Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Congeneric Dots, Series & Planars | Ligand Replacement Congeneric Series |
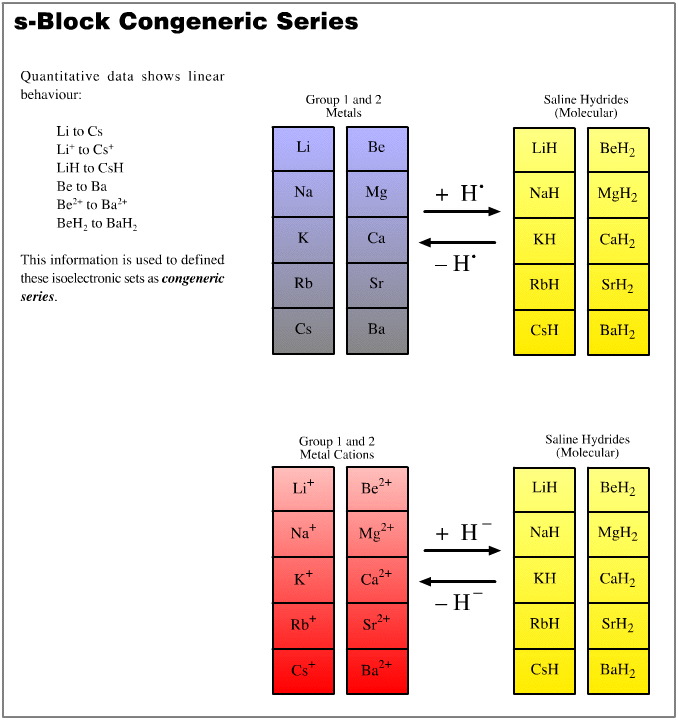
Quantifying Congeneric Behaviour
Congeneric (linear) structure and reactivity behaviour amongst the arrays of simple chemical species derived from the five hydrogen probe experiments is quantified in terms of physical data.
Linearity
Arrays can be checked for linear structure and linear behaviour traits using:
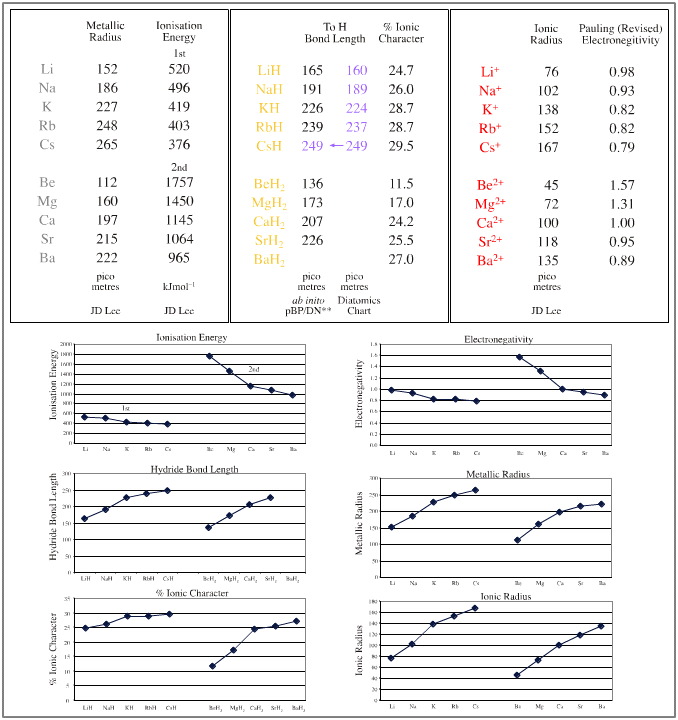
- Atomic radius, well
known data
- Ionic radius, well known data
- Ionisation energy, well known data
- Bond length, calculated at the HF 3-21G* level
or better
- Electronegativity, Revised Pauling, well known
data
- % Ionic-covalent character, calculated using the
Pauling equation, here
- pKa, well known data
We shall be looking for linear data, across series and over planars:
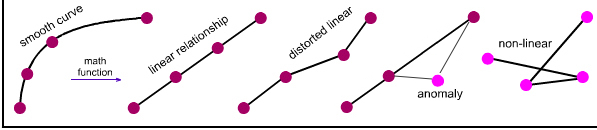
Note: 'curve' is considered to be linear, or more strictly linearisable, when it can be transformed into a straight line by the application of a simple function: 1/X, log(X), ex, COS(X), etc.
