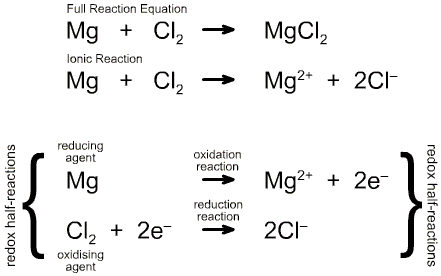| Nucleophiles and the Fluoride Ion |
Redox Chemistry Synthlet |
Redox
Chemistry
The chemogenesis
analysis identifies five distinct types of electronic reaction mechanism, here, and one of these is redox chemistry.
This page explores the different types of oxidation and reduction reaction
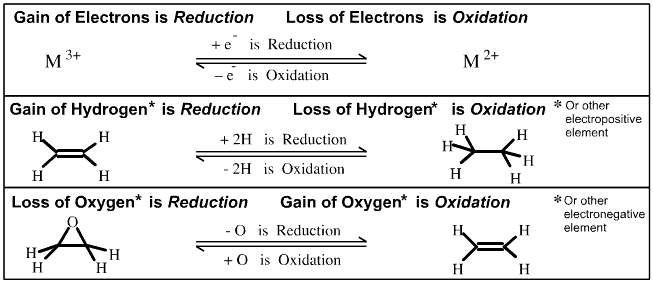
behaviour that lie behind the easily memorised (but over simplistic): OIL RIG, Oxidation
Is [electron] Loss and Reduction Is [electron] Gain.
Introduction to
Redox Chemistry
Redox chemistry is concerned
with net electron flow to and from a defined centre during
a chemical reaction. A defined centre may be:
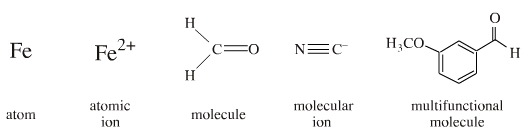
A defined centre is said to
be oxidised if the electron density decreases, and reduced if electron
density increases, during a reaction. The rule is:
Loss of electrons
equates with Oxidation
and
Gain of electrons
equates with Reduction
The oxidation of a defined
centre can be changed in two ways.
Firstly by
Single Electron Transfer (SET) to the defined centre (reduction) or
from the defined centre (oxidation). For example, the iron(III) ion,
Fe3+, can be reduced to iron(II), Fe2+. The reaction
can also occur in the oxidation direction.
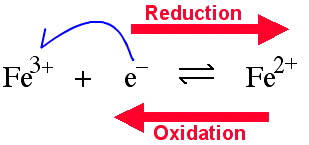
The reduction electron can
either be provided by a chemical reducing agent (often a metal) or electrochemically.
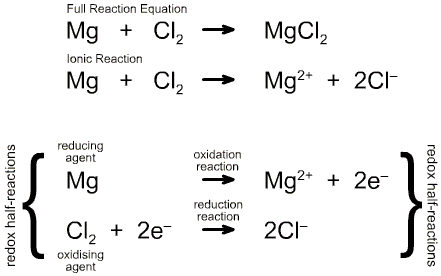
Electron flow by way of single
electron transfer oxidation and reduction can be predicted using standard
reduction potential data (below).
The second method of changing the oxidation number is by reversal of bond polarisation
at the defined centre.
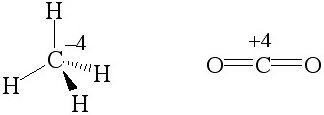
Hydrogen is electropositive
and it renders the carbon of methane, CH4, electron
rich and it is defined as having an oxidation number of -4. However,
the carbon of carbon dioxide has an oxidation number of +4 because oxygen
is more electronegative than carbon. (Each bond contributes once.)
