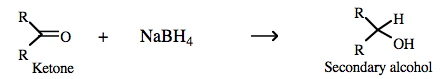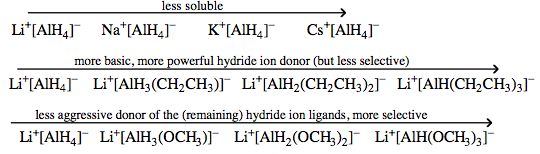Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
Lewis Acid/Base Interaction Matrix Database
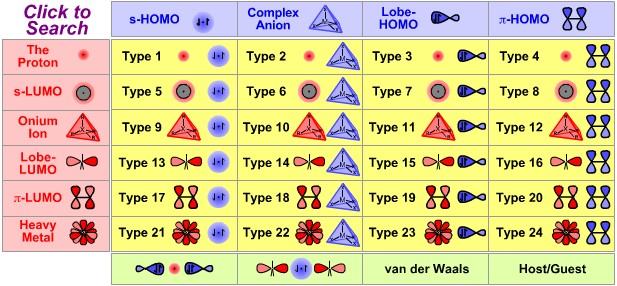 |
| Type 6 Lewis acid/base complex (generic) |
|
|
|
Beryllium silicate more here |
|
|
Borax more here |
|
|
Calcium tungstate more here |
|
|
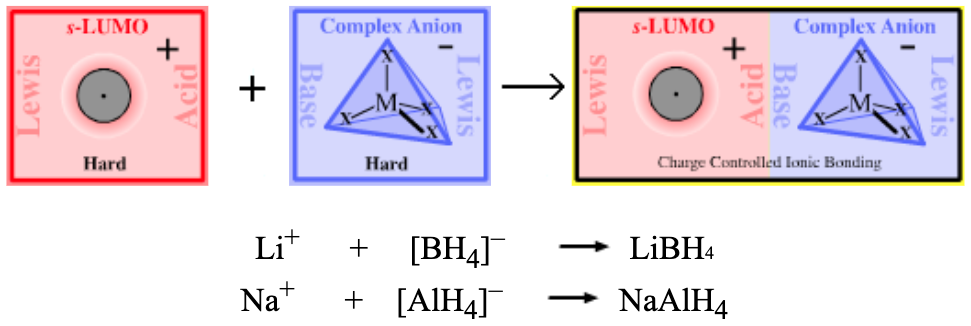
Lithium aluminium hydride more here |
|
|
Lithium borohydride more here |
|
|
Lithium tri-tert-butoxy aluminium hydride more here |
|
|
Pentasulfide dianion more here |
|
|
Potassium aluminium hydride more here |
|
|
Potassium borohydride more here |
|
|
Potassium buckide more here |
|
|
Potassium dichromate(VI) more here |
|
|
Potassium hexachloro platinate more here |
|
|
Potassium manganate(V) more here |
|
|
Potassium manganate(VI) more here |
|
|
Potassium nitrate more here |
|
|
Potassium perbromate more here |
|
|
Potassium perchlorate more here |
|
|
Potassium rhenium nonahydride more here |
|
|
Potassium tetrachloroplaninate(II) more here |
|
|
Sodium aluminium hydride more here |
|
|
Sodium argentocyanide more here |
|
|
Sodium aurocyanide more here |
|
|
Sodium borohydride more here |
|
|
Sodium chromate more here |
|
|
Sodium cyanoborohydride more here |
|
|
Sodium dichromate more here |
|
|
Sodium hexafluoroaluminate more here |
|
|
Sodium hexafluorosilicate more here |
|
|
Sodium perchlorate more here |
|
|
Sodium tetrafluoroborate more here |
|
|
Tetrasulfide dianion more here |
|
|
Trisulfide dianion more here |
|
|
Zeise's salt more here |