Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Photochemistry | The STAD Mechanistic Step |
Species/Species Interactions
This page looks at what happens when two chemical species approach each other in terms of charge and the frontier molecular (FMO) interactions. +/+, –/–, +/–, LUMO/HOMO, HOMO/HOMO, LUMO/LUMO, HOMO/SOMO, etc. and qualitatively analyzed in terms of the Klopman equation and FMO diagrams.Three Initial Points
First, species-plus-species interactions and complexations are represented throughout the chemogenesis web book with a forward slash "/".
Examples include:
Lewis acid/base
LUMO/HOMO
BF3/diethyl ether
H+/Brønsted base
species/species
radical/radical
SOMO/SOMO
Second, the species/species interaction plays a special role in the discussion of reaction chemistry... but perfect examples are actually rather rare, particularly amongst Lewis acid/base interactions.
Lewis acids and Lewis bases are reactive species which usually exist in a complexed form. During reactions, Lewis species are passed between conjugate Lewis species via substitution-transfer-abstraction-displacement (STAD) mechanistic steps, here. For example, the proton, H+, is never found as a free species, it is always passed between competing Brønsted bases. In the next section we will discuss the STAD mechanistic step in detail, however, any STAD mechanistic step can always be deconstructed into pairs of competing complexations:
X + Y-Z → X-Y + Z
Y/Z interaction vs. X/Y interaction
Third, a great deal of theoretical work was done in this area in the 1960s and early 1970s.
Woodward and Hoffmann explained the chemical reactivity of an apparently diverse set of reactions involving π-systems using FMO theory and pericyclic reactions, here.
Pearson proposed the hard, soft, [Lewis] acid, base (HSAB) principle.
Klopman developed his FMO approach to studying Lewis acid/base and radical reactivity.
This emerging work is reviewed in Ian Fleming's book, Frontier Orbitals and Organic Chemical Reactions.
The Klopman Equation
The Klopman equation separates the charge/charge and FMO/FMO contributions when two species, r and s, approach and interact with each other.

(Note: the equation can deal with both LUMO/HOMO and SOMO/SOMO interactions.)
The Klopman equation has two parts:
The charge/charge interaction is determined by the relative charges, q, associated with r and s, the Coulombic repulsion (or attraction), the polarity of the solvent and all the various solvation and desolvation energies. When a charged species (r–) reacts with a charged species (s+) the reaction will be kinetically fast due to a large electrostatic attraction between the two ions. However, ionic and polar species are strongly stabilised by polar solvents: water, alcohols, THF, DMSO, etc. The solvation term concerns the changes in solvation energies that occur when the r/s complex is formed. If the solvation term is larger than the charge/charge attraction, the various species will remain solution as ions.
The FMO/FMO interaction is dominated by the relative energies, E, of the occupied and unoccupied FMOs, m and n. The closer together the relative energies of the HOMO and the LUMO, the smaller the denominator (below the line), and so the larger the influence of the orbital coefficients, c, and the resonance integral. (Note that there is a problem with this very simple formulation: if the FMO energies are exactly equal as there will be a division by zero.)
Klopman goes on to suggest that some types of interaction are so dominated by charge/charge effects that they can be referred to as charge-controlled and that others are so dominated by the FMO/FMO interactions that they are FMO controlled. Examples:
- The interaction of the cesium cation, Cs+, with the fluoride anion, F–, to give cesium fluoride, CsF, is 89% ionic according to the Pauling equation In Klopman's parlance, this as a charge controlled interaction. Likewise, the bonding in solid ammonium tetrafluoroborate is charge controlled. Thus, a charge controlled interaction presents as ionic bonding.
Cs+/F– charge controlled
[NH4]+/[BF4]– charge controlled
- When two methyl radicals, H3C•, couple to give ethane, H3CCH3, there is zero charge difference between the two species, so the interaction will be dominated by the equal energy orbital/orbital interactions.
H3C•/H3C• FMO controlled
- However, most interactions exhibit a mixture of charge and FMO contributions, for example the interaction of a carbenium ion with a carbanion to generate a strong C–C bond will have a mixture of charge/charge and FMO/FMO contributions. And so will the interaction of borontrifluoride, delta+ boron, with trimethylamine, delta– nitrogen, to form the Lewis acid/base "dative" complex, F3B<–NMe3.
