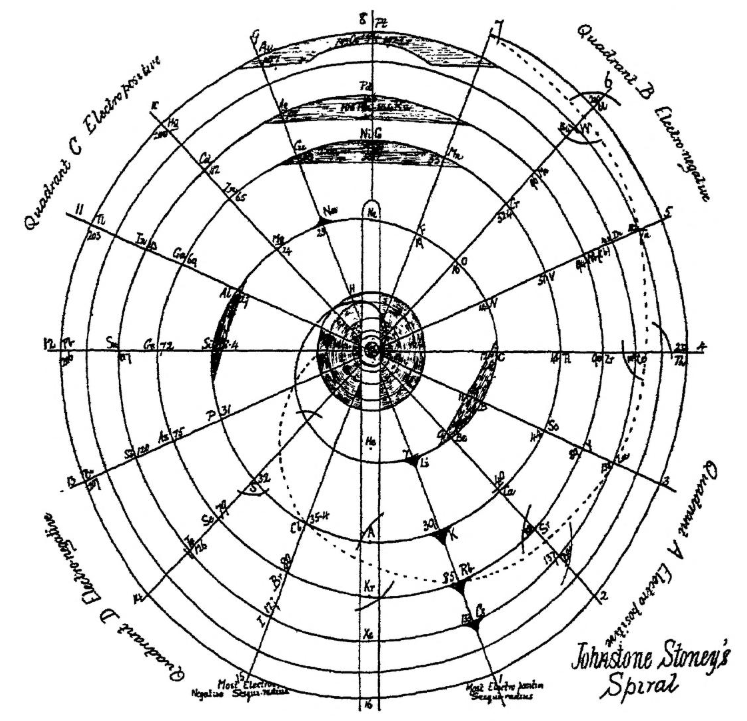
Stoney's Spiral
Johnstone Stoney's Spiral, taken from A. E. Garrett's The Periodic Law (page 167, 1909 pub. D. Appleton And Company). The reference is given – page 167 – is: Phil. Mag. [6], 4, pp 411 et seq.; Proc. Roy. Soc., 1888, p115.
Thanks to Roy Alexander for the tip!
1888
Stoney's Spiral Periodic Table
In the Proceedings of the Royal Society of London, Series A, Containing Papers of a Mathematical and Physical Character, Volume 85, Issue 580, Aug 1911, p. 472, there is an article On Dr. Johnstone Stoney's Logarithmic Law of Atomic Weights, by Lord Rayleigh (who co-discovered argon in 1894), who writes :
"In the year 1888, Dr. G. Johnstone Stoney communicated to the Society a memoir with title nearly as above, which, however, was not published in full. At the request of the author, who attaches great importance to the memoir, I have recently, by permission of the Council, consulted the original manuscript in the archives of the Society, and I propose to give some extracts, accompanied by a few remarks. The author commenced by plotting the atomic weights of the elements taken as ordinates against a series of natural numbers as abscissæ. But a curve traced through the points thus determined was found to be 'one which has not been studied by mathematicians.
