Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Classification of Matter | Lewis & Brønsted Models of Acidity |
The Chemogenesis Analysis: An Overview
Abstract... What It's All About... In a Nutshell... Executive Summary... Chemogenesis in 700 Seconds... Chemogenesis explores the nature of chemical structure and reactivity in logical steps, each described over one or two pages of this web book.
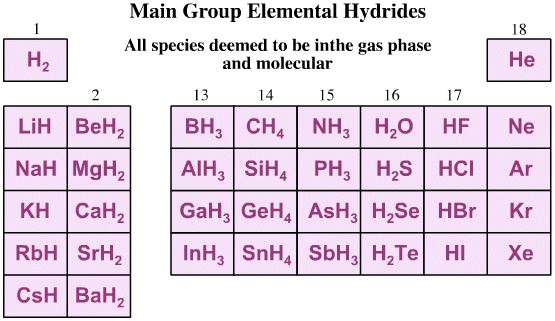
Main Group Element Hydrides
The story starts with the main group elements, as their hydrides.
This set includes many common chemicals with well known and understood properties and behaviours: hydrogen, methane, water, hydrogen bromide, argon, etc.
The Five hydrogen Probe Experiments
Five hydrogen probe experiments are performed upon the set of main group elemental hydrides:
- Add a proton: H+
- Remove a proton: H+
- Add a hydride: H–
- Remove a hydride ion: H–
- Remove a hydrogen radical: H•
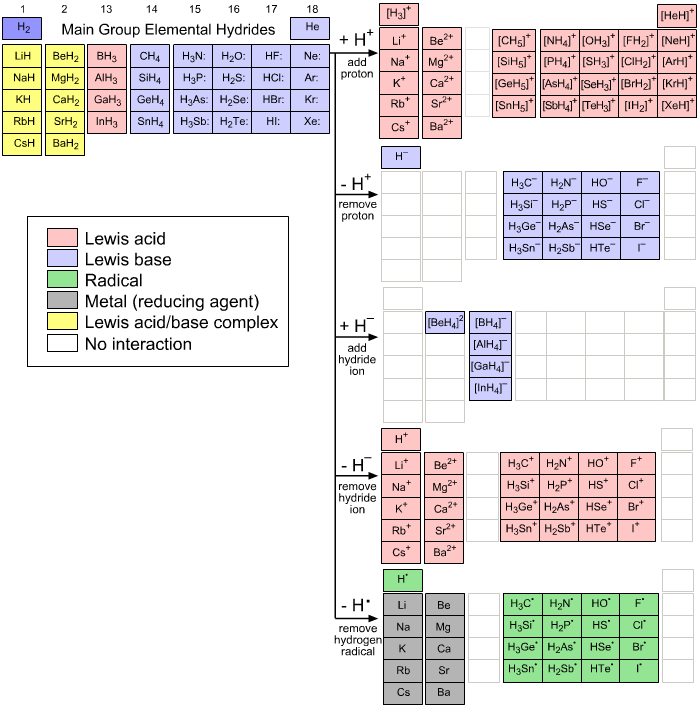
Observations:
- Due to periodicity, species
naturally collect into congeneric (of the same family) arrays.
- Observed array dimensions are: [1
x 1] [1 x 4]
[1 x 5] & [4 x 4]
- The species resulting from
the five hydrogen probe experiments present as:
Lewis acids
Lewis bases
Radicals
Complexes
Note the colour scheme: Lewis acids are red, Lewis bases are blue & radicals are green which will be used consistantly throughout this web book.
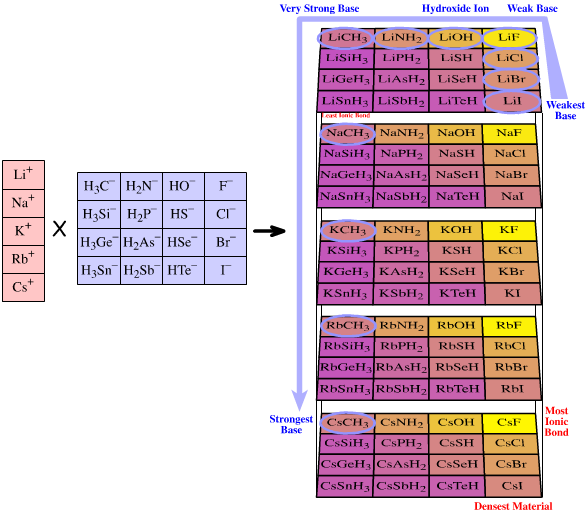
Congeneric Array Interactions
Concentrating on the Lewis acid and Lewis base arrays:
- It is well known that Lewis acids interact with Lewis bases to give Lewis acid/base complexes.
- So it should come as no
surprise that congeneric arrays of Lewis acids interact with congeneric arrays of Lewis bases to give congeneric arrays of Lewis acid/base complexes.
- The usual rules of array
algebra hold, so that
a [5 x 1] array of Lewis acids vs. a [4 x 1] array of Lewis bases gives a [5 x 4] array of Lewis acid/base complexes.
