Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Electronegativity | Tetrahedra of Material Type |
van Arkel-Ketelaar Triangles of Bonding
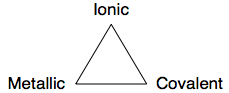
The chemical elements and binary compounds hold a special place in chemical understanding because they exhibit only a single type of strong chemical bond, of which there are three extreme types: ionic, metallic & covalent:
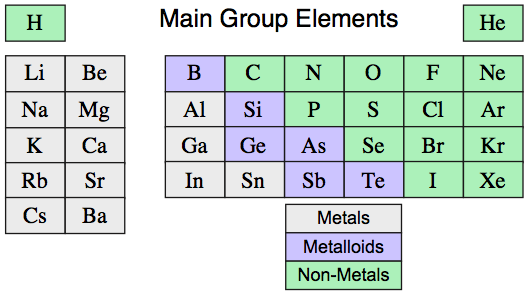
The Main Group Elements as Materials
The first 36 main group elements are well known materials that can be classified as: metals, metalloids and non-metals:
The chemical elements, by themselves, can undergo phase–change and allotropic interconversion processes:
|
gas ⇄ liquid
|
boiling/condensing |
|
liquid ⇄ solid
|
melting/freezing |
|
gas ⇄ solid
|
subliming/condensing |
|
solid
⇄ solid
|
allotrope
interconversion |
Simple Binary Compounds: Dielemental Materials
There are 36 main group elements and there are approximately 650 binary (dielemental) compounds (chemical substances) materials that are derived from the first 36 main group elements.
Compounds can be predicted using a combinatorial matrix (interaction table) of main group elements (a type of Karnaugh map):
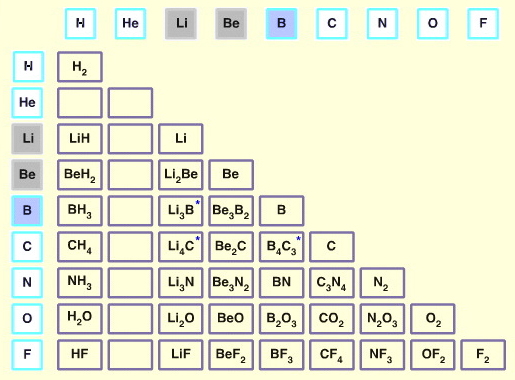
The number of binary compounds is approximately 650 because: 36+35+34+... +1 = 666, but helium and neon do not form any known compounds, and some element + element interactions give rise to more than one binary, for example there are several nitrogen-oxygen binaries:
NO, NO2, N2O, N2O4, N2O3 & N2O5
PCl3 & PCl5
IF, IF3, IF5 & IF7
etc.Of the 45 materials shown in the H to F half-matrix above, three do not exhibit the expected stoichiometry:
- Lithium carbide exists as Li2C2 (lithium acetylide) not Li4C
- Boron carbide exists as B4C not B4C3
- Lithium boride exists as: Li2B3, LiB2 or LiB0.8-1.0, not Li3B
The number of possible CnHm hydrocarbon (and borane, BnHm) homologs and isomers is uncountably huge, here. However, apart from methane, CH4, (and the transient species methylene, CH2, and methine, CH, here) all hydrocarbons contain more than one type of covalent bond: ethane, CH3CH3, has C-C and C-H covalent bonds, for example.
Grimm's Triangle
William B. Jensen's paper: Logic, History, and the Chemistry Textbook, J.Chem.Educ., 817-828, 75, 1998 discusses Grimm's "Dreieckeschema" from 1928 which uses the logic discussed above:
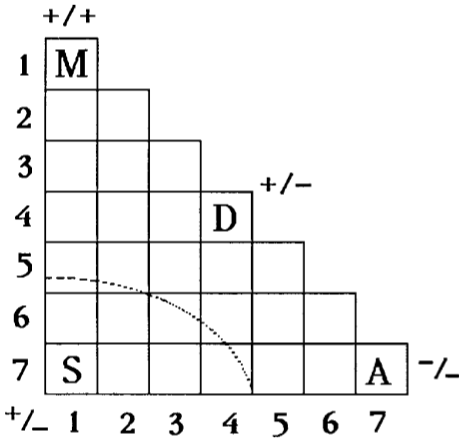
Grimm's 1928 "Dreieckeschema" produced by constructing intra- and inter-row binary combination matrices for the elements in the periodic table.
