Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Chemistry & Complexity: Linear Systems | Videos |
Chemical Systems Prone to Complexity
The science of chemistry – chemistry, the whole thing – is analysed in terms of systems thinking over three pages of this web book. This third page deals with non-linear (unpredictable, complex, chaotic) chemistry systems.
Real Compounds, Broken Terrains

Image by Erik Tanghe from Pixabay
Consider the first 36 main group elements, hydrogen to barium, not as gas-phase mono-atomic species, but as chemicals in bottles: as reagent chemicals in their standard (thermodynamically most stable) state at 298 Kelvin and 100 kPa (25°C & 1.0 atm) pressure:
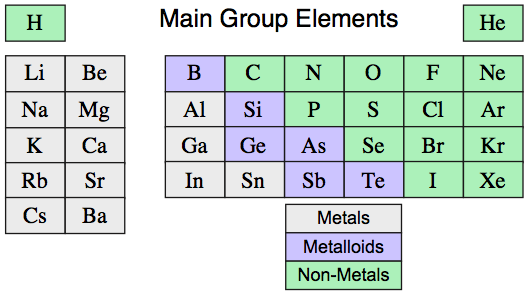
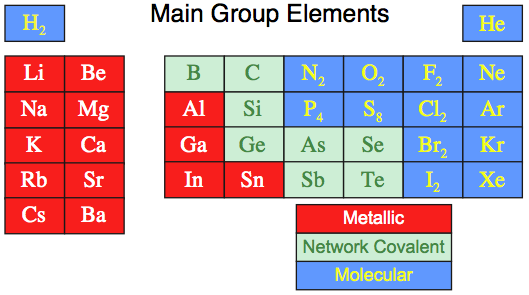
The bulk properties: structure, melting/boiling points, electrical & thermal conductivity conductivity, reactivity, etc., of the main group elements as materials are not predictable from first principles, and neither are they periodically related in a general way.
Consider the elements N, P, O & S in the standard state. All exist as molecules, but:
Nitrogen exists as N2
Phosphorus exists as P4
Oxygen exists as O2
Sulfur exists as S8Indeed, where is the regular change?, in:
Al (metal)
Si (network)
P4 (molecular)
S8 (molecular)
Cl2 (molecular)
Ar (single atom molecular)These are complicated materials.
At 25° and 1.0 atm (std. conditions, 298K & 100kPa), sulfur, due to bond length, bond angle, van der Waals radius, electronic and crystal packing considerations, prefers to exist in rings of exactly eight sulfur atoms, hence S8.
Phosphorus prefers to exists as four atom, P4, tetrahedra. Etc.
|
While it is relatively easy to explain after the fact "why" a particular allotropic form is stable under a particular set of conditions using the phase diagram, valence and molecular orbital theory... these arguments are invariably based on empirical (experimental) evidence and always end up being circular:
Should it have been the case that sulfur existed in S10 rings... well, we would have been able to explain that as well... |
Remove the standard state (298 Kelvin and 100 kPa) constraint, and things get far more involved as different elemental allotropes can be found which are stable (or meta-stable) under various conditions:
- Until 1991, only two carbon
allotropes were known: graphite and diamond. Since then many more have
been discovered, including: literally dozens of spherical and football
(rugby ball) shaped "buckyballs"
like C36, C60, C128,
etc., as well as various types of single & multi wall carbon nanotubes
(SWCNTs & MWCNTs).
- For many years it has been
predicted that hydrogen will form a metallic
phase at high pressure, however, this form of the element has not
yet been observed in the lab (writing in July 2005 and still in September 2010),
although there is plenty of indirect evidence that it exists deep inside
the gas giant planets Jupiter and Saturn. Hydrogen is the simplest element,
and if the phase diagram of any element is going to be predicted from
first principles it will be hydrogen (or helium).
- Oxygen has two allotropes
dioxygen, O2, and trioxygen or ozone, O3.
However, O2, is a diradical that can exist in several electronic spin states, each of which is a
distinct chemical species. Ground state triplet oxygen is paramagnetic
and is attracted to magnets, here.
Ozone is able to undergo 1,3-dipolar cycloaddition, a class of pericyclic reaction.
- In 1999 a new pentanitrogen
cation was announced, [N5]+, but unfortunately
not named (Christe et al, AngChem IntEd, 38, 2004-2009, 1999). I have
always wondered just how (un)stable the azide salt of this pentanitrogen
cation will be: "N-five azide", [N5]+ [N3]–. This material would be
a unique ionic allotrope.
- Napoleon's army had uniform
buttons made from metallic tin, Sn, but during the long march to Moscow
in 1812, the intense cold of the Russian winter transformed tin metal
into the mechanically weak network covalent allotrope, and the buttons
turned to powder...
- Etc., etc., etc... and we are just talking about elements.
Generally, it is not possible to deduce an element's equation of state or phase diagram from first principles; they must be experimentally determined.
As expected, chemical compounds are far more involved than the chemical elements. [At least they are usually are: the main group hydrides and congeneric series used in the chemogenesis analysis are notable exceptions.]
Oxides are particularly difficult. Consider, for example, the oxides of the period 2 elements: Li, Be, B, C, N, O, F & Ne. According to webelements, the set of period 2 oxides consists of:
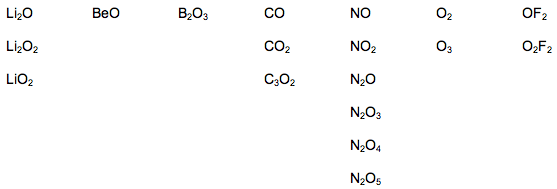
This set of chemical compounds clearly presents as an unpredictable "broken terrain". It is not possible to predict which period 2 oxides will exist from first principles. Indeed, it is not an impossible scenario that a new Period 2 oxide will be discovered. Should this is be the case, theory will be used to explain why the new compound is stable and how it is able to exist.
|
I predict that the carbon oxide C2O2, O=C=C=O, should exist from my knowledge of carbon dioxide, CO2 (O=C=O), and carbon suboxide, C3O2 (O=C=C=C=O), both of which are known. I can generate all three homologs in Spartan, a molecular modeling package employing high level (QM/HF/DF) theory. However, I know that chemists will take little or no notice of my prediction until it is verified by experiment, the new carbon oxide is made and its melting point determined. Prof. Harry Kroto predicted the existence of fullerene, C60 (Wikipedia), in 1985 after he observed an ion at m/e 720 by mass spectrometry, in a molecular beam experiment which created carbon clusters. This cluster had to contain 60 carbon atoms and he assigned the "football" structure, but scientists only took note – and awarded the Nobel prize – after the substance had been prepared in relative bulk in 1991. Thinking further... I suspect C2O2 will be susceptible to fragmentation, yielding two molecules of carbon monoxide, CO. Bother... |
• The set of all possible compounds is a huge, complex, broken terrain. It has been estimated that there are more that 1060 possible organic molecules with a molecular mass of 500 or less. Bohacek et. al., Med. Res. Rev. 16, 3-50, 1996
Amazingly, if just one molecule of each compound were to be produced, the total mass would nearly equal that of a million suns. Do the math:
- There will be more heavier isomers that lighter isomers, so assume the average molecular weight is close to 500.
