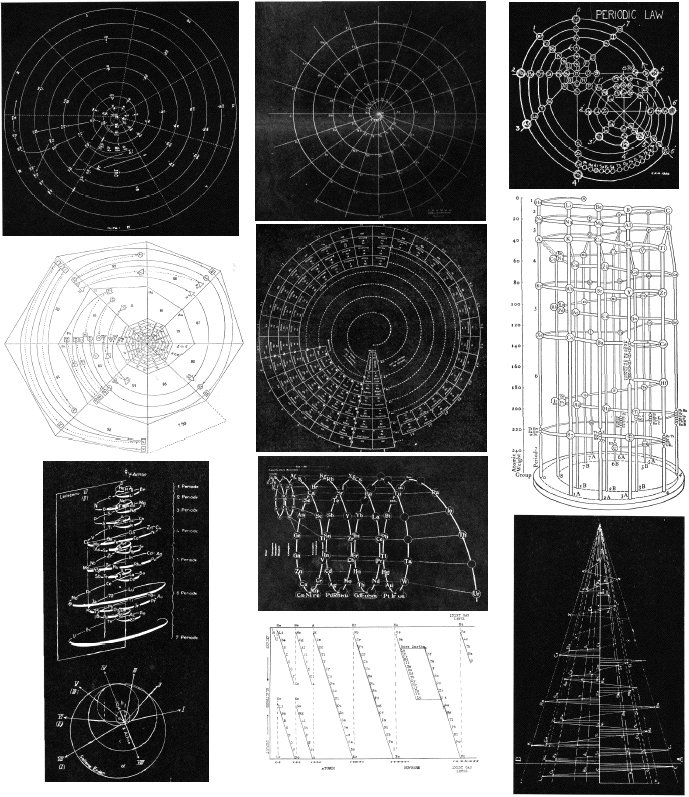
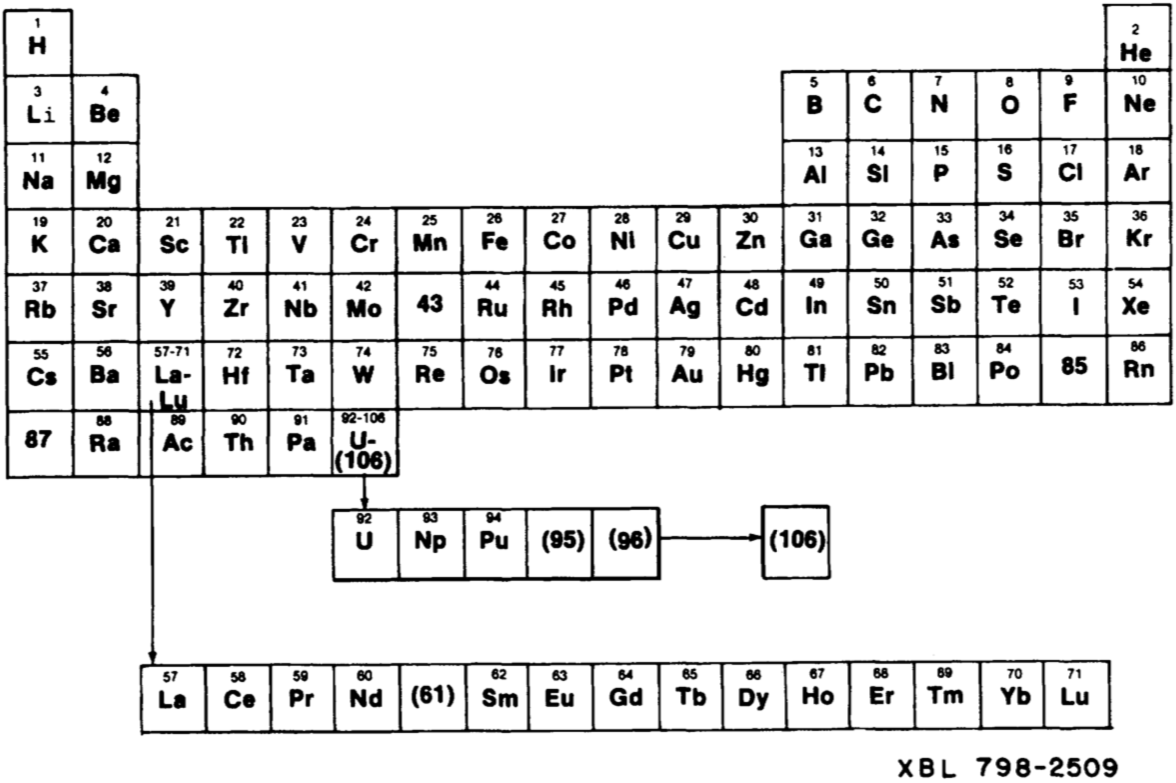
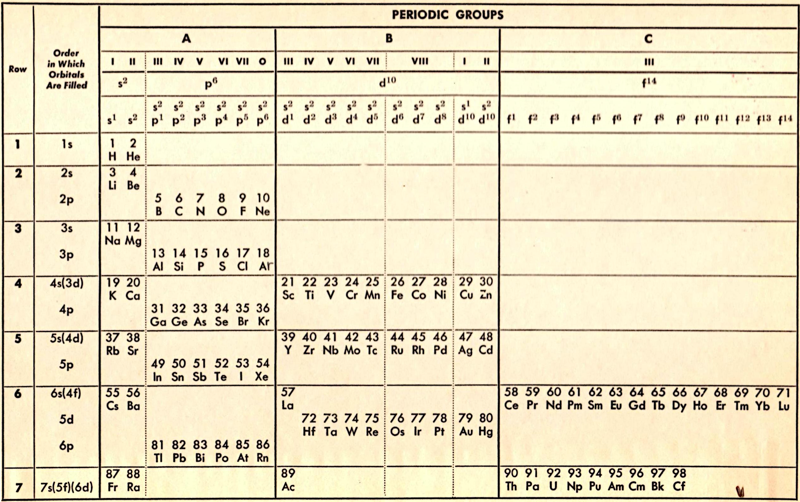
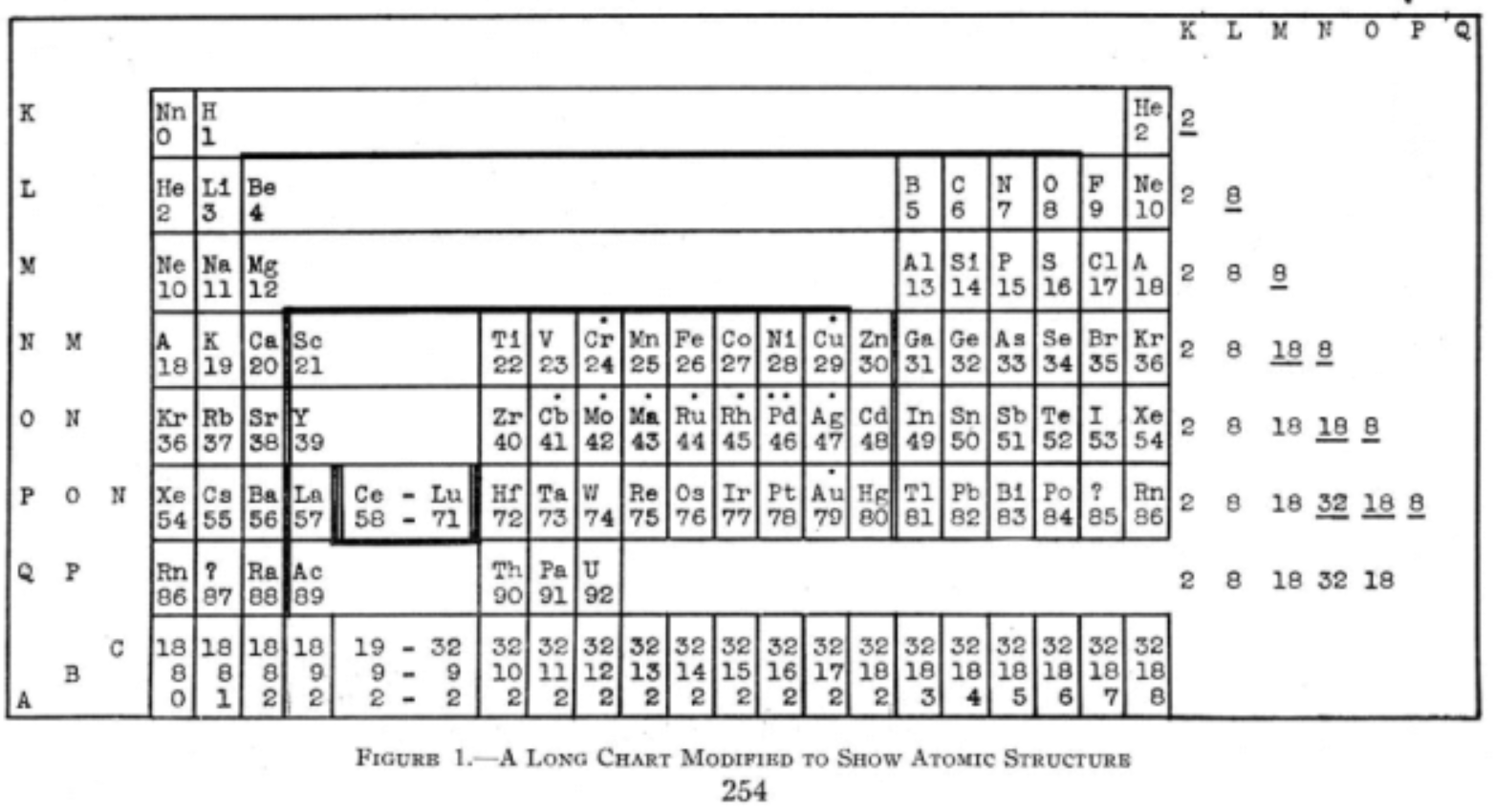
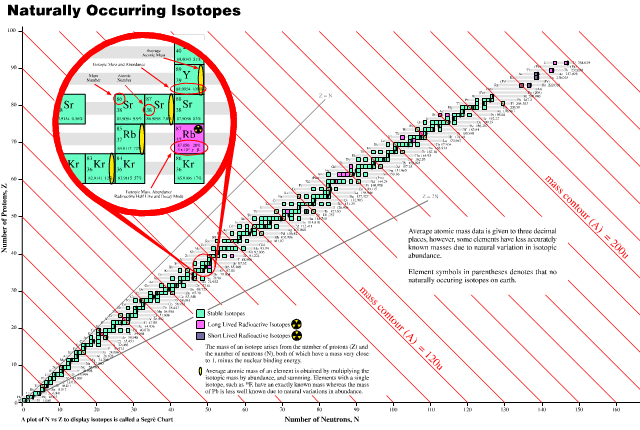
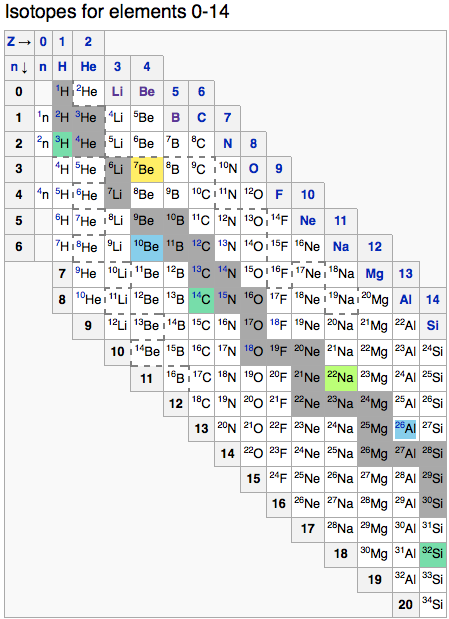
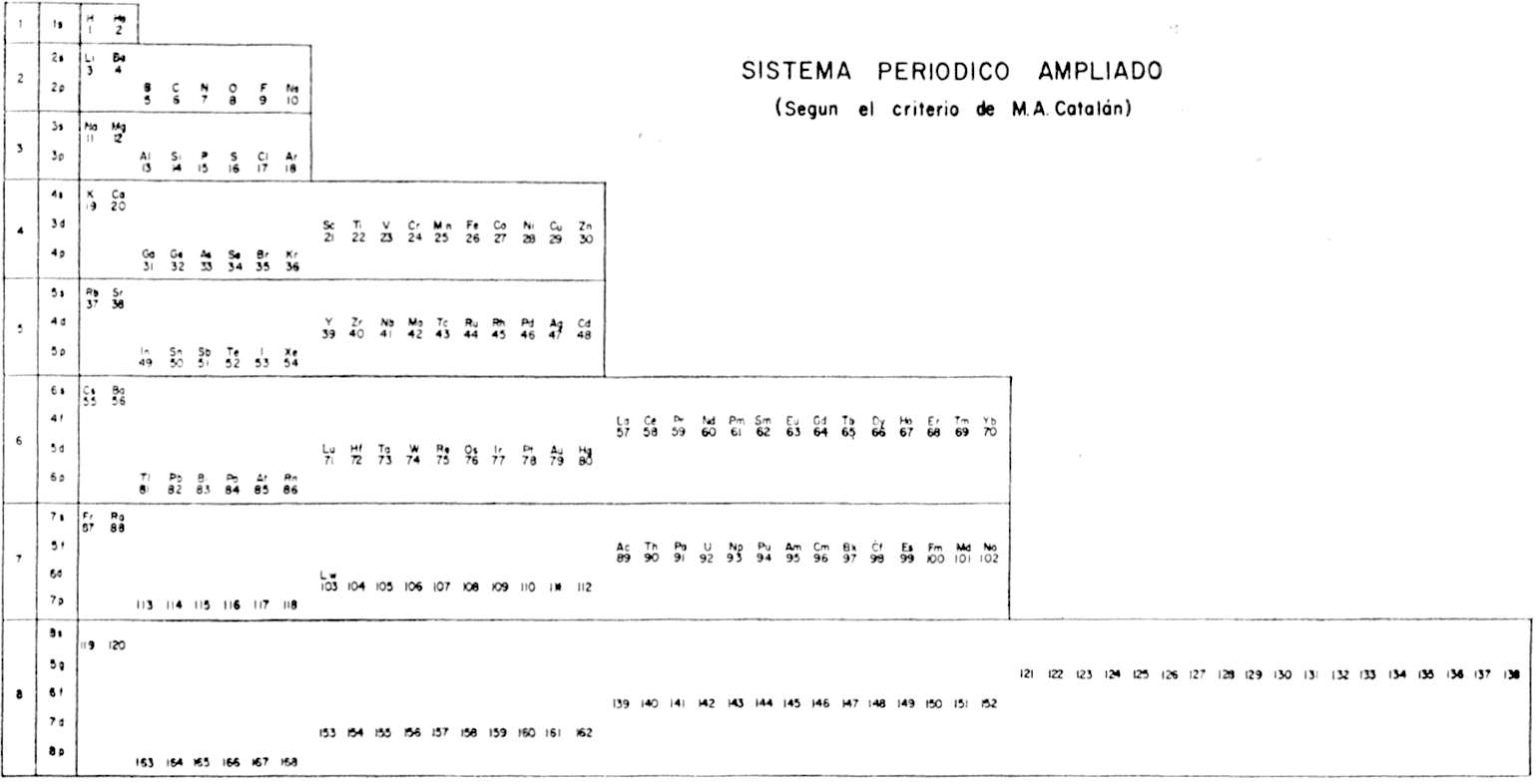
Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the years 1900 - 1949, by date:
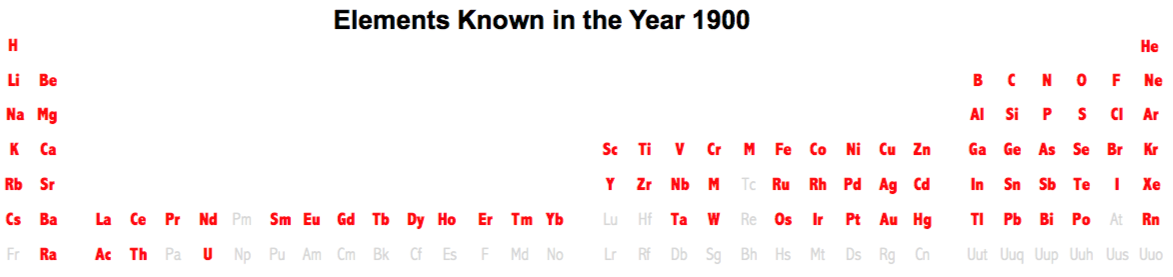
| Year: 1900 | PT id = 236, Type = formulation |
Elements Known in the Year 1900
Elements known in the year 1900, taken from this Wikipedia page:
| Year: 1900 | PT id = 1284, Type = formulation data element review |
History of the Discovery of the Group 18 (erstwhile Group 0) Elements
John Marks has provided a concise history of the discovery of the Group 18 elements and the element name"Nitron/Radon".
Radioactivity was discovered by Becquerel in 1896 and the Curies noted transferred radioactivity rather like the induction of electric or magnetic charge. Radon was discovered in 1900, by Dorn in Halle; Rutherford discovered thoron in 1899; and Debierne discovered actinon in 1903. The time-line is:
- 1868 Lockyer observed the spectrum of helium in the solar corona
- 1894 Ramsay discovers argon
- 1895 Ramsay isolates helium
- 1898 Ramsay discovers krypton, neon & xenon
- 1899 Curie observes an emanation from radium
- 1899 Rutherford observes an emanation from thorium
- 1900 Dorn identifies radon
- 1902 Rutherford & Soddy characterize thoron
- 1903 Rutherford & Soddy isolate radon
- 1903 Debierne observes an emanation from actinium
- 1904 Ramsay names the isotopic emanations exactinio, exradio & exthorio and surmises they are one element, probably an inert gas
- 1908 Professor Sydney Young’s "Stoichiometry" has a periodic table shows niton, Z = 86
- 1909 Ramsay characterizes niton as a group 0 inert gas
- 1910 Cameron's "Radiochemistry" describes the radioactive displacement law
- 1912 The name "niton" accepted by the International Commission for Atomic Weights
- 1913 Soddy expounds theory of isotopes
- 1913 Rydberg's periodic table has Nt (86) for the last inert gas
- 1919 Irving Langmuir's PT has Nt as the last inert gas
- 1922 Niels Bohr’s PT has Nt (86) as the last inert gas
- 1923 GN Lewis’s PT has Nt as the last inert gas
- 1924 CRC’s Handbook of Chemistry and Physics has niton as the last member of Group 0
So niton (from Latin nitens = shining) was noticed by the Curies in 1899 as an emanation from radium. That same year Rutherford noted an identical emanation from thorium, and in 1903 Debierne discovered the same emanation from actinium. All three ('radon', 'thoron' and 'actinon') were identified as an element by Ramsay in 1904 and characterized by him in 1909.
Ramsay named the element niton after its most prominent property viz. that it glowed in the dark.
With the introduction of Soddy's isotopes, it became clear that: thoron was Nt-220, radon was Nt-222 & actinon was Nt-219.
There are natural traces of other isotopes (e.g. Nt-217, Nt-218) from beta disintegration of astatine. So "radon" was just one isotope of niton.
The foregoing history of niton is uncontroversial and the name niton, Nt, for Z = 86 dates at least from Professor Young´s textbook of stoichiometry in 1908.
In 1912, the name 'niton' was adopted by the International Commission for Atomic weights. Rydberg's PT of 1913 has Nt as the last inert gas, as does Irving Langmuir's PT of 1919, Niels Bohr's PT of 1922, GN Lewis's PT of 1923 and even the CRC's Handbook of Chemistry and Physics in 1924.
John Marks concludes:
"Niton, Nt, for Z = 86, was thus established by its discoverers and accepted by the chemistry (and physics) establishment. Radon, Rn, is an error perpetuated by IUPAC [amongst its many sins].
"Radon is an isotope. We do not refer to hydrogen as 'protium', so why are we referring to niton as 'radon'?"
| Year: 1901 | PT id = 843, Type = element |
Discovery of Europium
Eu
Europium, atomic number 63, has a mass of 151.964 au.
Europium was first observed or predicted in 1896 by E.-A. Demarçay and first isolated in 1901 by E.-A. Demarçay.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
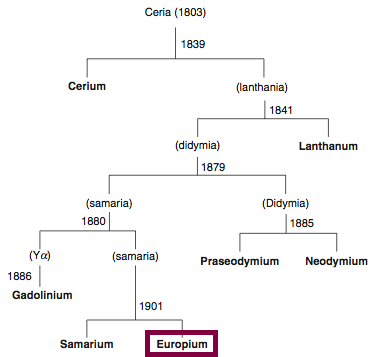
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1902 | PT id = 58, Type = formulation |
Brauner's Table
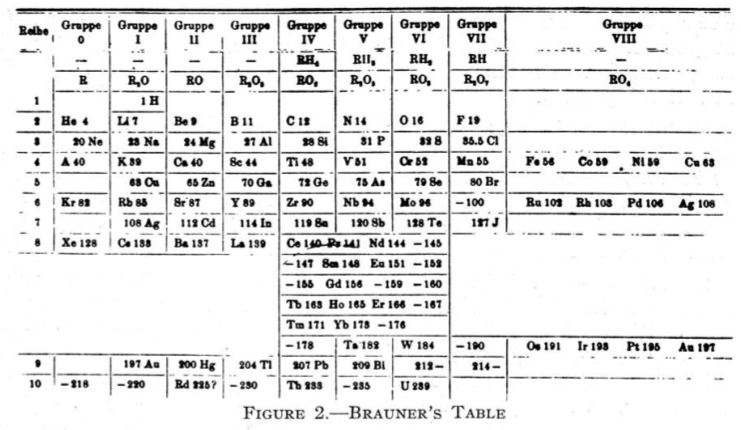
From Quam & Quam's 1934 review paper.pdf
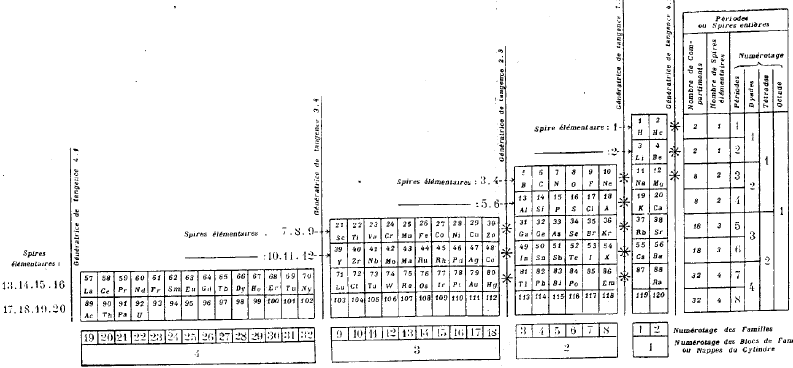
| Year: 1902 | PT id = 71, Type = formulation spiral |
Erdmann's Spiral Table
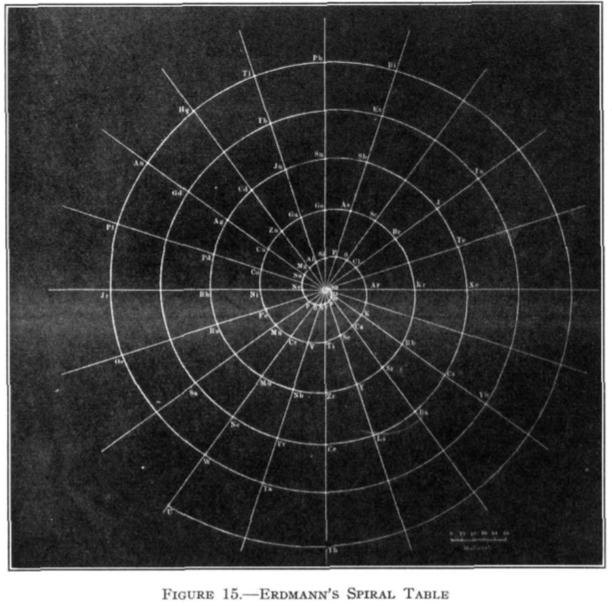
From Quam & Quam's 1934 review paper.pdf
| Year: 1902 | PT id = 365, Type = formulation |
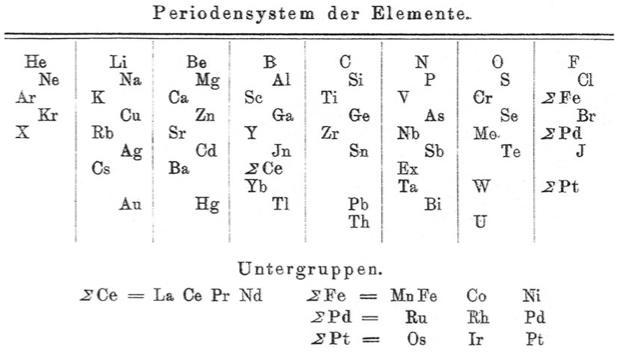
Blitz's Periodensystem der Elemente
Periodic Table of Biltz (1902) with an intraperiodic accommodation of the rare earths. Reproduced from Biltz, H., 1902. Ber. 35 (562), 4242:
| Year: 1902 | PT id = 869, Type = element |
Discovery of Actinium
Ac ![]()
Actinium, atomic number 89, has a mass of 227 au.
Radioactive element.
Actinium was first isolated in 1902 by F. O. Giesel.
| Year: 1904 | PT id = 366, Type = formulation |
Benedicks' Periodic Table
Periodic Table of Benedicks (1904) with an intraperiodic accommodation of the rare earths. Reproduced from Benedicks, C., 1904. Z. Anorg. Chem. 39, 41:
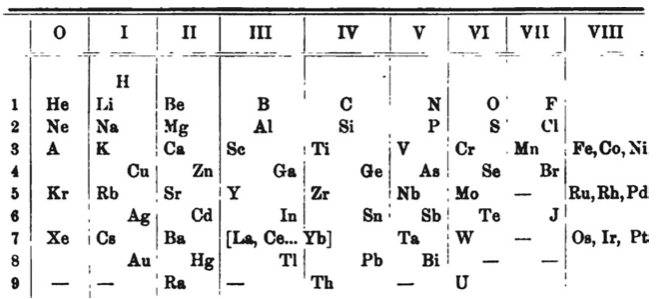
| Year: 1904 | PT id = 433, Type = formulation |
Mendeleev's 1904 Periodic Table
Mendeleev periodic table formulation from 1904.
This formulation was prepared to go with Mendeleev's article predicting that the ether (aether) would be found at the head of group zero in period zero. Also that dashes are left for six elements between H and He.
The predicted elements eka-boron (scandium), eka-aluminium (gallium) & eka-silicon (germanium) are present but the radioactive eka-manganese (technetium) is not. Also, the noble gas elements are on the left hand side of the formulation:

Thanks to Philip Stewart for the corrections and details.
| Year: 1904 | PT id = 546, Type = formulation |
Ramsay's Periodic Arrangement of The Elements
From from Scientific American in 1904,, an article by Sir William Ramsay discussing the Periodic Arrangement of The Element:
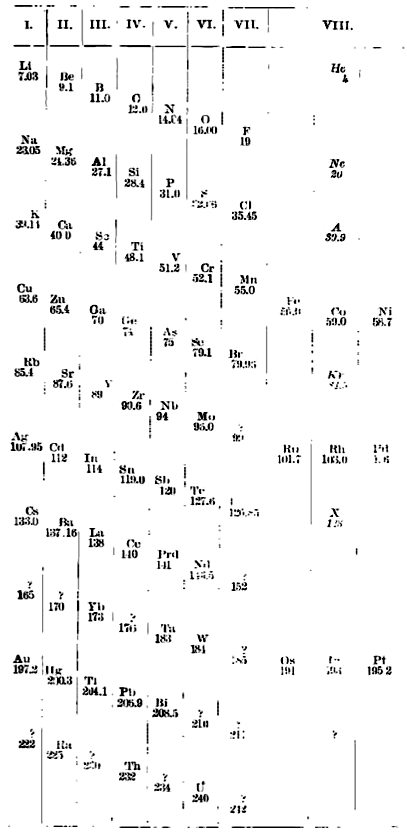
Redrawn by Mark Leach in 2019:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1905 | PT id = 64, Type = formulation |
Werner's Arrangement
Werner's Arrangement is the first modern looking PT formulation. It appeared before the structure of the atom was known, before the importance of atomic number was recognised and before quantum mechanics had been developed.
Berichte der Deutschen Chemischen Gesellschaft (1905), 38, 914-21 and J. Chem. Soc., Abstr. 88, II, 308-9 1905:
From Quam & Quam's 1934 review paper.pdf
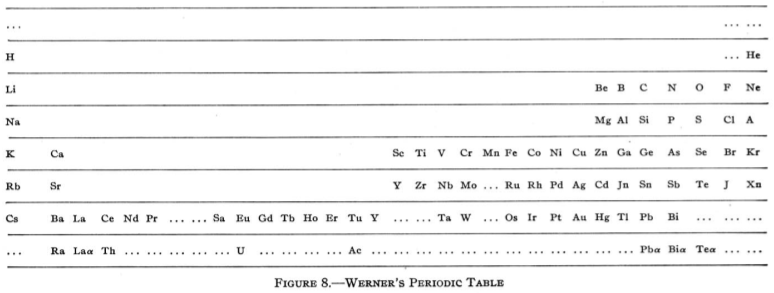
Eric Scerri comments that the interesting features are:
- A remarkably modern looking formulation in that it separates not only the transition metals but also the rare earths into separate blocks to give what we would now call a "long-form 32 column table". Except Werner guessed wrong as to how many rare earths exist, with the result that he shows 33 groups.
- This formulation is also interesting for showing an element between H and He and two elements before H.
- Werner computed the average gaps between atomic weights for the second through the fifth periods as 1.85, 2.4, 2.47 and 2.5, respectively.
- From this he extrapolated the gap for the first period as 1.5, which coincidentally was also half the difference between the atomic weights of H and He. Werner thus predicted a new element with atomic weight 2.5.
- Moseley's work of 1913 showed there were no elements before H and none between H and He.
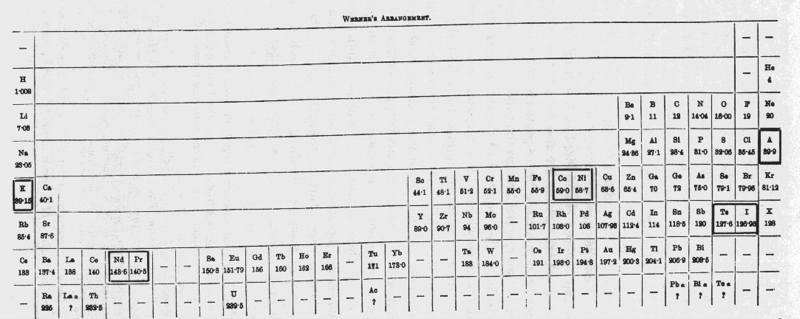
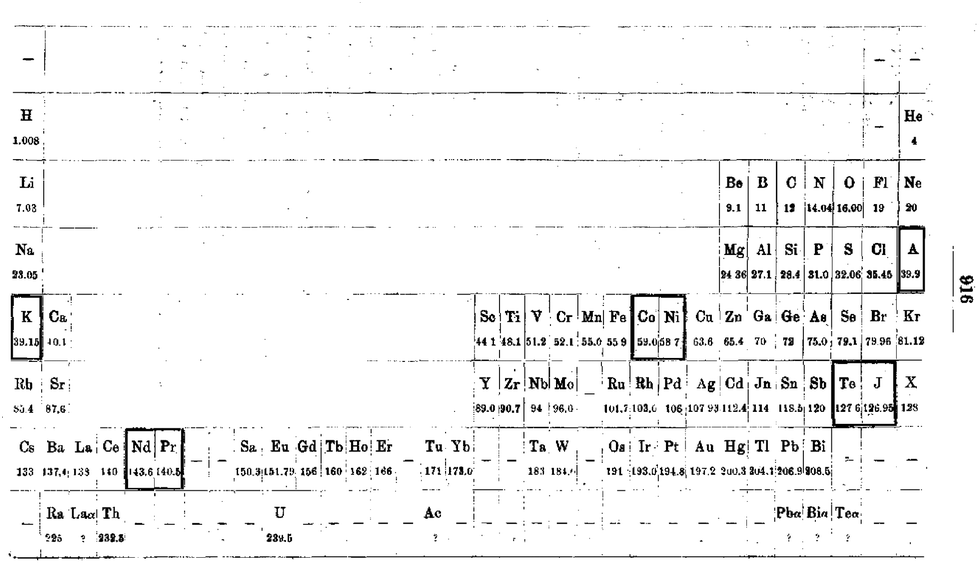
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
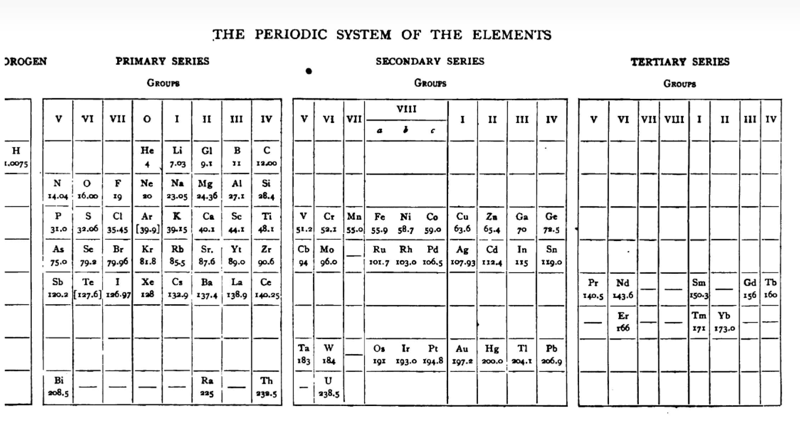
| Year: 1905 | PT id = 585, Type = formulation 3D spiral |
Gooch & Walker Periodic Table
Mazurs' reproduction (p. 82) of a periodic table formulation by Frank Austin Gooch and Claude Frederic Walker, from Outlines of Inorganic Chemistry, Macmillan, London and New York, p. 8/9, 1905 (ref Mazurs p.188):
Thanks to Laurie Palmer for the tip, and to Philip Stewart for the corrections and details.
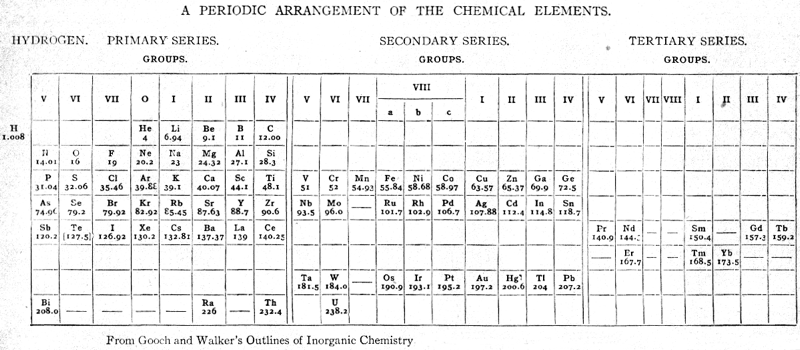
| Year: 1905 | PT id = 773, Type = formulation |
Gooch & Walker's Periodic System of The Elements
From a 1905 textbook by Gooch & Walker: Outlines of Inorganic Chemistry (see the Google Books scanned version pp273) comes an early 'right-step' periodic table. The formulation was reproduced in a 1917 textbook (lower image).
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1905 | PT id = 912, Type = formulation 3d spiral |
Gooch & Walker's Primary, Secondary, and Tertiary Series of Elements
This three dimensional formulation – clearly developed from the Crookes' vis generatrix model – is given a 1905 textbook by Gooch & Walker: Outlines of Inorganic Chemistry (see the Google Books scanned version pp273).
"The arrangement of the elements in three series of eight groups each may be represented by a model in which large and small wooden balls, on a spiral wire, represent the common and rare elements respectively; those balls falling in the same vertical column representing elements in the same groups":
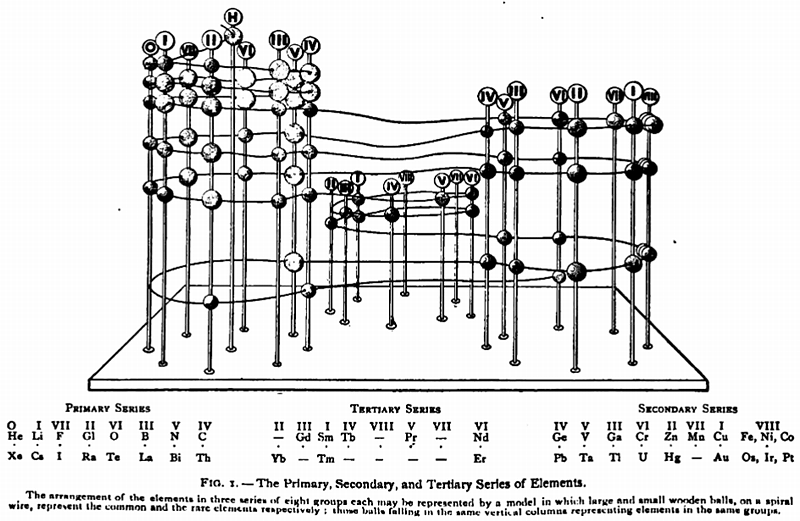
| Year: 1906 | PT id = 464, Type = formulation |
Mendeleev's 1906 Periodic Table
Mendeleev's periodic table of 1906, the last drawn up by Mendeleev himself, and published in the 8th edition of his textbook, Principles of Chemistry. Mendeleev died in 1907.
Mendeleev DI, Osnovy khimii (Principles of Chemistry), 8th edition, 1906, MP Frolova, Saint Petersburg.
- H retains the position of 1871
- The triad of Cu, Ag, Au is still duplicated.
- The noble gases are Group O
- This arrangement predates the concepts of atomic number and electron configuration
- Coronium is shown with a dash
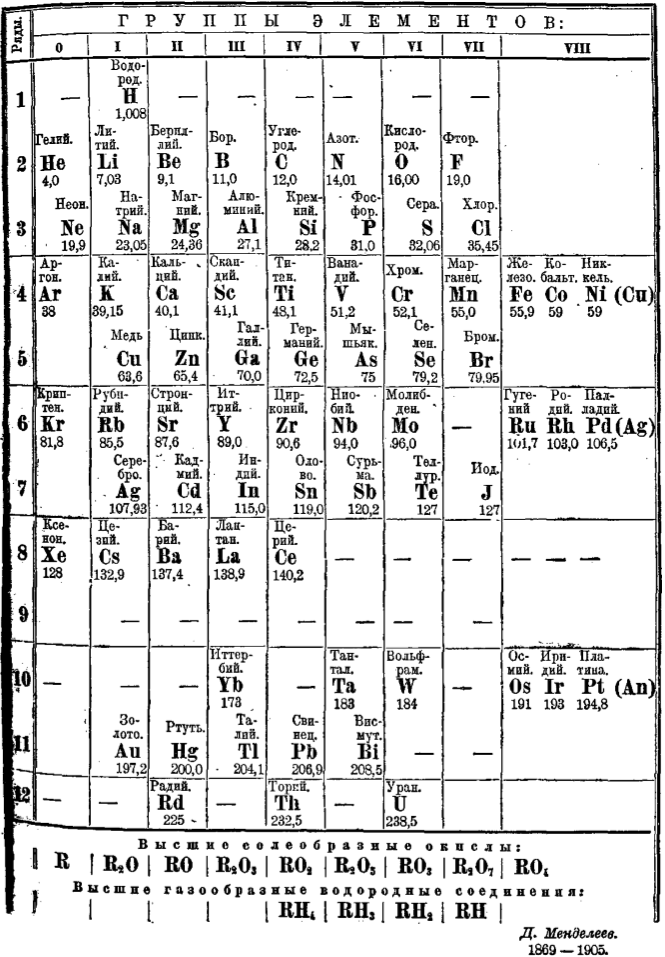
| Year: 1906 | PT id = 851, Type = element |
Discovery of Lutetium
Lu
Lutetium, atomic number 71, has a mass of 174.967 au.
Lutetium was first isolated in 1906 by C. A. von Welsbach and G. Urbain.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
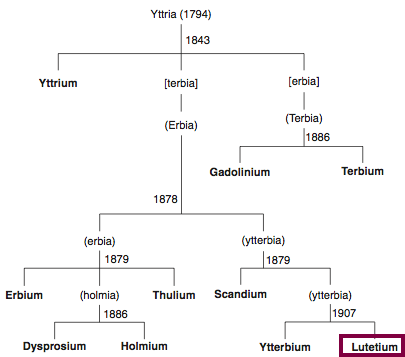
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1907 | PT id = 752, Type = formulation |
van den Broek Periodic Table 1
From Wikipedia: Antonius Johannes van den Broek (1870-1926) was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table (now called atomic number) corresponds to the charge of its atomic nucleus. The 1911 inspired the experimental work of Henry Moseley, who found good experimental evidence for it by 1913. van den Broek envisaged the basic building block to be the 'alphon', which weighed twice as much as a hydrogen atom.
Read more in Chapter 4, Antonius Van Den Broek, Moseley and the Concept of Atomic Number by Eric Scerri. This chapter can be found in the book: For Science, King & Country: The Life and Legacy of Henry Moseley (Edited by Roy MacLeod, Russell G Egdell and Elizabeth Bruton).
van den Broek's periodic table of 1907: Annalen der Physik, 4 (23), (1907), 199-203
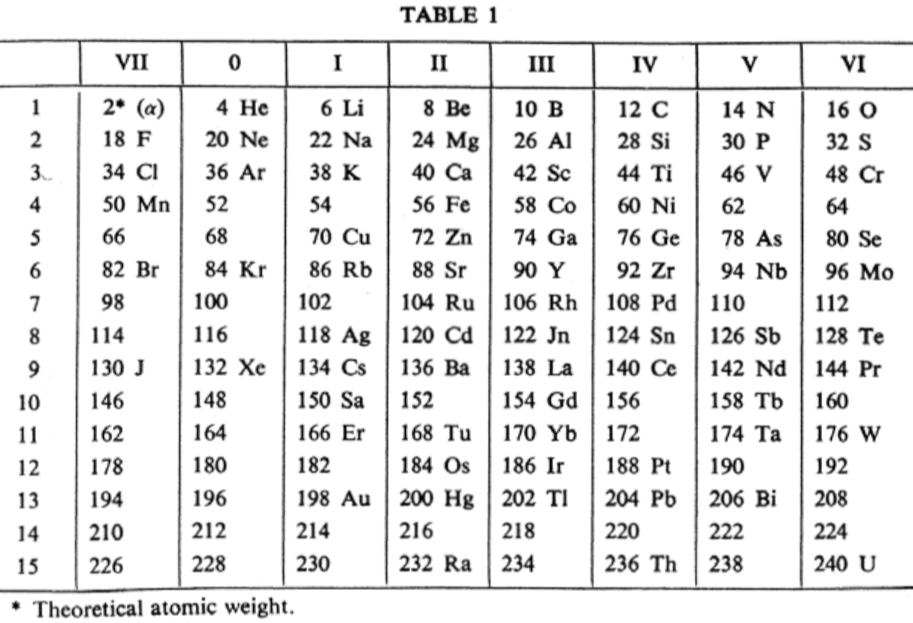
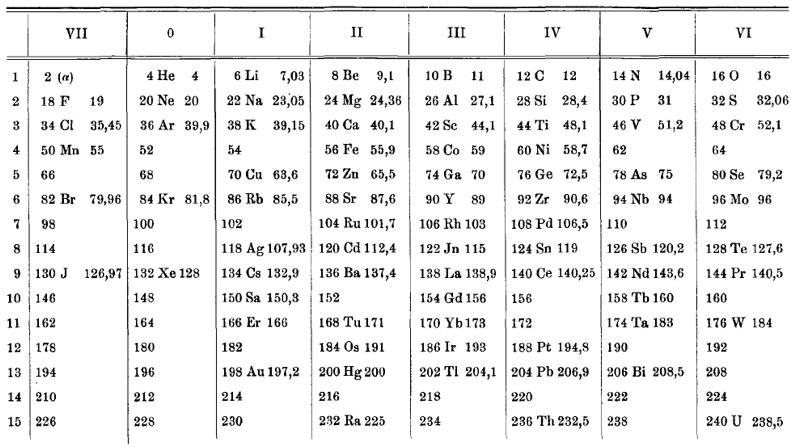
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1907 | PT id = 1105, Type = formulation |
Grouping of The Elements to Illustrate Refractivity
From C. Cuthbertson & E. Parr Metcalfe, Part III On The Refractive Indices of Gaseous Potassium, Zinc, Mercury, Arsenic Selenium and Tellurium, Phil. Trans. A: Mathematical & Physical Sciences, vol 207, pp135–148, 1907.
René Vernon writes:
"A curious periodic table which runs from group 12 on the left to group 13 on the right (see below). It seems to have done that way to bring out the pattern in multiples of refractivities i.e. x½ x 4 x 6 x10. The border around the elements in groups 15 to K-Rb-Cs in group 1 denotes this relatively strong regularity among the refractivity values. The L for iodine is a printer's error."
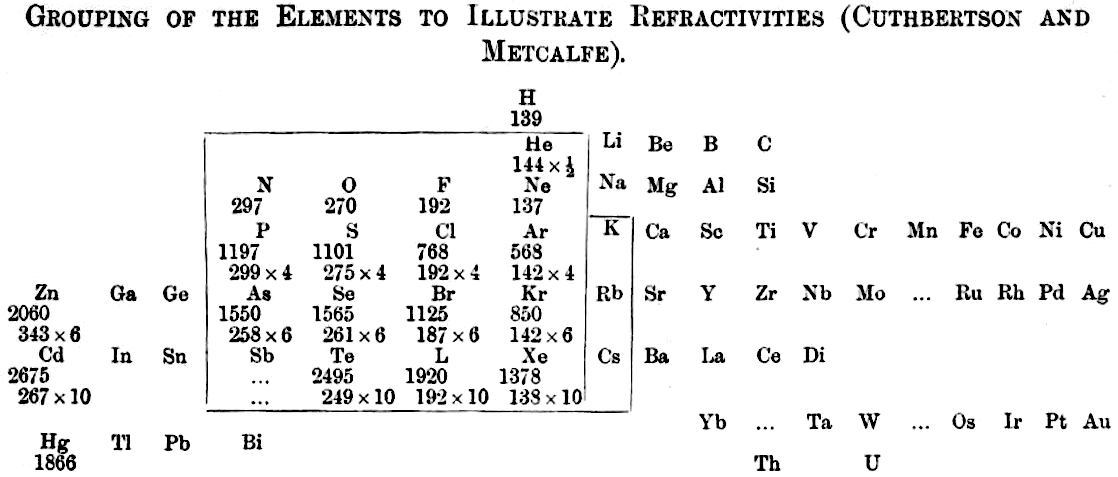
| Year: 1907 | PT id = 1332, Type = formulation review element |
Thompson's Electron Rings
After proposing, what became known as the plump-puddding model of the atom in 1904, J.J. Thompson developed the idea in his book The Corpuscular Theory of Matter, Archibale Constable, 1907(available as a scanned document online).
Thompson's Electron Rings are sumarised in this table:

The origional text reads (taken from pages 104-110). Note, for "corpusle" read "electron":



Thanks to Eric Scerri for the tip.
| Year: 1908 | PT id = 457, Type = formulation |
Young's Table
From Young's textbook Stoichiometry (1908):
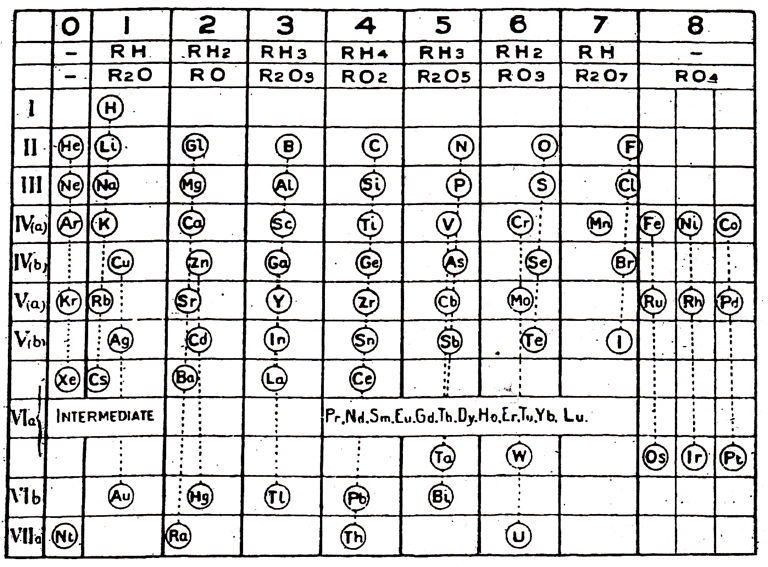
| Year: 1908 | PT id = 995, Type = formulation misc |
Ramsay's Periodic Table
William Ramsay with a section of his 1904 periodic table as a portrait in Vanity Fair.
From the Science History Institute:
In 1892 Ramsay's curiosity was piqued by Lord Rayleigh's observation that the density of nitrogen extracted from the air was always greater than nitrogen released from various chemical compounds. Ramsay then set about looking for an unknown gas in air of greater density, which – when he found it – he named argon.
While investigating for the presence of argon in a uranium-bearing mineral, he instead discovered helium, which since 1868 had been known to exist, but only in the sun. This second discovery led him to suggest the existence of a new group of elements in the periodic table. He and his coworkers quickly isolated neon, krypton, and xenon from the earth's atmosphere.

| Year: 1909 | PT id = 1032, Type = formulation |
Chemical News' Periodic Arrangement of the Elements
From Chemical News and Journal of Industrial Science, December 1909, a Periodic Arrangement of the Elements.
This formulation shows an element Np (mass 100 – Ogawa's nipponium), between Mo and Ru, a hypothesised element was later found to be the radioacive element technecium, discovered in 1937.
The formulation also has the Inactive Neutral Gases – the noble gases: St, RaEm, Z1 & Z2.
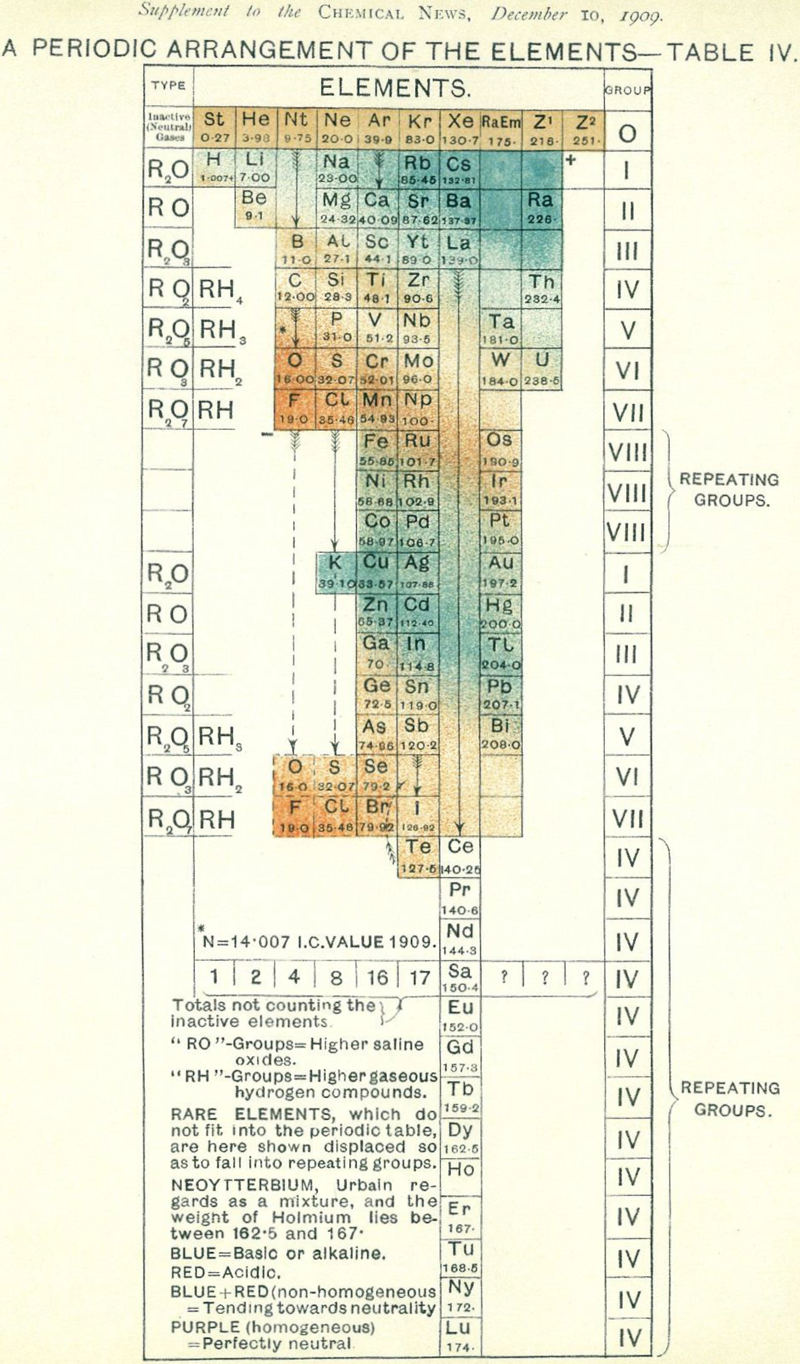
Many thanks to Sam Kidd for the tip!
| Year: 1911 | PT id = 67, Type = formulation |
Adams' Periodic Table
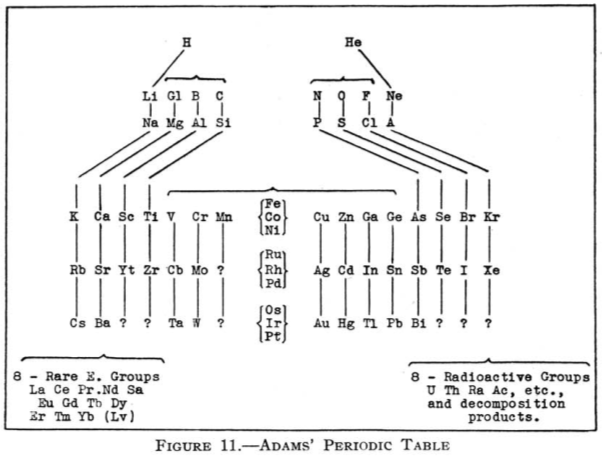
From Quam & Quam's 1934 review paper.pdf
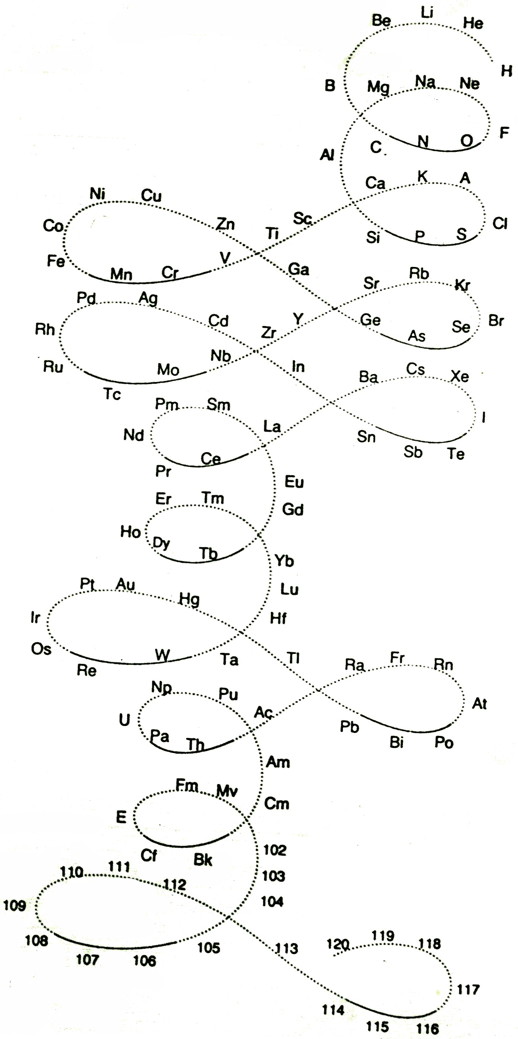
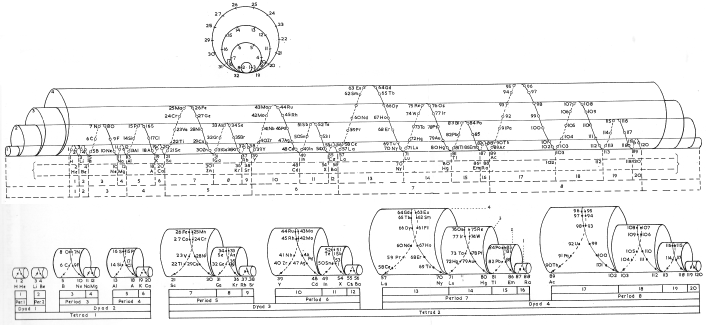
| Year: 1911 | PT id = 76, Type = formulation 3D spiral |
Emerson's Helix
From Quam & Quam's 1934 review paper.pdf:
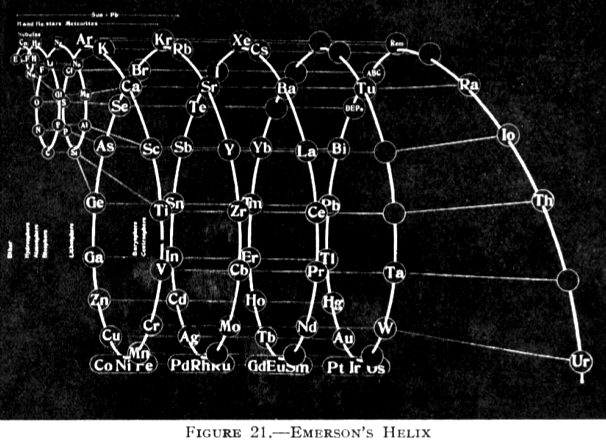
Another version of Emerson's Helix from "100 Years of Periodic Law of Chemical Elements, Nauka 1969, p. 74:
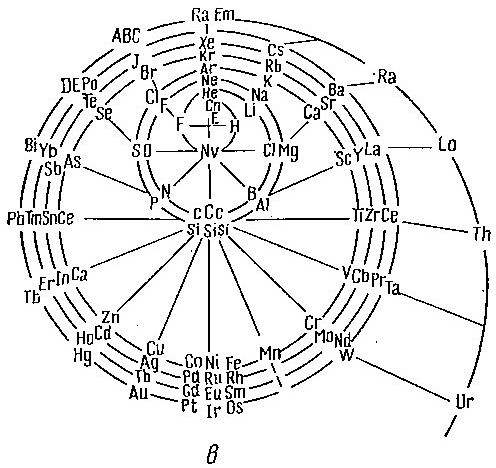
Thanks to Larry T for the tip!
| Year: 1911 | PT id = 288, Type = formulation spiral 3D |
Soddy's Three-Dimensional System
Soddy's three-dimensional system of 1911 (from van Spronsen):
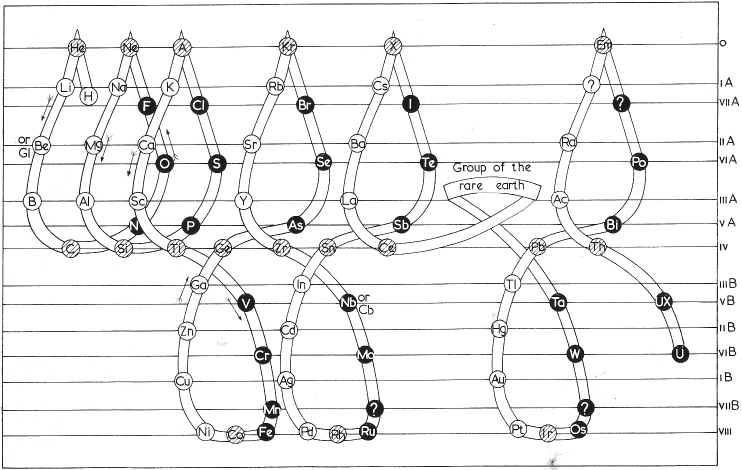
| Year: 1911 | PT id = 369, Type = formulation |
Baur's Periodic Table
Baur's periodic table, from Baur, E., 1911. Z. Phys. Chem. 76, 659:
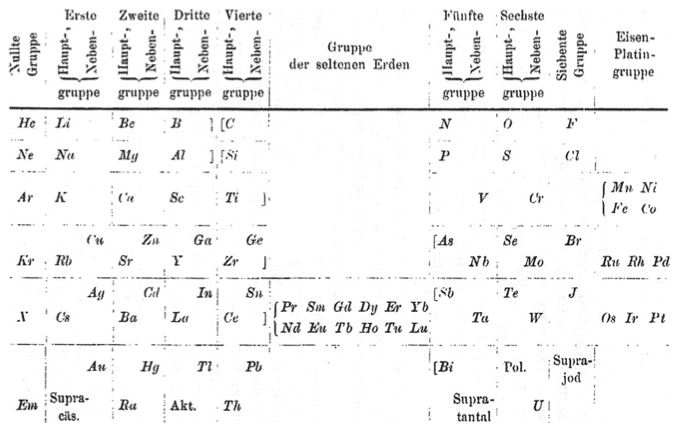
| Year: 1911 | PT id = 999, Type = formulation |
van den Broek's Periodic Table 2
From Wikipedia: Antonius Johannes van den Broek (1870-1926) was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table (now called atomic number) corresponds to the charge of its atomic nucleus. The 1911 inspired the experimental work of Henry Moseley, who found good experimental evidence for it by 1913. van den Broek envisaged the basic building block to be the 'alphon', which weighed twice as much as a hydrogen atom.
Read more in Chapter 4, Antonius Van Den Broek, Moseley and the Concept of Atomic Number by Eric Scerri. This chapter can be found in the book: For Science, King & Country: The Life and Legacy of Henry Moseley (Edited by Roy MacLeod, Russell G Egdell and Elizabeth Bruton).
van den Broek's periodic table of 1907: Annalen der Physik, 4 (23), (1907), 199-203


van den Broek's periodic table of 1911: Physikalische Zeitschrift, 12 (1911), 490-497); and also a paper in Nature the same year entitled: The Number of Possible Elements and Mendeléff's "Cubic" Periodic System, Nature volume 87, page 78 (20 July 1911)
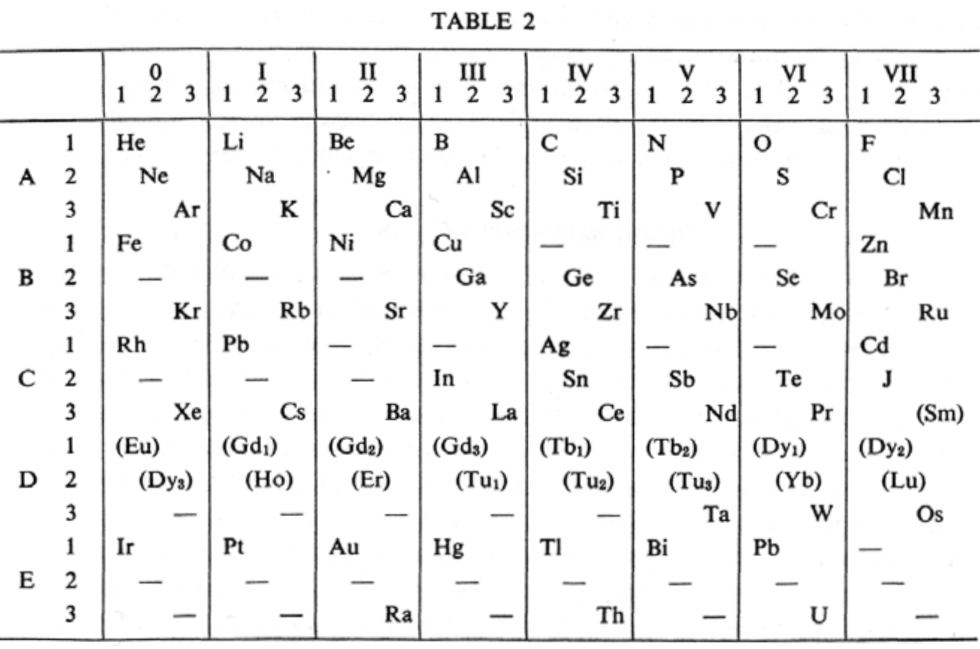
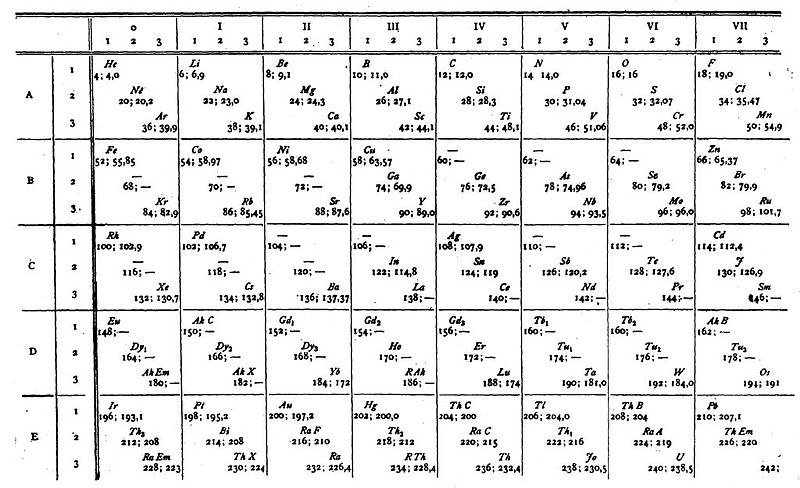
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1911 | PT id = 1296, Type = formulation |
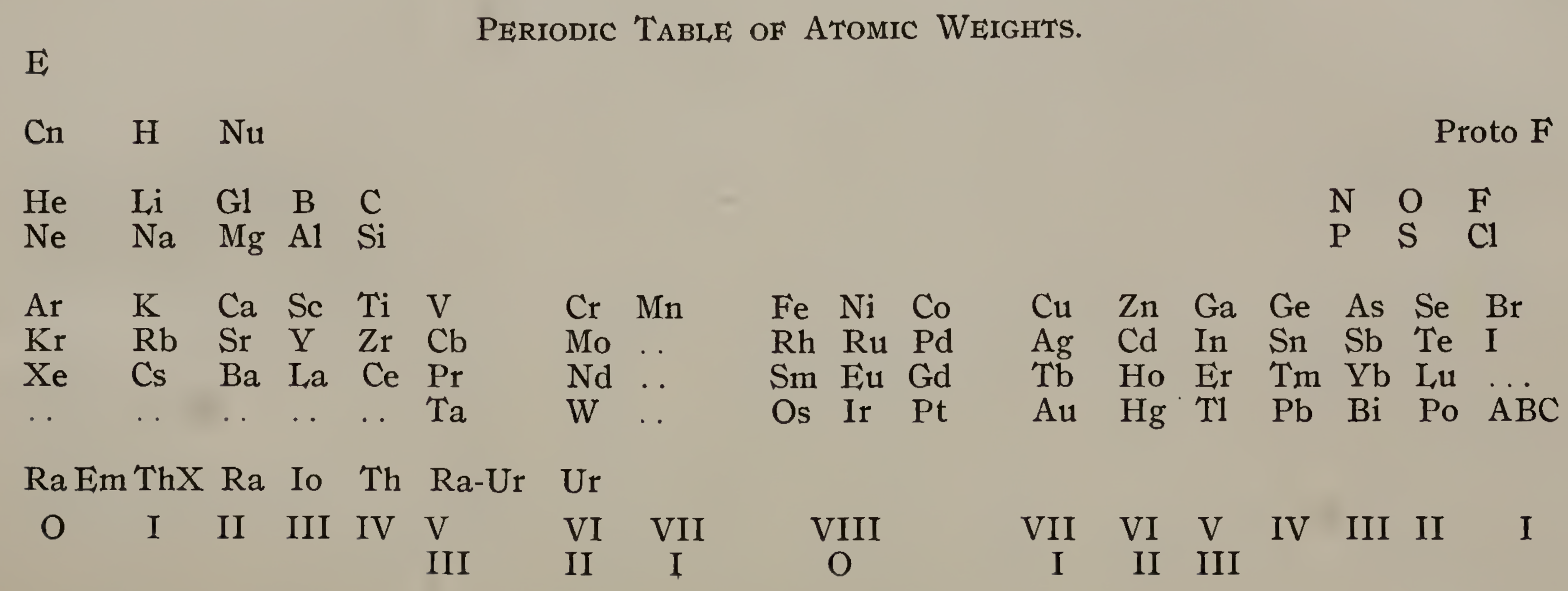
Emerson's Periodic Table of Atomic Weights
Emerson BK, Helix chemica: A study of the periodic relations of the elements and their graphic representation, American Chemical Journal, vol. 45, pp. 160–210 (1911). The formulation below appears on page 173; a scanned pdf version of the paper can be viewed here.
René Vernon writes:
Emerson includes two elements before hydrogen: "E" (either the luminiferous ether or the electron) and "Coronium". There are also two elements between hydrogen and helium: "Nebulium" and "Protofluorine".
This is the first time I have seen a PT showing four extra elements and where they are supposed to fit.
After La, Emerson incorporates 13 lanthanides (Ce to Lu) as transition elements into his 7th period.
Emerson missed dysprosium, between Tb and Ho.
"A, B and C" at the bottom right are supposed to be 'halogen emanations'.
Mark Leach adds that Emerson's very odd Periodic Table of Atomic Weights does not actually show any atomic weights.
| Year: 1913 | PT id = 13, Type = formulation |
Moseley's Periodic Law
Henry Moseley (1887-1915) subjected known elements to x-rays and was able to derive a relationship between x-ray frequency and number of protons.
From Scientific American:
"It was the clever young English physicist, Moseley, who discovered that the atomic number for each element was the number of external electrons in the atom.
"With this discovery came a law concerning the X-ray lines of any element in an X-ray target.
"Moseley's law states that the wavelength of these lines is inversely proportional to the square of the atomic number of the element. Therefore, if we know the atomic number of the element we are looking for, we can predict the wavelength of certain lines in its X-ray spectrum.
"If we set up our X-ray spectrograph so as to catch these lines where we expect them to fall, then, if the element is present in the target which we have chosen to use in our X-ray tube, we should know it. This provides one good way to identify difficult elements, but it is well to have another to use as a check. One of the best of these, and one which is almost as sensitive as the X-ray method, is that of positive ray analysis."
From his paper, The High Frequency Spectra of The Elements, H. G. J. Moseley, M. A. Phil. Mag. (1913), p. 1024, available here:
| Year: 1913 | PT id = 59, Type = formulation |
Rydberg's Table
René Vernon writes:
My source is the 1914 French translation of Rydberg’s 1913 German article.
- Rydberg 1913, Untersuchungen über das System der Grundstoffe, Lunds Univ. Årsskrift, (Acta Univers, Lundensis), vol. 9, no. 18, pp. 1-41
- — 1914, Recherches sur le système des éléments, Journal de Chimie Physique, vol. 12, pp. 585–639, https://doi.org/10.1051/jcp/1914120585

| Year: 1913 | PT id = 871, Type = element |
Discovery of Protactinium
Pa ![]()
Protactinium, atomic number 91, has a mass of 231.036 au.
Radioactive element: Pa is only found in tiny amounts in nature. Most samples are synthetic.
Protactinium was first observed or predicted in 1913 by O. H. Göhring and K. Fajans and first isolated in 1927 by A. von Grosse.
| Year: 1913 | PT id = 973, Type = formulation |
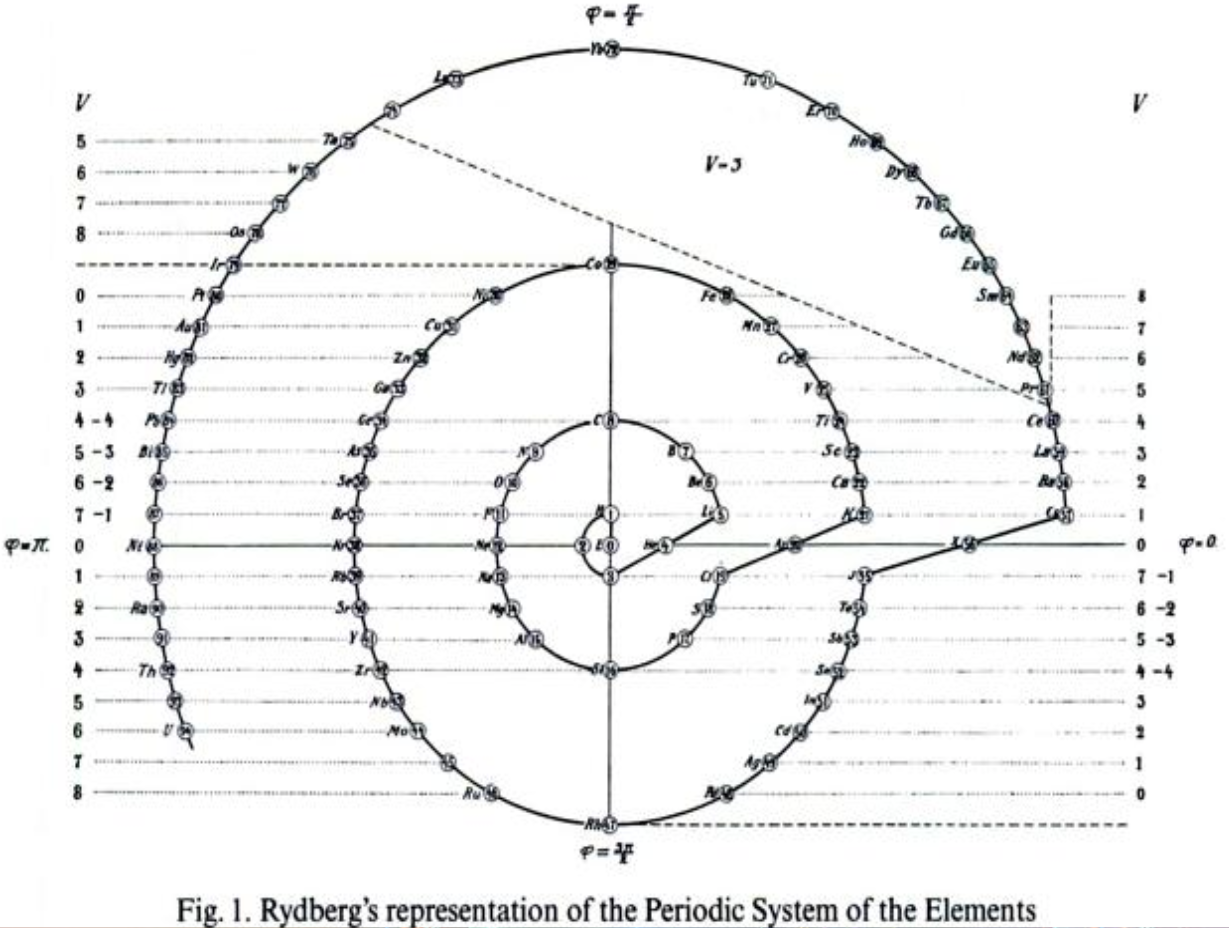
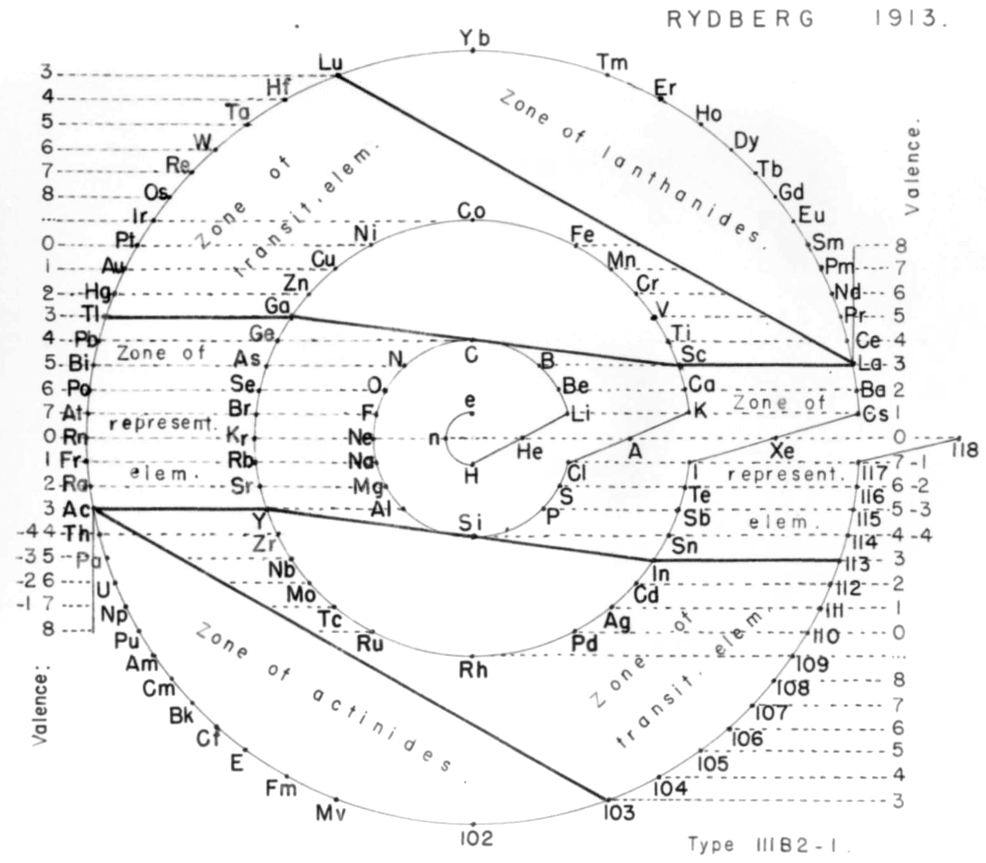
Rydberg's Periodic Table in style of Spiral with Four Revolutions
Periodic table in style of spiral with four revolutions circa 1913 (Original design) and 1957 (Date attributed to slide).
This table was originated by Swedish physicist Johannes Rydberg (1854-1919) in 1913 and classified by chemist Edward G. Mazurs as Type IIIB2-1 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957). The lower version of the table appears as Figure 63 on page 132 of Mazurs' 1957 publication.
| Year: 1913 | PT id = 1000, Type = formulation |
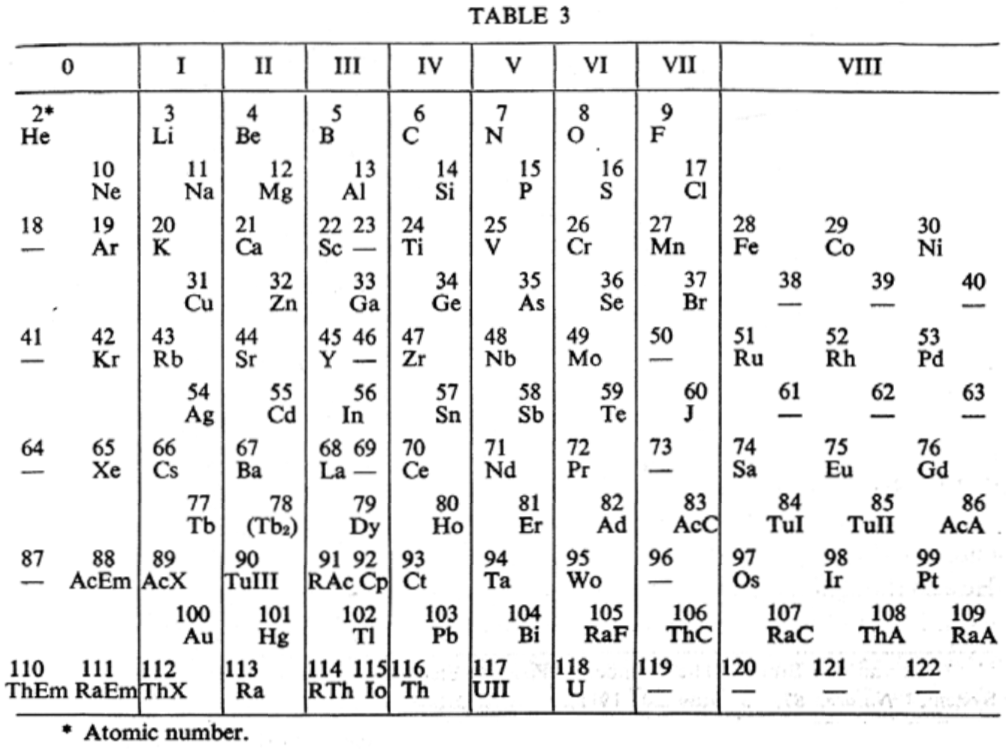
van den Broek's Periodic Table 3
From Wikipedia: Antonius Johannes van den Broek (1870-1926) was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table (now called atomic number) corresponds to the charge of its atomic nucleus. The 1911 inspired the experimental work of Henry Moseley, who found good experimental evidence for it by 1913. van den Broek envisaged the basic building block to be the 'alphon', which weighed twice as much as a hydrogen atom.
Read more in Chapter 4, Antonius Van Den Broek, Moseley and the Concept of Atomic Number by Eric Scerri. This chapter can be found in the book: For Science, King & Country: The Life and Legacy of Henry Moseley (Edited by Roy MacLeod, Russell G Egdell and Elizabeth Bruton).
van den Broek's periodic table of 1907: Annalen der Physik, 4 (23), (1907), 199-203


van den Broek's periodic table of 1911: Physikalische Zeitschrift, 12 (1911), 490-497); and also a paper in Nature the same year entitled: The Number of Possible Elements and Mendeléff's "Cubic" Periodic System, Nature volume 87, page 78 (20 July 1911)


