Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Species/Species Interactions | Unit & Compound Mechanistic Steps |
The Simplest Mechanistic Step: STAD
All chemical reactions can be broken down into "transfer" steps that involve a defined chemical species moving from one environment to another. Sometimes these steps are referred to as substitutions, sometimes as transfers, sometimes as abstractions and sometimes as displacements (even though they are mechanistically identical), hence the general term STAD.Introduction
The language used by chemists obscures the simplest mechanistic step. Indeed, the various chemistry sub disciplines are prone to describe the same process from different perspectives and to use different terminologies. Consider the hypothetical "X, Y, Z reaction".

To describe what is going on in this process we can say:
- X substitutes Z at the Y centre
- Y transfers from Z to X
- X abstracts Y from Z
- X displaces Z from Y
- X exchanges with Z at the Y centre
- X replaces Z at the Y centre
By way of example, Lewis acid/base chemistry usually discusses mechanisms in terms of nucleophilic and electrophilic substitution, while radical chemists prefer to use the term abstraction. Single electrons are transferred. There are no logical reasons for such differences.
The simplest possible mechanistic step occurs when a defined chemical species transfers from one environment to another.
This mechanistic process can be described in in terms of Substitution, Transfer, Abstraction or Displacement.
We shall use a single term, the acronym STAD.
We shall discuss come common mechanistic processes in terms of the STAD mechanistic step. Notice that each example is also defined with respect to one or more of the five reaction chemistries, here.
Click here to enlarge the table of STAD reactions. These are discussed one-at-a-time over the rest of this page:
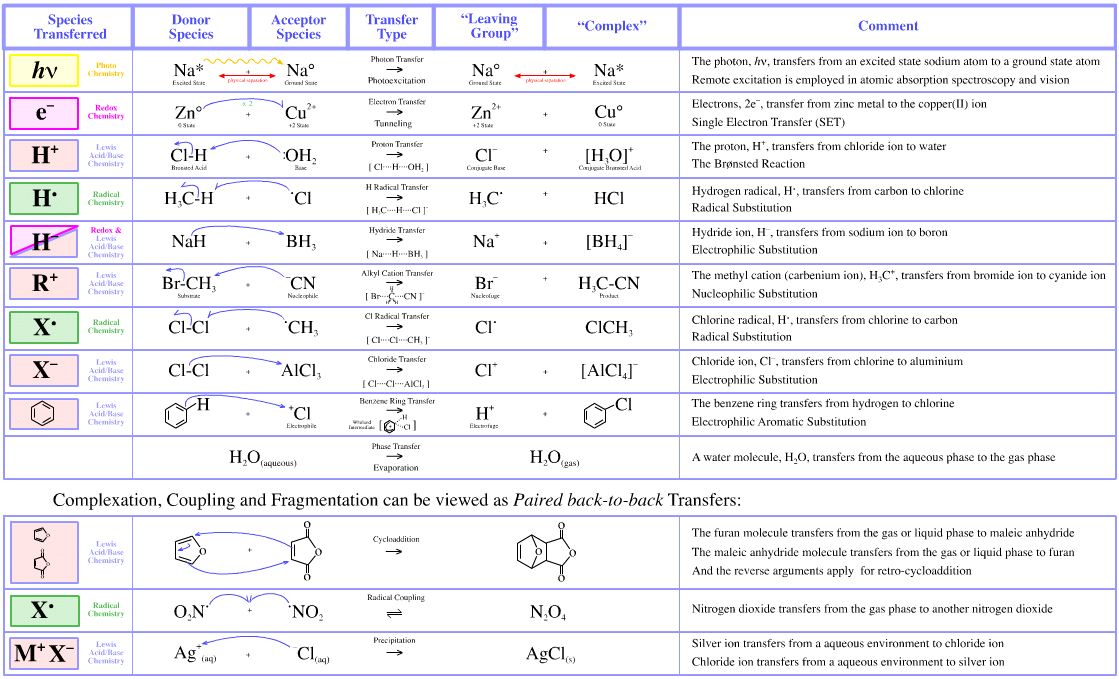
Electron Transfer
If zinc metal is added to a solution containing copper(II) ions, Cu2+, a redox reaction takes place and electrons transfer from the zinc to the copper to give a precipitate of copper metal. The zinc metal goes into solution as Zn2+.
