Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Tables from the years 2000 - 2009, by date:
| Year: 2000 | PT id = 449, Type = formulation 3D |
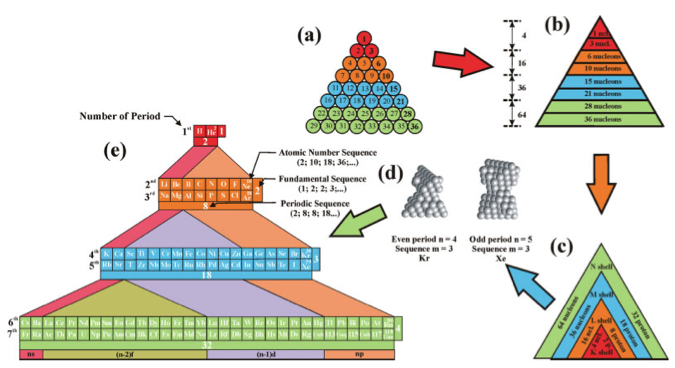
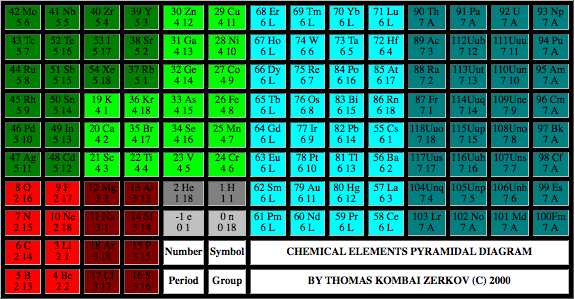
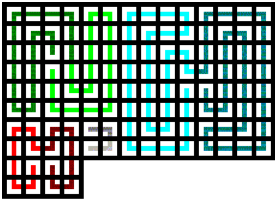
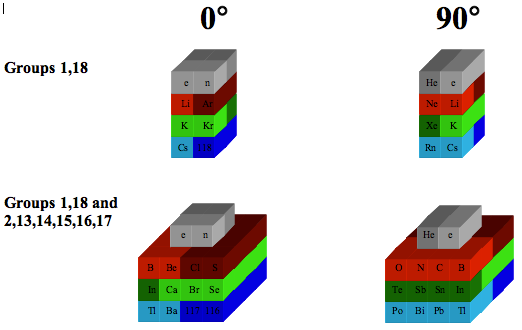
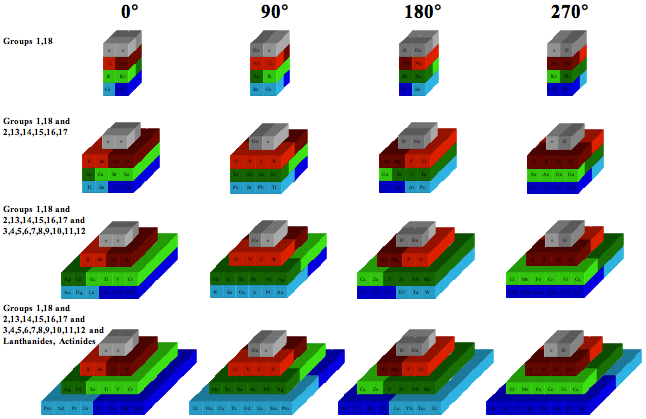
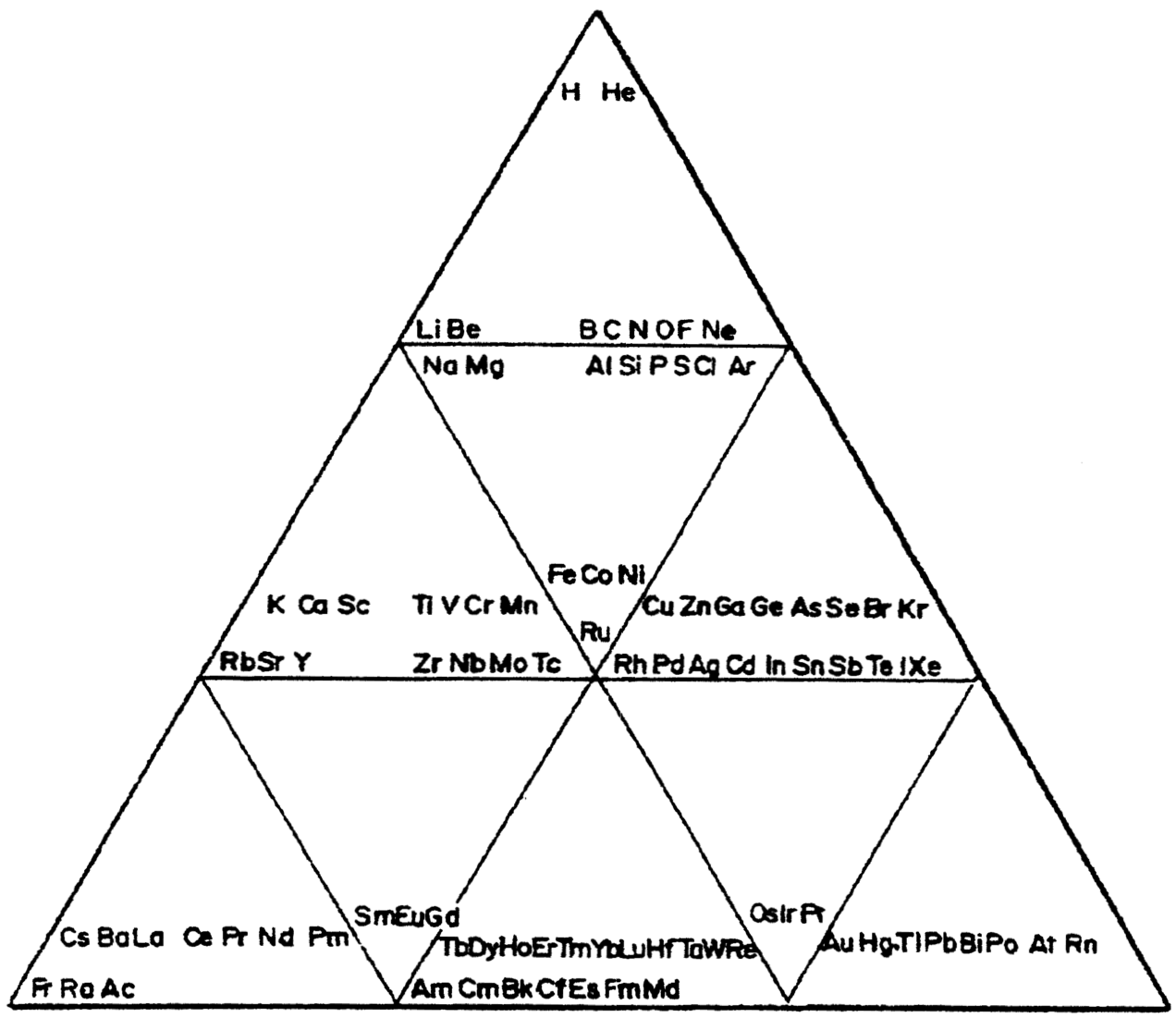
Chemical Elements Pyramidal Diagram
A Chemical Elements Pyramidal Diagram by Thomas Zerkov.
"The present work introduces a new arrangement of the chemical elements. Unlike the most popular existing arrangements, which are two-dimensional, this new arrangement is three-dimensional. It organizes the elements in a pyramidal structure of four levels, giving a clear spatial expression of different relations between the chemical elements. Since the three-dimensional structures are harder to perceive than the two-dimensional ones, the present work also suggests a two-dimensional table representation of the three-dimensional pyramidal diagram, where the four levels are all placed in a single plane, instead of one above the other."
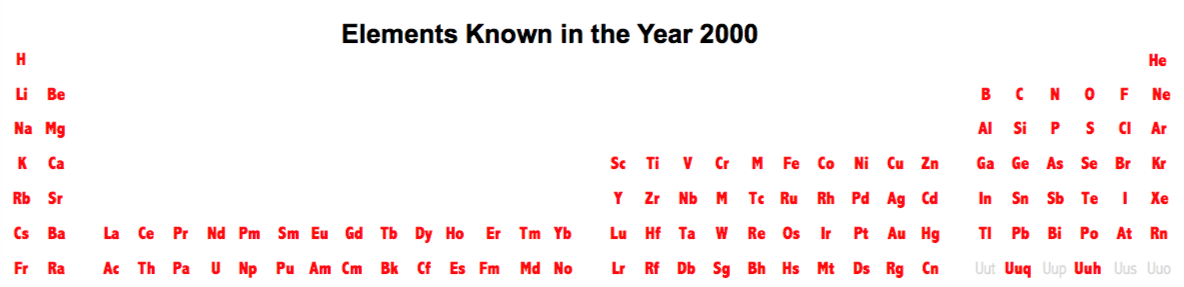
| Year: 2000 | PT id = 476, Type = formulation element |
Elements Known in the Year 2000
Elements known in the year 2000, taken from this Wikipedia page:
| Year: 2000 | PT id = 896, Type = element |
Discovery of Livermorium
Lv ![]()
Livermorium, atomic number 116, has a mass of 293 au.
Synthetic radioactive element.
Livermorium was first observed in 2000 by Y. Oganessian et al.
| Year: 2000 | PT id = 1240, Type = formulation data |
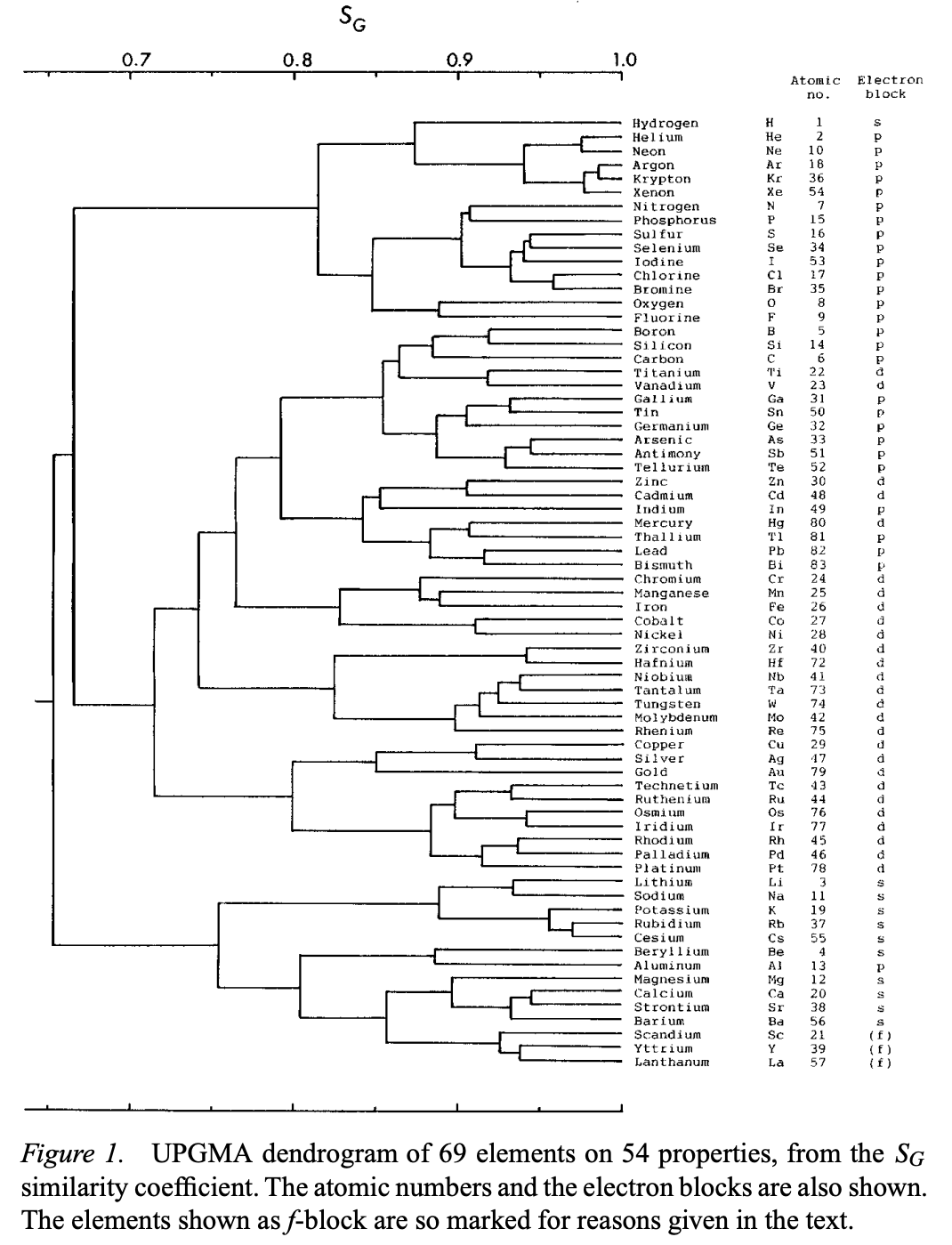
Sneath's Dendtogram
Sneath, P., Numerical Classification of the Chemical Elements and Its Relation to the Periodic System. Foundations of Chemistry 2, 237–263 (2000).
Abstract: "A numerical classification was performed on 69 elements with 54 chemical and physicochemical properties... Only 15 properties were scorable for the noble gases, but despite the paucity of properties reflecting chemical reactivity, analysis of the 69 elements on these properties still showed the major features seen from the full set."
Sneath writes:
"The UPGMA tree shows three major clusters at the 70% similarity level:
1. Hydrogen, noble gases, reactive nonmetals and halogens.
2. A large cluster of less reactive metals and metalloids, with a few nonmetals.
3. A cluster of highly reactive metals.These major clusters are almost consistent with the blocks based on electron shells. The first cluster belongs to the p-block plus hydrogen. The second belongs to the d-block together with a few p-block elements. The third consists of the s-block plus aluminum from the p-block.
Cluster 1: There are three subclusters. 1a. This contains hydrogen and the noble gases."
Thanks to René for the tip!
| Year: 2001 | PT id = 34, Type = formulation |
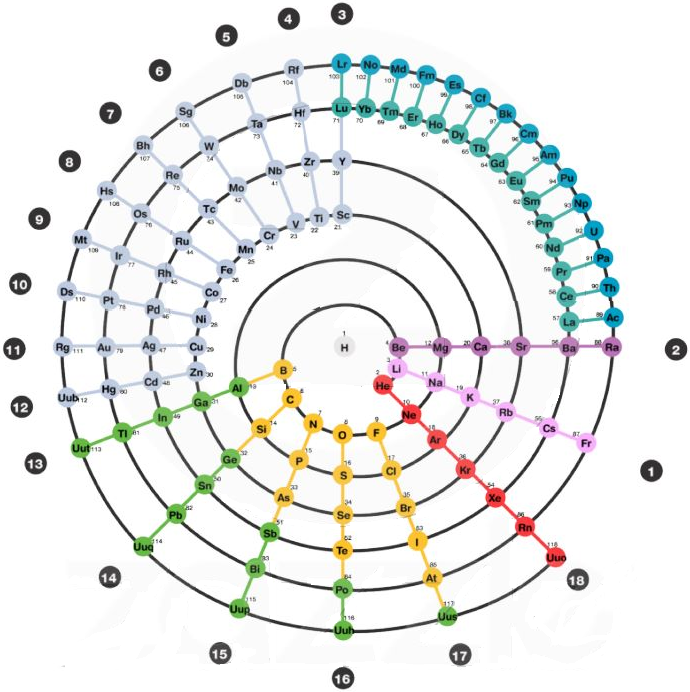
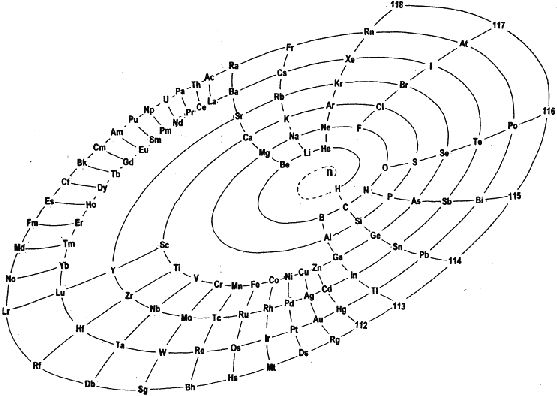
The Mayan Periodic Table
The Mayan Periodic Table of Elements, named for its similarity to the ancient Mesoamerican calendar, is based on electron shells. The shells are shown as concentric circles. Each row in the tabular form is shown as a ring.
