The
Periodic Table: What is it Showing?
The Periodic Table of the Chemical Elements is a cultural icon and an extraordinary object in science space. This page explores what the periodic table is in terms of the philosophy of science.
Introduction
The long (32 column) periodic table is – in this author's opinion – the preferred formulation:
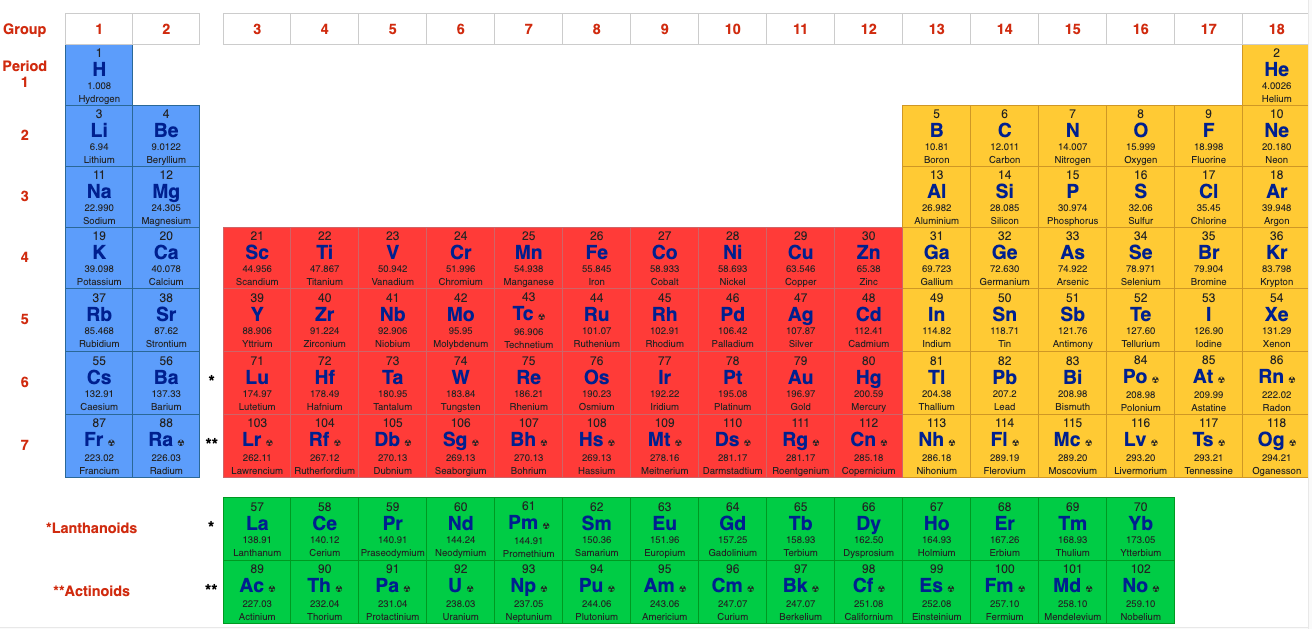
The 32 Column [Long Form] Periodic Table
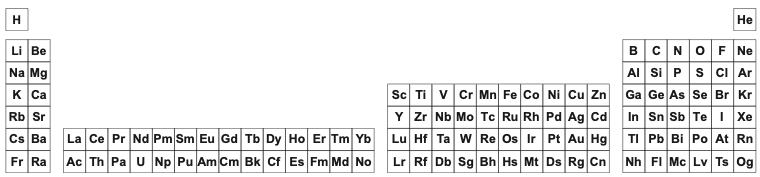
However, the long form is usually reduced to the 'classic' 18 column medium form periodic table, as used by WebElements and most other web sites & textbooks:
The WebElements 18 Column [Medium Form] Periodic Table
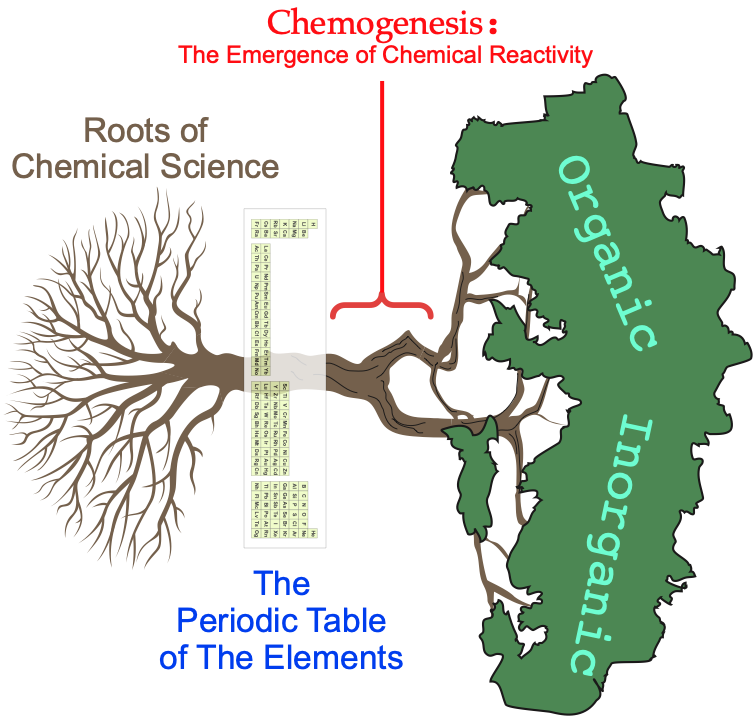
The chemogenesis web book explores how chemical reactivity emerges from the periodic table of the elements using a root-trunk-branch chemistry-tree metaphor, with the periodic table situated at the base of the trunk:
The question is: What actually is the periodic table, and what it is showing?
