Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| QNs to PTs | INTERNET Database of Periodic Tables |
The Periodic Table: What Is It Showing?
The Periodic Table of the Chemical Elements is both a cultural icon and an extraordinary object in science space. This page explores what the periodic table is in terms of the philosophy of science.
Introduction
The chemogenesis web book explores how chemical reactivity emerges from the periodic table of the elements using a root-trunk-branch chemistry-tree metaphor, with the periodic table situated at the base of the trunk.The question is: What actually is the periodic table, and what it is showing? It transpires that matters are a little more involved than they may at first appear...
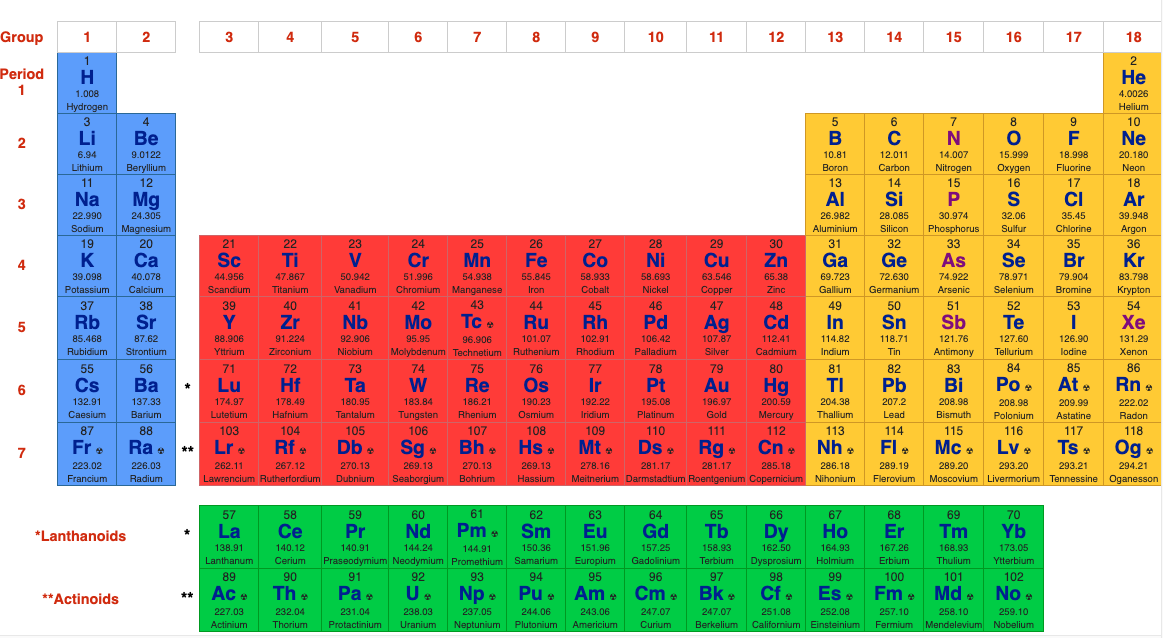
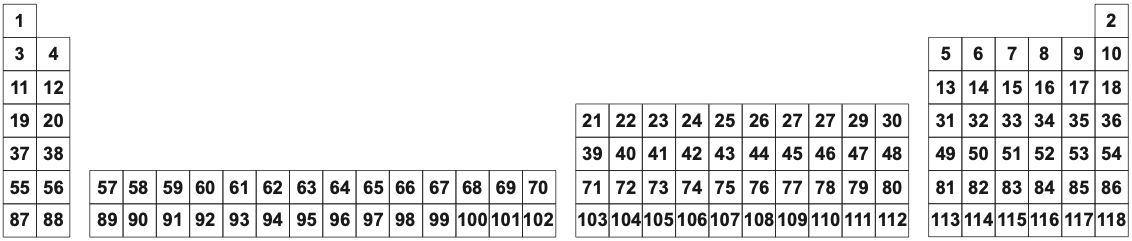
First, the long form 32 column periodic table (in this author's opinion, is the preferred formulation):
The 32 Column [Long Form] Periodic Table

But, the PT is usually reduced to the 'classic' 18 column medium form periodic table, as used by WebElements and most other web sites & textbooks:
The WebElements 18 Column [Medium Form] Periodic Table
There are many other possible formulation, as reviewed on the next page of this web book.
Philosophy | Chemistry | The Periodic Table
"[In 1913] when Henry Moseley experimentally determined and analysed the atomic inner shell X-ray spectra, and Niels Bohr invented his atomic model, the empirical and theoretical basis for the element number was found as the number of protons in the nucleus for the physicist, and as the number of electrons of the neutral atom for the chemist. "This reflects a conceptual difference:
"[Atomic number] Z has proven useful as an ordinal number to order the nuclei on an isotopic [Segrè] chart, and also to obtain a chemically meaningful order of the chemical elements." From: Physical Origin of Chemical Periodicities in the System of Elements, Pure and Applied Chemistry | Volume 91: Issue 12, 2019, Li & Schwarz et. al. |
Philosophers of chemical science consider the elements in distinct ways:
- Firstly, there is
the chemical element as the "basic substance",
that is the abstract or transcendental element, the essence of the element, the element as "a bearer of properties" but not having any actual
properties, except for atomic number Z. Chemical symbols,
such as H and Cu, and names are assigned to the element as the basic substance.
