Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Binary Material Synthlet | Overview of the Chemogenesis Analysis |
The Classification of Matter: Chemical Stuff
By definition, all material things are made of matter, and chemists are profoundly interested in the nature of material stuff.
The Four Aristotelian Elements
Western chemistry grew up around old alchemical ideas of Earth, Air, Fire, and Water – the so called Aristotelian elements – a concept that originated with the ancient Greeks and others, here.
From Philosophy of Chemistry by Martín Labarca:
"The elements as conceived by the ancient Greek philosophers. Empedocles elaborated the so-called 'doctrine of the four elements': water, air, earth and fire were the constituent parts of natural reality. According to Aristotle, these elements are determined by the characteristics of heat, cold, dryness, and humidity. Some of the pre-Socratic philosophers believed that such elements were made up of microscopic components of various shapes, which explained the diversity of their properties. The basic forms of such elements were that of the Platonic solids (tetrahedron, octahedron, icosahedron and cube). When a fifth Platonic solid, the dodecahedron, is discovered later, Aristotle posits the ether as the 'fifth element' or quintessence."
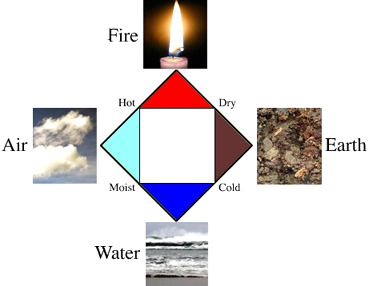
- Read more about the Four Elements in Carmen Giunta's Elements and Atoms: Case Studies in the Development of Chemistry.
- Fathi Habashi of Laval University discusses Zoroastra and The Theory of Four Elements, here, and Cambodia's Four Elements, here.
The Year 1800: Organic & Inorganic Matter
In the year 1800 some 27 chemical elements were known:
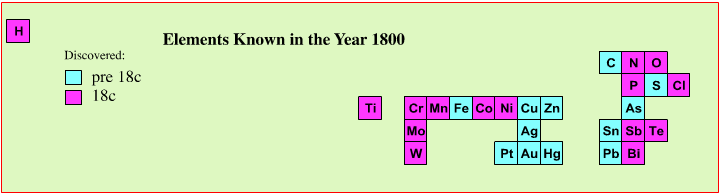
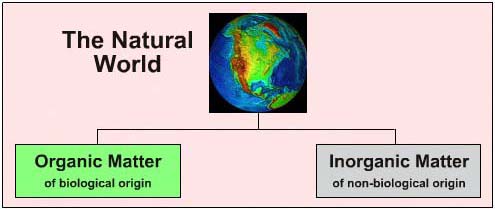
Ideas had moved on from the four Aristotelian elements, and it was thought that there were two distinct types of matter: organic and inorganic.
- Organic matter was associated with living things (biological origin: flora, fauna, food, us) and was assumed to possess a vital-force, an indefinable characteristic that separated living organisms and materials derived from living organisms from inanimate inorganic matter:
- Inorganic matter is of geological origin: minerals, rock, sea, air
New Ideas: Urea and an Unanswerable Challenge the Vital-Force Theory
In the nineteenth century chemical knowledge increased dramatically:
In 1804, Dalton proposed that matter was constructed from identical, indivisible atoms which combined with each other in constant, or stoichiometric, proportions.
In 1828 the classical distinction between organic and inorganic matter was resolved as evidence accumulated that organic materials could be synthesised in the laboratory from inorganic, non-living sources.
The crucial step occurred when the German chemist Friedrich Wohler heated ammonium cyanate, an inorganic salt, and produced the substance urea, H2NCONH2, that was identical to organic urea isolated from the urine of animals, and so was organic.
Wohler's synthesis of an organic chemical from inorganic starting materials was an unanswerable challenge to the vital-force theory.
By the end of the nineteenth century chemical methodology had become very sophisticated.
- Scientists understood that atom types could be classified and grouped into a periodic table of chemical elements, and by 1900 the only non-radioactive s, p or d-block element that remained to be discovered was rhenium, Re.
- Common pharmaceutical preparations such as opium, tobacco and coca were shown to have active ingredients that were discrete molecular entities (morphine, nicotine & cocaine, respectively) that could be purified to white crystalline materials (usually as the .HCl salt) of known chemical composition.
Three points:
- Most materials obtained from nature, organic and inorganic, are chemically complex, heterogeneous mixtures or composites.
- It is now generally recognised that many classically defined inorganic materials, such as limestone, coal and the oxygen in our atmosphere are actually of biological origin, produced over geological time scales.
