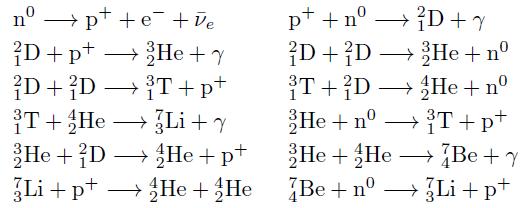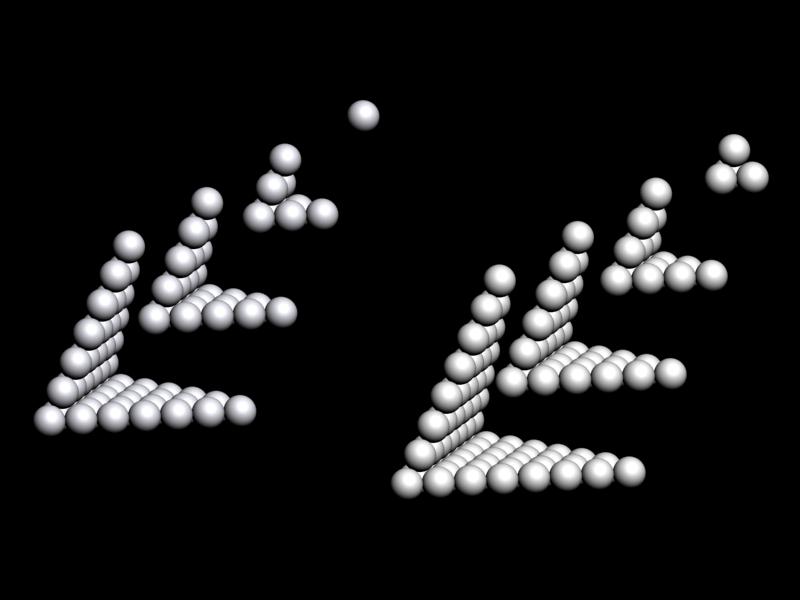Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Table formulations referencing Janet, by date:
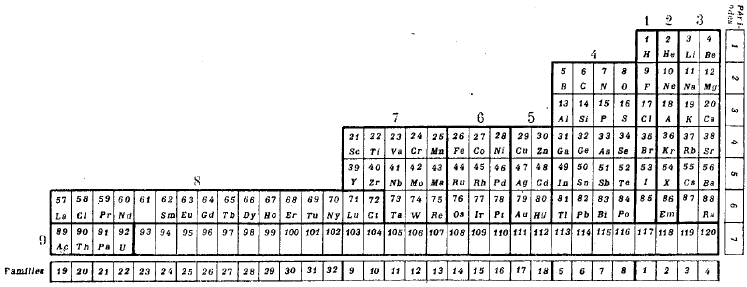
| Year: 1928 | PT id = 289, Type = formulation spiral 3D |
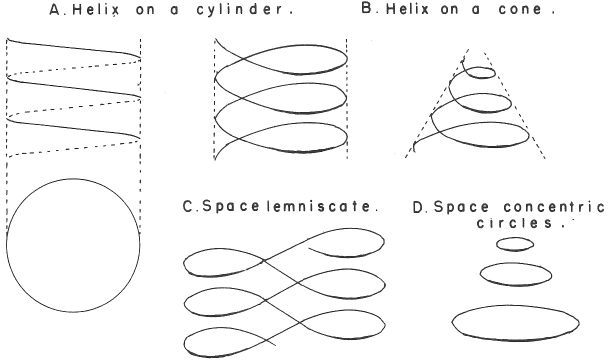
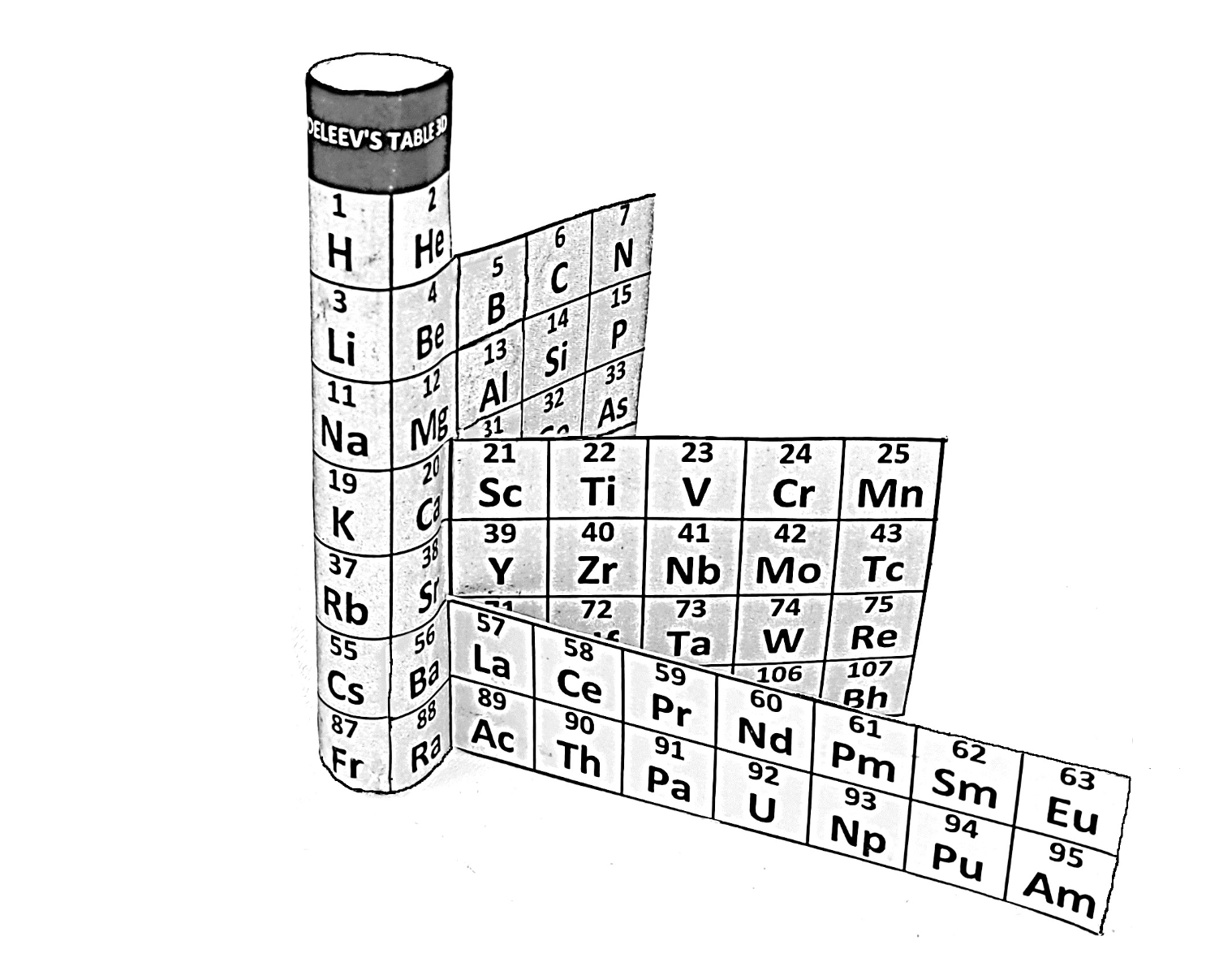
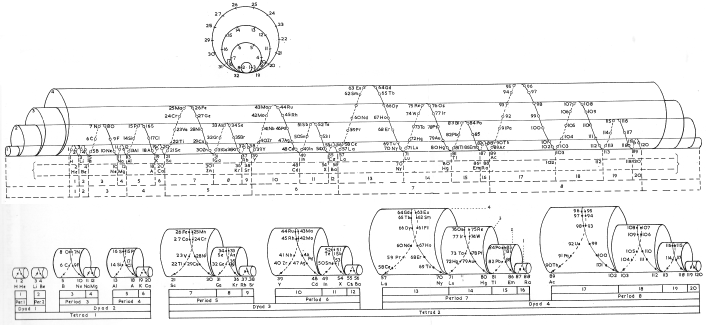
Janet's Three-Dimensional Spiral-Tube System
Janet's Three-Dimensional Spiral-Tube System of 1928 (from van Spronsen):
Click here for large diagram.
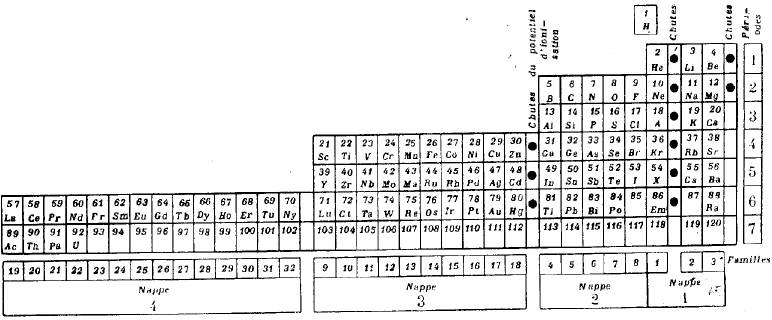
| Year: 1928 | PT id = 305, Type = formulation spiral |
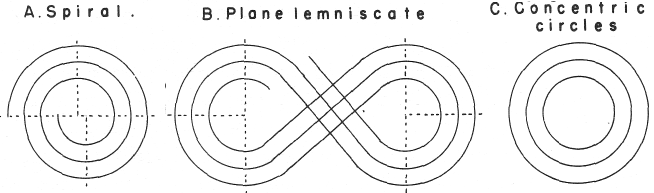
Janet's "Lemniscate" Formulation
From in The Helicoidal Classification of the Elements, Chemical News vol. 138, 21 June 1929, Fig. XI, p. 392:
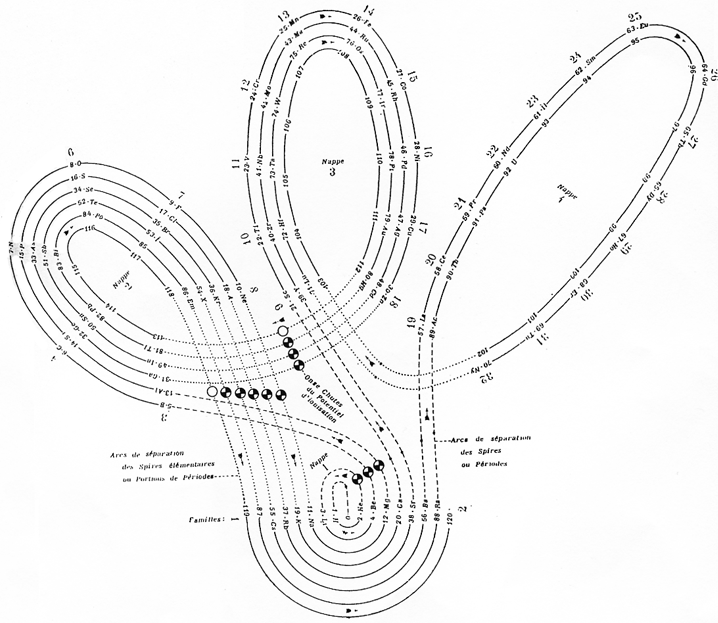
Philip Stewart points out that this formulation is an 'end on' view of the Janet Cylinder or Three-Dimensional Spiral-Tube System formulation, and the term "lemniscate" comes from Mazurs.
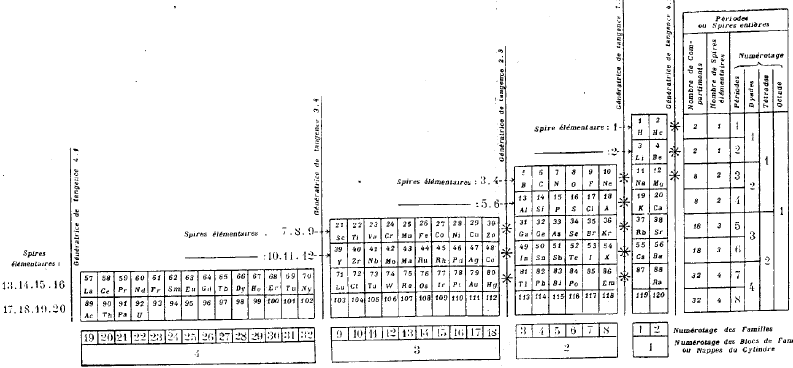
| Year: 1928 | PT id = 74, Type = formulation spiral |
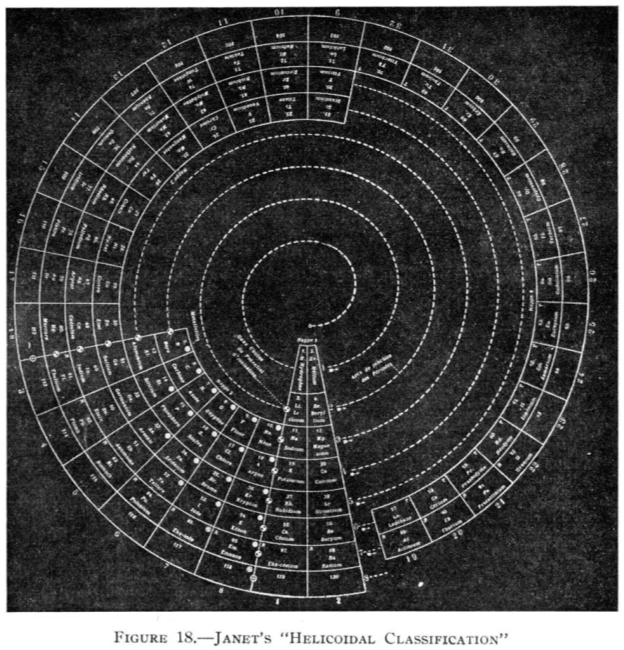
Janet's Helicoidal Classification
Janet's Helicoidal Classification, essentially his left-step formulation in its spiral version (ref. Charles Janet, La Classification Hélicoïdale des Éléments Chimiques. Beauvais: Imprimerie Départementale de l'Oise. 1928). Information supplied by Philip Stewart:
From Quam & Quam's 1934 review paper.pdf
| Year: 1928 | PT id = 152, Type = formulation |
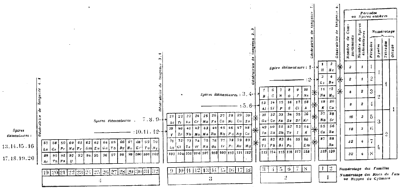
Janet's Left Step Periodic Table
There are the three versions of Janet's left step PT. He tried out versions I and II in his April 1928 paper, and rejected them in favour of version III in his paper of November of the same year. Each one was derived from a helix drawn on nested cylinders. Information supplied by Philip Stewart. Click each image for a larger image:
| Year: 1928 | PT id = 989, Type = formulation |
Corbino's Right-Step Periodic Table
Published in the same year as Janet's Left-Step formulation, Corbino OM (1928) Riv Nuovo Cimento 5:LXI (and from here) produced a Right-Step version.
Commenting on this formulation, Valery Tsimmerman writes:
"Corbino saw what Janet failed to see: If blocks shifted by corresponding value of quantum number l, then the rows represent electronic shells and Janet saw what Corbino fained to see, namely the Janet rule, also known as Madelung rule. Both used rectangular boxes, but neither noticed the perimeter rule."
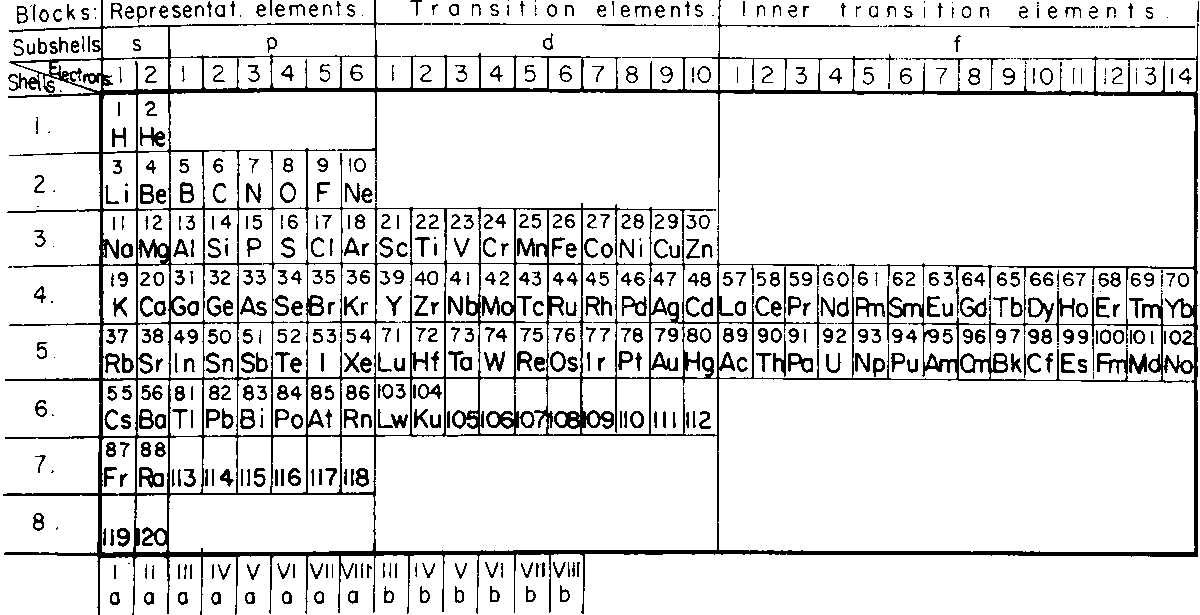

Thanks to Valery T for the tip!