It transpires that matters are a little more involved than they may at first appear...
Philosophy, Chemistry & The Periodic Table
Philosophers of chemical science consider the elements in two distinct ways:
- Firstly, there is
the chemical element as the basic element,
that is the abstract or transcendental element, the essence of the element, the element as "a bearer of properties" but not having any actual
properties, except for atomic number Z. (Historically, this was atomic weight, now it is Z.) Chemical symbols,
such as H and Cu, and names are assigned to the basic element.
- Secondly, there is the element as simple substance. A real physical 5.00g piece of copper metal has numerous, measurable, intrinsic properties such as: density, conductivity, colour, melting point, molar volume, etc.
Crucially, only the basic [transcendental] element survives in a compound. These two ideas – basic element vs. simple element – can be distinguished with the statement:
"Sodium's metallic properties and chlorine, the green gas, do not exist in the colourless, crystalline ionic salt, the substance sodium chloride."
In other words, the "silvery metal aspect of sodium" and the "green gas aspect of chlorine" do not exist in the binary compound.
These matters are discussed in a paper by Eric Scerri, HYLE--International Journal for Philosophy of Chemistry, Vol. 11, No.2 (2005), pp. 127-145: Some Aspects of the Metaphysics of Chemistry and the Nature of The Elements
Summarising Scerri's arguments:
- There is a metaphysical
view about the nature of the elements as basic substances and bearers
of properties that goes back to the ancient Greeks, long before the
discovery of atoms.
- Mendeleev insisted
that his periodic classification system concerned the elements as basic
substances possessing only one attribute, atomic weight.
- Paneth, one of
the founders of modern radiochemistry took Mendeleev's view about the
nature of basic and simple substance, but changed the basic/transcendental/abstract property
of an element from atomic weight/mass to atomic number, Z.
- Elements as basic substance represent natural kinds, a well understood philosophical position concerning the nature of classification. Elements as simple substances fail the natural kind test, due to the existence of isotopes and allotropes, etc.
Eric Scerri points out that the periodic table has, at times, been characterised as a:
- representation
- ordered domain
- classification
- system
- model
- law
- theory
This author agrees with Scerri that the periodic table is an ordered domain. But the periodic table is also a schema, a 'map', that that can be used to organise information, data & knowledge concerning the chemical elements.
Periodic Tables on Walls and In Books: What are they showing? Why is there a problem?Periodic tables use the design as an organising schema to list the chemical elements and to present physical data & material properties of the elements. In their simplest form, the periodic tables show elements with only the atomic number Z and the element symbol. Strictly, this is showing the elements as the basic [transcendental, abstract] substance. However, they may go on to state:
In this author's opinion, there has been a logical sleight-of-hand. The metaphysical periodic table of abstract, basic substances is being passed off as a periodic table of the material properties of simple substances, which is not the same thing at all. This causes confusion as to what exactly it is that a particular periodic table is showing. |
Morphing Multiple Periodic Tables
At least three periodic tables can be identified:
- The periodic table of basic elements with atomic number Z [and so atomic symbol: H, He, Li, etc.]
- The periodic table of gas phase atoms with their associated ionisation energies, spectra, etc.
- Then there is the periodic table of chemicals in bottles, the actual materials under room temperature and pressure [standard] conditions.
