Periodic Table |
 |
 |
 |
 |
 |
 |
 |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Books & Reviews about the Periodic Table of the Elements, by date:
| Year: 1885 | PT id = 1145 |
von Richter's Periodic System of the Elements
From page 244 of A Text-book of Inorganic Chemistry by Victor von Richter, Published by Blakiston (US ed. in English, 1885). The full text (scanned) is available from archive.org. The first edition was published in 1874 in German. von Richter was was from the Baltic region, in the the Russian empire at the time.
von Richter's work is almost certainly the first chemistry textbook based on the periodic system. Many (indeed most) modern Inorganic Chemistry texts follow this format, but NOT the Chemogenesis web book!
von Richter, writes:

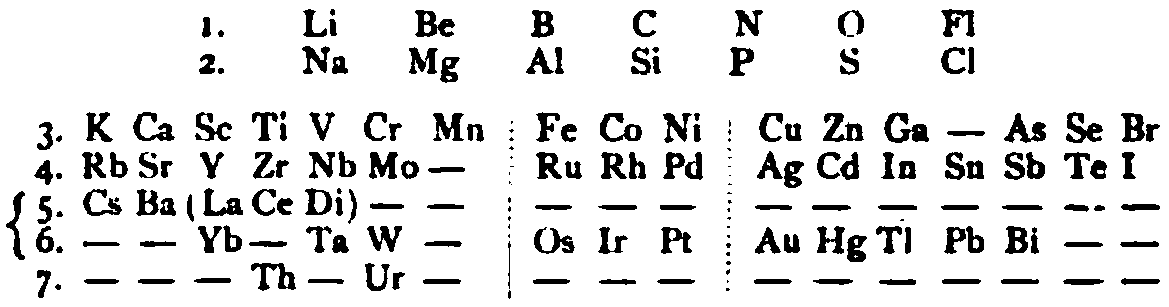

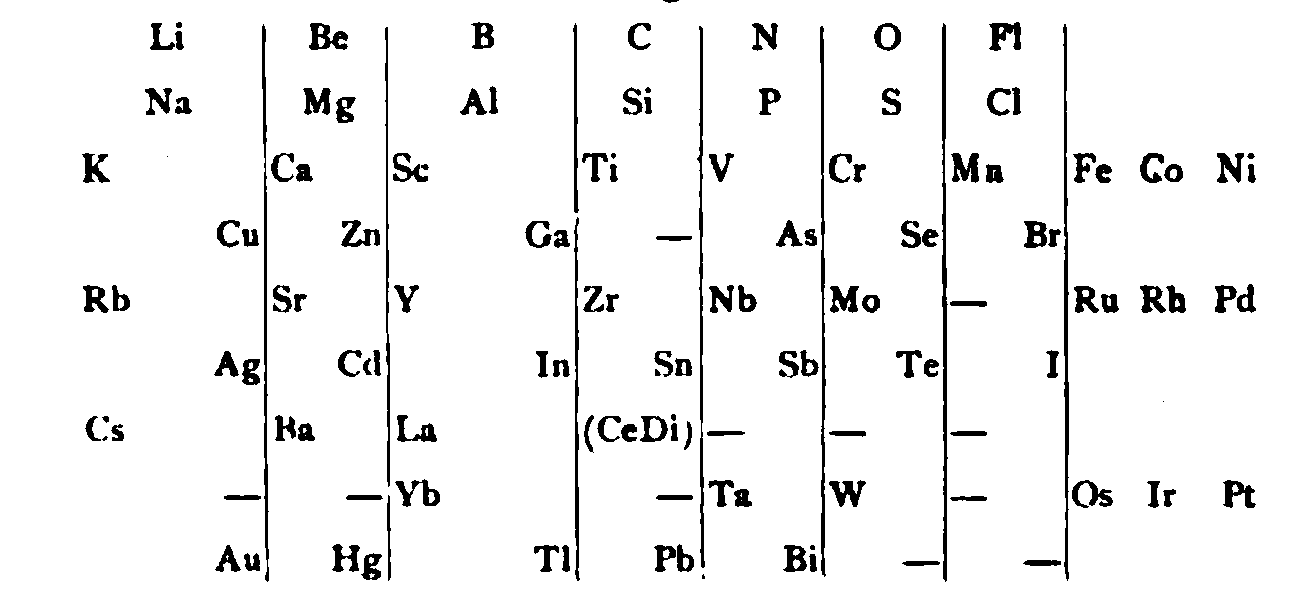
Thanks to René for the tip!
| Year: 1896 | PT id = 1134 |
Venable's The Development of The Periodic Law
The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896).
The full text (scanned) is available from archive.org.


Thanks to René for the tip!
| Year: 1900 | PT id = 1284 |
History of the Discovery of the Group 18 (erstwhile Group 0) Elements
John Marks has provided a concise history of the discovery of the Group 18 elements and the element name"Nitron/Radon".
Radioactivity was discovered by Becquerel in 1896 and the Curies noted transferred radioactivity rather like the induction of electric or magnetic charge. Radon was discovered in 1900, by Dorn in Halle; Rutherford discovered thoron in 1899; and Debierne discovered actinon in 1903. The time-line is:
- 1868 Lockyer observed the spectrum of helium in the solar corona
- 1894 Ramsay discovers argon
- 1895 Ramsay isolates helium
- 1898 Ramsay discovers krypton, neon & xenon
- 1899 Curie observes an emanation from radium
- 1899 Rutherford observes an emanation from thorium
- 1900 Dorn identifies radon
- 1902 Rutherford & Soddy characterize thoron
- 1903 Rutherford & Soddy isolate radon
- 1903 Debierne observes an emanation from actinium
- 1904 Ramsay names the isotopic emanations exactinio, exradio & exthorio and surmises they are one element, probably an inert gas
- 1908 Professor Sydney Young’s "Stoichiometry" has a periodic table shows niton, Z = 86
- 1909 Ramsay characterizes niton as a group 0 inert gas
- 1910 Cameron's "Radiochemistry" describes the radioactive displacement law
- 1912 The name "niton" accepted by the International Commission for Atomic Weights
- 1913 Soddy expounds theory of isotopes
- 1913 Rydberg's periodic table has Nt (86) for the last inert gas
- 1919 Irving Langmuir's PT has Nt as the last inert gas
- 1922 Niels Bohr’s PT has Nt (86) as the last inert gas
- 1923 GN Lewis’s PT has Nt as the last inert gas
- 1924 CRC’s Handbook of Chemistry and Physics has niton as the last member of Group 0
So niton (from Latin nitens = shining) was noticed by the Curies in 1899 as an emanation from radium. That same year Rutherford noted an identical emanation from thorium, and in 1903 Debierne discovered the same emanation from actinium. All three ('radon', 'thoron' and 'actinon') were identified as an element by Ramsay in 1904 and characterized by him in 1909.
Ramsay named the element niton after its most prominent property viz. that it glowed in the dark.
With the introduction of Soddy's isotopes, it became clear that: thoron was Nt-220, radon was Nt-222 & actinon was Nt-219.
There are natural traces of other isotopes (e.g. Nt-217, Nt-218) from beta disintegration of astatine. So "radon" was just one isotope of niton.
The foregoing history of niton is uncontroversial and the name niton, Nt, for Z = 86 dates at least from Professor Young´s textbook of stoichiometry in 1908.
In 1912, the name 'niton' was adopted by the International Commission for Atomic weights. Rydberg's PT of 1913 has Nt as the last inert gas, as does Irving Langmuir's PT of 1919, Niels Bohr's PT of 1922, GN Lewis's PT of 1923 and even the CRC's Handbook of Chemistry and Physics in 1924.
John Marks concludes:
"Niton, Nt, for Z = 86, was thus established by its discoverers and accepted by the chemistry (and physics) establishment. Radon, Rn, is an error perpetuated by IUPAC [amongst its many sins].
"Radon is an isotope. We do not refer to hydrogen as 'protium', so why are we referring to niton as 'radon'?"
| Year: 1909 | PT id = 1106 |
Garrett's The Periodic Law
A book reviewing The Periodic Law by A.E. Garrett, pub. D. Appelton & Co (1909). This work shows the state of knowledge in the first decade of the 20th century.
René Vernon writes:
"On page 43 Garrett notes that, '[Thomas] Carnelley was the first English chemist to work out in detail the manner in which the properties of the elements are periodic functions of their atomic weights. His papers on this subject appeared in the Philosophical Magazine between the years 1879 and 1885.' "

| Year: 1923 | PT id = 1256 |
Deming's Periodic Table With Commentry by Vernon
René Vernon writes:
Deming's 1923 periodic table is credited with popularizing the 18-column form.
I now see Deming used different thickness sloping lines to represent the different degrees of similarity between the main groups and their corresponding transition metal groups.
- The line between Li-Na and group 11 is dashed, denoting the weakest relationship.
- Be-Mg are in group 2 The line between Be-Mg and group 12 is not dashed, denoting a stronger relationship.
- B-Al are in group 3
- The line between B-Al and Ga-In-Tl is thicker yet.
When I plot up to 20 chemical properties v Z going down these options I get the following values for the average smoothness of the trendlines:
- 73.5% for Li-Na-Cu(+2)-Ag(+1)-Au(+3) versus 84% for Li-Na-K-Rb-Cs
- 70% Be-Mg over Zn versus 85% for Be-Mg-Ca-Sr-Ba
- 81% for B-Al-Ga-In-Tl versus 88% B-Al-Sc-Y-La
I would have thought the smoothness for the line between Li-Na and Cu would be < 70%, consistent with Deming’s dashed line. But the thickness of the line would depend on what Deming took into account when he drew it. The common wisdom about groups 1 and 11 is that their similarities are: "confined almost entirely to the stoichiometries (as distinct from the chemical properties) of the compounds in the +1 oxidation state." (Greenwood & Earnshaw 2002, p. 1177). Kneen et al. (1972, p. 521) say that, "the differences between the properties of the group IA and IB elements are those between a strongly and weakly electropositive metal." On this basis I follow Deming’s dashed line. I’ve appended some notes about Group 1 and Group 11.
- Main group 4 is C-Si-Ge-Sn-Pb
- The line between Si and Ti-Zr-Hf is thick
- The line between N-P and V is less thick
- The line between O-S and Cr is less thick again
- The line between F-Cl and Mn is dashed
I have [calculated] a smoothness for C-Si-Ti-Zr-Hf of 86% versus 70% for C-Si-Ge-Sn-Pb. Since Ti shows some transition metal chemistry but not C-Si, it is perhaps plausible to keep C-Si-Ge-Sn-Pb together (as Deming did ).
Deming was a smart author. Nigh on a century later and the metrics check out.
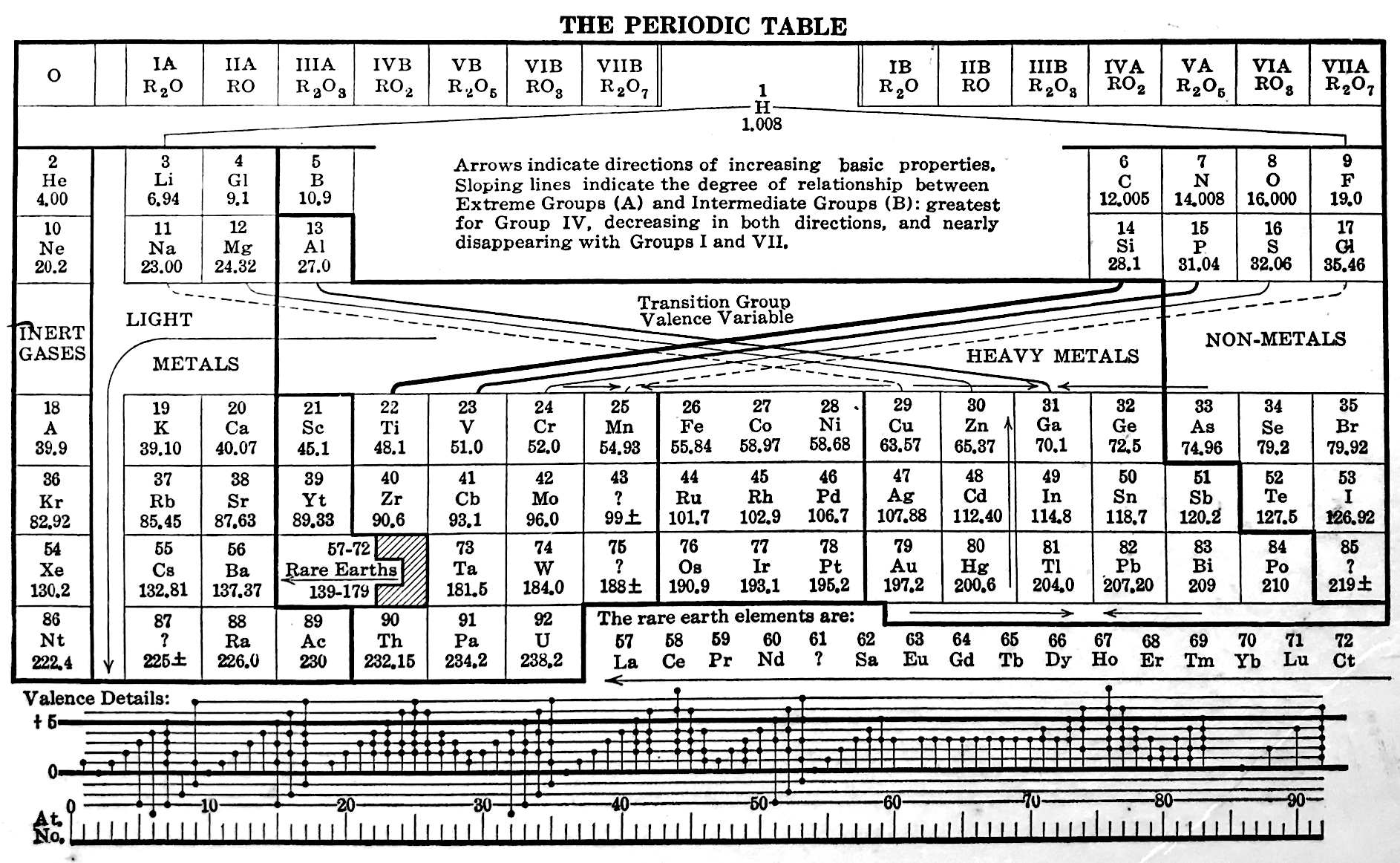
More about group 1 and group 11
There may be a little more to the relationship between Li-Na & Cu-Ag-Au, than is ordinarily appreciated. For example:
- The resulting composite "group" has two electropositive metals and three more electronegative metals so its overall nature is more nuanced then purely group 1 or purely group 11
- The ionic radii of Li+ and Cu+ are 0.76 and 0.77 Å, and there is at least some discussion in the literature about substitution phenomena (Vasilev et al. 2019, p. 2-15; Udaya et al. 2020, p. 98; Kubenova 2021 et al.)
- Group 1 and 11 metal atoms form clusters relatively easily including Au_42+, Ag_64+, Rb_75+, Na_43+ (Mile et al. 1991, p. 134; Wulfsberg 2000, p. 631).
- In an organometallic context, Schade & Scheyler (1988, p. 196) wrote that, "There is much evidence that differences between group 1 and group 11 metals are not of principal but rather gradual manner."
- Although most nonmagnetic metals exhibit superconductivity it is significant that the Group 1 and 11 metals do not become superconducting at very low temperatures (Rao & Gopalakrishnan 1997, p. 398).
- Gold forms intermetallic compounds with all alkali metals (Schwerdtfeger et al. 1989. p. 1769)
References
- Greenwood NN & Earnshaw A 2002, Chemistry of the Elements, 2nd ed., Butterworth Heinemann, Oxford
- Kubenova et al. 2021, "Some thermoelectric phenomena in copper chalcogenides replaced by lithium and sodium alkaline metals", Nanomaterials 2021, vol. 11, no. 9. article 2238, https://doi.org/10.3390/nano11092238
- Mile et al. 1991, "Matrix-isolation studies of the structures and reactions of small metal particles", Farady Discussions, vol. 92, pp. 129–145 (134), https://doi.org/10.1039/FD9919200129
- Rao CNR & Gopalakrishnan J 1997, New Directions on Solid State Chemistry, 2nd ed., Cambridge University Press, Cambridge
- Schade C & Schleyer PVR 1988, "Sodium, potassium, rubidium, and cesium: X-Ray structural analysis of their organic compounds", Advances in Organometallic Chemistry, vol. 27, Stone FGA & West R (eds), Academic Press, San Diego, pp. 169–278
- Schwerdtfeger et al. 1989, "Relativistic effects in gold chemistry. I. Diatomic gold compounds.", The Journal of Chemical Physics, vol. 91, no. 3, pp. 1762–1774. https://doi.org/10.1063/1.457082
- Udaya et al. 2020, Metal sulphides for lithium-ion batteries, in Inamuddin, Ahmer & Asiri (eds), Lithium-ion batteries: Materials and applications, Materials Research Forum, Millersville PA, pp. 91–122
- Vasiliev AN et al. 2019, Low-dimensional Magnetism, CRC Press, Boca Raton
- Wulfsberg 2000, Inorganic chemistry, University Science Books, Sausalito, CA
| Year: 1934 | PT id = 105 |
Quam & Quam's Graphical Representations of The Elements
Short Periodic Tables.pdf
Medium Periodic Tables.pdf
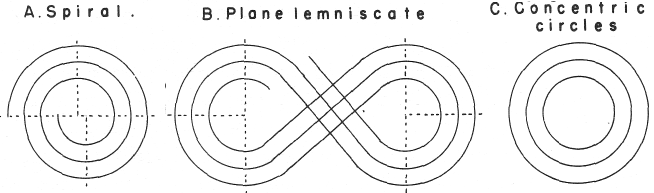
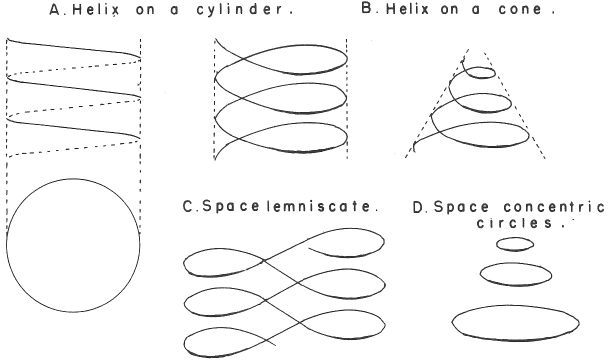
Spiral, Helical & Misc Periodic Tables.pdf
- Mendeléeff's Table (their spelling, 1872)
- Brauner's Table (1902)
- Rydberg Table (1913)
- Periodic Chart by Quam (1934)
- Rang's Periodic Table (1893)
- Werner's Periodic Table (1905)
- Courtines' Periodic Classification (1925)
- Bayley's Periodic System (1882)
- Adam's Periodic Chart (1911)
- Margary's Periodic Table (1921)
- Stareck's Natural Periodic System (1932)
- Baumhauer's Spiral (1870)
- Erdmann's Spiral Table (1902)
- Nodder's Periodic Table (1920)
- Partington's Periodic Arrangements of the Elements (1920)
- Janet's Helicodial Classification (1929)
- The Telluric Screw (1863)
- Crookes' Periodic Table model (1898)
- Emerson's Helix (1911)
- Periodic Table by Harkins and Hall (1916)
- Schaltenbrand's Periodic Table (1920)
- Rixon's Diagram of the Periodic Table (1933)
- Spring's Diagram (1881)
- Flavitzky's Arrangement (1887)
- Stephenson's Statistical Periodic Table (1929)
- Friend's Periodic System (1927)
- Many others, including: Vogel (1918), Stintzing (1916) and Caswell (1929) are discribed without the benefit diagrams.
| Year: 1940 | PT id = 1262 |
Hsueh & Chiang's Periodic Properties of the Elements
Hsueh & Chiang, Periodic Properties of the Elements, J. Chinese Chem. Soc., 5, 5, 253-275. See the PDF.
René Vernon writes:
"A mathematical expression of the periodic law was put forward in 1937 in an article by Chin-Fang Hsueh and Ming-Chien Chiang: J Chinese Chem Soc, 5, 263 (In English.) They derived a property equation from which the numerical magnitude of a property P is related to the atomic number Z of the element in question in terms of valence V, a function of the periodic factor y, the principal quantum number n, and two parameters a and p, which are constants for a given family of elements but different for different families."
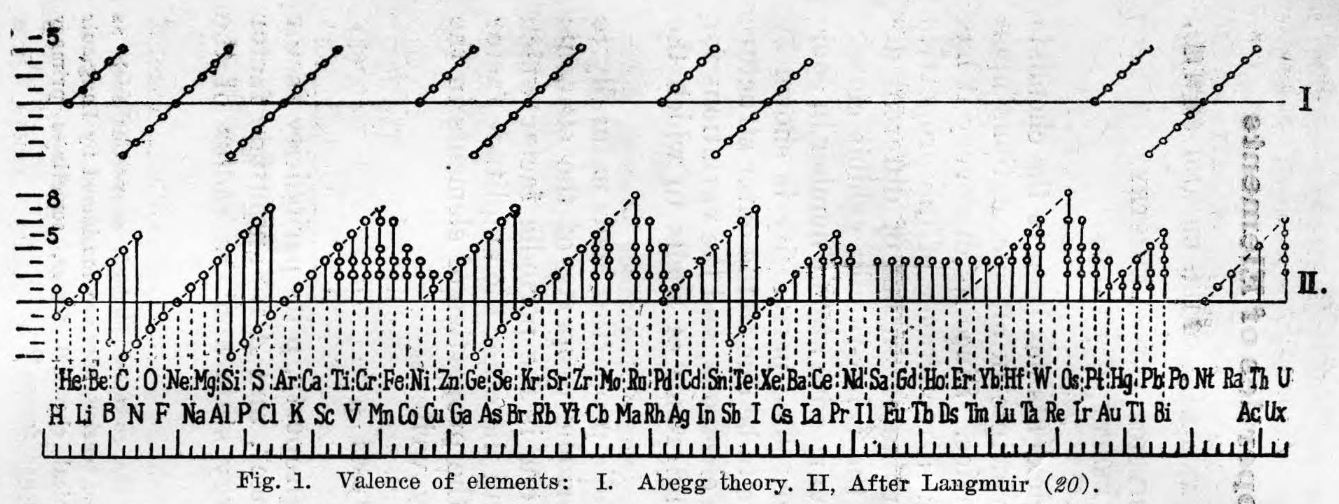
| Year: 1957 | PT id = 110 |
Mazurs' Graphical Representations of The Periodic System During 100 Years
Edward Mazurs, Graphical Representations of The Periodic System During 100 Years, University of Alabama Press, 1957.
There is an internet archive: Edward G. Mazurs Collection of Periodic Systems Images.
This book gives a very full analysis and classification of periodic table formulations. Most of the formulations are redrawn.
However, anybody who is seriously interested in periodic table formulations will want to see/read/own this book. Read more about Mazrus on the Elements Unearthed blog.
1955 |
Mazurs' Valence Periodic Table (1974, p.94) |
1955 |
Mazurs' Periodic Table (1974, p. 95) |
1955 |
Mazurs' 1955 Formulation (1974, p. 44) |
1958 |
Mazurs' 1958-73 Formulation (1974, endpaper) |
1965 |
Mazurs' 1965 Formulation (1974, p/ 134) |
1967 |
Mazurs' 1967 Formulation (1974. Inside front cover) |
1967 |
Mazurs' other 1967 Formulation (1974, p. 126) |
1967 |
Mazurs' another 1967 Formulation (1974, p. 134) |
1969 |
Mazurs' Perio |
1974 |
Mazurs' Version of Janet's "Lemniscate" Formulation (1974, p.80) |
1974 |
Marzus' Wooden Version of Mendeleev's Periodic Table (Chem. Heritage Foundn.) |
1974 |
Mazurs' PT Formulation Analysis (1974, pp.15-16) |
Many thanks to Philip Stewart for preparing the links table above.
| Year: 1959 | PT id = 1160 |
Mendoza's Neuvo Sistema Periodico
A memorial work, Ley De Configuraciones Electronicas, published posthumously in 1965 to honor Oswaldo Baca Mendoza (1908–1962 Cusco, Peru) and his 1959 Neuvo Sistema Periodico. Download the full PDF file (in Spanish).


