Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Ligand Replacement Congeneric Series | The Emergence of Organic Chemistry |
Congeneric Array Interactions
Congeneric arrays –sets of chemical species with linear structure and reactivity behaviour traits –can react with each other to produce new arrays which are also congeneric.
Congeneric Array Interaction Algebra
Congeneric interactions follow the usual rules of array algebra:
|
(1
x 1) x (1 x 1)
|
→
|
(
1 x 1)
|
dot
x dot
|
→
|
dot
|
|
|
(5
x 1) x (1 x 1)
|
→
|
(
5x 1)
|
series
x dot
|
→
|
series
|
|
|
(4
x 4) x (1 x 1)
|
→
|
(
4 x 4)
|
planar
x dot
|
→
|
planar
|
|
|
(5
x 1) x (4 x 1)
|
→
|
(
5 x 4)
|
series
x series
|
→
|
planar
|
|
|
(5
x 1) x (4 x 4)
|
→
|
(
5 x 4 x 4)
|
series
x planar
|
→
|
volume
|
For example, the proton x hydride ion (a dot x dot interaction) gives H2, a congeneric dot. Here is no other chemical species at all like H2 (other than its isotopic homologs D2, T2, HD, etc.):
(1 x 1) x (1 x 1) |
→ |
( 1 x 1) |
H+ + H– |
→ |
H2 |
Likewise, the Group 1 cations vs. the hydride ion (a series x dot interaction) gives rise to the Group 1 saline hydrides (a series).
(H–) x (Li+, Na+, K+, Rb+, Cs+) → (LiH, NaH, KH, RbH, CsH)
We have already seen these interactions with the hydrogen probe experiments, but the logic can be continued to generate a range of ionic and polar covalent materials.
We shall explore three (series x series) interactions to generate corresponding congeneric planars:
- Group 1 cations (Li+ to Cs+) vs. the Period 2 anions (H3C– to F–)
- Group 1 cations (Li+ to Cs+) vs. the Group 17 anions (F– to I–)
- Group 1 cations (Li+ to Cs+) vs. the methyl to tertiary butyl carbanions
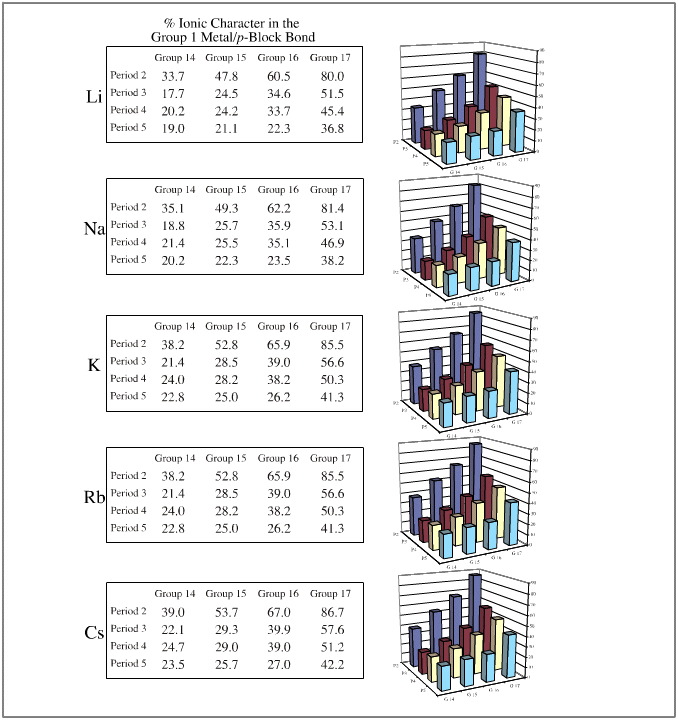
In each of these three cases, the congeneric planars are quantified with respect to the % ionic character of the formed bond as determined by the Pauling equation, here. This bond character is colour coded.
