Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Unit & Compound Mechanistic Steps | Addition Synthlet |
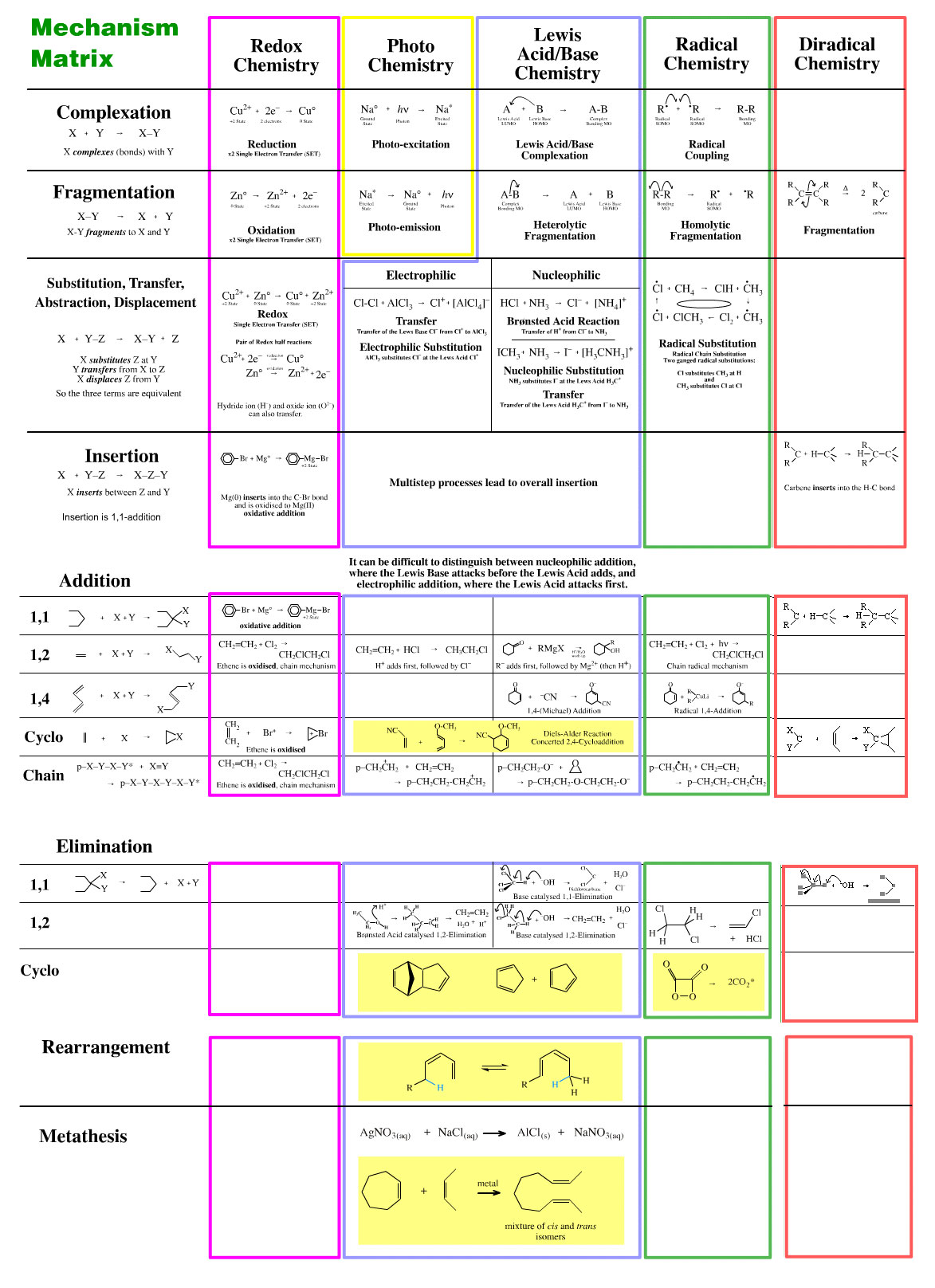
The Mechanism Matrix
The chemogenesis analysis identifies five types of electronic mechanism, here, and 10 types of unit & compound mechanistic step involving molecular fragments, here. It follows that it should be possible to arrange these into a 5 x 10 mechanism matrix. However – and very unfortunately – the resulting interaction schema is not as useful as hoped, although it is interesting to investigate why this is the case.