Each square represents a binary compound formed from the elements having the group numbers listed along the vertical and horizontal edges.
The letters indicate the general character of the resulting compounds:
A = Atommoleküle (molecular)
D = Diamantartige Stoffe (diamond like)
M = Metalle (metal)
S = Salze (salt)
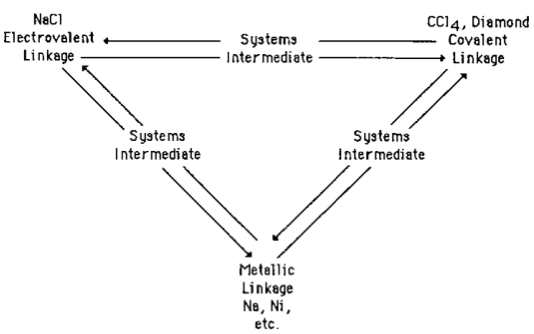
Fernelius & Robey's 1935 Bond-Type Triangle
Also in Jensen's paper is a mention of Fernelius & Robey's Bond-Type Triangle, Fernelius, W.C. and Robey, R.F. The Nature of the Metallic State. J. Chem. Educ. 1935, 12, 53–68:
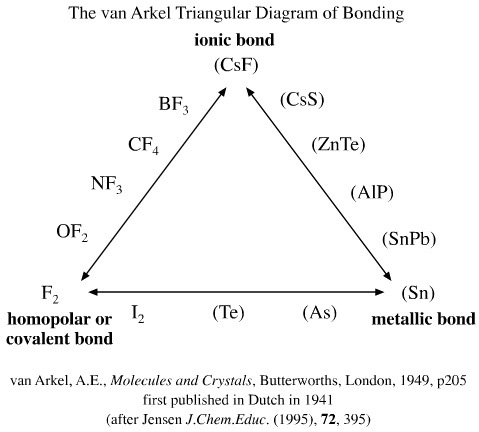
van Arkel's 1941 Triangle
Van Arkel recognised three extreme material and associated bonding types: ionic, metallic and covalent, and he placed these are the corners of an equilateral triangle. He then suggested intermediate species: for example, carbontetrafluoride, CF4, which has C-F bonds that are intermediate between ionic and covalent and are polar covalent. (We will be exploring these intermediate species further, particularly on the next page, here.)
The beauty of the van Arkel analysis is that real chemical species are used to provide hard empirical evidence. The triangle maps material type to theories of chemical structure.