In this reaction:
The zinc metal is oxidised to zinc(II) ions by the copper(II) ions.
The copper(II) ions are reduced to copper metal by the zinc metal.
The reaction proceeds via a double SET (single electron transfer) process.
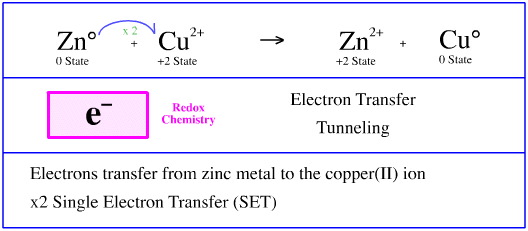
Proton (Lewis Acid) Transfer
The first point to note is that hydrogen chloride, HCl or HCl(g), is a gas.
Hydrogen chloride, the gas, is readily absorbed into water to give an acidic solution of hydrochloric acid.
The hydrogen chloride is a proton donating Brønsted acid. When hydrogen chloride gas dissolves in water the hydrogen chloride's proton transfers to water, H2O:, to give a solution consisting of oxonium ions, [OH3]+ and chloride ions, Cl–.
In this reaction:
- Hydrogen chloride is the Brønsted acid
- Water is the Brønsted base
- Oxonium ion, [OH3]+, is the conjugate Brønsted acid
- Chloride ion is the conjugate Brønsted base
- The oxonium ion, [OH3]+, is the Brønsted acidic species in all aqueous Brønsted acid solutions.
- When aqueous Brønsted acids are described as "H+ donors", the actual proton donor species is always [OH3]+.
Water will continue to absorb hydrogen chloride until it is 37% HCl by weight, a material is called concentrated hydrochloric acid. If concentrated hydrochloric acid is cooled to –15°C, crystals of the ionic salt [H3O]+/Cl– will precipitate.
Note that oxonium chloride, OH3Cl, is the oxygen analogue of ammonium chloride, NH4Cl. Both are type 11 Lewis acid/base complexes, here.
The transfer of H+ from Cl– to H2O: is a STAD process.
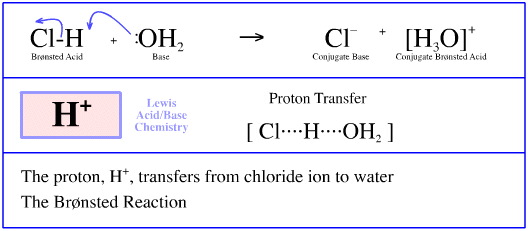
Hydrogen Radical Transfer
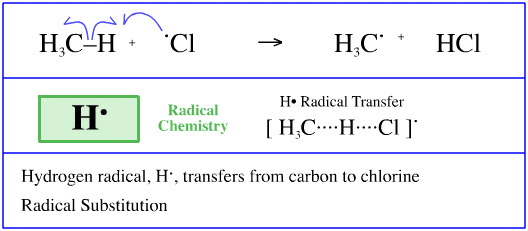
During the radical chlorination of methane, here, one of the propagation steps involves a hydrogen radical (a hydrogen atom) transferring from a methyl radical to a chlorine radical.
This process is commonly referred to hydrogen abstraction; it is also a STAD mechanistic process.
Hydride Ion (Lewis Base) Transfer
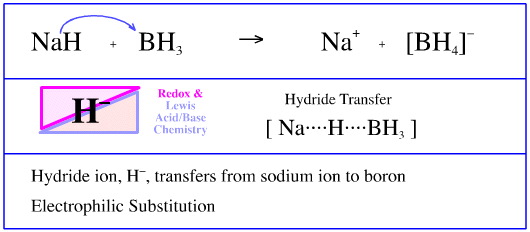
Sodium borohydride, NaBH4, can be prepared by reacting sodium hydride, NaH, with borane, BH3. (Actually, diborane, B2H6, is used.)
