Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Redox Chemistry Synthlet | Diradical Chemistry |
Radical Chemistry
Radicals are species with a single, unpaired electron. In molecular orbital theory, this state is represented as a singly occupied molecular orbital or SOMO. This page gives an outline of the formation and fate of radical species, including "chain" radical substitution and addition, and the radical oxidation of fats and oils (foodstuffs).
Formation of Radicals
Radical species can be electrically neutral, in which case they are sometimes referred to as free radicals.
Pairs of electrically neutral "free" radicals are formed via homolytic bond breakage. This can be achieved by heating in non-polar solvents or the vapour phase. At elevated temperature, all molecular species will dissociate into radicals.
Homolytic bond fission or fragmentation occurs when – in the language of Lewis theory – a two electron covalent bond breaks and one electron goes to each of the partner species:
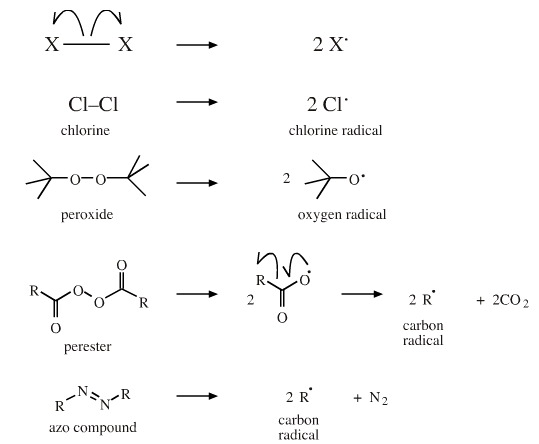
On heating, chlorine, Cl2, forms chlorine radicals, Cl•. Peroxides form oxygen radicals. Peresters fragment to acyl radicals, which lose carbon dioxide to give carbon radicals. Azo compounds eject nitrogen to give a pair of carbon radicals.
One radical coupling and homolytic bond fission process is commonly encountered while studying thermodynamics and equilibrium theory, although the radical fragmentation nature of the reaction is usually not discussed. That reaction is the equilibrium of nitrogen dioxide with dinitrogen tetroxide:
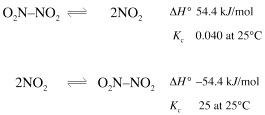
The data shows that the formation of the N2O4 dimer is an exothermic process (there is a negative delta H) and that the fragmentation is endothermic.
Applying Le Chatelier's principle to this system tells us that the coupling will be encouraged at lower temperature and fragmentation at high temperature.
This temperature relationship is true for all coupling and fragmentation processes.
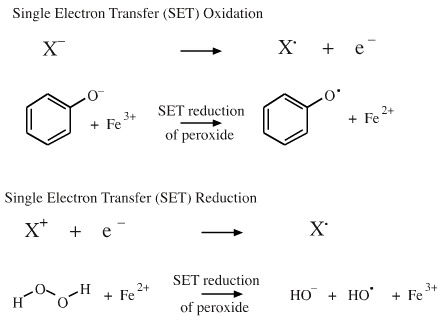
Neutral radicals can be produced in polar environments.
An electron can be removed from an anion, an oxidation process. For example, the phenoxide ion is oxidised by Fe3+ to give the phenyl oxygen radical and the Fe3+ is co-reduced to Fe2+.
The iron(II) ion, Fe2+, can reduce hydrogen peroxide to hydroxyl radical and hydroxide ion:
Radicals can be charged: when a neutral, spin-paired species gains a single electron it becomes a both a radical and an anion, a radical anion. Likewise, when a neutral, spin-paired species loses an electron it becomes a radical cation. Radical anions and radical cations are dual classified as both radicals and Lewis acid/base species. Reactivity may be charge controlled and be dominated by solvation energy effects.
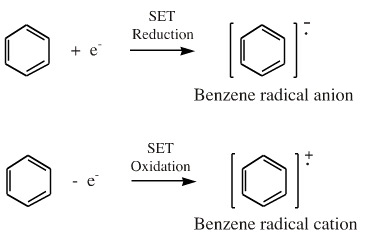
Radical cations and radical anions are known in the gas phase. They are routinely generated and studied in the complementary techniques of mass spectrometry and negative ion mass spectrometry.
The Triphenylmethyl Radical, Ph3C•
Triphenylmethyl chloride reacts with metallic silver to form the triphenylmethyl radical, Ph3C•, a species first observed in the year 1900, although its radical was not recognised as at the time.
