Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Main Group Elements & Hydrides | Congeneric Dots, Series & Planars |
Five Hydrogen Probe Experiments
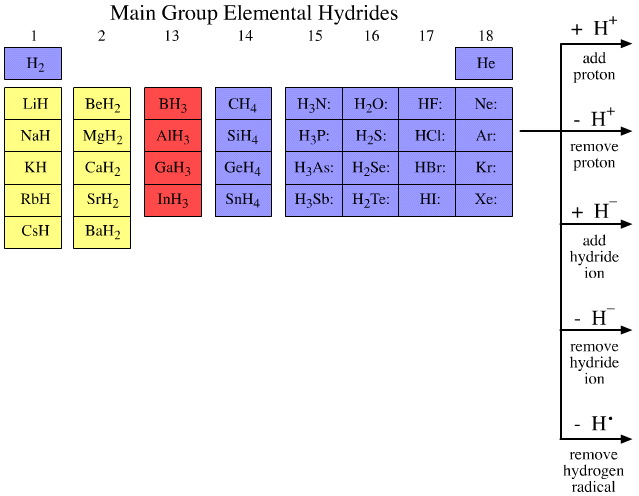
The Main Group Elemental Hydrides – as a set – are subjected to a series of experiments with nature's simplest chemical probes: H+, H– & H•. Many patterns of structure and reactivity behaviour are found:
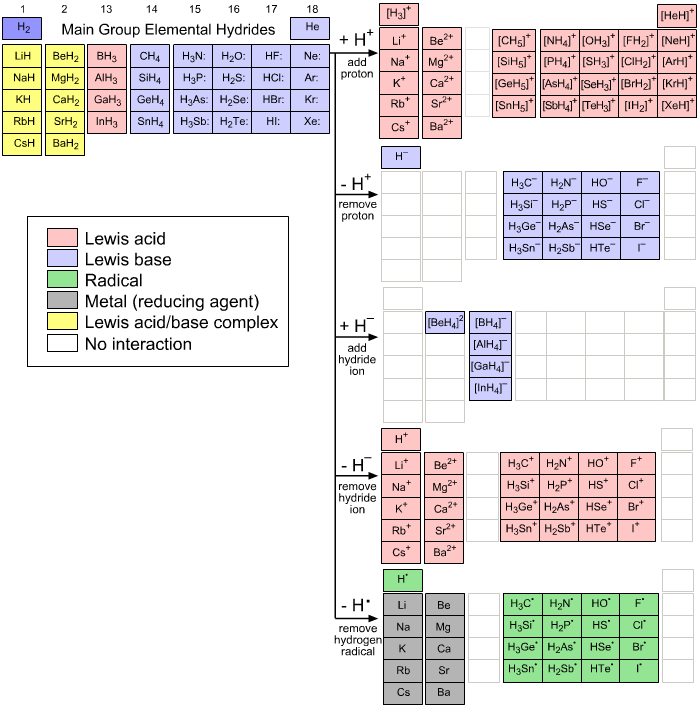
The Probe Experiments
The first 36 main group elemental hydrides, H2 to BaH2, are probed in five experiments involving hydrogen species. These five experiments are:
• Add a proton or two, H+
• Remove a proton, H+
• Add a hydride ion or two, H–
• Remove a hydride ion or two, H–
• Remove a hydrogen radical or two, H•
To and/or from the first 36 main group elemental hydrides:
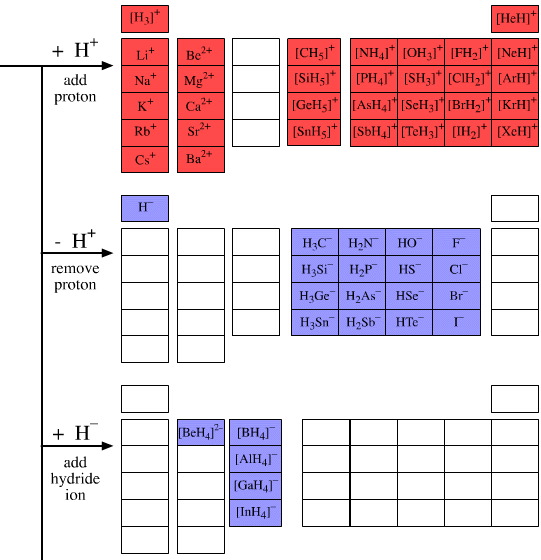
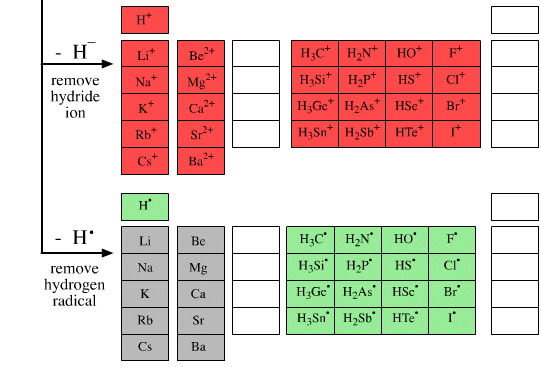
The Emergence of Congeneric Arrays
The main group elemental hydrides and the products of the five hydrogen probe experiments can be grouped into periodic "arrays", or as we shall call them "congeneric arrays". Twenty three such arrays emerge form the above analysis:
8 arrays are 1 x 1 dots
4 arrays are 1 x 4 series
6 arrays are 1 x 5 series (Group 1 and 2 cation series are counted only once)
5 arrays are 4 x 4 planars
Arrays always consist of sets of isoelectronic species:
"Two or more molecular entities are described as isoelectronic if they have the same number of valence electrons and the same structure, ie. number and connectivity of atoms, but differ in some of the elements involved." IUPAC
It follows that, as the species are isoelectronic they will have the same Lewis structure and/or frontier molecular orbital (FMO) topology, as discussed on this page of the Chemogenesis webbook.