The crucial step of this reaction involves a hydride ion transferring from sodium ion Lewis acid, Na+, to a borane Lewis acid.
This reaction could be regarded as an electrophilic substitution, with the borane electrophile substituting the sodium ion electrofuge (Lewis acid leaving group) from a hydride ion centre.
Or it can just be regarded as a STAD process.
Carbenium Ion (Lewis Acid) Transfer
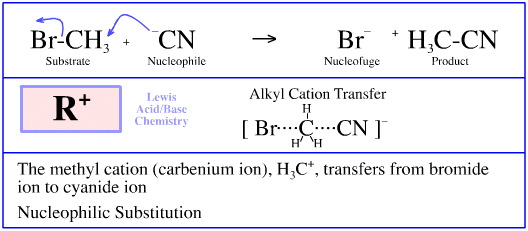
Cyanide ion, CN–, reacts with methyl bromide, CH3Br, to give methyl cyanide, CH3CN and bromide ion, Br–. This reaction is commonly referred to as a nucleophilic substitution. The argument is:
The cyanide ion is an electron rich Lewis base. The cyanide ion is also nucleophilic (nucleus or positive charge seeking) in that it is attracted to and "attacks" delta+ carbon centres, such as the delta+ carbon of methyl bromide.
The cyanide ion engages with the methyl bromide and forms a five centre (bipyramidal triangular) transition state (TS).
This TS rapidly collapses with the ejection of the nucleofugal (nucleus fleeing) bromide ion Lewis base. The reaction proceeds as a concerted, single-step process.
The product is methyl cyanide and bromide ion.
It is also quite reasonable to regard the mechanism as proceeding as a carbenium ion, H3C+, transferring between a pair of Lewis bases.
The reaction proceeds via a STAD mechanism.
Chlorine Radical Transfer
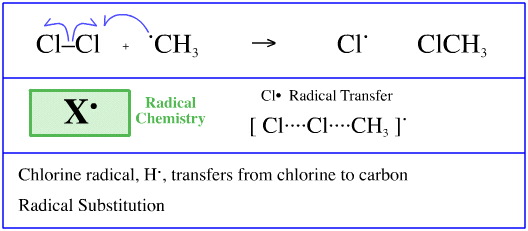
During the radical chlorination of methane, here, one of the propagation steps involves a methyl radical abstracting a chlorine radical from chlorine, Cl2, to give methyl chloride, CH3Cl, and leaving a chlorine radical.
This process can also be regarded as a chlorine radical transferring from a chlorine radical to a methyl radical. Another example of a STAD mechanistic step.
Chloride Ion (Lewis Base) Transfer
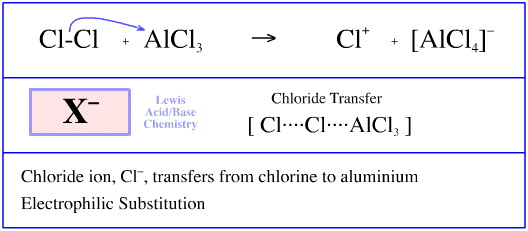
The first step of Friedel-Crafts chlorination of benzene involves adding chlorine to a solution (or a slurry) of anhydrous aluminium chloride, AlCl3, a powerfully halophilic (affinity for halogen anion) Lewis acid.
A reaction takes place whereby the aluminium chloride abstracts a chlorine anion from chlorine, Cl2, to form chlorenium aluminium tetrachloride, [Cl]+ [AlCl4]–.
The reaction can be regarded as electrophilic substitution, with the electrophilic Lewis acid AlCl3 displacing the electrofugal chlorenium ion Lewis acid, Cl+, from the chloride anion Lewis base centre.
Alternatively, the reaction can be viewed as chloride ion, Cl–, transferring between two Lewis acids, a STAD mechanism.
Note that the same mechanistic process occurs with Friedel-Crafts acylation, when aluminium chloride abstracts as chloride anion from an acyl chloride, RCOCl, to give an electrophilic acyl cation, RCO+, and an aluminum tetrachloride anion.
