Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Timeline of Structural Theory | Valence Shell Electron Pair Repulsion |
Modern Lewis Theory
- All beginning chemistry is taught in terms of Modern Lewis theory.
- All beginning chemistry is learned in terms of Modern Lewis theory.
- Most chemistry understood in terms of Modern Lewis theory.
- Most [not all!] chemists think in terms of Modern Lewis theory most of the time.
- The Chemogenesis Web Book uses Modern Lewis theory.
- So, what exactly IS Modern Lewis theory?
Electrons dance to a subtle & beautiful quantum mechanical tune.
The resulting patterns are both complicated & exquisite.
As chemists we try to understand and exploit the dance.
Our physicist friends attempt to understand the music theory.
Modern Lewis Theory & Stamp Collecting
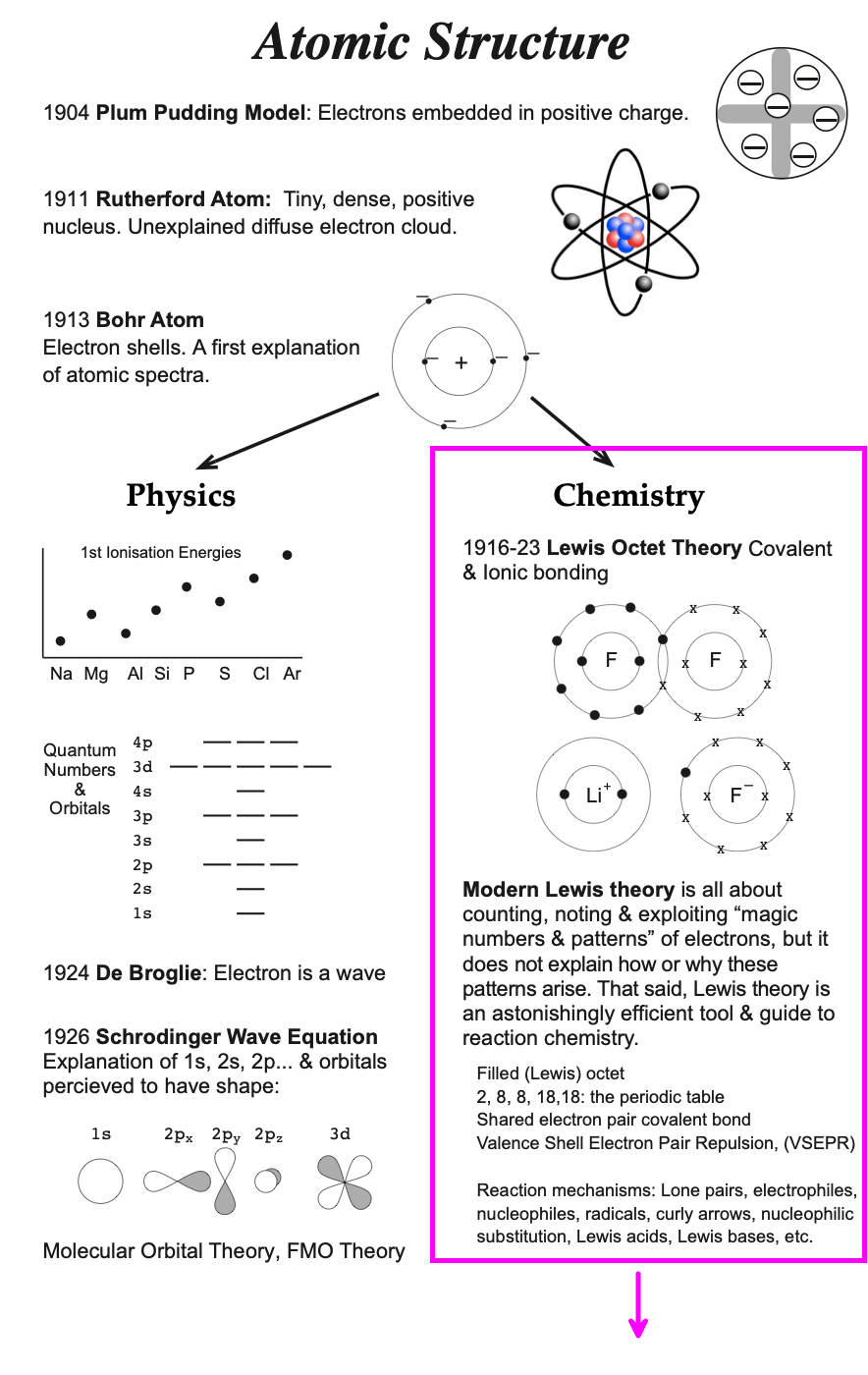
Introduced by Niels Bohr in 1913, the Bohr model is a quantum physics modification of the Rutherford model and is often referred to the Rutherford–Bohr model. (Bohr had conducted post-doctoral research work with Rutherford in Manchester, UK.) The model's key success lay in explaining (correlating) the Rydberg formula for the spectral emission lines of atomic hydrogen, but as a model/theory of the atom it was clearly not complete...
Greatly simplifying both the history & the science: In 1916 atomic theory bifurcated into physics and chemistry streams, a fork that continues to this day. |
In the early years of the 20th century at UC Berkeley under the leadership of G. N. Lewis, chemists were trying to understand structure, bonding & reactivity. Lewis and colleagues were actively debating the new atomic structure ideas, particularly the Rutherford & Bohr atoms, and postulated that the electron shell atomic model gave insights into their chemical problems.
The first Lewis paper to deal with the new structure & bonding ideas was published in the Journal of the American Chemical Society v. 38 (1916) [full paper, scanned version] The Atom and The Molecule. This was just three years after the introduction of the Rutherford-Bohr atomic structure model in which particle-like electrons were deemed to exist in shells around the small dense nucleus: two electrons in the first shell, 8 in the second, 8 in the third, etc.