- The isotopic composition of the nucleus is conserved, and the various nuclear properties move with the element as it undergoes chemical transformation and chemical reactions: average atomic mass, radioactivity, nuclear spin, electronegativity and because Z = n (the atomic number equals the principle quantum number in the neutral atom), the quantum numbers and electronegativity.
- Thirdly, there
is the element as simple substance. A real physical 5.00g piece of copper metal has numerous,
measurable, intrinsic properties such as: density, conductivity,
colour, purity, etc. Crucially, these properties are NOT conserved when the copper undergoes chemical reactions, such as dissolving in concentrated nitric acid.
[There is a language problem with this topic, and it concerns the use of the technical terms: 'basic' and 'simple'. These words are used in so many different contexts, that it can be very difficult to remember that here they have very specific meanings.]
Crucially, only the basic substance and the nuclear properties survive in a compound. These ideas can be illustrated with the statement:
"Sodium's metallic properties and chlorine, the green gas, do not exist in the colourless, crystalline ionic salt, sodium chloride."
In other words, the "silvery metal aspect of sodium" and the "green gas aspect of chlorine" do not exist in the binary compound sodium chloride.
These matters are discussed in a paper by Eric Scerri, HYLE--International Journal for Philosophy of Chemistry, Vol. 11, No.2 (2005), pp. 127-145: Some Aspects of the Metaphysics of Chemistry and the Nature of The Elements (2nd Ed. 2020 available)
Summarising Scerri's arguments:
- There is a metaphysical view about the nature of the elements as basic substances and bearers
of properties that goes back to the ancient Greeks, long before the
discovery of atoms.
- Mendeleev insisted
that his periodic classification system concerned the elements as basic
substances possessing only one attribute, atomic weight.
- Paneth, one of
the founders of modern radiochemistry took Mendeleev's view about the
nature of basic and simple substance, but changed the basic/transcendental/abstract property
of an element from atomic weight/mass to atomic number, Z.
- Elements as basic substance represent natural kinds, a well understood philosophical position concerning the nature of classification. Elements as simple substances fail the natural kind test, due to the existence of isotopes and allotropes, etc.
Eric Scerri points out that the periodic table has, at times, been characterised as a:
- representation, ordered domain, classification system, model, law and/or theory
This author agrees with Scerri that the periodic table is an ordered domain. But the periodic table is also a schema. Data, information & knowledge concerning the chemical elements can be mapped onto the periodic table.
Periodic Tables on Walls and In Books: What are they showing? Why is there a problem?Periodic tables use the design as an organising schema to list the chemical elements and to present physical data & material properties of the elements. In their simplest form, the periodic tables show elements with only the atomic number Z and the element symbol. Strictly, this is showing the elements as the basic [transcendental, abstract] substance. However, they may go on to state:
In this author's opinion, there has been a logical sleight-of-hand. The metaphysical periodic table of abstract, basic substances is being passed off as a periodic table of the material properties of simple substances, which is not the same thing at all. This causes confusion as to what exactly it is that a particular periodic table is, and what it is showing. |
Morphing Multiple Periodic Tables
At least three periodic tables can be easily identified (but as we will see there are actually many more):
- The periodic table of basic elements with atomic number Z and associated atomic symbol: H, He, Li, etc.
- The periodic table of gas phase atoms with their associated ionisation energies, spectra, etc.
- Then there is the periodic table of chemicals in bottles, the actual materials under standard [room] temperature and pressure conditions.