Benzene Ring (Lewis Base) Transfer
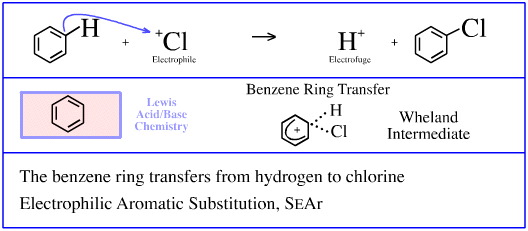
The second step of Friedel-Crafts chlorination of benzene involves the chlorenium ion (chlorine cation, Cl+) electrophilic Lewis acid substituting a proton at a benzene ring carbon.
The argument is that the electron poor (and hence electrophilic) chlorine cation, Cl+, is attracted to the electron rich π-system of the benzene ring.
A "Wheland" intermediate is formed in which the chlorenium ion complexes with the benzene.
The Wheland intermediate collapses and a hydrogen cation (proton) Lewis acid electrofuge is ejected leaving the chlorobenzene product, C6H5Cl.
This reaction can also be regarded as a benzene ring Lewis base (actually phenyl anion, [C6H5]–, Lewis base) transferring between a pair of Lewis acids, Cl+ and H+; a STAD mechanistic step.
Phase Transfer
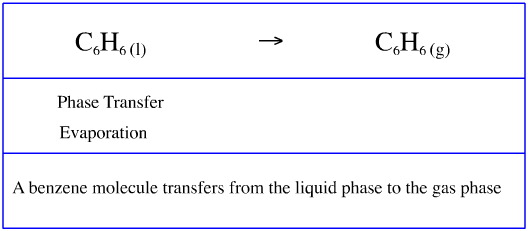
When a benzene molecule evaporates it transfers from an association with other benzene molecules in the liquid phase, C6H6(l), to an association with the gas phase.
There are a number of points to make about evaporation and similar processes which are of relevance here.
When discussing chemical equilibria systems, chemists are prone to discuss "physical" systems and processes and "chemical" systems and processes. However, on close examination the idea of the "physical" process is found to be misleading.
Throughout the Patterns in Reaction Chemistry project, we take the broad view of what it is that constitutes a chemical reaction:
Any process which can be represented with a chemical equation is regarded as being a chemical reaction.
This broad definition has many advantages. For example, chemical equations have the property that they can be balanced in terms of mass (stoichiometry) and energy. Certainly, the evaporation of benzene can be discussed in these terms.
Chemists have the propensity to define things with respect to standard states. For example, chemical thermodynamics defines heat of formation, H°f, and heat of reaction, H°r, with respect to the elements in their standard states which are all defined as having: H°f = 0.
In a similar way, chemists regard the vacuum (the gas phase) as being null and without effect.
(Remember, when a species is in the gas phase it only occasionally encounters other species or the sides of the container. For most of the time the species behaves as if it is alone and in a vacuum.)
The chemist's vacuum is referred to as a classical vacuum. And the view is encouraged by the way we draw molecules. Unless we explicitly include solvent molecules or solid phase neighbours, we are assuming that all species to be in the gas phase. This includes drawing and computer manipulating species in silico.
However, particle physicists regard the quantum vacuum as a seething ocean of virtual particles which form and disappear on very short time scales. Thus, in an absolute sense the quantum vacuum is just as active as any solvent.
Thus, if we take the physicists view point we can regard the seething quantum vacuum of the gas phase abstracting a benzene molecule from the liquid phase.
It follows that – with respect to a defined chemical species – we can regard all the following phase changes:
liquid ⇄ gas evaporation ⇄ condensation
solid ⇄ gas sublimation ⇄ condensation
solid ⇄ liquid melting ⇄ freezing
as transfer processes.
Two more phase transfer examples:
liquid ⇄ liquid extraction
solid ⇄ liquid dissolution
are not nearly so controversial.