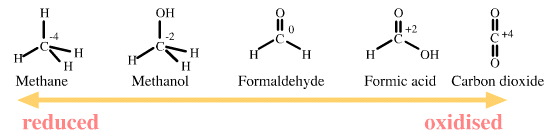
Carbon is able to exist in
several oxidation states:
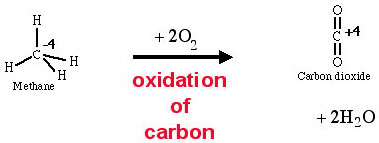
The combustion of methane
to carbon dioxide is an oxidation of carbon because the oxidation number
of carbon increases from -4 to +4
It follows that Redox Chemistry
can proceed by three types of redox reaction:
Classification of Redox
Types
A considerable number of oxidising
agents and reducing agents have been added to The
Chemical Thesaurus reaction chemistry database. These have been classified
into six general types of reducing agent and six general types of oxidising
agent, although (and please note) the classification is NOT as
clear-cut or rigorous as that carried out for Lewis acid and Lewis base
types.
Gain of electrons, gain
of hydrogen or metal, or loss of oxygen or halogen equates with reduction.
This can occur with various types of chemistry:
- Single Electron Transfer
Electron Donor Reducing Agent, here
- Hydride Complex Reducing
Agent, here
- Lewis Acid Hydride Donor
Reducing Agent, here
- Hydrogen Reducing Agent, here
- Dissolved Metal Reducing
Agent, here
- Miscellaneous Reducing Agent, here
Loss of electrons, loss
of hydrogen or metal, or gain of oxygen or halogen equates with oxidation.
This can occur with various types of chemistry:
- Single Electron Transfer
Electron Removal Oxidising Agent, here
- Hydrogen Removal Oxidising
Agent, here
- Per-Oxygen Oxidising Agent, here
- Oxidised Main Group Element
Oxidising Agent, here
- Oxidised Heavy Metal Oxidising
Agent, here
- Miscellaneous Oxidising
Agent, here
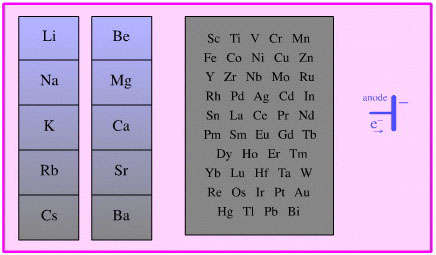
Single Electron Transfer
Electron Donor Reducing Agent
Species which act as donors
of electrons, including all electropositive elements including all metals
and anodes.
Electron Transfer Reactions in Standard Reduction Form
Temp 298K, Pressure 1.0 arm, All solutions 1.0 molar |
| +2.87 F2 → F– |
|
| +2.07 O3 → O2 |
|
| +1.81 Co3+ → Co2+ |
|
| +1.77 H2O2 → H2O |
|
| +1.77 NO2 → N2 |
|
| +1.70 MnO4– → MnO2 |
|
| +1.69 PbO2 → Pb4+ |
|
| +1.68 Au+ → Au |
|
| +1.66 Pb4+ → Pb2+ |
|
| +1.63 ClOH → Cl2 |
|
| +1.59 BrOH → Br2 |
|
| +1.51 MnO4– → Mn2+ |
|
| +1.46 PbO2 → Pb2+ |
|
| +1.45 IO– → I2 |
|
| +1.41 Au3+ → Au+ |
|
| +1.36 Cl2 → Cl– |
|
| +1.33 Cr2O72– → Cr3+ |
|
| +1.28 Ce4+ → Ce3+ |