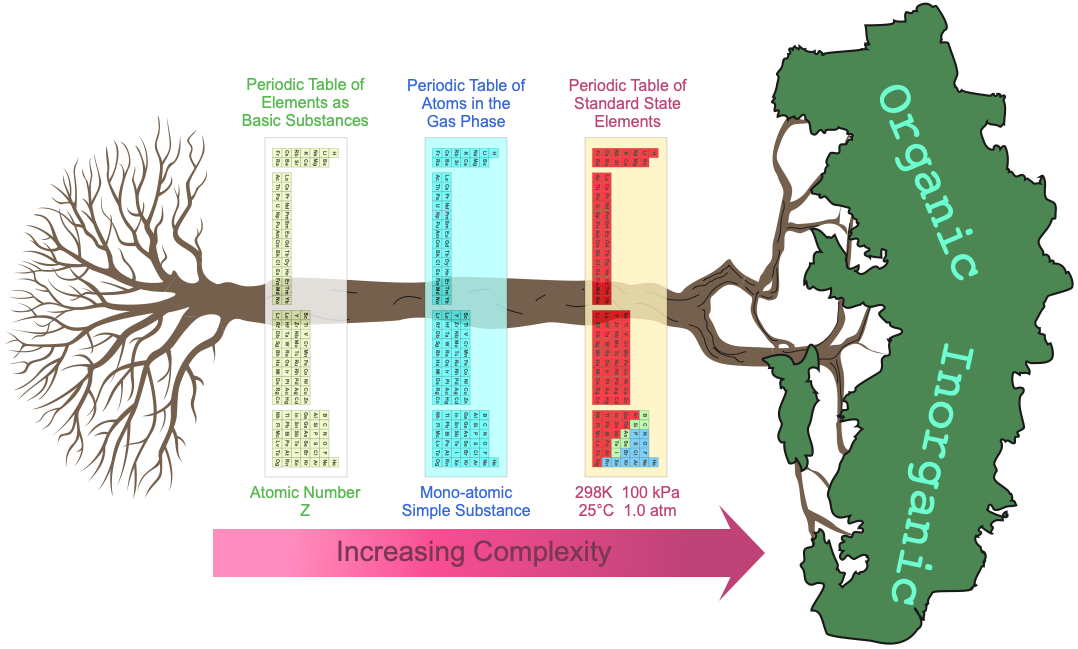
When moving across these three periodic tables, the system complexity and the number of properties dramatically increases.
- Elements in the periodic table of basic substances have only the property: atomic number Z.
- The gas phase atoms have properties of: ionisation energy, electron affinity, atomic radius, atomic emission spectrum, etc.
- The standard state elements have dozens of properties: electrical conductivity, density, molar volumes, colour, crystal structure, hydration enthalpy, etc. As substances, the elements may present as metals, molecular van der Waals materials, network covalent substances or intermediate metalloid.
And there are yet more periodic tables:
Periodic tables may show the phase (solid, liquid or gas) state of the elemental materials at the standard temperature of 25°C and at other temperatures, for example 2025°C (from pTable):
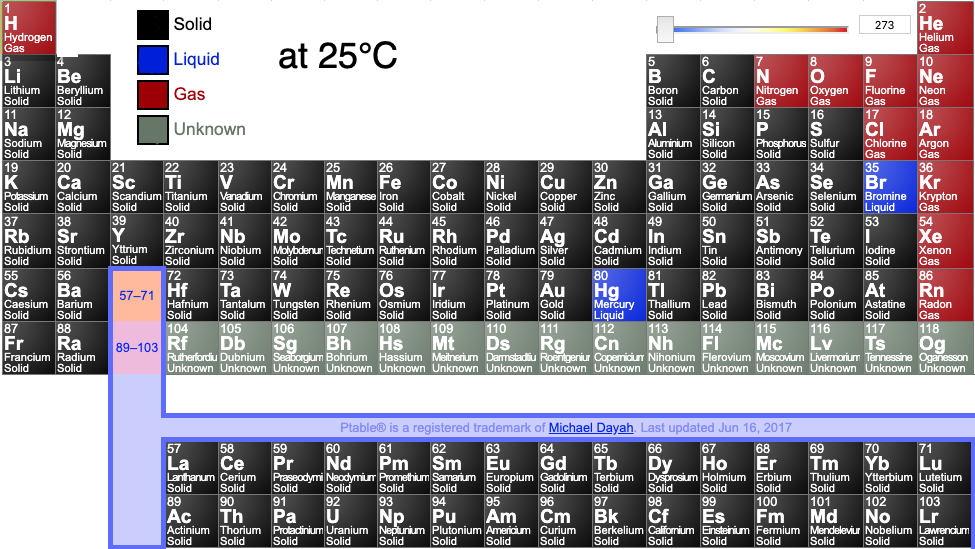
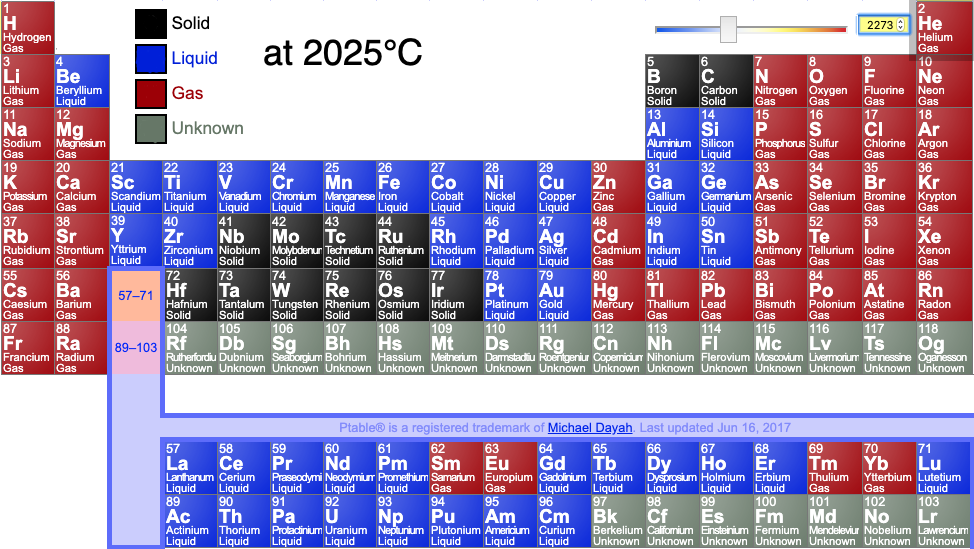
Or:
- Dates of discovery
- Countries in which the discovery was made
- Uses
- NMR properties
- etc...
