Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Congeneric Array Database | Lewis Acids & Lewis Bases |
Collecting
It All Together:
The Five Reaction Chemistries
When the set of chemical reactions is analyzed in terms of Lewis theory and FMO theory it is discovered that there are five general types of electronic reaction mechanism: Lewis acid/base, Radical, Photo, Diradical and Redox.
Theory & Empirical Evidence
If we review the various types of Lewis structure, FMO geometry, binary material type and reactivity behaviour encountered so far:
- MO structure of
diatomics and polyatomics give rise to: HOMOs, LUMOs, radicals, diradicals,
bonding and antibonding MOs.
- Elements in their
standard state behave as: oxidising agents, reducing agents and they
(generally) interact with each other via redox processes.
- Hydrogen probe
experiments generate: Lewis acids, Lewis bases, Lewis acid/base complexes radicals and metals, which are reducing agents.
- The π-systems
of organic chemistry can be defined in terms of: HOMOs, LUMOs, SOMOs, aromatic and antiaromatic
systems, cycloaddition, pericyclic reactivity.
- Lewis theory notes – but does not explain – that certain magic numbers of electrons
show stability: the Lewis octet, the 18 electron rule, the 4n + 2
rule, the
two electron chemical bond, Lewis acids, Lewis bases, radicals, diradicals, VSEPR.
- FMO theory gives
rise to: HOMOs, LUMOs, radicals, diradicals, bonding and antibonding
MOs.
- Spectroscopy identifies:
photoexcited states, AOs, MOs, radicals, singlet & triplet diradicals.
When this information, data & knowledge is sliced-'n'-diced, sorted and classified... when it is cogitated upon at length... it can be seen that there are five common and general types of reaction chemistry behaviour. |
- Lewis Acid/Base
Chemistry: LUMO/HOMO interactions
- Redox Chemistry: Loss or gain of electrons from a defined centre
- Radical Chemistry: SOMO interactions
- Diradical Chemistry: Singlet and triplet states
- Photochemistry: Excitation by photons and production of photons
Where each of the five reaction chemistries has a distinct electronic mechanism and associated electron accountancy, as discussed below:
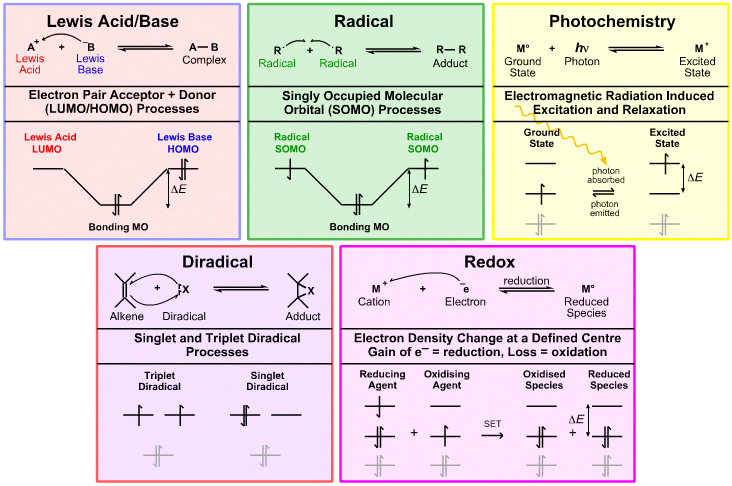
Lewis Acid/Base Reaction Chemistry
Lewis acid/base reaction chemistry concerns:
- Anions
- Cations
- Lone-pairs
- Ligands
- Spectator ions
- Electron pair donors & electron pair acceptors
- HOMOs & LUMOs
- Nucleophiles, nucleofuges, electrophiles, electrofuges, electrophilic & nucleophilic substitution, base catalysed eliminations
- Brønsted acidity, proton abstracting bases
- Adducts, complexes, Diels-Alder cycloaddition... and more...
- No other reaction chemistry is so broad, varied, or central to how we understand chemical reactivity.
The simplest Lewis acid plus Lewis base interaction is Lewis acid/base complexation. The simplest possible example of Lewis acid/base complexation process is the coupling of a proton Lewis acid, H+, with a hydride ion Lewis base, H–, to give dihydrogen, H2.
