Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| Poster | Nucleophiles & Bases |
Lewis Acid/Base Interaction Matrix Database
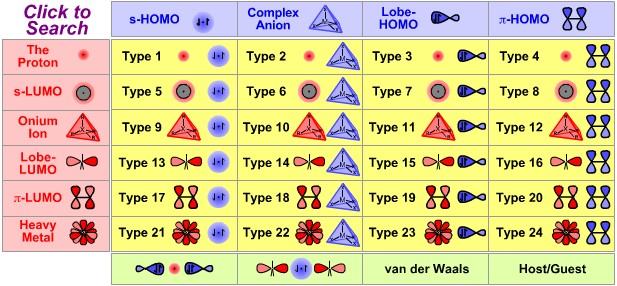 |
Type 20 Lewis Acid/Base Complexation Chemistry |
|
| π/π Interactions, including Diels-Alder Cycloaddition | |
 |
|
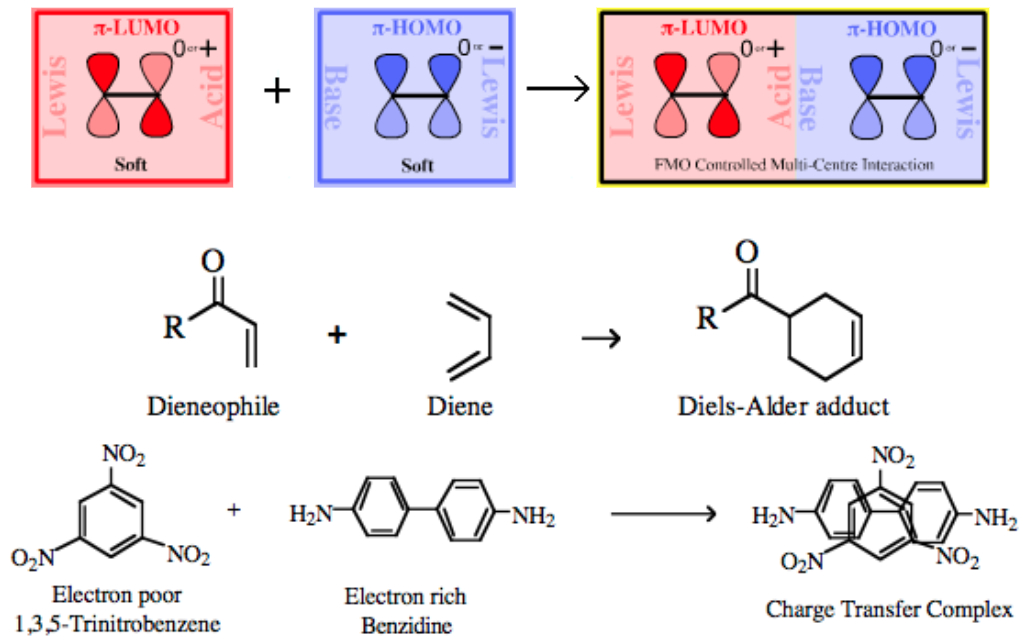
| Bonding: | If the π-LUMO Lewis acid and π-HOMO Lewis base species have suitable:
an orbital phase-symmetry controlled multi-centre π/π complexation reaction can take place with various the participating atoms rehybridizing to give a largely σ-bonded product. The transition state is deemed to proceed via a pericyclic intermediate, where "peri-" is a prefix meaning around or surrounding here. The classic pericyclic interaction is the Diels-Alder cycloaddition between a diene and a dienophile: 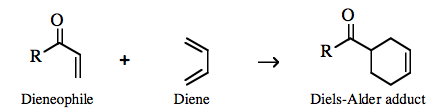
The orbital phase symmetry arguments required for cycloaddition to take place are interchangeable. Cycloaddition can either involve the HOMO of the diene + the LUMO of the dieneophile or the LUMO of the diene + the HOMO of the dieneophile: 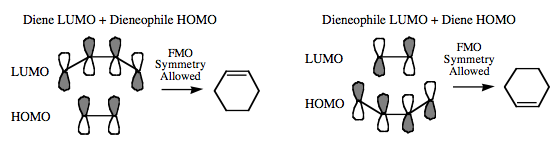
Pericyclic reactions are concerted: they take place in a single step. As a consequence, concerted processes provide allow for great Stereochemical control, and pericyclic processes are amongst the most useful of all synthetic methodologies available to the synthetic organic chemist.
Electron-poor δ+ π-systems can interact with electron-rich δ– π-systems. The initial attraction between is electrostatic (ionic) in nature to form a π/πcharge transfer complex. |
| Charge: | Complexes may be negatively charged, positively charged or they may be neutral. |
| Chemistry: | FMO Controlled Pericyclic Interactions Diels-Alder cycloaddition can be considered as multicentre π-LUMO plus π-HOMO Lewis acid/base Complexation chemistry. However, it is sometimes difficult to decide which species is acting as the Lewis acid and which is the Lewis base. In normal electron demand Diels-Alder cycloaddition chemistry the diene is electron rich, implying Lewis base character, and the alkene, the dieneophile, is electron deficient implying that the species is the Lewis acid: 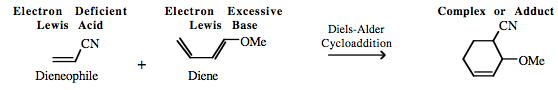
In reverse electron demand cycloaddition, the diene acts as the electron deficient Lewis acid and the dieneophile is the electron rich Lewis base: 
There are four general classes of pericyclic reaction process:
Charge Transfer Complexes π/π-Interactions can also lead to the formation of a charge-transfer complex, for example between the electron poor 1,3,5-trinitrobenzene and electron rich benzidine. 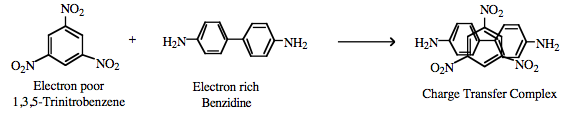
π/π-Interactions can also lead to the formation or conductive organic metals such as stacked TTF/TCNQ materials: 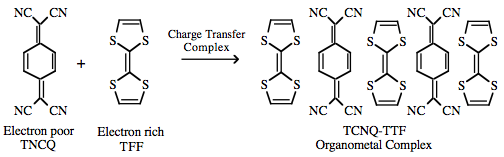 |
| Congeneric Series: | Few series. |
| Type 20 Lewis acid/base complex (generic) |
|
|
|
Benzidine/Trinitrobenzene charge transfer complex more here |
|
|
Cyclohexene more here |
|
|
Cyclopropane more here |
|
|
Diels-Alder adduct (generic) more here |
|
|
3,4-Dihydropyrazole more here |
|
|
Sulfolene more here |
|
|
TTF-TCNQ organometal complex more here |