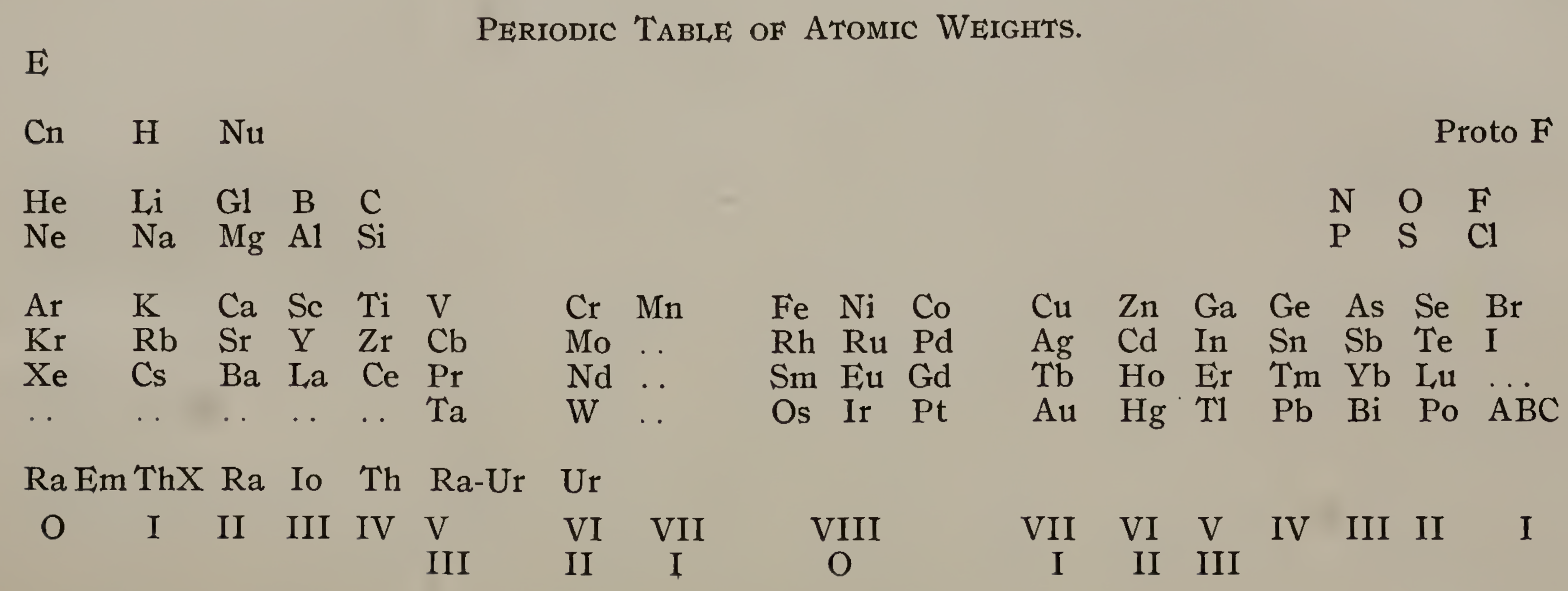
The INTERNET Database of Periodic Tables
The 8 Periodic Tables most recently added to the database:
2019
Ossmi LH & Chasib's Periodic Table
Al Ossmi LH & Chasib K F, A New Method of Elements Arrangement to Reattach the F–Block Elements of Lanthanides and Actinides in the IUPAC's Periodic Table of Elements, Med & Analy Chem Int J, 3, 4,2019, pp 1–18. DOI 10.23880/macij-16000148
The new 3-dimantional layout of the periodic table graphically shows the main body parts of the table and also these vertical and horizontal coordinators of Periods, Groups, and Nadas.
