Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Diradical Chemistry | Species/Species Interactions |
Photochemistry
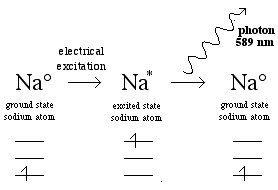
Photochemistry is concerned with the absorption, excitation and emission of photons by atoms, atomic ions, molecules, molecular ions, etc. The simplest photochemical process is seen with the absorption and subsequent emission of a photon by a gas phase atom such as sodium.Photochemical Mechanisms
When the sodium atom absorbs a photon it is said to be excited. After a short period of time, the excited state sodium atom emits a photon of 589 nm light and falls back to the ground state:
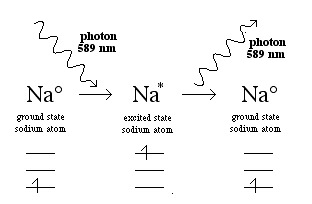
The atom can be excited by a flame, and this is the basis of the well known "flame test" of Group 1 and 2 metal salts (LiCl, NaBr, CaCl2, SrCl2, etc.) and atomic emission spectroscopy:
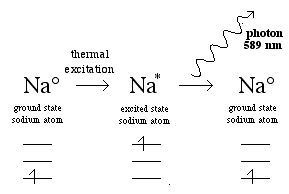
Or the sodium atoms can be electrically excited and this is the basis of energy efficient orange sodium street lights:
The Electromagnetic Spectrum
Photons have an energy which is dependent upon the wavelength of the light. The rule is:
Long wavelength light = low energy
Short wavelength light = high energy
When an opera diva shatters a glass with her singing voice, the glass absorbs resonant sound energy. To be "resonant" the frequency (or wavelength or energy) of the sound must exactly match that of the glass. And so it is with electromagnetic radiation and chemical species.
Spectroscopy is the study of absorption and emission of photons by atoms, ions and molecules. Studies reveal details of atomic, molecular and ionic structure. However, the revealed world is often strange because it is governed by quantum mechanics and subtle selection rules.
Photons of electromagnetic radiation of many wavelengths (or energies) interact with chemical species in a variety of ways.
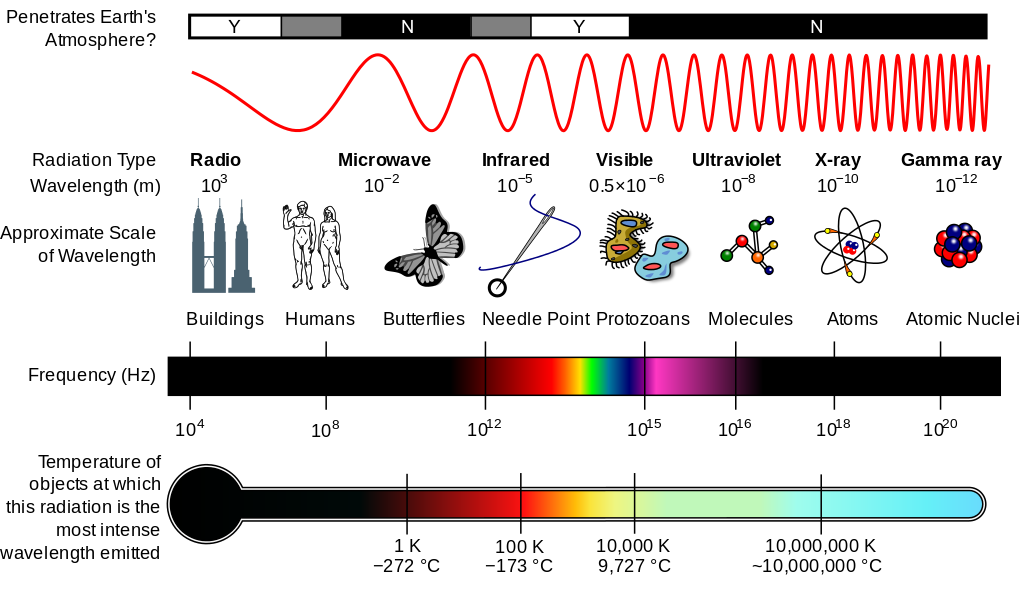
- In a strong magnetic field many atomic nuclei are made to resonate by radio waves. This is the nuclear magnetic resonance (NMR) effect.
- In the gas phase molecules are made to rotate by microwaves.
- Infrared radiation causes molecular bonds to stretch and vibrate. Infrared spectroscopy is often called "vibrational spectroscopy".
- Visible radiation induces low energy electronic transitions in atoms and molecules.
- Ultraviolet radiation causes high energy electronic transitions in molecules. The resulting excited states may relax via bond breakage. Traditionally most photo reaction chemistry has been performed with UV lamp sources. However, laser technology is extending the wavelength range of photoactivation.
- X-rays excite and eject inner shell electrons. This causes widespread ionisation and bond fragmentation.
- Gamma-Rays are highly penetrating ionising radiation which are generated during transitions in atomic nuclei.
Photochemical Reaction Processes
A photoexcited species, [X-Y]*, can relax (react) via a variety of pathways.
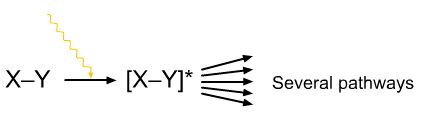
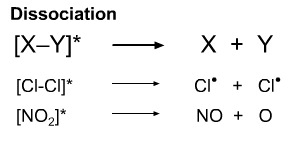
The excited state species may fragment to a pair of radicals or, in the case of nitrogen dioxide to nitric oxide and oxene.