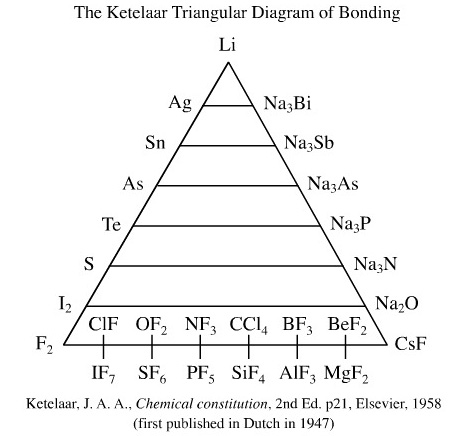
Ketelaar's 1947 Triangle
van Arkel's idea was developed by Ketelaar, another Dutchman:
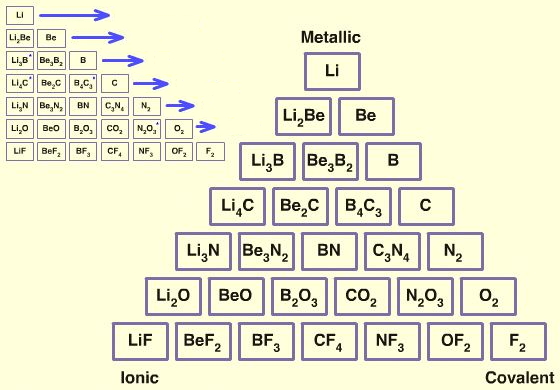
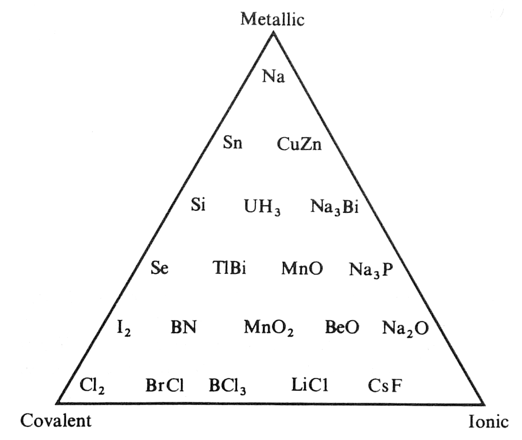
Triangle from the Main Group Interaction Matrix
A triangle of bonding is obtained from main group element interactions, above:
Triangle Rotations & Reflections
It transpires that the idea of a "triangle of bonding" with metallic, ionic and covalent extremes is very rich, and it can be extended in many ways.
The original van Arkel triangle has: covalent at the lower left, ionic at the top and covalent at the lower right, while Ketelaar has: covalent at the lower left, metallic at the top and, ionic at the lower right.
Geometrically, there are six ways to construct triangles through rotation and reflection:
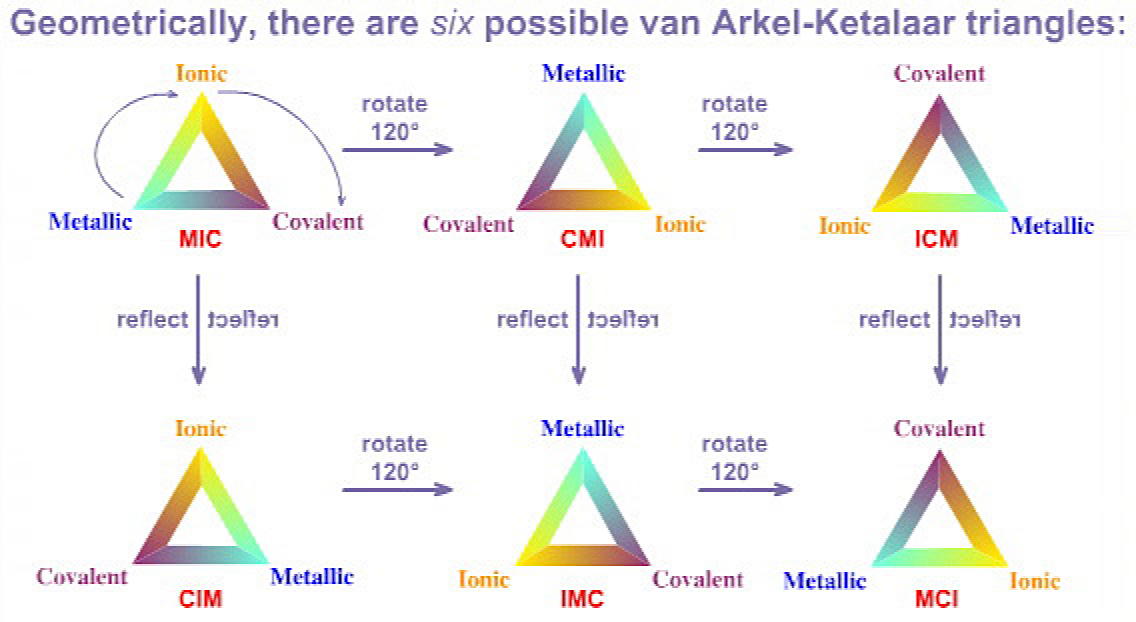
The original van Arkel triangle is CIM, the Ketelaar triangle is CMI, the main group element interaction matrix triangle is IMC,but it is more convenient to use the MIC formulation.
