Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Congeneric Array Interactions | Congeneric Array Database |
The Emergence of Organic Chemistry
Congeneric arrays – sets of chemical species with linear structure and reactivity behaviour traits – can react with each other to produce new arrays which are NOT congeneric.
A Bit of Personal History:
|
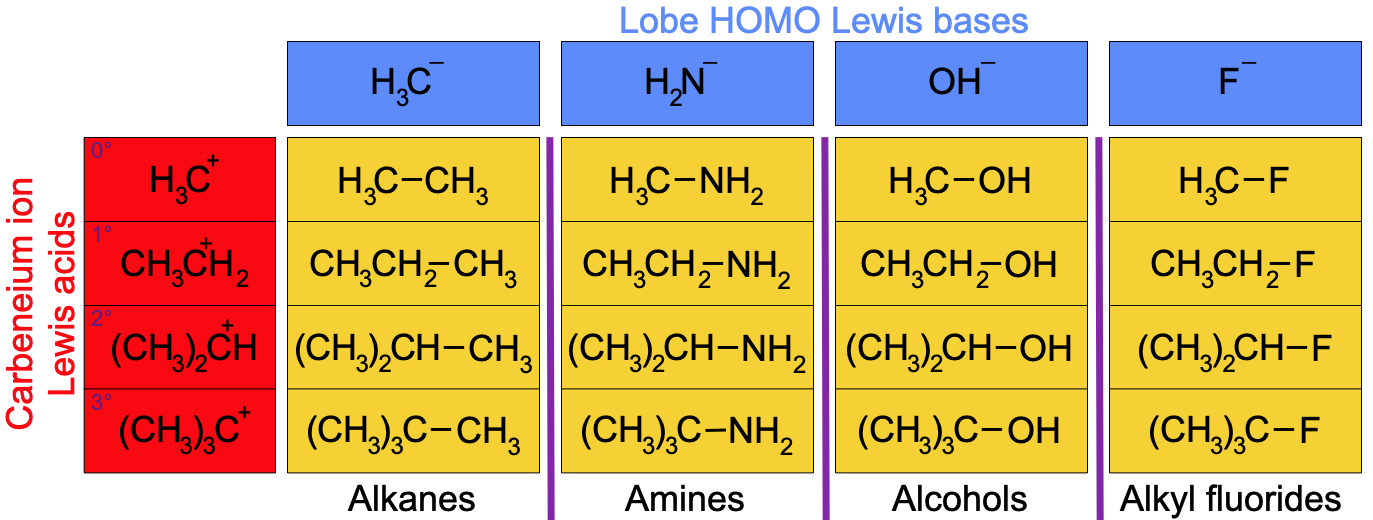
"In January 2000, I was in my office playing with the congeneric array interactions discussed over the previous few pages. I was using a graphics app to cut-n-paste the various species, when I built the following [4 x1] x [4x1] array interaction: [alkyl substituted carbenium ions] x [methyl anion to fluoride ion] [ H3C+ CH3CH2+ (CH3)2CH+ (CH3)3C+ ] x [ H3C– H2N– HO– F– ]
"I started to write how this was yet another congeneric planar with regularly changing properties of bond length (true), % ionic bond character (true) and reaction chemistry behaviour... when I suddenly realised that this was not the case at all. "The 16 species simply do NOT represent a 4x4 congeneric planar. Instead, some of the most common and distinct functional groups of organic chemistry emerged: alkanes; amines; methanol, 1°, 2° & 3° alcohols and 1°, 2° & 3° alkyl halides!