Read more and buy the poster and T-Shirts at MayanPeriodic.com.
| Year: 2001 | PT id = 43, Type = formulation |
Vertical Periodic Table
A vertical periodic table from apsidium.com:
| Year: 2001 | PT id = 353, Type = formulation |
Muradjan's Universal Periodic System
Muradjan's Universal Periodic System is a variation of the Janet formulation, however, it is worth visiting the web page and scrolling down as there is much interesting material:
| Year: 2001 | PT id = 511, Type = formulation spiral 3D |
ElemenTouch Periodic Table
Yoshiteru MAENO writes:
"I am a Physics Prof. at Kyoto University, Japan. My field of study is experimental superconductivity. I recently found the work by Schaltenbrand in 1920 on your website. One might say that Elementouch is a re-invention of Schaltenbrand's, but by arranging the element names helically on three cylinders, its usefulness has been improved":
| Year: 2001 | PT id = 1025, Type = data formulation review |
Wikipedia Periodic Table
The Wikipedia Periodic Table pages are astonishing, giving hyper-linked data about:
- Formulations
- History
- Discovery
- Elements
- Isotopes
- Personalities
- Compounds
- etc.
| Year: 2001 | PT id = 1062, Type = formulation |
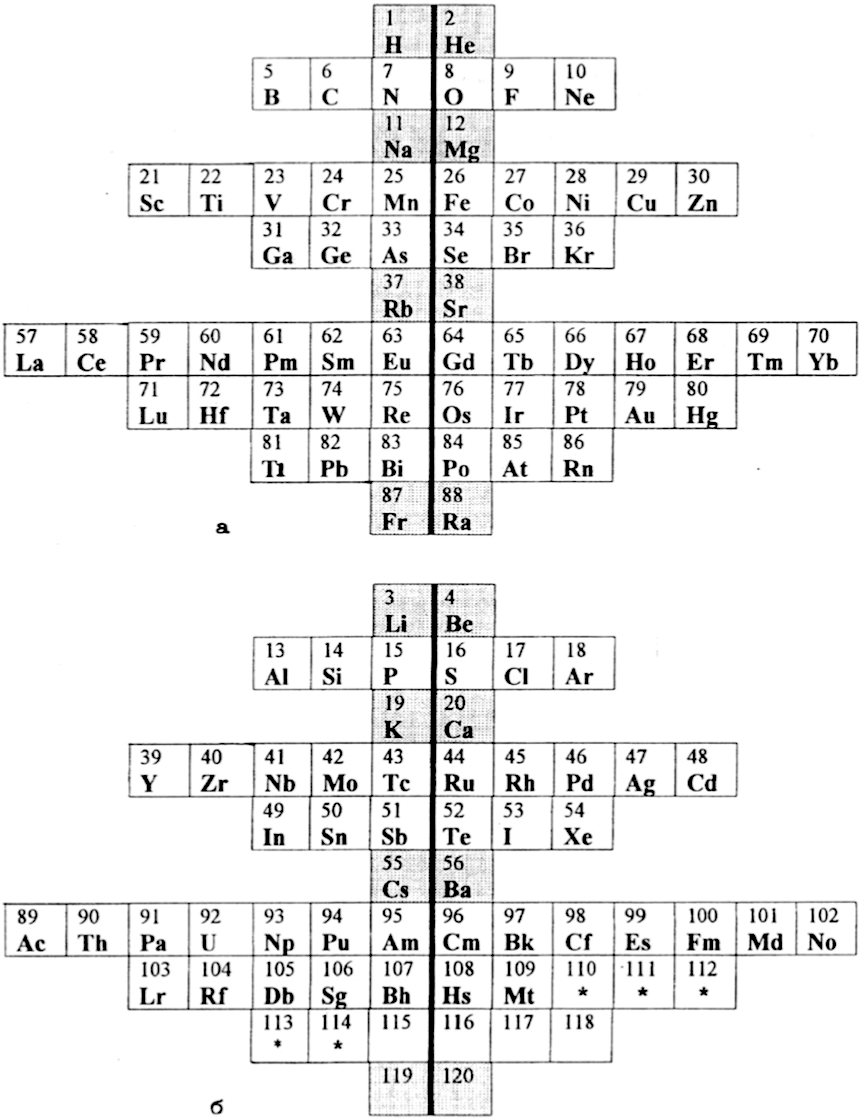
Gorbunov and Filippov's Doubled Periodic Table
Gorbunov, A. I., Filippov, G. G.: Fine Structure of D. I. Mendeleev Periodic Table: secondary periodicity, early and late elements. Khim-ya Tekhnol. 11, 43–45 (2001). (in Russian)
Naum S. Imyanitov (Foundations of Chemistry) writes:
"The two-table design is of particular interest. Atoms with odd n+l are located in the upper table, and the ones with even n+l are placed in the bottom table (Tables 5). The elements are divided by a vertical line of symmetry to the early and late ones both in the upper and bottom tables. The advantage of Tables 5 is a clear demarcation into subsets, with each subset having its own separate place in the table. The drawback is directly related to this advantage: this table does not reflect the similarity between members of different subsets."
| Year: 2001 | PT id = 1322, Type = review misc formulation |
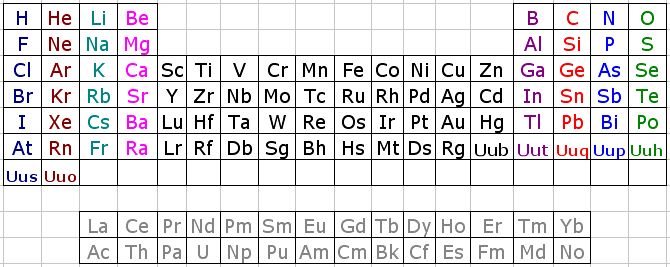
Oliver Sacks, Uncle Tungsten: Memories of Tungsten of a Chemical Beyond
René Vernon writes:
On the paperback cover of Oliver Sack's Uncle Tungsten (below) the periodic table shows a 16–wide set of elements at its base. This is quite unusual since this set is normally shown as being 15— or 14— elements wide. See, for example, the table found on the site of the International Union of Pure & Applied Chemistry which shows a 15–wide set of elements at its base.
It looks like the second pair are La and Ac, but what then are two immediately preceding elements?
I suspect they are probably the alkaline earth metals, Ba and Ra. This may be an homage to Mr Rare Earth^ aka Karl A. Gschneidner Jr (1930–2016), who wrote that:
...since Ba has a 4f06s2 configuration, these three elements are the first (Ba), mid (Eu), and end (Yb) members of the divalent 4f transition series.
The notion of 4f0 is not unprecedented; the IUPAC periodic table, with its 15-wide f-block presumably implies La as 4f0 5d1 6s2.
There is some good chemistry going on here, given the pronounced similarities between Ba and the lanthanides, and the alkaline earth metals generally with about 20 properties involved:
- Most of the physical properties of Eu and Yb, "such as the atomic volumes, metallic radii, melting and boiling points, heats of sublimation, compressibilities, and coefficients of expansion are more like those of the alkaline-earth metals, Ca, Sr, and Ba, than those of the rare-earth metals" (Pauling 1960, p. 418; Gschneidner 1964, p. 286).
- Liquid ammonia dissolves certain alkali, alkaline earth, and Ln metals, and... combines with them to form solid compounds. Those metals whose compound-forming ability has been confirmed are Li, Ca, Sr, Ba, Eu and Yb. (Mammano (1970, p. 367)
- The lanthanides are sometimes regarded as trivalent versions of the alkaline earth metals (Evans 1982).
- The electron configurations of lanthanide cations are similar to those of alkaline earth metal cations, as the inner f- orbitals are largely or completely unavailable for bond formation; (Choppin & Rizkalla 1994)
- The lanthanide trivalent cations are essentially spherical and present an environment very similar to alkali and alkaline earth ions towards complex formation... the standard electrode potentials for the lanthanides have similar values and are comparable with the redox potentials of alkaline earth metals (Sastri et al. 2003)
- Ba-Eu-Yb have cubic crystalline structures whereas the rest of the Ln are hexagonal, or rhombohedral in the case of Sm (Russell & Lee 2005)
- There is a close alloying similarity between the lanthanides and Ca, Sr and Ba (Artini 2007)
- Lanthanides are effective mimics of calcium and can stimulate or inhibit the function of calcium-binding proteins (Brayshaw 2019)
- Lanthanide cations can substitute for Ca2+ and Sr2+ cations in host materials for solid state lasers (Ikesue 2013)
- There is a knight’s move relationship between Ca and La:
- The ionic radius of Ca2+ is 114 pm; that of La3+ is 117 pm
- The similarity in sizes means La3+ will compete with Ca2+ in the human body, and usually win on account of having a higher valence for roughly the same hydrated radius
- The basicity of La2O3 is almost on par with CaO2 Freshly prepared La2O3 added to water reacts with such vigour that it can be quenched like burnt lime (CaO)
- The electronegativity of Ca is 1.0; that of La is 1.1.
Kudos to Oliver.
^Pecharsky 2016
Sources
- Artini C (ed.) 2017, Alloys and Intermetallic Compounds: From Modeling to Engineering, CRC Press, Boca Raton, p. 92
- Brayshaw et al. 2019, Lanthanides compete with calcium for binding to cadherins and inhibit cadherin-mediated cell adhesion, Metallomics, vol. 11, no. 5, 2019, pp. 914–924
- Choppin GR & Rizkalla EN 1994, Solution chemistry of actinides and lanthanides, Handbook on the Physics and Chemistry of Rare Earths, pp. 559–590(560)
- Evans WJ 1982, Recent advances in the low valent approach to f-element organometallic chemistry, in McCarthy GJ, Silber HB and Rhyne JJ (eds), The Rare Earths in Modern Science and Technology, vol. 3, Plenum Press, New York, pp. 61–70(62)
- Gschneidner KA 1965, in Seitz F & Turnbull D (eds), Solid State Physics, vol. 16, Academic Press, New York, p. 286
- Ikesue A, Aung YL, Lupei V 2013, Ceramic Lasers, Cambridge University Press, Cambridge, pp. 26, 28
- Mammano N 1970, Solid metal ammonia compounds, in Metal–Ammonia Solutions, Proceedings of an International Conference on the Nature of Metal–Ammonia Solutions: Colloque Weyl II, pp. 367-393 (367), https://doi.org/10.1016/B978-0-408-70122-8.50030-4
- Pauling L 1960, The Nature of the Chemical Bond, 3rd ed., Cornell University Press, Ithaca, p. 418
- Pecharsky V 2016, Karl A. Gschneidner Jr (1930–2016), Nature Materials, vol. 15, no. 1059, https://doi.org/10.1038/nmat4751
- Russell AM & Lee KL 2005, Structure-property relations in nonferrous metals, John Wiley & Sons, Hoboken, inside cover
- Sastri et al. 2003, Modern Aspects of Rare Earths and their Complexes, Elsevier, Amsterdam, pp. 377, 878

| Year: 2002 | PT id = 528, Type = formulation spiral 3D |
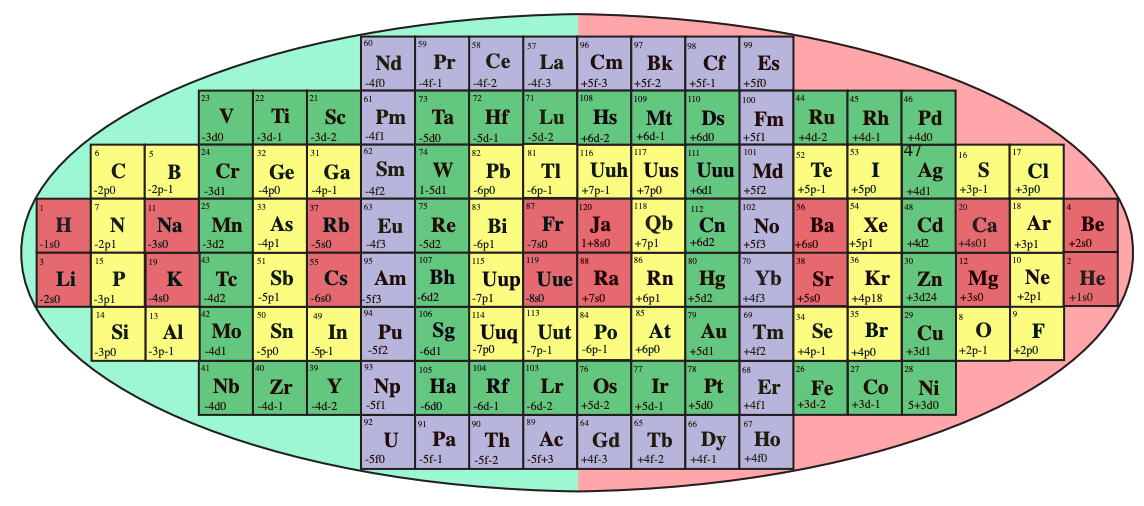
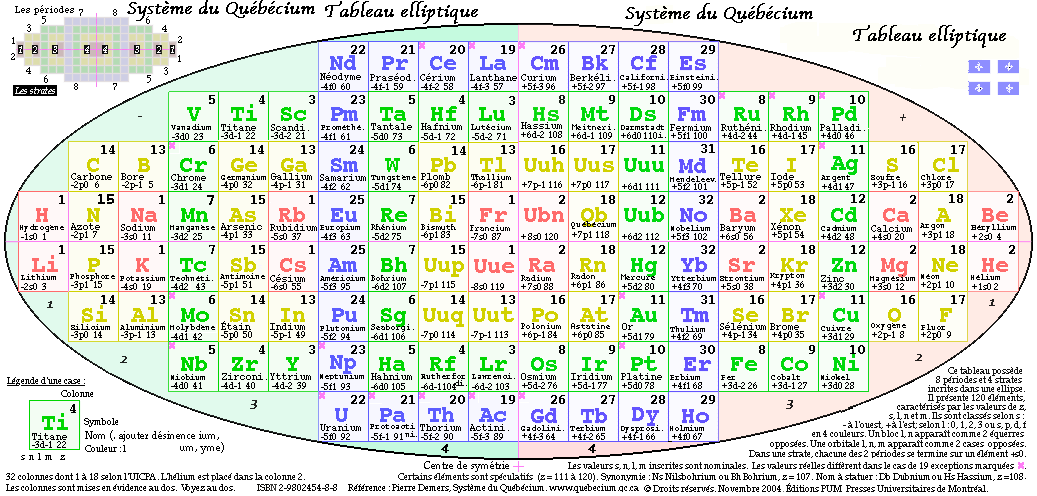
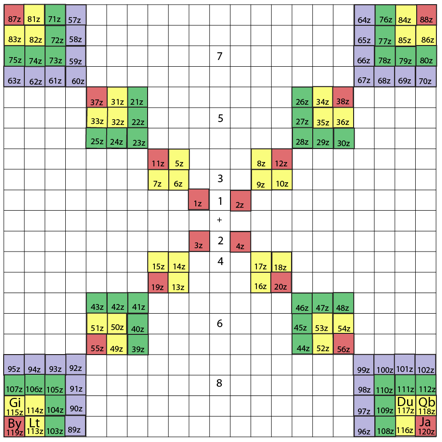
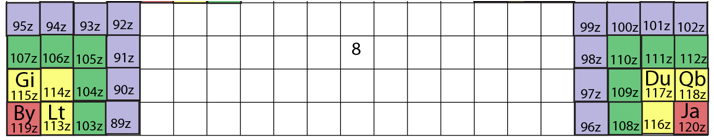
System Québécium Periodic Table
Using Google Translate of this page:
"To establish a new classification system components, Pierre Demers was assumed that the electronic structure of the atom contains one of my all others according to the equation Z = 117 to Z = 1. It is taking my electrons and removing them from my material that can reproduce all the elements and thus repeat the structure of your table. That is why this new organization is called the System Québécium":
| Year: 2002 | PT id = 714, Type = formulation |
Tetrahedral Twist: Chemistry Puzzle and Teaching Device
A twisting three dimensional puzzle apparatus for the study of chemistry and its history and based upon the Zmaczynski equilateral triangular model of the periodic table of the chemical elements. Each face of the pyramid has a series of equilateral shaped portions bearing portions of the periodic table of elements. The different segments can be rotated around in order to scramble the puzzle. Such portions can be constructed using same or similar technology that was used to design the Meffert PYRAMINX PUZZLE that is similar to the RUBIK'S CUBE design.
From a US Patent.
| Year: 2002 | PT id = 898, Type = element |
Discovery of Oganesson
Og ![]()
Oganesson, atomic number 118, has a mass of 294 au.
Synthetic radioactive element.
Oganesson was first observed in 2002 by Y. Oganessian et al.
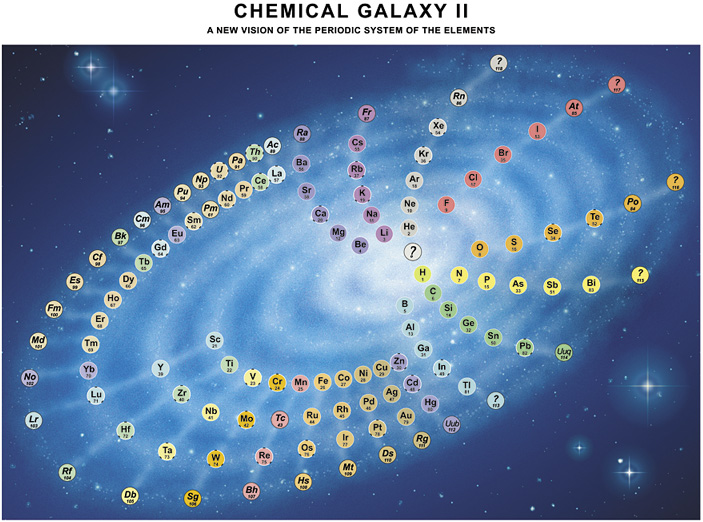
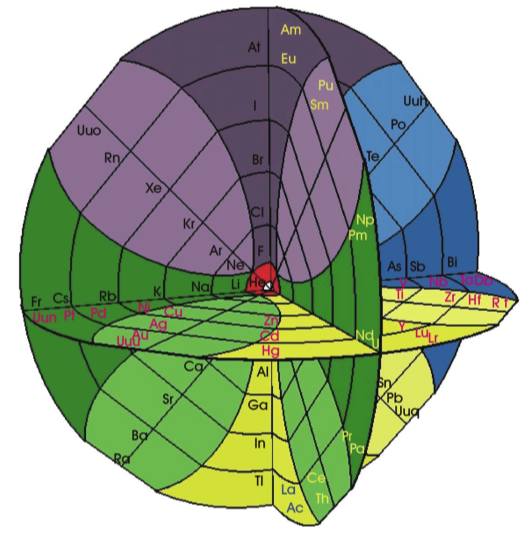
| Year: 2003 | PT id = 22, Type = formulation spiral |
Philip Stewart's Chemical Galaxy II
Philip Stewart's Chemical Galaxy II periodic table formulation, from here:
Click here for a larger version.
A simplified 'chemical galaxy':
| Year: 2003 | PT id = 44, Type = formulation 3D |
Denker's Cylinder With Bulges
John Denker fully discusses the logic behind a three dimensional periodic table that he describes as a "cylinder with bulges", here:
| Year: 2003 | PT id = 54, Type = formulation 3D misc |
Elephant Periodic Table
The periodic table does not map to an elephant very well:

Click on the poster below to go to a large version:
| Year: 2003 | PT id = 228, Type = formulation misc |
Elements by Orbital
From elsewhere in Mark Leach's Chemogenesis webbook:
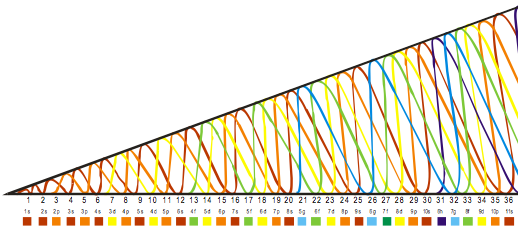
Madelung's Rule tells us that the orbitals fill in the order n + l (lowest first). This gives the sequence:
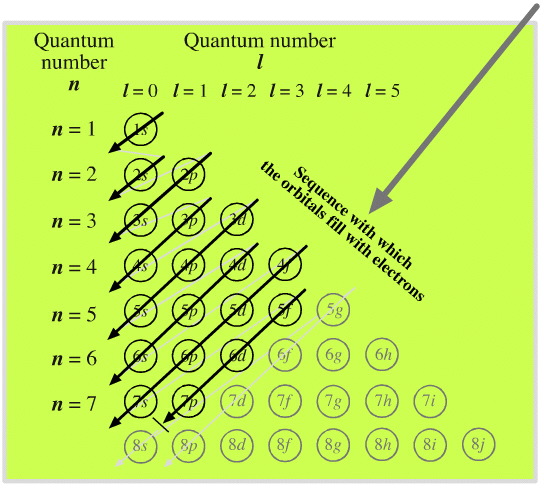
Electronic structure can be illustrated adding electrons to boxes (to represent orbitals). This representation shows the Pauli exclusion principle, the aufbau principle and Hund's rule in action.