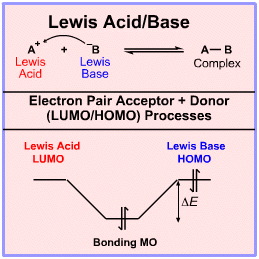
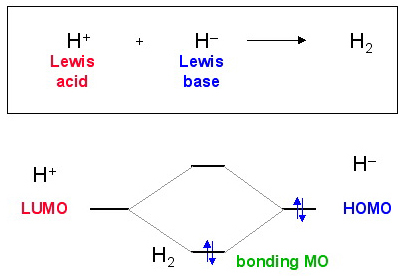
According to the Klopman analysis, here, Lewis acids react via their LUMO's and Lewis bases via their HOMOs.
Read more about Lewis acid/base reaction chemistry here.
Redox Reaction Chemistry
A considerable number of oxidising agents and reducing agents have been added to The Chemical Thesaurus reaction chemistry database. These have been classified into six general types of reducing agent and six general types of oxidising agent, although (and please note) the classification is NOT as clear-cut or rigorous as that carried out for Lewis acid and Lewis base types.
Gain of electrons, gain of hydrogen or metal, or loss of oxygen or halogen equates with reduction. This can occur with various types of chemistry:
- Single Electron Transfer Electron Donor Reducing Agent
- Hydride Complex Reducing Agent
- Lewis Acid Hydride Donor Reducing Agent
- Hydrogen Reducing Agent
- Dissolved Metal Reducing Agent
- Miscellaneous Reducing Agent
Loss of electrons, loss of hydrogen or metal, or gain of oxygen or halogen equates with oxidation. This can occur with various types of chemistry:
- Single Electron Transfer Electron Removal Oxidising Agent
- Hydrogen Removal Oxidising Agent
- Per-Oxygen Oxidising Agent
- Oxidised Main Group Element Oxidising Agent
- Oxidised Heavy Metal Oxidising Agent
- Miscellaneous Oxidising Agent
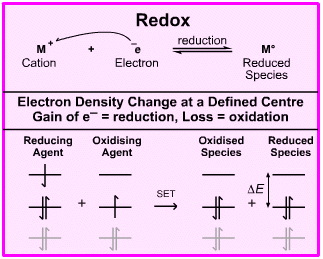
A typical example of a redox process would be the reaction of sodium and fluorine to give sodium fluoride, NaF. (The chemistry has been simplified to show ground state Na and F atoms reacting, rather than two moles of sodium reacting with molecular fluorine.)
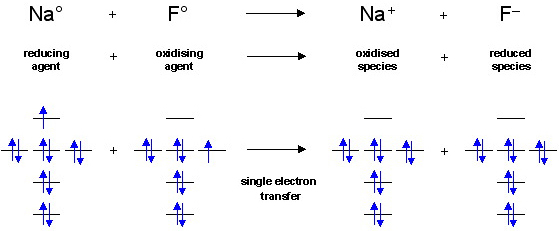
The electron transfers from the sodium's 3s AO to the fluorine's 2p orbital to give a sodium cation, Na+, and a fluoride anion, F–, which experience electrostatic attraction. They bond to give a material which is 81% ionic and 19% covalent (using the Pauling equation).
This redox reaction is a single electron transfer (SET) process.
The two crucial points are:
• When the reaction is defined with respect to the sodium atom the electron density decreases, and when defined with respect to the fluorine atom the electron density increases.
• For every reduction reaction which takes place there must be a concurrent oxidation.
To find out more about redox chemistry click here.
Radical Chemistry
Radicals are species with a single, unpaired electron. In molecular orbital theory, this state is represented as a singly occupied molecular orbital or SOMO. Radicals can be formed in various way, and they have various fates. Radicals are prone to undergo "chain" substitution and/or addition reactions.
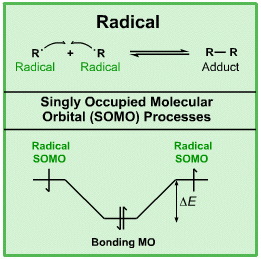
Lewis octet theory and electron accountancy were initially developed over the years 1913-1924, yet when radicals were discovered in the 1930s, they could be easily accommodated within the Lewis electron accountancy framework.