"While there are vertical (column) trends within each functional group type (as drawn) – the alcohols for example – the 16 species are simply NOT congeneric horizontally. "I spent several minutes just staring at the screen in amazement. "Over the previous few busy & creative weeks I had travelled from the main group elemental hydrides and the five hydrogen probe experiments to congeneric array interactions, where I had found – amongst other things – a congeneric volume consisting of 80 species. But when extending this congeneric array interaction logic to the one discussed here, the complexity and richness of organic chemistry had simply emerged before my eyes!" Mark Leach |
Explanation(s)
The products of the [carbenium ion] x [period 2 Lewis base hydride anion] interaction are: alkanes, amines, alcohols & alkyl fluorides. The formation of these chemically distinct species types marks the place in reaction chemistry space where organic chemistry breaks away from main group chemistry and assumes its own, distinct identity. The reasons why this particular planar bifurcates – forks – are three fold (at least):
Firstly:
The alkanes are not isoelectronic with respect to the corresponding amines and alcohols because the alkanes do not possess a functional group with a lone pair of electrons. The two relevant [4 x 1] congeneric series of Lewis bases run:
Note that there is an elemental frame shift between the two congeneric series, one runs C– to F– & the other N: to Ne:.
- Whereas carbanions are congeneric with respect to nitranions [amide ions].
- Alkanes are not congeneric with respect to amines.
This are an inevitable property of congeneric array interaction logic.
Secondly:
We know and understand the chemistry of alkanes, amines, alcohols & fluoroalkanes by their chemistry: aqueous solubility, chemical reactivity, etc. Many reaction pathways appear in aqueous/polar solvents over the pH range from: concentrated mineral acid (pH < 0) through neutral water (pH 7) to strong alkali solutions (pH > 14), and this reactivity experience dominates our understanding of these materials:
- Alkanes
are insoluble in acid, neutral water and alkali: they are chemically
inert with respect to these environments.
- Amines
are very soluble in aqueous mineral acids where they form the corresponding
ammonium salts. However, they are much less soluble in neutral water and/or alkaline
solutions. Indeed, amines are commonly separated from other organic
functions by extracting with aqueous acid, changing the pH to alkaline
(basic), and extracting back into a non-polar solvent. Caffeine extraction is a common laboratory practical.
- Alcohols
(of lower molecular weight) are fully miscible with water through hydrogen bonding.
- Alkyl fluorides are chemically inert, they are not soluble in aqueous acid or alkali.
[Note that under George Olah "super [Brønsted] acid" conditions – for example H+/[SbF6]– in liquid SO2 – alkanes, amines, alcohols & alkyl fluorides are all protonated and dissolve.]
Thirdly:
Differential reaction pathways start to appear. Alcohols are [often] able to undergo to undergo acid catalysed elimination (E2 mechanism) reactions to form the corresponding alkene + water:
CH3CH2OH ⇆ CH2=CH2 + H2O
This pathway is not available to methanol, but becomes ever easier with 1° < 2° < 3° alcohols.
Planars & Volumes in Organic Chemistry Space
If the carbenium ion Lewis acid, CH3+, is complexed with the period 2, 3 4 & 5 p-block hydride anions, a planar appears in organic chemistry space. However, as discussed above, the planar is not congeneric:
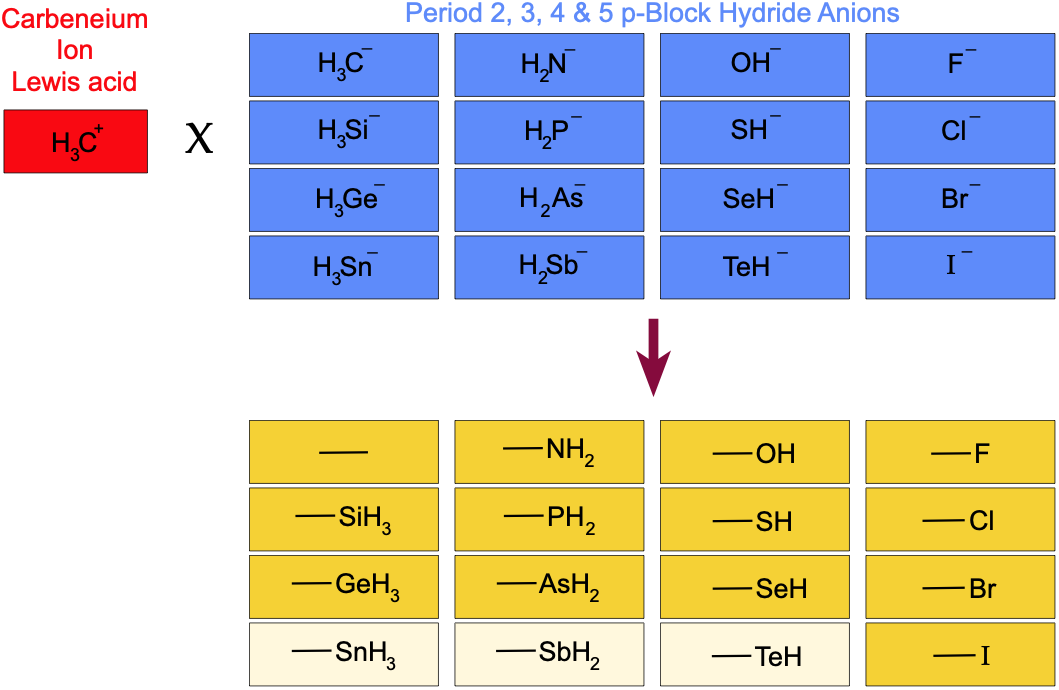
Note: Compounds with a light yellow background in the table above are not found with a google search. This methodology is not as exhaustive as a full chemical abstracts search.
Likewise, If the methyl, ethyl, isopropyl and tertary butyl carbenium ion Lewis acids are complexed with the period 2, 3 4 & 5 p-block hydride anions, a 4 x 4 x 4 volume appears.
However, and again, the planar is not congeneric over the whole object:
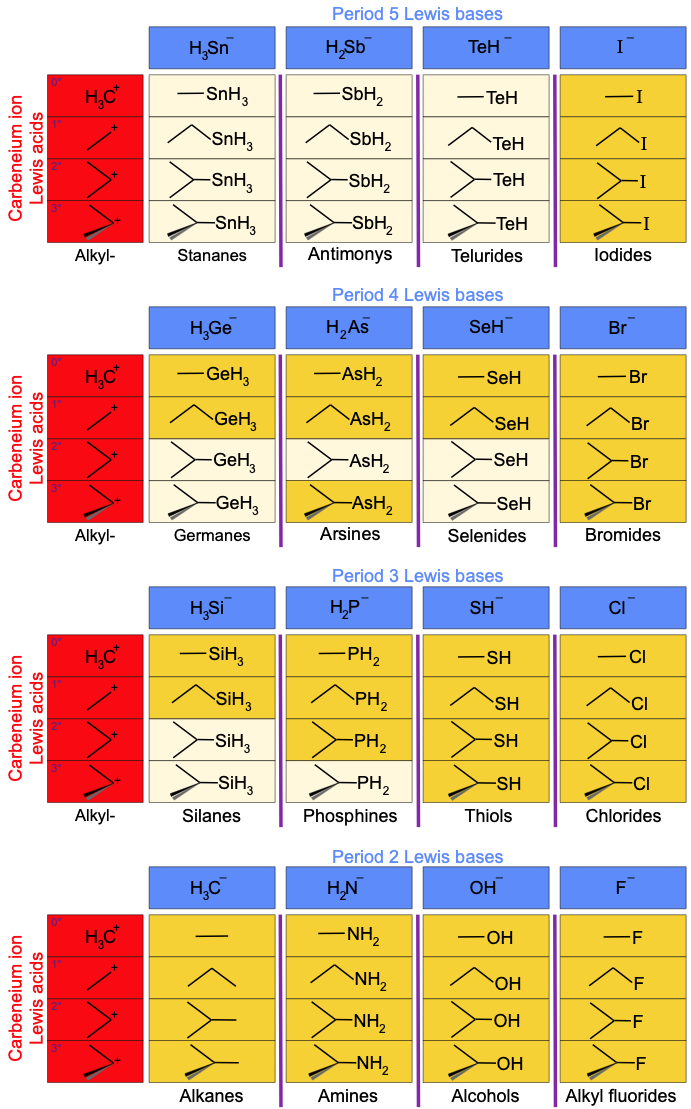
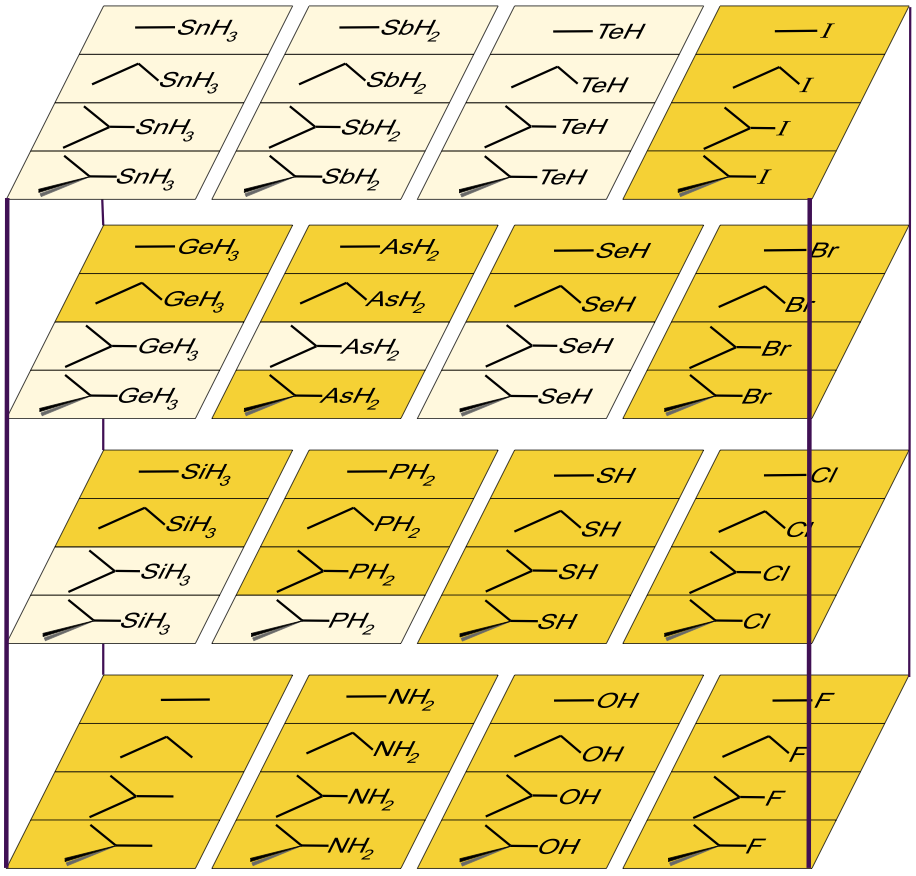
A cut-down version of the above volume – with the obscure (and unknown?) silicon, phosphorus, germanium, arsenic, selenium, tin, antimony and tellurium species removed – produces some of the most common reagents used in organic chemistry:
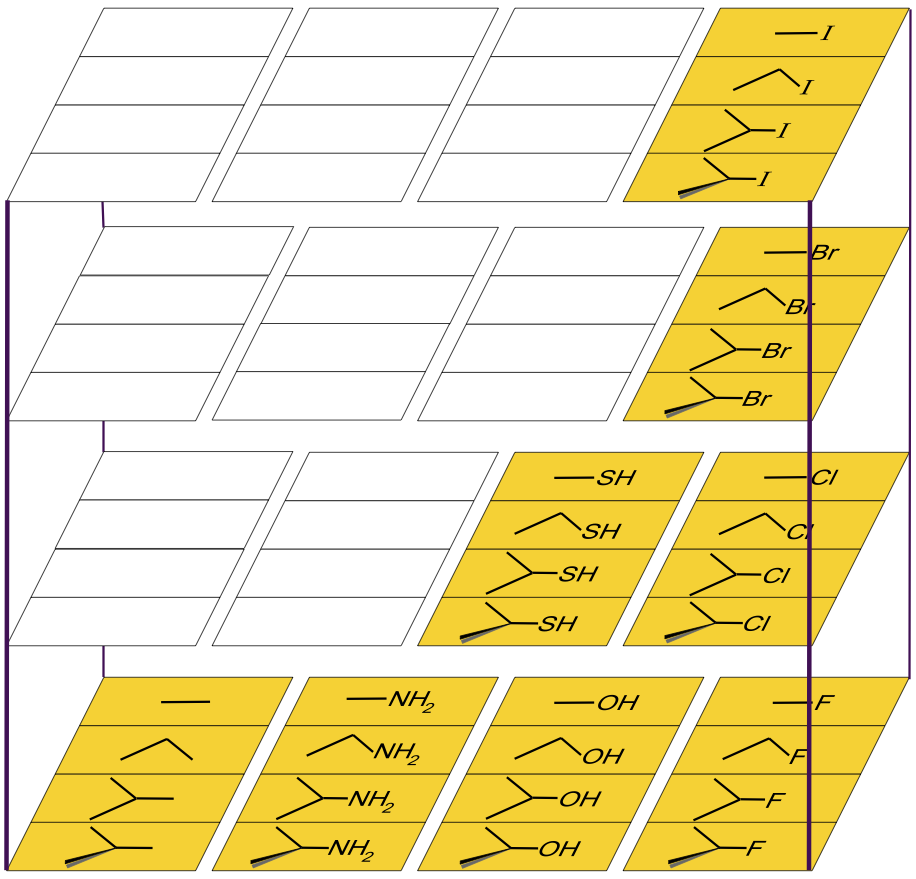
There are many reactivity trends to be seen in the above volume. One example: Alkyl halides are commonly used in SN2 reactions as 'alkylating agents' where the halogen anion, X–, acts as leaving group:
R-X + Nu– → R-Nu + X–
To add a methyl group, it is common to use iodomethane, CH3I. For ethyl, isopropyl and tertary groups it is better to use the alkyl bromide compounds, as the iodo compounds are too reactive/unstable. Generally, the chlorides are not reactive enough, and the fluorides are completely inert.
Furthermore, methyl iodide*, ethyl bromide* and isopropyl bromide* react via a concerted SN2 mechanism, the tertiary-butyl bromide* will react via the step-wise SN1 pathway.
* IUPAC names: Iodomethane, bromoethane, 2-bromopropane & 2-bromo-2-methylpropane