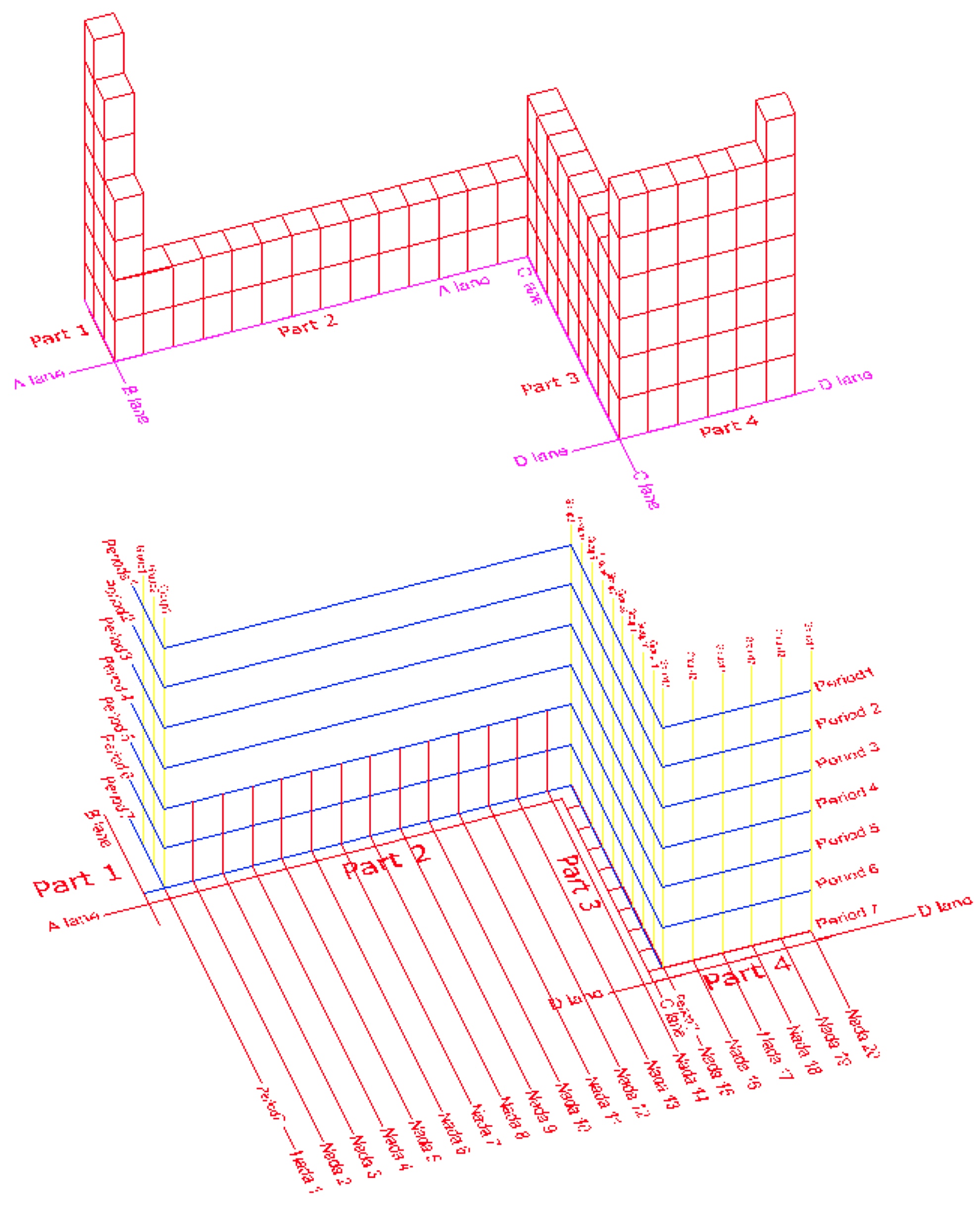
1911
Emerson's Periodic Table of Atomic Weights
Emerson BK, Helix chemica: A study of the periodic relations of the elements and their graphic representation, American Chemical Journal, vol. 45, pp. 160–210 (1911). The formulation below appears on page 173; a scanned pdf version of the paper can be viewed here.
René Vernon writes:
Emerson includes two elements before hydrogen: "E" (either the luminiferous ether or the electron) and "Coronium". There are also two elements between hydrogen and helium: "Nebulium" and "Protofluorine".
This is the first time I have seen a PT showing four extra elements and where they are supposed to fit.
After La, Emerson incorporates 13 lanthanides (Ce to Lu) as transition elements into his 7th period.
Emerson missed dysprosium, between Tb and Ho.
"A, B and C" at the bottom right are supposed to be 'halogen emanations'.
Mark Leach adds that Emerson's very odd Periodic Table of Atomic Weights does not actually show any atomic weights.