van den Broek's periodic table of 1913: Physikalische Zeitschrift, 14, (1913), 32-41
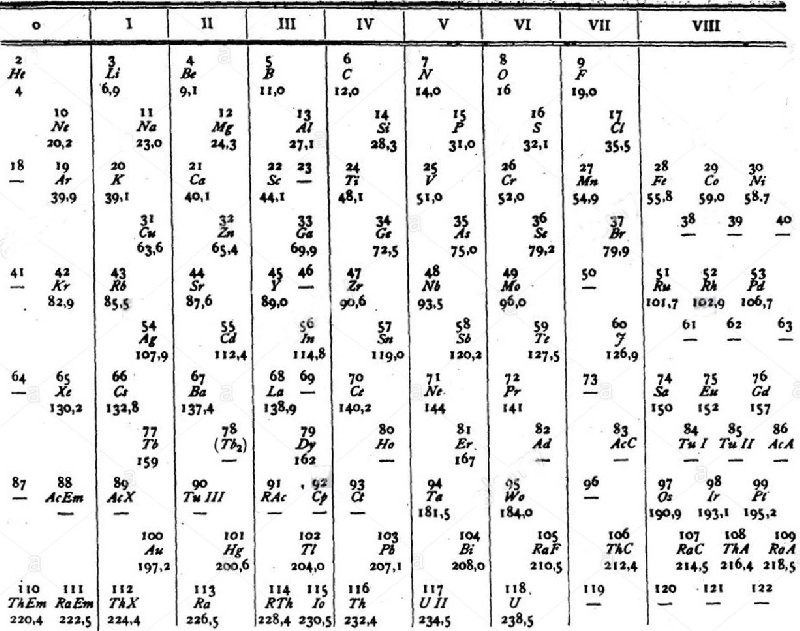
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1914 | PT id = 23, Type = formulation spiral |
Hackh's Spiral Periodic Table
Ingo Hackh's spiral periodic table of 1914, from Das Synthetisches System der Atome, Hamburg, Hephaestos.
Philip Stewart says:
"I believe that Hackh's 1914 spiral is of special interest it is the first spiral to take account of Mosley's atomic numbers, and the first to show successively larger pairs of coils. It is also interesting because H stands alone in the centre. I have only seen Mazurs' redrawn (as usual!) version, but Mazurs gives SciAm Supplement 1919 as one reference."
This is the Mazurs version:
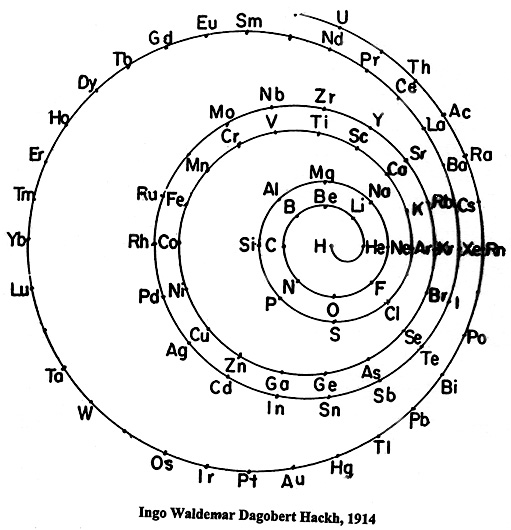
| Year: 1915 | PT id = 586, Type = formulation |
Crehore's Periodic System
[Part of] Crehore's periodic system, with the electronic configurations of the atoms, from Crehore, Gyroscopic Theory, p 323 (1915).
Crehore adopted Rydberg's ordinal number, implying the existance of two elements between hydrogen and helium.
Note the absence of groups VI and VII and that beryllium is shown as "Gl", glacinium.
From H. Kragh, Resisting the Bohr Atom, Perspectives in Physics, 13, (2011), 4-35:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1915 | PT id = 1233, Type = formulation 3D |
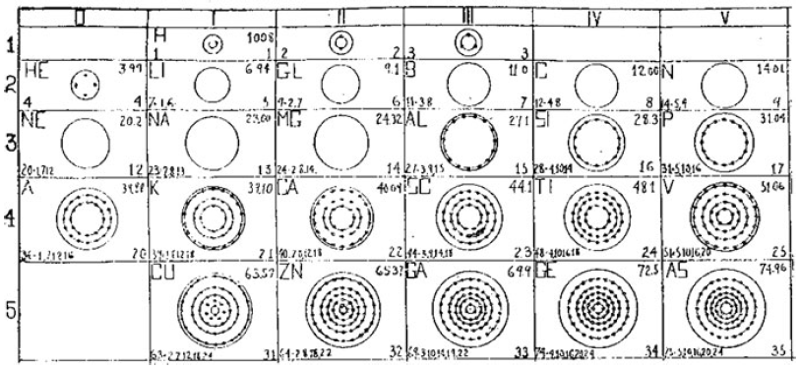
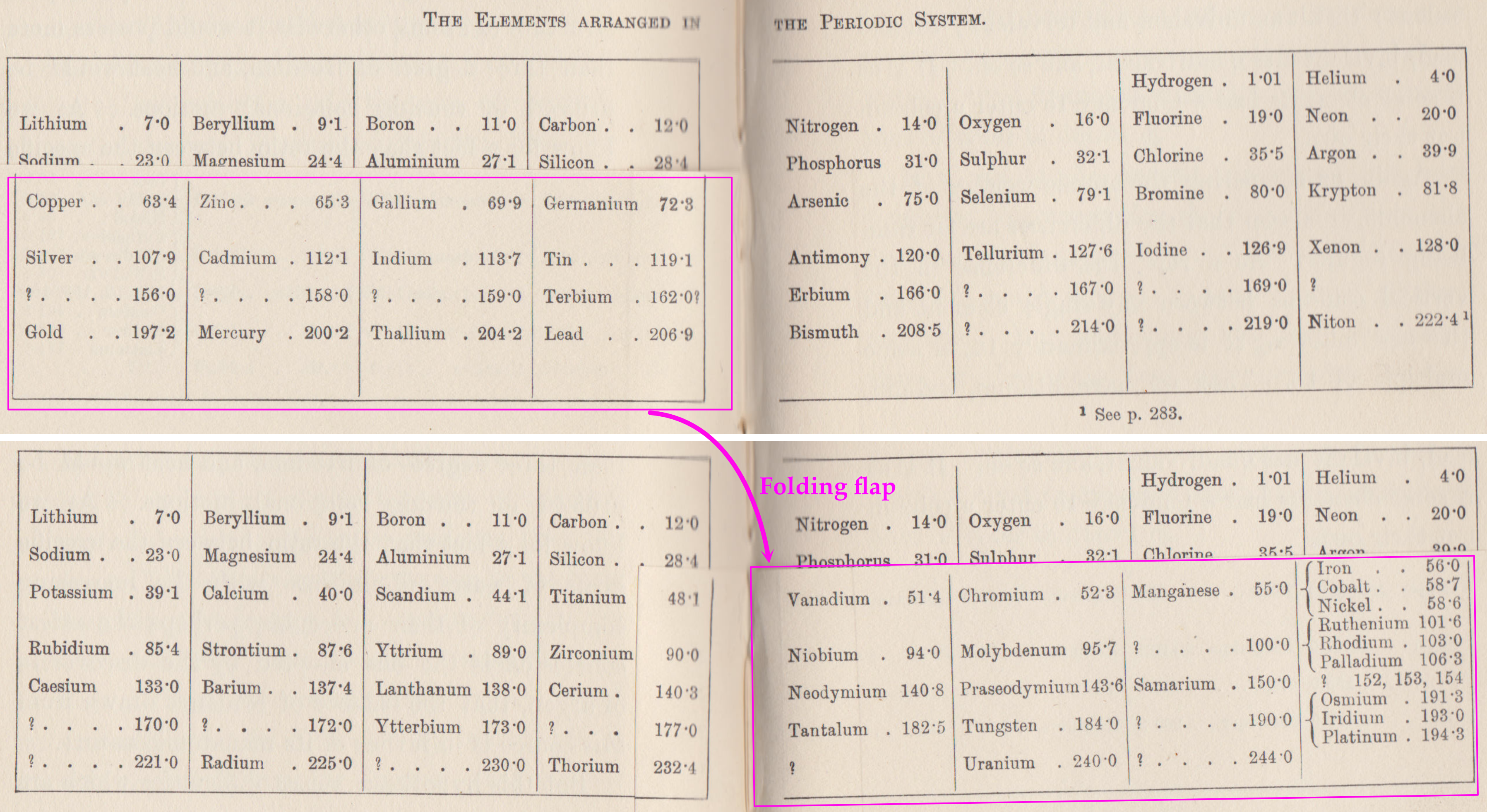
Ramsay's The Elements Arranged in The Periodic System (with movable flap)
From pages 220 & 221 of William Ramsay's book The Gases of The Atmosphere, McMillan (1915) comes a periodic table with a fold (or flap) that can be moved from page 220 to 221:
This periodic table is available as a Project Gutenberg ebook. The HTML version gives this dual representation:
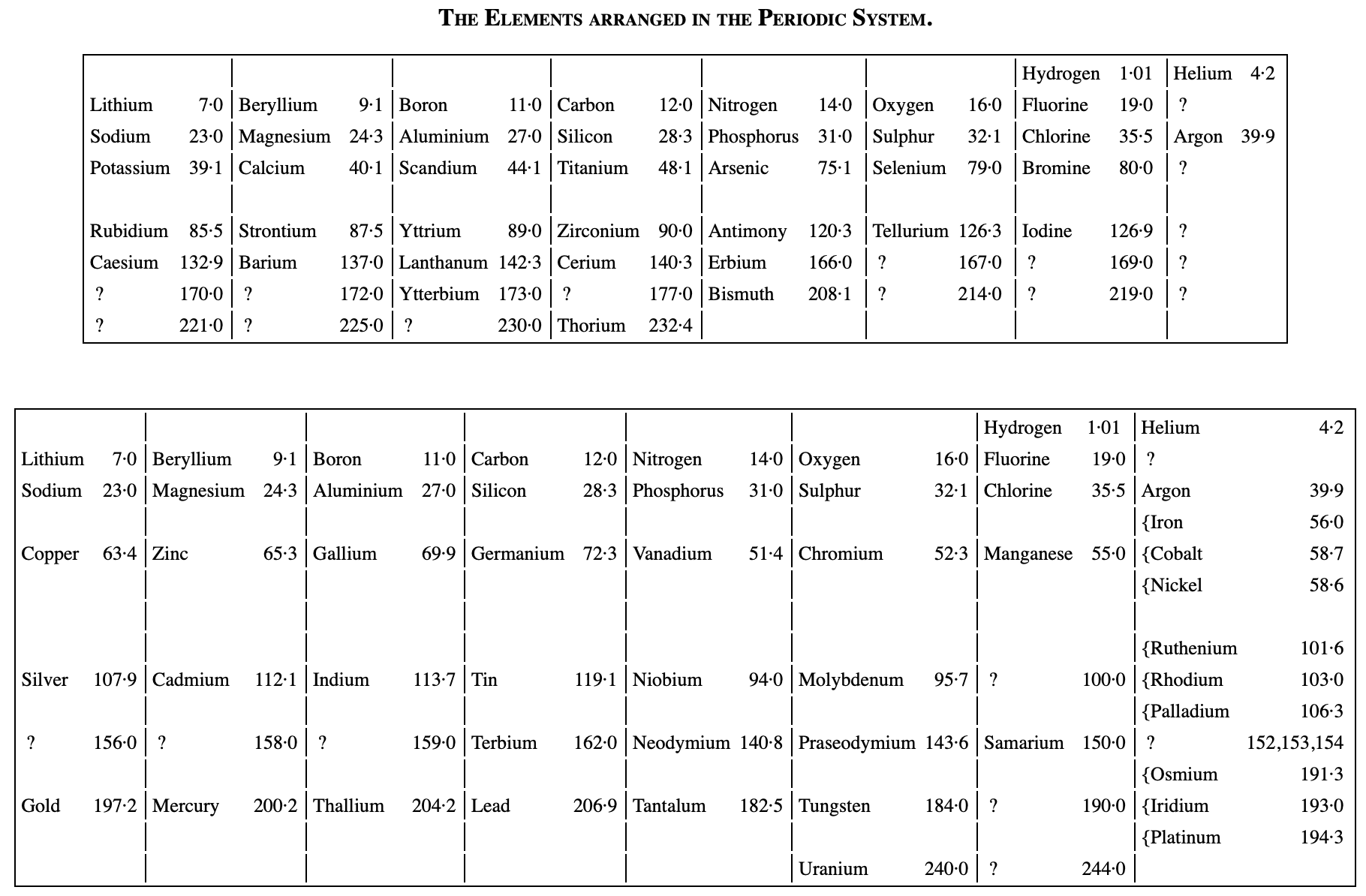
Thanks to John Marks for the tip!
| Year: 1916 | PT id = 77, Type = formulation 3D spiral |
Harkins & Hall's Periodic Table
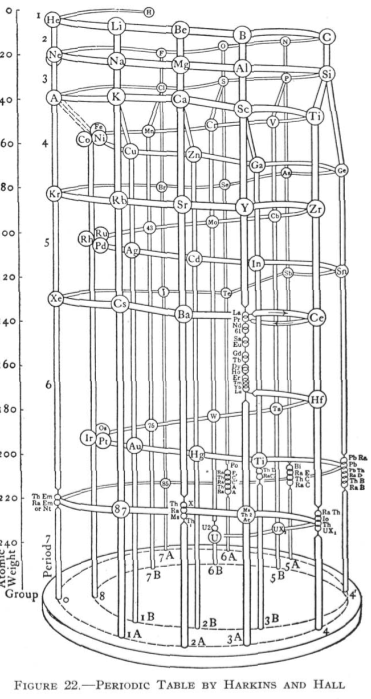
From Quam & Quam's 1934 review paper.pdf
| Year: 1916 | PT id = 541, Type = formulation |
Dushman's Periodic Table
By Dushman et al., a take on Mendeleeve's Periodic System:
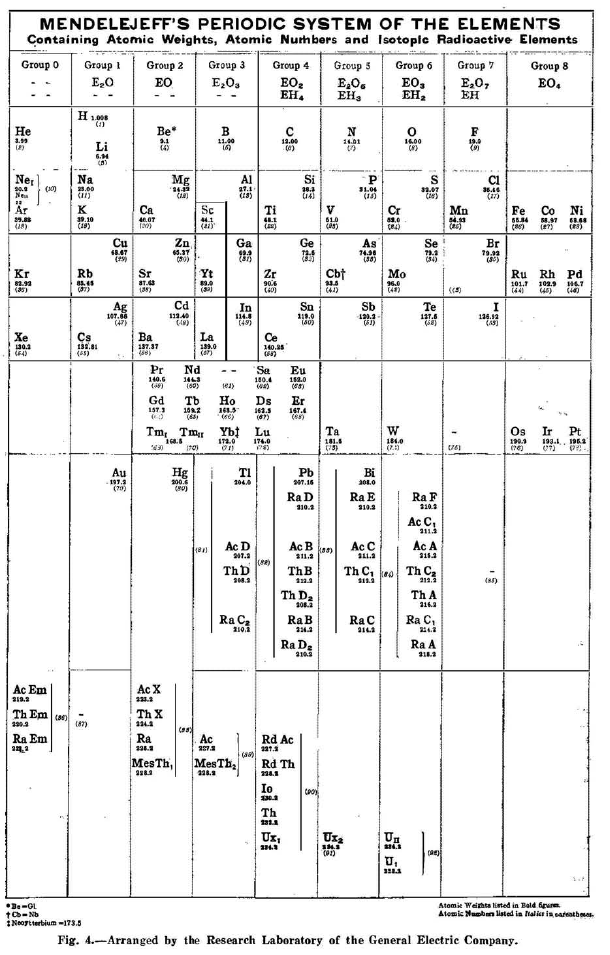
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1916 | PT id = 1214, Type = formulation |
Sommerfeld's Periodic Table
A periodic table by Arnold Sommerfeld, as an updated construction by Marks & Marks (2021).
John Marks writes:
"The reconstruction of Sommerfeld 1916 is derived from my reading of Henry Browse's translation of the third German edition of his Atomstruktur und Spektrallinien (Methuen 1923). Sommerfeld found the explanation of the greater (d– and f–) and lesser (s– and p–) periods in the solution of Kepler's ellipses using Schwarzschild's relativistic correction, communicated to him from the battlefront of WW1. Sommerfeld considered helium "an exception" but this is only an appearance deriving from defining periods as terminated by inert gases. In fact, the first period begins with hydrogen so the markers of periods are analogues of hydrogen, viz. the halogens."
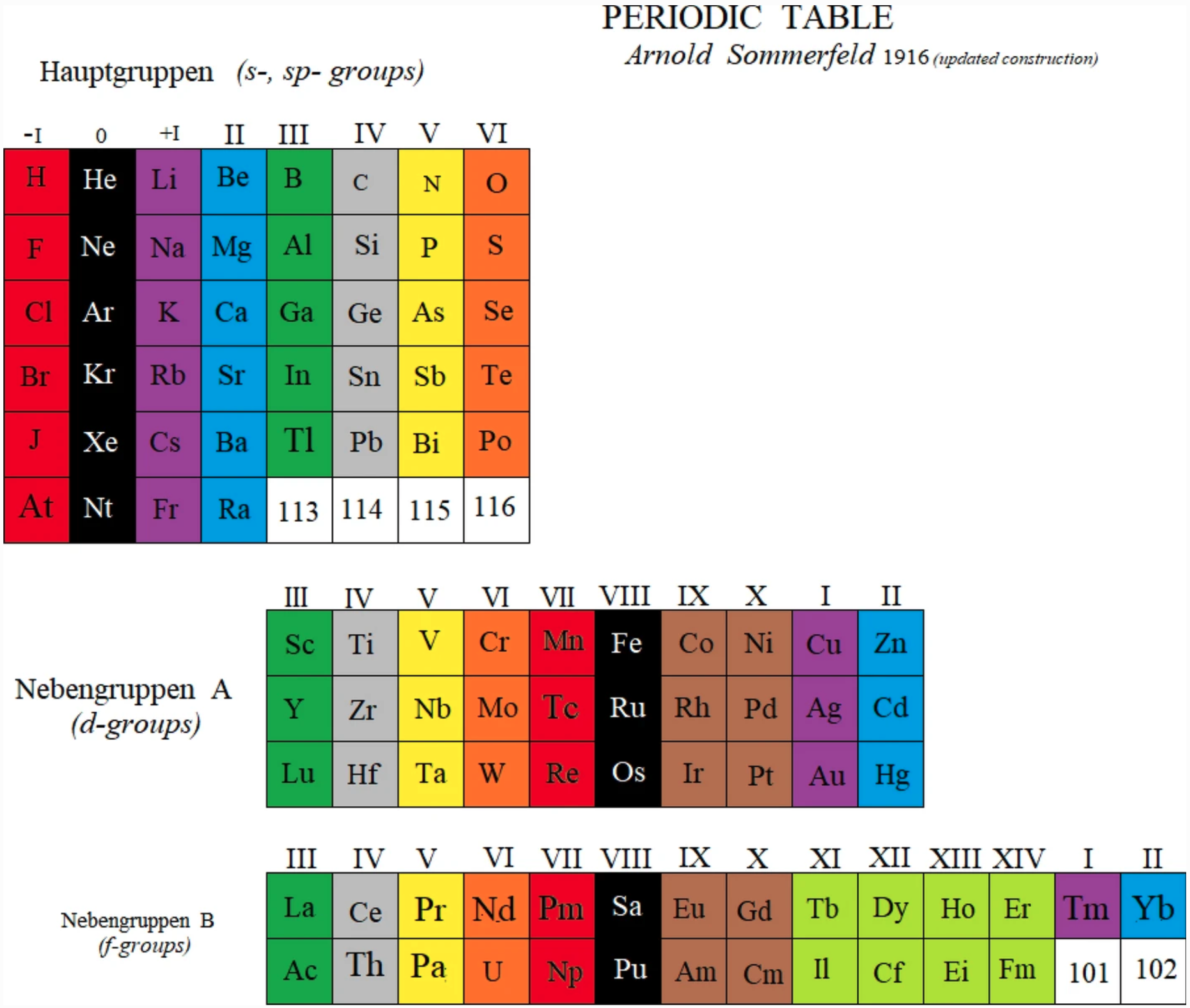
| Year: 1917 | PT id = 1155, Type = formulation |
Friend's Periodic Table (1917)
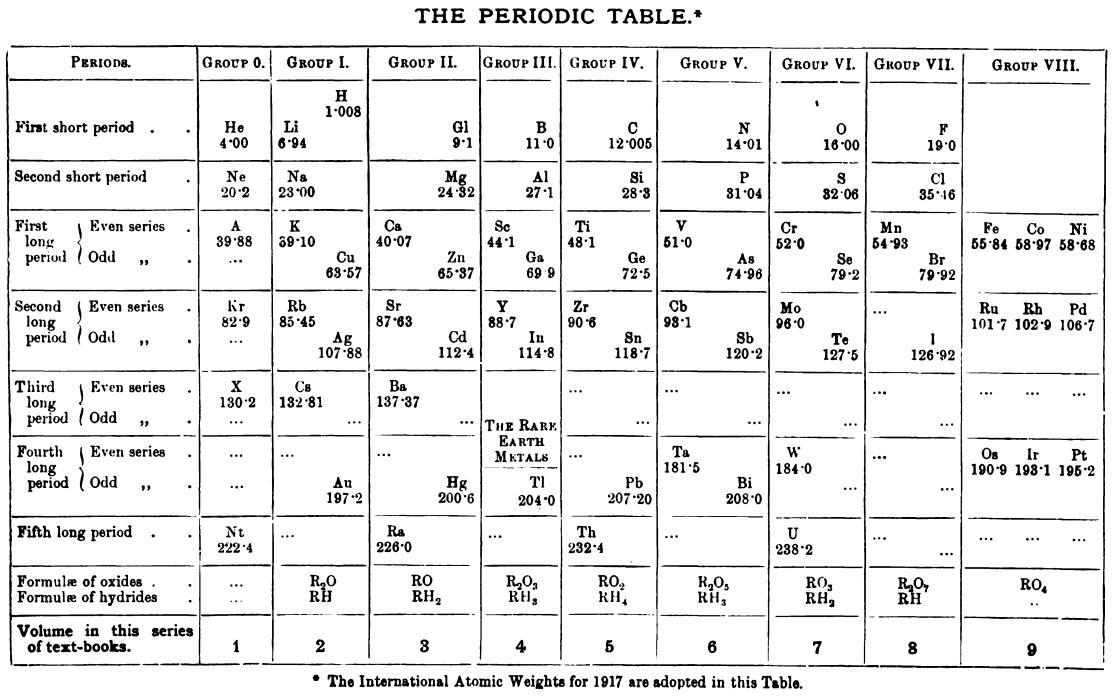
Thanks to René Vernon for the tip.
| Year: 1918 | PT id = 83, Type = formulation |
Hackh's Classification of the Elements
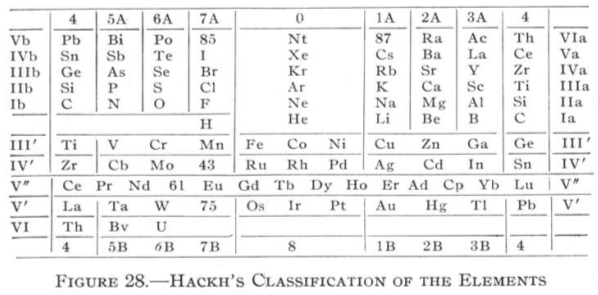
From Quam & Quam's 1934 review paper.pdf
| Year: 1918 | PT id = 367, Type = formulation |
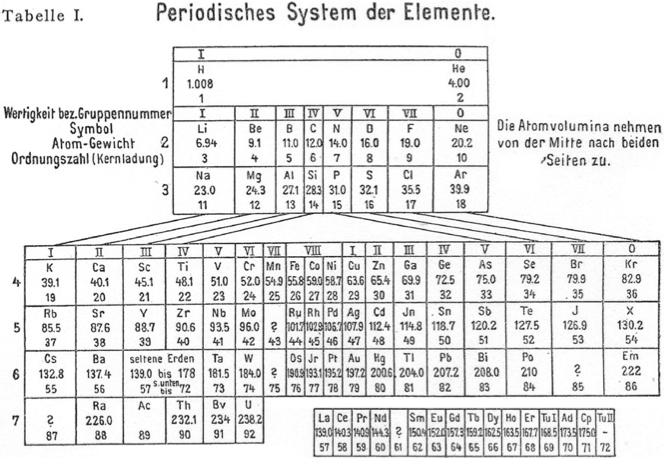
Meyer's Periodisches System der Elemente
Periodic Table of Meyer (1918) with an intraperiodic accommodation of the rare earths. Reproduced from Meyer, S., 1918. Phys. Z. 19, 178.
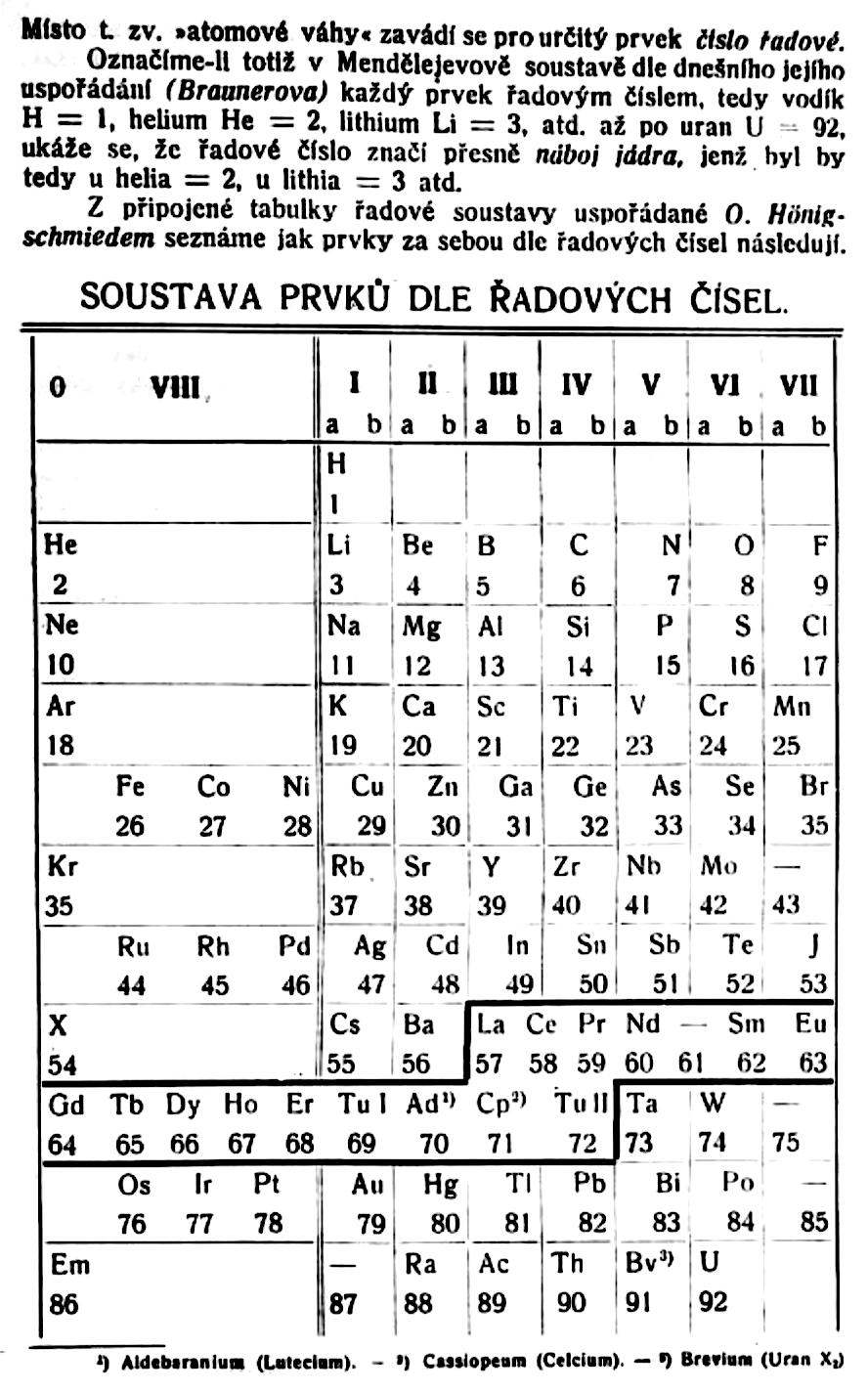
Philip Stewart has provided a bit more detail:
Stefan Meyer (1872-1949) was an Austrian physicist, no relation of Julius Lothar Meyer. He had a special interest in 'rare earth' and radioactive elements. He published several versions of the periodic table. In this definitive version of 1918, note elements 69-72. Tu I is 'thulium I', Ad is Aldeberanium (Yb), Cp is Cassiopeium (Lu) and Tu II is 'thulium II' (Hf).:
| Year: 1918 | PT id = 1260, Type = formulation |
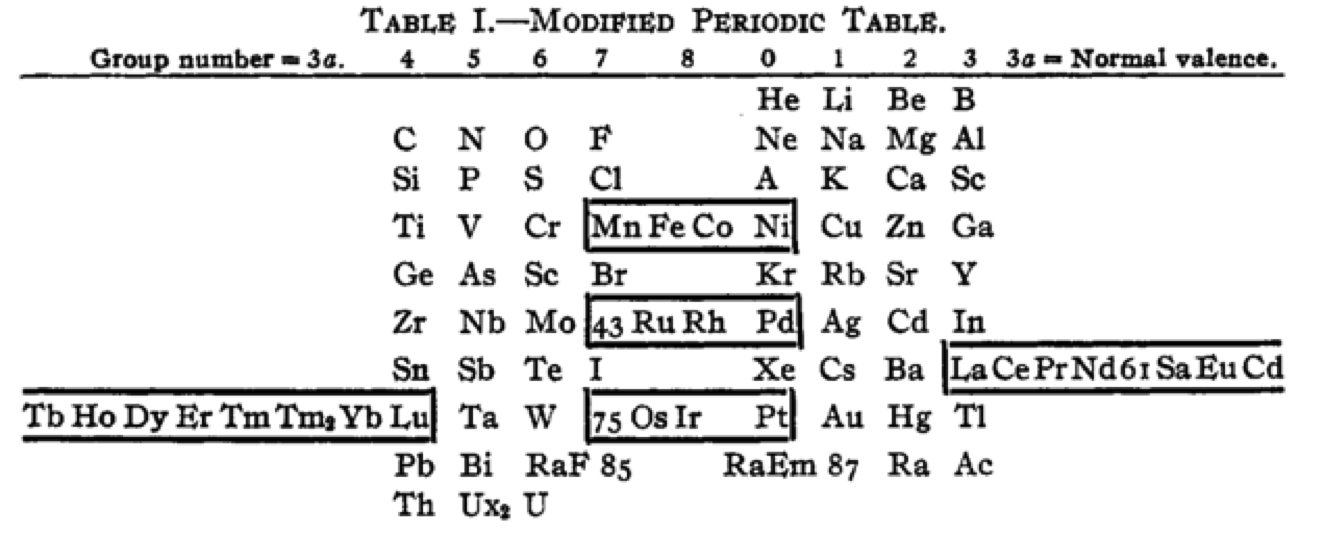
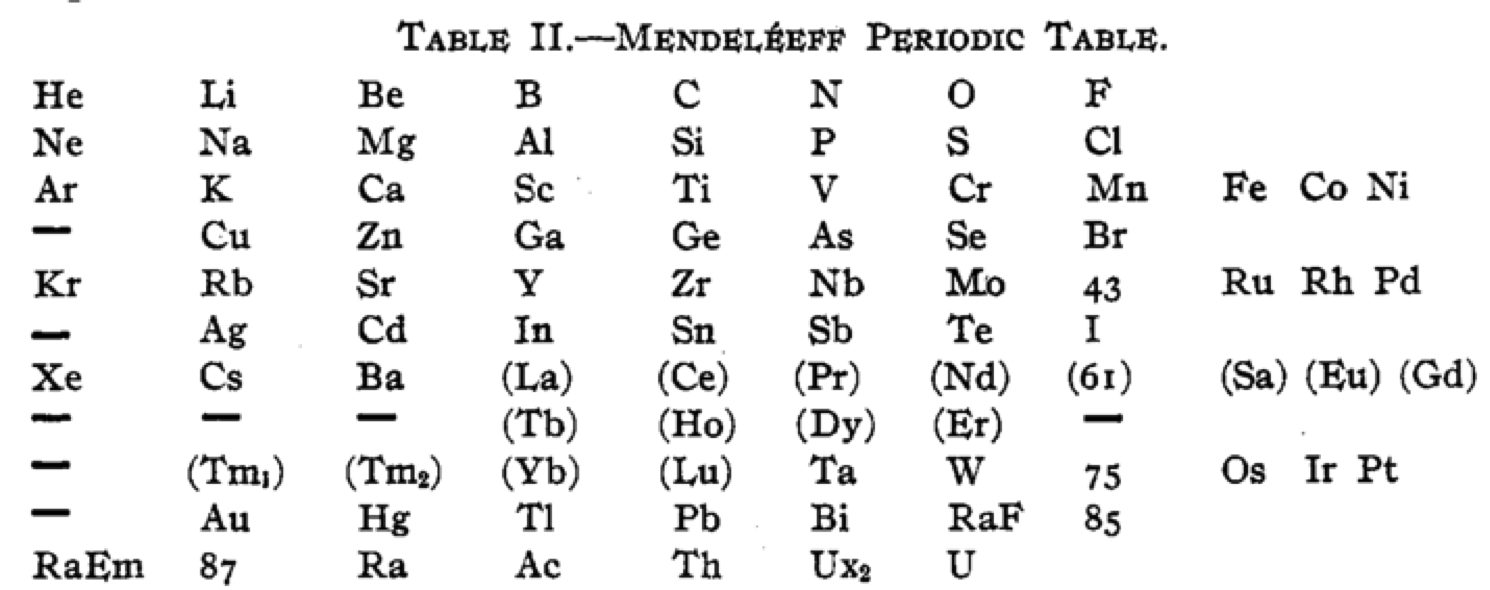
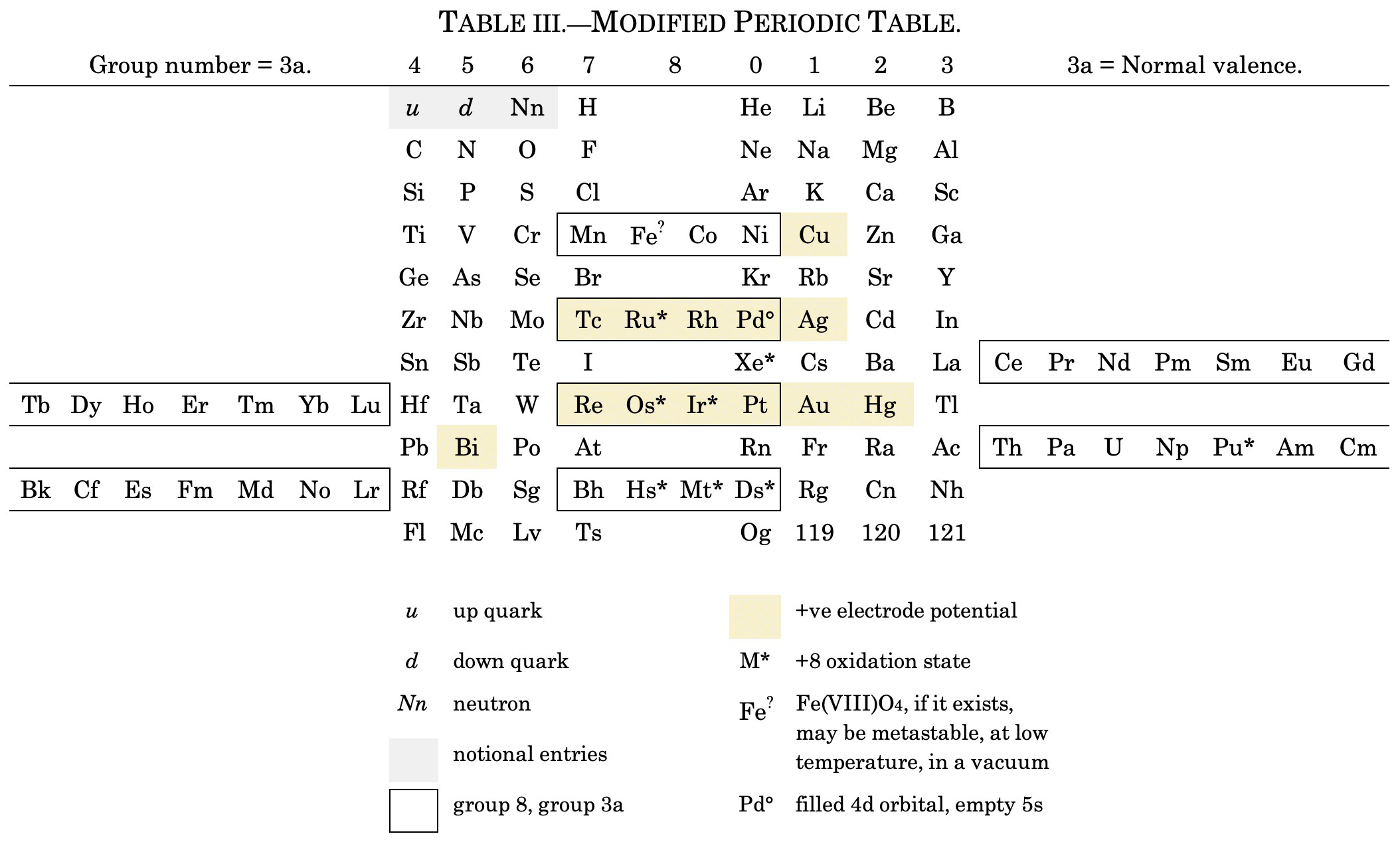
Cherkesov: Two Periodic Tables
von Bichowsky FR, The place of manganese in the periodic system, J. Am. Chem. Soc. 1918, 40, 7, 1040–1046 Publication Date: July 1, 1918 https://doi.org/10.1021/ja02240a008
René Vernon writes:
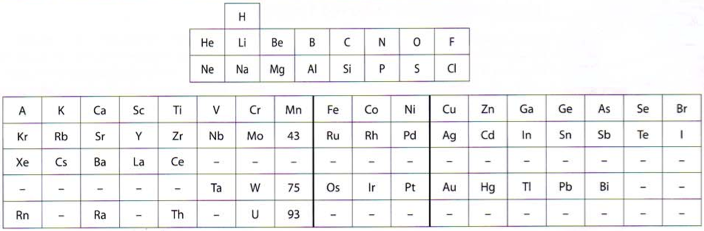
"In this curious article, von Bichowsky, a physical chemist (1889-1951), mounted an argument for regarding Mn as belonging to group 8 (see table 1 below) rather than group 7 (table 2). His article has effectively been assigned to the dustbin of history, having apparently gathered zero citations over the past 103 years.
"Items of note in his 24-column table:
- While Mn, 43 and 75 are assigned to group 8 they remain in alignment with group 7. Se is shown as Sc
- 14 lanthanides, from Ce to Yb, make up group 3a; If La and Lu are included, there are 16 Ln
- Gd is shown as Cd
- Positions of Dy and Ho have been reversed
- Tm and Tm2
- Po shown as "RaF"
- Ra shown as "RaEm"
- Pa shown as Ux2
von Bichowsky made his argument for Mn in group 8, on the following grounds:
- by removing the Ln from the main body of the table all of the gaps denoted by the dashes (in table 2) were removed
- the eighth group links Cr with Cu; Mo with Ag; and W with Au
- the symmetry of the table is greatly increased
- the triads are replaced by tetrads and a group of 16 Ln which accords better with "the preference of the periodic system for powers of two"
- about eight chemistry-based differences between Ti-V-Cr and Mn, including where Mn shows more similarities to Fe-Co-Ni, for example:
- divalent Ti, V, Cr cations are all powerful reducing agents, Cr being one of the most powerful known; divalent Mn, Fe, Co, Ni are either very mild reducing agents as divalent Mn or Fe, or have almost no reducing power in the case of divalent Co or Ni;
- metal titanates, vanadates and chromates are stable in alkaline solution and are unstable in the presence of acid whereas permanganates are more stable in acid than alkali; their oxidizing power is also widely different.
I can further add:
- Mn, Fe, and Co, and to some extent Ni, occupy the "hydrogen gap" among the 3d metals, having no or little proclivity for binary hydride formation
- the +2 and +3 oxidation states predominate among the Mn-Fe-Co-Ni tetrad (+3 not so much for Mn)
- in old chemistry, Mn, Fe, Co, and Ni represented the "iron group" whereas Cr, Mo, W, and U belonged to the "chromium group": Struthers J 1893, Chemistry and physics: A manual for students and practitioners, Lea Brothers & Co., Philadelphia, pp. 79, 123
- Tc forms a continuous series of solid solutions with Re, Ru, and Os
Moving forward precisely 100 years, Rayner-Canham (2018) made the following observations:
- Conventional classification systems for the transition metals each have one flaw: "They organise the TM largely according to one strategy and they define the trends according to that organisation. Thus, linkages, relationships, patterns, or similarities outside of that framework are ignored."
- There are two oxide series of the form MnO and Mn3O4 which encompass Mn through Ni. Here the division is not clear cut since there are also the series Mn2O3 for Ti-Cr and Fe; and MnO2 for Ti to Cr.
- Under normal condition of aqueous chemistry, Mn favours the +2 state and its species match well with those of the following 3d member, Fe.
Rayner-Canham G 2018, "Organizing the transition metals" [a chapter in] in E Scerri & G Restrepo, Mendeleev to Oganesson: A multidisciplinary perspective on the periodic table, Oxford University Press, Oxford, pp. 195–205
I've also attached a modern interpretation of von Bichowsky’s table. It's curious how there are eight metals (Fe aside) capable of, or thought to be capable of, achieving +8. I am not sure that a table of this kind with Lu in group 3 is possible, without upsetting its symmetry."
| Year: 1918 | PT id = 1300, Type = formulation |
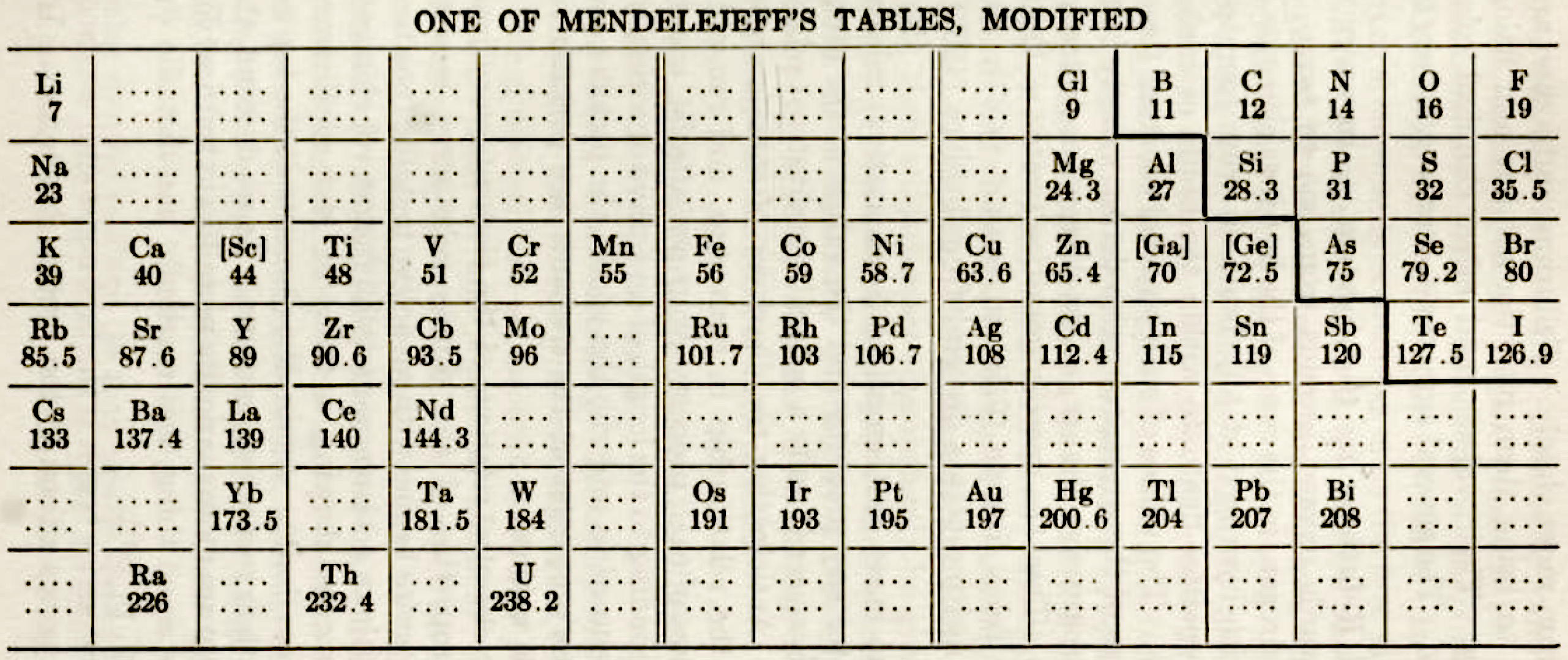
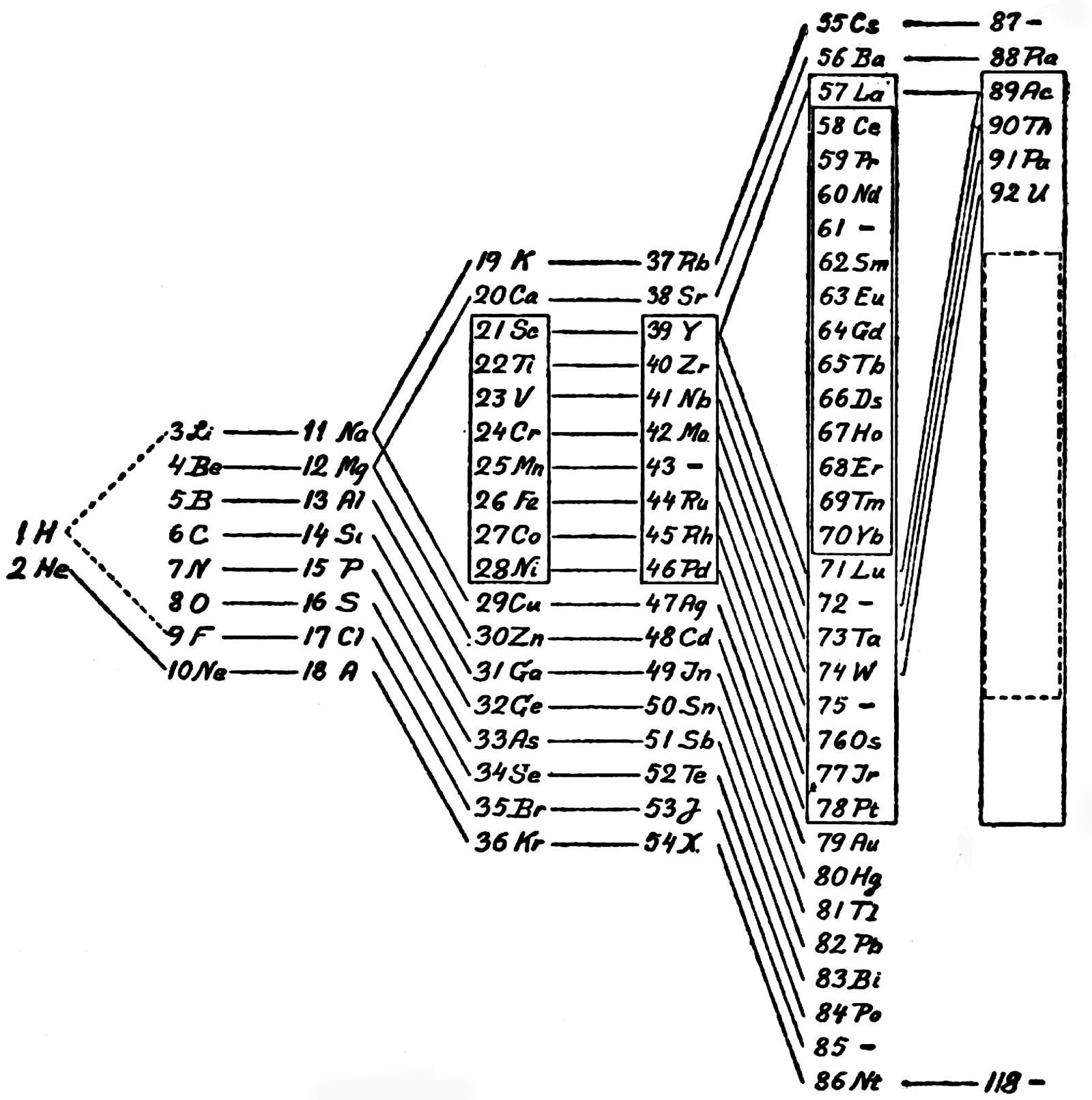
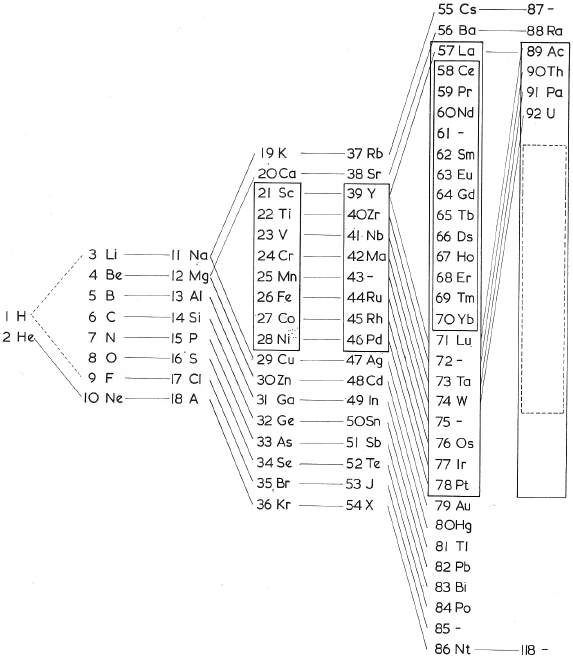
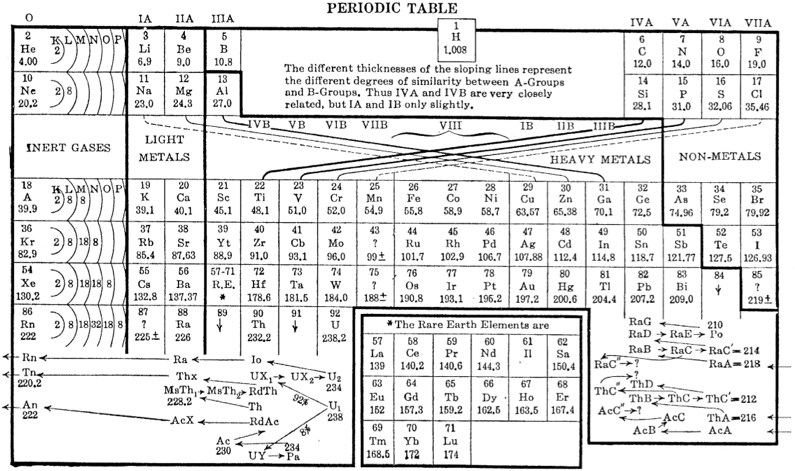
One of Mendelejeff's Tables, Modified
From Smith A 1918, General Chemistry for Colleges, 2nd ed., The Century Co., New York, p. 299
René Vernon writes:
- H is missing, as are the noble gases.
- Consequently, the period numbers are out by one apiece.
- Seven groups are on the left and seven are on the right (the ever present allure of symmetry).
- After La, Ce is placed under Zr, and Nd is placed under columbium/technetium.
- According to Smith the rest of the lanthanide elements do not fit into any series, because their valences and other chemical properties do not permit most of them to be distributed over so many different groups.
- Po is expected to be a metal which is what it turned out to be Smith has anticipated that astatine will be a metal. Nine decades later, Hermann, Hoffmann & Ashcroft (2013) predicted the same thing: Hermann, A.; Hoffmann, R.; Ashcroft, N. W. (2013). Condensed astatine: Monatomic and metallic. Physical Review Letters, 111 (11), 116404-1–116404-5
- While he does not discuss it, Smith appears to have allowed for missing elements between Li and Gl and between Na and Mg.
- The three elements inside square brackets are those predicted by Mendeleev.
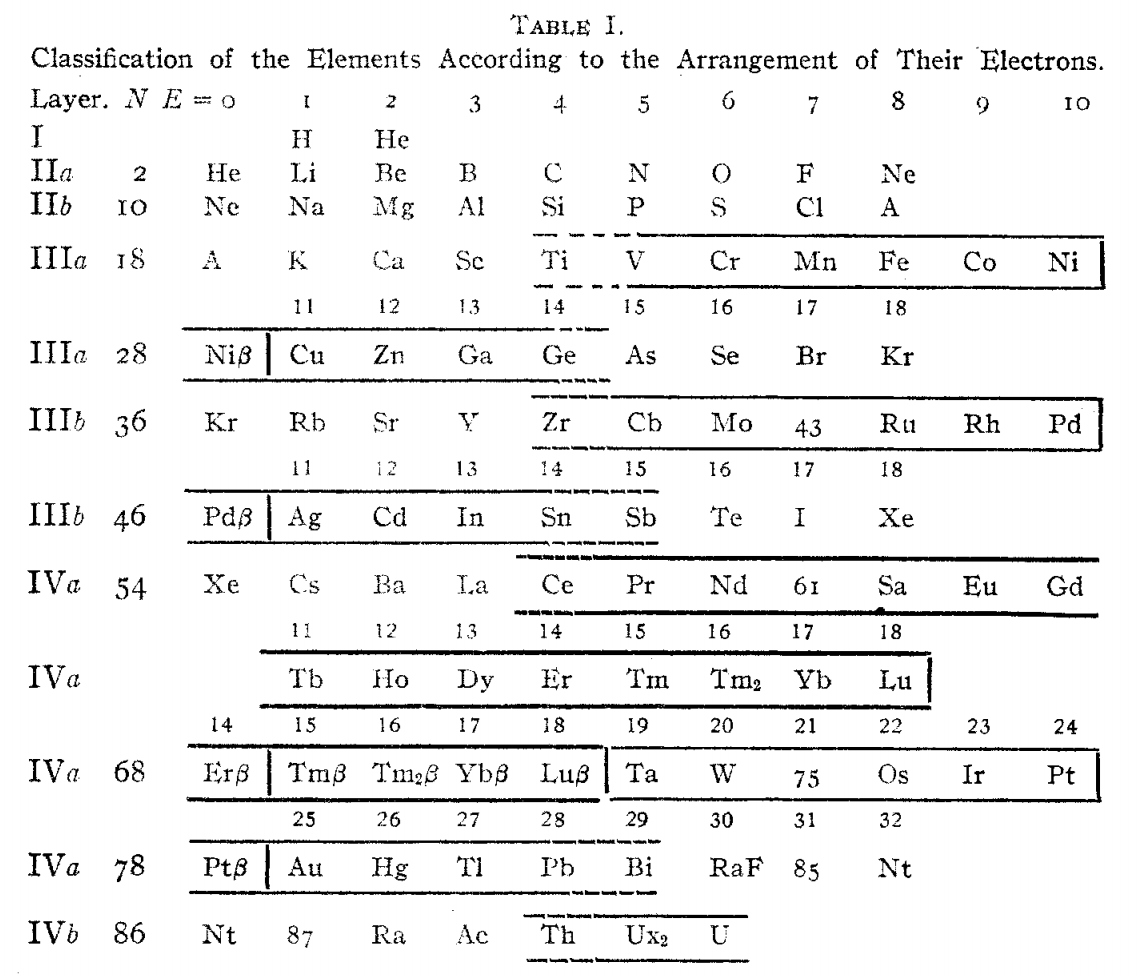
| Year: 1919 | PT id = 434, Type = formulation |
Langmuir's Periodic Table
From Irving Langmuir's theory of the Arrangement of Electrons in Atoms, J.Am.Chem.Soc., 41, 868 (1919), Langmuir's 1919 periodic table formulation.
This formulation seems to be the basis of Seaborg's formulations of 1939, 1942 & 1945:
| Year: 1919 | PT id = 547, Type = formulation |
Hackh's Classification of the Elements, Updated
From a Scientific American in March 1919, an article by Ingo W. D. Hackh discussing the classification of the elements.
Shown is a periodic table slightly updated from a version from two years before, and referenced by Quam & Quam:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1919 | PT id = 548, Type = formulation spiral |
Hackh's Periodic Spiral
From a Scientific American in March 1919, an article by Ingo W. D. Hackh discussing the classification of the elements.
Included is a periodic spiral, developed from Hackh's 1914 version:
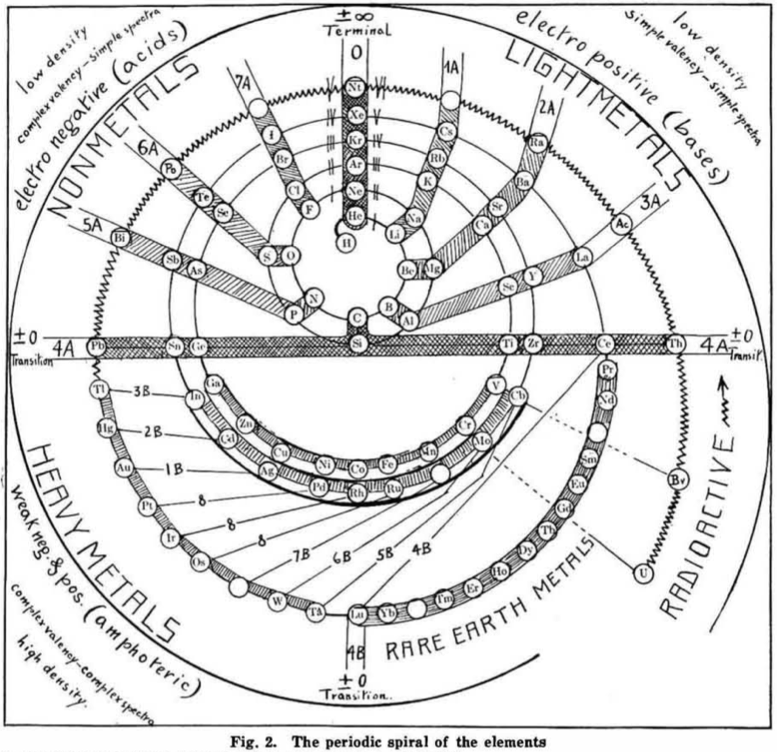
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1919 | PT id = 549, Type = formulation data |
Hackh's Periodic Chain
From a Scientific American in March 1919, an article by Ingo W. D. Hackh discussing the classification of the elements.
Included is a periodic chain showing the redox states of the elements:
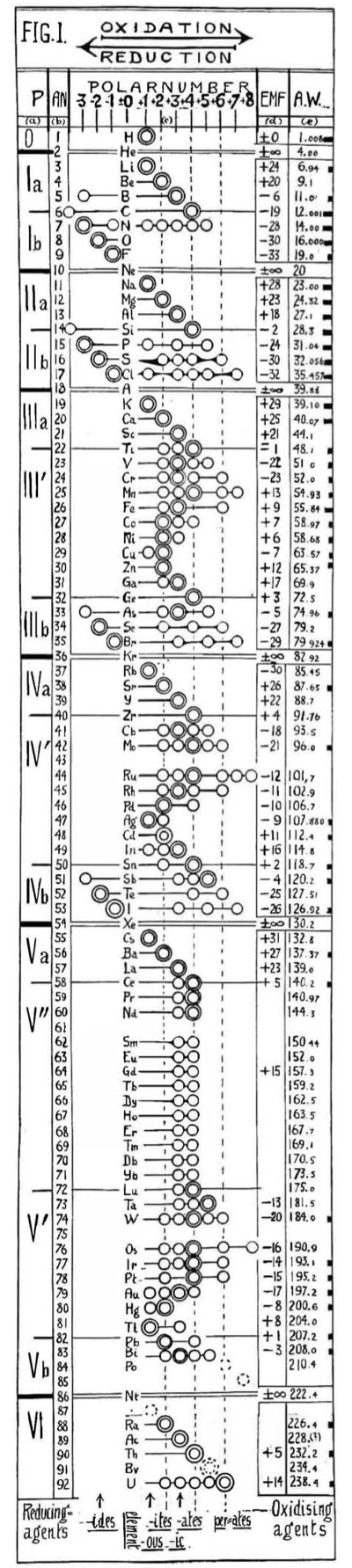
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1919 | PT id = 855, Type = element |
Discovery of Rhenium
Re
Rhenium, atomic number 75, has a mass of 186.207 au.
Rhenium was first observed or predicted in 1908 by M. Ogawa and first isolated in 1919 by M. Ogawa.
| Year: 1919 | PT id = 1293, Type = formulation |
Snyder's Fundamental Periodic Table of The Elements
Snyder MB 1919, The Fundamental Periodic Table of the Chemical Elements, filed in Congressional Library, Washington.
René Vernon writes:
"Notable for:
- Its attempted integration of the Ln and An into the short form of the periodic table
- Placement of H over He, Li and F
- Elements 108 = Pleon; 126 = Akron; 143 = Ultine"
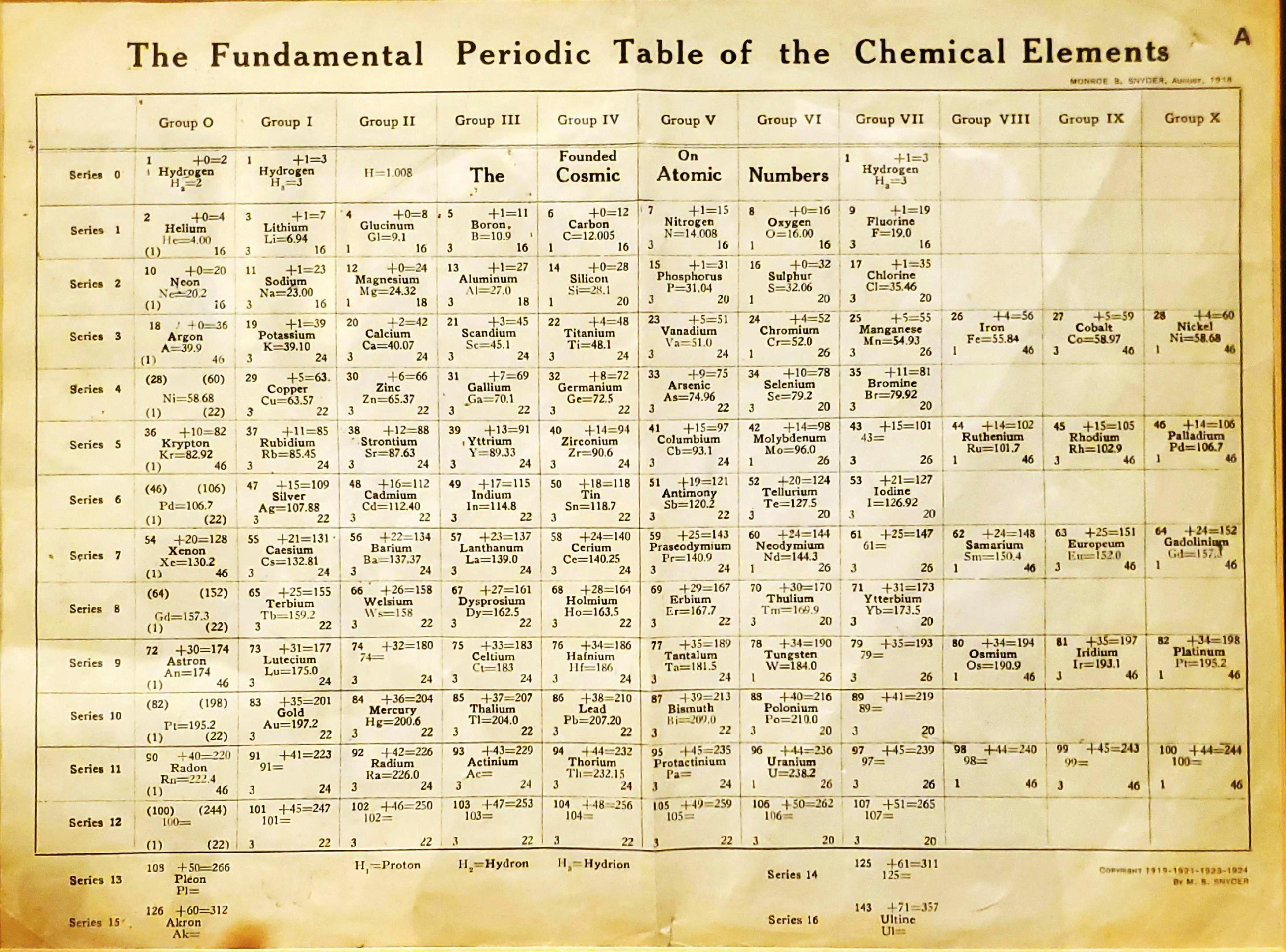
| Year: 1920 | PT id = 72, Type = formulation spiral |
Nodder's Periodic Table
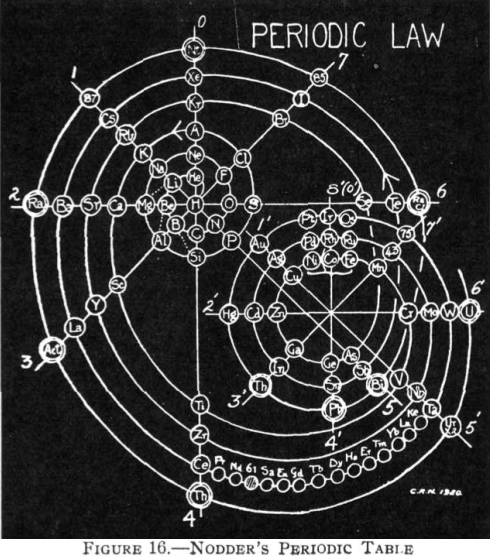
From Quam & Quam's 1934 review paper.pdf
| Year: 1920 | PT id = 73, Type = formulation spiral |
Partington's Periodic Arrangement of the Elements
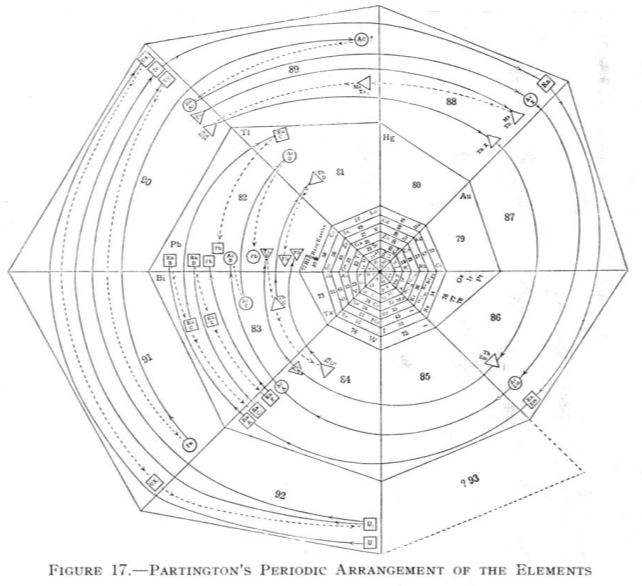
From Quam & Quam's 1934 review paper.pdf
| Year: 1920 | PT id = 78, Type = formulation 3D spiral |
Schaltenbrand's Helical Periodic Table
G. Schaltenbrand, Darstellung des periodischen Systems der Elemente durch eine räumliche Spirale, Z. anorg. allgem. Chem., 112, 221-4 (Sept. 1920)
From Quam & Quam's 1934 review:
"The elements are arranged in order of atomic weights on an eccentric spiral. The four sets of curves include positions of similar elements. The first small turn carries H and He; the remainder of the inert elements and the halogens are on successive small turns in analogous positions.
"On the next larger turn are found the alkali, alkaline-earth, and aluminum family elements.
"The long periods require larger turns and the period containing the rare-earth elements requires the longest turn of all. Elements of the same group are found in the same plane passing through the axis of the spiral."
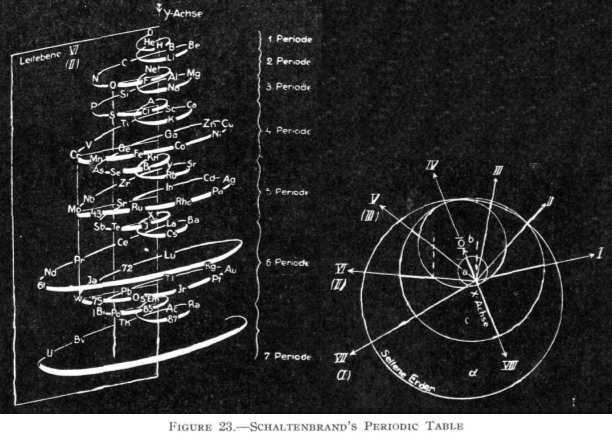
Commissioned in 2019 to match George Schaltenbrand's 1920 design for a helical gathering of the elements – albeit extended to all 118 current elements – and signed by Yuri Oganessian, it is almost certainly the most expensive periodic table in the world."