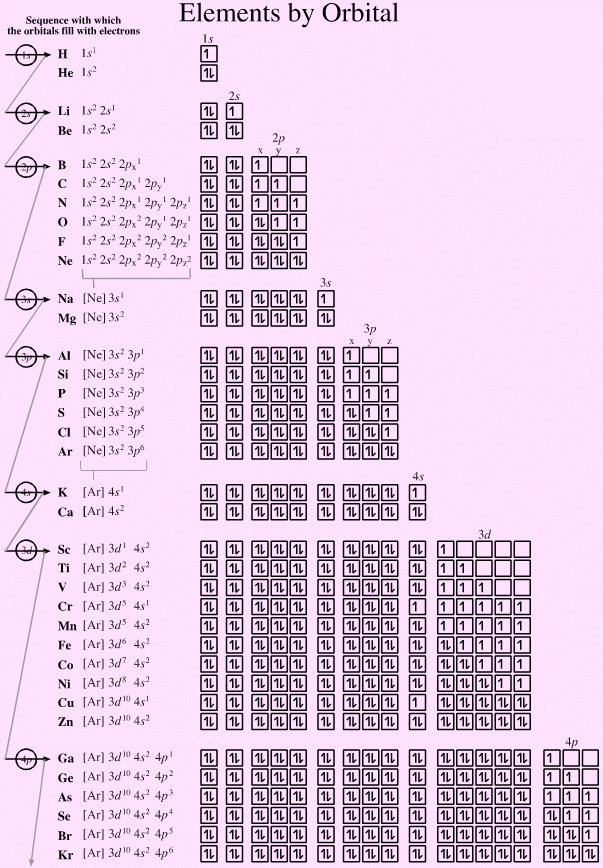
There are some subtle effects with the d block elements chromium, Cr, and copper, Cu. Hund's rule of maximum multiplicity lowers the energy of the 3d orbital below that of the the 4s orbital, due to the stabilisation achieved with a complete and spherically symmetric set of five 3d orbitals containing five or ten electrons. Thus,
- Chromium has the formulation: [Ar] 3d5 4s1 and not: [Ar] 3d4 4s2
- Copper has the formulation: [Ar] 3d10 4s1 and not: [Ar] 3d9 4s2
| Year: 2003 | PT id = 311, Type = formulation 3D |
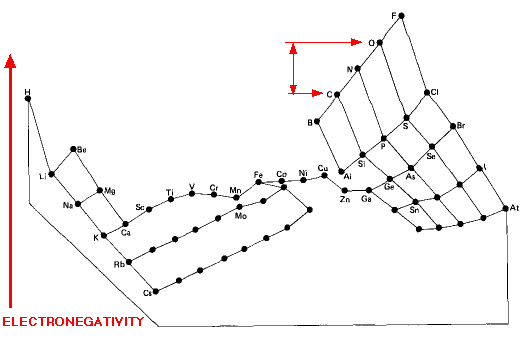
Electronegativity Periodic Table
"This image distorts the conventional periodic table of the elements so that the greater the electronegativity of an atom, the higher its position in the table", here:
| Year: 2003 | PT id = 343, Type = formulation |
Proper Place for Hydrogen in the Periodic Table
The Proper Place for Hydrogen in the Periodic Table, a paper by Marshall W. Cronyn of the Department of Chemistry, Reed College, Portlland.
Cronin writes in the Journal of Chemical Education Vol. 80, 94 -951: "After more than 130 years of construction, the place of hydrogen in the periodic table is still the subject of doubt, con- fusion, and inadequate explanation that appears to be little more than numerology..." and comes to the conclusion that hydrogen should be positioned above carbon:
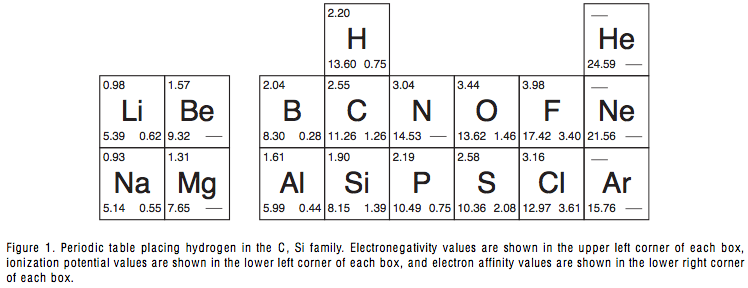
| Year: 2003 | PT id = 378, Type = formulation 3D |
Ukrainian Periodic Table
A Periodic Table from the Ukraine:

![]()
| Year: 2003 | PT id = 496, Type = formulation spiral 3D |
Bernard's Periodic Table of The Elements in Three Dimensional Form
Hinsdale Bernard's Periodic Table of The Elements in Three Dimensional Form, US Patent 7,297,000:

Roy Alexender, of the Desk Topper arrangement, has photoshopped a blurry photograph sent by Bernard along with a product mockup:


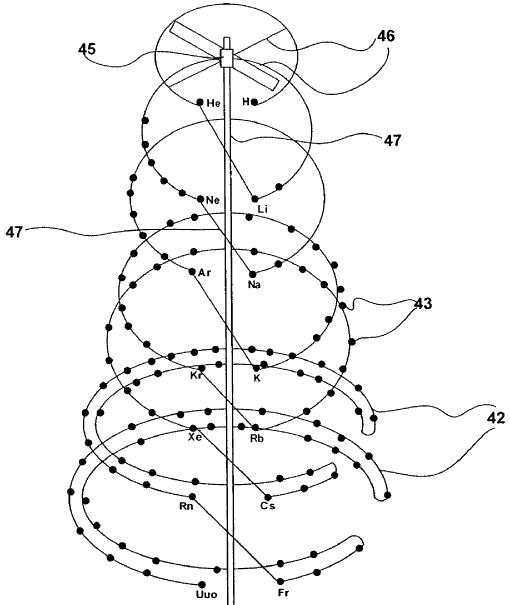
| Year: 2003 | PT id = 573, Type = formulation spiral |
Bird of Prey Periodic Table
From Edmond (Ned) Maurice Peyroux:
"I am a self-taught, underground cartoonist - around the end of 2005 I began studying ether physics, & mid 2006 orgone biophysics. End of 2008 I was going through old note & sketch books while compiling pieces for a poetry book, & came across a sketch I did in 2003 of the first 20 elements of the periodic table in a spiral. I had just begun studying the ether vortex model of the atom & thought a vortex model of the periodicity might be a fun experiment so I played with it more. I didn't remember what inspired the original concept sketch 5 years later, but my guess was I had stayed up too late watching public television again. It probably had to do with some 4-Dimension ring concepts I was playing with, but by 2008 I was thoroughly involved in 3-D biophysics & wasn't thinking back to earlier thought experiments I had done."
"The compositions are largely artistic, naturalistic, & most are like steps on a story board, showing transformation of the table, distorting from from rectangular to spiral, then splitting between metals & noble gases like the wings of a bird, flapping, then joining again to make the spiral (then the spiral inflates to make a flower, wilts into a spider's web) - there are many transitions I have in mind, but I my work is not limited to the periodic table.":
| Year: 2003 | PT id = 600, Type = formulation spiral |
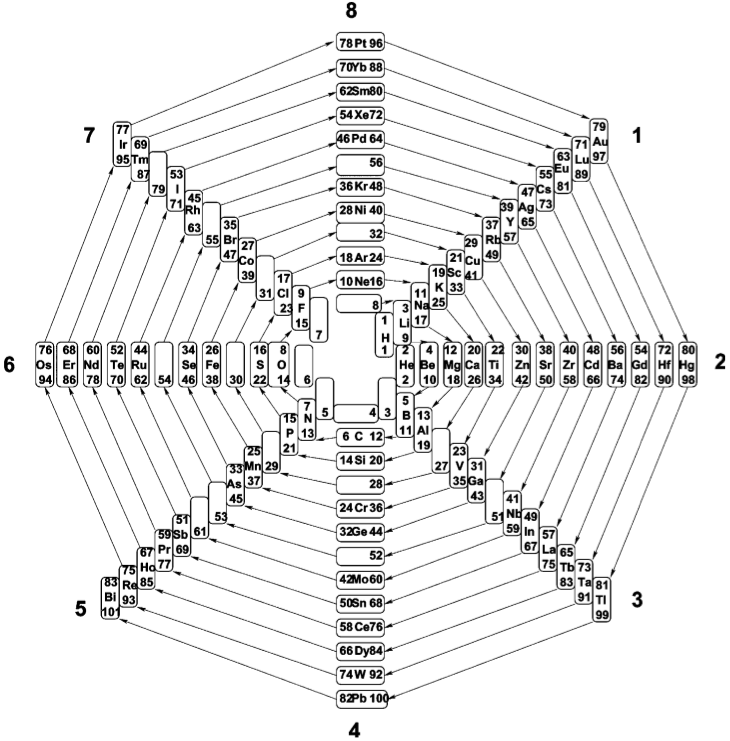
Eight-Group Periodic Table
From Number Patterns in Nature by Jan C.A. Boeyens, Crystal Engineering 6 (2003) 167–185.
The Eight-Group Periodic Table of the 81 stable elements, in spiral form. Available sites on the prime-number cross, starting from zero, number 102.,
| Year: 2003 | PT id = 893, Type = element |
Discovery of Nihonium
Nh ![]()
Nihonium, atomic number 113, has a mass of 284 au.
Synthetic radioactive element.
Nihonium was first observed in 2003 by Y. Oganessian et al. and K. Morita et al.
| Year: 2003 | PT id = 895, Type = element |
Discovery of Moscovium
Mc ![]()
Moscovium, atomic number 115, has a mass of 288 au.
Synthetic radioactive element.
Moscovium was first observed in 2003 by Y. Oganessian et al.
| Year: 2003 | PT id = 1082, Type = formulation 3D |
Two-Amphitheater Pyramid Periodic Table
From Chemical Education Journal (CEJ), Vol. 7, No. 2
A Novel Way of Visualization of the Periodic Table of the Elements by Alaa El-Deen Ali Mohamed, Alexandria University, Egypt.
The author writes:
"New form of the periodic table of the elements is given in this paper. This form can be seen as two amphitheater pyramids facing each other. The cubes that meet are s-elements (interior) then the p-elements then d-elements and the f-elements at last (exterior). The table can be represented by X-, Y- and Z-axes, where the Z-axis gives the number of the period that the element occupies. The table can be modeled by colored cubes helping in introducing the periodic table to the pupils early in the primary education."
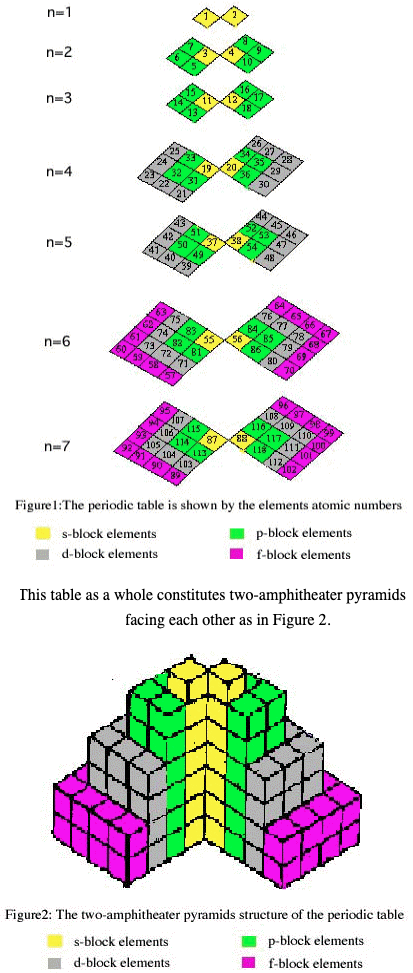
Thanks to René for the tip!
| Year: 2003 | PT id = 1150, Type = formulation data |
Stable Isotopes, Periodic Table of
From Boeyens, JCA 2003, J. Radioanal. Nucl. Chem., 257, 33 a periodic table of the 264 stable isotopes arranged as an 11 x 24 matrix.
Click the image to enlarge:
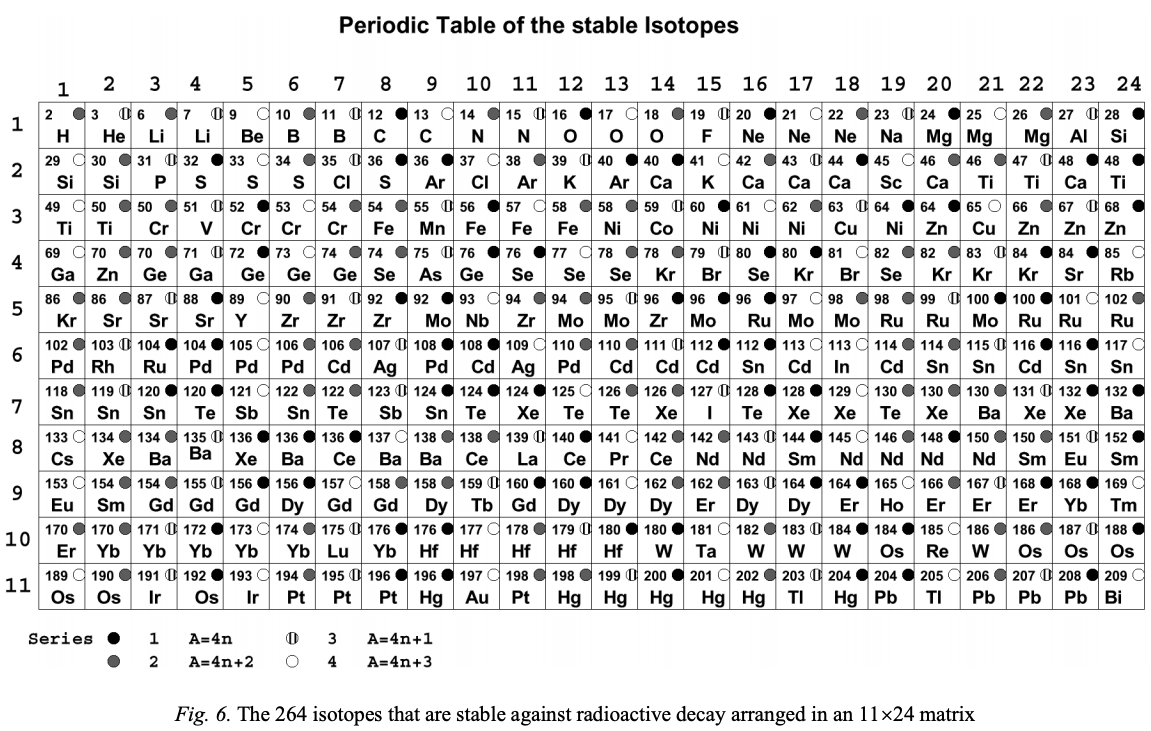
Thanks to René for the tip!
| Year: 2004 | PT id = 48, Type = formulation 3D spiral |
Rafael Poza Periodic Table (Click to Enlarge)
| Year: 2004 | PT id = 607, Type = formulation |
Piano Periodic Table
A Piano Periodic Table from Claude Bayeh:


| Year: 2004 | PT id = 1100, Type = formulation review |
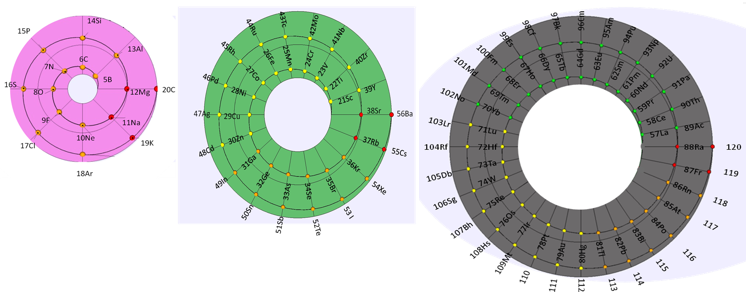
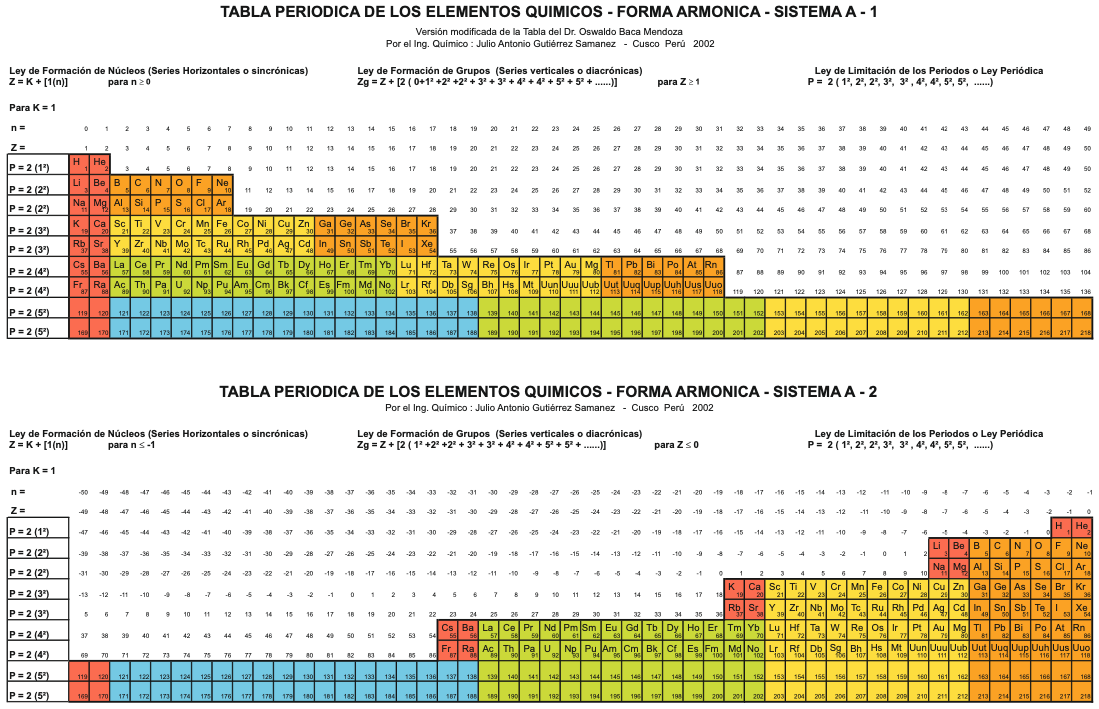
Sistema Periódico Armonico de Gutierrez-Samanez
A digitised 2004 book by book by Julio Gutiérrez-Samanez.
Julio writes:
"These matrix tables are inspired by the method used by the Peruvian chemist Oswaldo Baca Mendoza (1908-1962).
"The tables are read in this way: The Law of Formation of nuclei generates all the horizontal series Z, is dependent on n (series of integers numbers) and a constant K = 1. In the step-to-right tables (n) it will be equal to or greater than 0. In Janet's Left-Step table, (n) will be less than or equal to (-1). The values of this series Z will serve as a constant for the second law.
