Periodic Table |
 |
 |
 |
 |
 |
 |
 |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
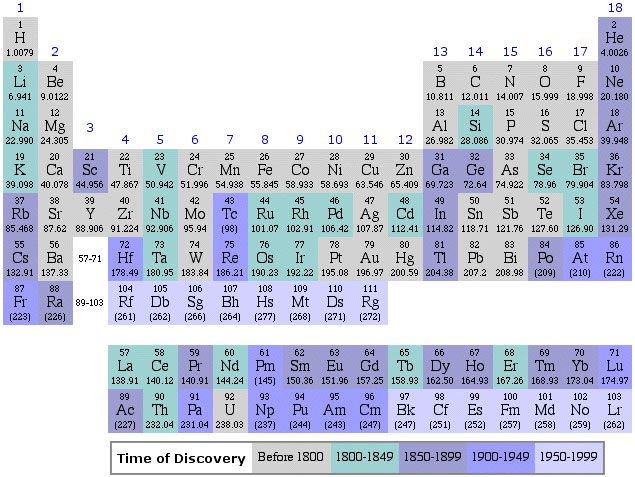
2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the years 1950 - 1999, by date:
| Year: 1950 | PT id = 14 |
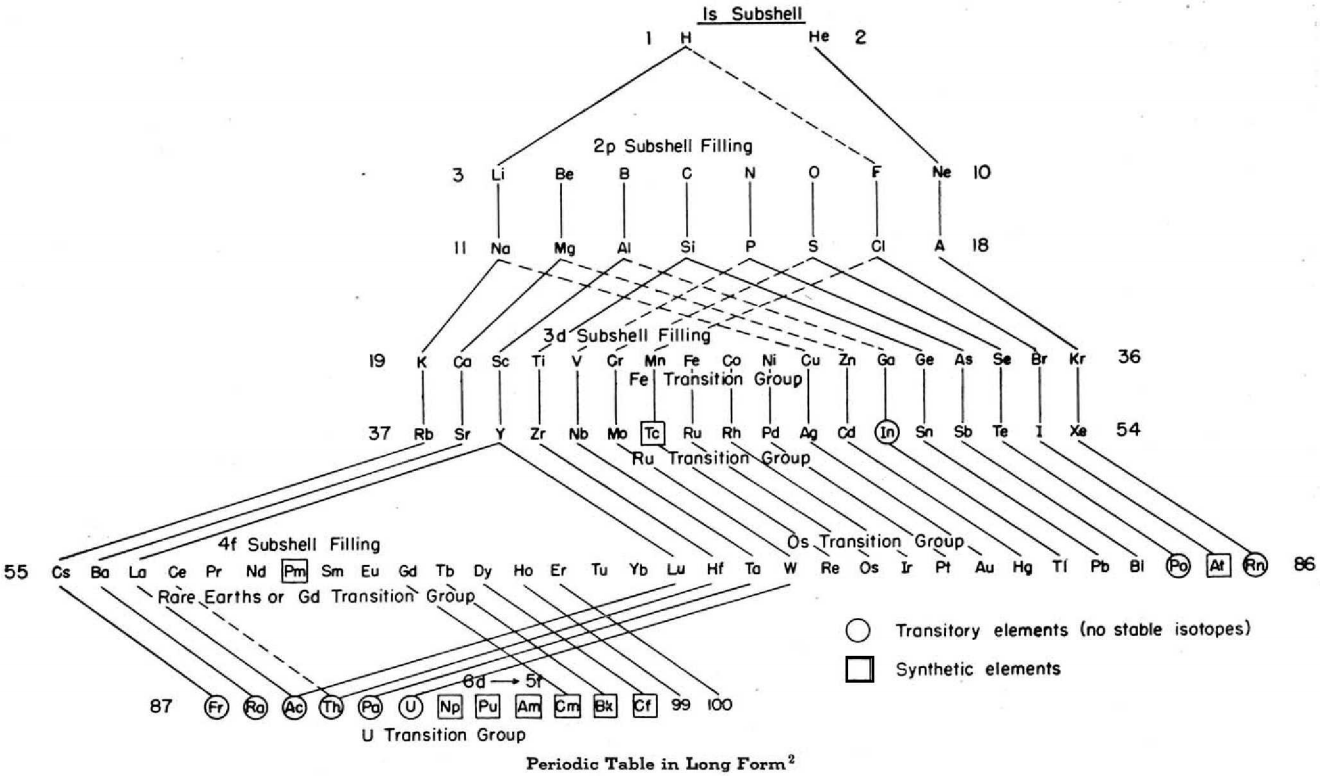
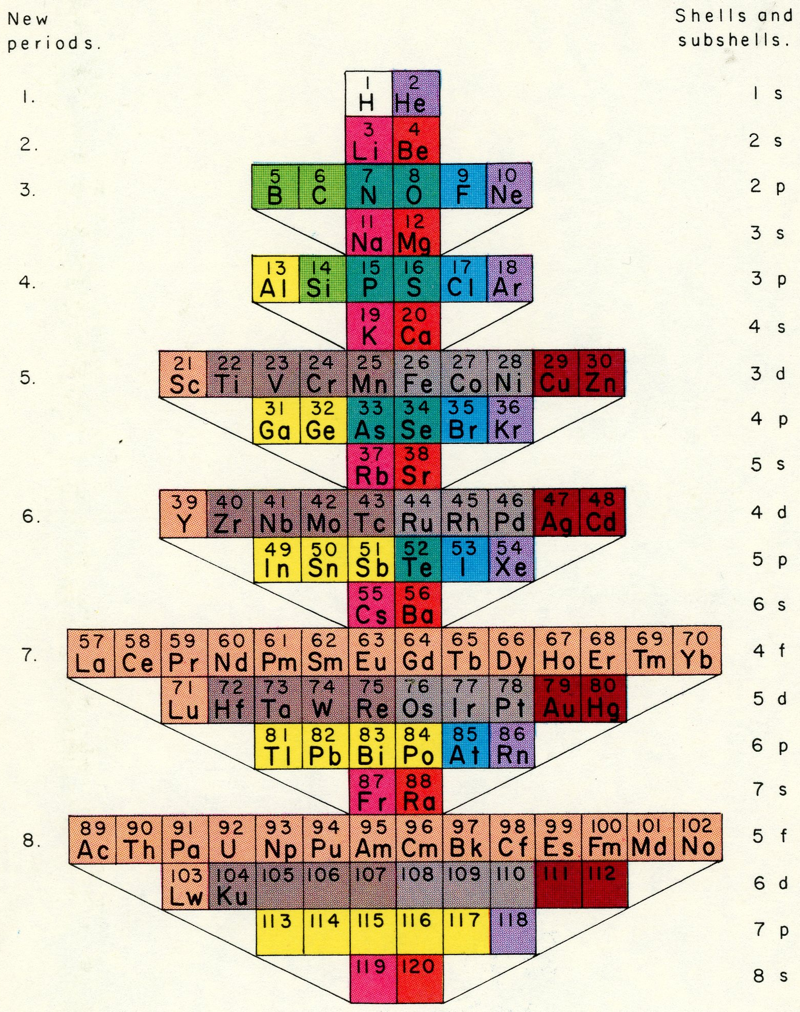
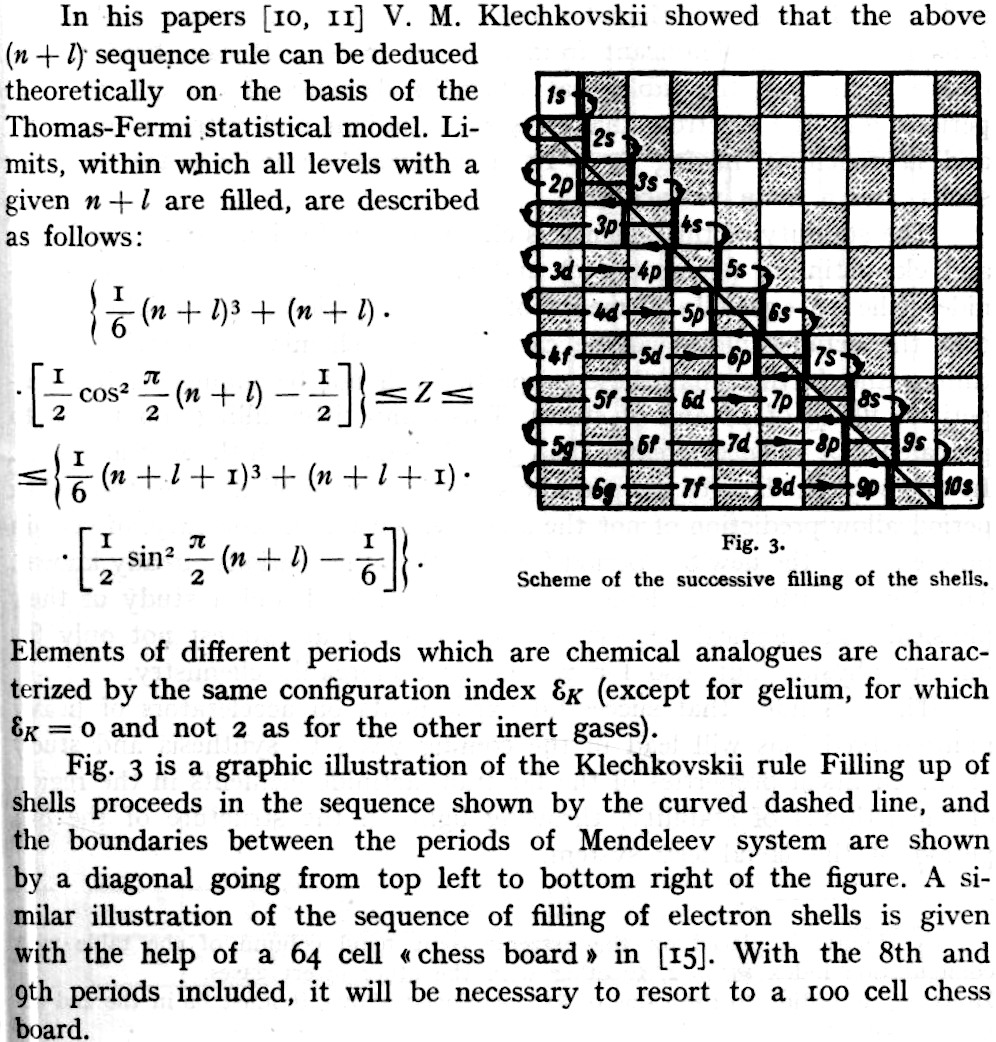
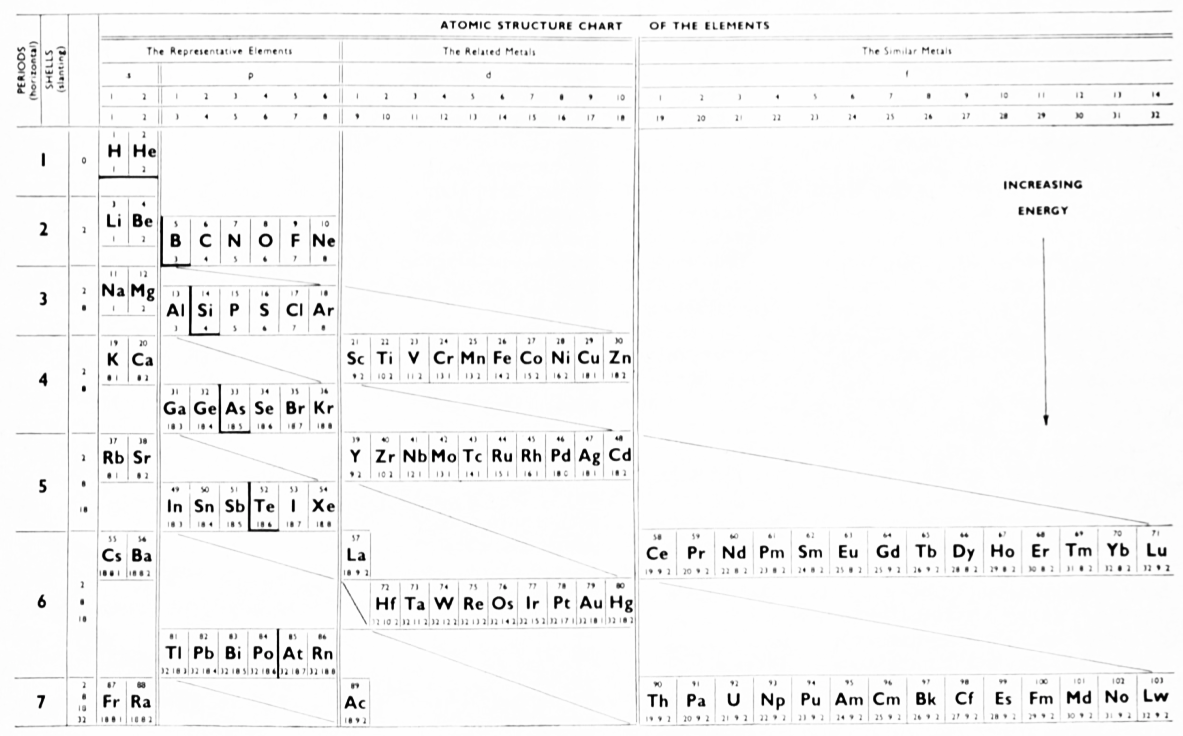
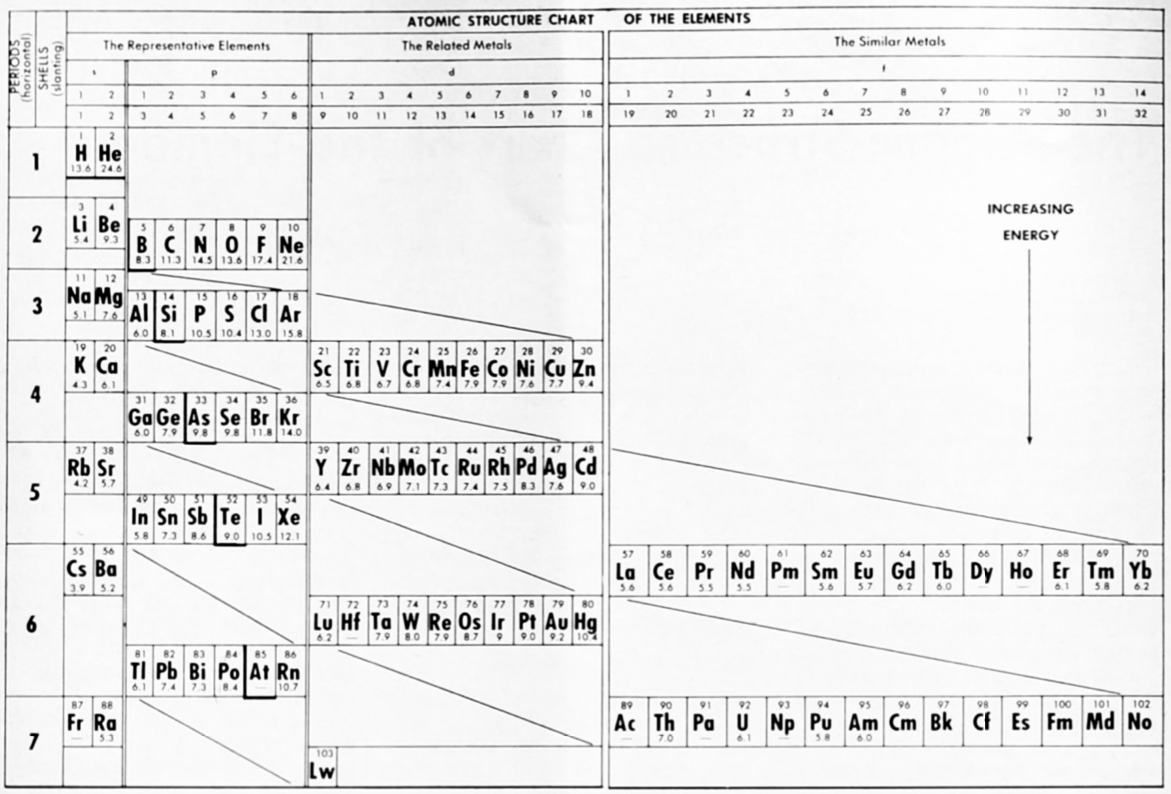
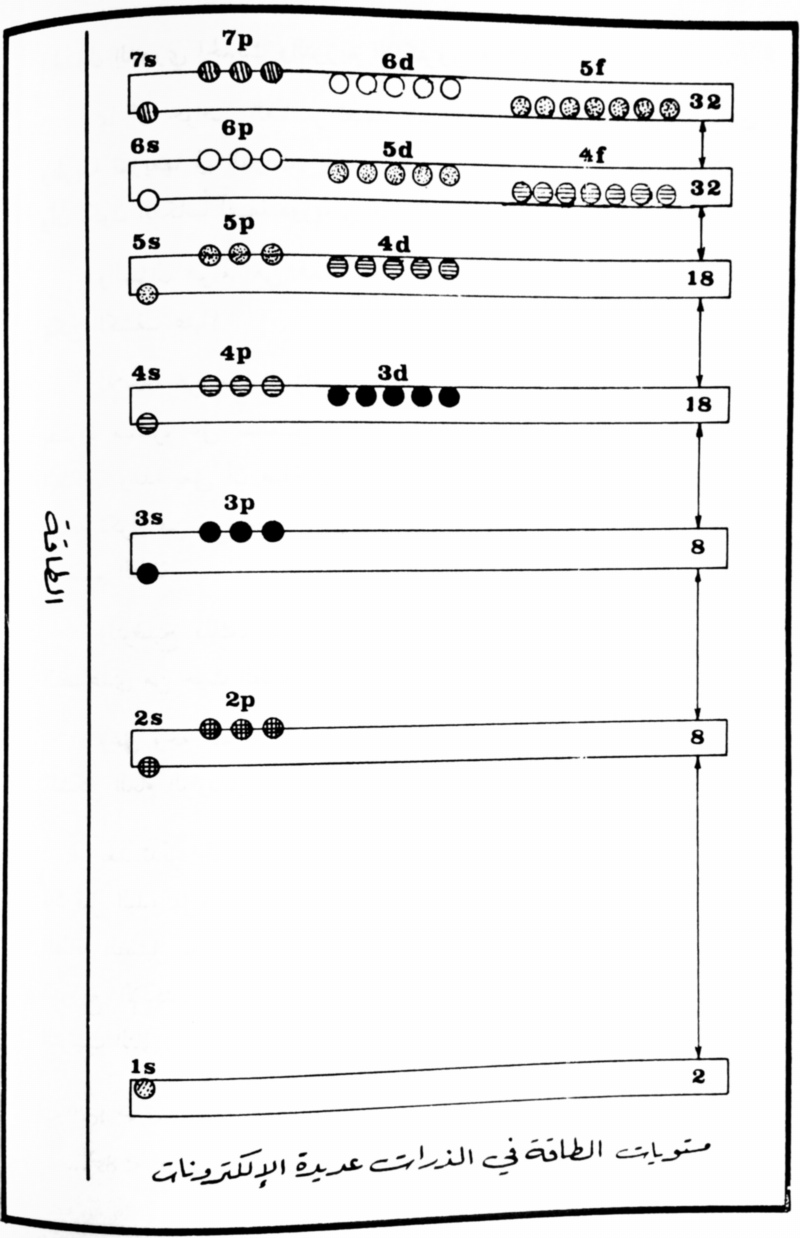
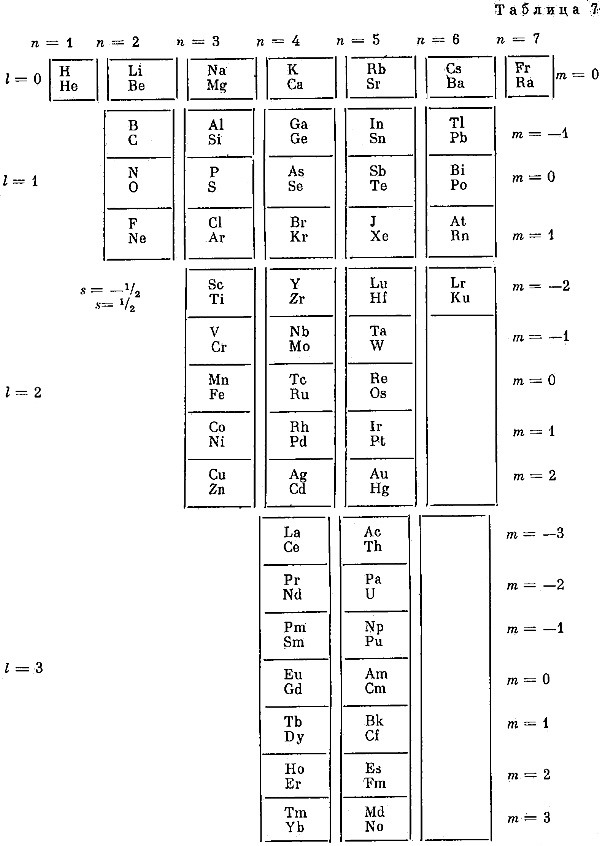
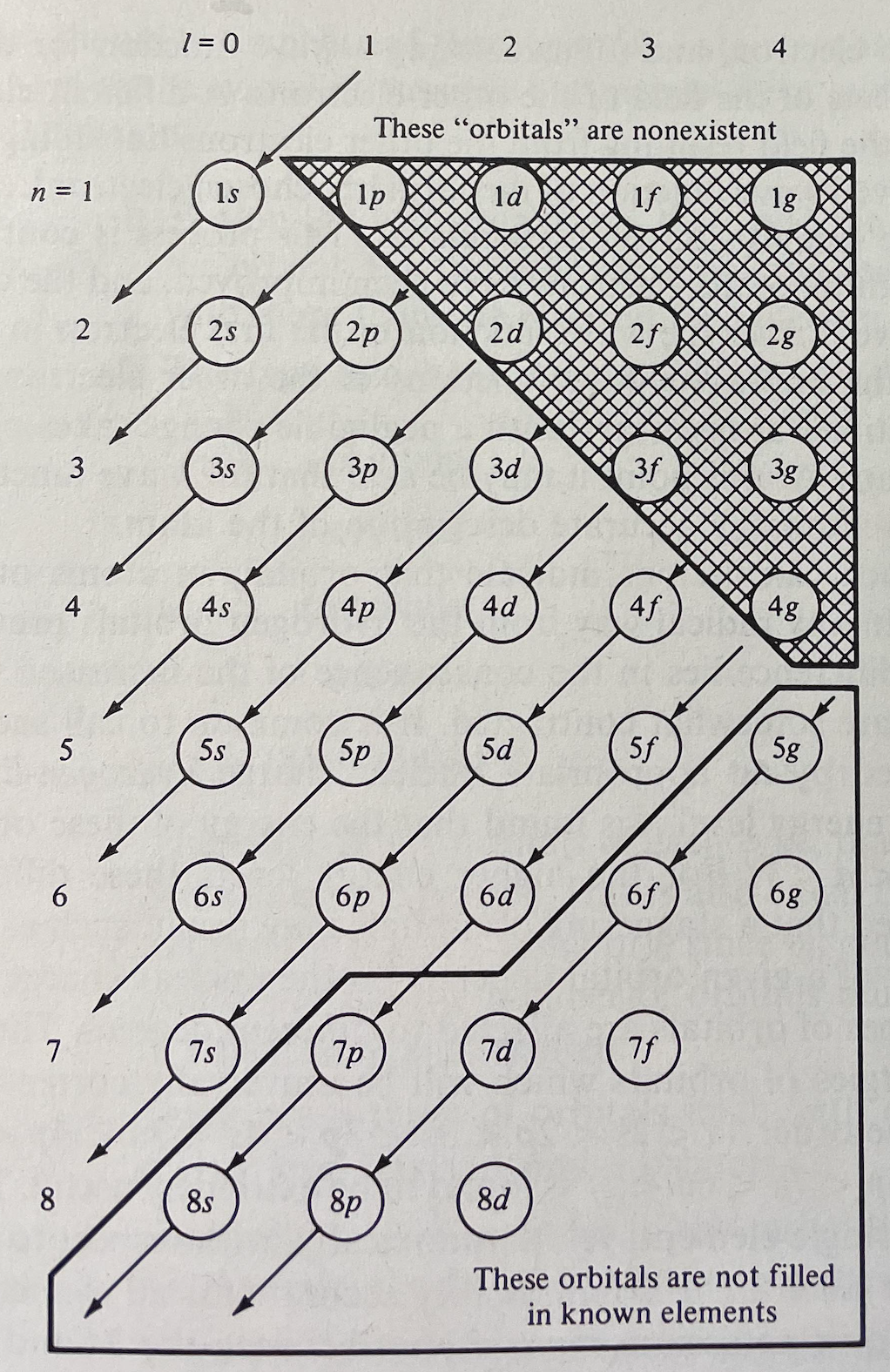
The modern periodic table is based on quantum numbers and blocks, here.
A periodic table can be constructed by listing the elements by n and l quantum number:
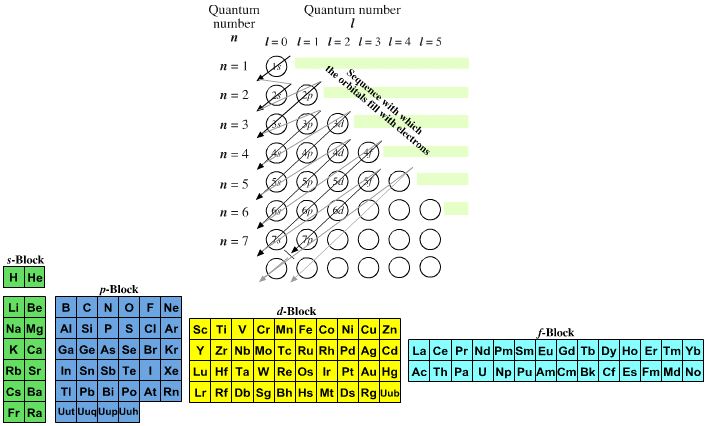
The problem with this mapping is that the generated sequence is not continuous with respect to atomic number atomic number, Z: Check out the sequence Ar to K, 18 to 19.
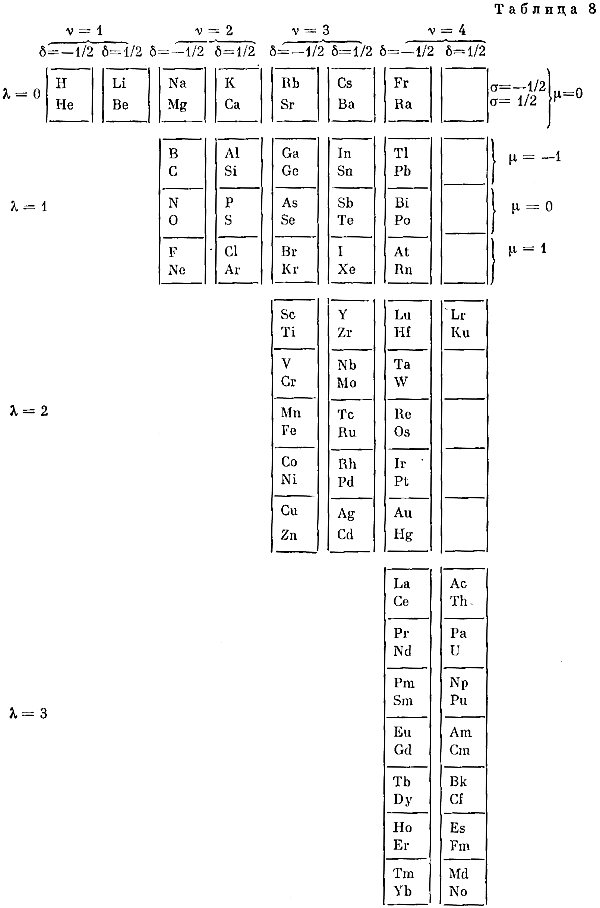
Named after a French chemist who first published in the formulation in 1929, the Janet or Left-Step Periodic Table uses a slightly different mapping:
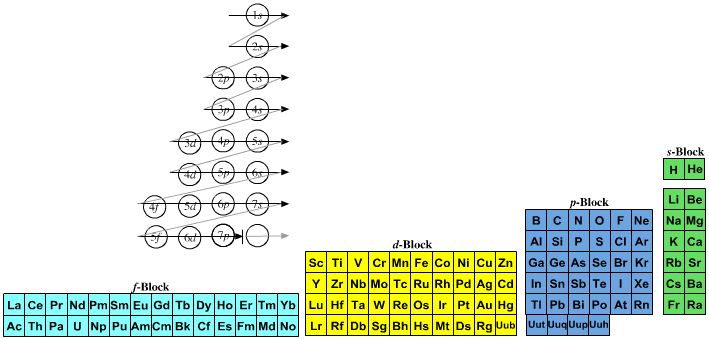
While the Janet periodic table is very logical and clear it does not separate metals from non-metals as well as the Mendeleev version, and helium is a problem chemically.
However, it is a simple mapping to go from the Janet or Left-Step periodic table to a modern formulation of Mendeleev's periodic table:
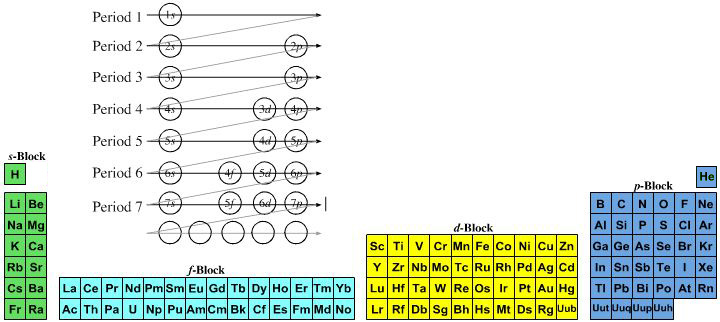
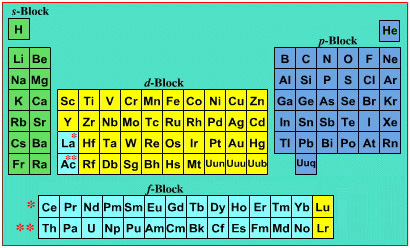
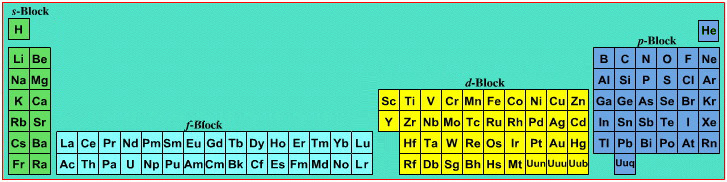
On this page web, "full" f-block included periodic tables are shown wherever possible, as above.
However, the periodic table is usually exhibited in book and on posters in a compressed form with the f-block "rare earths" separated away from the s-block, p-block and d-block elements:
However, the compression used introduces the well known problem known as a "fence post error".
The effect is that:
La and Ac: move from f-block to d-block
Lu and Lr: move from p-block to f-blockChemically, the elements can be fitted in and classified either way. Many thanks to JD for pointing the situation with the periodic table is a fence post error.
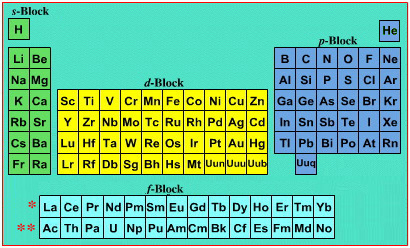
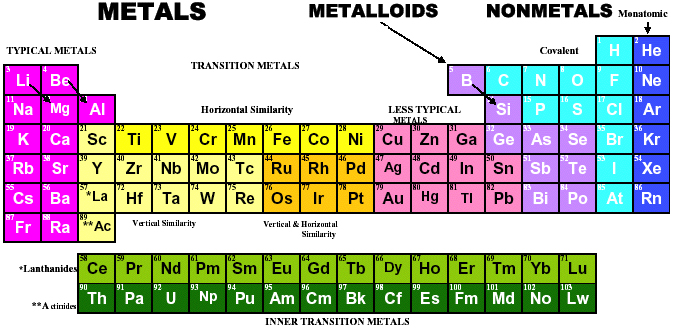
Mark Winter's Web Elements project, here, uses the formulation shown below:
Interestingly, the IUPAC periodic table separates out 15 lanthanides, La-Lu, and 15 actinides, Ac-Lr by leaving gaps in period 3 under Sc & Y:
This corresponds to:
By Mark Leach
| Year: 1950 | PT id = 153 |
Clark's Updated Periodic Table
John D Clark's 1950 chart. It looks as though the experience of producing the 1949 version for Life Magazine caused him to have a radical rethink. John D. Clark, A modern periodic chart of chemical elements. Science,111, 661-663 (1950). Information supplied by Philip Stewart.
| Year: 1950 | PT id = 287 |
Scheele's System
Scheele's system of 1950 (from van Spronsen):
| Year: 1950 | PT id = 475 |
Elements Known in the Year 1950
Elements known in the year 1950, taken from this Wikipedia page:
| Year: 1950 | PT id = 878 |
Discovery of Californium
Cf ![]()
Californium, atomic number 98, has a mass of 251 au.
Synthetic radioactive element.
Californium was first observed in 1950 by S. G. Thompson, K. Street, Jr., A. Ghiorso and G. T. Seaborg.
| Year: 1950 | PT id = 1080 |
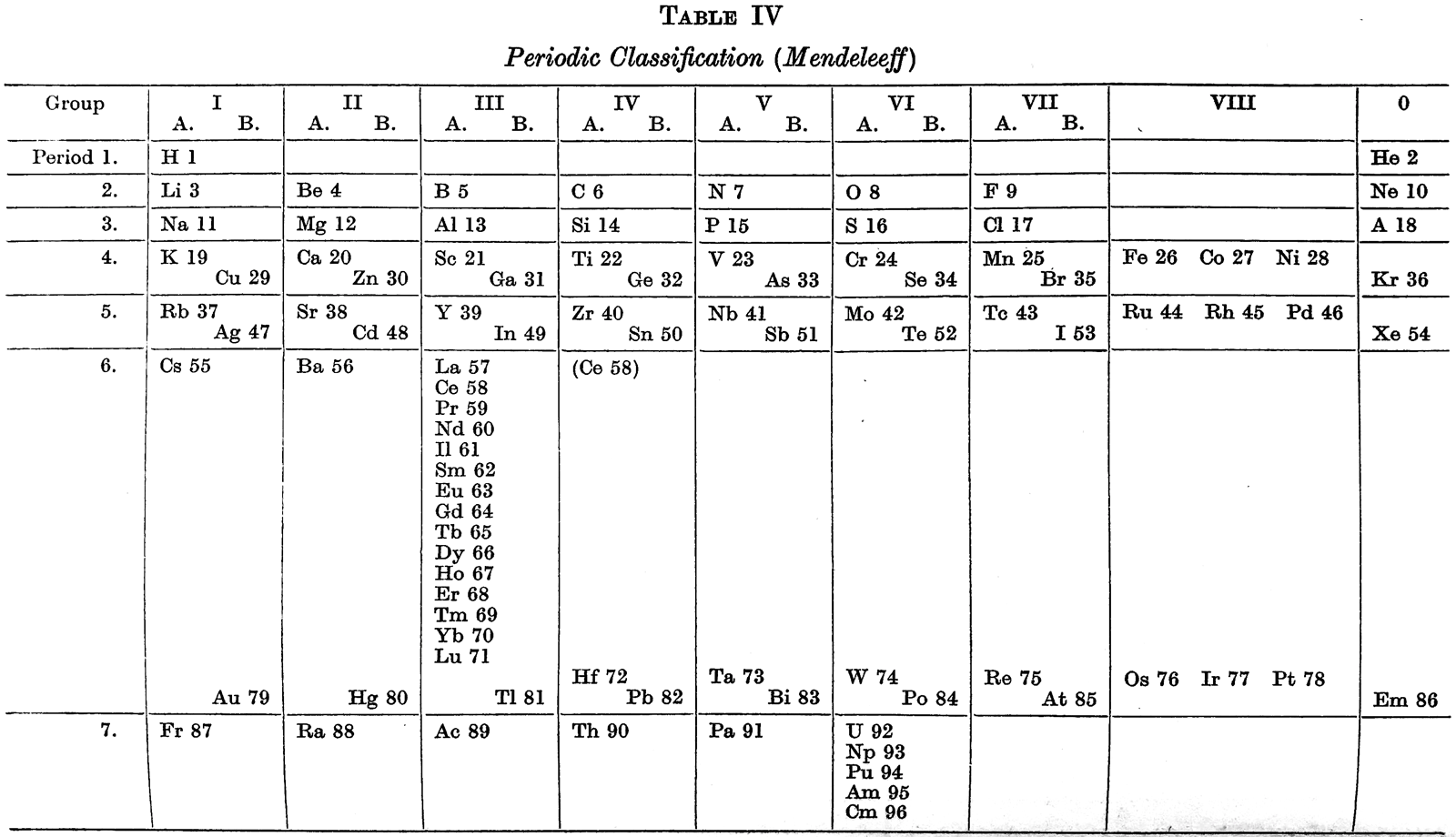
Sidgwick's Periodic Classification (Mendeleeff)
From N.V. Sidgwick, Chemical Elements and Their Compounds, vol. 1, Oxford University, London, p. xxviii (1950).
René Vernon writes:
"In this curious table the Lanthanides are located in group IIIA while the Actinides have been fragmented.
Instead:
• Ac, Th, and Pa are located in groups IIIA, IVA and VA under Lu, Hf, and Ta, respectively
• The uranides, U, Np, Pu, Am, and Cm, are located in group VIA, under W."
Sidgwick writes:
"This subgroup (VIA) consists of Cr, Mo, W, and U, to which the 'uranide' elements, Np, Pu, Am, and Cm (which might be assigned to any Group from III to VI) must now be added." (p. 998)
"...the trans-uranium elements 93–6... for the first time give clear evidence of the opening of the 'second rare earth series', the 'uranides', through the expansion of the fifth quantum group from 18 towards 32." (p. 1069)
"The question whether the fifth quantum group of electrons which is completed up to 18 in gold begins to expand towards 32, as the fourth does in cerium, has now been settled by the chemical properties of these newly discovered elements. In the Ln the beginning of the expansion is marked by the main valency becoming and remaining 3. With these later elements of the seventh period there is scarcely any sign of valencies other than those of the group until we come to uranium... Up to and including uranium, the group valency is always the stablest, but beyond this no further rise of valency occurs, such as we find in rhenium and osmium. Hence the point of departure of the new series of structures (corresponding to lanthanum in the first series) is obviously uranium, and the series should be called the uranides. (p. 1092):
| Year: 1950 | PT id = 1119 |
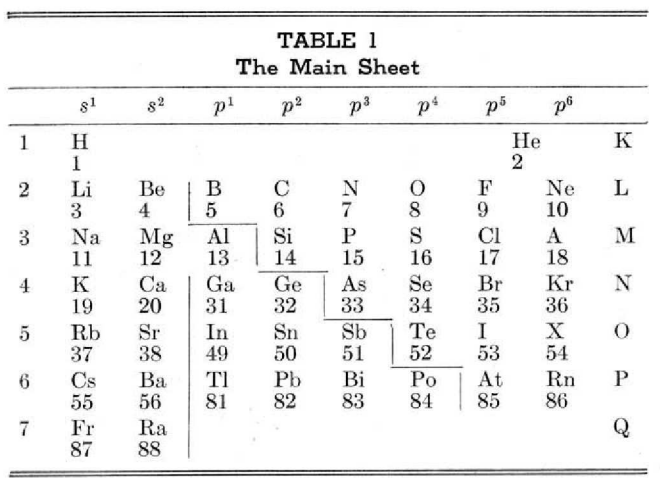
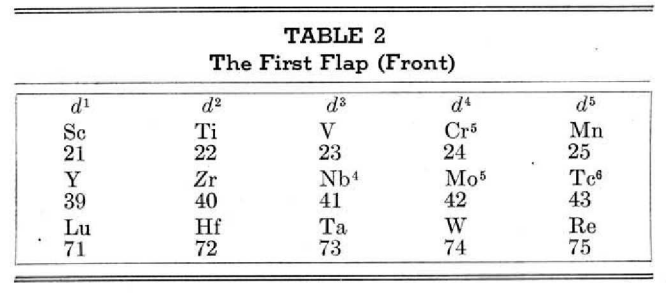
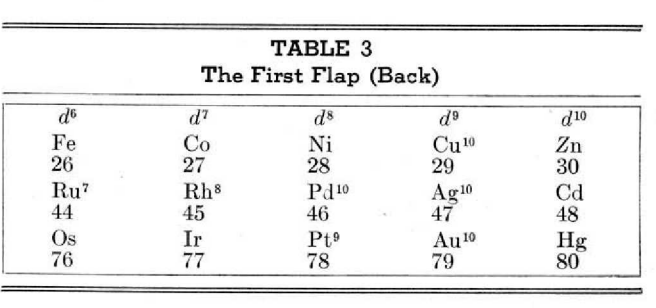
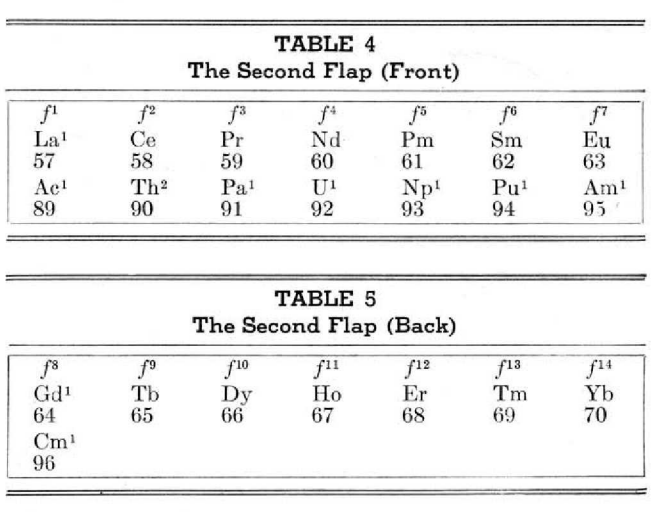
McCutchon's Simplified Periodic Classification of the Elements
McCutchon KB, A simplified periodic classification of the elements, Journal of Chemical Education, vol. 27, no. 1, pp. 17–19 (1950)
This 3-dimensional table has two double-sided flaps attached. The top flap is the f bock. Under that is the d block.
The superscripts denote the number of d electrons an element has. Thus, La1 is shown as being an f1 element. But it has a 1 superscript, meaning that the f electron count is reduced by 1 and the d electron count is 1.
René Vernon writes:
"On group 3, McCutchon cryptically says: The proposed arrangement brings out certain known facts about the tertiary elements which are rarely shown by other arrangements. For example, it suggests, correctly, that the resemblance between yttrium and lutecium is greater than that between yttrium and lanthanum. It classifies lanthanum but not lutecium as a rare earth, in accordance with their chemical properties (which also contradict spectrographic evidence at this point). It also demonstrates the tetravalence of both cerium and thorium, and that thorium and protactinium show a resemblance in chemical properties to zirconium and niobium, as well as to hafnium and tantalum."
I say "cryptically" because McCutchon presents no further evidence in support of his assertion that the resemblance between Y and Lu is greater than between Y and La. He may have had in mind the fact that Lu is more often found in ores of Y than is the case for La... and I don't understand his reference to spectrographic evidence.
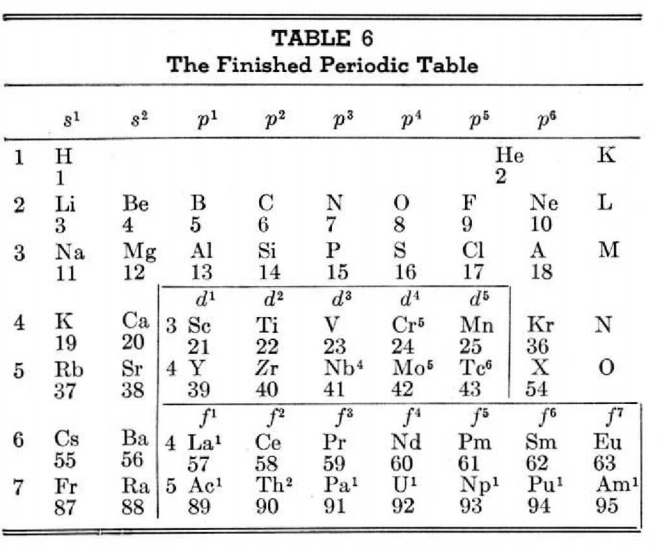
| Year: 1951 | PT id = 24 |
Longman's Mural from Festival of Britain
Edgar Longman's mural from the 1951 Festival of Britain Science Exhibition, restored by Philip Stewart:
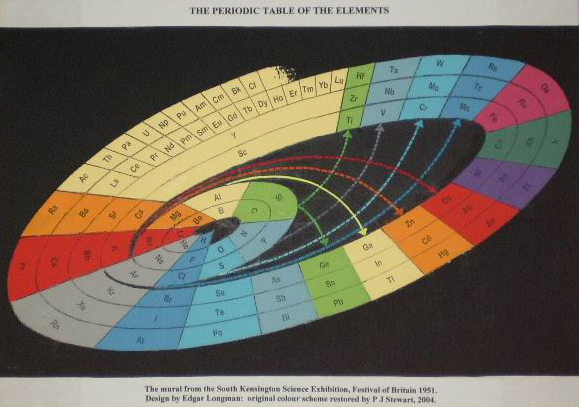

| Year: 1951 | PT id = 461 |
Mellor's Periodic Series of the Elements
Mellor's periodic series of the elements lists the rare earths as a vertical column below Y in Group III. Element Z=61, is identified as Il, illinium.
