Periodic Table |
 |
 |
 |
 |
 |
 |
 |
Lewis Acid/Base Interaction Matrix Database
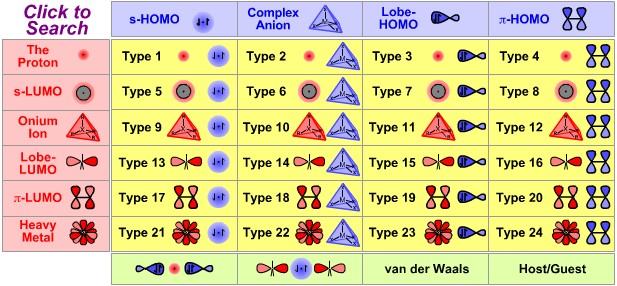 |
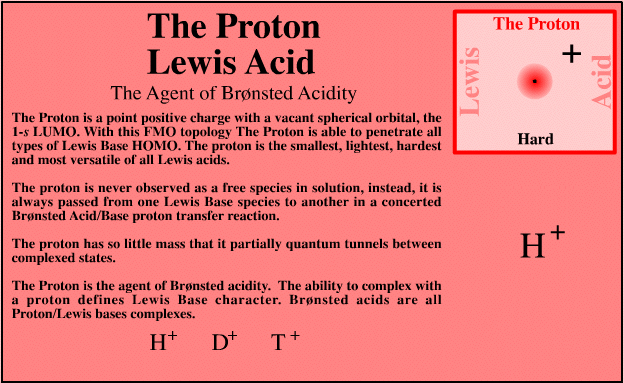
The Proton Lewis Acid
| The Proton | 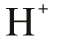
Search for proton Lewis acid species in The Chemical Thesaurus |
| FMO Topology: | The proton is a point positive charge with a vacant spherical orbital, the 1s LUMO. This geometry enables the proton to penetrate all types of Lewis base HOMO topology. |
| HSAB: | Intrinsically very hard |
| Chemistry: |
The proton is the smallest, lightest, hardest and most versatile Lewis acid. However, the proton is never observed free (in chemistry at least, high energy high vacuum physics is different). The proton is always passed or transferred from one Lewis base to another in a concerted Brønsted acid/base proton transfer reaction. The proton has so little mass that it (partially) quantum tunnels between complexed states, and the ability of a species to complex with a proton defines Lewis base character.
The Ka and pKa of are a measure of Brønsted acid strength with respect to water. As the Lewis acid H+ remains constant, the terms Ka and pKa are a measure of a conjugate (Lewis) base's affinity for H+ with respect to the standard Lewis base water, :OH2. |
| Congeneric Series: | H+ D+ T+ |
| Proton Lewis acid (generic) |
|
|
|
Deuteron more here |
|
|
Proton more here |
|
|
Tritium cation more here |
 |
 |
 |
| Poster | Nucleophiles & Bases |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.