By way of example, the 1916 paper contains this very modern looking diagram showing the formation of the ammonium ion:
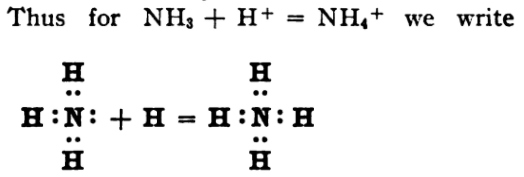
The diagram shows ammonia, NH3, with three "shared electron-pair covalent bonds" and a "lone pair of electrons" on the nitrogen. Plus, there is a "dative/coordinate covalent bond" in the ammonium ion, NH4+. [However, be warned... not all of this paper – with its odd looking cubic atoms, etc. – looks as "present-day" as the diagram above.]
In essence:
- Lewis theory observes, collects & exploits empirically derived patterns in the numbers of particle-like electrons – within the scope of the Bohr atom – that are seen when atoms interact, react & bond with each other.
- Lewis theory then uses these patterns to generate simple rules associated with stable (and by inference, unstable) atomic, molecular and ionic electronic configurations: full shells are associated with stability, whereas partially filled shells unstable and therefore reactive.
- Lewis theory promotes the rules & patterns, but makes no attempt to explain how or why the magic numbers of electrons associated with the patterns arise [it cannot]. Nor can Lewis theory explain anomalous behaviour... except by invoking a new pattern, which is readily does.
- With Lewis theory: the pattern is the science.
- Lewis theory has been taking this approach for more than 100 years, and we use the term "Modern Lewis Theory" for the collection of ideas.
Physics | Stamp Collecting | Lewis Theory Ernest Rutherford famously said: "All science is either physics or stamp collecting." Lewis theory is stamp collecting.
So it is with atoms, ions, molecules, molecular ions, materials, etc. As chemists we see many patterns in chemical structure and reactivity, and we try to draw conclusions and make predictions using these patterns:
But the Lewis approach is not complete and it only gives hints about the underlying quantum mechanics, a world observed through spectroscopy and understood through mathematics. |
Strikingly, the magic numbers of modern Lewis theory are generally (but not exclusively) small positive integers of even parity: 0, 2, 4, 6, 8...