- Total mass is (500 x 1060)/(6 x 1023) = 8 x 1035Kg
- The mass of the Sun = 2 x 1030Kg
- Therefore: (8 x 1035) / (2 x 1030) ~ 400,000 ~ half a million...
• The set of known compounds is strongly biased towards issues of biological, medical, military, commercial and/or academic interest. The three largest on-line chemical databases are:
CAS (the Chemical Abstracting Service) has a database of close to 127 million substances (as of June 2024), and about 10,000 more are added per day, that is more than three million a year.
Beilstein: 9 million organic substances
Gmelin: 2 million inorganic compoundsThe chemical industry produces vast numbers of compounds for testing, particularly in the areas of drug discovery and flavoring/fragrance. Industry probably has proprietary (secret) information on [intelligent guess here] half a billion chemical substances/compounds.
• The set of commercially available chemicals is represented by on-line databases.
The CAS developed CHEMCATS is a database of chemicals and suppliers. The database lists 8 million commercially available chemicals, 750 suppliers of commercial chemicals and 900 chemical catalogs
In the UK, the Available Chemicals Directory, hosted by the Chemical Database Service, lists 437,790 unique substances from 680 supplier catalogs, and the Screening Compounds Database lists 3.6 million compounds, but these are usually only available in very small quantities, down to 10 microgram quantities, from 20 or so specialist suppliers.
Of course, if you require a chemical that is not currently commercially available, somebody will happily make it for you, for a fee.
Conclusions:
Matter is complicated stuff and the sets of possible, known and available chemical compounds/substances form a broken terrain.
Chemistry is an experimental science. The existence of a compound can be predicted from theory, but until somebody actually makes the stuff, nobody will be interested.
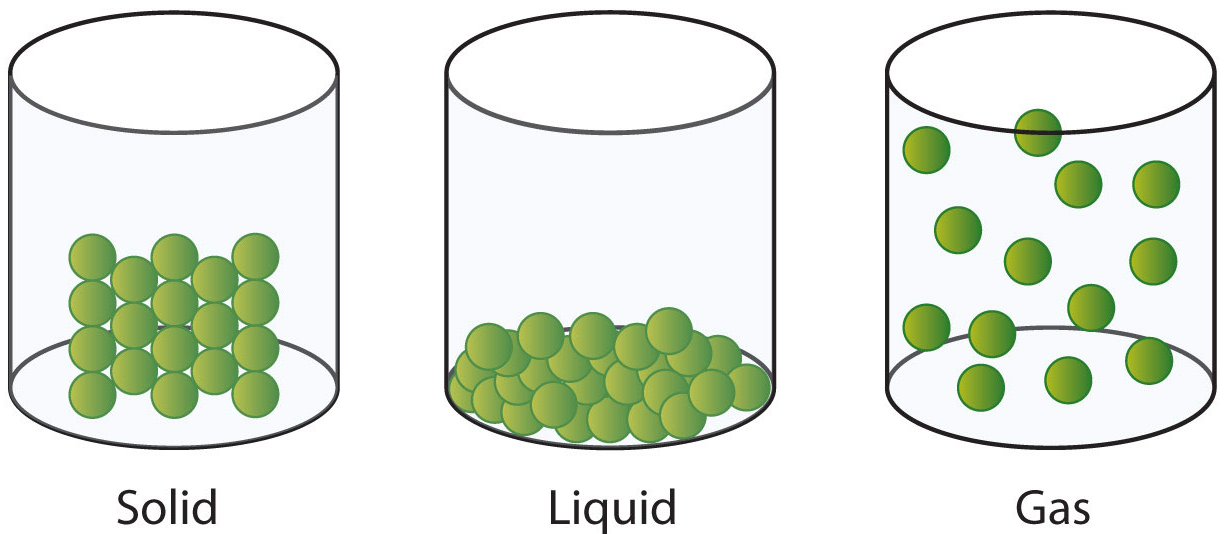
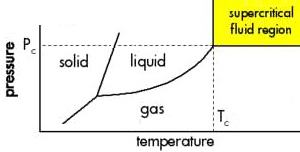
Gases, Liquids, Solids and the Phase Interaction Matrix
We learn about the solid, liquid & gaseous states of matter during in our very first science classes:
Solids:
• atomic,
ionic, molecular particles bonded together in a lattice
• particles are tightly
packed, usually in a regular pattern
• condensed
phase
• particles
vibrate – jiggle about – but generally do not move from place
to place
• samples
retain a fixed volume and shape
• not
easily compressed
• not
free flowing (unless granular, which is a special case)
Liquids:
• discrete
atomic or molecular particles
• particles
are packed closely together, but with no regular arrangement
• condensed
phase
• particles vibrate, and they are able to move about by "sliding
past" each other
• samples are fluids able assume the shape of the part of the container
which it occupies
• not easily compressed
• free flowing fluid: Navier-Stokes
Gases:
• discrete atomic or molecular particles
• particles are well separated and have no regular arrangement
• particles are able to move freely at high speeds, colliding with
container walls and each other
• samples are fluids able assume the shape and volume of the container
• compressible: PV = nRT
• free flowing fluid: Navier-Stokes
Later we learn to understand the physics and chemistry of gas, liquid and solid phases in terms of kinetic theory, thermodynamics and solution theory, as discussed in the section on linear chemistry here, and solid structure in terms of bonding theory, here and here.
So it is surprising to find out just how quickly complexity emerges in the gas-liquid-solid system when phases are mixed together.
Consider the Phase Interaction Matrix ('interaction table', a type of Karnaugh map):
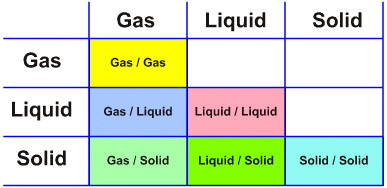
At first sight, there appear to be just six possibilities:
Gas/Gas A mixture of two gases
Gas/Liquid Mixture of a gas and a liquid
Liquid/Liquid Mixture of two liquids
Gas/Solid Mixture of a gas & a solid
Liquid/Solid Mixture of a liquid & a solid
Solid/Solid Mixture of two solids
But this is an illusion because phase interactions usually – but not always – lead to the formation of phase boundaries, heterogeneous systems and emergent complexity that is critically dependent upon the nature of the phase boundary surface.