H3C+/H3C– mixture of charge and FMO
F3B/Me3N: mixture of charge and FMO
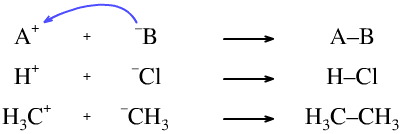
There are a number of possible charge combinations for Lewis acid/base interactions. Notice how in every case the curly arrow goes from the Lewis base to the Lewis acid:
The Lewis acid may carry a positive charge, and the Lewis base may carry a negative charge. For example, proton plus chloride ion or carbenium ion plus carbanion.
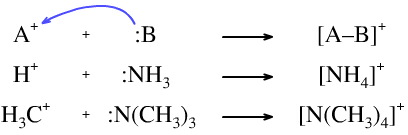
The Lewis acid may carry a positive charge and the Lewis base may be neutral. For example, the protonation of ammonia to give the ammonium ion or the alkylation of trimethylamine to give the tetramethylammonium ion:
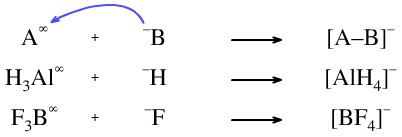
The Lewis acid may be neutral and the Lewis base may carry a formal negative charge. For example, the reaction of aluminium hydride with hydride ion to give the tetrahydroaluminate ion or between borontrifluoride and fluoride ion to give tetrafluoroborate. Please notice how we are adopting the "superscript infinity" symbol to indicate a neutral, vacant orbital, Lewis acid:
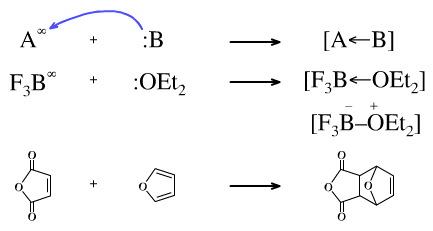
Both the Lewis acid and the Lewis base may be neutral. An example is the reaction of boron trifluoride with diethyl ether to give the so-called dative complex. There is actually nothing at all special about the "dative bond" even though it is often represented either by a "dative arrow" or by charges. The reason is due to the difficulty of representing this type of complexation using the usual lines-for-bonds system.
The cycloaddition of maleic anhydride (an electron poor, LUMO reacting, Lewis acid, π-system) with furan (electron rich, HOMO reacting, Lewis base, π-system) is a LUMO/HOMO interaction and can be considered within Lewis acid/base reaction chemistry space.
We will now look at charge/charge and FMO/FMO contributions in more detail. There are various combinations, as shown in this table. Click to enlarge:
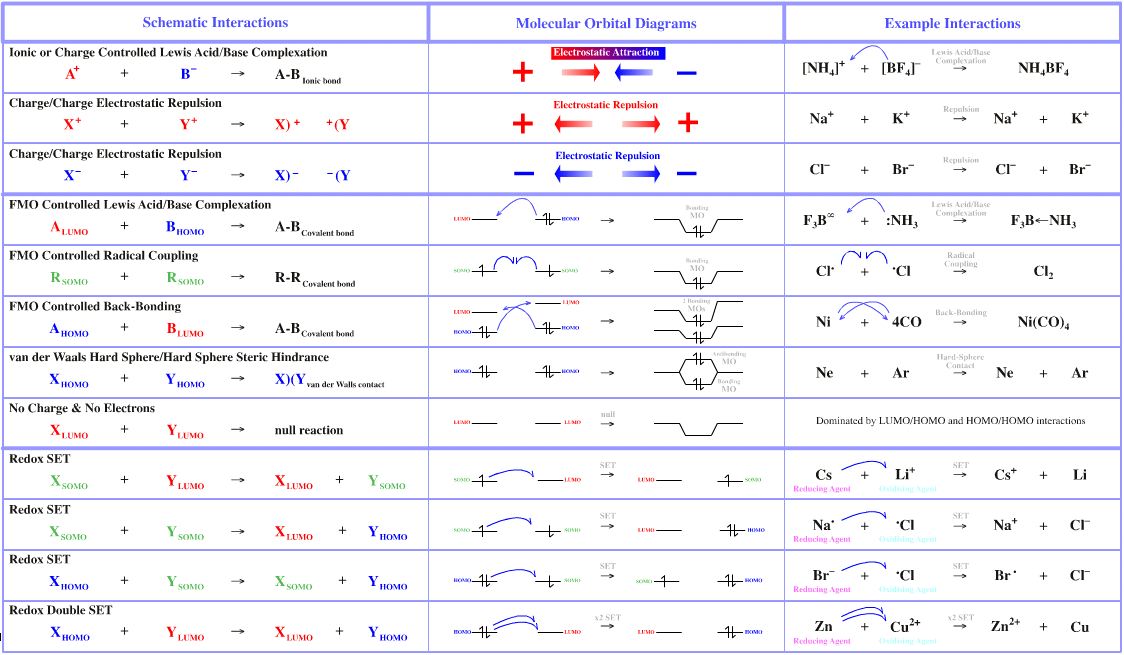
Ionic salts composed of onium ions and complex anions such as ammonium tetrafluoroborate ion are most ionic of all salts and have the most charge controlled interactions of any material.
All ionic materials exhibit charge-controlled bonding.
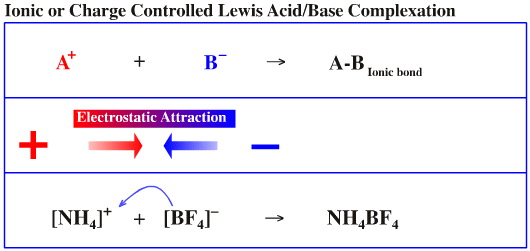
Positively charged species repel other positively charged species, and negatively charged species repel other negatively charged species. While this may seem like an obvious point to make, there are actually a number of implications.
Firstly, in chemistry (as opposed to physics*), systems are always charge neutral in that there are always equal numbers of positive and negative particles. In aqueous solution the charges can stabilise complicated structures.
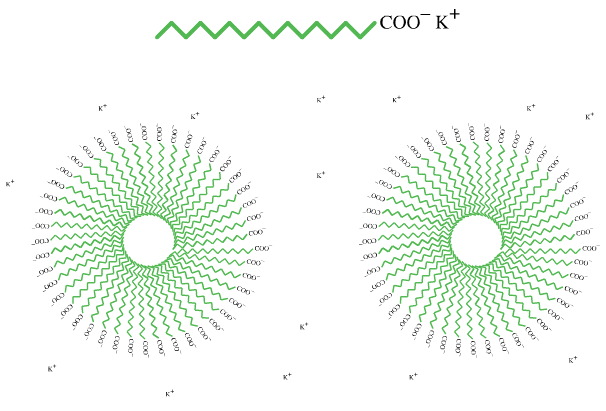
For example, it is well known that the sodium and potassium salts of long chain "fatty" acids (soaps) form micelles in which the non-polar alkyl chains clump together exposing the negatively charged, hydrophilic, carboxylate functions on the surface. Once formed, the individual negatively charged micelles repel each other even though the solution is net neutral due to the presence of stoichiometric potassium counter ions.

The opposite situation is found with tetraalkyl ammonium detergents in which the micelles have a positively charged surface.
* At CERN and other high energy physics labs, bunches of electrons or protons are held together in magnetic storage rings. Special techniques have to be employed to stop the bunches of similarly charged particles flying apart.
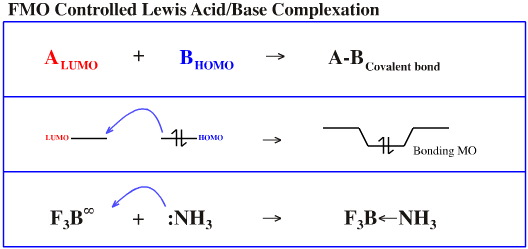
When close in energy and of similar topology (shape and phase), the LUMO and HOMO interact to give a bonding molecular orbital.
As discussed here, there are a variety of LUMO and HOMO topologies, some are compatible with each other, but others are not.