All of these many & various periodic tables morph into each other to give the compound object that is commonly known as [and presented as] The Periodic Table.
- Probably the periodic table in web space that best illustrates this multi-morphing propensity is Michael Dayah's dynamic pTable.
Periodic Table of The Elements as Basic Substances
The long form periodic table of basic, abstract, transcendental substances showing the only property, atomic number Z:
Periodic Table of Basic Substances (Atomic Number Z)
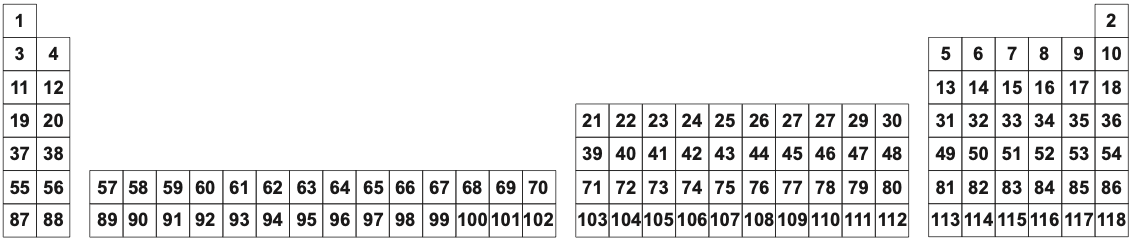
Element symbols and names are assigned to the atomic number Z:
Periodic Table of Basic Substances (Atomic Symbol)

In the basic substance formulation, oxygen atomic number 8 is "O" and not the common molecule "O2". Likewise, sulfur (Z = 16) is S and not the yellow solid S8.
The usual periodic table schema simply shows the element symbols in their respective periods, groups & blocks.
This, or an equivalent formulation, and there are many – see the next page of this web book – is the periodic table as Mendeleev would have intended it: a schema showing the elements as basic substances with their positions in the schema emphasising the periodic law:
"The periodic law is the principle that certain properties of elements occur periodically when arranged by atomic number. These similarities can be reflected best by a table, so that commonalties between elements appear both in rows and in columns of the table." Wikipedia
Author's Analysis & Comment Arranging the chemical elements by atomic number, Z, gives simple list:
By placing the chemical elements into a table formulation explicitly assigns group & period co-ordinates to the entities that make up the [periodic] table. For this to make any sense, some other property is being implicitly implied.
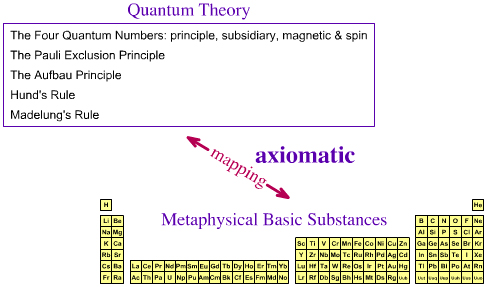
It follows, IMHO, that properties that pertain to the periodic table schema – group, period & block – are [must be] implicit properties of the basic element. Thus – in this author's opinion – the quantum numbers [see the previous page of this web book] must be a basic elemental property. However, it should be said not everybody agrees with this analysis! MRL's Basic Element Properties:
Z = 8 ∴ symbol = O ∴ electronic configuration = 1s2, 2s2, 2p4
|
Periodic Table of Gas Phase Atoms
The chemical elements as real, simple substances can be physically normalised by studying ground-state, monoatomic gas phase atoms of the material substance.
- For some elements
entering the monoatomic gas phase is trivial: the group 18 rare gases, He, Ar, Ne, Kr, Xe & Rn are already monoatomic, gas
phase entities under the standard conditions of 298K & 100kPa (25°C and 1.0 atm). In other words, they are naturally
in the desired state.
- Chlorine and oxygen are diatomic molecular
gases at room temperature, but thy can easily be converted into the atomic gas by heating at low pressure.
- Carbon boils
at 4027°C making it difficult to obtain a vapour of ground-state
carbon atoms – carbon gas, C(g) – but it is possible.
- Moving an element from its standard state to the gas phase equates the enthalpy of atomisation, ΔatH
The periodic table of ground state gas phase atoms is known, and it represents the periodic table of the very simplest simple substances:
Periodic Table of Gas Phase Atoms

Apart from the title, the graphic is exactly the same as the periodic table of basic substances, but this periodic table represents real chemical entities with actual, measurable, physical properties, including:
- Average atomic mass
- Atomic radius
- Accurate mass & abundance of the constituent isotopes
- Effective nuclear charge
- Electron affinity
- Electron binding energies
- Ionisation energies: 1st, 2nd, 3rd...