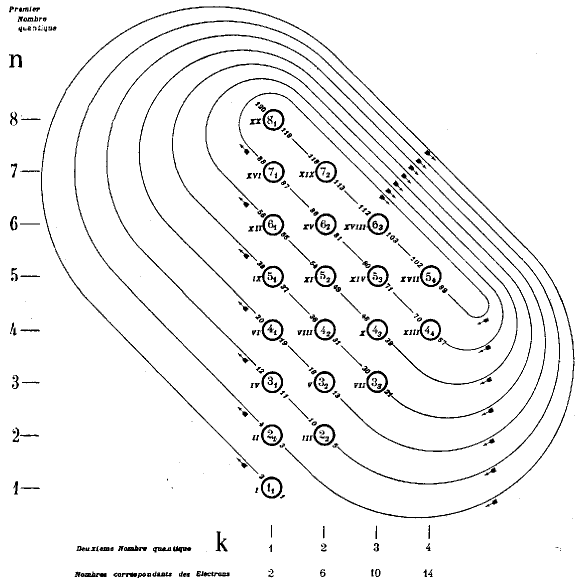
| Year: 1930 | PT id = 154, Type = formulation spiral |
Janet's Shell Filling Diagram
Janet produced six papers, in French, which are almost unobtainable as he had them privately printed and didn't distribute them properly. The shell-filling diagram dated from November 1930, six years before Madelung. Note that Janet uses Bohr's radial quantum number, k, which is l+1. In the text he formulates the n+k-1 rule. Information supplied by Philip Stewart.
| Year: 1934 | PT id = 105, Type = review formulation |
Quam & Quam's Graphical Representations of The Elements
Short Periodic Tables.pdf
Medium Periodic Tables.pdf
Spiral, Helical & Misc Periodic Tables.pdf
- Mendeléeff's Table (their spelling, 1872)
- Brauner's Table (1902)
- Rydberg Table (1913)
- Periodic Chart by Quam (1934)
- Rang's Periodic Table (1893)
- Werner's Periodic Table (1905)
- Courtines' Periodic Classification (1925)
- Bayley's Periodic System (1882)
- Adam's Periodic Chart (1911)
- Margary's Periodic Table (1921)
- Stareck's Natural Periodic System (1932)
- Baumhauer's Spiral (1870)
- Erdmann's Spiral Table (1902)
- Nodder's Periodic Table (1920)
- Partington's Periodic Arrangements of the Elements (1920)
- Janet's Helicodial Classification (1929)
- The Telluric Screw (1863)
- Crookes' Periodic Table model (1898)
- Emerson's Helix (1911)
- Periodic Table by Harkins and Hall (1916)
- Schaltenbrand's Periodic Table (1920)
- Rixon's Diagram of the Periodic Table (1933)
- Spring's Diagram (1881)
- Flavitzky's Arrangement (1887)
- Stephenson's Statistical Periodic Table (1929)
- Friend's Periodic System (1927)
- Many others, including: Vogel (1918), Stintzing (1916) and Caswell (1929) are discribed without the benefit diagrams.
| Year: 1936 | PT id = 777, Type = formulation data misc |
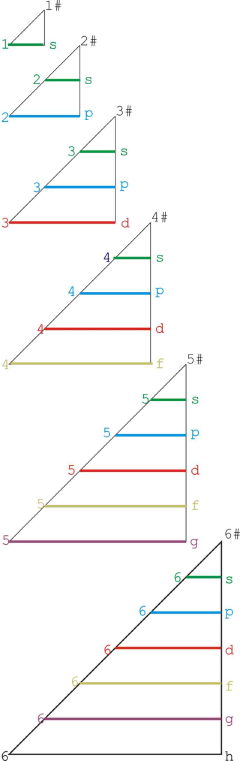
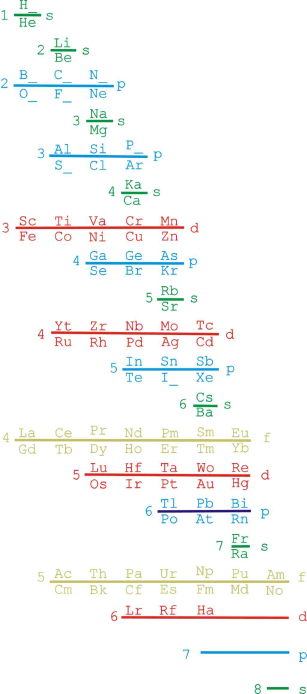
Orbital Filling With Electrons
Students of chemistry are often confused why the orbitals fill with electrons: 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p6... etc., because the 3d10 seems to be 'out of sequence'.
This 'out of sequence' difficulity is nicely explained if the orbitals are arranged in a slightly different way:
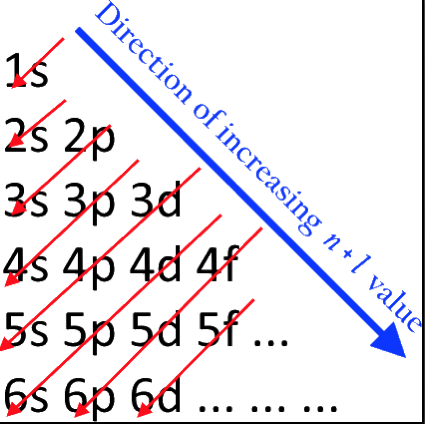
The aufbau principle states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels. For example, the 1s shell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible.
The order in which these orbitals are filled is given by the n + ![]() rule, also known as the Madelung rule (after Erwin Madelung), the Janet rule or the diagonal rule.
rule, also known as the Madelung rule (after Erwin Madelung), the Janet rule or the diagonal rule.
Orbitals with a lower n + ![]() value are filled before those with higher n +
value are filled before those with higher n + ![]() values. In this context, n represents the principal quantum number and ? the azimuthal quantum number. The values
values. In this context, n represents the principal quantum number and ? the azimuthal quantum number. The values ![]() = 0, 1, 2, 3 correspond to the s, p, d and f orbital lables.
= 0, 1, 2, 3 correspond to the s, p, d and f orbital lables.
Julio Gutiérrez Samanez writes:
"I send you the diagram below that reconciles quantum mechanics (diagram for filling the electronic cells) with the Janet table or LSPT. Explaining the duplication of periods with the duplication of the quantum number n, and the introduction of Tao (T) spin of the level or spin of the period, which explains the parity of the symmetric periods."
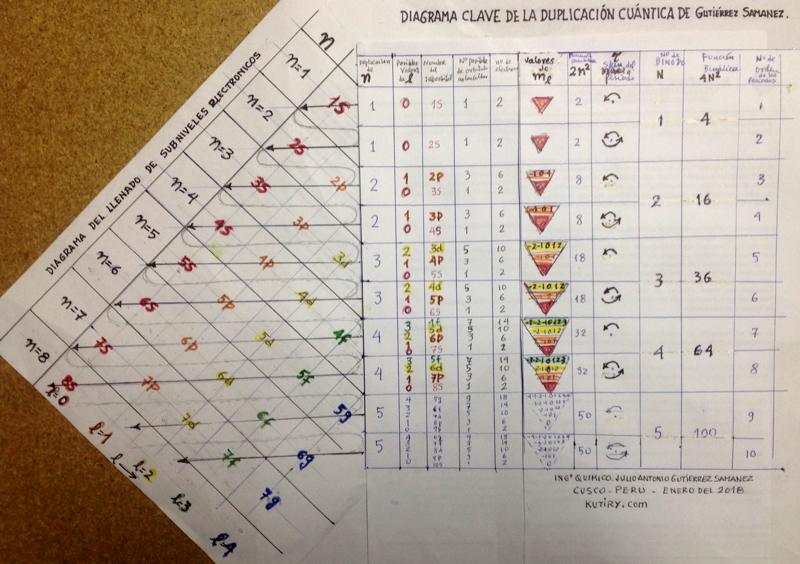
| Year: 1950 | PT id = 14, Type = formulation |
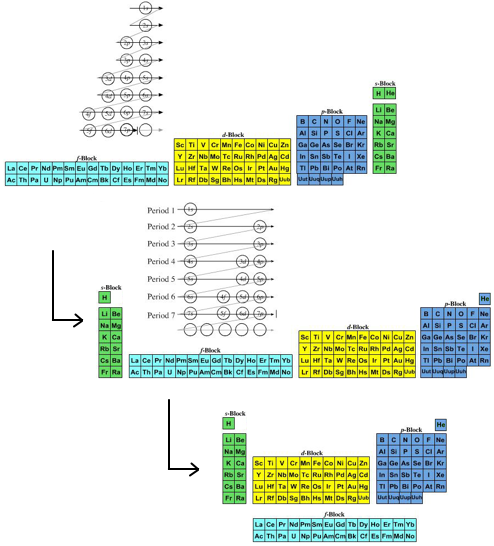
The modern periodic table is based on quantum numbers and blocks, here.
A periodic table can be constructed by listing the elements by n and l quantum number:
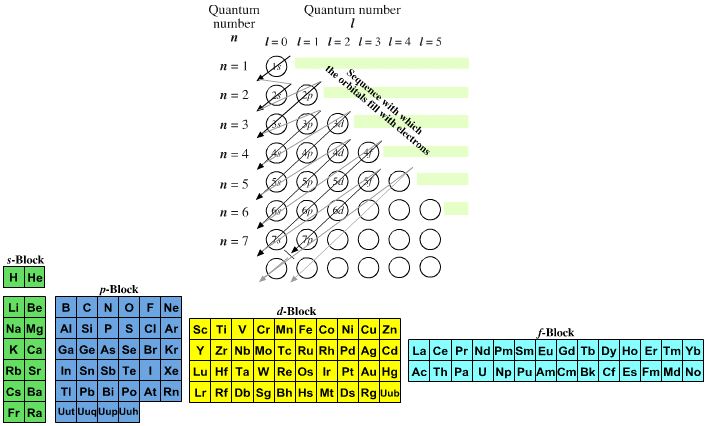
The problem with this mapping is that the generated sequence is not continuous with respect to atomic number atomic number, Z: Check out the sequence Ar to K, 18 to 19.
Named after a French chemist who first published in the formulation in 1929, the Janet or Left-Step Periodic Table uses a slightly different mapping:
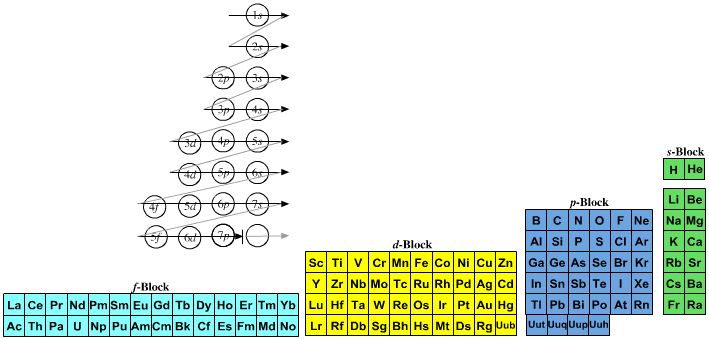
While the Janet periodic table is very logical and clear it does not separate metals from non-metals as well as the Mendeleev version, and helium is a problem chemically.
However, it is a simple mapping to go from the Janet or Left-Step periodic table to a modern formulation of Mendeleev's periodic table:
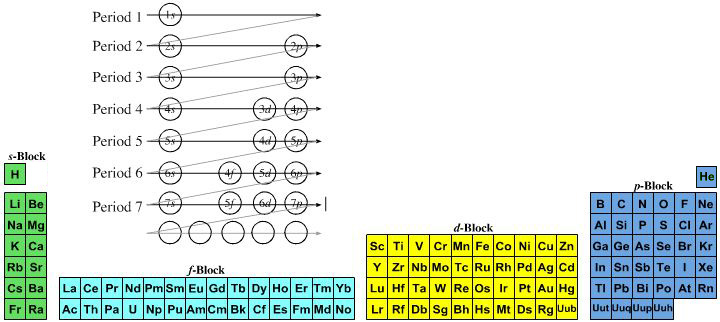
On this page web, "full" f-block included periodic tables are shown wherever possible, as above.
However, the periodic table is usually exhibited in book and on posters in a compressed form with the f-block "rare earths" separated away from the s-block, p-block and d-block elements:
However, the compression used introduces the well known problem known as a "fence post error".
The effect is that:
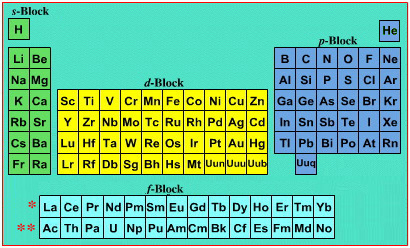
La and Ac: move from f-block to d-block
Lu and Lr: move from p-block to f-blockChemically, the elements can be fitted in and classified either way. Many thanks to JD for pointing the situation with the periodic table is a fence post error.
Mark Winter's Web Elements project, here, uses the formulation shown below:
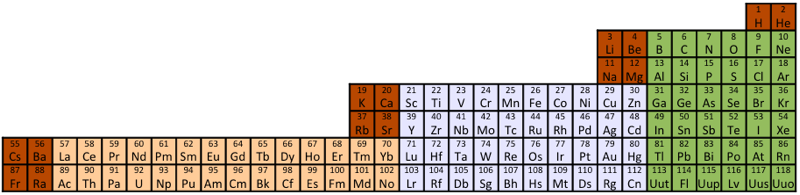
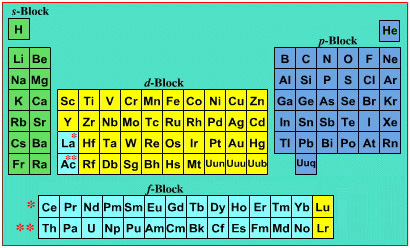
Interestingly, the IUPAC periodic table separates out 15 lanthanides, La-Lu, and 15 actinides, Ac-Lr by leaving gaps in period 3 under Sc & Y:
This corresponds to:
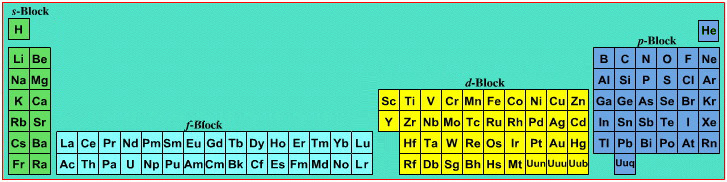
By Mark Leach
| Year: 1952 | PT id = 988, Type = formulation |
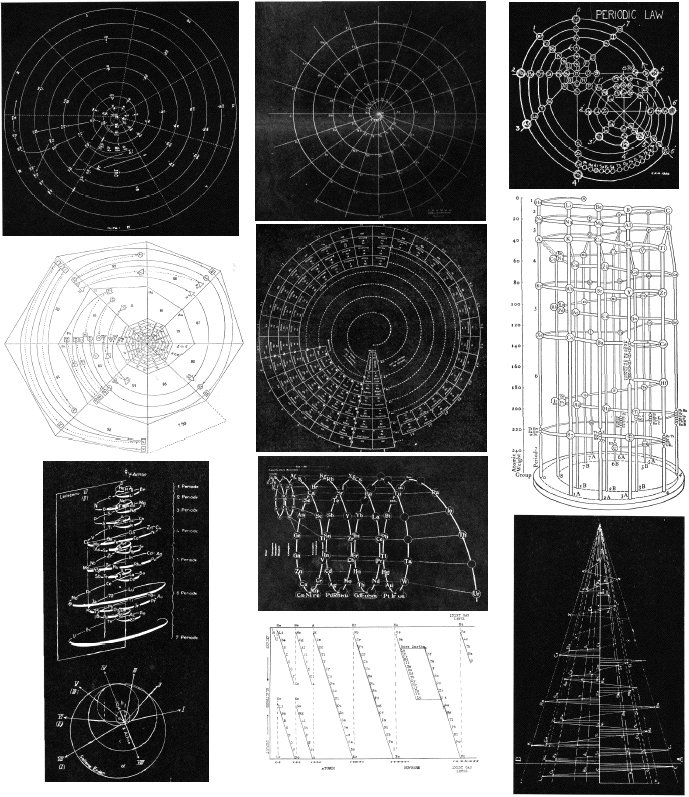
Hakala's Periodic Law in Mathematical Form
Reino Hakala published a paper, The Periodic Law in Mathematical Form, J.Phys.Chem., 1952, 56(2) 178-181. It is argued that: "Janet's [left-step] best meets these requirements".
