Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Quantifying Congeneric Behaviour | Congeneric Array Interactions |
Ligand Replacement Congeneric Series
Congeneric arrays of chemical species can be produced by changing the ligands about an atomic centre in a regular way. There are several approaches: ligands can be exchanged en masse or one-at-a-time. Carbon, with its propensity to form chains, gives rise to numerous homologous series.
Inorganic Species
Congeneric series can occur when a multivalent atomic centre, such as boron or aluminium, has its ligands exchanged in a regular manner. This can occur in two ways:
Firstly, an atomic centre can have a congeneric series of ligands changed en masse:
BF3 |
BCl3 |
BBr3 |
BI3 |
[AlF4]– |
[AlCl4]– |
[AlBr4]– |
[AlI4]– |
The symbiosis logic of C.K. Jørgensen suggests that Lewis acid and Lewis base atomic centres are symbiotically hardened by hard ligands and softened by soft ligands. The chemogenesis analysis fully supports this view, with the proviso that quantitative structure-reactivity behaviour will only be found when the ligands are congeneric:
Fluoride ion, F–, ligands are harder than bromide ion, Br–, ligands and therefore the Lewis acid centre of BF3 is symbiotically harder than the Lewis acid centre of BBr3.
Certainly, boron trifluoride has a high differential affinity for hard F–, to generate the spherically symmetric [BF4]– ion.
Likewise, BBr3 has a high affinity for the softer Br– Lewis base to yield the tetrahedral and spherically symmetric [BBr4]– species.
The ligand replacement is also seen amongst transition metals. Copper(I) & copper(II) both form halides, and according to web elements, these copper halides are known:
Pearson suggested that higher oxidation states are harder than lower oxidation states. As predicted, copper(II) iodide – the hardest cation with the softest anion – is the unknown copper halide.
Secondly, ligands can also be changed one-at-a-time:
CH4 |
CH3Cl |
CH2Cl2 |
CHCl3 |
CCl4 |
[NH4]+ |
[RNH3]+ |
[R2NH2]+ |
[R3NH]+ |
[R4N]+ |
Notice, when the ligands are changed one-at-a-time, the termini (ends) of the series end up being unrelated in a chemical reactivity sense, even though they remain isoelectronic:
Methane is a hydrocarbon but not a haloalkane. Carbon tetrachloride is a haloalkane but not a hydrocarbon.
The ammonium ion is a Brønsted acid but the tetraalkyl ammonium ion is not.
More that 100 ligand exchange congeneric series are listed in The Chemical Thesaurus reaction chemistry database, and these can be searched here.
Hydride Donor Complex Anions
This combinatorial approach has been exploited in the development of specialised and selective hydride donor reagents with anions of:
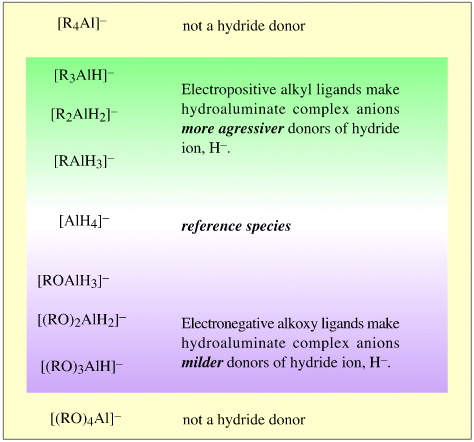
The counter ion cations can be: Li+, Na+, K+, Rb+ or Cs+. However, lithium salts are generally preferred because they are more soluble in diethyl ether, the usual solvent of choice for these aggressive and reactive reagents which must be used in an anhydrous environment.
The following is a selection of the many hydride donor reagents are available from the Sigma-Aldrich Chemical Company:
| LiAlH4 | lithium aluminium hydride |
| Li[(RO)3AlH] | lithium tris[(3-ethyl-3-pentyl)oxo]aluminohydride |
| NaAlH4 | sodium aluminium hydride |
| Na[Et2AlH2] | sodium diethyldihydroaluminium |
Organic Ligand Exchange Congeneric Series
Tse-Loc Ho, an organic chemist, argued that hydride ligands, H–, are soft compared with alkyl ligands, R–, so the carbenium ion, H3C+, is symbiotically softer than a trialkyl (tertiary) carbenium ion, R3C+. Likewise, the methyl carbanion, H3C–, is symbiotically softer than a tertiary alkyl carbanion, R3C–.
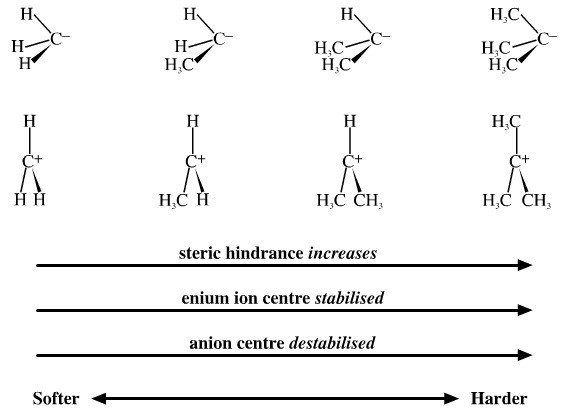
This argument is quite plausible: Methyl reactive intermediates: H3C–, H3C• or H3C+, are more polarisable and "forgiving" moieties than the trialkyl equivalents: R3C–, R3C• or R3C+ which implies that they are softer.