|
| +1.26 Tl3+ → Tl+ |
|
| +1.23 MnO2 → Mn2+ |
|
| +1.23 O2 → H2O |
|
| +1.20 IO3– → I2 |
|
| +1.20 Pt2+ → Pt |
|
| +1.17 Ag2O → Ag |
|
| +1.09 Br2 → Br– |
|
| +1.00 HNO2 → NO |
|
| +0.99 Pd2+ → Pd |
|
| +0.96 NO3– → NO |
|
| +0.94 NO3– → HNO2 |
|
| +0.91 Hg2+ → Hg+ |
|
| +0.88 NO3– → [NH4]+ |
|
| +0.85 Hg2+ → Hg |
|
| +0.80 NO3– → N2O4 |
|
| +0.80 Ag+ → Ag |
|
| +0.79 Hg+ → Hg |
|
| +0.77 Fe3+ → Fe2+ |
|
| +0.68 O2 → H2O2 |
|
| +0.62 I2 → I– |
|
| +0.54 Cu2+ → CuCl |
|
| +0.52 Cu+ → Cu |
|
| +0.45 H2SO3 → S |
|
| +0.40 Cd2+ → Cd |
|
| +0.40 O2 → HO– |
|
| +0.34 Cu2+ → Cu |
|
| +0.27 Hg2Cl2 → Hg |
|
| +0.25 PbO → Pb |
|
| +0.23 Ge2+ → Ge |
|
| +0.22 AgCl → Ag |
|
| +0.17 SO4– → H2SO3 |
|
| +0.17 S → H2S |
|
| +0.16 Cu2+ → Cu+ |
|
| +0.15 Sn4+ → Sn2+ |
|
| +0.12 CuCl → Cu |
|
| +0.10 Si → SiH4 |
|
| +0.06 P → PH3 |
|
| 0.00 H+ → H2 |
|
| -0.06 Fe3+ → Fe |
|
| -0.12 CO2 → CO |
|
| -0.13 Pb2+ → Pb |
|
| -0.14 Sn2+ → Sn |
|
| -0.18 V2+ → V |
|
| -0.20 CO2 → HCOOH |
|
| -0.23 In+ → In |
|
| -0.26 V3+ → V2+ |
|
| -0.27 Ni2+ → Ni |
|
| -0.28 Co2+ → Co |
|
| -0.28 H3PO4 → P(OH)3 |
|
| -0.34 Tl+ → Tl |
|
| -0.36 PbSO4 → Pb |
|
| -0.37 Se → SeH2 |
|
| -0.37 Ti3+ → Ti2+ |
|
| -0.40 Ga3+ → Ga+ |
|
| -0.40 In3+ → In+ |
|
| -0.41 Cr3+ → Cr2+ |
|
| -0.44 Fe2+ → Fe |
|
| -0.48 S → S2– |
|
| -0.49 CO2 → (COOH)2 |
|
| -0.50 P(OH)3 → HP(OH)2 |
|
| -0.51 Sb → SbH3 |
|
| -0.51 HP(OH)2 → P |
|
| -0.56 Ga3+ → Ga |
|
| -0.60 As → AsH3 |
|
| -0.61 U4+ → U3+ |
|
| -0.74 Cr3+ → Cr |
|
| -0.76 Zn2+ → Zn |
|
| -0.86 SiO2 → Si |
|
| -0.91 Cr2+ → Cr |
|
| -0.92 Se → Se2– |
|
| -1.14 Te → Te2– |
|
| -1.18 Mn2+ → Mn |
|
| -1.63 Ti2+ → Ti |
|
| -1.66 Al3+ → Al |
|
| -1.80 U3+ → U |
|
| -1.85 Be2+ → Be |
|
| -2.36 Mg2+ → Mg |
|
| -2.48 Ce3+ → Ce |
|
| -2.52 La3+ → La |
|
| -2.71 Na+ → Na |
|
| -2.87 Ca2+ → Ca |
|
| -2.89 Sr2+ → Sr |
|
| -2.90 Ba2+ → Ba |
|
| -2.92 Cs+ → Cs |
|
| -2.92 Rb+ → Rb |
|
| -2.93 K+ → K |
|
| -3.03 Li+ → Li |
|
| -3.10 N2 → HN3 |
|
Search the Chemical Thesaurus Reaction Chemistry Database here
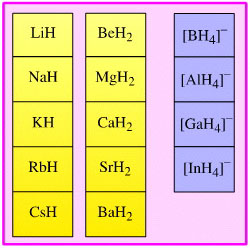
Hydride Complex Reducing
Agent
Complexes which act as donors
of basic, nucleophilic hydride ion.
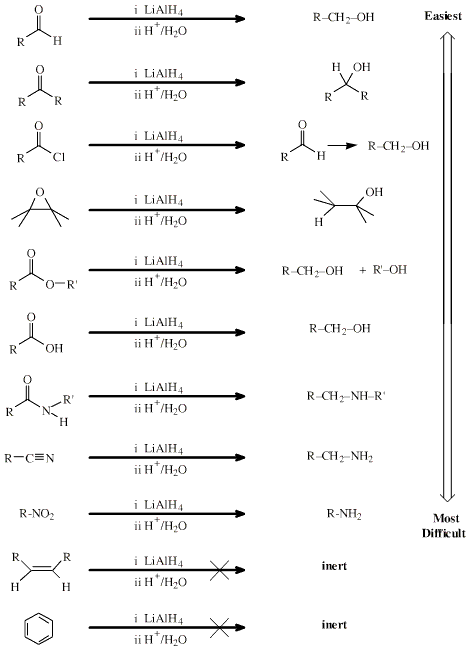
One of the most powerful hydride
complex reducing agents is lithium aluminium hydride (lithal), LiAlH4.