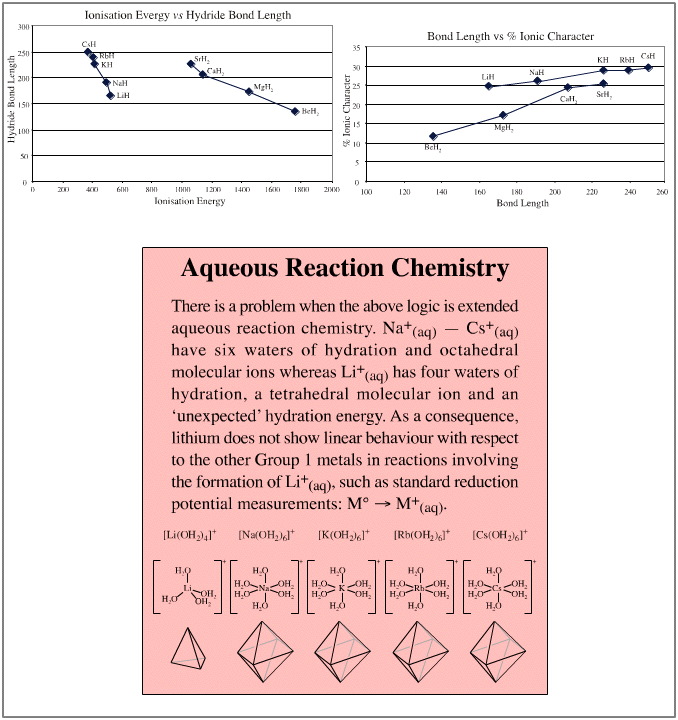
We will find that, yes, linear relationships are common within the data sets, both when parameters are plotted singly and when pairs of parameters are plotted against each other. The two dimensional plots can be most revealing.
Furthermore, we find that within a particular congeneric series it is quite reasonable to decide that one end is [Pearson HSAB] harder and the other is [Pearson HSAB] softer.
We are able to do this is because the term "hardness" is actually being used as a proxy for a physical parameter. Bond length is a good example, we find that:
Short "to–hydrogen" bond length equates with a harder conjugate centre
Long "to–hydrogen" bond length equates with a softer conjugate centre
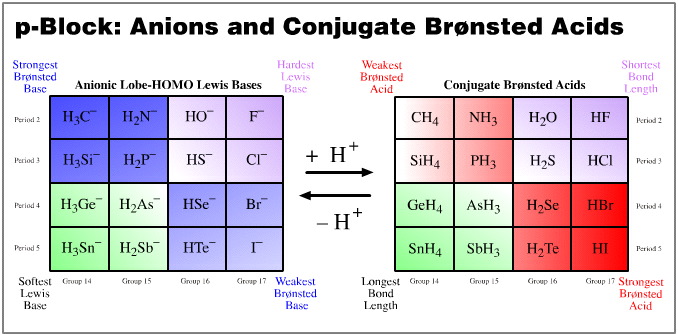
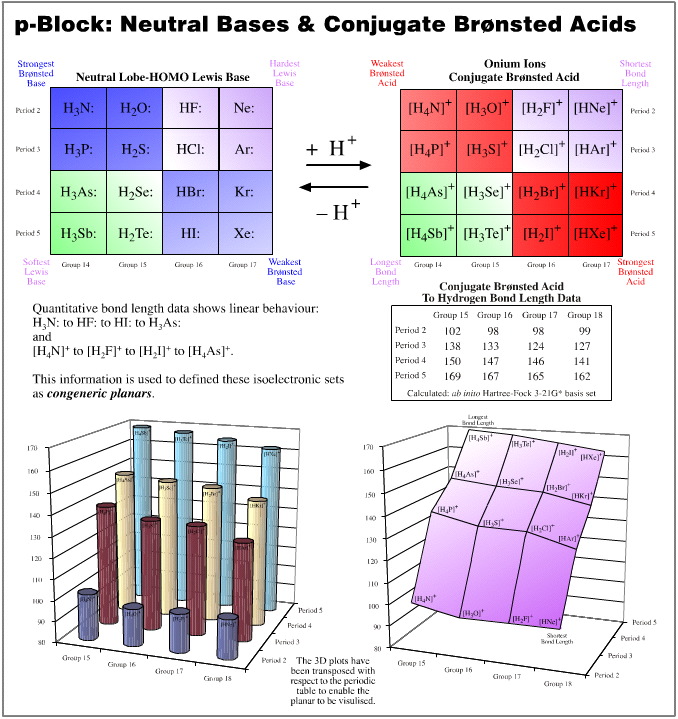
p-Block Anions and Conjugate Brønsted Acids
Of all the congeneric planars, the p-block anions and their conjugate acids, related as they are by protonation, are the most interesting. We shall study these planars by examining the pKa of the conjugate acids and the "to–hydrogen" bond lengths.