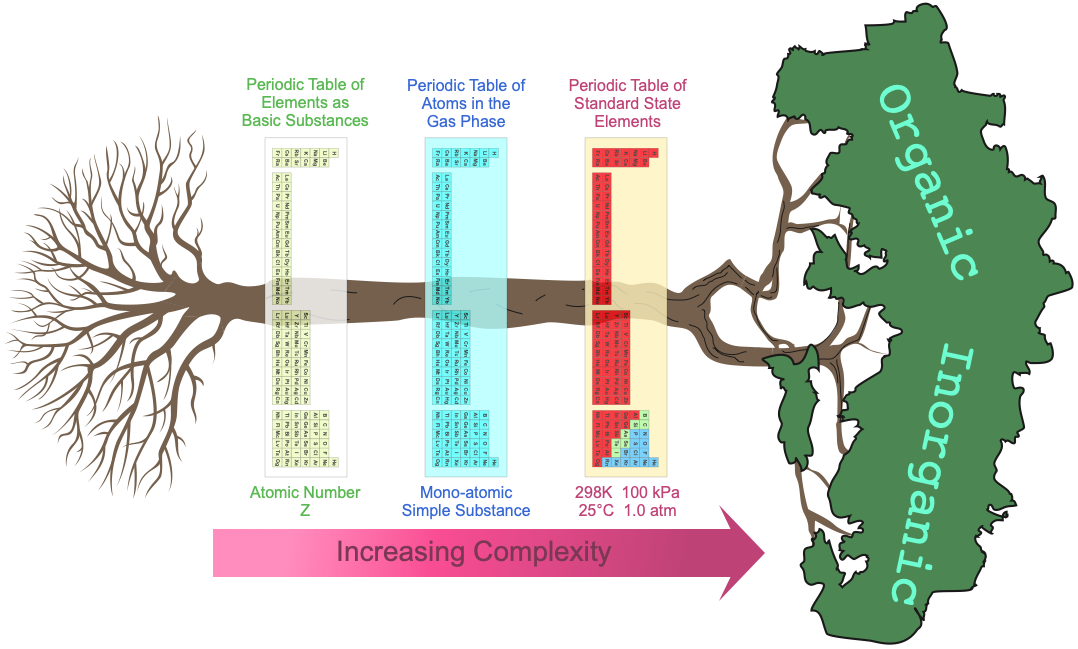
When moving across these three periodic tables, the system complexity and the number of properties dramatically increases.
- Elements in the periodic table of basic substances have only the property: atomic number Z.
- The gas phase atoms have properties of: ionisation energy, electron affinity, atomic radius, atomic emission spectrum, etc.
- The standard state elements have dozens of properties: electrical conductivity, density, molar volumes, colour, crystal structure, hydration enthalpy, etc. As substances, the elements may present as: metals, molecular van der Waals materials, network covalent substances and even intermediate metalloids.
And there are yet more periodic tables:
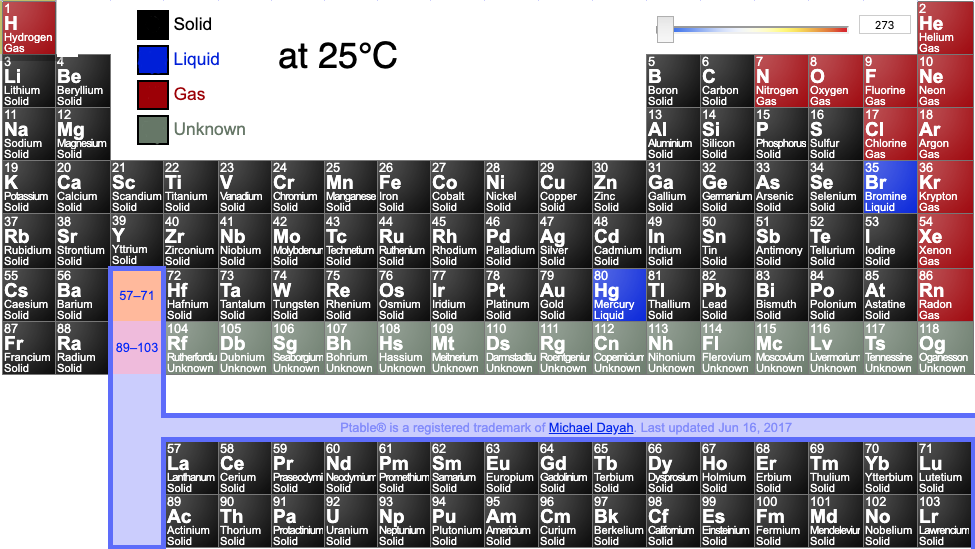
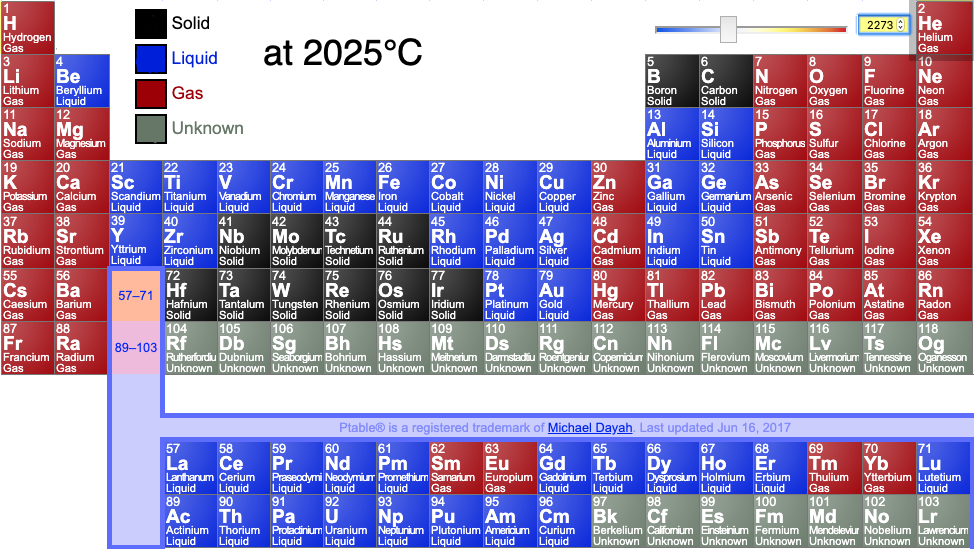
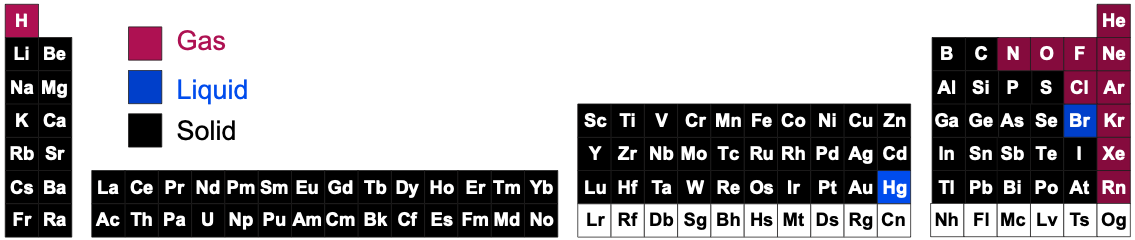
Periodic tables may show the phase (solid, liquid or gas) state of the elemental materials at the standard temperature of 25°C and at other temperatures, for example 2025°C (from pTable):
Or:
- Dates of discovery
- Countries in which the discovery was made
- Uses
- NMR properties
- etc...
All of these many & various periodic tables morph into each other to give the compound object that is commonly known as [and presented as] The Periodic Table.
- The periodic table in web space that best illustrates this multi-morphing propensity is Michael Dayah's dynamic pTable.
Periodic Table of The Elements as Basic Substances
The long form periodic table of basic, abstract, transcendental substances showing the only property, atomic number Z, behaving as an ordinal number:
Periodic Table of Basic Substances (Atomic Number Z)
Element symbols and names are assigned to the atomic number Z:
Periodic Table of Basic Substances (Atomic Symbol)

In the basic substance formulation, oxygen atomic number 8 is "O" and not the common molecule "O2". Likewise, sulfur (Z = 16) is S and not the yellow solid S8.
The usual periodic table schema simply shows the element symbols in their respective periods, groups & blocks.
This, or an equivalent formulation, is the periodic table as Mendeleev would have intended it: a schema showing the elements as basic substances with their positions in the schema emphasising the periodic law:
"The periodic law is the principle that certain properties of elements occur periodically when arranged by atomic number. These similarities can be reflected best by a table, so that commonalties between elements appear both in rows and in columns of the table." Wikipedia
Periodic Table of Conserved [Nuclear] Properties
Conserved nuclear properties are a consequence of the isotopic composition of the element which directly account for the relative atomic mass (defined as the ratio of the average mass of the atoms of a chemical element in a given sample compared to 1/12 of the mass of an atom of carbon–12.)
Relative atomic mass is conserved in chemical reactions:
Na (mass = 22.99) + Cl (mass = 35.45) → NaCl (mass = 22.99 + 34.45 = 58.44)
Likewise, radioactivity is transferred with the atoms. Radio-labelled sodium will react with chlorine to give radio-labelled sodium chloride.
There is a minor (but important) kinetic isotope effect or KIE. Heavier isotopes are very slightly less reactive than lighter isotopes, simply because they are less mobile. The KIE effect is most notable with hydrogen, where deuterium, 2H, has double the mass of protium, 1H. However, as a first approximation, KIE can be ignored.
In the element as the basic (abstract) substance, the atomic number Z is simply an ordinal (ordering) number. In the periodic table of conserved [nuclear] properties, Z is the charge on the atomic nucleus, in the neutral atom it is also the number of electrons, and hence, it directly determines the principle quantum number n.
The quantum numbers, n, ℓ, mℓ & ms, lead* to the corresponding electronic (orbital) configurations:
Neutral atoms: n = Z Ions: n = (Z – charge)
| symbol | Z | n = (Z - charge) | n | electronic structure |
| Na | 11 | 11 – 0 | 11 | 1s2, 2s2, 2p6, 3s1 |
| Na+ | 11 | 11- 1 | 10 | 1s2, 2s2, 2p6 |
| Cl | 17 | 17 – 0 | 17 | 1s2, 2s2, 2p6, 3s2, 3p5 |
| Cl– | 17 | 17 – –1 = 17 + 1 | 18 | 1s2, 2s2, 2p6, 3s2, 3p6 |
*Note that not all atomic electronic structures can be so easily deduced: the simplistic man-made rules, such as Bohr's aufbau principle & Madelung's rule, are fine for the lighter main group elements but they do not always give the correct results with heavier d-block elements.
Nuclear spin is an isotopic property that is exploited in NMR spectroscopy, particularly with the 1H and 13C isotopes. Nuclear spin is conserved and transferred with an atom, from the elemental substance into its compounds.