The peculiarities of Ce, Eu, Tb and Yb are not evident. U is positioned below W emphasizing its 6+ oxidation state.
From Michael Laing's paper: A Revised Periodic Table with the Lanthanides Repositioned, Found. Chem. (2005) 7: 203-233
| Year: 1951 | PT id = 677 |
Tomkeieff's Periodic Table Formulation Formula
A short letter to Nature in which Tomkeieff gives a formula to generate the periodic table:
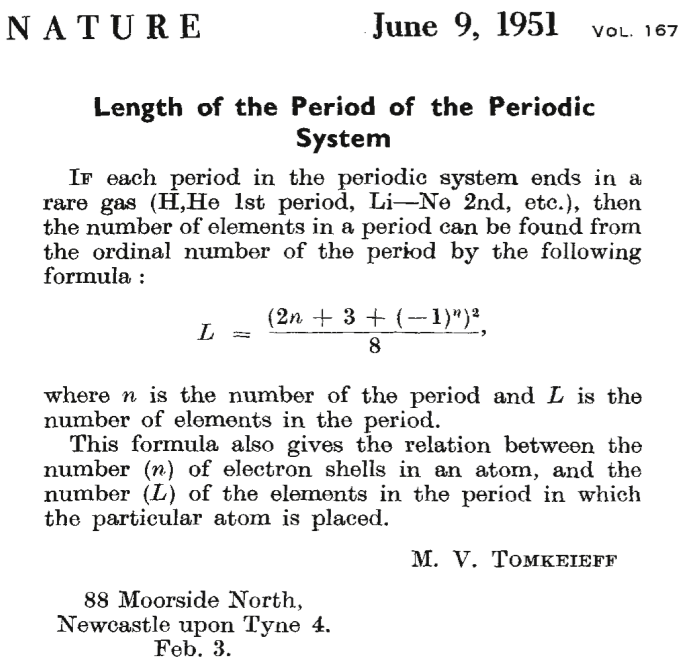
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1951 | PT id = 1245 |
Friend's Updated Periodic Table
René Vernon writes:
"This 1951 table succeeds Friend’s table of 1926. Notice how Pu, Am, and Cm have been assigned to group VIII. The splitting of the Ln across two periods is bizarre."
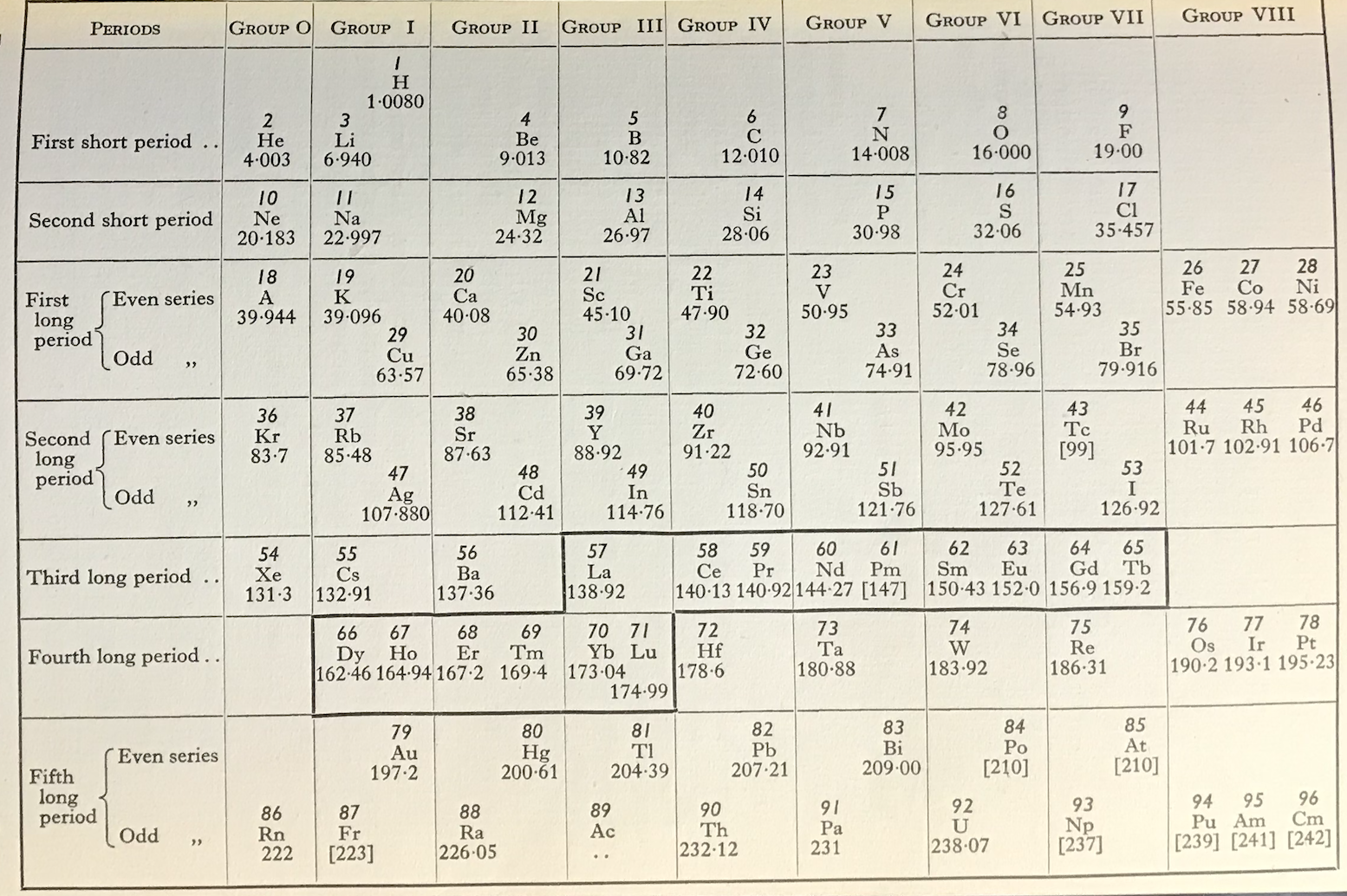
| Year: 1951 | PT id = 1272 |
Spedding's Rare Earths Periodic Table
Ref: Spedding FH 1951 The Rare Earths, Scientific American, vol. 185, no. 5, pp. 26–31
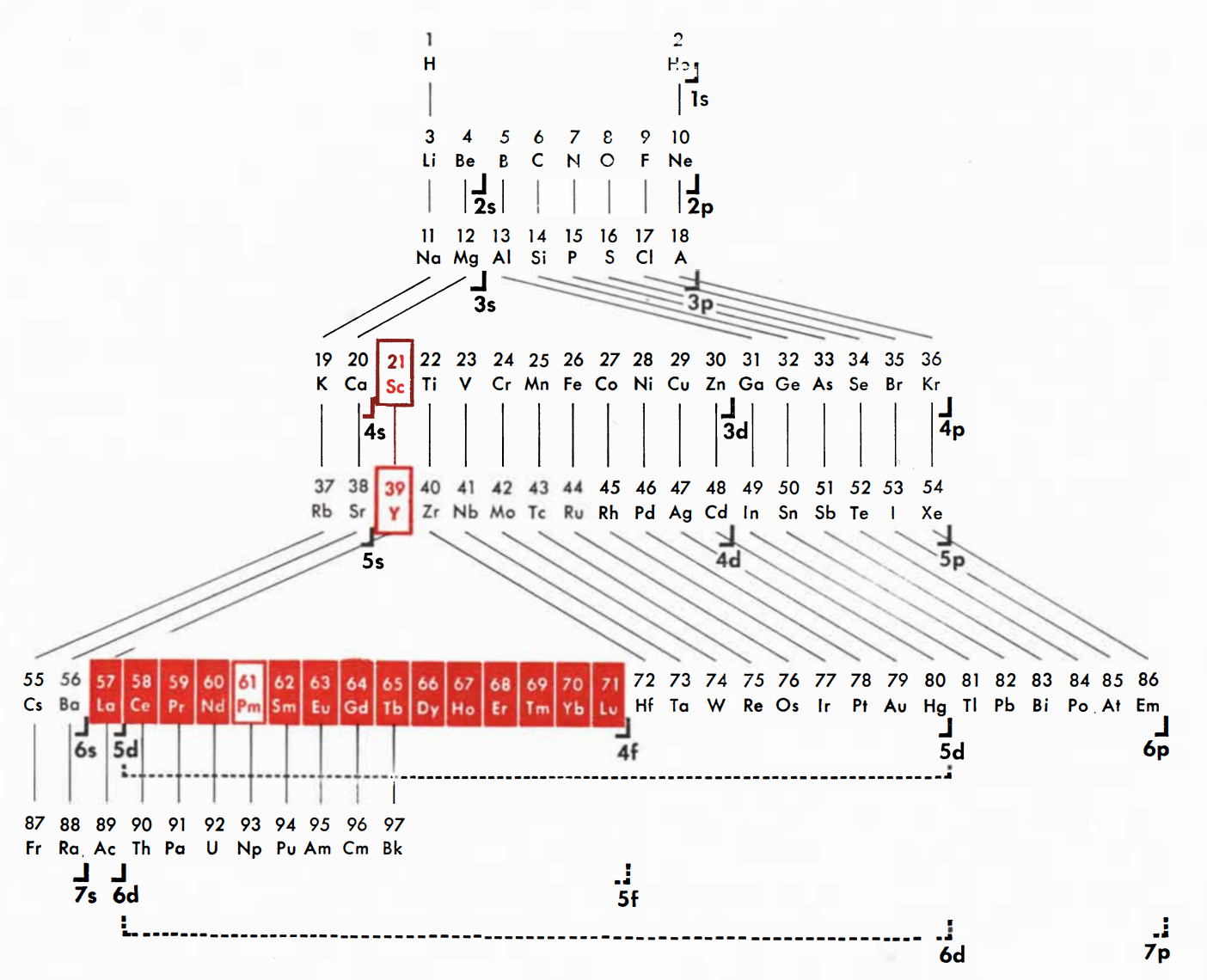
Thanks to René for the tip!
| Year: 1952 | PT id = 879 |
Discovery of Einsteinium
Es ![]()
Einsteinium, atomic number 99, has a mass of 252 au.
Synthetic radioactive element.
Einsteinium was first observed in 1952 by A. Ghiorso et al.
| Year: 1952 | PT id = 880 |
Discovery of Fermium
Fm ![]()
Fermium, atomic number 100, has a mass of 257 au.
Synthetic radioactive element.
Fermium was first observed in 1952 by A. Ghiorso et al.
| Year: 1952 | PT id = 988 |
Hakala's Periodic Law in Mathematical Form
Reino Hakala published a paper, The Periodic Law in Mathematical Form, J.Phys.Chem., 1952, 56(2) 178-181. It is argued that: "Janet's [left-step] best meets these requirements".
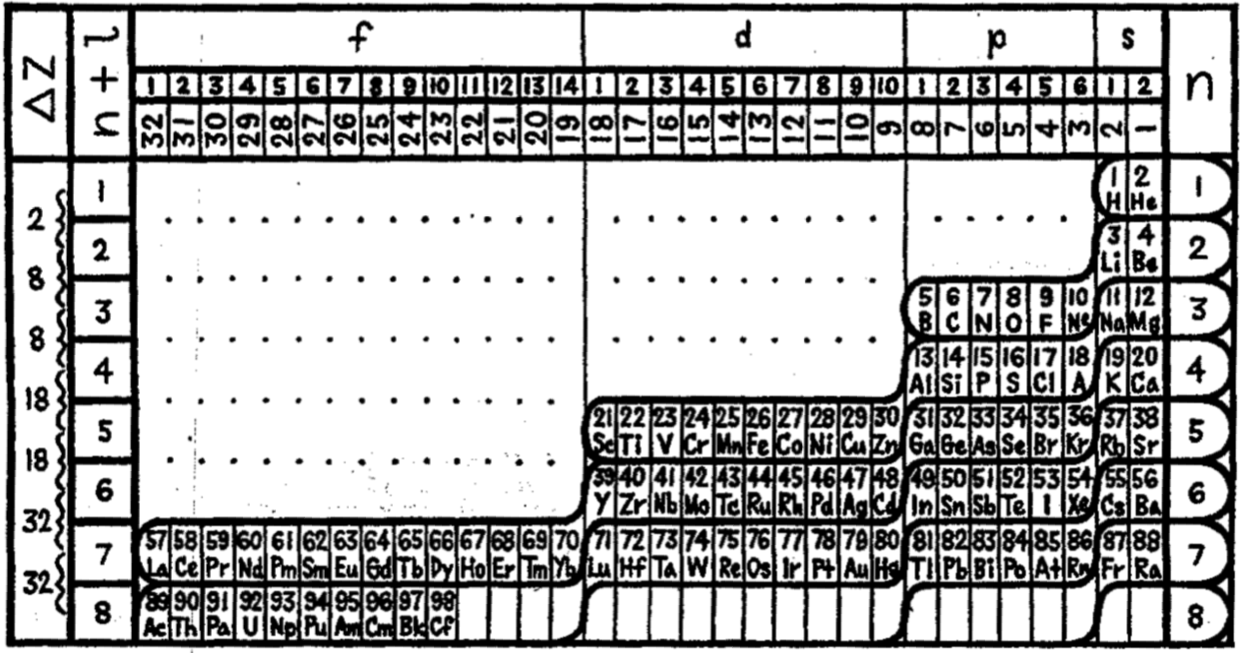
Thanks to René for the tip!
| Year: 1952 | PT id = 1054 |
Coryell's Periodic Table in Long Form
Charles D. Coryell The periodic table: The 6d-5f mixed transition group, J. Chem. Educ., vol. 29, no. 2, pp. 62–64 1952.
Coryell (1912–1971), was an American chemist involved in the discovery of promethium.
René Vernon writes:
"In Coryell's table, just two elements are shown as having two solid 'tie lines':
Yttrium: to La-Ac and to Lu-Ac
Silicon: to Ti-Zr-Hf and to Ge-Sn-Pb.
"These days Ti-Zr-Hf-Rf is deemed to make-up group 4 (rightly so given group 4 is the first to exhibit characteristic transition metal properties) whereas C-Si-Ge-Sn-Pb-Fl is deemed to make-up group 14.
The solid tie lines Coryell shows between Hf-Th, Ta-Pa, and W-U would now be rendered in broken form.
If Coryell's table was mapped to a 32- or 18-column form, group 3 would presumably be shown as bifurcating after Y.
The circle around indium is possibly a typo(?): indium has two stable isotopes, In-113 (4.29%) & In-115 (95.71%)... actually, In-155 has a half-life of 4.4x1014 years."
| Year: 1953 | PT id = 397 |
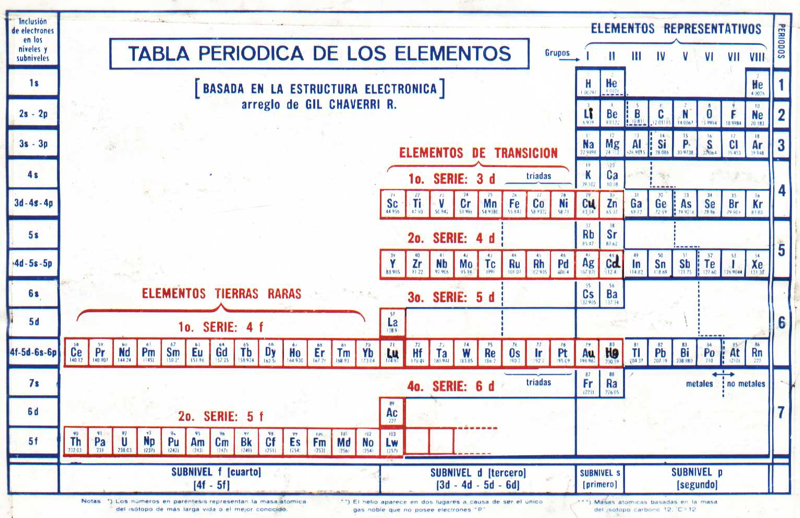
Chaverri-Rodríguez Tabla Periódica de los Elementos Químicos
Spanish to English translation from here.
Click here to see a larger version.
Originally published: Tabla Periódica de los Elementos. J. Chem. Educ. 1953, 30, 632-633
"The arrangement of the Periodic Table of the Elements according to Gil Chaverri-Rodríguez mainly takes into account the electronic structure of an element in determining the element's position in the table. It takes into account the different periods of elements have different length, because the first is of two elements, then followed by two periods of eight elements each, then two periods of 18 elements each, then a period of thirty-two elements and finally a seventh period incomplete.
"The table takes into account the important fact that, despite the variable length, the first two elements and the last six items in each period respectively have similar properties, forming the eight groups or columns of representative elements with similar chemical properties. The elements that constitute the Series Transition and Rare Earth Series are arranged in rows, in locations that correspond to how energy sublevels are filled that characterize these elements. Each element corresponds to a specific place and only in a box in the table, with no need to drop items off the table, at the foot of it, as in previous arrangements.
"With the information provided by the Board, you can deduce the electronic structure of a component, from its placement on the table, except the few cases that have small irregularities. In general, the Table is a settlement based on the electronic structure of chemical elements and this criterion determines its position in the array.":
| Year: 1953 | PT id = 407 |
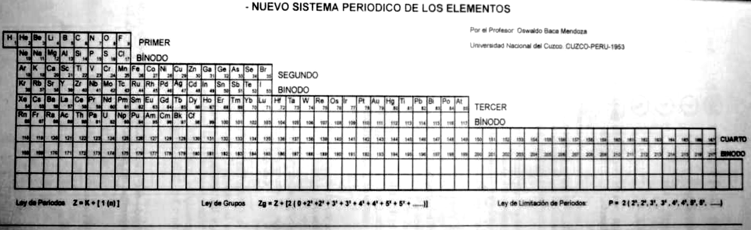
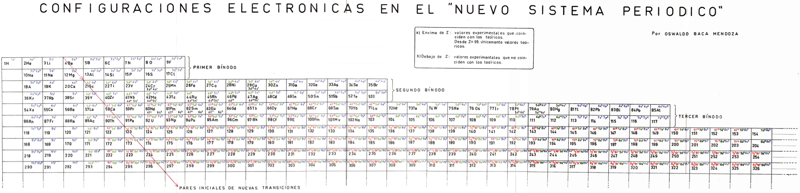
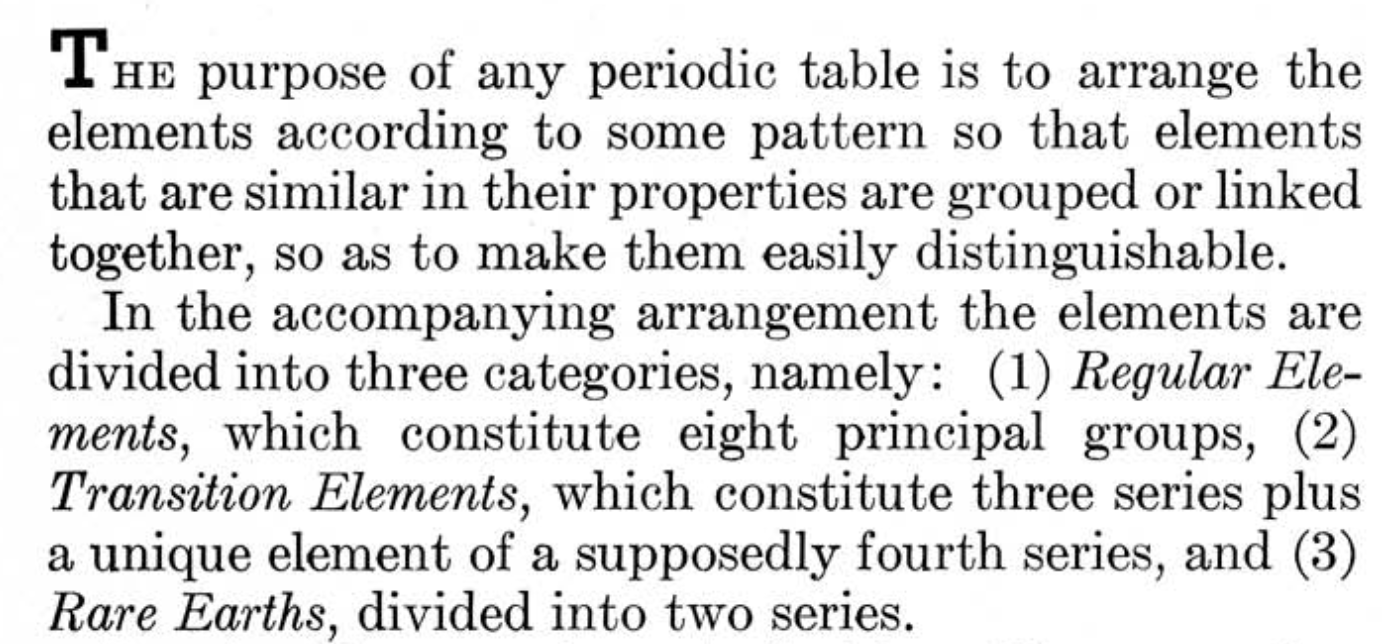
Mendoza Periodic Table
A paper (PDF here) titled "Generic Laws of The Chemical Elements: A New Periodic System" by the Peruvian Oswaldo Baca Mendoza. Click here for a large version:
| Year: 1953 | PT id = 1235 |
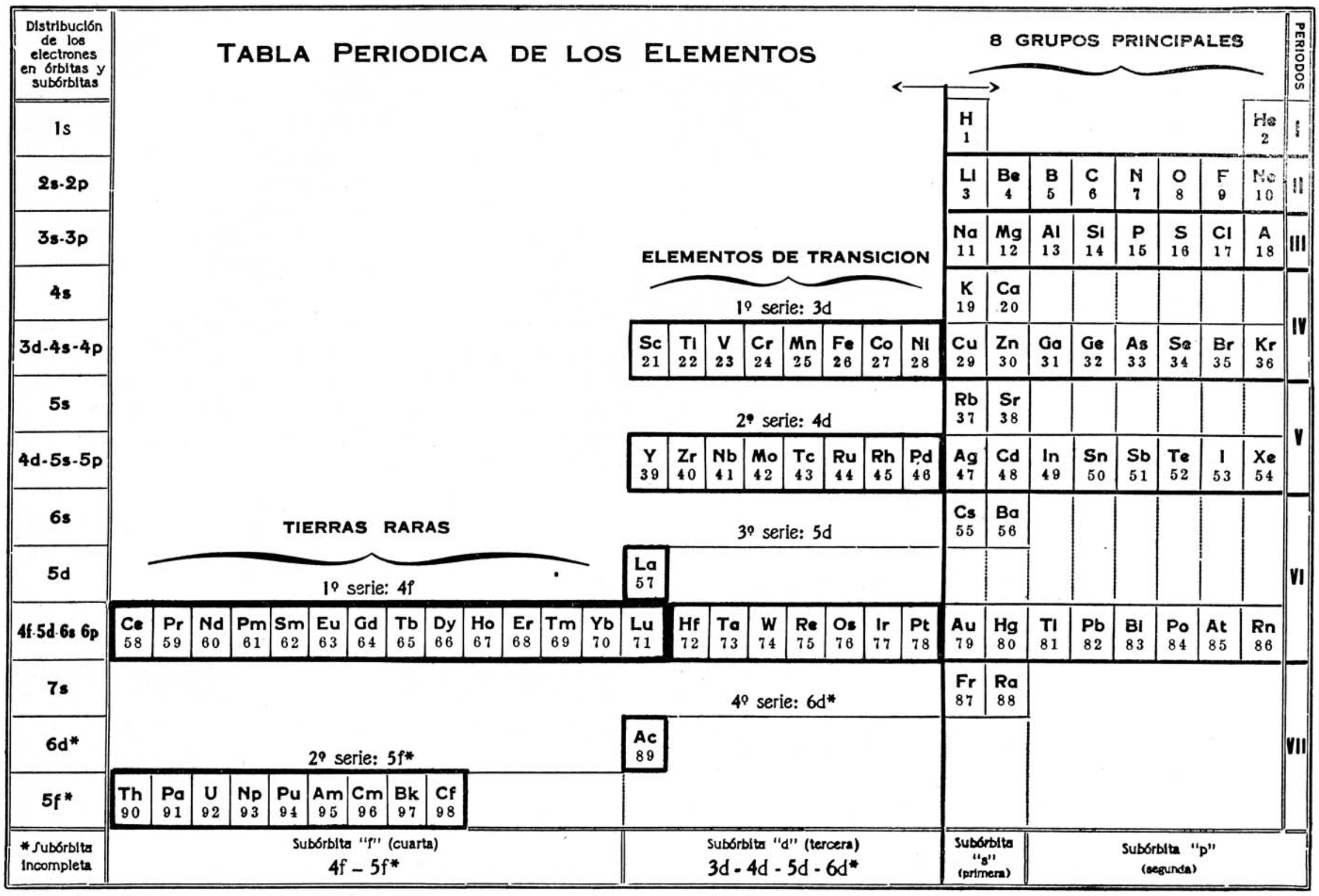
Chaverri's Tabla Periodica de Los Elementos
Gil Chaverri's Tabla Periodica de Los Elementos (Periodic Table of The Elements, J. Chem. Educ. 1953, 30, 12, 632):
| Year: 1954 | PT id = 628 |
Sanderson's "One More" Periodic Table
From Sanderson's paper: One More Periodic Table (J. Chem. Educ., 1954, 31 (9), p 481):
| Year: 1954 | PT id = 922 |
Sabo & Lakatosh's Volumetric Model of the Periodic Table
From the Russian Book: 100 Years of Periodic Law of Chemical Elements, Nauka 1969, p.87.
The caption says: "Volumetric Model of 18-period Long System of D.I.Mendeleev." after Sabo and Lakatosh (1954).
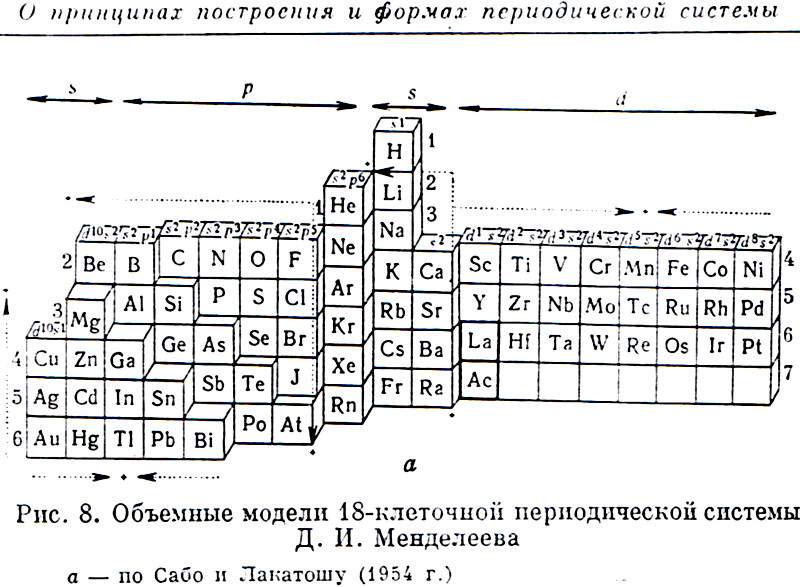
Thanks to Larry T for the tip!
| Year: 1954 | PT id = 1255 |
Ephraim's Periodic Classification
Ephraim F 1954, Inorganic Chemistry, 6th ed., Oliver and Boyd, London (revised by PCL Thorne and ER Roberts)
René Vernon writes that items of interest include:
- The position of H "Which [according to Ephraim] is difficult to place in this table in a satisfactory manner", outside of the main body of the periodic table, "remote from both Li and F, well removed from C, and above He and the inert gases"
- The old school location of B-Al in Group IIIa
- C-Si belong to both Ti-Zr-Hf-Th and Ge-Sn-Pb
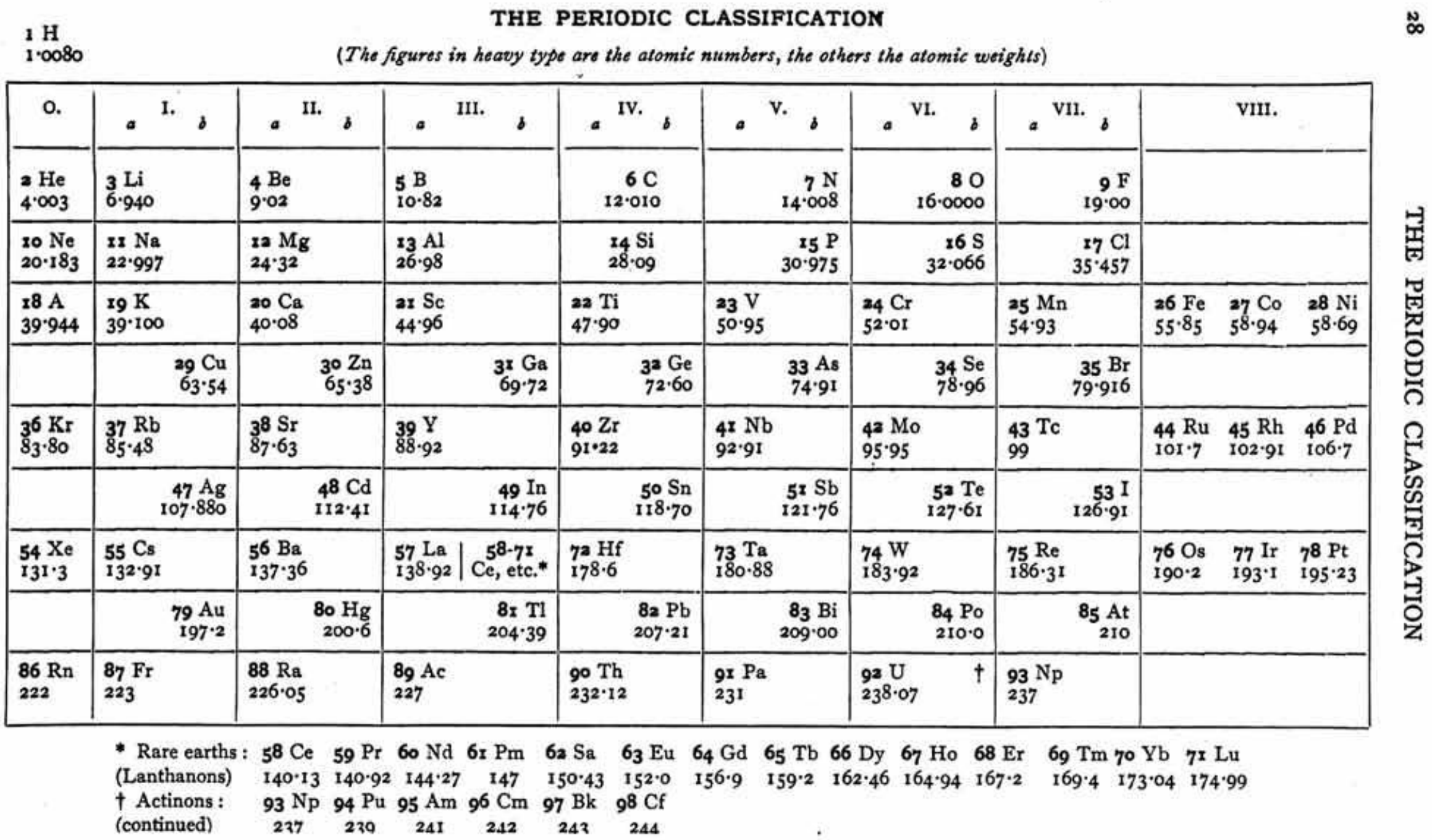
| Year: 1955 | PT id = 300 |
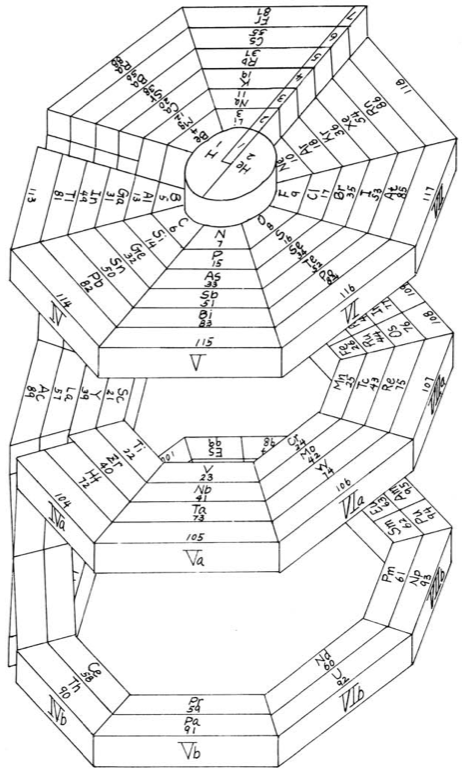
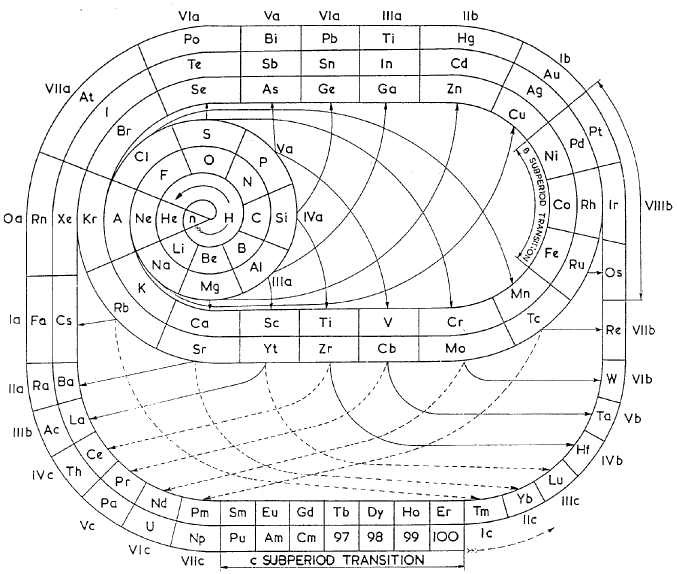
Mazurs' Valence Periodic Table
In his 1974 book Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press (2nd edition) Edward G. Mazurs presents a valence periodic table. He classifies this as a Subtype IIIC3-6a formulation:
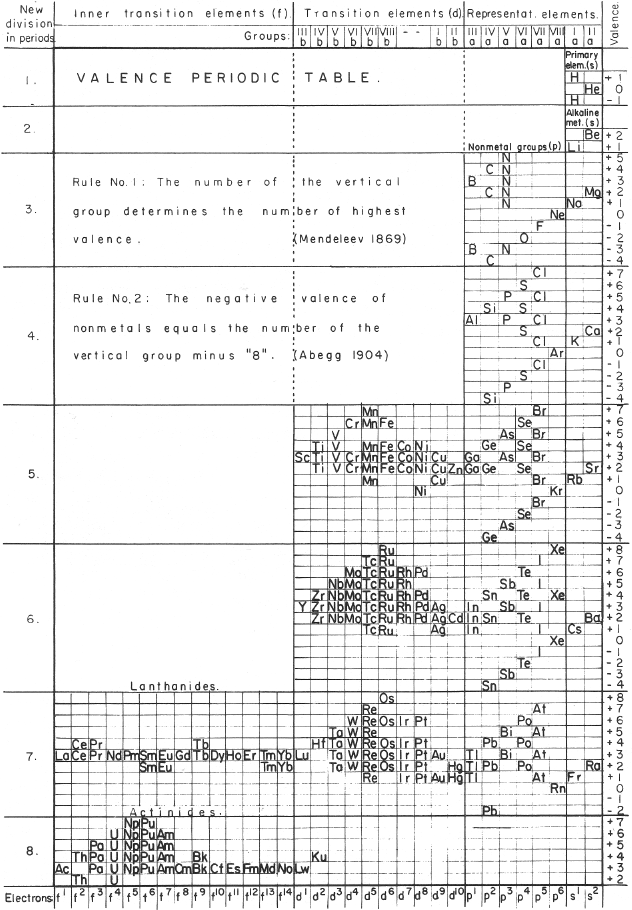
| Year: 1955 | PT id = 301 |
Mazurs' Valence Periodic Table
In his 1974 book Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press (2nd edition) Edward G. Mazurs presents a periodic table he classifies as a Subtype IIIC3-6b formulation:
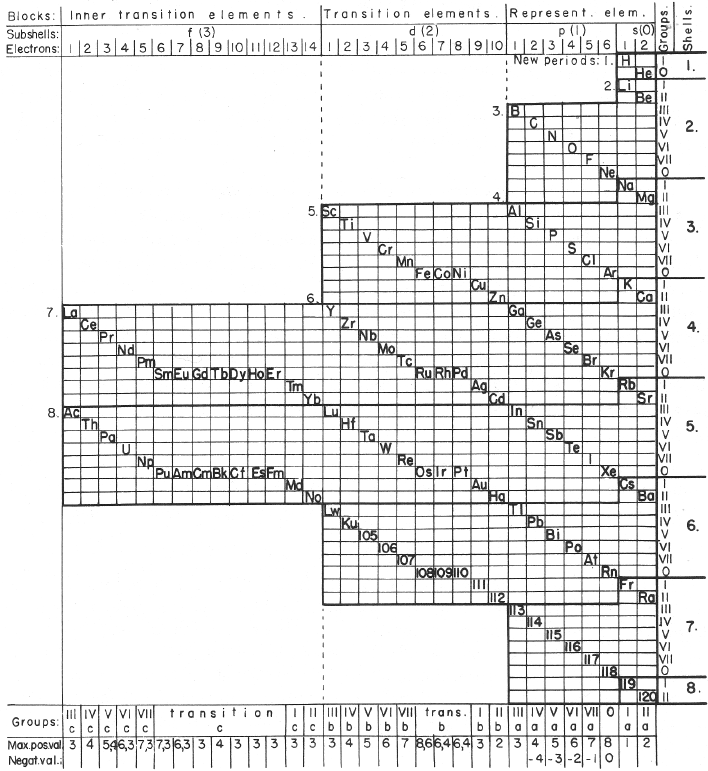
| Year: 1955 | PT id = 579 |
Krafft's Periodic Table (1955)
From The Ether and Its Vortices, p. 63, Carl Frederick Krafft
Thanks to Edmond Maurice Peyroux for the tip!
| Year: 1955 | PT id = 691 |
Mazurs' 1955 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
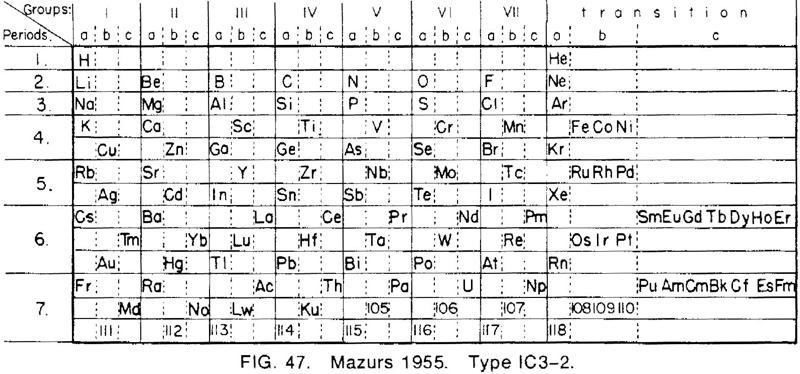
Thanks to Philip Stewart for the tip!
| Year: 1955 | PT id = 881 |
Discovery of Mendelevium
Md ![]()
Mendelevium, atomic number 101, has a mass of 258 au.
Synthetic radioactive element.
Mendelevium was first observed in 1955 by A. Ghiorso, G. Harvey, R. Choppin, S. G. Thompson and G. T. Seaborg.
| Year: 1955 | PT id = 1086 |
Element Hunters
A YouTube video, The Element Hunters.
The text accompanying the video says:
"Scientist in Berkeley discover new elements [Californium & Einsteinium] from hydrogen bomb debris in 1951 and then use the 60 inch Cyclotron to create Mendelevium, element 101. The team included Nobel Prize winner Glenn Seaborg and famed element hunter, Albert Ghiorso."
Thanks to Roy Alexander for the tip!
| Year: 1956 | PT id = 976 |
Remy's Long Period Form Periodic Table
From H. Remy's 1956, Treatise on Inorganic Chemistry, Vol. 1, (Introduction and main groups of the periodic table), Elsevier, Amsterdam, p. 4, is what Remy calls a "Long-Period Form of the Natural System of the Elements".
This is a semi-lanthanide/actinide formulation, with Th-Pa-U shown as 6d metals, and the remaining actinides (Np, etc.) shown as transuranic counterparts to Pm, etc. The layout of Remy's table was based on ideas by Haissinsky in competition with Seaborg's formulation of 1945.
In the appendix there is a second "Table II" version of this formulation with shorter periods.
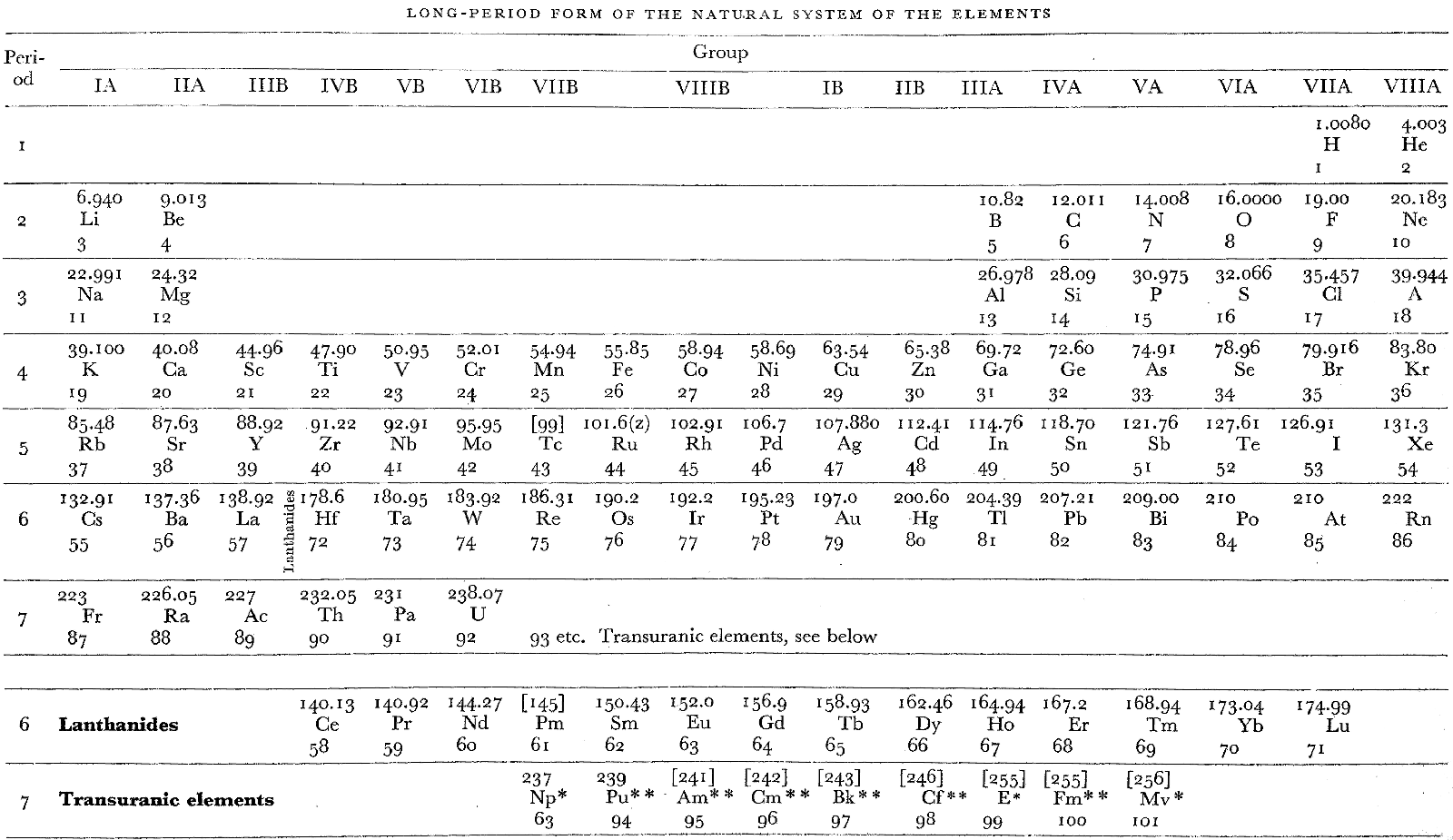
Thanks to René for the tip!
| Year: 1956 | PT id = 1009 |
Walker & Curthoys' New periodic Table Based of Stability of Atomic Orbitals
By W. R. Walker and G. C. Curthoys, A new periodic table based on the energy sequence of atomic orbitals, J. Chem. Educ., 1956, 33 (2), p 69.