Some points before we begin:
- Mixable fluid phases (gases and liquids) are very efficiently kneaded together by turbulence. Efficient intermingling makes a system homogeneous and simple. In other words, all parts are chemically identical.
However, using turbulent mixing to produce homogeneity is somewhat ironic because turbulence is itself a complex non-linear process that is here being used to smooth out the chaos and complexity associated with unstirred mixtures. One type of chaos destroying another.
- Gravity is
the opposite of turbulence and complexity. If there is a density difference
between two phases – and there usually is – AND one
or both phases are fluid, then gravity will act over time to separate
the phases. Mechanical agitation will speed this process.
- Two phase
systems can either consist of:
The same substance in different phases: ice and steam. In this situation the phase change processes are: melting, evaporation, sublimation, etc.
Two (or more different) substances: oil and water.
Examples of both types appear below. - Colloids consist of small particles of one phase dispersed in another phase. Colloidal size is typically 0.001 micron to 1 micron.
Milk and smoke are both colloids.
- The phase interaction processes discussed below are illustrated by phase separation methods and by technologies that exploit the mixed phase system under discussion, such as chromatography.
|
Gas/Gas: A mixture
of two or more gases such as air.
|
|
Gas/Liquid: Mixture of a gas and a liquid: clouds, foams, aerosols, sprays
|
|
Liquid/Liquid: Mixture of two liquids, which may be miscible or immiscible
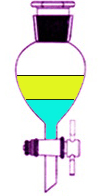
In terms of chromatography, solvents an be can be collected into an eluotropic series, non-polar-to-polar:
|
|
Gas/Solid: The mixture of a gas and a solid can take two forms: foam or smoke.
|
|
Liquid/Solid: The solid may be insoluble in the liquid, or it may be soluble, or an emulsion may form.
|
|
Solid/Solid: A mixture of two solids can result in a heterogeneous mixture or a homogeneous alloy.
In solid/solid alloys and heterogeneous mixtures it is not possible for mixing to occur because there is no mobile fluid phase. Therefore the only chemical reactions that can occur are temperature and/or pressure induced phase changes. Geologists and geochemists are particularly interested in how rocks are metamorphosed over geological time. The ice-water-steam system is exceedingly involved. This type of analysis is not new. Here is an old "like-dissolves-like" diagram from 1751 using old alchemical symbols: 
|
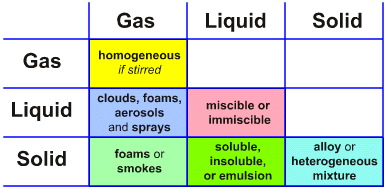
The rule of thumb is that homogeneous solutions are simple, whereas heterogeneous systems are complicated because they are dominated by the nature of the phase boundary. However, solutions – particularly aqueous solutions – can only be expected to be ideal (linear) when solute concentrations are below 0.1 molar. This situation is restricted to:
• Mixtures of gases (stirred)
• Mixtures of miscible liquids (stirred and where one component is < 0.1 molar)
• Solution of a solid in a solvent (stirred and where the solute is < 0.1 molar)
• Solid solutions (where one component is < 0.1 molar)At high concentrations, activity and fugacity "fiddle factors" have to be employed.
Linear behaviour is rare. Most phase interactions are non-linear, inherently complex and emergence abounds.
Isomers and Emergent Structure
As molecular size increases, so does complexity, and emergent new properties appear. Consider the linear and branched alkane alcohols, the alkanols. Above, it was argued that there is:
- One structural isomer of the C1 alcohol: methanol
- One C2 alcohol: ethanol
- Two C3 alcohols: 1-propanol & 2-propanol
- Four C4 alcohols: 1-butanol, 2-butanol, 2-methyl-1-propanol & 2-methyl-2-propanol
- Eight C5 alcohols...
However, some of the alkanols – the first example is 2-butanol, CH3CHOHCH2CH3 – have a chiral centre with four different groups attached about a tetrahedral carbon. The implication is that there are two ways (configurations) that the groups can attach to the tetrahedral centre and the molecule can exist as a pair of "handed" enantiomers or "stereoisomers". So, actually there is:
- One isomer – structural and stereo – of the C1 alcohol
- One C2 alcohol
- Two C3 alcohols
- Five C4 alcohols
- Eleven C5 alcohols
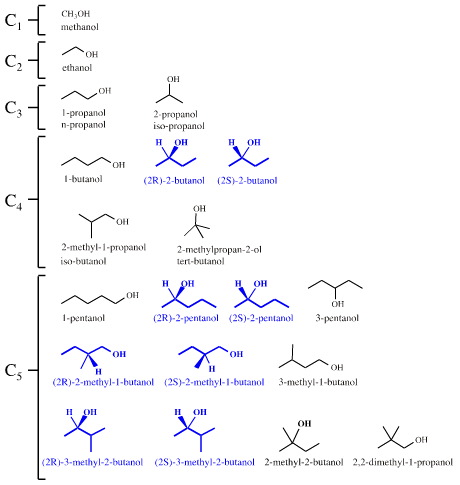
Enantiomers can only be distinguished in a chiral environment, but biology is very, very chiral and with just one exception (glycine): all amino acids, all nucleic acids, all sugars, all peptides, all proteins, all DNA strands and all organisms are chiral and are enantiomer selective. Biologically, (2R)-2-butanol is completely different to (2S)-2-butanol.
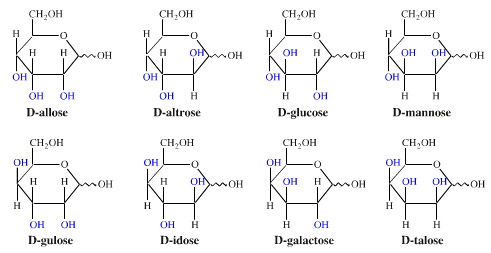
Molecules with more than one chiral centre exist as diastereoisomers (diastereomers), which unlike enantiomers are chemically distinguishable in an achiral (non-chiral) environment. Diastereomers have different melting points, different IR spectra, different reaction chemistry as well as different biology.