For electronegativity, this author uses the definition:
"Electronegativity is a measure, integrated over numerous physical parameters, of the power of a gas phase or bonded atom to attract electrons to itself."
Sodium is a large atom that is easily ionised. In reactions, sodium generally reacts to give the sodium ion, Na+. These features lead to the conclusion that sodium is an electropositive element.
Chlorine is a small atom with a high electron affinity and a propensity to form chloride ions, Cl–. These features lead to the conclusion that chlorine is an electronegative element.
Electronegativity is not a "simple" elemental substance property, like the metallic lustre of sodium or chlorine presenting as a green gas, but is intrinsic to every aspect of an element's chemistry and it travels with an atom. Electronegativity is a conserved property that is a consequence of Z.
Periodic Table of Gas Phase Atoms
The chemical elements as real, simple elemental substances can be physically normalised into a similar set by examining ground-state, monoatomic gas phase atoms of the material substance.
- For some elements
entering the monoatomic gas phase is trivial: the group 18 rare gases, He, Ar, Ne, Kr, Xe & Rn are already monoatomic, gas
phase entities under the standard conditions of 298K & 100kPa (25°C and 1.0 atm). In other words, they are naturally
in the desired state.
- Chlorine and oxygen are diatomic molecular
gases at room temperature, but thy can easily be converted into the atomic gas by heating at low pressure.
- Carbon boils
at 4027°C making it difficult to obtain a vapour of ground-state
carbon atoms – carbon gas, C(g) – but it is possible. Tungsten boils at 5930°C.
- Moving an element from its standard state to the gas phase equates the enthalpy of atomisation, ΔatH
The periodic table of ground state gas phase atoms is known, and it represents the periodic table of the very simplest simple substances:
Periodic Table of Gas Phase Atoms

Apart from the title, the graphic is exactly the same as the periodic table of basic substances, but this periodic table represents real chemical entities with actual, measurable, physical properties, including:
- Average atomic mass
- Atomic radius
- Accurate mass & abundance of the constituent isotopes
- Effective nuclear charge
- Electron affinity
- Electron binding energies
- Ionisation energies: 1st, 2nd, 3rd...
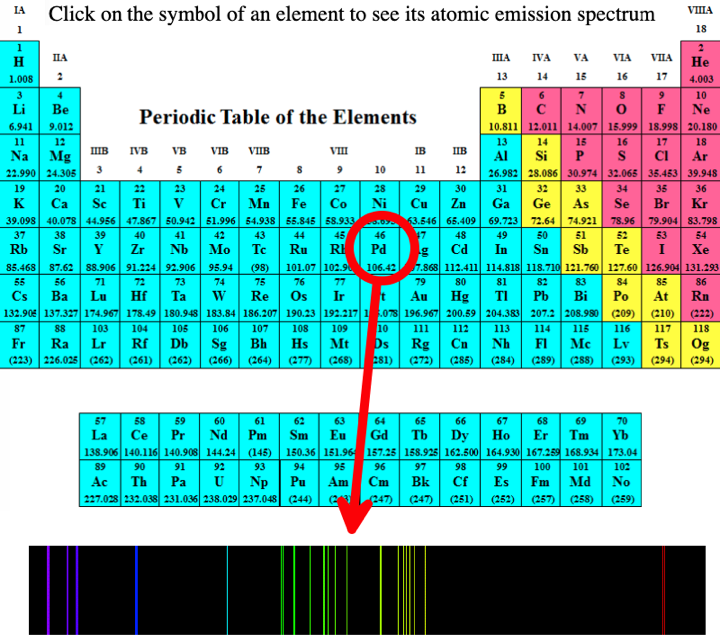
- Emission spectra. Penn State university has a dynamic spectral periodic table, here, that shows the atomic spectra of all the elements in the gas phase:
Many modern technologies utilise gas phase atoms, including:
- Sodium lamps for street lighting
- Caesium atomic clocks
- Most of the heavy and short lived man-made transuranic elements, 104 – 118, are only known as short lived gas phase atoms.
- Atomic adsorption spectroscopy (AAS) and ICP-OES [optical emission spectroscopy] use ground state gas phase atoms for elemental analysis.
- In mass spectrometry, atoms [or molecules] are transformed into gas atoms and then gas phase ions, usually M+ ions, in a vacuum. The ion are accelerated using a potential difference and the mass-to-charge ratio of the analyte particles are determined, the mass spectrum.
- When an atom [or molecule] is studied in silico (constructed or mathematically modelled in a computer) the atom is effectively being studied as an isolated gas phase species.
Crucially, when we consider the electronic structure of an atom [or the orbital structure of a molecule, for that matter], we are considering the atom to be an isolated entity, exactly as it would be in the gas phase. For example, when we say "sodium has a 1s2, 2s2, 2p6, 3s1 electronic structure", we are referring to the sodium the gas phase atom, Na(g), not sodium in its solid, metallic state, Na(s).