The abstract states:
"Since the theory of atomic and molecular orbitals has proven to be of such value in interpreting the data of inorganic chemistry, it is hoped that a new periodic table based on the energy sequence of atomic orbitals will be an aid to the further systematizing of chemical knowledge."
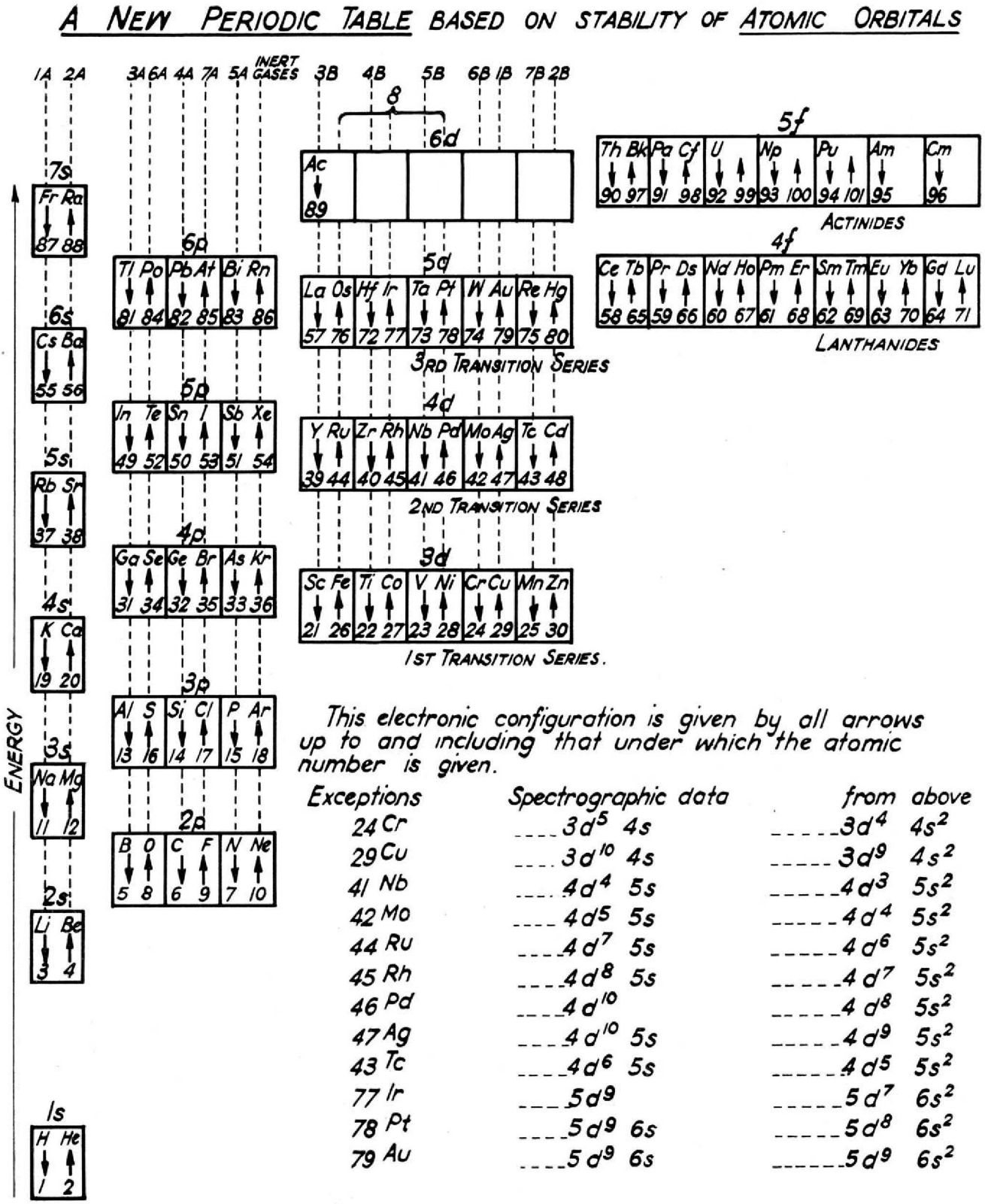
Thanks to René for the tip!
| Year: 1956 | PT id = 1216 |
Sistema Periodico de Los Elementos (after Antropoff)
Mario Rodríguez Peña, PhD translates the spanish text on the Archive.org website:
"Periodic System of Elements, type Antropoff., 1956 Antropoff's periodic table was designed in Bonn (Germany) in 1926: https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=26 It was disused after the WWII (1945) in most of the countries, except Spain. This was dated in 1956 because Mendelevium (101) was discovered and accepted by IUPAC in 1955 and in 1957, the element symbols of Argon (18), Xenon (54), Einstenium (99) and Mendelevium itself changed to the current Ar, Xe, Es and Md, respectively."
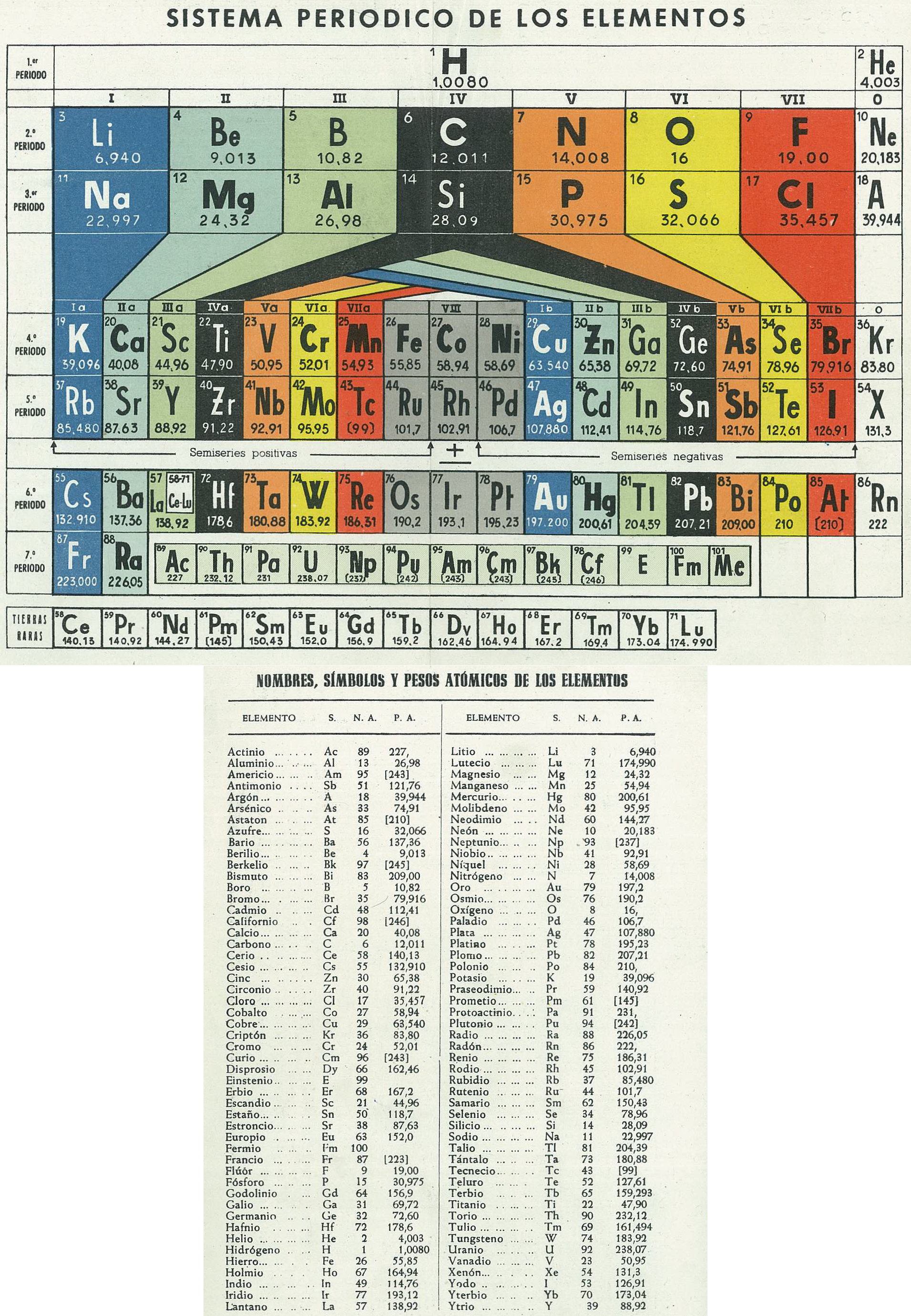
| Year: 1956 | PT id = 1227 |
Remy's Periodic Table II: The Short Period Presentation
Next to Remy's Long Form Periodic Table (H. Remy, Treatise on Inorganic Chemistry, Vol. 1, Introduction and main groups of the periodic table, Elsevier, Amsterdam, 1956, p. 4) is what Remy calls a "Short Period presentation" shown in the appendix, pages 838-939. The author comments:
"The form of presentation used in Table II in which the elements of the Long Periods are divided into two series, so that the short Periods determine the horizontal breadth of the system, is known as the Short Period presentation, as contrasted with the Long Period presentation in which the elements of the Long Periods are each time included in a single series.
"The Short Periods can then be broken up accordingly. Mendeléeff had already used the short-periodic and long-periodic mode of tabulation. The adjacent Table I sets it out as a form which is based directly on that already used by Mendeléeff, but completed by the insertion of the elements discovered subsequently."
Thanks to Mark Winter of WebElements for the tip!
| Year: 1957 | PT id = 1079 |
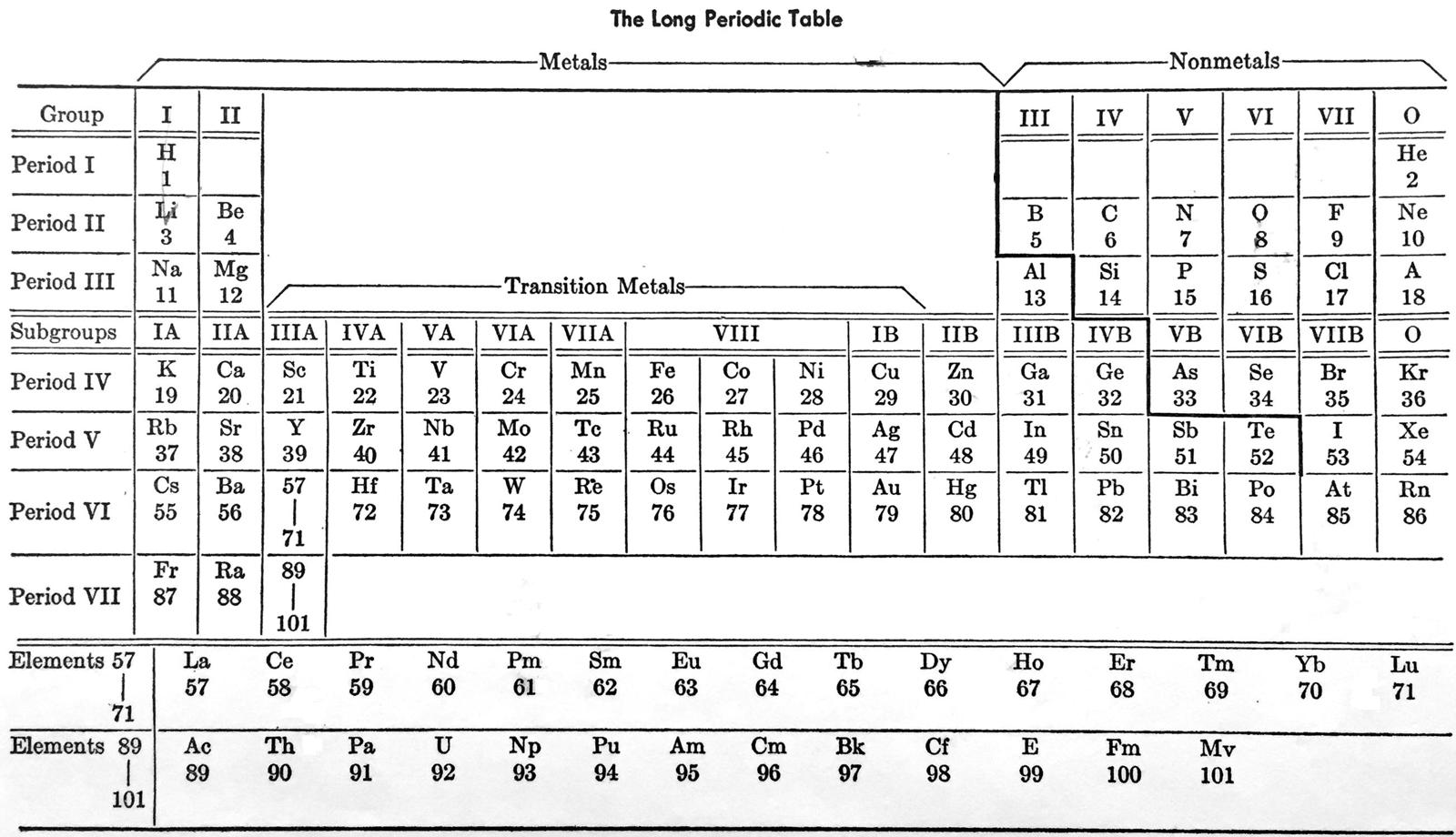
Laubengayer's Long Periodic Table
From A.W. Laubengayer, General Chemistry, revised ed., Holt, Reinhart and Winston, New York (1957).
René Vernon writes:
"In this busy table the author appears to show three of each of groups I to VII (e.g group I; group IA; group IB) and one group VIII, and one group 0, for a total of 23 groups and subgroups."
| Year: 1958 | PT id = 693 |
Mazurs' 1958-73 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
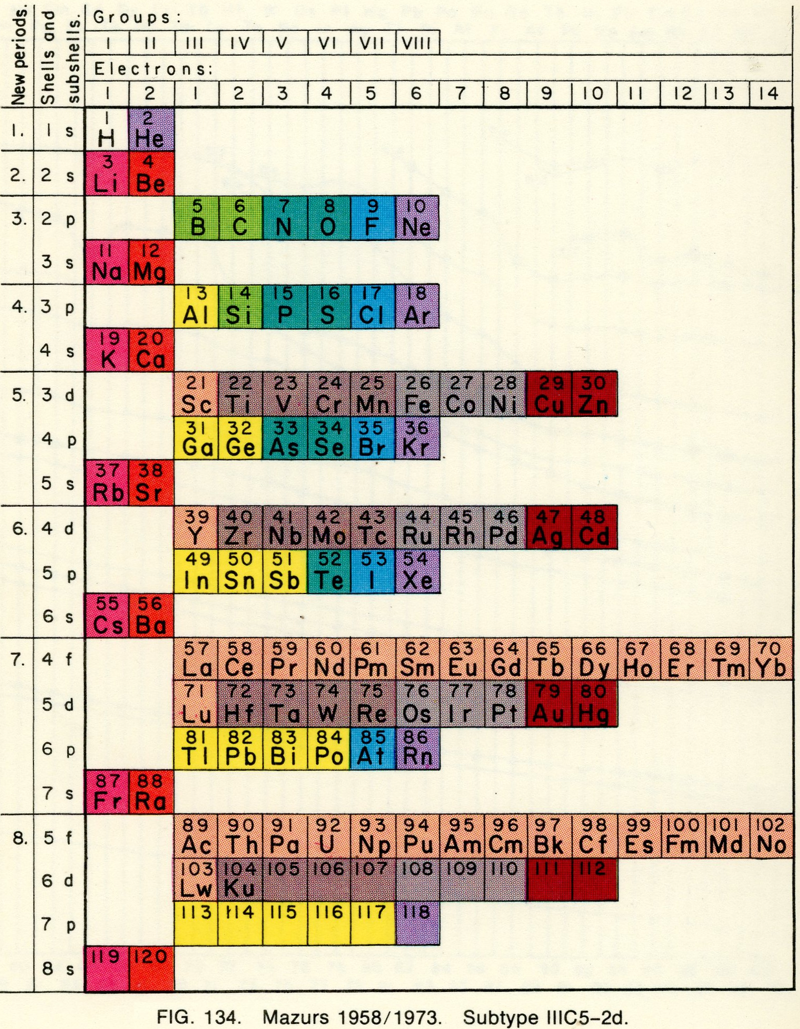
Thanks to Philip Stewart for the tip!
| Year: 1958 | PT id = 1148 |
Landau & Lifshitz's Periodic System of Mendeleev
L.D. Landau & E.M. Lifshitz, Quantum Mechanics (Volume 3 of A Course of Theoretical Physics), pages 255-258. (Note: First published in English in 1958, the link is to the 1963 3rd ed. of the English version translated from Russian.)
René Vernon writes:
The authors discuss aspects of the periodic system of D I Mendeleev. The electron configurations of hydrogen & helium are briefly noted. This is followed by three tables setting out the electron configurations of the s, p, d & f elements.
Some extracts from the text follow:
"The elucidation of the nature of the periodic variation of properties, observed in the series of elements when they are placed in order of increasing atomic number, requires an examination of the peculiarities in the successive completion of the electron shells of atoms." (p. 252)
"Many properties of atoms (including the chemical properties of elements...) depend principally on the outer regions of the electron envelopes." (p. 254)
"The elements containing complete d and f shells (or not containing these shells at all) are called elements of the principal groups; those in which the filling up of these states is actually in progress are called elements of the intermediate groups. These groups of elements are conveniently considered separately." (p. 254)
"We see that the occupation of different states occurs very regularly in the series of elements of the principal groups: first the s states and then the p states are occupied for each principal quantum number n. The electron configurations of the ions of these elements are also regular (until electrons from the d and f shells are removed in the ionisation): each ion has the configuration corresponding to the preceding atom. Thus, the Mg+ ion has the configuration of the sodium atom, and the Mg++ ion that of neon." (p. 255)
"Let us now turn to the elements of the intermediate groups. The filling up of the 3d, 4d, and 5d shells takes place in groups of elements called respectively the iron group, the palladium group and the platinum group. Table 4 gives those electron configurations and terms of the atoms in these groups that are known from experimental spectroscopic data. As is seen from this table, the d shells are filled up with considerably less regularity than the s and p shells in the atoms of elements of the principal groups. Here a characteristic feature is the 'competition' between the s and d states."
"This lack of regularity is observed in the terms of ions also: the electron configurations of the ions do not usually agree with those of the preceding atoms. For instance, the V+ ion has the configuration 3d4 (and not 3d24s2 like titanium) ; the Fe+ ion has 3d64s1 (instead of 3d54s2 as in manganese)."
"A similar situation occurs in the filling up of the 4f shell; this takes place in the series of elements known as the rare earths. † The filling up of the 4f shell also occurs in a slightly irregular manner characterised by the 'competition' between 4f, 5d and 6s states."
"† In books on chemistry, lutetium is also usually placed with the rare-earth elements. This, however, is incorrect, since the 4f shell is complete in lutetium; it must therefore be placed in the platinum group."
"The last group of intermediate elements begins with actinium. In this group the 6d and 5f shells are filled, similarly to what happens in the group of rare-earth elements." (p. 256–257)
The authors exclude lanthanum from the rare earths since the 4f shell has not started filling. Yet actinium and thorium are included by them with what we now call the actinoids even though these two metals have no f electrons. No explanation is provided for this puzzling lack of consistency with their categories.

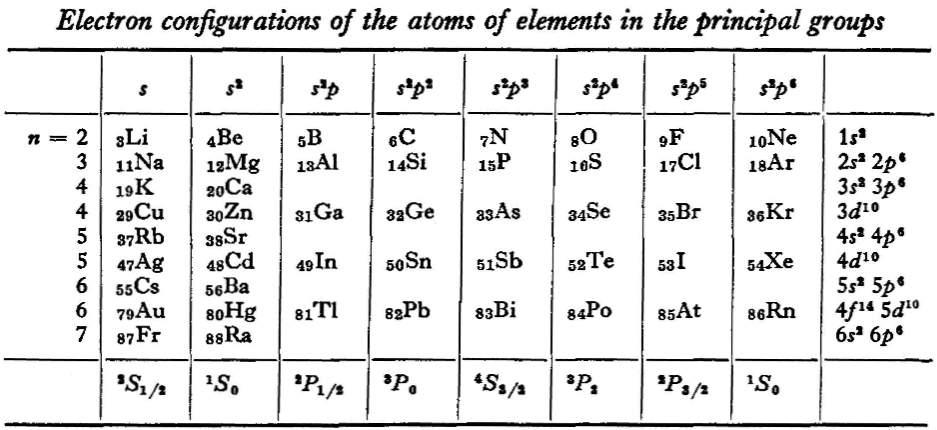
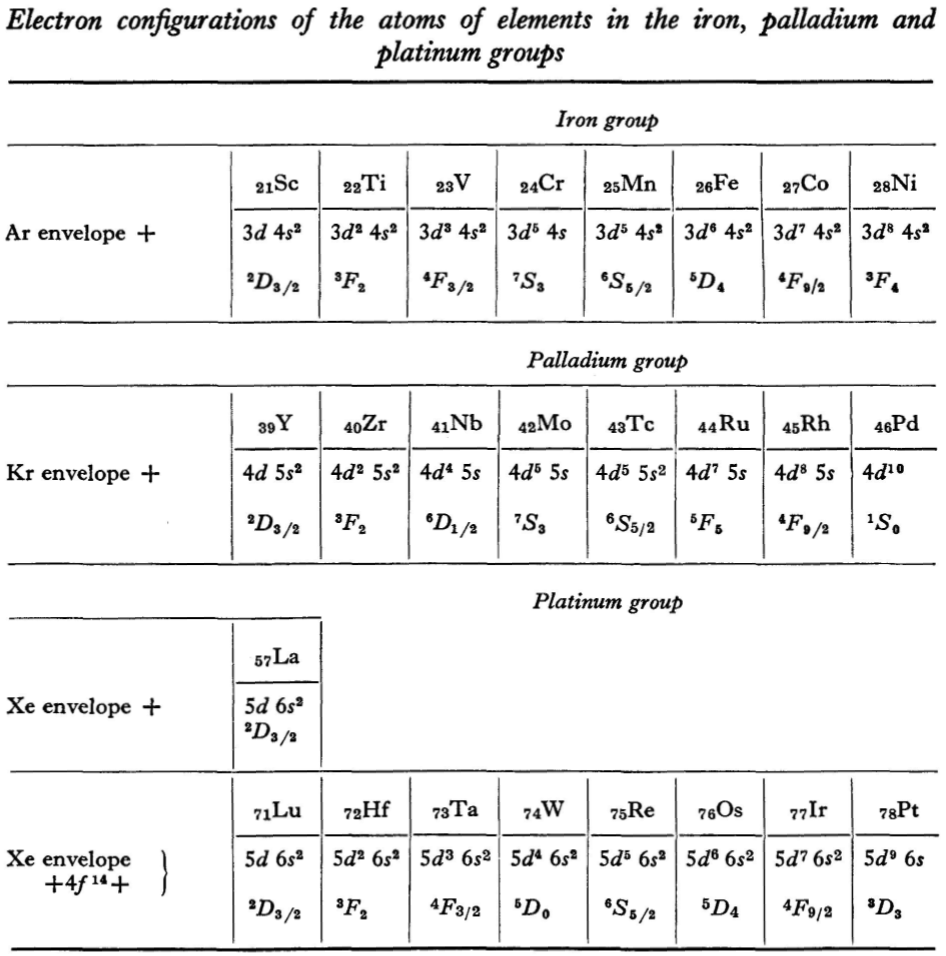
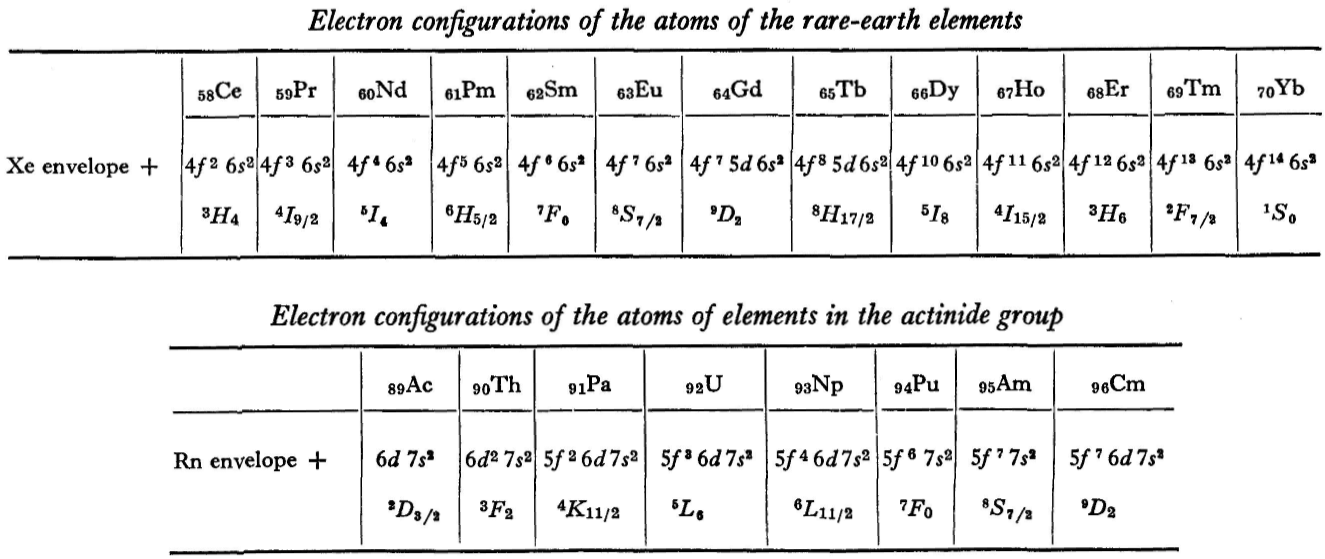
René Vernon writes: I have joined up their one note and three tables. (Curium was the last known element at their time of writing; transcurium elements are shown in parentheses.):
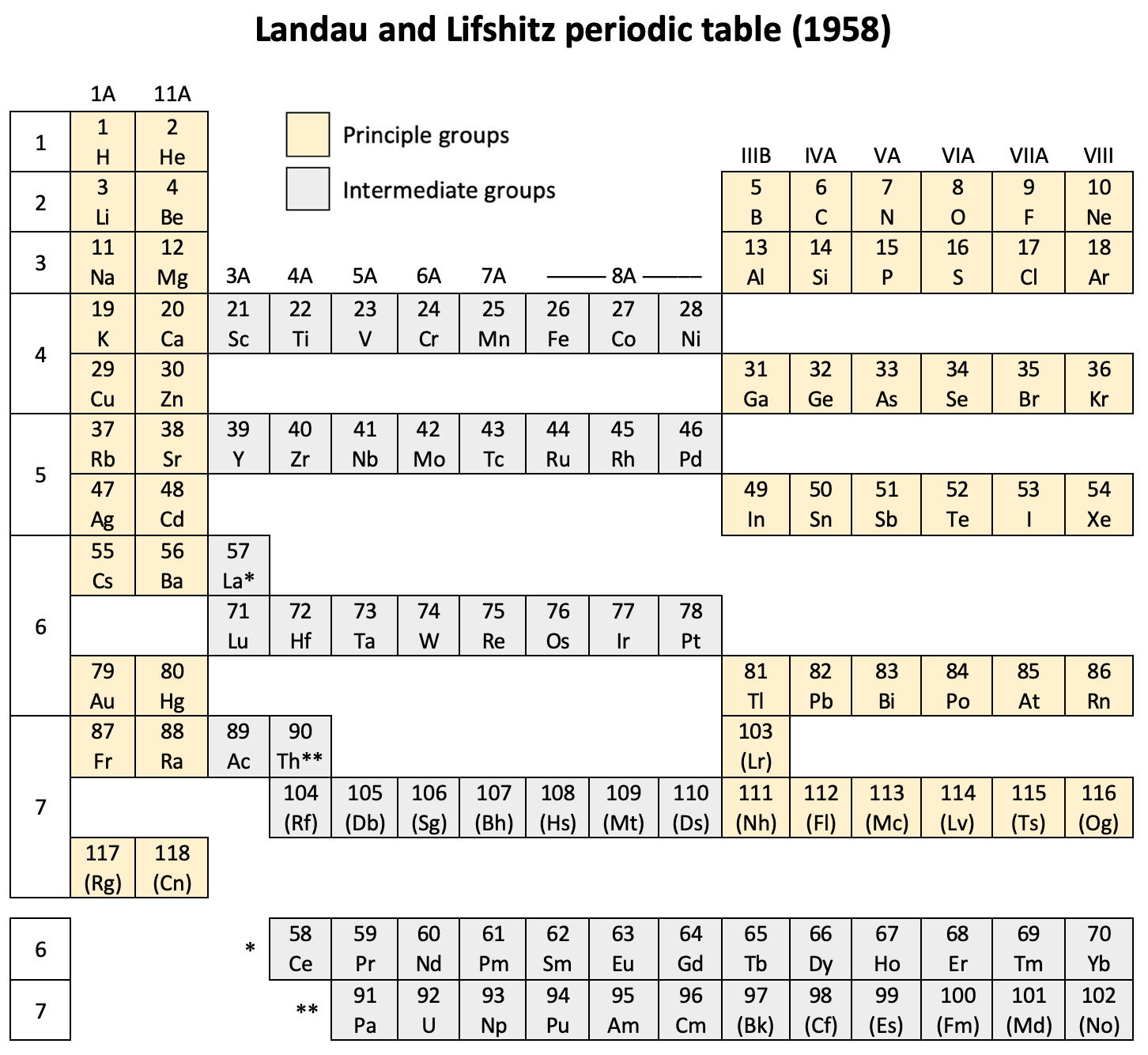
| Year: 1958 | PT id = 1263 |
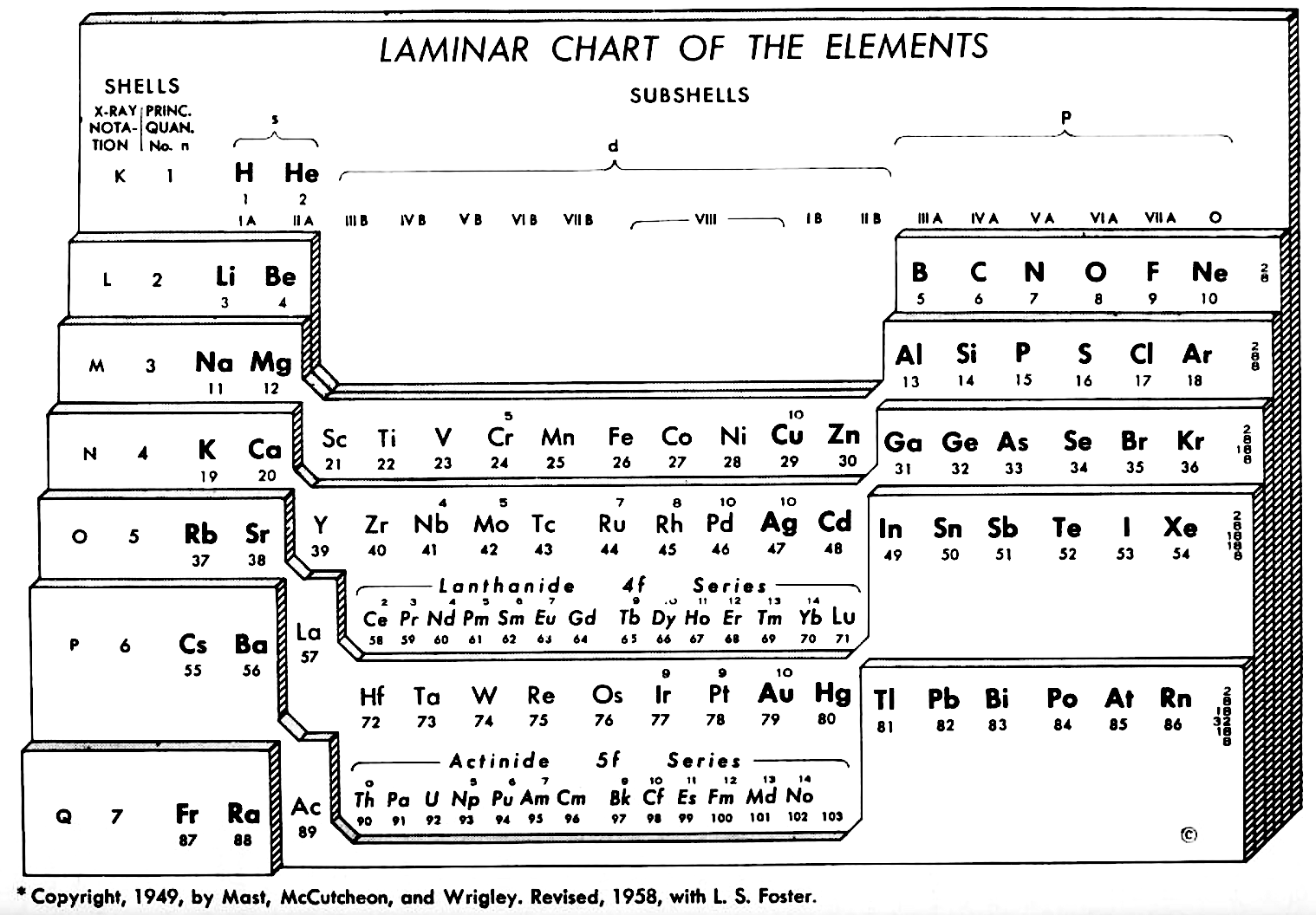
Weaver & Foster's Laminar Chart of the Elements
Weaver EC & Foster LS 1960, Chemistry For Our Times. 3rd ed., McGraw-Hill, New York, p. 382
René Vernon writes:
An earlier version of this table appeared in JChemEd in 1949. The authors then wrote:
"It is apparently difficult to give a proper idea of electronic configuration in two dimensions without spreading out vertically or horizontally, and thereby sacrificing the order of atomic number, or compactness, or both. In three dimensions it is entirely feasible, but the first reaction is to discard three dimensions as too awkward. The laminar chart here proposed seems to the authors to possess the advantages of both the two dimensional and three dimensional charts and to have none of their disadvantages.
"A minor feature of the table, introduced for reasons of expediency, is the artificial break between the first and the second main shell. Use is made of this space to print the traditional group headings, I A, III A, IVB, etc., which are firmly entrenched in the literature, and still find active use as classifying labels. Other objects in making the artificial break were to minimize the resemblance between hydrogen and the alkali metals and to emphasize helium's character as an inert gas (completed 1s subshell), rather than, as might otherwise be supposed, a member of the alkaline earth family.
"CONTOUR LAMINAR TABLE
"By another modification, constructing the Periodic Chart in the form of contour laminae, it is possible to represent actual energy levels without the necessity of referring to auxiliary tables. This is done by proportioning the rises between each subshell to correspond to the Pauling energy diagram. Thus, although the subshells having the same principal quantum number will be on the same contour lamina, they will not be on the same planar level. The recognition of these contour laminae is facilitated by the use of a different color for each one. A table of this type will then be more physically correct than the previous laminar models, and it is a question as to which form has the most practical utility.
"We believe that the laminar periodic tables, in either the original or a modified form, will greatly facilitate systematic teaching of the properties of the chemical elements. Students indoctrinated with the new system cannot fail to obtain a clearer and more lasting conception of the fundamental principles of inorganic chemistry."
Note the 4f and 5f series have been split into dyads of seven apiece. This is consistent with Shchukarev (1974, p. 118) who wrote that the filling sequence among the 4f metals is periodic, with two periods. Thus, after the occurrence of a half-full 4f subshell at europium and gadolinium, the filling sequence repeats with the occurrence of a full subshell at ytterbium and lutetium (Rokhlin 2003, pp. 4–5). A similar, but weaker, periodicity (Wiberg 2001, pp. 1643–1645) is seen in the actinoids, with a half-full 5f subshell at americium and curium, and a full subshell at nobelium and lawrencium.
Note that Zn, Cd, Lu and Hg have no electron numbers above them since the underlying shells were filled at Cu, Cd, Yb, and Au respectively.
- Rokhlin LL 2002, Magnesium Alloys Containing Rare Earth Metals: Structure and Properties, Taylor & Francis, London
- Shchukarev SA 1974, Neorganicheskaya khimiya, vol. 2. Vysshaya Shkola, Moscow (in Russian)
- Weaver EC & Foster LS 1960, Chemistry For Our Times. 3rd ed., McGraw-Hill, New York, p. 382
- Wiberg N 2001, Inorganic Chemistry, Academic Press, San Diego
- Wrigley AN, Mast WC & McCutcheon TP 1949, A laminar form of the periodic table, Part I, Journal of Chemical Education, 26(4), 216
- —— A laminar form of the periodic table, Part II, Journal of Chemical Education, 26(5), 248
| Year: 1959 | PT id = 931 |
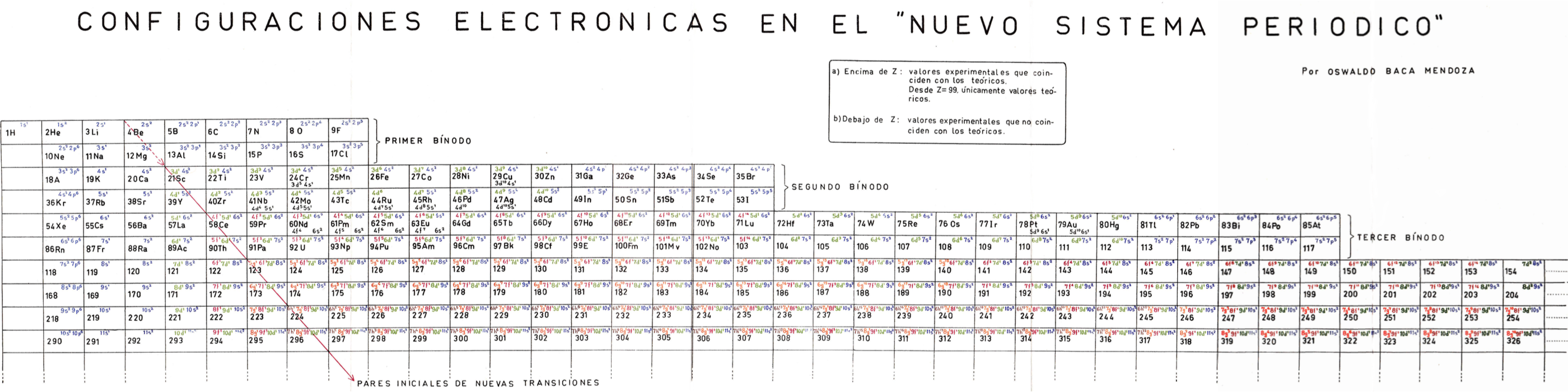
Mendoza's Nuevo Sistema Periodico
Dr. Oswaldo Baca Mendoza's Nuevo Sistema Periodico, presented to the VII Latin American Congress of Chemistry, held in Mexico from March 29 to April 3, 1959. Click image to enlarge:
| Year: 1959 | PT id = 1160 |
Mendoza's Neuvo Sistema Periodico
A memorial work, Ley De Configuraciones Electronicas, published posthumously in 1965 to honor Oswaldo Baca Mendoza (1908–1962 Cusco, Peru) and his 1959 Neuvo Sistema Periodico. Download the full PDF file (in Spanish).


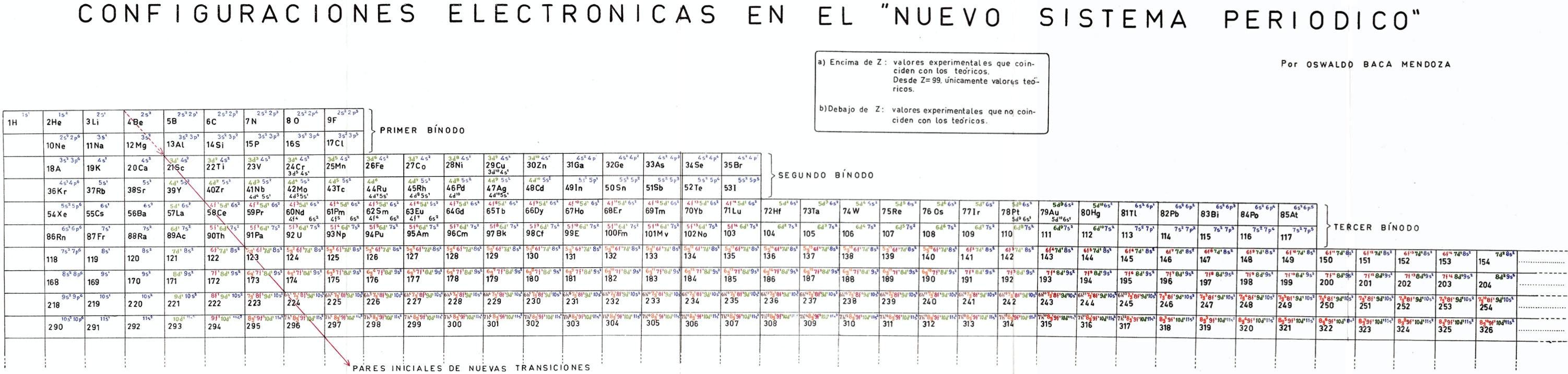
Thanks to Julio Gutierrez Samanez for the infomaton, etc.
| Year: 1960 | PT id = 53 |
Unfortunately, this wonderful formulation from a Union Carbide advertisement (1960) does not work; it is not (in this author's opinion) possible to wrap the PT onto a sphere:
| Year: 1960 | PT id = 444 |
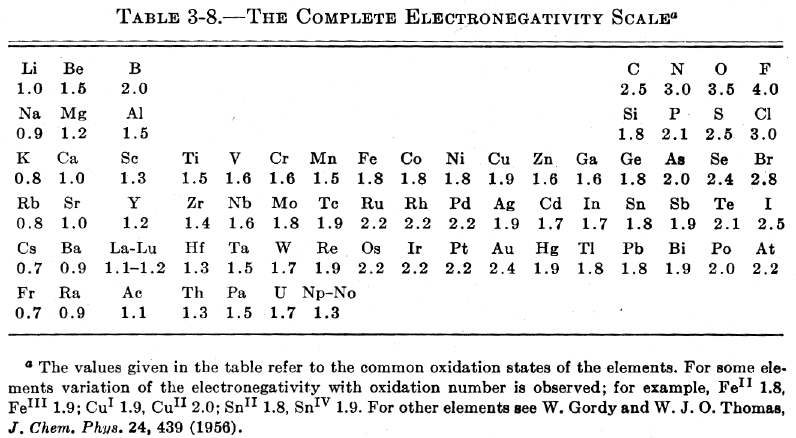
Pauling's Complete Electronegativity Scale
From The Nature of The Chemical Bond, 3rd Ed, pp 93, Pauling gives a periodic table showing the electronegativity of the elements.
Notice how the d block appears between groups 3 and 4 (13 & 14), rather than between groups 2 and 3 (2 & 13):
| Year: 1960 | PT id = 769 |
Sistema Periodico Degli Elementi
An Italian Periodic Table in Science Museum, Turin (Estimated date 1960).