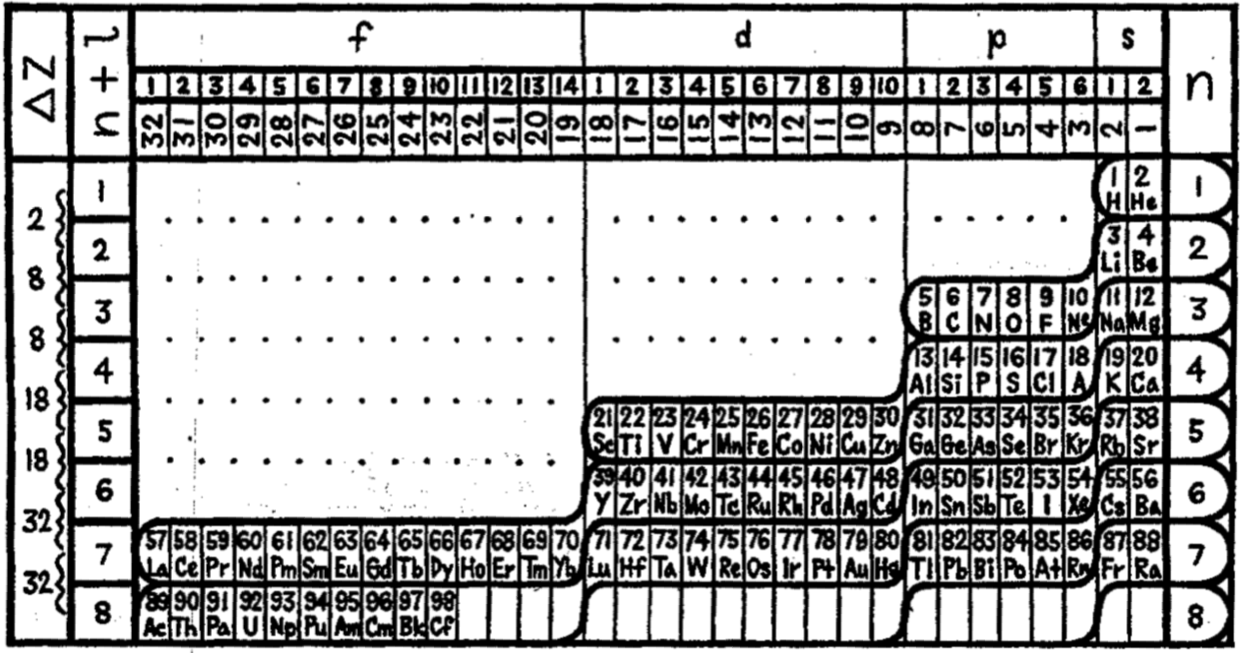
Thanks to René for the tip!
| Year: 1957 | PT id = 110, Type = review |
Mazurs' Graphical Representations of The Periodic System During 100 Years
Edward Mazurs, Graphical Representations of The Periodic System During 100 Years, University of Alabama Press, 1957.
There is an internet archive: Edward G. Mazurs Collection of Periodic Systems Images.
This book gives a very full analysis and classification of periodic table formulations. Most of the formulations are redrawn.
However, anybody who is seriously interested in periodic table formulations will want to see/read/own this book. Read more about Mazrus on the Elements Unearthed blog.
1955 |
Mazurs' Valence Periodic Table (1974, p.94) |
1955 |
Mazurs' Periodic Table (1974, p. 95) |
1955 |
Mazurs' 1955 Formulation (1974, p. 44) |
1958 |
Mazurs' 1958-73 Formulation (1974, endpaper) |
1965 |
Mazurs' 1965 Formulation (1974, p/ 134) |
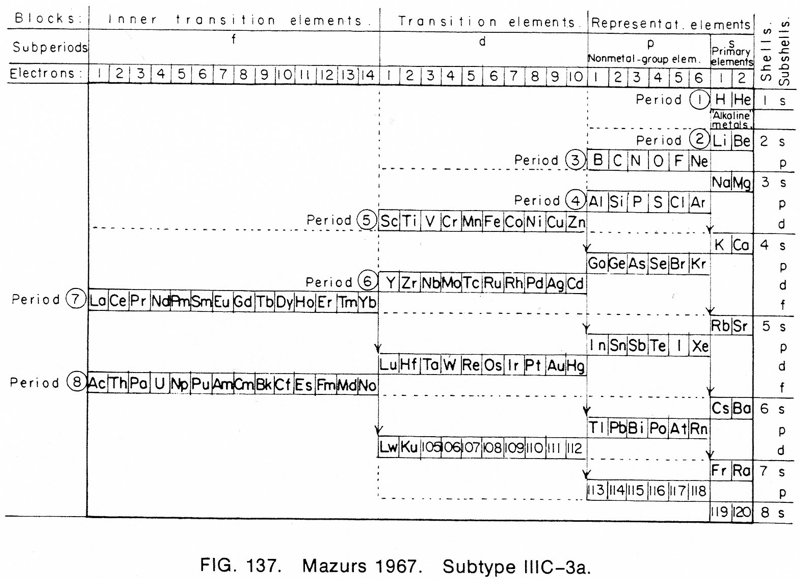
1967 |
Mazurs' 1967 Formulation (1974. Inside front cover) |
1967 |
Mazurs' other 1967 Formulation (1974, p. 126) |
1967 |
Mazurs' another 1967 Formulation (1974, p. 134) |
1969 |
Mazurs' Perio |
1974 |
Mazurs' Version of Janet's "Lemniscate" Formulation (1974, p.80) |
1974 |
Marzus' Wooden Version of Mendeleev's Periodic Table (Chem. Heritage Foundn.) |
1974 |
Mazurs' PT Formulation Analysis (1974, pp.15-16) |
Many thanks to Philip Stewart for preparing the links table above.
| Year: 1964 | PT id = 1271, Type = formulation |
Ternström's Periodic Table
Ref: A Periodic Table, Torolf Ternström, J. Chem. Educ. 1964, 41, 4, 190
René Vernon writes:
"Ternström gives us a triple-combo table drawing on the advantages of:
- the complete block system according to Werner (1905)
- a horizontal Bohr line-system according to Spedding (1951)
The outcome resembles the left step form of Janet (1928).
Some interesting features of Ternström's formulation are:
- a period 0 containing the neutrino and neutron
- element number "00" for "v" suggests the neutrino has neither nuclear charge nor mass while "0" for Nn implies no nuclear charge
- regular period lengths of 2-2-8-8-18-18-32-32
- hydrogen has no direct relationship with a group, only secondary relationships with groups A1 and A7
- germanium, a semiconductor, is counted as a metal
- all groups numbered, where A = representative; B = transition
- C = Ln/An; analogous "transition" groups in the d-block (B8) and the f-block (C6)
- double periodicity among the Ln and An; and
- 25 columns wide i.e 18 + 32 = 50/2 = 25"
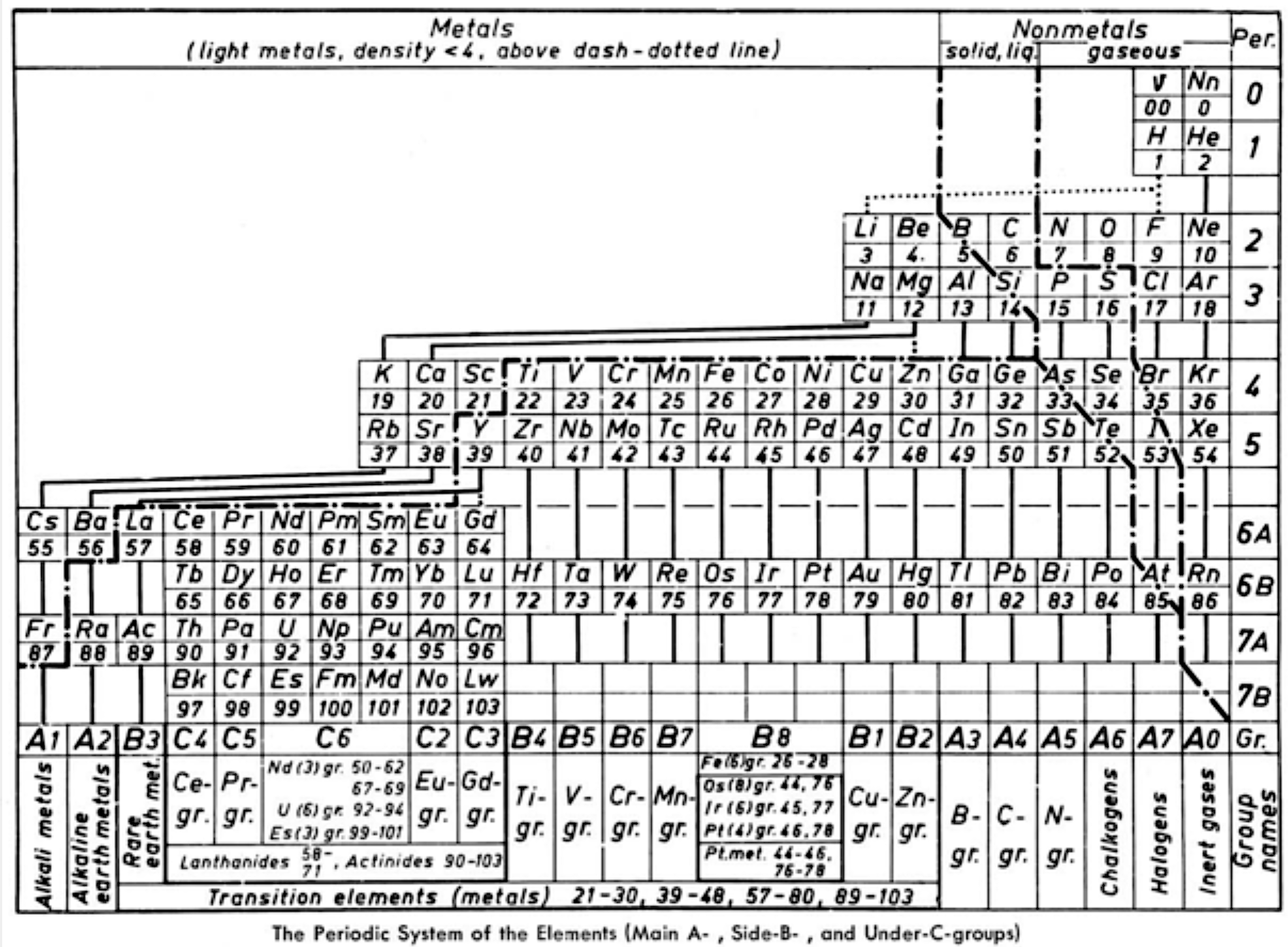
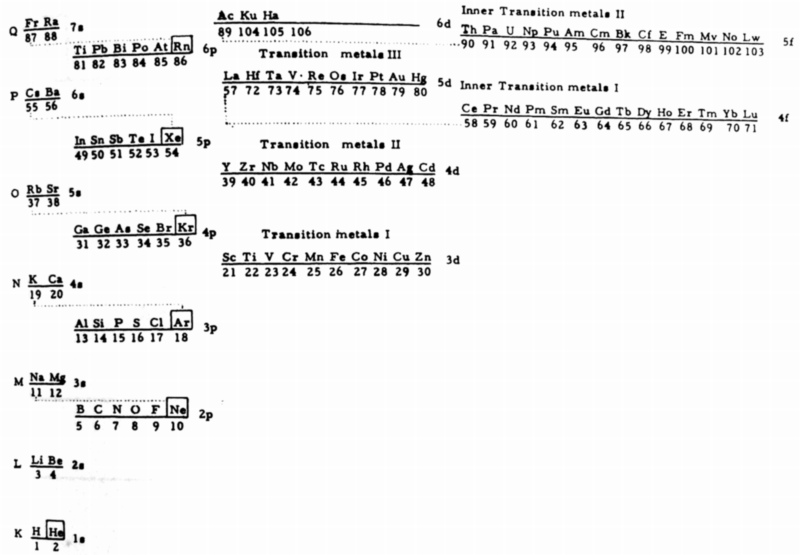
| Year: 1967 | PT id = 695, Type = formulation |
Mazurs' another 1967 Formulation
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press.
This formulation is the basis of Philip Stewart's Janet Rajeuni:
Thanks to Philip Stewart for the tip!
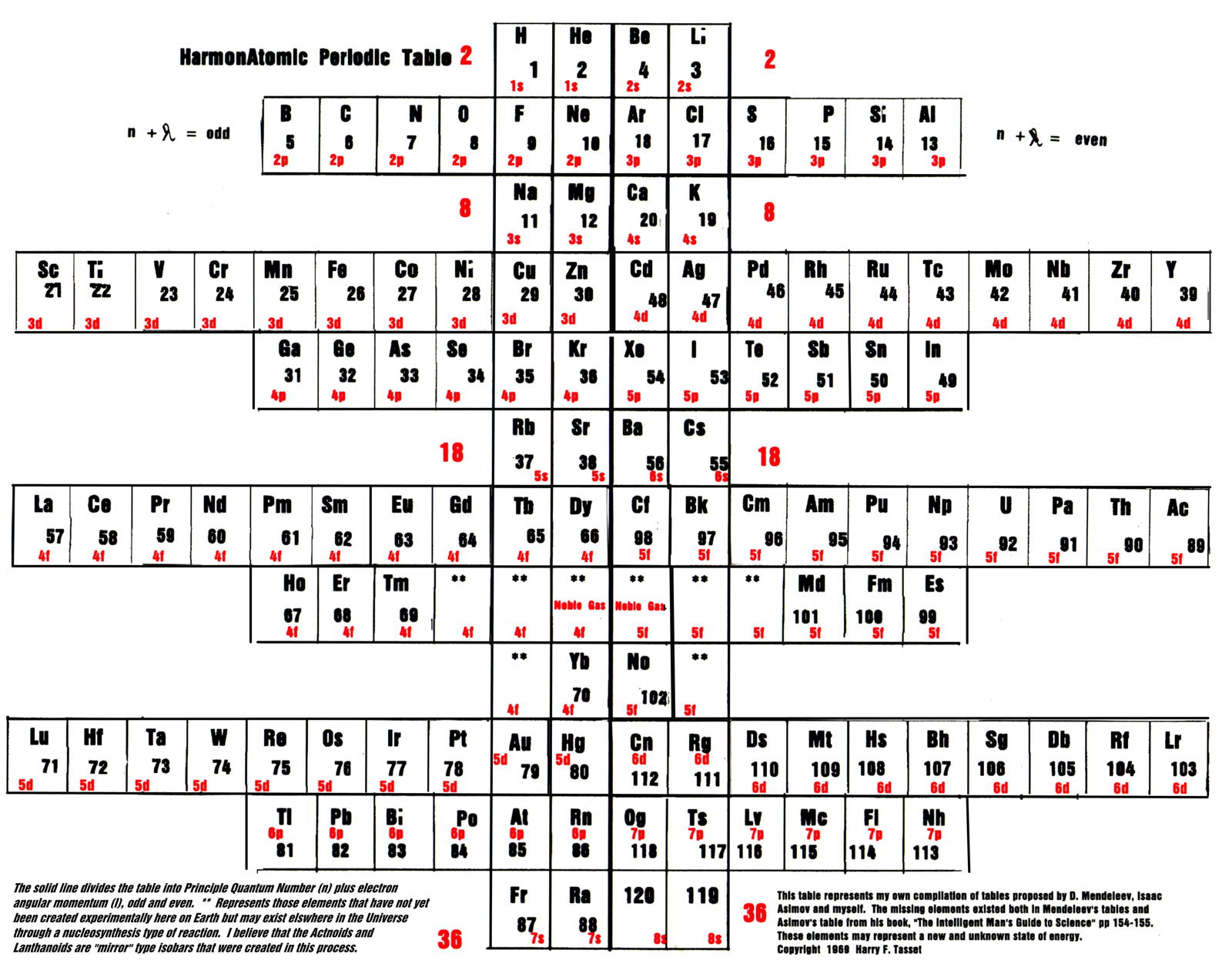
| Year: 1969 | PT id = 1126, Type = formulation |
Tasset's HarmonAtomic Periodic Table
Harry F. Tasset writes:
"The periodic table (below) was originally copyrighted by me in 1969 and represents those missing elements that were lost due to the influence of physicists who were trying to mold the table into a more 'suitable' form. While they did succeed for many years, innovators like Charles Janet (left step table) and Isaac Asimov (missing elements table) had other ideas.
"Below you will see that the laws of chemistry, first discovered by Mendeleev, triumphed."