Radicals have a single, unpaired electron they are able to couple together. For example, if hydrogen is heated to a few thousand °C, the H2 will be in equilibrium with 2H•
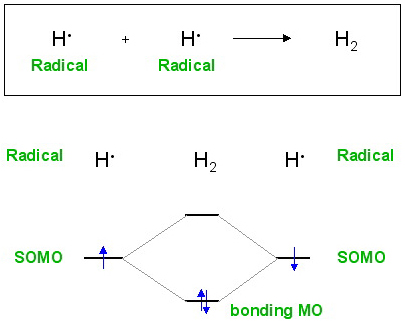
Read more about radical chemistry here.
Diradical Reaction Chemistry
Diradicals may appear to be rather obscure species, however, the oxygen, O2, we breath is a triplet diradical. An understanding of diradical structure and reactivity tells us that the simplistic electron pair covalent bonding of Lewis octet theory is not the whole story. Liquid oxygen is magnetic, a fact that can only be explained by understanding the nature of the bonding in O2.
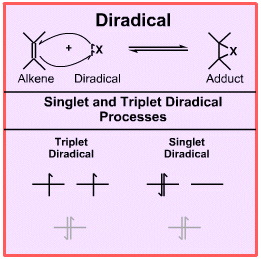
Although less common than Lewis acids, Lewis bases radicals and redox agents, diradicals are important species with distinctive FMO structure and reaction chemistry. Oxygen, O2, is the commonest diradical.
Diradicals have two electrons, each electron can be "spin-up" or "spin-down" and there are two degenerate (equal energy) orbitals. There are three ways in which the electrons can be accommodated, although only two of these are stable or meta-stable:
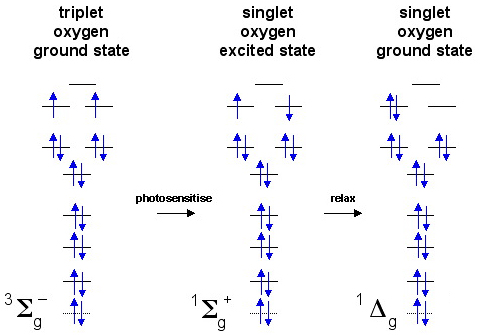
The excited state ( the middle state in the diagram above) is required to inter-convert the stable and meta-stable states. This is because It is a rule of quantum mechanics that an electron cannot move between orbitals and "spin-flip" at the same time, the two events must occur in a stepwise manner.
A species such as dioxygen, O2, can exist in a triplet state in which the two electron have the same spin and are in different orbitals. This is the ground state for dioxygen. This electronic configuration can explain why liquid oxygen is paramagnetic and blue. A triplet diradical acts as if it has two radical centres.
Dioxygen, O2, can be photoexcited to the meta-stable singlet state which has a pair of spin-paired electrons in one orbital and an empty orbital. In this state, the species has an electron pair and a vacant orbital (a reactive HOMO and LUMO) within the same molecule.
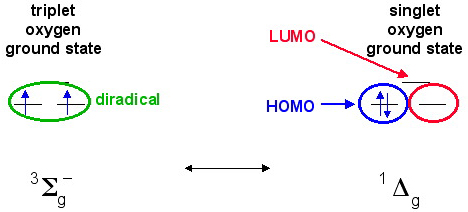
Singlet and triplet diradicals undergo subtly different reaction chemistry, as discussed here.
Photochemistry
Photochemistry is concerned with the absorption, excitation and emission of photons by atoms, atomic ions, molecules, molecular ions, etc. The simplest photochemical process is seen with the absorption and subsequent emission of a photon by a gas phase atom such as sodium.
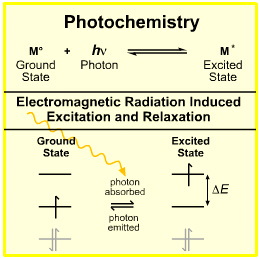
Photochemistry is concerned with how light interacts with matter and initiates chemical reactions, or conversely, how chemical reactions cause light to be emitted.