Some examples of the rules & patterns of modern Lewis theory:
- Atoms and atomic ions show particular stability when they have a full outer (or valence) shell of electrons and are isoelectronic with the noble gases: He, Ne, Ar, Kr & Xe:
Magic numbers in shells: 2, 8, 8, 18, 18, 36
Magic numbers in total: 2, 10, 18, 36, 54
For example, period 2 & 3 atoms & atomic ions: N3–, O2–, F–, Ne, Na+, Mg2+ & Al3+ all have 8 electrons in their outer shell and 10 electrons in total. Magic number in the valance shell = 8 & Magic number in total = 10.
- Stable atoms, ions, molecules and molecular ions associated with the lighter main group elements (H to Ar) react so that they obtain a full Lewis octet of valence shell electronic structure: Magic number = 8.
Sodium metal tends to react to give the sodium ion, Na+, a species that has a full octet of 8 electrons in its valence shell. Magic number = 8.
Fluorine reacts to give the fluoride ion, F–, a species that has a full octet of 8 electrons in its valence shell. Magic number 8.
- First introduced by Walther Kossel, the ionic bond can be understood within the Lewis model. The reaction between lithium and fluorine gives the ionic salt lithium fluoride, LiF, where the Li+ ion is isoelectronic with He and F– is isoelectronic with Ne, both ions have filled valence shells, magic numbers 2 & 8:
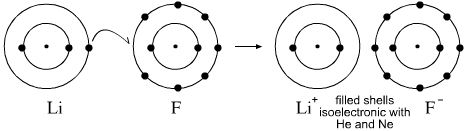
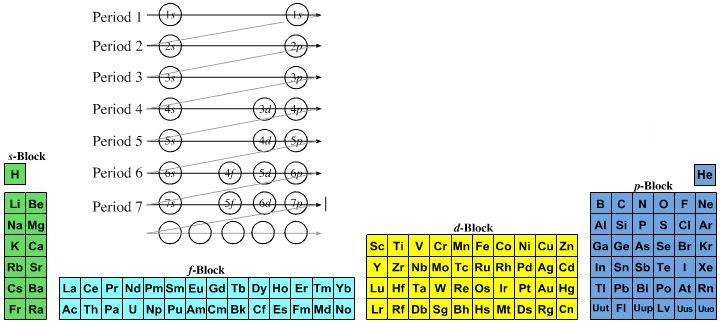
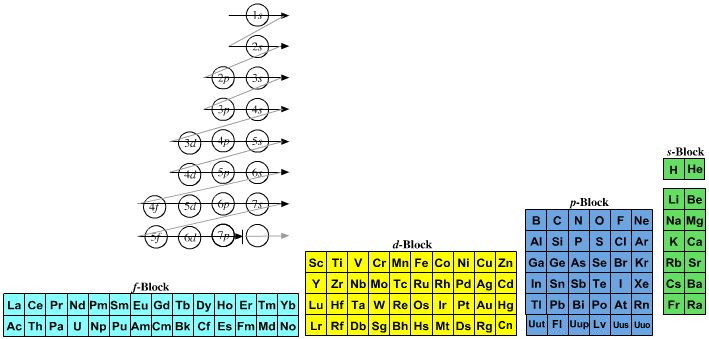
- The Bohr shell structure is used to explain the pattern of the periodic table:
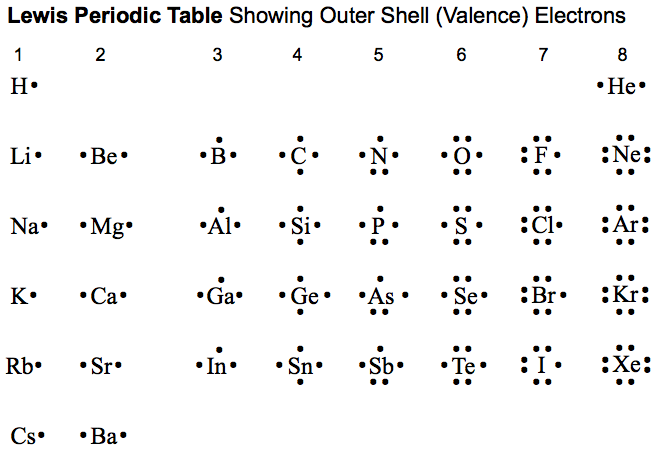
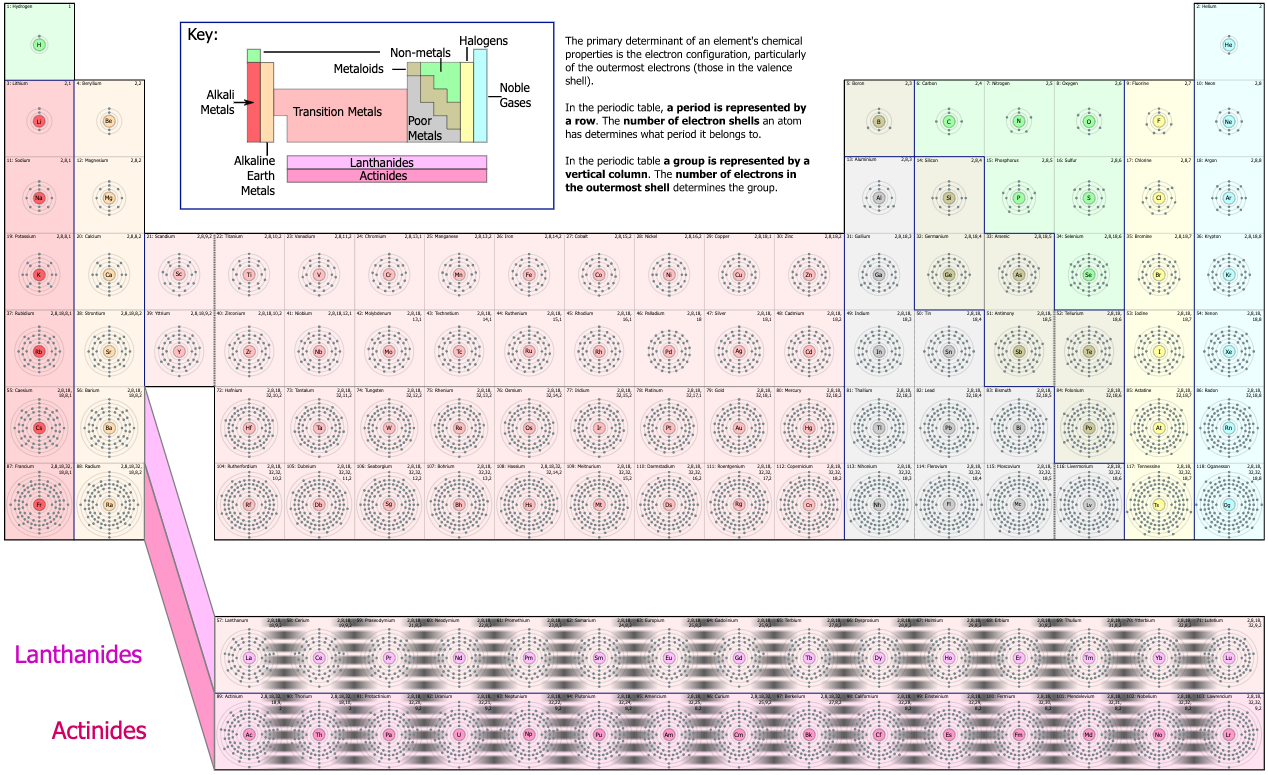
- Lewis theory has incorporated more recent quantum mechanical orbital/sub-shell logic: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10... etc. and labelled the s-block, p-block, d-block & f-block of the periodic table.
Magic numbers associated with sub-shells: s = 2, p = 6, d = 10 & f = 14.
The very idea of a "sub-shell" is Lewis thinking.
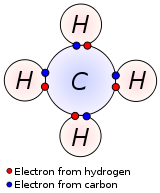
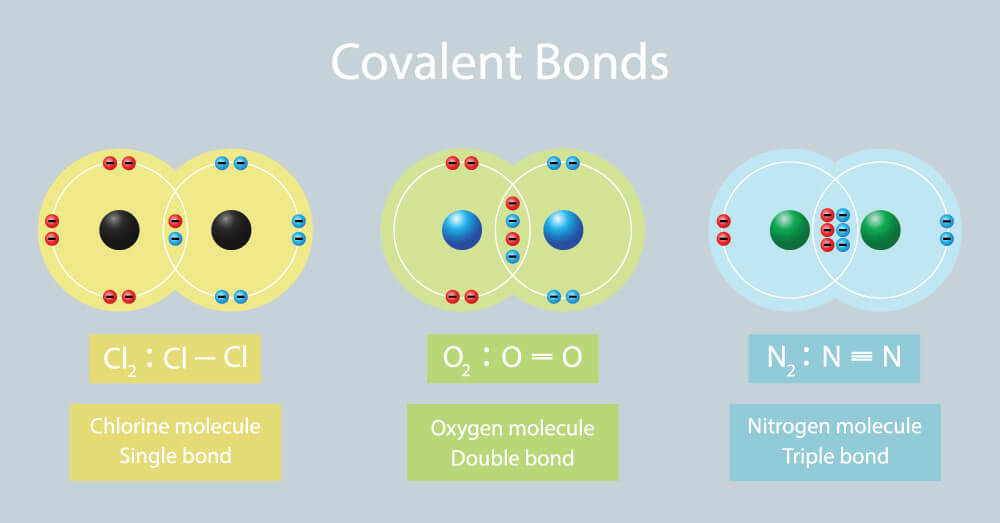
- A covalent bond, such as the bond seen in diatomic hydrogen, H2, consists of "a shared pair electrons": Magic number 2.