Click the image to enlarge
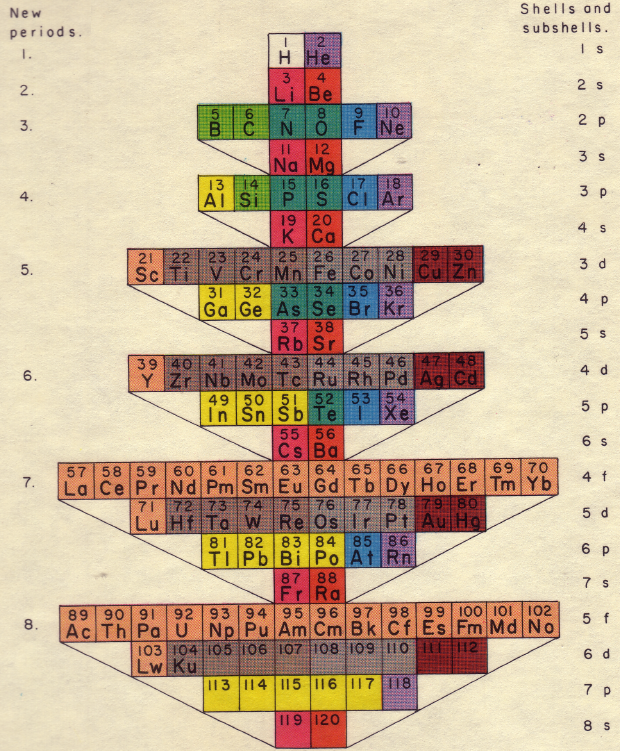
| Year: 1969 | PT id = 624, Type = formulation |
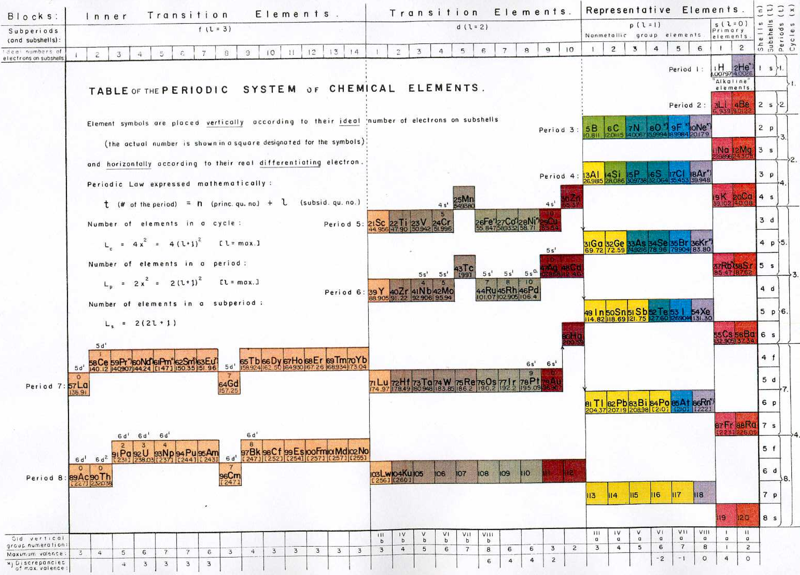
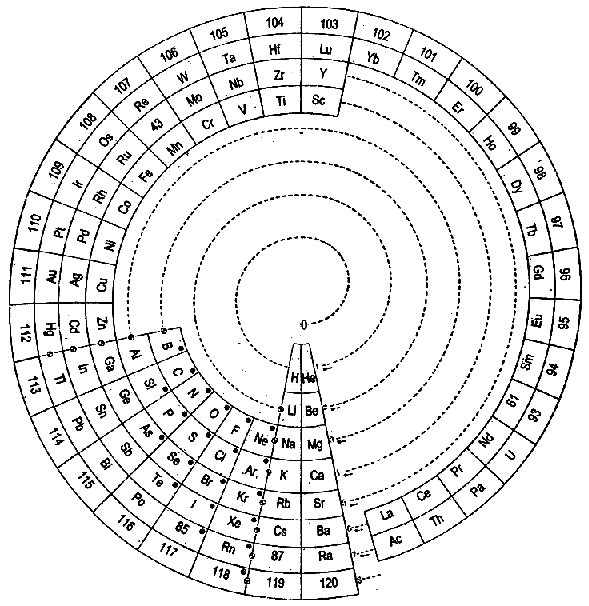
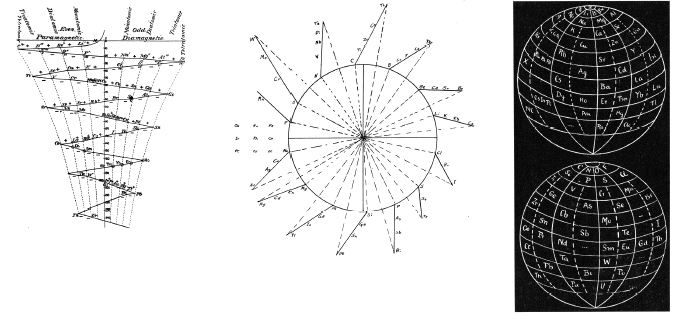
Mazurs Periodic System of Chemical Elements
A foldout from the Mazurs book, Graphical Representations of The Periodic System During 100 Years.
Mazurs said he drew it in 1967 and published it in 1969: ref. E Mazurs, A new numeration of periods in the periodic system and the Kessler Principle for the construction of the periodic table, Canad. Chem. Edu. 4(3), 21-23, 1969.
It is a Janet's modified system to show the irregularities – Lu, Cr, Pd etc. Click here for a larger version:
Thanks to Philip Stewart for the tip!
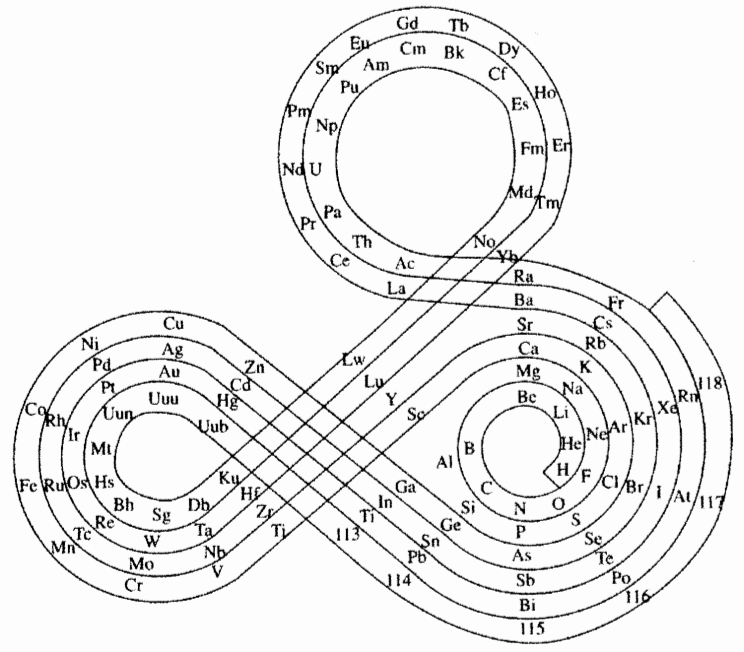
| Year: 1974 | PT id = 260, Type = formulation spiral |
Mazurs Version of Janet's "Lemniscate" Formulation
Janet's lemniscate formulation periodic table as modified by E.G. Mazur in his Graphic Representations of the Periodic System during One Hundred Years (1974), cited in Punyashloke Mishra's The Role of Abstraction in Scientific Illustration: Implications for Pedagogy (1999) republished in Carolyn Handa's Visual Rhetoric in a Digital World: A Critical Sourcebook", from the Island94 blog, here:
| Year: 1974 | PT id = 1058, Type = formulation spiral |
Mazurs' Redrawing of Stedman's Formulation
An spiral formulation by Mazurs, cited as being after Janet (1928). However, it is actually, it is after Stedman (1947).
In an article Bull. Hist. Chem., VOLUME 34, Number 2 (2009) O.T. Benfey writes:
"After we had developed our own [Periodic Snail] spiral design, we found that E. G. Mazurs had published a spiral with a separate protrusion for the lanthanides which, under the image, he misleadingly ascribed to Charles Janet in 1928, the same year that Janet had published a simple circular form also shown by Mazurs. The Mazurs diagram with the lanthanide protrusion was reprinted in [the journal] Chemistry. However, [Philip] Stewart informed me that the Mazurs figure bears no resemblance to the Janet diagram he indicated nor to any other of his designs. Detailed references given a few pages later by Mazurs suggested correctly that the spiral derives from Stedman and is so identified and depicted by van Spronsen. The Mazurs diagram is a mirror image of the Stedman spiral, updated to include elements discovered since 1947." [For references, see the article.]"
Mazurs (p. 77) writes:
"Subtype IIIA3–1a Helix on a modified cone. The transition and inner transition elements have special revolutions in the form of loops. This table, originated by Stedman in 1947 is not a successful one."
Thanks to René for the tip and information!
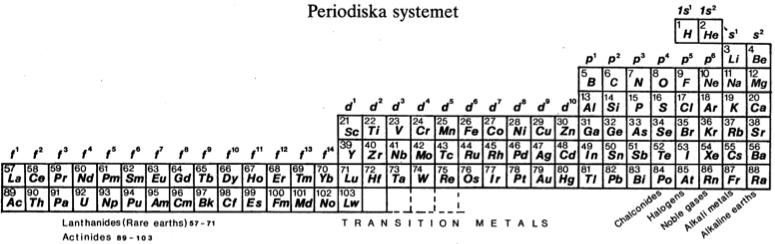
| Year: 1982 | PT id = 451, Type = formulation |
Periodiska Systems Rätta Form
Hanno Essén's Periodiska Systems Rätta formulation of the Periodic Table, published in the International Journal of Quantum Chemistry vol. XXI pp.717-726 (1982).
Essén's formulation is a variant of the Janet Left-Step formulation of 1928:
| Year: 1984 | PT id = 924, Type = formulation |
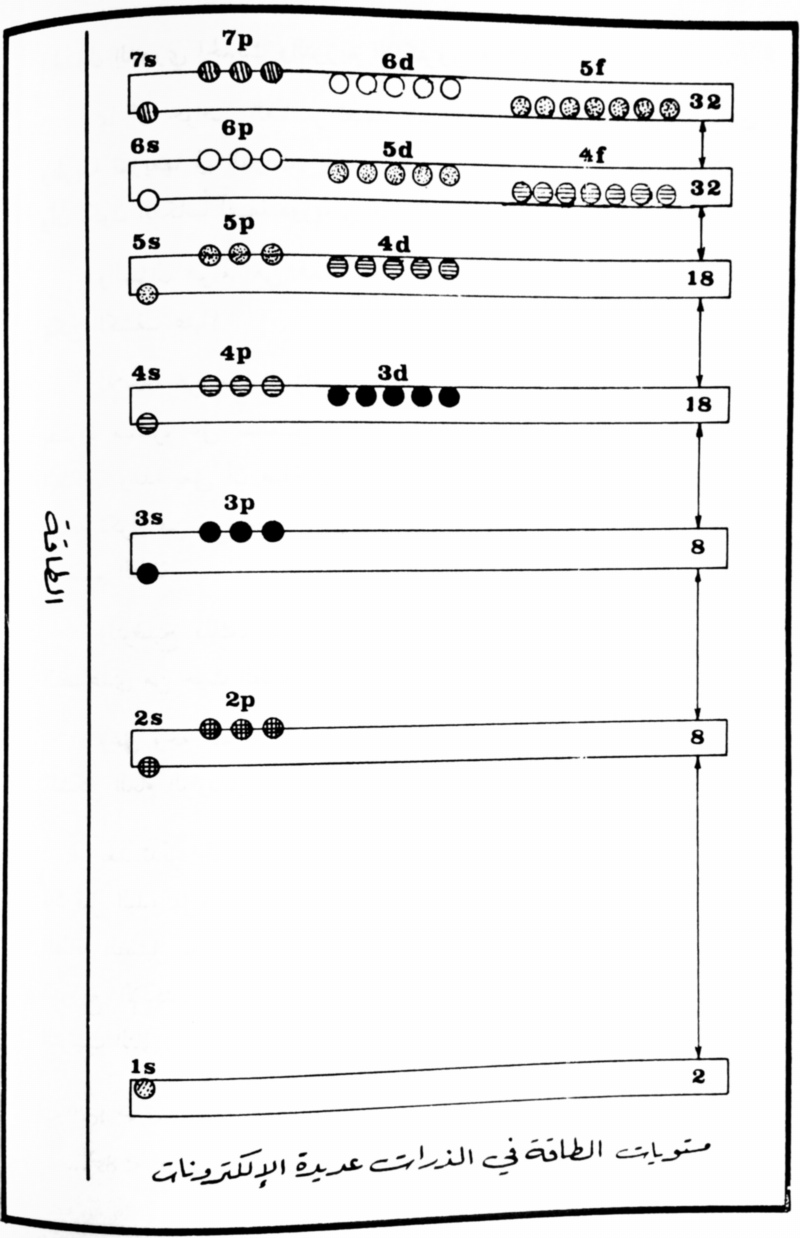
Arabic Periodic Tables
From Arabic introductory text published by the Royal Scientific Society, Amman, Jordan, 1984. Jeries A. Rihani, who provided the two images, writes:
"The first image shows a periodic table similar to that of the Janet left-step periodic table, but in an upside-down flipped format.The second image displays the comparative energy levels of orbitals of atoms of many electrons."
Thanks to Jeries A. Rihani for the tip!
| Year: 1992 | PT id = 935, Type = formulation |
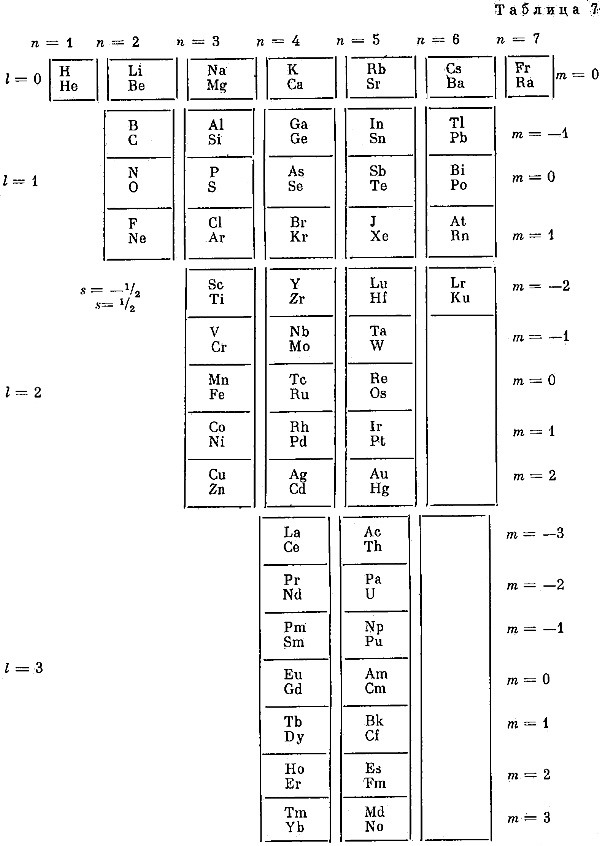
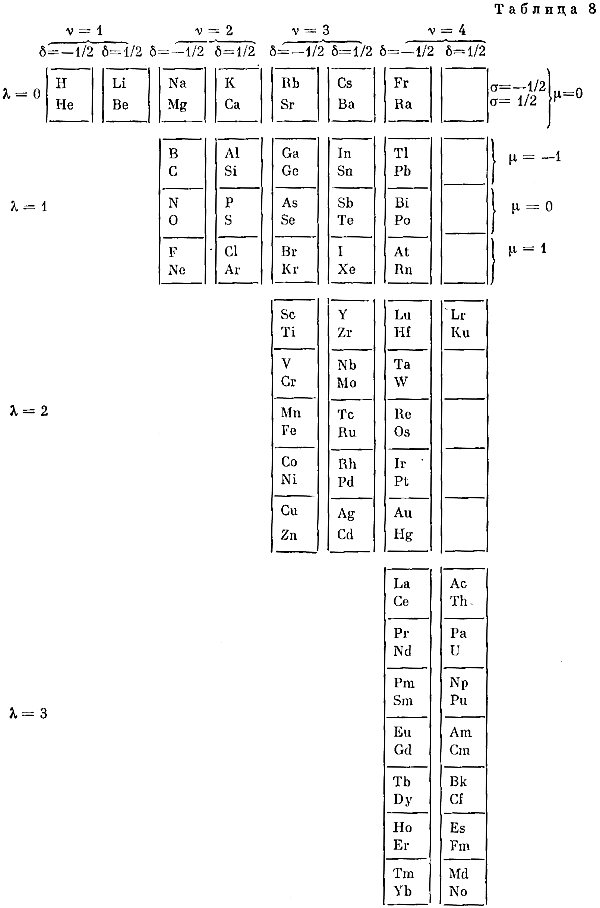
Fet's Periodic Tables
Two periodic tables by A.I. Fet from his book, "Mathematical Modeling in Biology and Chemistry. New Approach" Nauka, Sib.Dep., 1992.
Larry Tsimmerman writes:
"First formulation, Tab. 7, is precursor of Adomah PT with broken Z-sequence and questionable pairing of elements in accordance with "ml". Tab. 8 is a Janet LST shown vertically. Fet discusses Periodic Table in the light of Group Theory. (The book was sent to me by Eric Scerri and it was signed by Fet for Hefferlin)."
| Year: 2001 | PT id = 353, Type = formulation |
Muradjan's Universal Periodic System
Muradjan's Universal Periodic System is a variation of the Janet formulation, however, it is worth visiting the web page and scrolling down as there is much interesting material:
| Year: 2004 | PT id = 1100, Type = formulation review |
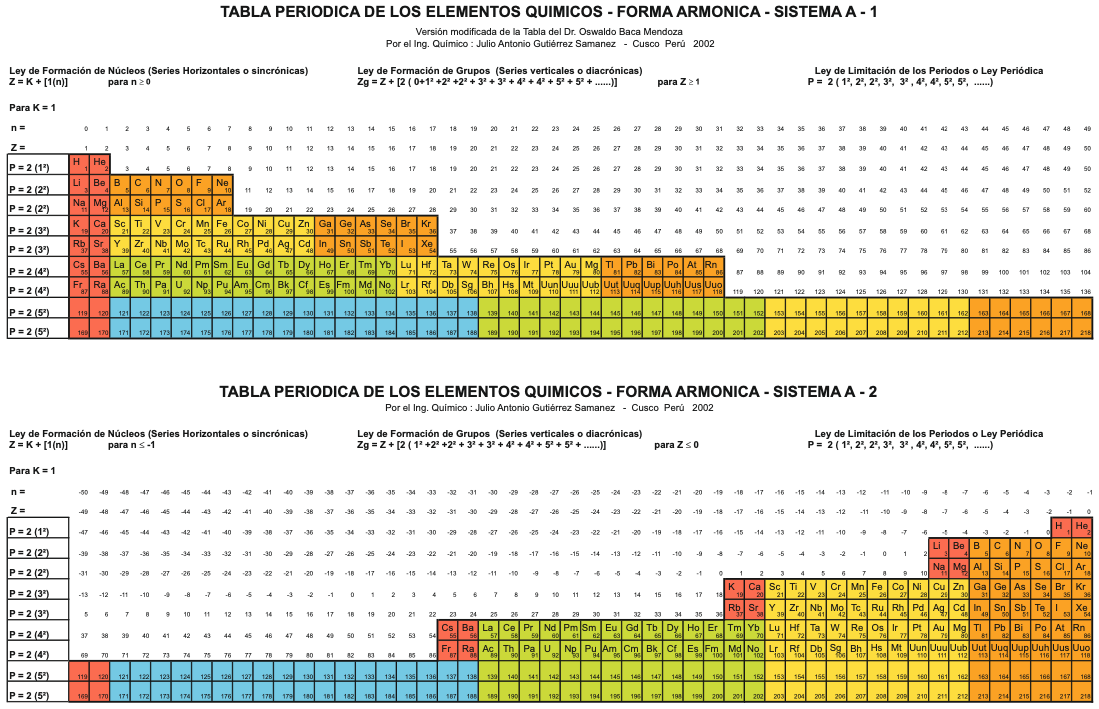
Sistema Periódico Armonico de Gutierrez-Samanez
A digitised 2004 book by book by Julio Gutiérrez-Samanez.