Introduction
Students of chemistry often get confused with the terms:
- Electrophilic addition
- Nucleophilic substitution
- Radical elimination
- and their various combinations
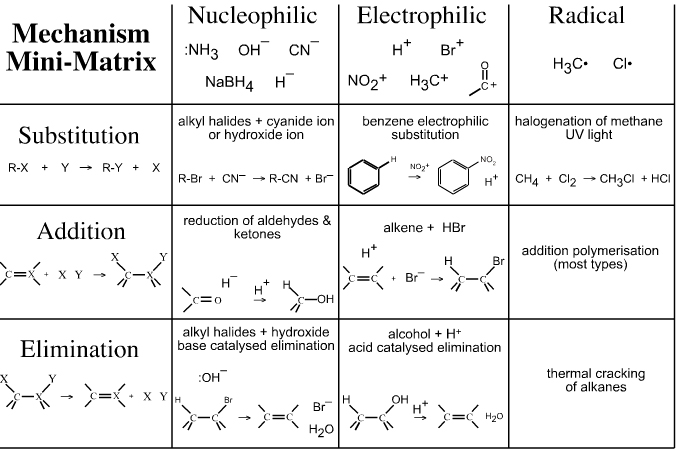
It is important to recognise that the terms substitution, addition and elimination can be seen with the structural changes that occur to the molecule:
- Substitution, one moiety replaces another
- Addition, a double bond becomes single bond
- Elimination, double bond formed
but that the terms electrophilic, nucleophilic and radical require a knowledge of the electronic mechanism that is occurring.
To aid understanding, the substitution, addition and elimination changes to molecular structure can be arranged against the electrophilic, nucleophilic and radical electronic mechanisms in a mechanism matrix so that any combination of substitution, addition & elimination AND electrophilic, nucleophilic & radical is possible:
The chemogenesis analysis identifies five distinct types of electron accountancy and five associated reaction chemistries, here:
Analysis of atom-to-atom mapping can be understood in terms of unit and compound mechanistic steps, here:
- Complexation
- Fragmentation
- Substitution
- Insertion
- Pericyclic processes
- Metathesis
- Addition
- Elimination
- Rearrangement
- Multistep name reaction
The five reaction chemistries can be arranged against the atom-to-atom mappings to give a matrix of mechanism types:

Alas and Alack, Where Is The Perfect Data?
Unfortunately, the mechanism matrix does not generate perfect data – far from it – and it is most instructive to examine some of the reasons why:
- Not all of the five
reaction chemistries have associated atom-to-atom mappings with the result
that there are many blank or null cells. For example:
Only photochemical excitation, emission and transfer (equivalent to complexation, fragmentation and STAD) are possible. A photoexcited species may undergo homolytic bond fission, but this is better classified as radical fragmentation, not as a photochemical process.
Diradicals do not abstract or displace other diradicals.
Rearrangements do not (obviously) occur by redox mechanisms.
- There is significant
duplication in the matrix.
Mg° inserting into a C–Br bond to give the Grignard reagent, R–Mg–Br, can be considered as an insertion, an oxidative process (Mg° –> Mg2+), a 1,1-addition or as a special case of complexation.
This duplication can be hidden by providing different examples for each instance, for example, use Grignard reagent formation for insertion and the Heck reaction for oxidative 1,1-addition.
- There is simply too
much data, too many examples and too many exceptions.
A schema should collect specific information into the general themes, provide a meta-view and simplify the system. The reader may reasonably feel that the mechanism matrix fails to achieve this.
- Name reactions are often
multistep processes that mix-and-match between the five reaction chemistries.