"This sudden transition may have some connection with the fact that no elements have been found on sesqui-radius 16, although the investigation in § 3 shows that the values of m corresponding to the stations on sesqui-radius 16 cannot be dispensed with.
"The vacant places here pointed out are now occupied by the since discovered inert gases. The anticipation is certainly a remarkable one, and it goes far to justify the high claims made for the diagram, as representing in a telling form many of the leading facts of chemistry."
Comment from Mark Leach:
"Notice how the electronegative elements are positioned top right & bottom right and the electropositive elements top left & bottom right."
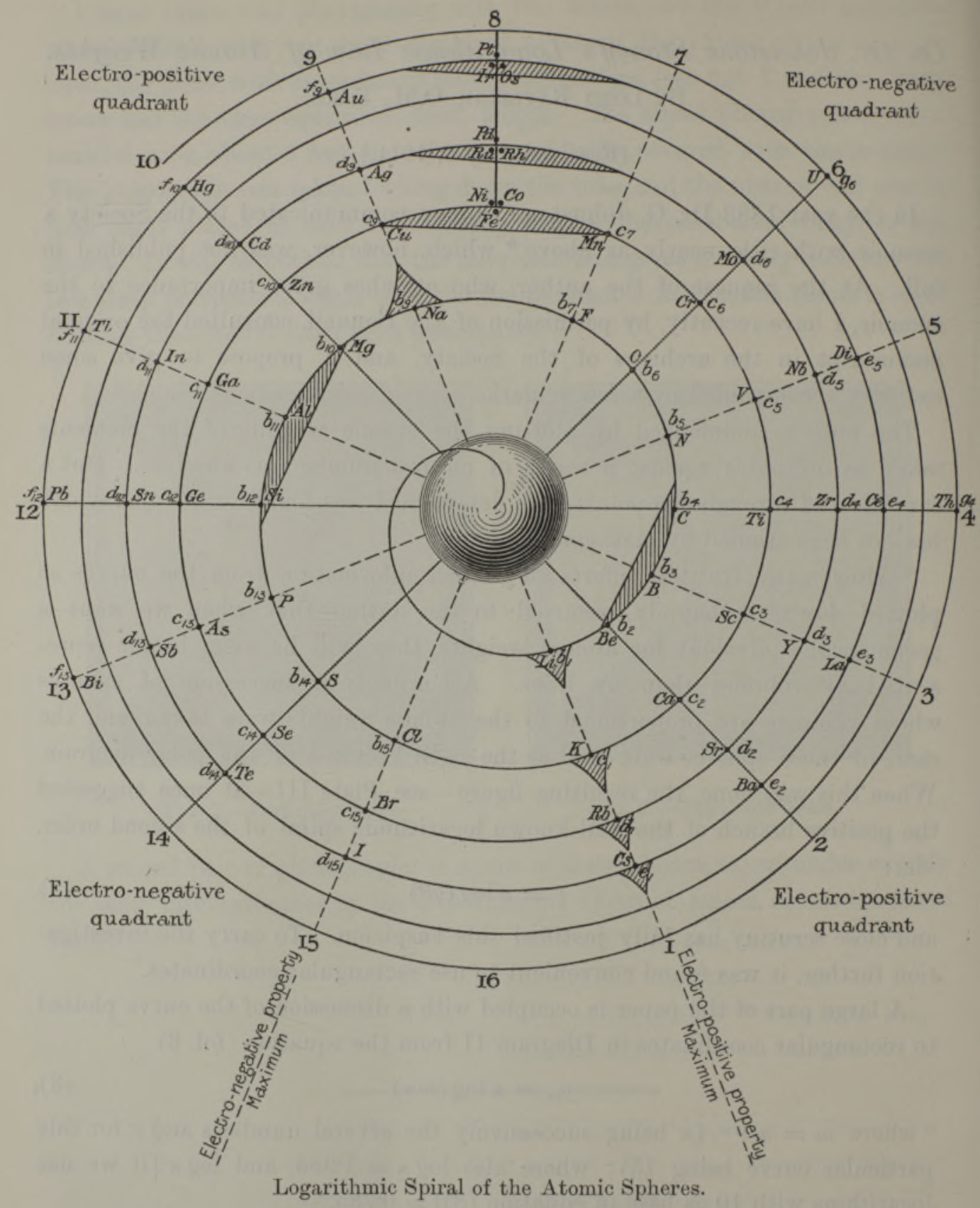
René Vernon writes:
"Stoney has another article in the September 1902 edition of the The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, called Law of Atomic Weights, pp. 411–415. At the back of the journal is an updated fold-out version of Stoney’s table, image attached.
- Ar, Kr and Xe fit on the spiral, and on spoke 16.
- Neon fits on the spiral but is instead on spoke 8.
- Helium is on spoke 18 but is not on the spiral.
- The circle in the middle represents H (p. 414).
"On the page after the updated spiral, there looks to be some printed content, but it is hidden by what looks to be a folded over page."
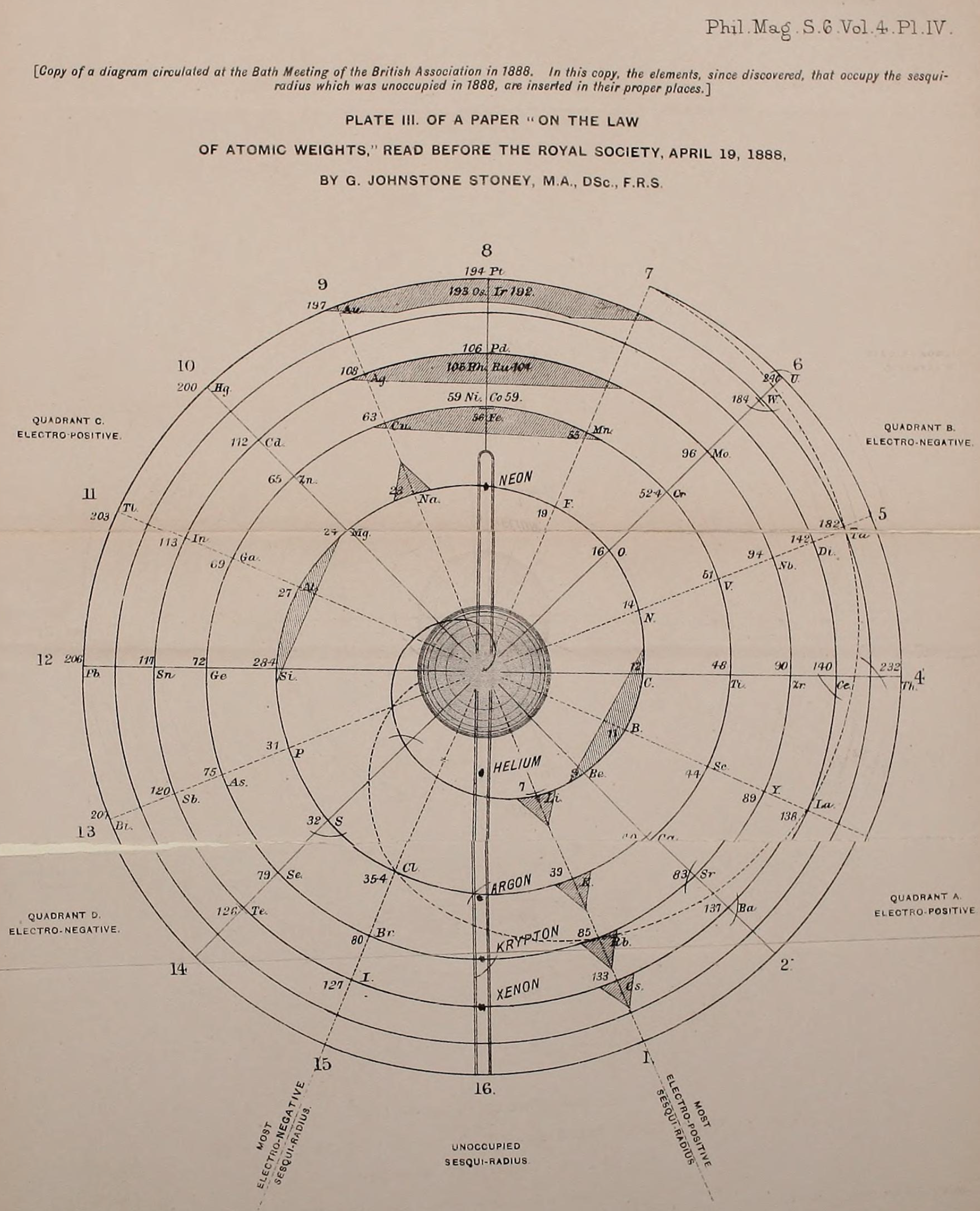
Thanks to René for the tip!