Liquid–liquid phase transfer extraction is used to transfer non-polar organic substances from aqueous solvents (water) into organic solvents. Alternatively, water is used to extract polar entities out of immiscible organic solvents.
Solid-liquid phase transfer occurs whenever a species dissolves into a solvent or precipitates from a solvent.
To sum up: phase transfers are chemical reaction processes which proceed via the STAD mechanism.
Photon Transfer
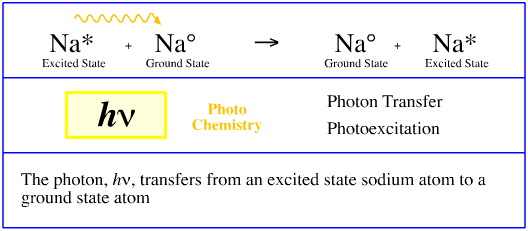
The system described above – where an excited state sodium atom transfers a photon to a remote ground state sodium atom which is excited – is exploited in the technique of atomic absorption spectroscopy (AA or AAS).
Atomic absorption spectroscopy is a common analytical technique that uses the absorption of light to measure the concentration of gas-phase atoms. Light from sodium atoms are used to measure the concentration of sodium atoms in an analytical sample.
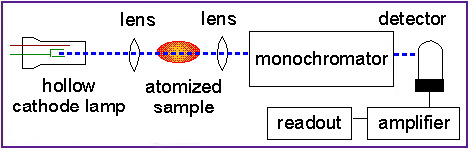
A sodium hollow cathode lamp is used to excite sodium atoms into emitting light at 589nm, the sodium D-line.
Since analytical samples containing sodium ions are usually liquids or solids, the analyte sample must be vaporised and atomised in a flame or a graphite furnace.
The AA instrument is arranged so that the sodium D-line light from the hollow cathode lamp passes through the flame or graphite furnace containing analyte atoms.
The analyte sodium atoms absorb the 589nm light and make transitions to higher electronic energy levels.
The concentration of sodium atoms is determined from the amount of absorption: the more sodium in the flame, the more the 589nm light from the hollow cathode lamp is attenuated.
Concentration measurements are usually determined by calibrating the instrument with standards of known concentration and determining the range over which the Beer-Lambert law can be assumed.
Photon transfer is exploited by all visual systems which detect photons emitted by excited state species, see here.
Radical Coupling is "Back-to-Back" Transfer
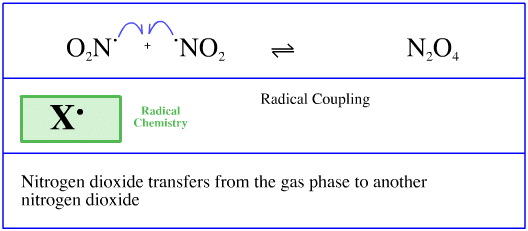
Consider again the radical coupling of nitrogen dioxide to dinitrogen tetroxide (introduced here).
Each of the nitrogen dioxide molecules transfers from an association with the [seething, classical] vapour phase to an association with a nitrogen dioxide molecule.
Thus, two paired or "back-to-back" transfers are occurring, and this is true whenever two species couple together.
The reverse argument is also true whenever a single species fragments into two species.
It follows that all couplings, complexations, decouplings and fragmentaions can be considered as paired or back-to-back STAD processes.
However, whether this level of mechanistic analysis is necessary or useful will be discussed in the next section, here. But a couple more examples follow.
Lewis Acid/Base Complexation is "Back-to-Back" Transfer
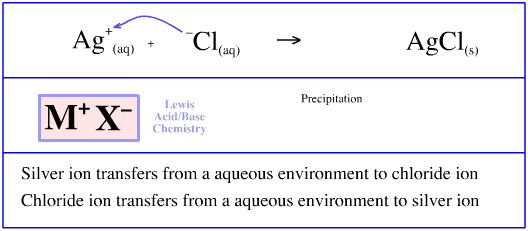
Silver ions in aqueous solution, Ag+(aq), complex with aqueous chloride ions, Cl–(aq), and precipitate as solid silver chloride, AgCl(s).