2020
University of UNAM Periodic Table
Mexico City, University of UNAM Chemistry Department
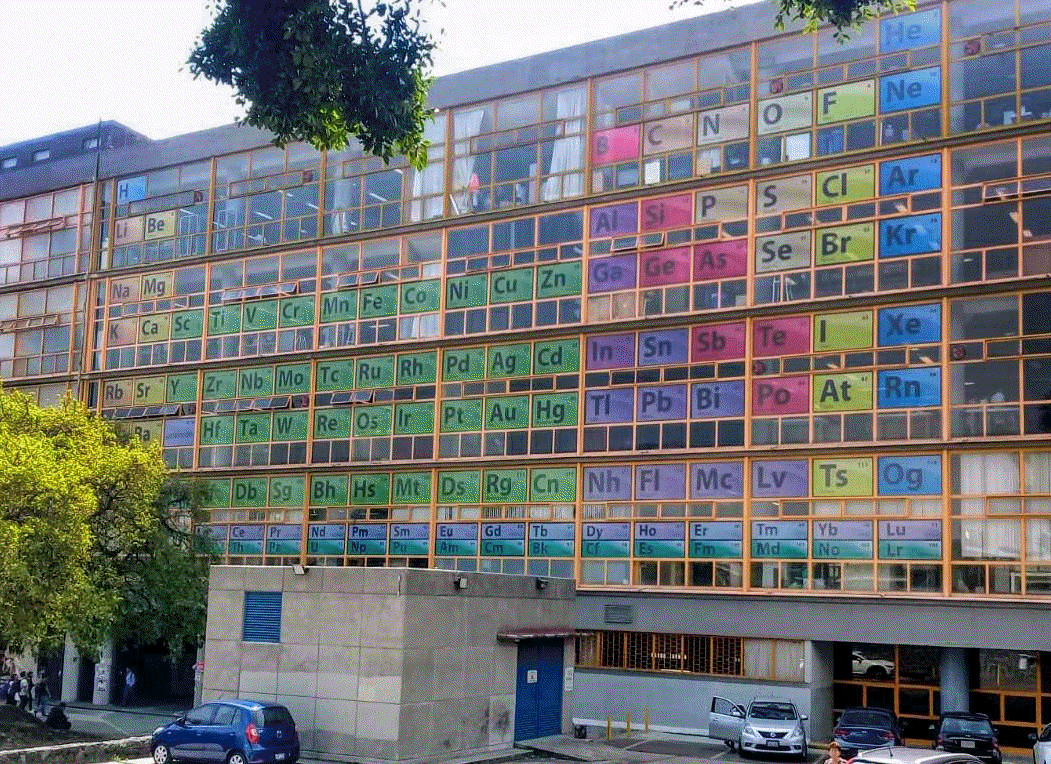
Thanks to Marcus for the tip!
2024
Periodic Table Regions
Permanent link to the comic: https://xkcd.com/2913/
Image URL (for hotlinking/embedding): https://imgs.xkcd.com/comics/periodic_table_regions.png
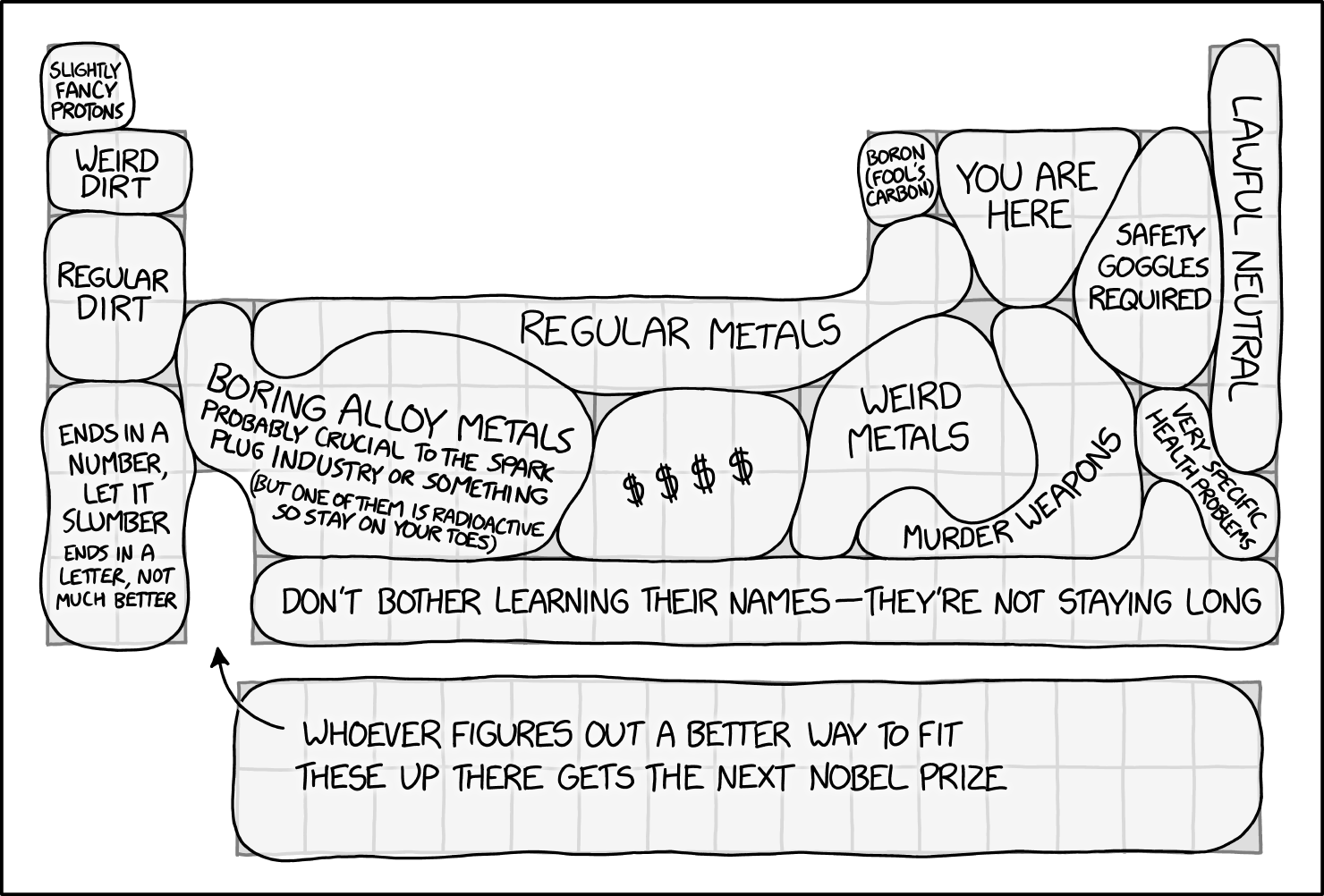
Thanks to Marcus for the tip!
1919
Snyder's Fundamental Periodic Table of The Elements
Snyder MB 1919, The Fundamental Periodic Table of the Chemical Elements, filed in Congressional Library, Washington.
René Vernon writes:
"Notable for:
- Its attempted integration of the Ln and An into the short form of the periodic table