Julio writes:
"These matrix tables are inspired by the method used by the Peruvian chemist Oswaldo Baca Mendoza (1908-1962).
"The tables are read in this way: The Law of Formation of nuclei generates all the horizontal series Z, is dependent on n (series of integers numbers) and a constant K = 1. In the step-to-right tables (n) it will be equal to or greater than 0. In Janet's Left-Step table, (n) will be less than or equal to (-1). The values of this series Z will serve as a constant for the second law.
"The Group Formation Law or vertical series. Its generate the numeric values of the columns either from left to right or from right to left. In system A -1: With the first law: n = 0, then Z = 1. The vertical series Zg = 1, 3 11, 19, 37, 55, 87 ... That is: 1H, 3Li, 11Na, 19K, 37Rb, 55Cs, 87Fr, 119, 169 ... Changing the values of n or Z, all the columns of the table will be obtained.
"In system A -2: With the first law: on the left, n = -1, then Z = 0. The vertical series Zg will be: 2He, 10Ne, 18Ar, 36Kr, 54Xe, 86Rn, 118Og, 168, 218. .. Similarly, changing the n or Z values, we can fills the columns of the table. In system B -1: With the first law: for n = 0, then Z = 1. The vertical series Zg will be; 1H, 3Li, 5B, 13Al, 21Sc, 39Y, 57La, 89Ac, 121, 171 ... By varying the values of n or Z, the entire table is filled. In system B -2: (Its mathematizes the Janet system). With the first law: on the left, for n = -1, then Z = 0.
"The vertical series Zg will be; 2He, 4Be, 12Mg, 20Ca, 38Sr, 56Ba, 88Ra, 120, 170, 220 ... By varying the values of n or Z, the entire table is filled. The third law of the limiting the periods or periodic law, appears graphically, by comparison between rows: For example: in table B -1, in column Z = 3, after 1H and 2He, en of the first horizontal line, the value 3 appears, which is already entered in the first column as 3Li, therefore, that part of the first horizontal row (from 3 to 50) is deleted.
"The same happens with the number 5 in column 3, which is already in the first column as 5B, therefore it will be deleted in the second row from 5 to 52. The same applies to pair 13, 21 of the column Z = 9, same, with the pair 39, 57 of the column Z = 19 and of the pair 89, 121 of the column Z = 33. For that reason the periods: P are duplicated function: 2 (1 ^ 2), 2 (1 ^ 2), 2 (2 ^ 2), 2 (2 ^ 2), 2 (3 ^ 2), 2 (3 ^ 2) .... = 2, 2, 8, 8, 18, 18, 32, 32 ... and the forms are exact and staggered. The colors represent the quantum functions: s (red), p (orange), d (yellow), f (green), g (blue)."
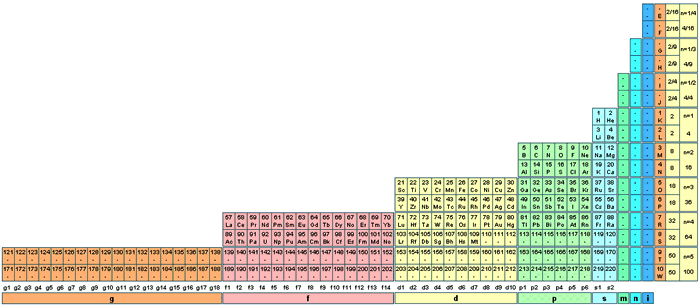
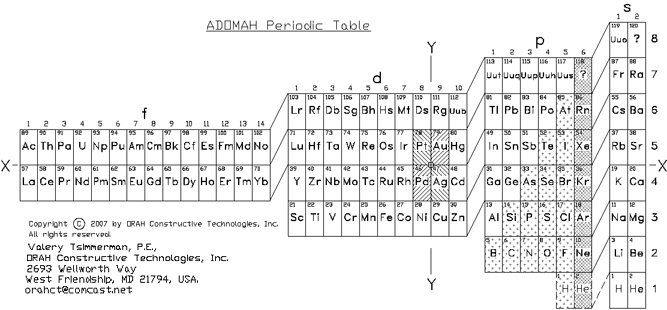
| Year: 2006 | PT id = 32, Type = formulation |
ADOMAH Periodic Table by Valery Tsimmerman
The ADOMAH periodic table is based on the Janet or left-step periodic table. It consists of four blocks (s, p, d & f) corresponding to quantum numbers l = 0,1,2,3. Blocks are separated, shifted and reconnected with each other via diagonal lines. This arrangement creates "layers" or "strata" that retain continuity in respect to atomic number Z, in addition to usual columns and rows. Therefore, numbers shown on the right hand side of the table may represent either quantum numbers n (electronic shells) if horizontal rows are followed, or n + l if "layers" or "strata" are followed.
This feature assists in creation of electronic configurations of the elements. Elements H and He are placed in two positions that reflect their dual nature and give proper consideration to atomic structure and chemical properties of those two elements. This feature also preserves triads He, Ne, Ar and H, F, Cl. Also, the elements are placed in rectangular "boxes", so any two of such "boxes" make up a square thus symbolising electron pairs. This also cuts table length in half. Unlike the Janet table, this table is assembled from bottom up in direction of increase of quantum number n, as well as atomic weight and energy. The ADOMAH table has symmetry and, assuming total number of elements 120, can be divided in four parts of 30 elements with center point located among precious metals.
| Year: 2006 | PT id = 42, Type = formulation review |
Henry Bent's Exploration into Janet's Left-Step Formulation
Henry Ben't detailed exploration into the Left-Step formulation of the periodic table is available as a book:
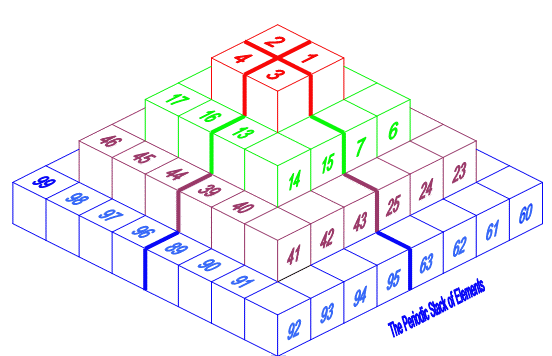
| Year: 2008 | PT id = 156, Type = formulation 3D |
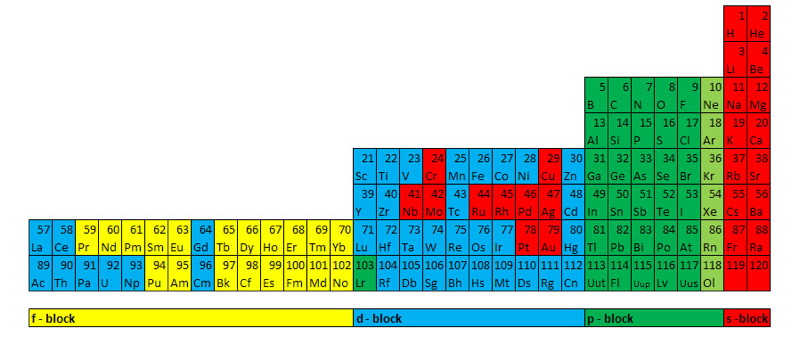
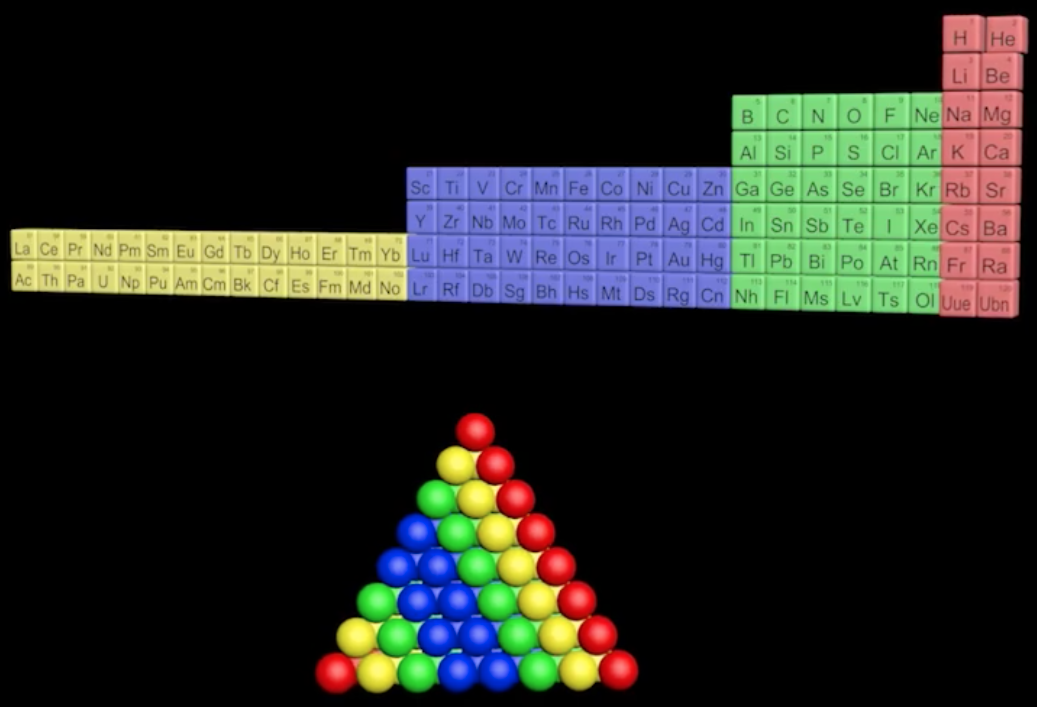
Pyramid (Stack) Periodic Table
The Janet Periodic Table of Elements (1928) may be re-arranged as a series of square matrices.
The matrices are of different sizes and each matrix organizes the atomic orbitals into square concentric rings. Each cell may be assigned an atomic number which also identifies a “most significant electron”. The matrices may be stacked vertically to form a periodic Pyramid Stack of Elements as shown below.
The sub-atomic particles may also be arranged as square matrices. These matrices may be stacked. Read more here.
Please send your comments to: rick_kingstone777@hotmail.com
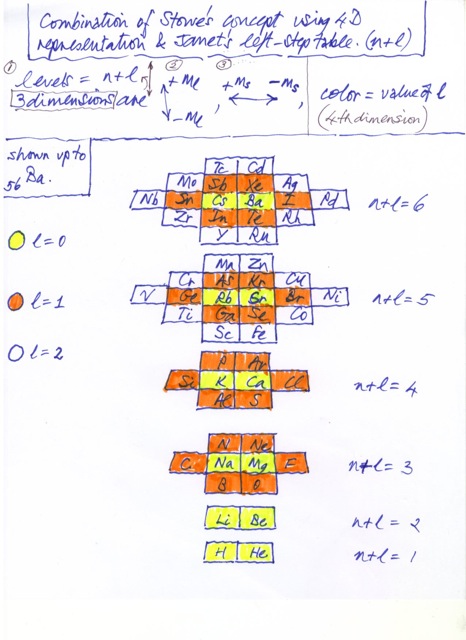
| Year: 2009 | PT id = 206, Type = formulation |
Janet Based Periodic Table Layout by Ivan Antonowitz
"Every element has its own unique Periodic Table which is a mixture of two Ideal Forms. However, the main point at the moment is what level of complexity would be suitable? I am trying to get the most minimalistic presentation of the essential features. Explaining the logic governing the 'reversals' is quite tricky, if not controversial, and others may have more conventional rationales and so better fill in the details."
Ivan Antonowitz
 |
 |
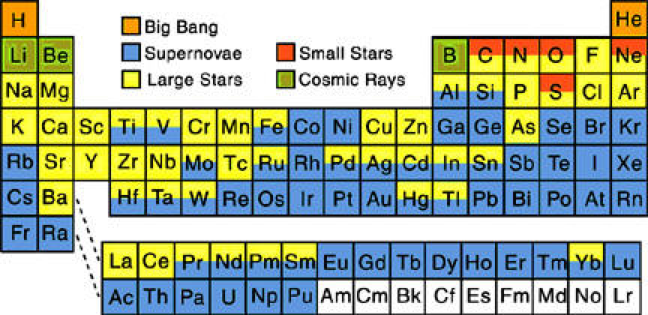
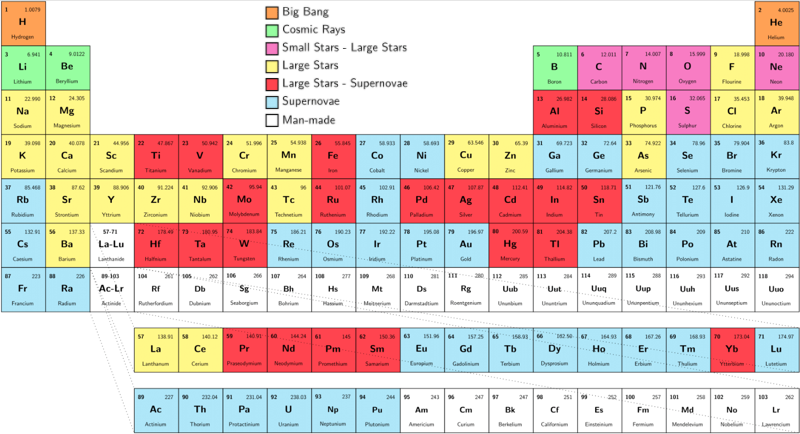
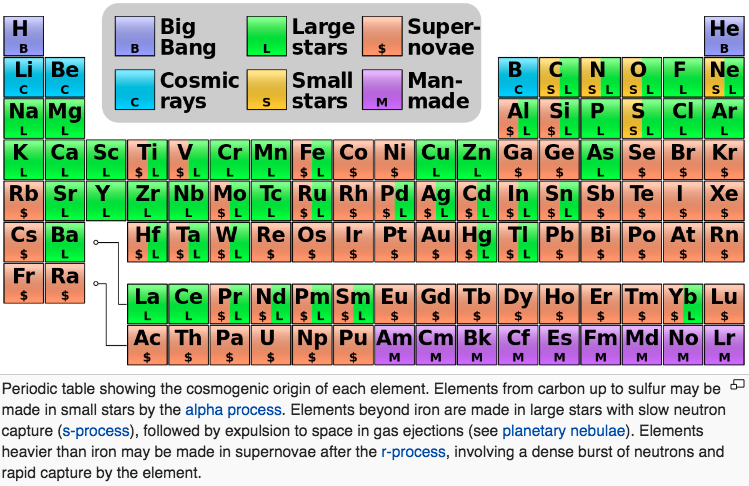
| Year: 2010 | PT id = 593, Type = data |
Nucleosynthesis Periodic Tables
The buildup of heavy elements from lighter ones by nuclear fusion.
Helium, and some lithium, was produced by cosmic (or primordial) nucleosynthesis from 2 to 20 minitues after the Big Bang, here and here:
From the Encyclopedia of Science:
Today most element-building nucleosynthesis takes place in stars.
Stellar nucleosynthesis converts hydrogen into helium, either by the proton-proton chain or by the carbon-nitrogen-oxygen cycle. As a star evolves, a contracting superdense core of helium is produced from the conversion of hydrogen nuclei into helium nuclei.
Eventually, the temperature and pressure inside the core become high enough for helium to begin fusing into carbon. If the star has more than about twice the Sun's mass, a sequence of nuclear reactions then produces heavier elements such as oxygen, silicon, magnesium, potassium, and iron. Successively heavier elements, as far as iron (in the most massive stars) are built up in later stages of stellar evolution by the triple-alpha process. The heaviest elements of all are produced by explosive nucleosynthesis in supernova explosions, by mechanisms such as the p-process, r-process, and s-process:
From FigShare (Athanasios Psaltis):
Our quest to explain the origin of the elements started in the late 1950's by two famous papers independently - E. M. Burbidge et al., Rev. Mod. Phys. 29, 547 (1957) & A.G.W. Cameron, Pub. Astron. Soc. Pac. 69, 201 (1957) - whose authors claimed that the elements are created in astrophysical environments. This is the well-known periodic table of elements, but where each element is labeled by the environment that is created (e.g Supernova explosion etc.).
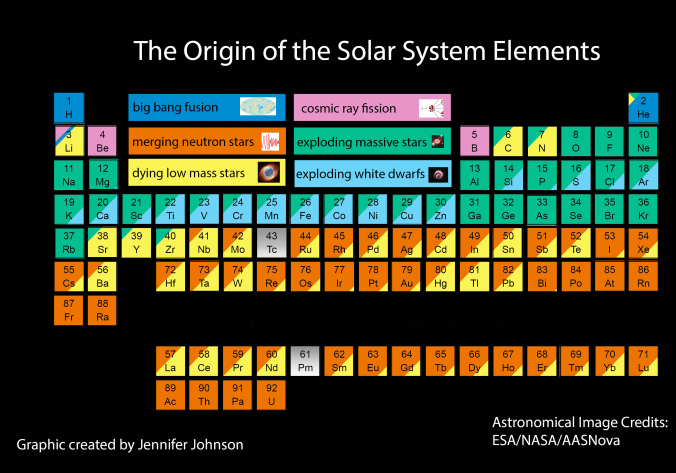
In 2017 the LIGO gravitional wave detector identified the merger of two neutron stars, an event which produces large quantities of gold, platinum etc. Thus, an updated periodic table of nucleosyntheis looks like this, from an interesting SDSS blog:
Conal Boyce has prepared a Janet Left-Step Nucleosynthesis Periodic Table. Conal writes:
"This formulation was created by mapping the Ivans/Johnson color-coding scheme onto a Janet grid, using Tsimmerman half-cells. Although several attempts to contact Professor Jennifer Johnson failed, I did receive enthusiastic feedback on this LST mapping from Professor Inese Ivans, and decided to make it public on that basis."
| Year: 2011 | PT id = 775, Type = formulation 3D |
Weise's Tetrahedron
Dmitry Weise shows how it is possible to go from the Janet [left-step] periodic table formulation, to a tetrahedral formulation.