The Jolly Triangle
It is also possible to select different combinations of species to represent metallic, ionic, covalent and intermediate material types. In his 1985 book, William Jolly gives a triangle of bonding with his own selection of species, a CMI formulation:
Allen's Quantitative Triangle
The Allen triangle of bonding (1992) uses configuration energy (CE): "the average one-electron valence shell energy of the ground-state free atom" as an atomic parameter. CE is used directly to quantify the metal-covalent edge, and the difference in CE is used as a measure of ionic bonding.
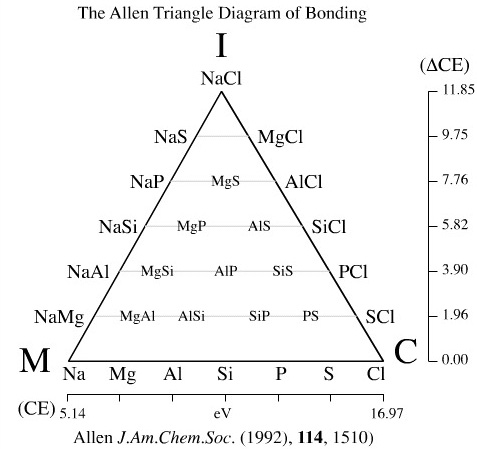
The Allen triangle of bonding is restricted to period 3 elements.
Jensen's Quantitative Triangle
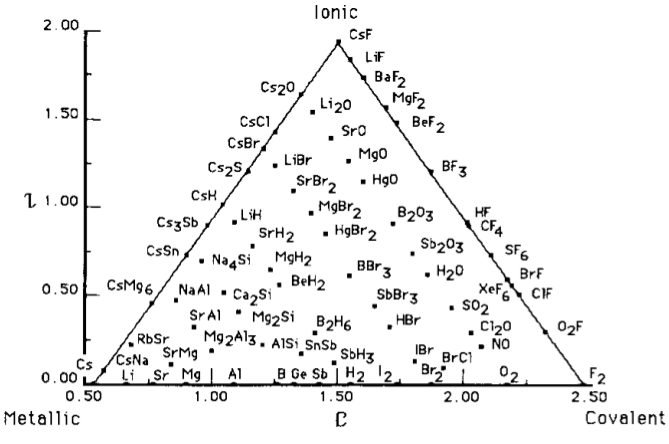
William Jensen reviewed the van Arkel-Ketelaar triangle literature in his 1995 paper: J.Chem.Educ. 395-398, 72, 1995. The paper introduces a triangle of bonding quantified in two dimensions using electronegativity data.
The x axis, the metallic-covalent edge, is the average electronegativity of two atoms.
The y axis, ionic character, is the difference in electronegativity.
The Quantified Jensen Triangle of Bonding uses an electronegativity scale introduced by Martynov & Batsanov. There are two versions, the first used compounds and the second bonds:
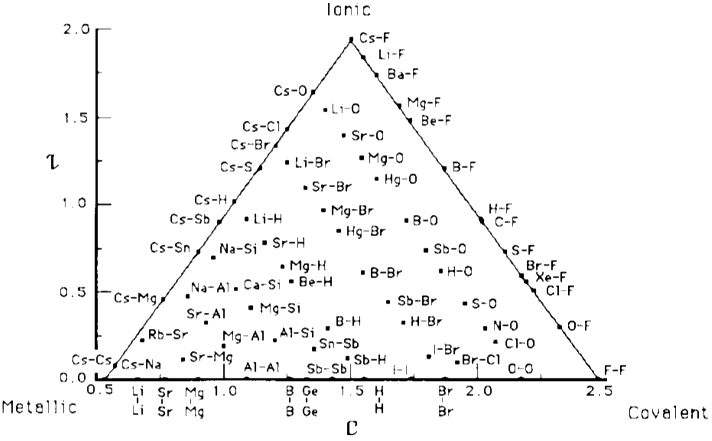
A version of the Jensen triangle using (revised) Pauling electronegativity, is reproduced below for the 481 main group binaries. (Only 31 of the first 36 main group elements have electronegativity data.) This diagram is captured from an Excel spreadsheet, and the original .xls file can be downloaded here.