- The modern chemical classification system says that to be "organic" a substances possess carbon hydrogen (C-H) chemical bonds, and that "inorganic" substances do not possess C-H bonds. Under this system, oxygen is inorganic. And, ironically, so is urea, H2NCONH2.
The Chemical Classification of Matter
Many chemistry textbooks provide a diagram In their introductory sections showing how matter can be classified into mixtures and pure substances, and then to heterogeneous and homogeneous mixtures, elements and compounds:
Matter, the stuff from which our physical world is formed, presents to us as various types of material. On a first analysis, the possible phases are:
- gaseous, such as air
- liquid, such as water
- solid, such as rock
- (there is a fourth phase, plasma)
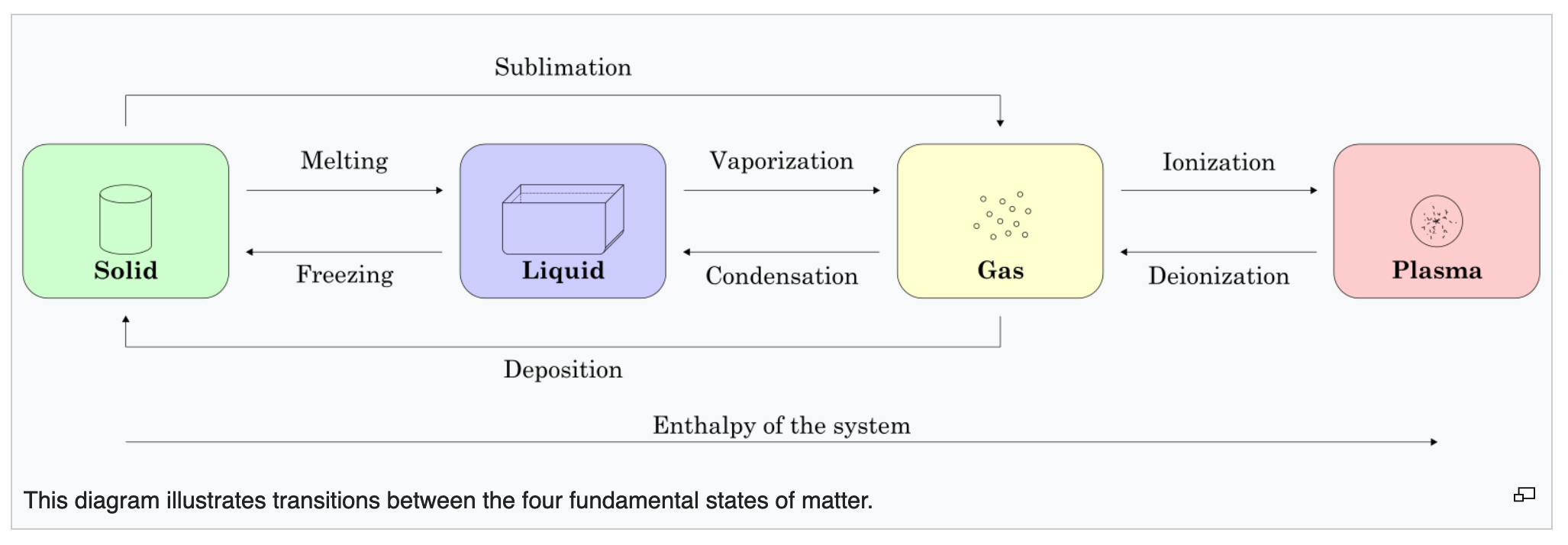
However, for classification purposes it is useful to divide materials into:
- mixtures: variable composition
- pure substances: stoichiometric composition
Physical techniques, such as: distillation, filtration, crush-&-sort, selective dissolution, chromatography, etc., can be used to separate the individual components of a mixture into chemically pure substances, and physical methods such as turbulent mixing can be used to blend pure substances together into mixtures:

The Chemical Classification of Matter: Updated
However, the above graphic is a little over simple for our purposes, and can be usefully expanded to a classification system is both derived from and is compatible with the classification system employed in The Chemical Thesaurus reaction chemistry database:

| Mixtures can be sub classified into four types: homogeneous, heterogeneous, colloidal and composite. |
|
Homogeneous Mixtures can all be regarded as solutions, and they can form in various ways:
By definition, any region of a homogeneous solution will be chemically identical to any other region so sampling is not an issue. A common way to insure that a homogeneous mixture remains homogeneous is by turbulent mixing. |
|
Heterogeneous Mixtures are agglomerates. In the natural world, nearly all matter is heterogeneous, apart from air, fresh clear water and various minerals such as quartz, rock salt, sulfur etc. However, scale is important: a 1.0 m3 sample of air will be homogeneous but the atmosphere as a whole is heterogeneous. Poorly stirred solutions where there is chemistry occurring, even simple heating, are liable to become heterogeneous.