1891
Mendeleev's Table In English
A table, from Wikipedia, showing the periodicity of the properties of many chemical elements, from the first English edition of Dmitrii Mendeleev's Principles of Chemistry (1891, translated from the Russian fifth edition).
It is worth noting that this 1981 formulation shows the presence of gallium and germanium that were not his original table.
1891
Wendt's Generation-Tree of the Elements
From page 244 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:
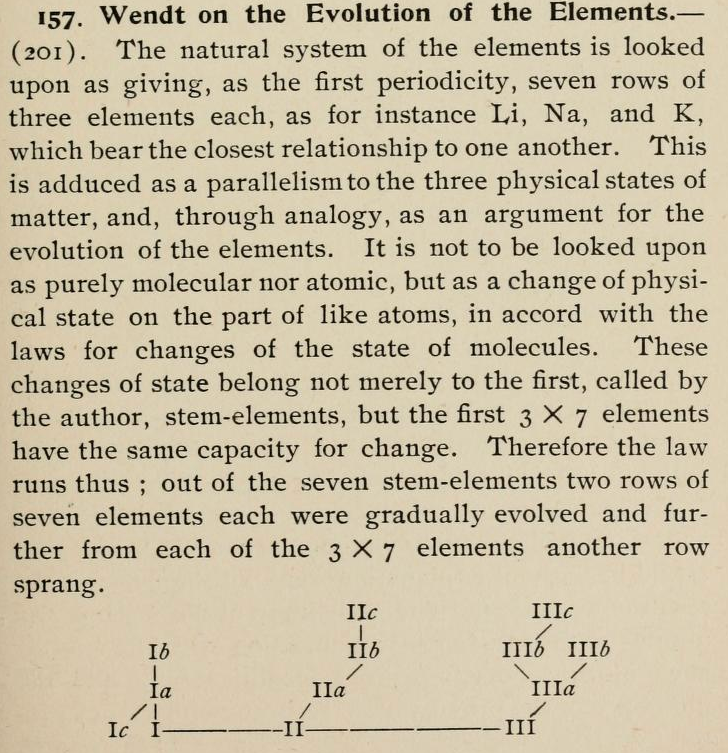
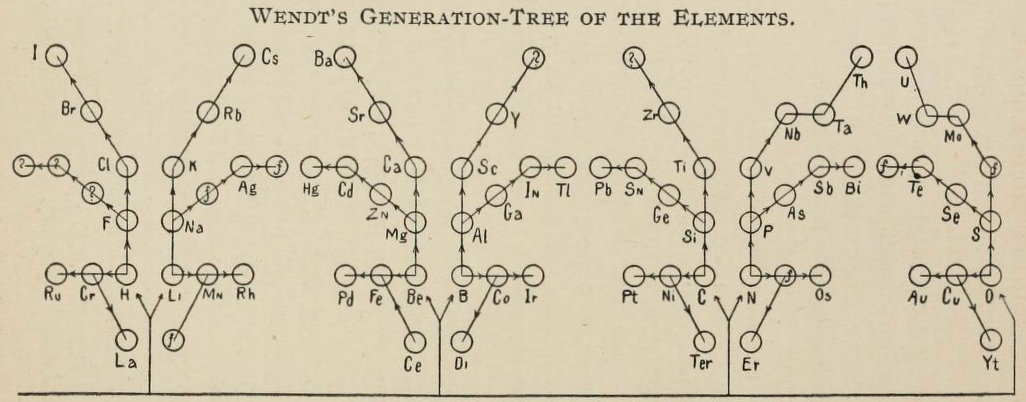
Thanks to René for the tip!
1892
Bassett's Vertical Arrangement
Bassett's Vertical Arrangement is actually designed to be a three dimensional formulation. Quam & Quam's review paper states:
"This table resembles Mendeléeff's vertical arrangement. The Cs period, however, starts far above the horizontal line of K and Rb, thereby giving space to the known and predicted elements of that period. The alkali metals appear in three horizontal lines. Co and Ni are arranged in order of their atomic weights.
"Bassett suggested cutting out the table and rolling it onto a cylinder of such circumference that similar elements would fall in line in Groups. For instance, Li, Na, K, Rb, and Cs would then fall on a line parallel to the axis of the cylinder."