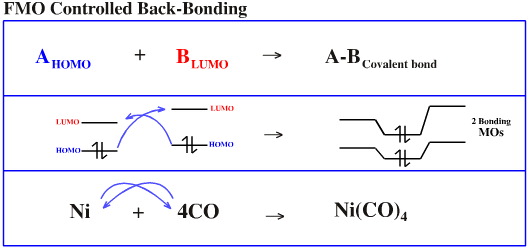
Many chemical bonds are stronger and less polar than simple LUMO/HOMO and VB resonance arguments would suggest due to the phenomena of back-bonding.
At its simplest level, FMO theory says that "the LUMO of the Lewis acid interacts with the HOMO of the Lewis base".
However... every species has both a HOMO and LUMO. A Lewis base has a LUMO and a Lewis acid has a HOMO, we just assume that these do not interact with each other... but they do, particularly when the Lewis acid is a transition metal.
The FMO level back-bonding argument is as follows:
The LUMO of species A overlaps with the HOMO of species B to form a (normal) bonding MO. However, if it should occur that A's HOMO and B's LUMO have the appropriate phase-symmetry, are geometrically correctly positioned and are close together in energy, they too will overlap to form a net bonding molecular orbital: the back-bond. Thus, both A and B are exhibiting both Lewis acid and Lewis base character.
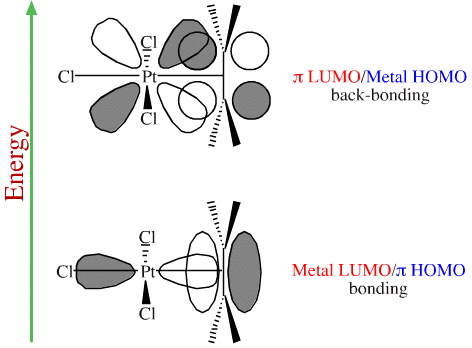
Consider the MO structure of Zeise's salt, a species with platinum/alkene bonding:
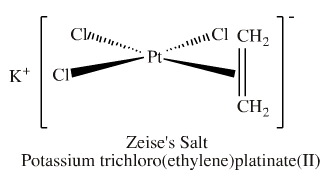
The platinum atomic centre is square planar. It has one ethene (ethylene) ligand and three chloride ligands.
The "forward" Pt/alkene organometallic bond involves the HOMO of the π-alkene system overlapping with a vacant p orbital LUMO on the transition metal.
The alkene lies perpendicular to the square planar complex which allows two occupied (HOMO) lobes of the metal's d orbital to overlap with the LUMO of the alkene to give a HOMO/LUMO back-bond.
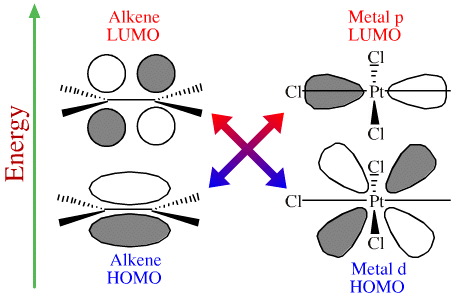
The result is that the Pt/alkene complex exhibits normal, forward bonding and back-bonding:
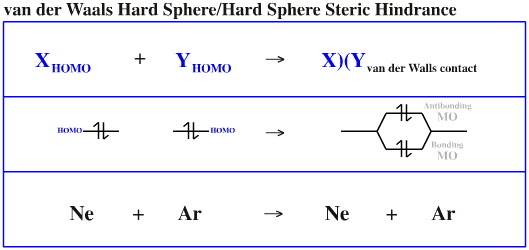
It is common to assume that species have a van der Waals surface which is constructed from closed electron shells.
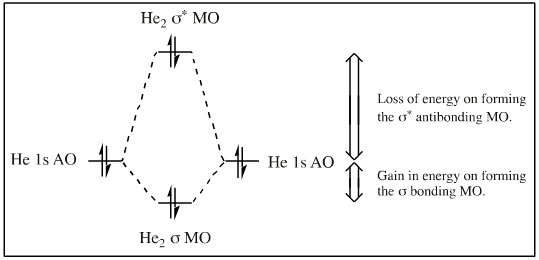
A helium atom as two electrons in its 1s AO (its HOMO). When two helium atoms approach each other there is a 1s + 1s HOMO/HOMO interaction. The effect is net antibonding and He2 does not form.