|
|
Colloids are defined thus:
|
|
Many real world solid materials are composites:
|
| Pure Substances, as noted by John Dalton, have a fixed stoichiometric composition with simple ratios of atoms: Ne, NaCl, O2, S8, CO2, C6H12O6, etc. Pure substances are also materials. |
Elemental substances are collections of atoms with the same proton number. Most elements consist of a mixture of isotopes. This is not usually an issue, however, isotopes can be separated (enriched or depleted) in various ways. It is difficult to say how exactly many elements there are because:
|
Binary compound substances consist of just two elements with the constraint [used here] that there is just one type of strong bond present: metallic, ionic or covalent.
|
| The Laing Tetrahedron of bonding and material type, discussed in detail here, appears on this page because pure elements and substances consisting of only two elements but with only one type of strong chemical bond – exhibit four extremes of material type: metals, ionic salts, molecular substances, network covalent materials, or they are intermediate between these four extremes. |
| Ternary and polyelemental compound substances include chloromethane, CH3Cl, methanol, CH3OH, and glucose, C6H12O6. There substances have multiple types of chemical bonds of varying polarity. |
| Chemical Substance Types |
| Network covalent materials have atoms arranged in an extended lattice of strong, "shared electron pair" covalent bonds. Materials are generally hard, refractory solid substances. They are poor electrical conductors, and they are not soluble in any solvent. Very high melting point (>1500°C). Chemically intractable materials. Examples include: diamond, C, boron, B, silica, SiO2, gemstones, etc. |
| Metallic elements are (in the Drude model) a lattice of cations immersed in a sea of mobile valence electrons that are delocalised over the entire crystal. Electrons are the agents responsible for the conduction of electricity and heat. Metals have a characteristic lustre, are often ductile and exhibit a huge range of melting points, from mercury, -39°C, to tungsten at 3200°C. All the elements on the left hand side of the periodic table are metals. Indeed, as the pure elemental substance the majority of the elements present as metals. |
| Alloys are "a partial or complete solid solution of one or more elements in a metallic matrix", from here. If can only be determined if an alloy is heterogeneous, homogeneous or stoichiometric by microscopic, physical and chemical examination. Read more a alloys elsewhere in the Chemogenesis web book, here. |
|
Molecular substances consist of discrete molecules. Materials held together internally by strong intramolecular (within molecule) "shared electron pair" covalent bonds, but when forming condensed solid or liquid phases, the molecules interact via weak intermolecular (between molecule) van der Waals forces:
Molecular materials exhibit a vast array of properties, but they are generally mechanically weak, have low electrical conductivity, have low melting and boiling points, and/or a susceptibility to sublime. Molecular materials usually soluble in (or miscible with) non-polar solvents. Hydrogen bonded molecular solids are often soluble in water. |
| Simple ionic salts, like sodium chloride, Na+ Cl–, have a crystal lattice with anions electrostatically attracted to adjacent cations and cations electrostatically attracted to adjacent anions. Simple ionic materials are insulators as solids, but are electrical conductors when molten and when dissolved in aqueous solution. Simple ionic materials may be soluble in water (and sometimes in dipolar aprotic solvents such as DMSO), but they are insoluble in non-polar solvents like hexane. Simple ionic materials have moderately high melting points, usually 300-1000°C. |
|
Molecular and complex salts have a crystal lattice anions and cations electrostatically attracted to each other, but the cations and anions are compound entities. Some properties of molecular and complex salts are dominated by the ionic nature of the material. For example, substances are more soluble in water than organic solvents, indeed, many complex ions are only stable in aqueous solution. Other properties are dominated by the molecular nature of the ions. For example, melting points tend to be low or substances decompose on heating. Solubility is often pH dependent. Examples include:
|
| Intermediate materials are between ionic, molecular and network. Examples include metal oxides, such as magnesium oxide and calcium oxide, as well as metal sulfides and phosphides. This topic is discussed in detail here. |
|
Polymers consist of a large number of identical monomer components linked together in a chain, and there maybe cross linking between chains. Properties such as melting point and crystallinity are determined more by chain length and the degree of cross linking than by the nature of the monomer entities or their bonding.
|
|
Glass, as defined by Wikipedia:
An interesting video of melting glass in a microwave oven. The secret is to make a spot red hot first: |
|
Minerals As defined by Wikipedia:
A slightly wider definition could/should read:
Minerals are of crucial important to chemists because – ultimately – all chemical substances are obtained from biological or geological sources. |
| Transient & Hypothesised Entity Types |
|
Atomic ions are ions of single atoms:
|
|
Molecular and complex ions are ionic compound entities:
|
| Free radicals, or simply radicals, are neutral molecular species with a single unpaired electron in their valence shell. Radicals are discussed in more detail here. |
|
Excited state species are transient atomic or molecular entities formed by moving a ground state electron to a higher energy orbital. The behaviour of the excited state species will be very different to the ground state species. |

The Nature of Substance: A Philosophical Question
The idea of chemical substance, of physical chemical stuff, holds a special place in chemistry.