Dmitry writes:
"Three-dimensional table of the periodic law can be constructed in the form of a tetrahedron having an inner order. A comparison of the tetrahedron shells and the table of elements shows, that one tetrahedron shell corresponds to 4 periods of the 2D table."
Jess Tauber adds:
"The spheres here also aren't labeled, but I explain how they get labeled in the text accompanying the pic. Each such period (except for s-only, which are obviously simpler) we have a 'switchback' configuration. Like a road going up a mountain back and forth to minimize verticality, or a parachute folded into a pack. There are 8 different ways to do this (4 basic types in 2 chirally opposite mappings). And the original Weise-style non-continuous tetrahedron is just another way to organize half tetrahedra."
| Year: 2011 | PT id = 414, Type = formulation 3D |
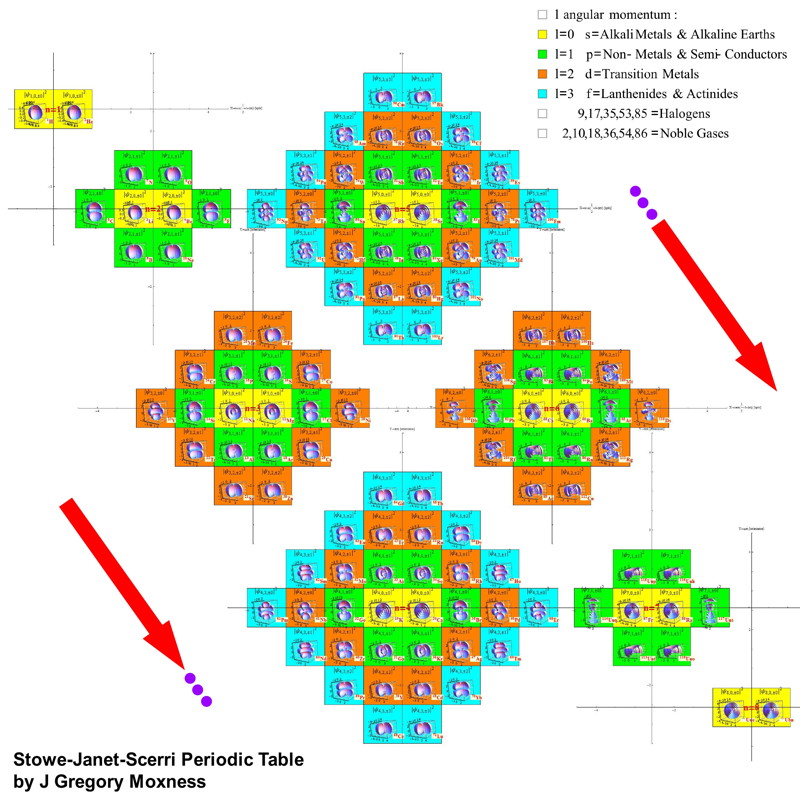
Stowe-Janet-Scerri Periodic Table
Eric Scerri made contact, writing: "Following the discussions on Periodic Table debate on the Chemistry Views website here, and as a result of recent turns, I have developed a new periodic table which I believe combines virtues of the Stowe table and also the Janet left-step table. I propose the name Stowe-Janet-Scerri Periodic Table. The explanation is posted on the Chemistry Views debate pages.
| Year: 2011 | PT id = 438, Type = formulation |
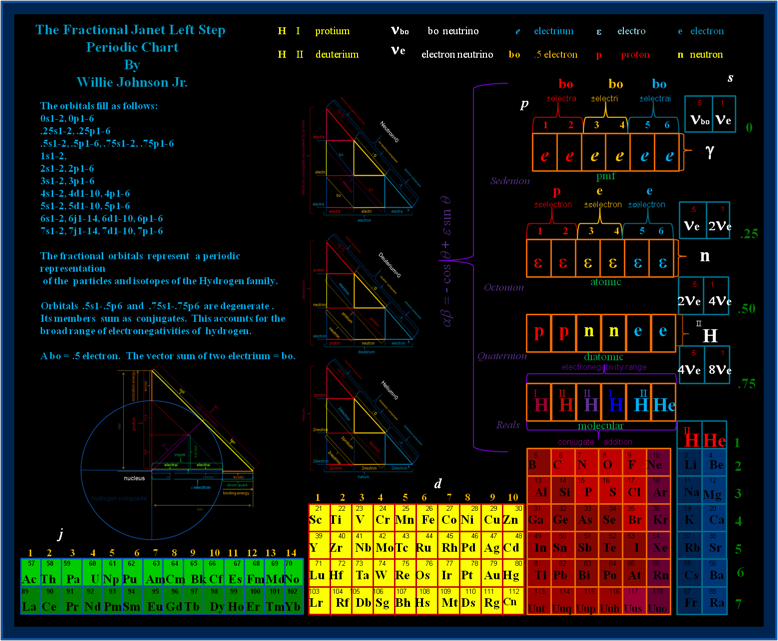
Fractional Janet Left-Step Periodic Chart
On Willie Johnson Jr.'s website – Gyroscopic Force Theory – can be found the Fractional Janet Left-Step Periodic Chart:
| Year: 2012 | PT id = 524, Type = formulation |
Compact Mendeleev-Moseley-Seaborg Periodic Table (CMMSPT)
A Compact Mendeleev-Moseley-Seaborg Periodic Table (CMMSPT).
This table can be found by two different ways:
- Via MMSPT - All terms of the MMSPT are shifted to the right side without spaces.
- Via Janet Periodic Table - The first row of the Janet PT is deleted. - We remove 2 from all others 118 terms.
These 2 transformations lead to the same table, with 7 rows and 32 columns. Blocks p (green), d (light grey), and f (light orange) are preserved.
The 14 terms of the s block (dark orange/red) are splited in "cascads".
This table can be seen in the A173592 sequence in the On-line Encyclopedia of Integer Sequences (OEIS). Row differences are 8, 8, 18, 18, 32, 32.
| Year: 2013 | PT id = 589, Type = formulation 3D |
4D Stowe-Janet-Scerri Periodic Table
By Jgmoxness who writes:
"I've replaced the standard periodic table in the 7th "Chemistry Pane" of my E8 visualizer with a 2D/3D/4D Stowe-Janet-Scerri version of the Periodic Table.
"Interestingly, it has 120 elements, which is the number of vertices in the 600 Cell or the positive half of the 240 E8 roots. It is integrated into VisibLie_E8 so clicking on an element adds that particular atomic number's E8 group vertex number to the 3rd E8 visualizer pane.
"The code is a revision and extension of Enrique Zeleny's Wolfram Demonstration":
| Year: 2013 | PT id = 610, Type = review |
Top 10 Periodic Tables
There are more than 1000 periodic tables hosted by the Chemogenesis Webbook Periodic Table database, so it can be a little difficult to find the exceptional ones.
Here we present – in our humble opinion – The ten most significant periodic tables in the database.
We present the best:
- Three best data rich periodic tables
- Five formulations which show the development of the modern PT
- One, of many, interesting alternative formulations
- One example of the periodic table being used as an infographic template
Three Excellent, Data Rich Periodic Tables
The first three of our top 10 periodic tables are classic element data repositories.
They all work in the same way: click on the element symbol to get data/information about the selected element. The three are Mark Winter's WebElements, Theo Gray's Photographic Periodic Table & Michael Dayah's Ptable.
- Since 1993 – and with its rather bland interface – WebElements has given access to vast quantities of in depth chemical data & information. This is the professional chemist's periodic table:
- Theo Gray's Photographic Periodic Table is undoubtedly the most attractive PT available in web space, but there is more. Clicking around the website gives access to a host of information, pictures & anecdotes from Theo's extraordinary and extensive collection of chemical elements:
- Ptable has a super-slick, and very fast interface. It is data/information rich and is available in 50 languages:
Five Formulations Showing The History & Development
The next five examples deal with history and development Periodic Table. The first is Dalton's 1808 list of elements, next is Mendeleev's 1869 Tabelle I, then Werner's remarkably modern looking 1905 formulation. This is followed by Janet's Left Step formulation and then a discussion of how and why the commonly used medium form PT formulation, is constructed.
- There are several early listings of chemical substances, including Valentinus' Alchemy Table and Lavoisier's Table of Simple Substances (1789). In 1803 Dalton proposed that matter consists of discrete atoms that combine in fixed ratios, stoichiometry, to form chemical elements. Thus, Dalton's list of chemical elements, plus mass data, must be included in any top ten listing:

- If you examine the periodic tables from Antiquity to 1899, you will see that from about 1830 onwards, proto-periodic tables were coming thick and fast. Significant developments include: Daubeny's Teaching Display Board of Atomic Weights (1831), Chancourtois Telluric Helix (1862) and Newlands octaves (1864).
But, it was Mendeleev's Tabelle I that was first near complete periodic table formulation of the then known elements (no Group 18 rare gasses, note). Crucially, Mendeleev identified gaps and was able to make predictions about the chemical properties of the missing substances. Plus, Mendeleev promoted his ideas with great energy:

- Werner's 1905 Periodic Table is remarkably modern looking. The formulation is a long form that separates transition metals and rare earths, but he guessed wrong on how many existed:

- Janet's Left Step formulation of 1928 is one for the purists as it clearly shows the chemical elements arranged into s, p, d & f-blocks of the recently developed quantum mechanical description of atomic structure:
- The modern (and commonly employed) periodic table is obtained by transforming Janet's Left Step into the modern long form periodic table by rearranging the blocks around. This transformational mapping is discussed in some detail here.
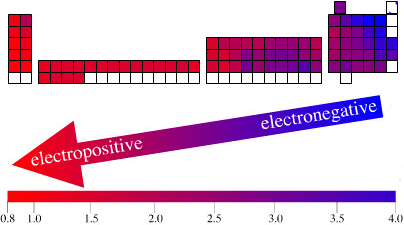
The long form and medium form PTs have electronegativity trending from top-right (electronegative) to bottom left (electropositive), and many aspects of periodicity corollate with electronegativity: atomic radius, first ionisation energy, etc.
Thus, the long form and medium form periodic tables are commonly used in the classroom:
An Alternative Formulation
The internet database contains many, many alternative formulations, and these are often spiral and/or three dimensional. These exemplified by the 1965 Alexander DeskTopper Arrangement. To see the variety of formulations available, check out the Spiral & Helical and 3-Dimensional formulations in the database:

Non-Chemistry PTs
The periodic table as a motif is a useful and commonly used infographic template for arranging many types of object with, from 50 to 150 members.
There are numerous examples in the Non-Chemistry section where dozens of completely random representations can be found:
- Adobe Illustrator Shortcuts
- Adult Positions
- Airline Customer Reviews
- Beer Styles
- And, chosen more or less at random, European Nations:
| Year: 2013 | PT id = 613, Type = formulation |
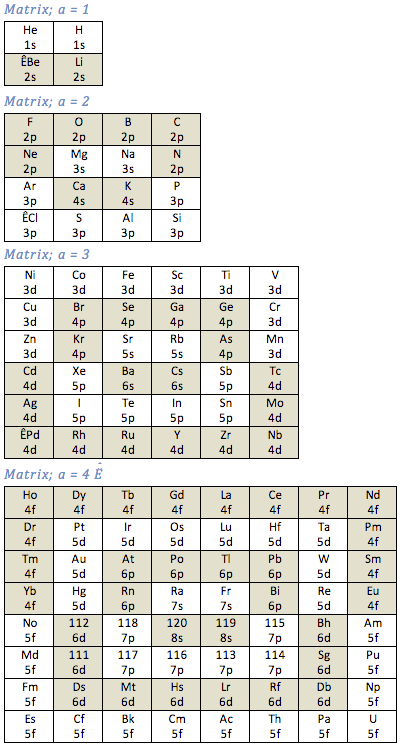
Matrix Series Periodic Table
By Richard Kingstone, a Matrix Series Periodic Table. Read more here.
The Janet Periodic Table (aka Left Step Table) may be re-arranged as a series of square matrices. Each element is represented as a cell and is identified by the atomic number (Z), shown as the upper number of each cell.
The quantum numbers (n, l, mL, mS) determine the location of an element within the table. The quantum pair (n, l ) are the lower numbers in each cell. Four matrices are required, each matrix is identified by a 'matrix number' (a) as shown below;:
| Year: 2014 | PT id = 638, Type = formulation |
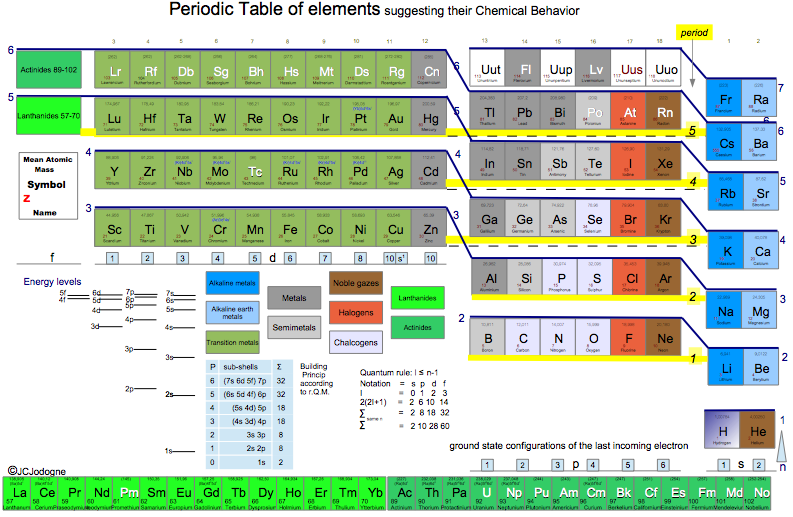
Jodogne's Janet New Color Periodic Table
By J.C. Jodogne, "a Janet type slightly modified to enhance shells, periods and to make determination of ground state configuration (orbitals) very easily to build". Click here to get the full size pdf.
| Year: 2014 | PT id = 642, Type = formulation 3D |
ADOMAH Periodic Table Glass Cube
Valery Tsimmerman, of the ADOMAH Periodic Table and the ADOMAH Tetrahedron, has now used these ideas to produce a beautiful glass cube:

This amazing object is available for sale from Grand Illusions:
A Note by Philip Stewart stewart.phi@gmail.com
The cube represents 120 chemical elements etched into a cube of Optical Crystal glass. The s, p, d, and f blocks of the Janet periodic table form four rectangles, which are slices of a regular tetrahedron, parallel with two of its edges and with two faces of the circumscribed cube. All four quantum numbers are made visible in this arrangement. You can see a 2-D version on the Perfect Periodic Table website, click on the "skyscraper" version on the right to see the tetrahedron, and go to Regular Tetrahedron at foot of page for details.
The regular tetrahedron is the only form in which slices are rectangles of different shape and identical perimeter. When each orbital is represented by a square of unit edge, the rectangles representing the blocks all have the same perimeter, which is twice the length of the edges of the tetrahedron (which are of course √2 times the edges of the cube): 18 units = 2(values of n + values of ml).
Block |
values of n |
values of ml |
s |
8 |
1 |
p |
6 |
3 |
d |
4 |
5 |
f |
2 |
7 |
Valery Tsimmerman, orahct@gmail.com, creator of the design, has written to me as follows:
"I just had some thoughts about the Perimeter Rule that is at the basis of the tetrahedral arrangement. Dimensions of the blocks are dictated by number of values of ml and number of values of n. We know that n governs quantization of energy. Recently I learned that quantization of the possible orientations of L with respect to an external magnetic field is often referred to as space quantization. (Serway, Jewett: Physics for Scientists and Engineers. 6th edition. p.1369).
"That is, ml stands for space quantization. Therefore, the Perimeter Rule reflects a direct relationship between energy and space. I think that this could have some significance. The beautiful thing about the Universe is that each type of symmetry is related to some conservation law. Symmetry in time is related to energy. Therefore, n is related to time also, so, in the Perimeter Rule we have relationship between time and space on quantum numerical level. The interesting thing is that ml can be positive and negative, while n can only be positive. Similarly, things can move in space in positive and negative directions, but time has only one direction. There is no negative time, just as there are no negative values of quantum number n."
Adomah is a variant of Adamah, Hebrew for 'dust of the earth', from which Adam was made (Genesis 2:7).
| Year: 2014 | PT id = 664, Type = formulation |
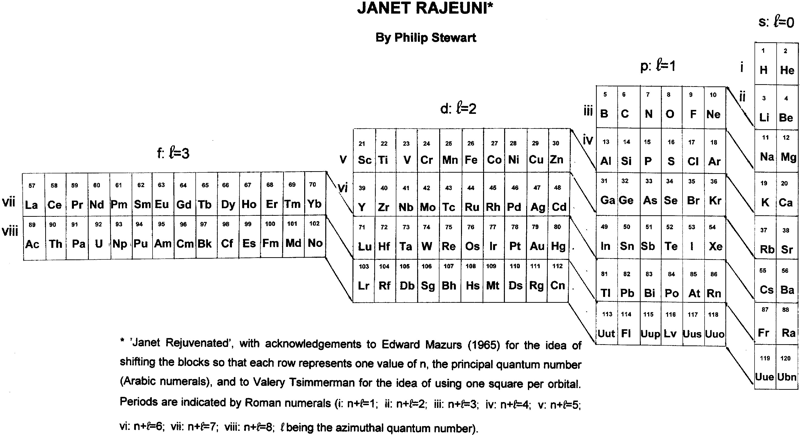
Janet Rajeuni
By Philip Stewart:
Janet Rejuvenated, with acknowledgement to Mazurs and to Valery Tsimmerman for the idea of using one square per orbital and of shifting the blocks so that each row represents one value of n, the principal quantum number.