Carbon, Emergent Behaviour, NKS & The Game of LifeThere is an additional reason for the emergence of organic chemistry as a distinct entity. We saw, here, that ligand exchange congeneric array interactions lead to the formation of linear and cyclic alkanes. The same logic extends to the methyl, ethyl, isopropyl and tertiary butyl series of carbenium ions used above. There are general types of array interaction behaviour. They may be:
oxide → hydroxide → water → oxonium ion O2– → OH– → H2O → H3O+
Chemistry is a generative science, in that successive interaction steps from the periodic table lead to reaction chemistry, and this is the approach of the chemogenesis argument. Language is also a generative system: letters → words → phrases → sentences → paragraphs → books → libraries → Internet One of the central findings of complex systems science – also known as complexity theory – is that emergent behaviour can develop in very simple generative systems called cellular automata. One of the best known and studied examples The Game of Life invented by the British mathematician John Conway in the late nineteen sixties.

The Game of Life consists of a two dimensional array of squares on a computer screen. The game is played over a series of steps or generations with two simple rules:
Cellular automata such as the The Game of Life often develop in bewilderingly involved ways, but reoccurring patterns can be seen. Games of Life have three common types of ending:
There are close analogies between The Game of Life and reaction chemistry in that larger chemical structures are always built using generative chemical steps which employ the interaction rules of reaction chemistry space (reaction mechanisms). There are differences:
But, just like Conway's Game of Life, the reaction chemistry system has a propensity to complexity. And there are structures in reaction chemistry space which are analogous with The Game of Life end games.