Note how the noble gases (as Group 0) are shown down the left hand side of the table:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1960 | PT id = 1012 |
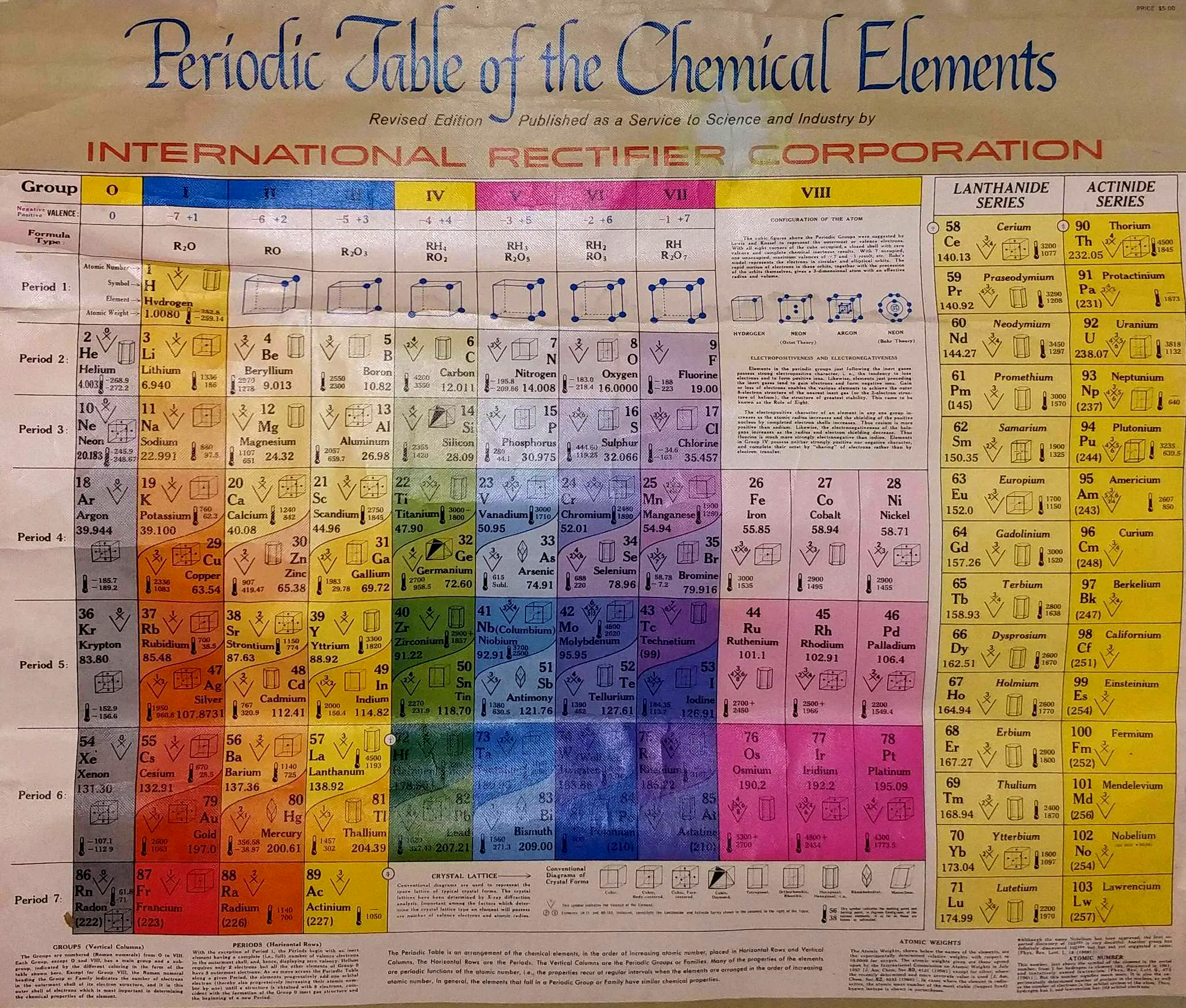
International Rectifier Corporation Periodic Table
International Rectifier Corporation was an American power management technology company manufacturing analog and mixed-signal ICs, advanced circuit devices, integrated power systems, and high-performance integrated components for computing. It is now part of Infineon Technologies.
The periodic table below was produced in the late 1950s to early 1960s. The earliest version we can find on the web dates from 1960.
Thanks to René for the tip!
| Year: 1960 | PT id = 1076 |
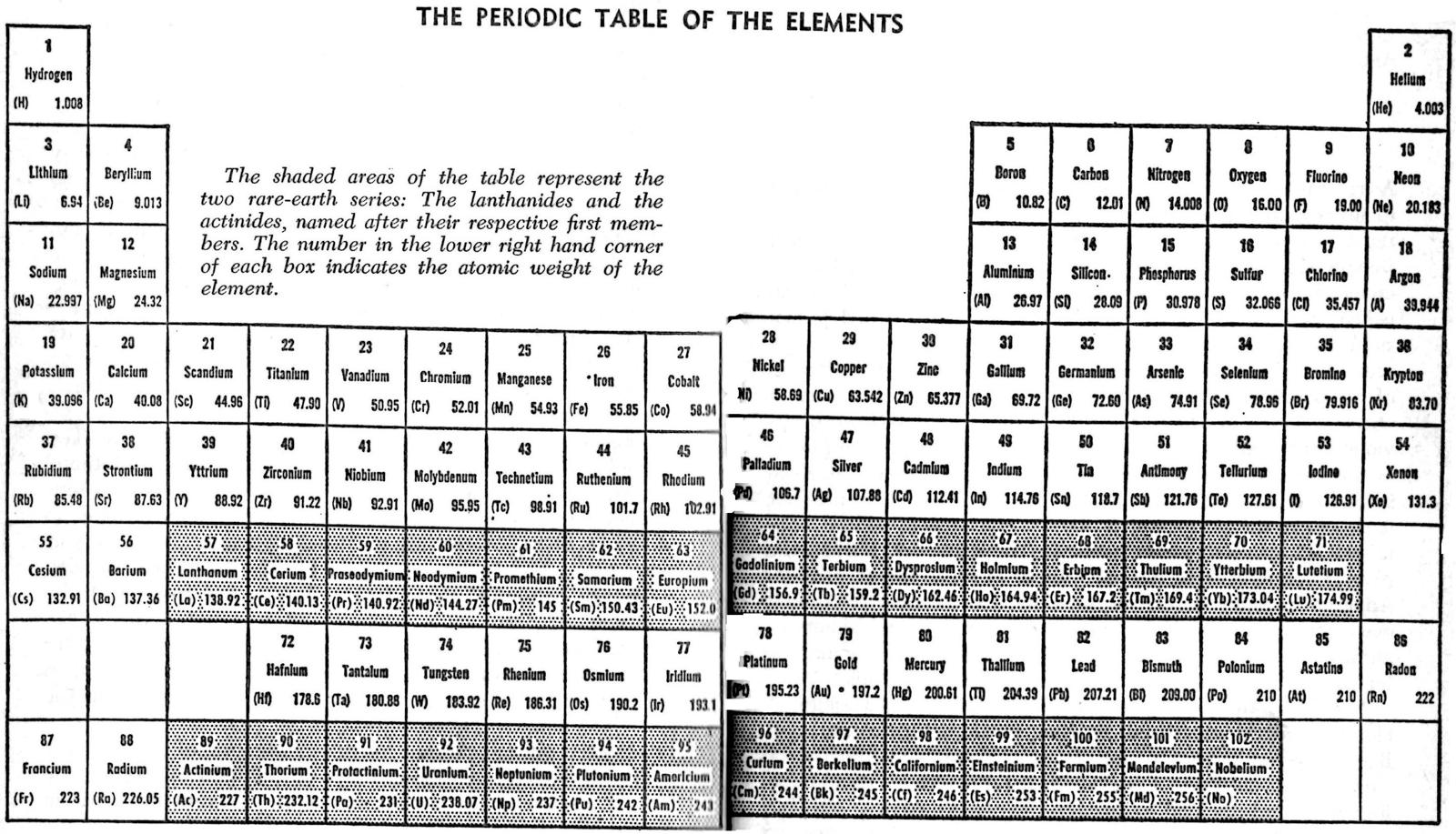
Asimov's Periodic Table of The Elements
Harry F. Tasset writes:
"As a Professor of Bio-Chemistry, Isaac Asimov wrote many volumes. One of the most interesting is The Intellegent Man's Guide to Science in which the attached periodic table appears: pages 154-155.
"The table suggests that there are still missing elements in the middle, not at the end.
"Published in 1960, this would have been a break with the thinking of most chemists and nuclear physicist of that time because there was no acceptable reason for their existence. My personal thoughts and research has lead me to the conclusion that Professor Asimov intended this to be a faithfull re-construction of the Mendeleev tables. When one takes the time to re-construct the Mendeleev table there are indeed empty spaces and more than just four."
| Year: 1961 | PT id = 508 |
Gamow's Wound Ribbon Periodic Table
From George Gamow's 1961 book, The Atom and Its Nucleus. There is an earlier 1948 version.
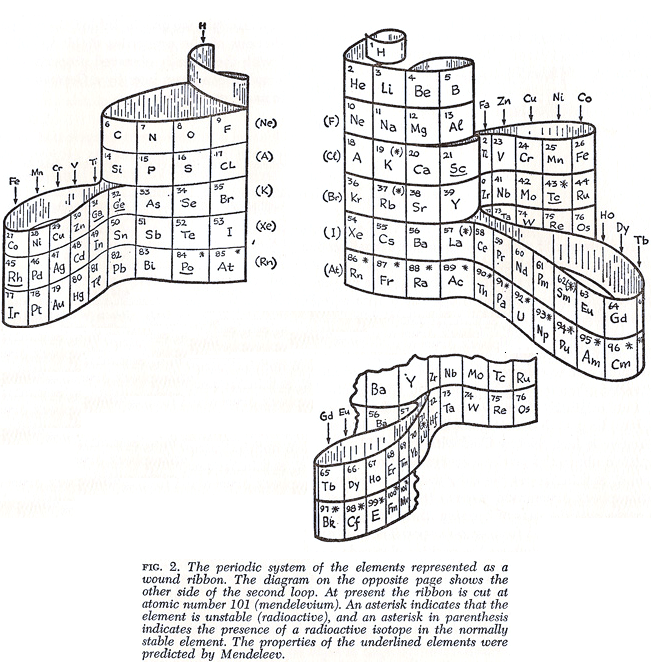
Thanks to Roy Alexander for the tip!
| Year: 1961 | PT id = 569 |
Chaverri's Tabla Periodica de Los Elementos
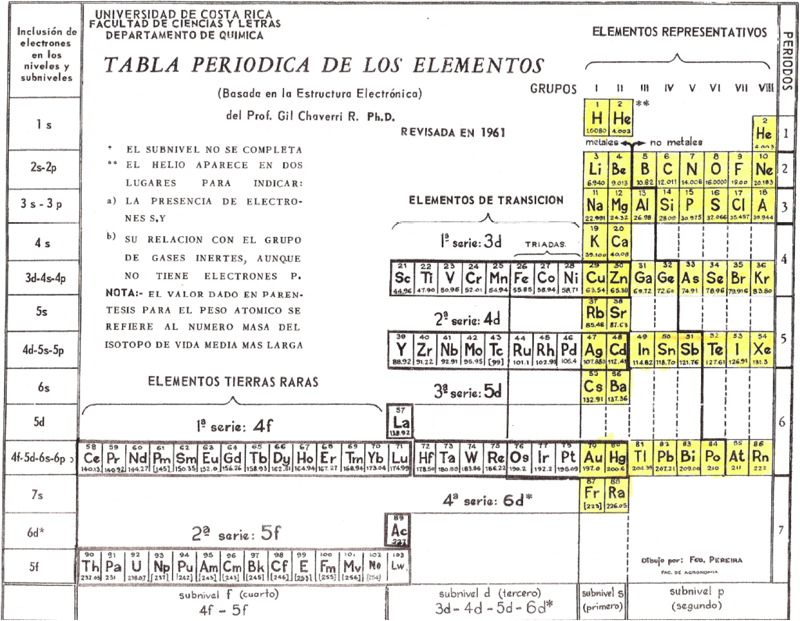
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1961 | PT id = 883 |
Discovery of Lawrencium
Lr ![]()
Lawrencium, atomic number 103, has a mass of 262 au.
Synthetic radioactive element.
Lawrencium was first observed in 1961 by A. Ghiorso, T. Sikkeland, E. Larsh and M. Latimer.
| Year: 1961 | PT id = 1251 |
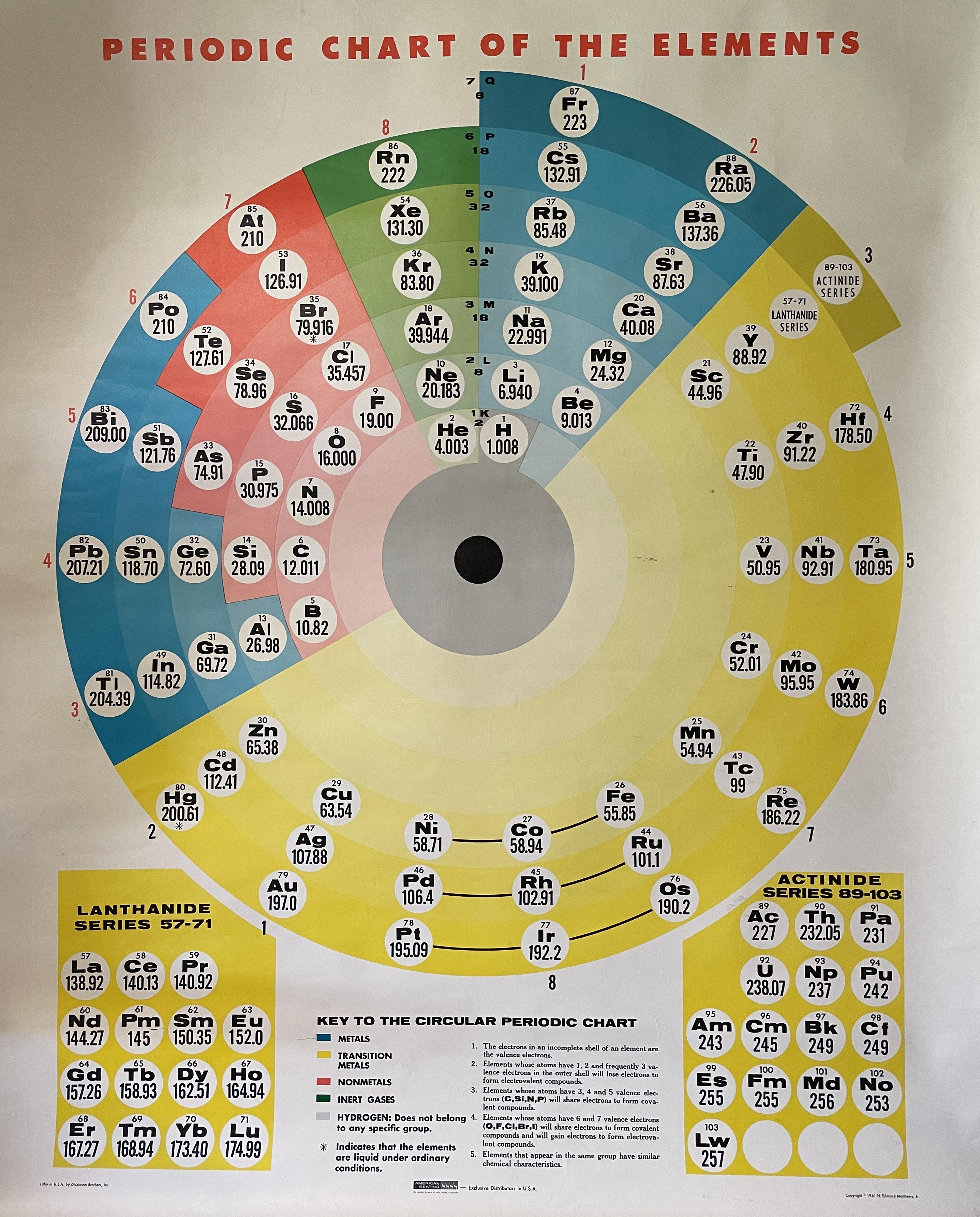
Circular Periodic Chart of The Elements
Chris R. Hagness writes:
"I own a wall chart that doesn't seem to be in your database of periodic tables. It's a circular 'periodic chart of the elements' published by Dickinson Brothers, Inc. in USA, distributed by American Seating, copyrighted 1961 by H. Edmund Matthews, Jr.
"I have never been able to find any information on this on the internet. Not on Edmund Matthews, no old catalogs from American Seating, no reference to this particular circular periodic table. I've never seen another of these anywhere, and I've been looking on and off for 10 years. I can't imagine this would have been a popular selling item. If you have any info, that would be appreciated!"
| Year: 1962 | PT id = 1177 |
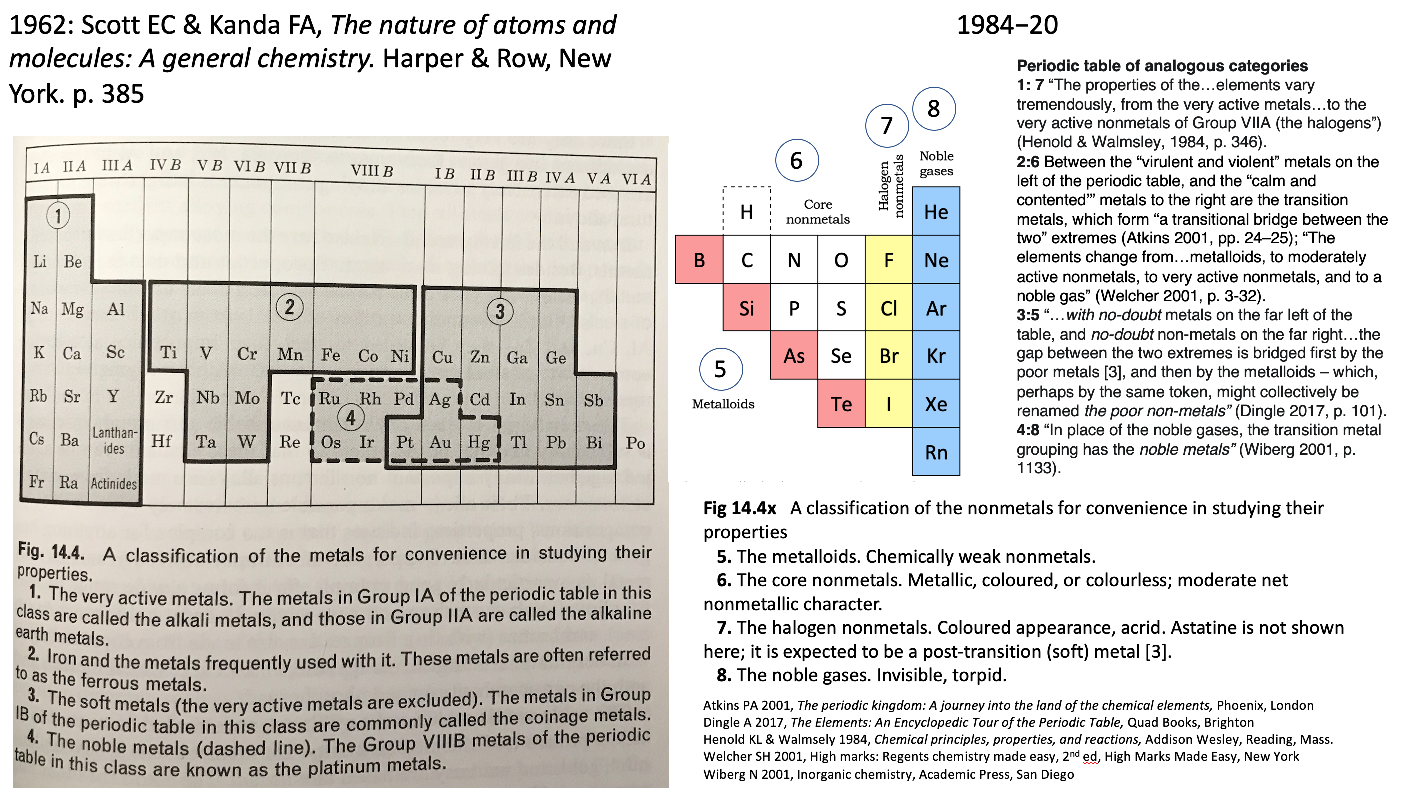
Scott & Kendal Periodic Table
René Vernon shows an extract from Scott E.C. & Kendal F.A., The Nature of Atoms & Molecules: A General Chemistry. Harper & Row, New York, 1962 pp 385, categorising the metals.
Rather than providing a holistic treatment of the nonmetals, the authors take a group-by-group approach.
Items of interest: Al over Sc; the split between groups 3 and 3; and the inclusion of Pt with the soft metals.
On the right is my add-on for the nonmetals, plus extracts from the literature speaking to the analogies between the four metal and four nonmetal categories.
| Year: 1963 | PT id = 412 |
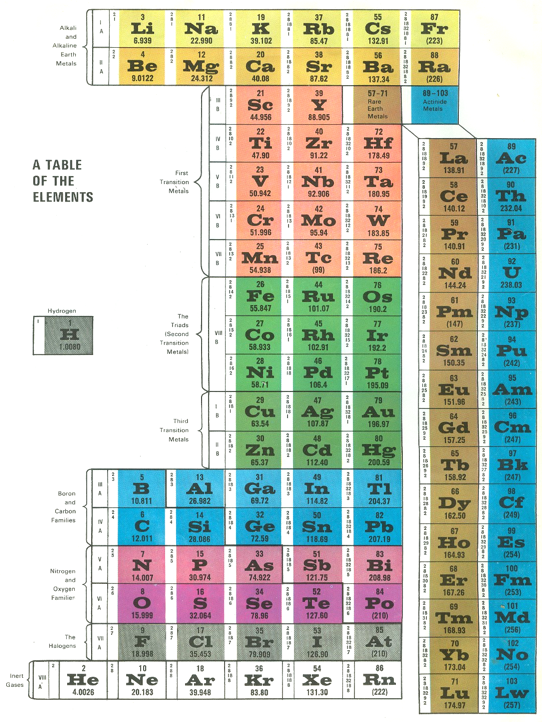
Life Science Library Periodic Table
An periodic table in the Life Science Library book, Matter, by Ralph E. Lapp (1963).
The PT is arranged vertically instead of having the usual horizontal format. It is also probably the first book to show pictures of nearly every element, arranged by family:
| Year: 1963 | PT id = 990 |
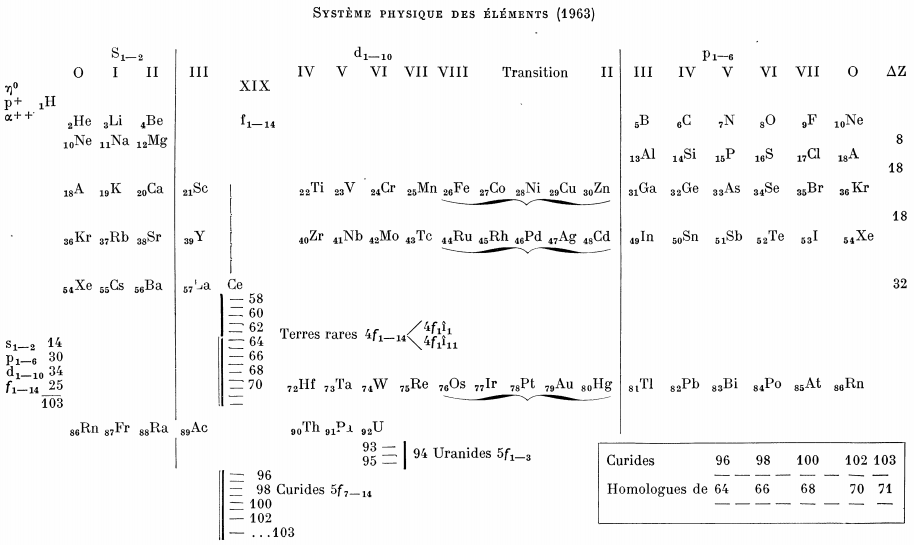
Bedreag's Système Physique Des Éléments
From Le Journal De Physique Et Le Radium, 24, pp27 (1963).
After a short historical account of the evolution of the periodic system Bedreag analyses some properties of various groups of elements: density, spectra, ionic radii, ionization potentials and so on, arguments are given in favour of the division of the transuranic elements into "uranides" and "curides".
Thanks to René for the tip!
| Year: 1963 | PT id = 1033 |
Royal Military College of Science Three-dimensional Spiral
From a Science Museum blog, Rajay Shah writes:
"Supported by poles and twisting around itself in a snake-like manner, this object is one of many weird and interesting forms of the periodic table. It was built at the Royal Military College of Science in 1963. The Science Museum asked for this model to be made for them to display in their new chemistry gallery after the original model was seen at an exhibition held by the Physical Society.":


| Year: 1963 | PT id = 1249 |
Hutton's Periodic Table of The Elements
Hutton, K 1963, Chemistry: The Conquest of Materials, Penguin Books. Harmondsworth, Middlesex, pp. 38–39
René Vernon writes:
"Hutton shows:
- H over F
- the lanthanides under Y
- fifteen uranides under W (Hutton says they have, "properties increasingly similar to one another".)
Hutton refers to group 6A (Cr, Mo, W) as the "steel hardening" elements".
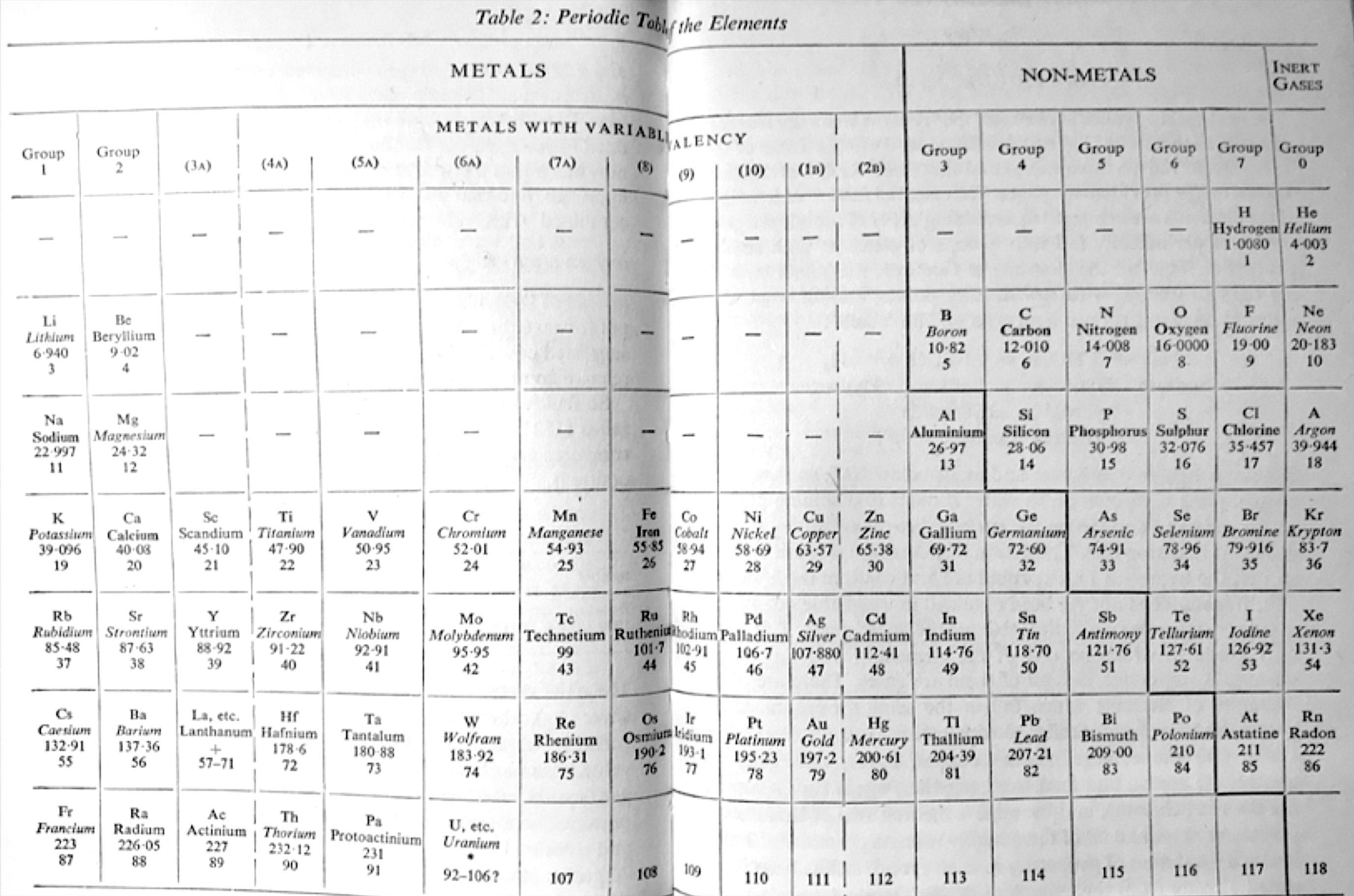
| Year: 1964 | PT id = 33 |
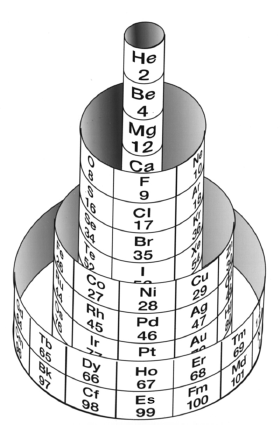
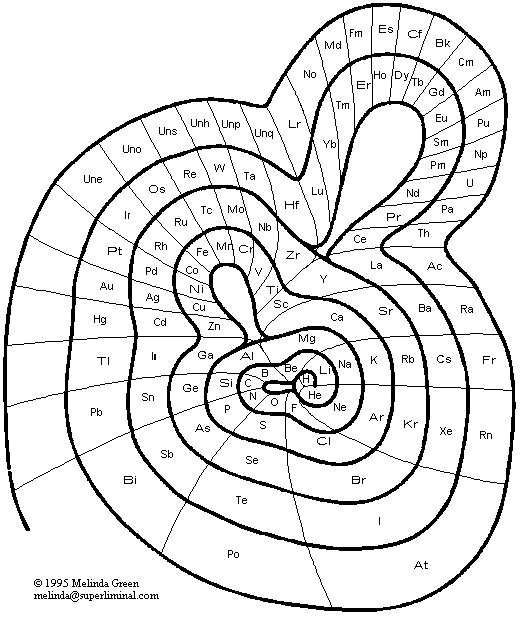
Benfey's Spiral Periodic Table or Periodic Snail
Spiral Periodic Table by Otto Theodor Benfey:
From Wikipedia:
"One of the Benfey's publications in Chemistry was a model of an extended periodic table, sometimes referred to as the periodic snail. First published in 1964, it explicitly showed the location of lanthanides and actinides. The elements form a two-dimensional spiral, starting from hydrogen, and folding their way around two peninsulars, the transition metals, and lanthanides and actinides. A superactinide island is already slotted in."
Read more here in an article by OTB: Bull. Hist. Chem., VOLUME 34, Number 2 (2009)
| Year: 1964 | PT id = 514 |
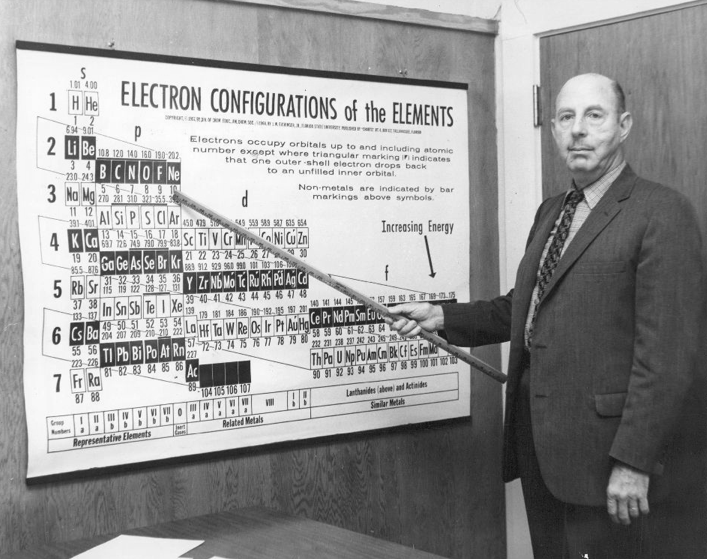
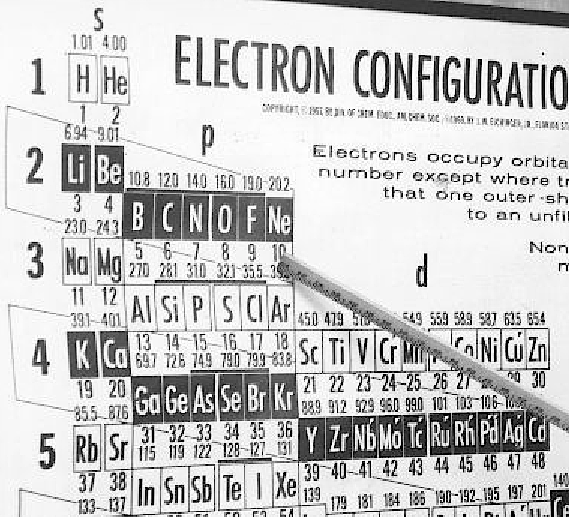
Eichinger Periodic Table
A 1964 photograph of Dr Jack Eichinger of Florida State Univ with his Periodic Table:

| Year: 1964 | PT id = 732 |
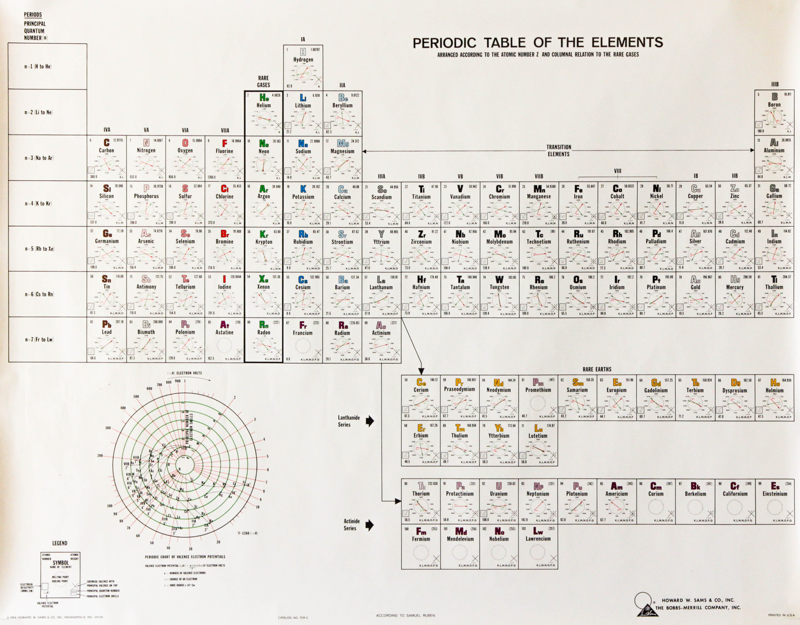
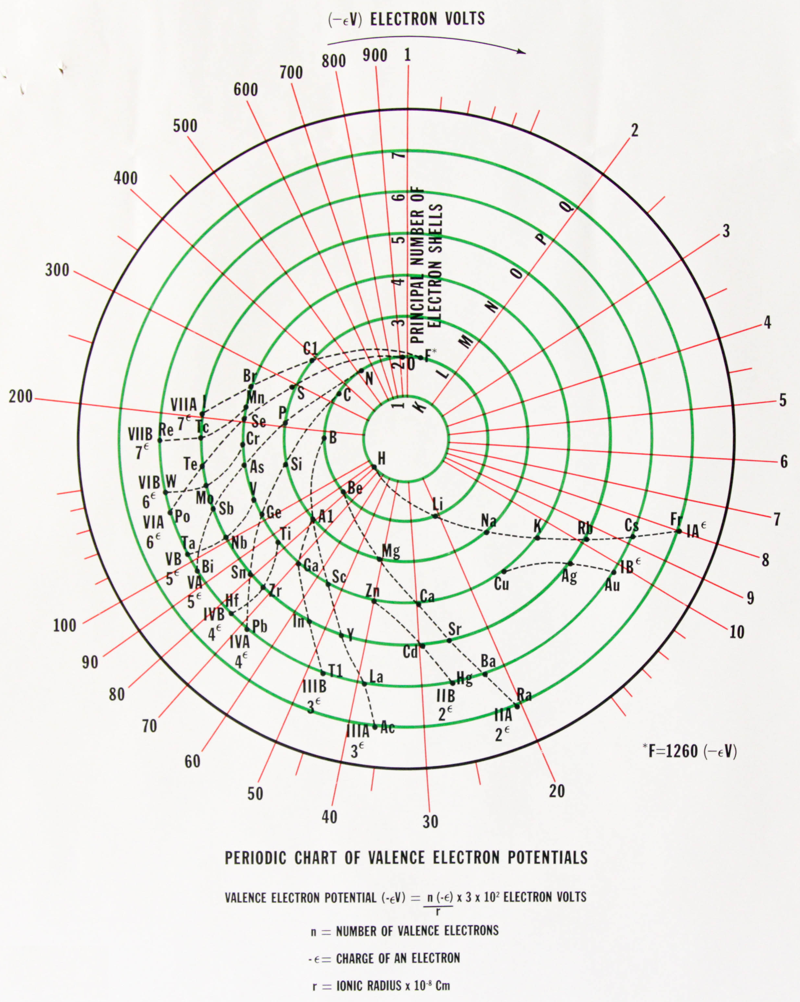
Samuel Ruben Periodic Table
An interesting periodic table from 1964, found at an estate sale. The text says that the elements are: "arranged according to the atomic number Z and column relation to the rare gases", and is by Samuel Ruben (wikipedia).
Click here to see the full size version.
Thanks to Rachel Helling for the tip!
| Year: 1964 | PT id = 960 |
Lee's Quantum Number Periodic Table
In his book Concise Inorganic Chemistry (pp. 22, 5th Ed, Blackwell Science, 1996), J.D. Lee gives a representation of "Quantum numbers, the permissible number of electrons & the shape of the periodic table".
Note: JD Lee taught Inorganic Chemistry to the curator of this database of periodic tables while at university:
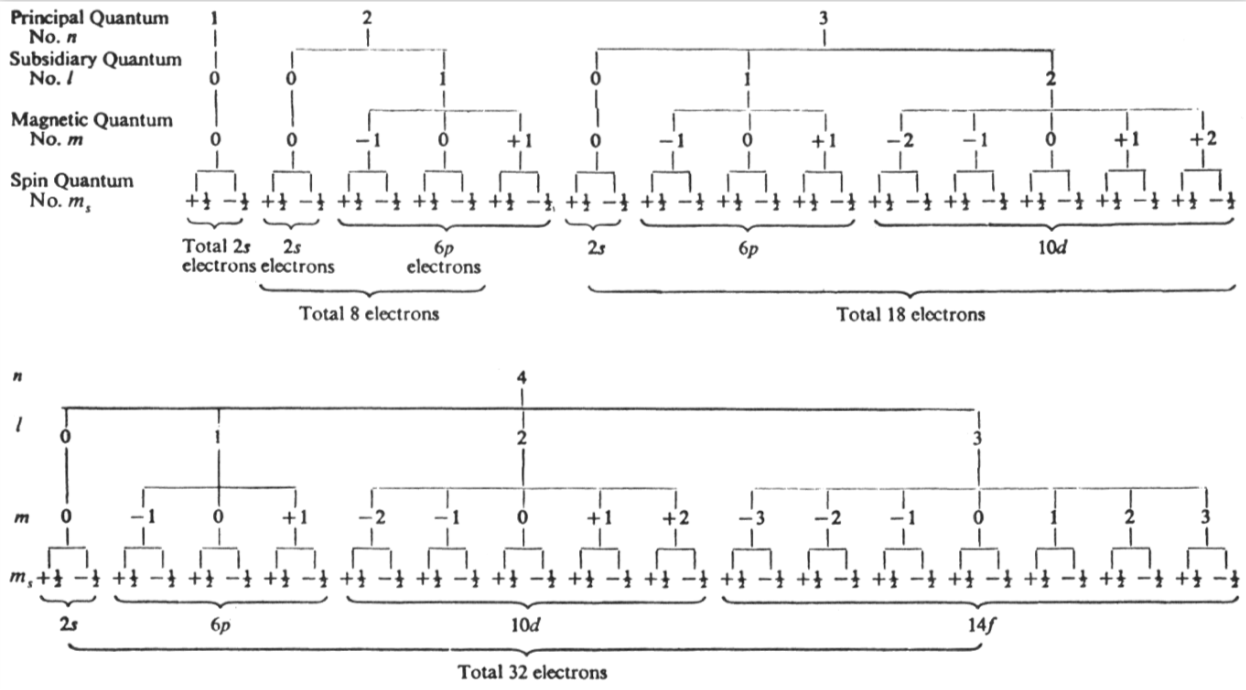
| Year: 1964 | PT id = 1006 |
Haward's Periodic Table
Roger Hayward created this periodic table for the book: Pauling & Hayward, p4, The Architecture of Molecules, W H Freeman and Company, San Francisco (1964).
From The Pauling Blog:
"By the end of the 1950s, Roger Hayward had retired from his professional work as an architect at the same time that his career as an illustrator was reaching its peak. Hayward signed a contract in the early 1960s that helped to solidify his position as a technical artist. The contract that Hayward signed was with W.H. Freeman & Company, a San Francisco-based publishing house that rose out of relative obscurity primarily by publishing Linus Pauling's hugely popular textbook, General Chemistry."
Thanks to René for the tip!
| Year: 1964 | PT id = 1271 |
Ternström's Periodic Table
Ref: A Periodic Table, Torolf Ternström, J. Chem. Educ. 1964, 41, 4, 190
René Vernon writes:
"Ternström gives us a triple-combo table drawing on the advantages of:
- the complete block system according to Werner (1905)
- a horizontal Bohr line-system according to Spedding (1951)
The outcome resembles the left step form of Janet (1928).
Some interesting features of Ternström's formulation are:
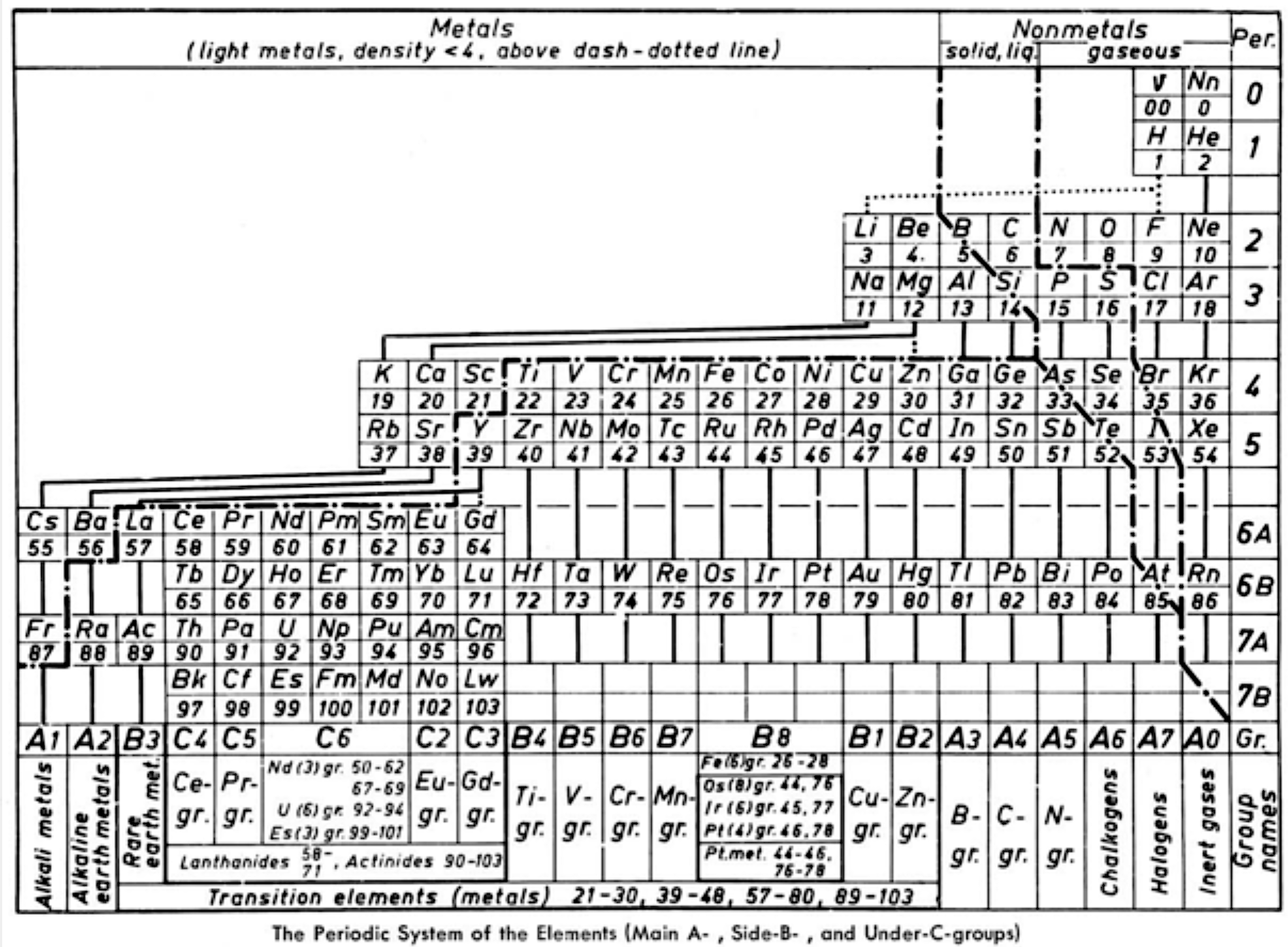
- a period 0 containing the neutrino and neutron
- element number "00" for "v" suggests the neutrino has neither nuclear charge nor mass while "0" for Nn implies no nuclear charge
- regular period lengths of 2-2-8-8-18-18-32-32
- hydrogen has no direct relationship with a group, only secondary relationships with groups A1 and A7
- germanium, a semiconductor, is counted as a metal
- all groups numbered, where A = representative; B = transition
- C = Ln/An; analogous "transition" groups in the d-block (B8) and the f-block (C6)
- double periodicity among the Ln and An; and
- 25 columns wide i.e 18 + 32 = 50/2 = 25"
| Year: 1965 | PT id = 21 |
Alexander Arrangement of Elements
The Alexander Arrangement of Elements is a 3D periodic table concept based on strict adherence to the Periodic Law, and, like the first representation of elements in periods by de Chancourtois, connects every element data box in unbroken order.