Periodic Table Chemical of Substances Under Standard Conditions
Under standard conditions, 298K and 100kPa (25°C & 1.0 atm), the chemical elements as simple substances – real chemicals, chemical reagents, chemicals in bottles – present as:
- Gases, liquids or solids
- Metals, metalloids
or non-metals
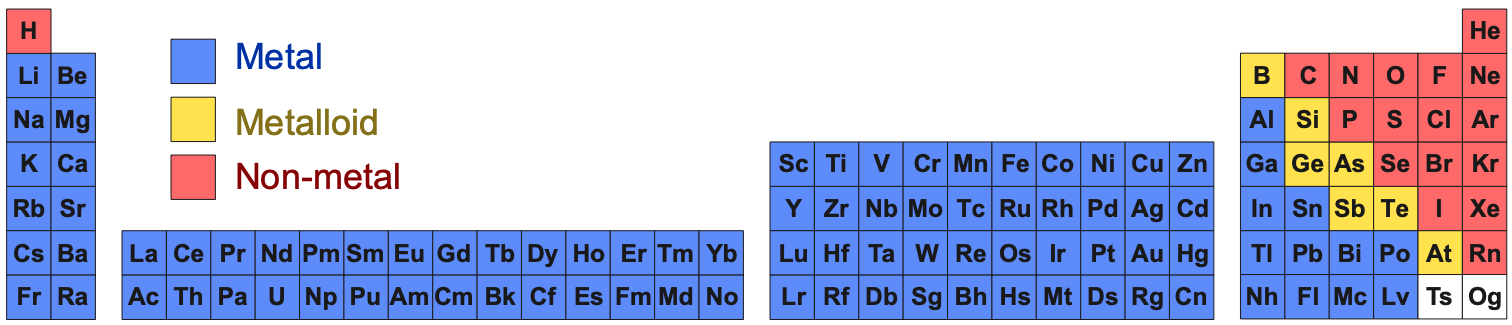
- Metallic, network covalent or molecular materials (read more here):
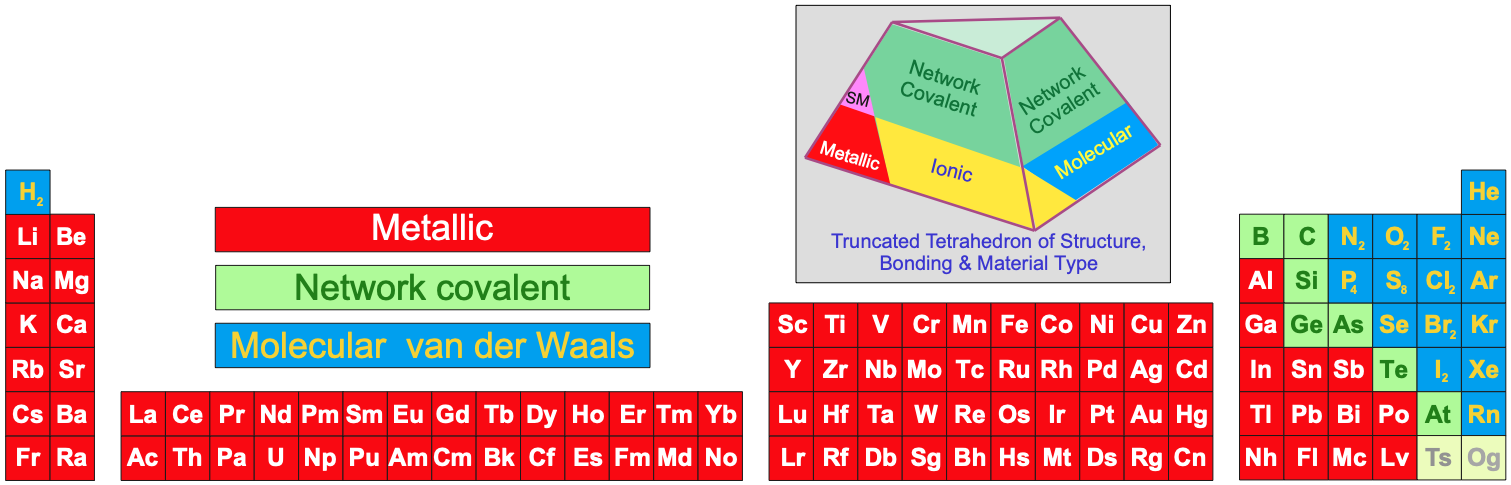
Note:
- Several Group 3, 4, 5 & 6 (13, 14, 15 & 16) elements have multiple allotropes (structural forms). There are many allotropes of B, P, S, Si etc. There are many metallic phases of Pu, etc.
- Carbon has an uncountable number of allotropes: graphite, diamond, buckminsterfullerene C60, single walled nanotubes, multi-walled nanotubes, etc. There is a wikipedia page of ~100 famous diamonds, each unique.