Lithal selectively reduces some functional groups more easily than others:
Search the Chemical Thesaurus Reaction Chemistry Database here
Lewis Acid Hydride Donor
Reducing Agent
Hydrogen rich Lewis acids which
complex with a Lewis base function and then transfer hydride ion to the
function.
Borane, BH3,
and its dimer, diborane, B2H6,
exhibit a range of reducing ability which is complementary to lithal:
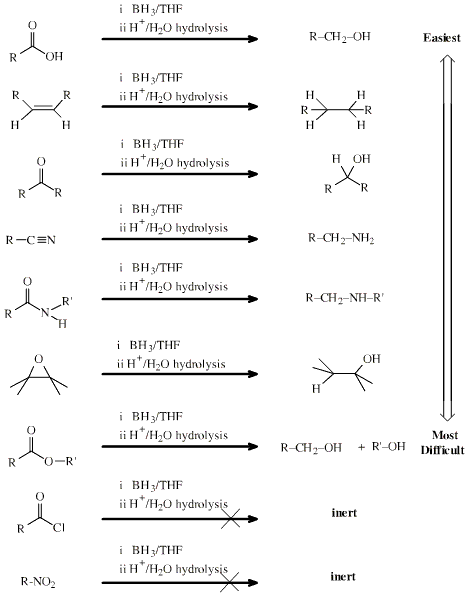
Search the Chemical Thesaurus Reaction Chemistry Database here
Hydrogen Reducing Agent
Hydrogen is a reducing agent,
although it is nearly always used with a transition metal catalyst to
aid addition of hydrogen to a function.
Many functional groups are
reduced by hydrogen plus catalyst:
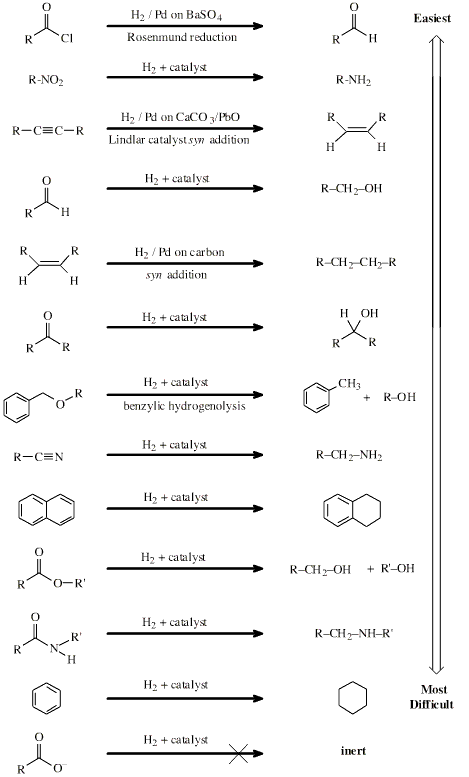
Search the Chemical Thesaurus Reaction Chemistry Database here
Dissolved Metal Reducing
Agent
There are a number of reduction
methodologies which use a metal dissolved in a polar solvent: aqueous
acid, water, ammonia, alcohols, amines, etc. Careful choice of metal and
solvent can result in very selective reduction.
