Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
Lewis Acid/Base Interaction Matrix Database
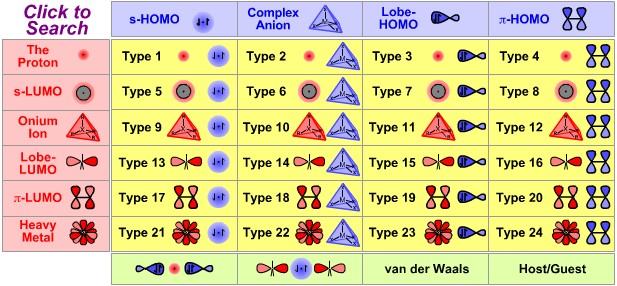 |
| Type 14 Lewis acid/base complex (generic) |
|
|
|
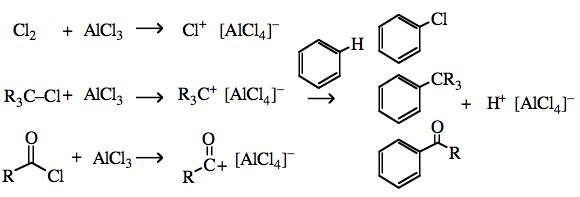
Acyl cation/tetrachloroaluminate complex (generic) more here |
|
|
Bromine tetrabromoferrate more here |
|
|
Carbenium ion/tetrachloro aluminate complex (generic) more here |
|
|
Chlorine perchlorate more here |
|
|
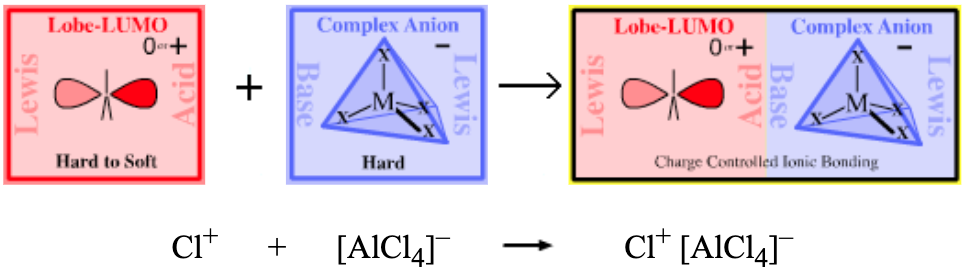
Chlorine tetrachloroaluminate more here |
|
|
N5 hexafluoroarsinate more here |
|
|
Nitronium tetrafluoroborate more here |
|
|
Xenon fluoride hexafluoroplatinate(V) more here |