Roy Alexander, a Brooklyn born science museum exhibit and teaching aid designer, has told me in a personal communication: "I came up with the idea (being ignorant of anything but the flat Sargent Welch charts) in 1965. I wasn't able to patent [the downslant in the p-block] until 1971." (U.S.Patent #3,581,409)
At the time Roy had no idea that others had employed a similar technique to build a 3D table - including the very first periodic table developer, de Chancourtois, who is often credited with being the original discoverer of the periodicity of elements and the originator of the three-dimensional method of element arrangement and representation.
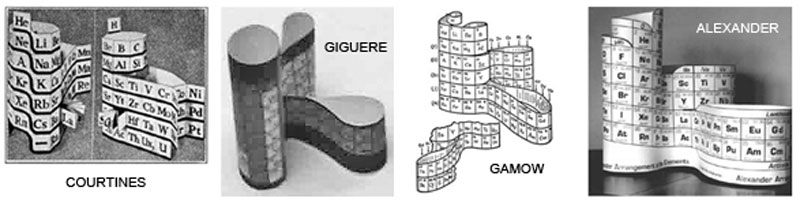
These 3D forms attempt to return the Seaborg separated f-block to its proper position in the table rather than remaining exiled. This, and contemporary attitudes about Hydrogen as being in more families than one - is uniquely addressed in Roy's 3D models.
Subsequent study of the Periodic Law and the periodic table's value in education convinced Roy that the basic rationale for developing the Alexander Arrangement of Elements was only one of the many good reasons for producing it for the public to share, so he sought and was granted a U.S. patent on the p-block downslant in order to manufacture and market the AAEs as teaching/learning aids.
Roy Alexander's goal of introducing the AAE into classrooms, laboratories, chemistry textbooks, and reference material remains the same today, but rather than replacing the conventional charts, its niche in education is at the very point that a lesson on arrangement of atoms into a chart begins. Element sequencing (vs. 24 breaks/gaps) credits the chart as well as the Periodic Law, which establishes subsequent confidence in the common flat charts, much as the world globe establishes the reality, and flat printed projections - maps - are vital (and relished) for convenience.
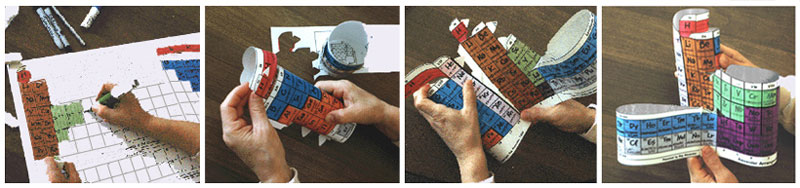
The first commercial production of Alexander Arrangements was in 1995, when Roy pioneered by constructing a website - periodictable.com - for marketing. Three versions were printed: two versions for student entry of element symbols, the larger die-cut for easier assembly.
An even larger model was produced with basic element data printed in the boxes, also die cut. These were printed on white card stock, with black ink.
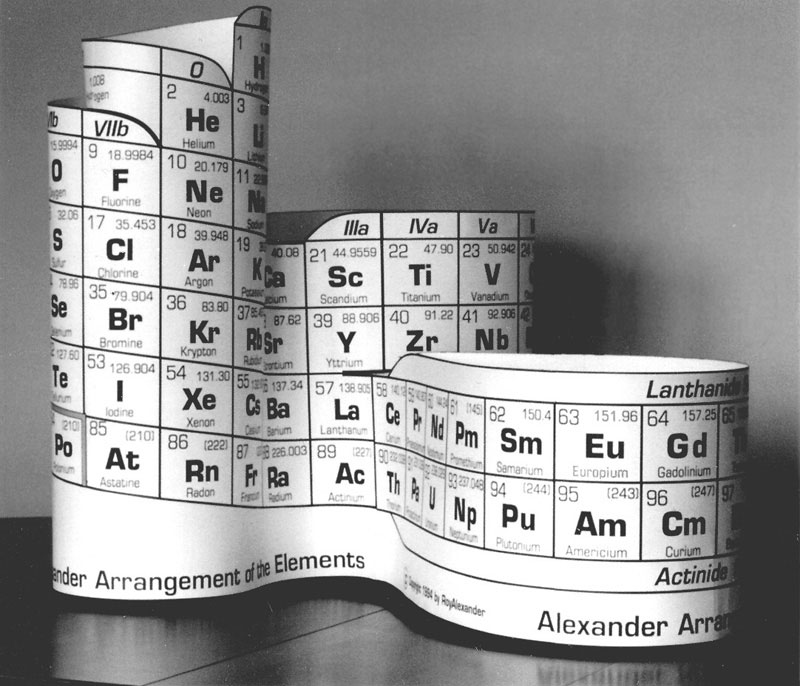
Another version (below) was produced in conjunction with ATMI's annual report in 2000. This was added to Roy's product offerings, called the DeskTopper, and is still available. They are die cut to form a 7.25" high model with the f-block position attached after La, but can be altered to put La on the f-block. (See AAE Features at the top of this page.)

Besides the hands-on educational application, the DeskTopper can be used as a pen & pencil caddy, and flattened without losing the continuity of the element data. This flattened form has suggested design of a Braille periodic table of the same format, and this is also being pursued.
Marketing the Alexander Arrangements was moved to AllPeriodicTables.com in cooperation with Theodore Gray in 2006, who purchased the PeriodicTable.com domain name and funded the production of Roy's newest model, illustrated with Theo's amazing element photos.

For the first time, the elements beyond those naturally occurring have been omitted from a modern periodic table, simplifying initiation to chemistry. This factor denies the concept of obsolescence, and this version has been called the Forever Periodic Table. Details of this new 3D periodic table model kit have been placed at 3DPeriodicTable.com.

Further AAE information and images may be found at the Alexander Arrangement website.
| Year: 1965 | PT id = 525 |
Giguère's Periodic Table
Paul Giguère's Periodic Table formulation, "The 'new look' for the periodic system". Chemistry in Canada vol. 18 (12): 36–39 (see p. 37):
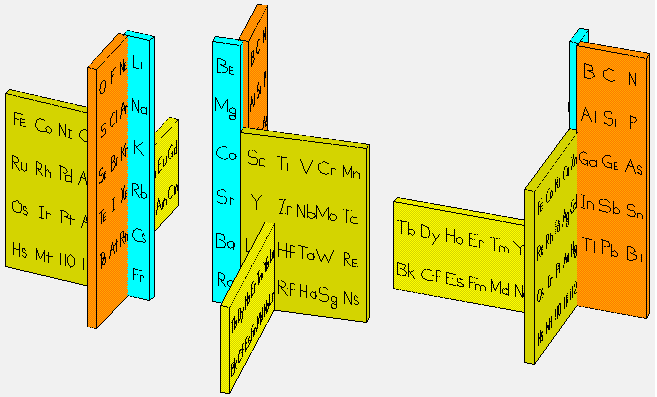
| Year: 1965 | PT id = 692 |
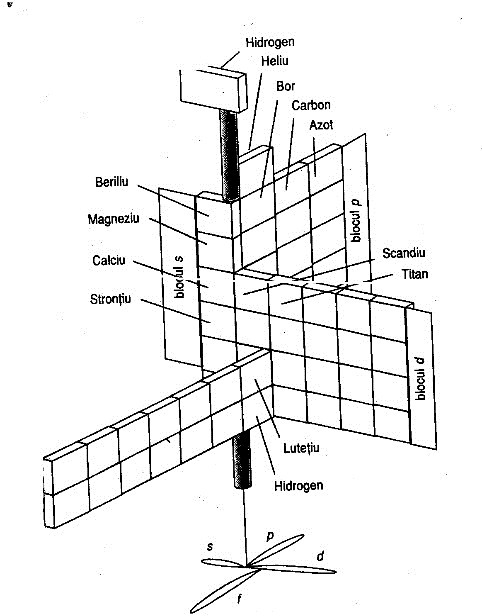
Mazurs' 1965 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
Thanks to Philip Stewart for the tip!
| Year: 1965 | PT id = 713 |
Dutch Periodic Table
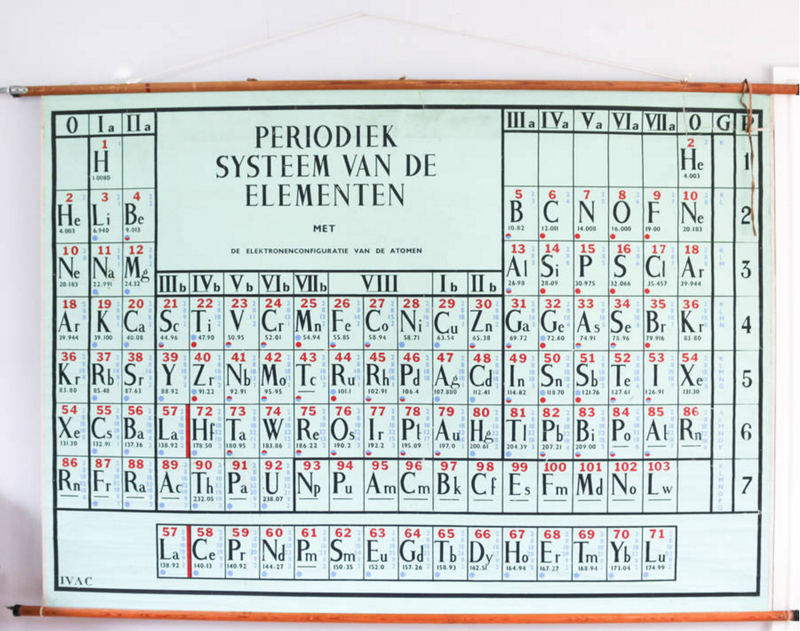
A Dutch periodic table formulation, Periodiek Systeem van de Elementen, probably from the mid-nineteen sixties: Element 103 Lr (shown as Lw), discovered 1961, is listed but Rf 104, discovered in 1964 is not shown.
Note how this formulation shows the noble gases, He-Rn, both on the left-side and the right-side.
This historic and original periodic table is listed for sale (Nov. 2015) on the Not On The Hight Street website.
| Year: 1966 | PT id = 248 |
Periodic Table of Ions
From Concept of Chemical Periodicity: from Mendeleev Table to Molecular Hyper-Periodicity Patterns E. V. Babaev and Ray Hefferlin, here.
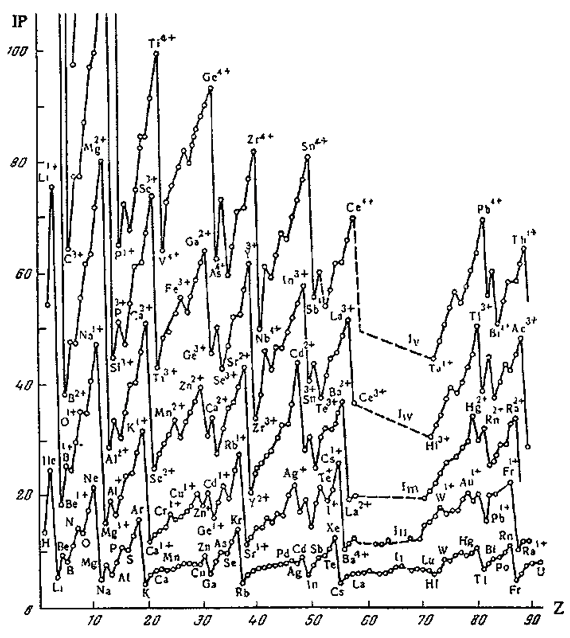
"One intriguing problem that arises from with the periodic table of atoms is the possibility of constructing periodic systems of ions, V. K. Grigorovich, Periodic Law of Mendeleev and Electronic Structure of Metals, Nauka Publ.: Moscow, 1966 (in Russian). An atom can be completely or partially ionized to a cation by removing electrons or transformed into an anion by the addition of new electrons. The energy required for a few consecutive ionisations of atoms is plotted against the atomic number. One can see that the curves are periodic, and hence it is possible to construct periodic tables for mono-, di-, and multi- charged cations. If we look at the dispositions of the maxima and minima of the curves and compare them with those for atoms, it becomes evident that the magic numbers of electrons for ions are the same as for neutral atoms. Therefore, the number of electrons (but not the charge of the nucleus) is responsible for the periodicity of ions."
| Year: 1966 | PT id = 443 |
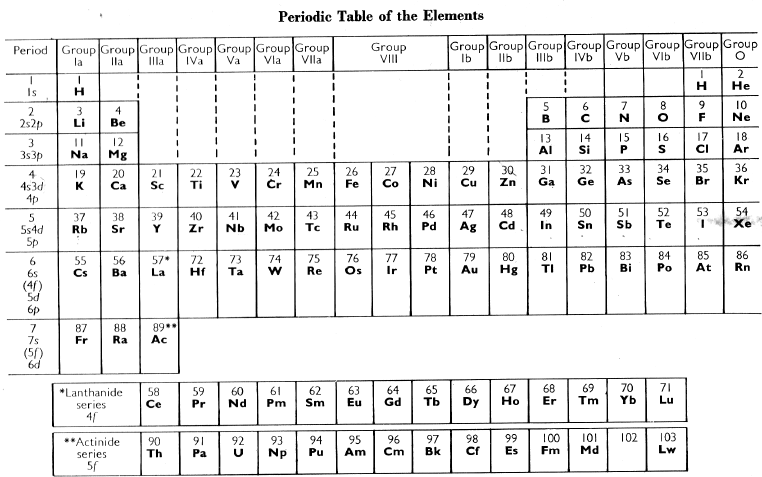
Cotton and Wilkinson Periodic Table of The Elements
From the Advanced Inorganic Chemistry 2nd Ed. textbook by Cotton and Wilkinson:
| Year: 1966 | PT id = 882 |
Discovery of Nobelium
No ![]()
Nobelium, atomic number 102, has a mass of 259 au.
Synthetic radioactive element.
Nobelium was first observed in 1966 by E. D. Donets, V. A. Shchegolev and V. A. Ermakov.
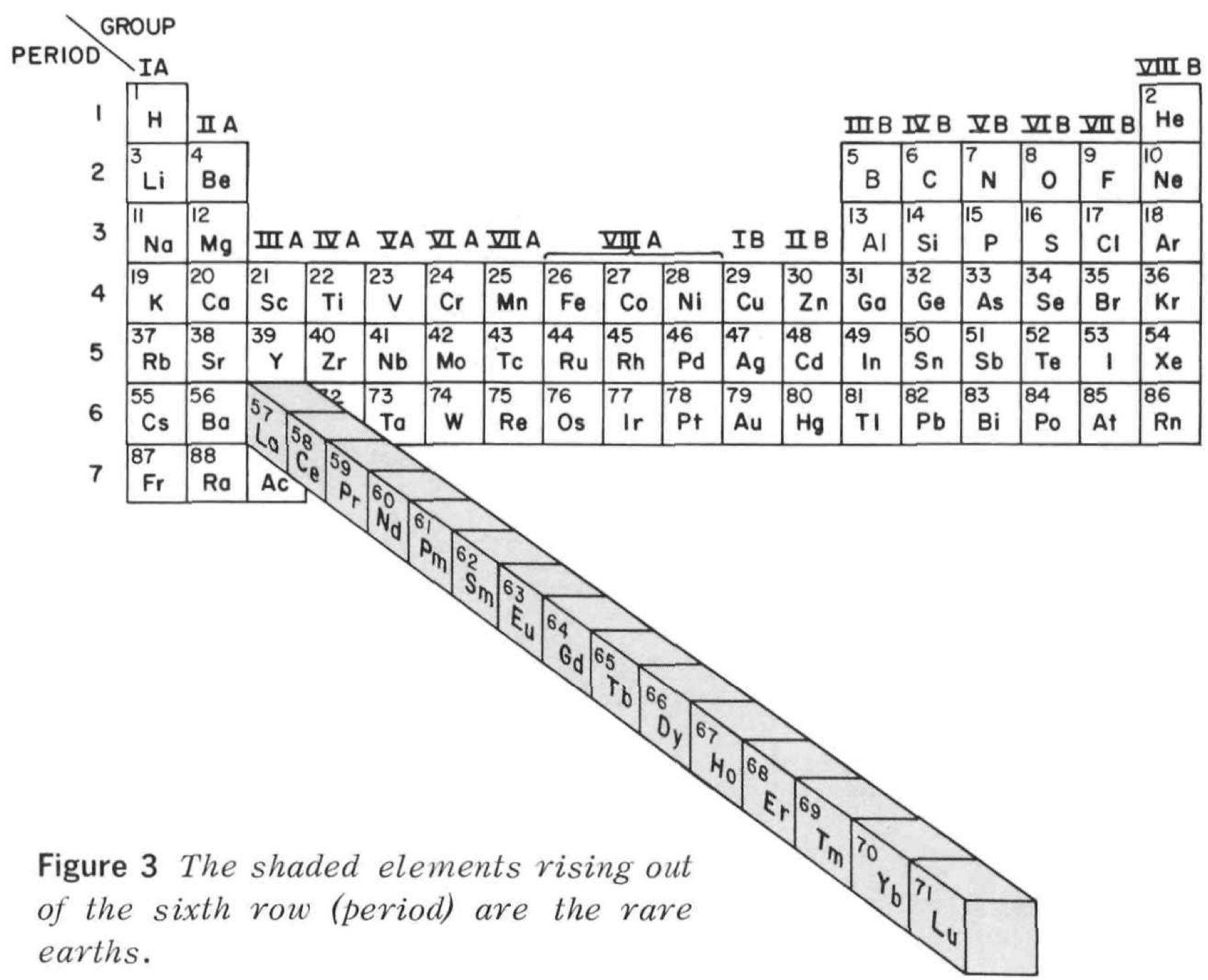
| Year: 1966 | PT id = 1265 |
Rare Earth Pop Out Periodic Table
From Rare Earths, The Fraternal Elements by Karl A. Gschneidner Jr., United States Atomic Energy Commission Division of Technical Information Library of Congress Catalog Card Number: 65-60546 1964; 1966 (Rev.)
There is an interesting point made in the text concerning the term "Rare Earths":
"The name rare earths is actually a misnomer for these elements are neither rare nor earths. They are metals, and they are quite abundant. Cerium, which is the most abundant, ranks 28th in the abundances of the naturally occurring elements and is more plentiful than beryllium, cobalt, germanium, lead, tin, or uranium. The least abundant naturally occurring rare earth, thulium, is more plentiful than cadmium, gold, iodine, mercury, platinum, or silver. Indeed, 25% of the elements are scarcer than thulium."
Thanks to René for the tip!
| Year: 1967 | PT id = 230 |
Elements of The Standard Model
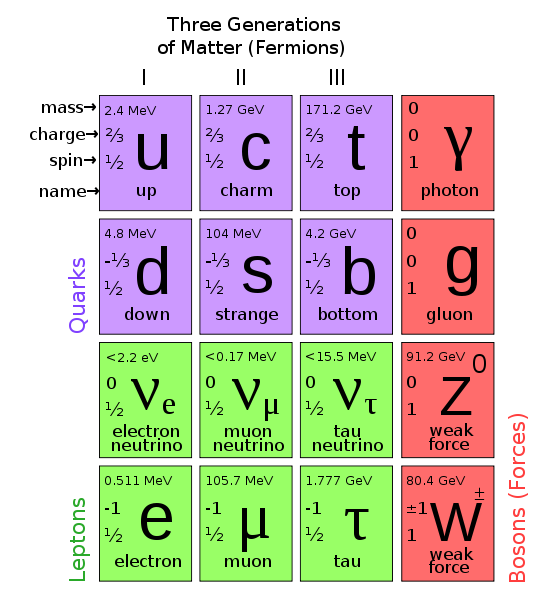
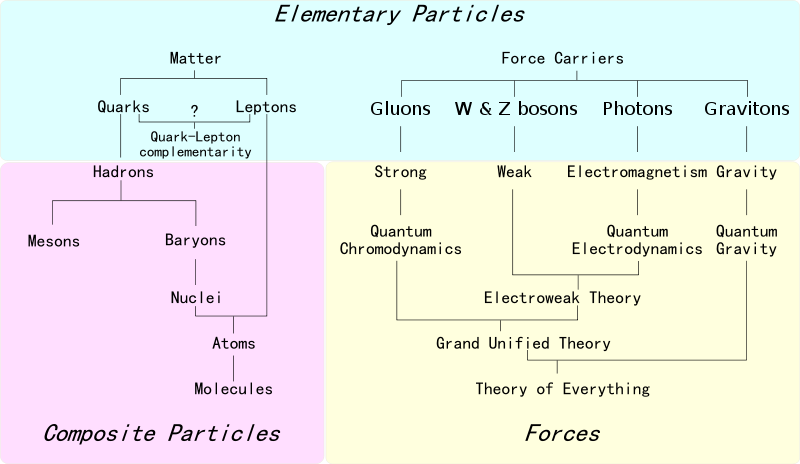
The first step towards the Standard Model of particle physics was Glashow's 1960 discovery of a way to combine the electromagnetic and weak interactions. In 1967, Weinberg & Salam incorporated the Higgs mechanism, giving the standard model its modern form of: quarks leptons and bosons.
These diagrams are the periodic tables of elementry particle physics:
| Year: 1967 | PT id = 298 |
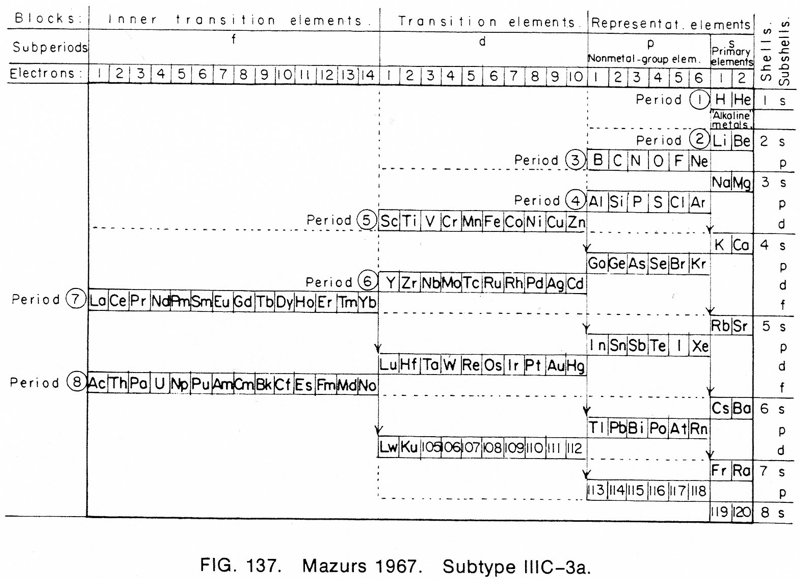
Mazurs' 1967 Formulation
From the front cover of Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
Thanks to Philip Stewart for the tip!
| Year: 1967 | PT id = 462 |
Sanderson's Periodic Table of the Chemical Elements
Sanderson's 1967 formulation has both the d-block elements and the f-block elements totally removed from the body of the table thus allowing the elements in the Major Groups of Periods 4, 5 and 6 to be grouped with the "typical" elements of Periods 2 and 3.
The Inner Transition elements are from Ce to Yb, i.e., in this 'rational' arrangement there are only thirteen lanthanide metals.
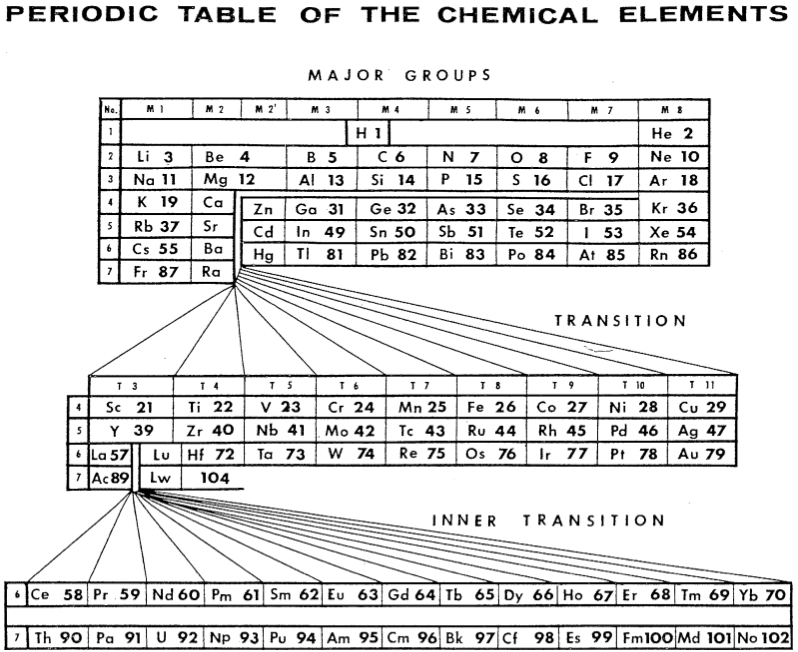
From Michael Laing's paper: A Revised Periodic Table with the Lanthanides Repositioned, Found. Chem. (2005) 7: 203-233
| Year: 1967 | PT id = 694 |
Mazurs' other 1967 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
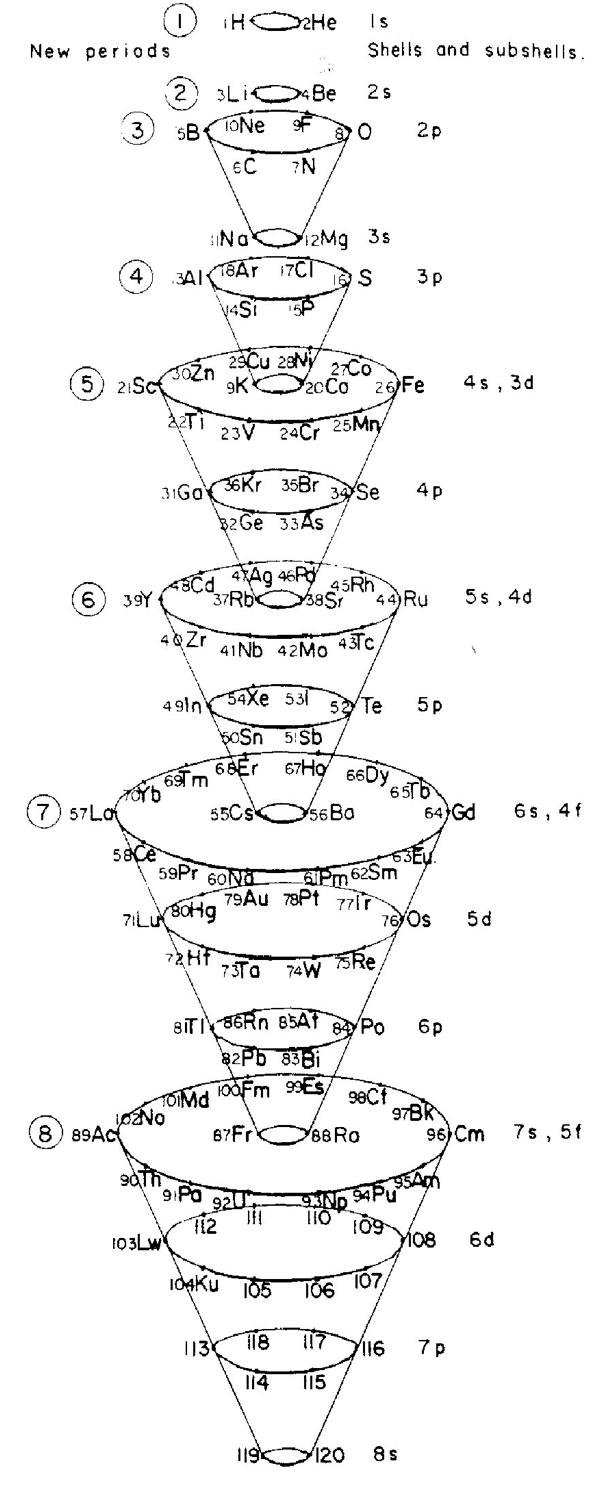
Thanks to Philip Stewart for the tip!
| Year: 1967 | PT id = 695 |
Mazurs' another 1967 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press.
This formulation is the basis of Philip Stewart's Janet Rajeuni:
Thanks to Philip Stewart for the tip!
| Year: 1968 | PT id = 441 |
Merck Index Periodic Chart of The Elements
From the 8th Edition of the Merck Index:
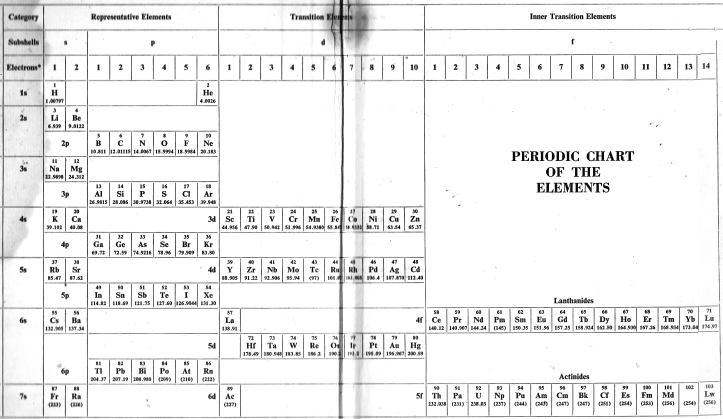
| Year: 1969 | PT id = 15 |
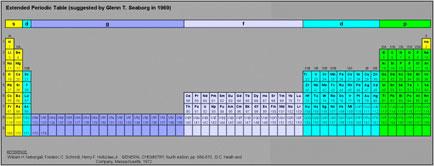
Glen T. Seaborg's g-Block Formulation
An long periodic table – developed by Glenn T. Seaborg in 1969 – containing the yet-to-be-discovered g-block elements can be constructed. For the full version and discussion, go to Jeries Rihani's pages, here and here.
| Year: 1969 | PT id = 16 |
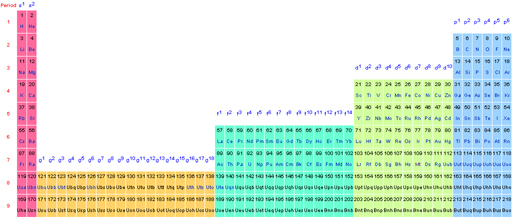
Wikipedia Extended Periodic Table
There is an extended Seaborg periodic Table on Wikipedia, here:
| Year: 1969 | PT id = 520 |
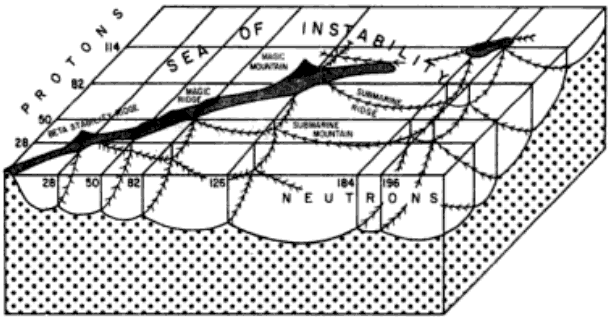
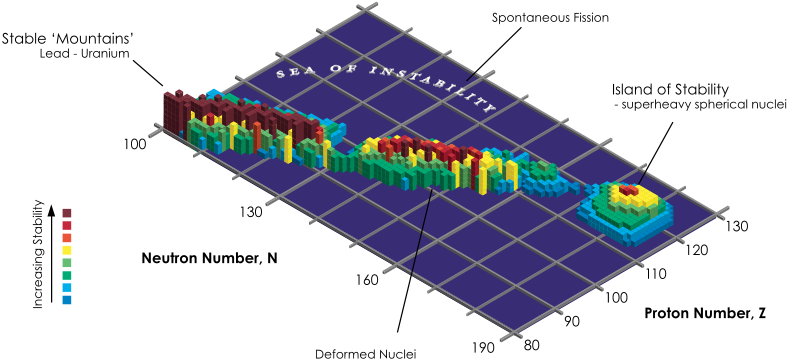
Island of Stability
From Wikipedia: The island of stability in nuclear physics describes a set of as-yet undiscovered isotopes of transuranium elements which are theorized to be much more stable than others. The possibility was proposed by Glenn T. Seaborg in the late 1960s: Prospectd for Further Considerable Extension of the Periodic Table, J.Chem.Educ., 46, 626-633 (1969) and reprinted in Modern Alchemy: Selected Papers of Glenn T. Seaborg (1994).
The hypothesis is that the atomic nucleus is built up in "shells" in a manner similar to the structure of the much larger electron shells in atoms. In both cases, shells are just groups of quantum energy levels that are relatively close to each other.
| Year: 1969 | PT id = 624 |
Mazurs Periodic System of Chemical Elements
A foldout from the Mazurs book, Graphical Representations of The Periodic System During 100 Years.
Mazurs said he drew it in 1967 and published it in 1969: ref. E Mazurs, A new numeration of periods in the periodic system and the Kessler Principle for the construction of the periodic table, Canad. Chem. Edu. 4(3), 21-23, 1969.
It is a Janet's modified system to show the irregularities – Lu, Cr, Pd etc. Click here for a larger version:
Thanks to Philip Stewart for the tip!
| Year: 1969 | PT id = 625 |
van Spronsen Periodic Table
From the van Spronsen book, The Periodic System of Chemical Elements: A History of the First Hundred Years:
Thanks to Philip Stewart for the tip!
| Year: 1969 | PT id = 884 |
Discovery of Rutherfordium
Rf ![]()
Rutherfordium, atomic number 104, has a mass of 267 au.
Synthetic radioactive element.
Rutherfordium was first observed in 1969 by A. Ghiorso et al. and I. Zvara et al.
| Year: 1969 | PT id = 1010 |
Dash's Quantum Table of the Periodic System of Elements
Harriman H. Dash, A quantum table of the periodic system of elements, International Journal of Quantum Chemistry, vol. 3, no. S3A, supplement: Proceedings of the International Symposium on Atomic, Molecular, and Solid?state Theory and Quantum Biology, 13/18 January 1969, pp. 335–340.
The abstract reads:
"The shortcomings of the long form of the periodic table of the chemical elements and the evident need for updating this format are briefly reviewed. To the question 'what format?' quantum physics provides an unequivocal answer. The foundations for the design of a quantum table are outlined. These are based on the principal quantum number as derived from the Schroedinger wave equation, the law of second order constant energy differences, and the coulomb–momentum interaction. These concepts are all combined into a single format which optimally and explicitly relates periodicity to atomic structure and the physical, chemical, and biological properties of the elements. This relationship emphasizes the unity and universality of all sciences."
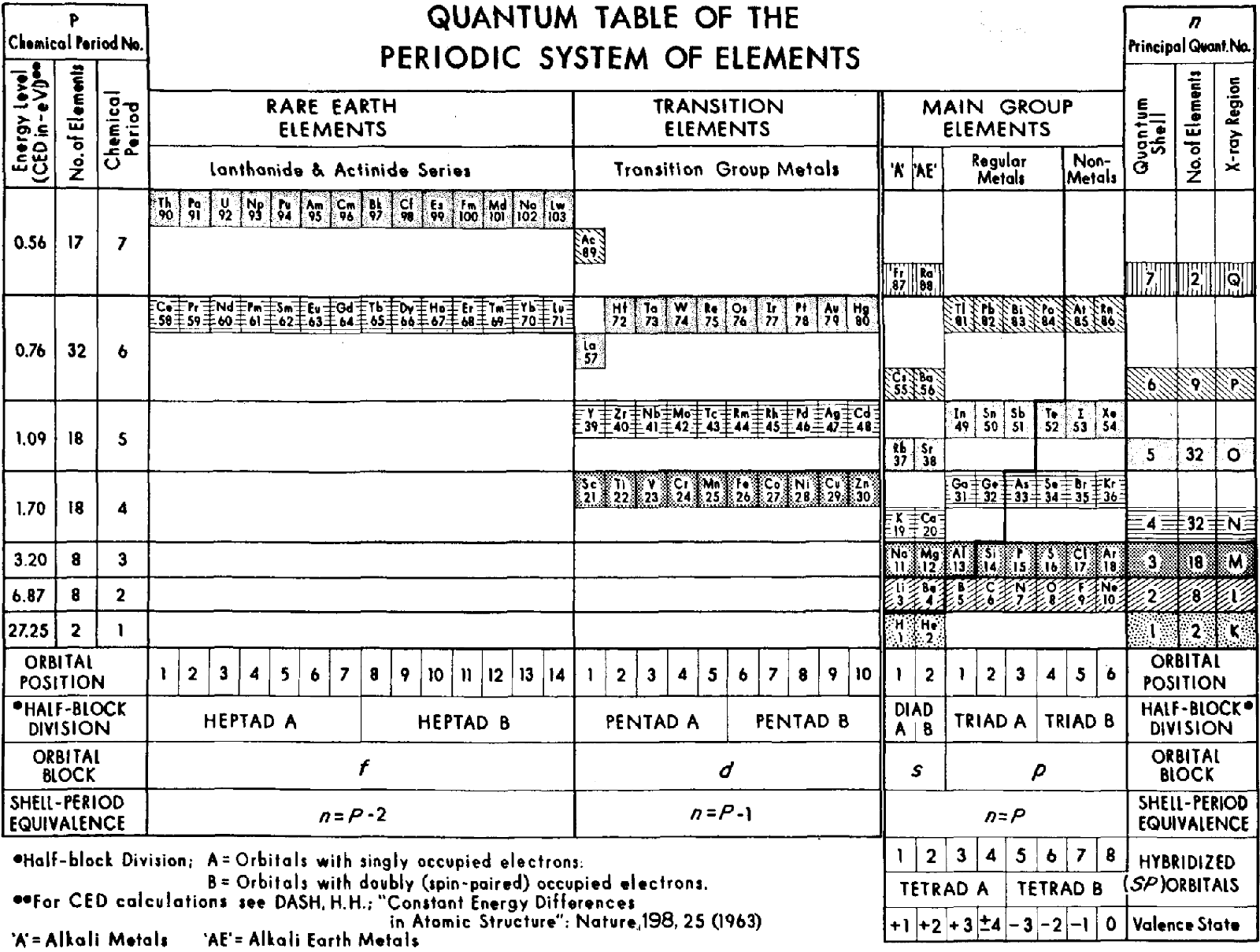
Thanks to René for the tip!
| Year: 1969 | PT id = 1126 |
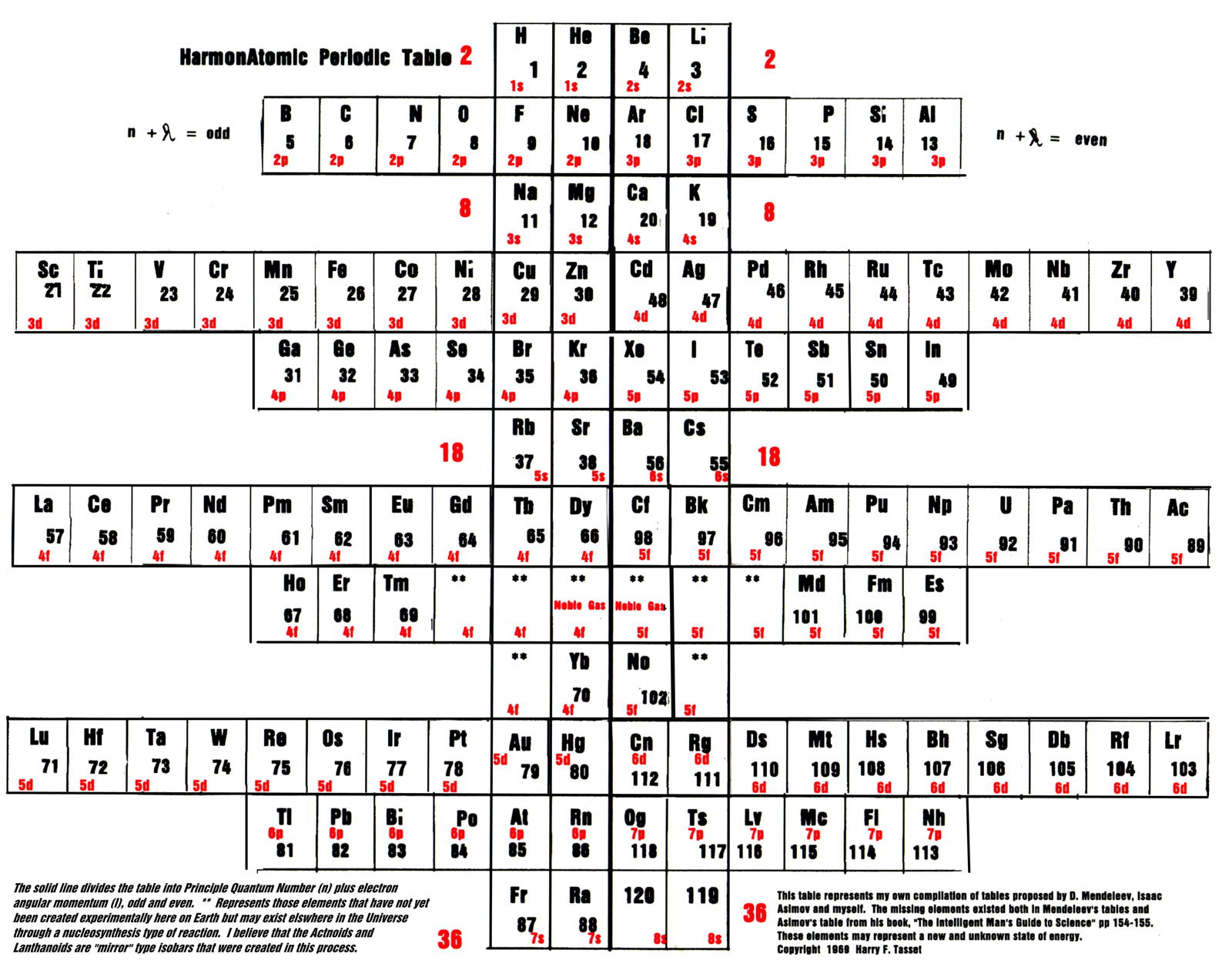
Tasset's HarmonAtomic Periodic Table
Harry F. Tasset writes:
"The periodic table (below) was originally copyrighted by me in 1969 and represents those missing elements that were lost due to the influence of physicists who were trying to mold the table into a more 'suitable' form. While they did succeed for many years, innovators like Charles Janet (left step table) and Isaac Asimov (missing elements table) had other ideas.
"Below you will see that the laws of chemistry, first discovered by Mendeleev, triumphed."
Click the image to enlarge
| Year: 1969 | PT id = 1270 |
Seel-Klechkovskii Version of Madelung's Rule for Orbital Filling
Seel F., Bild der Wissenschaft, 6, 44 (1969), a monthly popular scientific journal.
- Klechkovskii VM, Dokl. Akad. Nauk. SSSR, 135. 855 (1980). [In Russian]
- Klechkovskii VM, Zh. Eksperim. i. Teoret. Fiz., 41. 465 (1961). [In Russian]
Thanks to René for the tip!
| Year: 1969 | PT id = 1273 |
Martin's Crystal Structure Periodic Table
Ref: Martin JW 1969, Elementary Science of Metals, Wykeham Publications, London
René Vernon writes:
Note the unusual placement of La-Ac in two places, under Y and before Ce-Th. On another aspect, Martin writes:
"The non-metals, which occupy the top right-hand corner of the Periodic Table... form about one-sixth of all elements, and they are characterized by having melting-points and boiling points below about 500°C, and by having their solid and liquid phases not conducting electricity. About two-thirds of all elements are metals, and a further one sixth have properties intermediate between those of metals and non-metals."
His approach to the question of which elements are metals and non-metals, and which are intermediate may be the most useful "rough-and-ready" rubric I've seen. It is remarkable for its use of four criteria.
