Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
Lewis Acid/Base Interaction Matrix Database
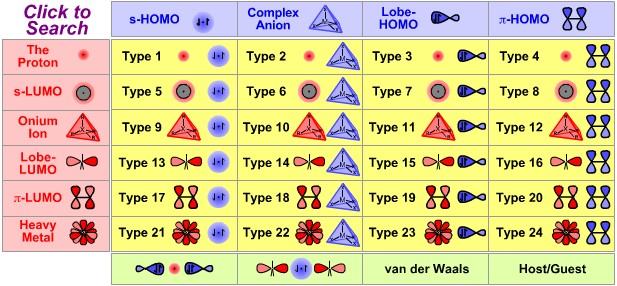 |
| Type 9 Lewis acid/base complex (generic) |
Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
 |
| Type 9 Lewis acid/base complex (generic) |