| Year: 1920 | PT id = 292, Type = formulation 3D |
Kohlweiler's System
Kohlweiler's system of 1920 (from van Spronsen):
| Year: 1920 | PT id = 1070, Type = formulation |
Black & Conant's Periodic Classification Of The Elements
From N.H. Black NH & J.B. Conant's Practical Chemistry: Fundamental Facts and Applications to Modern Life, MacMillan, New York (1920)
Eric Scerri, who provided this formulation writes (personal communication):
"Notice conspicuous absence of H. And, Conant was the person who gave Kuhn his first start in the history of science at Harvard."
René Vernon tells us that Conant and his coauthor write:
"The position of H in the system has been a matter of some discussion, but it is not of much consequence. It seems to be rather an odd element. Perhaps the best place for it is in group IA as it forms a positive ion." (p. 350)
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1920 | PT id = 1075, Type = formulation |
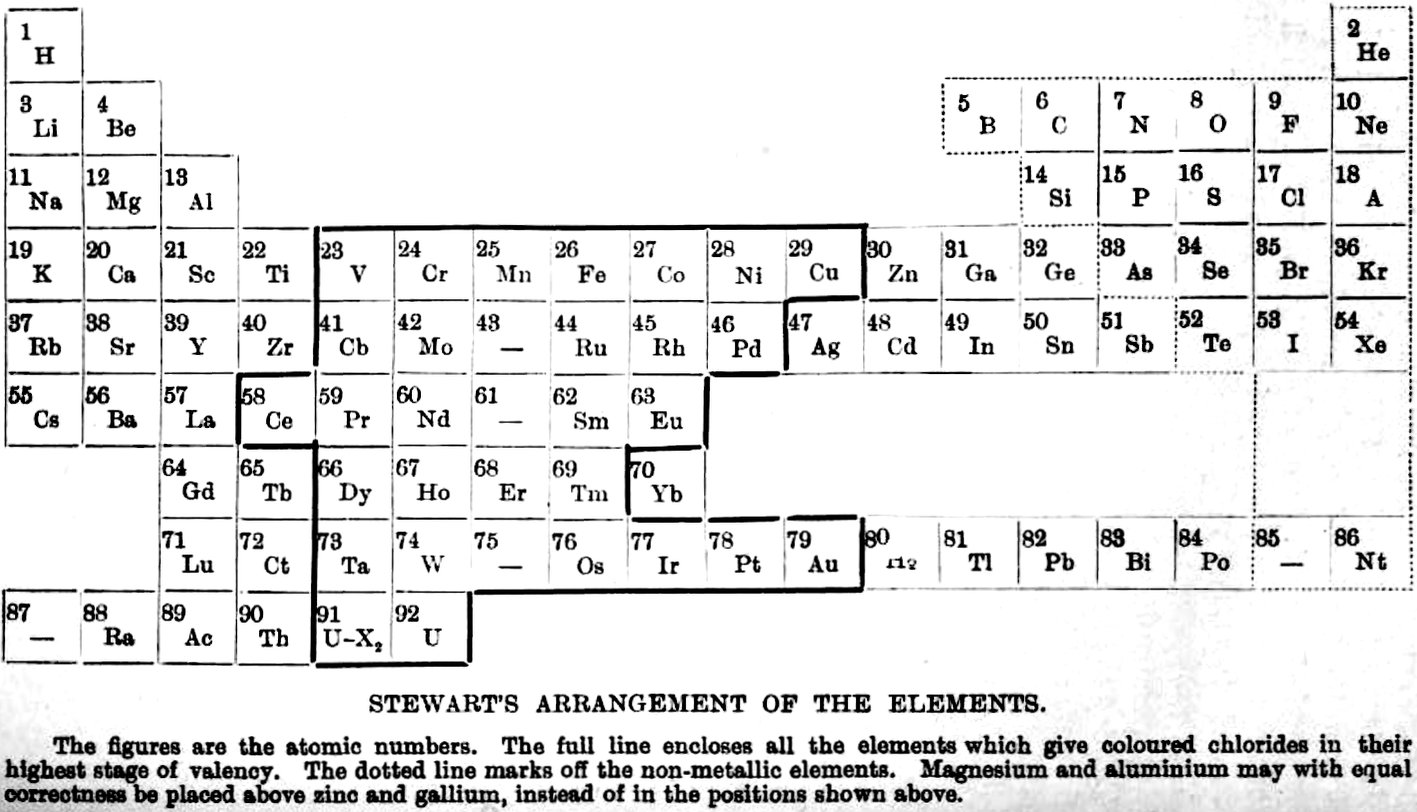
Stewart's Arrangement of The Elements
From A.W. Stewart, Recent Advances in Physical and Inorganic Chemistry, 3rd ed., Longmans, Green and Co., London (1920)
René Vernon writes:
"Stewart discusses the 'forced symmetry' of Mendeleev's table, and the distinction between 'facetious symmetry' (as he calls it) and the actual correlation of facts (as he saw them at that time)."
Extracts:
237. Mendeleev... objected strongly to the employment of graphic methods of expressing the Periodic Law, on the ground that such methods did not indicate the existence of a limited and definite number of elements in each period.
239. The Periodic Table, as laid down by Mendeleeff in his writings, exhibits a symmetry which was one of its greatest assets. For some psychological reason, symmetry has an attraction for the human mind; and we are always apt to prefer a regular arrangement to one in which irregularities pre- dominate. Psychological peculiarities are, however, undesirable guides in the search for truth; and a careful examination of the Table in the light of our present knowledge will suffice to show that it can boast of no such symmetry as we are led to expect from the text-books of our student days.
For example, owing to the omission of some of the rare earth elements and by the insertion of blanks, the Table in its original form attained a very high degree of regularity; but since there are, as we know from the X-ray spectra results, only sixteen elements to fill the eighteen vacant spaces in the Table, it is evident that the symmetry of Mendeleeff s system is purely factitious.
Further, in order to produce the appearance of symmetry, Mendeleeff was forced to place copper, silver, and gold in the first group, although there is no known oxide Au2O and the stable chloride of gold is AuCl3.
These examples are well-known, and are mentioned here only for the purpose of enforcing the statement that the symmetry of Mendeleef's system cannot be sustained at the present day. Fascinating though its cut-and-dried regularity may be, we cannot afford to let symmetry dominate our minds when in actual fact there is no symmetry to be found.
240. The most superficial examination shows that, instead of being a symmetrical whole, the Table is really pieced together from a series of discrete sections.
250. The first attempt to arrange all the elements in a periodic grouping took the form of a three-dimensional model the Telluric Helix of de Chancourtois and it is not surprising that from time to time attempts have been made to utilize the third dimension as an aid to classification. It cannot be said that much light has been thrown on the matter by these essays; but some account of them must be given here for the sake of completeness.
251. The main drawback to the spiral representation appears to be that in it no new facts are brought to light, and there is no fresh collocation of the allied elements which might give it an advantage over the ordinary forms of classification. Also, in most cases it is more difficult to grasp as a whole.
253 ...if we have to choose between factitious symmetry and actual correlation of facts, we must decide in favour of the latter, discomforting though the choice may be.
255. The following new grouping seems worth considering. Although it has many good points, it is not to be regarded as a final solution, but is put forward mainly in the hope that an examination of it may suggest some more perfect system.
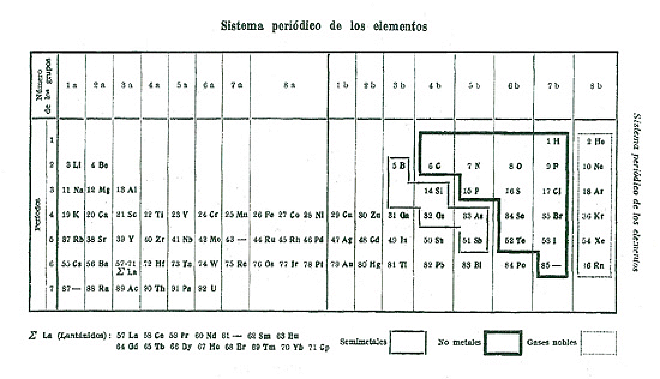
| Year: 1921 | PT id = 68, Type = formulation |
Margary's Periodic Table
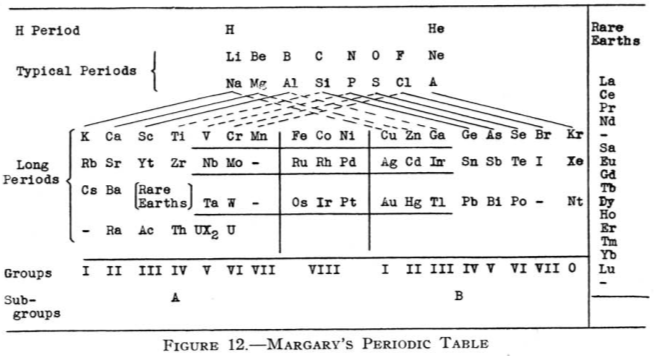
From Quam & Quam's 1934 review paper.pdf
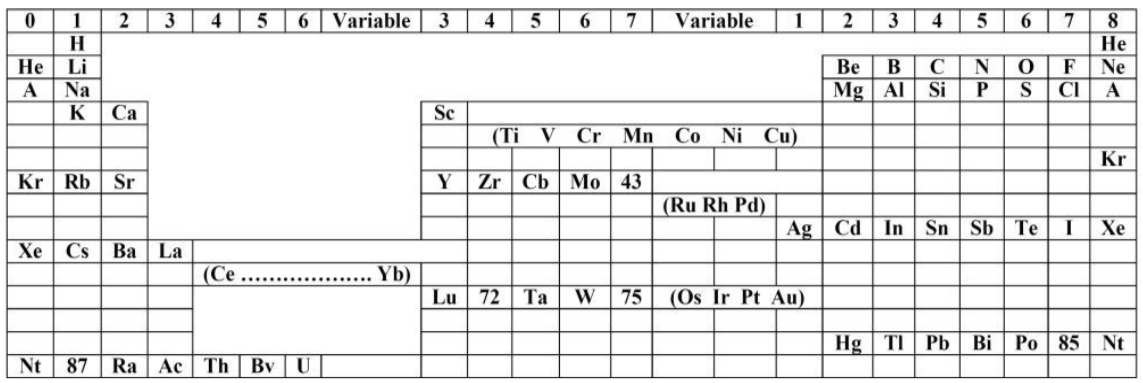
| Year: 1921 | PT id = 1020, Type = formulation |
Bury's Periodic Arrangement based on Langmuir's Theory
Using Langmuir's theory of the arrangement of electrons in atoms, J.Am.Chem.Soc., 41, 868 (1919), Charles R. Bury formulated a Periodic Arrangement: C.R. Bury, Langmuir's theory of the arrangement of electrons in atoms and molecules, J. Am. Chem. Soc., 43, 1602-1609 (1921).
This formulation seems to be the basis of Seaborg's formulations of 1939, 1942 & 1945.
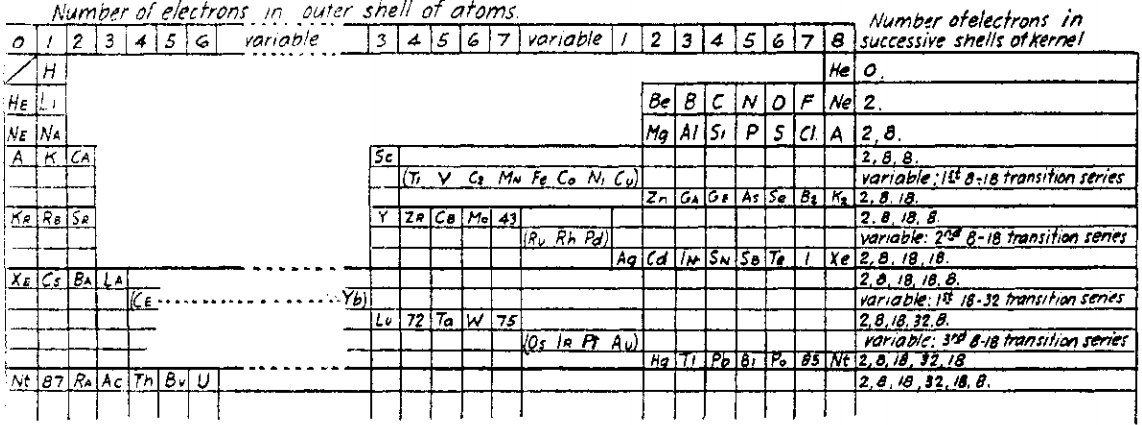
Ricardo R Contreras, Avances en Química, 14(1), 41-60 (2019), has re-drawn the Bury PT and writes [Google Translate]:
"This version emphasizes periods and electronic configurations.
"There is a long period in which the metals of titanium to copper are found, which he calls transition elements. [This formulation] leaves spaces for the element atomic number 43, technetium, discovered by Perrier Segre in 1937; for the element 72, hafnium, discovered in 1932 by D. Coster and G. von Hevesey; for the element 87, the eka-cesium, which corresponds to francium (Fr), discovered in 1939 by the French physicist Marguerite C. Perey (1909-1975) and, at the end of the group of halogens, for the element 85, the astatine (At), synthesized for the first time in 1940 by American physicists Dale R. Corson (1914-2012), Kenneth R. MacKenzie (1912-2002) and the Italian-American physicist Emilio G. Segrè (1905-1989) at the University of Berkeley (California), bombarding bismuth with particles.
"Bury uses 'A' as the symbol argon, 'Nt' (niton) for radon (Rn) and, the symbol 'Bv' (brevium) for proctactinium (Pa)."
| Year: 1921 | PT id = 1192, Type = formulation |
Formánek's Periodic Table
Formánek J. 1921, Short Outline of Inorganic Chemistry (in Czech), 2nd ed., Ministerstvo zemedelstvi CSR, Praha. p. 281
René Vernon writes:
Here is an eight column table with some interesting features.
Main groups 0, Ia, IIa, Vb, VIb, and VIIb, correspond to what we have today:
- 0 Noble gases
- Ia Alkali metals
- IIa Alkaline earths
- Vb Pnictogens
- VIb Chalcogens
- VIIb Halogens
Main group IIIa is B-Al-Sc-Y... Ac whereas these days B-Al have been moved over Ga on electronic grounds. This happened despite the fact that the average trend line for chemical and physical properties v Z going down B-Al-Sc-Y... Ac is more regular.
In main group IV, notice how C and SI are positioned in the middle of the cell, unlike their neighbours to either side. The group thus bifurcates after Si into a Ti branch and a Ge branch. This is quite reasonable since there is not much difference in the average trendlines going down either option. In any case, C-Si came to be moved over Ge again on electronic grounds.
He survived the electronic revolution, staying over Ne.
| Year: 1921 | PT id = 1237, Type = formulation |
Margary's Modified Table
Ivan D. Margary B.A. (1921) XXXVI. A modification more in accord with atomic structure, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 42:248, 287-288.
An old school table showing group 3 as B-Al-Sc-Yt-Rare earths.
Thanks to René for the tip!
| Year: 1922 | PT id = 285, Type = formulation |
Bohr's System
Niels Bohr's system of 1922 (Theory of Spectra and Atomic Constitution, Cambridge University Press) and as reproduced by van Spronsen:
| Year: 1922 | PT id = 852, Type = element |
Discovery of Hafnium
Hf
Hafnium, atomic number 72, has a mass of 178.49 au.
Hafnium was first isolated in 1922 by D. Coster and G. von Hevesy.
| Year: 1922 | PT id = 1129, Type = formulation |
Aston's Periodic Table of The Elements
ISOTOPES F.W. ASTON, M.A., D.S.C., A.I.C., F.R.S., London, 1922, Edward Arnold & Co.
Harry F. Tasset writes:
"Francis Aston was a chemist and a physicist who pioneered the discovery of the isotopes. He was a Fellow of the Royal Society and worked with J.J. Thomson. He started his work with the mass spectrometer. His periodic table was published in 1922 and is remarkable because it was one of the first attempts to group the rare earth elements. His success in separating the isotopes was rewarded with the Nobel Prize in Chemistry in 1922."
Click image to enlarge:
Thanks to Harry F. Tasset for the tip and info!
| Year: 1923 | PT id = 360, Type = formulation |
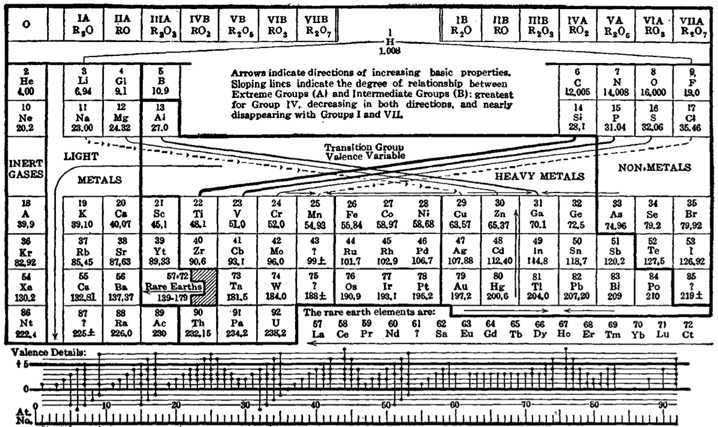
Deming Periodic Table
H.G. Deming used the long periodic table in his textbook General Chemistry, which appeared in the USA for the first time in 1923 (Wiley), and designated the first two and the last five Main Groups with the notation "A", and the intervening Transition Groups with the notation "B".
The numeration was chosen so that the characteristic oxides of the B groups would correspond to those of the A groups. The iron, cobalt, and nickel groups were designated neither A nor B. The Noble Gas Group was originally attached (by Ueming) to the left side of the periodic table. The group was later switched to the right side and usually labeled as Group VlllA.
This version of the periodic table was distributed for many years by the Sargent-Welch Scientific Company, Skokie, Illinois, USA.:
| Year: 1923 | PT id = 456, Type = formulation |
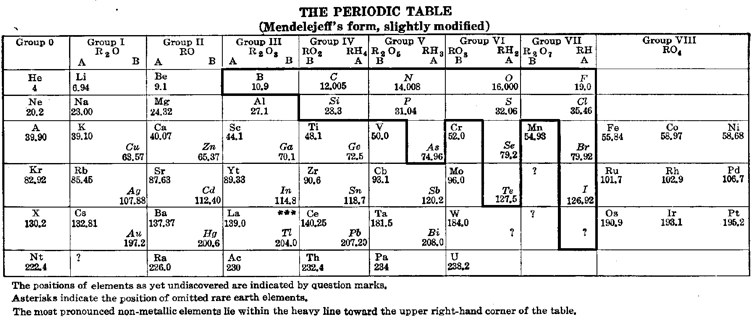
Deming’s Other 1923 Periodic Table: Mendeleev style
Deming's "other" 1923 periodic table: a Mendeleev style formulation with an unusual metal-non-metal dividing line:
| Year: 1923 | PT id = 941, Type = formulation |
Lewis' Periodic Table
From G.N. Lewis' book: VALENCE and the Structure of Atoms and Molecules, The Chemical Catalog Company (1923).
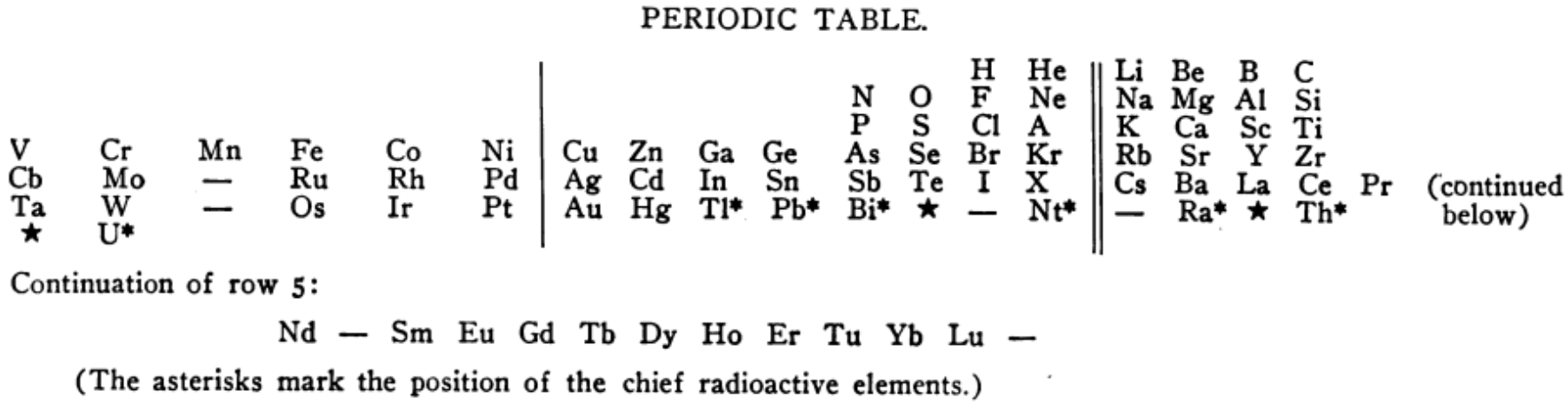
| Year: 1923 | PT id = 1198, Type = formulation |
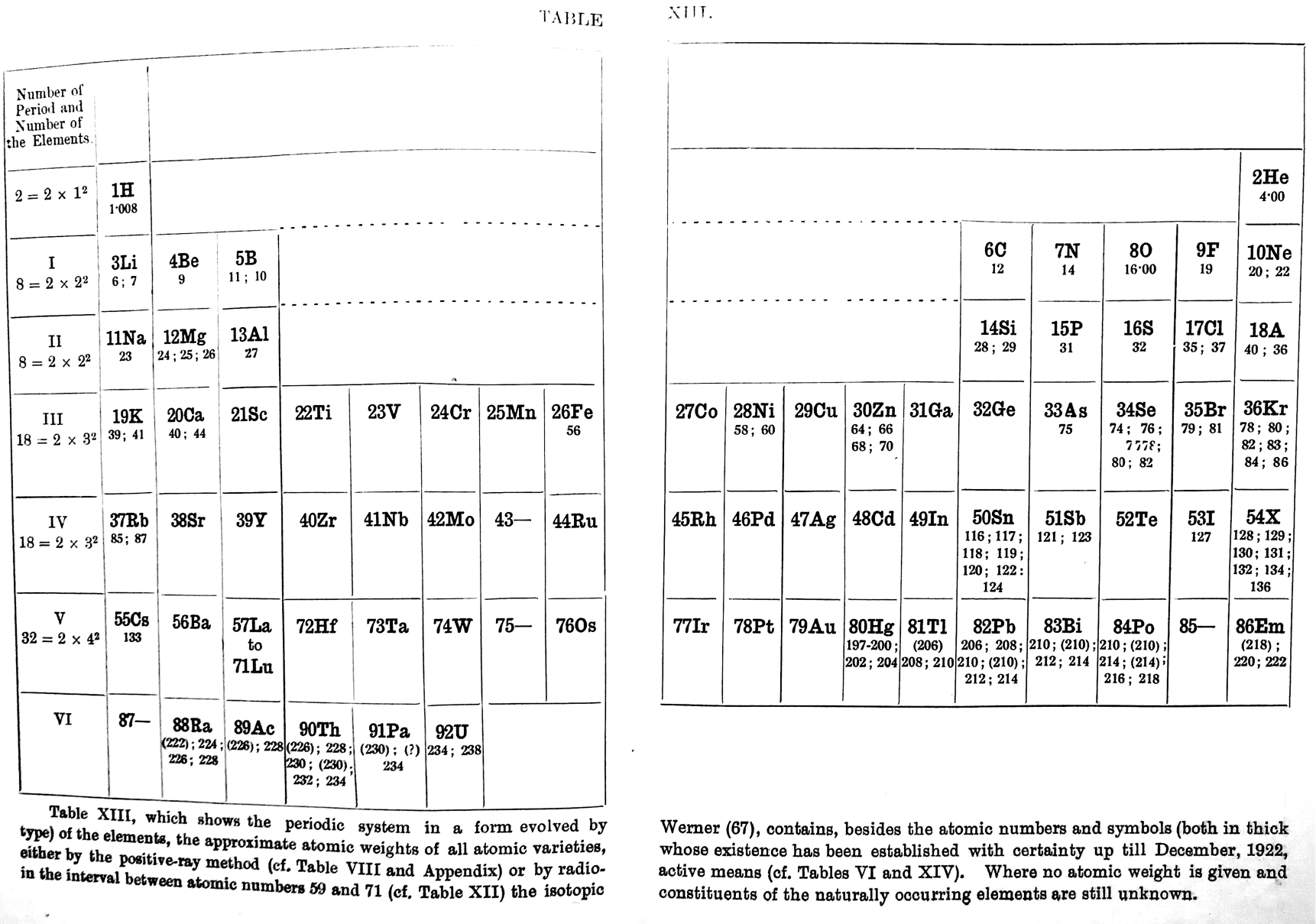
Fajans' Periodic Table
Fajans K., Radioactivity and the latest developments in the study of the chemical elements, trans. TS Wheeler, WG King, 4th German edition, Methuen & Co., London, pp. 116-117, 1923.
René Vernon writes: "An addition to the long list of tables with B-Al over Sc."
| Year: 1923 | PT id = 1256, Type = formulation review |
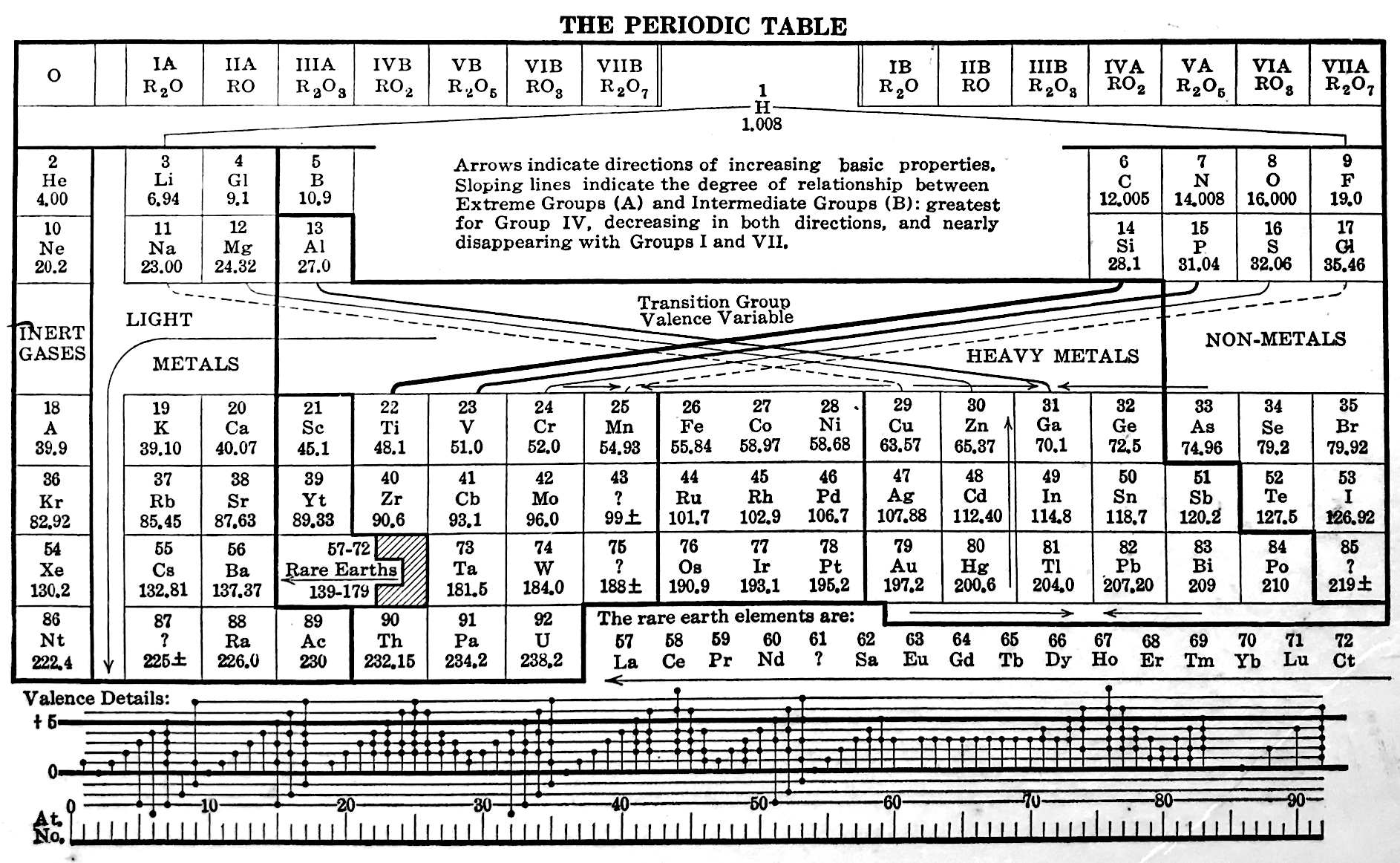
Deming's Periodic Table With Commentry by Vernon
René Vernon writes:
Deming's 1923 periodic table is credited with popularizing the 18-column form.
I now see Deming used different thickness sloping lines to represent the different degrees of similarity between the main groups and their corresponding transition metal groups.
- The line between Li-Na and group 11 is dashed, denoting the weakest relationship.
- Be-Mg are in group 2 The line between Be-Mg and group 12 is not dashed, denoting a stronger relationship.
- B-Al are in group 3
- The line between B-Al and Ga-In-Tl is thicker yet.
When I plot up to 20 chemical properties v Z going down these options I get the following values for the average smoothness of the trendlines:
- 73.5% for Li-Na-Cu(+2)-Ag(+1)-Au(+3) versus 84% for Li-Na-K-Rb-Cs
- 70% Be-Mg over Zn versus 85% for Be-Mg-Ca-Sr-Ba
- 81% for B-Al-Ga-In-Tl versus 88% B-Al-Sc-Y-La
I would have thought the smoothness for the line between Li-Na and Cu would be < 70%, consistent with Deming’s dashed line. But the thickness of the line would depend on what Deming took into account when he drew it. The common wisdom about groups 1 and 11 is that their similarities are: "confined almost entirely to the stoichiometries (as distinct from the chemical properties) of the compounds in the +1 oxidation state." (Greenwood & Earnshaw 2002, p. 1177). Kneen et al. (1972, p. 521) say that, "the differences between the properties of the group IA and IB elements are those between a strongly and weakly electropositive metal." On this basis I follow Deming’s dashed line. I’ve appended some notes about Group 1 and Group 11.
- Main group 4 is C-Si-Ge-Sn-Pb
- The line between Si and Ti-Zr-Hf is thick
- The line between N-P and V is less thick
- The line between O-S and Cr is less thick again
- The line between F-Cl and Mn is dashed
I have [calculated] a smoothness for C-Si-Ti-Zr-Hf of 86% versus 70% for C-Si-Ge-Sn-Pb. Since Ti shows some transition metal chemistry but not C-Si, it is perhaps plausible to keep C-Si-Ge-Sn-Pb together (as Deming did ).
Deming was a smart author. Nigh on a century later and the metrics check out.
More about group 1 and group 11
There may be a little more to the relationship between Li-Na & Cu-Ag-Au, than is ordinarily appreciated. For example:
- The resulting composite "group" has two electropositive metals and three more electronegative metals so its overall nature is more nuanced then purely group 1 or purely group 11
- The ionic radii of Li+ and Cu+ are 0.76 and 0.77 Å, and there is at least some discussion in the literature about substitution phenomena (Vasilev et al. 2019, p. 2-15; Udaya et al. 2020, p. 98; Kubenova 2021 et al.)
- Group 1 and 11 metal atoms form clusters relatively easily including Au_42+, Ag_64+, Rb_75+, Na_43+ (Mile et al. 1991, p. 134; Wulfsberg 2000, p. 631).
- In an organometallic context, Schade & Scheyler (1988, p. 196) wrote that, "There is much evidence that differences between group 1 and group 11 metals are not of principal but rather gradual manner."
- Although most nonmagnetic metals exhibit superconductivity it is significant that the Group 1 and 11 metals do not become superconducting at very low temperatures (Rao & Gopalakrishnan 1997, p. 398).
- Gold forms intermetallic compounds with all alkali metals (Schwerdtfeger et al. 1989. p. 1769)
References
- Greenwood NN & Earnshaw A 2002, Chemistry of the Elements, 2nd ed., Butterworth Heinemann, Oxford
- Kubenova et al. 2021, "Some thermoelectric phenomena in copper chalcogenides replaced by lithium and sodium alkaline metals", Nanomaterials 2021, vol. 11, no. 9. article 2238, https://doi.org/10.3390/nano11092238
- Mile et al. 1991, "Matrix-isolation studies of the structures and reactions of small metal particles", Farady Discussions, vol. 92, pp. 129–145 (134), https://doi.org/10.1039/FD9919200129
- Rao CNR & Gopalakrishnan J 1997, New Directions on Solid State Chemistry, 2nd ed., Cambridge University Press, Cambridge
- Schade C & Schleyer PVR 1988, "Sodium, potassium, rubidium, and cesium: X-Ray structural analysis of their organic compounds", Advances in Organometallic Chemistry, vol. 27, Stone FGA & West R (eds), Academic Press, San Diego, pp. 169–278
- Schwerdtfeger et al. 1989, "Relativistic effects in gold chemistry. I. Diatomic gold compounds.", The Journal of Chemical Physics, vol. 91, no. 3, pp. 1762–1774. https://doi.org/10.1063/1.457082
- Udaya et al. 2020, Metal sulphides for lithium-ion batteries, in Inamuddin, Ahmer & Asiri (eds), Lithium-ion batteries: Materials and applications, Materials Research Forum, Millersville PA, pp. 91–122
- Vasiliev AN et al. 2019, Low-dimensional Magnetism, CRC Press, Boca Raton
- Wulfsberg 2000, Inorganic chemistry, University Science Books, Sausalito, CA
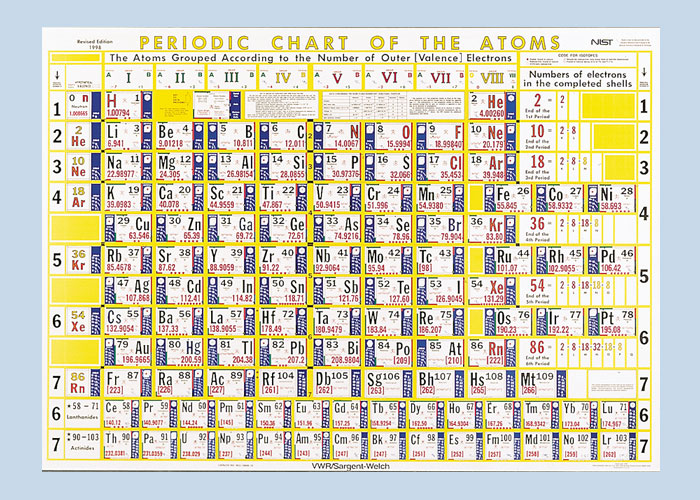
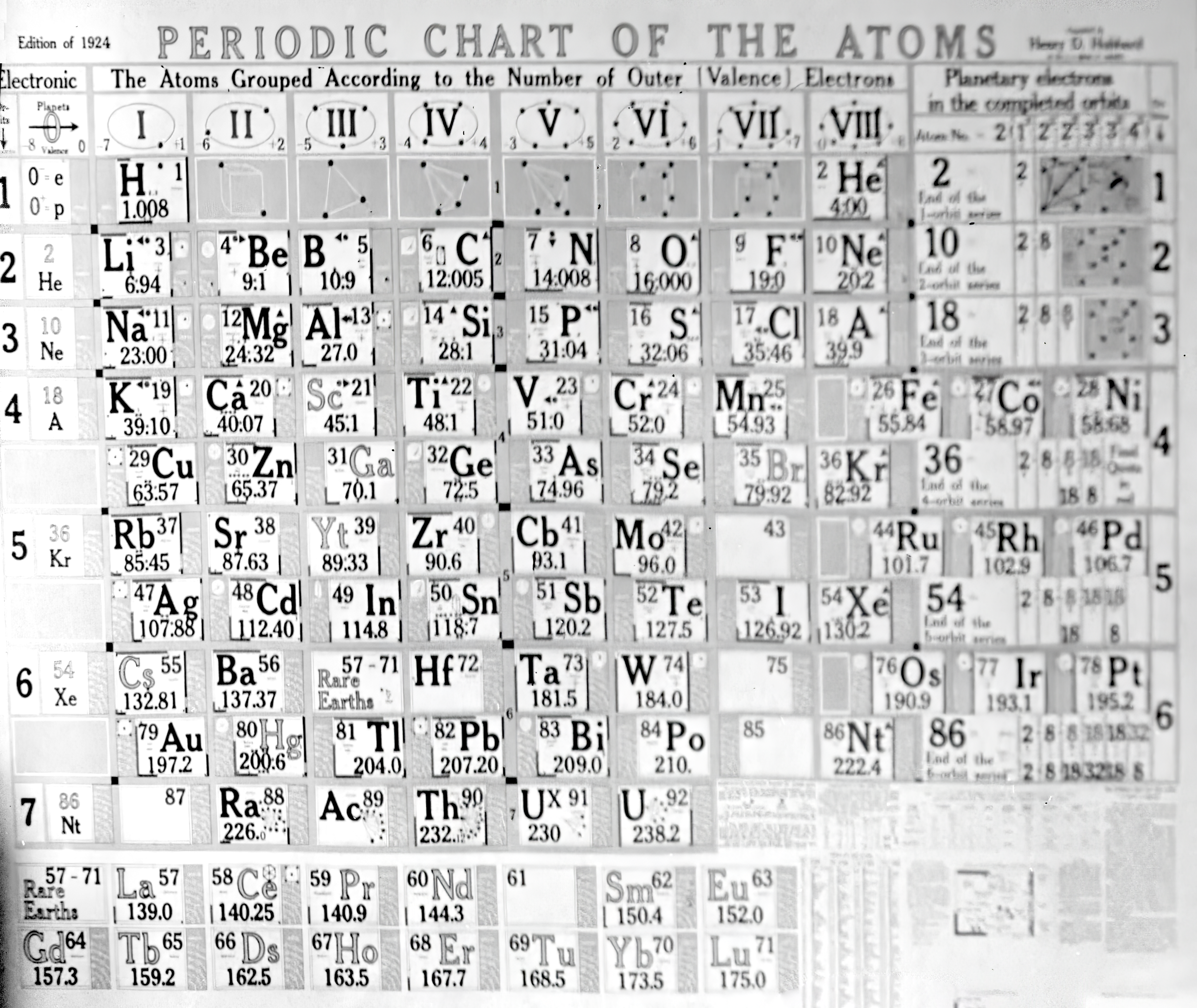
| Year: 1924 | PT id = 31, Type = formulation |
Hubbard Periodic Chart Of The Atoms
The American classic Henry Hubbard Periodic Chart Of The Atoms went through 12 editions.
A 1924 original on a dining room wall:

The current Sargent Welch version of the Henry Hubbard Periodic Table:
| Year: 1924 | PT id = 208, Type = formulation |
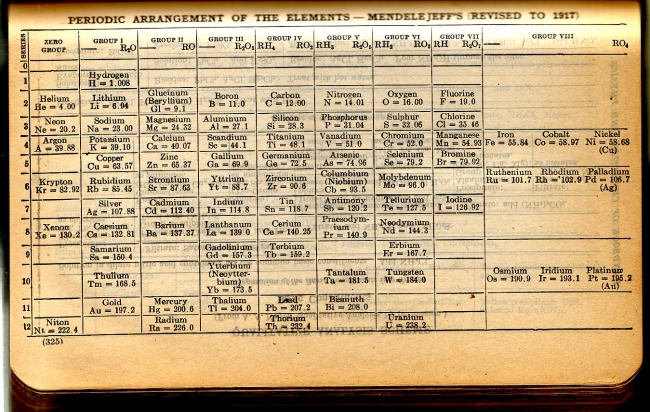
CRC Periodic Table
A periodic table from the 1924 CRC Handbook of Chemistry and Physics showing 79 elements. The text says "Revised To 1917". From here.
| Year: 1924 | PT id = 1342, Type = formulation |
Hubbard and His Periodic Table
Henry D. Hubbard in front of his 1924 periodic table from wikimedia:

Thanks to Doug Simpson for the tip!
| Year: 1925 | PT id = 84, Type = formulation 3D |
Friend's Periodic Sphere
J. A. N. Friend, "The periodic sphere and the position of the rare earth metals", Chem. News., 130, 196-7 (Mar., 1925).
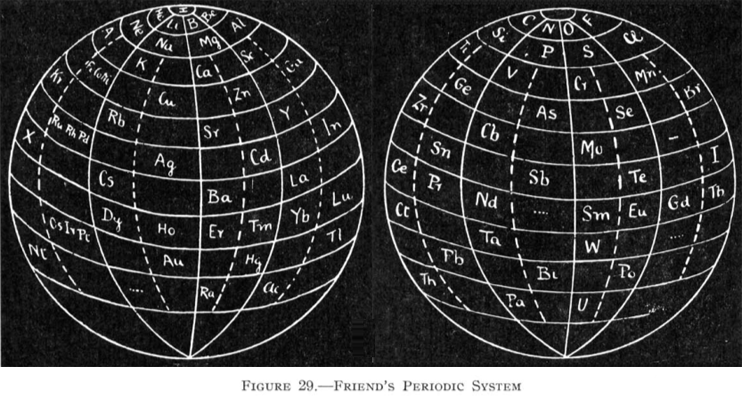
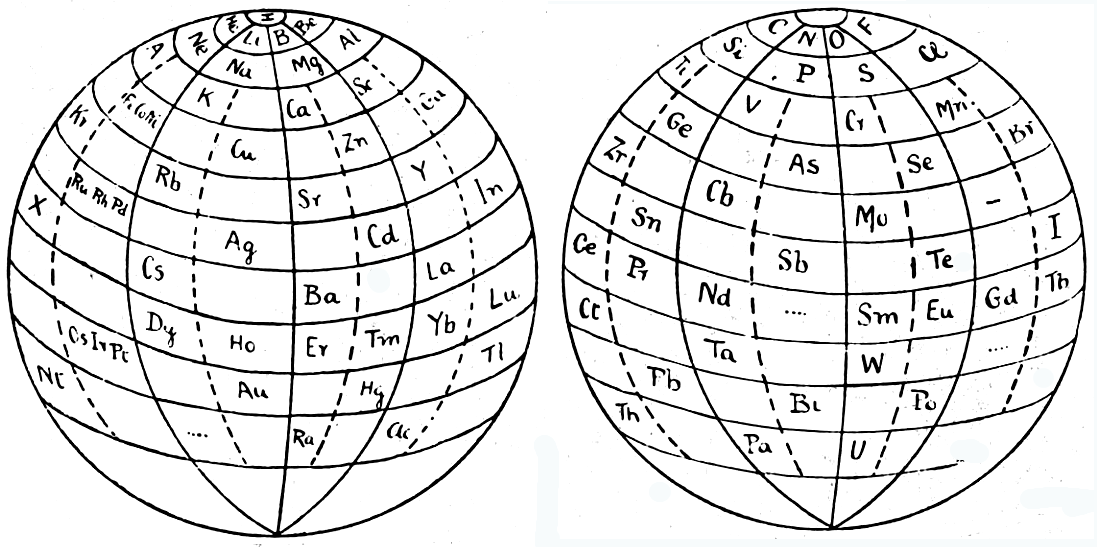
From Quam & Quam's 1934 review paper.pdf
| Year: 1925 | PT id = 308, Type = formulation |
Noddack Periodic Table
Ida Noddack studied the periodic table in the first half of the 20th century and was the co-discoverer of the last non-radioactive element to be isolated, rhenium. Later she worked on nuclear fission. In 1925 presented Noddack her formulation:
From Ida Noddack and the Missing Elements by Fathi Habashi, Education in Chemistry (March 2009)
| Year: 1925 | PT id = 460, Type = formulation |
Deming's (Updated) Periodic Table
This 1925 table has the Heavy Metals spread out, and the Rare Earth Elements (fifteen, including La and Lu) withdrawn into a box that is divorced from the body of the table. Ce, Gd, Yb form a vertical triad.
Th is assigned to Group IV below Hf.
From Michael Laing's paper: A Revised Periodic Table with the Lanthanides Repositioned, Found. Chem. (2005) 7: 203–233
| Year: 1925 | PT id = 735, Type = formulation 3D spiral |
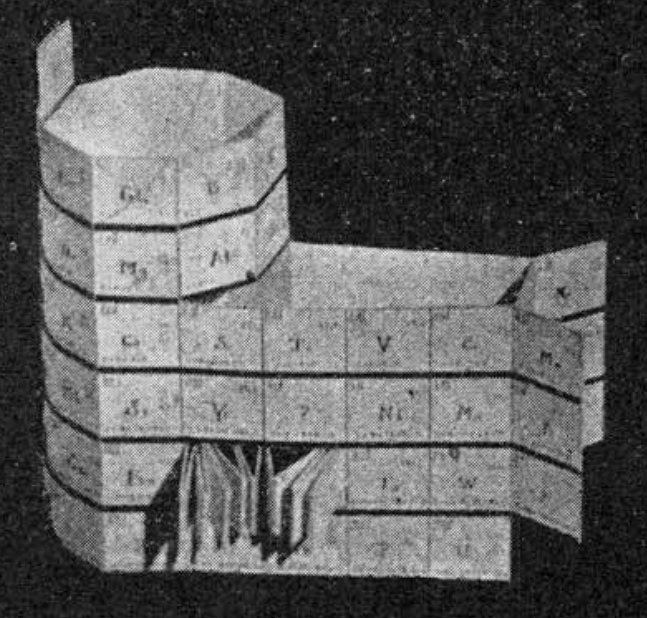
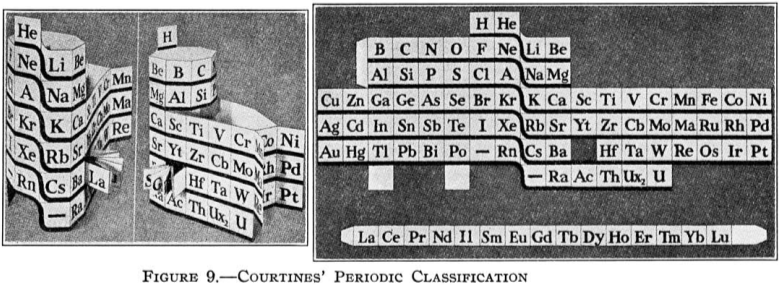
Courtines' Model of the Periodic Table or Periodic Classification
Published in J. Chem. Ed., 2, 2, 107-109 in 1925 by M. Courtines of the Laboratory of Experimental Physics, College of France, Paris.
Q&Q write:
"The unfolded tower arrangement appears much like a modernised Chauvierre chart cut on a line between Ni and Cu, Cu, with the right part fitted to the left in order of increasing atomic numbers. The rare-earth elements, however, are placed on a novel accordion-like folded strip with ends made secure just below Xt and between Ba and Hf. The author describes in detail the method of folding the chart into a tower-like cylindrical model. H is folded back to show its lack of relationship other groups of elements. In the space for each symbol, electron arrangements and isotopes are also enumerated."
And, in what appears to be a 'top down' view of the above 3D formulation, Courtine M 1926, Oùen est la physique, Gauthier-Villars et Cie, Paris:
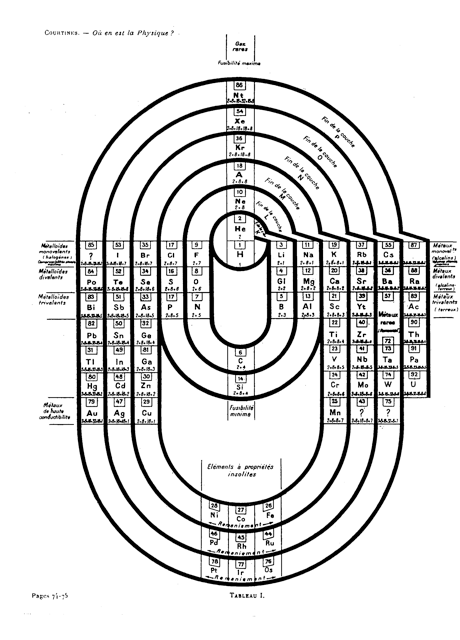
Thanks to Eric Scerri for the tip & René Vernon's additions!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1925 | PT id = 926, Type = formulation |
Sommerfeld's Electon Filling Diagram
Arnold Sommerfeld diagram appears in an issue of Memoirs and Proceedings of the manchester Literary and Philosophical Society for 1925-26. volume 70, p. 141-151.
Eric Scerri writes:
"The electron groupings are not exactly the same as what is believed to exist today but it amounts to the same order of filling. For example p orbitals were thought to consist of two groups of 2 and 4 electrons, rather than 2, 2, 2 as believed today. Similarly d orbitals were thought to be formed of two groups of 4 and 6 electrons. With that in mind you will see that Sommerfeld was the first to propose an aufbau filling system: The occupation of 4s before 3d or as represented here the 2 electrons in orbit 11 followed by the 4 and 6 from orbits 3,s and 3,3.
"Sommerfeld does indicate sub-shells. They are just not the same groupings as the current ones. For example 2,1 and 2,2 indicates subshells within the 2nd main shell. Similarly the 3rd shell is presented as 3,2 and 3,3. The totals are of course the same, namely 6 for what we now call p orbitals and 10 for what we call d orbitals. All this came before the discovery of the 4th or spin quantum number. This is in keeping with Bohr's original assignment of shells and sub-shells.
"The discovery of sub-structure to electron shells was not an 'all or nothing' development, but a gradual and almost organic evolution."
Eric has a new book out – A Tale of Seven Scientists and a New Philosophy of Science – in which the gradual evolution of electronic structure involving Bohr, Sommerfeld, Bury, Main Smith, Pauli and others is traced out.
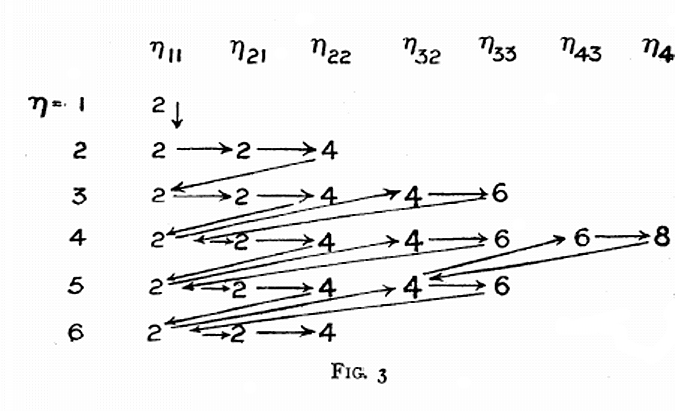
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed
| Year: 1925 | PT id = 1035, Type = formulation 3D |
Model of the Periodic System of de Chancourtois
From the Science Museum in the UK collection, a model of the Periodic System of de Chancourtois from 1862:
"Model demonstrating the telluric screw periodic system of Alexander-Emile Beguyer de Chancourtois proposed in a paper published in 1862.
"This model, made by the Science Museum in 1925, provides a rare physical realisation of arguably the earliest periodic system of for the elements. It was devised by the French geologist, Alexander-Emile Beguyer de Chancourtois in 1862, 7 years prior to Dmitri Mendeleev's periodic table.
"De Chancourtois arranged the elements in the order of their atomic weights along a helix which was traced on the surface of a vertical cylinder, with an angle of 45 degrees to its axis. The base of the cylinder was divided into 16 equal parts (the atomic weight of oxygen), and the lengths of the spiral corresponding to the weights of the elements were found by taking the one-sixteenth part of a complete turn as a unit":

| Year: 1926 | PT id = 26, Type = formulation |
Antropoff's Periodic Table
The Andreas von Antropoff periodic table, restored by Philip Stewart on the basis of the article 'Eine neue Form des periodischen Systems der Elementen'. Zeitschrift für angewandte Chemie 39, pp. 722-725, 1926:
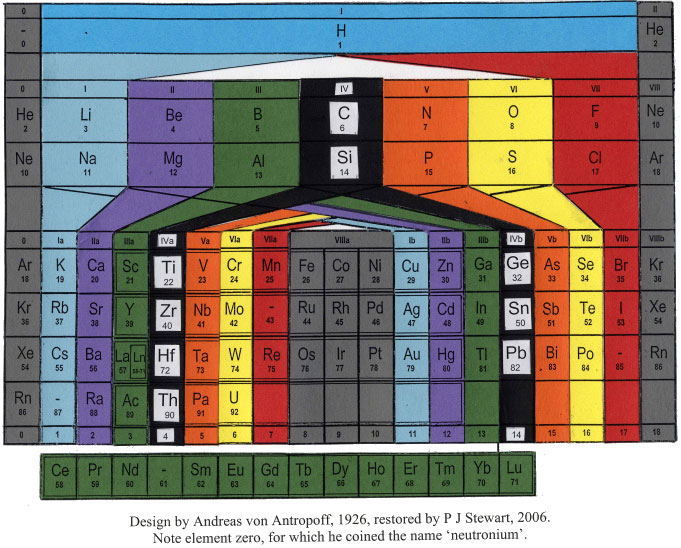
This formulation has a satisfying balance compared to most other tables and was the most popular wall-chart in German schools for many years but quickly disappeared after von Antropoff was disgraced in 1945 for his Nazi activities: he presided over the raising of the swastika over Bonn University in 1933. But he put science above politics and was a stout defender of Einstein's theories.
A recently restored wall version of the von Antropoff formulation from the University of Barcelona, origionally painted in 1934 (thanks to Philip Stewart & Claudi Mans):