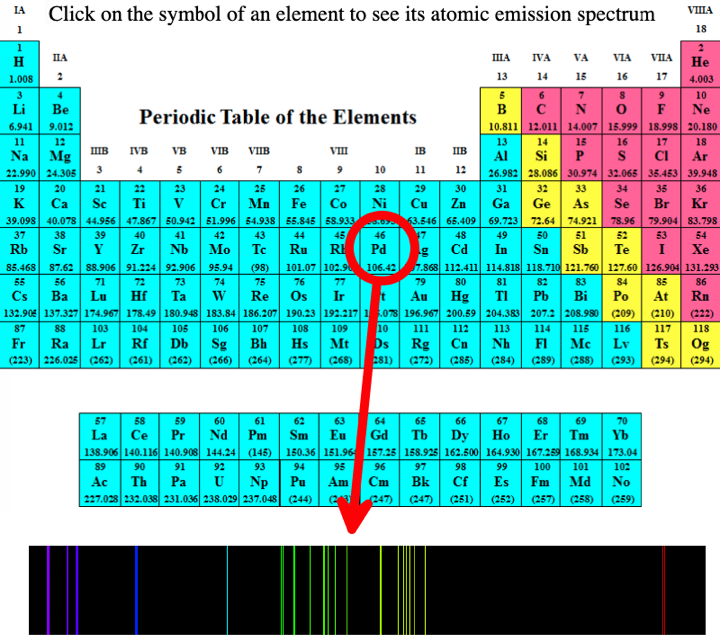
- Emission spectra. Penn State university has a dynamic periodic table, here, that shows the atomic spectra of all the elements in the gas phase:
Many modern technologies utilise gas phase atoms, including:
- Sodium lamps for street lighting
- Caesium atomic
clocks
- Most of the heavy and short lived man-made transuranic
elements, 104 – 118, are only known as isolated gas phase atoms.
- Atomic adsorption spectroscopy (AAS) and ICP-OES for elemental analysis.
- When an atom is studied in silico (constructed or mathematically modelled in a computer) the atom is effectively an isolated gas phase species.
Crucially, when we consider the electronic structure of an atom [or the orbital structure of a molecule, for that matter], we are considering the atom to be an isolated entity, exactly as it would be in the gas phase.
Periodic Table Chemical of Substances Under Standard Conditions
Under standard conditions, 298K and 100kPa (25°C & 1.0 atm), the chemical elements as simple substances – chemical reagents, real chemicals in bottles – present as:
- Gases, liquids
or solids
- Metals, metalloids
or non-metals
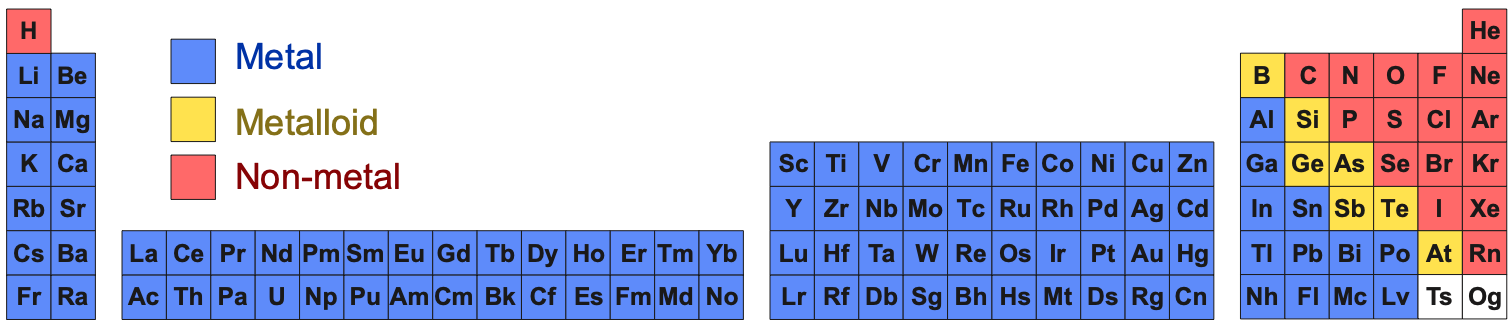
- Metallic, network covalent or molecular materials (read more here):
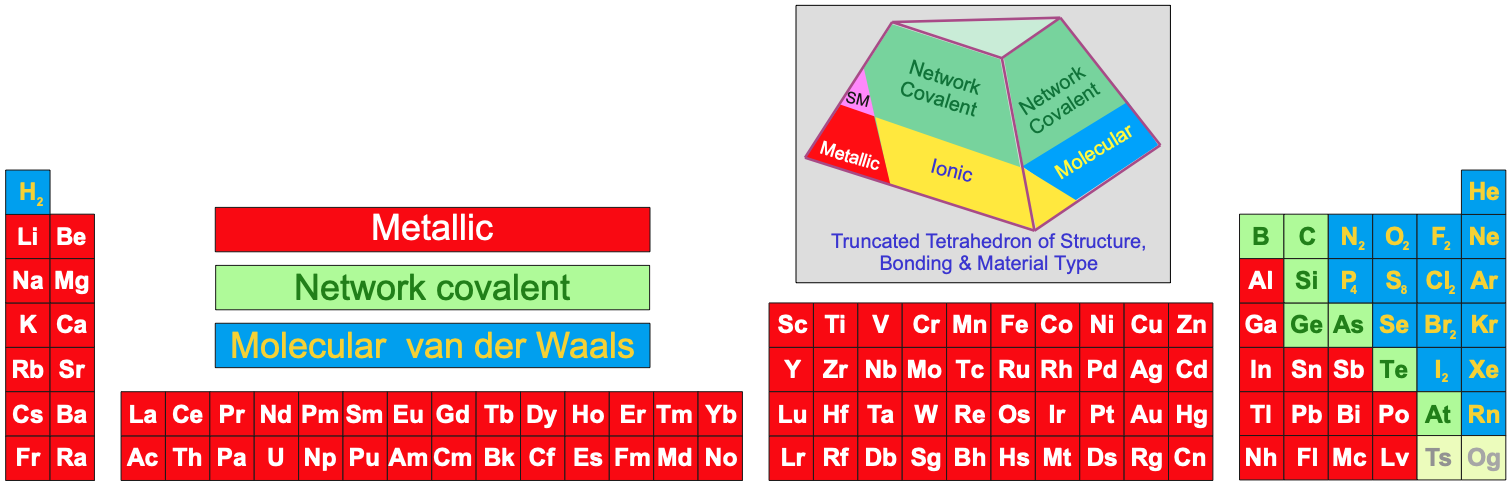
Note:
Several Group 4, 5 & 6 (14, 15 & 16) elements have multiple allotropes (structural forms).
Carbon can exist as graphite, diamond, C60, single walled nanotubes, etc.
The P4 form of phosphorus is defined as the standard state of the element.
The structure of astatine is actually unknown, but it may be isostructural with iodine
or it may be a metalloid.