The simplest photochemical process is the thermal or electrical excitation of an atom, such as a sodium atom in a flame, to an exited state species which subsequently emits a photon as the electron falls back to the ground state:
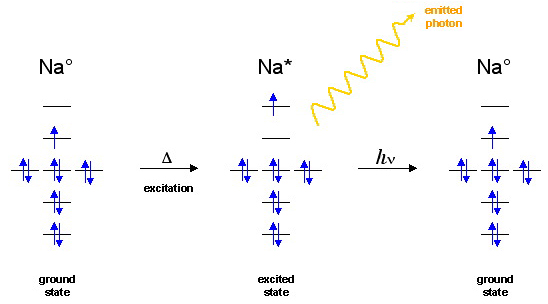
The electron goes from the LUMO (which is in this case also a SOMO) to the LUMO+1. After a very brief time, the electron falls back to the ground state and emits a photon. The energy of the photon will be equal to the energy difference between the energy levels.
Molecules are able to undergo more involved relaxation mechanisms than atoms and this can be used to initiate reaction chemistry, as discussed here.
Five Reaction Chemistry Paradigms
Each of the five reaction chemistries can be considered to be a paradigm, where a paradigm is a particular theoretical framework or way of thinking. The result is that it can be difficult to think in terms of more than one of the five reaction chemistries at the same time.
Consider the hydride ion, H–. The hydride ion is a Lewis base and as such the the hydride ion can be qualitatively compared with other nucleophilic and proton abstracting Lewis bases:
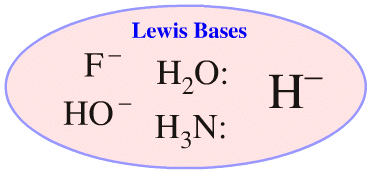
We can ask questions such as "is the hydride ion a good proton abstractor, is it a good nucleophile and does it ever act as a spectator ion?" When asking questions like these we can compare the hydride ion with other Lewis bases such as ammonia, dialkyl ethers, fluoride ion and hydroxide ion. (Note that the hydride ion is not congeneric with these other Lewis bases.)
The hydride ion, H–, is also a reducing agent and it can be compared with other reducing agents such as the electron, electron donating metals and electron donating metal ions:
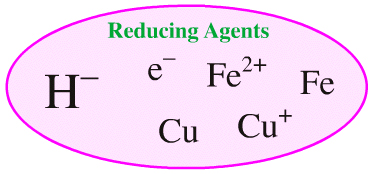
But, the set of all Lewis bases cannot be compared with the set of all reducing agents as illustrated by a Venn diagram:
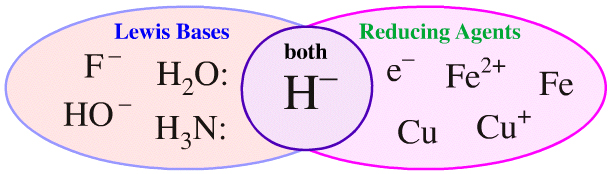
Of the species above, only the hydride ion is both a Lewis base and a reducing agent. While it is possible to compare and contrast Lewis bases with other Lewis bases, and it is possible to compare and contrast reducing agents with each other, it is not possible to compare and contrast the set of Lewis bases and the set of reducing agents at the same time.
Likewise,
• The proton, H+, is a Lewis acid and an oxidising agent.
• Dioxygen, O2, is a diradical and an oxidising agent.
• Photochemical methods can convert triplet diradicals into singlet diradicals.
• The benzene radical cation, [C6H6]+•, is both a radical and a Lewis acid.
• Photochemical and redox methods can be used to produce radicals.
There are many examples like this in reaction chemistry space.
Additional Reaction Chemistries
The identification of the five reaction chemistries is a major component of the chemogenesis analysis, but it is not the whole story. There are reaction chemistry processes and/or "viewpoints" which simply do not fit with the chemogenesis argument, and it is interesting to consider these. First, and of vast importance, comes "thermochemistry":
Thermochemistry is a superset. All mechanistic reaction chemistry exists "within" thermodynamics. Put bluntly, thermochemistry is mechanism independent, and this web book largely about mechanism.
Hydrogen chloride adds to ethylene to give ethyl chloride:
HCl + CH2=CH2 → CH3CH2Cl
Under different conditions this reaction can proceeds via radical addition or ionic (Lewis acid/base) addition.