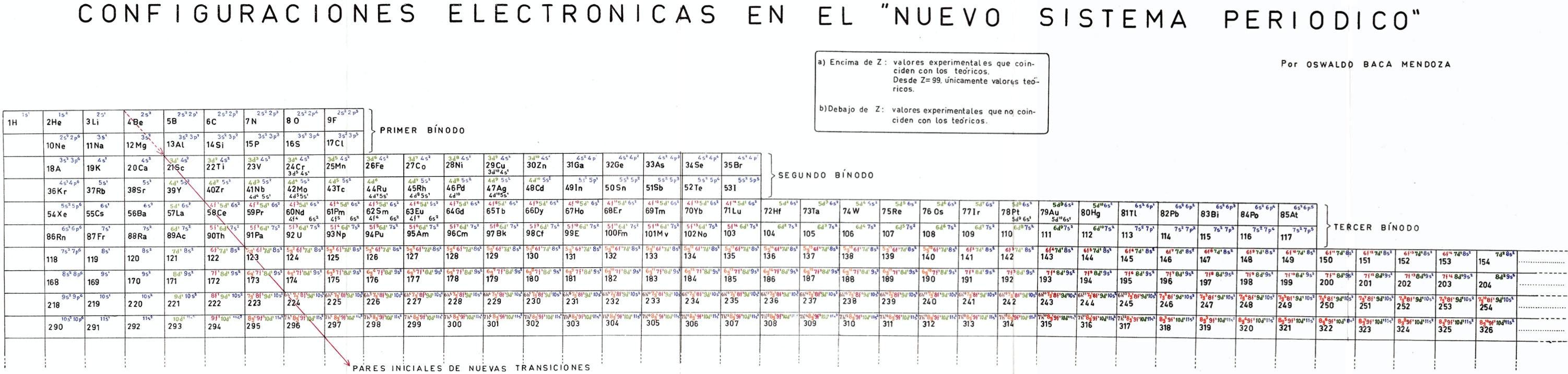
Thanks to Julio Gutierrez Samanez for the infomaton, etc.
| Year: 1963 | PT id = 911 |
Galaxy of Elements [Discovered] by Swedish Scientists
Uploaded by request of Fathi Habashi, an historical video on elements discovered by Swedish scientists.
The film is in the Swedish film database, where it is named: Atomernas vintergata and is dated 1963:
| Year: 1966 | PT id = 1265 |
Rare Earth Pop Out Periodic Table
From Rare Earths, The Fraternal Elements by Karl A. Gschneidner Jr., United States Atomic Energy Commission Division of Technical Information Library of Congress Catalog Card Number: 65-60546 1964; 1966 (Rev.)
There is an interesting point made in the text concerning the term "Rare Earths":
"The name rare earths is actually a misnomer for these elements are neither rare nor earths. They are metals, and they are quite abundant. Cerium, which is the most abundant, ranks 28th in the abundances of the naturally occurring elements and is more plentiful than beryllium, cobalt, germanium, lead, tin, or uranium. The least abundant naturally occurring rare earth, thulium, is more plentiful than cadmium, gold, iodine, mercury, platinum, or silver. Indeed, 25% of the elements are scarcer than thulium."
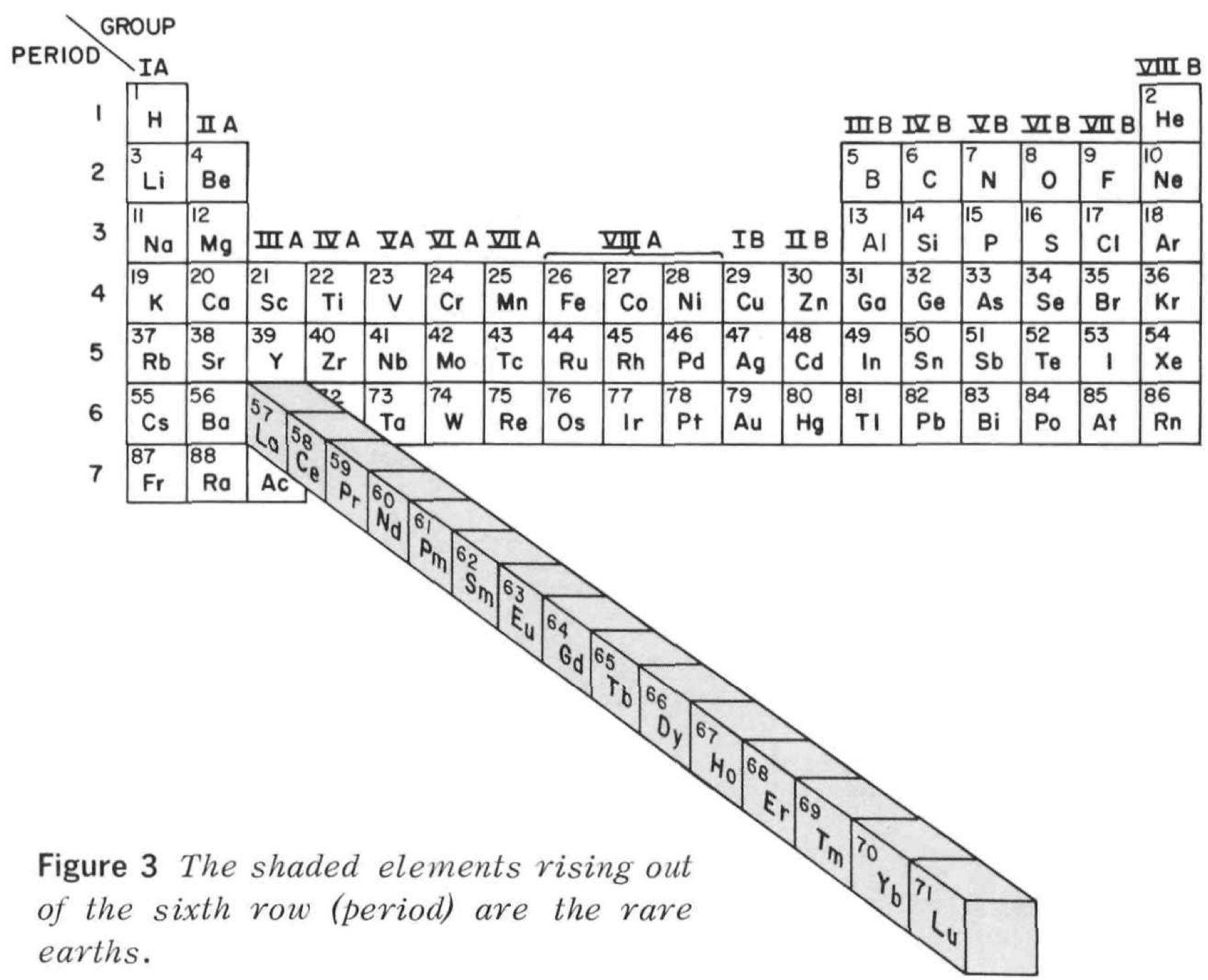
Thanks to René for the tip!
| Year: 1969 | PT id = 109 |
J. W. van Spronsen, The Periodic System of Chemical Elements: A History of the First Hundred Years, Elsevier 1969
This book gives a good review and discussion of periodic table formulations. Anybody who is seriously interested in periodic table formulations will want to see/read/own this book.
| Year: 1969 | PT id = 1146 |
Mendeleevian Conference, Periodicity and Symmetries in the Elementary Structure of Matter
Atti del Convegno mendeleeviano : periodicità e simmetrie nella struttura elementare della materia : Torino-Roma, 15-21 settembre 1969 / [editor M. Verde] Torino : Accademia delle Scienze di Torino ; Roma : Accademia Nazionale dei Lincei, 1971 VIII, 460 p.
Google Translate: Proceedings of the Mendeleevian Conference: periodicity and symmetries in the elementary structure of matter: Turin-Rome, 15-21 September 1969 / [editor M. Verde] Turin: Turin Academy of Sciences; Rome: National Academy of the Lincei, 1971 VIII, 460 p.
From the Internet Archive, the scanned book. Papers are in Italian & English.
For the 100th Anniversary of Mendeleev's iconic periodic table, a conference was held to look at (review) the elementary structure of matter. The 1960s saw huge developments in particle physics, including the theory of quarks. Papers were presented by many notable scientists including John Archibald Wheeler and the Nobel laureates: Emilio Segrè & Murray Gell-Mann.Thanks to René for the tip!
| Year: 1975 | PT id = 744 |
Primo Levi's Elements
Primo Levi's elements, from his book The Periodic Table:
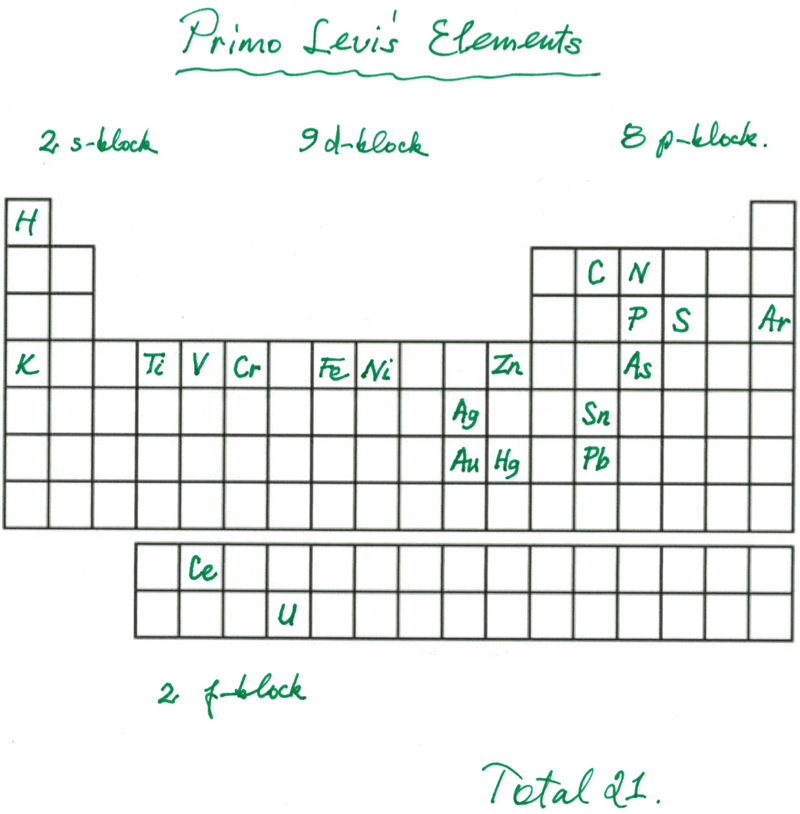
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1979 | PT id = 994 |
Seaborg's "How the Periodic Table Evolved Over 40 Years" (1939 – 1979)
From the C&EN paper THE PERIODIC TABLE: Tortuous path to man-made elements 57, 1979, pp 46-52.
Until World War II, the three heaviest known elements – thorium, protactinium & uranium – were believed to be related to hafnium, tantalum & tungsten respectively. Similarly, elements 93 to 100 were expected to fit neatly into the periodic table:
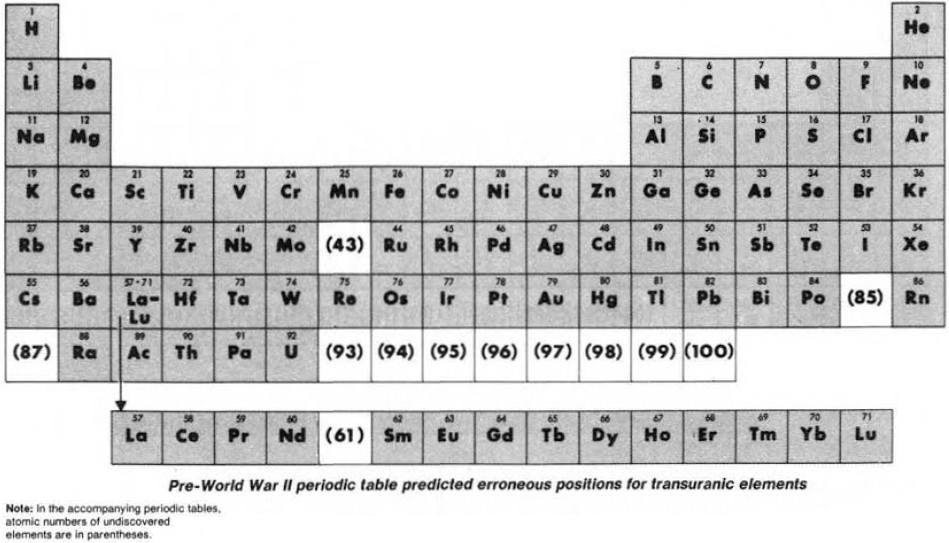
Synthesis and study of the transuranic elements – neptunium & plutonium – indicated that these new elements were "cousins" of uranium and in 1944 should be placed into a new "uranide" group.
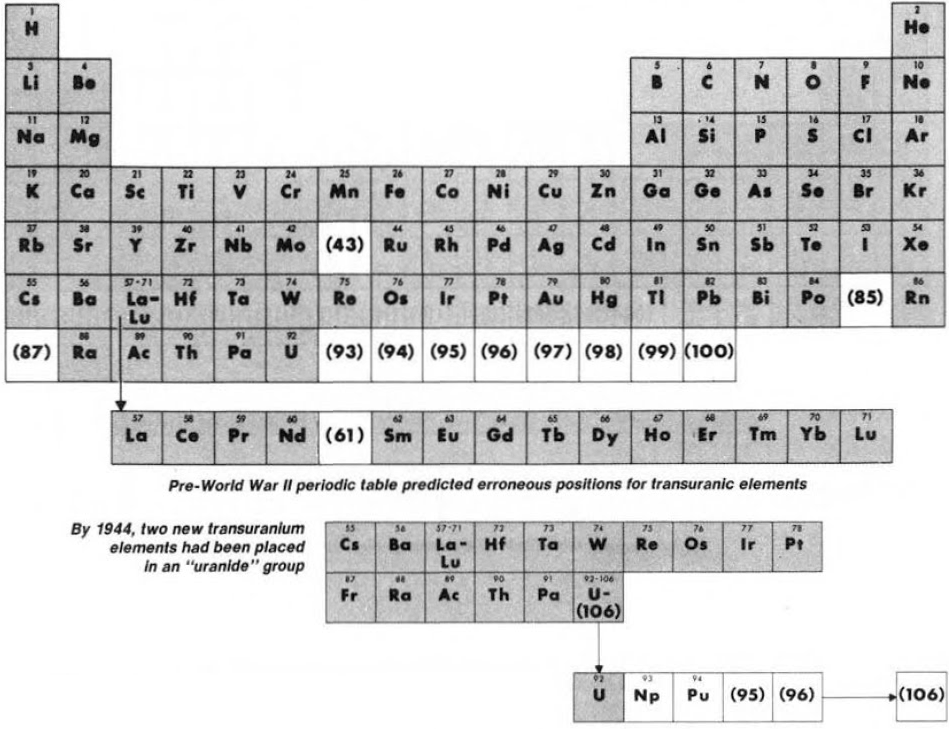
Subsequently (1944/45), Seaborg advanced the theory that elements heavier than actinium actually constitute a distinct "actinide" group that mirrors the lanthanide rare-earth group:
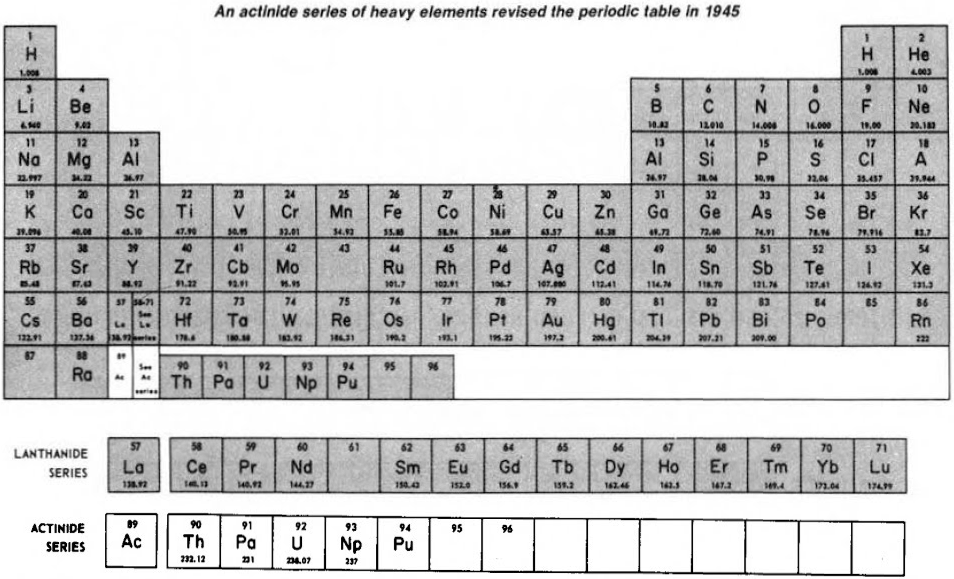
Finally, Seaborg postulated what a future periodic table, up to Z = 168, may look like:
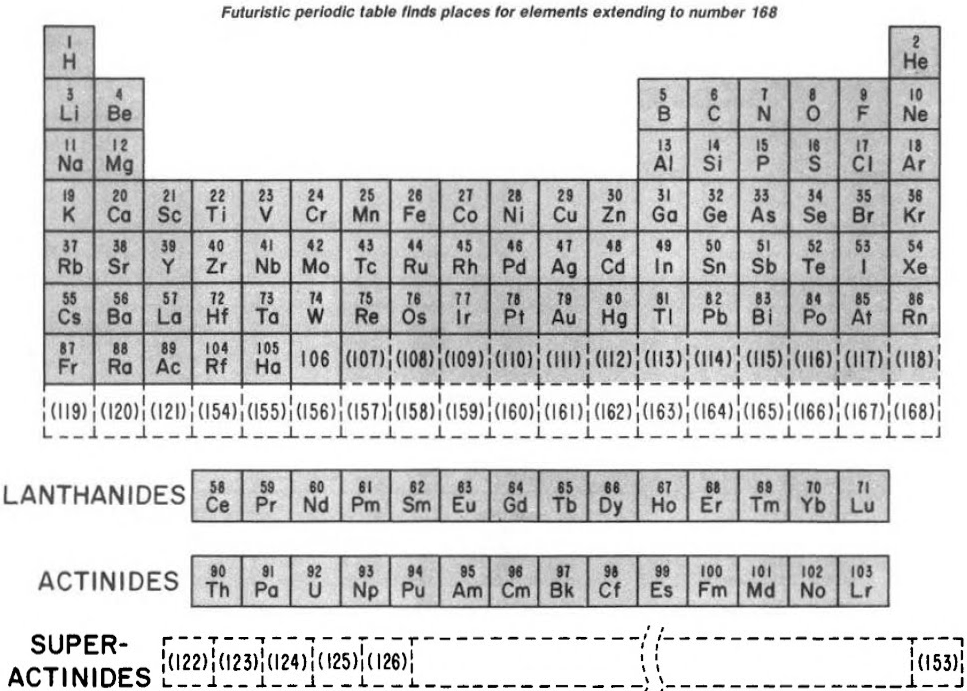
| Year: 1992 | PT id = 1045 |
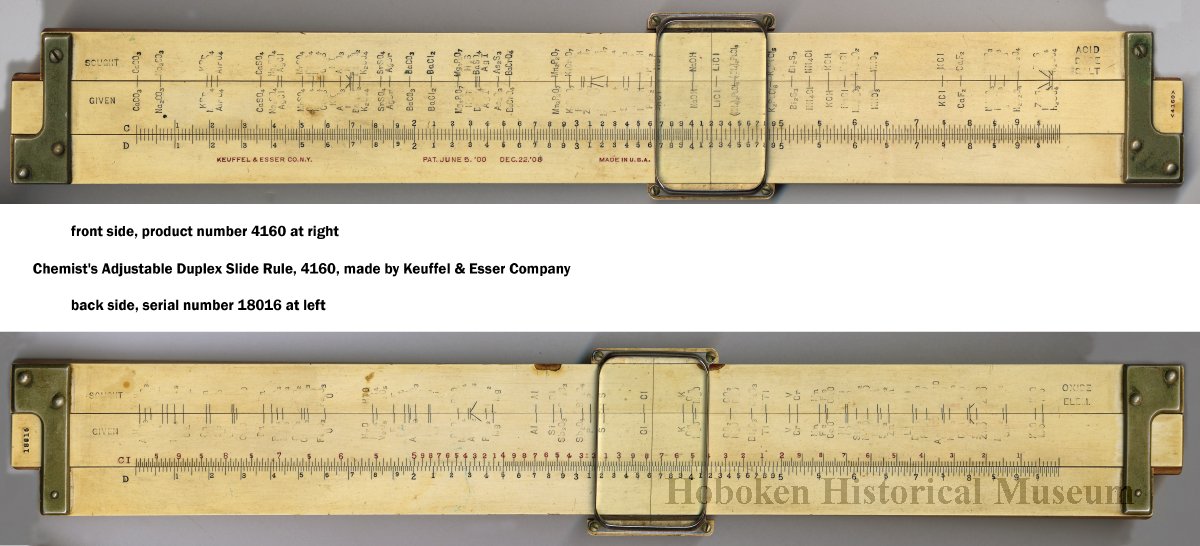
Chemical Slide Rules
The first chemical slide rules are of interest here because they are, in effect, early periodic tables. But the are more than this, as they can be used for performing chemical calculations. Writing in Bull. Hist. Chem. 12 (1992) (and here), William D. Williams of Harding University writes:
"An article by George Bodner in the Winter 1990 issue of the Bulletin described a rare chemical slide rule designed by Lewis C. Beck and Joseph Henry - their little-known Improved Scale of Chemical Equivalents. [My] paper attempts to place this slide rule in context by describing its origins, as well as some of its predecessors and successors."
Some chemical slide rules mentioned in the text:
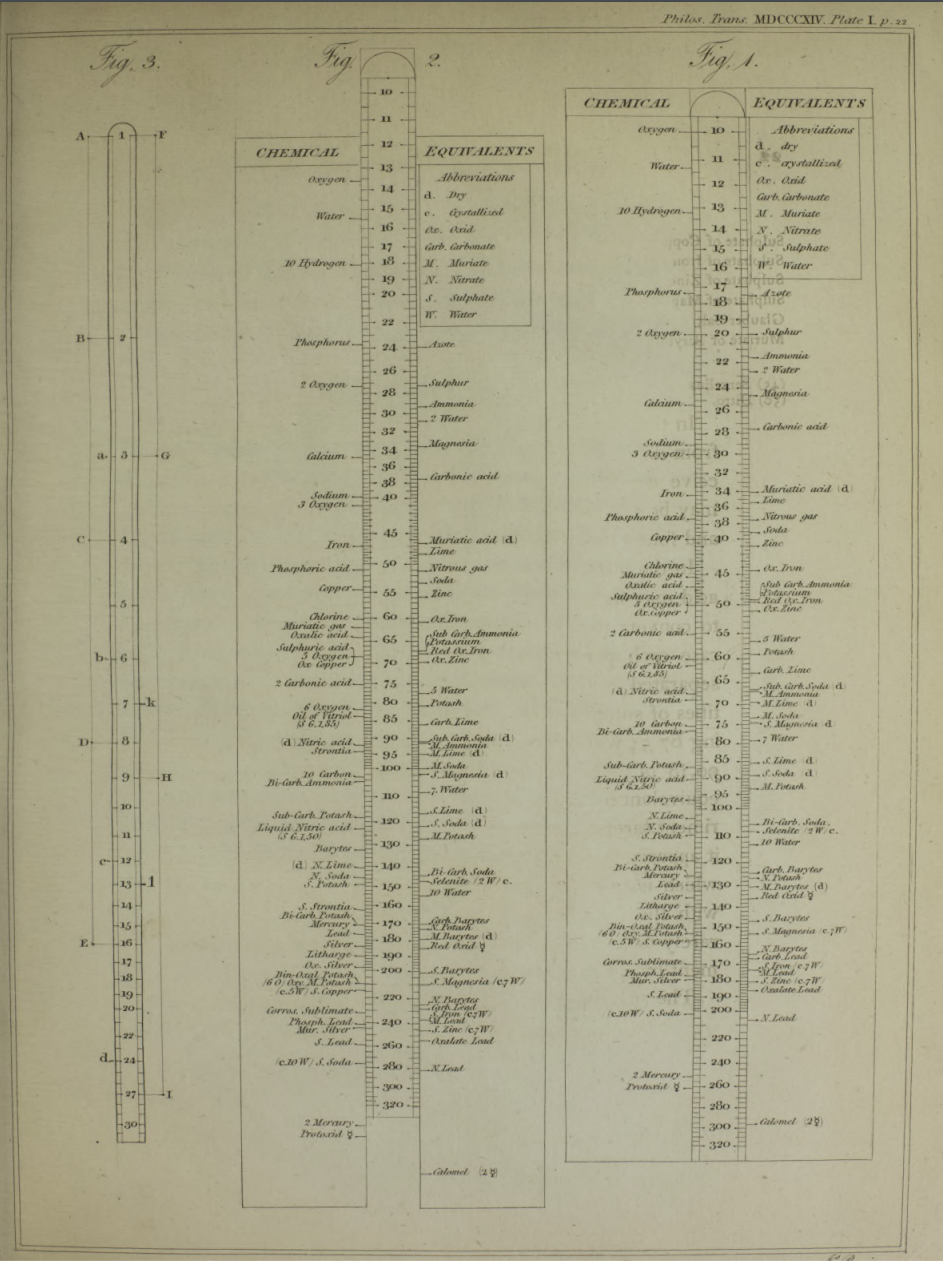
- Chemist's Adjustable Duplex Slide Rule made by Keuffel & Esser Co., n.d., ca. 1936-1940. Here are the full instructions for use.
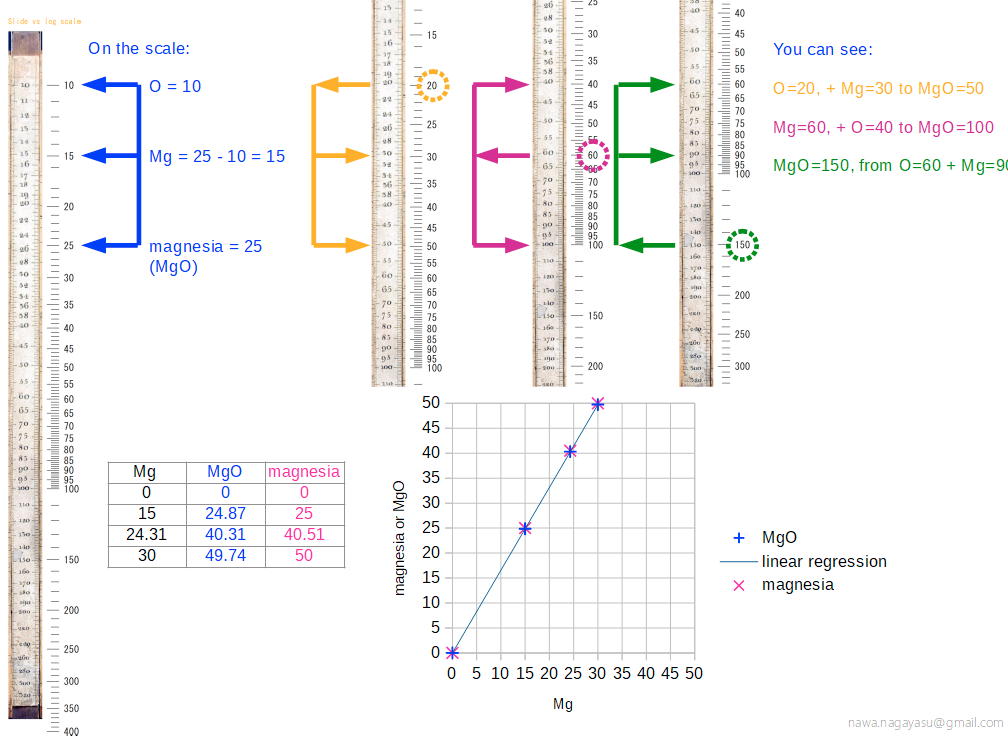
Nagayasu Nawa writes and provides an explanation as how Wollaston's chemical equivalents slide rules should be used:
"It is very interesting slide rule for me. Because we actually used slide rule in 1960s. There were not the electronic calculator in the world. I think it would be used as a simple slide rule of The Law of Definite Proportions by J.L. Proust 1799."
- '10 water', for example, may be hydrating water in chemical compound
- 'Chlorine' may be HClO: HCl(35) + O(10) = HClO(45), etc.
Click image to enlarge:
Thanks to Nawa for the tip!
| Year: 1996 | PT id = 107 |
Elements & Atoms: Case Studies in the Development of Chemistry
Carmen Giunta of Le Moyne College Department of Chemistry has collected many of the original papers plus commentary dealing with eighteenth and nineteenth century science in a web book called Elements and Atoms: Case Studies in the Development of Chemistry. This web resource is highly recommended:
| Year: 1996 | PT id = 944 |
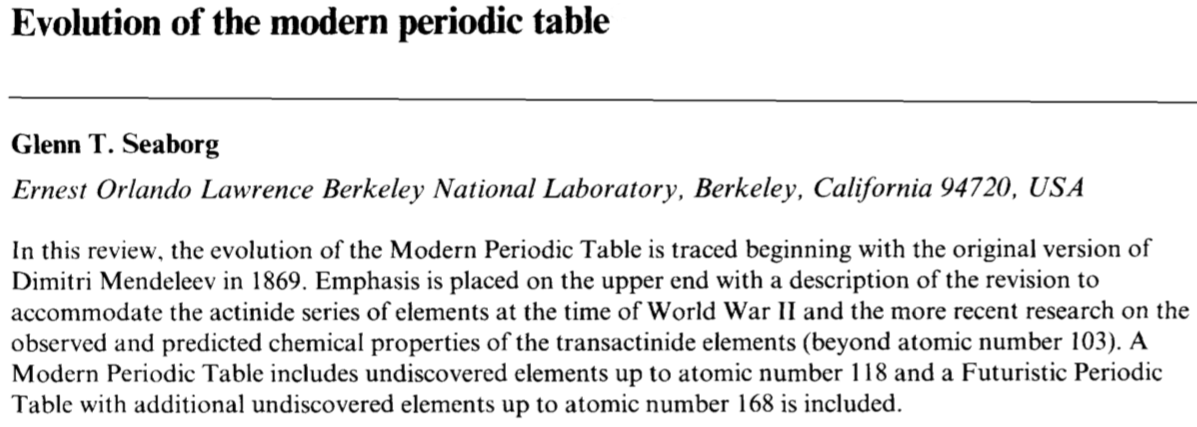
Seaborg's Evolution of the Modern Periodic Table
By Glenn T. Seaborg, from J. Chem. SOC., Dalton Trans., 1996, Pages 3899-3907:
"In this review, the evolution of the Modern Periodic Table is traced beginning with the original version of Dimitri Mendeleev in 1869.Emphasis is placed on the upper end with a description of the revision to accommodate the actinide series of elements at the time of World War II and the more recent research on the observed and predicted chemical properties of the transactinide elements (beyond atomic number 103).A Modern Periodic Table includes undiscovered elements up to atomic number 118 and a Futuristic Periodic Table with additional undiscovered elements up to atomic number 168 is included."
| Year: 1996 | PT id = 250 |
Concept of Chemical Periodicity
Concept of Chemical Periodicity: from Mendeleev Table to Molecular Hyper-Periodicity Patterns E. V. Babaev and Ray Hefferlin
The paper "The Concepts of Periodicity and Hyper-Periodicity: from Atoms to Molecules" was published as a the book: Concepts in Chemistry: a Contemporary Challenge. (Ed. D.Rouvray). Research Studies Press, London, 1996, pp. 24-81.
The website, here, is the original text of this paper (copyright by the authors).
The text deals with periodicity in isotopes, atoms and materials.
| Year: 1999 | PT id = 108 |
Dave Trapp' Development of the Periodic Chart
Dave Trapp has an excellent discussion of the development of the periodic table on his Development of the Periodic Chart pages, part of his Sequim Science web site.
Dave Trapp also has a web site dealing with the origin of the names of the elements:
| Year: 2000 | PT id = 972 |
Jensen Article: The Periodic Law and Table
From William (Bill) Jensen's website, an article: The Periodic Law and Table (written for Britannica on Line, Encyclopaedia Britannica: Chicago, lL, 2000, but never published. ).
| Year: 2001 | PT id = 1025 |
Wikipedia Periodic Table
The Wikipedia Periodic Table pages are astonishing, giving hyper-linked data about:
- Formulations
- History
- Discovery
- Elements
- Isotopes
- Personalities
- Compounds
- etc.
| Year: 2004 | PT id = 1100 |
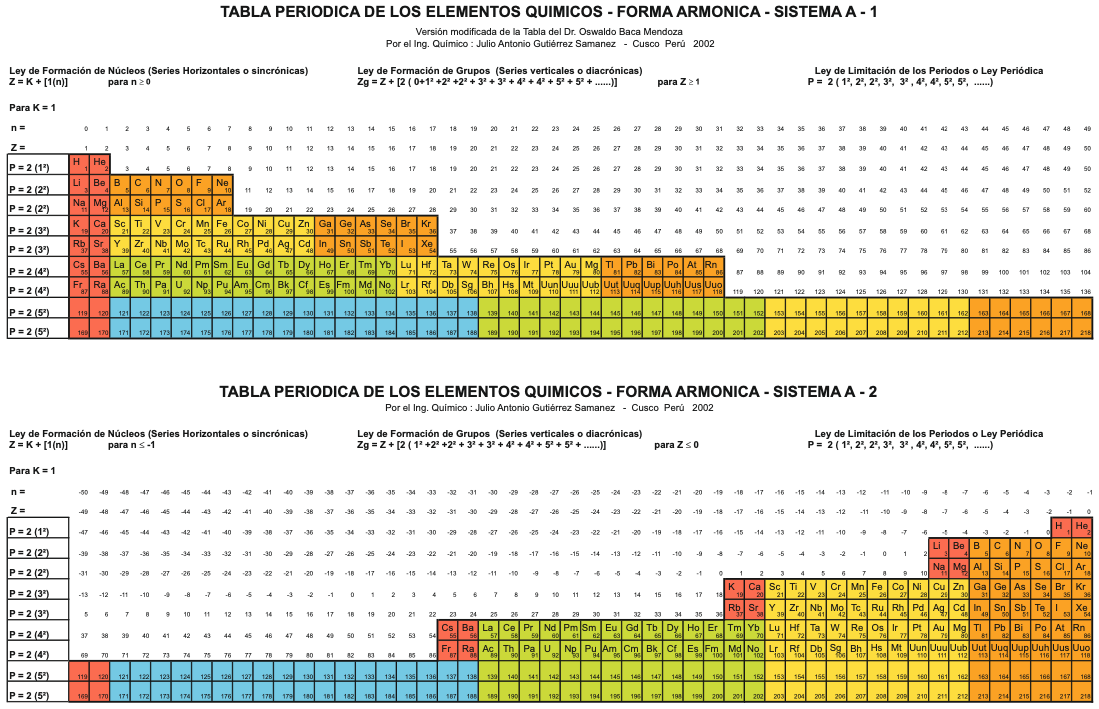
Sistema Periódico Armonico de Gutierrez-Samanez
A digitised 2004 book by book by Julio Gutiérrez-Samanez.
Julio writes:
"These matrix tables are inspired by the method used by the Peruvian chemist Oswaldo Baca Mendoza (1908-1962).
"The tables are read in this way: The Law of Formation of nuclei generates all the horizontal series Z, is dependent on n (series of integers numbers) and a constant K = 1. In the step-to-right tables (n) it will be equal to or greater than 0. In Janet's Left-Step table, (n) will be less than or equal to (-1). The values of this series Z will serve as a constant for the second law.
"The Group Formation Law or vertical series. Its generate the numeric values of the columns either from left to right or from right to left. In system A -1: With the first law: n = 0, then Z = 1. The vertical series Zg = 1, 3 11, 19, 37, 55, 87 ... That is: 1H, 3Li, 11Na, 19K, 37Rb, 55Cs, 87Fr, 119, 169 ... Changing the values of n or Z, all the columns of the table will be obtained.
"In system A -2: With the first law: on the left, n = -1, then Z = 0. The vertical series Zg will be: 2He, 10Ne, 18Ar, 36Kr, 54Xe, 86Rn, 118Og, 168, 218. .. Similarly, changing the n or Z values, we can fills the columns of the table. In system B -1: With the first law: for n = 0, then Z = 1. The vertical series Zg will be; 1H, 3Li, 5B, 13Al, 21Sc, 39Y, 57La, 89Ac, 121, 171 ... By varying the values of n or Z, the entire table is filled. In system B -2: (Its mathematizes the Janet system). With the first law: on the left, for n = -1, then Z = 0.