"The Group Formation Law or vertical series. Its generate the numeric values of the columns either from left to right or from right to left. In system A -1: With the first law: n = 0, then Z = 1. The vertical series Zg = 1, 3 11, 19, 37, 55, 87 ... That is: 1H, 3Li, 11Na, 19K, 37Rb, 55Cs, 87Fr, 119, 169 ... Changing the values of n or Z, all the columns of the table will be obtained.
"In system A -2: With the first law: on the left, n = -1, then Z = 0. The vertical series Zg will be: 2He, 10Ne, 18Ar, 36Kr, 54Xe, 86Rn, 118Og, 168, 218. .. Similarly, changing the n or Z values, we can fills the columns of the table. In system B -1: With the first law: for n = 0, then Z = 1. The vertical series Zg will be; 1H, 3Li, 5B, 13Al, 21Sc, 39Y, 57La, 89Ac, 121, 171 ... By varying the values of n or Z, the entire table is filled. In system B -2: (Its mathematizes the Janet system). With the first law: on the left, for n = -1, then Z = 0.
"The vertical series Zg will be; 2He, 4Be, 12Mg, 20Ca, 38Sr, 56Ba, 88Ra, 120, 170, 220 ... By varying the values of n or Z, the entire table is filled. The third law of the limiting the periods or periodic law, appears graphically, by comparison between rows: For example: in table B -1, in column Z = 3, after 1H and 2He, en of the first horizontal line, the value 3 appears, which is already entered in the first column as 3Li, therefore, that part of the first horizontal row (from 3 to 50) is deleted.
"The same happens with the number 5 in column 3, which is already in the first column as 5B, therefore it will be deleted in the second row from 5 to 52. The same applies to pair 13, 21 of the column Z = 9, same, with the pair 39, 57 of the column Z = 19 and of the pair 89, 121 of the column Z = 33. For that reason the periods: P are duplicated function: 2 (1 ^ 2), 2 (1 ^ 2), 2 (2 ^ 2), 2 (2 ^ 2), 2 (3 ^ 2), 2 (3 ^ 2) .... = 2, 2, 8, 8, 18, 18, 32, 32 ... and the forms are exact and staggered. The colors represent the quantum functions: s (red), p (orange), d (yellow), f (green), g (blue)."
| Year: 2005 | PT id = 28, Type = formulation |
Laing's Revised Periodic Table with the Lanthanides Repositioned
Michael Laing's "Revised Periodic Table with the Lanthanides Repositioned", from Foundations of Chemistry 7:203-233.
- The new system emphasizes oxidation states.
- The elements Eu and Yb fall directly below Ba, indicating the common oxidation state of 2+.
- Elements La, Gd, Lu fall in a column below Y, all having the dominant oxidation state 3+.
- Ce and Tb fall between Zr and Hf in keeping with their 4+ oxidation state. Pm (Z=61) lies immediately below Tc (Z=43), both without stable naturally occurring isotopes.
- The easily attained oxidation states of 2+ for Am and No, and 4+ for Bk are evident from the analogous positioning of the actinides.
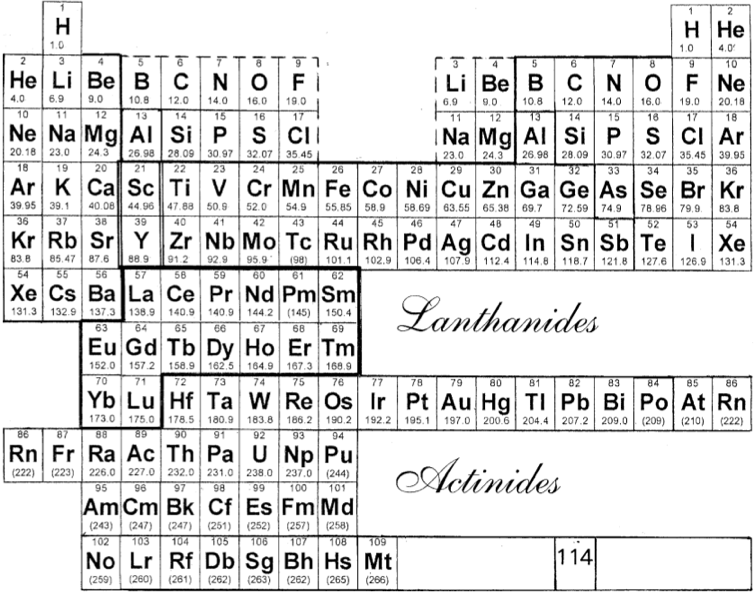
Philip Stewart's modification of the Laing formulation:
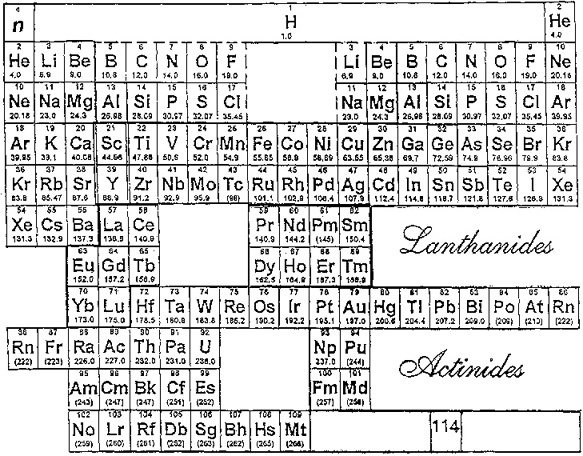
Philip Stewart says (in a personal communication):
"It seems wrong to suggest an analogy between Pr to Sm and Dy to Tm with the V, Cr, Mn, Fe groups. I have pushed them to the right to suggest that those lanthanides are like the old group VIII (including the coinage metals); like them they cannot use all their outer electrons in bonding (with the exception of Ru viii and Os viii. I have treated the actinides differently to take account of Pa v and U vi. It's ability to lose the juxtaposition of Tc and Pm, but it is physical rather than chemical anyway."
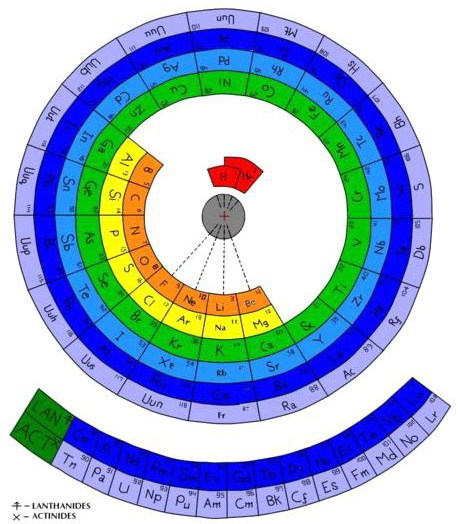
| Year: 2005 | PT id = 37, Type = formulation spiral |
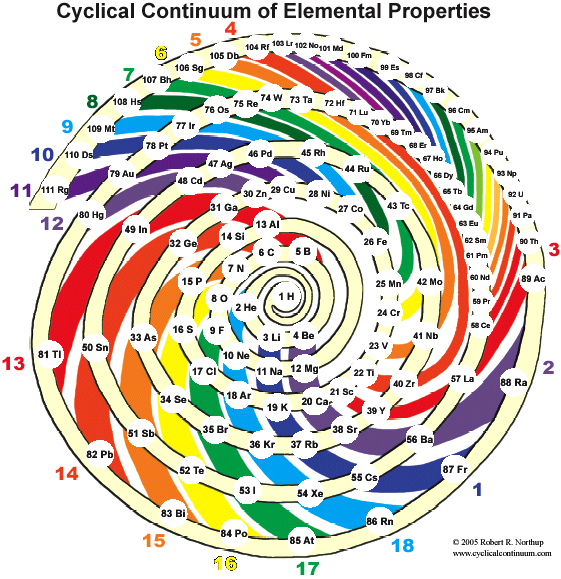
Cyclical Continuum of Elemental Properties by Robert R. Northup
The Cyclical Continuum of Elemental Properties Periodic Table by Robert R. Northup
"The Cyclical Continuum of Elemental Properties is a user-friendly teaching tool that is intended to accompany the Periodic Table of Elements. Hydrogen is shown at the center, atomic numbers and symbols form an unbroken spiral, and element groups 1 through 18 (noble gases, alkali metals, halogens, etc.) are displayed by colored arcs. Beginning chemistry students can visually see the continuity of atomic numbers in the Cyclical Continuum as a way to introduce and orient them to the Periodic Table. Advanced chemistry students can test their understanding of the Periodic Table's organization by applying that knowledge to interpretation of the Cyclical Continuum."
Read more and buy the poster at the Cyclical Continuum web site.
| Year: 2005 | PT id = 41, Type = formulation |
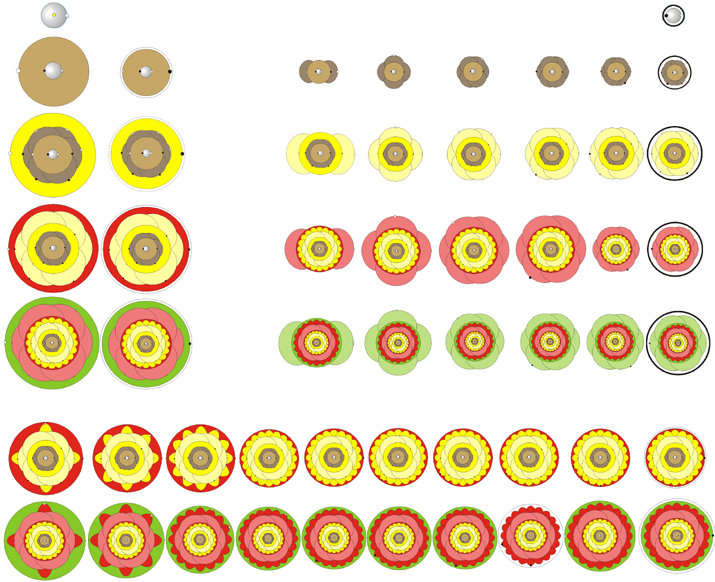
AtomFlowers by Boy Boer
A periodic table that gives a representation of the electron orbitals that look like flowers:

| Year: 2005 | PT id = 242, Type = formulation misc non-chem spiral |
Painting of The Elements
From Gabrielle David's website, here, a painting called Elements, inspired by Melinda Green's Periodic Fractal formulation of 1995:
- The tiniest ball in the center is hydrogen, the next helium, lithium, etc.
- Colors indicate the chemical group.
| Year: 2005 | PT id = 347, Type = formulation spiral 3D |
Pyramid Format Periodic Table
From Wikipedia, this Pyramid Format Periodic Table is Based on a graphic from Scholten J."Secret Lanthanides", 2005, ISBN 90-74817-16-5;
| Year: 2005 | PT id = 634, Type = formulation |
Górski's Atomic Core Based Periodic System
From Go?rski A., Pol. J. Chem., 79, 1435 (2005), an atomic core based periodic system of the chemical elements. In this version of the Periodic Table, He is placed close to H and simultaneously above Be (and not above Ne):
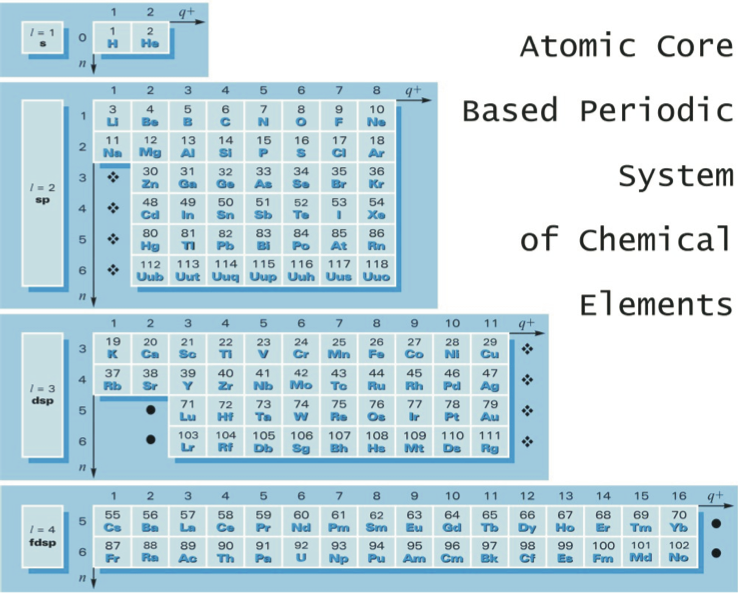
| Year: 2005 | PT id = 1242, Type = formulation |
Rich's Periodic Chart Exposing Diagonal Relationships
Rich, R. L. (2005). Are Some Elements More Equal Than Others? Journal of Chemical Education, 82(12), 1761. doi:10.1021/ed082p1761.
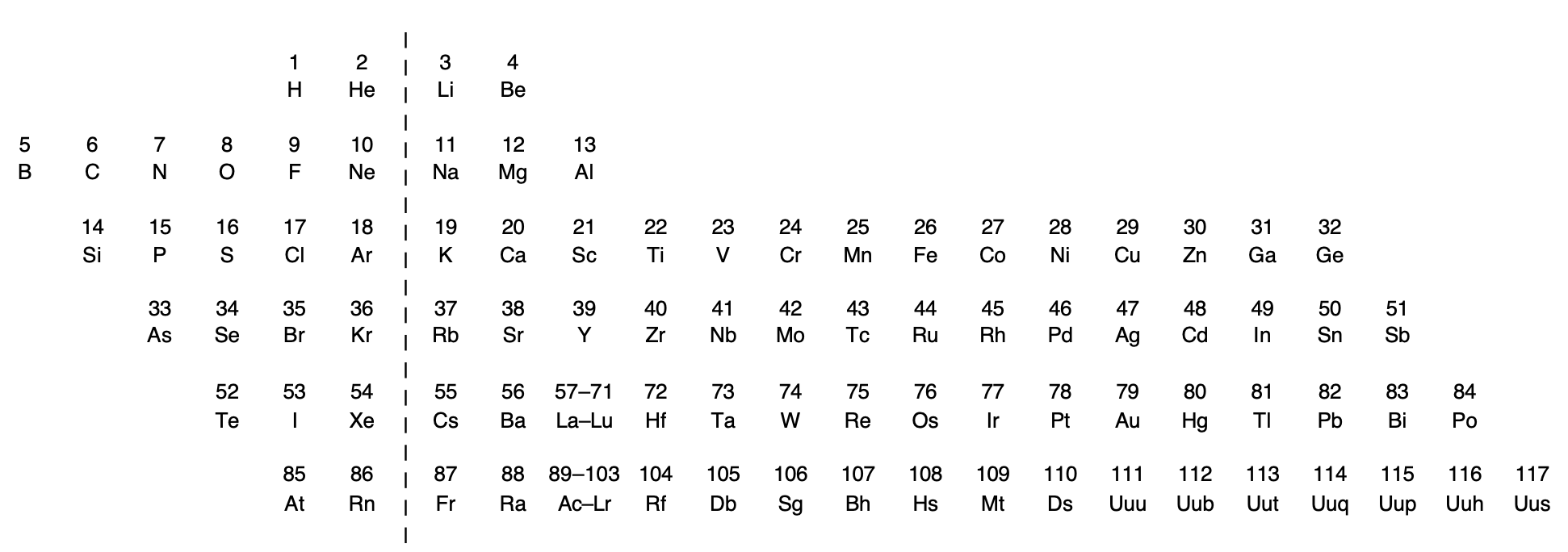
Thanks to René for the tip!
| Year: 2006 | PT id = 17, Type = formulation |
Where Should Hydrogen Go?
There are four possible positions for hydrogen:
- A Group 1 element, above Li, because it forms H+ ions.
- A Group 17 element, above F, because it forms H- ions.
- Above and between boron and carbon because it is of intermediate electronegativity.
- In the top middle, because nowhere else is ideal.
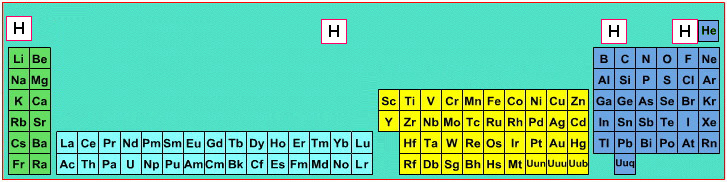
By Mark Leach
| Year: 2006 | PT id = 20, Type = formulation |
Eric Scerri's Triad Periodic Table
Eric Scerri says, "I have recently developed a new periodic table with some very nice features. I am now shifting my allegiance from the left-step table to this one."
- New design based on the fundamental nature of triads, and on atomic number triads in particular.
- H,F,Cl is a new perfect atomic number triad not featured in the usual medium-long form table. There are also many chemical arguments for placing H among the halogens rather than the alkalis.
- Note the regularity regarding period lengths. 8, 8, 18, 18, 32, 32 ...
- All period lengths repeat without fail, unlike in the medium-long form.
- Also note the bi-lateral symmetry assuming the rare earths are given as a footnote.
Read the paper on the philosophy of science web site.