Consider the biologically important D-aldohexose sugars, a set which includes glucose. Each D-aldohexose has three variable chiral centres, which leads to a set of eight (23) D-diastereomers, each with a unique and distinct melting point, IR and H-NMR spectra, reaction chemistry and biochemistry:
As molecular size and complexity increases, further degrees of freedom emerge. For example, molecular structure cannot be fully described in terms of bond lengths, three atom [X-Y-Z] bond angles and chiral centre configurations. As illustrated by hydrogen peroxide, HOOH, here and specifically, here, the four atom dihedral angle emerges as an ever more important parameter:
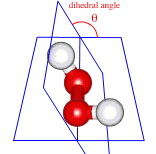
Variations in dihedral angle lead to conformational isomers, and variations in dihedral angle with time lead to rotormers and dynamic molecular structures. For example, at room temperature, cyclohexane, C6H12, exists as dynamic mixture of interchanging chair, boat and in-between twisted boat conformations, with attached groups adopting axial or equatorial positions:

Most pharmaceutical agents are smallish molecules (GFW = 50 - 300) that are able to enter enzyme active sites or hormone receptor sites where they "mimic" – more technically, agonise (positive effect) or antagonise (negative effect) – the local biosystem in some way.
These days much drug discovery research involves modelling how substrate molecules and synthetic agonists & antagonists interact with and effect the active site, a process that involves understanding the conformation(s) and dynamic conformational changes adopted by molecules while in or bound to the active site.
An example of the software available to pharma industry scientists is Omega by OpenEye Scientific Software. Omega is able to "generate multi-conformer structure databases so that conformational expansion of drug-like molecules can be performed". Below are two [of thousands of possible] overlaid conformers of the cholesterol-lowering medication Lipitor:
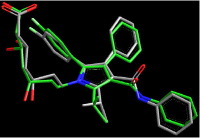
Conclusion:
As molecular structures grow in size and complexity, emergent properties like diastereoisomerism and dynamic conformation become ever more important.
Mechanistic Pathways
All reaction mechanisms can be deconstructed into sequences of STAD (substitution-transfer-abstraction-displacement) steps, as discussed here, although it is usually more useful to consider reaction mechanisms in terms of unit mechanistic steps and sequences of unit steps, as discussed here:
Complexation
Fragmentation
Substitution
Insertion
Pericyclic processes
Metathesis
Addition
Elimination
Rearrangement
Name Reactions
Many factors determine when an particular mechanism is able to come into play, and mechanisms can compete with each other. This is nicely illustrated by the common undergraduate laboratory preparation of tertiary butyl chloride (2-chloro-2-methylpropane), (CH3)3CCl:
Method: Tertiary butanol (2-methylpropan-2-ol), (CH3)3COH, is carefully mixed with concentrated hydrochloric acid in a separating funnel. After a few minutes an upper organic layer appears. After 30 minutes the lower aqueous layer is discarded, and the upper organic layer is washed with aq. base/water, dried and distilled to give tertiary butyl chloride, (2-chloro-2-methylpropane), (CH3)3CCl.
The yield of tertiary butyl chloride is always low. One reason is because the reaction proceeds via a first order nucleophilic substitution, SN1, mechanism, a pathway that has a tertiary carbenium ion (3° carbocation, R3C+) species as an intermediate. The 3° carbenium ion can react in two ways: either it can complex with chloride ion nucleophile, Cl–, to give (CH3)3CCl, or it can lose a proton, H+, to form methylpropene.
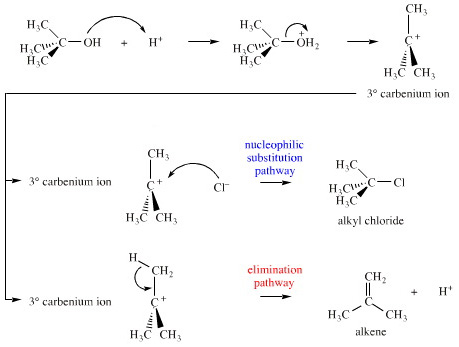
The ratio of alkyl chloride to alkene is dependent on everything: temperature, solvent polarity, mode of addition, relative and absolute concentrations, rate of stirring, etc.
Reactions can give fast forming kinetic products, or the more stable thermodynamic products. This effect is illustrated with the sulfonation of naphthalene giving 1-naphthalene sulfonic acid and/or 2-naphthalene sulfonic acid:
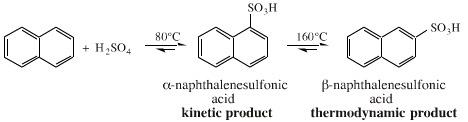
Under moderate temperature conditions (~80°C) and short reaction times the 1-naphthalene sulfonic acid is formed. This is the kinetic or "fastest forming" product.
At higher temperatures (~160°C), the less sterically hindered and more stable the 2-naphthalene sulfonic acid is formed.
Molecular conformation can influence chemical reactivity.
Alkyl chlorides can undergo base catalysed E2 elimination to give an alkene and HCl. The base commonly used is ethoxide ion in a solvent of ethanol.
There is a rule – explainable in terms of frontier molecular orbital (FMO) theory – that the H and the Cl atoms must be "diaxial" (at 180° with respect to each other) before the E2 mechanism can occur. This constraint is not an issue with small molecules like 2-chloropropane, CH3CHClCH3, where bonds are free to rotate, but things are rather more involved with chlorocyclohexanes.
Consider neomenthyl chloride (below). There is a large, bulky isopropyl group, –CH(CH3)2, that prefers to sit in an equatorial position about the cyclohexane ring, an influence that dominates the ring conformation. One effect of this conformation is that the H and the Cl are forced into axial positions that are 1,2 relative to each other. The result is fast base mediated E2 elimination to give 1-isopropyl-4-methylcyclohexene:
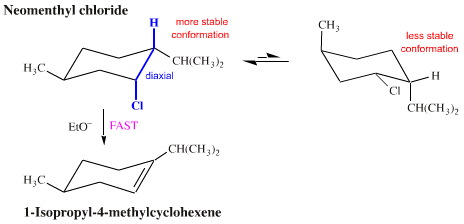
However, in menthyl chloride, a diastereoisomer of neomenthyl chloride, the chlorine is held in an equatorial position by the bulky isopropyl function. In this situation, it is only when the ring (occasionally) flips into another conformation that the chlorine finds itself 1,2-diaxial with respect to a hydrogen. The result is that menthyl chloride undergoes base mediated E2 elimination at only 1/600th of the rate of neomenthyl chloride, and the product is a different isomer, 3-isopropyl-6-methylcyclohexene:
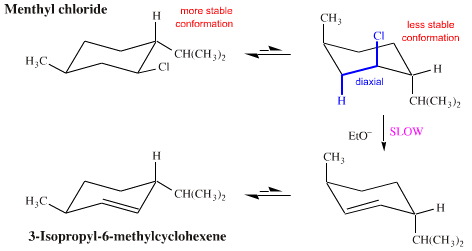
Ph.D. students with research projects looking at target synthesis or synthetic methodology learn to explore how configuration and conformation influence competing mechanistic pathways.