- The P4 form of phosphorus is defined as the standard state of the element, even though this is actually not the most stable allotrope under standard conditions.
- The structure of astatine is unknown. It may be isostructural with iodine or it may be a metalloid.
The chemical elements as material substances have many properties, including [from WebElements, copper]:
- Properties at standard conditions 25°C and 1.0 atm: crystal structure, molar volume, hardness, etc.
- Phase changes under non-standard conditions: boiling point, allotropes, etc.
- History
- Biology
- etc.
|
Abundance of
elements (Earth's crust) |
Hardness - Vickers |
NMR relative sensitivity |
Quantum Mechanics, Emission Spectra & The Axiomatic Periodic Table
In the first half of twentieth century, much effort was expended trying to make the periodic table of the elements axiomatic ("self-evident" or "unquestionable"), ie. trying to fully understand the Mendeleev system in terms of quantum mechanics.
In 1929 Paul Dirac famously claimed this situation had been fully and completely achieved in principle:
"The underlying physical laws necessary for the mathematical theory of a large part of physics and the whole of chemistry are thus completely known and the difficulty is only that the exact application of these laws leads to equations much too complicated to be soluble."
P.A.M. Dirac, Proc.R.Soc.Lond.Ser.A 123 (1929) 714
We certainly teach school and university students that "the periodic table is fully understood in terms of electronic theory", and this line of reasoning is advanced elsewhere in this web book, here and the HyperPhysics site, here.
The argument is put forward that:
- Each gas phase element produces a unique set of spectral lines.
- The spectral lines act as a fingerprint for the presence of an element (and the signature is quantitative).
- The pattern of spectral lines can be fully understood in terms of quantum mechanics via the Schrödinger wave equation, and its developments: the relativistic Dirac equation, the Lamb shift and Quantum Electrodynamics (QED).
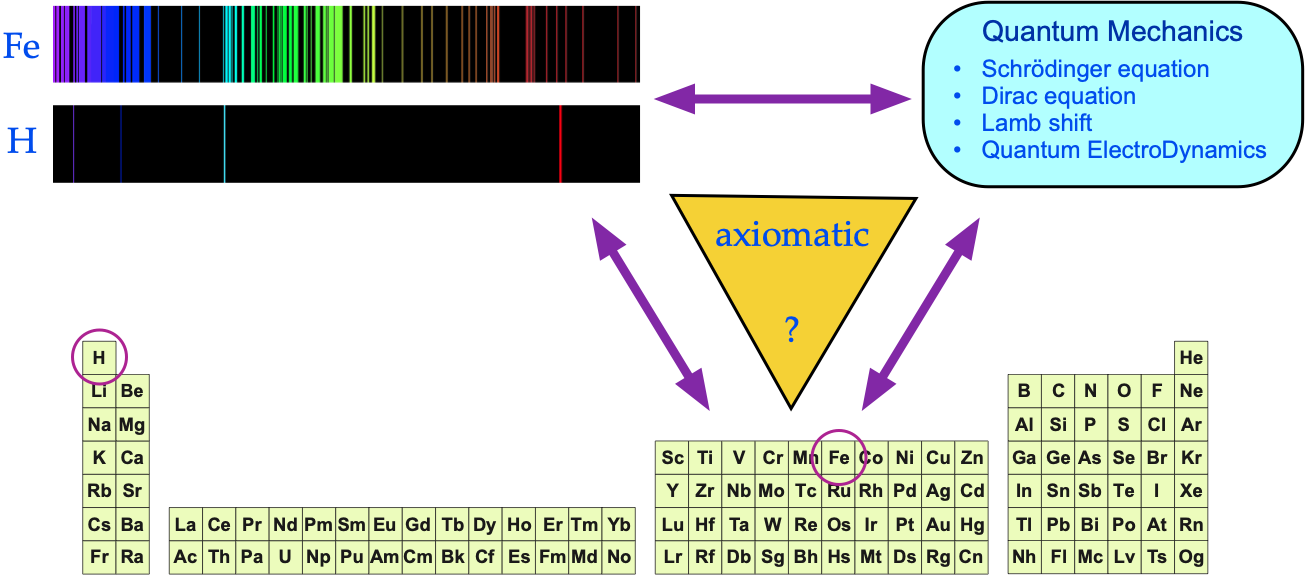
Yes, there is an empirical (experimental) correspondence between gas phase atoms and their spectra, but is there a 1-to-1-to-1 correspondence between: the periodic table, atomic spectra & quantum mechanics?
Eric Scerri: Chemist | Philosopher | Theorist | Author

Eric Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006. Read an interview with the author, here, and a review of the book here. |
disputes the full and complete axiomatic mapping between theory and the periodic table:
"Electronic configurations are not [fully] reduced to quantum mechanics nor can they be derived from any other theoretical approach. They are obtained by a mixture of spectroscopic observations and semi-empirical methods like Bohr's aufbau scheme". Has The Periodic Table Been Fully Axiomatized? Erkenntnis, 47, 229-243, 1997
The reason for the discrepancy concerns difficulty of dealing with multi-electron atoms (ions and molecules).