Eric Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006. Read an interview with the author, here, and a review of the book here.
| Year: 2006 | PT id = 32, Type = formulation |
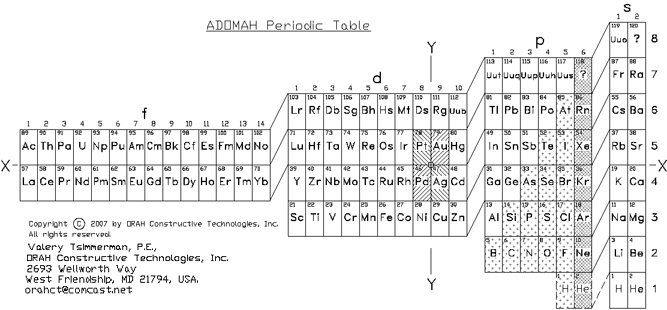
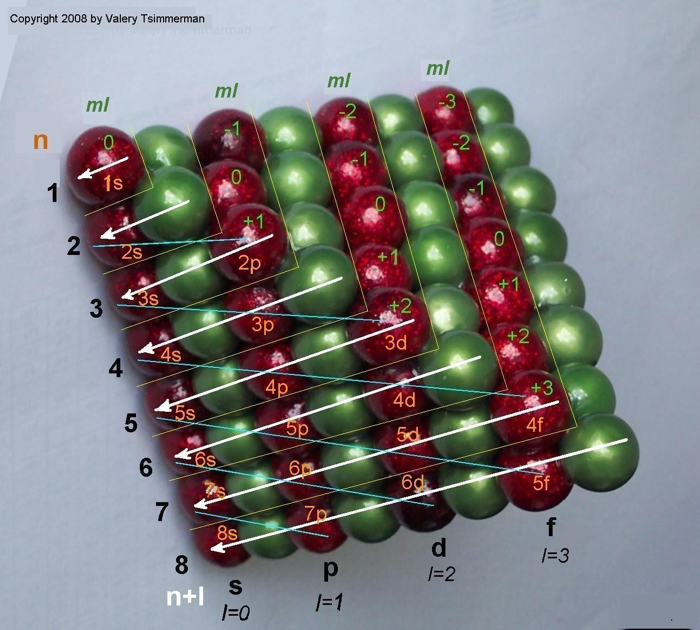
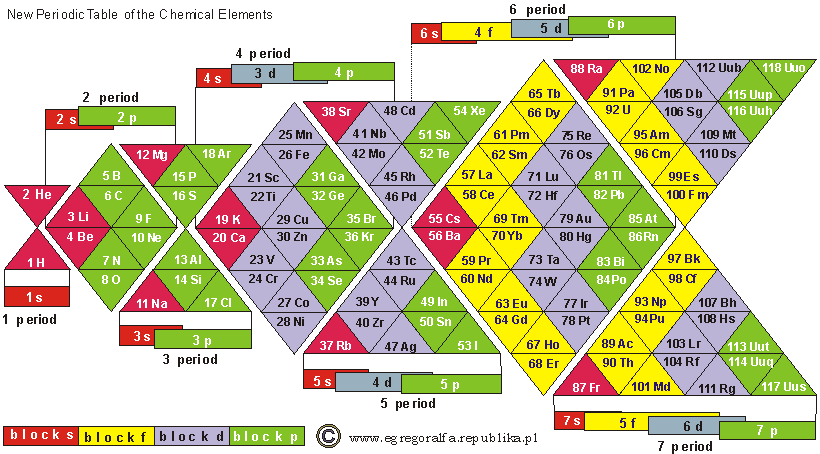
ADOMAH Periodic Table by Valery Tsimmerman
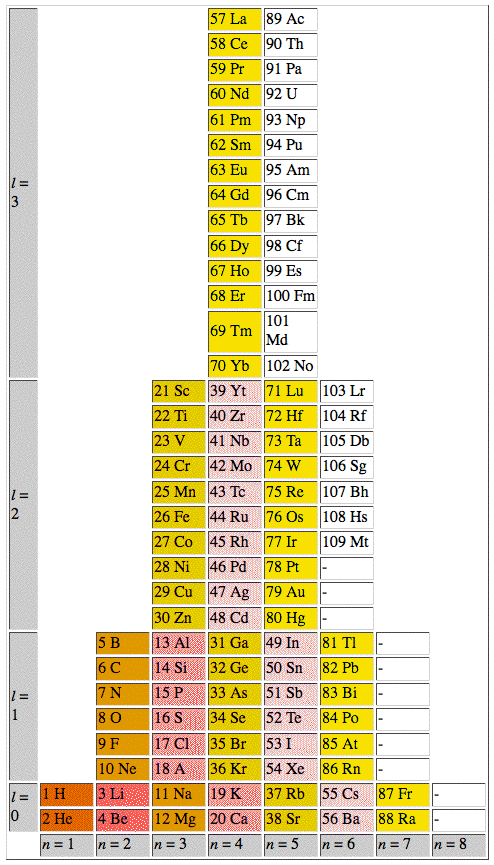
The ADOMAH periodic table is based on the Janet or left-step periodic table. It consists of four blocks (s, p, d & f) corresponding to quantum numbers l = 0,1,2,3. Blocks are separated, shifted and reconnected with each other via diagonal lines. This arrangement creates "layers" or "strata" that retain continuity in respect to atomic number Z, in addition to usual columns and rows. Therefore, numbers shown on the right hand side of the table may represent either quantum numbers n (electronic shells) if horizontal rows are followed, or n + l if "layers" or "strata" are followed.
This feature assists in creation of electronic configurations of the elements. Elements H and He are placed in two positions that reflect their dual nature and give proper consideration to atomic structure and chemical properties of those two elements. This feature also preserves triads He, Ne, Ar and H, F, Cl. Also, the elements are placed in rectangular "boxes", so any two of such "boxes" make up a square thus symbolising electron pairs. This also cuts table length in half. Unlike the Janet table, this table is assembled from bottom up in direction of increase of quantum number n, as well as atomic weight and energy. The ADOMAH table has symmetry and, assuming total number of elements 120, can be divided in four parts of 30 elements with center point located among precious metals.
| Year: 2006 | PT id = 35, Type = formulation spiral |
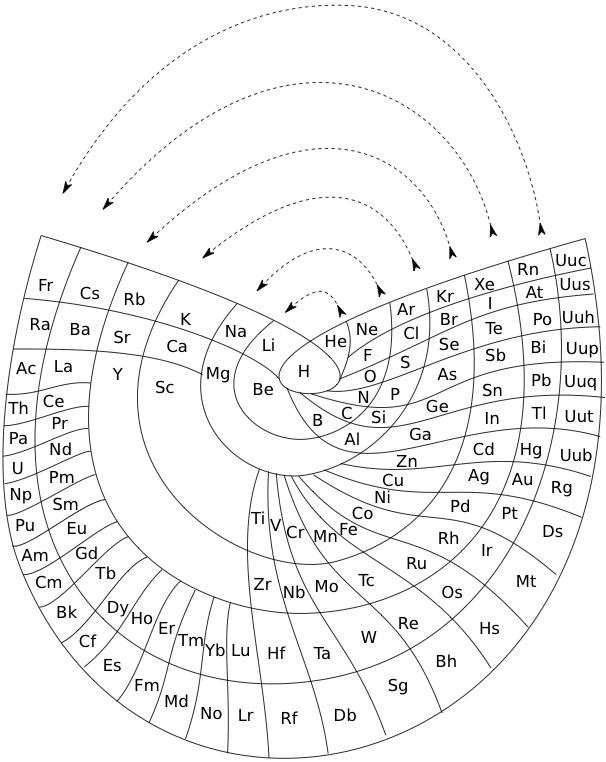
The Wikipedia Alternative Periodic Table
On the Wikipedia there is another circular form of periodic table:
| Year: 2006 | PT id = 42, Type = formulation review |
Henry Bent's Exploration into Janet's Left-Step Formulation
Henry Ben't detailed exploration into the Left-Step formulation of the periodic table is available as a book:
| Year: 2006 | PT id = 56, Type = formulation |
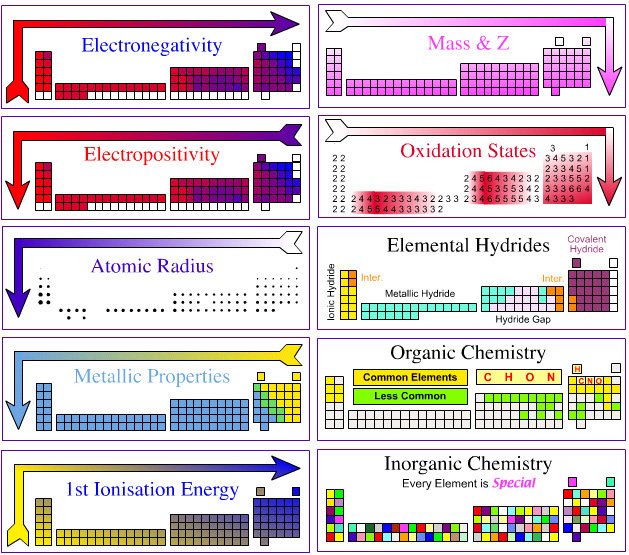
Reaction Chemists' Periodic Table
OK, so which Is The Best formulation of The Periodic Table?
Personally as a reaction chemist, my preferred periodic table is the 'long' form shown below, with hydrogen above and between boron and carbon, although clearly other scientists have other ideas.
All periodic tables show the increase in mass and atomic number, Z, but only the long form unambiguously shows the general top-right-to-bottom-left trends in electronegativity, atomic radius, metallic properties and first ionisation energy.
Electronegativity is absolutely crucial to the understanding of structure, bonding, material type (van Arkel-Ketelaar triangle and Laing tetrahedron) and chemical reactivity, and it underpins much of the chemogenesis analysis.
| Year: 2006 | PT id = 199, Type = formulation |
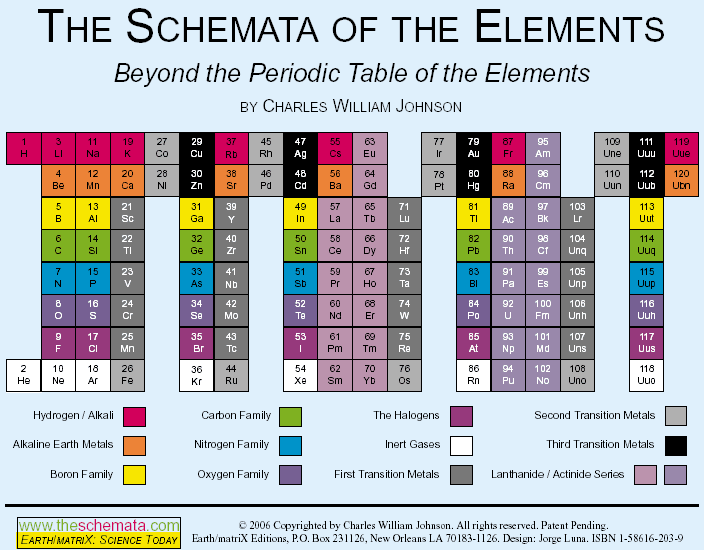
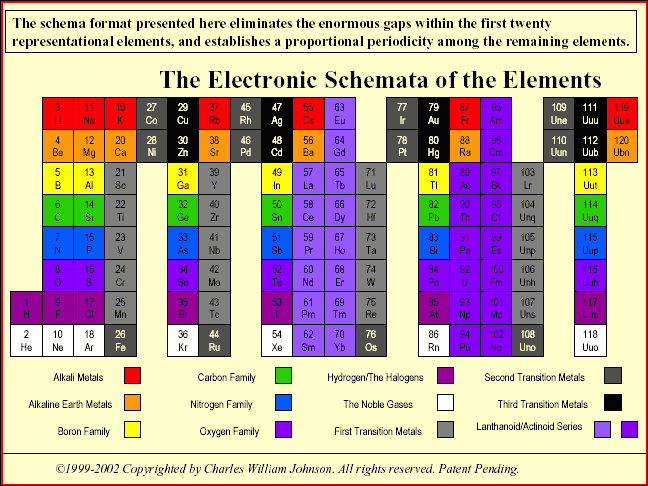
"The conventional periodic table reflects what is called the aufbau design, which represents a progression of numbers; in this case, that of the atomic number of the elements. The table, however, contradicts the aufbau concept in reality, because there are large gaps within among the primary (representative) elements, as well as in relation to the tertiary elements (transition and inner transition elements). The latter case, the Lanthanoids and the Actinoids, lie completely outside of the main body of the periodic table, thereby effectively breaking down the aufbau design... more..." from here by Charles William Johnson:
The Neutronic Schemata: Specialized Schemata of the Elements
| Year: 2006 | PT id = 282, Type = formulation |
Various Periodic Tables
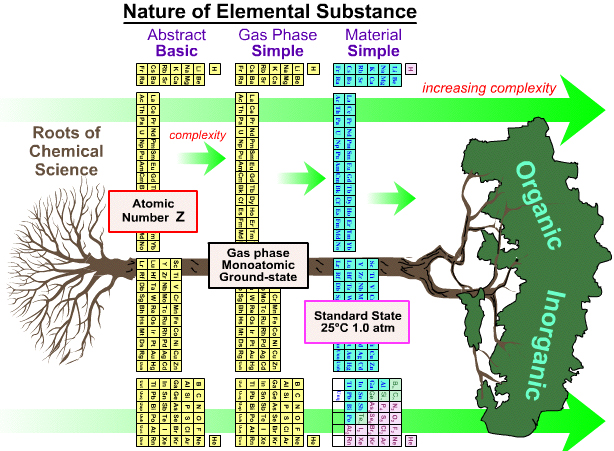
As discussed on this page of the Chemogenesis webbook, the periodic table is ambiguous as to what it is showing.
Does the PT show the element as the abstract 'basic substance', or gas phase atoms or the material substance?
By Mark Leach
| Year: 2006 | PT id = 535, Type = formulation 3D |
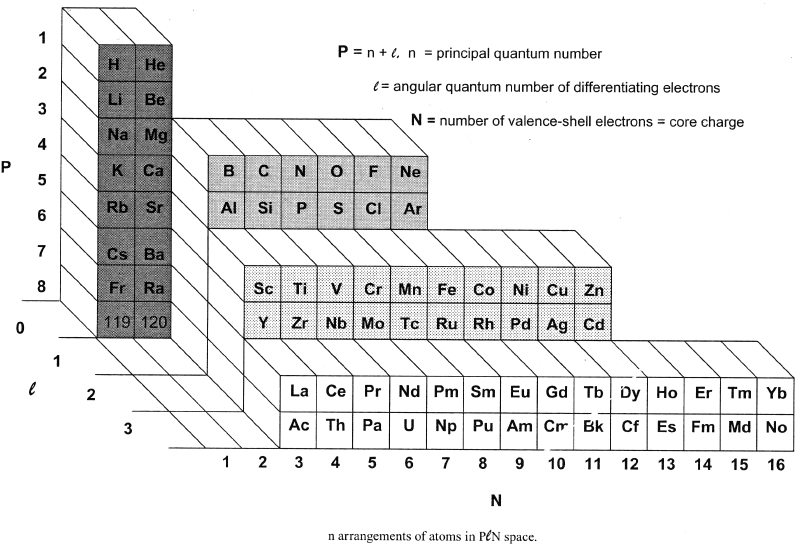
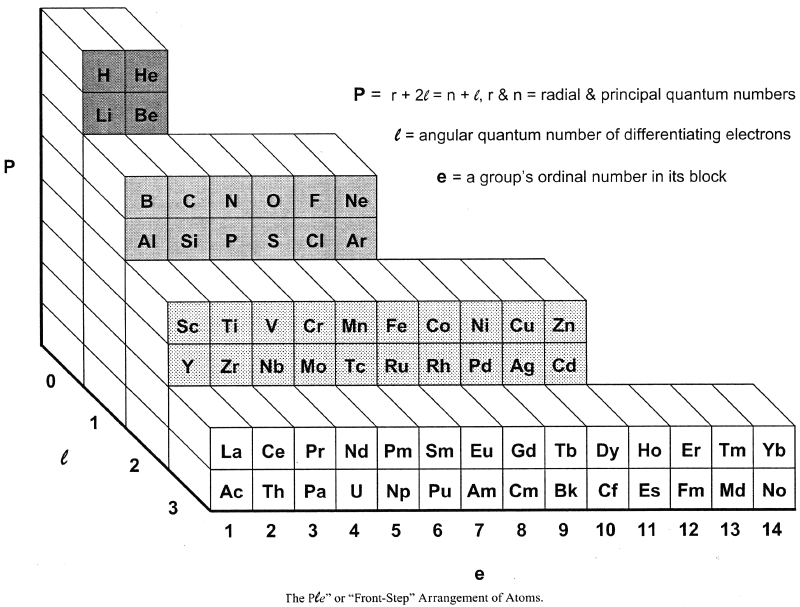
Bent's PlN and Ple (Front Step) Periodic Tables
In his book, New Ideas in Chemistry from Fresh Energy for the Periodic Law, here, Henry Bent introduces the PlN and Ple (Front Step) Periodic Tables, Figs 50 & 52:
| Year: 2006 | PT id = 563, Type = formulation spiral |
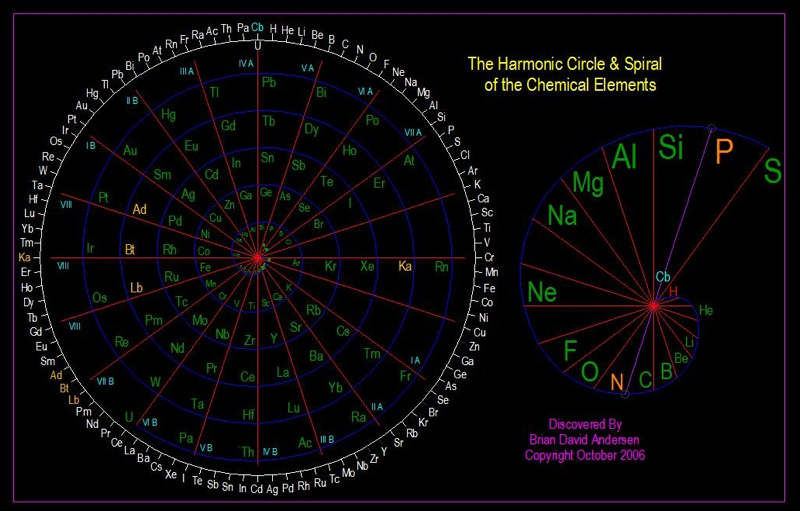
Harmonic Circle & Spiral of the Chemical Elements
Brian David Andersen of Tri-Vortex Technology (Researcher/Inventor/Scientist), Subtle Energy Products trivortex.com:
| Year: 2006 | PT id = 1078, Type = formulation spiral |
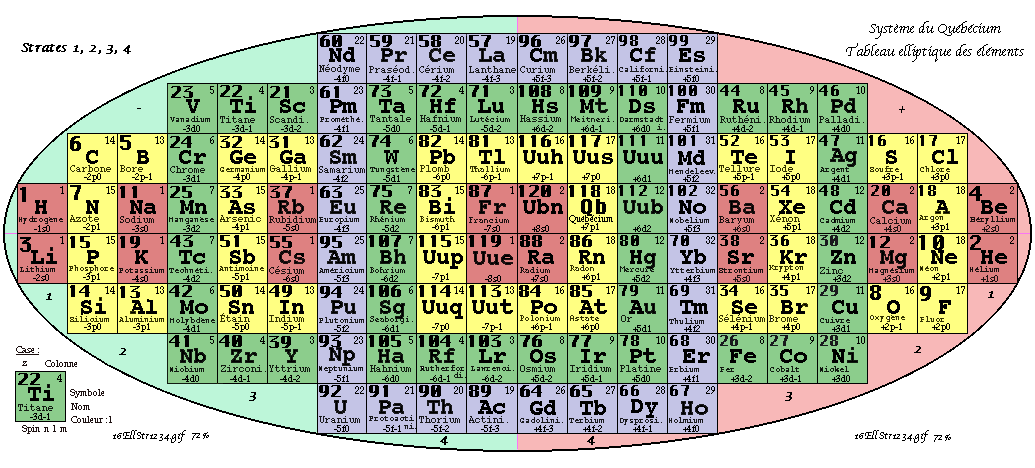
Demers' Système du Québécium
Updated from this 2002 entry comes: Quebec System, Numbers and geometry in the classification of the elements by Pierre Demers.