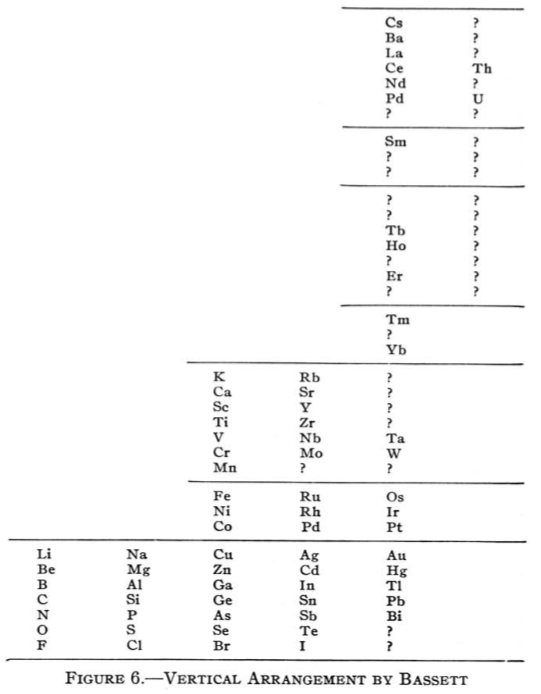
From Quam & Quam's 1934 review paper.pdf
1892
Bassett Dumb-Bell Form
The Basset 'dumb-bell' formulation, ref. H. Basset, Chem. News, 65 (3-4), 19 (1892).
The image is from Concept of Chemical Periodicity: from Mendeleev Table to Molecular Hyper-Periodicity Patterns E. V. Babaev and Ray Hefferlin, here.
1893
Rang's Periodic Arrangement of The Elements
P.J.F. Rang's The Periodic Arrangement of the Elements, Chemical News, vol. 67, p. 178 (1893)
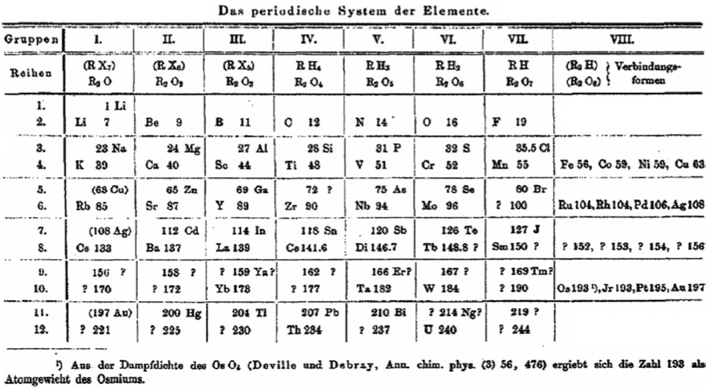
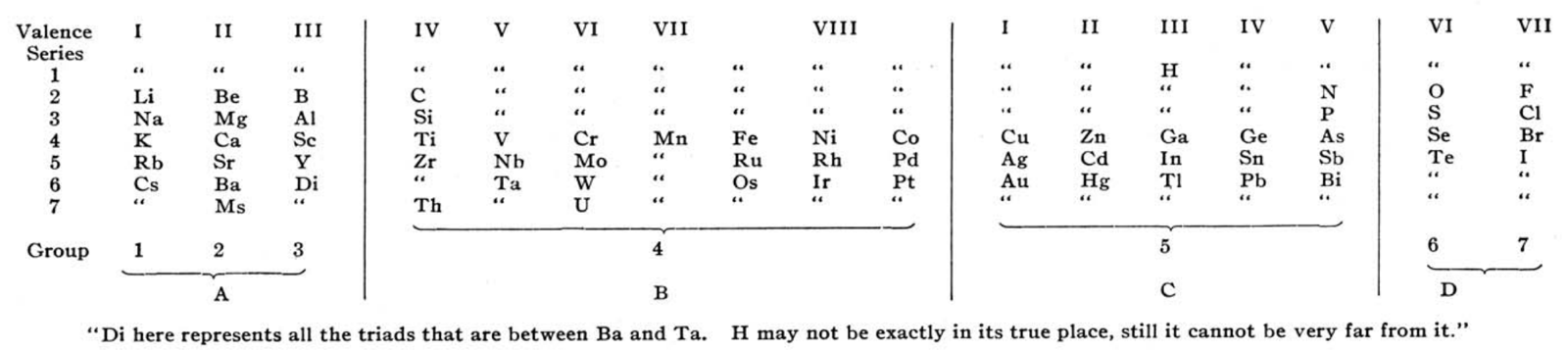
Observing that that Rang's table has four 'groups': A, B, C & D, René Vernon writes:
- Group A contains the strongest positive elements; group D the strongest negative elements. At such an early date, it's odd to see groups 1 to 3 categorised together.
- Group B are the elements with high melting points; "they are all remarkable for their molecular combinations" (presuamably, a reference to multiple oxidation states). At one side of group B are the "anhydro-combinations", probably referring to the simple chemistry of Ti, Zr, [Hf] Nb and Ta being dominated by insoluble oxides. At the other side are the "amin, carbonyl, and cyanogen combination", probably a reference to the group VIII carbonyls, as metal carbonyls had only just been discovered. Ni is shown after Fe, rather than Co.
- Group C includes the "heavy metals that have low melting points"; an early reference to frontier or post-transition metals, as a category.
- Rang says: ...if groups A and D be split up vertically in respectively three and two parts, the table presents seven vertical groups, and horizontally seven more or less complete series. Each group in each of the series 2 and 3 are represent by one element... The octave appears both horizontally and vertically in the table.
- Rang's reference to Di as representing all the triads between Ba and Ta kind of works since Hf would go under Zr, and that would leave 15 Ln or five sets of three. Thus, something like this:

Gd occupies the central position among the Ln. This arrangement won't fit however unless Rang envisaged all 15 Ln occupying the position under Y. - The location of H over | Ga | In | Tl, appears strange... but the electronegativity of H (2.2) is closer to B (2.04) than it is to C (2.55).