Tertiary alkyl bromides will readily dissociate in polar solvents and undergo first order Sn1 nucleophilic substitution, whereas methyl bromide will only undergo concerted, second order Sn2 nucleophilic substitution. The alkyl ligands appear to have hardened the carbon centre making the C-Br bond labile with respect to the hydrogen ligated methyl function.
Tertiary butyl lithium, tBuLi, is a stronger base and is less nucleophilic than methyl lithium, MeLi.
However, symbiotic hardening and softening effects are obscured by other factors such as the substantial steric hindrance which occurs about bulky tertiary alkyl carbon centres and the fact that alkyl functions strongly stabilise carbenium ion, R3C+, centres and strongly destabilise carbanion centres, R3C–, compared with H3C+ and H3C–.
It is simply not possible to disentangle symbiosis from steric hindrance and charge stabilisation. We just note that the
H3C > RH2C > R2HC > R3C
series – whether cationic, anionic or radical – show regular structural and reactivity trends and so are congeneric.
The author has performed numerous ab initio calculations to try and find some structural parameter such as bond length which quantifies the assertion that methyl anions, radicals and enium ions are softer that the alkylated analogues. However, it does not seem to be possible to disentangle bonding preference from steric and charge stabilisation effects. While the author agrees with the Ho analysis, he argues that it is not possible in principle to provide quantitative proof.
This point is stressed because we will be returning to the methyl, 1°, 2° & 3° carbenium, radical and carbanion congeneric series which are so important to organic chemistry.
There are many examples of ligand exchange congeneric series in organic chemistry, often based on exchanging hydrogen and alkyl ligands around carbon, nitrogen, oxygen, silicon, phosphorus, sulfur, etc. centres. These are all listed in The Chemical Thesaurus.
Chloroacetic Acids
The chloroacetic acids are congeneric with electronegative chlorine atoms rendering the carboxylic acid function a stronger Brønsted acid. pKa data shows the species to be congeneric.
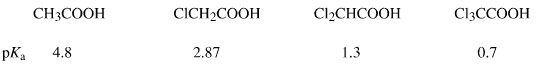
Alkanes: Melting Points & Boiling Points
The linear alkanes and cycloalkanes can be considered ligand replacement congeneric series. Boiling point data show these series to be congeneric.
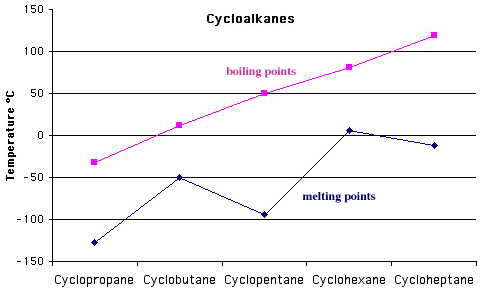
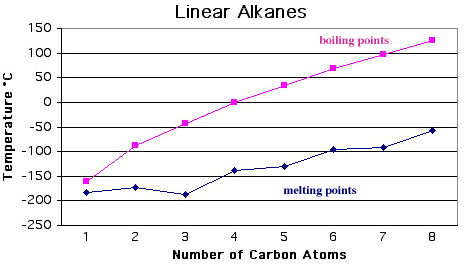
Notice, that with both the linear and cyclic alkanes, the boiling points (liquid to gas phase change) are linear with respect to carbon number, but melting points (solid to liquid phase change) are not. The reason is due to packing and crystallinity effects which are important in the ordered solid phase, but which not found in the disordered liquid or gas phases.
So, ordered to disordered phase changes likely to exhibit non-linear effects whereas disordered to disordered phase changes are not.
Now, some readers may be surprised that we are using melting point and boiling point examples while discussing chemical reactions.
The reason is that while some authors like to differentiate between physical processes, such as phase change, and chemical reaction processes, there is no real difference. When a water molecule moves from the liquid phase to the gas phase it [the defined water molecule] is transferring from one chemical environment to another, in other words it is undergoing a chemical reaction.
Throughout the Chemogenesis web book and The Chemical Thesaurus reaction chemistry database, we use a very broad definition of what it is that constitutes a chemical reaction. In essence, it comes down to whether the process can be described in the form or a chemical reaction equation with an arrow:
X + Y → Z or Y → Z
Using a broad definition of what it is that constitutes a chemical reaction solves many problems, and causes few.
 |
 |
 |
| Quantifying Congeneric Behaviour | Congeneric Array Interactions |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.