Reactions of this type, photolysis, are important in gas phase and atmospheric chemistry. Many species can be induced to photodissociate.
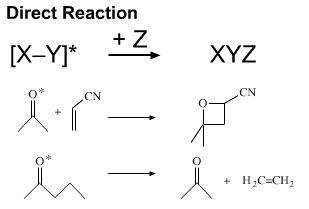
The photoexcited state may undergo reactions unavailable to the ground state species. For example, a photoexcited ketone can undergo a 2+2 cycloaddition with an alkene to give an oxetane (a cyclic ether with a four membered ring), a Paterno-Büchi reaction.
Woodward and Hoffmann showed that photo initiated 2+2 cycloadditions were complementary to the more common 2+4 thermal cycloadditions, such as Diels-Alder cycloaddition.
Photoexcited species may also be able to fragment. For example, 2-pentanone (also called methyl propyl ketone) can be photoexcited to a species which eliminates ethene (ethylene) to give propanone (acetone).
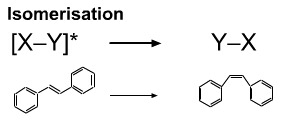
Photoexcited species may undergo isomerisation. For example, trans-stilbene can be photoexcited to a state that allows free rotation around the alkene double bond. The photoexcited species is able to relax back to the ground state at any time, and if this happens when the excited stilbene is in the cis conformation, ground state cis-stilbene will be formed.
This is photo induced trans-to-cis isomerism exploited in the visual system where the conversion of all-trans-retinal to 11-cis-retanal is the main photon detector. With time, the 11-cis-retanal thermally relaxes back to the all-trans-retinal configuration. Retinal is derived from retinol, also known as vitamin A.
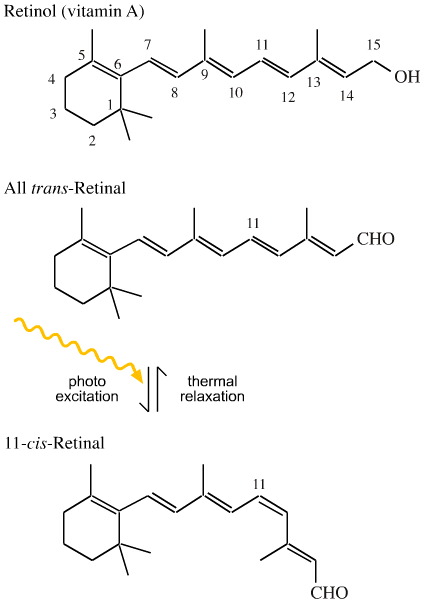
For more information on the visual system, try here.
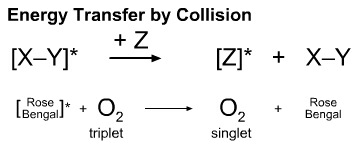
An excited state species can transfer energy to a ground state species. This process is used to produce singlet oxygen. A dye, usually rose bengal, is photoexcited with UV light, here. The photoexcited is able to transfer energy to triplet oxygen, which is converted to singlet oxygen. Singlet oxygen has a different reactivity spectrum compared with the triplet species.
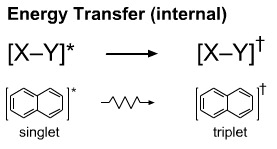
The energy state may undergo intersystem crossing and relax in an intramolecular manner. For example, the S1(excited singlet) state of naphthalene can convert into the T1 (excited triplet) state.
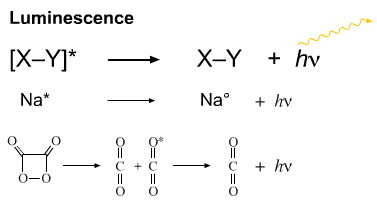
Excited state species can emit light in a process called luminescence. If the process is quick, the process is known as fluorescence and if it is delayed it is known as phosphorescence.
If the excited state is produced chemically, the system can be used to produce "chemical light":
The four membered ring system shown above (oxalic per-anhydride) is a carbon dioxide dimer. The ring strained species is able to undergo a retrocycloaddition to produce two molecules of carbon dioxide, one of which is highly excited. This excited state species quickly emits a photon of UV light. If there is a fluorescent dye present, this photon of UV light can be converted into a photon of visible light. This chemistry in used in the popular "light sticks".
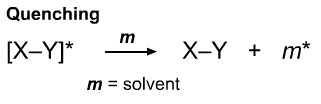
In the liquid state, the excited state species may be quenched in which the energy of the excited state species is converted into vibrational energy (heat).
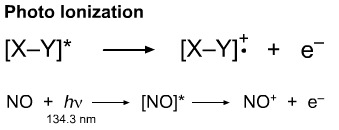
Photoionisation processes are of great importance high in the atmosphere where pressures are low and short wavelength UV radiation from the sun has a high flux.
Further Reading
C.E. Wayne and R.P. Wayne, Photochemistry, Oxford Chemistry Primers, 39, OUP (1996).
J.G. Calvert and J.N. Pitts Jr., Photochemistry, Wiley (1966).
For Photo Cycloaddition, chapter 6 of: I. Fleming, Frontier Orbitals and Organic Chemical Reactions, Wiley (1976)
 |
 |
 |
| Diradical Chemistry | Species/Species Interactions |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.