Conclusion:
The previous page of this web book, here, introduced the idea of the mechanism matrix. However, the proposed schema – which looked plausible – simply cannot be mapped to the complex terrain of reaction chemistry space.
Also, the chemistry presented in textbooks simply does not reflect the reality of low yield reactions where products are often accompanied by large quantities of ill defined black gunk.
Non-Equilibrium Thermodynamics
While systems at equilibrium and approaching equilibrium are predictable and linear, as discussed here, non-equilibrium systems are a rich source of complexity, emergent behaviour and chaos.
There are two main ways of producing a non-equilibrium thermodynamic state in a chemical system: use electrical or photon stimulation rather than thermal (heat) energy to excite the system, or, heat a system locally, but do not stir.
Photon Stimulation
One of the central ideas of equilibrium thermodynamics is that of the Boltzmann distribution of particles between two (or more) states, i and j. The rule is that at thermal equilibrium there will always be more species in the lower energy state i than the higher energy state j, here.
However, if species are excited from i to j is by a light source with a wave length/energy that exactly matches the energy difference i and j (j – i), then a population inversion will be set up with more species in the higher energy state j. This is a non-equilibrium system, and the Boltzmann equation does not hold.
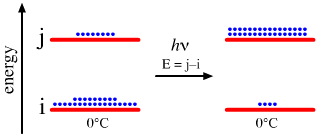
• Laser operation requires population inversion. The laser "works" by species in the higher energy state falling to the lower state and emitting a photon of energy j – i. This photon stimulates adjacent species in the higher state to emit their photon as well – as predicted by Einstein – producing a cascade of coherent light, where "coherent" light is all of the same frequency and is in phase.
• According to classical equilibrium thermodynamics, it should not be possible to form ozone, O3, from dioxygen, O2:
3O2 → 2O3
because the Gibbs free energy for this process is positive and anti- spontaneous at all temperatures. The reaction is thermodynamically "disallowed" on both enthalpy and entropy grounds. In fact, the reverse ozone to oxygen process, 2O3 → 3O2, has a negative Gibbs free energy at all temperatures.
However, ozone can be formed when O2 is exposed to short wavelength UV light, the process generates the ozone layer high in the atmosphere.
O2 + UV photon → 2O•
O• + O2 → O3
Photochemistry, here, and other methods of non-thermal excitation are widely exploited.
Local Heating
If a layer of fluid is heated from below, the density at the bottom layer becomes less than at the top layer due to thermal expansion. Experiments, carried by Bernard in 1900, showed that hexagonal convection cells develop, here:
An image of Bernard cells, captured of from Georgia Tech:
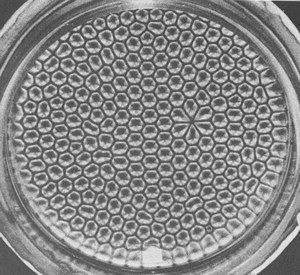
The appearance of Bernard cells is an example of order out of chaos. The local heating causes entropy to increase, but the density inversion induces complex & non-linear behaviour: convection cells that arrange into a regular hexagonal lattice, classic emergent behaviour. This is exactly analogous to the one dimensional waves of slowing and speeding found on highways at high traffic densities. As discussed in the intro to this section here.
The implication of Bernard convection is that local heating can cause complex emergent behaviour. To eliminate such effects it is necessary to stir (turbulently mix) the fluid and render the system homogeneous.
The Sun also heats our planet with thermal radiation, but the atmosphere is large and it has a spherical geometry, so it cannot be mixed to homogeneous. Indeed, on Earth, the Bernard cells present as ocean currents and weather systems.
Life on EarthOur planet is in a non-equilibrium thermodynamic situation with respect to the Sun. The Sun heats us and bombards us with high energy photons (light) that are exploited by photosynthetic systems in cyanobacteria and plants to convert carbon dioxide into glucose and oxygen. The implications of non-equilibrium thermodynamic systems generating complexity are both extraordinary and profound, as eloquently summed up by Prof. Peter Atkins of Physical Chemistry textbook fame on the BBC:
The simplest living cells consists of just a few hundred chemicals: amino acids, sugars, metabolites, intermediates etc., as well as the molecular biology apparatus for replication and protein synthesis. However, the various enzyme catalysed biochemical processes are not at thermodynamic equilibrium. The emergent complexity of the cell is due to it being a non-equilibrium system. A dead cell that is at equilibrium. |
For a technical discussion of non-equilibrium thermodynamics go to the Wikipedia page, here.
Conclusion:
Non-equilibrium systems are prone to develop in complex ways that exhibit emergent behaviour.
Diffusion Controlled Processes
If the following reagents are added together in a flask:
• Malonic acid - 0.2 M/L
• Sodium bromate - 0.3 M/L
• Sulfuric acid - 0.3 M/L
• Ferroin - 0.005 M/L
and the reaction mixture is stirred vigorously, an oscillating Belousov-Zhabotinsky reaction (BZR) is set up:
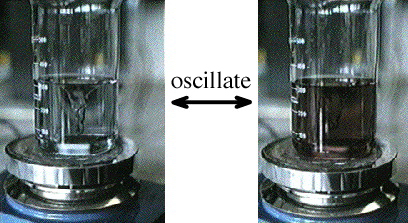
The BZ reaction is an auto-catalytic reduction-oxidation (redox) process. Each step of the multi-step reaction sequence generates a catalyst that speeds up the counter reaction:
As the reduction step proceeds it produces more and more catalyst to speed up the oxidation, and vice versa.
The reduced state reaction is red while the oxidised state is a pale brown.
