Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Radical Chemistry | Photochemistry |
Diradical Chemistry
Diradicals may appear to be rather obscure species, however, the oxygen, O2, we breath is a triplet diradical. An understanding of diradical structure and reactivity tells us that the simplistic electron pair covalent bonding of Lewis octet theory is not the whole story. Liquid oxygen is magnetic, a fact that can only be explained by understanding the nature of the bonding in O2, here.
Introduction to Diradical Reaction Chemistry
Examples of diradicals include:
Oxygen, O2
Methylene (carbene), CH2
Dichorocarbene, CCl2
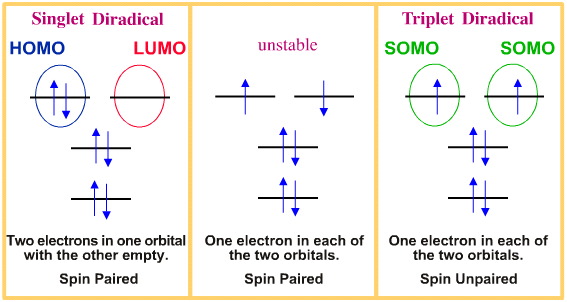
Diradicals are species with a pair of degenerate (equal energy) molecular orbitals and two electrons. There are three possible diradical arrangements: the singlet state, the triplet state and an unstable intermediate state.
Some Technical Stuff
The terms "singlet", "doublet" and "triplet" concern the degeneracy of the electronic state.
|
Degeneracy = 2S+1, where S is the total electron spin angular momentum. An electron has a spin of +1/2 or –1/2, and an orbital can contain up to two electrons but they must be of opposite spin: the Pauli exclusion principle. Singlet diradicals have a pair of electrons, one spin-up and one spin-down [+1/2 and –1/2], in one orbital with the second, equal energy orbital, empty.
A simple free radical, R•, is a doublet.
Triplet diradicals have two "spin-up" electrons in adjacent, degenerate (equal energy) orbitals.
Thanks to Prof. Paul Percival of the Simon Fraser University and TRIUMF for help with this section (personal communication). |
Differential Reactivity
Carbenes undergo addition reactions with alkenes. The singlet and triplet states exhibit subtly different reaction chemistry:
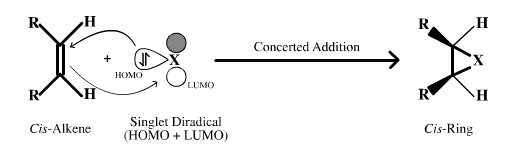
Singlet Diradicals
- Singlet diradical species behave as if they have both a Lewis base (HOMO) centre and a Lewis acid (LUMO) centre. For carbenes, nitrenes and oxenes these two centres occur at the same atom.
- Singlet carbene, CH2, has two electrons in a Lobe-HOMO Lewis base centre and a vacant p-orbital LUMO. The two centres react with alkenes in a concerted, single-step manner and so give rise to stereospecific products.
- Singlet diradicals undergo 1,1-addition reactions with cis-alkenes with retention of relative stereochemical configuration:
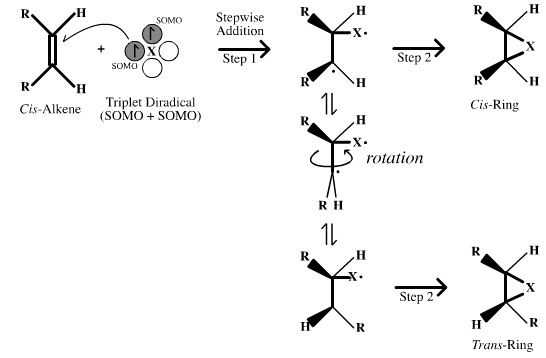
Triplet Diradicals
- Triplet Diradicals have two non-spin paired electrons which behave as a pair of radical (SOMO) centres.
- These two centres react with an alkene in a stepwise fashion. This means that molecular rotation can occur around the "single" bond between the reactions steps.
- The result is that triplet diradicals give stereo mixed addition products:
Forming Carbenes
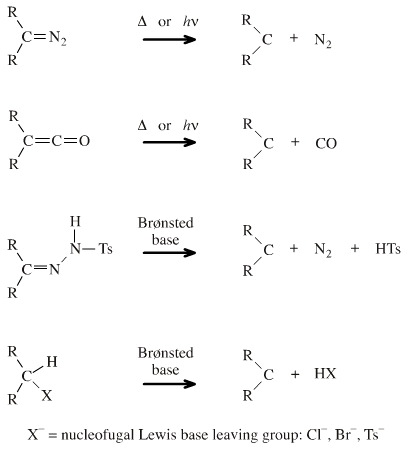
Carbenes, R2C, are often called reactive intermediates and they only exist transiently. There are several methods of production:
Triplet Oxygen
Ground state oxygen, O2, is a triplet diradical, a property which can explain why liquid oxygen is paramagnetic and attracted to the poles of a magnet:

Singlet Oxygen
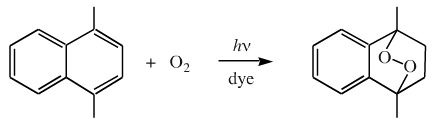
Triplet oxygen, the formal form, can be converted into singlet oxygen with UV light and a dye such as rose bengal, discussed here.
The singlet oxygen can undergo cycloaddition reactions, for example with 1,4-dimethyl naphthalene:
 |
 |
 |
| Radical Chemistry | Photochemistry |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.