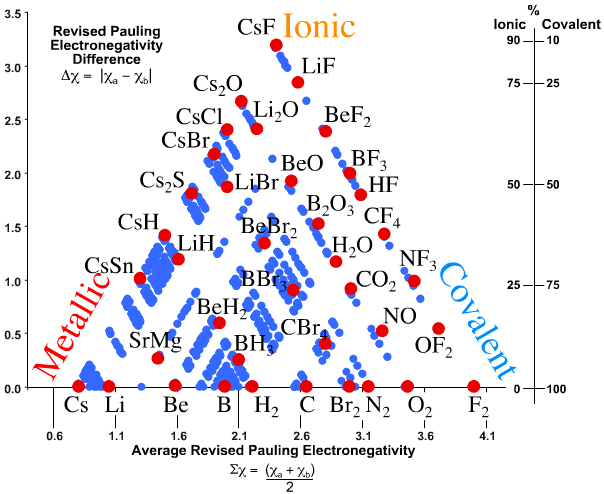
Norman's Quantitative Triangle
N.C. Norman developed a triangle of bonding that, rather like the Allen version, is quantitative in two dimensions and which (like Jensen) uses electronegativity. However, and very confusingly(!), the Norman Triangle of Bonding uses Allen electronegativity rather than Martynov & Batsanov or revised Pauling electronegativity. And yes, that is the same Allen who used configuration energy rather than electronegativity.
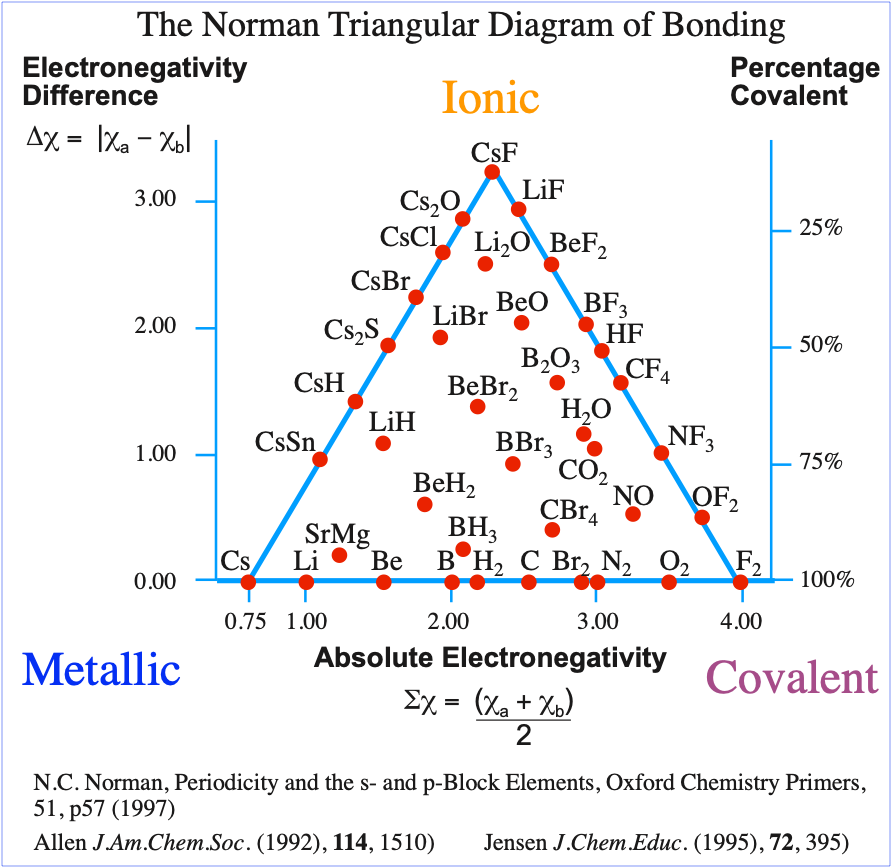
The astute reader will have noticed how similar the Allen configuration energy (CE) triangle is to the Jensen, Norman and other electronegativity triangles of bonding.