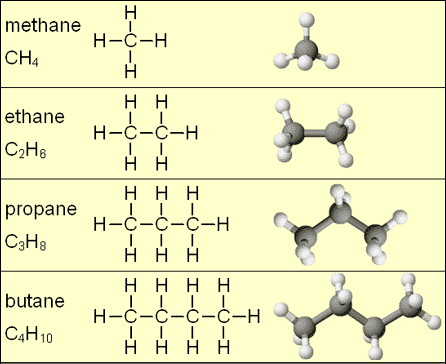
- Methane, CH4, has four shared electron pair covalent bonds. This gives carbon a total of [Magic number] 8 electrons in its valence shell, and each or the four hydrogens have [Magic number] 2 electrons in their valence shells:
- The structure of methane with its four shared electron pair covalent bonds can be extended to the alkane homologous series:
- Ammonia, H3N:, has a "lone pair of electrons" in its valence shell: Magic number 2.
- Ethene, H2C=CH2, is said [in Lewis theory] to have a "double covalent bond": Magic numbers (2 + 2)/2 = 2.
- Nitrogen, N2, N≡N, has a "triple covalent bond": Magic numbers (2 + 2 + 2)/2 = 3.
- Lewis bases (proton abstractors & nucleophiles) react via their "available electron pair": Magic number 2.
- Lewis acids such as borane, BH3, react by "accepting" a lone pair of electron into their valence shell in order to fill their octet: Magic numbers 6 + 2 = 8.
For example, the Lewis acid borane, BH3, reacts with the Lewis base ammonia, NH3, to form a Lewis acid/base complex with a dative covalent bond in which both the boron and the nitrogen atoms now have a "full octets":
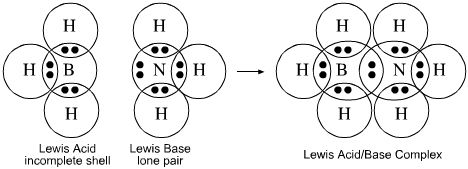
- Curly arrows represent the movement of an electron pair: Magic number 2.
- Ammonia, :NH3, and phosphine, :PH3, are isoelectronic in that they have the same Lewis valence shell structure. Both have three covalent bonds and a lone pair of electrons: Magic numbers (3 x 2) + 2 = 8.
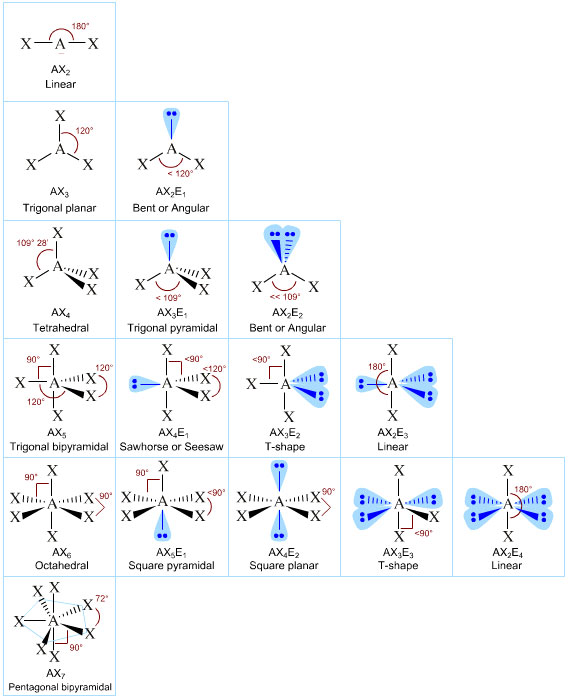
- The shapes of molecules and molecular ions can be predicted using valence shell repulsion, VSEPR. The technique involves counting the number of electrons in valence shell of the central atom, dividing by 2, grouping the electron pairs and bonding and non-bonding (lone pairs) and noting that the electron pairs mutually repel.
- The Lewis-VSEPR approach is so successful that its ideas can be projected onto physical models of atoms which can build molecular structures:

It is astonishing how well these physical "balls & sticks" achieve their objective of modeling molecular and network covalent material structure.
The approach can also be modelled in software, where the approach is called molecular mechanics. Atoms and bonds are parametrised in terms of classical force fields.
- The Sn2 mechanism involves a nucleophilic Lewis base, with its lone-pair of electrons, displacing a nuclefugal Lewis base leaving group at a δ+ carbon centre: net change 0.
- Lewis theory is used to explain most types of reaction mechanisms, including Lewis acid/base, redox reactions, radical, diradical & photochemical reactions by locating and counting the electrons.
Whenever a curly arrow is used in a reaction mechanism, Lewis theory is being evoked. Do not be fooled by the sparse structural representations employed by organic chemists, curly arrows and inter-converting resonance structures are pure Lewis theory:
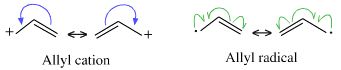
Lewis theory is very accommodating and is able to 'add-on' those bits of chemical structure and reactivity that it is not very good at explaining itself.