- After Lewis acid/base
complexation and heterolytic bond fission, "ionic" mechanisms
bifurcate into electrophilic and nucleophilic subtypes, for example: both
electrophilic and nucleophilic aromatic substitutions, SEAr
and SnAr, are known.
- The mechanism matrix does not explicitly accommodate pericyclic reactivity.
Pericyclic ReactivityA classification decision was made early in the development chemogenesis argument when Diels-Alder cycloaddition, here, was deemed to be a Lewis acid/base complexation between a π-LUMO Lewis acid and a π-HOMO Lewis base to give a type 20 complex, see here. The decision to consider cycloaddition within Lewis acid/base chemistry is at odds with the view that cycloaddition is a pericyclic reaction process, and that pericyclic reactivity is a distinct, sixth type of reaction chemistry.
Pericyclic reactivity comes into its own when it is realised addition, elimination and rearrangement processes are always better understood in terms of FMO analysis. There is no conflict between the chemogenesis and the Fleming views, in fact an understanding of both positions leads to a better understanding of the system as a whole. The Diels-Alder reactions can be considered to be both a Lewis acid/base complexation and a pericyclic process, in the same way that a hydride ion can be considered to be both a nucleophilic Lewis base and a reducing agent, here. |
- Many reactions – particularly some of the most common main group chemical reactions – do not proceed by "obvious" mechanisms at all. Consider the combustion reaction of hydrogen with oxygen to give water:
2H2 + O2 → 2H2O
What is the mechanism of this reaction? The reaction is clearly a "redox process" because the hydrogen is oxidised and the oxygen is reduced, and the overall reactivity can be predicted using standard reduction potential data. But how exactly does the combustion reaction occur... remembering that each discrete step must be a STAD process?
The mechanism of the 2H2 + O2 → 2H2O reaction (shown below) has at least 11 discrete steps involving: radicals (H•, HO• and HOO•), diradicals (O2 and •O•), the neutral species H2 and HOOH (hydrogen peroxide is an intermediate species in the combustion of hydrogen and oxygen!) and the species, m and m*, which carry away the kinetic energy of gas phase complexation (coupling), as discussed here:
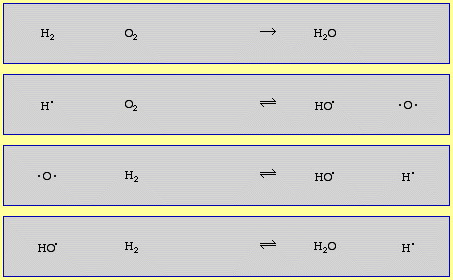
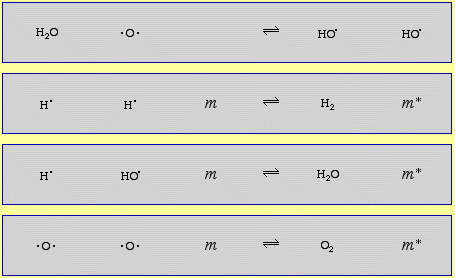
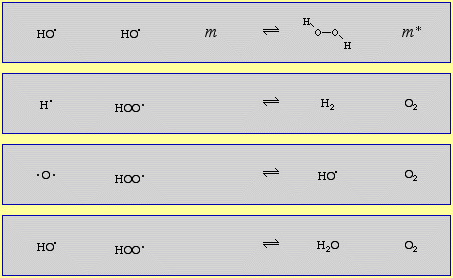
The combustion of methane, CH4, with oxygen has several dozen steps.
Likewise, consider the thermal elimination of hydrogen chloride, HCl, from 1,2-dichloroethane (ethylene dichloride, EDC) to give chloroethene (vinyl chloride monomer, VCM):
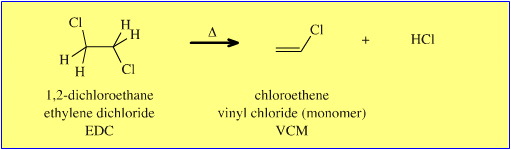
The mechanism of the solution phase, [Brønsted] base catalysed elimination reaction is clear. In a concerted manner, the [Brønsted] base abstracts a beta-hydrogen, a π-bond forms, and a chloride ion (nucleofugal, Lewis base leaving group) is ejected. For the reaction to proceed, the molecule must adopt an anti (diaxial) conformation so that there is a 180° dihedral angle between the H and the Cl, a requirement can be rationalised using FMO theory.