Perhaps we can then parse the elements as follows
Non-metals (16) = 15.5%
Fluids: H, N, O, F, Cl, Br; He, Ne, Ar, Kr, Xe, Rn 2
Solids: P, S, Se*, IIntermediate (16) = 15.5%
Metalloids: B, Si, Ge, As, Sb, Te
Near metalloids: C, At 3
Sub-metalloids: Al, Ga, In, Tl; Sn, Pb; Bi; PoMetals (71) = 68.9%
Be,^ Zn^
All the rest^ Borderline intermediate
Dingle (2017, The Elements: An Encyclopedic Tour of the Periodic Table, Quad Books, Brighton, p. 101) puts the situation this way:
"...the gap between the two extremes [of metals and nonmetals] is bridged... by the poor metals, and... the metalloids – which, perhaps by the same token, might collectively be renamed the poor non-metals.”
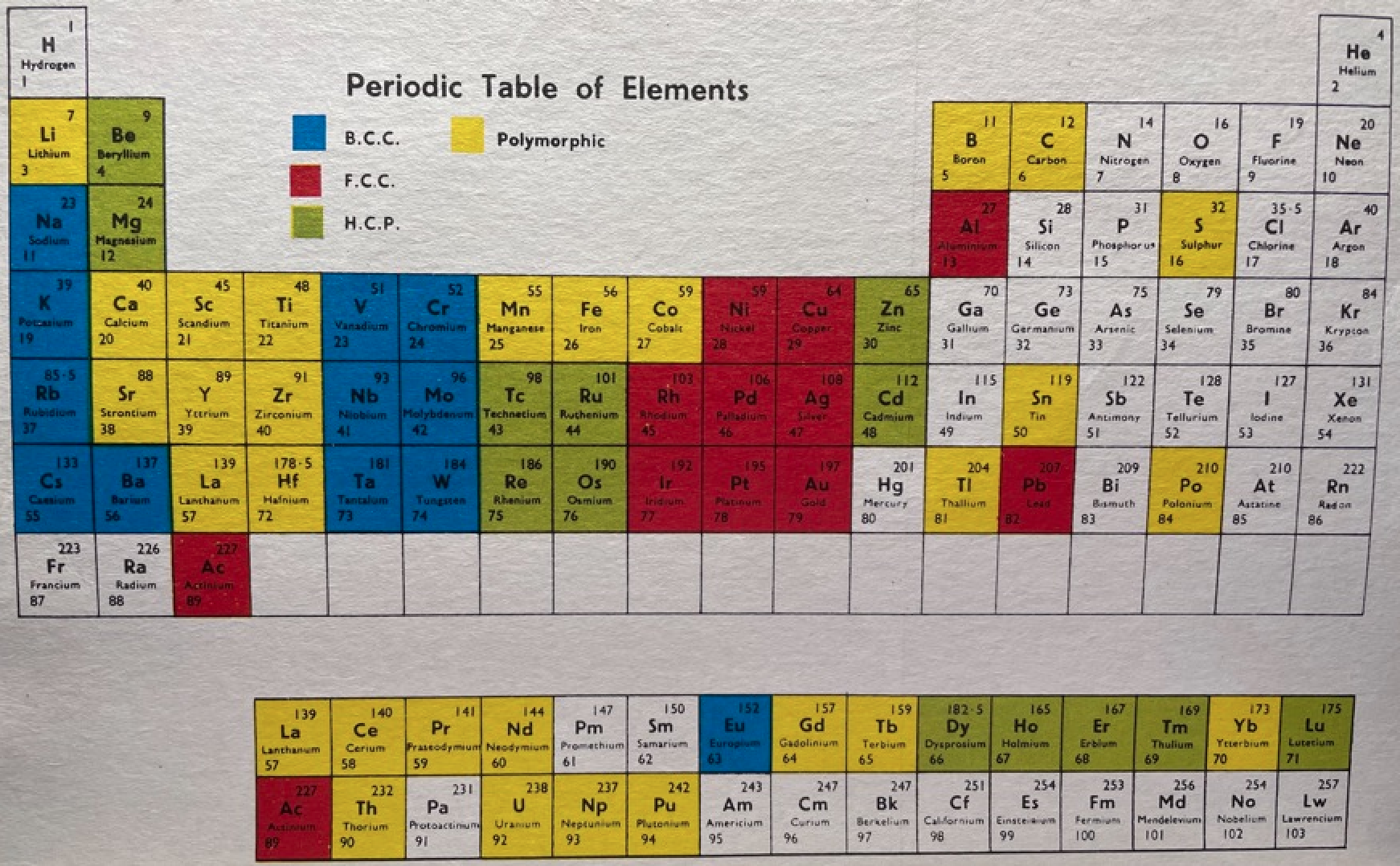
Redrawn by Vernon:
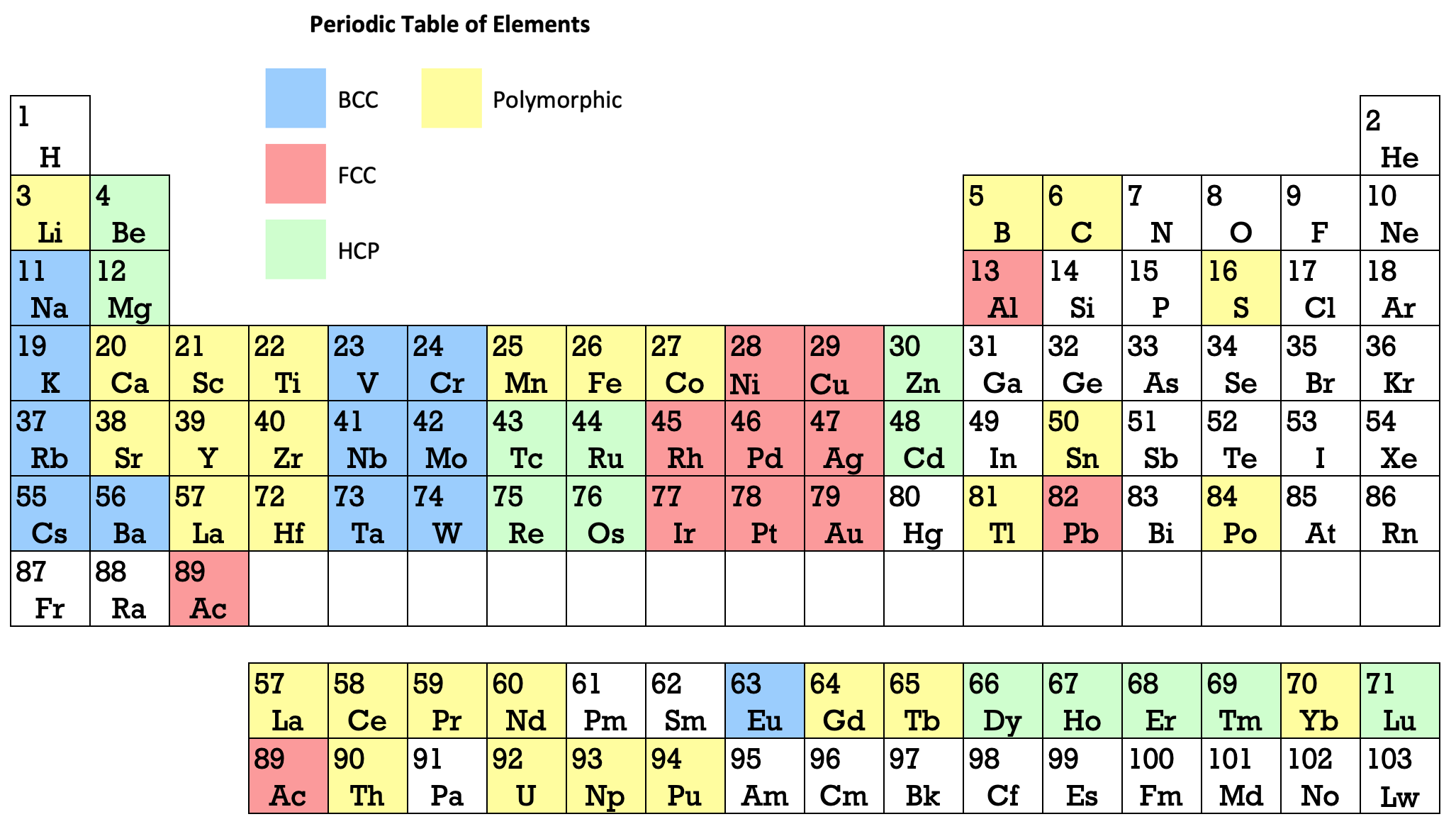
Thanks to René for the tip!
| Year: 1970 | PT id = 186 |
Monument to the Periodic Table
Monument to the periodic table, in front of the Faculty of Chemical and Food Technology of the Slovak University of Technology in Bratislava, Slovakia. The monument honors Dmitri Mendeleev, and is by the artist Karol Lacko, academic sculptor born in 1938 in Spiská Noá Ves, and who died in 2007. (Many thanks to Fathi Habashi for finding this information.)
| Year: 1970 | PT id = 321 |
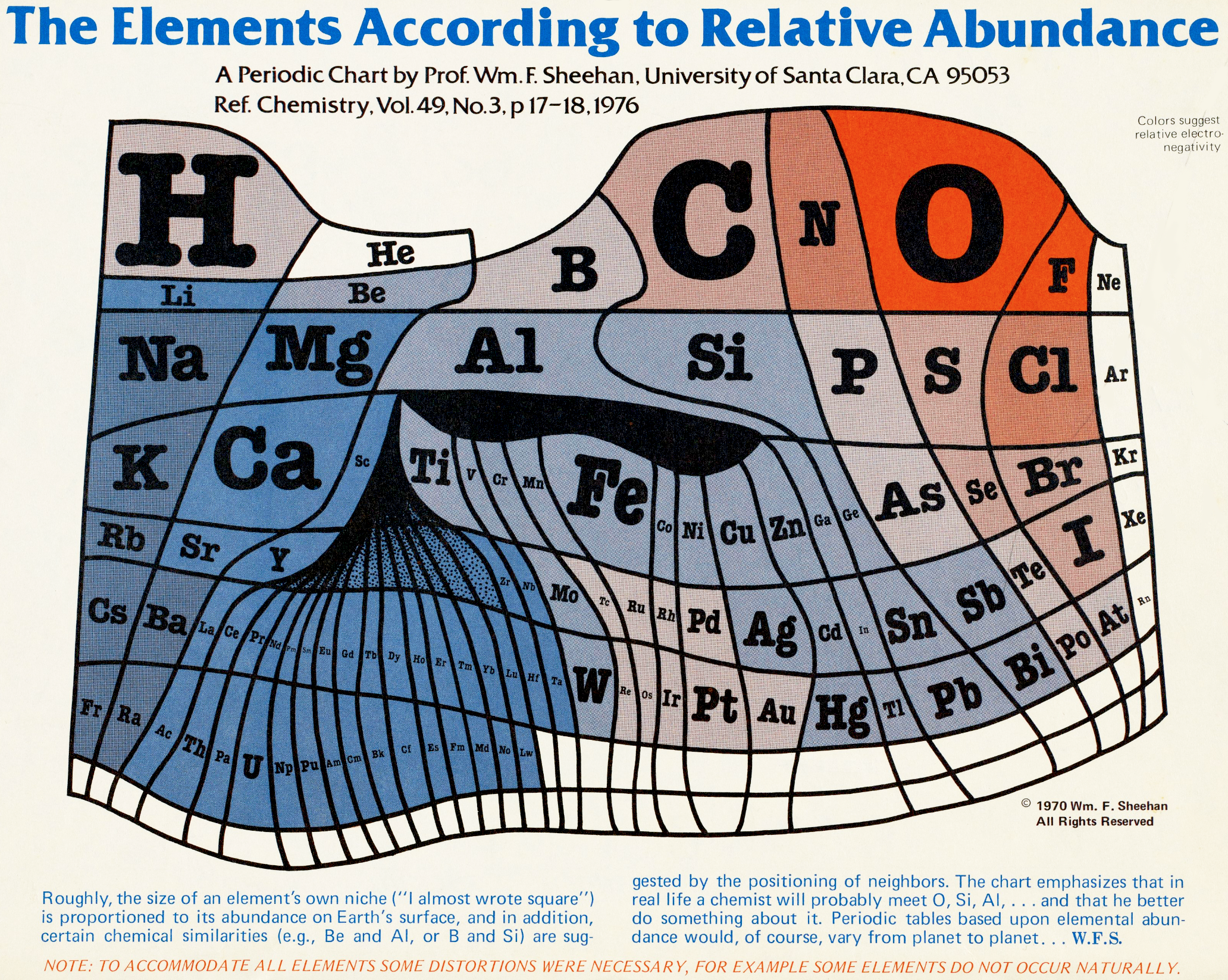
Abundance of the Elements
A 1970 periodic table by Prof. Wm. F. Sheehan of the University of Santa Clara that claims to show the elements according to relative abundance at the Earth's surface. [However, we dispute the relative areas given to the various elements; there is almost no helium at the Earth's surface, for example.] Click the image to enlarge:
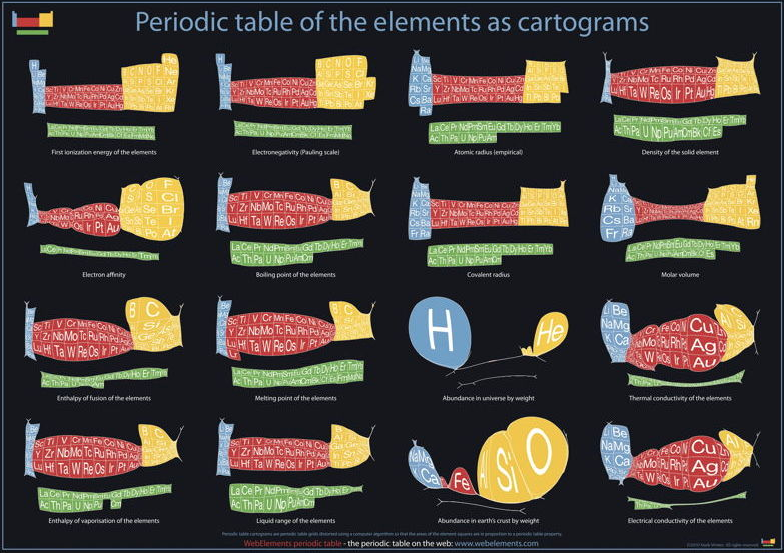
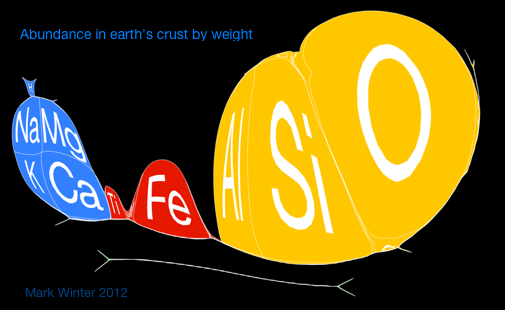
Below are some cartiogram representations, including the relative abundance of the elements in the Earth's crust, from Mark Winter's WebElements website:
| Year: 1970 | PT id = 885 |
Discovery of Dubnium
Db ![]()
Dubnium, atomic number 105, has a mass of 268 au.
Synthetic radioactive element.
Dubnium was first observed in 1970 by A. Ghiorso et al. and V. A. Druin et al.
| Year: 1970 | PT id = 1007 |
Pauling's "General Chemistry" Periodic Table
From Linus Pauling's General Chemistry (3rd Ed.). Notice that the noble gases apear twice, at the beginning and the end of each period.
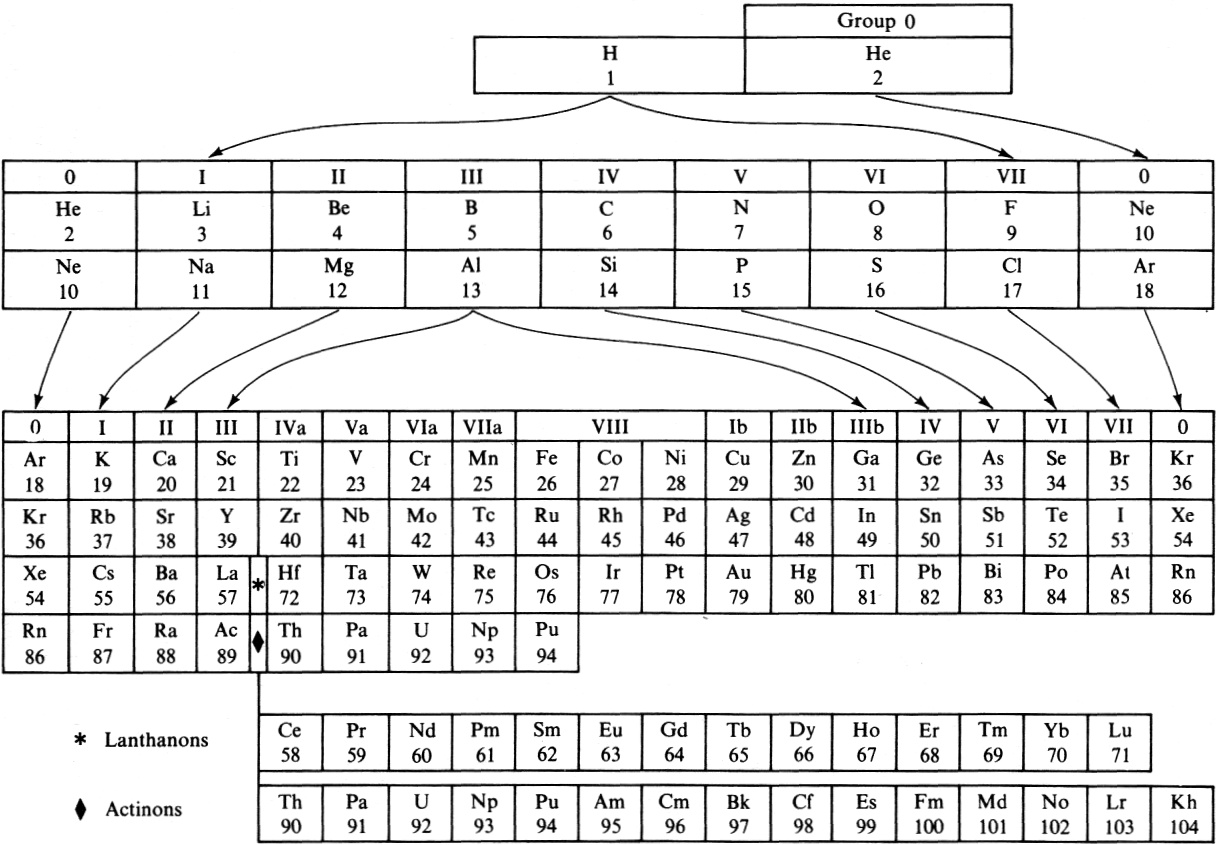
Thanks to René for the tip!
| Year: 1970 | PT id = 1055 |
Luder's Atomic-Structure Chart of the Elements
W.F. Luder, The Atomic-Structure Chart of the Elements, Canadian Chemical Education, April 1970, pp13-16.
Eric Scerri writes:
"A very nice article from 1970. This deals with the group 3 question in a very dear and simple fashion."
"I corresponded with the author in the 1980s. Also, I have always wondered where Jensen got some of his information for his article on group 3. I think it's from this and other articles by Luder. He published a number of articles in Journal of Chemical Education."
| Year: 1970 | PT id = 1188 |
Energy Level Diagram of Electron Shells & Subshells of the Elements
Figure 5-11 from page 128 of Linus Pauling's General Chemistry, W.H. Freeman, San Francisco 1970 (Dover Edition 1988):
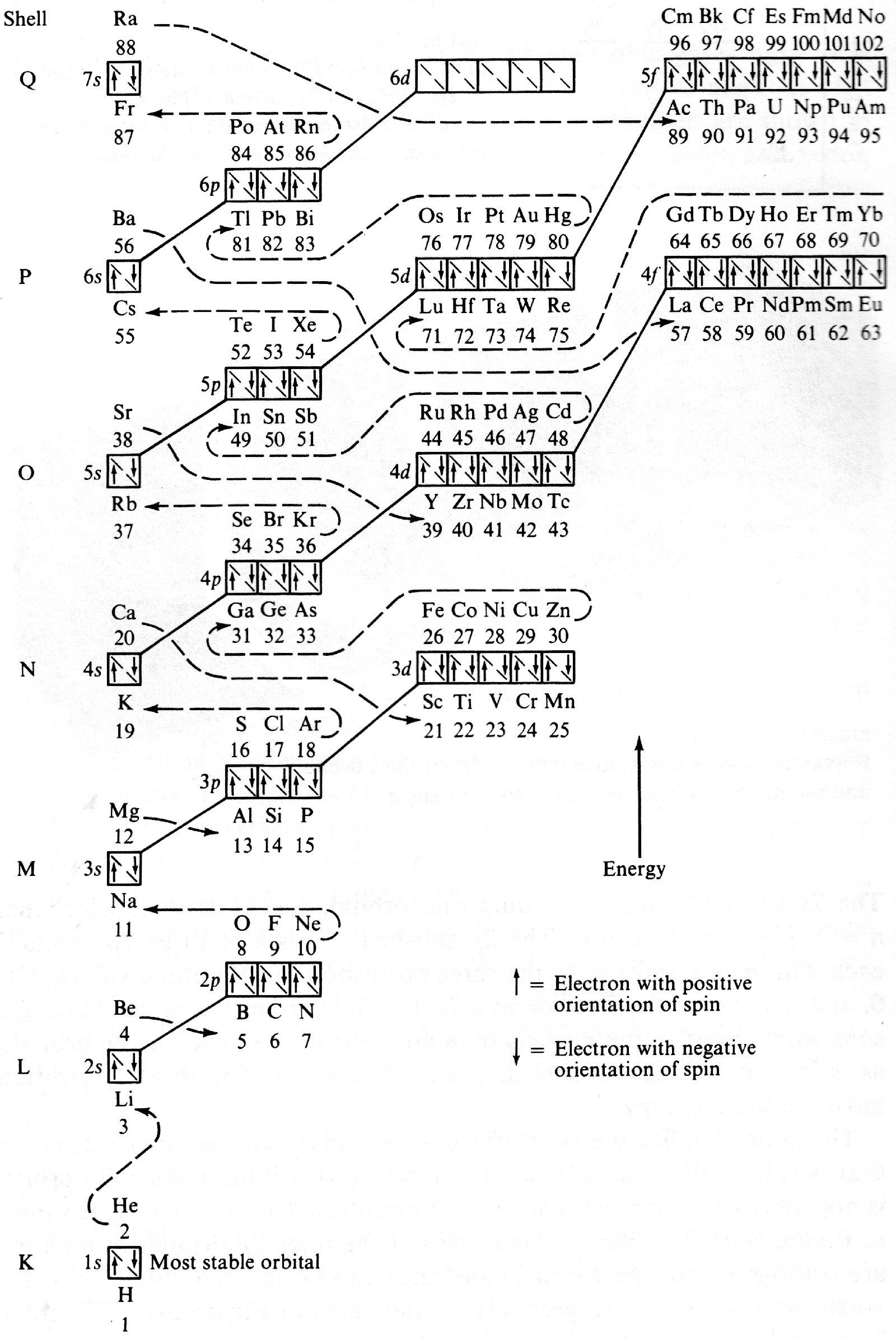
| Year: 1971 | PT id = 205 |
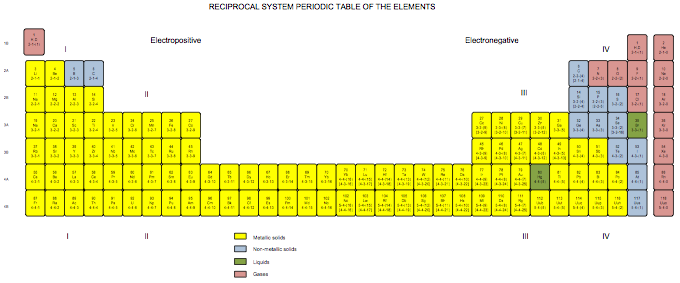
Satz Reciprocal System Periodic Table
Developed in 1971 for my book The Unmysterious Universe, this periodic table is based on Dewey B. Larson's Reciprocal System of theory. The numbers below the symbols indicate the rotational displacement (spin numbers) of the atoms. The Roman numerals indicated divisions; the rows, 1B to 4B, are referred to as "groups" rather than as "periods." Note that we have the same trouble positioning hydrogen as does everyone else; here, I've put it over both the alkali metals and the halogens, because it acts both as electropositive (e.g., with respect to water) and electronegative (with respect to carbon).
Click here for larger PDF file.
Ronald W. Satz, Ph.D.
Transpower Corporation
www.transpowercorp.com
transpower.wordpress.com
transpower@aol.com
| Year: 1971 | PT id = 672 |
Clark, John O. E. Periodic Table
Thanks to René Vernon who found this formulation, and writes:
"Here's a strange table I found in the following book: Clark Jonh O.E. 1982, Chemistry (The Hamlyn Publishing Group, Feltham, Middlesex) ISBN 0600001245. The colour coding is exasperating. The way the table is laid out is bizarre. The copy I have is a reprint of the original 1971 edition so I have to wonder if the graphic designer was drawing inspiration from the trippy 60s."
| Year: 1971 | PT id = 1269 |
Goldanskii's Chess Board Version of The Madelung Rule (For Orbital Filling)
Ref: Goldanskii, V I: The Periodic System of D I Mendeleev and Problems of Nuclear Chemistry pp 137-162 ex: Verde M (ed.): 1st International Conference on the Periodic Table, Vincenzo Bona, Torino 1971.
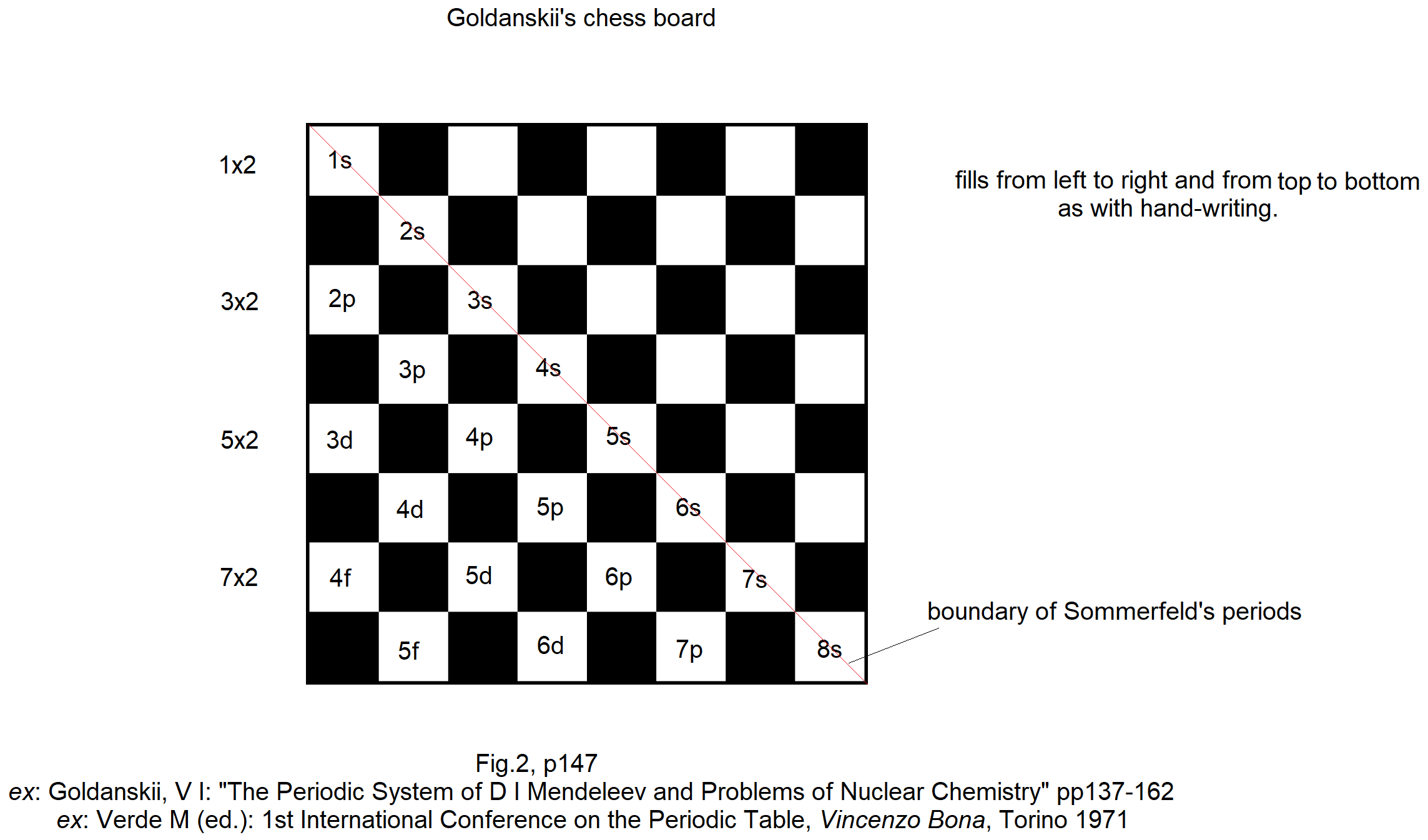
Thanks to John Marks for the tip!
| Year: 1972 | PT id = 452 |
Octagonal Prismatic Periodic Table
In the Journal of Chemical Education (1972), Tang Wah Kow of New Method College Hong Kong, presents an octagonal prismatic periodic table:
| Year: 1974 | PT id = 260 |
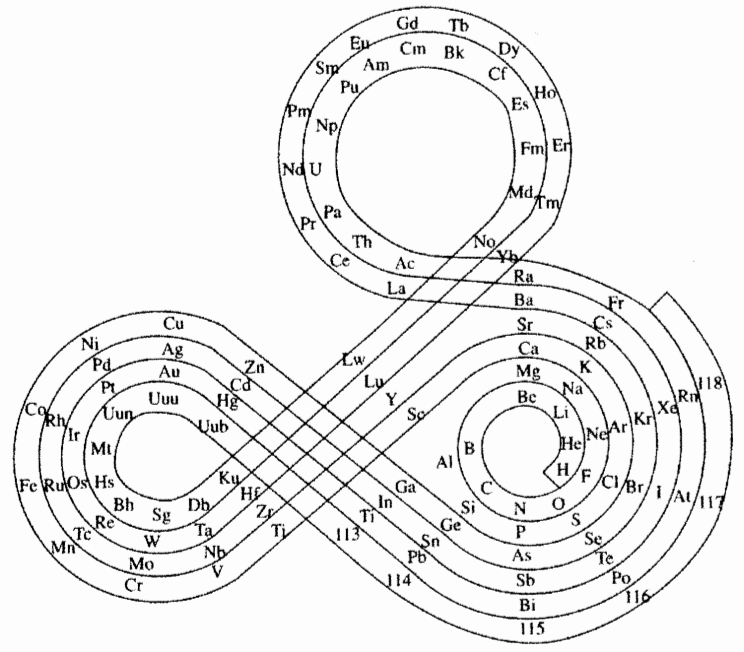
Mazurs Version of Janet's "Lemniscate" Formulation
Janet's lemniscate formulation periodic table as modified by E.G. Mazur in his Graphic Representations of the Periodic System during One Hundred Years (1974), cited in Punyashloke Mishra's The Role of Abstraction in Scientific Illustration: Implications for Pedagogy (1999) republished in Carolyn Handa's Visual Rhetoric in a Digital World: A Critical Sourcebook", from the Island94 blog, here:
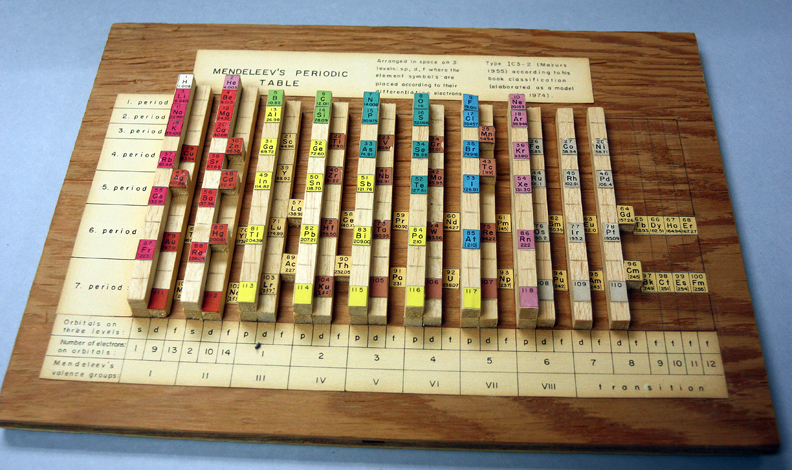
| Year: 1974 | PT id = 267 |
Mazurs Wooden Version of Mendeleev's Periodic Table
There is a posting in the The Elements Unearthed blog by David V Black concerning a view of the Marzus archive:
"My biggest discovery this week has been a collection in our archives of the notes of Edward Mazurs, who wrote the definitive work on classifying different systems of periodic tables in 1957 with a revised edition in 1974 (Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press). He collected articles and wrote extensive, detailed notes on every version of the periodic table he could find as it developed from its start in the early 1860s with the work of de Chancourtois through 1974. All of those notes have been donated to Chemical Heritage Foundation and fill up ten binders, with meticulous drawings, charts, tables, and frequent additions and changes. There are also some pieces of the original artwork prepared for the book, and a wooden model of the periodic table Mazurs built himself. "
| Year: 1974 | PT id = 299 |
Mazurs' PT Formulation Analysis
In his 1974 book Edward G. Mazurs (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press gives a comprehensive analysis of periodic table formulations.
Mazurs identifies most PT formulations as being:
- Spiral
- Plane lemniscate
- Concentric circles
- Helix on a cylinder
- Helix on a cone
- Space lemniscate
- Space concentric circles
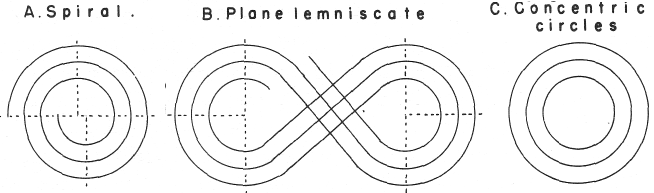
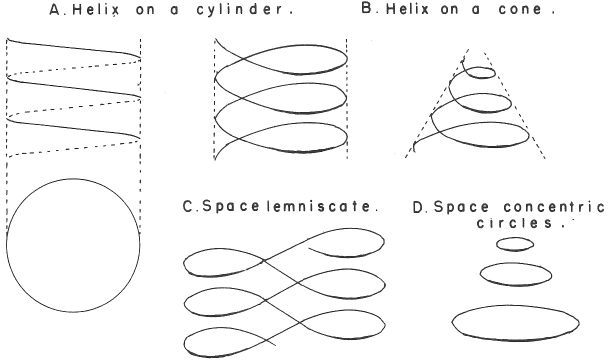
| Year: 1974 | PT id = 886 |
Discovery of Seaborgium
Sg ![]()
Seaborgium, atomic number 106, has a mass of 271 au.
Synthetic radioactive element.
Seaborgium was first observed in 1974 by A. Ghiorso et al.
| Year: 1974 | PT id = 1058 |
Mazurs' Redrawing of Stedman's Formulation
An spiral formulation by Mazurs, cited as being after Janet (1928). However, it is actually, it is after Stedman (1947).
In an article Bull. Hist. Chem., VOLUME 34, Number 2 (2009) O.T. Benfey writes:
"After we had developed our own [Periodic Snail] spiral design, we found that E. G. Mazurs had published a spiral with a separate protrusion for the lanthanides which, under the image, he misleadingly ascribed to Charles Janet in 1928, the same year that Janet had published a simple circular form also shown by Mazurs. The Mazurs diagram with the lanthanide protrusion was reprinted in [the journal] Chemistry. However, [Philip] Stewart informed me that the Mazurs figure bears no resemblance to the Janet diagram he indicated nor to any other of his designs. Detailed references given a few pages later by Mazurs suggested correctly that the spiral derives from Stedman and is so identified and depicted by van Spronsen. The Mazurs diagram is a mirror image of the Stedman spiral, updated to include elements discovered since 1947." [For references, see the article.]"
Mazurs (p. 77) writes:
"Subtype IIIA3–1a Helix on a modified cone. The transition and inner transition elements have special revolutions in the form of loops. This table, originated by Stedman in 1947 is not a successful one."
Thanks to René for the tip and information!
| Year: 1975 | PT id = 164 |
Hyde's Periodic Relationships of The Elements
J. Franklin Hyde was an industrual chemist. His PT formulation is available from the Gelest website:
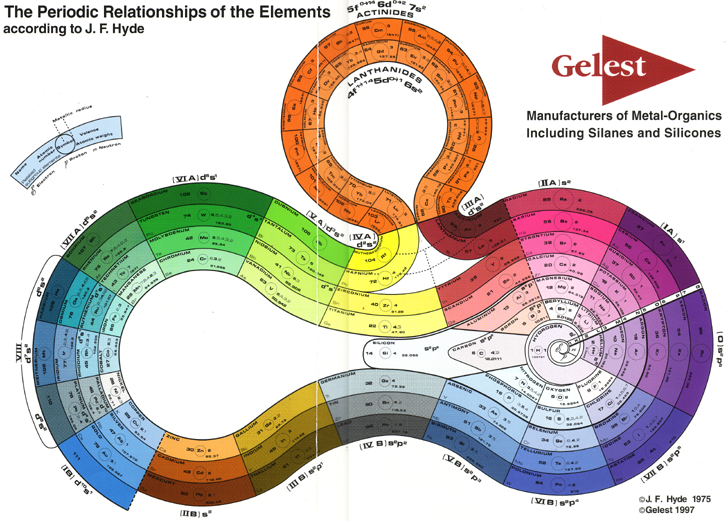
| Year: 1975 | PT id = 746 |
Russian Periodic Table(s)
Eric Scerri writes:
"The periodic tables [below], and data, are from some Russian books given to me by the late Ray Hefferlin, when I visited him a few years ago in Tennessee. Sorry, I can't give any source details as the inserts got separated from the book."
The captions say: "Fig. XVII. Block-type periodic table" and "U.L.Kulakov, Classification of the chemical elements on the new background".
Looking at the graphics style, we are guessing they date from the mid-1970s (MRL)
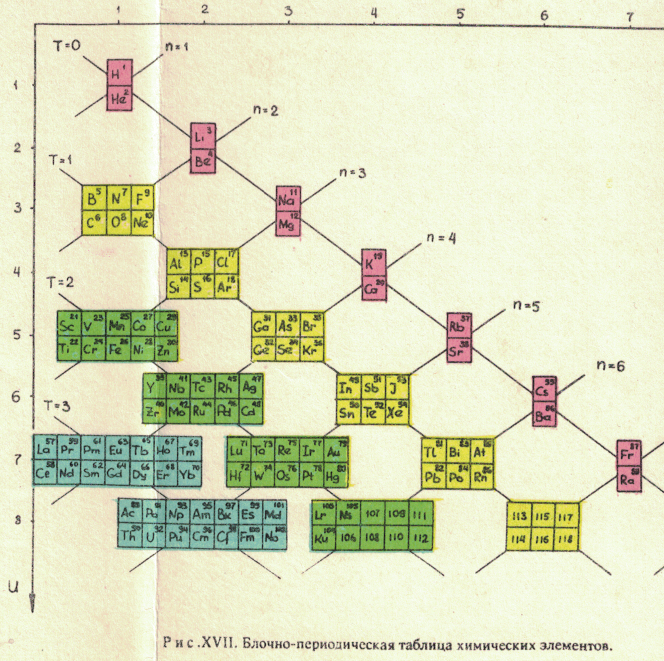
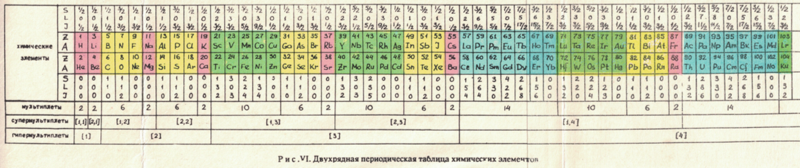
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
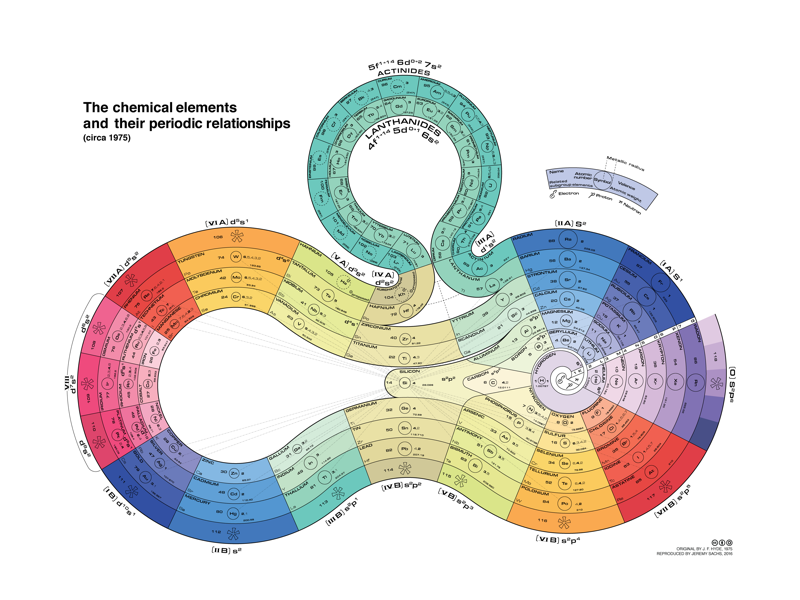
| Year: 1975 | PT id = 748 |
Hyde's Periodic Relationships of The Elements (updated)
I received an email from Jeremy Sachs saying:
"Gelest don't seem to offer [this periodic table formulation] anymore, and because their version heavily modifies Hyde's original table, I've reproduced the 1975 version of his table with the permission of his surviving relatives."
Click here to see the full size version.
| Year: 1975 | PT id = 1167 |
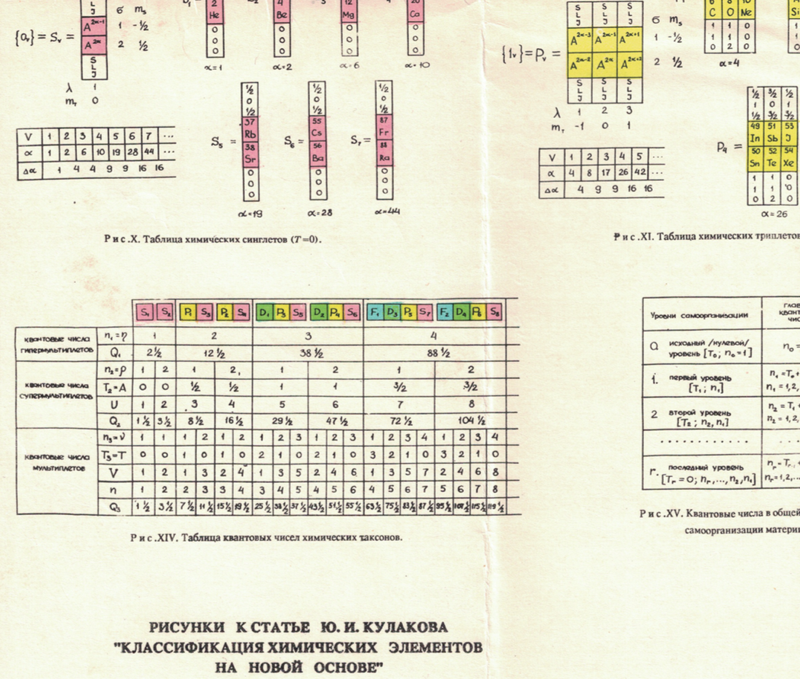
Shukarev's Periodic System
Shukarev SA 1975, "On the image of the periodic system with the use of fifth move of late a-elements", Collection of Scientific and Methodological Articles on Chemistry. M.: Higher School, no 4, pp 3-12 (in Russian).