Demers writes:
"First, I present the Periodic Table of Elements, and I proceed to improve it. By an arithmetic and geometrical analysis, I make appear important symmetries of order four which exist in the arrangement of the atoms between them. I confirm these results with an original discussion of irregular atoms. I will try, in a work that will follow, to interpret the symmetries of the classification by the symmetries of the atom which are associated with the precession cones of the kinetic moments and orbitals."
Read [much] more on this page of links:
Thanks to Conal for the tip!
| Year: 2007 | PT id = 47, Type = formulation |
A new periodic table formulation by James Rota here.
| Year: 2007 | PT id = 103, Type = formulation |
Jelliss' Periodic Table
Jelliss' Periodic Table, more information here:
| Year: 2007 | PT id = 149, Type = formulation spiral |
Wikipedia Circular Periodic Table of The Elements
Wikipedia circular periodic table of the elements here:
| Year: 2007 | PT id = 160, Type = formulation spiral 3D non-chem |
Gyroscopic Periodic Table
From the Garuda Biodynamics web site: "The Gyroscopic Periodic Table has been a natural progression developed from a study of Soil Science, Dr Steiner's Agriculture and Medical Courses, Astronomy and Astrology."
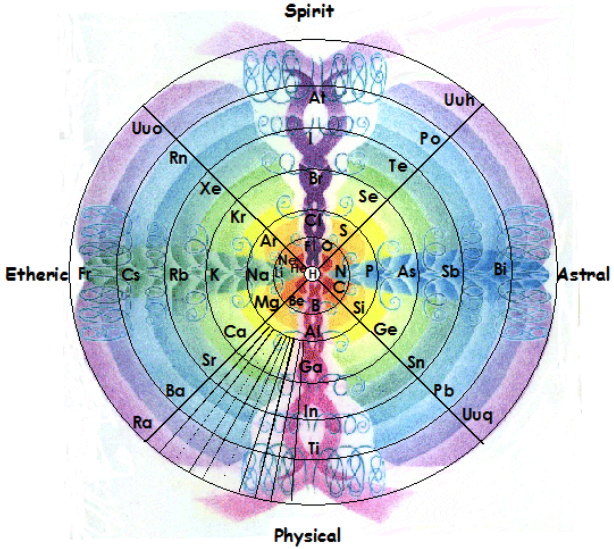
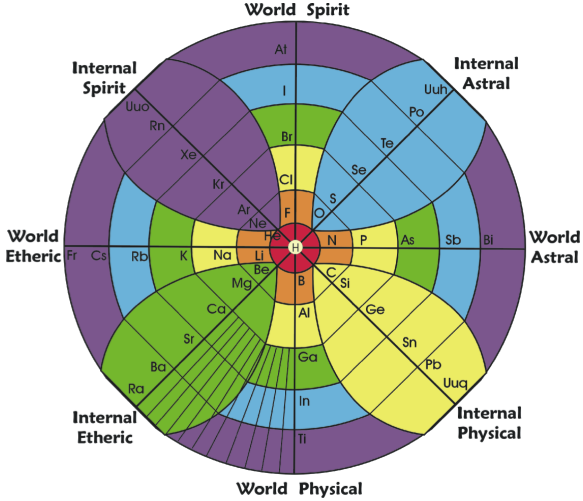
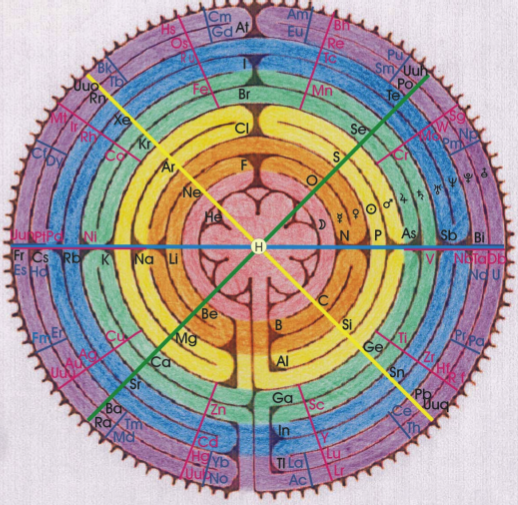
| Year: 2007 | PT id = 202, Type = formulation 3D |
Second Life Periodic Table
From the Useful Chemistry blog: "Further adding to the set of chemistry tools in Second Life, Hiro Sheridan has created a 3D periodic table with rotating atoms. Although not directly proportional, the relative sizes of the spheres are in the correct order. Clicking on them provides basic information about the corresponding element. The 3D periodic table is available on the Chemistry Corner on Drexel Island."
| Year: 2007 | PT id = 307, Type = formulation misc |
University of Jaén (Spain) Wall Mural Periodic Table
From November of 2007 a large Periodic Table placed on the main facade of Sciences Building in the University of Jaén (Spain) welcome everybody.
The table was made in honor of Mendeleev on the 100 aniversary of his death and on the occasion of the Spanish Year of Science according to the concept and design of the Spanish Chemist Antonio Marchal Ingrain, who was inspired in a postage stamp launched that year in Spain.
The artistic mural is composed of 117 tiles of 20 x 30 cm, one for each of the elements known to date, reaching a final dimensions of 2.8 x 3.6 meters. Apart from the traditional information with which students are familiar, such as the atomic number, atomic mass and the chemical symbol of the element, each of the ceramics incorporate information concerning the meaning of its name in Latin or Greek, the year and the name of the person or group of people who discovered it or isolated.
Dr. Antonio Marchal, UNIVERSITY OF JAÉN, SPAIN
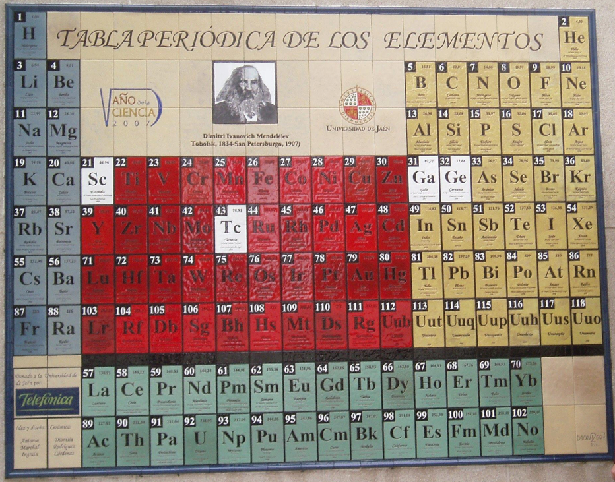
| Year: 2007 | PT id = 778, Type = formulation data |
Mechanical Engineer's Periodic Table
Avallone EA, Baumeister T & Sadegh AM (eds) 2007, Marks' Standard Handbook for Mechanical Engineers, 11th ed., McGraw-Hill, New York, p. 6-6. Click here for a larger version.
This mech eng PT has a couple of odd features: hydrogen is in Group 17 above fluorine and the lanthanides are split:
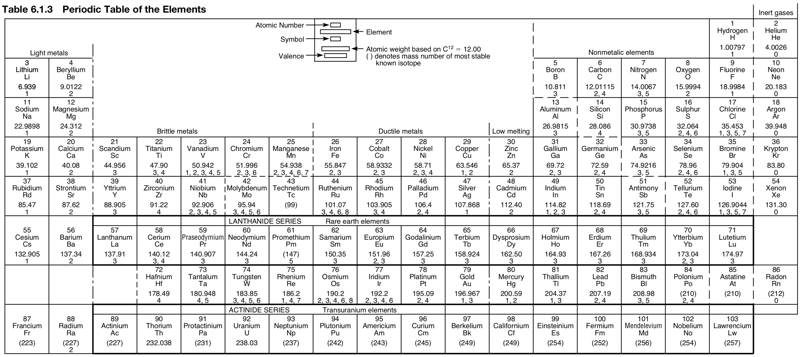
Thanks to René for the tip!
| Year: 2007 | PT id = 1021, Type = formulation 3D spiral |
Bent & Weinhold's 2D/3D Periodic Tables
From a paper by Henry Bent & Frank Weinhold, J. Chem. Educ., 2007, 84, 7, 1145 and here. The authors write in the abstract:
"The periodic table epitomizes chemistry, and evolving representations of chemical periodicity should reflect the ongoing advances in chemical understanding. In this respect, the traditional Mendeleev-style table appears sub-optimal for describing a variety of important higher-order periodicity patterns that have become apparent in the post-Mendeleevian quantal era. In this paper we analyze the rigorous mathematical origins of chemical periodicity in terms of the quantal nodal features of atomic valence orbitals, and we propose a variety of alternative 2D/3D display symbols, tables, and models.":
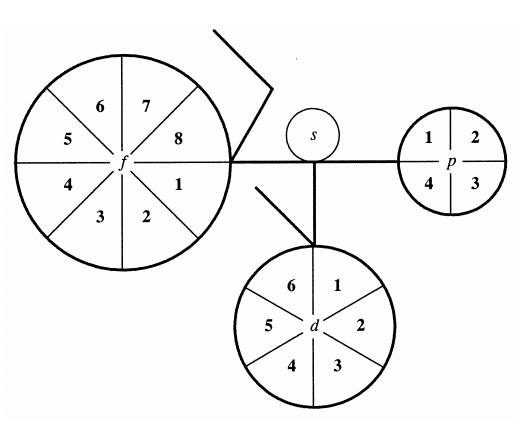
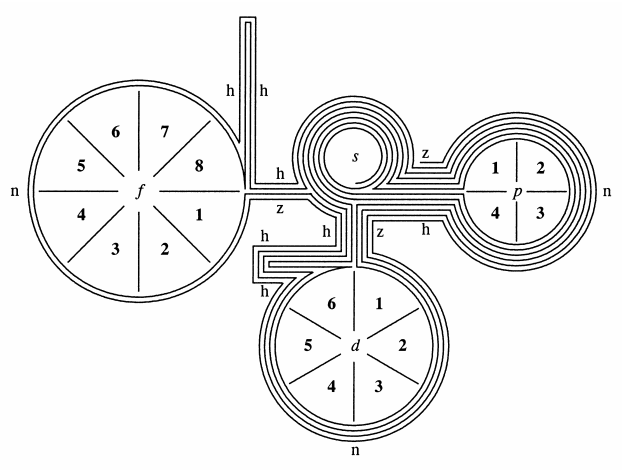
Thanks to René for the tip!
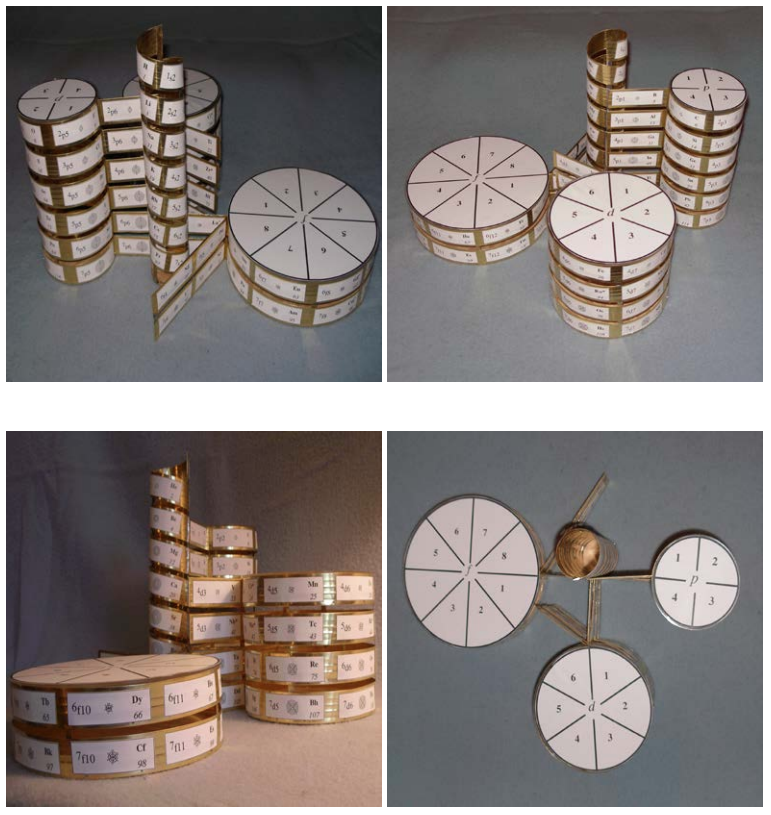
| Year: 2007 | PT id = 1201, Type = formulation |
Orthogonal Dimension Periodic Table
Novel Visualization of the Periodic System of Elements. The Orthogonal Dimension. Click here to read the paper.

ABSTRACT: The Periodic system of elements is presented in a novel way such that rare earth and actinides, and the triads Fe/Co/Ni, Ru/Rh/Pd, Os/Ir/Pt, and Hs/Mt/Uun, are shown orthogonal within the table, and not separately as accompanying rows. The new graphic presentation facilitates the visual orientation, eliminates the now prevailing crunching of the elements in the middle of the table, and avoids the cognitive confusion of scattered positioning of the <<a>> and <<b>> element subgroups sometimes widely separated within the same row. A characteristic meandering pattern emerges whenever switch occurs between the horizontal and orthogonal dimension of the Periodic system; the inner meaning of these switches remains to be elucidated.
| Year: 2007 | PT id = 1282, Type = formulation |
Seeger-Quadbeck Periodic Table
Seeger-Quadbeck H-J 2007, World of the Elements Elements of the World, Wiley-VCH, Wienheim, inside cover.
René Vernon, who provided the graphic, writes:
"An example of a rarely seen 32-column table. The categorisation scheme is interesting.
- Hydrogen is a category of its own.
- The semimetals include selenium and astatine.
- There is no separate category for the halogen nonmetals.
- On page 100 the author refers to the inner occupation of the TM and Ln/An being particularly clear."

| Year: 2007 | PT id = 1364, Type = formulation |
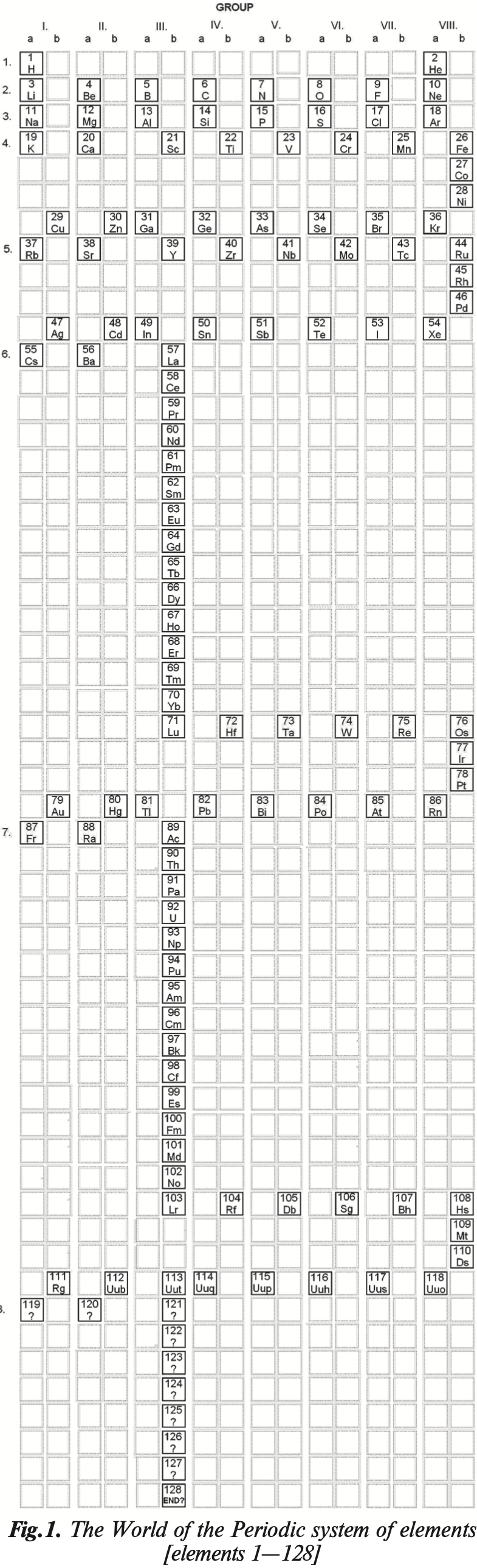
Distribuzione Peridica Degli Elemnti
Distribuzione Peridica Degli Elemnti – Periodic Distribution of Elements – by Leonello Paoloni.
From La Chimica nella Scuola, Sept. 2007, pp 111.
"Fig. 2. The periodic distribution of elements correlates with the group distribution of atomic electrons. Groups are formed so that all elements of the same family are found in corresponding positions (for example, the halogens are always in the second-to-last position in a group of six elements)." (Google Translate)
Thanks to Eric Scerri for the tip!
