Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| The HSAB Principle | The Five Hydrogen Probe Experiments |
The Main Group Elemental Hydrides
The chemogenesis story starts with the periodic table, simplified to the first 36 main group (s & p-block) elements, hydrogen to barium. The main group elements are then normalised to the corresponding main group elemental hydrides, a set that includes such well known species as: hydrogen, water, ammonia, methane, lithium hydride, xenon and hydrogen chloride. Patterns in structure and reaction behaviour are noted.
The First 36 Main Group Elements: Hydrogen to Barium
Initially we shall limit our initial discussions to s and p-block elements, to first 36 main group elements, hydrogen to barium:
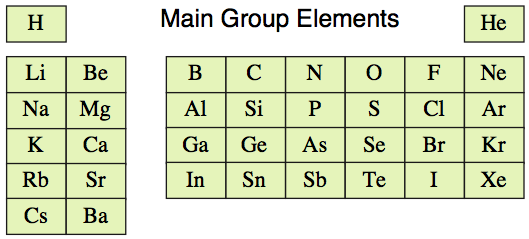
However, as chemical substances in their standard state (100kPa pressure & 298K – 1.00atm & 25°C) the main group elements present as a diverse and complicated collection of substances:
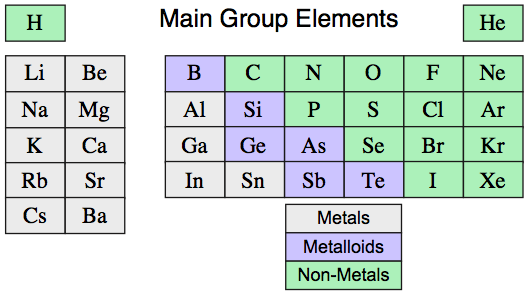
As discussed previously in this web book – see the pages on the Classification of Matter and the Tetrahedron of Structure, Bonding & Material Type pages – elemental and binary substances exhibit four extreme material types [metallic, ionic, molecular & network covalent], however the elements present as just: metallic, molecular & network covalent (no pure element is ionic):
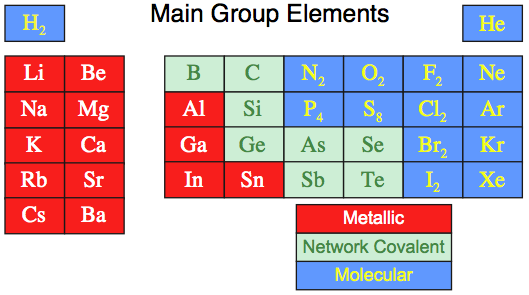
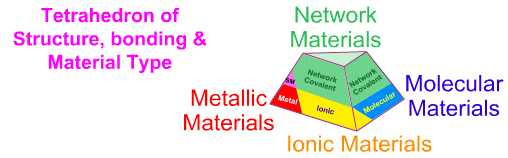
When this analysis is applied to the set of the first 36 main group elements we find:
16 elements are molecular, 15 are metallic, 5 are network covalent.
However, even this is a simplification because several elements have metallic and non-metallic allotropes that are intermediate between metallic and network, and are metalloid or semi-metallic in nature: C, Si, Ge, As, Sn, Sb & Te.
Thus, as a set, even the first 36 main group elements exhibit considerable complexity.
Normalisation
Data normalisation is a common procedure in science, here.
- It is convenient to normalise length data – inch, foot, millimetre, cubit, light year – with respect to the metre.
- Chemists routinely normalise the "amount of substance" data with respect to the mole (or mol).
The question is:
Can the diversity associated with the elements in their standard state be removed? Is it possible to normalise the first 36 main group elements so that they can be meaningfully compared and contrasted with each other?
One way to normalise the elements is to examine the gas phase mono-atomic species, here. While this tells us much about the physics of atoms and atomic structure, it tells us little about the reaction chemistry of chemical elements as substances and reagents.
The approach taken by the chemogenesis analysis is to saturation bond each of the first 36 main group elements with a common bonding partner, and then to explore the chemistry of the resulting binary compounds. There are several candidate bonding partner elements, including:
- Hydrogen
- Lithium
- Fluorine
- Oxygen
to give the corresponding: hydrides, lithides, fluorides and oxides
By far the most interesting bonding partner is hydrogen. This gives rise the corresponding set of 36 main group elemental hydrides.