From Quam & Quam's 1934 review paper.pdf
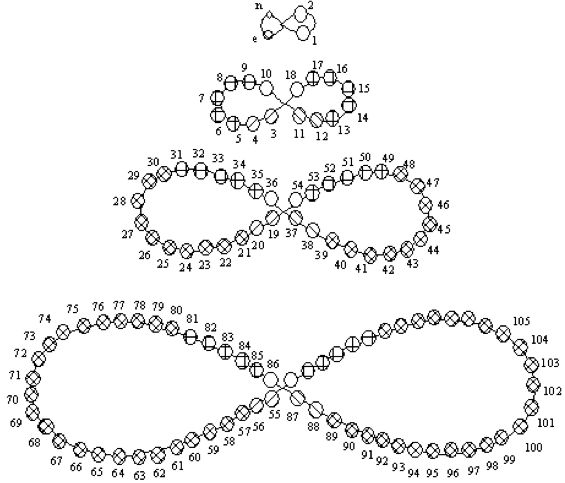
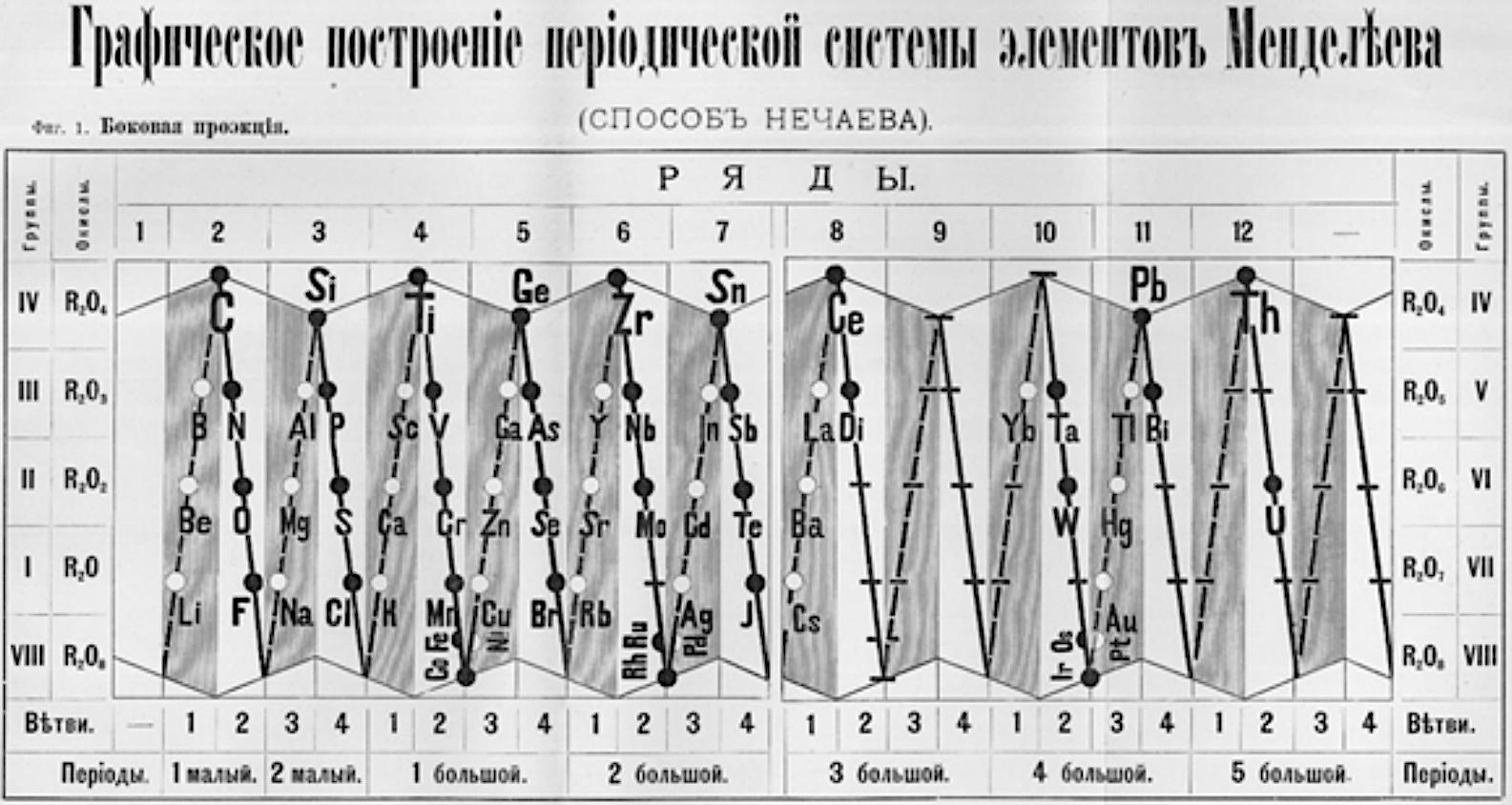
1893
Nechaev's Truncated Cones
René Vernon (who found this formulation) writes:
This weird and wonderful table appears in Teleshov & Teleshova (2019, p. 230). It is attributed by them to Nechaev (1893) and is apparently discussed by Ipatiev (1904):
- The caption accompanying the table is: "Scanning of the projection of rotational bodies in the form of truncated cones as used in Nechaev's spatial construction of the periodic system, 1893."
- Looking at the table it seems to anticipate, after a fashion, the double periodicity noticed by later authors.
- Alternatively, if turned on its side, it would be just five columns wide.
- Between Ce (ignoring Di) and Yb, there are spaces for 12 missing elements, which is one too many.