Hess's law states that these routes are thermodynamically equivalent; the thermodynamics "simply does not care" about the mechanistic route taken, although careful analysis of thermochemical data does give clues to the mechanism taking place. Peter Sykes tells the story of the elucidation of organic reaction mechanisms beautifully.
Thermochemistry is a measured, universal, empirical science. (Einstein said that thermodynamics is the one scientific theory that will never be overturned.)
Mechanistic chemistry is inferred, it is theory, it is a human construct.
But, and absolutely crucially, an understanding of thermodynamics can be used to understand mechanistic behaviour ands vice versa.
And, the human brain finds it easier to think in terms of kinetic and mechanistic theory and to construct mental models which predict behaviour... rather than remembering look-up tables of data.
- Boyle's empirical law tells us that when the volume of a gas is reduced the pressure proportionately increases (at constant temperature).
- Kinetic theory says that because there is less volume for the fixed number of particles to bounce around in, and if the law of conservation of energy is to hold, then the pressure must increase. (Speaking for myself, when I think about a gas law problem I use a mental kinetic model to make predictions about behaviour.)
- Boyle's law, the ideal gas law, equations of state, the Gibbs function, etc. mathematically model the thermochemical world, but as humans we like to think of the world mechanistically.
- Sometimes mechanistic predictions can be very subtle, and theory has reached it pinnacle with pericyclic chemistry, which coincidentally discussed immediately below.
Chemists study and design reaction systems. If a reaction proves commercially important, it may be scaled up and used industrially. Engineers (generally) have no interest in mechanism, but they do want to know about the thermodynamics: Is the reaction exothermic? Is heating, cooling or pressure required? Is the product purified by distillation or crystallisation?
Phase change processes. Chemistry textbooks sometimes distinguish between "physical change" and "chemical change" and consider the boiling of water and the burning of hydrogen as different types of process:
H2O(l) → H2O(g) physical change
2H2 + O2 → 2H2O chemical change
But such distinctions are meaningless:
- During a phase change, individual species transfer between chemical environments, just like they do in any reaction, see here.
- Chemical reactions are a type of phase change.
- Phase changes can be described by chemical equations which can be balanced in terms of mass and energy.
- Phase change processes are included in The Chemical Thesaurus reaction chemistry database, here.
This web book is concerned with electronic and mechanistic chemical reactivity.
Change of coordination number occurs when an atomic centre gains or loses ligands. PCl3 reacts with Cl2 to give PCl5; hexaaquacopper(II) ion reacts with chloride ion to give tetrachlorocopper(II) ion, etc.
The coordination numbers change:
- Phosphorus from 3 to 5
- Copper from 6 to 4
- It is tempting to think of the copper reaction as a redox process, but it is not. Copper is in the +2 oxidation state in both [Cu(H2O)6]2+ and [CuCl4]2–.
These reactions cannot be adequately described in terms of either redox or Lewis acid/base reactivity. However, they can be considered as 1,1-addition or 1,1-elimination proc
esses, and often as insertion reactions, here. Many inorganic textbooks call such reactions oxidative addition (Oxad) processes, and the reverse as reductive eliminations.
Pericyclic chemistry is associated with certain π-system cyclisation and rearrangement reactions. Pericyclic reactivity does not fit well with the five reaction chemistries, even though Diels-Alder cycloaddition can be classified as a Lewis acid/base complexation interaction. Pericyclic reactivity is discussed in some detail here and here.
Radioactive decay and nucleosynthesis processes are outside the scope of this web book, but they are included in The Chemical Thesaurus reaction chemistry database, here. Likewise, with quark, boson and hadron interaction.
Biochemical and life science processes are outside the scope of this web book, although a few example biochemical processes have been added to The Chemical Thesaurus reaction chemistry database, here. Biochemical reaction processes are tightly controlled feedback loops.
OK, can you think of any reaction processes that do not fit in with the five reaction chemistry analysis... or the exceptions' discussed above? If you do know of any, *please* get in contact with your suggestions/ideas, here.
 |
 |
 |
| Congeneric Array Database | Lewis Acids & Lewis Bases |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.