Closed-shell/closed-shell repulsion can be equated with VSEPR theory and it is an essential ingredient in all classical mechanical or ball-and-stick models of molecular structure. In these models atoms are considered to be spheres with distinct size and rigidity and sigma-bonds are deemed able to rotate freely and bend only slightly.
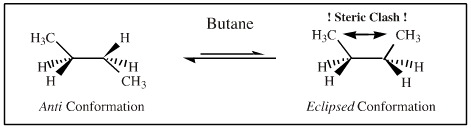
For example, sigma-bonded molecular structures such as butane are able to adopt a multitude of different conformation states where each state has a distinct associated energy. (The various conformational states are dynamically populated according to Boltzmann mechanics.)
Butane has slightly restricted rotation about the 2-3 carbon-carbon bond due to the van der Waals size of the hydrogen and methyl groups. The lowest energy conformation is the anti conformation in which the large methyl groups are as far away from each other as possible. At room temperature butane exists as a dynamic mixture of all possible conformations but with a statistical excess of molecules in the anti conformation.
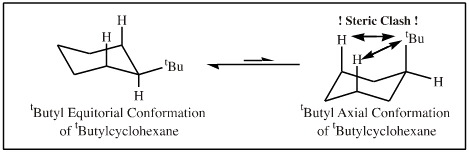
Another example from organic chemistry, tertiary butylcyclohexane exists almost exclusively with the large bulky tertiary butyl function equatorial and away from the axial hydrogen atoms.
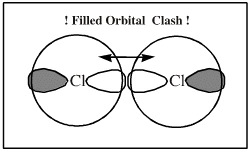
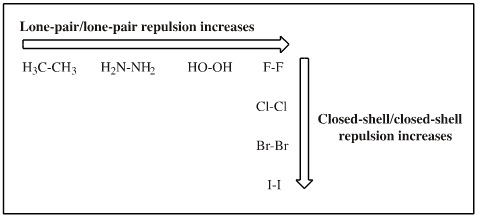
Closed-shell/closed-shell repulsion can be held responsible for the weakened bonding observed in the heavier dihalogens, Cl2, Br2 and I2 where the ever increasing numbers of filled shells which inhibit the atoms approaching.
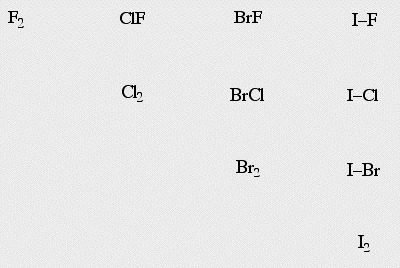
The effect is seen with the reaction chemistry. When added together, the halogens react together to give interhalogens. Thus, the interhalogens are more stable than the halogens.
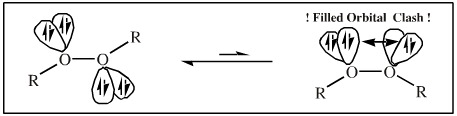
Homonuclear period 2 bonds, for example the O-O peroxide bond, are weak due to intramolecular lone-pair/lone/pair repulsion. Peroxides prefer to exist in an anti-conformation, and the O-O bond is weak.
These two effects can be considered to combine and contribute to periodicity:
van der Waals Attractions & Hydrogen Bonding
There are several types of van der Waals attraction and/or hydrogen bonding that hold molecular materials together in a condensed phase (liquid & solid). Indeed, molecular materials are sometimes referred to as van der Waals materials.
There are three subtypes of van der Waals attraction:
- dipole/dipole
- dipole/induced-dipole
- spontaneous-dipole/induced-dipole (induced-dipole/induced-dipole)
It is tempting to consider these forces to be of different strengths, but the interaction distance is more important. The spontaneous-dipole/induced-dipole van der Waals attractions, also known as London dispersion forces (LDF), are surprisingly strong but they only act at very short range. It is as if the surface of neutral molecules like methane is 'sticky'.