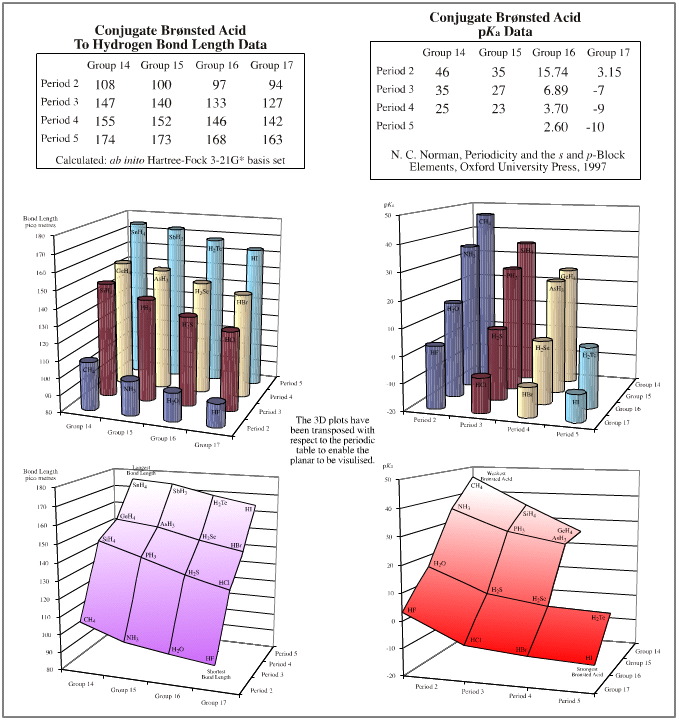
pKa data is linear over the planar. Proton donating power ranges from the strong Brønsted acid hydrogen iodide, to methane, CH4, a species that many would not consider to be a Brønsted acid at all. But the conjugate base to methane is the super base methyl lithium. Alkyl lithium reagents (of which methyl lithium is a member) are extraordinarily powerful proton abstractors.
"To-hydrogen" bond length is linear over the planar. The shortest bond length is seen with HF, and the longest with SnH4.
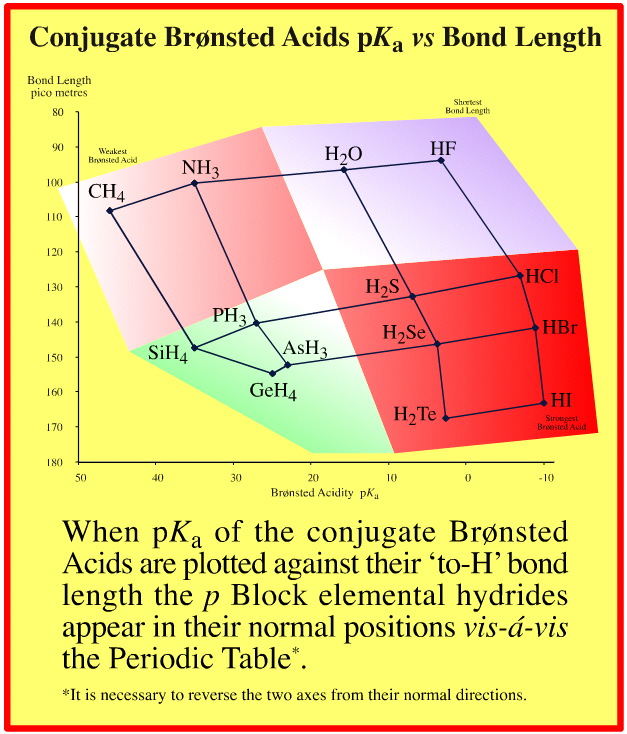
Observe: the two data sets are orthogonal (at 90°) to each other:
Brønsted proton donation-abstraction behaviour runs from top-left-to-bottom-right
"To-hydrogen" bond length runs from bottom-right-to-top left
This is one of the keys to unlocking the chemogenesis story.