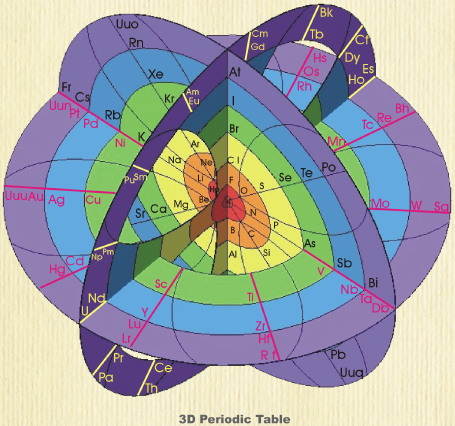
| Year: 2008 | PT id = 87, Type = formulation 3D spiral |
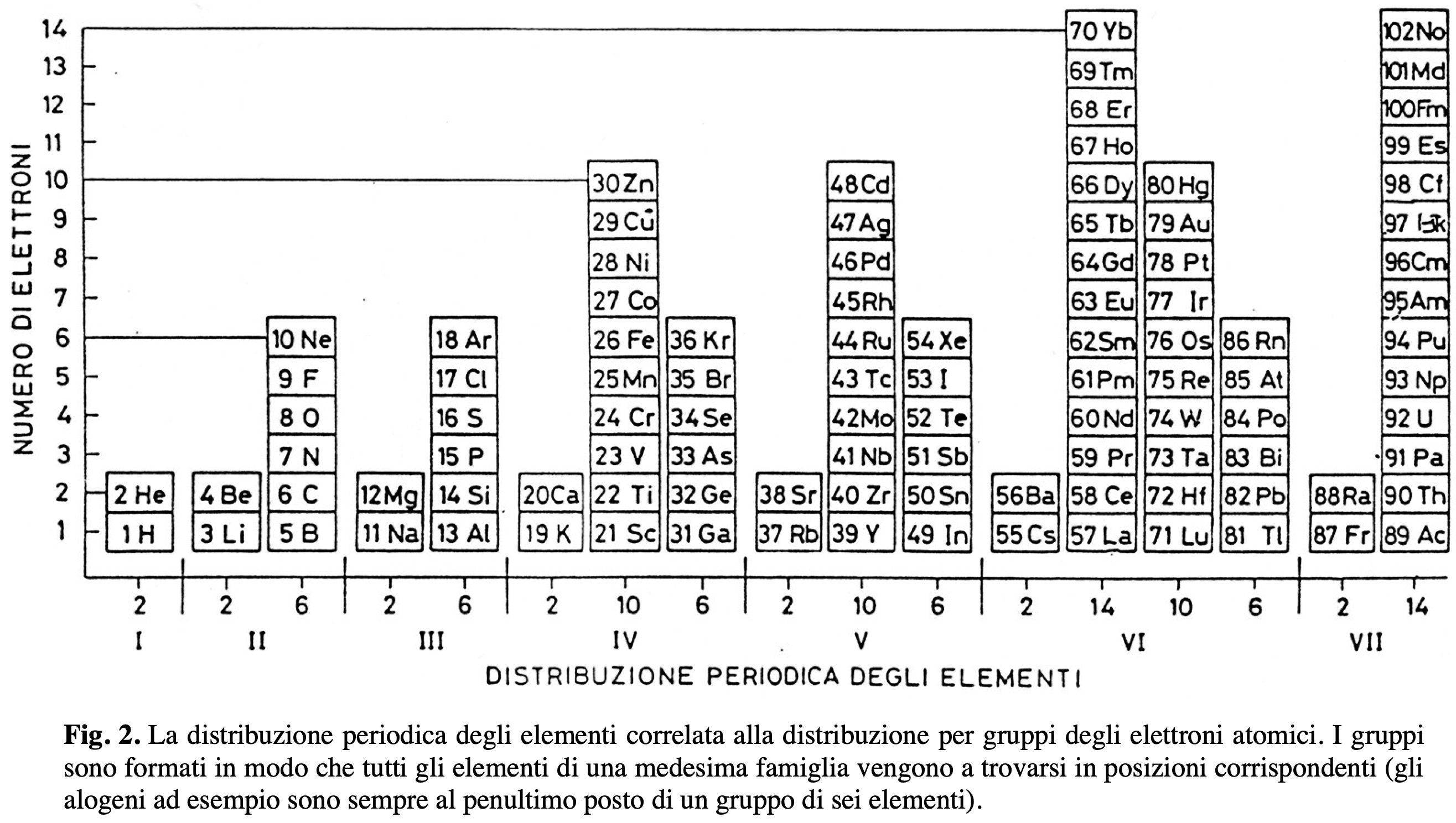
Rafael Poza's Elements and the Magnetosphere
| Year: 2008 | PT id = 88, Type = formulation 3D |
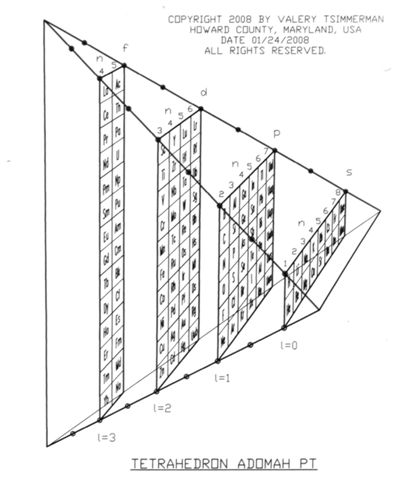
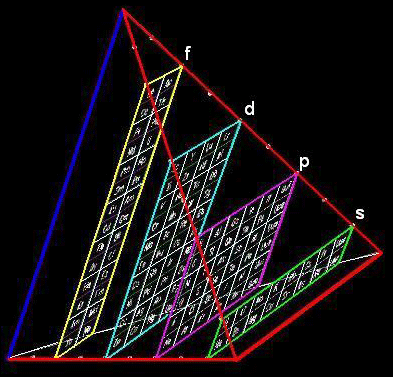
ADOMAH Tetrahedron
Valery Tsimmerman has developed various periodic table formulations, available at perfect perioidic table.com.
| Year: 2008 | PT id = 102, Type = formulation |
Bydgoszcz's Periodic Table
Bydgoszcz's Periodic Table, web site:
| Year: 2008 | PT id = 155, Type = formulation spiral 3D |
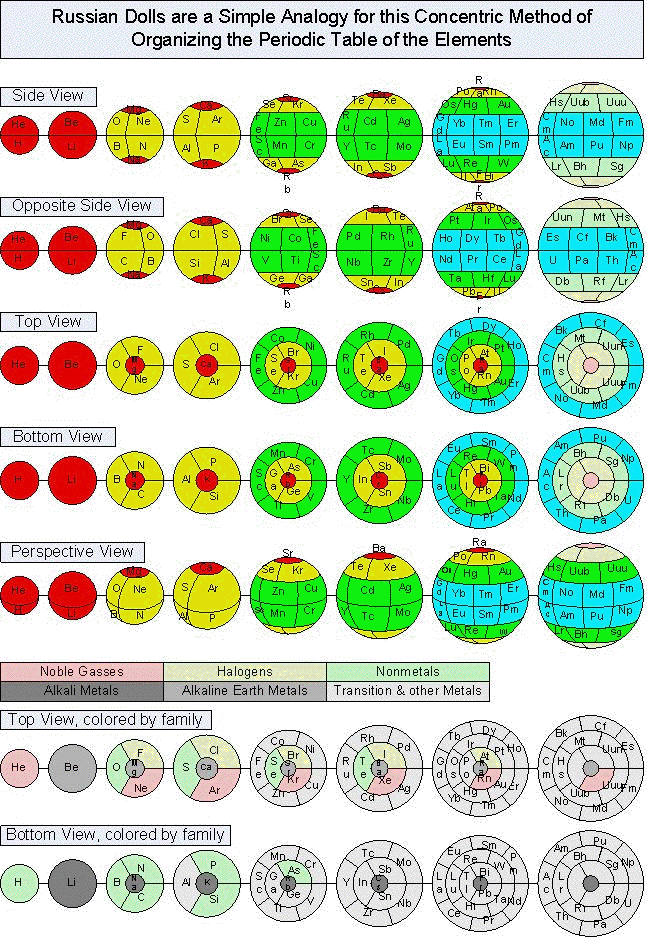
Tomás A. Carroll's Spherical & Russian Doll Formulations
Tomás A. Carroll has devised a spherical formulation of the Periodic Table, and from this a nested Russian Doll formulation.
Tomás writes: "I accept your veiled challenge that it is not possible to formulate a spherical periodic table and propose two solutions for your consideration. The EXCEL spreadsheet shows exactly how I transformed the quantum numbers from the standard 4D Cartesian coordinates to spherical coordinates in 3D, using two different centers. I included cylindrical coordinates too, just for fun."

| Year: 2008 | PT id = 156, Type = formulation 3D |
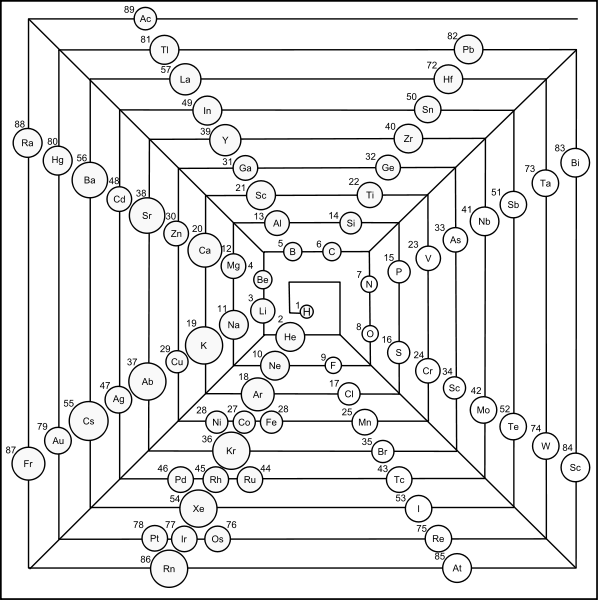
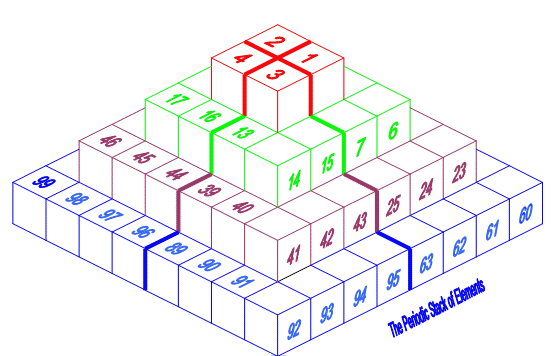
Pyramid (Stack) Periodic Table
The Janet Periodic Table of Elements (1928) may be re-arranged as a series of square matrices.
The matrices are of different sizes and each matrix organizes the atomic orbitals into square concentric rings. Each cell may be assigned an atomic number which also identifies a “most significant electron”. The matrices may be stacked vertically to form a periodic Pyramid Stack of Elements as shown below.
The sub-atomic particles may also be arranged as square matrices. These matrices may be stacked. Read more here.
Please send your comments to: rick_kingstone777@hotmail.com
| Year: 2008 | PT id = 159, Type = formulation spiral |
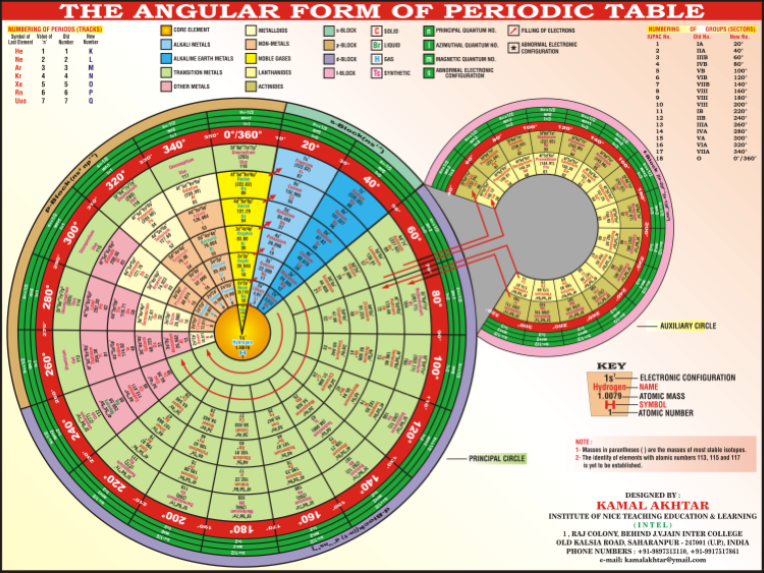
Angular Form of the Periodic Table by Kamal Akhtar
"The complete periodic table is consists of two circles, principal circle and auxiliary circle. The principal circle is consist of seven tracks (periods) and eighteen sectors (groups). The auxiliary circle is consist of only two tracks, inner track and outer track. There is no division of sectors in auxiliary circle." Read more in a word.doc. View the full size PT.
KAMAL AKHTAR
INSTITUTE OF NICE TEACHING EDUCATION AND LEARNING
1, RAJ COLONY, BEHIND J.V. JAIN INTER COLLEGE
OLD KALSIA ROAD, SAHARANPUR-247001 (U.P.), INDIA
| Year: 2008 | PT id = 167, Type = formulation spiral |
Jan Scholten's Periodic table (Spiral Format)
A spiral format periodic table by Jan Scholten:
| Year: 2008 | PT id = 175, Type = formulation spiral misc |
Spiral Periodic Table
A spiral periodic table available as a poster, binder, cup, T-shirt, etc. by Vectoria:
| Year: 2008 | PT id = 212, Type = formulation |
Trinity College Dublin Periodic Table
A periodic table from the Trinity College Dublin physics dept. website:
| Year: 2008 | PT id = 243, Type = formulation |
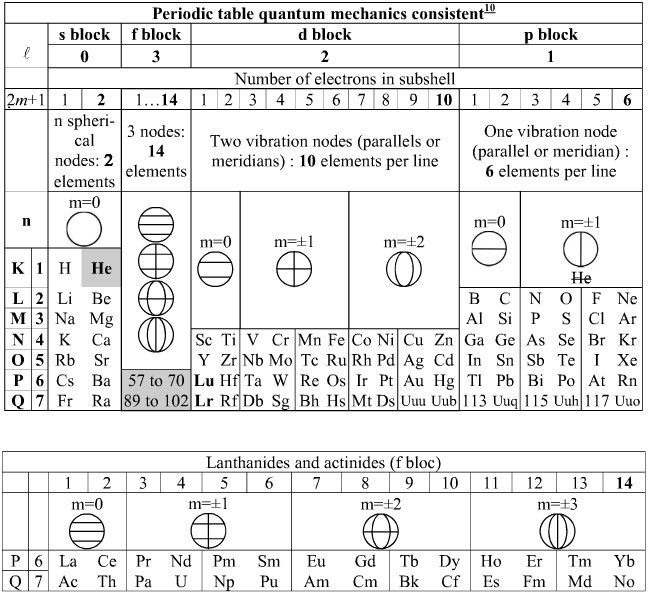
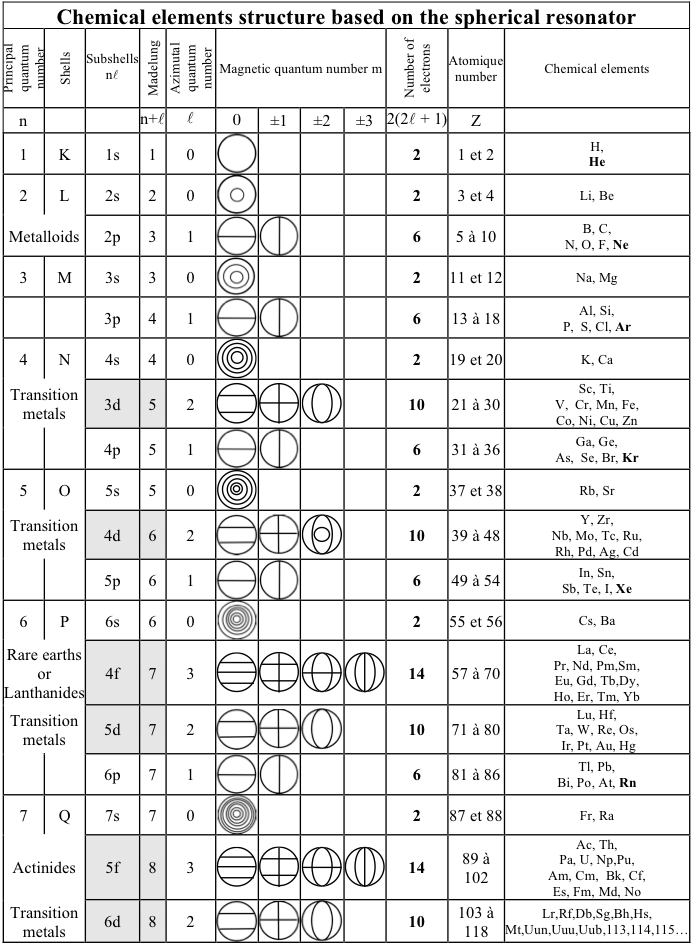
Bernard Schaeffer's Quantum Mechanics Consistent Periodic Table
My graphic representation of the orbitals needed for the periodic table is without brilliant colors, but much simpler. It shows the nodes of vibration of the spherical resonator (a spherical musical instrument) also called spherical harmonics appearing in the spherical solution of the Schrödinger equation. It may be noticed that the atom is also a spherical resonator, not of sound but of the de Broglie waves.
The spherical harmonics (feminine word in french!) have been discovered by Legendre two centuries ago, see my book. Only the plane nodes of vibration are shown. The nodes of the orbitals are a 3D equivalent of the Chladni figures (also discovered two centuries ago) on a vibrating plate: "Aufbau" with spherical harmonics.
The random electronic exceptions in the subshells don't appear. The spherical nodes of the orbitals are represented only for the s subshells. This is a much simpler representation than the usual 3D representations. It can be used to represent the entire periodic table as I have shown earlier. The elements are in regularly increasing atomic numbers.
Bernard Schaeffer's Quantum Mechanics Consistent periodic table from here:
| Year: 2008 | PT id = 313, Type = formulation |
Nuevo Modelo Mathemático Tabla Periódica
Julio Antonio Gutiérrez Samanez presents his Periodic Table formulation ideas in a 2006 PDF paper (in Spanish):
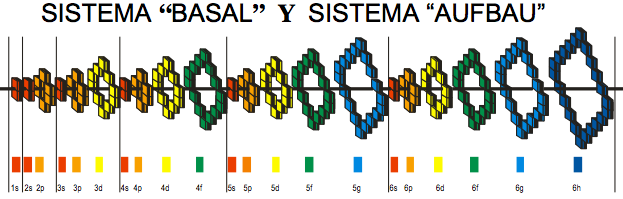
| Year: 2008 | PT id = 361, Type = formulation review |
Mathematical Formulas Describing the Sequences of the Periodic Table
Mathematical formulas describing all of the sequences of the chemical elements are derived from double tetrahedron face-centered cubic lattice model. More here.