- Placement of H over He, Li and F
- Elements 108 = Pleon; 126 = Akron; 143 = Ultine"
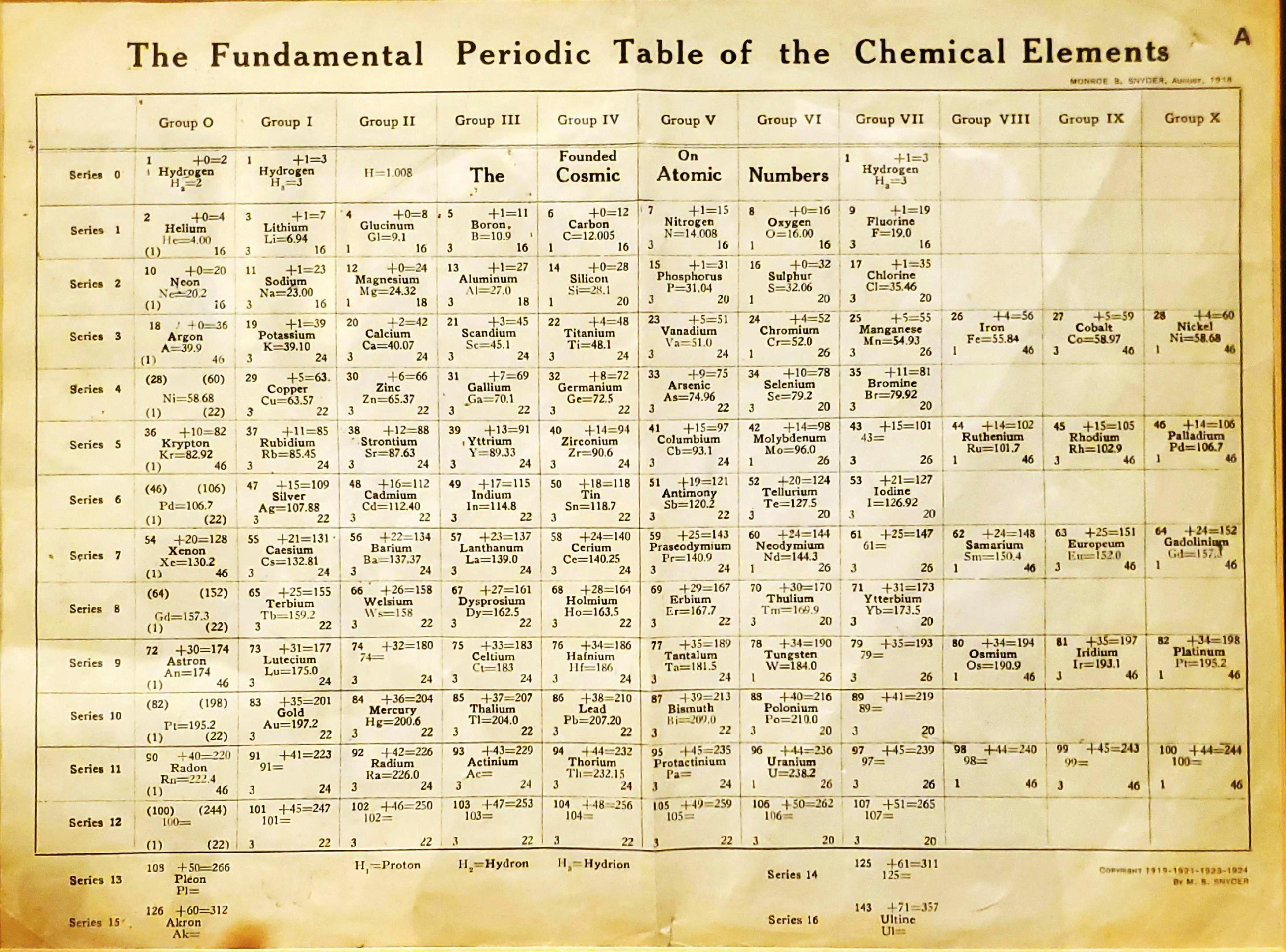
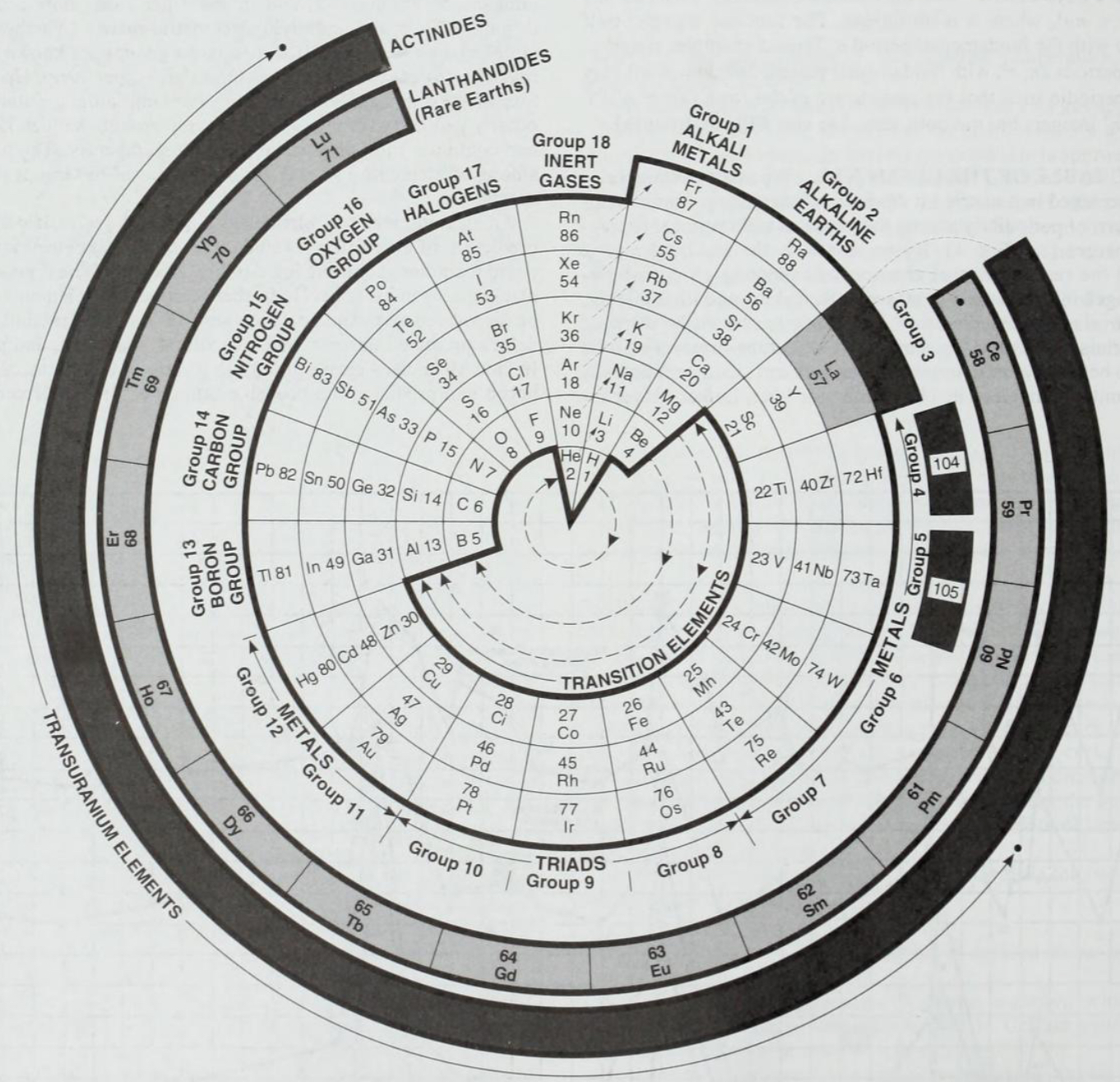
1995
Considine's Polar Periodic Table
From: Considine DM (ed.) 1995, Van Nostrand’s Encyclopedia of Science, 8th ed. New York, p. 2376
René Vernon writes:
"A nice design but of quite limited practical utility for quick reference or detailed chemical analysis."
2023
Bala's Shape of the Periodic Table
Gavin J. Bala has produced a nice and detailed look at The Shape of The Periodic Table (.PDF) that reviews the science:

1936
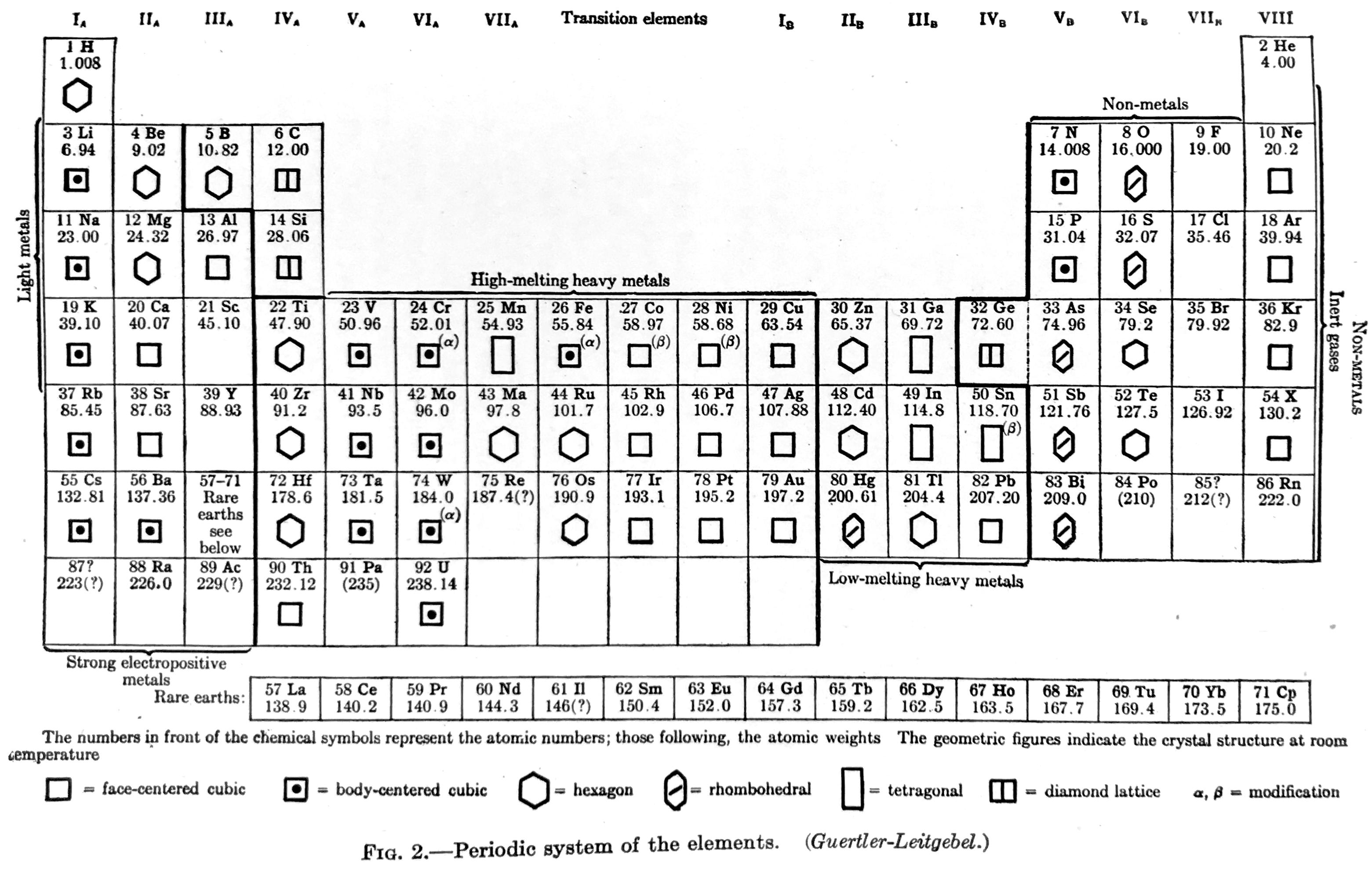