The point is that species can be expected (and ONLY be expected) to have similar, mutually predictable, reaction chemistry if they are isoelectronic.
Isoelectronic arrays usually – but do not always – exhibit linear reactivity traits, something we shall explore in detail over the next few pages.
Arrays which are linear in both structure and reactivity are defined as being:
congeneric (or "of the same family" or here)
The 23 arrays associated with the main group elemental hydrides and generated by the five hydrogen probe experiments exhibit a few, general types of reactivity behaviour:
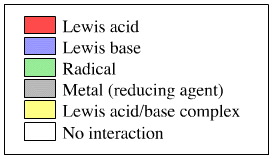
9 arrays are Lewis acids
8 arrays are Lewis bases
2 arrays are Lewis acid/base complexes
2 arrays are radicals
2 arrays are metallic
There are also many null interactions
These are colour coded:
Just Why Are The Main Group Elemental Hydrides and The Five Hydrogen Probe Experiments So Interesting?
- All
the species generated by the five hydrogen probe experiments are common
with well established structure and reaction chemistry.
- The species
generated by the five hydrogen probe experiments make excellent surrogates
for other cations, anions and radicals. For example, the [BH4]– ion has many similarities with the [BF4]– ion.
- Protons,
H+, hydride ions, H–, and hydrogen radicals,
H•, make excellent structural and reactivity probes because they
are electronically simple moieties. Hydrogen interactions minimise the
often convoluted orbital/orbital interactions observed with heavier
cations, anions and radicals.
Hydrogen species have such a low mass that they are able to partially quantum tunnel between species (states). Transfer reactions involving H+, H– and H• are kinetically fast and the thermodynamic product product is formed. Complicating activation energy effects are minimal.
For example:
The Brønsted acid reaction, proton or H+, transfer, is a thermodynamically controlled process.
The mechanistically equivalent nucleophilic substitution, R+ transfer, is kinetically controlled, and:
• The reaction may require heating in a polar solvent.
• Side reactions are likely.
• The kinetics may be first order, SN1, or second order, SN2.
- The supply
and removal of H+ to/from a species equates with Brønsted
acidity and the pKa of a Brønsted
acid gives information about both the Brønsted acid and about the conjugate [Brønsted AND Lewis] base.
The fact that methane has a pKa 46 tells us about the proton donating ability of CH4 AND about the proton abstracting ability of the conjugate base, H3C–. There is a rule:
- Weak Brønsted
acids have strong conjugate Brønsted bases.
- Strong Brønsted acids have weak conjugate Brønsted
bases.
- Methane is such a weak protonating [Brønsted] acid that many would not consider it to be acidic at all. But the conjugate base the methyl anion, H3C–, in the form of methyl lithium, LiCH3, is amongst the strongest proton abstracting [Brønsted] bases known. It is a "super-base".
- Weak Brønsted
acids have strong conjugate Brønsted bases.
- The element
to hydrogen bond length, X-H, is a subtle and versatile proxy for a
kind of reactivity.
- Subtle, because the parameter often tells us something about the intrinsic [Pearson HSAB] hardness of an atomic centre X, a term which will be strictly
defined later, here.
- Versatile, because the X-H bond can be disconnected , as indicated by the disconnection arrow =>, in three ways to give information about conjugate anions, X–, cations, X+, and radicals, X•.
- Subtle, because the parameter often tells us something about the intrinsic [Pearson HSAB] hardness of an atomic centre X, a term which will be strictly
defined later, here.
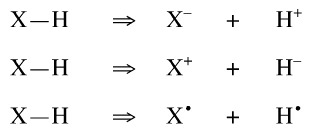
- The X–H molecular
distance (bond-length) parameter is difficult to obtain by physical
experiment because hydrogen atoms cannot be seen in x-ray crystal
analysis, the most common direct method of determining the three dimensional
structure of molecules.
To observe hydrogen atoms, and hence X–H distances, it is necessary to perform neutron diffraction experiments.
However, X–H distances can be readily and accurately calculated using ab initio molecular orbital (MO) software such as Spartan by Wavefunction.
Five Probes | Ten Probe Experiments
The five hydrogen probe experiments discussed above are a subset of five probes, hν, e–, H+, H–, H• and ten probe (add & remove each probe) experiments.