The chemical elements as material substances have many properties, including [from WebElements: Cu]:
- Properties at standard conditions 25°C and 1.0 atm: crystal structure, molar volume, hardness, etc.
- Phase changes under non-standard conditions: boiling point, allotropes, etc.
- History
- Biology
- etc.
|
Abundance of
elements (Earth's crust) |
Hardness - Vickers |
NMR relative sensitivity |
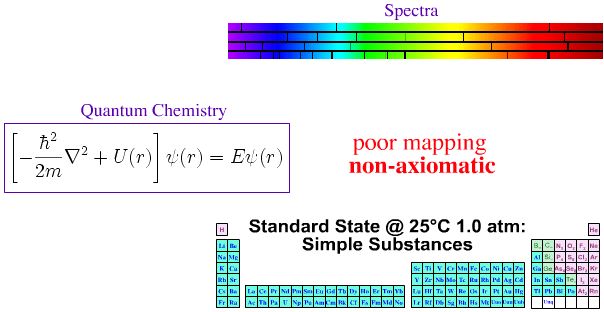
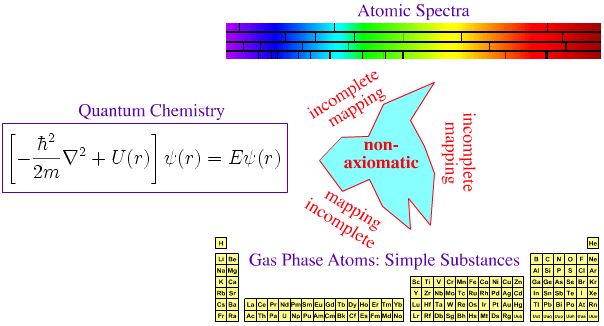
Quantum Mechanics, Atomic Emission Spectra & The Periodic Table
In the first half of twentieth century, much effort was expended trying to make the periodic table of the elements axiomatic ("self-evident" or "unquestionable"), ie. trying to fully understand the Mendeleev system in terms of quantum mechanics.
In 1929 Paul Dirac famously claimed this situation had been fully and completely achieved in principle:
"The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble."
P.A.M. Dirac, Proc.R.Soc.Lond.Ser.A 123 (1929) 714
We certainly teach school and university students that "the periodic table is fully understood in terms of electronic theory", and this line of reasoning is advanced elsewhere in this web book, here and the HyperPhysics site, here.
The argument is put forward that:
- Each gas phase element produces a unique set of spectral lines.
- The spectral lines act as a fingerprint for the presence of an element (and the signature is quantitative).
- The pattern of spectral lines can be fully understood in terms of quantum mechanics via the Schrödinger wave equation, and its developments: the relativistic Dirac equation, the Lamb shift and Quantum Electrodynamics (QED).