Perhaps it was the disgrace of von Antropoff which led Linus Pauling to borrow his design, without acknowledgement, for his 1949 book, General Chemistry (and subsequently in later editions of The Chemical Bond).
The PT below is scanned in from Pauling's The Nature of The Chemical Bond, 3rd ed., 1960:
| Year: 1926 | PT id = 104, Type = formulation spiral |
Monroe & Turner's Spiral
Monroe and Turner's spiral, in which they correctly place the actinides. Information supplied by Philip Stewart.
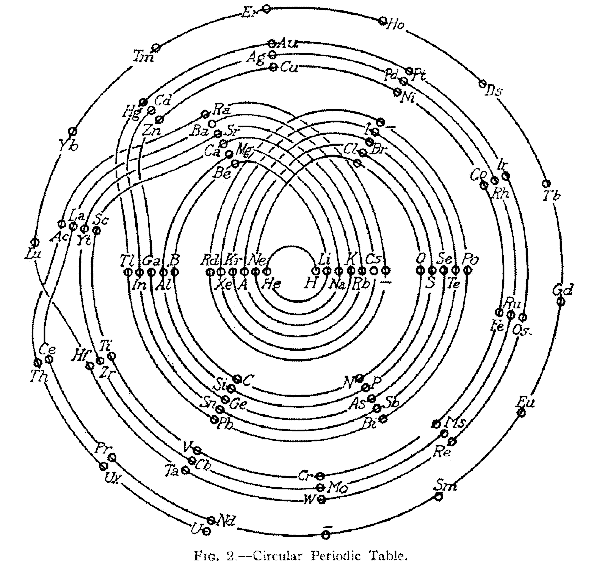
Ref. is C J Monroe and W D Turner A new Periodic Table of the Elements, J Chem Ed, 3, 1058-65, 1926
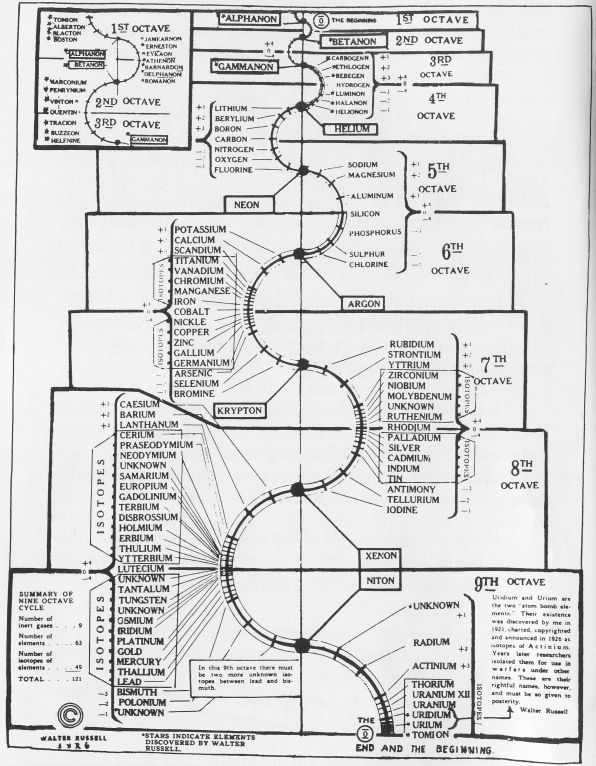
| Year: 1926 | PT id = 147, Type = formulation |
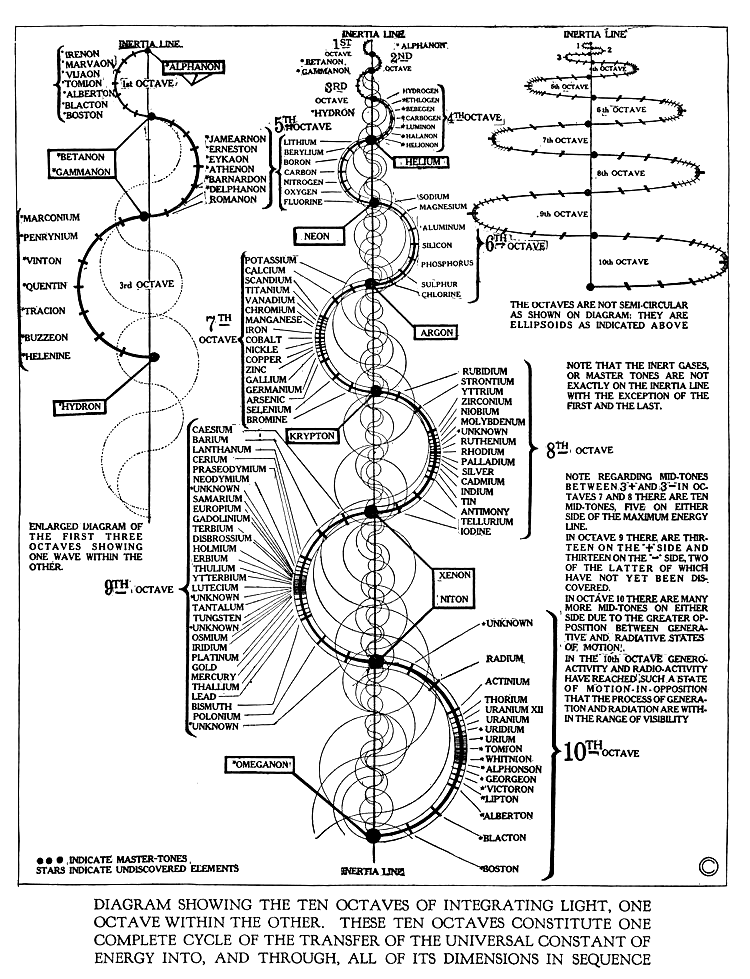
Walter Russell's Periodic Chart of The Elements 1
Walter Russell's Periodic Chart of The Elements 1. View other formulations and an interview here:
| Year: 1926 | PT id = 148, Type = formulation spiral |
Walter Russell's Periodic Chart of The Elements 2
Walter Russell's Periodic Chart of The Elements 2. View other formulations and an interview here:
| Year: 1926 | PT id = 550, Type = formulation |
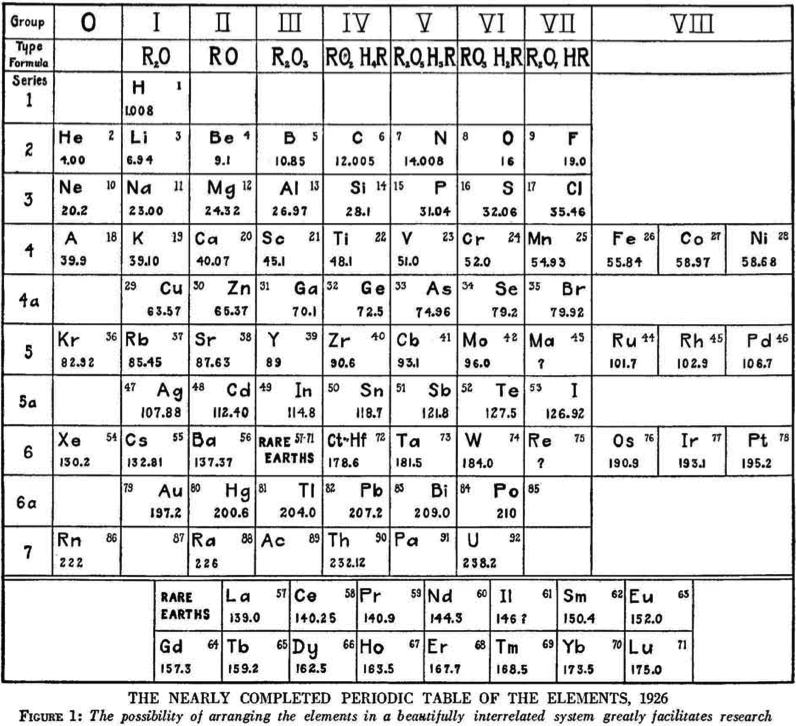
Hopkins' Nearly Completed Periodic Table of The Elements
From a Scientific American of March 1927, an article by B.S. Hopkins discussing the building blocks of the universe.
Included is The Nearly Completed [Hubbard Type] Periodic Table of the Elements from 1926.
As Eric Scerri pointed out: "Notice element, 43, masurium, according to Noddack, Noddack and Berg, and later synthesized as Tc":
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
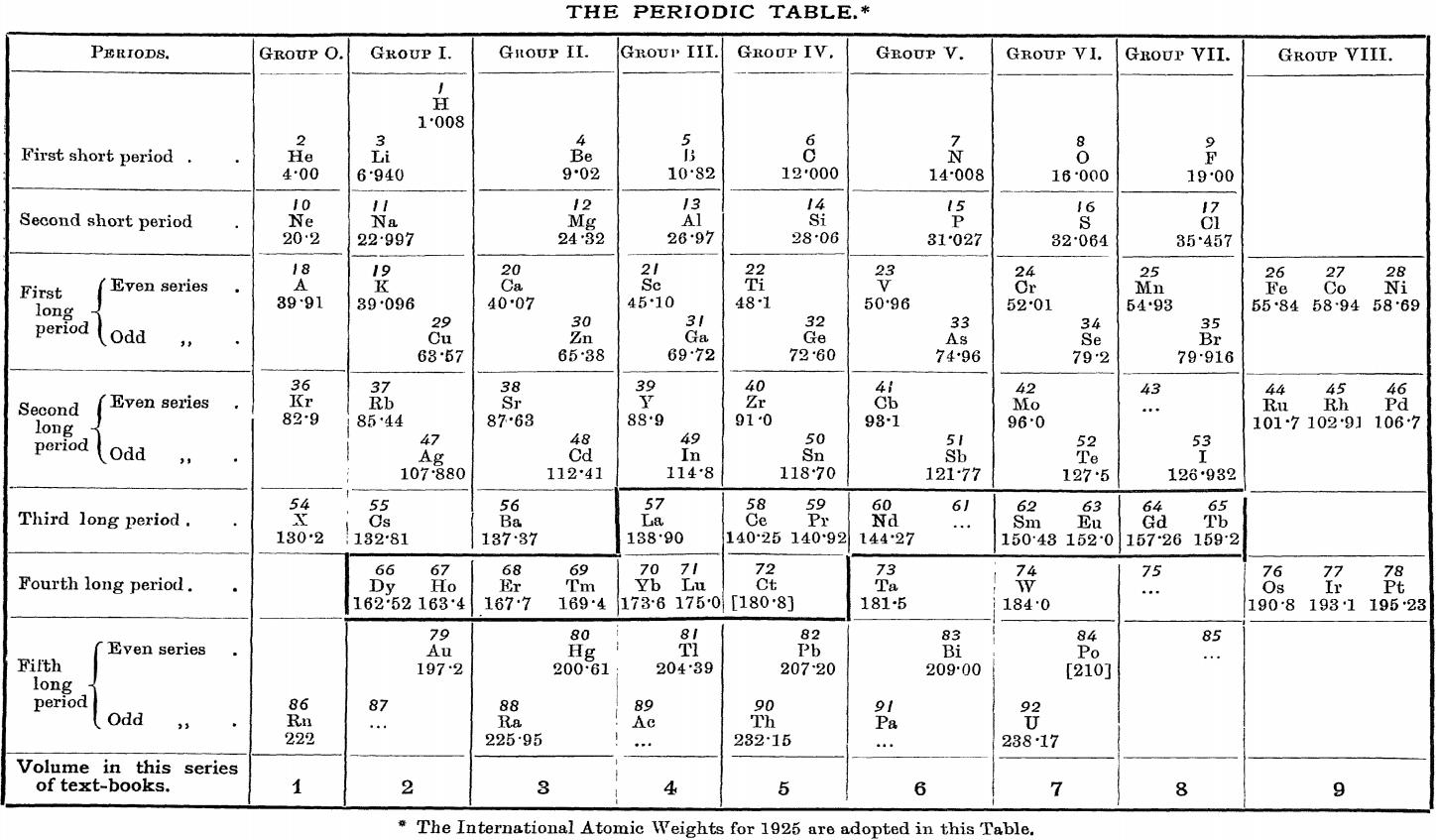
| Year: 1926 | PT id = 1156, Type = formulation |
Friend's Periodic Table (1926)
Vallance RH & Eldridge AA, A Text-Book of Inorganic Chemistry, Vol. VII, Part III, Chromium and its Congeners, JN Friend (ed.) Charles Griffin & Company, London (1926), front paper.
René Vernon (who found this formulation) writes:
"I can't recall seeing a table in which the lanthanoids were allocated in quite such a manner: across seven groups. And, 16 such lanthanoids shown. Even curiouser, Argon = A; xenon = X; are shown in group 0. Wonderful nomenclature from nearly a century ago."
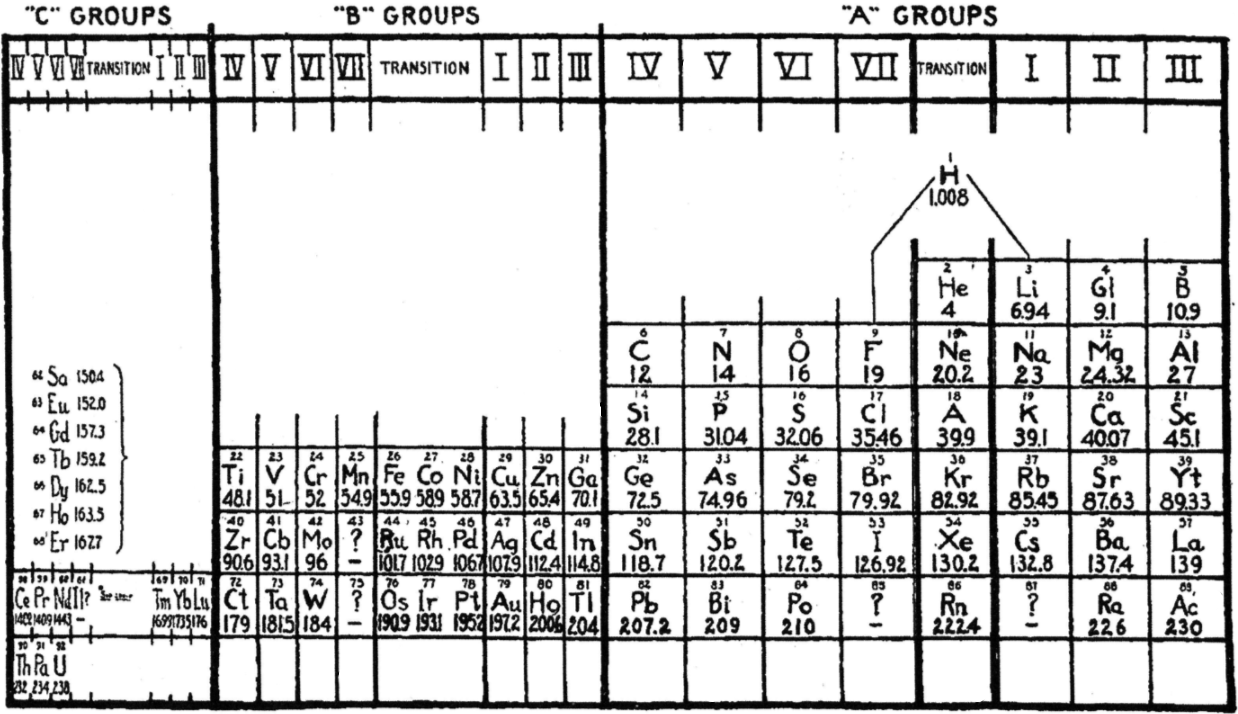
| Year: 1927 | PT id = 1015, Type = formulation |
LeRoy's Periodic Table
R.H. LeRoy, Teaching the Periodic Classification of Elements, School Science and Mathematics 1927, 27: 793-799. This formulation thulium in group IC and has the actinides in the C groups, analogous to the lanthanides, two decades before Seaborg.
René adds:
"This 1927 formulation has several remarkable features.
"The lighter and heavier lanthanides and actinides are shown in numbered C groups i.e. C4, C5, C6, C7 and C1, C2, and C3. The 14 remaining elements between C7 and C1 are labelled as transition elements, analogous to the old chemistry notion of the ferromagnetic and platinum metals in IUPAC groups 9 to 11 being labelled as transition elements. There is no known Tm(I) although this would not be inconceivable. Nd is in group C6, which doesn't quite work since there is no Nd(VI) although such an oxidation state is not inconceivable given the existence of Pr(V). in group C7, Pm(VII) is not known. For the actinides, Md(I) has been reported but not confirmed.
"B-Al-Sc-Y-La-Ac are shown as main group metals; that would be consistent with their chemistry. While Sc-Y-La-Ac are routinely classified as transition metals their chemistry is largely that which would be expected of main group metals following the alkaline earths in IUPAC group 2.
"The author refers to the noble gases as 'transitional'. The noble gases bridge the most reactive groups of elements in the periodic table – the alkali metals in group I and the halogens in group VII. That's a concept that's rarely referred to these days even though it's still quite valid.
"Ga-In-Tl are shown as B3 metals, falling just after Zn-Cd-Hg in group B2, and Cu-Ag-Au in group B1. That doesn't work for Ga etc, which are nowadays regarded as main group metals.
"H is shown floating above the A elements, and in the transitional zone, with links to F and to Li."
Thanks to John Marks for the tip, and to René for the comments/analysis!
| Year: 1928 | PT id = 74, Type = formulation spiral |
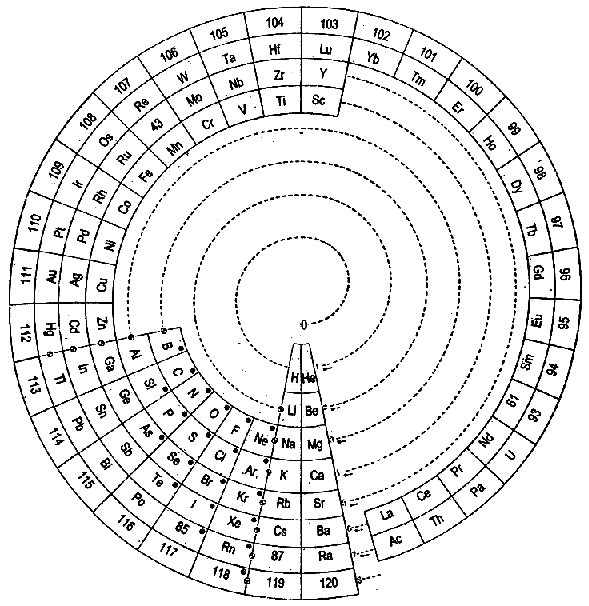
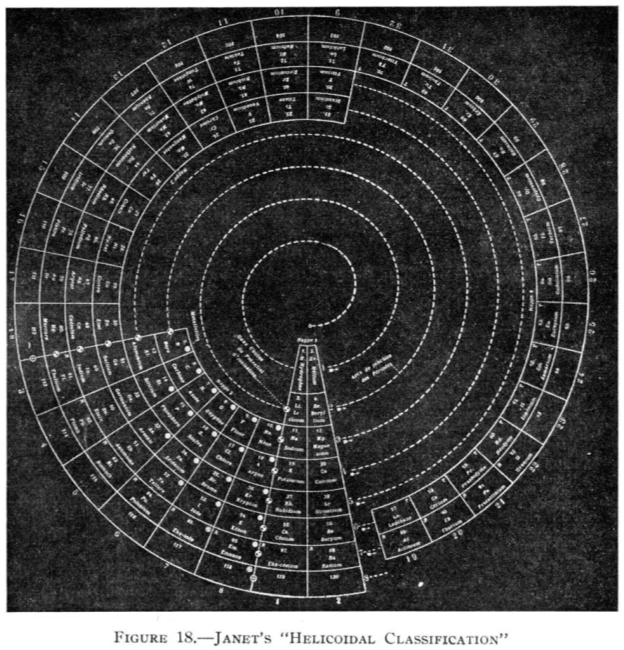
Janet's Helicoidal Classification
Janet's Helicoidal Classification, essentially his left-step formulation in its spiral version (ref. Charles Janet, La Classification Hélicoïdale des Éléments Chimiques. Beauvais: Imprimerie Départementale de l'Oise. 1928). Information supplied by Philip Stewart:
From Quam & Quam's 1934 review paper.pdf
| Year: 1928 | PT id = 152, Type = formulation |
Janet's Left Step Periodic Table
There are the three versions of Janet's left step PT. He tried out versions I and II in his April 1928 paper, and rejected them in favour of version III in his paper of November of the same year. Each one was derived from a helix drawn on nested cylinders. Information supplied by Philip Stewart. Click each image for a larger image:
| Year: 1928 | PT id = 289, Type = formulation spiral 3D |
Janet's Three-Dimensional Spiral-Tube System
Janet's Three-Dimensional Spiral-Tube System of 1928 (from van Spronsen):
Click here for large diagram.
| Year: 1928 | PT id = 305, Type = formulation spiral |
Janet's "Lemniscate" Formulation
From in The Helicoidal Classification of the Elements, Chemical News vol. 138, 21 June 1929, Fig. XI, p. 392:
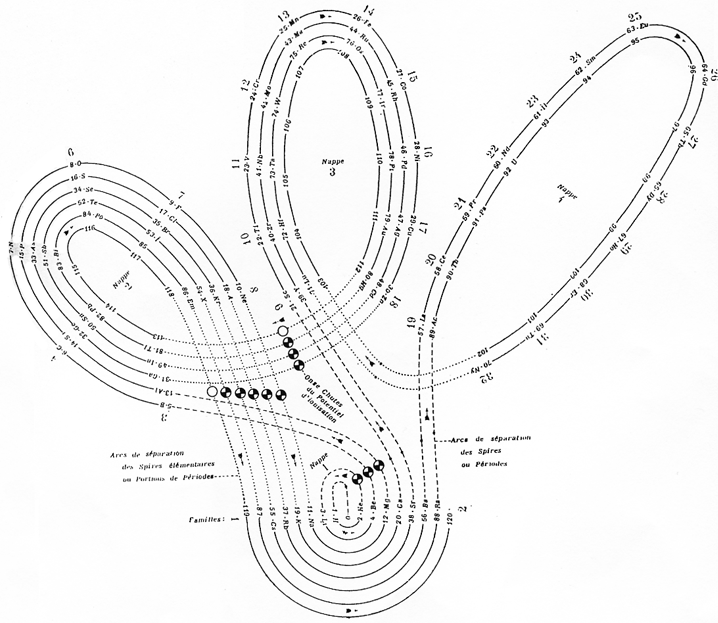
Philip Stewart points out that this formulation is an 'end on' view of the Janet Cylinder or Three-Dimensional Spiral-Tube System formulation, and the term "lemniscate" comes from Mazurs.
| Year: 1928 | PT id = 594, Type = formulation |
Riesenfeld Periodic Table
From here, using Google Translate:
This table is from the book "Practical Inorganic Chemistry" Publisher EH Riesenfeld Labor, Barcelona (1950). It is a reprint of the second edition (1943) which in turn is a translation of a German edition, its seventh edition in 1928. This suggests that Riesenfeld is himself the author of it.
It is a pre-Seaborg table in the sense that the actinides are known throughout the period July. It also does not include the Tc since it was discovered in 1937. These facts support the dating of the table. But the most interesting thing about it is that to make the separation between subgroups and major groups Be cut after the first period and after the Al in the second. Which leaves isolated in group B without any element 2b below it:
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1928 | PT id = 989, Type = formulation |
Corbino's Right-Step Periodic Table
Published in the same year as Janet's Left-Step formulation, Corbino OM (1928) Riv Nuovo Cimento 5:LXI (and from here) produced a Right-Step version.
Commenting on this formulation, Valery Tsimmerman writes:
"Corbino saw what Janet failed to see: If blocks shifted by corresponding value of quantum number l, then the rows represent electronic shells and Janet saw what Corbino fained to see, namely the Janet rule, also known as Madelung rule. Both used rectangular boxes, but neither noticed the perimeter rule."

Thanks to Valery T for the tip!
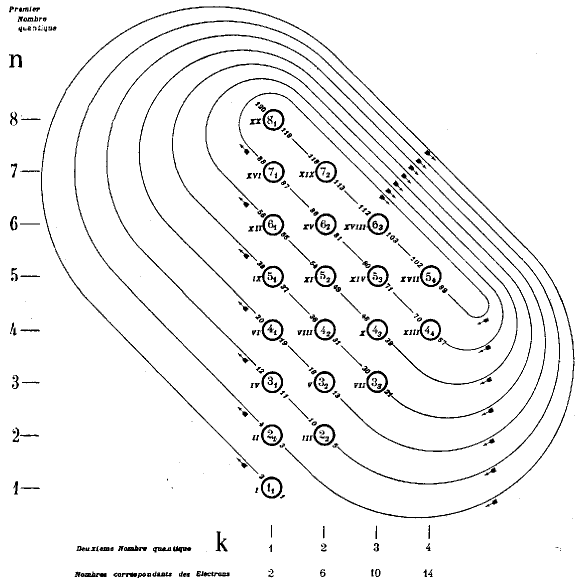
| Year: 1930 | PT id = 154, Type = formulation spiral |
Janet's Shell Filling Diagram
Janet produced six papers, in French, which are almost unobtainable as he had them privately printed and didn't distribute them properly. The shell-filling diagram dated from November 1930, six years before Madelung. Note that Janet uses Bohr's radial quantum number, k, which is l+1. In the text he formulates the n+k-1 rule. Information supplied by Philip Stewart.
| Year: 1930 | PT id = 696, Type = formulation |
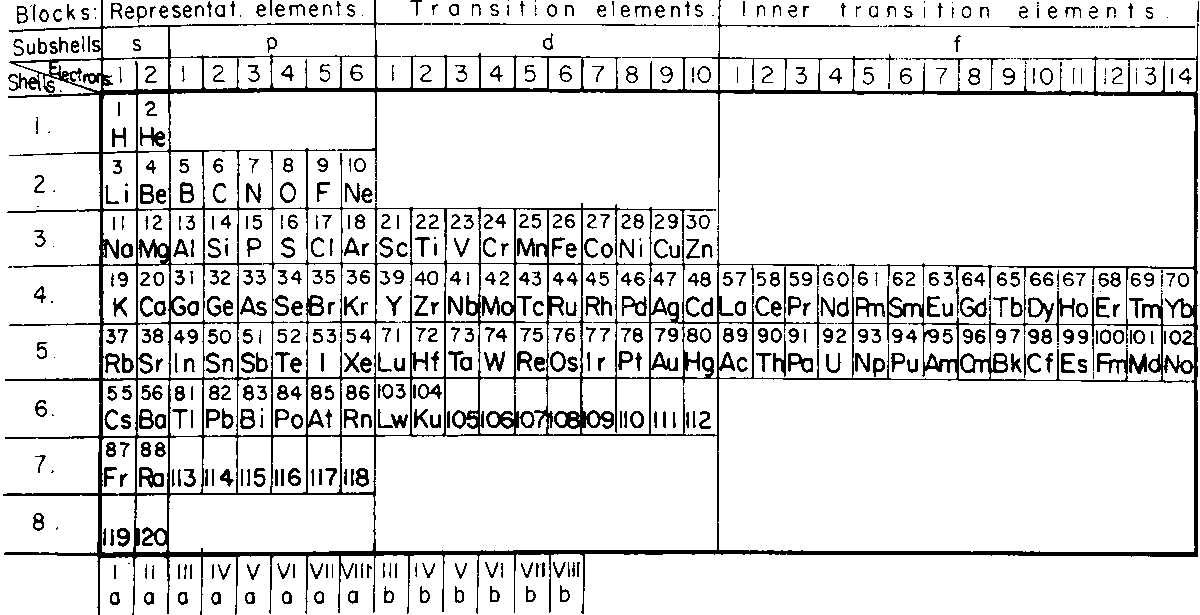
Gardner & Mazzucchelli's Periodic System Elaborated as Electronic Configuration
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
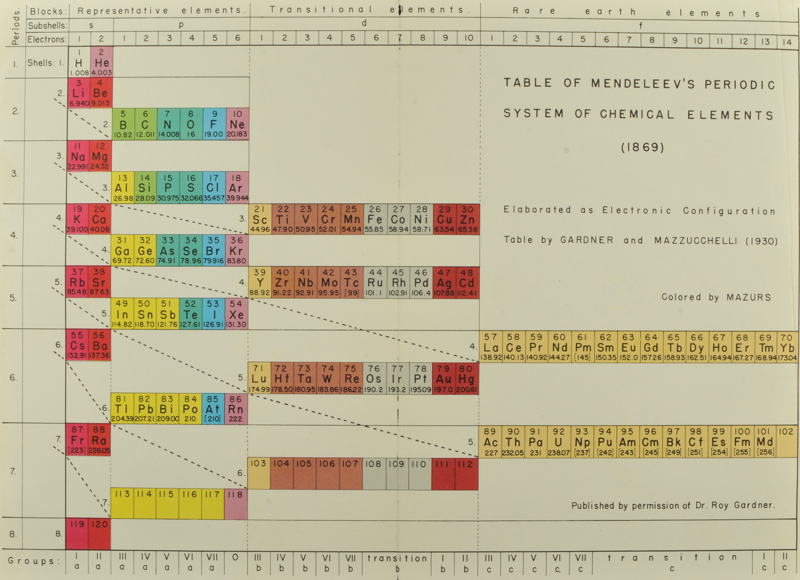
Thanks to Philip Stewart for the tip!
| Year: 1930 | PT id = 1264, Type = formulation |
Gardner's Table of Electronic Configurations of the Elements
A table of electronic configurations of the elements. Nature 125, 146 (1930). https://doi.org/10.1038/125146a0
Abstract:
"MR. ROY GARDNER gave an interesting paper on A Method of Setting out the Classification of the Elements at a recent meeting of the New Zealand Institute. The paper included the accompanying Table, which shows the distribution of electrons into groups corresponding to the principal quantum numbers for all the elements and at the same time preserves the most essential features of the two-dimensional arrangement of Mendeleef. Elements having the same complete groups (that is, all stable groups of 8 or 18) are placed in the same horizontal row, and the vertical columns include elements with the same number of electrons in the incomplete outer groups. The electronic configurations are those given by Sidgwick ("Electronic Theory of Valency", 1927). An asterisk marks elements for which the 'normal' atom is thought to have only one electron in the outermost group, but as practically all these give divalent ions, the point is of minor interest chemically. Distribution of electrons into k-subgroups is unnecessary; these have at present little significance for chemical purposes, and in any case the subgroups are considered to be filled in order to the maxima 2, 6, and 10."
René Vernon writes:
In this table Gardner emphasises the existence of four types of elements:
- those with all "groups" complete
- those with one incomplete group
- those with two incomplete groups (transition elements)
- those with three incomplete groups (rare earth elements)
The upper limits of existence of covalencies of 8, 6, and 4 are marked by heavy horizontal lines.
Note:
- there are nine groups of d-block elements [as we would now call them], and but 13 f-block elements
- La and Lu are treated as d-block elements
- while Yb is counted as an f-block element it was later realised (1937) that the 4f shell is full at Yb, hence it is not clear where Gardner would have placed it (Yb)—seemingly in the 0 column
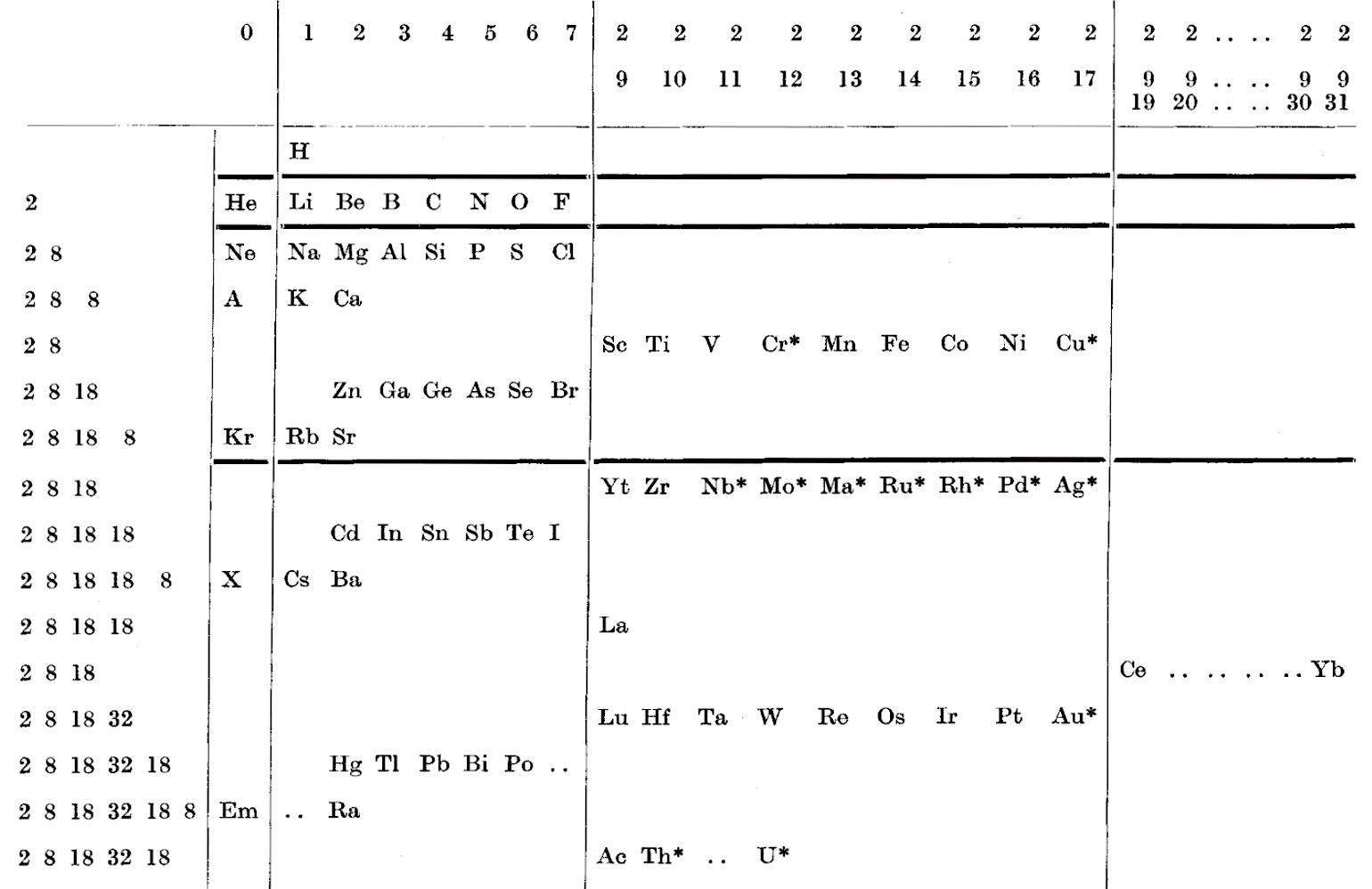
| Year: 1931 | PT id = 1017, Type = formulation |
LeRoy's Periodic Table
R.H. LeRoy, Teaching the Periodic Classification of Elements, School Science and Mathematics 1927, 27: 793-799. This formulation thulium in group IC and has the actinides in the C groups, analogous to the lanthanides, two decades before Seaborg.
René adds:
"This 1927 formulation has several remarkable features.
"The lighter and heavier lanthanides and actinides are shown in numbered C groups i.e. C4, C5, C6, C7 and C1, C2, and C3. The 14 remaining elements between C7 and C1 are labelled as transition elements, analogous to the old chemistry notion of the ferromagnetic and platinum metals in IUPAC groups 9 to 11 being labelled as transition elements. There is no known Tm(I) although this would not be inconceivable. Nd is in group C6, which doesn't quite work since there is no Nd(VI) although such an oxidation state is not inconceivable given the existence of Pr(V). in group C7, Pm(VII) is not known. For the actinides, Md(I) has been reported but not confirmed.
"B-Al-Sc-Y-La-Ac are shown as main group metals; that would be consistent with their chemistry. While Sc-Y-La-Ac are routinely classified as transition metals their chemistry is largely that which would be expected of main group metals following the alkaline earths in IUPAC group 2.
"The author refers to the noble gases as 'transitional'. The noble gases bridge the most reactive groups of elements in the periodic table – the alkali metals in group I and the halogens in group VII. That's a concept that's rarely referred to these days even though it's still quite valid.
Ga-In-Tl are shown as B3 metals, falling just after Zn-Cd-Hg in group B2, and Cu-Ag-Au in group B1. That doesn't work for Ga etc, which are nowadays regarded as main group metals.
"H is shown floating above the A elements, and in the transitional zone, with links to F and to Li."

Thanks to John Marks for the tip, and to René for the comments/analysis!
| Year: 1932 | PT id = 69, Type = formulation |
Stareck's Natural Periodic System
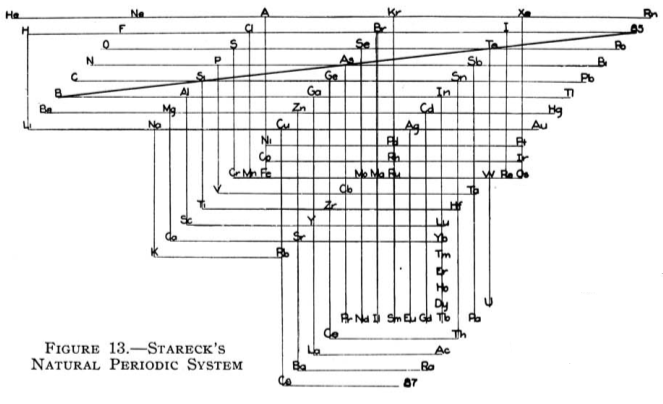
From Quam & Quam's 1934 review paper.pdf
| Year: 1932 | PT id = 1051, Type = formulation |
Bacher & Goudsmith's Periodic System and Index
R.F. Bacher RF and S.A. Goudsmith, Atomic Energy States, McGraw-Hill, New York, p. xiii. 1932:
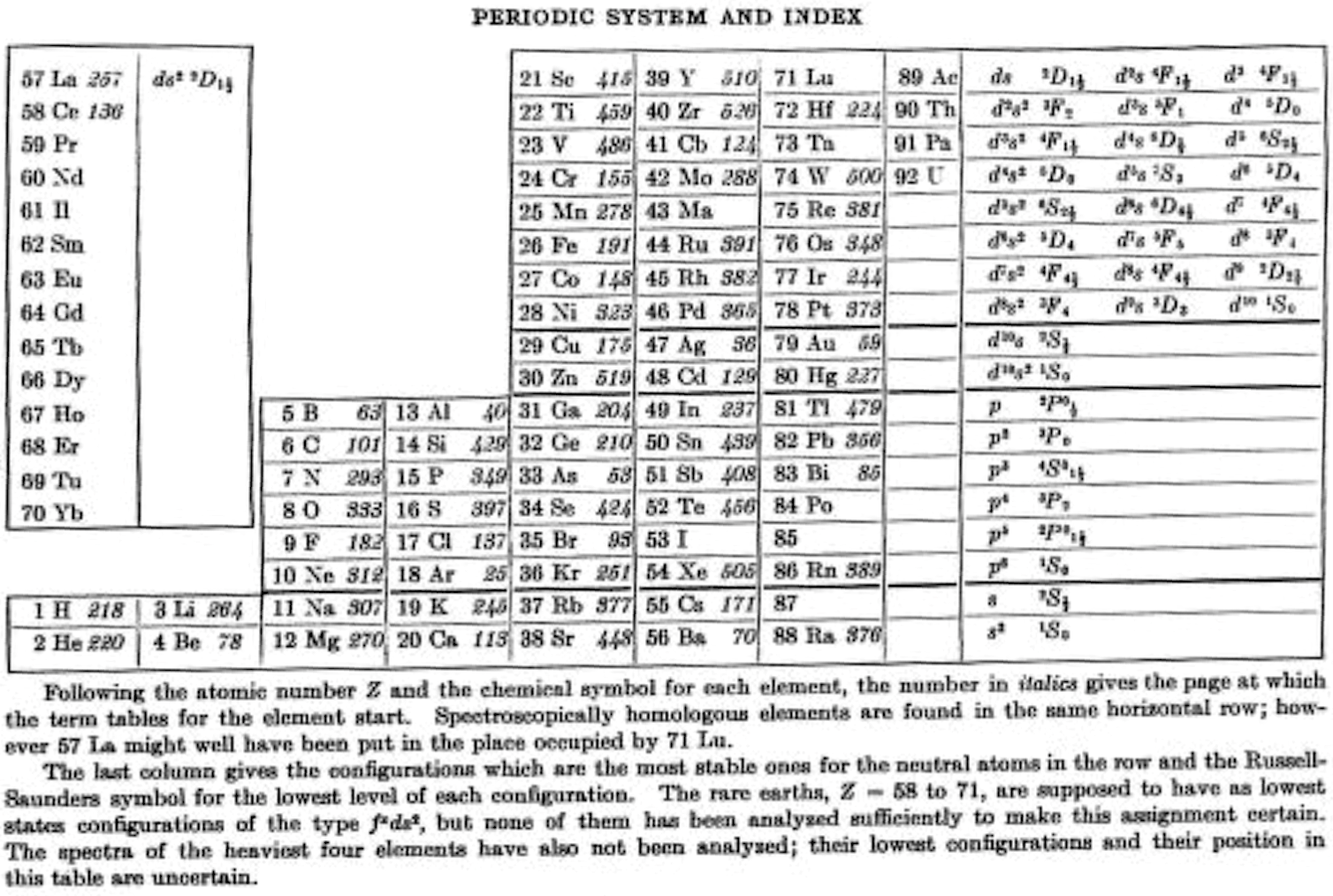
Thanks to René for the tip!
| Year: 1932 | PT id = 1211, Type = formulation |
Bejerrum's Periodic Table
Bjerrum N, Inorganic chemistry, trans. (1936) from the 3rd Danish edition (1932) by N Bjerrum and RP Bell, William Heinemann, London
René Vernon observes:
- There are split blocks everywhere in Bjerrum's periodic system: s once; f once; d twice; p twice.
- As per old chemistry: B and Al are over Sc
- The group numbering is interesting: eight groups and eight sub-groups
- Bjerrum says the metals fall naturally into two groups: the light metals with a density below 4 gm/cm^3; the heavy metals with a density above 7 gm/cm^3, many of which form coloured salts
- Bjerrum refers to the transition metals as being those in subgroups 8a, 8b and 8c
| Year: 1933 | PT id = 60, Type = formulation |
Quam's Periodic Chart
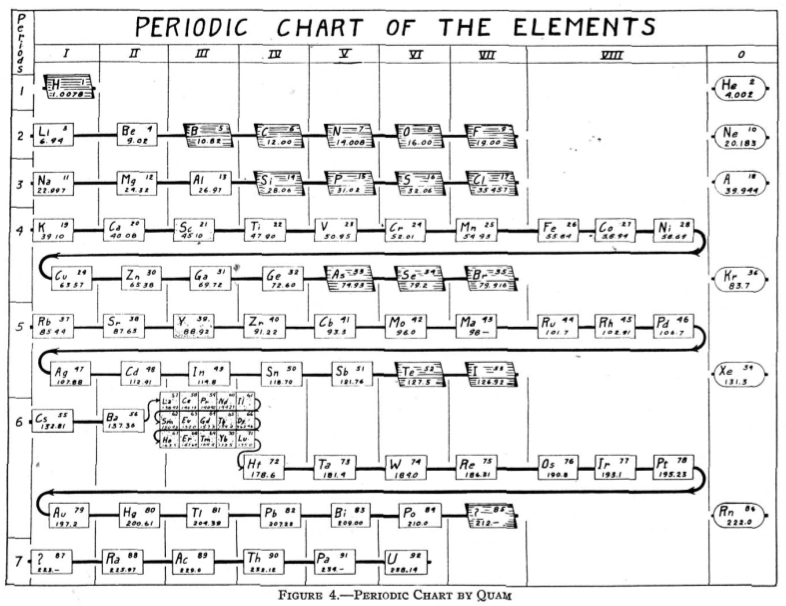
From Quam & Quam's 1934 review paper.pdf
| Year: 1933 | PT id = 79, Type = formulation |
Rixon's Diagram of the Periodic Table
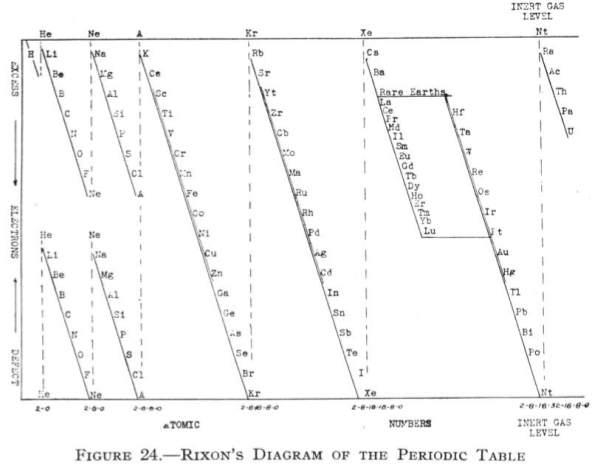
From Quam & Quam's 1934 review paper.pdf
| Year: 1933 | PT id = 86, Type = formulation spiral |
Clark's Periodic Arrangement of The Elements
Origionally developed in 1933:
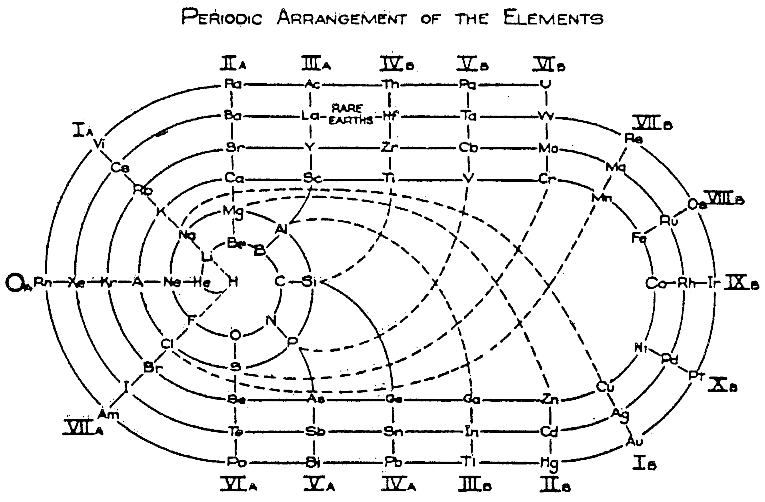
| Year: 1933 | PT id = 187, Type = formulation 3D |
Chicago Museum of Science & Industry Periodic Table
The [Chicago] Museum of Science and Industry (MSI) opened to the public in 1933. The building that the Museum of Science and Industry now occupies however, has a rich history going back to its construction for the 1893 World's Columbian Exposition.
The Special Exhibits Hall – Alexander Graham Bell Memorial Suite – had a huge Periodic Table with the ninety-two elements arrayed in colorful and orderly fashion. These "building blocks of the universe" stood beneath the great central dome of the Museum.
Steve Rosengard, Assistant Curator, Collections Department, Museum of Science & Industry writes:
"After doing a bit of digging, it looks as though the original table was in the Great Hall within the Hall of Science at the 1933-34 World's Fair. Because of prior negotiations, virtually everything inside the Hall of Science was designed by MSI draftsmen so that it could be re-used in the Museum afterwards. The records show that MSI took in the table but had it redesigned and rebuilt by Shaw Naess and Murphy (E.M. Weymer Co. was a subcontractor) in 1938-39. One of the pages from the booklet from the Fair states the '[p]]articular credit is extended to Dr. B.S. Hopkins, of the University of Illinois, for assistance in arranging the collection.' The term assistance is a bit misleading because from the other papers in the file, it's very clear that Hopkins basically did the design entirely on his own. In terms of funding, I would assume that Rand McNally made some contribution beyond the loan of the globe on top since it was known as the Rand-McNally Periodic Table, but I have found no records supporting this."
Some historical images are available from the Chicago Postcard Museum.