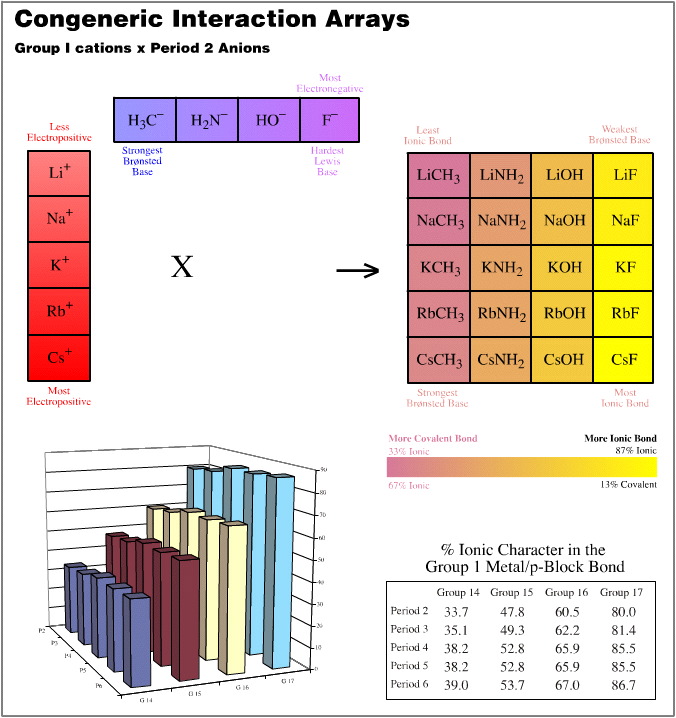
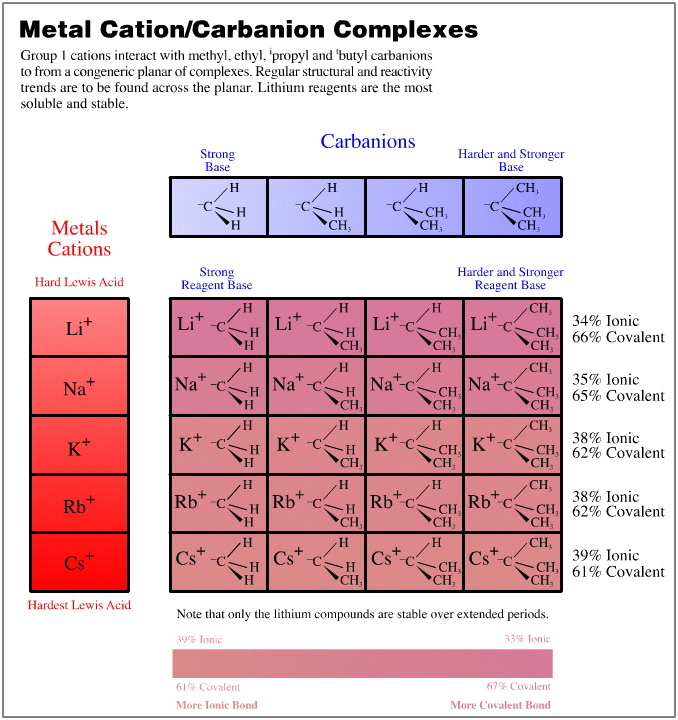
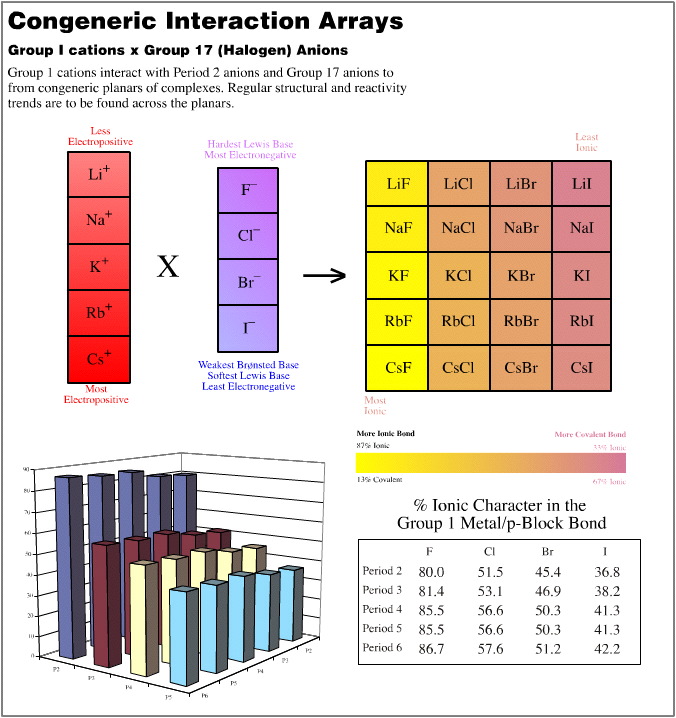
The (5 x 1) x (4 x 4) Congeneric Array Interaction: A Congeneric Volume
The logic of congeneric array interaction can be continued to produce a congeneric volume:
|
(5
x 1) x (4 x 4)
|
→
|
(
5 x 4 x 4)
|
series
x planar
|
→
|
volume
|
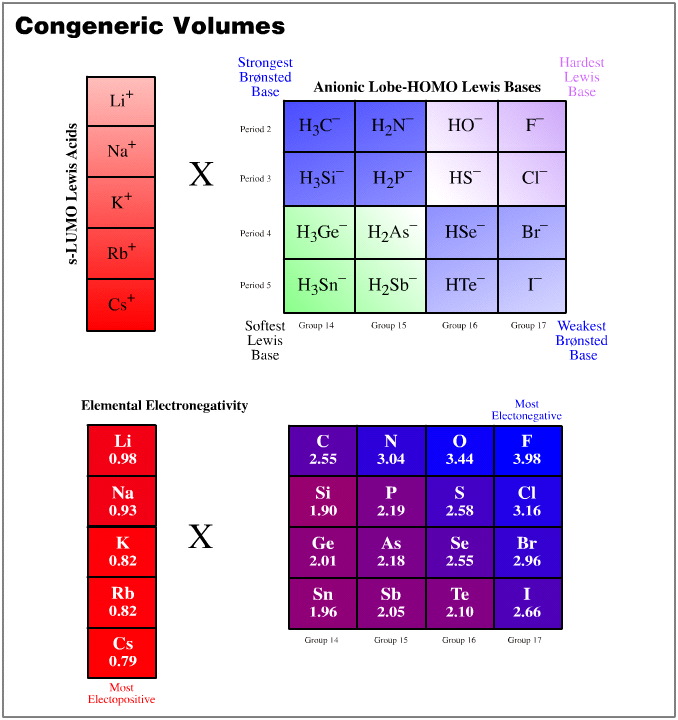
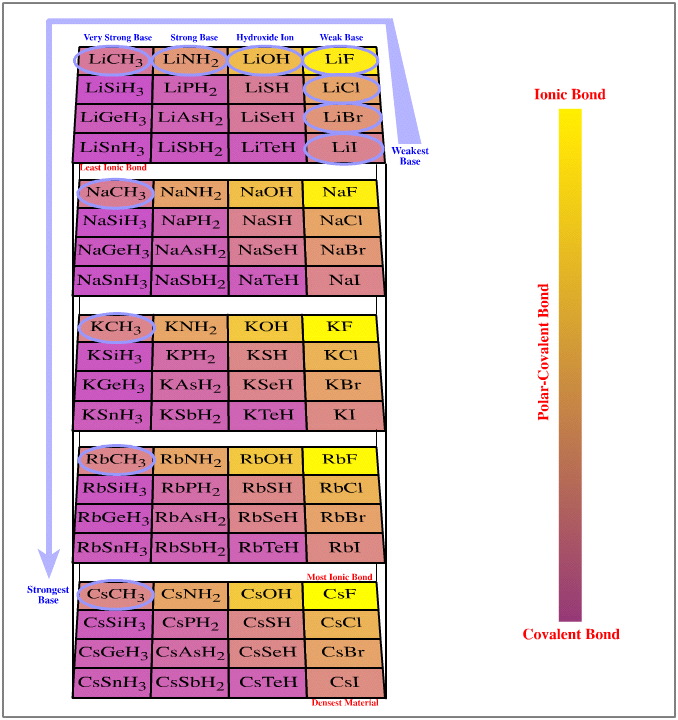
One such congeneric volume is discovered when the Group 1 cations, Li+ to Cs+, are arranged against the anionic Lewis bases of the type H3C– , F– and I–.
The resulting congeneric volume has regular changing bond polarisation properties over the volume:
- Over the set of compounds, the least [Brønsted] basic compound is lithium iodide, LiI. (The iodide ion is the weakest proton abstractor because its conjugate Brønsted acid is hydrogen iodide and that is the strongest Brønsted acid.) Proton abstracting ability increases to lithium fluoride, to methyl lithium and to methyl cesium. Thus, Brønsted base strength increases over three connected vertices of the congeneric volume.
- The Li-Sn bond of LiSnH3 will be the most covalent (21% ionic) and the cesium fluoride bond will be the most ionic (89% ionic). These species are found at opposite corners of the congeneric volume.
- All the Group 1 (and Group 2) halides have excellent optical properties and can be used as lenses and prisms from the UV to infrared. Of these, cesium iodide is often the optimum material because it is able to pass infrared light of the longest wavelengths, to 70 microns.
- The density of aqueous solutions can be increased by dissolving salts, particularly the Group 1 halides. The aqueous solutions with the highest specific gravity are prepared by saturating water with cesium iodide.
 |
 |
 |
| Ligand Replacement Congeneric Series | The Emergence of Organic Chemistry |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.