The image has been re-drawn and commented upon.
| Year: 1976 | PT id = 945 |
Seaborg's Futuristic Periodic Table
A Futuristic Periodic Table Showing Predicted Locations of a Large Number of Transuranium Elements (Atomic numbers in parentheses) by Glenn Seaborg in 1976. Internal reference number: XBL 751-2036
| Year: 1977 | PT id = 442 |
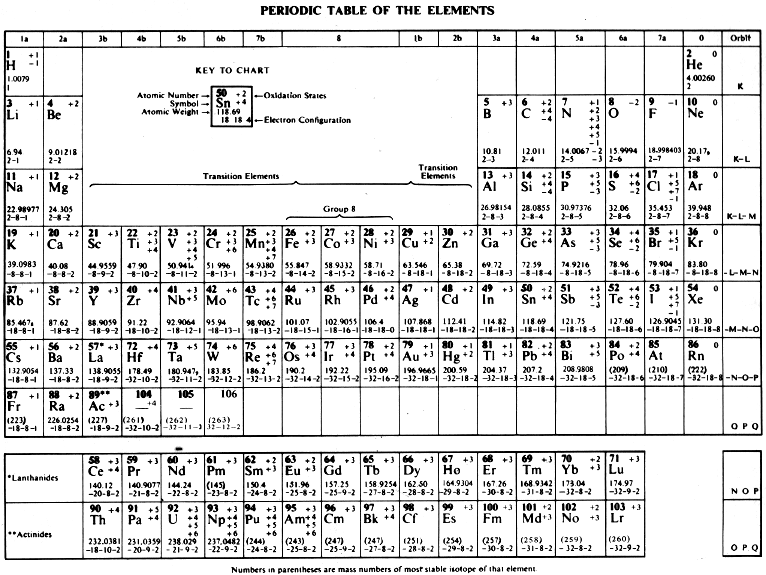
CRC Handbook Periodic Table of The Elements
From the 58th Edition of the CRC Handbook of Chemistry and Physics:
| Year: 1977 | PT id = 754 |
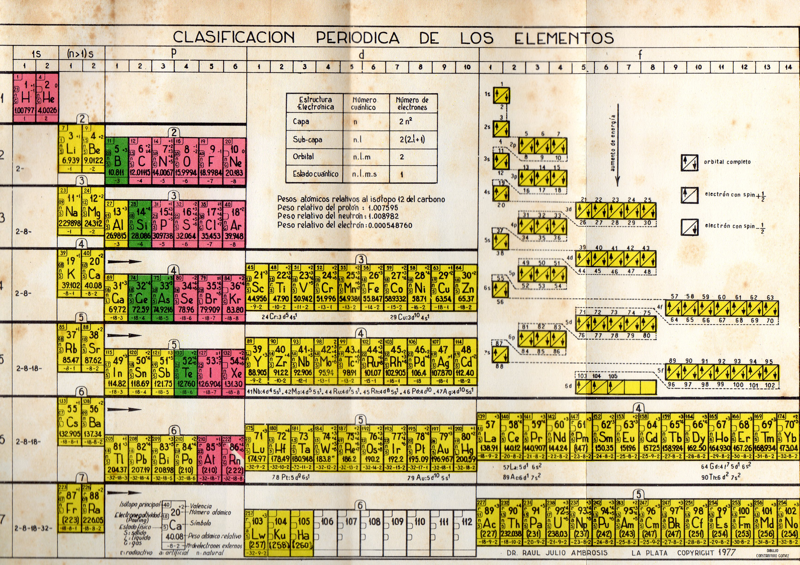
Ambrosis' Clasification Periodica de los Elementos
By Dr Raúl Julio Ambrosis, who taught Chemistry at the National University of La Plata in Argentina. The source is the Clasificación Periódica de los Elementos, Buenos Aires, Ediciones Marymar, 1977.
Click here for the full size version:
| Year: 1979 | PT id = 471 |
Mann's Spiral Periodic Table
From AT Mann:
"I designed a spiral periodic table which was published first in my book The Divine Plot: Astrology, Reincarnation, Cosmology and History (George Allen & Unwin, London, 1986) which attempts to correlate the PT with astrological understanding of the inherent properties of the signs and planets":
| Year: 1979 | PT id = 994 |
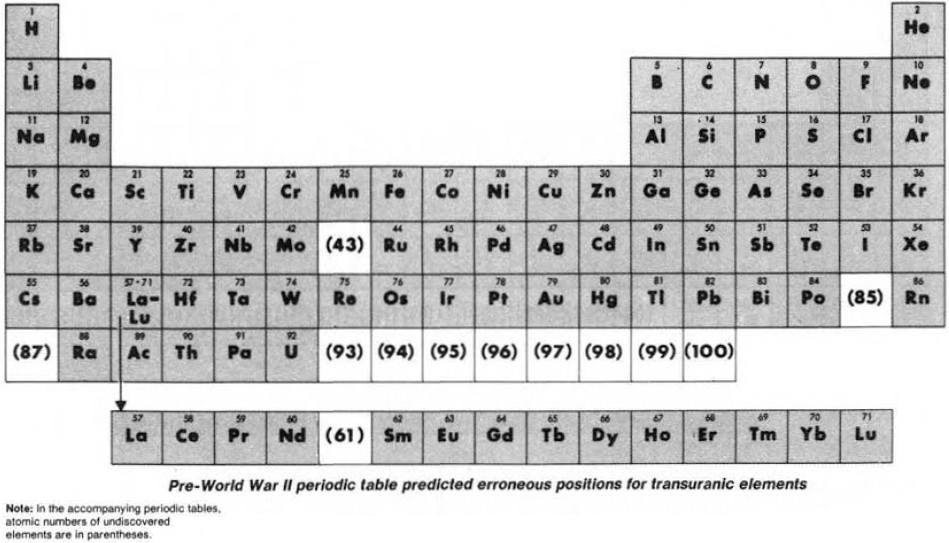
Seaborg's "How the Periodic Table Evolved Over 40 Years" (1939 – 1979)
From the C&EN paper THE PERIODIC TABLE: Tortuous path to man-made elements 57, 1979, pp 46-52.
Until World War II, the three heaviest known elements – thorium, protactinium & uranium – were believed to be related to hafnium, tantalum & tungsten respectively. Similarly, elements 93 to 100 were expected to fit neatly into the periodic table:
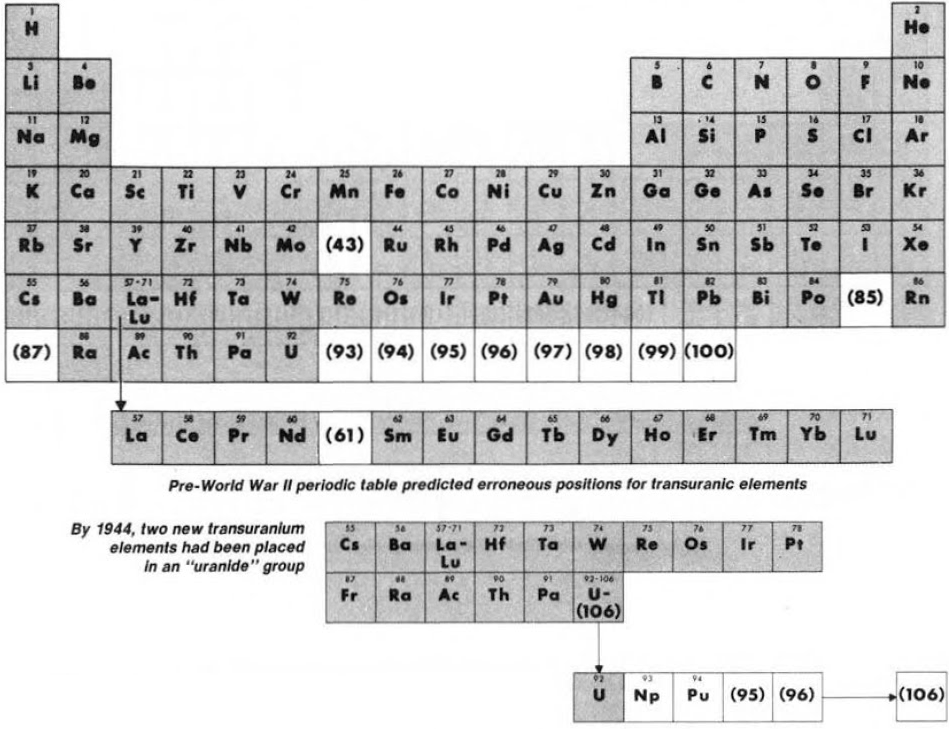
Synthesis and study of the transuranic elements – neptunium & plutonium – indicated that these new elements were "cousins" of uranium and in 1944 should be placed into a new "uranide" group.
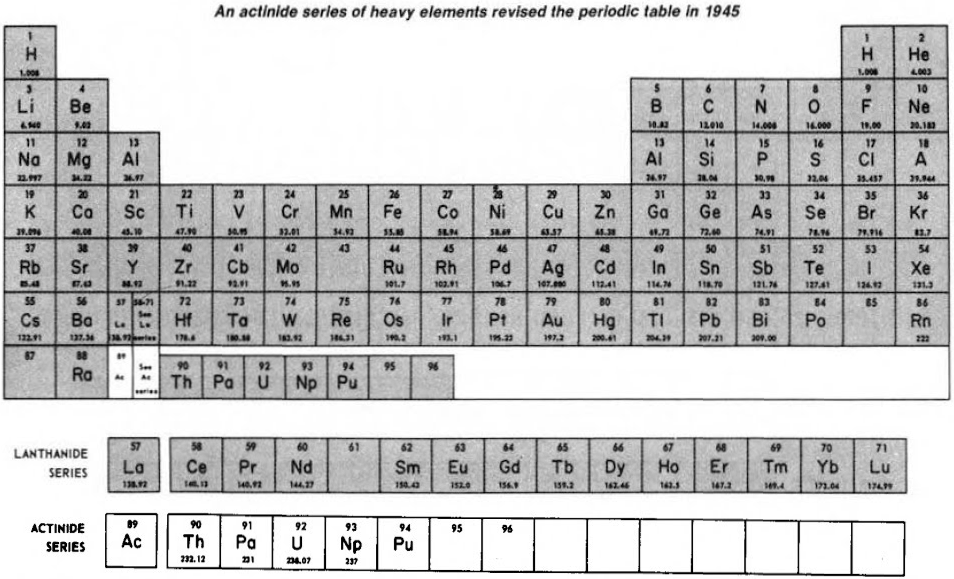
Subsequently (1944/45), Seaborg advanced the theory that elements heavier than actinium actually constitute a distinct "actinide" group that mirrors the lanthanide rare-earth group:
Finally, Seaborg postulated what a future periodic table, up to Z = 168, may look like:
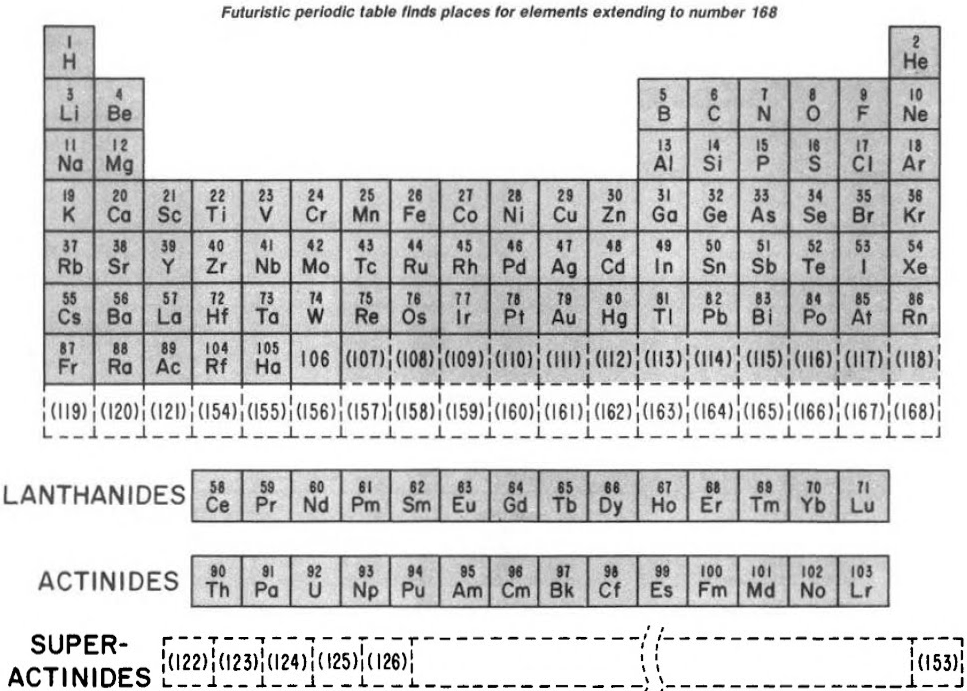
| Year: 1980 | PT id = 158 |
Periodic RoundTable
Gary Katz says: "The Periodic RoundTable is a unique three-dimensional model of the Periodic Table, an elegant spatial arrangement of the chemical elements that is both symmetrical and mathematical. It is the ultimate refinement of Mendeleev's scheme, one that will take us into the twenty-first century and beyond. The Periodic RoundTable possesses such a high degree of order because it is based exclusively on the system of ideal electronic configuration, which in turn is the basis of periodicity among the elements. In the Periodic RoundTable the electron shells are filled in the same order as the elements themselves appear, demonstrating a holistic relationship between the chemistry of the elements and the orbital descriptions of their electrons."
| Year: 1981 | PT id = 887 |
Discovery of Bohrium
Bh ![]()
Bohrium, atomic number 107, has a mass of 272 au.
Synthetic radioactive element.
Bohrium was first observed in 1981 by G.Münzenberget al.
| Year: 1982 | PT id = 49 |
Cement Chemist's Periodic Cube
Periodic table designed in the style of a cube by J. Francis Young, Professor of Civil and Ceramic Engineering, University of Illinois. This table was published by Instruments for Research and Industry and includes instructions for assembly into a 3-D model.
More information, including high resolution files, at the Science History Institute.
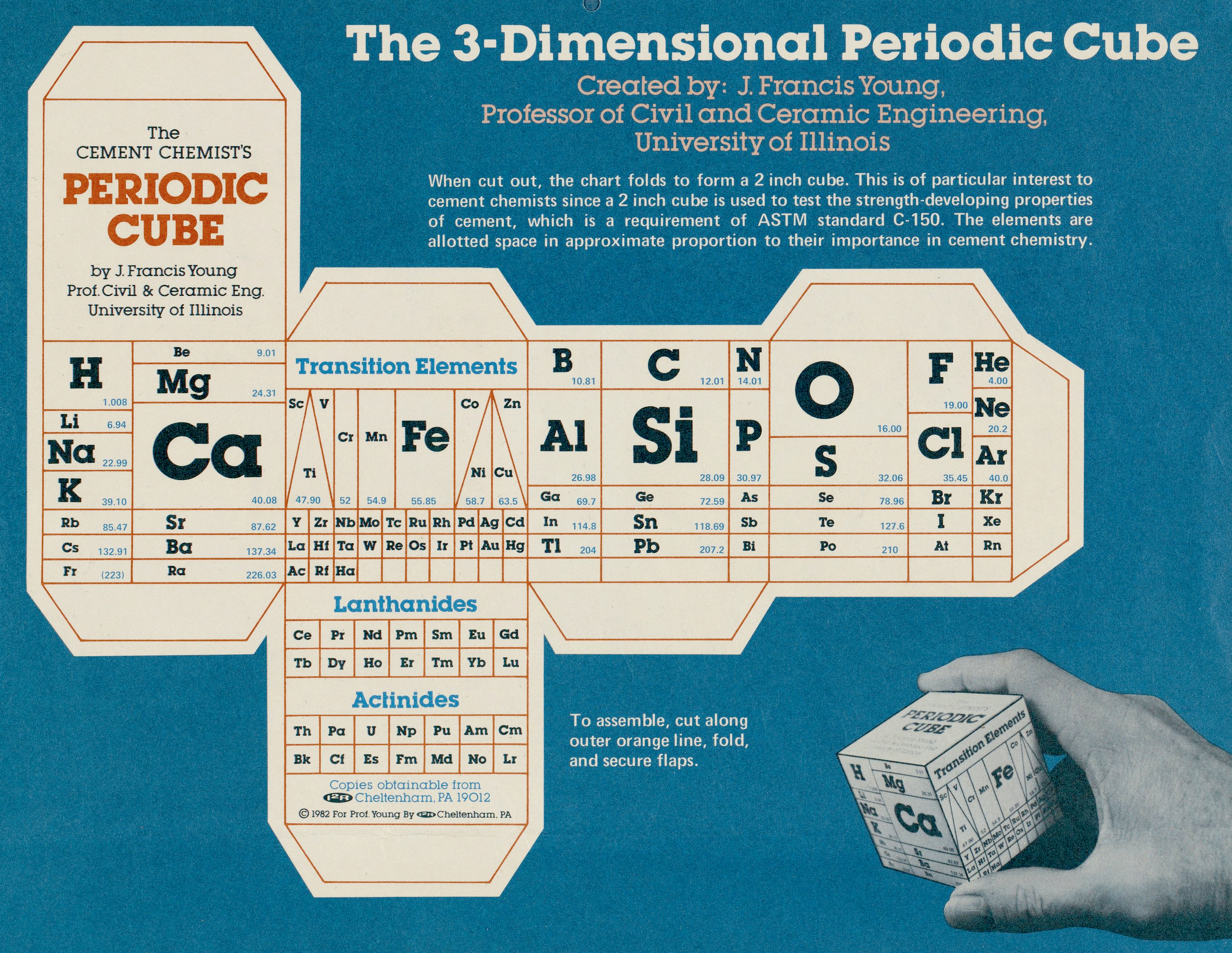
Thanks to René Vernon for the tip!
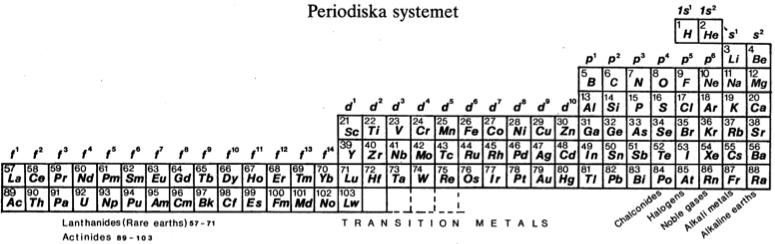
| Year: 1982 | PT id = 451 |
Periodiska Systems Rätta Form
Hanno Essén's Periodiska Systems Rätta formulation of the Periodic Table, published in the International Journal of Quantum Chemistry vol. XXI pp.717-726 (1982).
Essén's formulation is a variant of the Janet Left-Step formulation of 1928:
| Year: 1982 | PT id = 889 |
Discovery of Meitnerium
Mt ![]()
Meitnerium, atomic number 109, has a mass of 276 au.
Synthetic radioactive element.
Meitnerium was first observed in 1982 by G. Münzenberg, P. Armbrusteret al.
| Year: 1983 | PT id = 50 |
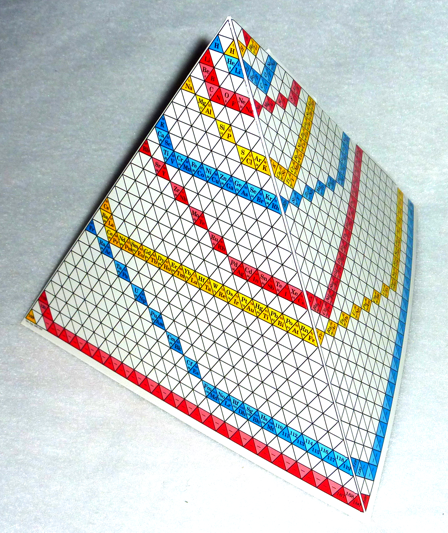
Periodic Pyramid
Periodic table designed in the style of a pyramid by Charles E. Gragg. This table was published by Instruments for Research and Industry and includes instructions for assembly into a 3-D model.
More information, including high resolution files, at the Science History Institute.

Thanks to René Vernon for the tip!
| Year: 1984 | PT id = 888 |
Discovery of Hassium
Hs ![]()
Hassium, atomic number 108, has a mass of 270 au.
Synthetic radioactive element.
Hassium was first observed in 1984 by G. Münzenberg, P. Armbruster et al.
| Year: 1984 | PT id = 924 |
Arabic Periodic Tables
From Arabic introductory text published by the Royal Scientific Society, Amman, Jordan, 1984. Jeries A. Rihani, who provided the two images, writes:
"The first image shows a periodic table similar to that of the Janet left-step periodic table, but in an upside-down flipped format.The second image displays the comparative energy levels of orbitals of atoms of many electrons."
Thanks to Jeries A. Rihani for the tip!
| Year: 1984 | PT id = 1258 |
Cherkesov: Two Periodic Tables
Cherkesov AI 1984, Ionization energy of 1-6 p-electrons and formation enthalpies of lutetium and lawrencium halides. Position of these elements in Periodic system, Radiokhimiya, vol. 26, no. 1, p. 53?60 (in Russian), https://inis.iaea.org/search/search.aspx?orig_q=RN:16012913
René Vernon writes:
"Two Russian offerings, the first is Mendeleev style, including He over Be and the integration of the Ln and An into the main body of the table.
"The second is the first time I have seen a genuine right step table, albeit at the expense of the numbers going backwards, and the non-intuitive group numbering scheme. Bonus marks for out-of-the box thinking."
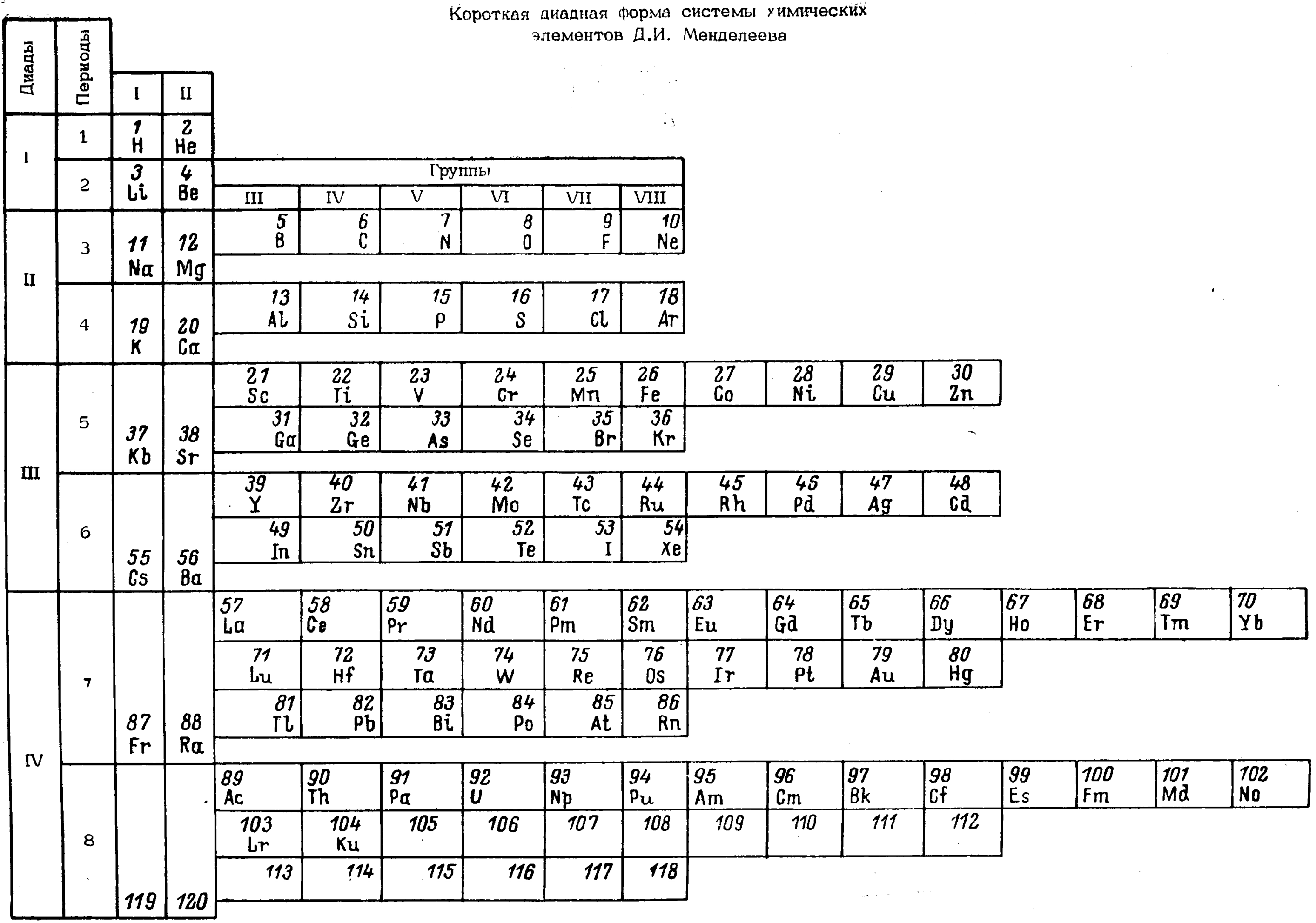
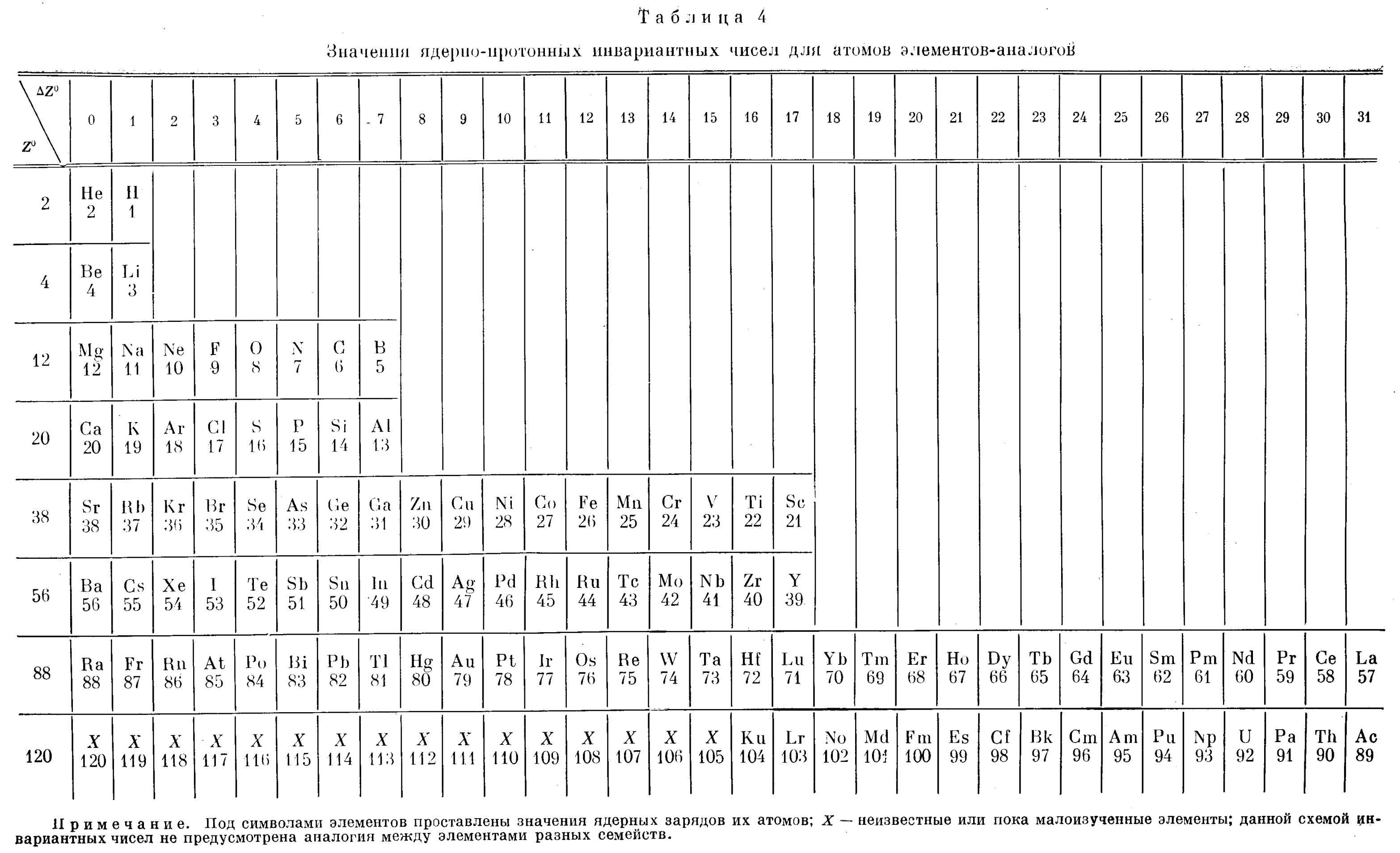
| Year: 1985 | PT id = 383 |
Jodogne's Tableau des Éléments
Jean-Claude Jodogne's Tableau des Éléments. Click here for a full size version:
| Year: 1987 | PT id = 1039 |
Step-Pyramid Form of the Periodic Chart
By Bill (William) Jensen, a Step-Pyramid form of the periodic chart.
This formulation is an updated version of the charts by Thomsen (1895) and Bohr (1922) with more elements, including placeholders up to 118, electronic configuration lables, etc. Read more on the Science History Institute website.
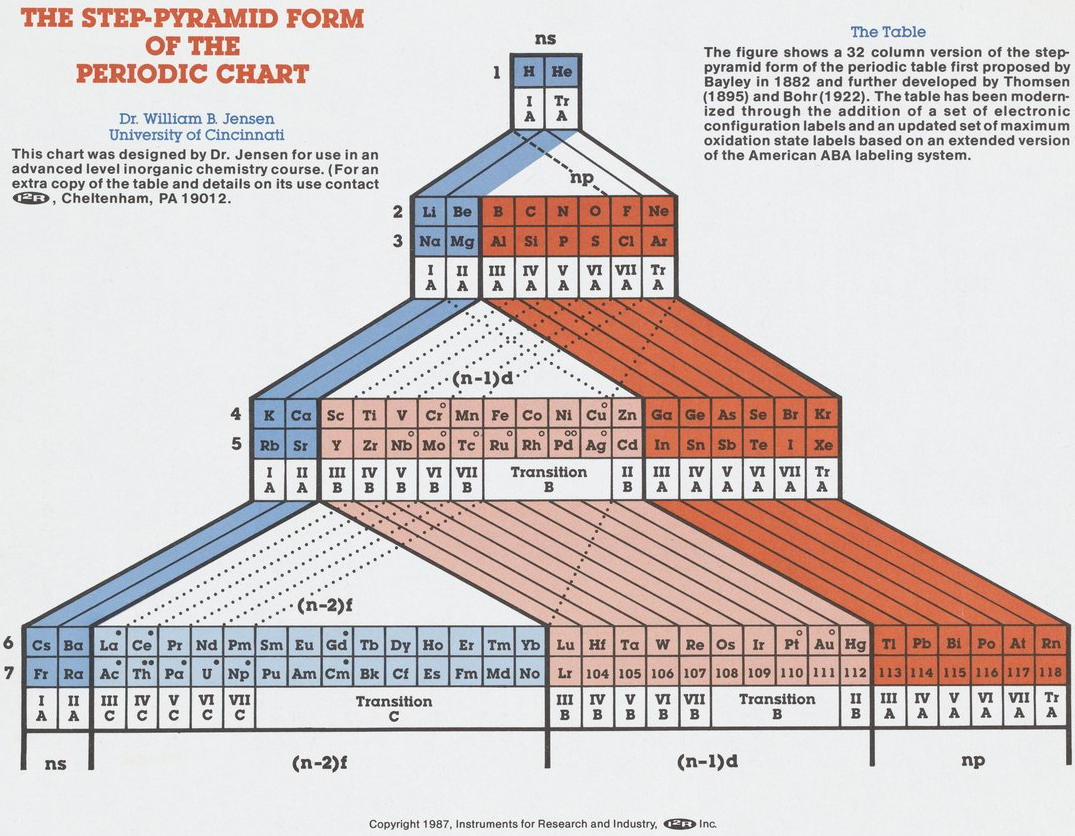
Thanks to René for the tip!
| Year: 1987 | PT id = 1115 |
Variation of Orbital Radii with Atomic Number
From Jour. Fac. Sci., Hokkaido Univ., Ser. IV. vol. 22, no. 2, Aug., 1987, pp. 357-385, The Connection Between the Properties of Elements and Compounds; Mineralogical-Crystallochemical Classification of Elements by Alexander A. Godovikov & Yu Hariya.
The analyses of the variations of the orbital atomic radii values (rorb) with the increase of the atomic number (Z) allow establishment of the following recurring regularities of their change:
Click image below to enlarge:
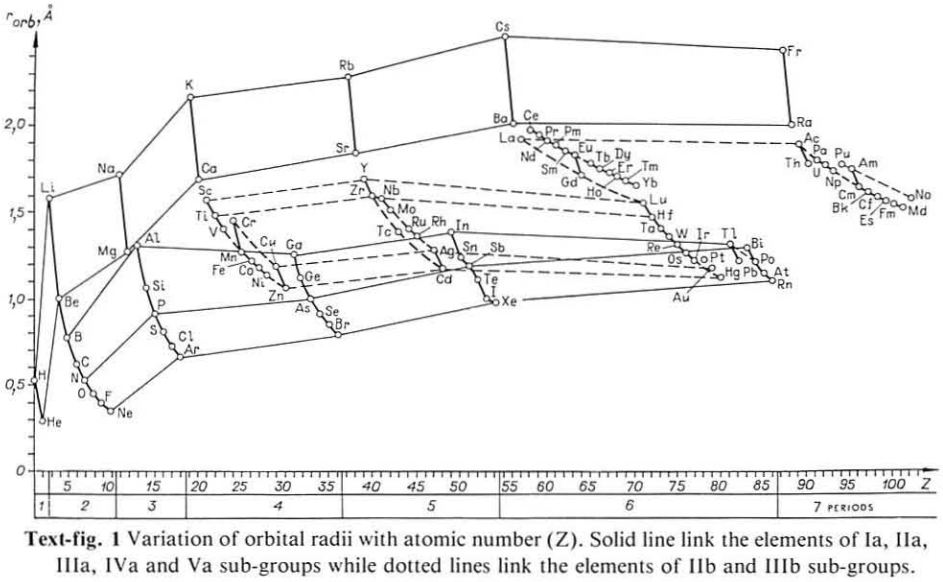
Thanks to René for the tip!
| Year: 1987 | PT id = 1116 |
Mineralogical-Crystallochemical Classification of Elements
From Jour. Fac. Sci., Hokkaido Univ., Ser. IV. vol. 22, no. 2, Aug., 1987, pp. 357-385, The Connection Between the Properties of Elements and Compounds; Mineralogical-Crystallochemical Classification of Elements by Alexander A. Godovikov & Yu Hariya.
Any mineralogical-crystallochemical classification of elements must provide answers to the following queries:
- Which type of compounds certain elements will prefer to form under given conditions of mineral genesis (elementary substance, chalcogenide, oxide, oxysalt, etc.,)
- Whether the element will play a role of a cation or anion of a certain valency
- Which type of chemical bond the resulting mineral compound will have
Click images below to enlarge:
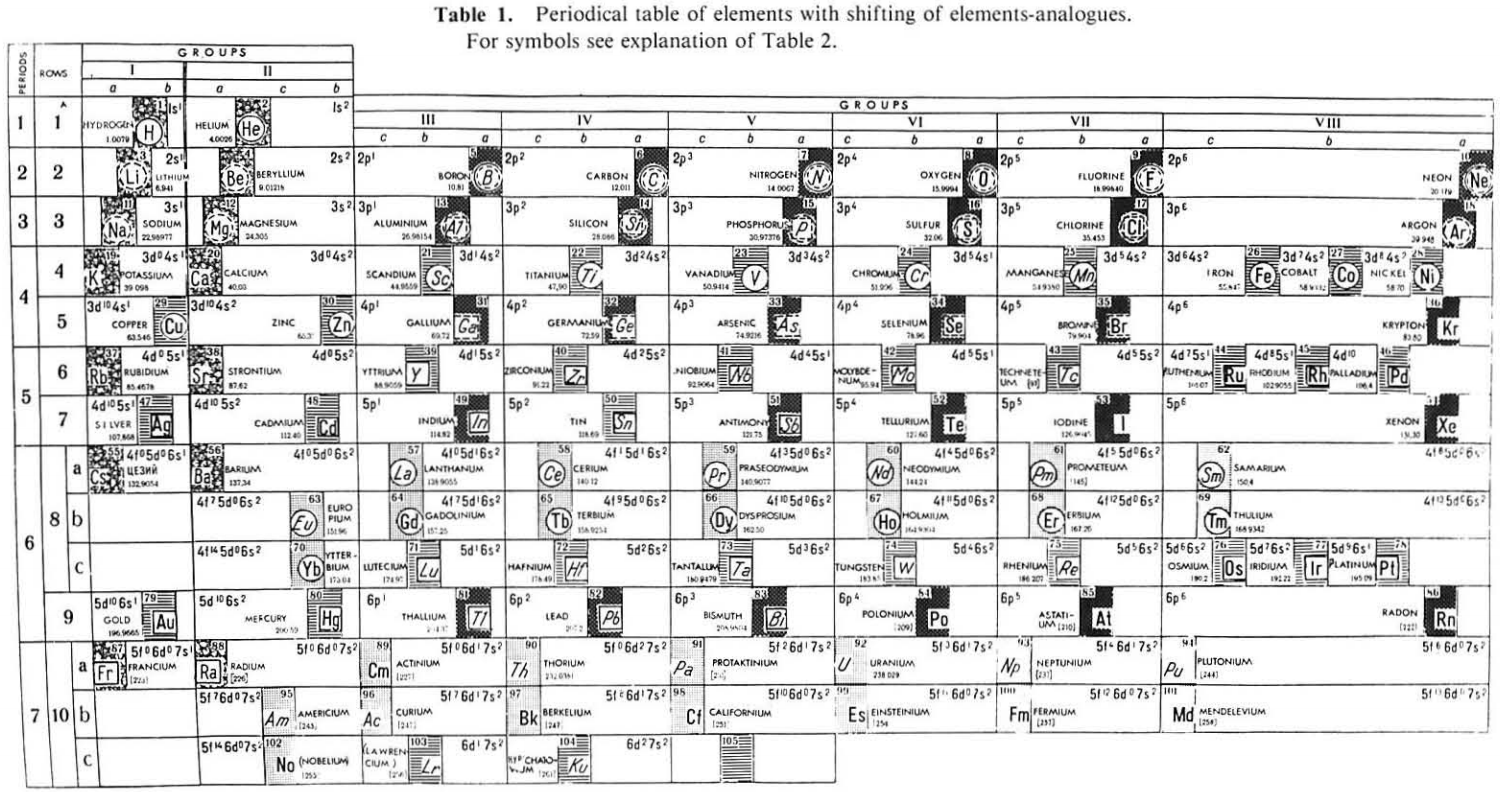
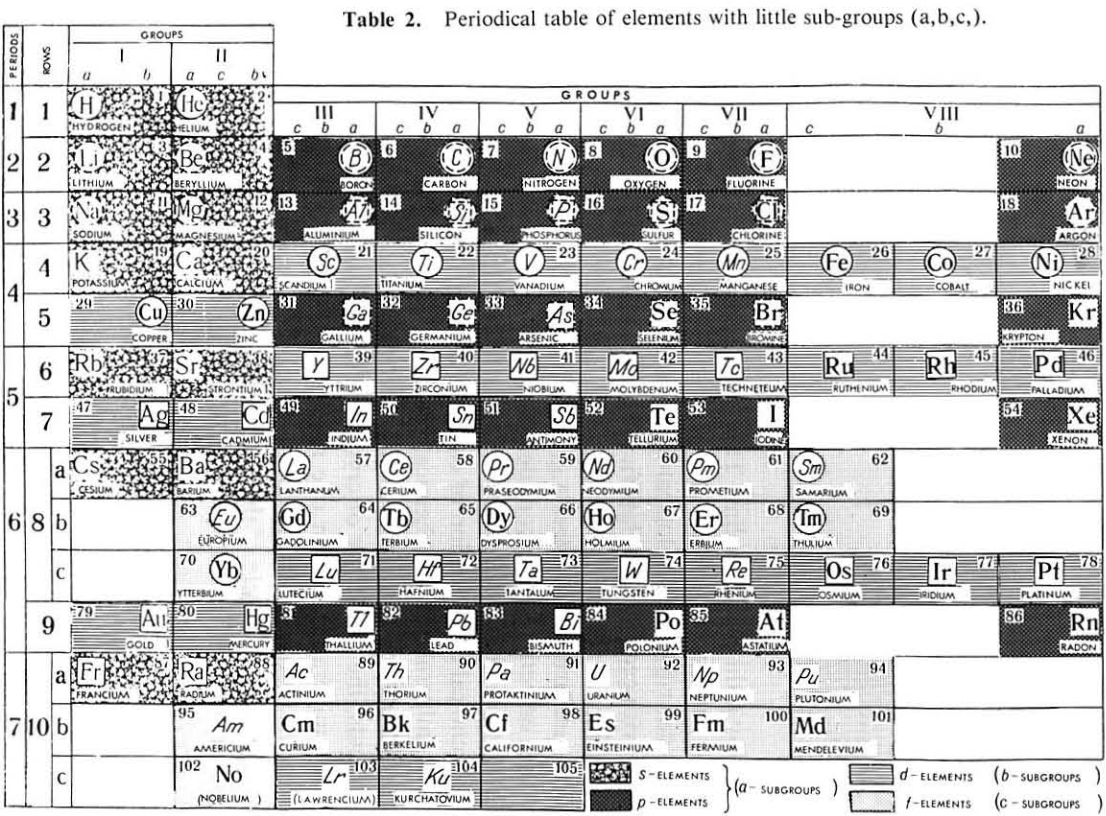
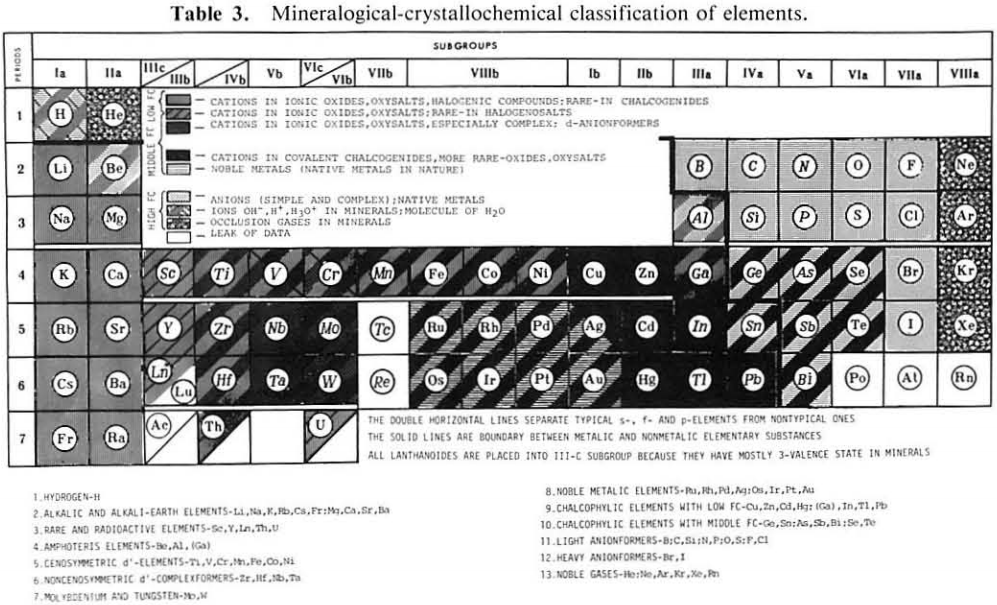
Thanks to René for the tip!
| Year: 1988 | PT id = 52 |
Click here for the full size version, and here for a discussion about this formulation.
| Year: 1989 | PT id = 38 |
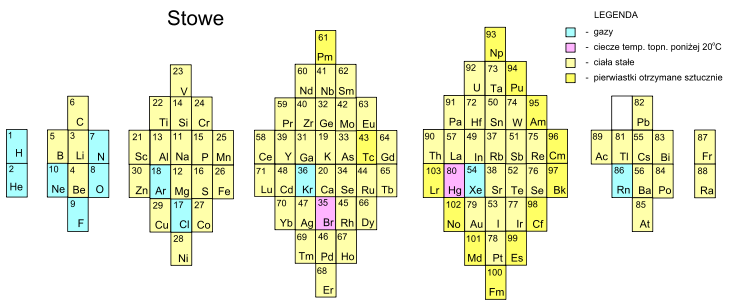
Stowe's A Physicist's Periodic Table
The Physicist's Periodic Table by Timothy Stowe is a well know formulation for those interested in such things, but for a long time its origin was been lost. Eric Scerri has rediscovered the original formulation: a 1989 publication by the company Instruments Research and Industry (I2R) Inc:
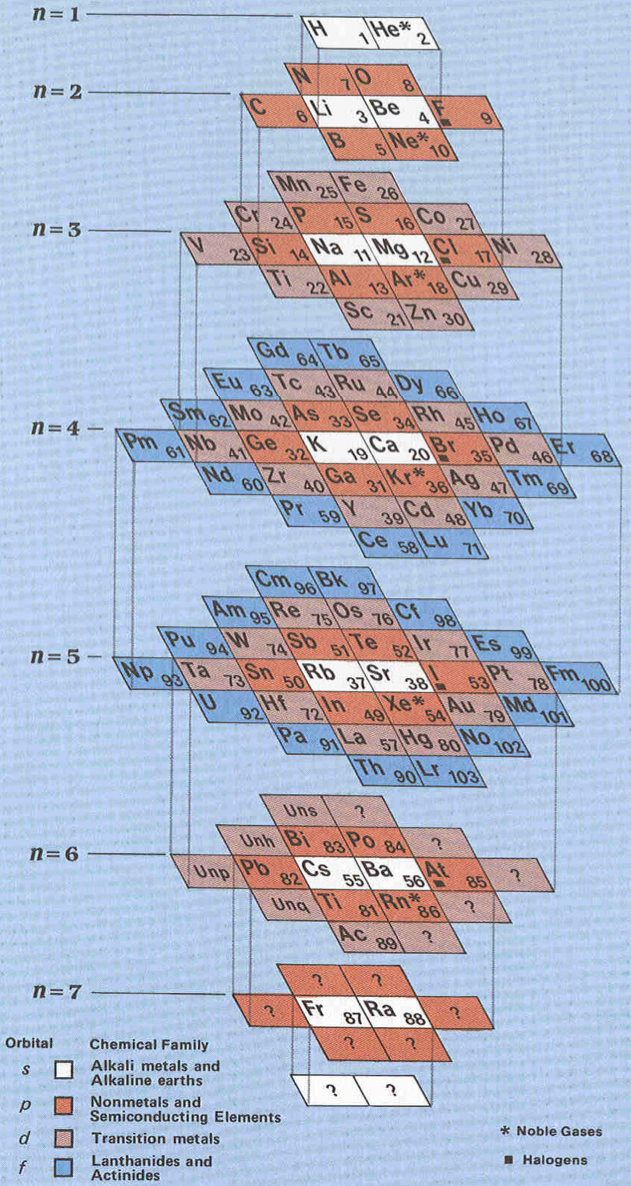
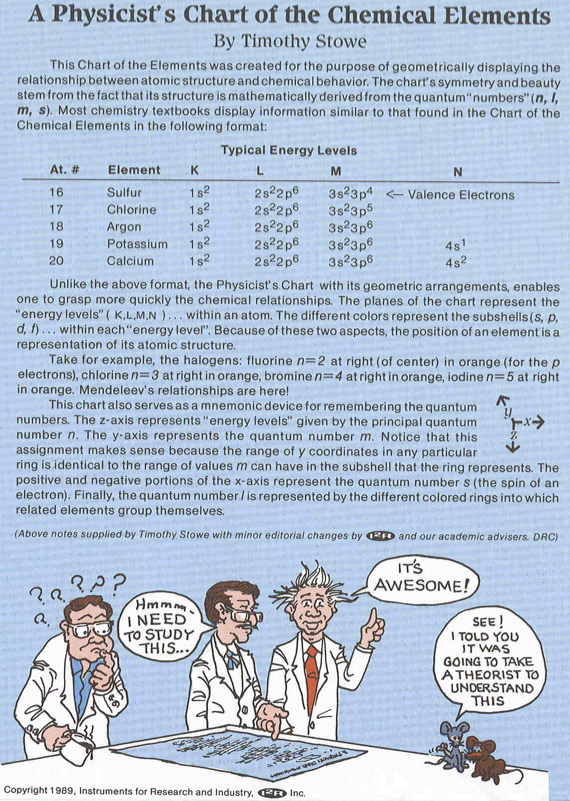
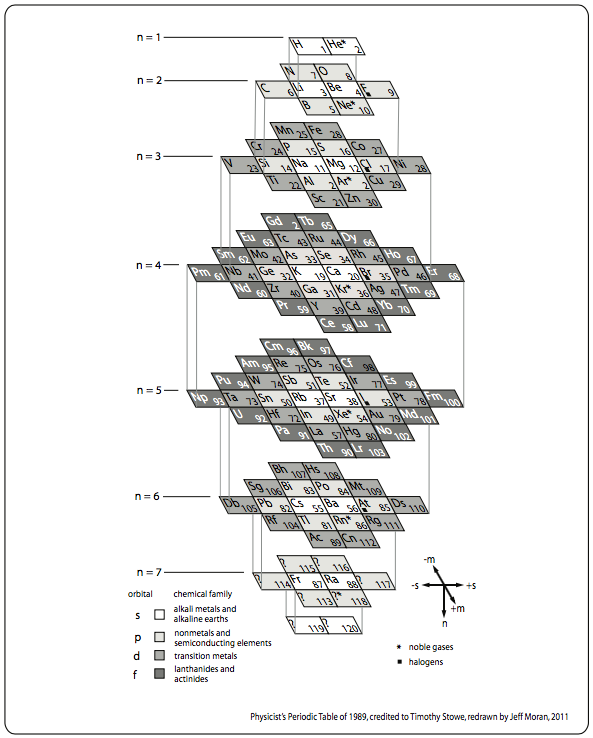
From Wikipedia, this Stowe Format Periodic Table is Based on a graphic from Scholten J."Secret Lanthanides", 2005, ISBN 90-74817-16-5;
Eric Scerri has developed an updated version of the Stowe formulation, here.
| Year: 1989 | PT id = 222 |
Electron Shell Periodic Table
A modified form of a periodic table showing known and predicted electron shells.