In this reaction the silver ion, Ag+, transfers from an association with water, H2O:, to an association with chloride ion, Cl–, in a solid silver chloride matrix, AgCl(s).
The chloride ion, Cl–, transfers from an association with water, H2O, to an association with silver ion, Ag+, in a solid silver chloride matrix, AgCl(s).
As stated above, all couplings, complexations, decouplings and fragmentaions can be considered as paired or back-to-back STAD processes. But whether this level of analysis is strictly necessary will be discussed in the next section, here.
Cycloaddition is "Back-to-Back" Transfer
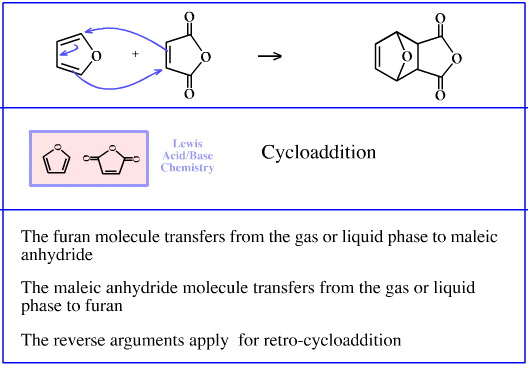
Furan reacts with maleic anhydride to give a Diels-Alder cycloaddition adduct. This type of reaction is usually carried out in a non-polar solvent such as toluene.
The furan molecule transfers from the toluene liquid phase to an association with the maleic anhydride, and the maleic anhydride transfers from the toluene liquid phase to an association with furan.
Again, a pair of back-to-back STAD processes.
Comparing Electron Transfer and Proton Transfer
There are two very important STAD processes which involve the transfer of low mass species, e– and H+.
These low mass entities, e– and H+, transfer so readily – the reactions have very low activation energies – that the thermodynamic equilibrium states are quickly reached. The two processes are:
- Single Electron Transfer (SET) redox reactions
- Proton transfer or Brønsted acidity
The similarity of these two STAD processes is such that both can be described by:
- Full reaction equations
- Net ionic equations
- Paired half-reactions
- Half-reactions in standard form
- The half reactions in standard form can quantified and related to the deltaG, the Gibbs free-energy of the process.
Typical Redox and Typical Brønsted Acid Reactions
We will compare and contrast:
- Zinc metal reacting
with aqueous copper sulfate: a typical, simple SET redox process
- Hydrogen chloride reacting with sodium hydroxide, a typical, simple Brønsted acid reaction
First the full equations with all species represented:
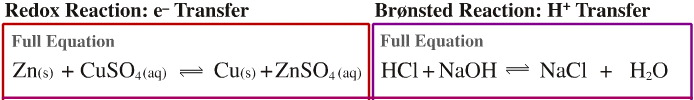
- Zinc metal is a solid
and it reacts with aqueous copper sulfate solution to give copper metal
and an aqueous solution of zinc sulfate. The copper sulfate solution
is blue and the zinc sulfate solution is colourless. The reaction can
be followed by the disappearance of the blue colour.
- Hydrogen chloride is a proton donating Brønsted acid and sodium hydroxide is a proton accepting Brønsted base. Hydrogen chloride will react with sodium hydroxide to give sodium chloride and water. If equimolar quantities of acid and alkali are used, the resulting mixture of sodium chloride and water will have a neutral pH of 7.0.
Please note that "hydrogen chloride", the compound HCl, is a gas. Hydrogen chloride dissolves in water to give an acidic aqueous solution called hydrochloric acid. Very confusingly, both hydrogen chloride gas and aqueous solutions of hydrochloric acid are referred to as "H Cl".