- Imagine soccer balls covered in Velcro: they only stick together when they touch and are moving slowly.
All molecules exhibit London dispersion forces and the strength is proportional the size/surface area of the molecule. In addition, some molecules have dipole-dipole, hydrogen bonding, etc., which increase the total amount of interaction between the molecules.
- Consider iodine chloride, ICl and bromine, Br2. Both have a mass of about 160 and are 70-electron systems, but ICl is polar and Br2 is non-polar. The materials have rather similar boiling points, 97° and 59° respectively, showing that the dipole/dipole attraction makes only a minor contribution. (Many thanks to members of the ChemEd list for the above points.)
Molecular materials may also be hydrogen bonded, where a hydrogen bond involves a proton being shared between two Lewis bases, usually with oxygen, nitrogen or fluorine atomic centres, as discussed here.
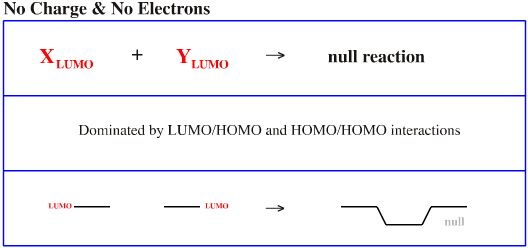
This is the null interaction. If there are no electrons there is no interaction.
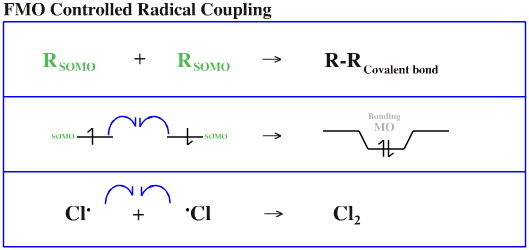
Radical coupling and fragmentation is considered here.
Notice the use of the fishhook arrow to indicate the moment of a single electron.
Electron Transfer
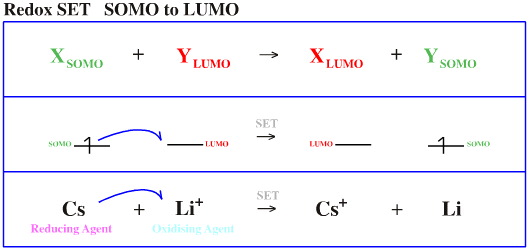
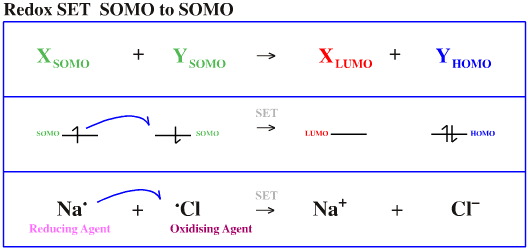
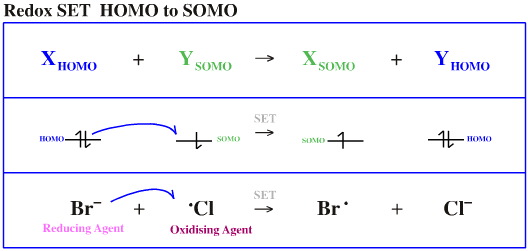
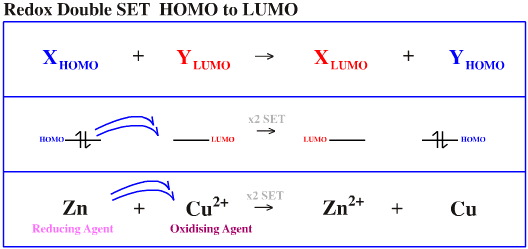
Species do not just interact with each other in a bonding or non-bonding way. Electrons can also be transferred between orbitals by single electron transfer (SET):
SOMO to LUMO
SOMO to SOMO
HOMO to SOMO
HOMO to LUMO (x2 SET)
The species which donates the electron(s) is the reducing agent and the species which receives the electron is the oxidising agent. Examples of each are given below:
 |
 |
 |
| Photochemistry | The STAD Mechanistic Step |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.