But if the reaction system is not stirred, diffusion control takes over. This can be observed when the reaction mixture is prepared as a thin film, 0.5 - 1 mm deep, to avoid any vertical mixing, and waves of reaction can be seen propagating through the unstirred mixture:

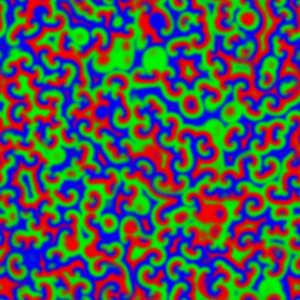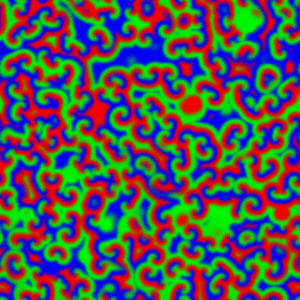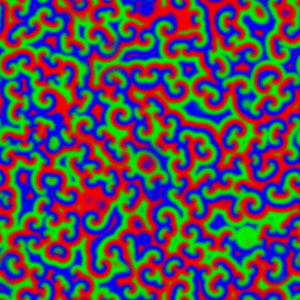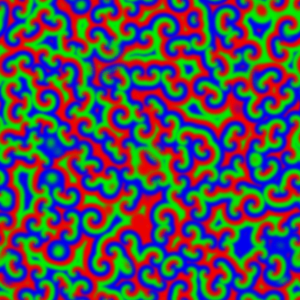
For a chemical reaction between X and Y to proceed, the chemical species first have to meet each other in the reaction medium. In an unstirred reaction medium, the rate at which the various chemical species move, meet and react is limited – and so controlled – by the speed that the species diffuse through the reaction medium. The effect the BZ type auto-catalysis is to cause the rather dramatic moving waves, and these are made visible by the colour change.
There are many resources about the BZ reaction and other non-linear process on the web:
- Rubin R. Aliev at MUSC
- Peter Ruoff's at University of Stavanger, Norway
- The Non-Linear Kinetics group at Leeds University
- A google search, here
An example of order out of chaos, is a mercury 'beating heart' where the various processes lead to a regular pulse. Such processes are exceeding rare in chemistry space, and are always of interest:
Molten materials and super saturated solutions have a thermodynamic tendency for form crystals, however, crystal growth can be highly complex.
The following images and accompanying text are captured from www.materials.leeds.ac.uk/themes/Non-Equilibrium.htm:
"Many pure metals, alloys [and other materials] solidify by the growth of complex crystals called dendrites. Unlike most inorganic crystal which display a faceted interface, dendrites exhibit a smooth, continuously variable interface, which displays multiple branching. This leads to the formation highly complex growth patterns."

The left image shows a theoretically modelled dendritic crystallisation that is is described as "seaweed". The image to the right shows the "as-solidified microstructure of Cu undercooled by 280 K". From here.
"Dendrites may undergo a kinetically induced instability which results in repeated multiple tip splitting, or doublon formation":

Heterogeneous catalyst efficiency can be boosted if the active material is deposited on a solid support with a fractal surface:
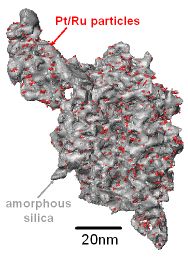
"Surface render of Ru10Pt2 catalyst particles (shown in red) anchored on and within a disordered porous silica support (grey). Data was obtained via tomography, an electron microscopy technique that generates a three-dimensional volume from a series of two-dimensional projections taken at different angles. A fractal analysis (performed using standard box-counting algorithms) revealed that the fractal dimension of the surface to be 2.4 +/- 0.1. The number of catalyst particles accessible to reactant gas molecules will therefore depend on their size according to this fractal distribution of surface sites, strongly influencing catalytic activity and selectivity." EPW Ward, T J V Yates and P A Midgley, Department of Materials Science, University of Cambridge.
Conclusion:
Chemists usually like to work with turbulently mixed, homogeneous solutions so as to avoid diffusion controlled artifacts. Materials scientists are not so lucky, and they often have to work under diffusion controlled conditions.
Experiential Design: Time, Gravity, Geometry, Temperature, Chemical Potential, Physical Separation
Chemists use clever experimental design – implemented using laboratory equipment constructed from glass, stainless steel, rubber tubing and Teflon – that exploit time, gravity, geometry, differential temperature, chemical potential, physical separation, etc., to influence the outcome of reaction and separation processes, usually to maximise the % yield or separation of a particular material or compound and/or to minimise the formation of unwanted by-products and contaminants.
There as a number of standard pieces of laboratory equipment: flasks, condensers, syringes, pipettes, burettes, separating funnels, filters, Bunsen burners, Dean & Stark traps, etc., (in different sizes). Bespoke equipment is developed as well. This lab apparatus dramatically increases the complexity of reaction chemistry space.
As a first approximation, the reaction system is the system universe, here. But as soon as the reaction system is constrained to a physical experiment, the reaction system is bound by the experimental design.
Some examples – taken from literally thousands of possible examples – of how intelligent design is used to optimise a reaction system space in various ways:
The Bunsen Burner
Methane burns in air, but in the Bunsen burner, methane is premixed with air to create a mixture that burns with a hot, smokeless flame. The Bunsen burner is carefully designed and constructed to maximise localised heating.

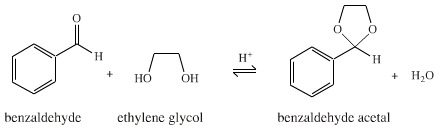
The Dean & Stark Trap
Benzaldehyde reacts with ethylene glycol (1,2-dihydroxyethane) to form the cyclic acetal (2-phenyl-1,3-dioxolane). The reaction is an equilibrium process, and the cyclic acetal product can be formed in 100% yield if the water by-product is removed as it is formed:
One very convenient and elegant method for removing the water from the reaction is to use an azeotroping solvent, such as toluene, with a Dean & Stark trap:
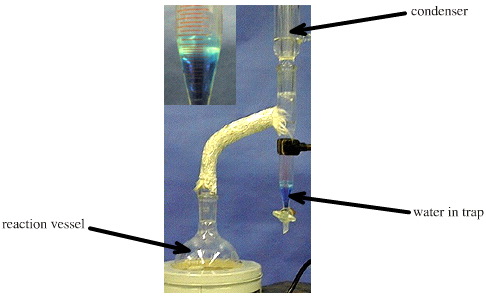
The water produced in the reaction is co-distilled with the refluxing toluene solvent with which it forms a vapour phase azeotrope. The toluene/water vapour phase complex rises into the condenser where it cools and condenses. However, as liquids toluene and water are immiscible and they separate into two phases. The denser water (containing a blue dye in the photo above) collects in the "trap", and the toluene - with water removed - returns to the main reaction vessel.
With this experimental design: gravity, temperature are geometry are exploited.
• Gravity is required to set up two density gradients:
• The hot vapour phase azeotrope rises into the condenser.
• Immiscible water and toluene separate in the trap.Indeed, the Dean & Stark apparatus would not work in the zero gravity of the International Space Station.
• Temperature and temperature differences are required to:
- Overcome the activation energy and speed up the reaction.