Consider the mechanism of electrophilic aromatic substitution, SEAr:
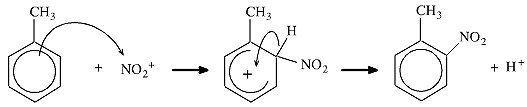
The diagram above is pure Lewis theory:
- The toluene is assumed to have a sigma-skeleton that can be described with VSEPR.
- The benzene π-system is added to the sigma-skeleton.
- The curly arrows are showing the movement of pairs of electrons, pure Lewis.
- The Wheland intermediate is deemed to be non-aromatic because it does not possess the magic number six π-electrons (below).
- The Drude model of metallic structure involves a lattice of metal cations in a sea of delocalised particle-like electrons.
- Etc., etc., etc.
Lewis theory is electron accountancy:
Count the electrons & look for the patterns and note the magic numbers.
Lewis theory is numerology:
Lewis theory cannot explain where the magic numbers come from, other than suggesting that the patterns themselves are the reason for the patterns:
"Bromine is below chlorine in the periodic table, therefore Br2 reacts to give Br–."
Lewis theory is eclectic: it greedily begs, borrows, steals and assimilates numbers, patterns & ideas from deeper, predictive theories and incorporates them into itself. "Oh, there's a pattern... we'll have that..."
Consider Hückel aromaticity which has a sound molecular orbital theory foundation. Aromaticity is predicted by the 4n+2 π-electron rule, giving the number π-electrons associated with aromaticity as: 2, 6, 10, 14, 18...
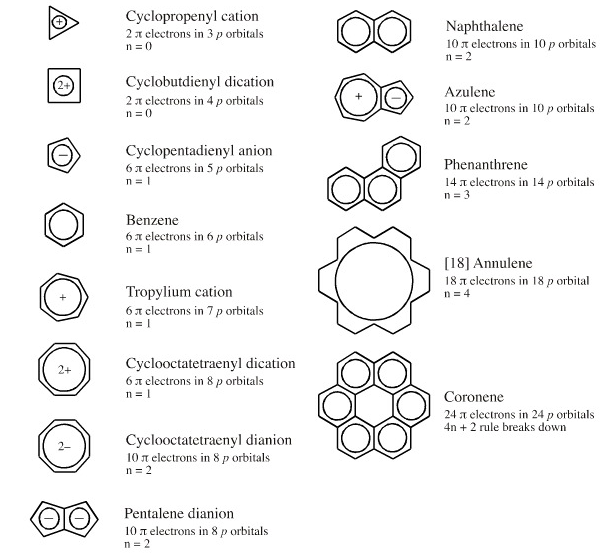
The way Hückel aromaticity is often invoked in chemical education is pure Lewis:
"Benzene has three double covalent bonds, hence three π–bonds, hence 6 π–electrons... which is one of the magic Hückel aromatic numbers (2, 6, 10, 14...) so benzene is aromatic":
Liar, liar, Pants on Fire
Lewis theory is also naughty... it tells fibs!
Dioxygen
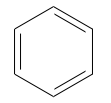
Consider the Period 2 diatomic molecular species: fluorine, oxygen and nitrogen, F2,, O2, & N2.
Every school student (and the biologist who drew the diagram below) 'knows' that:
Fluorine has a single covalent bond: F–F
Oxygen has a double covalent bond: O=O
Nitrogen has a triple covalent bond: N≡N
But this is a lie... a damnable lie.
Dioxygen is actually a diradical that can exist in singlet & triplet states. Ground state triplet oxygen is paramagnetic and liquid oxygen attracted to the poles of a magnet:

Ground state triplet oxygen, O2, should be represented as: •O–O• or ↿O–O↿.
Thus, there is no F2 O2 N2 pattern. Dioxygen simply does not fit the Lewis pattern.
This inconvenient fact is simply ignored by Lewis theory and many Lewis theory centric textbooks because the Lewis analysis is just so pretty... even though the diradical structure of O2, is inevitable in the molecular orbital description of dioxygen.
Madelung's Rule
Another example of naughty Modern Lewis theory: Madelung's Rule.
Madelung's rule states that atomic orbitals fill with electrons with the sequence: n + ℓ, where n is the principal quantum number and ℓ is the subsidiary quantum number. The rule is used to explain orbital filling and why the 4s orbital has a lower energy than the 3d orbital, and thus it invoked to explain 'why' the periodic table its characteristic appearance.