- Pulling Yb back by one position would have done the trick.
"... We would also like to mention one more version of the periodic table, namely the one offered by V. Ipatiev. Ipatiev's version was one of the first to have been applied in a school textbook, and is also concise and accompanied by a detailed methodological commentary. More specifically, Ipatiev is important in directing our attention to the fact that an essential feature common to all elements should be chosen if the elements are to be systematized. Furthermore, Ipatiev also offered another crucial insight in arguing that this selected feature must satisfy certain conditions, namely: 1) it must be measurable, 2) it must be common to all elements and 3) it must be paramount, i.e. that all the remaining properties of the elements must depend on it [Ipatiev]."
References:
Ipat'ev, V. & Sapozhnikov, A. (1904). Kratkij kurs himii po programme voennyh uchilishh [A concise course in chemistry for military academies]. Sankt-Peterburg: tip. V. Demakova.
Nechaev N. P. (1893). Graficheskoe postroenie periodicheskoj sistemy jelementov Mendeleeva. Sposob Nechaeva [Graphic construction of Mendeleev's periodic system of elements. Nechaev's way]. Moskva: tip. Je. Lissnera i Ju. Romana
Teleshov S, Teleshova E.: The international year of the periodic table: An overview of events before and after the creation of the periodic table. In V Lamanauskas (ed.).: Science and technology Education: Challenges and possible solutions. Proceedings of the 3rd International Baltic Symposium on Science and Technology Education, BalticSTE2019, Šiauliai, 17-20 June, 2019. pp. 227-232, (2019)
1894
Discovery of Argon
Ar
Argon, atomic number 18, has a mass of 39.948 au.
Argon is a noble gas.
Argon was first isolated in 1894 by Lord Rayleigh and W. Ramsay.
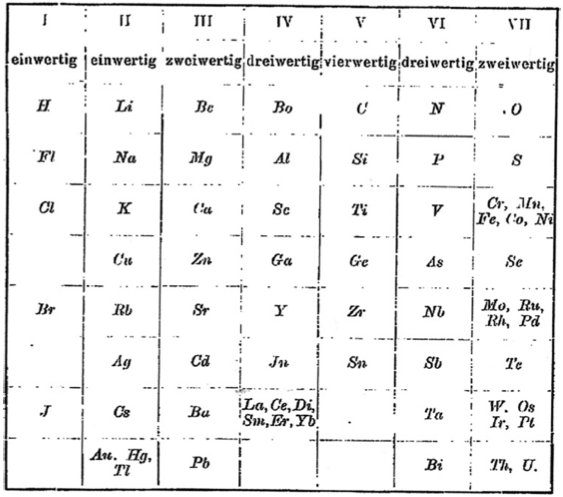
1895
Retger's Periodic Table
Periodic Table of Retgers with an intraperiodic accommodation of the rare earths. Retgers, J.W., 1895. Z. Phys. Chem. 16, 644:
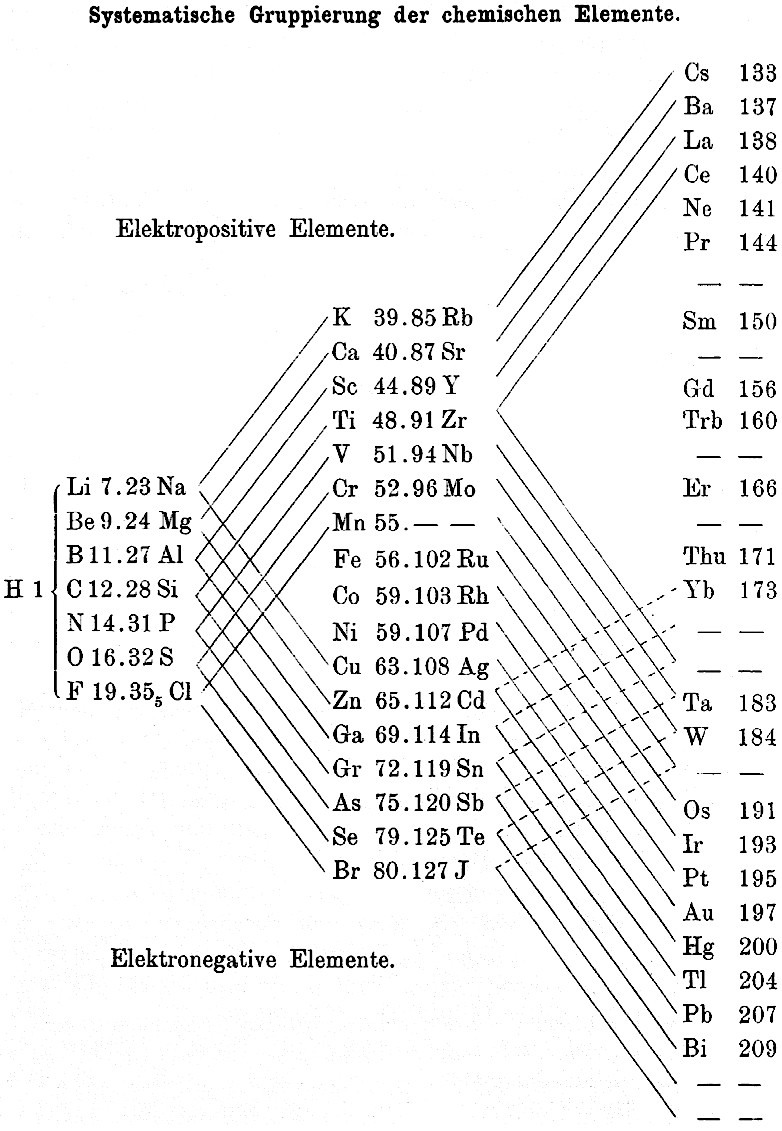
1895
Thomsen's Systematic Arrangement of the Chemical Elements
In 1895 the Danish thermochemist Hans Peter Jørgen Julius Thomsen proposed (Thomsen, J., 1895. Z. Anorg. Chem. 9, 190 & Chemical News, 72, 89–91, p. 90) a pyramidal/ladder representation.