"The vertical series Zg will be; 2He, 4Be, 12Mg, 20Ca, 38Sr, 56Ba, 88Ra, 120, 170, 220 ... By varying the values of n or Z, the entire table is filled. The third law of the limiting the periods or periodic law, appears graphically, by comparison between rows: For example: in table B -1, in column Z = 3, after 1H and 2He, en of the first horizontal line, the value 3 appears, which is already entered in the first column as 3Li, therefore, that part of the first horizontal row (from 3 to 50) is deleted.
"The same happens with the number 5 in column 3, which is already in the first column as 5B, therefore it will be deleted in the second row from 5 to 52. The same applies to pair 13, 21 of the column Z = 9, same, with the pair 39, 57 of the column Z = 19 and of the pair 89, 121 of the column Z = 33. For that reason the periods: P are duplicated function: 2 (1 ^ 2), 2 (1 ^ 2), 2 (2 ^ 2), 2 (2 ^ 2), 2 (3 ^ 2), 2 (3 ^ 2) .... = 2, 2, 8, 8, 18, 18, 32, 32 ... and the forms are exact and staggered. The colors represent the quantum functions: s (red), p (orange), d (yellow), f (green), g (blue)."
| Year: 2004 | PT id = 111 |
Rouvray & King's The Periodic Table: Into the 21st Century
D. H Rouvray and R. B. King (ed.), The Periodic Table: Into the 21st Century, Research Studies Press 2004.
| Year: 2004 | PT id = 113 |
Peter van der Krogt's Elementymology & Elements Multidict
Peter van der Krogt's Elementymology & Elements Multidict, the web site for element names, origins (etymology) of element names and translations into other languages.
| Year: 2006 | PT id = 42 |
Henry Bent's Exploration into Janet's Left-Step Formulation
Henry Ben't detailed exploration into the Left-Step formulation of the periodic table is available as a book:
| Year: 2006 | PT id = 106 |
Eric Scerri's The Periodic Table & Its Significance
Eric Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006. Read an interview with the author, here, and a review of the book here.
| Year: 2008 | PT id = 266 |
Elements Unearthed
The Elements Unearthed is a blog by David V Black concerning "Our Discovery and Usage of the Chemical Elements".
| Year: 2008 | PT id = 333 |
Braille Guidebook Interactive Periodic Table Study Set
Azer's Interactive Periodic Table Study Set is designed to make learning about the Periodic Table of the Elements accessible to students with visual impairments or blindness.
The tangible materials included with this study set complement APH's Periodic Table of the Elements Reference Chart and allow students to enhance their understanding of concepts consistent with the National Science Standards.
Inspired by Samir Azer, a science teacher at the Kentucky School for the Blind, this set can assist in the instruction and demonstration of concepts related to the arrangement of the periodic table, atomic structure, ionic and covalent bonding, and balancing of chemical equations to students who benefit from a hands-on, interactive model.
Special attention was given to make the materials tactually discriminable and visually appealing to the target population, yet appropriate for all students regardless of visual acuity:
| Year: 2008 | PT id = 361 |
Mathematical Formulas Describing the Sequences of the Periodic Table
Mathematical formulas describing all of the sequences of the chemical elements are derived from double tetrahedron face-centered cubic lattice model. More here.
J. Garai, Department of Earth Sciences, Florida International University. International Journal of Quantum Chemistry, Vol 108, 667-670 (2008):
| Year: 2008 | PT id = 201 |
Periodic Table Radio Show "The Music of Matter"
Periodic Table Radio Show "The Music of Matter" featuring John Emsley, Oliver Sacks & Eric Scerri
| Year: 2008 | PT id = 203 |
Allperiodictables.com
Roy Alexander, inventor of the "Desk-Topper" 3-dimensional formulation has developed a rich periodic table resource.. available at Allperiodictables.com.
| Year: 2008 | PT id = 227 |
Chemistry In Its Element
Introducing Chemistry in its element, a tour of the periodic table.
A leading scientist or author tells the stories behind the elements in a five minute podcast.
Podcasts to Download:
etc...
| Year: 2009 | PT id = 200 |
Scerri's Selected Papers on The Periodic Table
Edited by Eric Scerri (University of California, Los Angeles, USA)
Published by: Imperial College Press in London
The book contains key articles by Eric Scerri, the leading authority on the history and philosophy of the periodic table of the elements. These articles explore a range of topics such as the historical evolution of the periodic system as well as its philosophical status and its relationship to modern quantum physics. In this present volume, many of the more in-depth research papers, which formed the basis for this publication, are presented in their entirety; they have also been published in highly accessible science magazines (such as American Scientist), and journals in history and philosophy of science, as well as quantum chemistry. This must-have publication is completely unique as there is nothing of this form currently available on the market.
Contents:
- Chemistry, Spectroscopy, and the Question of Reduction
- The Electronic Configuration Model, Quantum Mechanics and Reduction
- The Periodic Table and the Electron
- How Good is the Quantum Mechanical Explanation of the Periodic System
- Prediction and the Periodic Table
- Löwdin's Remarks on the Aufbau Principle and a Philosopher's View of Ab Initio Quantum Chemistry
- Mendeleev's Legacy
- The Role of Triads in the Evolution of the Periodic Table: Past and Present
- The Past and Future of the Periodic Table
- The Dual Sense of the Term "Elements", Attempts to Derive the Madelung Rule, and the Optimal Form of the Periodic Table, If Any
Readership: Academic readers: philosophers and science historians, science educators, chemists and physicists. 200pp (approx.) Pub. date: Scheduled Fall 2009
200pp (approx.) Pub. date: Scheduled Fall 2009
ISBN 978-1-84816-425-3
1-84816-425-4 US$88 / £66
| Year: 2009 | PT id = 251 |
Gray's The Elements
As Theo (modestly... ) says:
"Much anticipated (by me at least), this is the definitive be-all, end-all book of the elements. Like my poster, it contains beautiful photographs of all the chemical elements, shining out from a deep black background. But unlike my poster, it's not limited to just one picture per element. Instead each element gets a whole 2-page spread. At 10" x 20" (25cm x 50cm), each spread is as large as the whole place mat version of my poster! And several of the more popular elements even get two spreads. There are literally hundreds and hundreds of photos in this book, nearly all of them taken by myself and my co-author Nick Mann of objects in my collection."
Read more here.
| Year: 2009 | PT id = 253 |
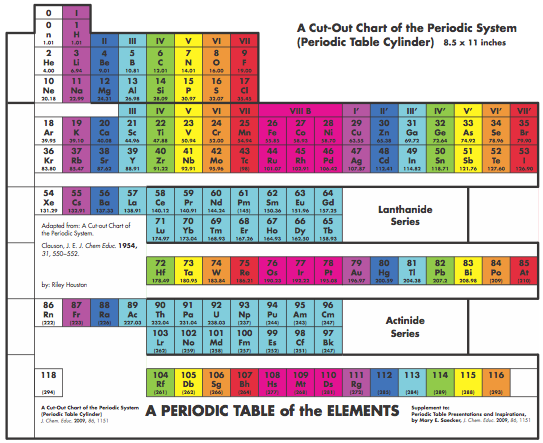
Graphic Representations of the Periodic System
Mary E. Saecker writes an article in Chemical Education Digital Library, Periodic Table Presentations and Inspirations: Graphic Representations of the Periodic System, that reviews some periodic table formunations.
The paper contains a link to this pdf file which gives templates and instructions for several print, cut-out & build periodic table formulations:
Supplement to: Periodic Table Presentations and Inspirations by Mary E. Saecker, J. Chem. Educ., 2009, 86, 1151.
Construction Directions A Cut-Out Chart of the Periodic System (Periodic Table Cylinder)
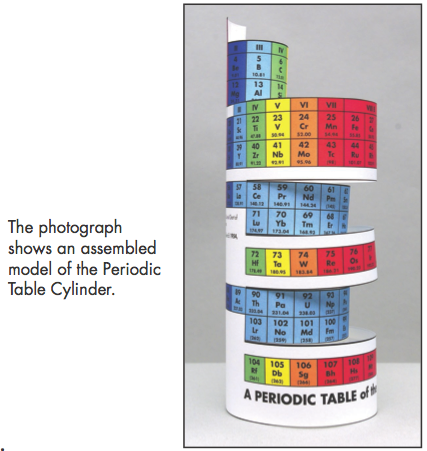
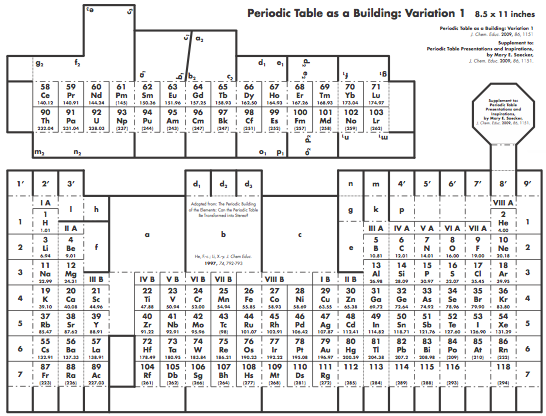
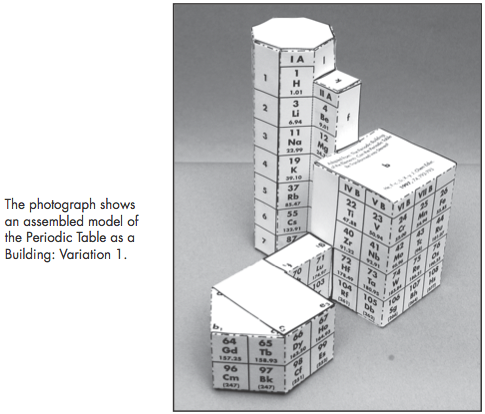
| Year: 2010 | PT id = 302 |
Before & After Mendeleev: Periodic Table Videos
Two videos by the Chemical Heritage Foundation:
- Part 1 Before Mendeleev (17min) covers the events leading up to Mendeleev's invention of the periodic table, including the work of several precursors such as de Chancourtois, Newlands, Odling, Hinrichs, and Meyer.
- Part 2 Mendeleeve & Beyond (20 min). The second part covers Mendeleev's working out of his periodic system and the work of his successors, as well as some interesting questions such as whether the periodic table can be entirely deduced from quantum mechanics and the mystery of the Knight's Move pattern of properties.
The videos feature interviews with Dr. Eric Scerri of UCLA, with added narration, animations, illustrations, photos, captions, etc. by David V. Black as well as publication artwork and notes by Edward G. Mazurs.
| Year: 2010 | PT id = 316 |
Disappearing Spoon
The Disappearing Spoon is a 2010 book by Sam Kean:
"The Periodic Table is one of our crowning scientific achievements, but it's also a treasure trove of passion, adventure, betrayal, and obsession. The fascinating tales in The Disappearing Spoon follow carbon, neon, silicon, gold, and every single element on the table as they play out their parts in human history, finance, mythology, conflict, the arts, medicine, and the lives of the (frequently) mad scientists who discovered them: Why did a little lithium help cure poet Robert Lowell of his madness? And how did Gallium (Ga, 31) become the go-to element for laboratory pranksters?"
"The Disappearing Spoon has the answers, fusing science with the classic lore of invention, investigation, discovery, and alchemy, from the Big Bang through the end of time."
| Year: 2010 | PT id = 370 |
Rare Earths in the Periodic Table
CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0):