It transpires that the term 'substance' can have – in this author's opinion, at least – at least three rather different meanings: abstract substance, real substance & specific substance...
OK, I can imagine a reader thinking: "What is the author going on about now? Is this of any importance???"
Yes, it is as will become clear...
Consider abstract, real & specific substance with respect to the substance water:
- Abstract water is the idea of water. Abstract water is imagined water. Abstract water is transcendental. Abstract water has no properties unless they are explicitly assigned. Abstract water consists of H2O molecules and it has a reactivity profile, for example abstract water is formed by reacting abstract hydrogen, H2, with abstract oxygen, O2.
- Real water is water the material substance. Real water is a liquid and it is wet. Real water has a literature melting point and boiling point, 0°C and 100°C at 1.00 atm pressure. Real water can never be completely free from impurities. Real water has hydrogen and oxygen isotopes, 2H and 17O, and very small quantities of the radioactive isotopes 3H and 18O. Real water is assumed to be a homogeneous single phase.
- Thirdly, there is the specific water. This is the water as understood by the analytical chemist where every sample is unique. Consider the analytical lab attached to a municipal water works, a supplier of bottled water or the steam generation plant of a power station. Samples of water will be collected every hour (or whatever) and will be given a unique sample ID. Numbered samples will be tested optically for clarity and colour, inorganics will be tested for by AA or ICP and organics will be determined by GCMS. There will be analytically limits, and only if the specific sample passes the various analytical tests will the batch (with a batch number) of water be passed for use.
The problem is that teachers of chemistry and authors of chemistry textbooks, this author included, tend to talk about chemical substances in different ways in different situations.
When a lecturer/teacher/author writes: 2Na + 2H2O → 2NaOH + H2, they are considering water in a very general and abstract sense. We are being asked to imagine the process. It is not stated if solid, liquid or gaseous water is used. There is no glassware, no reagents. Just the notion of sodium, the notion of water, the notion of sodium hydroxide and the notion of hydrogen... these are idealised chemical entities, not real substances.
Practicing chemists tend to get around this issue by use of the term "reagent", where the reagent is the actual chemical in the bottle. Chemists learn to read the data on the reagent bottle to get all sorts of information: molecuar formula, name(s), molecular weight, melting/boiling point, hazards, etc. about the chemical in use.
When the 2Na + 2H2O → 2NaOH + H2 reaction is carried out in the labatory with actual chemical reagents, the experence is of a spectular and dangereous and full safty precautions should be taken:
An chemistry class may sample a local river every six hours and analyse the specific samples using atomic absorption spectroscopy, AAS. To get meaningful results all reagents must be of the highest (and known) purity. It is assumed, almost certainly correctly, that the river various samples will be quantifiably different because rivers are continuously changing, dynamic systems.
Crucial to the abstract-real-specific substance logic is whether the stuff in question is homogeneous or heterogeneous– whether it is a single phase or has phase boundaries. Chemists love their chemical stuff to be homogeneous: be it pure substance or a homogeneous mixture (solution). Solids are dissolved, solutions are filtered & stirred in an effort to ensure homogeneity.
- Abstract substance occupies a universe of infinite scale and so is phase independent by default, unless phase is explicitly introduced. When discussing Henry's law or Raoult's law the liquid-vapour interphase is assumed to be the infinite surface of a boundless ocean.
- Real substance has phase and phase boundaries by inspection. 500ml of liquid in a flask consists of the liquid phase plus liquid-air, liquid-glass and glass-air phase boundaries. In a given situation it may be possible to discount the phase boundaries, for example it is usually possible to ignore the evaporation of water during a titration.
- Specific substance is assumed to be homogeneous within a batch (within analytical limits), but is assumed to be heterogeneous between batches, but to still be within limits. This is actually true of any [non-bespoke] commercial product.
- There is a discussion about phase boundaries elsewhere in this Chemogenesis web book.
Consider diamond, the well known allotrope of carbon.