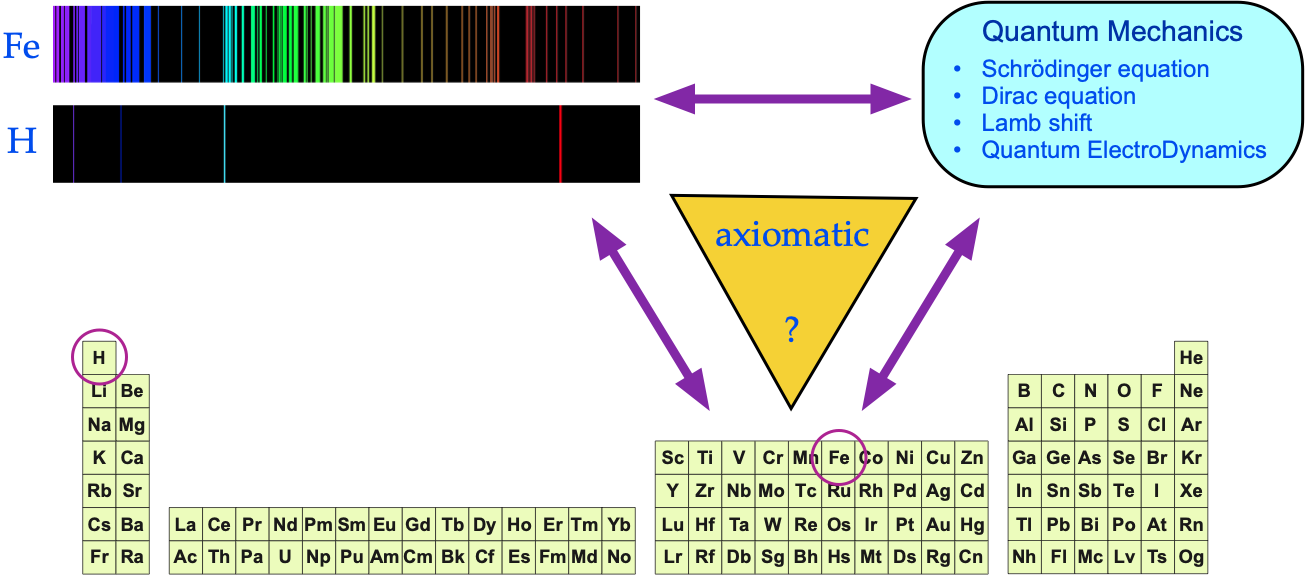
Yes, there is an empirical (experimental) correspondence between gas phase atoms and their spectra, But is there a 1-to-1-to-1 correspondence between QM, spectra and the periodic table?
Eric Scerri: Philosopher, Theorist, Chemist, Author
Eric Scerri disputes the full and complete axiomatic mapping between theory and the periodic table:
"Electronic configurations are not [fully] reduced to quantum mechanics nor can they be derived from any other theoretical approach. They are obtained by a mixture of spectroscopic observations and semi-empirical methods like Bohr's aufbau scheme".
Has The Periodic Table Been Fully Axiomatized? Erkenntnis, 47, 229-243, 1997
|
Eric Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006. Read an interview with the author, here, and a review of the book here. |
The reason for the discrepancy concerns multi-electron atoms, ions and molecules.
- The Schrödinger
wave equation can only be solved analytically for one electron systems
like the hydrogen-atom, H•, and other one electron systems: He+,
Li2+, Be3+, etc., Wikipedia. For multielectron
systems, approximations in the math have to be made to deal with electron-electron
interactions and correlations. Multi-electron atoms are complex
objects, in the systems sense. The mathematical
techniques employed to describe chemical systems are usually pragmatic
rather than rigorous, and they are often semiempirical: ie partially
based on experimental data.
From the Wikipedia: "For atoms with two or more electrons, the governing equations can only be solved with the use of methods of iterative approximation. Orbitals of multi-electron atoms are qualitatively similar to those of hydrogen, and in the simplest models, they are taken to have the same form. For more rigorous and precise analysis, numerical approximations must be used. Atomic orbitals are often expanded in a basis set of Slater-type orbitals which are orbitals of hydrogen-like atoms with arbitrary nuclear charge Z."
- The effect is to produce nice fast computer code that efficiently predicts atomic & molecular energies, geometries and spectra, etc., but at the expense of the theory being fully axiomatic: formally the logic of the underlying theory becomes blurred. As a result, we get a useful model but not a mathematical proof.
On this page we identify three different periodic tables:
- Periodic table of basic substances
- Periodic table of gas phase atomic simple substances
- Periodic table of standard state material simple substances
Are any of these periodic tables axiomatized with respect to theory?
Note that a distinction has been introduced between "quantum chemistry", the techniques, methodologies and computer software used by physical chemists and chemical physicists, and the underlying quantum mechanics in the form of quantum electrodynamics (QED), the most accurate and precise theory known to humankind. However, chemical problems are simply too involved [currently] to be studied by QED, although in principle they can be.
Q: Has The Periodic Table of Chemical Substances Under Standard Conditions Been Axiomatized?The elements-as-chemicals periodic table – real simple material substances under standard conditions of temp & pressure – is certainly not axiomatized. Quantum chemistry calculations cannot predict the equation of state of an element. The Schrödinger wave equation without mathematical approximation cannot be used to predict that sulfur exists in S8 rings, that copper is a reddish coloured metal or that mercury is a liquid at room temperature.