| Year: 2011 | PT id = 581 |
Dufour's Periodic Tree: Two Short Films
Elsewhere in this database we can see the 1990 Dufour's Periodic Tree, now two short films have been made about this 3D formulation, here & here:
Eric Scerri Letter from Ben Ged Low on Vimeo.
Five Foot 3D Model from Ben Ged Low on Vimeo.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2011 | PT id = 623 |
Scerri's Very Short Introduction To The Periodic Table
A book by Eric Scerri, The Periodic Table: A Very Short Introduction.
- Considers the fundamental nature of the periodic table to the physical sciences
- Explores the history of the discovery of trends among elements, to the construction of various forms of the table, and the growth of understanding of its meaning
- Touches on key ideas about both early atomic theory and quantum mechanics, showing how they have proved key to the meaning of the table
- Ideal for those who are curious to learn more about the periodic table and essential for any student of physics and chemistry
- Part of the Very Short Introduction series - over three million copies sold worldwide:
| Year: 2011 | PT id = 379 |
Curious Lives of the Elements: Periodic Tales
Periodic Tales: The Curious Lives of the Elements by Hugh Aldersey-Williams and published by Viking, ISBN: 9780670918119.
Everything is made of them, from the furthest reaches of the universe to this book that you hold in your hands, including you.
Like you, the elements have lives: personalities and attitudes, talents and shortcomings, stories rich with meaning. You may think of them as the inscrutable letters of the periodic table but you know them much better than you realise.
Welcome to a dazzling tour through history and literature, science and art. Here you'll meet iron that rains from the heavens and noble gases that light the way to vice. You'll learn how lead can tell your future while zinc may one day line your coffin. You'll discover what connects the bones in your body with the Whitehouse in Washington, the glow of a streetlamp with the salt on your dinner table.
From ancient civilisations to contemporary culture, from the oxygen of publicity to the phosphorus in your pee, the elements are near and far and all around us. Unlocking their astonishing secrets and colourful pasts, Periodic Tales will take you on a voyage of wonder and discovery, excitement and novelty, beauty and truth. Along the way, you'll find that their stories are our stories, and their lives are inextricable from our own.
| Year: 2012 | PT id = 516 |
Eric Scerri's Lecture on The Periodic Table
A lecture by Eric Scerri at the Oscar Peterson auditorium of Concordia University, in Montreal.
The topic is the history and iconic nature of the Periodic Table, in high quality video, about one hour:
| Year: 2012 | PT id = 543 |
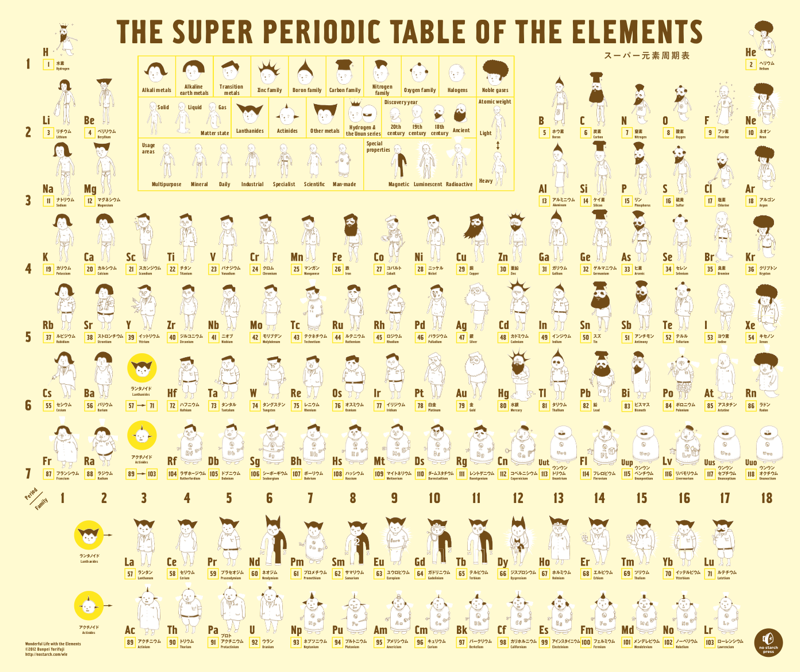
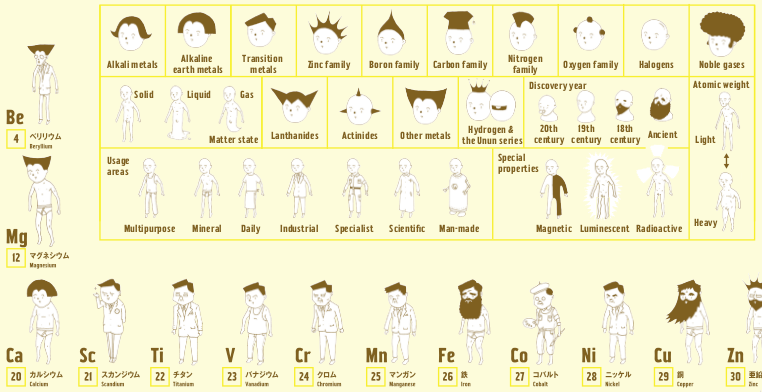
Wonderful Life with the Elements
From the Japanese artist Bunpei Yorifuji comes Wonderful Life with the Elements, an illustrated guide to the periodic table that gives chemistry a friendly face, available from Amazon.
In this super periodic table, every element is a unique character whose properties are represented visually: heavy elements are fat, man-made elements are robots, and noble gases sport impressive afros. Every detail is significant, from the length of an element's beard to the clothes on its back. You'll also learn about each element's discovery, its common uses, and other vital stats like whether it floats - or explodes - in water.
There is also a full review with more images from Wired.
| Year: 2012 | PT id = 558 |
Scientific American: The Quest for the Periodic Table
From Scientific American, a series of original articles (scanned) dealing with the development of the periodic table dating from 1861 to 1998.
Edited by Eric Scerri.
| Year: 2012 | PT id = 1102 |
Eric Scerri Lecture, Dedicated to Fernando Dufour
Dr. Eric Scerri from the Chemistry Department at UCLA giving a distinguished invited lecture at the Oscar Peterson auditorium of Concordia University, in Montreal. The topic is the history and iconic nature of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2012 | PT id = 484 |
Eric Scerri.com
ericscerri.com is the personal internet domain and website of Eric Scerri: chemist and leading philosopher of science specializing in the history and philosophy of the periodic table. He is founder and editor-in-chief of the international journal Foundations of Chemistry, which publishes academic papers concerned with the PT, and is the author of the respected book: The Periodic Table and Its Significance (Oxford University Press, 2007).
The website has links to all of Eric's extensive publications, including online video lectures and interviews and external links.
| Year: 2012 | PT id = 485 |
A Tale of 7 Elements
A new book by Eric Scerri: A Tale of 7 Elements about seven 'missing' elements: protactinium, hafnium, rhenium, technetium, francium, astatine, promethium:
| Year: 2012 | PT id = 501 |
Books on the Chemical Elements and the Periodic Table/System
From Eric Scerri's forthcoming book A Tale of Seven Elements (Oxford University Press, 2013) and used by permission of the author, is the most complete and up-to-date list of Books on the Chemical Elements and the Periodic Table/System, including some titles in foreign languages.
Additional books in other languages can be found listed in Mazurs, 1974
- H. Alderesey-Williams, Periodic Tales, Viking Press, 2011
- N.P. Agafoshin, Ley Periódica y Sistema Periódico de los Elementos de Mendeleiev, Ed. Reverté S.A., Barcelona, 1977
- I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966
- P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995
- O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953
- P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002
- P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004
- I. Barber, Sorting The Elements: The Periodic Table at Work, Rourke Publishing, Vero Beach, Florida, US, 2008
- R. Baum (ed), Celebrating the Periodic Table, Chemical & Engineering News, A Special Collector's Issue, September 8, 2003
- H.A. Bent, New Ideas in Chemistry from Fresh Energy for the Periodic Law, Author House, Bloomington IN, 2006
- J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007
- J. C.A. Boeyens, D.C. Lavendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008
- N. Bohr, Collected Works Vol 4. The Periodic System (1920-1923), Nielsen J Rud (Editor), North Holland Publishing Company, 1977
- T. Bondora, The Periodic Table of Elements Coloring Book, Bondora Educational Media Publications, 2010
- D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964
- P.A. Cox, The Elements, Oxford University Press, Oxford, 1989
- P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002
- H. Dingle and G.R. Martin, Chemistry and Beyond: Collected Essays of F.A. Paneth, Interscience, New York, NY, 1964
- S. Dockx, Theorie Fondamentale du Systeme Periodique des Elements, Office Internationale de Librairie, Bruxelles, 1950
- A. Ducrocq, Les éléments au pouvoir, Julliard, Paris, 1976
- A. Ede, The Chemical Elements, Greenwood Press, Westport, CT, 2006
- J. Emsley, The Elements, 3rd edition. Clarendon, Oxford University Press, 1998
- J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001
- P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004
- D.E. Fisher, Much Ado About (Practically) Nothing, The History of the Noble Gases, Oxford University Press, New York, 2010
- I. Freund, The Study of Chemical Composition: An Account of its Method and Historical Development, Dover Publications, Inc., New York, NY, 1968
- J. García-Sancho & F. Ortega-Chicote, Periodicidad Química, Trillas, México, 1984
- A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909
- L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988
- L. Gonik, C. Criddle, The Cartoon Guide to Chemistry, Harper Resource, New York, 2005
- M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, Basic Books, New York, 2004
- T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009
- D. Green, The Elements, The Building Blocks of the Universe, Scholastic Inc. New York, 2012
- R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989
- D.L. Heiserman, Exploring the Chemical Elements and their Compounds, McGraw-Hill New York, 1991
- S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002
- F. Hund, Linienspektren und Periodisches System der Elemente, Verlag von Julius Springer, Berlin, 1927
- W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869-1905, Dover, Mineola, NY, 2005
- S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010
- D.M. Knight, Classical Scientific Papers, Chemistry Second Series, American, Elsevier, New York, NY
- P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982
- H.M. Leicester and H.S. Klickstein, A Source Book in Chemistry 1400-1900, 1st Edition, McGraw-Hill Book Company Inc., London, 1952
- M.F. L'Annunziata, Radioactivity, Introduction and History, Elsevier, 2007
- S.E.V. Lemus, Clasificación periódica de Mendelejew, Guatemalan Ministry of Public Education, Guatemala, 1959
- P. Levi, The Periodic Table, 1st American Edition. Schocken Books, New York, NY, 1984
- R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997
- J. Marshall, Discovery of the Elements, Pearson Custom Publishing, 1998
- E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974
- D. Mendeleeff, An Attempt Towards A Chemical Conception of the Ether, translated by G. Kamensky. Longmans, Green, and Co., London, 1904
- D. Mendeleeff, The Principles of Chemistry, translated by G. Kamensky, 5th Edition, vol. 2. Longmans, Green, and Co., London, 1891
- L. Meyer, Modern Theories of Chemistry, 5th Edition, translated by P.P. Bedson, Longmans, Green, and Co., London, 1888
- L. Meyer, Outlines of Theoretical Chemistry, 2nd Edition, translated by P.P. Bedson and W.C. William. Longmans, Green, and Co., London, 1899
- F. Mohr, (E), Gold Chemistry, Wiley-VCH, 2009
- D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003
- I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Norfolk, UK, 1997
- R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010
- M.J. Pentz, (General Editor), The Periodic Table and Chemical Bonding, Open University Press, Bletchley, Buckinghamshire, UK, 1971
- I.V. Peryanov, D.N. Trifonov, Elementary Order: Mendeleev's Periodic System, translated from the Russian by Nicholas Weinstein, Mir Publishers, Moscow, 1984
- J.S.F. Pode, The Periodic Table, John Wiley, New York, NY, 1971
- R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972
- R.J. Puddephatt and P.K. Monaghan, The Periodic Table of the Elements, 2nd edition. Oxford University Press, Oxford, 1986
- H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007
- E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930
- R. Rich, Periodic Correlations, Benjamin, New York, 1965
- J. Ridgen, Hydrogen, The Essential Element, Harvard University Press, Cambridge, MA, 2002
- H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998
- D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004
- D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006
- G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900
- G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904
- O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001
- R.T. Sanderson, Periodic Table of the Chemical Elements, School Technical Publishers, Ann Arbor, MI, 1971
- S. E. Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009
- E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007
- E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, London and Singapore, 2009
- E.R. Scerri, A Very Short Introduction to the Periodic Table, Oxford University Press, Oxford, 2011; Also translated into Spanish and Arabic.
- E.R. Scerri, Le Tableau Périodique, Son Histoire et sa Signification, EDP Sciences, 2011, (translated by R. Luft); Japanese Translation by Hisao Mabuchi et. al.
- C. Schmidt, Das periodische System der chemischen Elementen, Leipzig, 1917.
- G.T. Seaborg, W.D. Loveland, The Elements Beyond Uranium, Wiley, New York, 1990
- M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992
- H.H. Sisler, Electronic Structure, Properties, and the Periodic Law, Reinhold, New York, 1963
- P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999
- R.S. Timmreck, The Power of the Periodic Table, Royal Palm Publishing, 1991
- M. Tweed, Essential Elements, Walker and Company, New York, 2003
- F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896
- M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960
- B.D. Wilker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003
- J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969
- T. Zoellner, Uranium, Penguin Books, London, 2009
- A. Zwertska, The Elements, Oxford University Press, Oxford, 1998
Works by D. I. Mendeleev
- Nauchnyi arkhiv. Periodicheskii zakon, t. I, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1953
- Periodicheskii zakon. Dopolnitel'nye materialy. Klassiki nauki, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1960
- Periodicheskii zakon. Klassiki nauki, ed. B. M. Kedrov. Moscow: Izd. AN SSSR, 1958
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2013 | PT id = 566 |
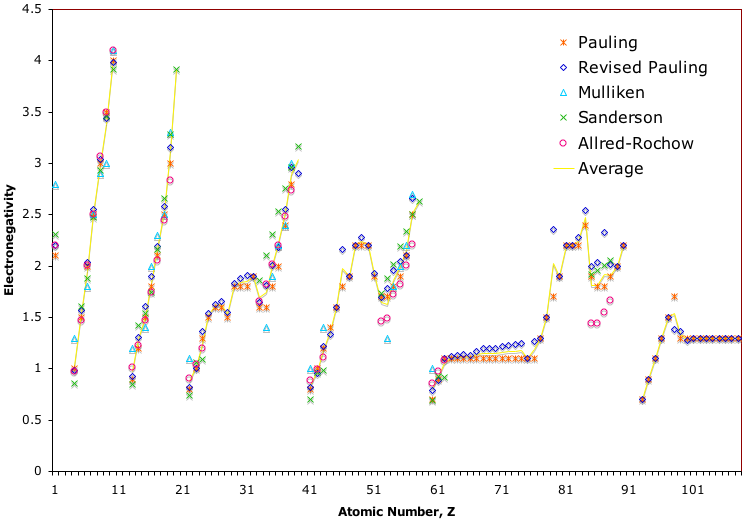
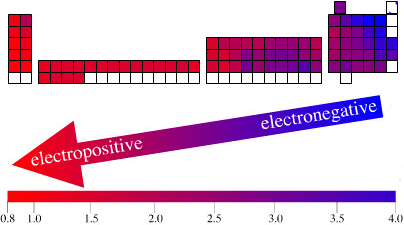
Electronegativity Chart (Leach)
From Mark R Leach's paper, Concerning electronegativity as a basic elemental property and why the periodic table is usually represented in its medium form, Journal & PDF.
Due to the importance of Pauling's electronegativity scale, as published in The Nature of The Chemical Bond (1960), where electronegativity ranges from Cs 0.7 to F 4.0, all the other electronegativity scales are routinely normalised with respect to Pauling's range.
When the Pauling, Revised Pauling, Mulliken, Sanderson and Allred-Rochow electronegativity scales are plotted together against atomic number, Z, the similarity of the data can be observed. The solid line shows the averaged data:
| Year: 2013 | PT id = 591 |
30 Second Elements
30 Second Elements The 50 most significant elements, each explained in half a minute. A book Edited by Eric Scerri and published by Ivy Press.
"30 Second Elements presents you with the foundations of chemical knowledge, distilling the 50 most significant chemical elements into half-a-minute individual entries, using nothing more than two pages, 300 words and one picture. Divided into seven chapters, it includes the atomic details of the other 68 elements and the relationships of all 118, as well as biographies of the chemists who transformed scientific knowledge and unlocked the mysteries of life itself. Illustrated with explosive graphics, here is the quickest way to know your arsenic from your europium".
The curator of this website is a contributor:
| Year: 2013 | PT id = 596 |
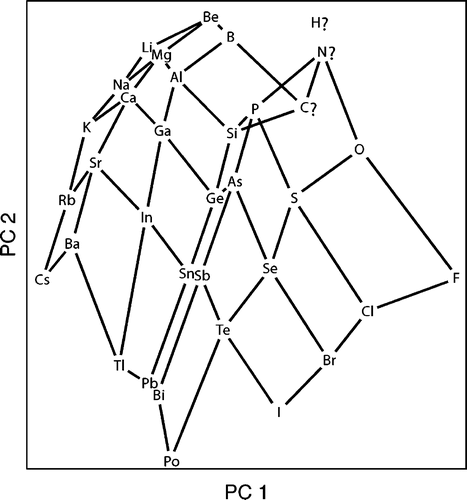
From Periodic Properties to a Periodic Table Arrangement
A paper in J.Chem. Ed.: From Periodic Properties to a Periodic Table Arrangement
Emili Besalú, Departament de Química i Institut de Química Computacíonal i Catàlisis, Universitat de Girona, C/Maria Aurèlia Capmany, 69, 17071 Girona, Catalonia, Spain.
J. Chem. Educ., 2013, 90 (8), pp 1009-1013 DOI: 10.1021/ed3004534 Publication Date (Web)
"A periodic table is constructed from the consideration of periodic properties and the application of the principal components analysis technique. This procedure is useful for objects classification and data reduction and has been used in the field of chemistry for many applications, such as lanthanides, molecules, or conformers classification. From the information given, the whole procedure can be reproduced by any interested reader having a basic background in statistics and with the help of the supplementary material provided. Intermediate calculations are instructive because they quantify several concepts the students know only at a qualitative level. The final scores representation reveals an unexpected periodic table presenting some interesting features and points for discussion."
| Year: 2013 | PT id = 608 |
Twitter @periodic_table
The Twitter feed from Mark Winter of WebElements:
| Year: 2013 | PT id = 610 |
Top 10 Periodic Tables
There are more than 1000 periodic tables hosted by the Chemogenesis Webbook Periodic Table database, so it can be a little difficult to find the exceptional ones.
Here we present – in our humble opinion – The ten most significant periodic tables in the database.
We present the best:
- Three best data rich periodic tables
- Five formulations which show the development of the modern PT
- One, of many, interesting alternative formulations
- One example of the periodic table being used as an infographic template
Three Excellent, Data Rich Periodic Tables
The first three of our top 10 periodic tables are classic element data repositories.
They all work in the same way: click on the element symbol to get data/information about the selected element. The three are Mark Winter's WebElements, Theo Gray's Photographic Periodic Table & Michael Dayah's Ptable.
- Since 1993 – and with its rather bland interface – WebElements has given access to vast quantities of in depth chemical data & information. This is the professional chemist's periodic table:
- Theo Gray's Photographic Periodic Table is undoubtedly the most attractive PT available in web space, but there is more. Clicking around the website gives access to a host of information, pictures & anecdotes from Theo's extraordinary and extensive collection of chemical elements:
- Ptable has a super-slick, and very fast interface. It is data/information rich and is available in 50 languages:
Five Formulations Showing The History & Development
The next five examples deal with history and development Periodic Table. The first is Dalton's 1808 list of elements, next is Mendeleev's 1869 Tabelle I, then Werner's remarkably modern looking 1905 formulation. This is followed by Janet's Left Step formulation and then a discussion of how and why the commonly used medium form PT formulation, is constructed.
- There are several early listings of chemical substances, including Valentinus' Alchemy Table and Lavoisier's Table of Simple Substances (1789). In 1803 Dalton proposed that matter consists of discrete atoms that combine in fixed ratios, stoichiometry, to form chemical elements. Thus, Dalton's list of chemical elements, plus mass data, must be included in any top ten listing:

- If you examine the periodic tables from Antiquity to 1899, you will see that from about 1830 onwards, proto-periodic tables were coming thick and fast. Significant developments include: Daubeny's Teaching Display Board of Atomic Weights (1831), Chancourtois Telluric Helix (1862) and Newlands octaves (1864).
But, it was Mendeleev's Tabelle I that was first near complete periodic table formulation of the then known elements (no Group 18 rare gasses, note). Crucially, Mendeleev identified gaps and was able to make predictions about the chemical properties of the missing substances. Plus, Mendeleev promoted his ideas with great energy:

- Werner's 1905 Periodic Table is remarkably modern looking. The formulation is a long form that separates transition metals and rare earths, but he guessed wrong on how many existed:

- Janet's Left Step formulation of 1928 is one for the purists as it clearly shows the chemical elements arranged into s, p, d & f-blocks of the recently developed quantum mechanical description of atomic structure:
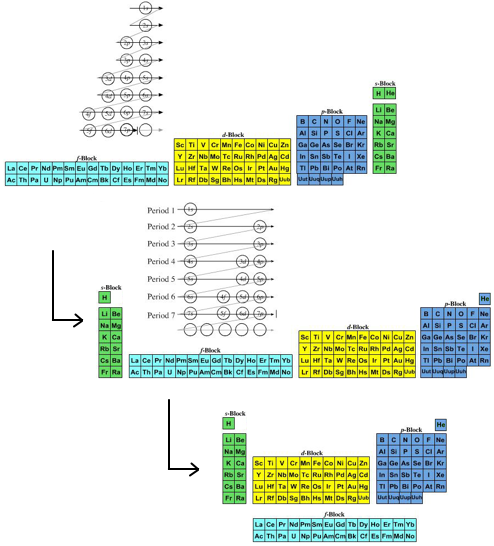
- The modern (and commonly employed) periodic table is obtained by transforming Janet's Left Step into the modern long form periodic table by rearranging the blocks around. This transformational mapping is discussed in some detail here.
The long form and medium form PTs have electronegativity trending from top-right (electronegative) to bottom left (electropositive), and many aspects of periodicity corollate with electronegativity: atomic radius, first ionisation energy, etc.
Thus, the long form and medium form periodic tables are commonly used in the classroom:
An Alternative Formulation
The internet database contains many, many alternative formulations, and these are often spiral and/or three dimensional. These exemplified by the 1965 Alexander DeskTopper Arrangement. To see the variety of formulations available, check out the Spiral & Helical and 3-Dimensional formulations in the database:

Non-Chemistry PTs
The periodic table as a motif is a useful and commonly used infographic template for arranging many types of object with, from 50 to 150 members.
There are numerous examples in the Non-Chemistry section where dozens of completely random representations can be found:
- Adobe Illustrator Shortcuts
- Adult Positions
- Airline Customer Reviews
- Beer Styles
- And, chosen more or less at random, European Nations:
| Year: 2014 | PT id = 655 |
Rogue Elements: What's Wrong with the Periodic Table
An article in New Scientist by Celeste Biever (news editor at Nature), Image by Martin Reznik
Weights gone awry, elements changing position, the ructions of relativity – chemistry's iconic chart is far from stable, and no one knows where it will end
IF IMITATION is the sincerest form of flattery, the periodic table has many true admirers. Typefaces, types of meat and even the Muppets have been ordered in its image. For chemists, knowing an element's position in the periodic table, and the company it keeps, is still the most reliable indicator of its properties – and a precious guide in the search for new substances. "It rivals Darwin's Origin of Species in terms of the impact of bringing order out of chaos," says Peter Edwards of the University of Oxford.
The origins of the periodic table lie in the 19th century, when chemists noticed that patterns began to emerge among the known chemical elements when they... click here to continue:
| Year: 2015 | PT id = 686 |
Elements: A Series of Business Radio Programs/Podcasts
A series of BBC World Service Radio Programs, available as MP3 Podcasts, talking about the chemical elements with a strong business/technology bias, rather than the more usual chemical or historical approach:
Thanks to Marcus Lynch for the tip!
| Year: 2015 | PT id = 689 |
Oliver Sacks' Table of Elements
From Radiolab (a podcast):
"As a young boy, neurologist, author and Radiolab favorite Oliver Sacks pored over the pages of the Handbook of Physics and Chemistry, fantasizing about the day that he, like the shy gas Xenon, would find a companion with whom to connect and share. That companion turned out to be the Periodic Table of the Elements itself, a relationship he's never outgrown. He introduces us to the elements that he's known and loved."
| Year: 2015 | PT id = 709 |
Mystery of Matter: Search for the Elements
The Mystery of Matter: Search for the Elements is a multimedia project about one of the great adventures in the history of science: the long (and continuing) quest to understand what the world is made of – to identify, understand and organize the basic building blocks of matter. In a nutshell, the project is about the human story behind the Periodic Table of the Elements.
The centerpiece of the project is a three-hour series that premieres Aug. 19, 2015 on PBS. The Mystery of Matter introduces viewers to some of history's most extraordinary scientists:
- Joseph Priestley and Antoine Lavoisier, whose discovery of oxygen – and radical interpretation of it – led to the modern science of chemistry
- Humphry Davy, who made electricity a powerful new tool in the search for elements
- Dmitri Mendeleev, whose Periodic Table brought order to the growing gaggle of elements
- Marie Curie, whose groundbreaking research on radioactivity cracked open a window into the atom
- Henry Moseley, whose investigation of atomic number redefined the Periodic Table
- Glenn Seaborg, whose discovery of plutonium opened up a whole new realm of elements, still being explored today.
The Mystery of Matter will show not only what these scientific explorers discovered but also how, using actors to reveal the creative process through the scientists' own words, and conveying their landmark discoveries through re-enactments shot with replicas of their original lab equipment. Knitting these strands together into a coherent, compelling whole is host Michael Emerson, a two-time Emmy Award-winning actor best known for his roles on Lost and Person of Interest. Eric Scerri appears as the expert.
| Year: 2016 | PT id = 1049 |
Mystery of Matter: Three Videos
From Alpha-Omega, three videos about the discovery of the Periodic Table.
The Mystery of Matter: Search for the Elements is an exciting series about one of the great adventures in the history of science: the long and continuing quest to understand what the world is made of. Three episodes tell the story of seven of history's most important scientists as they seek to identify, understand and organize the basic building blocks of matter.
The Mystery of Matter: Search for the Elements shows us not only what these scientific explorers discovered but also how, using actors to reveal the creative process through the scientists' own words and conveying their landmark discoveries through re-enactments shot with replicas of their original lab equipment.
Knitting these strands together is host Michael Emerson, a two-time Emmy Award-winning actor.
Meet Joseph Priestley and Antoine Lavoisier, whose discovery of oxygen led to the modern science of chemistry, and Humphry Davy, who made electricity a powerful new tool in the search for elements.
Watch Dmitri Mendeleev invent the Periodic Table, and see Marie Curie's groundbreaking research on radioactivity crack open a window into the atom.
The Mystery of Matter: Search for the Elements brings the history of science to life for today's television audience.:
| Year: 2017 | PT id = 1120 |
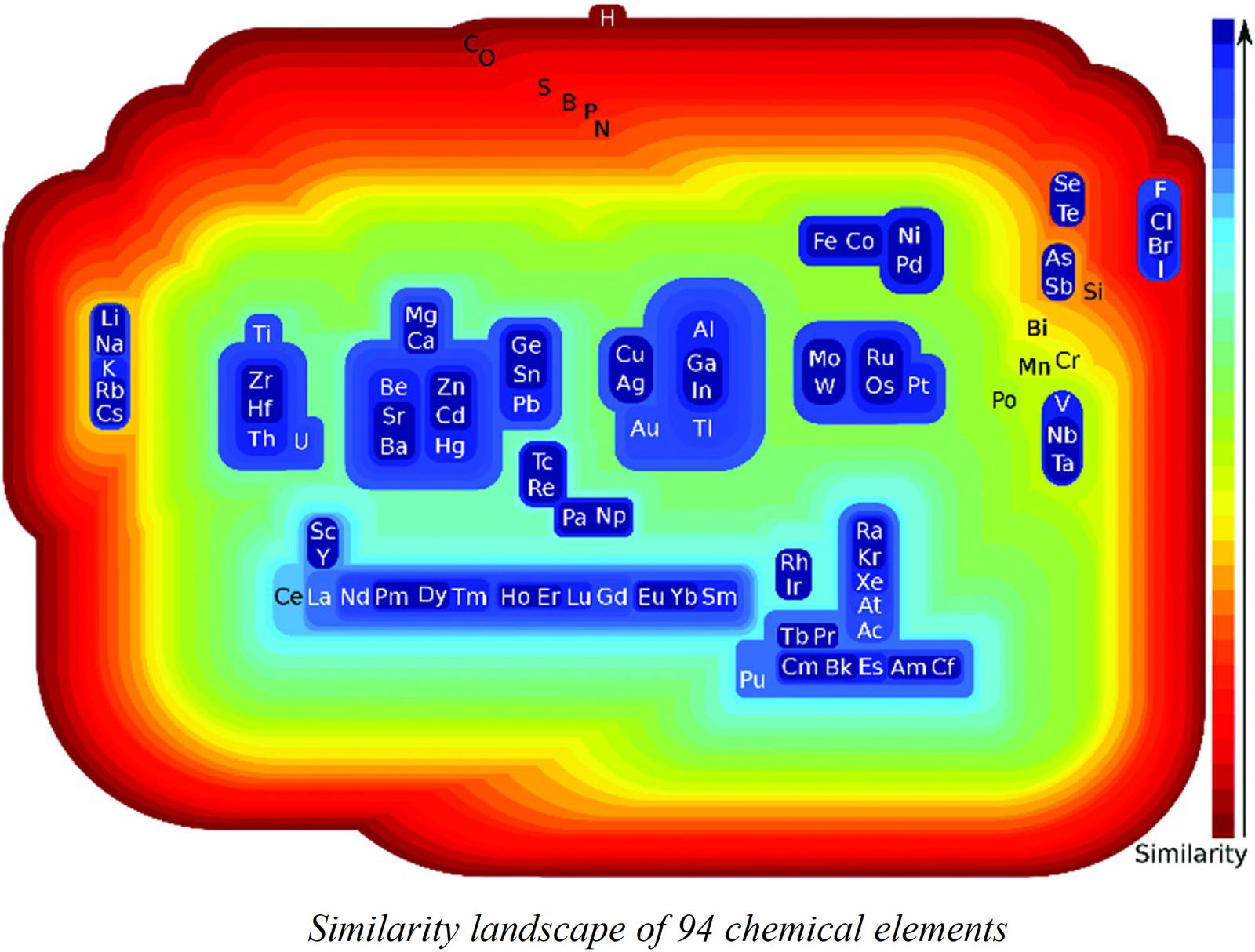
Restrepo's Similarity Landscape
Building Classes of Similar Chemical Elements from Binary Compounds and Their Stoichiometries by Guillermo Restrepo, Chapter 5 from: Elements Old and New: Discoveries, Developments, Challenges, and Environmental Implications p 95-110.
From the abstract:
Similarity is one of the key concepts of the periodic table, which was historically addressed by assessing the resemblance of chemical elements through that of their compounds. A contemporary approach to the similarity among elements is through quantum chemistry, based on the resemblance of the electronic properties of the atoms involved. In spite of having two approaches, the historical one has been almost abandoned and the quantum chemical oversimplified to free atoms, which are of little interest for chemistry. Here we show that a mathematical and computational historical approach yields well-known chemical similarities of chemical elements when studied through binary compounds and their stoichiometries; these similarities are also in agreement with quantum chemistry results for bound atoms. The results come from the analysis of 4,700 binary compounds of 94 chemical elements through the definition of neighbourhoods for every element that were contrasted producing similarity classes. The method detected classes of elements with different patterns on the periodic table, e.g. vertical similarities as in the alkali metals, horizontal ones as in the 4th-row platinum metals and mixed similarities as in the actinoids with some transition metals. We anticipate the methodology here presented to be a starting point for more temporal and even more detailed studies of the periodic table.
Thanks to René for the tip!
| Year: 2017 | PT id = 947 |
Gray's Wooden Periodic Table Table Video
Theo Gray collects elements and has put together this awesome Periodic Table Table. This week Reactions explores the science and chemistry going on inside this periodic table.
Step into his office at Wolfram Research, and you'll see a silicon disc engraved with Homer Simpson, a jar of mercury, uranium shells and thousands of other chemical artifacts. But his real DIY masterpiece is the world's first "periodic table table". Within this masterfully constructed table-top lay samples of nearly every element known to man, minus the super-radioactive ones.
Theo Gray is 2011 winner of the ACS Grady Stack Award for Interpreting Chemistry for the Public. The Periodic Table Table is a testament to Theo's love for chemistry -- as well as his Ebay buying habits -- and is full of fascinating stories.
| Year: 2017 | PT id = 753 |
Habashi Book: The Periodic Table & Mendeleev
By Fathi Habashi, a small book:

| Year: 2018 | PT id = 900 |
Race to Invent the Periodic Table
From PBS Digital Studios, a short-but-fast-moving video about the development of the periodic table during the 19th century, and a discussion about gallium:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 2018 | PT id = 907 |
Alphabet of Chemistry
A BBC World Service radio program, first broadcast Tue 23 Jan 2018.
The Russian chemist Dmitri Mendeleev attempted nothing less than to pull apart the fabric of reality and expose the hidden patterns that lie beneath everything in existence, from shoes and ships and sealing wax to cabbages and kings. The result was something known to almost everyone who has ever been to school: the Periodic Table of the elements. But why this particular arrangement? And why is it still the foundation of chemistry?
The presenter Quentin Cooper is joined by:
- Hugh Aldersey-Williams, who since he was a teenager, has collected samples of elements and has drawn on his samples and knowledge to write Periodic Tales: The Curious Lives of the Elements.
- Michael Gordin, Professor of History at Princeton University and the author of A Well-Ordered Thing: Dmitri Mendeleev and the Shadow of the Periodic Table.
- Ann Robinson, Historian at the University of Massachusetts studying the development of the periodic table.
- Eugene Babaev, Professor of Chemistry at Moscow State University who maintains both Russian and English websites on Mendeleev and his work.