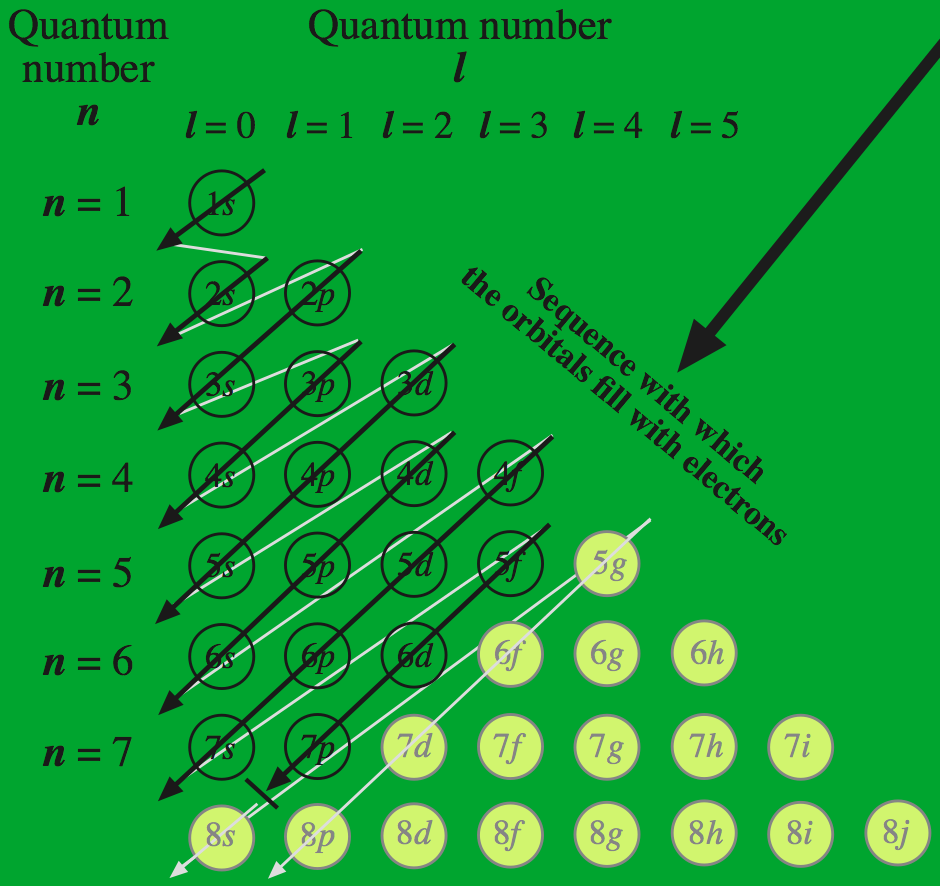
Madelung's rule is just so beautiful. The pattern is so convincing.

But in actuality, Madelung's is only a rather crude "rule of thumb" with many exceptions. When examined closely, it fails with a third of the transition elements. There are many quantum mechanical subtleties to atomic structure: nuclear screening, electron repulsion, spin-orbit coupling, relativistic effects, etc. The greater the number of electrons [as with the heavier transition elements] the more considerations there are... and the more over-simple rule breaks down.
Madelung's Rule looks like (and smells like) authentic quantum mechanics with its use of principle & subsidiary quantum numbers, but it is actually stamp collecting and not very good stamp collecting at that. Madelung's Rule makes over simple predictions about patterns of electronic structure... but the predictions often turn out to be inexplicably incorrect. Yet, within Madelung's Rule the logic looks so beautiful, so pretty, so perfect!
And Yet... And Yet... And Yet...
Modern Lewis theory works remarkably well with the lighter main group elements – and thus nearly all organic chemistry employs – which is why modern Lewis theory is taught to school and university students the world over.
The approach works less and less well as the number of electrons increases.
Patterns
Consider the pattern:

Now zoom out slightly:
The right hand side "does not fit the pattern" of the first image. Is an anomaly?
Zoom out a bit more and the anomaly seems to disappear:

Or does it? There is a purple patch on the upper right hand side does not seem to fit the pattern and so may be anomaly:

But zooming right out we see that everything is part of a larger regular pattern:

(Thanks Digital Flowers at DryIcons)
When viewing the larger scale the overall pattern emerges and everything becomes clear. Of course, the Digital Flowers pattern is trivial, whereas the interactions of electrons and positive nuclei are astonishingly subtle, more like this:





On close inspection, Roy Lichtenstein's Girl Crying looks like a regular pattern of dots, but zoom out and the full complex picture emerges.
This situation is like learning about chemical structure and reactivity using Lewis theory.
First we learn about the Lewis octet and how atoms lose & gain electrons so that they have 8 electrons in their valence shell – magic number 8 – and we come to believe that the pattern of chemical reactivity can be explained in terms of the very useful Lewis octet model.
Then we encounter phosphorous pentachloride, PCl5, and discover that it has 10 electrons in its valence shell. Is PCl5 an anomaly?
No! just get over it...
The fact is that the pattern generated through the Lewis octet model is just too simple.
Look, PCl5 has five bonding pairs and Lewis theory's VSEPR technique predicts the molecule to have a trigonal bipyramidal geometry with 120° & 90° bond angles, and it does:
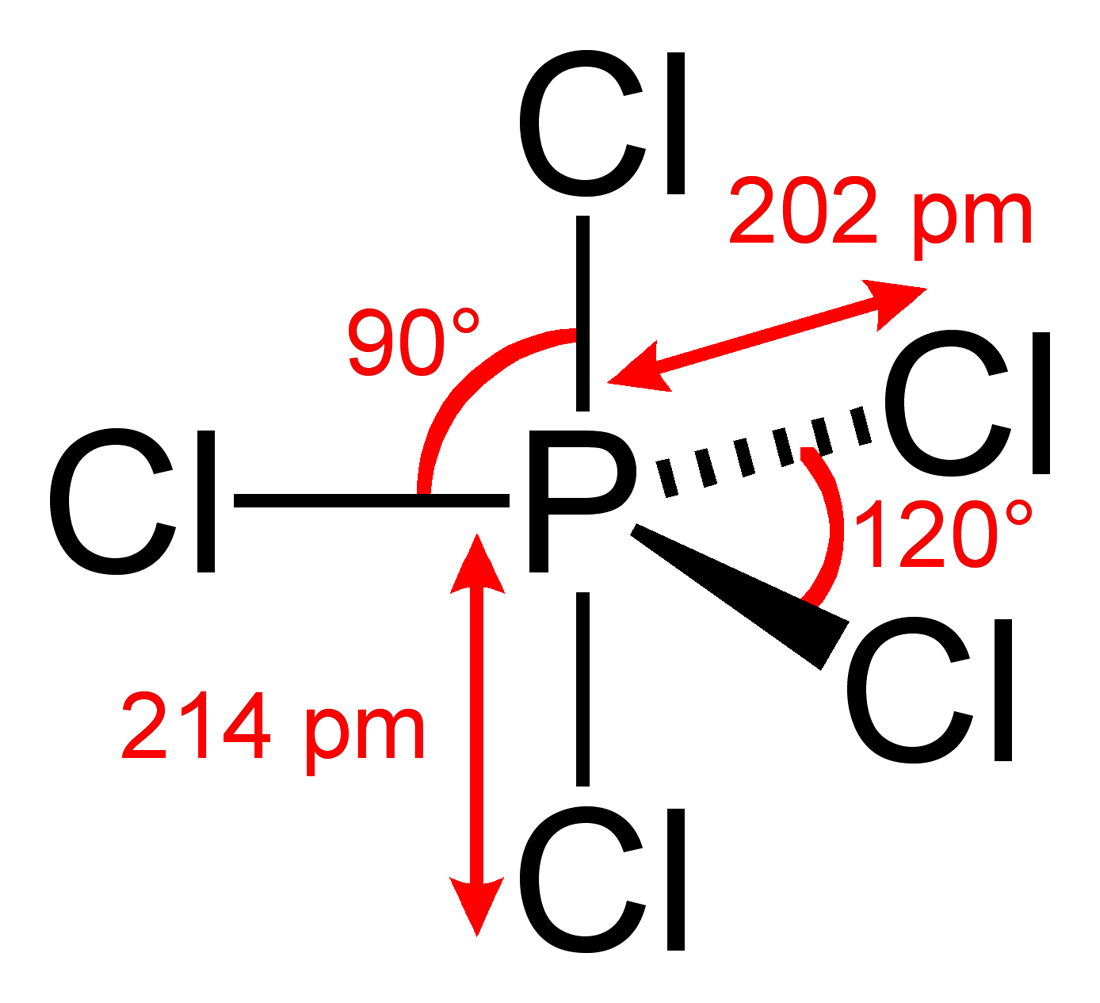
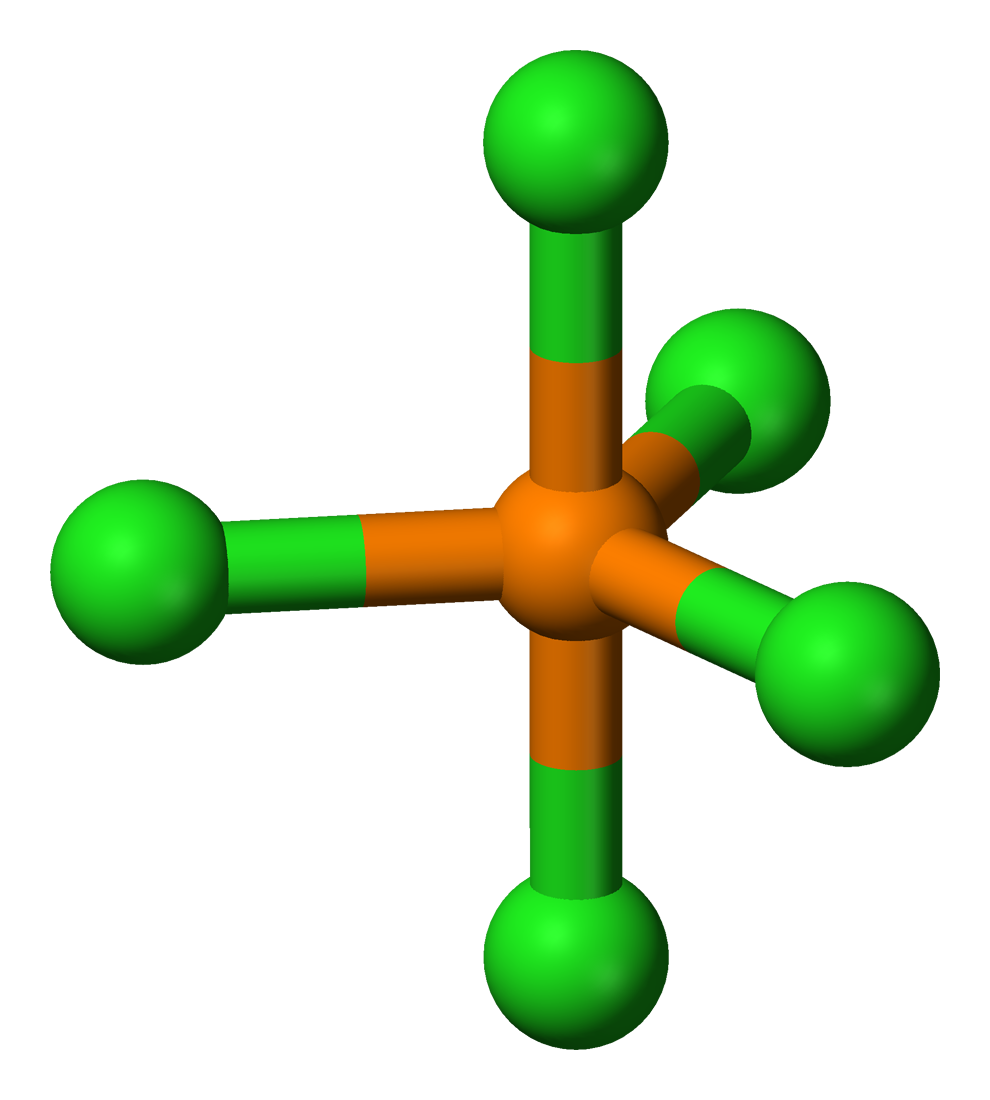
The chemist's approach to understanding chemical structure & reactivity is to count the electrons and take note of the patterns.
This is Modern Lewis theory.
A Bit of Philosophy: Realist vs. Antirealist
It is possible to take two positions with respect to a particular theory: realist or antirealist.