- Form the refluxing reaction mixture and produce the vapour phase azeotrope.
- Condense the vapour phase azeotrope into the two immiscible liquids, water and toluene.
• The geometry of the Dean & Stark trap physically separates the water generated in the reaction and so drives the equilibrium position to the formation of the acetal.
A experimental set up with a simple reflux condenser would not drive the equilibrium to the right by removing the water.
Time
Temporal (time) considerations usually involve exploiting differential rates of reaction, where "rate" is measures of moles of material transformed per unit time.
Cyclopentadiene is a reagent commonly used in the study cycloaddition chemistry (here), and organotransition metal chemistry.
However, the cyclopentadiene monomer is in equilibrium with the dimer. At room temperature the dimer is stable and over just a few hours the cyclopentadiene monomer will completely dimerise.
Therefore, before use the cyclopentadiene dimer must be cracked to the cyclopentadiene monomer:
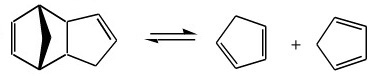
To use cyclopentadiene in a reaction it is necessary to heat the dimer at reflux (170°C) for a couple of hours to "crack" the dimer to the monomer. The pure monomer is distilled off and used immediately, and before it has time to re-dimerise. The rate of dimerisation can also be reduced by dilution in a suitable solvent.
Chemical Potential (Reagent Strength)
Benzene is nitratrated to nitrobenzene with nitrating mixture, a mixture of concentrated sulfuric acid and concentrated nitric acid. Nitrating mixture produces as equilibrium concentration of nitronium ion, [NO2]+, the electrophilic agent which partakes in the electrophilic aromatic substitution of benzene.
However, vary in their reactivity towards nitration. Activated aromatics like phenol are nitrated far more easily than benzene and the reaction can be carried out with dilute nitric acid. Other aromatic compounds require more forcing conditions (or are not stable in nitrating mixture) and for these situations the highly potent nitronium tetrafluoroborate can be used. This ionic reagent consists of the tetraborate ion, [BF4]–, stabilised salt or nitronium ion, [NO2]+.
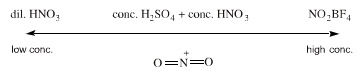
The chemical potential of the nitrating agents increases:
dilute nitric acid << nitrating mixture << NO2BF4
Another example. It is a common procedure in synthetic chemistry carry out the nucleophilic substitution of a nucleofugal leaving group at an acyl carbon centre. (This mechanism is also referred to as addition-followed-by-elimination and the tetrahedral mechanism.) It is known that leaving group ability – the ease with which X– is lost – correlates with the pKa of the leaving group's conjugate Brønsted acid. With this knowledge in mind it is possible to develop a range of substituted acyl species which under go ever more facile substitution with a nucleophile.
Below are shown methyl ester (least reactive), anhydride, mixed anhydride & acid chloride (most reactive):
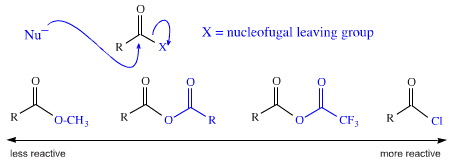
Reagents with greater chemical potential partake in more exothermic reactions. For example, a methyl ester can be converted into an ethyl ester by adding excess ethanol and a bit of H+ catalyst (a couple of drops of conc. H2SO4 or 0.1g of para toluene sulfonic acid). No heat will be generated as the Keq is about 1.0. However, if ethanol is added directly to acid chloride will be a great fizzing as the reaction gets hot because it is very exothermic.
Conclusion:
Reaction process environments can be designed chemically, spatially, thermally and temporally.
For most reaction systems, chemists are able to prepare (or buy) a reagent of suitable power and reactivity.
Synthesis
For a chemical species to "exist", two conditions must hold:- The species must be
stable over a defined time scale and/or set of conditions. For example, benzyne is an interesting
an useful chemical species that "exists", but it is only transiently
stable at room temperature and is only known as a reactive intermediate
in solution. These days many chemical reagents are available that are
long lived, but that are not stable when exposed to air and/or moisture.
Examples of such compounds would be lithium aluminium hydride, LiAlH4,
and methyl cyanoacrylate, otherwise known as "Superglue".
- There must be a method of making – synthesizing– the species in question. In other words there must be a suitable reaction mechanism (atom-to-atom mapping) available AND there must be practical reaction conditions available to make the compound AND under these conditions there must not be any alternative reaction mechanism available which will render any intermediates or the final product unstable.
A pertinent paper titled "What Is the Smallest Saturated Acyclic Alkane that Cannot Be Made?" (de Silver & Goodman, J. Chem. Inf. Model. 2005, 45, 81-87), examines this very question with respect to branched alkanes, exemplified by the structures below:
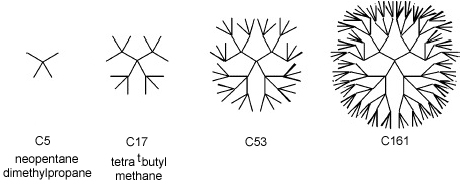
The C5 (five carbon) molecule above, neopentane, is well known but the C17 homolog (3,3-bis-(1,1-dimethylethyl)-2,2,4,4-tetramethylpentane) is unknown. The paper's authors write: "[The C17 molecule] has never been synthesized, and studies show that it must be extremely strained. However, this is not a proof that it is impossible to prepare. Indeed, molecules which include this carbon framework have been synthesized.. and their existence suggests preparation [of C17] may be possible."
It was argued above that the set of compounds is a complex, broken terrain. That being the case, the synthetic route to a specific compound must involve a complex path through the broken terrain exploiting intermediate compounds to act as way points up the mountain.
Synthesis is analogous with the well known children's game of Snakes & Ladders – or Chutes & Ladders – where ability to produce a material or compound is a synthetic ladder, and lack of stability is an anti-synthetic snake. In reaction chemistry space there are few ladders but many, many snakes.
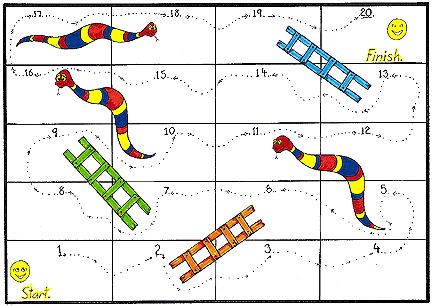
Consider a couple of related examples from industrial inorganic chemistry: the extraction of the metals iron, Fe, and titanium, Ti, from their oxide ores, Fe2O3 and TiO2. Both oxide ores are common and both can be reduced by carbon at high temperature.
