Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| The Five Hydrogen Probe Experiments | Quantifying Congeneric Behaviour |
Congeneric Dots, Series & Planars
Congeneric behaviour – linear or predictable structure and reactivity traits, an extension of the well known idea of periodicity – is explored amongst the products of the hydrogen probe experiments, as discussed on the previous page. Sets of chemical species with similar structure and/or behaviour are identified.
Congeneric Behaviour Amongst Simple Species
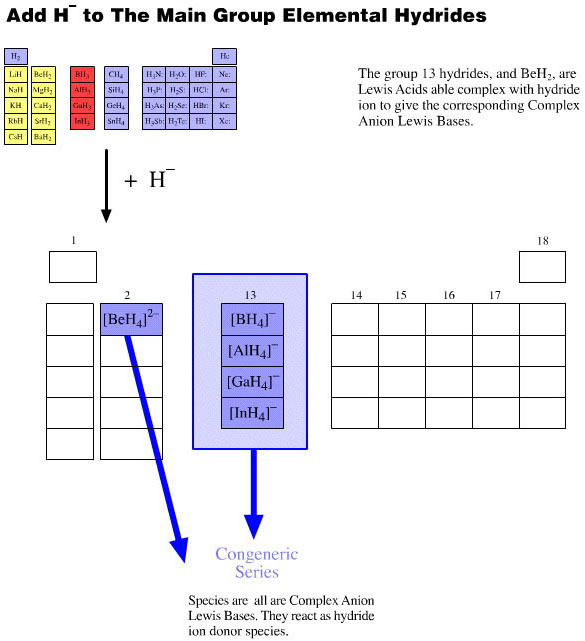
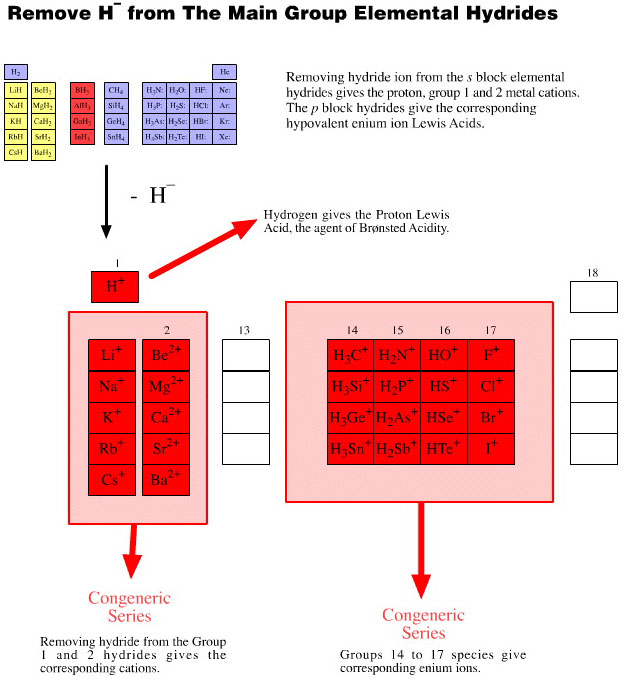
The main group elemental hydrides and the products of the hydrogen probe experiments can be explored for congeneric dots, series and planars, for types of reactivity behaviour and for regions linear reactivity:
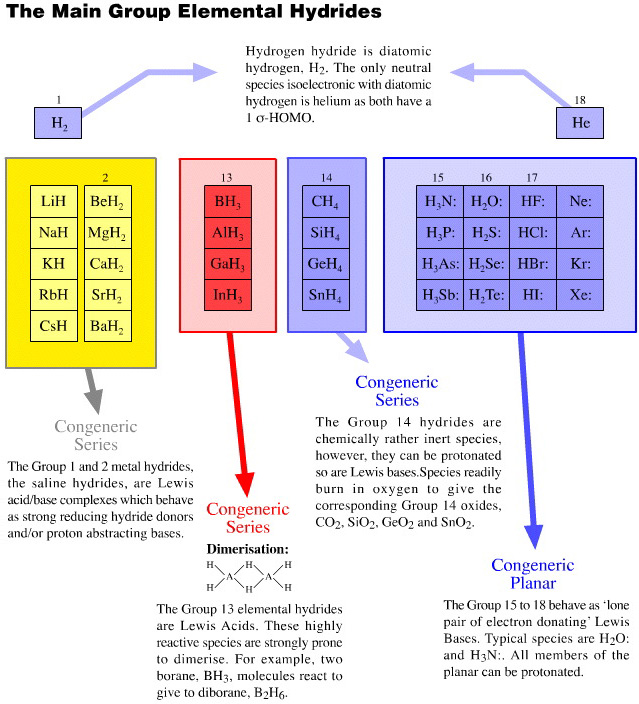
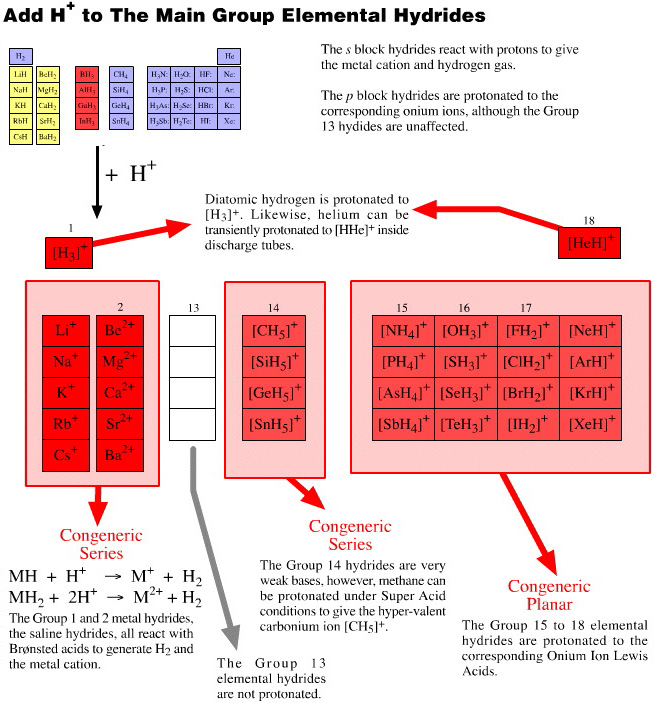
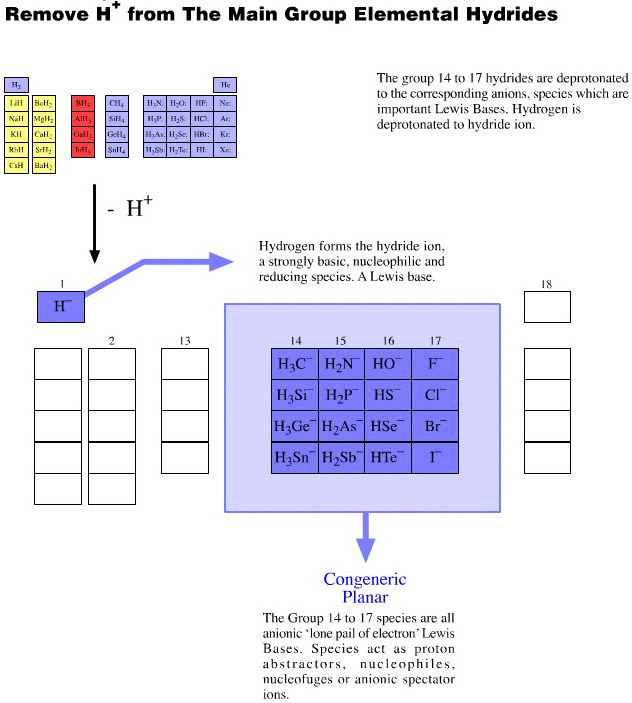
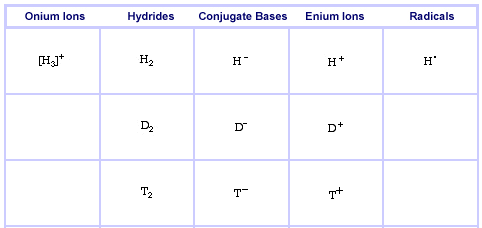