The triphenylmethyl radical adopts a propeller-like conformation, it is non-planar due to steric hindrance imposed by the bulky phenyl ligands. There is extensive delocalisation which stabilises the radical centre.
The triphenylmethyl radical reacts with a number of reagents, including oxygen to give the peroxide, iodine to give the iodide, nitric oxide to give the nitroso compound:
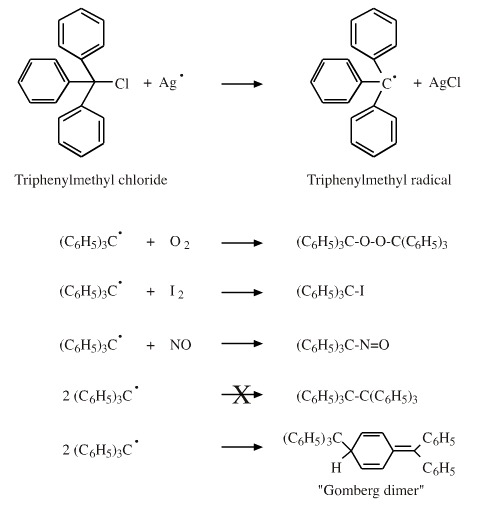
Triphenylmethyl radicals couple to the Gomberg dimer, rather than the hexaphenyl ethane, Ph3C-CPh3, as Gomberg originally proposed. The reason is that it is energetically more favourable for the dimeric compound to lose aromatic stabilisation from one ring than to form the sterically strained hexaphenylethane structure.
Radicals from the Hydrogen Probe Experiments
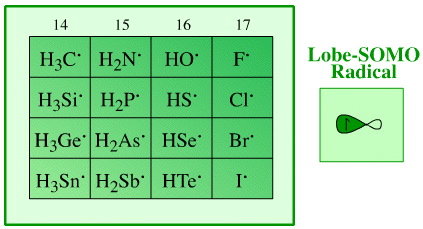
The hydrogen probe experiments, here, generate the hydrogen radical and a congeneric planar of p-block radicals.
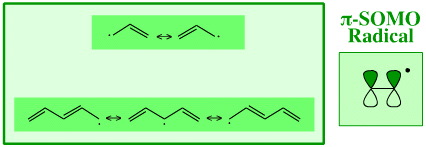
Radicals are also associated with linear π-systems with 3 carbon atoms, the allyl radical, and five carbon atoms, the pentadiene radical.
Radical Reactions
Reactions involving electronically neutral radicals are frontier orbital controlled processes, here, and solvation energy effects are unimportant. Many radical reactions can be explained in terms of bond enthalpy data. Radical reactions can involve:
Bond forming and bond breaking
Substitution
Insertion
Addition
Elimination
Rearrangement
Single electron transfer (SET)
Radical bond forming reactions – radical couplings – are rather rare processes.
The reason is because radicals are normally present at low concentrations in a reaction medium, and it is statistically more likely they will abstract a hydrogen, or undergo another type of a substitution process, rather than reacting with each other by coupling. And as radicals are uncharged, there is little long range coulombic attraction between two radical centres.
Radical substitution and addition reactions tend to proceed as chain reaction processes, often with many thousands of identical propagation steps. The propensity for chain reactivity gives radical chemistry a distinct feel compared with polar Lewis acid/base chemistry where chain reactions are less common.
Chain Radical Substitution
Methane can be chlorinated with chlorine to give chloromethane and hydrogen chloride. The reaction proceeds as a chain, radical, substitution mechanism:
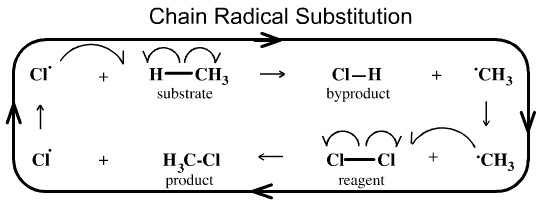
A chlorine radical abstracts a hydrogen from methane to give hydrogen chloride and a methyl radical. The methyl radical then abstracts a chlorine atom (a chlorine radical) from Cl2 to give methyl chloride and a chlorine radical... which abstracts a hydrogen from methane... and the cycle continues...
