Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
| Year: 1864 | PT id = 8, Type = formulation |
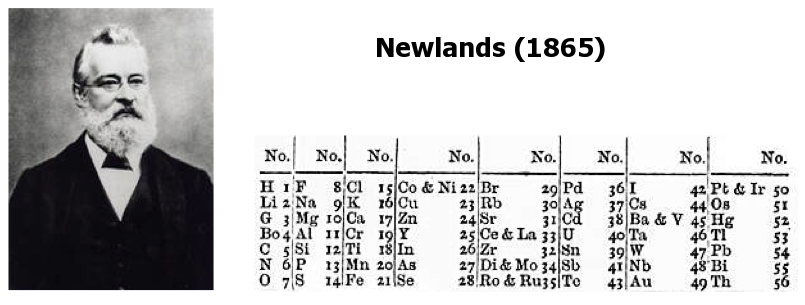
Newlands' Octaves
One of the first attempts at a periodic table that arranged the known elements by atomic weight and chemical property, was by John Newlands and is known as "Newlands Octaves".
Newland noticed that if he broke up his list of elements into groups of seven – starting a new row with the eighth element – the first element in each of those groups had similar chemistry.
Note: In the tables below, Newlands Octaves go downwards: H to O, F to S, Cl to Fe, etc.
|
H 1
|
F 8
|
Cl 15
|
Co & Ni 22
|
Br 29
|
Pd 36
|
I 42
|
Pt & Ir 50
|
|
Li 2
|
Na 9
|
K 16
|
Cu 23
|
Rb 30
|
Ag 37
|
Cs 44
|
Os 51
|
|
G 3
|
Mg 10
|
Ca 17
|
Zn 24
|
Sr 31
|
Cd 38
|
Ba & V 45
|
Hg 52
|
|
Bo 4
|
Al 11
|
Cr 19
|
Y 25
|
Ce & La 33
|
U 40
|
Ta 46
|
Tl 53
|
|
C 5
|
Si 12
|
Ti 18
|
In 26
|
Zr 32
|
Sn 39
|
W 47
|
Pb 54
|
|
N 6
|
P 13
|
Mn 20
|
As 27
|
Di & Mo 34
|
Sb 41
|
Nb 48
|
Bi 55
|
|
O 7
|
S 14
|
Fe 21
|
Se 28
|
Ro & Ru 35
|
Te 43
|
Au 49
|
Th 56
|
- Seeing the word octave applied to this table may lead one to think that Newlands recognised periods of eight elements with repeating properties, as we do with the modern periodic table, for example: Li Be B C N O F Ne.
- However, each sequence of Newlands' octaves contain only seven elements. Count the elements in the columns! In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not yet been discovered.
- To Newlands, H to F & F to Cl are octaves of eight elements, the eighth element repeating the properties of the first.
There are seven notes in a musical octave: A B C D E F G, after which you start again with A'; similarly for Newlands, seven elements H Li G Bo C N O, then the 8th is F and you start again. [Note that Newlands treated H as a halogen.] More here.
A B C D E F G A
Philip Stewart's musical representation:
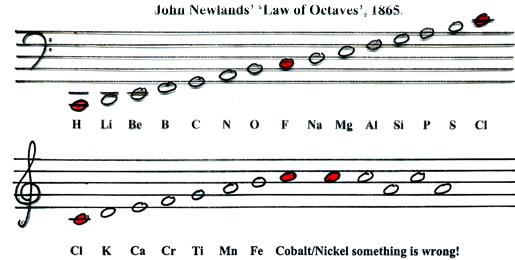
- To Newlands, H to F is an octave of eight elements.
- Today we say Li to Ne & Na to Ar are periods of eight elements, and that that Li and Na are in different periods. Indeed, the Li to Na series consists of nine elements.
- In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not been discovered.
Read more about Newland's Octaves, including a commentary on the original papers in Carmen Giunta's Elements and Atoms: Case Studies in the Development of Chemistry.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.