Extraction of Iron
The overall iron(III) oxide plus carbon reduction reaction is:
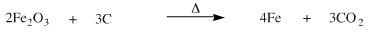
This reaction process represents a "synthetic ladder": at high temperature (1200°C) the reducing ability of the carbon and the entropy driven loss of gaseous CO2 is able to drive a redox reaction to give liquid iron metal. (The pig iron produced by a blast furnace must be further purified to steel and wrought iron, but this is not the issue here.)
Extraction of Titanium
Titanium(IV) oxide can also be reduced by carbon to titanium metal and carbon dioxide, however, the hot titanium reacts with carbon to form titanium carbide, TiC.
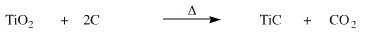
Even small amounts of TiC impurity make titanium metal very brittle and unusable. The titanium carbide cannot be removed, and its formation is an "anti-synthetic snake". As a result, the simple, direct carbon reduction reaction pathway cannot be used.
An alternative pathway is employed by industry, the Kroll process. The carbon reduction reaction is carried out in the presence of chlorine, Cl2, to form titanium(IV) chloride, TiCl4, and carbon dioxide. A great advantage of this method is that titanium tetrachloride is a low boiling point molecular liquid, bp 136°C, which can be distilled to very high purity. In the second synthetic step, titanium tetrachloride is reduced with sodium or magnesium metal to give high purity titanium metal and sodium (or magnesium) chloride.
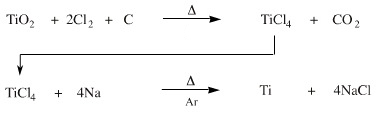
That is not quite the end of the story because there are other anti-synthetic snakes: titanium tetrachloride is readily hydrolysed by water, at elevated temperature the titanium metal is rapidly oxidised by air back to titanium(IV) oxide and hot titanium even reacts with nitrogen, N2, to form titanium(III) nitride, TiN. As a result, air and moisture have to be rigorously excluded, and the second-step processes are carried out under an atmosphere of inert argon.
The major downside of the two-step synthetic route to titanium is that it is expensive, not because of the use of argon which can be recycled, but because two moles of chlorine and four moles of sodium are consumed for each mole of metal produced; the process is said to have a poor atom economy. However, titanium is so useful as a material that the expense of the extraction process can be adsorbed.
The thirteen-step synthesis of vitamin A, by O. Isler et al (1947), shows a long and complex synthetic pathway:
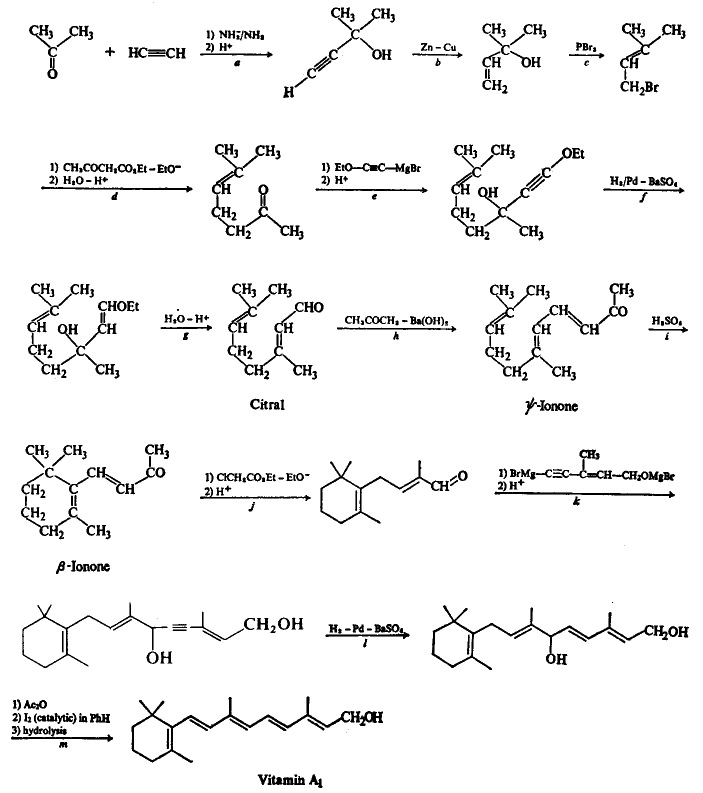

O. Isler et al, Helv. Chima. Acta, 30, 1911(1947)
R. O. C. Norman, Principles of Organic Synthesis, Chapman and Hall, 1978, pp 711
The reason why the total synthesis of natural products is so tantalising to the cognoscenti is that Nature throws up some extraordinary structures, and the biosynthetic route – if known – is usually little guide to developing a successful laboratory route.
And, of course, the beauty of the natural product is that there can be no doubt that the species really does exist, so lack of stability arguments cannot be used!
R.B.Woodward, the greatest synthetic chemist of the 20th century, was heard to say while his research group was attempting a particularly difficult synthesis "If we can't make strychnine, we'll take strychnine."
Or, as more put generally by the scientific philosopher, and advocate of Darwin, T.H. Huxley (1825-1895): "The great tragedy of science - the slaying of a beautiful hypothesis by an ugly fact"
Conclusion:
The set of possible compounds is a broken terrain, and the individual species that occupy this space can act as stepping stones or way points, from which multi-step synthetic routes are constructed.
Within the broken terrain there will be compounds which in principle exist but which in practice cannot be made, however, this can never be proved.
This brings in some philosophy. Karl Popper introduced the idea of falsifiability in science, the notion that a scientific idea can only proved to be false, not true: "All crows are black because [insert your theory here]". Find one white crow, and the black crow theory is false.
Synthesis of unnatural target compounds turns this idea round because there are many ways not to make something. It follows that it is impossible to prove that a compound cannot be synthesized, only that it can.
Finding a synthetic route – a series of synthetic ladders – in reaction chemistry space that avoids all of the anti-synthetic snakes is very difficult, and in the 20th century target synthesis represented the pinnacle of chemical science. Will this continue in the 21st century?
Chemistry & Complexity: Summing Up
Chemistry is – and will remain – an experimental science because predictions can seldom be made from first principles due to the inherent complexity.
When predictions are made from theory they always need to be verified in the laboratory.
There is an excellent & recent RSC paper (2008) that discusses these matters:
R. Frederick Ludlow & Sijbren Otto
Systems Chemistry
Chem. Soc. Rev., 2008, 37, 101-108
 |
 |
 |
| Chemistry & Complexity: Linear Systems | Videos |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.