Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
Lewis Acid/Base Interaction Matrix Database
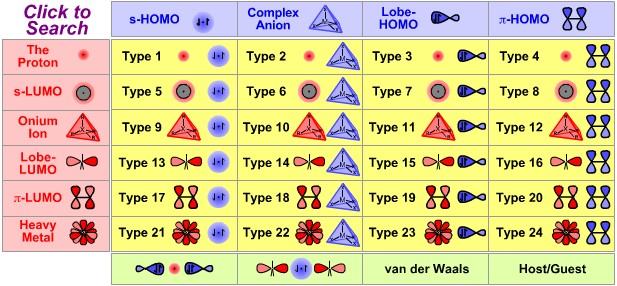 |
Type 1 Lewis Acid/Base Complexation Chemistry |
|
| 1σ2 Complexes | |
 |
|
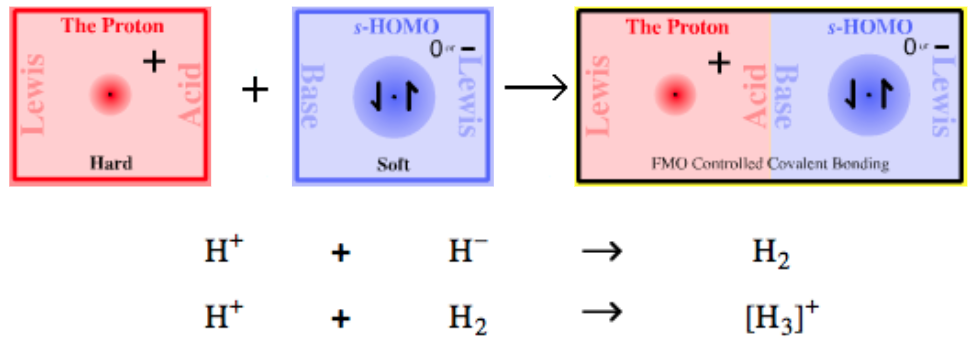
| Bonding: | Type 1 complexes, typified by hydrogen H2, are covalently bonded. The bonding is frontier molecular orbital (FMO) controlled. Hydrogen, H2, has a 1σ2 MO structure, ie they have two electrons in their 1σ molecular orbital. Find out more about the bonding diatomic species elsewhere in this webbook, here. |
| Charge: | Complexes can be neutral, H2, or positively charged [H3]+. |
| Chemistry: | Protons complex with hydride ions to form molecular hydrogen, H2, a uniquely simple and much studied diatomic molecule. The H+ + H– → H2 reaction is not reversible: H2 does not act as a proton donor (although at high temperature, when exposed to high energy UV radiation or when absorbed onto a metallic surface, H2 can homolytically dissociate: radical cleavage). As protons and hydride ions do not exist as independent species, they require "delivery" by donor complexes, ie reagents. Protons, H+ ions, are supplied by Brønsted acids and hydride ions by hydride donor complexes. For example, hydrogen chloride an H+ donor reacts with sodium hydride an H– donor to give diatomic hydrogen and sodium chloride: HCl + NaH → H2 + NaCl [H3]+, the product of H+ and H2, is the simplest possible triatomic molecular ion – it has only two electrons – and is of considerable theoretical interest.
(In this author's opinion the [H3]+ ion should be called the 'hydronium ion', and [OH3]+ should be the 'oxonium ion'.) |
| Congeneric Series: | Few series. |
| Type 1 Lewis acid/base complex (generic) |
|
|
|
Deuterium more here |
|
|
Duteronium ion more here |
|
|
Helonium ion more here |
|
|
Hydrogen more here |
|
|
Hydrogen deuteride more here |
|
|
Hydronium ion, [H3]+ more here |