Van Wert Periodic table (after Guertler-Leitgebel)
Van Wert LR, An Introduction to Physical Metallurgy, McGraw-Hill, New York, 1936, pp 17. Van Wert says the periodic table is after "Guertler-Leitgebel", which is presumably Guertler WM & Leitgebel M 1929, Vom Erz zum metallischen Werkstoff: Leitlinien und Rüstzeug der metallurgischen und metallkundlichen Wissensgebiete, Akademische Verlagsgesellschaft, m.b.H., Leipzig
From René Vernon who writes:
In this almost symmetrical presentation, Van Wert divides the periodic table metals into:
Strongly Electropositive: Groups 1 to 3, Ln
High-melting Heavy Metals: Transition metals
Low-melting Heavy Metals: Post-transition metalsIf the 15 Rare Earths had been shown as 14, and moved one cell to the left we would have a perfectly symmetrical table.
Elsewhere (p. 38) Van Wert refers to the noble metals as follows:
"With respect to corrosion, the noble metals — gold, the platinum metals, and to a less degree, silver — are in a class by themselves. They are comparatively chemically inert to all common corrodents; only silver is appreciably attacked by sulphur gas."
Van Wert's table also refers to non-metals and to inert gases. On page 7 mention is made of the metalloids:
"There are a few elements, also, that partake of the nature of both metals and nonmetals, under many—indeed, under most—conditions they seem metallic enough, but on occasion their behavior is decidedly nonmetallic. These metalloids, as they are sometimes called, add a further difficulty in the attempt to frame a satisfactory definition of the metallic state."
By 1936, it was known that metalloids had a predominately nonmetallic chemistry (Newth 1894, pp. 7??8; Friend 1914, p. 9). So, on the nonmetal side of house are metalloids; "nonmetals"; and noble gases. Separating out the halogens from the nonmetals yields: metalloids; "nonmetals"; halogens; noble gases.
The net result is four types of metals and four of nonmetals = more symmetry.