- The Schrödinger
wave equation can only be solved analytically for one electron systems
like the hydrogen-atom, H•, and other one electron systems: He+,
Li2+, Be3+, etc., Wikipedia. For multielectron
systems, approximations in the math have to be made to deal with electron-electron
interactions and correlations. Multi-electron atoms are complex
objects, in the systems sense. The mathematical
techniques employed to describe chemical systems are usually pragmatic
rather than rigorous, and they are often semiempirical: ie partially
based on experimental data.
- From Wikipedia: "For atoms with two or more electrons, the governing equations can only be solved with the use of methods of iterative approximation. Orbitals of multi-electron atoms are qualitatively similar to those of hydrogen, and in the simplest models, they are taken to have the same form. For more rigorous and precise analysis, numerical approximations must be used. Atomic orbitals are often expanded in a basis set of Slater-type orbitals which are orbitals of hydrogen-like atoms with arbitrary nuclear charge Z."
The effect is to produce nice fast computer code that can efficiently predict properties such as atomic & molecular energies, geometries and spectra, etc., but at the expense of the theory being fully axiomatic: formally the logic of the underlying theory becomes blurred. As a result, we get a useful model but not a mathematical proof.
- Indeed, there is a profound difference between "quantum chemistry", the techniques, methodologies and computer software used by physical chemists and chemical physicists, and the underlying quantum mechanics in the form of quantum electrodynamics (QED), the most accurate and precise theory known to humankind. Chemical problems are simply too involved [currently] to be studied by QED, although in principle they could be.
- So, while it may be possible to explain and model the spectra of the gas phase elements using the methods of quantum chemistry the analysis is not of the form of a mathematical proof.
Read more here: Physical Origin of Chemical Periodicities in the System of Elements, Pure and Applied Chemistry | Volume 91: Issue 12, 2019, Li & Schwarz et. al.
Furthermore, there is even an issue with the concept of the atomic orbital:
From: Philosophy of Chemistry by Martín Labarca: "Orbitals cannot be observed since, strictly speaking, they do not exist." The debate [about orbitals] is a manifestation of a problem that has profound consequences for the teaching of chemistry, since the teachers of the discipline cannot do without the concept of orbital in their teaching [including in this web book!]. Some authors have pointed out that this position collides with the assumption that quantum mechanics has the last word on the subject: only the concept of wave function is legitimate; the term 'orbital' has no real-world reference. In particular, the realistic view of orbitals adopted by chemistry teachers turns out to be inconsistent with their own position when they introduce quantum mechanics as the underlying explanatory theory of chemical phenomena. This problem is explicitly pointed out by Scerri when he posed the question: "Can orbitals be real in chemistry but not in physics?" It seems quite clear that this paradoxical situation has negative consequences for a deep understanding of the discipline for the following reasons:
|
Basic & Simple Substance | Theoretical & Practical Chemistry: Does Any of This stuff Matter?
The reader may consider that the concept of element-as-basic-substance and element-as-simple-substance to be an arcane distraction. However, the analysis does actually have implications for how we understand and teach the subject of chemistry.
Beginning chemistry students always have access to a periodic table, but unfortunately this may not the PT they actually need. Students are expected to know and understand that all of the reaction equations involving chemical elements will likely involve the chemical substance, the chemical in the bottle, the reagent. Thus, reactions of hydrogen involve and should show molecular hydrogen, H2 and should therefore be written as:
2H2 + O2 → 2H2O
and NOT:
2H + O → H2O
Likewise: nitrogen is N2, oxygen O2, fluorine F2, chlorine Cl2, bromine Br2 & iodine I2. Reactions involving these entities should always be written so as to show the dimeric species. However, somehow students are also expected to know that molecular sulfur, S8, and phosphorus, P4, are usually written as involving S and P.
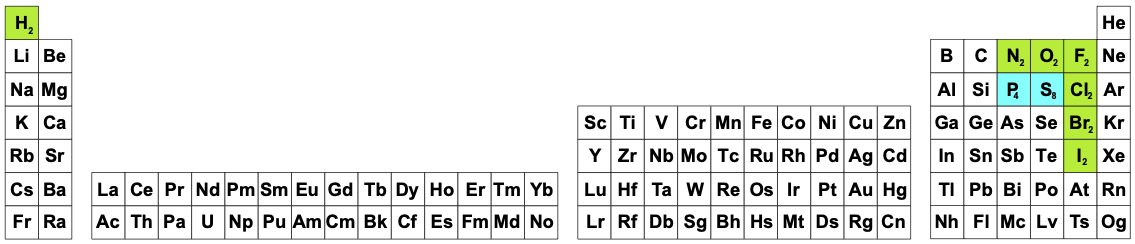
 |
 |
 |
| QNs to PTs | INTERNET Database of Periodic Tables |
© Mark R. Leach 1999-
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.