The five simplest probes are:
Photon, hν
Electron, e–
Proton (hydrogen cation), H+
Hydride ion (hydrogen anion), H–
Hydrogen radical (hydrogen atom), H•
Each of these five probe species can – in principle – be added or removed form a species, which results in 10 possible probe experiments, however, any single species not be able to partake in all 10 of the probe experiments.
We will be using methane, CH4, as our subject molecule:
Add A Photon to Methane

Adding a photon gives an excited state methane, which may relax in a number of ways. The effect will depend upon the wavelength (energy) of the photon. Infrared radiation of the correct wavelength will cause the methane molecule to vibrate. Ultra violet radiation of the correct wavelength will result in electronic excitation, or possibly ionisation.
Geometry optimisation using density function theory at the pBP/DN** level shows excited state methane to be tetrahedral.
The study of electromagnetic radiation interacting with matter is the science of spectroscopy and is outside the scope of this web book.
Remove A Photon from Methane
A photon can only be removed from an excited species. Thus, the above process can be deemed to occur when an excited methane loses a photon (ie, the reverse of the methane plus photon reaction).
Add An Electron to Methane
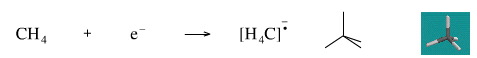
Adding an electron to methane gives the methane radical anion. The species is an anion because it has an excess electron and it is a radical because the extra electron is unpaired.
The methane radical anion is known in the gas phase. Radical anions are routinely generated and studied in the technique of negative ion mass spectrometry.
Geometry optimisation using density function theory at the pBP/DN** level shows the methane radical anion to be tetrahedral.
Remove An Electron from Methane
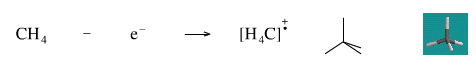
Removing an electron from methane gives the methane radical cation. The species is a cation because is has one electron less that is required for neutrality, and it is a radical because it has an unpaired electron.
Radical cations are routinely generated in the gas phase and studied in the technique of mass spectrometry.
Geometry optimisation using density function theory at the pBP/DN** level shows the methane radical cation to be tetrahedral.
Add A Proton to Methane
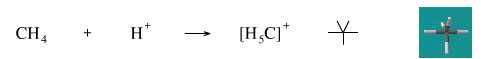
Methane (and other alkanes) can be protonated in solution by George Olah type "super-acids", for example H+[SbF6]–. Protonated methane is called the carbonium ion.
Geometry optimisation using density function theory at the pBP/DN** level shows the carbonium ion to be trigonal bipyramidal.
Remove A Proton from Methane
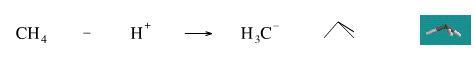
While it is not possible to remove a proton from methane to generate the methyl anion, H3C–, the reverse reaction is well know. Methyl lithium, CH3Li, is a well known strong base which violently reacts with water to give methane.
Geometry optimisation using density function theory at the pBP/DN** level shows the carbanion to be trigonal pyramidal. The same geometry is predicted with VSEPR.
Add A Hydride Ion to Methane
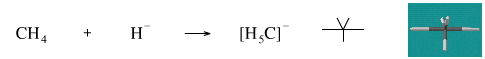
This reaction is not known for methane. However borane, BH3, reacts with hydride ion to give the tetrahydroborate ion, [BH4]–.
Geometry optimisation using density function theory at the pBP/DN** level shows the [H5C]– carbonium ion to be trigonal bipyramidal.
Remove A Hydride Ion from Methane
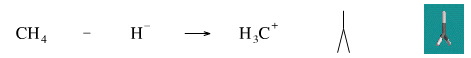
While it is not possible to remove a hydride ion from methane to generate the methyl cation (the carbenium ion), the reverse reaction is well know. Methyl iodide reacts with lithium aluminium hydride [a source of hydride ion] to give methane.
Geometry optimisation using density function theory at the pBP/DN** level shows the [CH3]+ carbenium ion to be planar. The same geometry is predicted with VSEPR.
Add A Hydrogen Radical to Methane
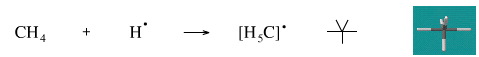
This reaction is not known for methane.
Geometry optimisation using density function theory at the pBP/DN** level shows the H5C• radical to be trigonal bipyramidal.
Remove A Hydrogen Radical from Methane
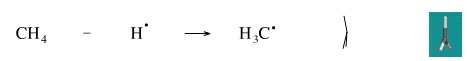
A hydrogen radical can be abstracted from methane by a chlorine radical to give a methyl radical, and this is one of the steps in the free radical halogenation of methane to methyl chloride.
Geometry optimisation using density function theory at the pBP/DN** level shows the methyl radical, H3C•, to be planar. Experiment shown it to be slightly distorted to trigonal pyramidal.
 |
 |
 |
| Main Group Elements & Hydrides | Congeneric Dots, Series & Planars |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.