Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
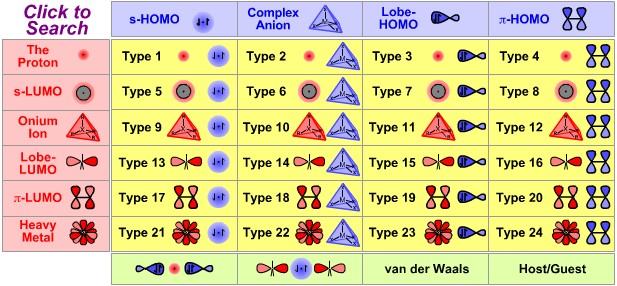
Lewis Acid/Base Interaction Matrix Database
 |
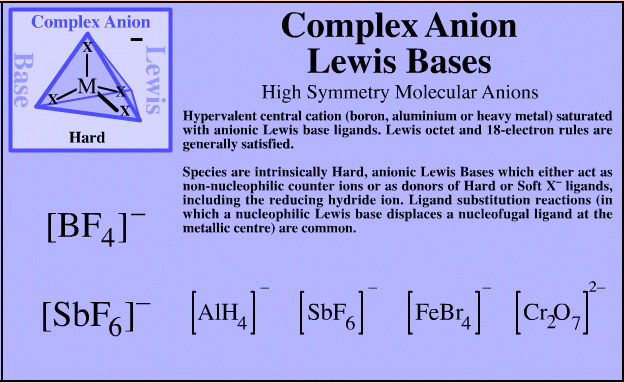
Complex Anion Lewis Bases
| High Symmetry Molecular Anions | 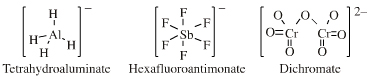
Search for complex anion Lewis bases species in The Chemical Thesaurus |
| FMO Topology: | Complex anion Lewis bases have a hypervalent central cation (boron, aluminium or heavy metal) saturated with anionic Lewis base ligands. Lewis octet and 18-electron rules are generally satisfied. The HOMO shows high spherical symmetry. |
| Charge: | Negative. |
| HSAB: | Intrinsically hard. |
| Chemistry: |
Complex anion Lewis base species behave as charged hard spheres that form ionic charge-controlled complexes (ie act as non-nucleophilic counter ions), or they behave as donors of hard/soft ligands, X–. Ligand substitution – in which a nucleophilic Lewis Base displaces a nucleofugal ligand – is common and ligand symbiosis considerations/effects are very important. There are four subclasses of complex anion Lewis base: X = Halogen anion which gives rise to the synthetically useful non-basic, non-nucleophilic, non-interfering anionic spectator counter ions:
X = Hydride ion which gives rise to species which act as donors of nucleophilic hydride ion, [BH4]– and [AlH4]–, as long as there is not a Brønsted Acidic proton available or H2 is generated. M = Heavy metal (Fe or Cr as opposed to B or Al). Such complex anions are much studied in classical inorganic coordination chemistry. Transition metals centres often exhibit multiple oxidation states. These are better considered as type 23 Lewis acid/base complexes. X = Oxygen heavy metal species with oxygen ligands are commonly used as oxidising agents. |
| Congeneric Series: |
Families of ligand replacement congeneric series are common: 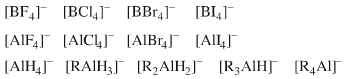 |
| Complex anion Lewis base (generic) |
|
|
|
Alkoxy aluminium hydride ion (generic) more here |
|
|
Alkoxy borohydride ion (generic) more here |
|
|
Alkyl aluminium hydride ion (generic) more here |
|
|
Alkyl borohydride ion (generic) more here |
|
|
Aluminium hexafluoride ion more here |
|
|
Aurocyanide ion more here |
|
|
Beryllate ion more here |
|
|
Bromine hexafluoride ion more here |
|
|
Chlorine tetrafluoride ion more here |
|
|
Dialkoxyaluminium hydride ion (generic) more here |
|
|
Dialkoxyborohydride ion (generic) more here |
|
|
Dialkylaluminium hydride ion (generic) more here |
|
|
Dialkylborohydride ion (generic) more here |
|
|
Dichlorosilver(I) ion more here |
|
|
Dicyanosilver(I) ion more here |
|
|
Germanium hexachloride dianion more here |
|
|
Germanium hexafluoride dianion more here |
|
|
Hexafluoroantimonate(V) more here |
|
|
Hexafluoroarsinate ion more here |
|
|
Hexafluoropalladium(IV) more here |
|
|
Hexafluoroplatinate ion more here |
|
|
Hexafluorosilicate ion more here |
|
|
Hexahydroiron(II) ion more here |
|
|
Iodine hexafluoride ion more here |
|
|
Iodine octafluoride ion more here |
|
|
Lead hexachloride dianion more here |
|
|
Lead hexafluoride dianion more here |
|
|
Manganate(V) ion more here |
|
|
Manganate(VI) ion more here |
|
|
Metaborate ion more here |
|
|
Orthoborate ion more here |
|
|
Pentahydrocobalt(I) ion more here |
|
|
Perbromate ion more here |
|
|
Perchlorate ion more here |
|
|
Periodate ion more here |
|
|
Permanganate ion more here |
|
|
Perxenate ion more here |
|
|
Phosphorus hexafluoride ion more here |
|
|
Platinum(II) tetrachloride ion more here |
|
|
Platinum(IV) hexachloride ion more here |
|
|
Scandium(III) hexafluoride ion more here |
|
|
Tetrabromoaluminate more here |
|
|
Tetrabromoborate more here |
|
|
Tetrabromoferrate ion more here |
|
|
Tetrachloroaluminium ion more here |
|
|
Tetrachloroborate more here |
|
|
Tetrachlorocopper(I) ion more here |
|
|
Tetrafluoroaluminate more here |
|
|
Tetrafluoroberyllate ion more here |
|
|
Tetrafluoroborate ion more here |
|
|
Tetrahydroaluminate more here |
|
|
Tetrahydroborate ion more here |
|
|
Tetrahydrogallinate ion more here |
|
|
Tetrahydroindate ion more here |
|
|
Tetrahydronickel(II) ion more here |
|
|
Tetrahydroxyaluminate ion more here |
|
|
Tetrahydroxyborate ion more here |
|
|
Tetrahydrozinc(II) ion more here |
|
|
Tetraiodoaluminate more here |
|
|
Tetraiodoborate more here |
|
|
Tin hexachloride dianion more here |
|
|
Tin hexafluoride dianion more here |
|
|
Tin pentachloride ion more here |
|
|
Trialkoxyaluminium hydride ion (generic) more here |
|
|
Trialkoxyborohydride ion (generic) more here |
|
|
Trialkylaluminium hydride ion (generic) more here |
|
|
Trialkylborohydride ion (generic) more here |
|
|
Vandate(V) ion more here |
|
|
Xenate ion more here |
|
|
Xenon heptafluoride ion more here |
|
|
Xenon octafluoride ion more here |
|
|
Zintl ion more here |
 |
 |
 |
| Poster | Nucleophiles & Bases |
© Mark R. Leach 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.