| Year: 2018 | PT id = 919 |
Mendeleev to Oganesson: A Multidisciplinary Perspective on the Periodic Table
Since 1969, the international chemistry community has only held conferences on the topic of the Periodic Table three times, and the 2012 conference in Cusco, Peru was the first in almost a decade. The conference was highly interdisciplinary, featuring papers on geology, physics, mathematical and theoretical chemistry, the history and philosophy of chemistry, and chemical education, from the most reputable Periodic Table scholars across the world. Eric Scerri and Guillermo Restrepo have collected fifteen of the strongest papers presented at this conference, from the most notable Periodic Table scholars. The collected volume will contain pieces on chemistry, philosophy of science, applied mathematics, and science education.
Eric Scerri is a leading philosopher of science specializing in the history and philosophy of chemistry and especially the periodic table. He is the author of numerous OUP books including A Tale of Seven Scientists and a New Philosophy of Science (2016) and The Periodic Table: A Very Short Introduction (2012). Scerri has been a full-time lecturer at UCLA for the past eighteen years where he regularly teaches classes in history and philosophy of science.
Guillermo Restrepo is a chemist specializing in mathematical and philosophy of chemistry with more than sixty scientific papers and book chapters on these and related areas. Restrepo was a professor of chemistry at the Universidad de Pamplona (Colombia) between 2004 and 2017, and since 2014 has been in Germany as an Alexander von Humboldt Fellow at Leipzig University and more recently as researcher at the Max Planck Institute for Mathematics in the Sciences.
Preface
1. Heavy, Superheavy...Quo Vadis?
2. Nuclear Lattice Model and the Electronic Configuration of the Chemical Elements
3. Amateurs and Professionals in Chemistry: The Case of the Periodic System
4. The Periodic System: A Mathematical Approach
5. The "Chemical Mechanics" of the Periodic Table
6. The Grand Periodic Function
7. What Elements Belong in Group 3 of the Periodic Table?
8. The Periodic Table Retrieved from Density Functional Theory Based Concepts: The Electron Density, the Shape Function and the Linear Response Function
9. Resemioticization of Periodicity: A Social Semiotic Perspective
10. Organizing the Transition Metals
11. The Earth Scientist's Periodic Table of the Elements and Their Ions: A New Periodic Table Founded on Non-Traditional Concepts
12. The Origin of Mendeleev's Discovery of the Periodic System
13. Richard Abegg and the Periodic Table
14. The Chemist as Philosopher: D. I. Mendeleev's "The Unit" and "Worldview"
15. The Philosophical Importance of the Periodic Table
| Year: 2018 | PT id = 923 |
Timelines, of The Periodic Table
By Steven Murov, a chronology of the events that have resulted in our present periodic table of the elements and a celebration of the 150th anniversary of the Mendeleev (birthday, 02/08/1834) periodic table (1869).
Recursively, the Murov website has many links to this [Chemogenesis] website.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 2019 | PT id = 1024 |
5 Periodic Tables We Don't Use (And One We Do)
SciShow says:
"From Mendeleev's original design to physicist-favorite "left-step" rendition, the periodic table of elements has gone through many iterations since it was first used to organize elements 150 years ago - each with its own useful insights into the patterns of the elements":
| Year: 2019 | PT id = 1026 |
Papers of Mendeleev, Odlings, Newlands & Chancourtois from the 1860s
Peter Wothers from the University of Cambridge with Sir Martyn Poliakoff, of the University of Nottingham discuss the discovery/development of the periodic table in the 1860s with the original publications.
Links to some of the formulations discussed in the video:
- Mendeleeve Table I
- Mendeleeve Table II
- Odlings' Formulation
- Newlands Octaves
- Chancourtois' Teluric Helix
| Year: 2019 | PT id = 1057 |
Meyer's NYT Graphic
A nice graphic by Alex Eben Meyer in the New York Times accompanying an article about the periodic table and some of Sir Martyn Poliakoff ideas.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1059 |
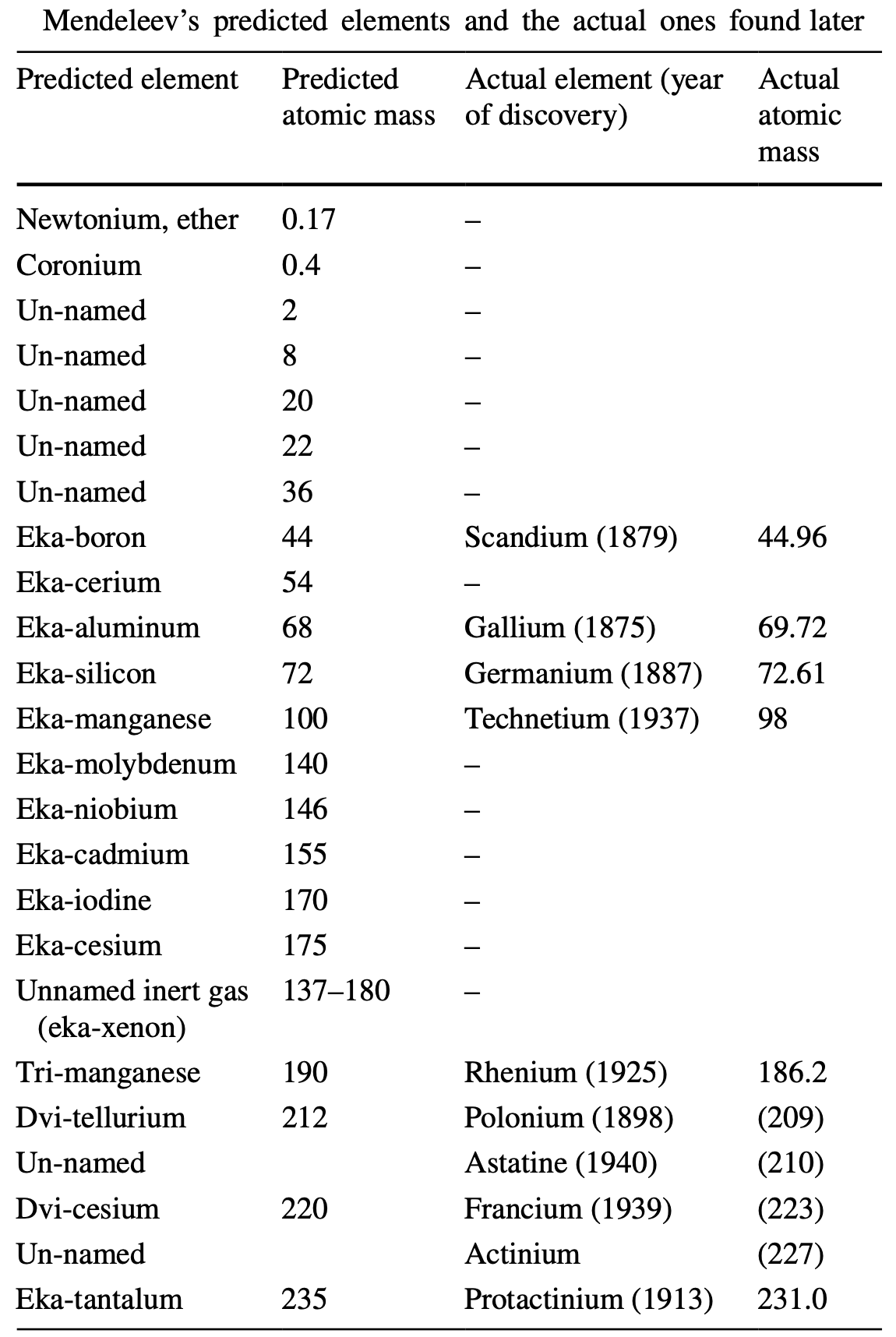
Where Mendeleev Was Wrong
A paper by Gábor Lente, Where Mendeleev was wrong: predicted elements that have never been found, from ChemTexts https://doi.org/10.1007/s40828-019-0092-5.
As is well known, Mendeleev sucessfully predicted the existance of several elements, but he was not always correct.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1060 |
Bloomberg Businessweek Special Issue: The Elements
A Bloomberg Businessweek Special Issue on The Elements.
Using state of the art [2019] web graphics, and packed with interesting business stories:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1061 |
Bloomberg Businessweek: Why the Periodic Table of Elements Is More Important Than Ever
A Bloomberg Businessweek article on the chemical elements: Mendeleev's 150-year-old periodic table has become the menu for a world hungry for material benefits. (This story is from Bloomberg Businessweek's special issue The Elements.)
Thanks to Roy Alexander for the tip!
| Year: 2019 | PT id = 1065 |
Mendeleev 150
Mendeleev 150 is the 4th International Conference on the Periodic Table. The event welcomed nearly 300 guests from over 30 countries and has become one of the key events of IUPAC's International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1068 |
C&EN No Agreement
From C&EN: The periodic table is an icon. But chemists still can't agree on how to arrange it:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1084 |
Scerri's The Periodic Table: Its Story & Its Significance 2nd Edition
The 2nd Edition of Eric Scerri's well regarded book, The Periodic Table: Its Story & Its Significance has been published by Oxford University Press and is available at all good bookshops, including online.
See Eric's website EricScerri.com and Twitter Feed.


| Year: 2019 | PT id = 1094 |
Physical Origin of Chemical Periodicities in the System of Elements
From de Gruyter: Physical origin of chemical periodicities in the system of elements, Chang-Su Cao, Han-Shi Hu, Jun Li* and W. H. Eugen Schwarz*, Pure Appl. Chem. 2019; 91(12).
Published Online: 2019-11-30 | DOI: https://doi.org/10.1515/pac-2019-0901 (open access)
Abstract:
The Periodic Law, one of the great discoveries in human history, is magnificent in the art of chemistry. Different arrangements of chemical elements in differently shaped Periodic Tables serve for different purposes. "Can this Periodic Table be derived from quantum chemistry or physics?" can only be answered positively, if the internal structure of the Periodic Table is explicitly connected to facts and data from chemistry.
Quantum chemical rationalization of such a Periodic Tables is achieved by explaining the details of energies and radii of atomic core and valence orbitals in the leading electron configurations of chemically bonded atoms. The coarse horizontal pseudo-periodicity in seven rows of 2, 8, 8, 18, 18, 32, 32 members is triggered by the low energy of and large gap above the 1s and nsp valence shells (2 ≤ n ≤ 6 !). The pseudo-periodicity, in particular the wavy variation of the elemental properties in the four longer rows, is due to the different behaviors of the s and p vs. d and f pairs of atomic valence shells along the ordered array of elements. The so-called secondary or vertical periodicity is related to pseudo-periodic changes of the atomic core shells.
The Periodic Law of the naturally given System of Elements describes the trends of the many chemical properties displayed inside the Chemical Periodic Tables. While the general physical laws of quantum mechanics form a simple network, their application to the unlimited field of chemical materials under ambient 'human' conditions results in a complex and somewhat accidental structure inside the Table that fits to some more or less symmetric outer shape. Periodic Tables designed after some creative concept for the overall appearance are of interest in non-chemical fields of wisdom and art.
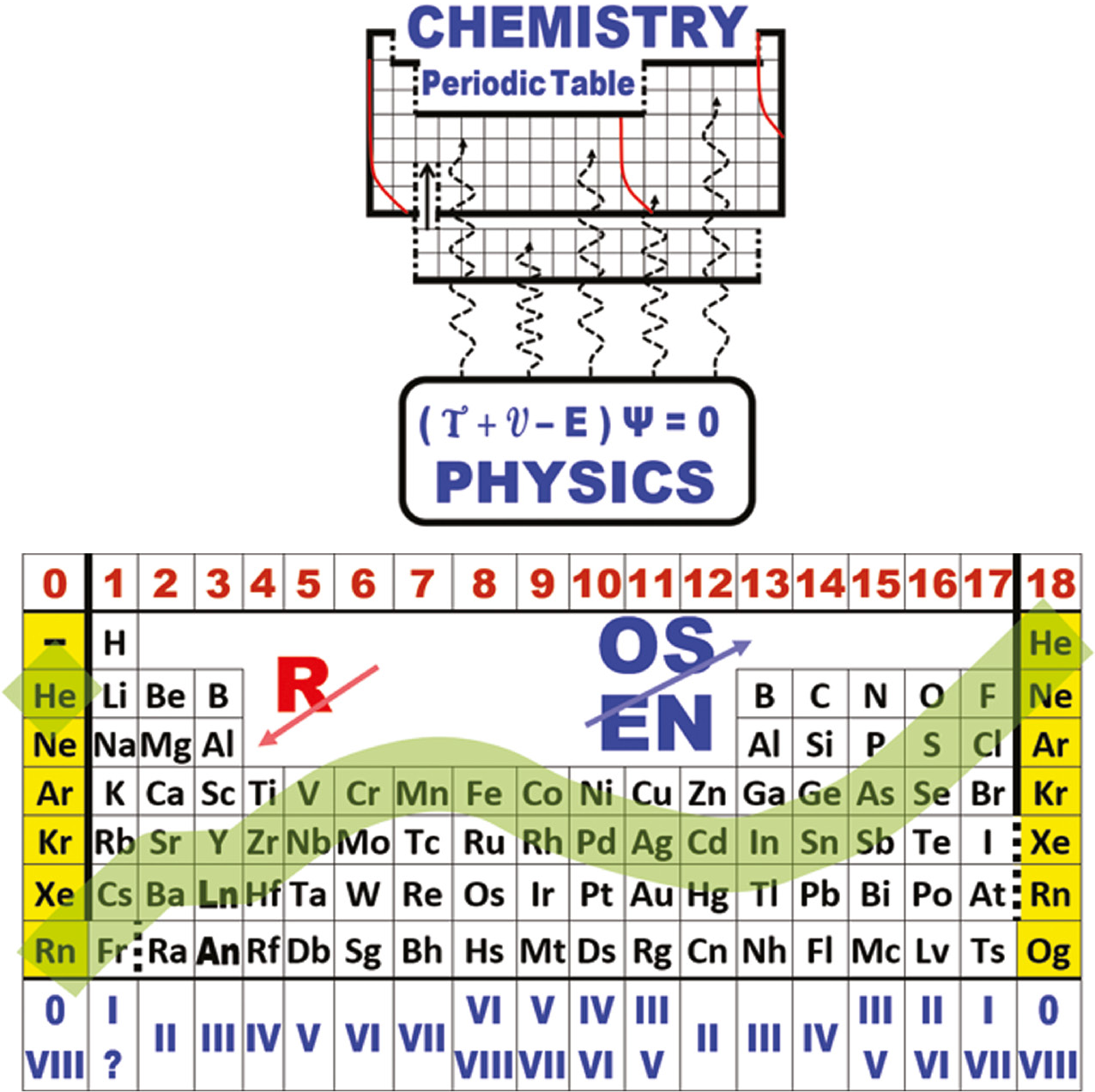
| Year: 2019 | PT id = 1101 |
International Year of the Periodic Table with Eric Scerri
Interview with leading expert on the periodic table and UCLA professor, Eric Scerri, to celebrate the International Year of the Periodic Table.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1128 |
Elemental Podcast
Radio New Zealand Podcast: Elemental, A journey through the periodic table of elements with chemistry professor Allan Blackman, from AUT, and Alison Ballance
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 1143 |
Schmiermund's The Discovery of the Periodic Table of Chemical Elements
Torsten Schmiermund's book: The Discovery of the Periodic Table of Chemical Elements (Springer, in German)
Google Translate:
150 years ago, in 1869, D. I. Mendeleev and L. Meyer independently published their ideas on the arrangement of the chemical elements in a periodic system. The United Nations and UNESCO therefore declared 2019 the "International Year of the Periodic Table". The question arises what is so special about this "simple table". Embark on a short journey into the history of the periodic table with the author. Get to know its predecessors and see how the Periodic Table of the Elements has evolved over the years. Discover the periodic properties of the elements. Find out what makes the periodic table so interesting and timeless, and see what other ideas there are and have been.
The author, Torsten Schmiermund has worked as a chemical engineer in the chemical industry for many years.

| Year: 2019 | PT id = 962 |
Möbius-Escher Periodic Table
A comment article in Nature by Prof. Eric Scerri about quantum mechanics and the periodic table:
"Can quantum ideas explain chemistry's greatest icon? Simplistic assumptions about the periodic table lead us astray.
"Such has been the scientific and cultural impact of Dmitri Mendeleev's periodic table of the elements that many people assume it is essentially complete. [But] in its 150th year, can researchers simply raise a toast to the table's many dividends, and occasionally incorporate another heavy synthetic element?
"No – this invaluable compilation is still not settled. The placements of certain elements, even hydrogen and helium, are debated."
The article is accompanied by a fantastic illustration by Señor Salme with ideas from the Möbius strip and M.C. Escher:
| Year: 2019 | PT id = 983 |
International Year of the Periodic Table (in Paris and Moscow)
Prof. Martyn Poliakoff of The University on Nottingham and Periodic Videos at the opening of the International Year of the Periodic Table:
| Year: 2019 | PT id = 1001 |
Kultovoy's Periodic Table Book
Nicolay Kultovoy, website, as sent me a copy of his Periodic Table book, entitled [Google Translate]: Book 5. Part 11-08. A single quantum mechanical model of the structure of the atomic nucleus and the periodic table of chemical elements of D.I. Mendeleev.
In a mixture of Russian & English, the PDF of the book can be viewed here.
Chapter 1. Triune (electrons, nucleons, chemical elements) quantum mechanical model of Colt. Three
1.1 the Rules of filling of the orbits of electrons.
1.2 Pyramidal lattice.
1.3 models with cubic sieve.
1.4 models with face-centered lattice.
1.5 quantum Mechanical form of the periodic table of chemical elements.
1.6 Stowe-Janet-Scerri Periodic Table.
Chapter 2. A lattice model of the nucleus. Model 62
2.1 Berezovsky G. N.
2.2 I. Boldov
2.4 Konovalov.
2.5 Manturov V.
2.6 Semikov S. A.
2.7 alpha-partial model of the atomic nucleus.
2.8 Burtaev V.
Chapter 3. Various lattice (crystal) model of the nucleus of an atom. One hundred five
3.0 Luis Pauling.
3.1 Valery Tsimmerman. ADOMAH Periodic Table. Model 3-2.
3.2 Klishev B. V. Model 3-1.
3.3 Garai J. Model 3-1.
3.4 Winger E Model 4-2.
3.5 Norman D. Cook. Model 4-1.
3.6 Gamal A. Nasser. Model 4-1.
3.7 D. Asanbaeva Model 4-1.
3.8 Datsuk V. K.
3.9 Bolotov B.
3.10 Djibladze M. I.
3.11 Dyukin S. V.
3.12 A. N. Mishin.
3.13 M. M. Protodyakonov
3.14 Dry I. N.
3.15 Ulf-G. Meißner.
3.16 Foreign works.
Chapter 4. Long-period periodic table. One hundred eighty one
4.1 long-Period representation of the periodic table.
4.2 Artamonov, G. N.
4.3 Galiulin R. V.
4.4 E. K. Spirin
4.5. Khoroshavin L.
4.6 Step form proposed by Thomsen and Bohr.
4.7 Symmetrical shape of the periodic table.
Chapter 5. Construction of a periodic table based on the structure of orbitals. Two hundred twenty one
5.1 construction of the periodic table on the basis of orbitals.
5.2 Short V. M.
5.3 Kulakov, the Novosibirsk table of multiplets.
Chapter 6. Atomic structure. Two hundred forty eight
6.1 Table of isotopes.
6.2 the structure of the orbitals.
| Year: 2019 | PT id = 1004 |
St Catharine's College: Celebrating the Periodic Table
The United Nations have proclaimed 2019 to be the International Year of the Periodic Table of Chemical Elements since it is the 150th anniversary of the publication of Dmitri Mendeleev's first Periodic Table. But was it really the first?
St Catharine's College, Cambridge, in the UK, is proud to exhibit its fine collection of material relating to the early development of the Periodic Table. Starting from the first list of elements which emerged around the time of the French Revolution in the late 1780s, and the first list of atomic masses drawn up by Manchester chemist John Dalton, we explore why six different chemists from around the world each came up with their own versions of the iconic table in the 1860s.