- Abstract diamond is a network covalent material consisting of an extended lattice of covalently bonded carbon atoms. Abstract diamond is hard and has a high melting point. Abstract diamond is colourless and it has a reactivity profile: abstract diamond burns in oxygen to give carbon dioxide. Abstract diamond is the notion of diamond, it is the idealised or transcendental substance.
- Real diamond can either be a natural mineral or it may be synthetic, produced in a lab or industrially. Real diamonds (natural or synthetic) may not be be completely colourless, they may have a blue, pink or yellow colour tint. Real diamonds contain 1.1% carbon-13 (13C), they have a refractive index 2.4175–2.4178, a specific gravity 3.52 (+/- .01) and a Mohs hardness of 10.
- There are many specific diamonds, indeed there is a Wikipedia page listing famous diamonds. These diamonds have individual: weights, cuts, colours, inclusions, settings and even names.
So the abstract diamond, the allotrope of carbon, notion of diamond is rather different to the notion of real diamond, the synthetic grit used for hardening machine parts or a bag of small colour and sized matched gem stones used by jewelers, and this is rather different to the large specific diamonds, of extraordinary value with individual names.
As discussed elsewhere in this web book, chemists may attempt the synthesis of a chemical that cannot be made in principle. Such a synthetic target can only be abstract. It is thought hydrogen forms a metallic phase at high pressure, but until proved conclusively the notion of metallic hydrogen is abstract.
Ions are abstract entities. Sodium chloride exists, but the sodium ion and chloride ion are imagined. The proton, H+, does not actually exist in water: it is hypothesised. The aluminium ion in water may be represent as: Al3+, Al3+(aq) or [Al(H2O)6]3+.
Notes From The Laboratory BenchI quickly learned while doing my Ph.D. in organic chemistry that the problem was the practical nature of my chosen research project.
Question: If a proposed reaction fails in the research lab, is it because the reaction scheme is flawed or because the experiment was poorly executed? |
Chemical Database DesignThe issue occurs with the design of The Chemical Thesaurus Reaction Chemistry Database. When I first started to develop this project I naively assumed that there would be one entry per chemical, but this soon proved impossible.
|
What is going on?In large part getting to grips with chemistry involves understanding that the idealised, abstract, essential, transcendental chemical entities we hear about in class and read about in books – including this web book – are actually complicated, difficult to use*, real, material, substances in the laboratory.
|
The Classification of Matter in The Chemical Thesaurus Reaction Chemistry Database
The web based Chemical Thesaurus reaction chemistry database, here, holds information about "chemical entities", and their interactions and reactions.
The database does not just include "real" matter, as discussed above, but also generic entities that are idealised objects that are representative of real matter. This is because chemists often discuss structure and reactivity in terms of hypothetical generic entities and the idea is used throughout The Chemical Thesaurus reaction chemistry database. Examples of generic entities include:
- 2° alcohol
- aldehyde
- carboxylic acid
- p-block element
- nucleophile
- electrofugal leaving group
- oxidising agent
- spectator ion
The term "entity" is used because it is inclusive and so can be used to group together "all objects of chemical interest" including:
- Atoms, isotopes, molecular substances & discrete molecules, photons, metals, alloys, ionic salts, network materials,electrons, ions, radicals, reactive intermediates, generic species such as nucleophile, and even specialist apparatus like the Dean & Stark trap.
No other term is so general:
- The sodium ion, Na+, is a chemical species but not a substance or a material.
- Diamond is a material and it is a substance, but not a species.
- Aldehydes and nucleophiles are hypothetical, generic objects.
- The Dean & Stark trap is glassware.
Formally [ie built into the database schema] The Chemical Thesaurus sub-classifies chemical entities:
- Real, neutral, >99% pure substance or homogeneous mixture of substances of known composition: sodium chloride, methane, iron, aluminium, 1.00 mol/L NaCl(aq), etc.
- Real charged ion: sodium ion Na+, chloride ion Cl–, carbenium ion, H3C+
- Real transient species: methyl radical H3C•, benzyne
- Real mineral of approximate composition: apatite, calcite, gypsum
- Real biological entity that may be of approximate composition: coconut oil, fumarate hydratase
- Real commercial material of approximate composition: dynamite, glass
- Generic neutral entity: aldehyde (generic), cyclic ester (generic), metallic alloy (generic)
- Generic charged entity: acyl cation (generic), carbenium ion (generic), vinyl anion (generic)
- Reaction generic: nucleophile (generic), [free] radical (generic)
 |
 |
 |
| Binary Material Synthlet | Overview of the Chemogenesis Analysis |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.