Notice how this formulation identifies the electropositive & electronegative elements with respect to the periodic table, thirty years before Linus Pauling.
1895
Discovery of Helium
He
Helium, atomic number 2, has a mass of 4.003 au.
Helium is a noble gas, and is the second most abundant element in the universe after hydrogen.
Helium was first observed or predicted in 1868 by P. Janssen and N. Lockyer from solar spectra, and first isolated in 1895 by W. Ramsay, T. Cleve, and N. Langlet.
1896
Richards' Classification of The Elements
This is how the periodic table looked in 1896 in an article by Theodore Richards the pioneer of atomic weight measurement.
Notice all those elements at the bottom that could not be classified, explicitly listed including He and Ar :
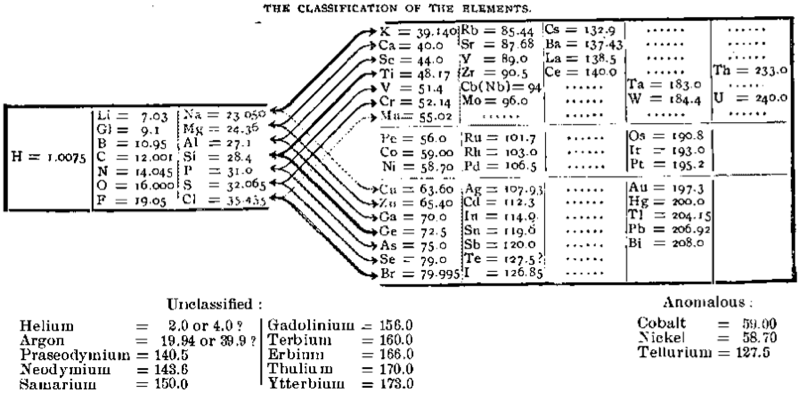
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
1896
Ramsay's Elements Arranged in the Periodic System
From The Gases of the Atmosphere, The History of Their Discovery by William Ramsay (and from the Gutenberg Project.)
The author writes pp 220-221:
"In 1863 Mr. John Newlands pointed out in a letter to the Chemical News that if the elements be arranged in the order of their atomic weights in a tabular form, they fall naturally into such groups that elements similar to each other in chemical behaviour occur in the same columns. This idea was elaborated farther in 1869 by Professor Mendeléeff of St. Petersburg and by the late Professor Lothar Meyer, and the table may be made to assume the subjoined form (the atomic weights are given with only approximate accuracy):—"
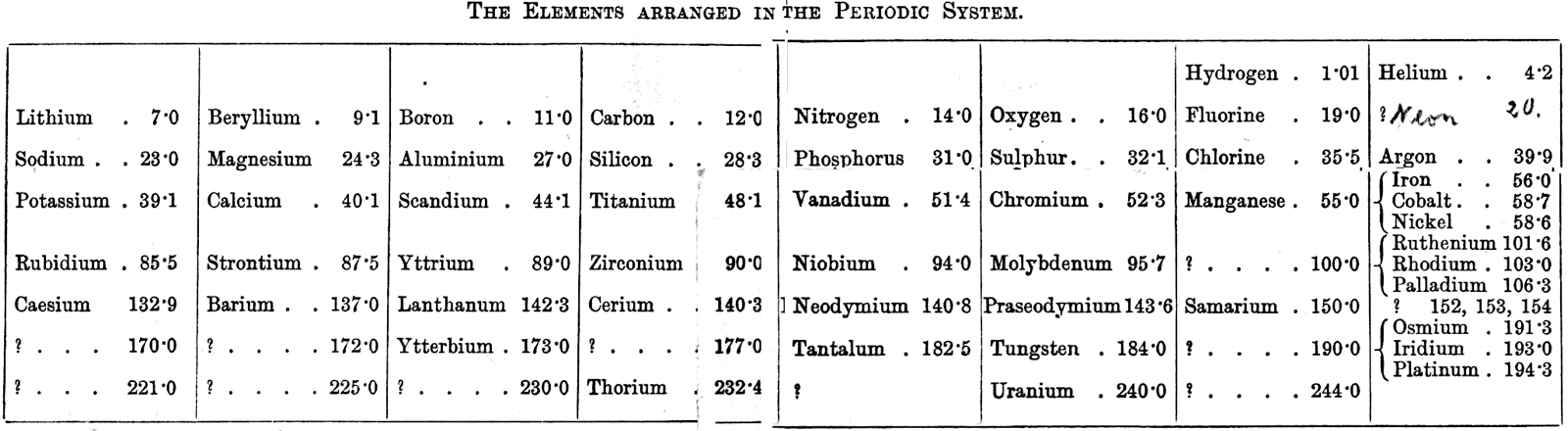
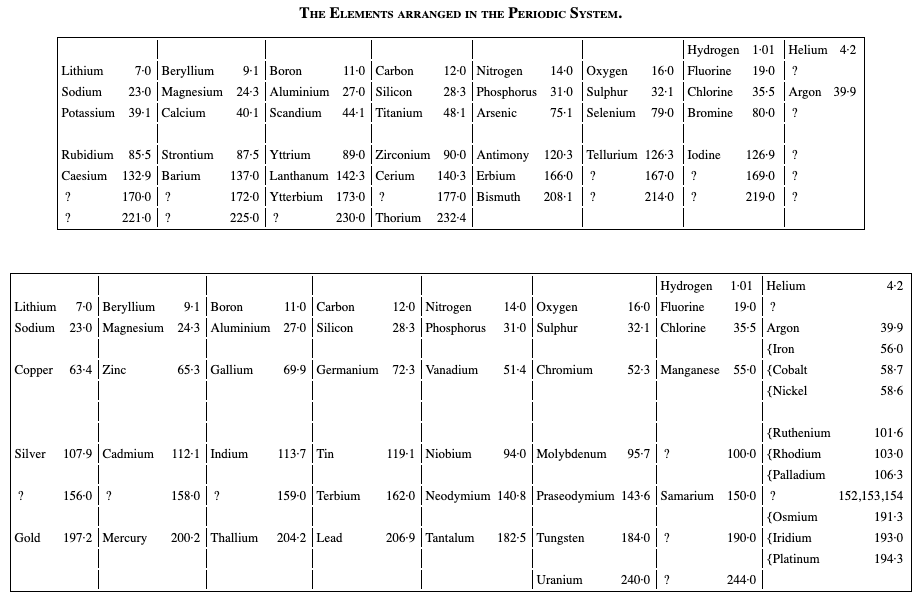
Thanks to René for the tip!