From the RSC Website:
"Curated by periodic table superfan Peter Wothers, the main body of the exhibition is a staggering collection of historic books that trace the creation of chemistry's roadmap.
"This is an unprecedented record of the periodic table's origins, from early alchemical texts through to original copies of Antoine Lavoisier's 1789 Elementary Treatise of Chemistry – the first true list of elements – and notes on the discoveries of (among others) John Newlands, Julius Lothar Meyer through to Dmitri Mendeleev".
Celebrating the periodic table – the first edition of Mendeleev's textbook from Chemistry World on Vimeo.
| Year: 2019 | PT id = 1022 |
Evolution and Understanding of the d-Block Elements in the Periodic Table
Edwin C. Constable's review, Evolution and understanding of the d-block elements in the periodic table:
| Year: 2019 | PT id = 1023 |
Nucleosynthesis of the Heavy Elements
A PBS video explaining how neutron star mergers lead to the formation of heavy elements, and how a merger only 80 million years before the formation of the solar system, 4.5 billions years ago, seeded the Earth wth the heavy elements of the periodic table:
| Year: 2020 | PT id = 1098 |
What Is A Chemical Element?
A Collection of Essays by Chemists, Philosophers, Historians, and Educators Edited by Eric Scerri and Elena Ghibaudi published by Oxford University Press
- A collection of 14 edited papers from historians of chemistry, philosophers of chemistry, and chemists with epistemological and educational concerns
- Contains educational debates concerning how to teach and present the concept of elements
- Provides a beneficial, scholarly, unique, and understandable overview of the current debate on the chemical elemen.
The concept of a chemical element is foundational within the field of chemistry, but there is wide disagreement over its definition. Even the International Union for Pure and Applied Chemistry (IUPAC) claims two distinct definitions: a species of atoms versus one which identifies chemical elements with the simple substances bearing their names. The double definition of elements proposed by the International Union for Pure and Applied Chemistry contrasts an abstract meaning and an operational one. Nevertheless, the philosophical aspects of this notion are not fully captured by the IUPAC definitions, despite the fact that they were crucial for the construction of the Periodic Table. Although rich scientific literature on the element and the periodic table exists as well as a recent growth in the philosophy of chemistry, scholars are still searching for a definitive answer to this important question: What is an element?
Eric Scerri and Elena Ghibaudi have teamed up to assemble a group of scholars to provide readers an overview of the current state of the debate on chemical elements from epistemological, historical, and educational perspectives. What Is A Chemical Element? fills a gap for the benefit of the whole chemistry community-experimental researchers, philosophers, chemistry educators, and anyone looking to learn more about the elements of the periodic table.
Foreword
Introduction
CHAPTER 1: The many questions raised by the dual concept of 'element' Eric R. Scerri
CHAPTER 2: From simple substance to chemical element Bernadette Bensaude-Vincent
CHAPTER 3: Dmitrii Mendeleev's concept of the chemical element prior to the Periodic Law Nathan M. Brooks
CHAPTER 4: Referring to chemical elements and compounds: Colourless airs in late eighteenth century chemical practice Geoffrey Blumenthal, James Ladyman, and Vanessa Seifert
CHAPTER 5: The Changing Relation Between Atomicity and Elementarity: From Lavoisier to Dalton Marina P. Banchetti-Robino
CHAPTER 6: Origins of the Ambiguity of the Current Definition of Chemical Element Joseph E. Earley
CHAPTER 7: The Existence of Elements, and the Elements of Existence Robin F. Hendry
CHAPTER 8: Kant, Cassirer, and the Idea of Chemical Element Farzad Mahootian
CHAPTER 9: The Operational Definition of the Elements: A Philosophical Reappraisal Joachim Schummer
CHAPTER 10: Substance and Function: The case of Chemical Elements Jean-Pierre Llored
CHAPTER 11: Making elements Klaus Ruthenberg
CHAPTER 12: A formal approach to the conceptual development of chemical element Guillermo Restrepo
CHAPTER 13: Chemical Elements and Chemical Substances: Rethinking Paneth's Distinction Sara N. Hjimans
CHAPTER 14: The dual conception of the chemical element: epistemic aspects and implications for chemical education Elena Ghibaudi, Alberto Regis, and Ezio Roletto
Appendix: Reference list on the philosophy of chemistry Index.

| Year: 2020 | PT id = 1149 |
Scerri's Periodic Table of Books About The Periodic Table & The Chemical Elements
From Eric Scerri, a periodic table of books about the periodic table & the chemical elements... many by Eric Scerri himself.
Eric Scerri, UCLA, Department of Chemistry & Biochemistry. See the website EricScerri.com and Eric's Twitter Feed.
There is no particular connection between each of the elements and the book associated with it in the table, with the exception of: H, He, N, Ti, V, Nb, Ag, La, Au, Ac, U, Pu & Og.
The following is a list of references for each of the 118 books featured on Periodic Table of Books About The Periodic Table & The Chemical Elements. Books published in languages other than English are. They include the Catalan, Croatian, French, German, Italian, Norwegian & Spanish languages:
| 1 | H | J. Ridgen, Hydrogen, the Essential Element, Harvard University Press, Cambridge, MA, 2002. |
| 2 | He | W.M. Sears Jr., Helium, The Disappearing Element, Springer, Berlin, 2015. |
| 3 | Li | K. Lew, The Alkali Metals, Rosen Central, New York, 2009. |
| 4 | Be | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish) |
| 5 | B | E.R. Scerri. The Periodic Table, Its Story and Its Significance, 2nd edition, Oxford University Press, New York, 2020. |
| 6 | C | U. Lagerkvist, The Periodic Table and a Missed Nobel Prize, World Scientific, Singapore, 2012. |
| 7 | N | W.B. Jensen, Mendeleev on the Periodic Law: Selected Writings, 1869–1905, Dover, Mineola, NY, 2005. |
| 8 | O | M. Kaji, H. Kragh, G. Pallo, (eds.), Early Responses to the Periodic System, Oxford University, Press, New York, 2015. |
| 9 | F | E. Mazurs, Graphic Representation of the Periodic System During One Hundred Years, Alabama University Press, Tuscaloosa, AL, 1974. |
| 10 | Ne | T. Gray, The Elements: A Visual Exploration of Every Known Atom in the Universe, Black Dog & Leventhal, 2009. |
| 11 | Na | N.C. Norman, Periodicity and the s- and p-Block Elements, Oxford University Press, Oxford, 2007. |
| 12 | Mg | M. Gordin, A Well-Ordered Thing, Dimitrii Mendeleev and the Shadow of the Periodic Table, 2nd edition, Basic Books, New York, 2019. |
| 13 | Al | S. Kean, The Disappearing Spoon, Little, Brown & Co., New York, 2010. |
| 14 | Si | P.A. Cox, The Elements, Oxford University Press, Oxford, 1989. |
| 15 | P | J. Emsley, The 13th Element: The Sordid Tale of Murder, Fire, and Phosphorus, Wiley, New York, 2002. |
| 16 | S | P. Parsons, G. Dixon, The Periodic Table: A Field Guide to the Elements, Qurcus, London, 2014. |
| 17 | Cl | P. Levi, The Periodic Table, Schocken, New York, 1995. |
| 18 | Ar | B.D. Wiker, The Mystery of the Periodic Table, Bethlehem Books, New York, 2003. |
| 19 | K | H. Alderesey-Williams, Periodic Tales, Viking Press, 2011. |
| 20 | Ca | P. Strathern, Mendeleyev's Dream, Hamish-Hamilton, London, 1999. |
| 21 | Sc | D. Scott, Around the World in 18 Elements, Royal Society of Chemistry, London, 2015. |
| 22 | Ti | E. W. Collings, Gerhard Welsch, Materials Properties Handbook: Titanium Alloys, ASM International, Geauga County, Ohio, 1994. |
| 23 | V | D. Rehder, Bioinorganic Vanadium Chemistry, Wiley-Blackwell, Weinheim, 2008. |
| 24 | Cr | K. Chapman, Superheavy, Bloomsbury Sigma, New York, 2019. |
| 25 | Mn | E.R. Scerri, E. Ghibaudi (eds.), What is an Element? Oxford University Press, New York, 2020. |
| 26 | Fe | M. Soon Lee, Elemental Haiku, Ten Speed Press, New York, 2019. |
| 27 | Co | J. Emsley, Nature's Building Blocks, An A-Z Guide to the Elements, Oxford University Press, Oxford, 2001. |
| 28 | Ni | T. James, Elemental, Robinson, London, 2018. |
| 29 | Cu | E.R. Scerri, The Periodic Table, Its Story and Its Significance, Oxford University Press, New York, 2007. |
| 30 | Zn | H. Rossotti, Diverse Atoms, Oxford University Press, Oxford, 1998. |
| 31 | Ga | P. Ball, A Very Short Introduction to the Elements, Oxford University Press, 2004. |
| 32 | Ge | I. Asimov, The Building Blocks of the Universe, Lancer Books, New York, 1966. |
| 33 | As | J. Browne, Seven Elements that Changed the World, Weidenfeld and Nicholson, London, 2013. |
| 34 | Se | N. Raos, Bezbroj Lica Periodnog Sustava Elemenata, Technical Museum of Zagreb, Croatia, 2010. (Croatian) |
| 35 | Br | P. Strathern, The Knowledge, The Periodic Table, Quadrille Publishing, London, 2015. |
| 36 | Kr | A. Ede, The Chemical Element, Greenwood Press, Westport, CT, 2006. |
| 37 | Rb | A. Stwertka, The Elements, Oxford University Press, Oxford, 1998. |
| 38 | Sr | E.R. Scerri, A Tale of Seven Elements, Oxford University Press, New York, 2013. |
| 39 | Y | H.-J. Quadbeck-Seeger, World of the Elements, Wiley-VCH, Weinheim, 2007. |
| 40 | Zr | M. Fontani, M. Costa, M.V. Orna (eds.), The Lost Elements, Oxford University Press, New York, 2015. |
| 41 | Nb | M. Seegers, T. Peeters (eds.), Niobium: Chemical Properties, Applications and Environmental Effects, Nova Science Publishers, New York, 2013. |
| 42 | Mo | E.R. Scerri, Selected Papers on the Periodic Table, Imperial College Press, Imperial College Press, London and Singapore, 2009. |
| 43 | Tc | A. Dingle, The Periodic Table, Elements with Style, Kingfisher, Richmond, B.C. Canada, 2007. |
| 44 | Ru | G. Rudorf, Das periodische System, seine Geschichte und Bedeutung für die chemische Sysytematik, Hamburg-Leipzig, 1904. (German) |
| 45 | Rh | I. Nechaev, G.W. Jenkins, The Chemical Elements, Tarquin Publications, Publications, Norfolk, UK, 1997. |
| 46 | Pd | P. Davern, The Periodic Table of Poems, No Starch Press, San Francisco, 2020. |
| 47 | Ag | C. Fenau, Non-ferrous metals from Ag to Zn, Unicore, Brussells, 2002. |
| 48 | Cd | J. Van Spronsen, The Periodic System of the Chemical Elements, A History of the First Hundred Years, Elsevier, Amsterdam, 1969. |
| 49 | In | M. Tweed, Essential Elements, Walker and Company, New York, 2003. |
| 50 | Sn | M.E. Weeks, Discovery of the Elements, Journal of Chemical Education, Easton PA, 1960. |
| 51 | Sb | P. Wothers, Antimony Gold Jupiter's Wolf, Oxford University Press, Oxford, 2019. |
| 52 | Te | W. Zhu, Chemical Elements in Life, World Scientific Press, Singapore, 2020. |
| 53 | I | O. Sacks, Uncle Tungsten, Vintage Books, New York, 2001. |
| 54 | Xe | E.R. Scerri, (ed.), 30-Second Elements, Icon Books, London, 2013. |
| 55 | Cs | M. Jacob (ed.), It's Elemental: The Periodic Table, Celebrating 80th Anniversary, Chemical & Engineering News, American Chemical Society, Washington D.C., 2003. |
| 56 | Ba | J. Marshall, Discovery of the Elements, Pearson Custom Publishing, New York,1998. |
| 57 | La | K. Veronense, Rare, Prometheus Books, Amherst, New York, 2015. |
| 58 | Ce | N. Holt, The Periodic Table of Football, Ebury Publishing, London, 2016. |
| 59 | Pr | S. Alvarez, C. Mans, 150 Ans de Taules Périodiques a la Universitat de Barcelona, Edicions de la Universitat de Barcelona, Barcelona, 2019. (Catalan) |
| 60 | Nd | L. Garzon Ruiperez, De Mendeleiev a Los Superelementos, Universidad de Oviedo, Oviedo, 1988. (Spanish) |
| 61 | Pm | P. Ball, A Guided Tour of the Ingredients, Oxford University Press, Oxford, 2002. |
| 62 | Sm | S. Esteban Santos, La Historia del Sistema Periodico, Universidad Nacional de Educación a Distancia, Madrid, 2009. (Spanish). |
| 63 | Eu | A. E. Garrett, The Periodic Law, D. Appleton & Co., New York, 1909. |
| 64 | Gd | M.S. Sethi, M. Satake, Periodic Tables and Periodic Properties, Discovery Publishing House, Delhi, India, 1992. |
| 65 | Tb | M. Eesa, The cosmic history of the elements: A brief journey through the creation of the chemical elements and the history of the periodic table, Createspace Independent Publishing Platform, 2012. |
| 66 | Dy | P. Depovere, La Classification périodique des éléments, De Boeck, Bruxelles, 2002. (French). |
| 67 | Ho | F. Habashi, The Periodic Table & Mendeleev, Laval University Press, Quebec, 2017. |
| 68 | Er | W.J. Nuttall, R. Clarke, B. Glowacki, The Future of Helium as a Natural Resource, Routledge, London, 2014. |
| 69 | Tm | R.D. Osorio Giraldo, M.V. Alzate Cano, La Tabla Periodica, Bogota, Colombia, 2010. (Spanish). |
| 70 | Yb | P.R. Polo, El Profeta del Orden Quimico, Mendeleiev, Nivola, Spain, 2008. (Spanish). |
| 71 | Lu | E.R. Scerri, A Very Short Introduction to the Periodic Table, 2nd edition, Oxford University Press, Oxford, 2019. |
| 72 | Hf | D.H. Rouvray, R.B. King, The Mathematics of the Periodic Table, Nova Scientific Publishers, New York, 2006. |
| 73 | Ta | P. Thyssen, A. Ceulemans, Shattered Symmetry, Oxford University Press, New York, 2017. |
| 74 | W | P.W. Atkins, The Periodic Kingdom, Basic Books, New York, NY, 1995. |
| 75 | Re | D.G. Cooper, The Periodic Table, 3rd edition. Butterworths, London, 1964. |
| 76 | Os | E. Lassner, W.-D. Schubert, Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds, Springer, Berlin, 1999. |
| 77 | Ir | J.C.A. Boeyens, D.C. Levendis, Number Theory and the Periodicity of Matter, Springer, Berlin, 2008. |
| 78 | Pt | R. Hefferlin, Periodic Systems and their Relation to the Systematic Analysis of Molecular Data, Edwin Mellen Press, Lewiston, NY, 1989. |
| 79 | Au | R.J. Puddephatt, The Chemistry of Gold, Elsevier, Amsterdam, 1978. |
| 80 | Hg | D.H. Rouvray, R.B. King, The Periodic Table Into the 21st Century, Research Studies Press, Baldock, UK, 2004. |
| 81 | Tl | R.E. Krebs, The History and Use of Our Earth's Chemical Elements, Greenwood Publishing Group, Santa Barbara, CA, 2006. |
| 82 | Pb | E. Torgsen, Genier, sjarlataner og 50 bøtter med urin - Historien om det periodiske system, Spartacus, 2018. (Norwegian). |
| 83 | Bi | K. Buchanan, D. Roller, Memorize the Periodic Table, Memory Worldwide Pty Limited, 2013. |
| 84 | Po | D. Morris, The Last Sorcerers, The Path from Alchemy to the Periodic Table, Joseph Henry Press, New York, 2003. |
| 85 | At | T. Jackson, The Elements, Shelter Harbor Press, New York, 2012. |
| 86 | Rn | R.J.P. Williams, J.J.R. Frausto da Silva, The Natural Selection of the Chemical Elements: The Environment and Life's Chemistry, Clarendon Press, Oxford, 1997. |
| 87 | Fr | G. Rudorf, The Periodic Classification and the Problem of Chemical Evolution, Whittaker & Co., London, New York, 1900. |
| 88 | Ra | L. Van Gorp, Elements, Compass Point Books, Manakato, MN, 2008. |
| 89 | Ac | G.T. Seaborg, J.J. Katz, L.R. Morss, Chemistry of the Actinide Elements, Springer, Berlin, 1986. |
| 90 | Th | G. Münzenberg, Superheavy Elements - Searching for the End of the Periodic Table, Manipal Universal Press, India, 2018. |
| 91 | Pa | A. Castillejos Salazar, La Tabla Periòdica: Abecedario de la Quimica, Universidad Autonoma de Mexico, D.F. Mexico, 2005. (Spanish). |
| 92 | U | T. Zoellner, Uranium, Penguin Books, London, 2009. |
| 93 | Np | J. Barrett, Atomic Structure and Periodicity, Royal Society of Chemistry, London, 2002. |
| 94 | Pu | J. Bernstein, Plutonium, Joseph Henry, Washington DC, 2007. |
| 95 | Am | S. Hofmann, Beyond Uranium, Taylor & Francis, London, 2002. |
| 96 | Cm | H.M. Davis, The Chemical Elements, Ballantine Books, New York, 1961. |
| 97 | Bk | P.González Duarte, Les Mils Cares de la Taula Periòdica, Universitat Autonoma de Barcelona, Bellaterra Barcelona, 2005 (Catalan). |
| 98 | Cf | R. Rich, Periodic Correlations, Benjamin, New York, 1965. |
| 99 | Es | E. Rabinowitsch, E. Thilo, Periodisches System, Geschichte und Theorie, Stuttgart, 1930. (German). |
| 100 | Fm | P.K. Kuroda, The Origin of the Chemical Elements, and the Oklo Phenomenon, Springer-Verlag, Berlin, 1982. |
| 101 | Md | G. Villani, Mendeleev, La Tavola Periodica Degli Elementi, Grandangolo, Milan, 2016. (Italian). |
| 102 | No | J. Russell, Elementary: The Periodic Table Explained, Michael O'Mara, London, 2020. |
| 103 | Lr | P. Enghag, Encyclopedia of the Elements, Wiley-VCH, Weinheim, 2004. |
| 104 | Rf | R.J. Puddephatt, The Periodic Table of the Elements, Oxford University Press, Oxford, 1972. |
| 105 | Db | L. Ohrström, The Last Alchemist in Paris, Oxford University Press, New York, 2013. |
| 106 | Sg | N.N. Greenwood, E. Earnshaw, Chemistry of the Elements, 2nd edition, Elsevier, Amsterdam, 1997. |
| 107 | Bh | R. Luft, Dictionnaire des Corps Simples de la Chimie, Association Cultures et Techniques, Nantes, 1997. (French) |
| 108 | Hs | Science Foundation Course Team, The Periodic Table and Chemical Bonding, The Open University, Milton Keynes, 1971. |
| 109 | Mt | W.W. Schulz, J. Navratil, Transplutonium Elements, American Chemical Society, Washington D.C., 1981. |
| 110 | Ds | I. Nechaev, Chemical Elements, Lindsay Drummond, 1946. |
| 111 | Rg | F. Hund, Linienspektren und Periodisches System Der Elemente, Springer, Berlin, 1927. |
| 112 | Cn | F.P. Venable, The Development of the Periodic Law, Chemical Publishing Co., Easton, PA, 1896. |
| 113 | Nh | O. Baca Mendoza, Leyes Geneticas de los Elementos Quimicos. Nuevo Sistema Periodico, Universidad Nacional de Cuzco, Cuzco, Peru, 1953 (Spanish). |
| 114 | Fl | B. Yorifuji, Wonderful Life with the Elements, No Starch Press, San Francisco, 2012. |
| 115 | Mc | D.I. Mendeléeff, The Principles of Chemistry, American Home Library, New York, 1902. |
| 116 | Lv | A. Lima-de-Faria, Periodic Tables Unifying Living Organisms at the Molecular Level: The Predictive Power of the Law of Periodicity, World Scientific Press, Singapore, 2018. |
| 117 | Ts | H.B. Gray, J.D. Simon, W.C. Trogler, Braving the Elements, University Science Books, Sausalito, CA, 1995. |
| 118 | Og | E.R. Scerri, G. Restrepo, Mendeleev to Oganesson, Oxford University Press, New York, 2018. |
| Year: 2020 | PT id = 1152 |
Rayner-Canham's The Periodic Table: Past, Present, and Future
A book by Geoff Rayner-Canham, The Periodic Table: Past, Present, and Future.
https://doi.org/10.1142/11775 | August 2020
Contents:
- About the Author
- Introduction
- The Periodic Table Exploration Begins!
- Isotopes and Nuclear Patterns
- Selected Trends in Atomic Properties
- First Period Problems
- The Group 3 Problem
- Categorizations of the Elements
- Isoelectronicity
- Group and Period Patterns among the Main Group Elements
- Patterns among the Transition Metals
- Group (n) and (n+10) Relationships
- Chemical "Knight's Move" Relationship
- Isodiagonality
- Lanthanoids, Group 3, and Their Connections
- Actinoid and Post-Actinoid Elements
- Pseudo-Elements
- Index
| Year: 2020 | PT id = 1153 |
Scerri's Periodic Table of Books About The Periodic Table & The Chemical Elements by ERS
From Eric Scerri, a periodic table of books about the periodic table & the chemical elements... by Eric Scerri, including translations.
Eric Scerri, UCLA, Department of Chemistry & Biochemistry. See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2020 | PT id = 1162 |
Workshop on Teaching 3d-4s Orbitals Presented by Dr. Eric Scerri
Dr. Eric Scerri, UCLA Department of Chemistry & Biochemistry, discusses many of the issues concerning the periodic table: the aufbau principle, Madelung's rule, the electronic and anomalous electronic structures of the transition elements, the Sc2+ ion, the Janet Left Step, Group 3: Sc, Y, Lu, Lr vs. Sc, Y, La, Ac, atomic spectroscopy, etc.
Many of the topics that concern those of us interested in the periodic table are discussed.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2020 | PT id = 1176 |
Interview with Chemist, Dr. Eric Scerri
From Academic Influence. We met with Dr. Eric Scerri to discuss the nature of science, the philosophy of chemistry, and much more. Enjoy!
| Year: 2021 | PT id = 1179 |
Chemogenesis In 700 Seconds
The Chemogenesis analysis – by Mark Leach – tells the story of how chemical structure and reactivity emerge from the periodic table of the elements. This video is a rapid dash through the story... which unfolds over many pages of the Chemogenesis Web Book.
| Year: 2021 | PT id = 1181 |
Understanding Periodic and Non-periodic Chemistry in Periodic Tables
Cao C, Vernon RE, Schwarz WHE and Li J (2021). Front. Chem. 8:813. https://doi.org/10.3389/fchem.2020.00813
Abstract:
The chemical elements are the "conserved principles" or "kernels" of chemistry that are retained when substances are altered. Comprehensive overviews of the chemistry of the elements and their compounds are needed in chemical science. To this end, a graphical display of the chemical properties of the elements, in the form of a Periodic Table, is the helpful tool. Such tables have been designed with the aim of either classifying real chemical substances or emphasizing formal and aesthetic concepts. Simplified, artistic, or economic tables are relevant to educational and cultural fields, while practicing chemists profit more from "chemical tables of chemical elements."
Such tables should incorporate four aspects:
(i) typical valence electron configurations of bonded atoms in chemical compounds (instead of the common but chemically atypical ground states of free atoms in physical vacuum);
(ii) at least three basic chemical properties (valence number, size, and energy of the valence shells), their joint variation across the elements showing principal and secondary periodicity;
(iii) elements in which the (sp)8, (d)10, and (f)14 valence shells become closed and inert under ambient chemical conditions, thereby determining the "fix-points" of chemical periodicity;
(iv) peculiar elements at the top and at the bottom of the Periodic Table.
While it is essential that Periodic Tables display important trends in element chemistry we need to keep our eyes open for unexpected chemical behavior in ambient, near ambient, or unusual conditions. The combination of experimental data and theoretical insight supports a more nuanced understanding of complex periodic trends and non-periodic phenomena.
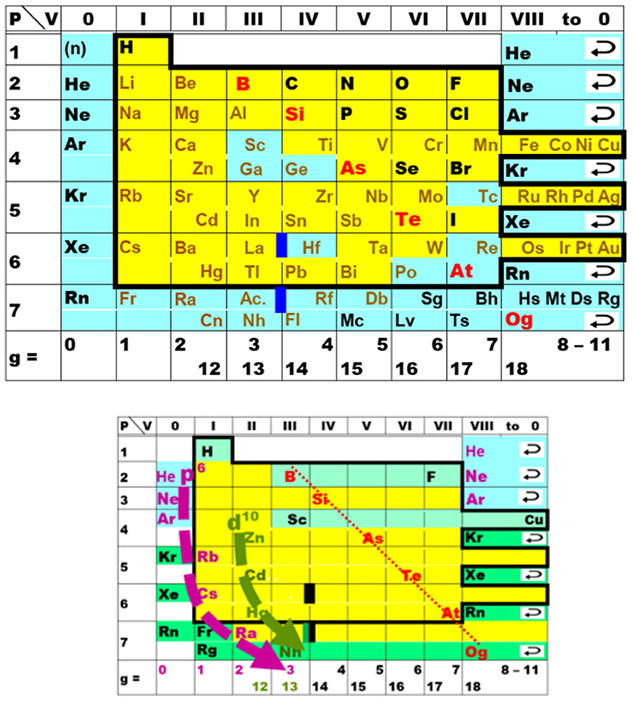
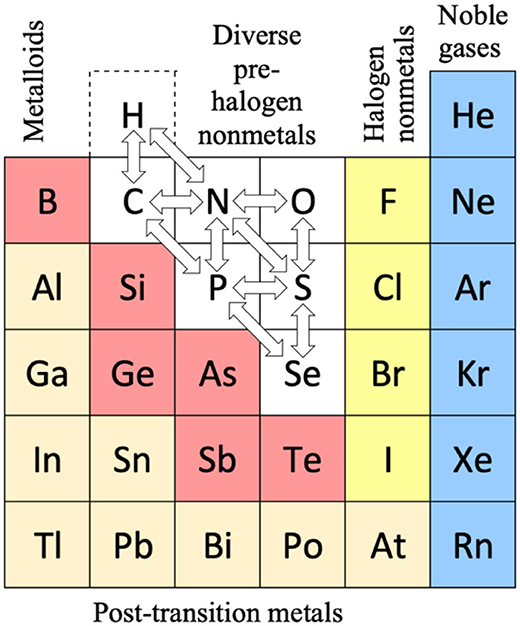
Thanks to René Vernon for the tip.
| Year: 2021 | PT id = 1185 |
150 Years of the Periodic Table
Based on the ACS 2019 symposium "150 Years of the Periodic Table". Provides an overview of how the periodic table has evolved over the past 150 years Written by leading experts in the field: Editors: Giunta, Carmen, Mainz, Vera V., Girolami, Gregory S. (Eds.)
This book provides an overview of the origins and evolution of the periodic system from its prehistory to the latest synthetic elements and possible future additions. The periodic system of the elements first emerged as a comprehensive classificatory and predictive tool for chemistry during the 1860s. Its subsequent embodiment in various versions has made it one of the most recognizable icons of science.
Based primarily on a symposium titled "150 Years of the Periodic Table" and held at the August 2019 national meeting of the American Chemical Society, this book describes the origins of the periodic law, developments that led to its acceptance, chemical families that the system struggled to accommodate, extension of the periodic system to include synthetic elements, and various cultural aspects of the system that were celebrated during the International Year of the Periodic Table.
Contributors include: Ann Robinson, Gary Patterson, Gisela Boek, Mary Virginia Orna, Simon Cotton, Jay Labinger, Kit Chapman, Virginia Trimble, Eric Scerri, William Jensen, Pekka Pyykko, Daniel Rabinovich, Carmen Giunta, Gregory Girolami, Vera Mainz
| Year: 2021 | PT id = 1191 |
Provisional Report on Discussions on Group 3 of The Periodic Table
Provisional Report on Discussions on Group 3 of The Periodic Table by Eric Scerri, De Gruyter | 2021
DOI: https://doi.org/10.1515/ci-2021-0115
The following article is intended as a brief progress report from the group that has been tasked with mak-ing recommendations to IUPAC about the constitu-tion of group 3 of the periodic table (https://iupac.org/project/2015-039-2-200). It is also intended as a call for feedback or suggestions from members of IUPAC and other readers.