The Main Group Elemental Hydrides
The 36 of the main group elemental hydrides, H2 to BaH2, are all well known species with non-controversial structure and reaction chemistry. Indeed, the set includes such common chemical species as water, H2O, methane, CH4, ammonia, NH3, hydrogen sulfide, H2S, hydrogen, H2, and neon, Ne.
Of more importance – and crucial to the normalisation argument – is that all the main group elemental hydrides can be found in the gas phase where they are molecular.
This "gas phase & molecular" point is crucial for two reasons:
- Most reaction
chemistry involves molecules and/or molecular environments (the gas
phase or in solution).
- Gas phase and molecular chemical species are simple to model, both on paper and in silico. This makes it possible to carry out computer aided virtual reaction chemistry.
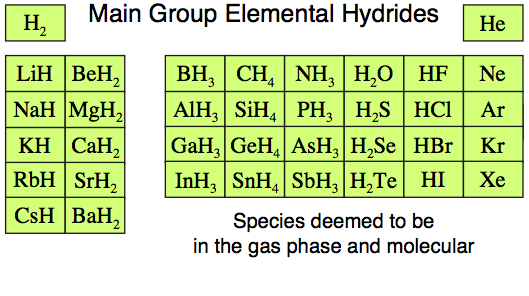
Some points to note:
- The Group
1 and 2 saline hydrides – lithium hydride to barium hydride, LiH
to BaH2 – are known to exist as ionic solids rather than as discrete
molecules. However, the saline hydrides and other ionic materials can
be studied in the gas phase molecular entities by laser
ablation of the crystal surface.
- Molecular lithium hydride, LiH,
is a very common species for theoretical study because it is the simplest
species with a heteronuclear bond, a bond between two dissimilar elements. The lithium hydride 'molecule' has only four electrons.
- The Group
13 hydrides such as borane, BH3, dimerise to B2H6.
- These seeming
exceptions to the molecular and in the gas phase argument are
actually core components of the chemogenesis argument, as will become
apparent.
- An additional reason for restricting the current discussion to s and p-block elemental hydrides, as discussed above, is that the d and f-block elements form non-stoichiometric hydrides which can vary in composition. (These interesting materials are being studied for the storage of hydrogen.)
Patterns In Reaction Chemistry Space
The main group elemental hydrides show patterns of reaction chemistry behaviour which correspond with the s-block and p-block construction of the periodic table in that they show real and rich periodicity.
- The main group elemental
hydride species are shown below in coloured blocks that correspond to
classification as Lewis acids, Lewis bases and Lewis acid/base complexes.
- While these classifications may seem a little odd and arbitrary, they will be shown to be an intrinsic part of the self-consistent chemogenesis classification system:
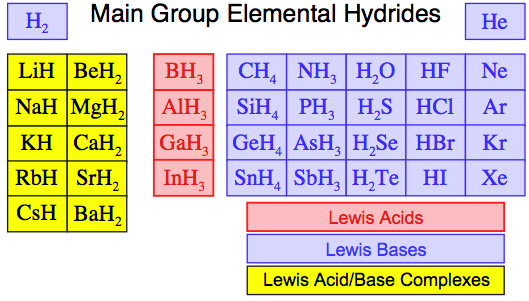
The Main Group Elemental Hydrides as: Lewis Acids, Lewis Bases and Lewis Acid/Base Complexes
[For a revision of the difference between the "Lewis" and "Brønsted" definitions of acids and bases, look here, and for a discussion of frontier molecular orbitals, look here.]
- Lewis acids are RED
- Lewis bases are BLUE
- Lewis acid/base complexes are YELLOW
In the original language of Lewis acid/base chemistry:
- A Lewis base is "a species with an electron pair".
- A Lewis acid is " an electron pair acceptor".
- A Lewis acid interacts with a Lewis base to give a complex with a polar covalent, two electron chemical bond.
In the 1960s, with the development of frontier molecular orbital (FMO) theory, this can be modified to:
- A Lewis base interacts [with a Lewis acid] via its highest occupied molecular orbital or HOMO.
- A Lewis acid interacts [with a Lewis base] via its lowest unoccupied MO or LUMO.
- HOMO + LUMO interactions give rise to a bonding molecular orbital, MO.
In the analysis presented here:
A Lewis acid's LUMO interacts with a Lewis base's HOMO to give a Lewis acid/base complex with a net bonding molecular orbital:LUMO + HOMO → LUMO/HOMO → Bonding MO
There is a self-consistent colour scheme running through the Chemogenesis web book.
 |
 |
 |
| The HSAB Principle | The Five Hydrogen Probe Experiments |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.