1898
Crookes' vis generatrix
Model of Crookes’ vis generatrix of 1898, built by his assistant, Gardiner. From: Proc. R. Soc. Lond. 63, 408.
The vertical scale represents the atomic weight of the elements from H = 1 to Ur = 239.
Missing elements are represented by a white circle. Similar elements appear underneath each other:
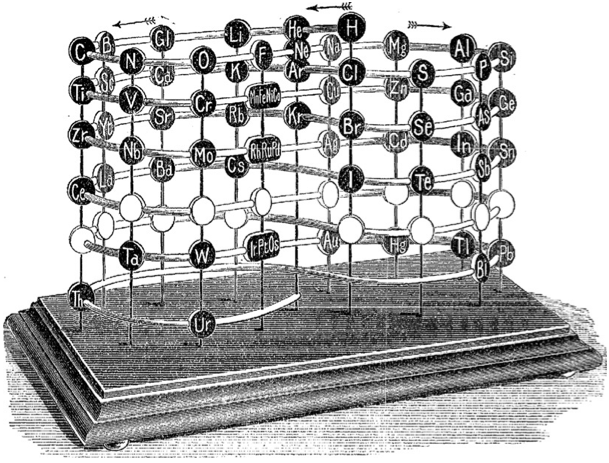
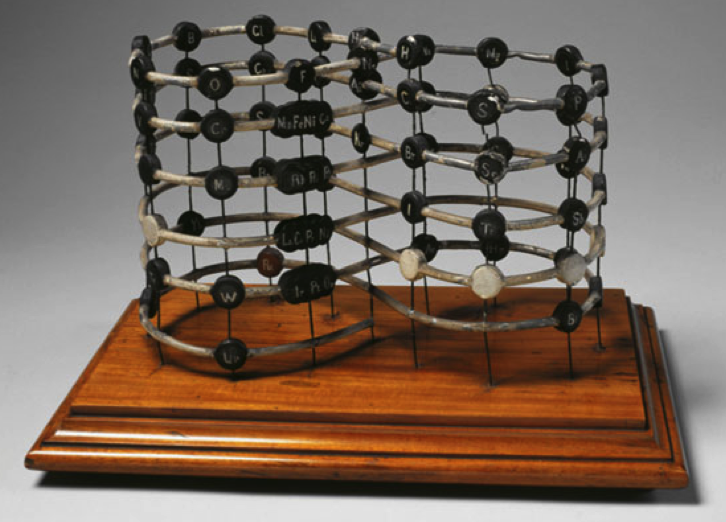
1898
Discovery of Neon
Ne
Neon, atomic number 10, has a mass of 20.18 au.
Neon is a noble gas. It is present in the atmosphere, 1 part in 65000.
Neon was first isolated in 1898 by W. Ramsay and W. Travers.
1898
Discovery of Krypton
Kr
Krypton, atomic number 36, has a mass of 83.798 au.
Krypton is a noble gas.
Krypton was first isolated in 1898 by W. Ramsay and W. Travers.
1898
Discovery of Xenon
Xe
Xenon, atomic number 54, has a mass of 131.293 au.
Xenon is a noble gas.
Xenon was first isolated in 1898 by W. Ramsay and W. Travers.
1898
Discovery of Polonium
Po ![]()
Polonium, atomic number 84, has a mass of 209 au.
Radioactive element.
Polonium was first observed or predicted in 1898 by P. and M. Curie and first isolated in 1902 by W. Marckwald.
1898
Discovery of Radium
Ra ![]()
Radium, atomic number 88, has a mass of 226 au.
Radioactive element.
Radium was first observed or predicted in 1898 by P. and M. Curie and first isolated in 1902 by M. Curie.
1899
Discovery of Radon
Rn ![]()
Radon, atomic number 86, has a mass of 222 au.
Radon is a noble gas and it is a radioactive element.
Radon was first observed or predicted in 1899 by E. Rutherford and R. B. Owens and first isolated in 1910 by W. Ramsay and R. Whytlaw-Gray.