| Year: 2021 | PT id = 1193 |
Eric Scerri's Articles, as Listed on Muck Rack
| Year: 2021 | PT id = 1213 |
Mendeleyev Revisited
An Open Access paper: Marks, E.G., Marks, J.A. Mendeleyev revisited. Found Chem 23, 215-223 (2021).
https://doi.org/10.1007/s10698-021-09398-4
"Despite the periodic table having been discovered by chemists half a century before the discovery of electronic structure, modern designs are invariably based on physicists' definition of periods. This table is a chemists' table, reverting to the phenomenal periods that led to the table's discovery. In doing so, the position of hydrogen is clarified."
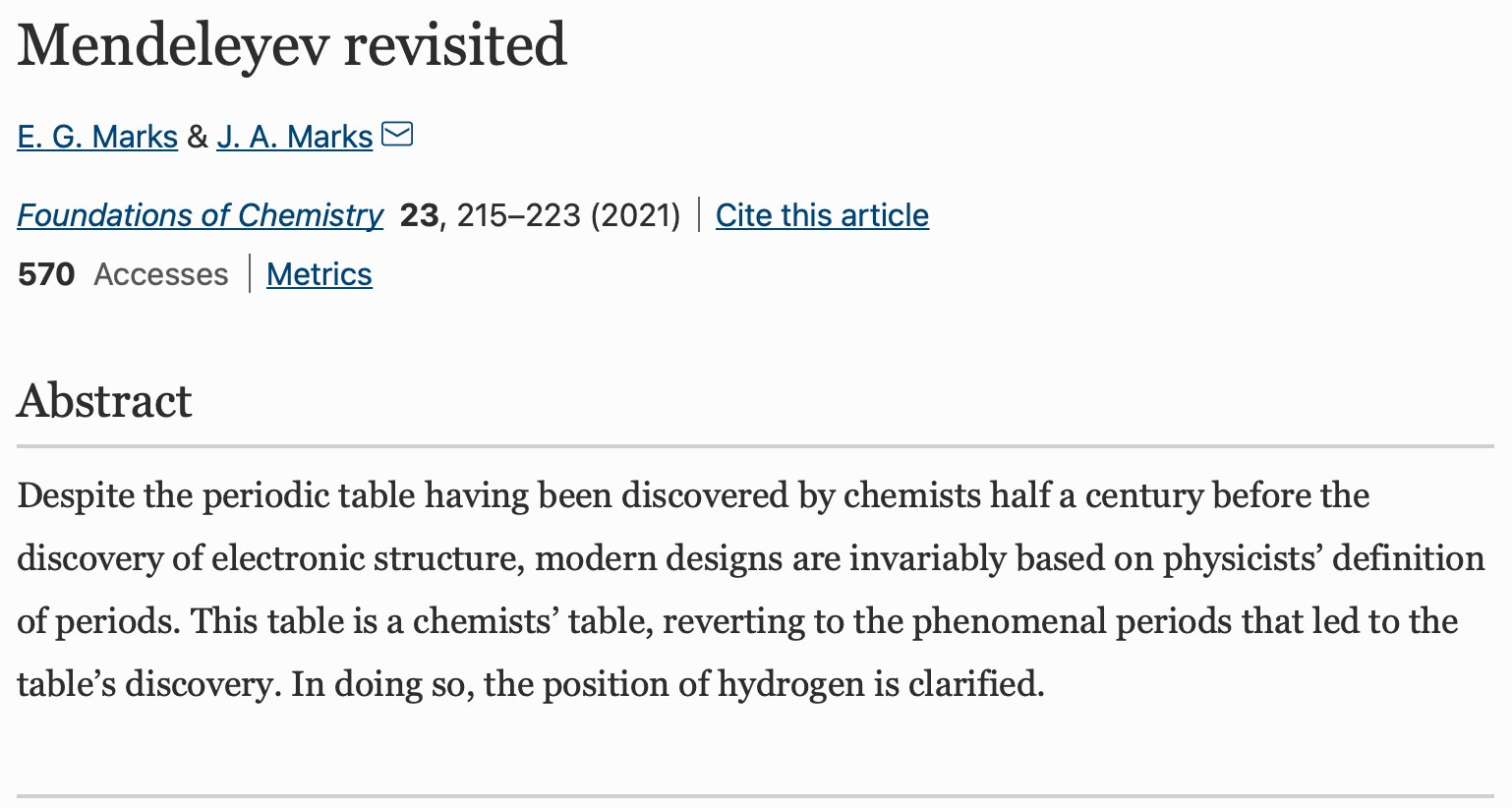
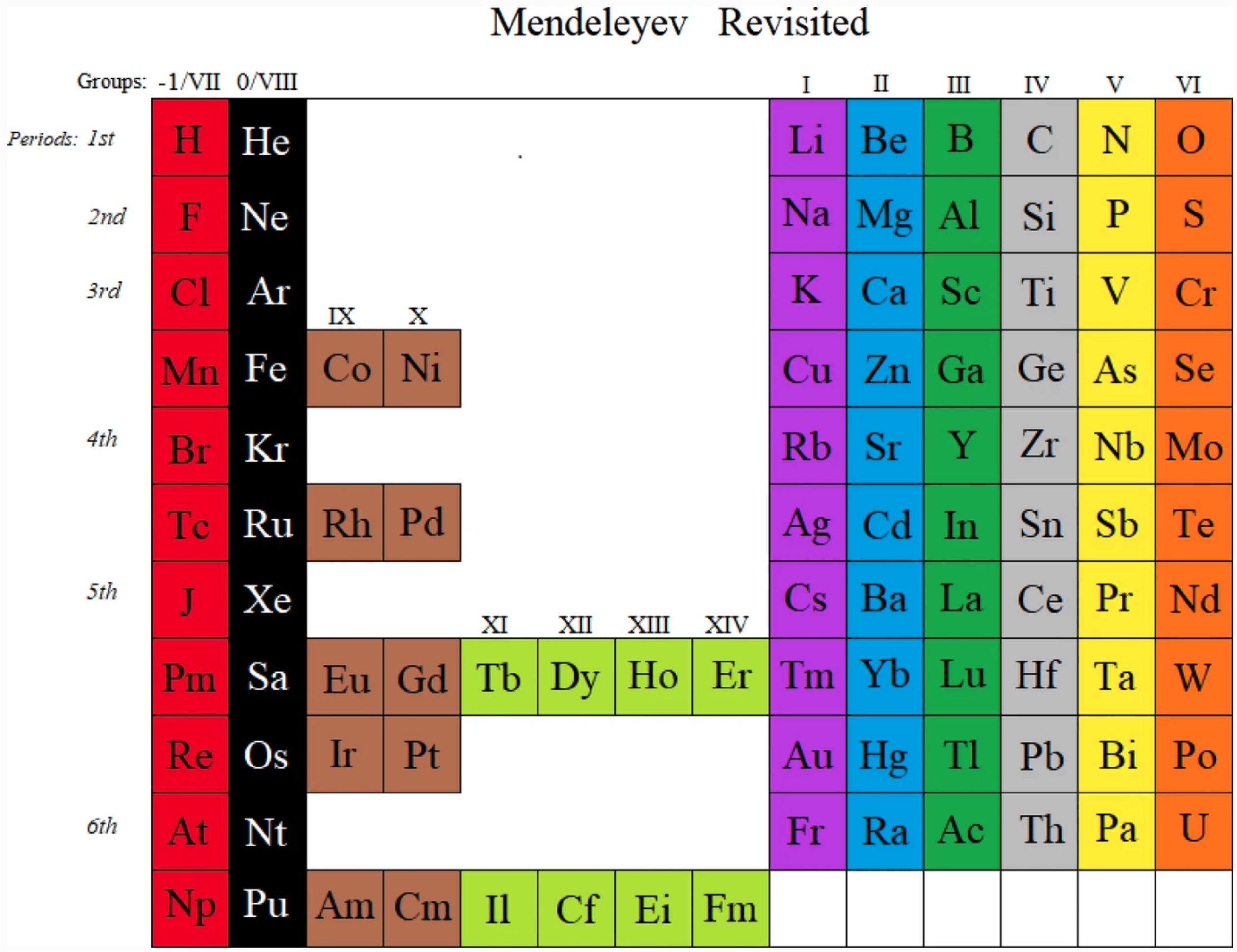
| Year: 2021 | PT id = 1232 |
The Periodic Table: Is it Perfect, is it Fractured or is it Broken?
A video from Mark Leach, who writes:
The periodic table is an icon of science. Indeed, all chemical matter is made from periodic table stuff. The periodic table of the elements is often presented as being:
- With 118 elements the periodic table is now complete
- The periodic table is perfectly described (fully explained) by the application of four quantum numbers with the and some simple rules
- Chemical structure & reactivity can be deduced from the periodicity of the Groups & Periods
However, the chemistry of the chemical elements is actually a little more complicated than this. So, where & why does the predictability 'break'?
| Year: 2021 | PT id = 1248 |
Meyer or Mendeleev: Who created the periodic table?
An article in Academic Influence by Eric Scerri: Meyer or Mendeleev: Who created the periodic table?
| Year: 2022 | PT id = 1253 |
Gorodkov's Periodic System of Periodic Systems
Mikhail Gorodkov writes:
"I hope you'll find attached version of Periodic System not only funny but also informative!"
| Year: 2022 | PT id = 1276 |
Periodic Table of Periodic Tables
René Vernon has collected the PT formulations in this PT database and classifed them as:
Short, Triangular, Medium, Long, Continious, Folding, Spatial & Unclassified
and tabulated them by date. The 'working document' is currently a word file.
| Year: 2023 | PT id = 1286 |
Six Stages of The Convergence of The Periodic System
Bran, A.M., Stadler, P.F., Jost, J. et al. The six stages of the convergence of the periodic system to its final structure. Commun Chem 6, 87 (2023). https://doi.org/10.1038/s42004-023-00883-9
Abstract (abridged):
"We show, by analysing the space between 1800 and 2021, that the system has converged towards its current stable structure through six stages, respectively characterised by the finding of elements (1800–1826), the emergence of the core structure of the system (1826–1860), its organic chemistry bias (1860–1900) and its further stabilisation (1900–1948), World War 2 new chemistry (1948–1980) and the system final stabilisation (1980–)."
Periodic tables representative of each period in history. Families of similar elements (sets sharing colour) shown in each table summarise the patterns and do not necessarily imply continuity nor simultaneity of the families throughout the period:
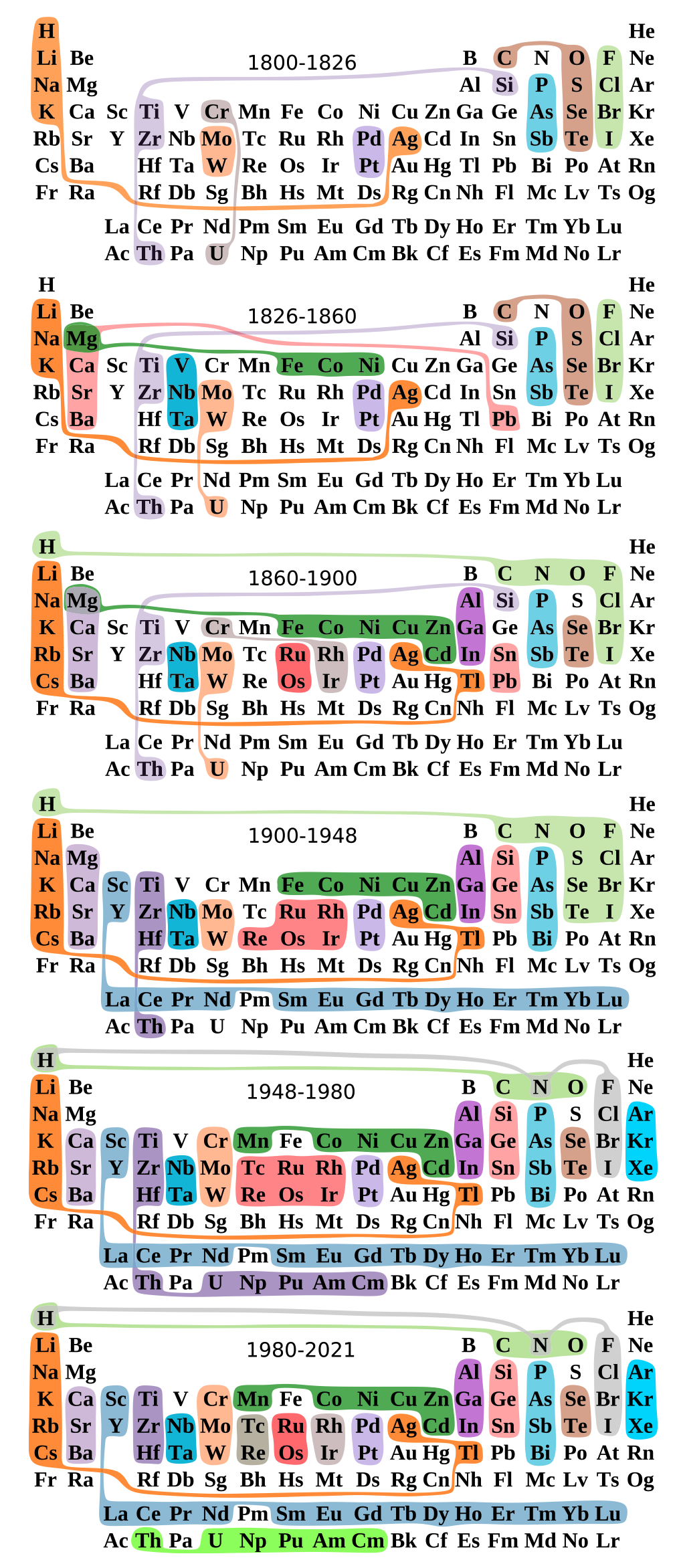
| Year: 2023 | PT id = 1289 |
Chemdex: Valence & Oxidation Number Trends
From Mark Winter's review paper Chemdex: quantification and distributions of valence numbers, oxidation numbers, coordination numbers, electron numbers, and covalent bond classes for the elements Dalton Trans., 2024,53, 493-511 https://doi.org/10.1039/D3DT03738J.
The images below show the Valence number (VN) and oxidation number (ON) proportions as percentages for the elements; and Periodic tables displaying valence number proportions (%). (There are few data for Pm and no data for Fr and elements beyond Es.)
The position of H and the group numbers are addressed in the paper.
| Year: 2023 | PT id = 1291 |
Bala's Shape of the Periodic Table
Gavin J. Bala has produced a nice and detailed look at The Shape of The Periodic Table (.PDF) that reviews the science:

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.

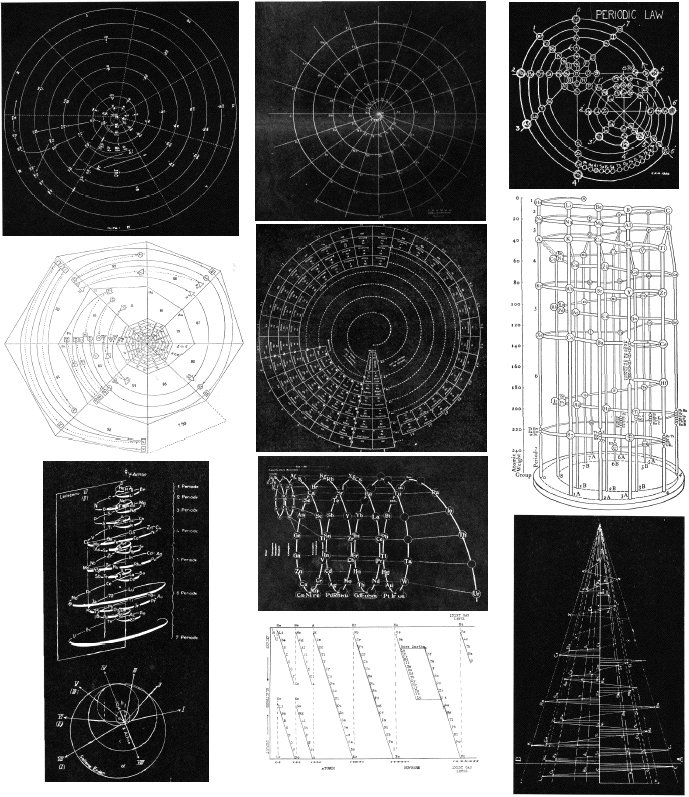
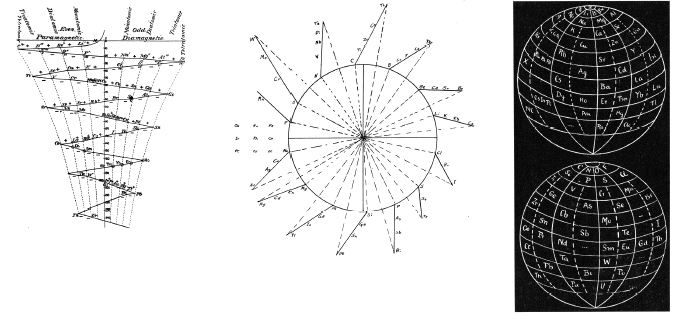

 dic System of Chemical Elements
dic System of Chemical Elements