The main objection people make to Janet is that He is placed at the head of the alkaline earth metals although it behaves as a noble gas. The essential answer is that electronic structure explains behaviour and not vice versa; like Ne (and unlike Ar, Kr, Xe and Rn), He has a complete shell. Similarly H, like C, is half way between a full and an empty shell, unlike the alkali metals and the halogens. I suggest a new argument: nobody finds it strange that the p block has a row of non-metals at its head (and that half its members are non-metals), so why not the s block?
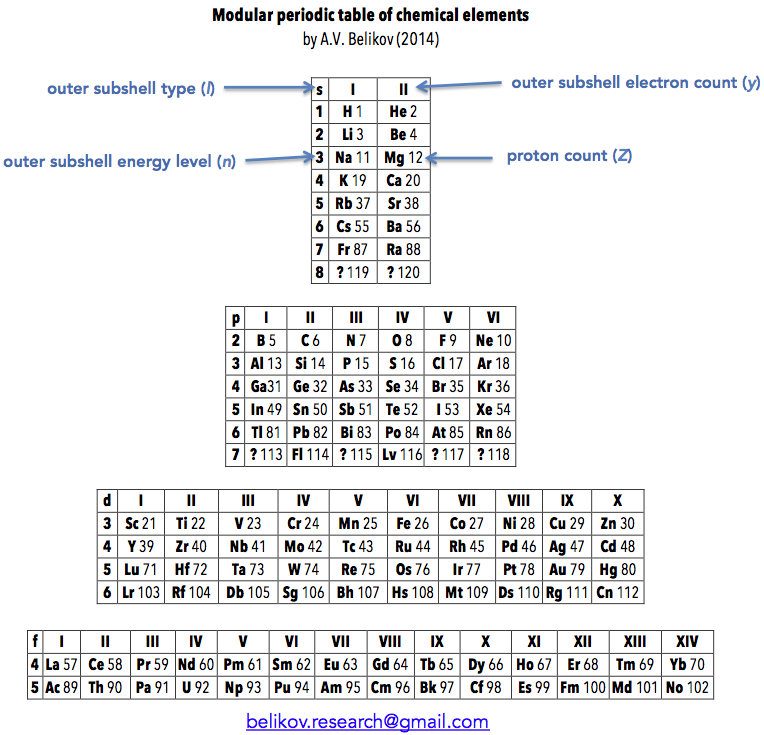
| Year: 2014 | PT id = 680, Type = formulation |
Belikov's Modular Periodic Table of Chemical Elements
"I call this version of the Modular Periodic Table of Chemical Elements. I got the idea for it some time between 2005 and 2007, during the chemistry course at my university, in attempt to rationalize the clumsy common version I was being taught. I showed it to my chemistry teacher, but he didn't seem to be impressed much, so it went into the drawer. Recently I decided to resurrect it and publish somewhere. So I had a look on the web and found your excellent database, with hundreds of versions. After the first shock, I realized that only few are actually similar to my version. These are well known Janet's table and ADOMAH table. So, it appeared to me that the idea to group elements strictly according to filling of their atomic shells is not new. However, the way I have done it is slightly different from the mentioned tables. For example, s,p,d and f blocks of elements are completely autonomous and can be placed wherever desired (hence the name 'Modular'). This reflects the notion that there is little in common in chemical behavior between the elements in different blocks. Also, outer subshell type, energy level and electron count are clearly labeled, so that these parameters can be quickly determined for each element.
"Overall, I think that this version of periodic table allows easier understanding and transition from IUPAC table and could be implemented in school and university textbooks."
Aleksey Belikov
| Year: 2014 | PT id = 681, Type = formulation |
Jodogne's Janet New Colour Periodic Table
"This Periodic Table(click here for larger version) incorporates the Real Aufbau of Professor Pyykkö based on relativistic Quantum Mechanics, with Z continuity horizontally and vertically, by means of taking into account the ground level - energy increase upward - of the last incoming electron (the lower side of the element case is the level guide mark). A large yellow line indicates period. A gradual color for H induces a manifold chemical behavior."
J.C.Jodogne
| Year: 2016 | PT id = 724, Type = formulation |
NAWA's byobu-Janet Periodic Table
NAWA, Nagayasu: A Japanese schoolteacher and periodic table designer presents a Janet form periodic table in the traditional Japanese "byobu" style:
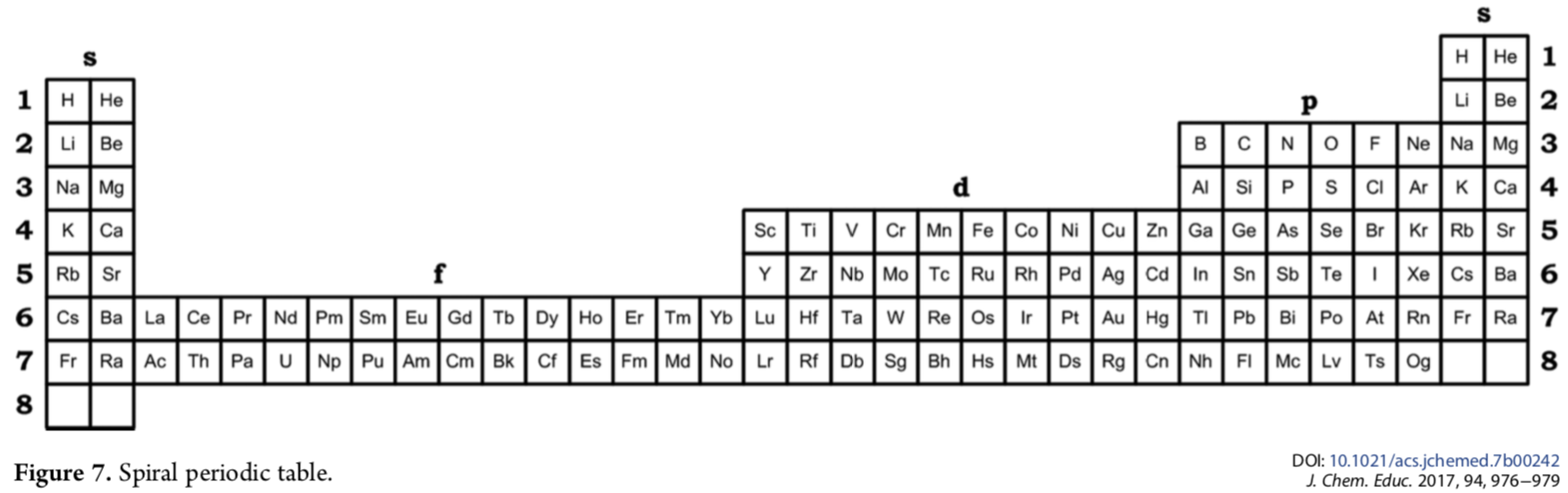
| Year: 2017 | PT id = 917, Type = formulation 3d spiral |
Kurushkin's Spiral Periodic Table
Mikhail Kurushkin has a way of constructing the standard long form periodic table from the Janet Left-Step formulation.
Mikhail writes in his J.Chem.Educ paper DOI: 10.1021/acs.jchemed.7b00242; J. Chem. Educ. 2017, 94, 976?979
"Addition of another s-block to the left of the left-step periodic table [enables it] to be rolled into a spiral so that the left and right s-blocks are merged together and the number of elements is exactly 118. The resulting periodic table is called the "spiral" periodic table, which is the fundamental representation of periodicity":
| Year: 2017 | PT id = 761, Type = formulation 3D |
Stewart's Chemosphere
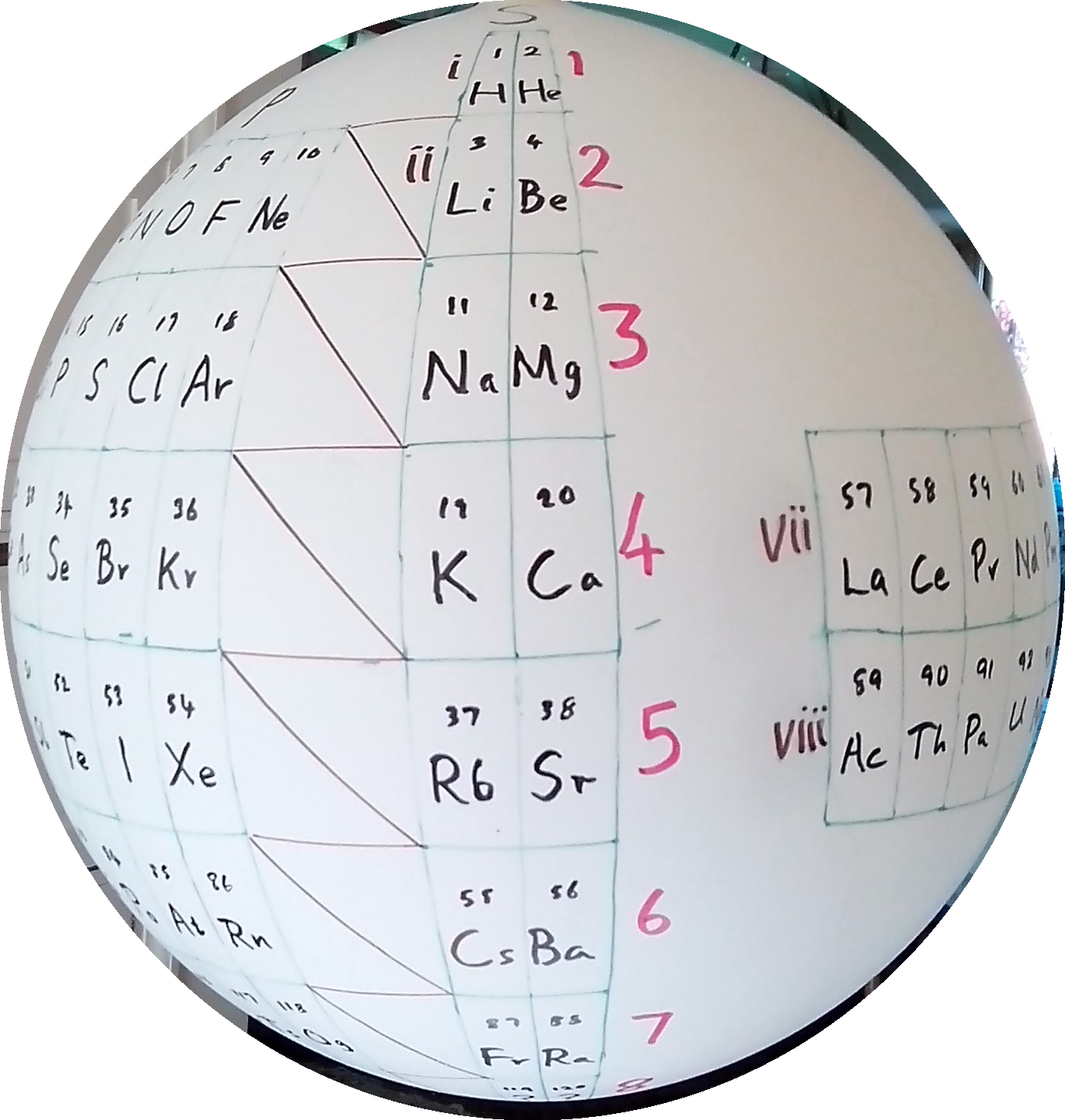
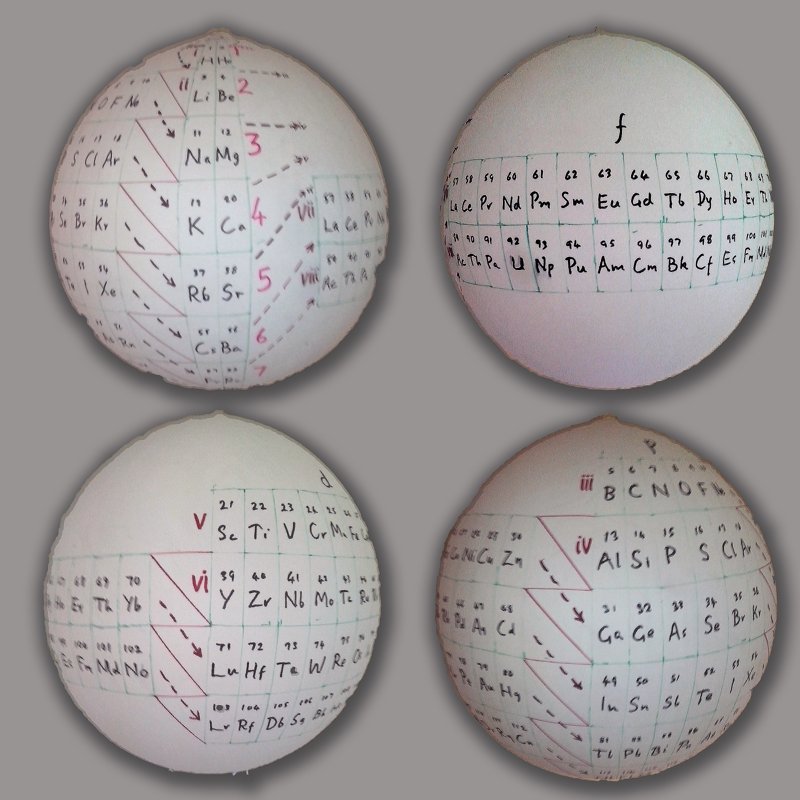
P J Stewart, a good friend of the periodic table database, has mapped a PT onto a sphere.
PJS writes: "It is Janet Rajeuni 2014 wrapped round a sphere, going back to Mazurs 1965, and Tsimmerman 2006. Arabic numerals indicate shells (values of principal quantum number); Roman numerals indicate periods."
| Year: 2018 | PT id = 774, Type = formulation data |
First Ionisation Energy to the Standard Form Periodic Table
There is debate amongst the cognoscenti about the 'best' representation of the periodic table, and how this 'best' formulation can be explained by [rationalized by] quantum mechanics (QM).
Many feel that the Janet PT formulation, the 'Left Step', is the ideal QM PT, but this formulation does not show periodicity very well, and there are issues with the placement of H, He, Be which spill over into questions about their placement in the standard form PT (the periodic table used in classrooms and textbooks around the world).
However, it is possible to get to the conventional standard form PT directly from the first ionisation energy data, where the 1st ionisation energy is the energy required to convert a gas phase atom (M) into its gas phase positive ion plus electron.
M(g) → M+(g) + e–
The process involves:
- taking the 1st ionisation data plot for the elements H to Xe (Z = 1 to 36)
- rotate 90° clockwise and stretch
- move the atoms horizontally into columns
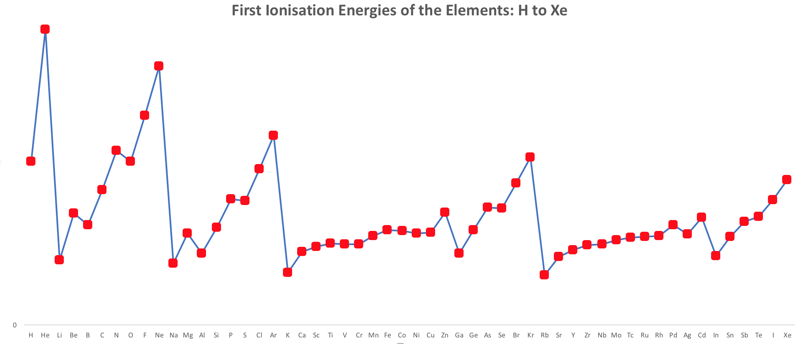
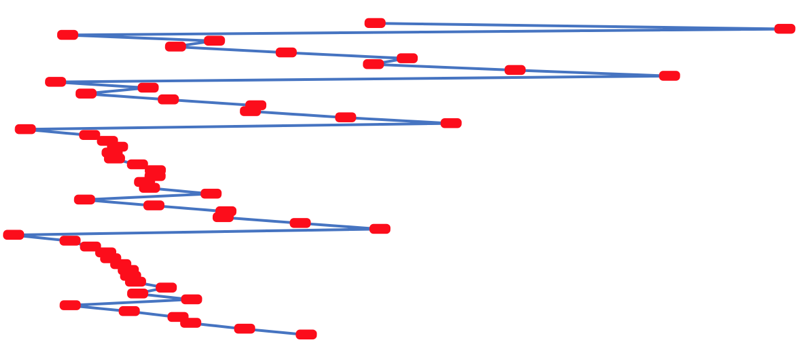
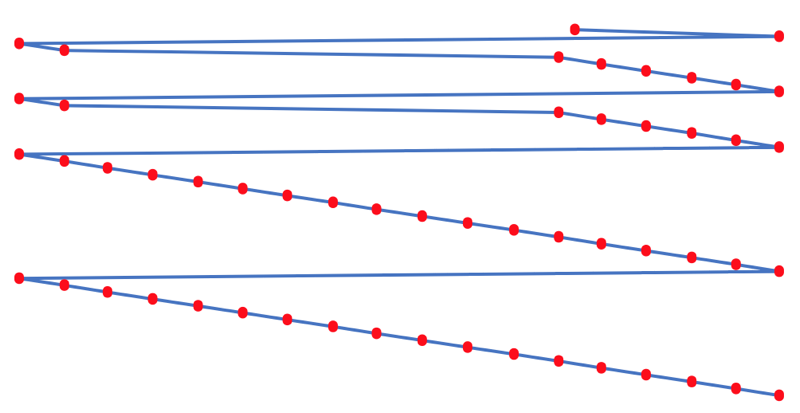
Note that a similar logic can be applied to atomic radius and electronegativity data.