Thanks to Roy Alexander for the info!
| Year: 1933 | PT id = 646, Type = formulation non-chem |
After Crookes: The Periodic Law
The Crookes three dimensional periodic table of 1898, here, has been adapted with the addition of two elements 'Adyarium' and 'Occultium' between hydrogen and helium, as presented to Theosophical Society (see bottom right hand corner).
Looking into this, we found the following:
INTRODUCTION TO THE THIRD EDITION By C. JINARAJADASA
This work contains a record of clairvoyant investigations into the structure of matter. The observations were carried out at intervals over a period of nearly forty years, the first in August 1895 and the last in October 1933. The two investigators, Annie Besant (1847-1933) and C. W. Leadbeater (1847-1934) were trained clairvoyants and well equipped to check and supplement each other's work.
Method of Investigation: The method is unique and difficult to explain. Many have heard of the word "clairvoyance" (clear-seeing), connoting the cognition of sights and sounds not perceived by ordinary people. In India the term Yoga is sometimes related to faculties that are beyond ordinary cognition. It is stated in Indian Yoga that one who has trained himself "can make himself infinitesimally small at will". This does not mean that he undergoes a diminution in bodily size, but only that, relatively, his conception of himself can be so minimized that objects which normally are small appear to him as large. The two investigators had been trained by their Eastern Gurus or Teachers to exercise this unique faculty of Yoga, so that when they observed a chemical atom it appeared to their vision as highly magnified.:
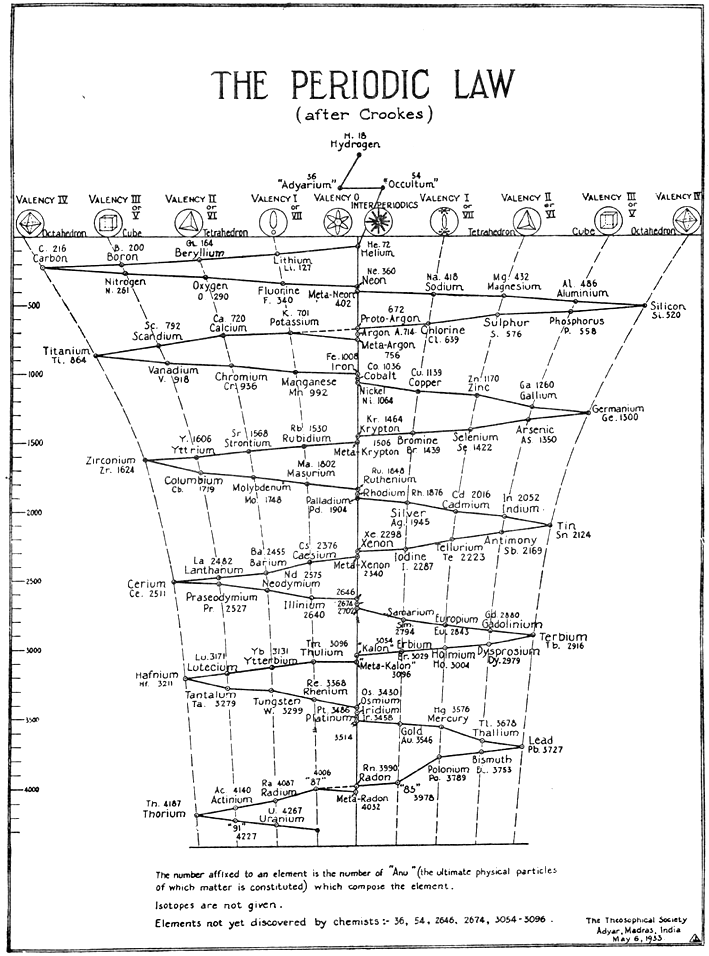
Thanks to Roy Alexander for the tip!
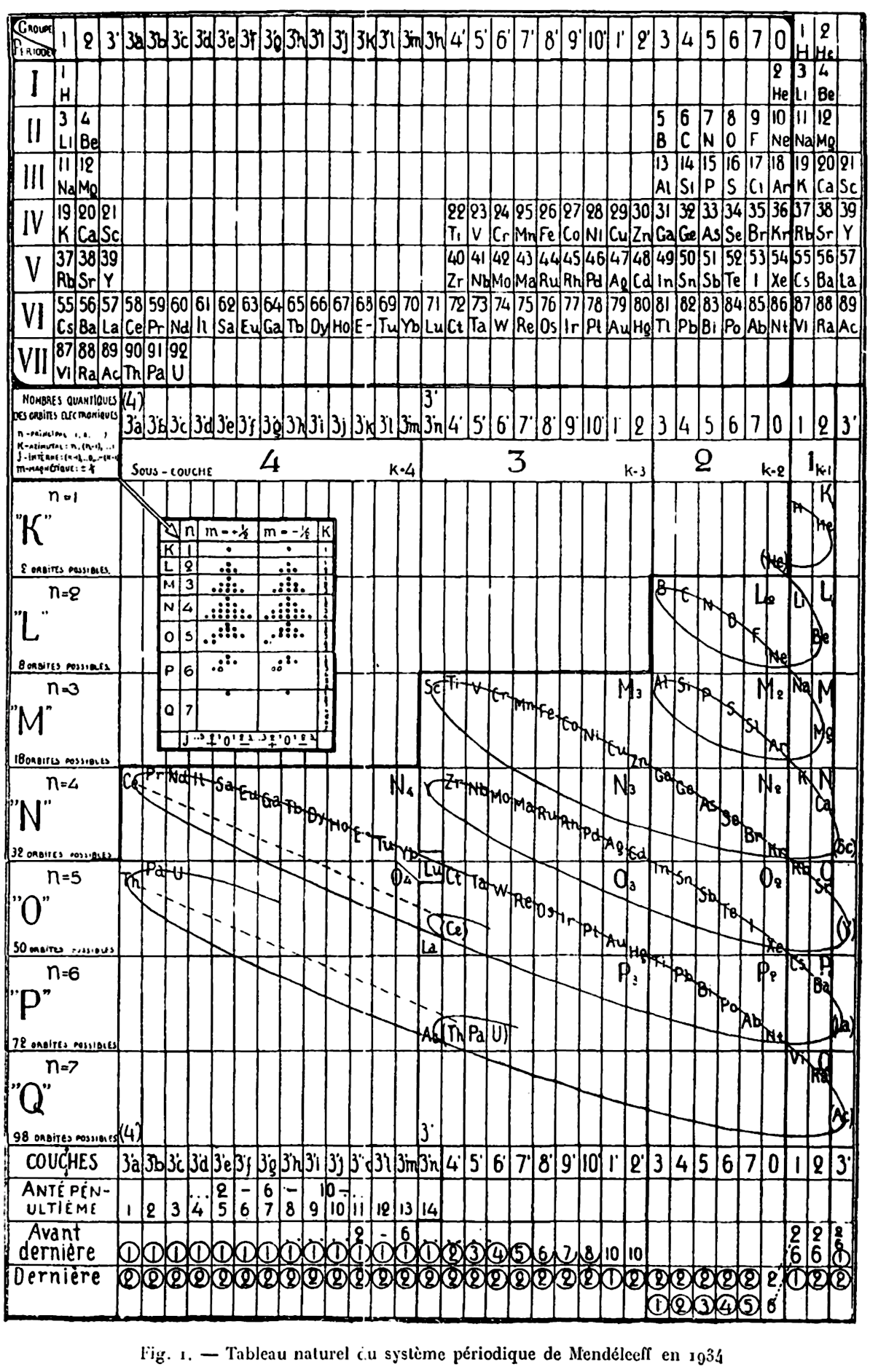
| Year: 1934 | PT id = 105, Type = review formulation |
Quam & Quam's Graphical Representations of The Elements
Short Periodic Tables.pdf
Medium Periodic Tables.pdf
Spiral, Helical & Misc Periodic Tables.pdf
- Mendeléeff's Table (their spelling, 1872)
- Brauner's Table (1902)
- Rydberg Table (1913)
- Periodic Chart by Quam (1934)
- Rang's Periodic Table (1893)
- Werner's Periodic Table (1905)
- Courtines' Periodic Classification (1925)
- Bayley's Periodic System (1882)
- Adam's Periodic Chart (1911)
- Margary's Periodic Table (1921)
- Stareck's Natural Periodic System (1932)
- Baumhauer's Spiral (1870)
- Erdmann's Spiral Table (1902)
- Nodder's Periodic Table (1920)
- Partington's Periodic Arrangements of the Elements (1920)
- Janet's Helicodial Classification (1929)
- The Telluric Screw (1863)
- Crookes' Periodic Table model (1898)
- Emerson's Helix (1911)
- Periodic Table by Harkins and Hall (1916)
- Schaltenbrand's Periodic Table (1920)
- Rixon's Diagram of the Periodic Table (1933)
- Spring's Diagram (1881)
- Flavitzky's Arrangement (1887)
- Stephenson's Statistical Periodic Table (1929)
- Friend's Periodic System (1927)
- Many others, including: Vogel (1918), Stintzing (1916) and Caswell (1929) are discribed without the benefit diagrams.
| Year: 1934 | PT id = 290, Type = formulation spiral 3D |
Romanoff's System
From Revue Scientifique 1934, V. Romanoff's paper (pages 661–665) Le Système Périodique de Mendéléeff Par Représentation Graphique.
Dr. Erik Strub writes:
"The article's Fig. 1 is the first [formulation] (to my knowledge) which contains a "classical" representation of the periodic system in which an Actinoide series is placed beneath the Lanthanoides and not beneath the d block elements:
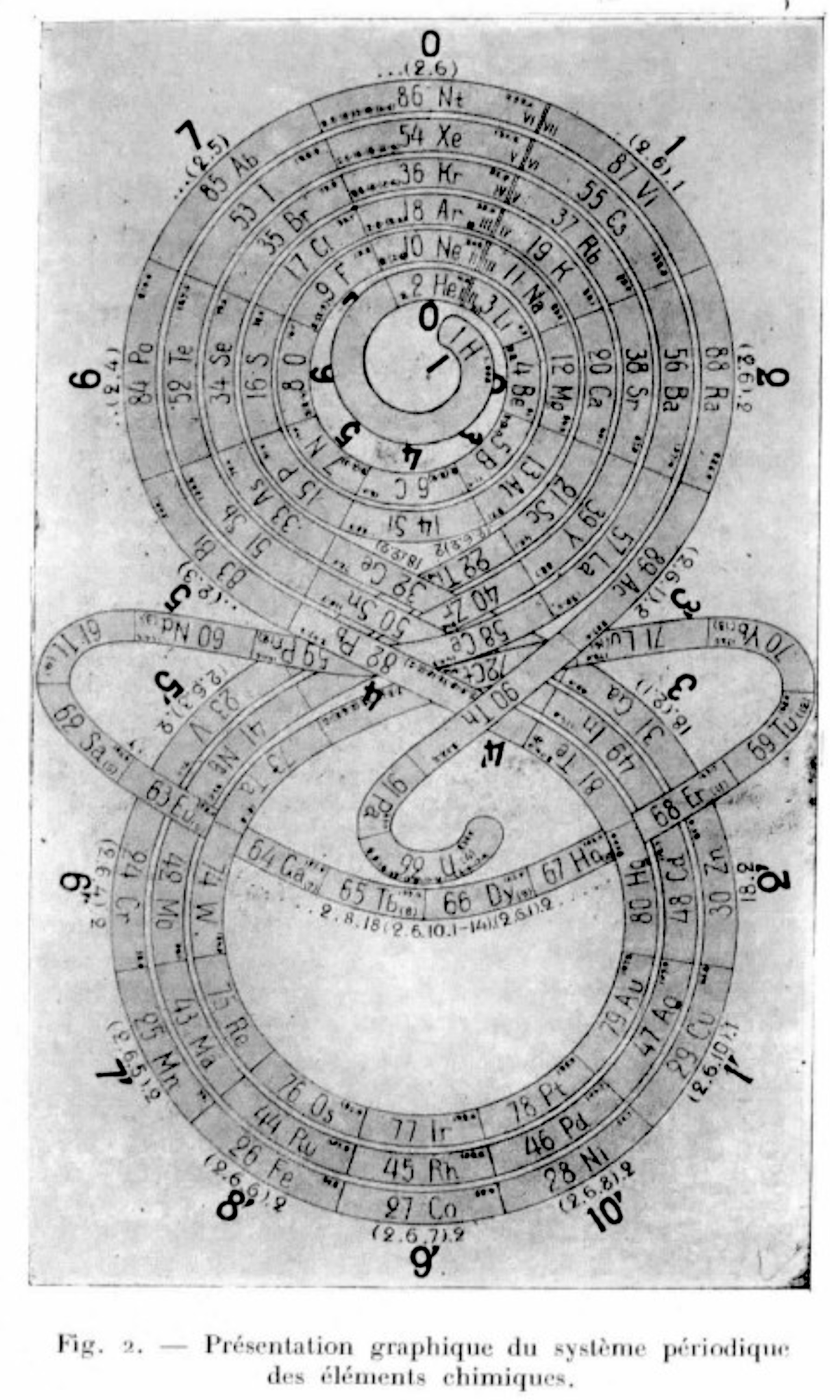
Romanoff's System of 1934 (from van Spronsen):
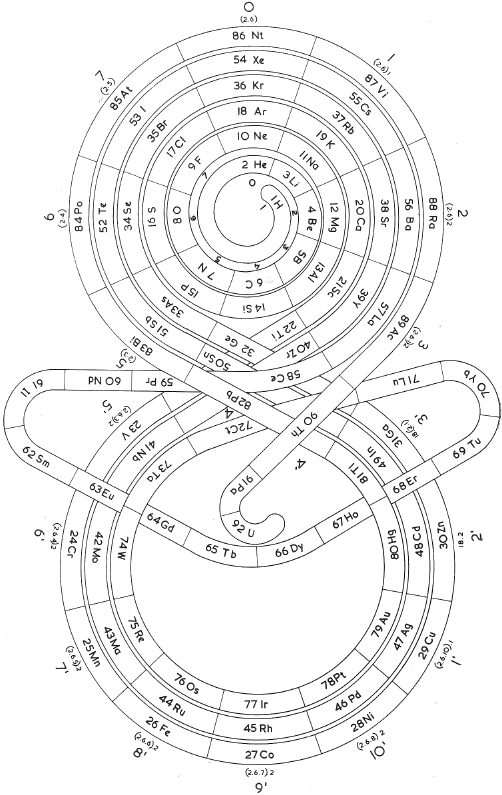
| Year: 1934 | PT id = 296, Type = formulation misc |
Leningrad Monument To The Periodic Table
Leningrad monument to the periodic table, located near to the main chamber of weights and measures, 1934 (from van Spronsen):

From Wikipedia:
| Year: 1934 | PT id = 435, Type = formulation |
Brazilian Version of The Hubbard Periodic Chart Of The Atoms
A Brazilian Version of the American classic Henry Hubbard Periodic Chart Of The Atoms from a lecture theater in Rio, rediscovered by Martyn Poliakoff of PeriodicVideos.com and The University of Nottingham. From the early 1930s:

- Ma – Masurium (43) Disputed claim to discovery of technetium.
- Cb – Columbium (41) Former name of niobium
- Ab – Alabamine (85) Discredited claim to discovery of astatine.
- Il – Illinium (61) Discredited also
- Sa – Samarium (62) Current symbol is Sm
- Sp – Spectrium (70) Suggested name for ytterbium
- Cb – Columbium (41) Former name of niobium (also called Pelopium)
The current Sargent Welch version of the Henry Hubbard Periodic Table:
| Year: 1934 | PT id = 465, Type = formulation data |
White's Periodic Table
The periodic table of White shows the normal state electronic configurations, from H.E. White. Introduction to Atomic Spectra. New York: McGraw-Hill, 1934,
p. 85, Table 5.4..
Helium is clearly associated with H, and placed above Be in accord with the s2 electron configuration of the free atom.
| Year: 1935 | PT id = 40, Type = formulation |
Zmaczynski's Triangular Periodic Table
A Triangular Periodic Table by Emil Zmaczynski:
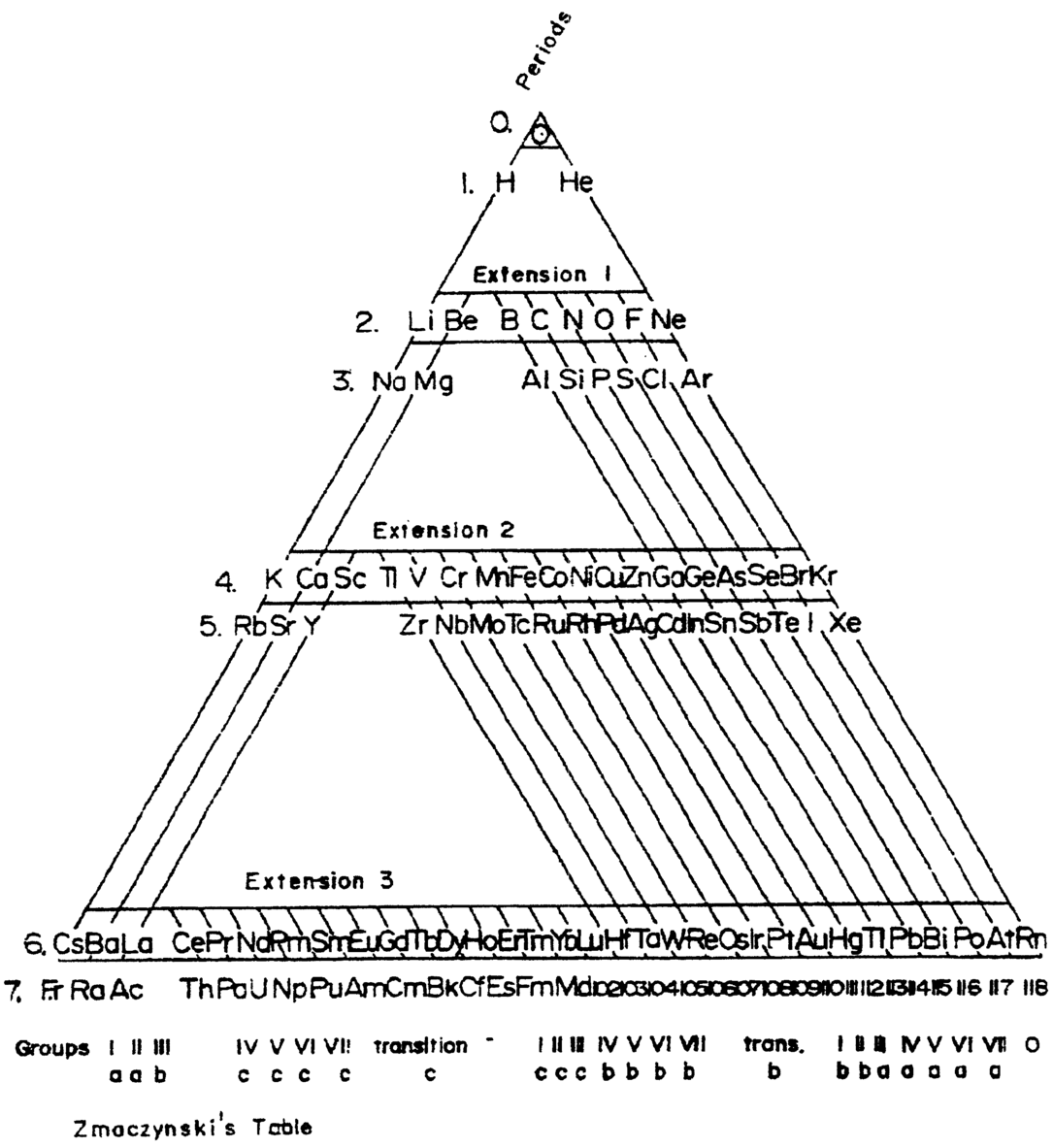
| Year: 1935 | PT id = 1011, Type = formulation |
Rysselberghe's Periodic Table
Pierre Van Rysselberghe J. Chem. Educ. vol. 12, no. 10, pp. 474—475 1935.
The author writes:
"The usual relationships between analogous elements are preserved and are in fact emphasized by this new arrangement. The only missing regularity is the natural succession of atomic, numbers, but all periodic classifications have to sacrifice it on account of the rare earths. Moreover, it can easily be restored by reading the horizontal lines n the order indicated by the numbers written on the left of the heavy frame line. Each horizontal line is limited by the frame of the table. For instance, K and Ca on the one hand, Cu and Zn on the other hand, form two distinct horizontal lines, as shown by the different numbers given to these groups. They are at the same level because the valence electrons have the same quantum numbers."
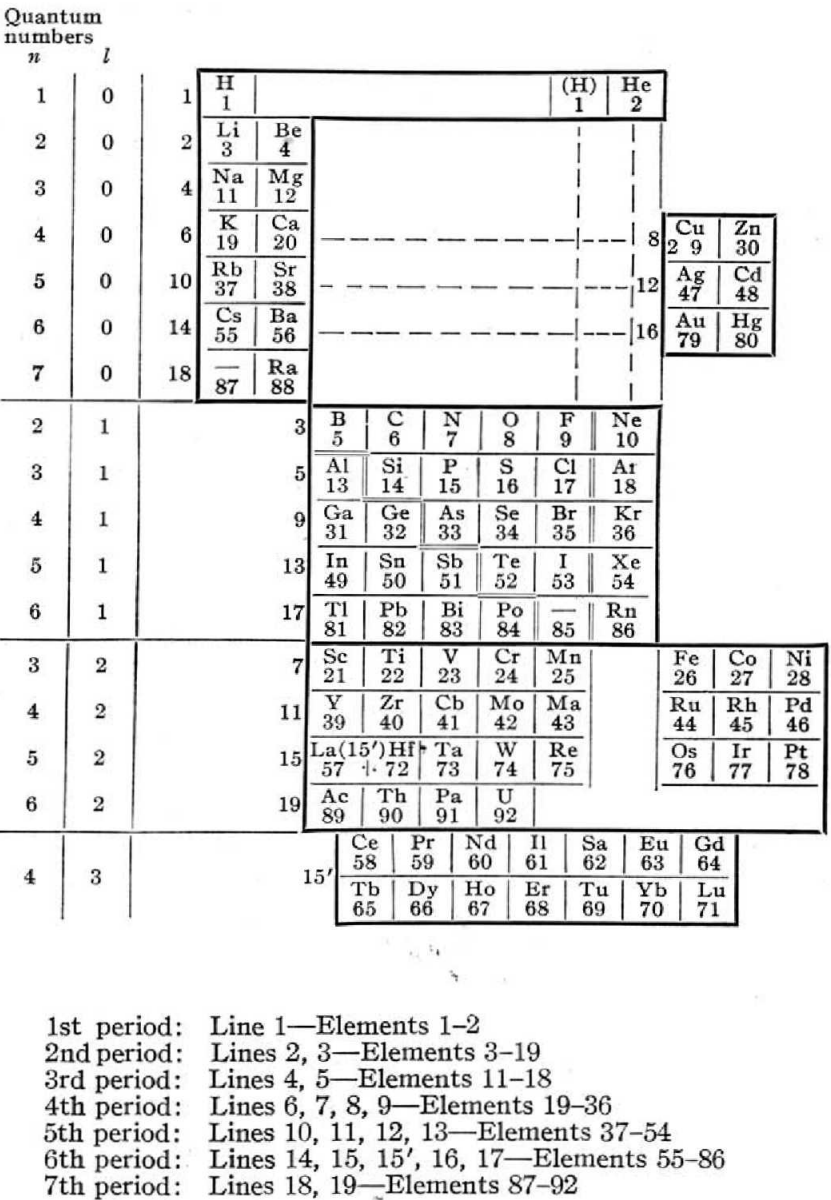
Thanks to René for the tip!
| Year: 1936 | PT id = 595, Type = formulation |
Nekrasov Periodic Table
From here, using Google Translate:
The shape of the table is presented by Bohr effect of considering the properties of the elements as simple substances and for reactions to occur with the intervention of such substances. But for the study of compounds and reactions that occur between them, the key factor is the electron configurations of atoms in states of valence to them on the given compounds.
It follows that a more complete picture of the periodic table would be when you take into account the peculiarities of atoms in both its neutral state and in all its particular valence states. This is the proposal of Boris Nekrasov, a member of the Academy of Sciences in Moscow.
Nekrasov distinguishes three types of analogies between elements Total analogs are those in which the analogy is shown in all its valence, all analogs compared to the valence valences except for the group corresponding to the number that can be called characteristic and analogous to the valence characteristic .
Thus, in the table shown here distinguish the elements entirely analogous joined by continuous lines, such as Na and K.
Those analogies in all except the characteristic valences joined by dotted lines. This is the case of Na and Cu in both cases if you lose an electron (valence feature) your setup is different. In the first case is 8 (1s2, 2P6) and the second 18 (3s2, 3p6, 3d10).
Lastly presenting exclusively analogies valence are connected with dashed lines. This is the case both S and Cr +6 elements have their valence electron configuration similar in the last layer 8 (2s2, 2p6) for the S and 8 (3s2, 3p6) for Cr.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1936 | PT id = 777, Type = formulation data misc |
Orbital Filling With Electrons
Students of chemistry are often confused why the orbitals fill with electrons: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p6... etc., because the 3d10 seems to be 'out of sequence'.
This 'out of sequence' difficulity is nicely explained if the orbitals are arranged in a slightly different way:
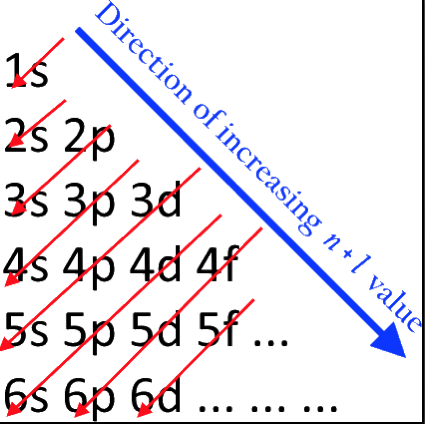
The aufbau principle states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels. For example, the 1s shell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible.
The order in which these orbitals are filled is given by the n + ![]() rule, also known as the Madelung rule (after Erwin Madelung), the Janet rule or the diagonal rule.
rule, also known as the Madelung rule (after Erwin Madelung), the Janet rule or the diagonal rule.
Orbitals with a lower n + ![]() value are filled before those with higher n +
value are filled before those with higher n + ![]() values. In this context, n represents the principal quantum number and ? the azimuthal quantum number. The values
values. In this context, n represents the principal quantum number and ? the azimuthal quantum number. The values ![]() = 0, 1, 2, 3 correspond to the s, p, d and f orbital lables.
= 0, 1, 2, 3 correspond to the s, p, d and f orbital lables.
Julio Gutiérrez Samanez writes:
"I send you the diagram below that reconciles quantum mechanics (diagram for filling the electronic cells) with the Janet table or LSPT. Explaining the duplication of periods with the duplication of the quantum number n, and the introduction of Tao (T) spin of the level or spin of the period, which explains the parity of the symmetric periods."
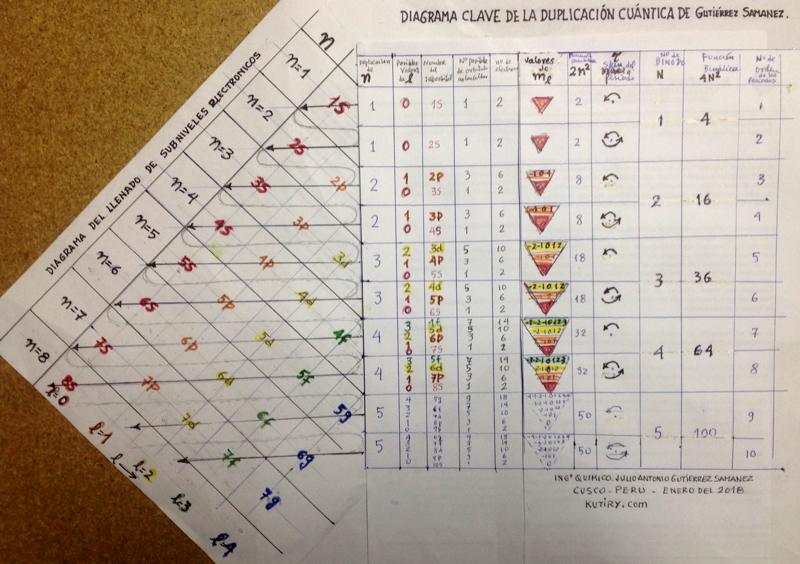
| Year: 1936 | PT id = 909, Type = formulation spiral |
Libedinski's Periodic Classification of The Elements
Simón Libedinski: PERIODIC CLASSIFICATION OF THE ELEMENTS, from his book: Dialectical Materialism, in Nature, in Society and in Medicine, Ediciones Ercilla, Santiago de Chile, 1938, pp 56-57:
"Mendeleev's Table, like that of Werner and others, are not, however, more than flat projections of the actual ordering of the elements. There is as much difference between Mendeleev's Table and the real group as there is between the planisphere and a rotating globe. A rational representation, starting from the simplest element – the negative electron –, would be a spiral line that, surrounding said central point, first gave a small turn, touching only two bodies: hydrogen and helium. From here it would jump to a much larger orbit, in which it would touch eight bodies and then another equal, also of eight. From here, another jump to a much larger orbit, comprising eighteen bodies, and then another equal; from this point one jumps to another orbit, again augmented, comprising thirty-two bodies (including rare earths); and when this round is over, the last one begins, to vanish a short distance.
"In the dialectical grouping of the elements, which I have the satisfaction of exposing, the classic arrangement of the same is respected. Only the arrangement changes, which instead of being rectilinear, is spiral. So I managed to suppress the anomaly of the double columns, and comfortably incorporate the important group of rare earths. I can not give my graphic the name of Tabla, because it is just the opposite: it aims to give the idea of ??space, and of movement in space. The double columns of the Classic Table can be found here as well, but only if you look through the whole, considered as a planetary system of conical shape, with the electron at the vertex. Effectively: column 1 coincides, through space, with column 1a; column 4 with column 4 bis, etc. The dialectical grouping also allows us to easily appreciate the remarkable dialectical character of the properties of matter: these properties are repeated periodically. These are the "returns" to qualities or previous properties, but not exactly equal to those, but only similar: and this resemblance, only to a certain extent. The difference is that that quality, those properties or some characteristic, are exalted to each dialectical return."
Contributed by Julio Antonio Gutiérrez Samanez, Cusco, Peru, March 2018 (using Google Translation)
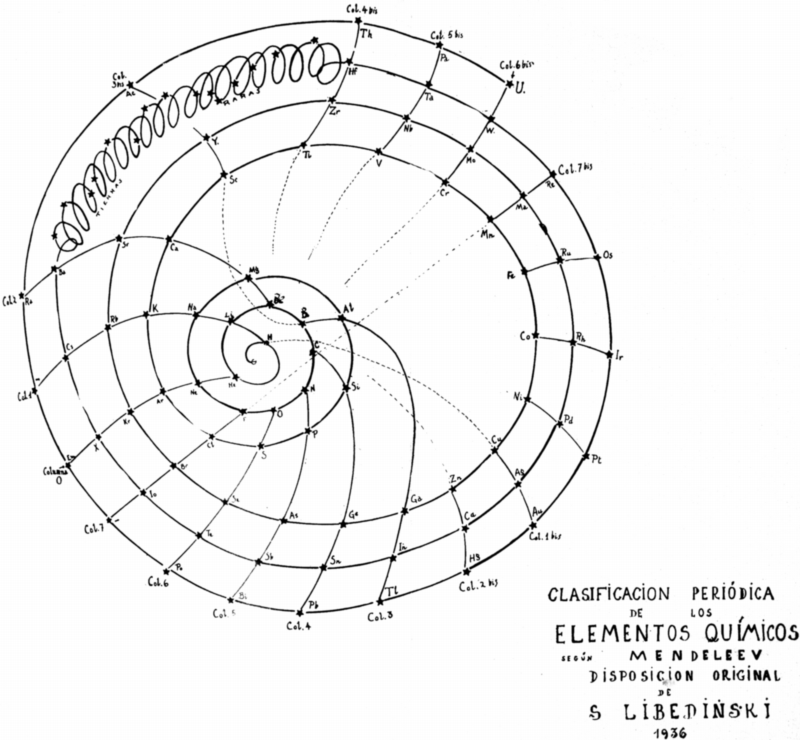
| Year: 1936 | PT id = 1290, Type = formulation |
Van Wert Periodic table (after Guertler-Leitgebel)
Van Wert LR, An Introduction to Physical Metallurgy, McGraw-Hill, New York, 1936, pp 17. Van Wert says the periodic table is after "Guertler-Leitgebel", which is presumably Guertler WM & Leitgebel M 1929, Vom Erz zum metallischen Werkstoff: Leitlinien und Rüstzeug der metallurgischen und metallkundlichen Wissensgebiete, Akademische Verlagsgesellschaft, m.b.H., Leipzig
From René Vernon who writes:
In this almost symmetrical presentation, Van Wert divides the periodic table metals into:
Strongly Electropositive: Groups 1 to 3, Ln
High-melting Heavy Metals: Transition metals
Low-melting Heavy Metals: Post-transition metalsIf the 15 Rare Earths had been shown as 14, and moved one cell to the left we would have a perfectly symmetrical table.
Elsewhere (p. 38) Van Wert refers to the noble metals as follows:
"With respect to corrosion, the noble metals — gold, the platinum metals, and to a less degree, silver — are in a class by themselves. They are comparatively chemically inert to all common corrodents; only silver is appreciably attacked by sulphur gas."
Van Wert's table also refers to non-metals and to inert gases. On page 7 mention is made of the metalloids:
"There are a few elements, also, that partake of the nature of both metals and nonmetals, under many—indeed, under most—conditions they seem metallic enough, but on occasion their behavior is decidedly nonmetallic. These metalloids, as they are sometimes called, add a further difficulty in the attempt to frame a satisfactory definition of the metallic state."
By 1936, it was known that metalloids had a predominately nonmetallic chemistry (Newth 1894, pp. 7??8; Friend 1914, p. 9). So, on the nonmetal side of house are metalloids; "nonmetals"; and noble gases. Separating out the halogens from the nonmetals yields: metalloids; "nonmetals"; halogens; noble gases.
The net result is four types of metals and four of nonmetals = more symmetry.
| Year: 1937 | PT id = 25, Type = formulation spiral |
Pozzi Spiral Periodic Table
A spiral periodic table formulation constructed by E.C. Pozzi in 1937, from here.
Note the "Strong Positive, Strong Negative, Weak Positive and Weak Negative" corners:
| Year: 1937 | PT id = 286, Type = formulation |
Zmaczynski's Fan-Shaped System
Zmaczynski's fan-shaped system of 1937 (from van Spronsen):
| Year: 1937 | PT id = 823, Type = element |
Discovery of Technetium
Tc ![]()
Technetium, atomic number 43, has a mass of 98 au.
Radioactive element: Tc is only found in tiny amounts in nature. Most samples are synthetic.
Technetium was first isolated in 1937 by C. Perrier and E. Segrè. The element had been predicted by Mendeleev in 1871 as eka-manganese.
| Year: 1939 | PT id = 627, Type = formulation spiral |
Periodic Chart in Spiral Form
Periodic Chart in Spiral Form from K. Gordon Irwin, Periodicity Patterns of The Elements J.Chem.Ed. 1939 16 (7), 335 DOI: 10.1021/ed016p335
Thanks to René Vernon for the reference:

| Year: 1939 | PT id = 867, Type = element |
Discovery of Francium
Fr ![]()
Francium, atomic number 87, has a mass of 223 au.
Radioactive element.
Francium was first observed in 1939 by M. Perey.
| Year: 1939 | PT id = 943, Type = formulation |
XBL 769-10601, Periodic Table Before World War II
An internal document of the Lawrence Berkeley Laboratory XBL 769-10601 shows a late 1930s (pre World War II) periodic table.
Note that this formulation erroneously predicts positions for transuranium elements:
- thorium is shown in the group below hafnium
- protactinium below tantalum
- uranium below tungsten
| Year: 1939 | PT id = 1056, Type = formulation |
Foster's Periodic Arrangement
L.S. Foster, "Why not modernise the textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9, pp. 409–412, https://pubs.acs.org/doi/10.1021/ed016p409
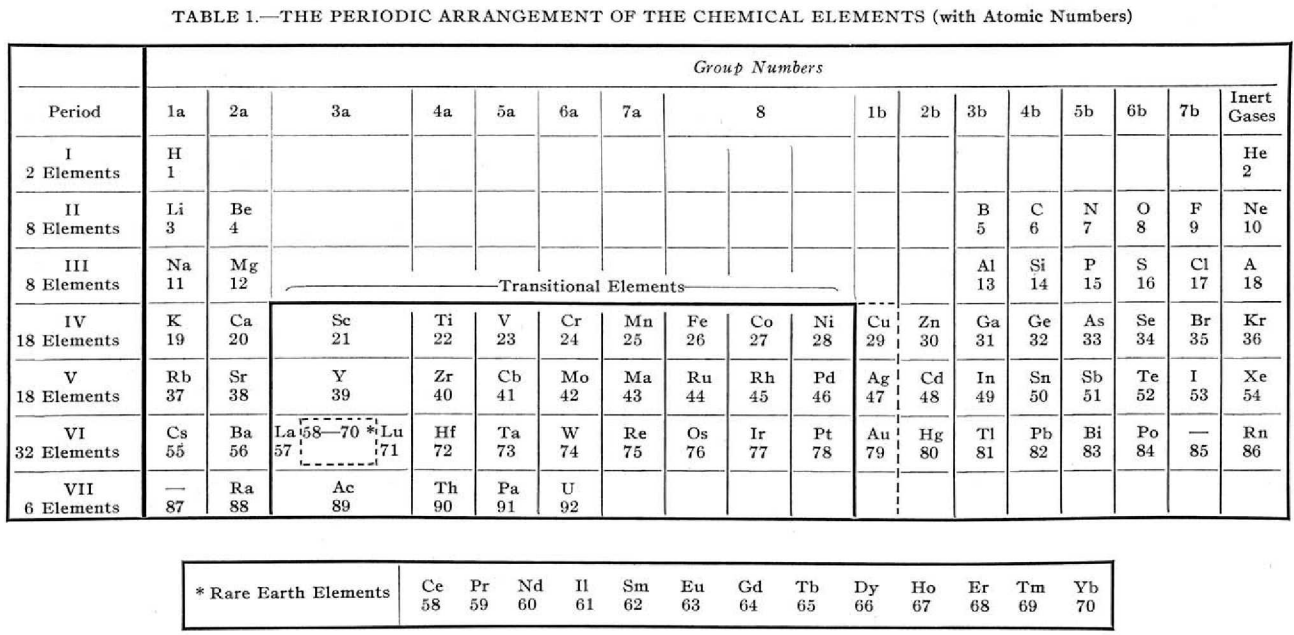
Foster writes:
"The [above] modern periodic table is simply an orderly array of the elements with all unnecessary ornamentation omitted, has been found highly satisfactory for instructional purposes.
"The transitional elements, with two unfilled electron shells, are separated from the non-metallic elements.
"The rare-earth elements, defined as those with three incomplete electron shells, are shown to be those of atomic numbers 58 to 70, while La and Lu, which have only two incomplete electron shells are classified as transitional elements.
"Copper, silver, and gold act as transitional elements except when the state of oxidation is one."
René Vernon writes:
Foster couldn't show the coinage metals – with their full d10 complements – as transitional elements, but by adding a broken line around them he was showing they had the capacity to act as if they were.
I tried to work out how he distinguished La & Lu from Ce to Yb. Foster seems to be saying that La 5d1 6s2, has incomplete 5th and 6th (ie. 6p) shells.
Same for Lu 4f14 5d1 6s2 having incomplete 5th and 6th shells. Whereas, for example, Ce 4f1 5d1 6s2 has incomplete 4th, 5th and 6th shells. Presumably this was in the years before the fact that the 4f shell became full at Yb was widely appreciated. So, strictly speaking, group 3a should have read:
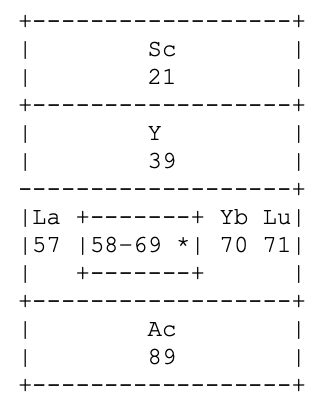
On the other hand, Yb3+ has an f13 configuration, so it does meet his three unfilled shells criterion. Had he known, he probably would've put a broken line around Yb to indicate its full f14 complement but that it normally acted as a rare earth, with an incomplete 4f shell; whereas neither La nor Lu have this capacity.
Good to see Foster put so much thought into organising his table, and his experience with using it for instructional purposes.
Van Spronsen does not mention Forster's table. Mazurs has a reference to Foster's table but lumps it in with the other medium-long tables, not appreciating its subtlety.
Mark Leach writes:
This formulation is very much like the XBL 769-10601, Periodic Table Before World War II used by Seaborg and the Manhattan project and is a precursor to the modern periodic table.
| Year: 1940 | PT id = 763, Type = formulation 3D |
Gamow [First] Ribbon Periodic Table
George Gamow is well known for his Gamow 1961 ribbon formulation. It appeared in a 1948 book: One, Two, Three... Infinity, but it first appeared in 1940 in 'The Birth and Death of the Sun' (Viking, N. Y.).
Conal Boyce writes:
"The 1940 version of the wound ribbon (in The Birth and Death of the Sun, Figure 12) appears to be the earliest. Gamow re-used it in two editions of another book, 1, 2, 3...Infinity (1948, 1953), as is. He redrew it from scratch for the 1961 edition of 1, 2, 3...Infinity, adding about a dozen new items, notably Np through No, on a new loop. (Unfortunately, in the 1961 version he introduced 4 or 5 goofy errors, including the non-existent 'Fa' for Ga, and a misplaced 'Ba' where Sr belongs, etc.) Most significantly, in another one of his 1961 publications, a book entitled The Atom and Its Nucleus, he swapped the left and right halves of the diagram (see pp. 10-11, Figure 2), so that the noble gas column could be seen as the backbone of the whole structure. He calls it out as such on page 9."
Thanks to Conal and Philip Stewart for the tip!
| Year: 1940 | PT id = 865, Type = element |
Discovery of Astatine
At ![]()
Astatine, atomic number 85, has a mass of 210 au.
Radioactive element.
Astatine was first observed or predicted in 1940 by R. Corson, R. MacKenzie and E. Segrè.
| Year: 1940 | PT id = 873, Type = element |
Discovery of Neptunium
Np ![]()
Neptunium, atomic number 93, has a mass of 237 au.
Radioactive element: Np is only found in tiny amounts in nature. Most samples are synthetic.
Neptunium was first observed in 1940 by E.M. McMillan and H. Abelson.
| Year: 1940 | PT id = 874, Type = element |
Discovery of Plutonium
Pu ![]()
Plutonium, atomic number 94, has a mass of 244 au.
Radioactive element: Pu is only found in tiny amounts in nature. Most samples are synthetic.
Plutonium was first observed in 1940 by Glenn T. Seaborg, Arthur C. Wahl, W. Kennedy and E.M. McMillan.
| Year: 1940 | PT id = 1262, Type = formulation review |
Hsueh & Chiang's Periodic Properties of the Elements
Hsueh & Chiang, Periodic Properties of the Elements, J. Chinese Chem. Soc., 5, 5, 253-275. See the PDF.
René Vernon writes:
"A mathematical expression of the periodic law was put forward in 1937 in an article by Chin-Fang Hsueh and Ming-Chien Chiang: J Chinese Chem Soc, 5, 263 (In English.) They derived a property equation from which the numerical magnitude of a property P is related to the atomic number Z of the element in question in terms of valence V, a function of the periodic factor y, the principal quantum number n, and two parameters a and p, which are constants for a given family of elements but different for different families."
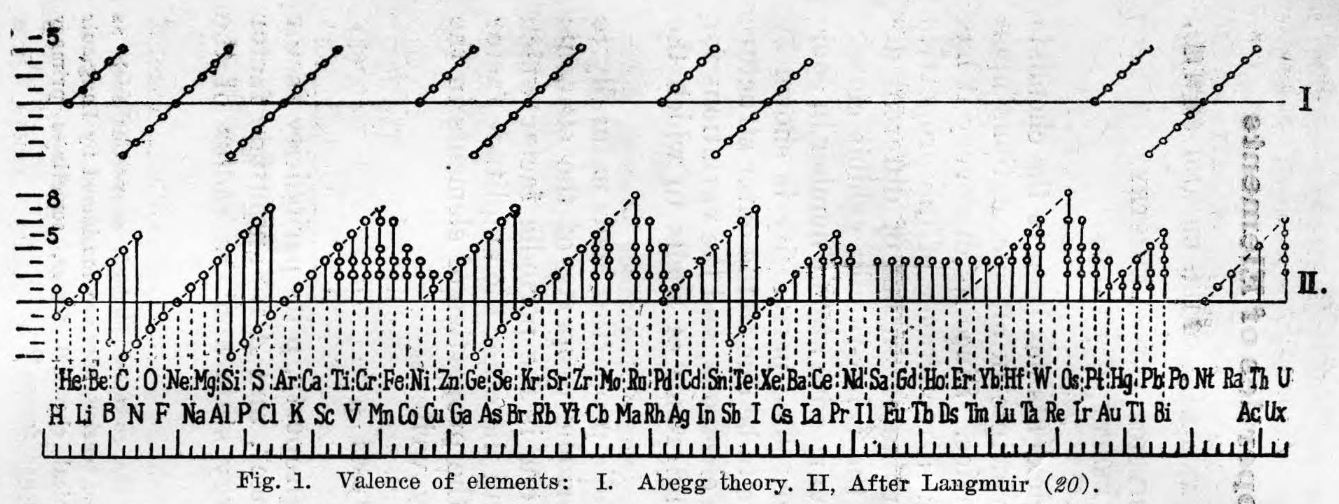
| Year: 1942 | PT id = 521, Type = formulation |
Seaborg's Periodic Table of 1942
In his Priestly Medal Address, The Periodic Table: Tortuous Path to Man-Made Elements printed in C&EN April 16, 1979 and reprinted in Modern Alchemy: Selected Papers of Glenn T. Seaborg (1994), page 181, Glenn Seaborg describes how the extension of the PT, caused the discovery of the transuranic elements, plutonium and neptunium, resulted in a new "uranide" group.
The formulation below is the working (and at the time top-secret) formulation used by the Manhatten atomic bomb project. The Lawrence Berkeley Laboratory internal reference number for this document is XBL 798-2509.
Like the 1939 formulation, XBL 769-10601, the formulation below erroneously predicts positions for the heaviest elements:
- thorium is shown in the group below hafnium
- protactinium below tantalum
- uranium below tungsten
| Year: 1942 | PT id = 565, Type = formulation data |
Paneth's Table
Published by Paneth in 1942 in an article in Nature in which he suggests that newly discovered elements such as Z = 43 should be given names by their discoverers. The other highlighted elements (below) had also not yet been named.
Element 43 had been discovered 9 years earlier but had not been given an official name because there was reluctance to consider synthetic elements on the same footing as naturally occurring ones. This changed as a result of Paneth's article.
For more information see Eric Scerri's, A Tale of Seven Elements, OUP, 2013.
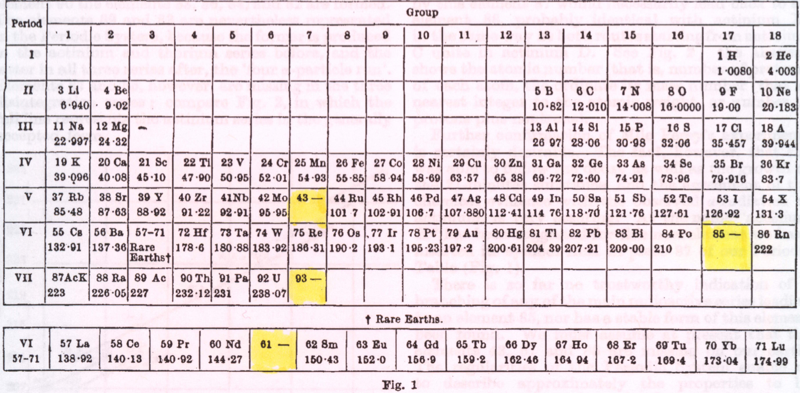
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1942 | PT id = 762, Type = formulation |
Barber & Taylor Periodic Table
The periodic table that appears on the inside of the front cover of: Barber, H.H., Taylor, T.I. Semimicro Qualitative Analysis, Harper, 1942. Click here for a larger version.
Conal Boyce writes:
"This is actually the Gardner/Mazzucchelli 1930 formulation, a colored version can be found here in the database. The periodic table below is found on the inside cover of Barber & Taylor's Semimicro Qualitative Analysis (1953[1942]), it is printed without attribution or source. Thanks to Philip Stewart for identifying the source.":
Thanks to Conal for the tip!
| Year: 1942 | PT id = 1081, Type = formulation spiral |
Kipp (& Mazurs') Periodic Table in Style of Spiral and Plane Lemniscate
Kipp, Friedrich, and Edward G. Mazurs. "Periodic Table in Style of Spiral and Plane Lemniscate". Glass, circa 1942–1957. Edward G. Mazurs Collection of Periodic Systems Images, Box 1. Science History Institute, Philadelphia. https://digital.sciencehistory.org/works/nz806022g
Periodic table in style of spiral and plane lemniscate 1942 (Original design) circa 1957 (Date attributed to slide).
This table was originated by Friedrich Kipp in 1942 and classified by chemist Edward G. Mazurs as Type IIB2-2 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957).A version of this table appears as Figure 49 on page 122 of Mazurs' 1957 publication.
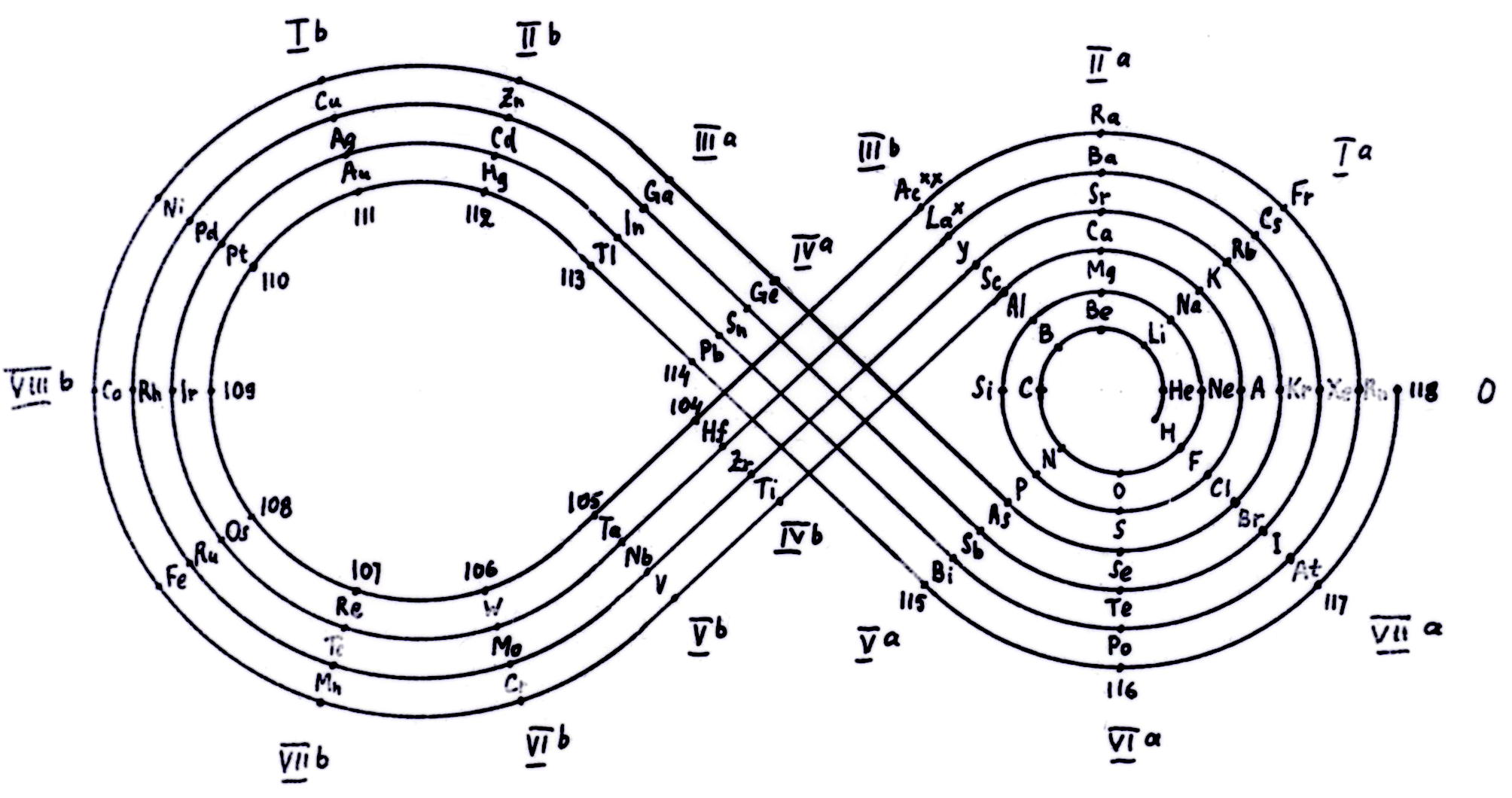
Thanks to Dhr. J.G. van Gils for the tip!
| Year: 1943 | PT id = 294, Type = formulation 3D |
Finke's Spatial System
Finke's spatial system of 1943 (from van Spronsen):
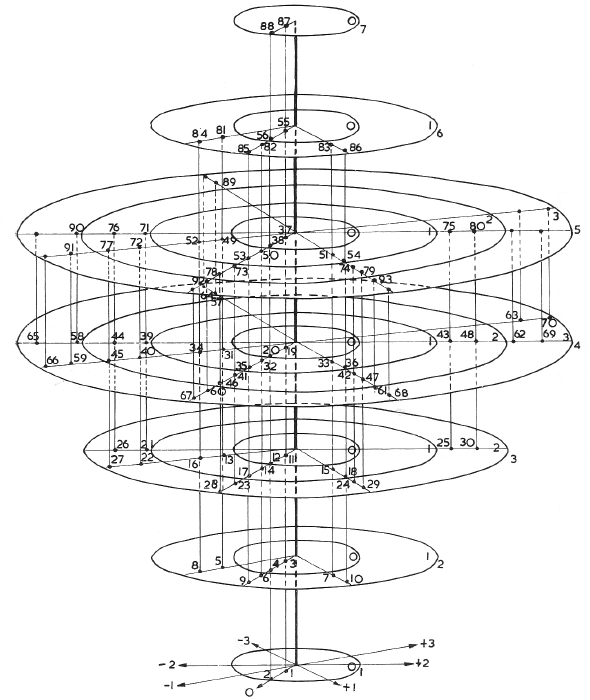
| Year: 1943 | PT id = 771, Type = formulation |
Luder's Electron Conguration Periodic Table
W.F. Luder's Electron Configuration as The Basis of the Periodic Table, J. Chem. Educ., 1943, 20, 21-26:
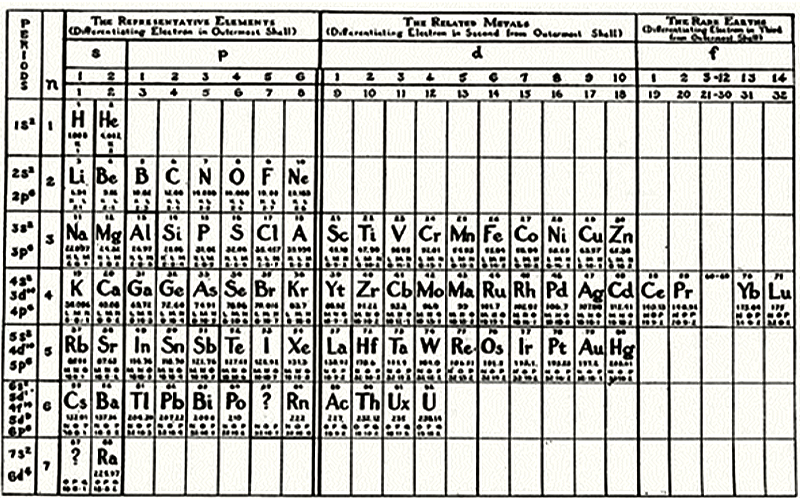
| Year: 1944 | PT id = 293, Type = formulation misc |
Müller's Tree System
In 1944 Müller produced a formulation based on Darwin's tree of life (from van Spronsen):
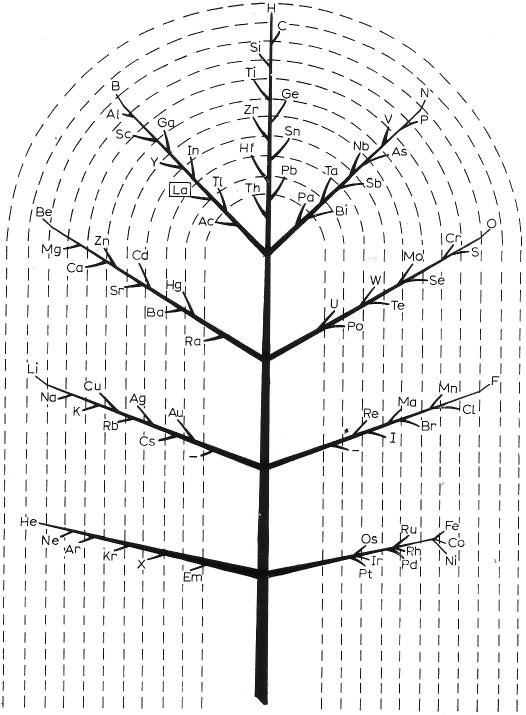
| Year: 1944 | PT id = 875, Type = element |
Discovery of Americium
Am ![]()
Americium, atomic number 95, has a mass of 243 au.
Synthetic radioactive element. It is used in smoke detectors, and so – surprisingly – is present most houses and buildings.
Americium was first observed in 1944 by G. T. Seaborg, R. A. James, O. Morgan and A. Ghiorso.
| Year: 1944 | PT id = 876, Type = element |
Discovery of Curium
Cm ![]()
Curium, atomic number 96, has a mass of 247 au.
Synthetic radioactive element.
Curium was first observed in 1944 by G. T. Seaborg, R. A. James and A. Ghiorso.
| Year: 1944 | PT id = 1238, Type = formulation |
Emerson's Long Chart Modified to Show Atomic Structure
Edgar I. Emerson 1944, A chart based on atomic numbers showing the electronic structure of the elements, J. Chem. Educ. 1944, 21, 5, 254.
A WWII chart showing neutronium over He, and a split d-block.
Thanks to René for the tip!
| Year: 1944 | PT id = 1305, Type = formulation spiral |
Emerson's Spiral Formulation
Emerson EI, 1944, A new spiral form of the periodic table, JChemEd., vol. 21, no. 3, pp. 111–115
René Vernon writes:
Emerson says that the elements in the A groups are called the representative elements because, as Eble states, they "include metals, nonmetals, inert elements, liquids, and gases." Eble RL, 1938, Atomic structure and the periodic table, JChemEd., vol. 15, p. 575
Note the inclusion in Emerson’s table of the neutron as element 0. Astonishingly, Emerson writes: "Element 0, possibly neutron [sic], is considered as a noble gas. Because of its probable chemical inertness and extreme density it might not be detected in a sizeable amount until some future scientist succeeds in sampling the center of the Earth." (p. 111)
(Mark Leach adds: The date is 1944 when the Manhattan Project was in full swing and nothing was being published about nuclear physics and/or neutron interactions. This idea may have come from some type of Popular Science story?)
Other features:
- The A and B groups are diametrically opposed in their positioning. "The electron structure of H either as H+ or H– finds a counterpart in the structure of element 0 or 2." (p. 113)
- The cell spaces for Be and Mg have been stretched on account of uncertainty as to whether they belong to group 2 or group 12. "The break between the periphery of the loops of the spiral along the spaces allotted to Mg and the transition metals of the fourth period serves to indicate that Be and Mg are not to be considered as a kind of prototype of these groups." (p. 111)
- "The C group is shown as a separate segment. If one were not concerned by plane representation the rare earths could be represented as a loop or bulge above the surface of the plane. One might imagine that this group of metals is a sort of hernia of nature that has been excised so as to maintain a flat surface." (p. 112–113)

| Year: 1945 | PT id = 231, Type = formulation misc |
Segrè Chart of Elements & Isotopes
The Segrè chart of elements and isotopes arranges atomic nuclei by numbers or protons and numbers of neutrons and is a table of nuclides. There are various ways the axes can be arranged. From elsewhere in this chemogenesis web book:
And from Wikipedia:
| Year: 1945 | PT id = 522, Type = formulation |
Seaborg's Periodic Table of 1945
From his Priestly Medal Address, The Periodic Table: Tortuous Path to Man-Made Elements printed in C&EN April 16, 1979 and reprinted in Modern Alchemy: Selected Papers of Glenn T. Seaborg (1994), page 181.
Seaborg describes how "the theory was advanced that [the] new elements heavier than than actinium might constitute a second series similar to the series of 'rare-earth' or 'lanthanide' elements":
| Year: 1945 | PT id = 578, Type = formulation |
Krafft's Periodic Table (1945)
From Ether and Matter, p. 86, Carl Frederick Krafft:
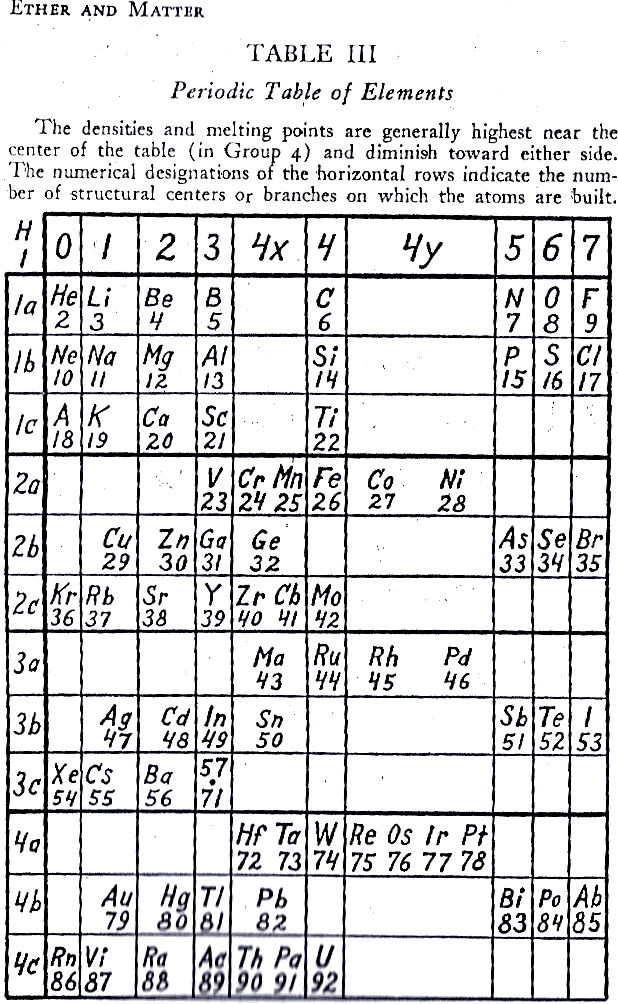
Thanks to Edmond Maurice Peyroux for the tip!
| Year: 1945 | PT id = 841, Type = element |
Discovery of Promethium
Pm ![]()
Promethium, atomic number 61, has a mass of 145 au.
Radioactive element: Pm is only found in tiny amounts in nature. Most samples are synthetic.
Promethium was first observed or predicted in 1942 by S. Wu, E.G. Segrè and H. Bethe and first isolated in 1945 by Charles D. Coryell, Jacob A. Marinsky, Lawrence E. Glendenin, and Harold G. Richter.
| Year: 1945 | PT id = 1118, Type = formulation 3D |
Talpain's Gnomonic Classification of the Elements
Talpain PL 1945, Gnomonic classification of elements, J.Phys. Radium 6, 176-181 (in French), https://doi.org/10.1051/jphysrad:0194500606017600
Talpain writes:
"To overcome the drawbacks presented by the various tables in rows and columns into which the classification of chemical elements is usually inserted, the author proposes a diagram in space, having the form of a double pyramid constructed according to a simple arithmetic law, inspired by Greek surveyors. Under these conditions, all the bodies belonging to the same chemical family are placed on the same column, and all those which have similar physical properties (magnetic, electrical, radioactive, crystallographic, rare earths, etc.) are grouped together. This same diagram also makes it possible to represent the electronic structure of the atoms, the quantified states of the electrons, the energy levels and the spectral lines of hydrogen. Perhaps spectroscopists will be able to use it to also represent the lines of other bodies."
Thanks to René for the tip!
| Year: 1946 | PT id = 776, Type = formulation |
Achimof's System
Van Spronsen, on p. 157, says:
"Achimov's system took the form of a cross-section of a pyramid. He based his system on the principle that the lengths of the periods and the analogies in properties between the elements of these periods must be clearly demonstrated."
Achimov EI 1946 Zhur. Obshchei Khim., vol. 16, p. 961
Thanks to René for the tip!
| Year: 1946 | PT id = 1072, Type = formulation |
Yost & Russell's Periodic System
From D.M. Yost & H. Russell, Systematic Inorganic Chemistry of the Fifth-and-Sixth-group Nonmetallic Elements, Prentice-Hall, 1946, New York, p. 406.
René Vernon writes:
"Features of this peculiar periodic system:
- There are 11 main groups
- Group 3 features B-Al, followed by Sc-Y-La-Ac
- Group 4 bifurcates after C-Si into a Ti branch and a Ge branch; seemingly the Ti branch bifurcates after Zr into a Ce branch and a Hf branch
- Group 8 is Fe-Ru-Os
- Group 9 is Co-Rh-Ir
- Group 10 is Ni-Pd-Pt
- There are only 13 rare earths, running from Pr to Lu.
| Year: 1946 | PT id = 1088, Type = formulation |
Harrington's Crystal Chemistry of the Periodic System
R.H. Harrington, The Modern Metallurgy of Alloys, John Wiley & Sons, New York, p. 143 (1946)
René Vernon writes:
- The planar arrangement differs from the plan of his solid model, 'only as necessary to clarify the presentation'
- One the major features is that only two groups, at Si and Y, are considered to be 'truly' branched and that the latter 'is not usually considered in this manner'
- The smaller symbols, such as V under P, aren't necessary but are 'merely offered for consideration'
- Si shows a greater resemblance to Ge than it does to the closer Ti, while Y similarly shows greater resemblance to Lu than to La
- Stedman first drew his first version of this table sixteen years ago (= 1931)
- The neutron is included in group 0.
- Argon is still A; niobium Cb
- There's a blank space for Pm (discovered 1945).
- The main groups are recognisable, with the exception of group 3 as B-Al-Sc-Y-La. The other side of the table lists B-Al as being analogous to Sc-Y-La, rather than Ga-In-Tl.
The former option works better than the latter in terms of the quantitative smoothness of chemico-physical trend lines going down the group."
"The numbers below each element symbol refer to the crystal: 1 = FCC, 9 = graphite structure, 11 = orthorhombic, etc. Extra numbers are for structures at higher temperatures.
"The wriggly lines between groups 3 and 4, and 11 and 12 refer to a gradation between the classes involved. Wikipedia calls these linking or bridging groups
"Harrington's class names are novel. [Who would have thought of the elements of groups 1 to 3 as being called the "salts of electrons"?] Then again, "in view of the extensive role that electrons play as anions" Dye (2015) asked: "where should electrons be placed in the periodic table?" (Note: In 1946 Achimof tried answering this, with an electron as element -1 above H and a neutron as element 0 above He.)
"Aluminium appears in group 3 and group 13 since, according to Harrington, it has the crystalline structure of a true metal. This is not quite true since its crystalline structure shows some evidence of directional bonding.
"For the transition metals as "wandering bonds", Harrington writes that the metallic bond is spatially undirected and that it may operate between any given atom and an indefinite number of neighbours" (p. 145). Since A-metals are better called, in his mind, "salts of electrons" [and B-metals show signs of significant directional bonding] the transition metals are therefore called by him as wandering bonds. This becomes confusing, however, given d electrons in partially filed d-orbitals of transition metals form covalent bonds with one another.
"Counting boron as a pseudo metals looks strange.
"Germanium is counted as a metal: "...the electrical conductivit[y]... [is] sufficiently high to show that the outer electrons are very loosely held and the linkage must be partly metallic in character." (p. 148). In fact the electrical conductivity of high purity germanium, which is a semiconductor, is around 10–2S.cm–1. Compare this with antimony, at 3.1 x 104S.cm–1
"Tin has brackets around it to show its "renegade" status, "with its white form behaving largely as would a True Metal, whereas its grey form is more non-metallic than metallic." White tin actually has an irregularly coordinated structure associated with incompletely ionised atoms.
"Thallium and lead have brackets around them since their crystalline structures are supposedly like those of true metals. This is not quite right. While both metals have close-packed structures they each have abnormally large inter-atomic distances that have been attributed to partial ionisation of their atoms.
"The B-subgroup metals are divided into pseudo metals and hybrid metals. The pseudo metals (groups 11 and 12) behave more like true metals than non-metals. The hybrid metals As, Sb, Bi, Te, Po, At – which other authors would call metalloids – partake about equally the properties of both. According to Harrington, the pseudo metals can be considered related to the hybrid metals through the carbon column.
"The location of the dividing line between metals and nonmetals, running as it does through carbon to radon is peculiar. The line is usually shown running through boron to astatine."
| Year: 1947 | PT id = 85, Type = formulation spiral |
Stedman's Design
In his article Stedman says:
Thanks to René for the tip!
| Year: 1947 | PT id = 291, Type = formulation spiral 3D |
Stedman's Conic System
D. F. Stedman, A Periodic Arrangement of the Elements, Canadian Journal of Research, 1947, 25b(3): 199-210, https://doi.org/10.1139/cjr47b-023
Stedman's conic system from van Spronsen:
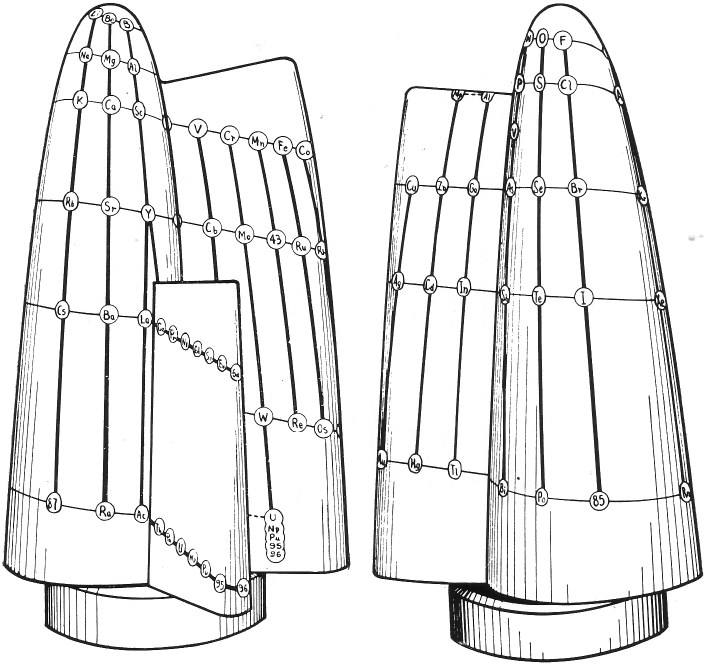
From c&en:
| Year: 1947 | PT id = 1166, Type = formulation |
Ageev's Crystalline Structures of The Elements
Ageev NV 1947, The nature of the chemical bond in metal alloys (Izdvo Akad. Nauk SSSR, Moscow/Leningrad, p. 10
René Vernon writes:
"In this curious 18-column table, showing the crystalline structures of the elements, Ageev locates the predominately non-metallic groups on the left and the remaining groups on the right.
"It's odd that he located boron and aluminium on the far left over gallium, rather than over scandium. I suppose he did this so that gallium, indium, and thallium would not be mistaken for d-block metals.
"Reading from left to right then, Ageev's table could be said to be made up of five blocs:"
[1] the nonmetallic bloc
[2] the alkaline bloc
[3] the inner transition bloc
[4] the transition metal block
[5] a post-transition metallic bloc
| Year: 1947 | PT id = 1243, Type = formulation |
Science Service: Two Periodic Tables
A two-sided Science Service periodic table from 1947. The one is listed as "After Bohr", the other as "After Mendeleeff".
René Vernon writes:
"Here’s a slightly odd table (with two sides):
| Year: 1948 | PT id = 1142, Type = formulation |
Hakala's Electronic Orbital Filling
Hakala, R.W., Letter to The Editor, J. Chem. Ed. 25, 229, 1948

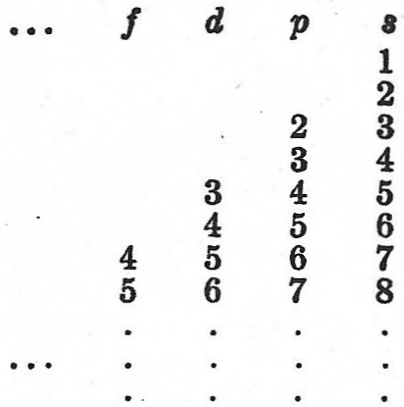
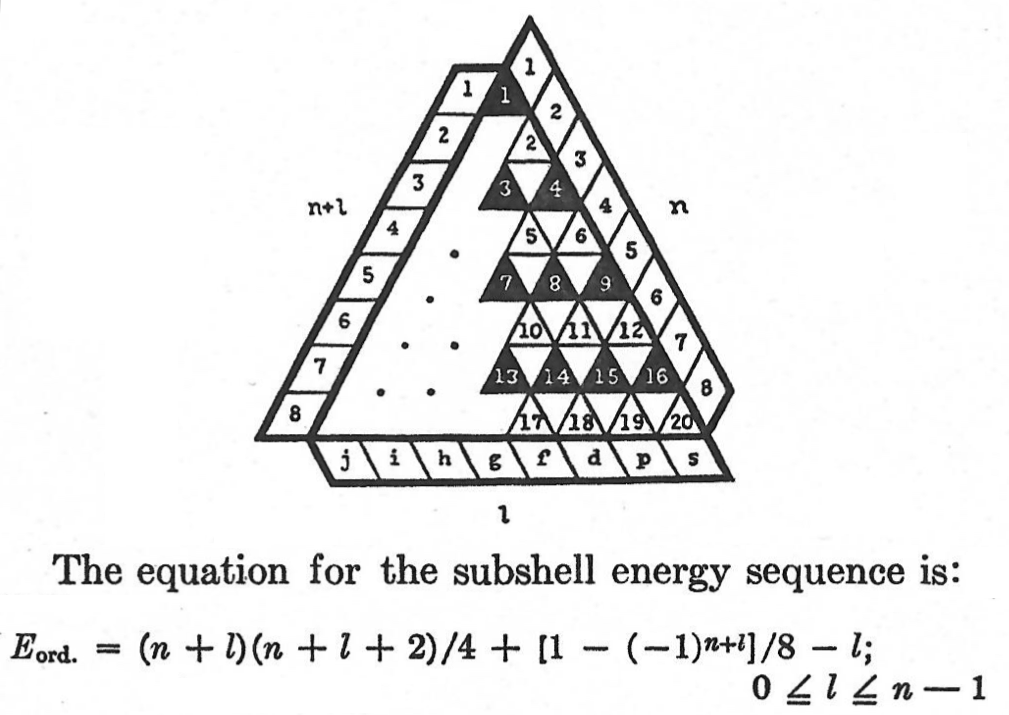
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1949 | PT id = 27, Type = formulation |
Pauling's Formulation
Linus Pauling borrowed von Antropoff 1926 design, without acknowledgement, for his 1949 book, General Chemistry (and subsequently in later editions of The Chemical Bond).
The periodic table below is scanned in from Pauling's The Nature of The Chemical Bond, 3rd ed., 1960:

| Year: 1949 | PT id = 276, Type = formulation spiral |
Clark's Periodic Arrangement of The Elements
Origionally developed in 1933, the colour version of Clark's arrangement was used the the May 1949 edition of Life Magazine, part of a 16 page feature on the atom.
This periodic table formulation was the model for Longman's 1951 Festival of Britain mural. Information supplied by Philip Stewart.
| Year: 1949 | PT id = 295, Type = formulation 3D |
Wrigley's Lamina System
In two papers: A.N. Wrigley, W.C. Mast, and T.P. McCutcheon, "A Laminar Form of the Periodic Table, part 1," Journal of Chemical Education 26 (1949): 216-218 and A.N. Wrigley, W.C. Mast, and T.P. McCutcheon, "A Laminar Form of the Periodic Table, part 2: Theoretical Development, and Modifications," Journal of Chemical Education 26 (1949): 248-250 a Laminar Periodic Table is introduced. (Thanks to Ann E. Robinson for this informaton & references.)

This formulation was discussed and re-drawn by van Spronsen in 1969:
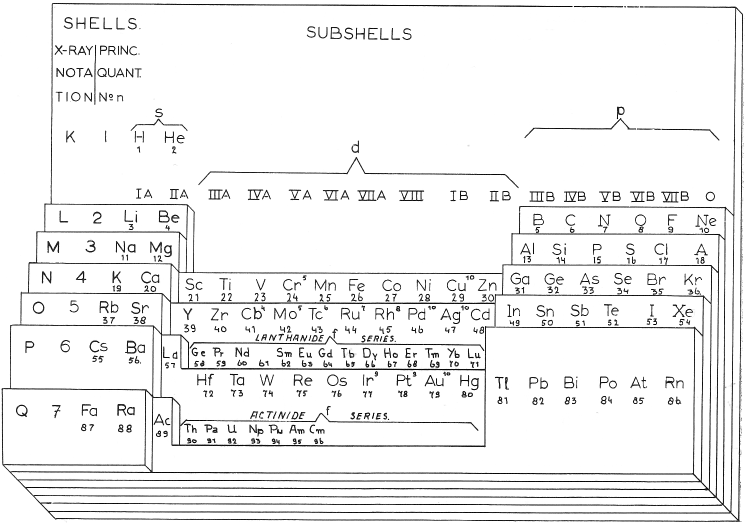
There is a Russian publication "100 Years of Periodic Law of Chemical Elements", Nauka 1969. On page 87 there is a formulation that appears to be a version of the van Spronsen re-drawing. The caption says: "Volumetric Model of 18-period Long System of D.I.Mendeleev." after Riggli (1949). (Thanks to Larry T for this.)
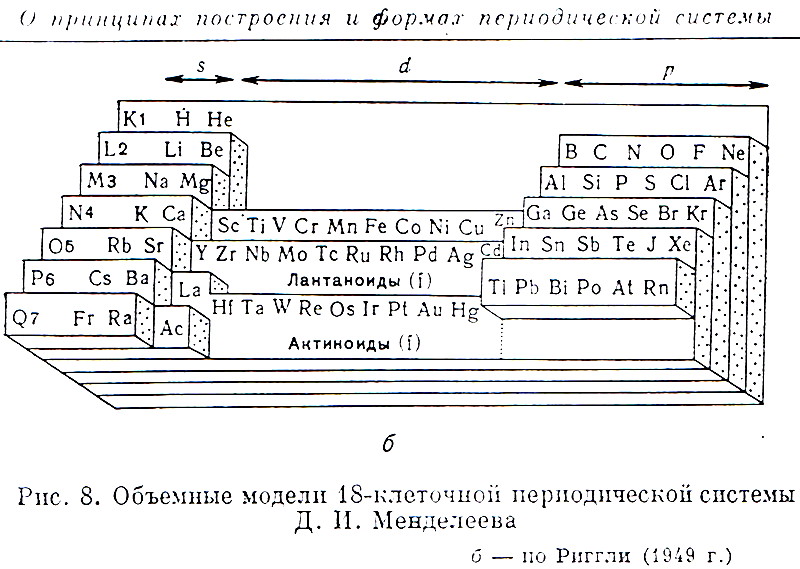
| Year: 1949 | PT id = 698, Type = formulation |
Antropoff's Representation of the Periodic System Revised by Fritz Scheele
Andreas von Antropoff's 1926 representation of the Periodic System, revised by Fritz Scheele in 1949, to include the lanthanides and actinides.
The table was reconstituted, using von Antropoff s colour scheme, by P J Stewart, November 2007:
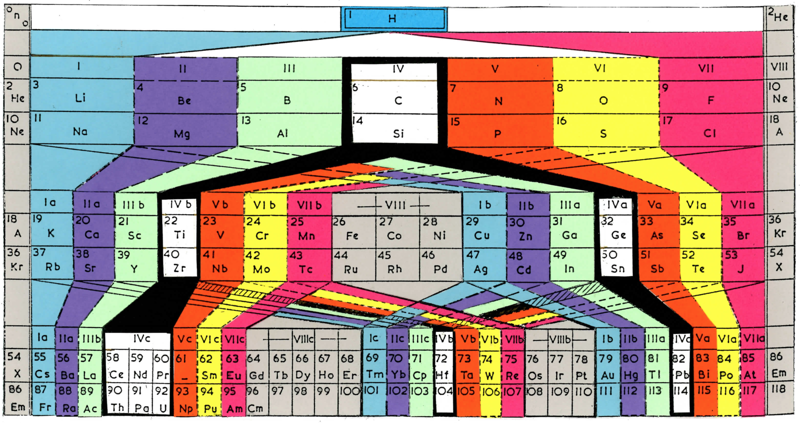
Thanks to Philip Stewart for the tip!
| Year: 1949 | PT id = 877, Type = element |
Discovery of Berkelium
Bk ![]()
Berkelium, atomic number 97, has a mass of 247 au.
Synthetic radioactive element.
Berkelium was first observed in 1949 by G. Thompson, A. Ghiorso and G. T. Seaborg.
| Year: 1949 | PT id = 921, Type = formulation 3D |
Riggli's Volumetric Model of the Periodic Table
From the Russian Book "100 Years of Periodic Law of Chemical Elements", Nauka 1969, p.87.
The caption says: "Volumetric Model of 18-period Long System of D.I.Mendeleev." after Riggli (1949).

Thanks to Larry T for the tip!
| Year: 1949 | PT id = 1018, Type = formulation 3D |
Scherer's Student Model of Spiral Periodic Chart
George A. Scherer, New Aids for Teaching the Periodic Law, School Science and Mathematics, vol. 49, no. 2 (1949).
René Vernon writes:
"This is a Left-Step periodic table with a split d-block, that can be rearranged into a cylinder. Students were expected to keep a copy of the two halves of the table in their note books, for reassembly as required. It was a clever way of introducing the 32-column form, and the transition from 2D to 3D (that faded into obscurity)":
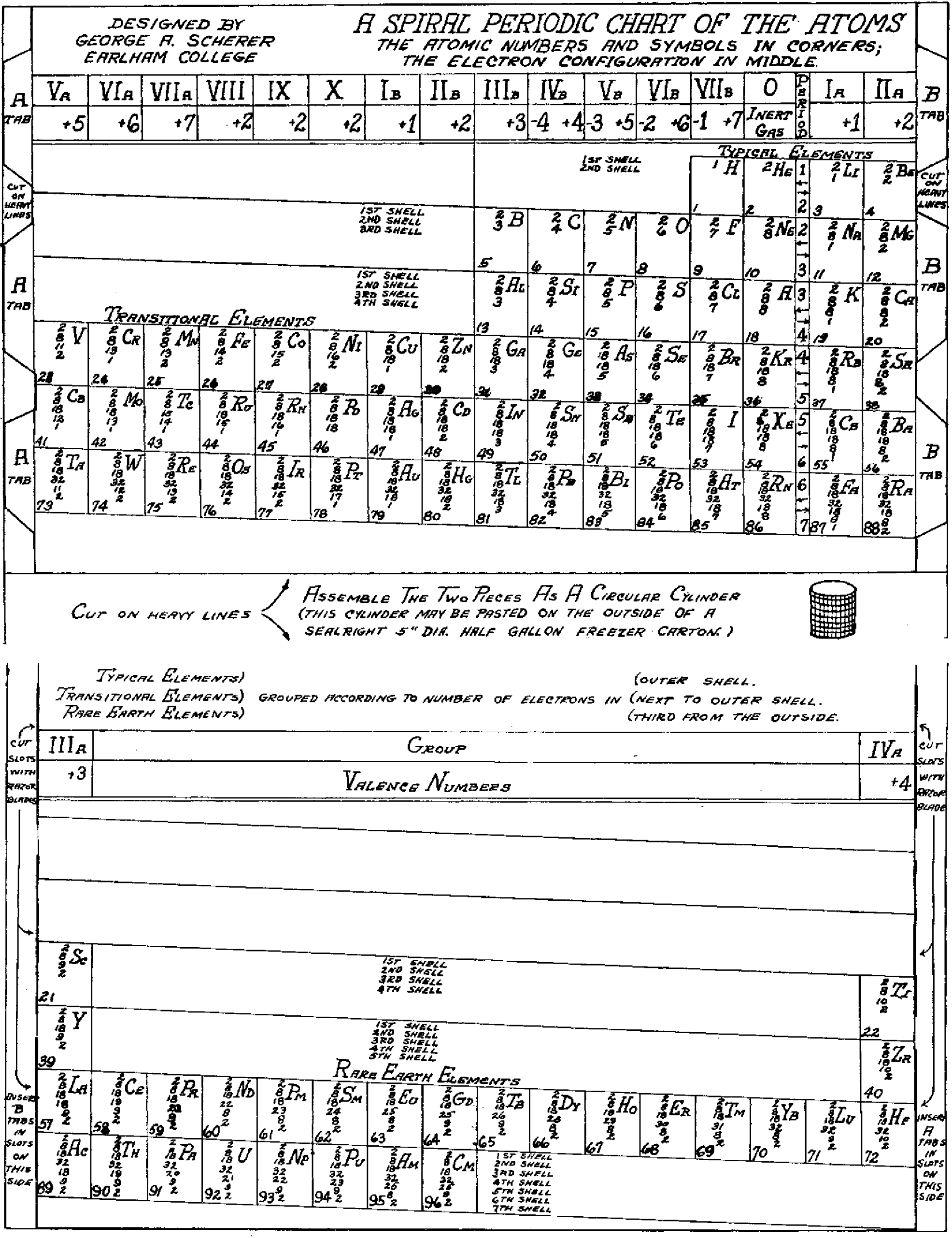
Thanks to René for the tip!
| Year: 1949 | PT id = 1052, Type = formulation |
Catalán's Periodic System/Sistema Periodico Ampliado
Two versions of Catalán's Periodic System/Sistema Periodico Ampliado. The first from Moore, Charlotte E., Atomic Energy Levels, National Bureau of Standards, Circular no. 467 (Washington, D. C.: U. S. Government Printing Office, 1949), vol. 1, Table 25. and the second as referenced here: http://www.miguelcatalan.net/pdfs/bibliografia/biblio09.pdf.
René Vernon, who provided the graphics, writes:
"I feel the footnote along the base of the first table could merit better attention being drawn to it. It says:
This arrangement is by Catalán. The electrons indicated in column two that are connected by braces have approximately the same binding energy. Consequently, for some elements one type of electron is preferred over another in the normal configuration, as for example, Cr, Cb, Pd, La, Ac, Th.
"The connecting braces hone in on the source of much of the controversy concerning notions of an ideal, optimal, better, this or that, or fundamental periodic table. I can't recall seeing a table with such a feature. For the second table, turning it on its side (attached) reminds of the ADOMAH [formulation].
Click on the images to enlarge:
Thanks to René for the tip!
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.