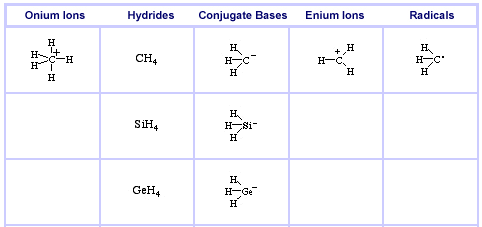
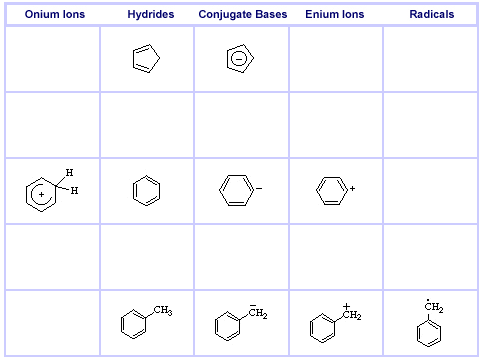
Onium Ions, Hydrides, Conjugate Bases, Enium Ions, Radicals and their Congeneric Series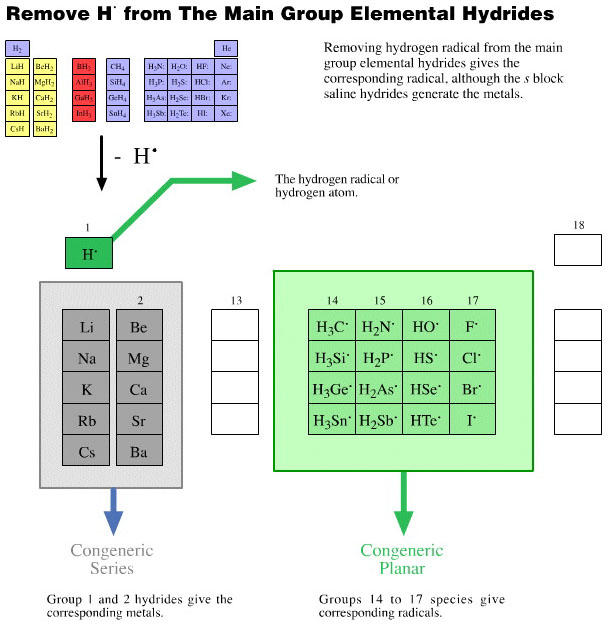
The hydrogen probe experiments generate several, general types of reactive species and associated reaction behaviour: onium Ions, anionic conjugate bases, enium Ions, radicals, metals and metal cations.
A word about onium ions and enium ions:
Onium Ions are hypervalent cations: the ammonium ion, [NH4]+, and protonated water, [OH3]+, also called the oxonium ion. All the onium ions produced by the hydrogen probe experiments are proton donating Brønsted acids. As cations, onium ions require anionic counter ions. The products are invariably ionic salts.
As part of the unfolding chemogenesis story, ionic salts – like ammonium chloride – are considered to be one of several types of Lewis acid/base complex. It follows from that onium ions are Lewis acids. Indeed, all positively charged species are Lewis acids.
Enium Ions are hypovalent cations. They are obtained by removing a negative ligand from a neutral species. Enium ion species are classic Lewis acids; like the isostructural Lewis acid borane, BH3, enium ions "only have six electrons in their valence shell", to use the arguments of Lewis octet theory.
The name "enium" comes from the George Olah who identified two types of "carbocation":
[CH5]+ is the carbonium ion
[CH3]+ is the carbenium ion
We follow the Olah system: Hypervalent cations are called onium ions and hypovalent cations are called enium ions. For example, Cl+ is the chlorenium ion.
But, we do not go so far as to rename the proton, H+, the hydrenium ion.
Collections of congeneric series from the periodic table groups show how onium ion, anionic conjugate base, enium Ion and radical characteristics have common features.
These collections of congeneric species show the rich patterns of behaviour that can be found within reaction chemistry space.