In his 1992 paper, Allen argues that configuration energy, CE, is a fundamental atomic property and is the "missing third dimension to the periodic table", but that electronegativity is simply an "ad hoc" parameter.
More plausibly, it can be argued that Allan's work shows that CE and electronegativity are in some way equivalent; a much more useful idea.
MRL's Quantitative Triangle
My personal take on the van Arkel-Ketelaar triangle is to use a single period, for example: Li to F, and to quantify the ionic-covalent edge in terms of % ionic (or covalent) character using electronegativity data and the Pauling equation:
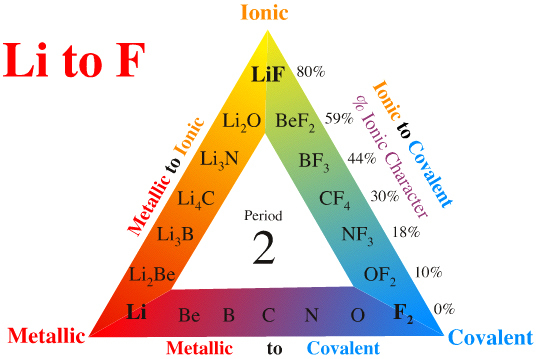
While the % ionic-covalent character numbers are useful – and they certainly do seem to be a good indication of polarity and reactivity – not too much should be read into them.
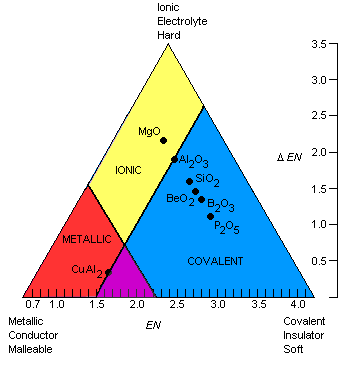
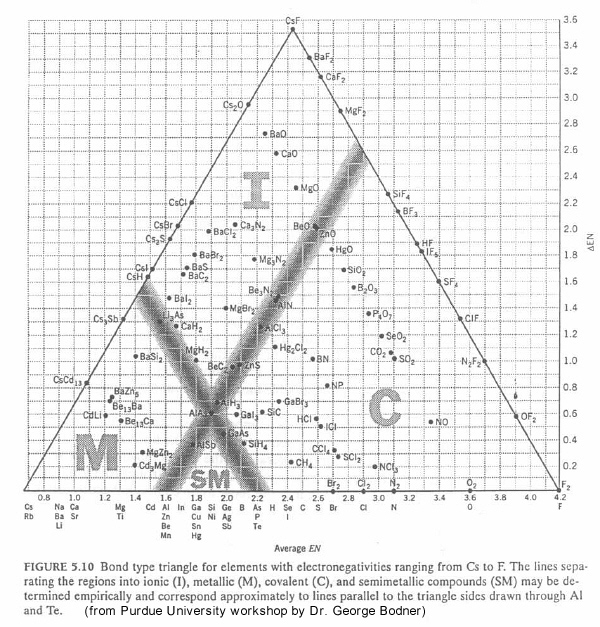
Purdue Triangle
From Purdue University chemistry department:
"The bonding between atoms in a solid is determined by a combination of two factors: the magnitude of the electronegativities of the atoms in the solid and the differences between these electronegativities. As a result, the bond-type triangle shown in the figure below can be used to predict the classification in which a solid should fall. Compounds with metallic bonds should be metallic solids, those with ionic bonds should be ionic solids, and those with covalent bonds should be either molecular or network covalent solids.":

Electrical Conductivity: Metallic Bonding
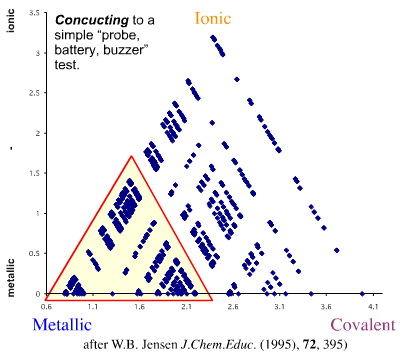
In his 1995 paper, Jensen pointed out that it is possible to explore the metallic region of the van Arkel-Keteleaar triangle using a simple "probe, battery, buzzer" conductivity test. Materials within the area shown below are "conducting" to this simple test, materials outside are "insulating".