The process is a little more involved, and three steps are involved: initiation, propagation and termination:
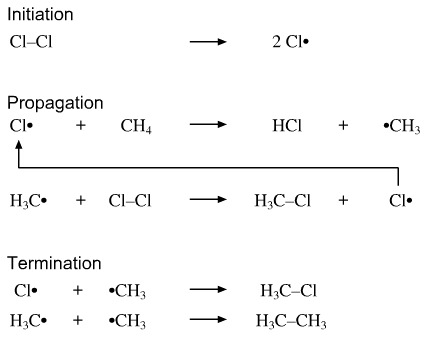
Initiation is used to form the low concentration of radical required to start the chain reaction. A special chemical, usually a peroxide, perester or azo compound which undergoes homolytic bond fission on heating is usually used. In the case of chlorination, chlorine may be used as the initiator.
Propagation describes the chain reaction itself. There may be thousands of identical propagation steps.
Termination is the process by which radical centres are quenched. Termination occurs when two radical centres come into contact and couple together.
Chain Radical Addition
Many polymers are made by the chain radical addition of substituted alkenes:
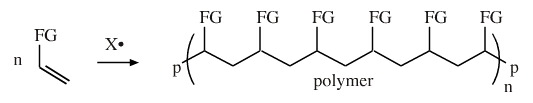
The "FG" refers to functional group. If FG = H, the alkene starting material is ethene (ethylene) and the product is polyethylene.
FG = H, alkene = ethylene, polymer = polyethylene
FG = Cl, alkene = vinyl chloride, polymer = PVC
FG = CN, alkene = acrylonitrile, polymer = polyacrylonitrile (orlon)
FG = C6H5, alkene = styrene, polymer = polystyrene
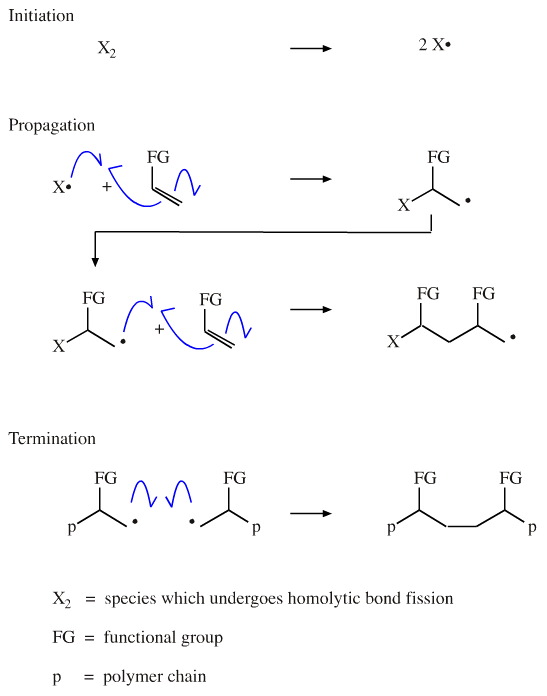
Like chain radical substitution, chain radical addition proceeds by the steps of initiation, propagation and termination:
Radical Oxidation of Fats and Oils
The radical oxidation of fats and oils in the food industry is important because oxidised foodstuffs are liable to become rancid.
The oxidation reaction starts with a radical (which may be photochemically generated, which is why we are advised to keep food in the dark) abstracting an allylic hydrogen from an unsaturated alkene function.
The allylic position is adjacent to an alkene function and is susceptible to abstraction of a hydrogen because the allylic radical is surprisingly stable, here.
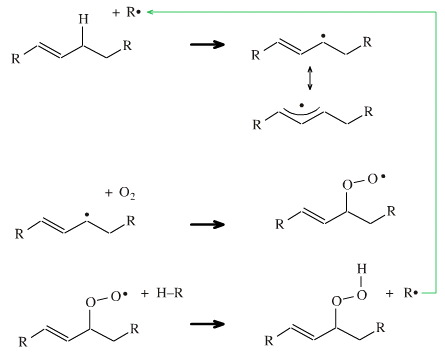
The allylic radical couples with oxygen, O2, (a diradical) to give a peroxide radical.
The peroxide radical is able to abstract a hydrogen from a neighbouring hydrocarbon to form the hydroperoxide and another radical centre which propagates the chain reaction.
The point is that the hydroperoxide compound is able to decompose to a zoo of species, including aldehydes and ketones with breakage of the hydrocarbon chain. When this occurs the fat or oil has a nasty taste and is said to be "rancid".
Fats and oils are regularly tested by food chemists to find the degree of peroxide present. The peroxide number is a measure of the degradation of the oil.
 |
 |
 |
| Redox Chemistry Synthlet | Diradical Chemistry |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.