And...
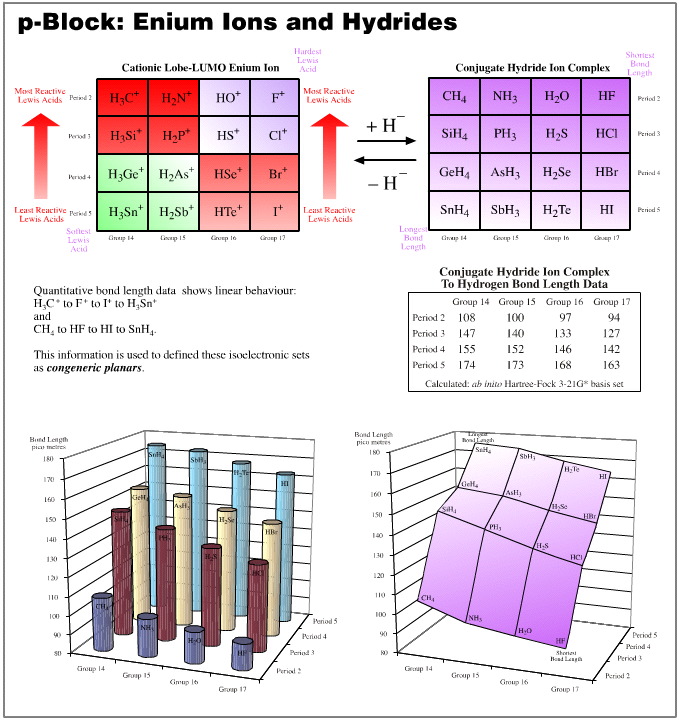
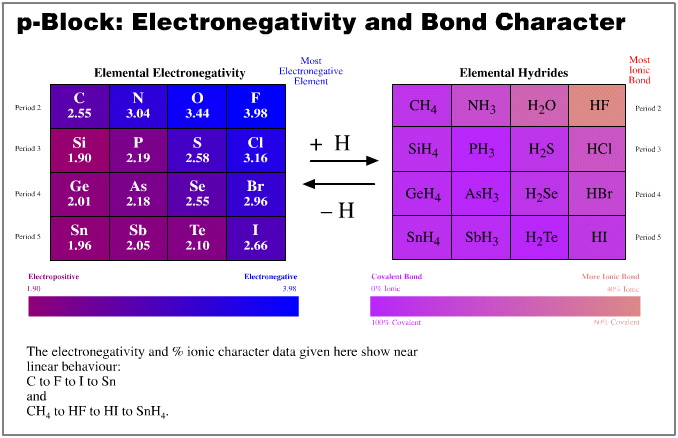
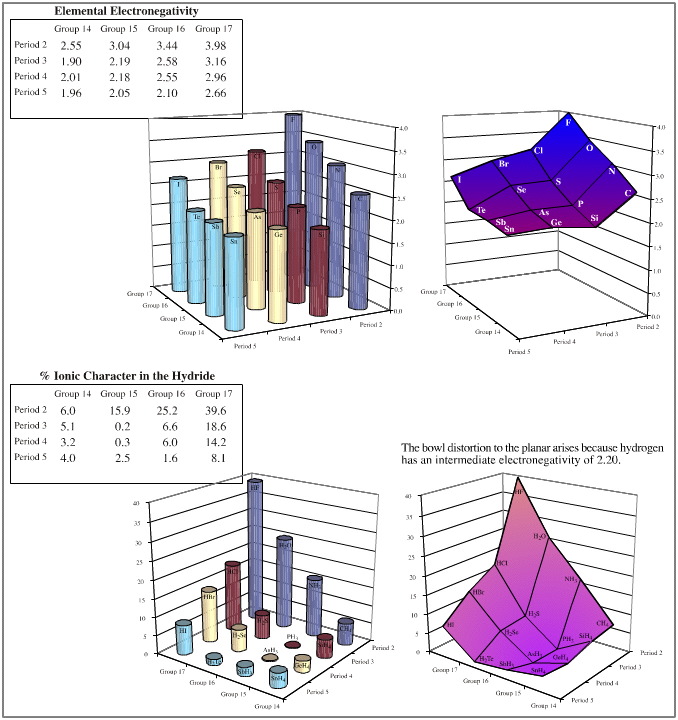
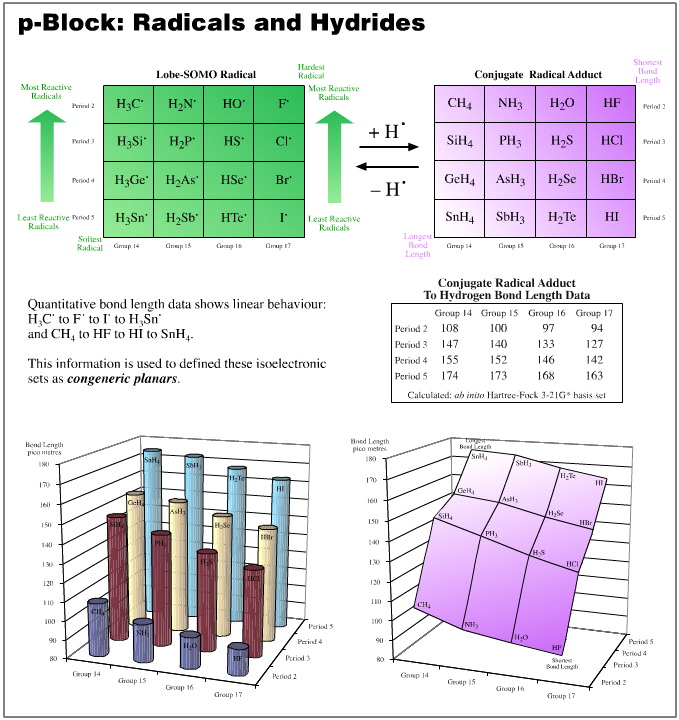
The above examples show that, yes, the congeneric series and planars generated from the five hydrogen probe experiments do exhibit linear or distorted linear structure and reaction behaviour traits. And so yes, the series and planars discussed are congeneric.
There is actually one place where the data does show a slight anomaly. Electronegativity data shows that the period 2 elements: Si, P, S, Cl are slightly less electronegative than expected. This is reflected in the corresponding distortion in the elemental hydride bond length data.
The Hard Soft [Lewis] Acid Base Principle
In the 1960s, Ralph Pearson introduced his hard soft [Lewis] acid base (HSAB) principle, here. As part of his analysis, Pearson suggested that hard-to-soft trends could be found amongst groups 15, 16 and 17 of the periodic table. The idea was extended by Tse Lok Ho who used realistic chemical species and coined the term congeneric.
 |
|||||
Bi |
Sb |
As |
P |
N |