Q: Has The Periodic Table of Gas Phase Atoms Been Axiomatized? Multi-electron atoms, even as isolated gaseous atoms, are too complex to be understood fully and exactly... although modern quantum chemistry mathematical modelling techniques do give very good and useful answers. There is no formal proof of one-to-one-to-one correspondence between quantum chemistry methodology, atomic spectra and the periodic table of gas phase atomic simple substances, although the predictions are useful:
Q: Has The Periodic Table of Metaphysical Basic Elements Been Axiomatized?The essential, metaphysical basic elements do not have properties other than atomic number, group and period... so we can can ignore spectra and simply concentrate on the pattern of the periodic table schema.
It is proposed here that yes, the periodic table of metaphysical basic substances is axiomatic with respect to theory, in that the pattern of the periodic table can be deduced from the quantum theory directly.
SummaryThe techniques of quantum chemistry cannot/do not completely – axiomatically – describe gas phase atoms (except H•, He+, Li2+, etc.), because multi-electron systems are too complex to be described analytically. However, the various quantum chemistry techniques/models are good enough for the periodicity of the metaphysical basic substances to be mapped to the periodic tables of gas phase and material simple substances. The periodic table of metaphysical, essential, basic elemental substances can be reduced to quantum numbers and simple rules (the old quantum theory)... but Richard Feynman told us (here): "I think I can safely say that nobody understands quantum mechanics." In other words, we cannot unpick quantum mechanics and ask "where it all comes from": it is simply how our world works. The relationship between the old quantum theory and the Schrödinger wave equation is mysterious... |
The Periodic Law, The Nature of Periodicity & Electronegativity
The periodic law is a property of the periodic table. As a consequence, periodicity and periodic trends get mapped to the element-the-basic-substance along with block, period & group.
Average atomic mass maps closely – but not exactly – to atomic number, and there are anomalies. However, most commentators would agree with the statement that "generally atomic mass increases with atomic number, Z" and this is a classic manifestation of the periodic law.
Electronegativity is a parameter of huge importance to understanding and predicting chemical structure and reactivity.
There is a clear electronegativity trend across the periodic table in its long form from the (Group 17) top-right where the most electronegative elements are found to the bottom-left where there are electropositive elements. This trend is a manifestation of the periodic law.
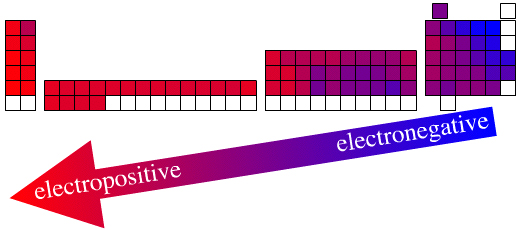
This author holds that while the actual elemental electronegativity data, for example (revised Pauling):
H 2.20
Li 1.00
F 3.98
O 3.44
Cl 3.16, etc.is a property of the simple elemental substance, the periodic trend is a manifestation of the periodic law that is inherent to the periodic table.
Like atomic number, Z, electronegativity is an atomic property that is conserved in molecules and ionic substances.
It follows that relative electronegativity is a basic property and not a simple property.
From the underlying quantum patterns element Z = 9 [Period 2, Group 17, fluorine, F] is electronegative, and indeed *must* be the most electronegative element. It is just how quantum mechanics works (and we do not understand QM in terms of a deeper theory).
Thus, the relative electronegativity of element Z = 9, for example, is a basic (essential) elemental property that comes from periodicity and the periodic law, even though the absolute electronegativity of the simple (real) elemental substance is 3.98.
Basic & Simple Substance, Theoretical & Practical Chemistry: Does Any of This stuff Matter?
The reader may consider that the concept of element-as-basic-substance and element-as-simple-substance to be an arcane distraction limited to the study of the periodic table. However, the idea is actually rather general and has implications for how we understand and teach the subject of chemistry.
Beginning students of chemistry always have access to periodic tables, but unfortunately not the PT they actually need.
Students are expected to know that in all equations hydrogen is molecular should [nearly always] be written as H2. Likewise, nitrogen is N2, oxygen O2, fluorine F2, chlorine Cl2, bromine Br2 and iodine I2, and should always be written as the dimeric species. But somehow students are expected to know that molecular sulfur, S8, and phosphorus, P4, should be written as S and P.
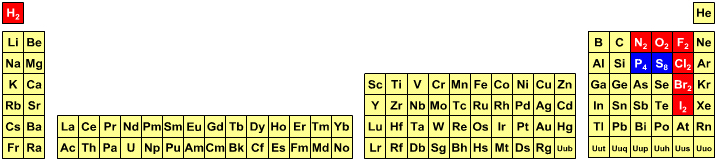
These matters are explored further elsewhere in the Chemogenesis web book.
| Quantum Numbers to Periodic Tables |
Periodic
Table Formulations
|
© Mark R. Leach 1999-
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions or periodic table representations not shown on this page
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.