Zintl Phases
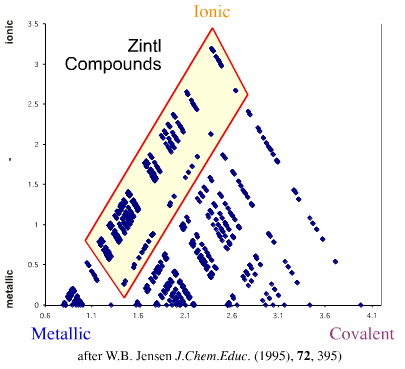
Jensen also points out that "Zintl phases", Wikipedia, can be mapped triangle of bonding. Known since the 1930's, "Zintl phases" are formed in liquid ammonia. Zintl phases on the web can be found here.
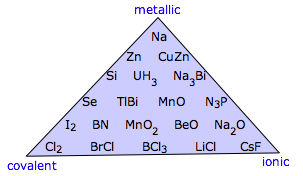
Heyes' Version of Ketelaar's Triangle
From the website (no longer extant) of Dr S.J. Heyes, University of Oxford:
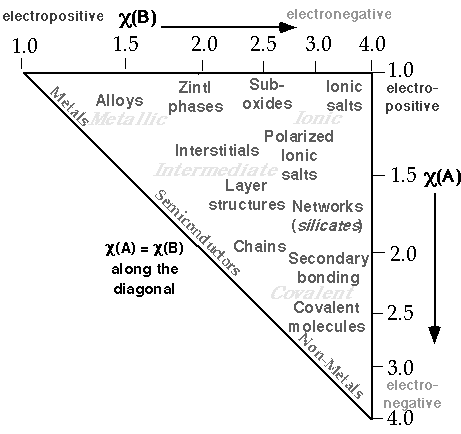
Stephen Lower's Virtual Textbook Version
Read more in Stephen Lower's Chem1 Virtual Textbook: States of Matter & Solids
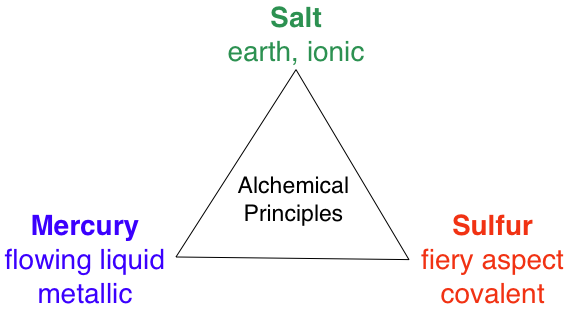
Triangle of Alchemical "Principles"
Ed Lisic, Professor of Chemistry at the Tennessee Tech University, made contact and wrote:
"Mercury, sulfur and salt are the three alchemical "principles", the Tria Prima, and they fit perfectly into a van Arkel-Ketelaar triangle.
- Sulfur is for fiery aspect
- Salt is for earth
- Mercury is for the flowing liquid aspect
"Sulfur, or covalent would be red, Ionic would be yellow or green, and metallic would be blue."
On this page little has been said about the materials intermediate between:
Metallic and covalent
Metallic and ionic
Covalent and ionic
This topic is developed on the next page, here. There is a software gadget here.
 |
 |
 |
| Electronegativity | Tetrahedra of Material Type |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.