- Congeneric interaction logic can even give rise volumes of chemical species, where the chemistry varies in a regular (linear) way over the array:
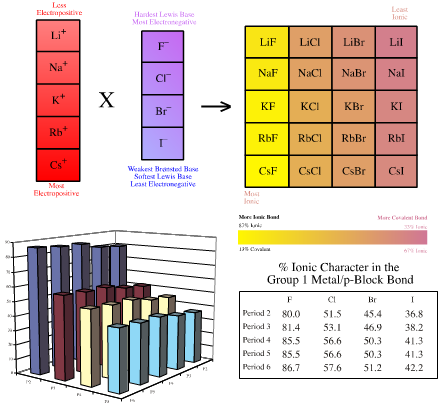
Several pages chemogenesis web book are spent exploring how linear structural and reactivity traits can be found with respect to atomic ionic radii, bond length, electronegativity, % ionic bond character and pKa.
FMO Theory
At this point we introduce some simple frontier molecular orbital (FMO) theory: Theory, diatomics, polyatomics & π-systems.
FMO theory identifies that reactive chemical species interact with each other via a rather limited set of frontier molecular orbitals, the FMOs:
- HOMO or Highest Occupied Molecular Orbital
- LUMO or Lowest Unoccupied Molecular Orbital
- SOMO or Singly Occupied Molecular Orbital
Collecting It All Together...
- Using data from the hydrogen probe experiments
- Taking information about the MO structure of diatomic and polyatomic species
- Considering pericyclic interactions
- Slicing 'n' dicing data in spreadsheets and The Chemical Thesaurus reaction chemistry database, etc...
It is found that there are five general types of reactive species and associated electronic chemical reactivity behaviour:
- Lewis acids,
Lewis bases & Lewis acid/base complexes
- Electron pair acceptor Lewis acids react via their LUMO
- Electron pair donating Lewis bases react via their HOMO
- Lewis
acid/base complexes have a bonding MO resulting from a HOMO/LUMO interaction
- Radicals
- Radicals have SOMOs
- Radicals
couple via SOMO/SOMO interactions to give a bonding MO
- Diradicals
- Triplet diradicals have two SOMOs
- Singlet diradicals
have a HOMO plus a LUMO
- Photochemically
[and otherwise] activated species
- Highly excited
SOMO states
- Highly excited
SOMO states
- Oxidising and
reducing (redox) species
- Pairs of single
electrons can transfer from a HOMO to a LUMO to give a LUMO + HOMO
Zn + Cu2+ → Zn2+ + Cu
There are other redox behaviours as well.
- Pairs of single
electrons can transfer from a HOMO to a LUMO to give a LUMO + HOMO
Types of Lewis acid and Lewis base
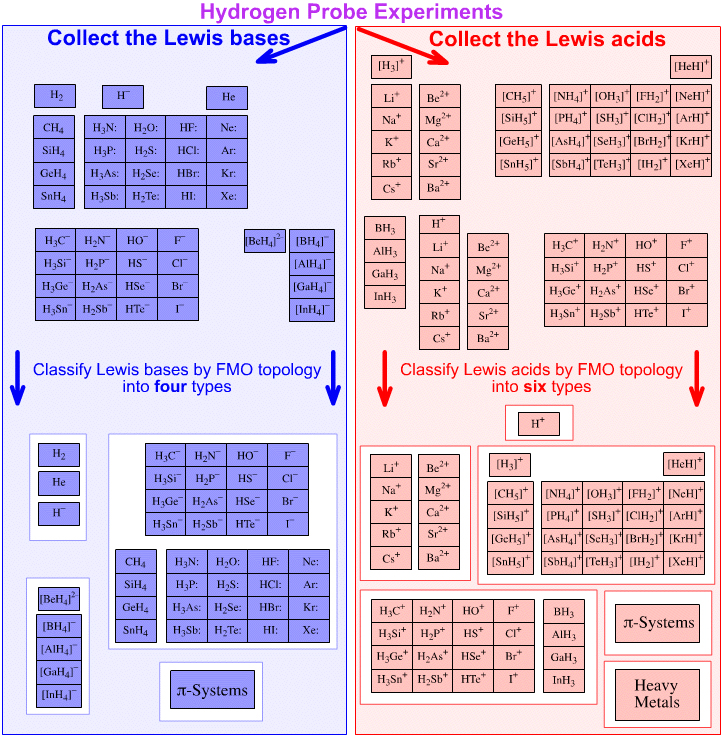
The next step is to take the arrays of Lewis acid and Lewis base species generated by five hydrogen probe experiments and sort them by frontier molecular orbital type, topology (3D geometrical shape + phase information) and reactivity behaviour.