Pearson, R.G., Hard and Soft Acids and Bases, JACS 85, 3533-3539 (1963) |
Te |
Se |
S |
O |
||
I |
Br |
Cl |
F |
||
R3Sb: |
R3As: |
R3P: |
R3N: |
Ho, T.-L., The Hard Soft Acids Bases (HSAB) Principle and Organic Chemistry Chemistry Reviews 75, 1-20 (1975) | |
H3C– |
H2N– |
HO– |
F– |
||
I– |
Br– |
Cl– |
F– |
||
H3C+ |
(CH3)H2C+ |
(CH3)2HC+ |
(CH3)3C+ |
||
Neither Pearson or Ho used quantitative data to back up their ideas, and with good reason!
As is discussed elsewhere in this web book, Pearson classified some 90 species as hard, borderline & soft Lewis acids and bases, here. But, no physical parameter correlates with behaviour over Pearson's sets. And this is the problem with the Pearson hard soft [Lewis] acid base (HSAB) classification system: the set of species is simply too large and diverse.
However, the chemogenesis approach starts with the isoelectronic series and planars (arrays) derived from the five hydrogen probe experiments and asks whether linear structural and reaction behaviour trends can be found in the isolated arrays.
 |
 |
 |
| Congeneric Dots, Series & Planars | Ligand Replacement Congeneric Series |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.