- The realist believes that a particular theory is a true and actual representation of the physical world in which we live.
- The anti-realist believes that a theory is simply a useful abstraction that does NOT represent the physical world in any meaningful way, even though the conclusions generated by the theory may be very useful.
An instrumentalist is an anti-realist who simply uses the toolbox of conceptual and mathematical techniques to describe and understand the physical world.
However, the problem goes deeper than this. Chemistry has an educational problem. Many of the structure and reactivity ideas/concepts in chemistry are taught to school and/or university students as if it is real, even though they are not.
Consider how the VSEPR analysis is used to explain/predict the shape of the methane, CH4,, molecule:
"A carbon atom has four electrons in its valence (outer shell). When covalently bonded to four hydrogen atoms, there is a Lewis octet of eight electrons in the carbon's valence shell arranged as four bonding pairs. These four bonding pairs [which can be regarded as negative charge clouds] repel each other to give a perfectly symmetrical tetrahedral molecule with 109.5° bond angles."
The above argument is 100% realist in that it asks us to actually believe that pairs of point like charged particles conveniently arrange themselves in a physical valence shell. The viewpoint is classical mechanical. We only have to study a small amount of quantum mechanics – and to discover that the electron is better considered as a wave – to realise that the VSEPR analysis is utter and complete tosh. BUT VSEPR WORKS!
- Does VSEPR always work? No.
- Is VSEPR real? No.
- Does VSEPR work often enough to be useful? Oh yes.
[What interests your author is how such a ridiculously simple set of rules can possibly work as well as they do.]
So, even though theories are all we have, they should be used but not believed. Do not inhale.
This is crucial, because when tested to the extreme all chemical theories are found wanting, a situation that always confuses the realist. The cynical anti-realist knows model breakdown is inevitable because theories are not real.
Now, chemistry teachers and textbook authors [yes, we are all guilty] are prone to present arguments about the nature of chemical structure and bonding without adding any anti-realist provisos... which confuses students when new facts come along, like PCl5. (But hey, we were confused when we were learning this stuff.)
For example, most of the structure and bonding ideas in textbooks, and this web book is no exception, are expressed in terms of Lewis/VSEPR ideas.
Lewis theory is a fantastic model and it works nearly all the time. In fact, Lewis theory is so good that most chemists can get away with assuming that it is true, that it is real.
Lewis Theory | Quantum Mechanics
Quantum mechanics and Lewis theory are both concerned with patterns. However, quantum mechanics is active and is concerned with the causation of the patterns, whereas Lewis theory is passive and only exploits the observed patterns.
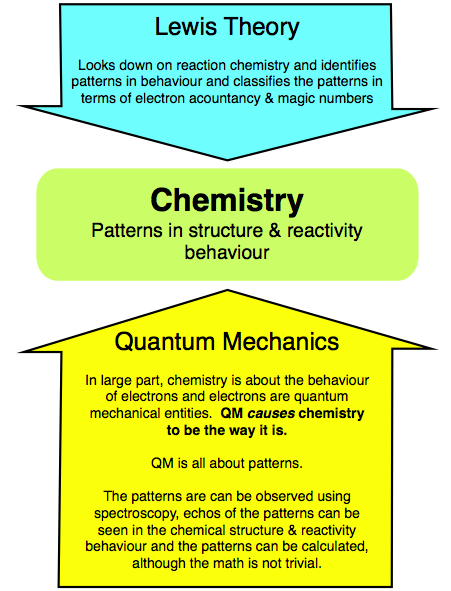
We observe patterns of structure & reactivity behaviour through experiment. Lewis theory looks down on the empirical evidence, identifies patterns in behaviour and classifies the patterns in terms of electron accountancy & magic numbers. Lewis theory gives no explanation for the patterns.
In large part, chemistry is about the behaviour of electrons and electrons are quantum mechanical entities. Quantum mechanics causes chemistry to be the way it is. The quantum mechanical patterns are can be:
- Observed using spectroscopy.
- Echoes of the underlying quantum mechanics can be seen in the chemical structure & reactivity behaviour patterns.
- The patterns can be calculated, although the mathematics is not trivial.
Not Lewis Theory
Small diatomic molecules rotate in the gas phase, but the motion is not classical, but quantum mechanical and only a few discrete values are allowed. Consider the rotational spectroscopy of hydrogen chloride, HCl, molecule:
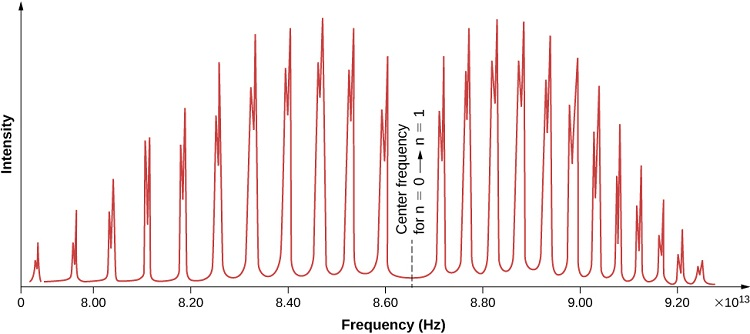
From Libre Texts, Chemistry: Absorption spectrum of hydrogen chloride (HCl) from the n=0 to n=1 vibrational levels. The discrete peaks indicate a quantization of the angular momentum of the molecule. The bands to the left indicate a decrease in angular momentum, whereas those to the right indicate an increase in angular momentum.
This spectrum is also discussed on the Hyperphysics page.
While there is no point in attempting to using any type of classical Lewis theory to help explain the above spectrum, the pattern is exquisitely described using quantum mechanics.
 |
 |
 |
| Timeline of Structural Theory | Valence Shell Electron Pair Repulsion |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.