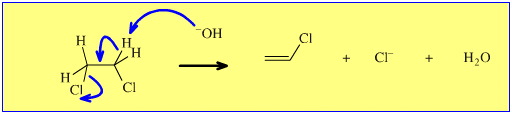
However, the industrial process is carried out by pyrolysing EDC at high temperature, in the gas phase and under pressure (reaction 1, below). The reaction proceeds by a number of discrete radical reaction steps.
- First a chlorine radical is formed by thermal fission of a C–Cl bond (2) and this is the initiation step.
- The chlorine radical propagates via a two step chain elimination sequence (3).
- There are numerous possible side reactions, some of which are given below (4):
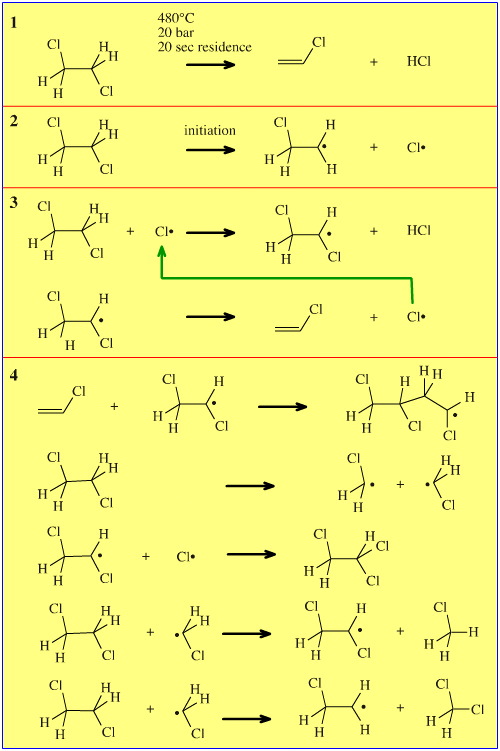
Overall, the industrial process has been optimised to give a 99% conversion of EDC to VCM + HCl. While this sounds like an excellent percentage conversion – and it is – a large VCM plant may produce 5,000 tons of VCM per day, and that means 50 tons per day of by-product (largely waste) is formed. One of the biggest process problems, but not eluded to on the diagram above, is the build up of coke (carbon).
Likewise, the Haber ammonia synthesis reaction:
3H2 + N2 ⇄ 2NH3
is, overall, a redox process. But the "gas phase" reaction has a solid phase catalyst consisting of small crystallites of Fe with small amounts of K and Al2O3.
The reaction and associated mechanism takes place at the heterogeneous gas-solid interface where mechanisms are both very involved and very difficult to study. (There is an excellent heterogeneous catalysis web book by Per Stoltze.)
In fact, most "real world reactions" such as the combustion of hydrogen and oxygen, the Haber ammonia synthesis or VCM production: very, very, very involved!
Indeed, the mechanism matrix marks the end of the chemogenesis story. The mechanism matrix marks the places in reaction chemistry space where the traditional organic, inorganic and industrial methodologies assume their distinct identities and explode with information and data. The amount of reaction chemistry information published in the primary literature (journals), in patents and held in industrial databases is quite simply staggering.
The RDMS To The Rescue
Reaction chemistry space in toto is not well modeled with linear books, even hyper-linked web books like the chemogenesis web book.
It is much more efficient to use a relational database management system (RDMS) to arrange [database] tables of chemical species, chemical reaction and reactions mechanism into a coherent whole.
The Chemical Thesaurus reaction chemistry database another free offering from meta-synthesis was ported to the web in January 2006:
 |
 |
 |
| Unit & Compound Mechanistic Steps | Addition Synthlet |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.