At this point we slide into the analysis three additional types of Lewis acid and Lewis base:
- electron rich π-system Lewis acids
- electron poor π-system Lewis bases
- heavy metal Lewis acids
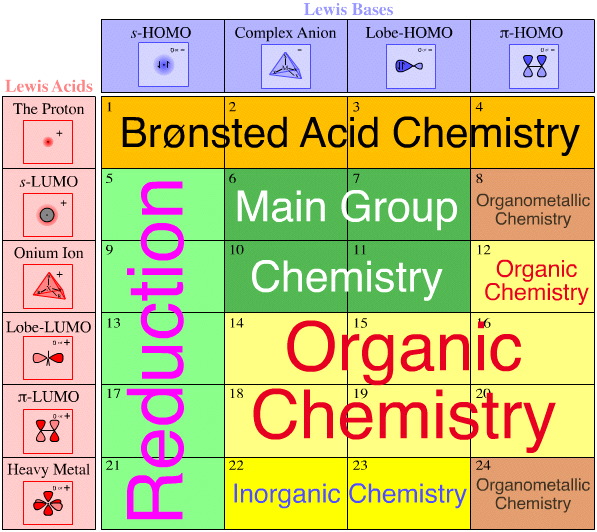
In toto, it transpires that there are Four general types of Lewis base and Six general types of Lewis acid:
|
Four General Types of Lewis Base:
|
Six General Types of Lewis acid:
|
Congeneric arrays are always found within a Lewis acid or Lewis base type, but never crossing types.
The Lewis Acid/Base Interaction Matrix
Each of the four types of Lewis base and six types of Lewis acid exhibits distinct electronic structure and characteristic reaction chemistry behaviour. It follows that the four types of Lewis bases interact with the six types of Lewis acid to produce a matrix of Lewis acid/base complexes.
Crucially, each and every cell of the Lewis acid/base interaction matrix encapsulates and exhibits distinct electronic structure and characteristic reaction chemistry behaviour:
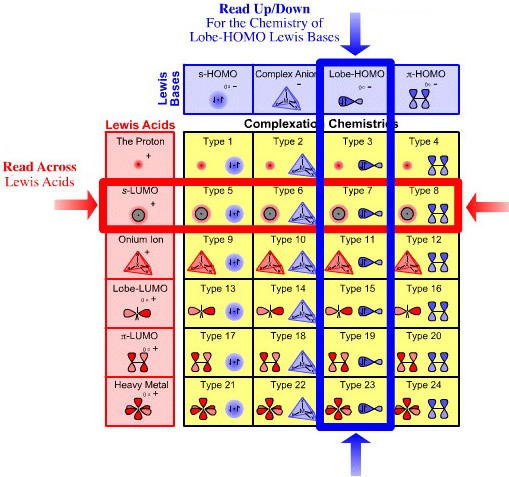
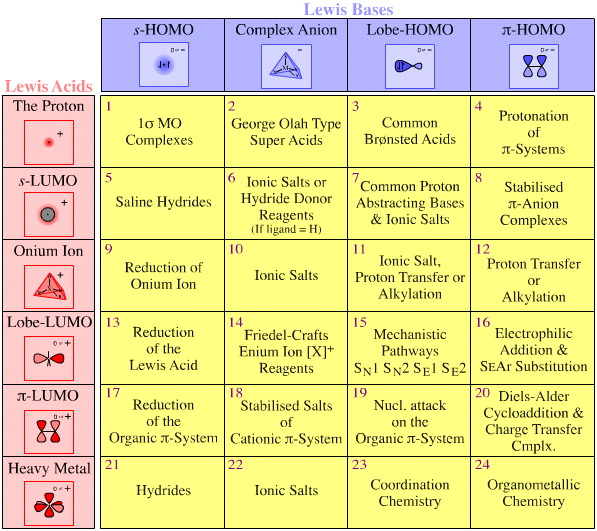
For example, an s-LUMO Lewis acid such as the sodium ion, Na+, interacts with a Lobe-HOMO Lewis bases such as the hydroxide ion, HO–, to give sodium hydroxide, a Type 7 Lewis acid/base complex.
The point is that most of the basic, proton abstracting reagents and many of the neutral inorganic salts used in the chemistry laboratory are Type 7 Lewis acid/base complexes, including:
methyl lithium, LiCH3
potassium hydroxide, KOH
sodium carbonate, Na2CO3
sodium hydrogen carbonate, NaHCO3
sodamide, NaNH2
lithium fluoride, LiF
calcium hydroxide, Ca(OH)2
sodium sulfide, Na2S
sodium cyanide, NaCN
magnesium oxide, MgO
barium sulfate, BaSO4
- Diels-Alder cycloaddition (and pericyclic chemistry in general) is Type 20 reactivity.
- Organometallic chemistry is associated with Type 7, Type 8 & Type 24 interactions.
- Classical transition metal complexation is associated with Type 23.
- Electrophilic aromatic substitution Type 16.
- Etc, etc, etc.
Like the periodic table, the Lewis acid/base interaction matrix is a schema and an extraordinary object with many properties. For example, the vast majority of the reaction chemistry taught to school and university students can be mapped to the Lewis acid/base interaction matrix with the effect that the chemistry has context, rather than existing as isolated facts.
Each cell of the matrix is discussed in detail, and each cell represents distinct chemistry:
Prof. Roald Hoffmann, who won the Nobel prize for his work FMO theory, wrote in a personal communication:
|
 |
 |
 |
| Classification of Matter | Lewis & Brønsted Models of Acidity |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.