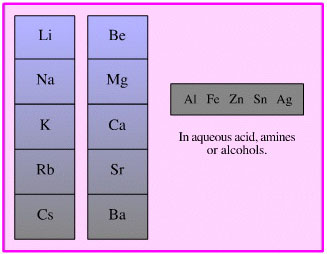
An example of a metal, such
as sodium, acting as a reducing agent in an organic reaction is the Birch
reduction of aromatic compounds, such as naphthalene, in a weakly
protic solvent. The metal provides electrons and the solvent provides
protons:
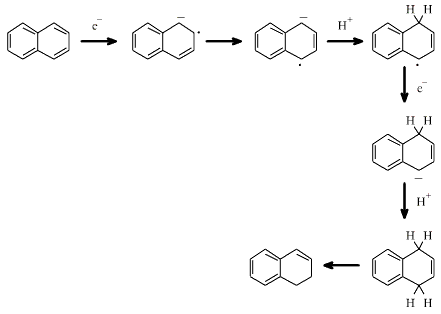
Search the Chemical Thesaurus Reaction Chemistry Database here
Miscellaneous Reducing Agent
There are a number of reducing
agents which act with mechanisms which do not fit into any general pattern.

Search the Chemical Thesaurus Reaction Chemistry Database here
Single Electron Transfer
Electron Removal Oxidising Agent
Species which accept electrons,
including: electronegative elements (halogens), metal cations and cathodes.
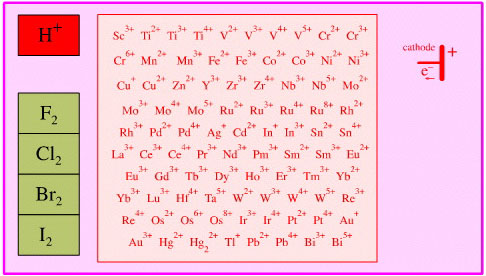
Search the Chemical Thesaurus Reaction Chemistry Database here
Hydrogen Removal Oxidising
Agent
There are a number of reagents
which remove hydrogen from hydrogen rich species. For example, sulfur
and selenium can convert cyclohexane to benzene.

Search the Chemical Thesaurus Reaction Chemistry Database here
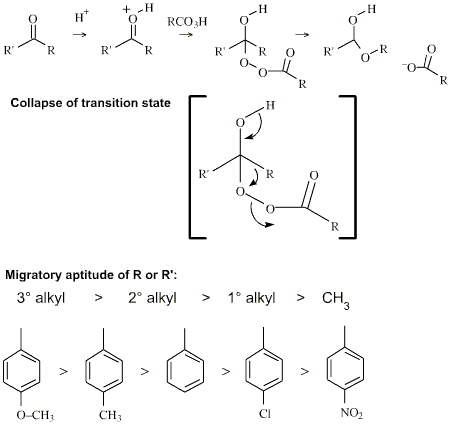
Per-Oxygen Oxidising Agent
As well as oxygen, there are
oxygen rich species which are powerful oxidising agents, including: ozone,
hydrogen peroxide and per-acids.
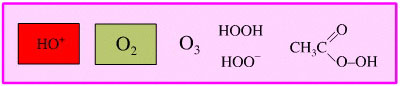
An example of such a reaction
is the Baeyer-Villiger oxidation of ketones to esters:
Search the Chemical Thesaurus Reaction Chemistry Database here
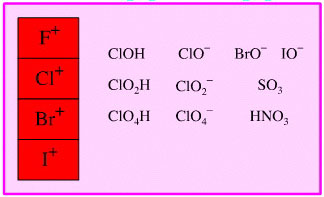
Oxidised Main Group Element
Oxidising Agent
Highly oxidised main group
elements, including the halogen (enium) cations can act as oxidising agents.
Search the Chemical Thesaurus Reaction Chemistry Database here
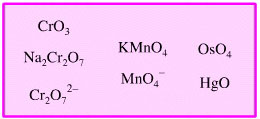
Oxidised Heavy Metal Oxidising
Agent
Highly oxidised heavy metals
and oxidised heavy metal ions can act as oxidising agents:
Search the Chemical Thesaurus Reaction Chemistry Database here
Miscellaneous Oxidising
Agent
There are a number of oxidising
agents which act with mechanisms which do not fit into any general pattern:
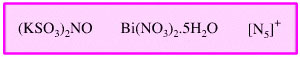
Search the Chemical Thesaurus Reaction Chemistry Database here
| Nucleophiles and the Fluoride Ion |
Redox Chemistry Synthlet |
© Mark R. Leach 1999 –
Queries,
Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,
please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open
access web book is an ongoing project and your input is appreciated.