Net Ionic Equations
Both the redox reaction and the Brønsted acid reaction can be simplified to the "net ionic equation" in which spectator counter ions are removed:
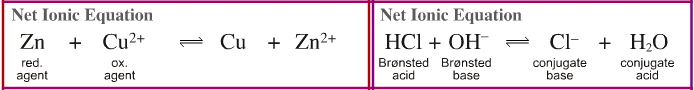
- In the redox reaction the sulfate ion, SO42–, is the spectator ion. When this species is removed, the reaction is simplifies to Zn + Cu2+ → Zn2+ + Cu. It now becomes clear the oxidation state of the zinc increases from 0 to +2 and so the zinc is oxidised. Likewise, the oxidation state of the copper reduces from +2 to 0 and so the copper is reduced. We can go further:
The copper is reduced from +2 to 0 by the zinc metal, so the zinc metal is the reducing agent.
The zinc metal is oxidised from 0 to +2 by the copper(II) ion so the Cu2+ is the oxidising agent.
- The hydrogen chloride plus sodium hydroxide reaction can also be viewed in net ionic form. The sodium cation, Na+, is the spectator ion. The deconstruct net ionic equation takes the form:
HCl + HO– → Cl– + H2O
HCl is the proton donating Brønsted acid
Hydroxide ion is the proton accepting Brønsted base
Water, H2O, is the conjugate Brønsted acid
Chloride ion is the conjugate Brønsted base
Paired Half Reactions
The redox and Brønsted acid reactions can be further deconstructed into half reactions. It is in this form that the transferred species appears for the first time:

- In the redox reaction the zinc is oxidised from Zn° to Zn2+. To balance this reaction in terms of charge two electrons must be added to the right hand side of the equation.
In a similar manner, the copper is reduced from Cu2+ to Cu° and this half reaction must be balanced with two electrons on the left hand side of the equation.
Note that if the two half reactions are added together the electrons – which appear on both sides – are canceled out and the net ionic equation is obtained.
- The HCl + HO– reaction can also be deconstructed into a pair of Brønsted acid half reactions. Again, the transferred species, H+, appears for the first time in the half reactions.
The HCl is deemed to dissociate to H+ and Cl–.
The H+ reacts with the HO– to give water.
Half Reactions in Standard Form
Half reactions are useful because they can be arranged in a standard form so that large numbers of similar reaction processed can be compared, contrasted and most importantly, quantified.

- By convention, SET redox processes are arranged as reduction reactions with the oxidised species plus the electrons on the left hand side of the equation and the reduced species on the right.
Reactions are all compared with the hydrogen redox reaction
2H+ + 2e– → H2
over a platinum electrode which is defined has having an E° of 0.00V.
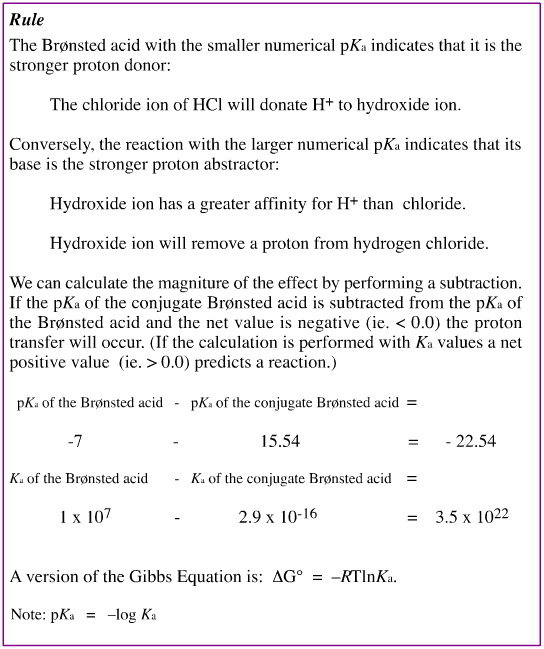
- Brønsted acid reactions are all compared as proton donations. Thus, the Brønsted acid appears on the left hand side of the equation and H+ plus the conjugate [Brønsted] base on the right hand side.
All reactions are quantified by standardising the proton acceptor as water, H2O:, and having the conjugate Brønsted acid as the oxonium ion, [OH3]+.
The standard measure is the equilibrium constant, Ka
Or its "minus log" form, pKa.
Electron Transfer

Proton Transfer
 |
 |
 |
| Species/Species Interactions | Unit & Compound Mechanistic Steps |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.