J. Garai, Department of Earth Sciences, Florida International University. International Journal of Quantum Chemistry, Vol 108, 667-670 (2008):
| Year: 2008 | PT id = 468, Type = formulation spiral 3D |
Teluric Helix from Gutierrez Samanez
The Teluric Helix from Gutierrez Samanez is inspired by the telluric helix Chancortois (1864) with the difference that the sequence of the elements are rolled into a cone shape rather than a cylinder:
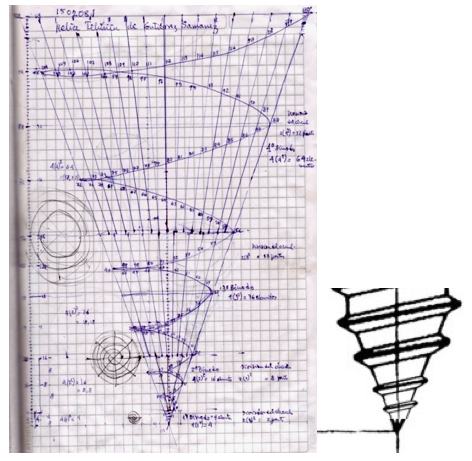
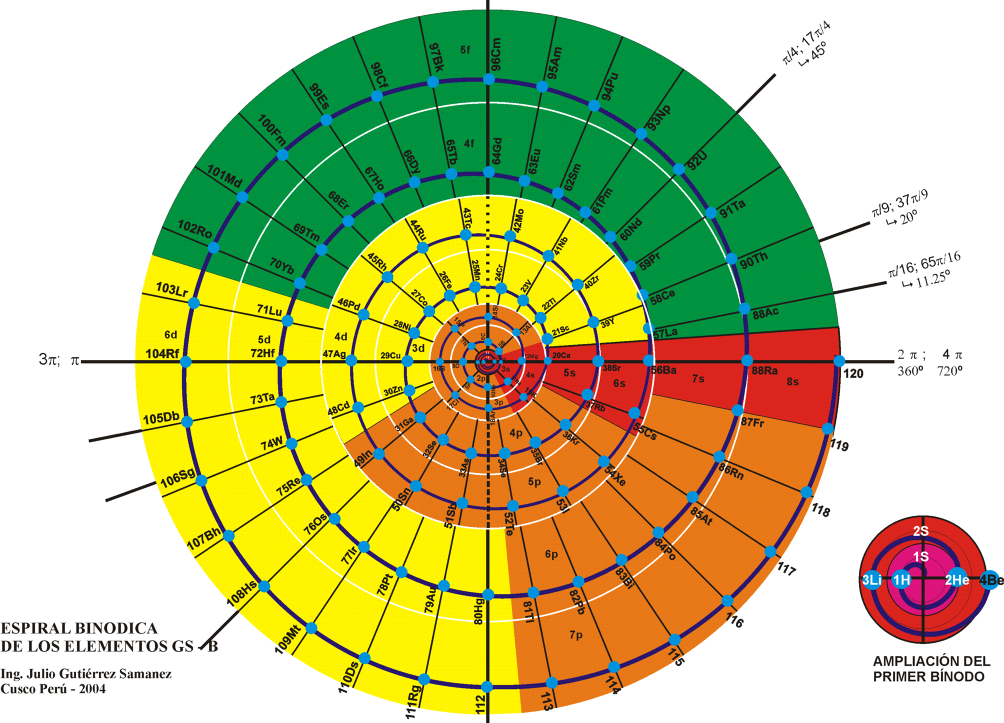
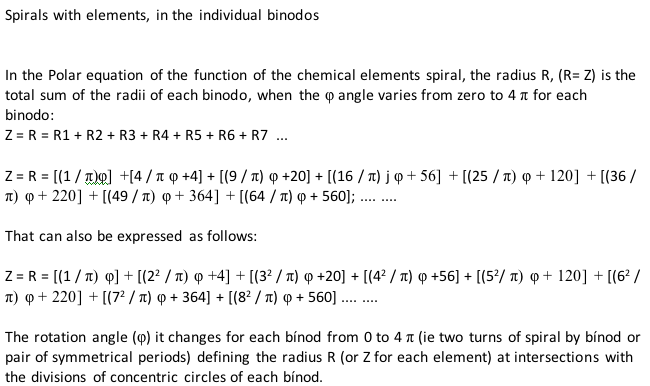
| Year: 2008 | PT id = 1302, Type = formulation |
Franklyn's Periodic Table
Franklyn writes on sciencemaddness.org: Electronic Orbital Periodicity Mendelevian grouping is only one possible organizational scheme, regardless of the schematic choice. A table is useful only to the extent that it provides easy reference to data and comparison. Most everyone who has considered arranging elements in tabular form has pondered what layout best serves the purpose. Below is a table I once made to determine the electronic shell and orbital structure of any element at a glance. Everything to the left and above the elements position indicates the complete full orbitals for those shells. Actually you can see the goup memebers run diagonally from upper left to lower right This arrangement shows that the progression of successive electrons is not straight forward with regard to placement within the atoms. The Mendelevian sequence begining with period 6 through the Lanthanides back to period 6 transition metals until Radon, continuing with period 7 ending with the first member of the Actinides, is as follows:
- shell 6, s orbital - Cesium, Barium (Cs, Ba)
- shell 5, d orbital - Lanthanum (La)
- shell 4, f orbital - Cerium to Lutecium (Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu)
- shell 5, d orbital - Hafnium to Mercury (Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg)
- shell 6, p orbital - Thallium to Radon (Tl, Pb, Bi, Po, At, Rn)
- shell 7, s orbital - Francium, Radium (Fr, Ra)
- shell 6, d orbital - Actinium (Ac)
- shell 5, f orbital - Thorium (Th)

Thanks to René Vernon for the tip!
| Year: 2009 | PT id = 189, Type = formulation misc |
Russian Periodic Table
A modern Russian periodic table using the Mendeleeve formulation:
An older version of the same formulation (date unknown, 1950s?), from here:
| Year: 2009 | PT id = 204, Type = formulation |
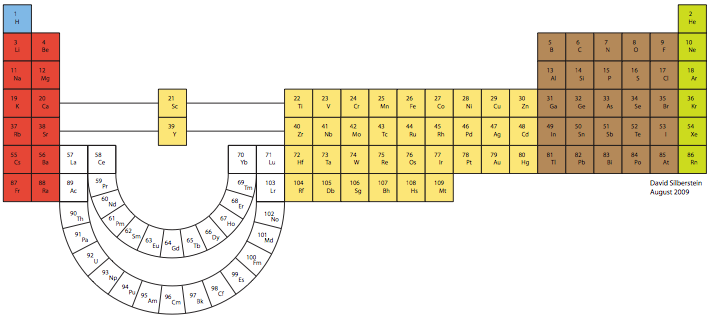
Silberstein Periodic Table
The organization of the periodic table that follows is based on the principle that, as the
position of Lanthanum, Actinium, Lutetium, and Lawrencium is debated with regard to the
elements in Group III, all four of these elements can be placed in an “extended” Group III and
still have the correct arrangement on the periodic table. Although Scandium and Yttrium appear
to be separated from the rest of the transition metal elements, they in fact should be considered to
retain their original positioning as in a short-form table; that is, they are immediately to the right
of Calcium and Strontium and immediately to the left of Titanium and Zirconium. The curving of
the rare earth elements is merely a tool to denote the position of Lanthanum, Actinium, Lutetium,
and Lawrencium in Group 3, with the remainder of the rare earth elements placed outside of an
existing group, or rather creating their own group. View larger pdf file.
David Silberstein, August 2009
| Year: 2009 | PT id = 206, Type = formulation |
Janet Based Periodic Table Layout by Ivan Antonowitz
"Every element has its own unique Periodic Table which is a mixture of two Ideal Forms. However, the main point at the moment is what level of complexity would be suitable? I am trying to get the most minimalistic presentation of the essential features. Explaining the logic governing the 'reversals' is quite tricky, if not controversial, and others may have more conventional rationales and so better fill in the details."
Ivan Antonowitz
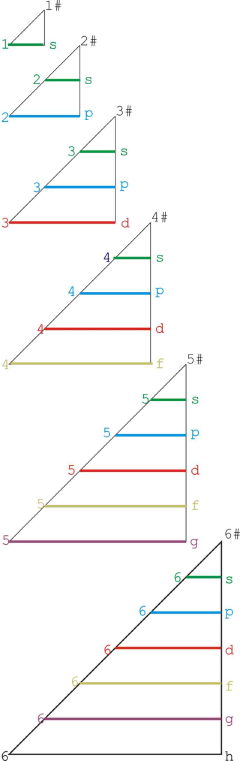 |
 |
| Year: 2009 | PT id = 239, Type = formulation |
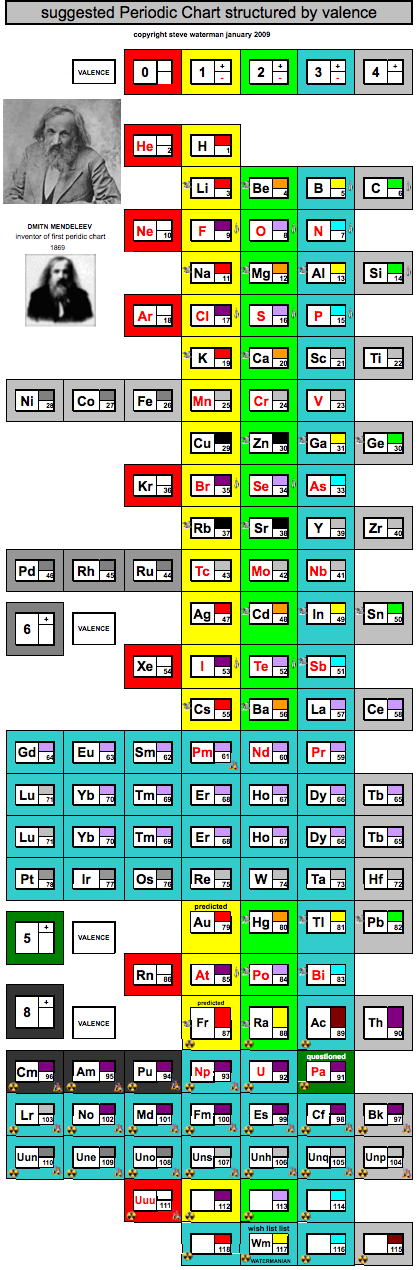
Periodic Chart Structured by Valence
A periodic chart structured by valence, developed by Steve Waterman:
| Year: 2009 | PT id = 249, Type = formulation misc non-chem spiral 3D |
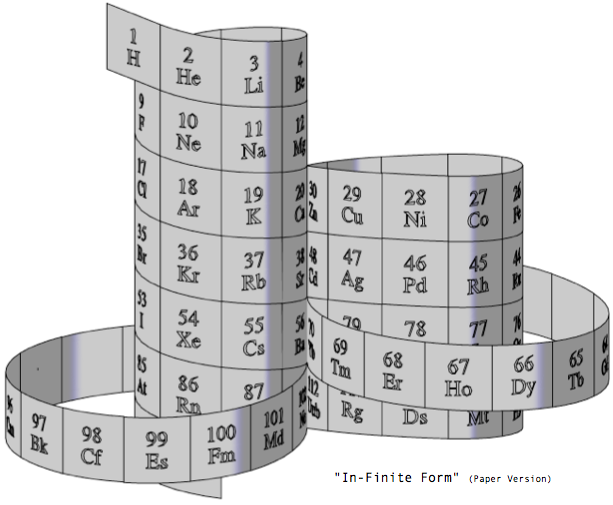
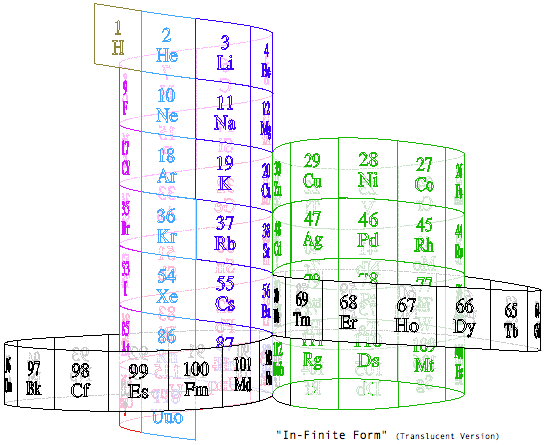
Steve Jensen's "In-Finite Form"
"I'm a figurative sculptor, living in Minneapolis MN. A few years ago, while looking at a two dimensional version of the periodic table, I too wondered if it would be possible to create a Periodic Table without any visual breaks in its numerical sequence. Although I had never seen anything other than the rectangular flat table, I thought I might be able to solve this spatial continuity problem three dimensionally. I also wanted to limit myself to using a 3-D "line" that had no sudden changes in direction. After coming up with what I thought was a new and unique sculptural resolution, I put the project aside. Only recently (after re-building my paper model out of a translucent material) did I do some research on the web, and immediately recognized the strong likeness between my version and the Alexander Arrangement. Even more surprising was my models' visual similarity to Crookes' figure eight design from some 111 years ago.
"Although there are obviously many inventive and well thought out responses to this design challenge, I believe that my solution is a unique one, and an improvement over some of the previous three dimensional forms. The "line" of my model allows for contiguous numerical placement of all the symbols (while maintaining group continuity along its vertical axis), even as the shape of its plan view makes visual reference to the well-known symbol for infinity. What's more, in my version, the Lanthanide & Actinide series do not occupy a separate field but are fully integrated into the continuous linear flow. This piece, which I've entitled "In-Finite Form" speaks to the mystery of the endless flow of space, even as it folds back onto itself within the confines of a finite system."
| Year: 2009 | PT id = 253, Type = formulation misc spiral 3D review |
Graphic Representations of the Periodic System
Mary E. Saecker writes an article in Chemical Education Digital Library, Periodic Table Presentations and Inspirations: Graphic Representations of the Periodic System, that reviews some periodic table formunations.
The paper contains a link to this pdf file which gives templates and instructions for several print, cut-out & build periodic table formulations:
Supplement to: Periodic Table Presentations and Inspirations by Mary E. Saecker, J. Chem. Educ., 2009, 86, 1151.
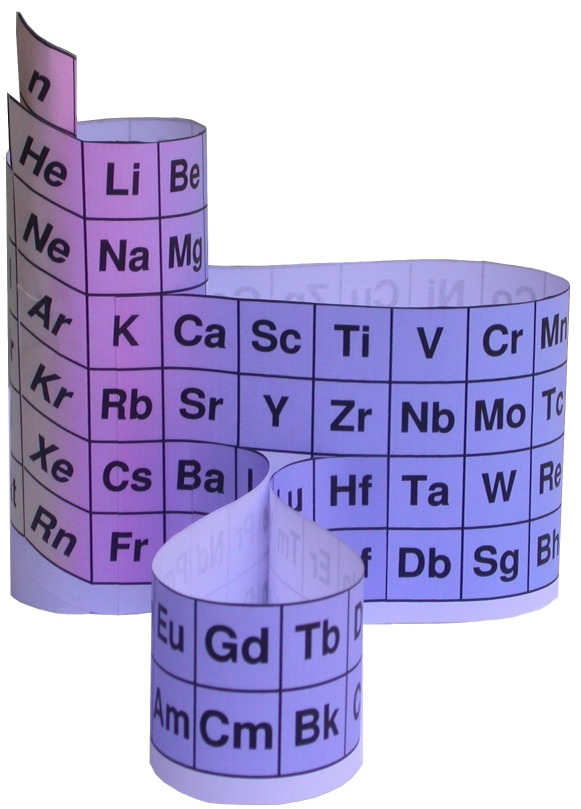
Construction Directions A Cut-Out Chart of the Periodic System (Periodic Table Cylinder)
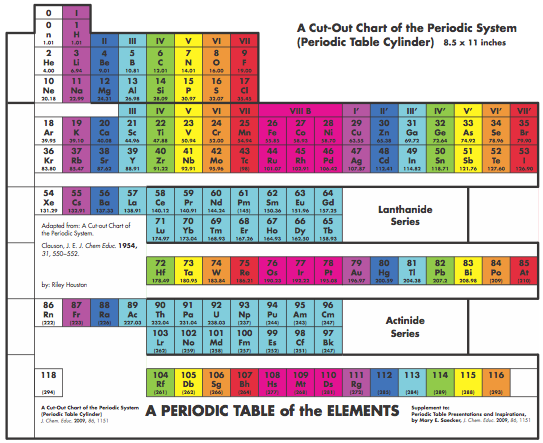
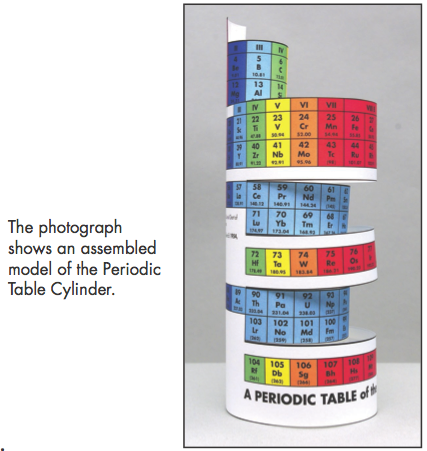
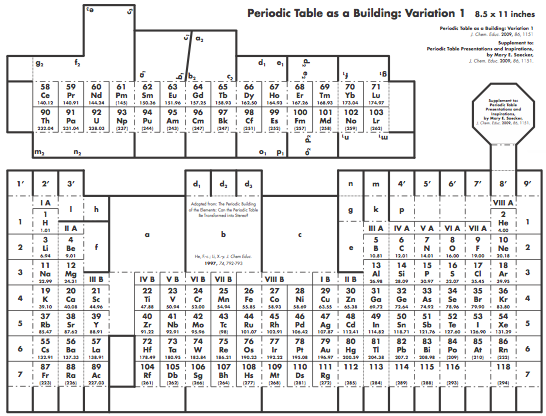
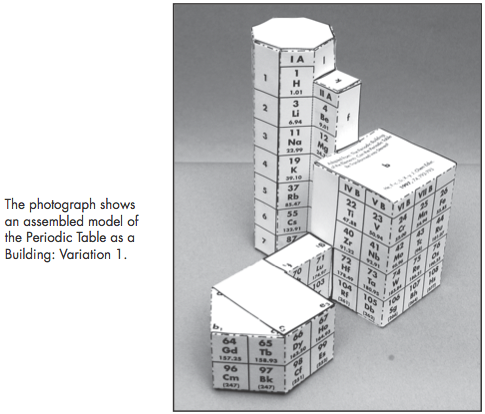
| Year: 2009 | PT id = 259, Type = formulation 3D |
Nasco's Periodic Table Toss-Up Ball
Toss some fun around the classroom with this 15" inflatable ball challenging students to name 118 elements from the Periodic Table. Two or more players toss the ball to each other, giving the element name for the number and symbol on which their left thumb lands. Answer sheet and instructions included. Grade 6 to adult.
| Year: 2009 | PT id = 314, Type = formulation non-chem |
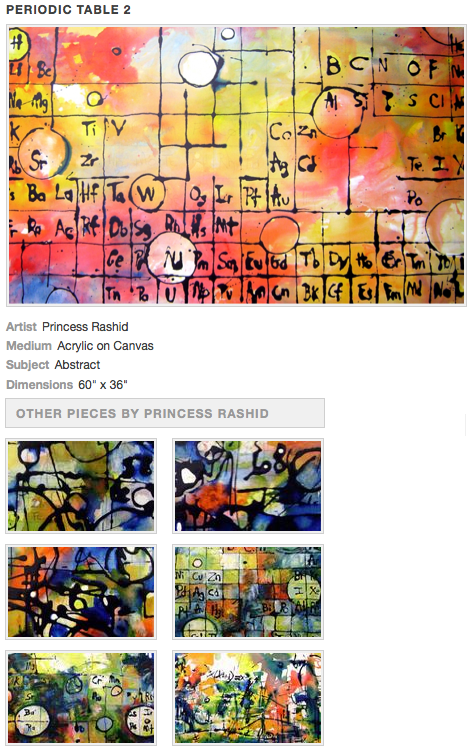
Acrylic on Canvas Painting by Princess Rashid
A series of acrylic on canvas abstract periodic table paintings by Princess Rashid:
| Year: 2009 | PT id = 346, Type = formulation 3D |
Russian MedFlower Periodic Table
Google Russian to English translation:
From Secology.Narod.RU: "Must also give up the basic heuristic principle of Mendeleev and follow him. Forget about the group, we will not argue with what period begins, but just consistently and continuously to build all the elements in a row in ascending order, and fold this series into a spatial helix, in the corporeal form, allowing the convergence of such chemical elements in the vertical..."
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.









.SVG-1.png)