From G.T. Seaborg, Lawrence Berkeley National Laboratory, 1989. From the Encyclopedia Britanica website:
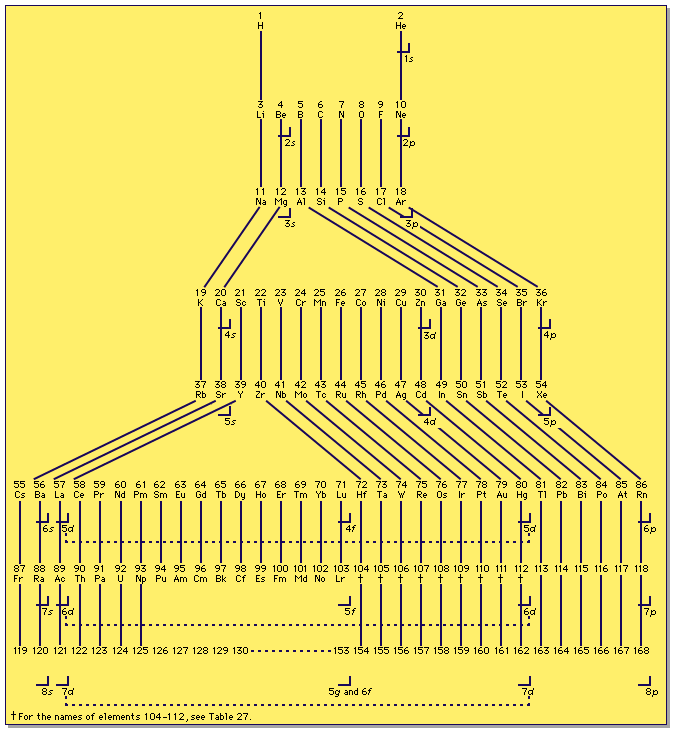
| Year: 1989 | PT id = 463 |
Laing's Modification of The Periodic Table of the Chemical Elements
Laing's modification of the periodic table. This arrangement has the lanthanide series (La to Lu) deliberately aligned with La below Y in Group 3 and with Ce below Zr in Group 4.
This places Pm below Tc, thus linking their common non-existence in Nature.
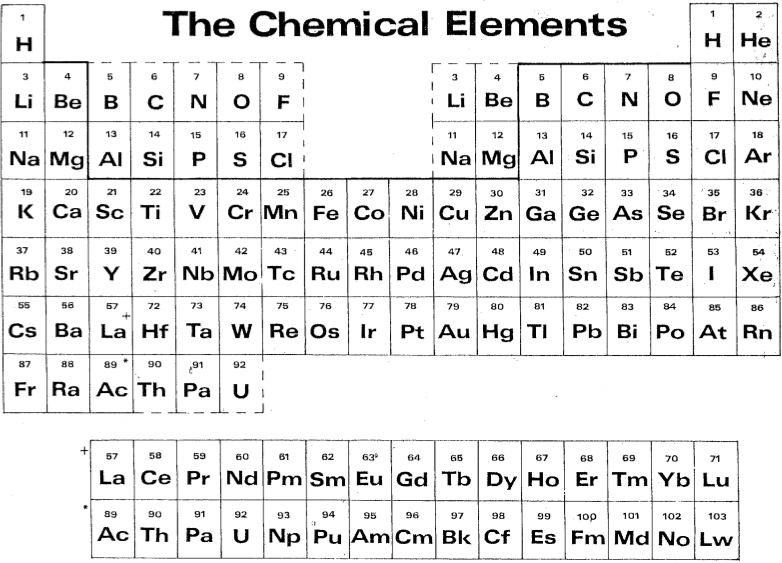
From Michael Laing's paper: A Revised Periodic Table with the Lanthanides Repositioned, Found. Chem. (2005) 7: 203–233
| Year: 1990 | PT id = 39 |
Dufour's Periodic Tree
The Dufour Periodictree periodic table formulation, from here:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1990 | PT id = 436 |
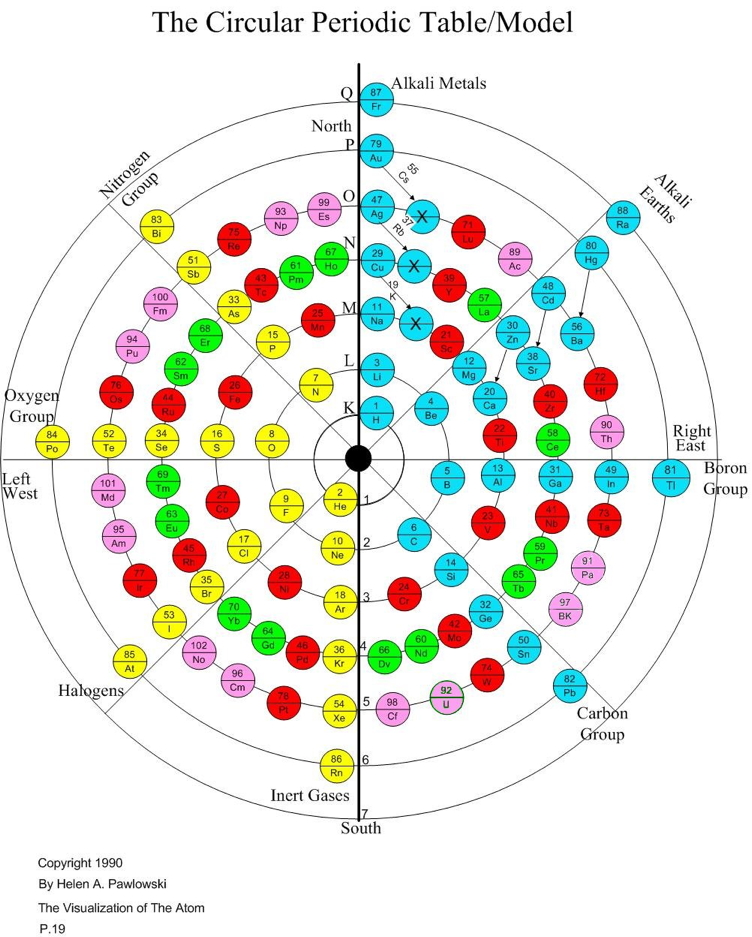
Pawlowski Circular Periodic Table
On John Pratt's website there is an article that is both an introduction to Helen Pawlowski's model of the atom and to her Circular Periodic Table, as well as a book review of her book The Visualization of the Atom (Riverton, UT: Pawlowski Family Trust, 1990). First Helen and her work are introduced, then the model's strengths and weaknesses are summarized:
| Year: 1990 | PT id = 716 |
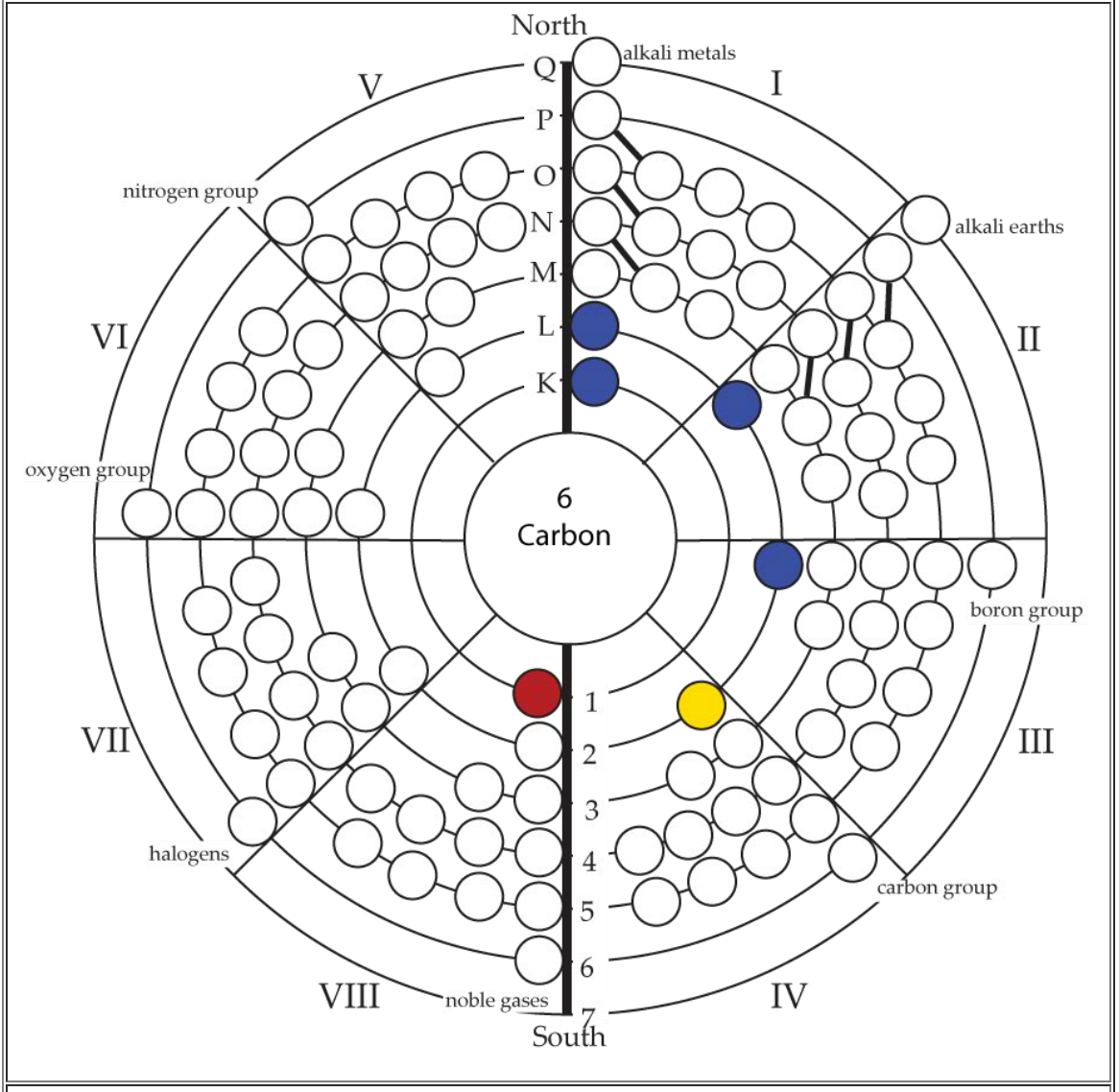
Circular Model of the Atom: Opposition in the Elements
The Circular Model of the Atom is a circular periodic table that shows atomic structure in addition to periodicity. Unlike any other periodic table or model, it demonstrates that the atomic structure has an inherent dipole magnet that create positve and negative fields and elemental qualities at the atomic level.
The Circular Model of the Atom was created by Helen A. Pawlowski in the 1980s, and published in her work, Visualization of the Atom.
Her brother, Paul A. Williams extended many of Helen's ideas with his examination of the standard model using Helen's Circular Atom Model. This website contains some of Helen's ideas and Paul's writings.
| Year: 1992 | PT id = 935 |
Fet's Periodic Tables
Two periodic tables by A.I. Fet from his book, "Mathematical Modeling in Biology and Chemistry. New Approach" Nauka, Sib.Dep., 1992.
Larry Tsimmerman writes:
"First formulation, Tab. 7, is precursor of Adomah PT with broken Z-sequence and questionable pairing of elements in accordance with "ml". Tab. 8 is a Janet LST shown vertically. Fet discusses Periodic Table in the light of Group Theory. (The book was sent to me by Eric Scerri and it was signed by Fet for Hefferlin)."
| Year: 1992 | PT id = 1045 |
Chemical Slide Rules
The first chemical slide rules are of interest here because they are, in effect, early periodic tables. But the are more than this, as they can be used for performing chemical calculations. Writing in Bull. Hist. Chem. 12 (1992) (and here), William D. Williams of Harding University writes:
"An article by George Bodner in the Winter 1990 issue of the Bulletin described a rare chemical slide rule designed by Lewis C. Beck and Joseph Henry - their little-known Improved Scale of Chemical Equivalents. [My] paper attempts to place this slide rule in context by describing its origins, as well as some of its predecessors and successors."
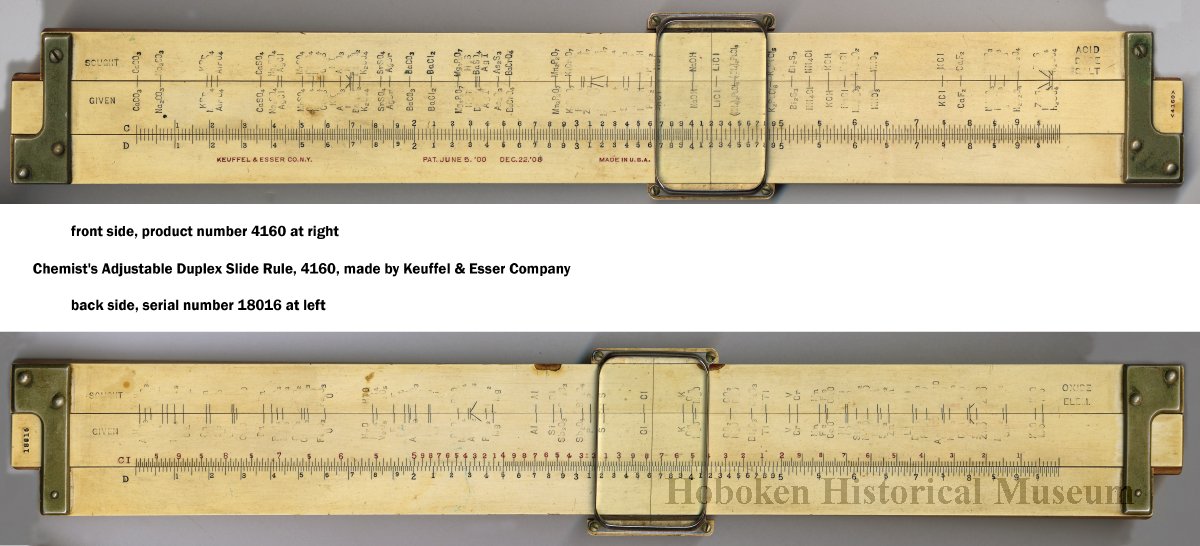
Some chemical slide rules mentioned in the text:
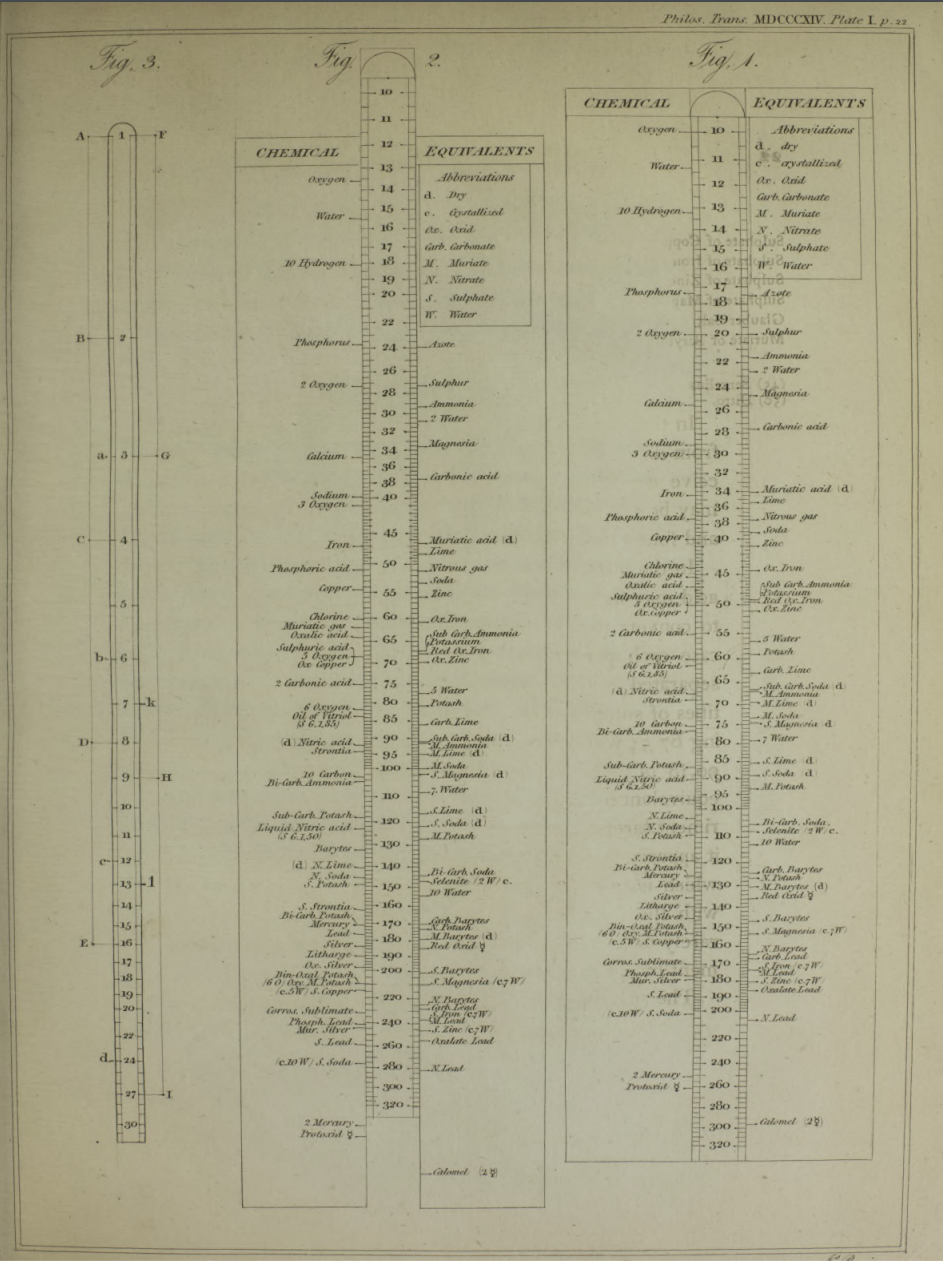
- Chemist's Adjustable Duplex Slide Rule made by Keuffel & Esser Co., n.d., ca. 1936-1940. Here are the full instructions for use.
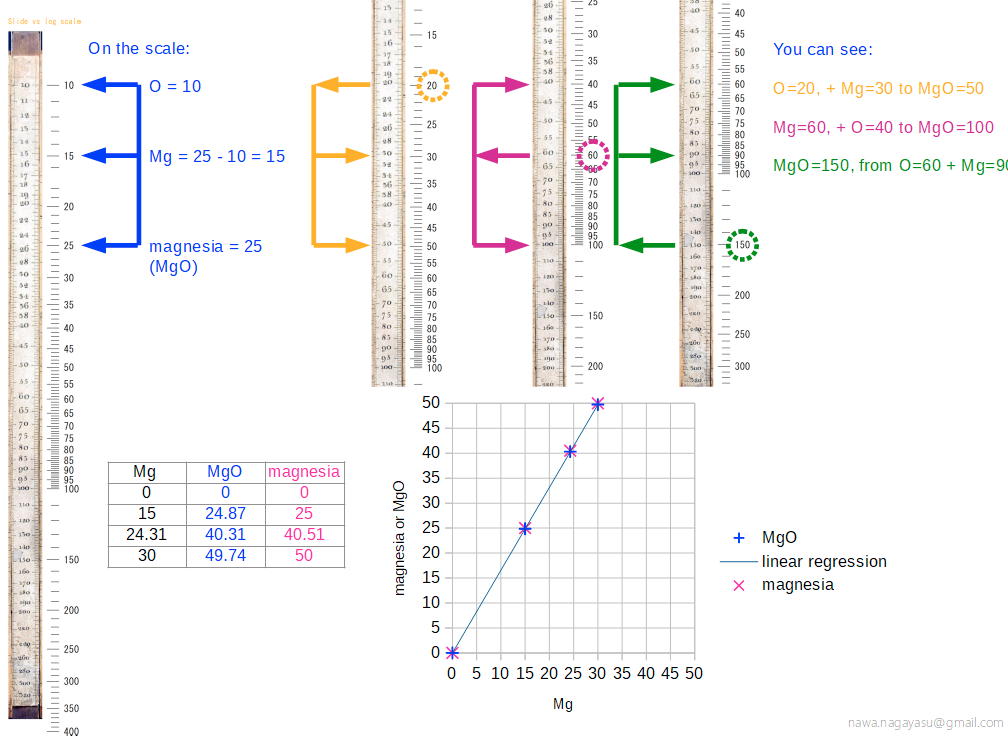
Nagayasu Nawa writes and provides an explanation as how Wollaston's chemical equivalents slide rules should be used:
"It is very interesting slide rule for me. Because we actually used slide rule in 1960s. There were not the electronic calculator in the world. I think it would be used as a simple slide rule of The Law of Definite Proportions by J.L. Proust 1799."
- '10 water', for example, may be hydrating water in chemical compound
- 'Chlorine' may be HClO: HCl(35) + O(10) = HClO(45), etc.
Click image to enlarge:
Thanks to Nawa for the tip!
| Year: 1992 | PT id = 1091 |
Magarshak & Malinsky's Three Dimensional Periodic Table
Y. Magarshak & J. Malinsky's Three Dimensional Periodic Table from Nature, 360, 114-115 (1992).
M&M say:
"We believe that our three dimensional representation is a useful tool for visualizing properties of chemical elements and is in complete accord with quantum mechanics."
Thanks to René for the tip!
| Year: 1993 | PT id = 905 |
Chemistry Imagined: The Periodic Table
From Roald Hoffmann & Vivian Torrence's book, Chemistry Imagined: Reflections of Science, a picture entitled The Periodic Table:

Thanks to Marcus Lynch for the tip!
| Year: 1993 | PT id = 1268 |
Huheey's Version of The Madelung Rule (For Orbital Filling)
Huheey, J.E., Keiter, E.A., Keiter, R.L.: Inorganic Chemistry: Principles of Structure and Reactivity. 4th edn. HarperCollins College Publishers (1993), p. 22
René Vernon comments: "A peculiar depiction of the Madelung Rule order of filling diagram."
| Year: 1994 | PT id = 18 |
Fathi Habashi aruges in Chemistry in Education (1994) that aluminium, Al, should be placed above scandium and next to magnesium. There is more information about this formulation here:
| Year: 1994 | PT id = 890 |
Discovery of Darmstadtium
Ds ![]()
Darmstadtium, atomic number 110, has a mass of 281 au.
Synthetic radioactive element.
Darmstadtium was first observed in 1994 by S. Hofmann et al.
| Year: 1994 | PT id = 891 |
Discovery of Roentgenium
Rg ![]()
Roentgentium, atomic number 111, has a mass of 280 au.
Synthetic radioactive element.
Roentgenium was first observed in 1994 by S. Hofmann et al.
| Year: 1994 | PT id = 1016 |
f-Block Elements 3D Periodic Table
From conference in Helsinki on the f-Block Elements to commemorate the bicentennial of Johan Gadolin's 1794 analysis of Yittria.
Pekka Pykkö writes to say:
"We used [this formulation] in Helsinki in 1994 on the cover of ICFE-2 conference proceedings. Who invented it or where it was copied from, I do not know. Anyway, all the hundreds of participants received it from us":
Claude Piguet's paper, Chimia 73 (2019) 165–172, also uses this 3D version of the standard periodic table. The text says: "Periodic table highlighting the location of Rare Earths (red elements). The elements shown in blue correspond to the actinide series":
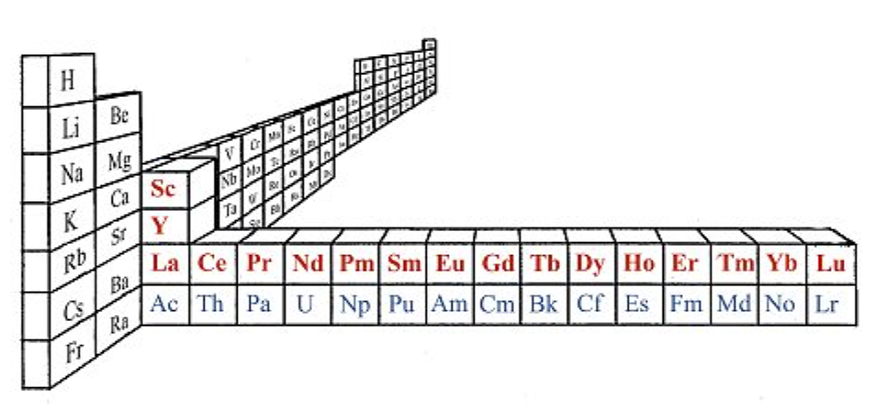
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1994 | PT id = 1159 |
Treplow's Periodic Table of The Atoms
R.S. Treplow, J. Chem. Educ. 1994, 71, 12, 1007: The Periodic Table of Atoms: Arranging the Elements by a Different Set of Rules.
"Although periodic tables differ greatly in their appearance, examination shows they are all designed according to a common set of conventions. This paper reviews those conventions and asks how the table would look under a different set of rules."
Ground-state multiplicity vs. atomic number for elements 1 to 103. Subblocks are labeled S, P, D & F. Lines connecting the dots show the "ideal" pattern. Atoms not on the lines are "nonideal" (where ideal refers to Madelung's rule):
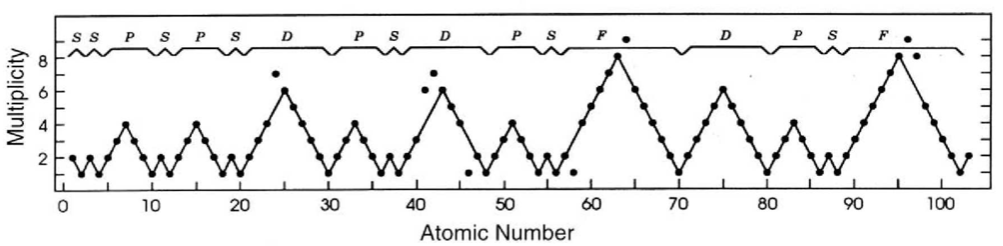
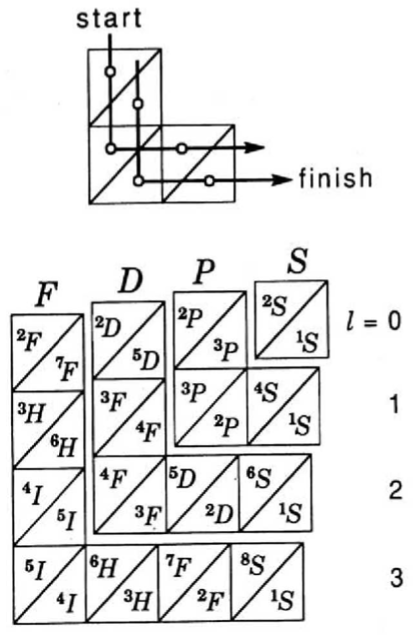
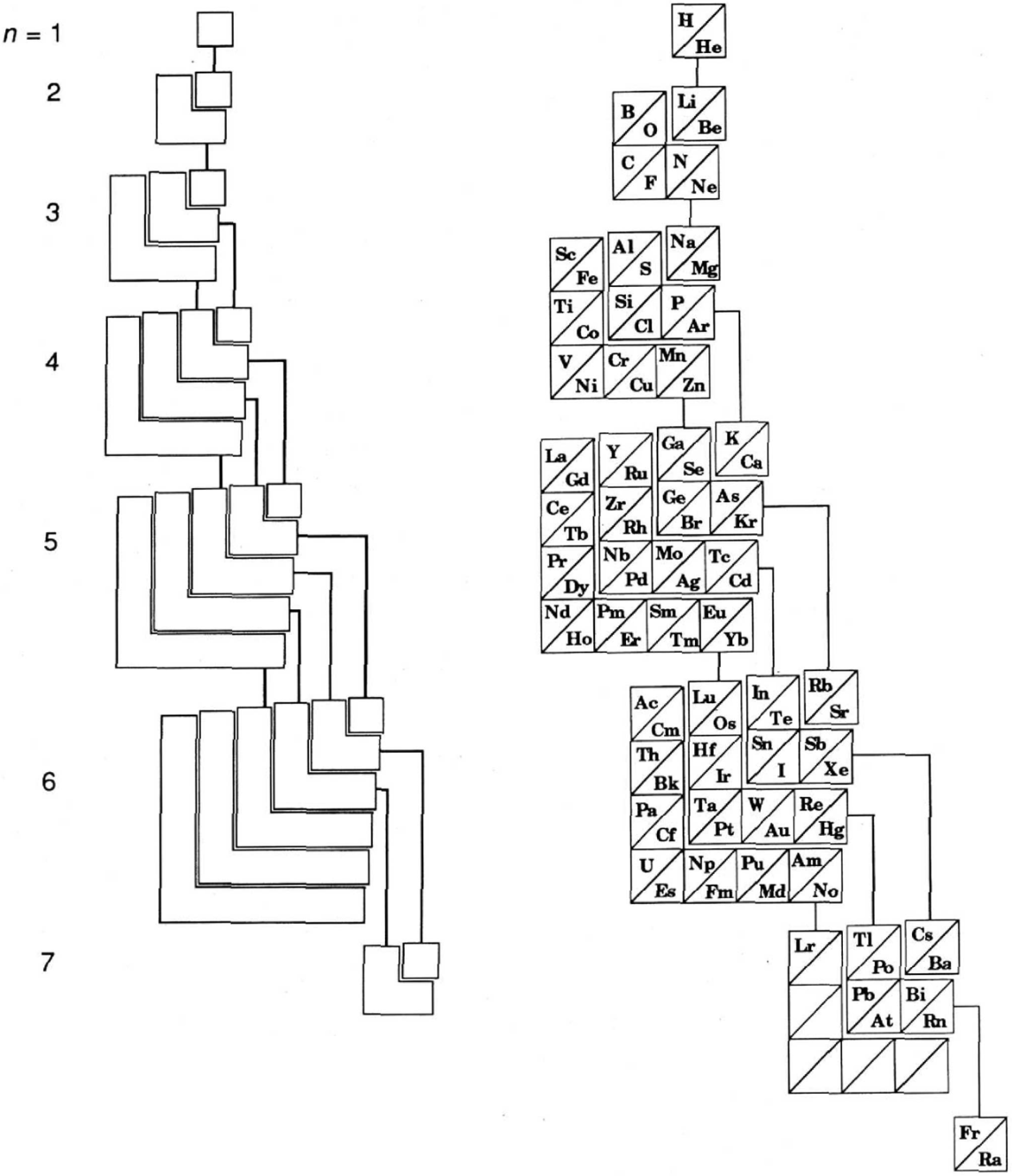
Thanks to René Vernon for his help.
| Year: 1995 | PT id = 45 |
Helical Periodic Table
Tarquin Publications sell a make-your-own three dimensional, helical periodic table.
| Year: 1995 | PT id = 241 |
Melinda Green's Periodic Fractal of The Elements
Melinda Green writes: "This is an alternative version of the standard chemistry Periodic Table of the Elements that I developed. In high school I learned the basic concept of element families and how they were arranged into columns to show the periodicity in their electrical properties. I was fascinated with the idea, but immediately wondered whether there might be better ways of graphically showing those relationships." read lots more here
| Year: 1995 | PT id = 1130 |
Klein's Periodic Table of The Elements
Klein DJ, Similarity and Dissimilarity in Posets, Journal of Mathematical Chemistry, 18(2), 321–348 (342) (1995)
The relevance of partially ordered sets (or posets) in a wide diversity of contexts in chemistry is emphasized, and the utility of distance functions (or metrics) on such posets is noted. First a notion of "scale similarity" is introduced to make comparisons within certain so-called "scaled" posets, for which there is formulated natural "comparators", which in turn lead to associated distance functions. Beyond taking note of several chemically relevant examples of these "scaled" posets and their consequent associated similarity measures, a second chemically relevant class of so-called "shifted" posets is similarly developed, with examples. Even further extension of some aspects of the current approach is indicated, and finally the multi-posetic character of chemical periodic law is suggested.
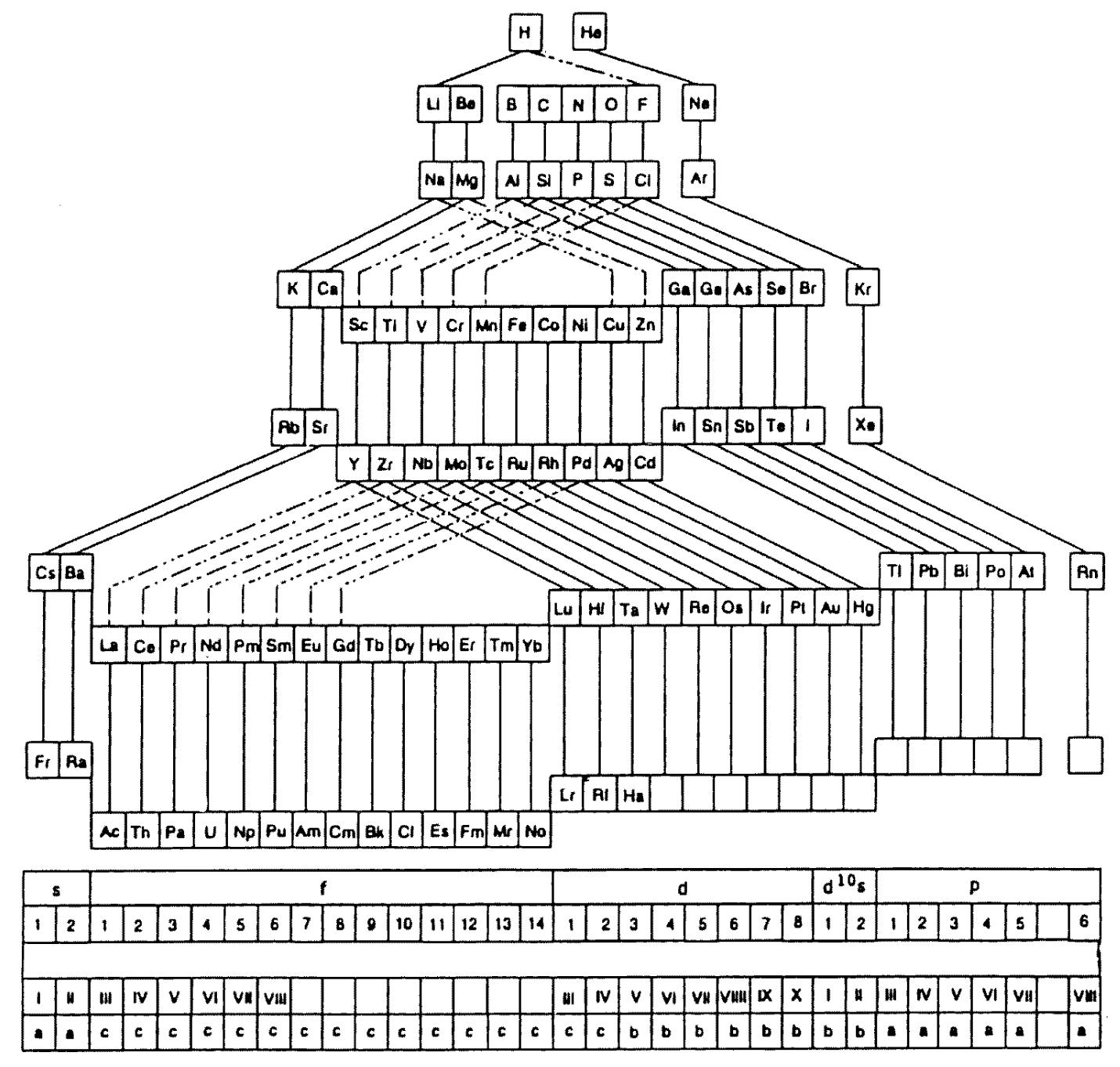
Thanks to René for the tip!
| Year: 1995 | PT id = 1292 |
Considine's Polar Periodic Table
From: Considine DM (ed.) 1995, Van Nostrand’s Encyclopedia of Science, 8th ed. New York, p. 2376
René Vernon writes:
"A nice design but of quite limited practical utility for quick reference or detailed chemical analysis."
| Year: 1996 | PT id = 238 |
First Ionisation Energy of The Elements
Periodic trend for ionization energy, for example Mg → Mg+ + e–
Each period begins at a minimum for the alkali metals, and ends at a maximum for the noble gases. From Wikipedia:
Based on data from: Martin, W. C.; Wiese, W. L. (1996). Atomic, Molecular, & Optical Physics Handbook. American Institute of Physics. ISBN 156396242X.
| Year: 1996 | PT id = 892 |
Discovery of Copernicium
Cn ![]()
Copernicum, atomic number 112, has a mass of 285 au.
Synthetic radioactive element.
Copernicium was first observed in 1996 by S. Hofmann et al.
| Year: 1996 | PT id = 966 |
ChemEasy Table of Periodic Properties of the Elements & more...
From Facebook, the ChemEasy Table of Periodic Properties of the Elements & more:
Thanks to Roy Alexander fo the tip!
| Year: 1997 | PT id = 19 |
Bayley-Thomsen-Bohr Periodic Table
A formulation adapted by Eric Scerri from tables developed by Thomas Bayley, Jørgen Thomsen and Neils Bohr that depicts the symmetrical nature of the periodic law.
Eric Scerri, The Evolution of the Periodic System, American Scientist, November-December issue, 1997, 546-553
| Year: 1997 | PT id = 345 |
Doyle Periodic Table of The Elements
| Year: 1997 | PT id = 380 |
Good Periodic Table of The Elements
From the Good Periodic Table website:
"The Geometric Organisation Of Dimension, aka 'G.O.O.D', Periodic Tables primary function acts as an identifier of relationships between like particles of matter. This operates utilising the original Sample process first discovered by Mendeleev; were atoms that are linked in a straight line hold a unique relationship as compared to the rest of the atoms on the table."
| Year: 1997 | PT id = 975 |
Homage to The Elements
Eulalia Bosch writes:
"As a curator of the Eugènia Balcells Foundation, I would like to share with you the project to celebrate the 2019 UN decreed International Year of the Periodic Table (IYPT).
"Eugènia Balcells included the mural Homage to The Elements in her exhibition FREQUENCIES at the Santa Monica Art Center in Barcelona in 2009. The exhibit incorporates the spectrum of light that identifies each element. The result is not just another presentation of the periodic table, but a tribute to the set of elements that, in their intertwining, make up the material world and to those spectra that, as Eugènia Balcells like to say are: 'the voice of matter'.
"Over the last few years, the mural Homage to The Elements has also been incorporated at the Pascual Vila Research and Development Center of the CSIC in Barcelona, at the Science Museum, CosmoCaixa, in Barcelona and we are finishing the formalities for its installation in the Universities of Tarragona and Girona. It has also been acquired by the Technische Universität Berlin, and by the Friedrich-Alexander-Universität Erlangen-Nürnberg, both in Germany. In the city of NY, where Eugènia lived for more than thirty years, the mural has found its place at the Maxine Greene High School for Imaginative Inquiry, located at the Martin Luther King Educational Campus in New York, in front of the Lincoln Center, the great ally artistic ally of the School.
"The Eugènia Balcells Foundation wants to actively collaborate in the celebration proposed by the United Nations offering to the educational world the mural Homage to The Elements, this sign that represents universal unity, and records the human knowledge acquired to this day."

| Year: 1998 | PT id = 246 |
Wheel of Motion Periodic Table
The Wheel of Motion (WoM) representation of the periodic table of elements shows the periodic nature of the elements, as developed in the Reciprocal System of Physical Theory (RST).
It was originally developed by Douglas Bundy in 1998, a member of the International Society of Unified Science (ISUS).
| Year: 1999 | PT id = 36 |
Moran's Spiral Periodic Table
Jeoff Moran's spiral periodic table can be found at periodicspiral.com.
See an article in the New York Times:
| Year: 1999 | PT id = 894 |
Discovery of Flerovium
Fl ![]()
Flerovium, atomic number 114, has a mass of 289 au.
Synthetic radioactive element.
Flerovium was first observed in 1999 by Y. Oganessianet et al.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.