The Congeneric Array Database
Search for congeneric arrays using the database page, here. For example:
The Hydrogen Series, including Deuterium & Tritium, here:
The Group 14 Series, here:
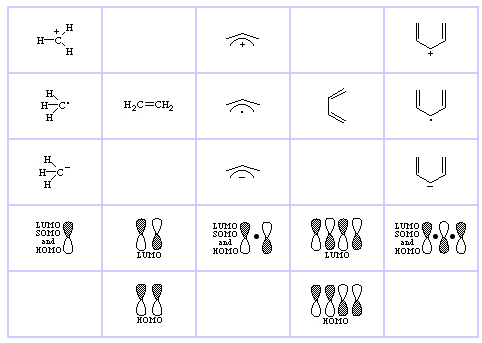
Linear Alkene Series, Methane (for comparison), Ethene, Propene & Butadiene, here:
Cyclic π-System Series: Cyclopentadiene, Benzene & the Benzyl System, here:
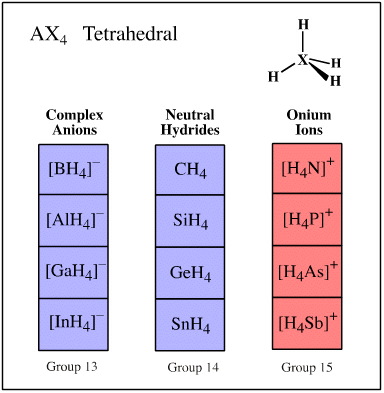
Grouping Congeneric Series by Geometry
Valence shell electron pair repulsion (VSEPR) theory recognises that the spatial arrangement of bonds around an atomic centre is correlated with the number of ligands and electron lone pairs, and that these are to be found at or near the vertices of regular polyhedra.
Ligand-electron pair configurations can be represented with the AXE system. Several geometries are commonly found:
- AX5 trigonal bipyramidal
- AX4 tetrahedral
- AX3 trigonal planar
- AX3E trigonal pyramidal
- AX2E2 angular
- AXE3 (only one ligand)
Examples of these sets are shown below:
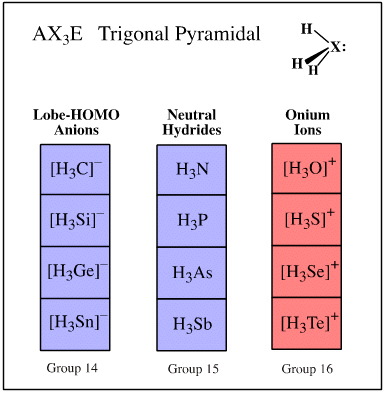
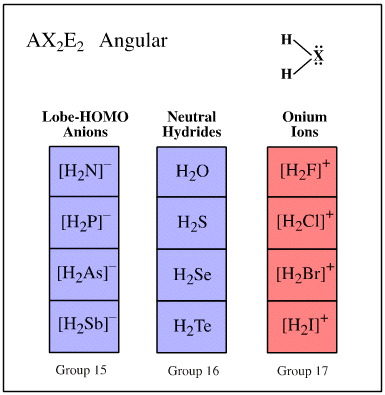
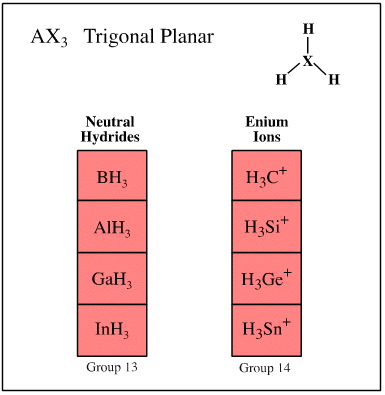
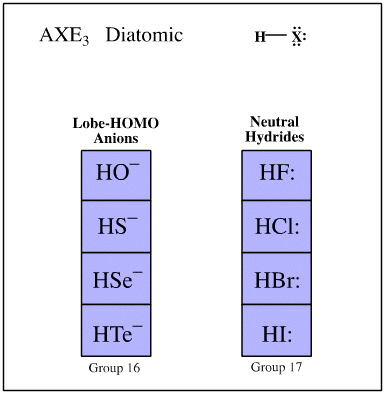
Search for congeneric arrays, here.
 |
 |
 |
| The Five Hydrogen Probe Experiments | Quantifying Congeneric Behaviour |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.