The emergent behaviours of recursive systems have been exhaustively explored in Steven Wolfram's New Kind of Science (NKS).
The above logic has been expanded into a full paper, click here.  |
Isomers & Emergence
Consider the growing alkane chains: methane, ethane, propane, etc... and look at the associated isomers. There are some beautiful examples of emergent behaviour within this system:
We will add a hydroxy, –OH, functional group for clarity:
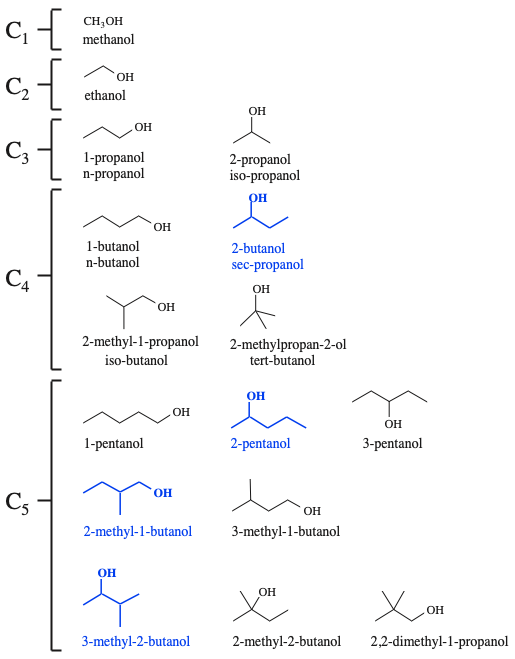
The is one structural isomer of the C1 alcohol (methanol), there is one structural isomer of the C2 alcohol (ethanol), there are two structural isomers of the C3 alcohol (1-propanol & 2-propanol), there are four structural isomers of the C4 alcohol and there are eight structural isomers of the C5 alcohol...
But, what about the alcohols in blue in the diagram above? What is special about these species? These molecules have a chiral centre, and each can exist as a pair of d/l or R/S of optical isomers.
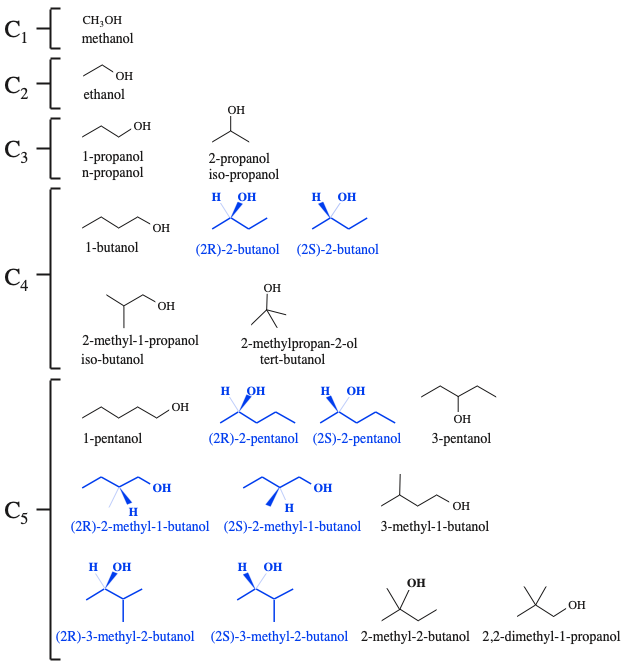
There is one C1 alcohol, there is one C2 alcohol, there are two C3 alcohols, there are five C4 alcohols and there are eleven C5 alcohols... as the number of carbon atoms increases the number of possible isomers explodes!
Systems which exhibit emergent behaviour are inherently complex, and experience shows that it is not possible to make predictions about how such systems will evolve.
The subject of complexity and emergent behaviour in chemistry is discussed here.
 |
 |
 |
| Congeneric Array Interactions | Congeneric Array Database |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.