However, there are issues about the measurement of atomic radius, because atoms are 'soft at their edges', and gas phase atomic radius is not precisely defined. And, electronegativity is a derived parameter.
By Mark Leach
| Year: 2018 | PT id = 779, Type = formulation 3D |
Stowe-Janet-Scerri Periodic Table (Extended)
Stowe's A Physicist's Periodic Table was published in 1989, and is a famous & well respected formulation of the periodic table.
Since 1989 quite a number of elements have been discovered and Jeries A. Rihani has produced an updated and extended version in 2017. This has been further updated, below. Click for the full size .pdf version:
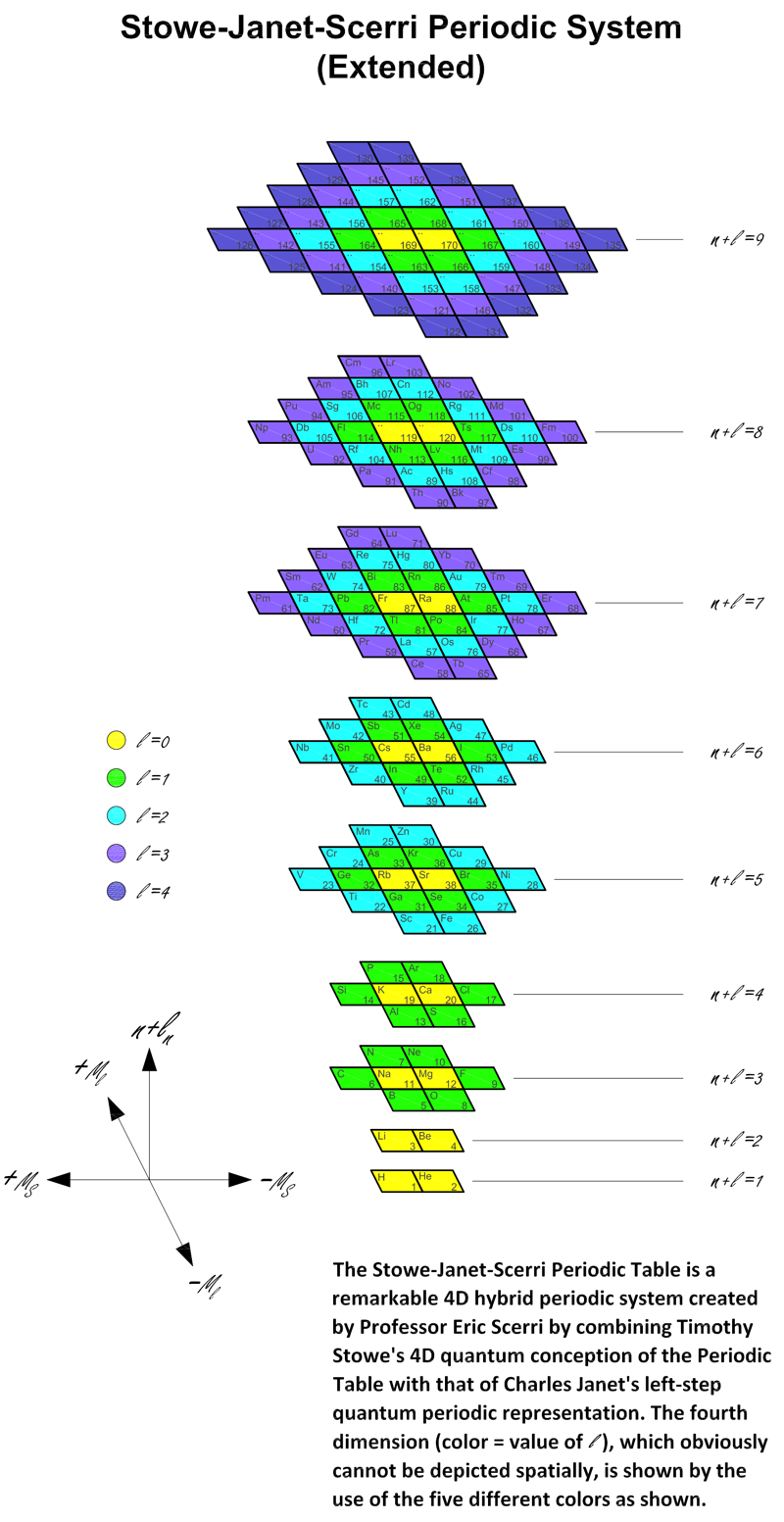
| Year: 2018 | PT id = 920, Type = formulation 3d |
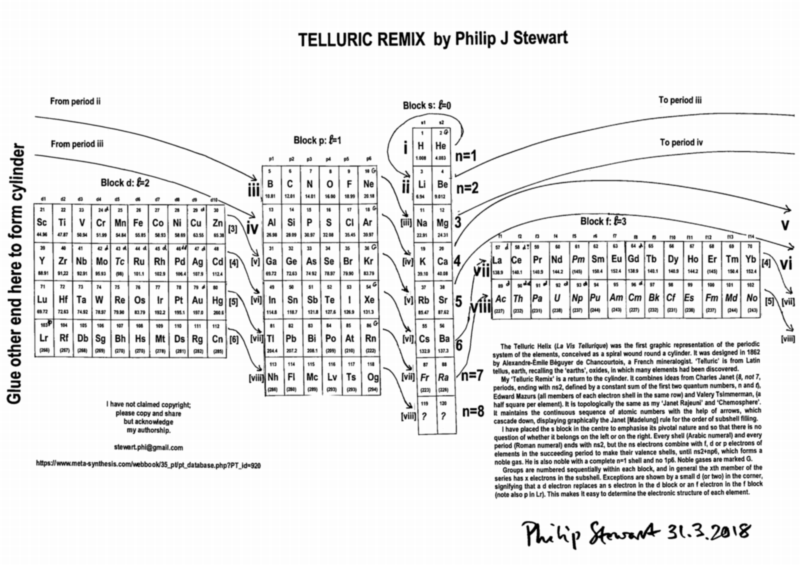
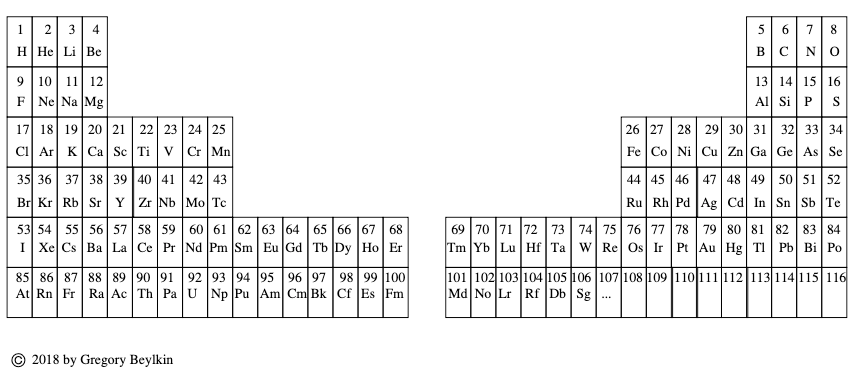
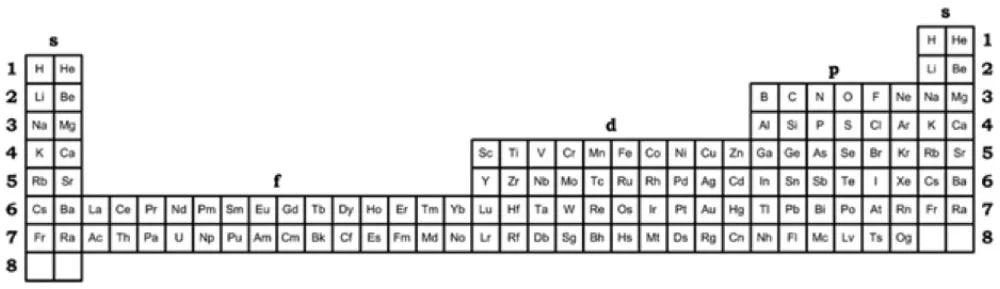
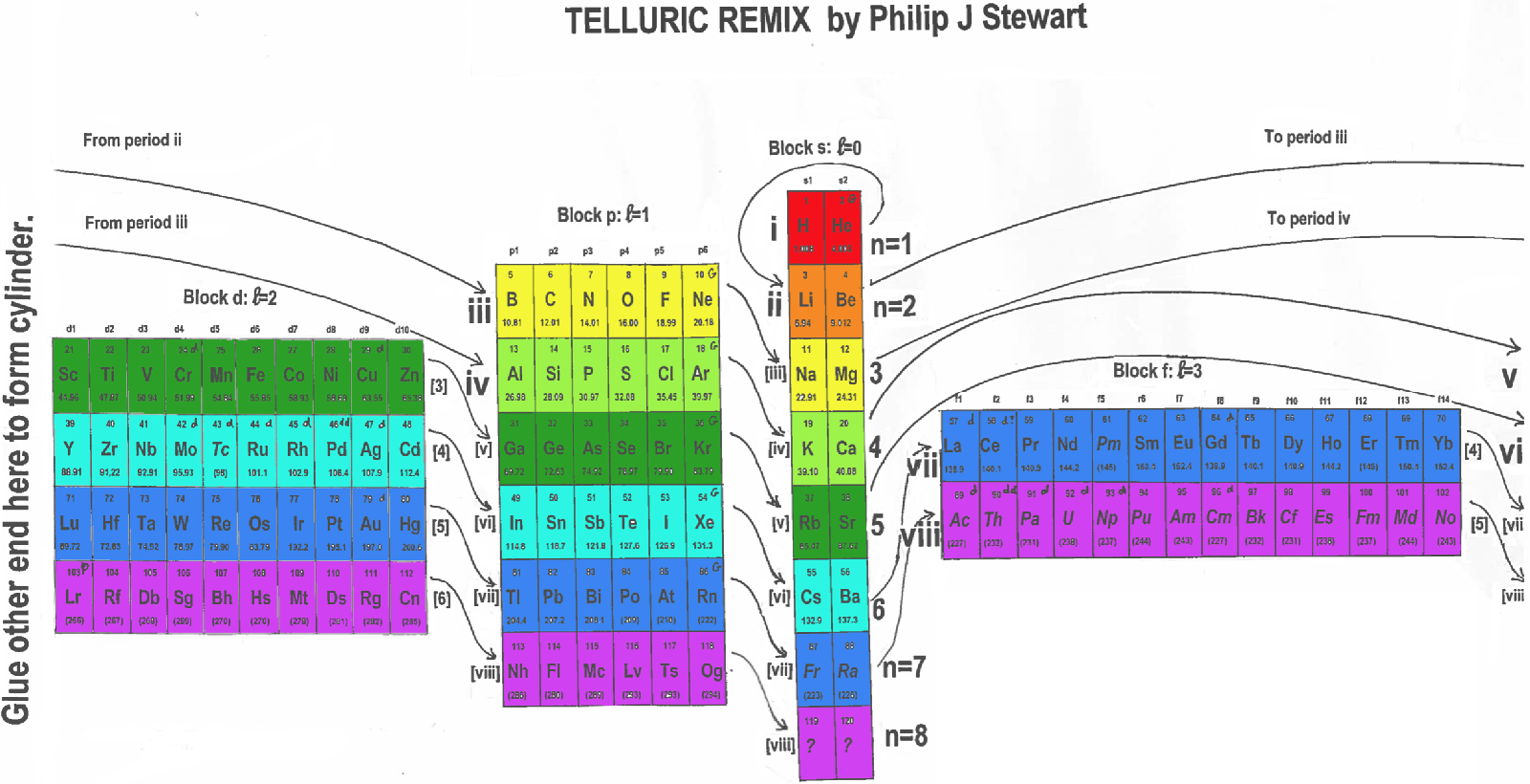
Telluric Remix
Philip Stewart writes:
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version.
The printable version is available (click here for the full size version) to make your own:
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2018 | PT id = 936, Type = formulation 3D |
Sistema Periódico Binodico
By Julio Antonio Gutiérrez Samanez, who writes:
"Sistema Periódico Binodico. Nuevo Paradigma Matematizado. I have followed the work of the wise Mendeleev, of Emil de Chancourtois, of Charles Janet; inspired by the work of my countryman Dr. Oswaldo Baca Mendoza. It is in Spanish but soon I will have the English version."
| Year: 2018 | PT id = 1202, Type = formulation |
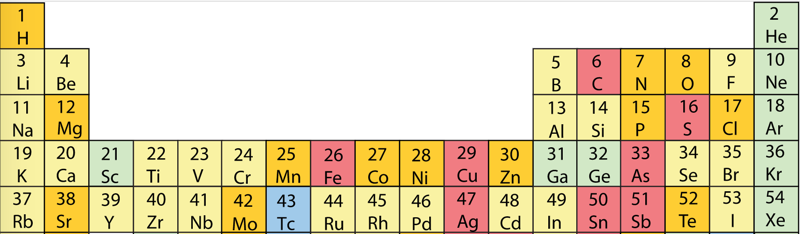
Beylkin's Periodic Table of The Elements
René Vernon writes: Beylkin's Periodic Table of The Elements has 4n2 periods, where n = 2,3..., and shows symmetry, regularity, and elegance, more so than Janet's left step table.
Beylkin (an applied mathematician) writes:
"Let us take a continuous strip of paper and, on one side of the strip, write all the elements in the order of their atomic numbers. We then form a spiral with the strip such that the two most chemically distinct groups, the group of halogens (in which we include hydrogen) and the group of noble gases, are properly aligned. By flattening the strip on a plane and folding it in the middle, we obtain the new periodic table..."
Other features:
- H is over F, which is a smoother fit in terms of physicochemical trends down the group
- He is over Ne, which is a smoother fit etc
- group 3 has lanthanum in it
- the modern relationships Ti-Zr-Hf, V-Nb-Ta, Cr-Mo-W, and Mn-Tc-Re can still be traced
- the lanthanides and actinides are integrated into the main body of the table
- 15 lanthanides and 15 actinides(!)
- the old school arrangement of B-Al-Sc-Y-La can still be traced, as can the less smooth alternative B-Al-Sc-Y-Lu
- the 1s "block" starts at H; the s block proper at Li; p at B; d at Sc; f at Ce
There are four new(ish) groups: Ti-Zr-Ce-Th, V-Nb-Pr-Pa, Cr-Mo-Nd-U and Mn-Tc-Pm-Np. For the actinide elements of these groups, the resemblance of the earlier actinides to their lighter transition metal congeners is well known. For the lanthanide elements, Johansson et al. (2014) wrote a nice article about Ce and its cross-road position. For Pr, Nd, and Pm, all of these are known in multiple oxidations states (+2, +3, +4 excl. Pm, and +5 for Pr only), just as the transitions metals are so known.
- Beylkin G 2018, The periodic table of the elements with 4n2 n = 2,3... periods, https://arxiv.org/pdf/1901.02337.pdf
- Eric 2006, https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=20
- Johansson, B., Luo, W., Li, S. et al. 2014, Cerium; crystal structure and position in the periodic table. Sci Rep 4, 6398. https://doi.org/10.1038/srep06398
- Gregory Beylkin: https://en.wikipedia.org/wiki/Gregory_Beylkin
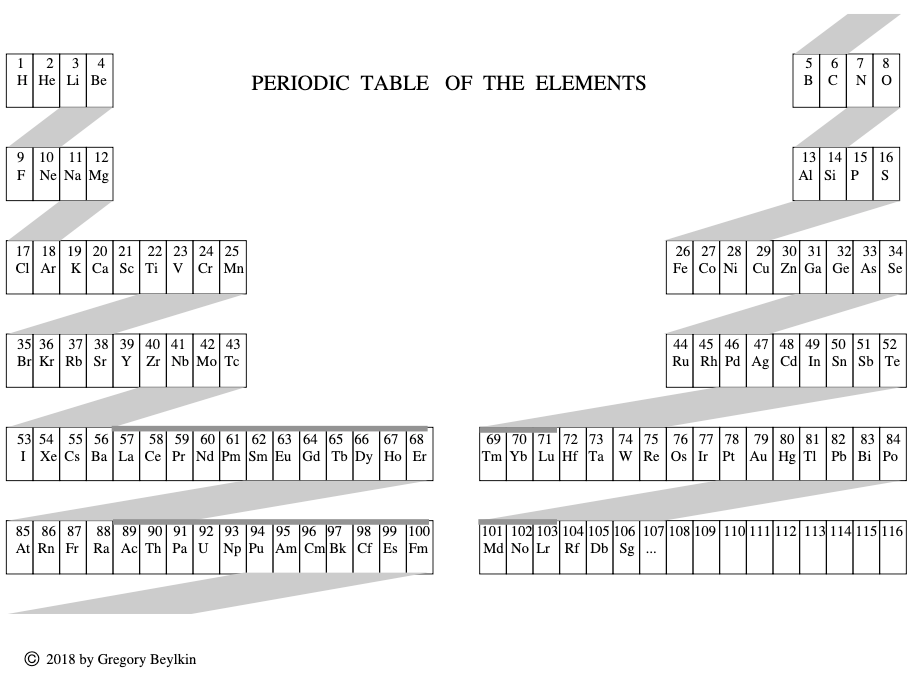
| Year: 2018 | PT id = 951, Type = formulation |
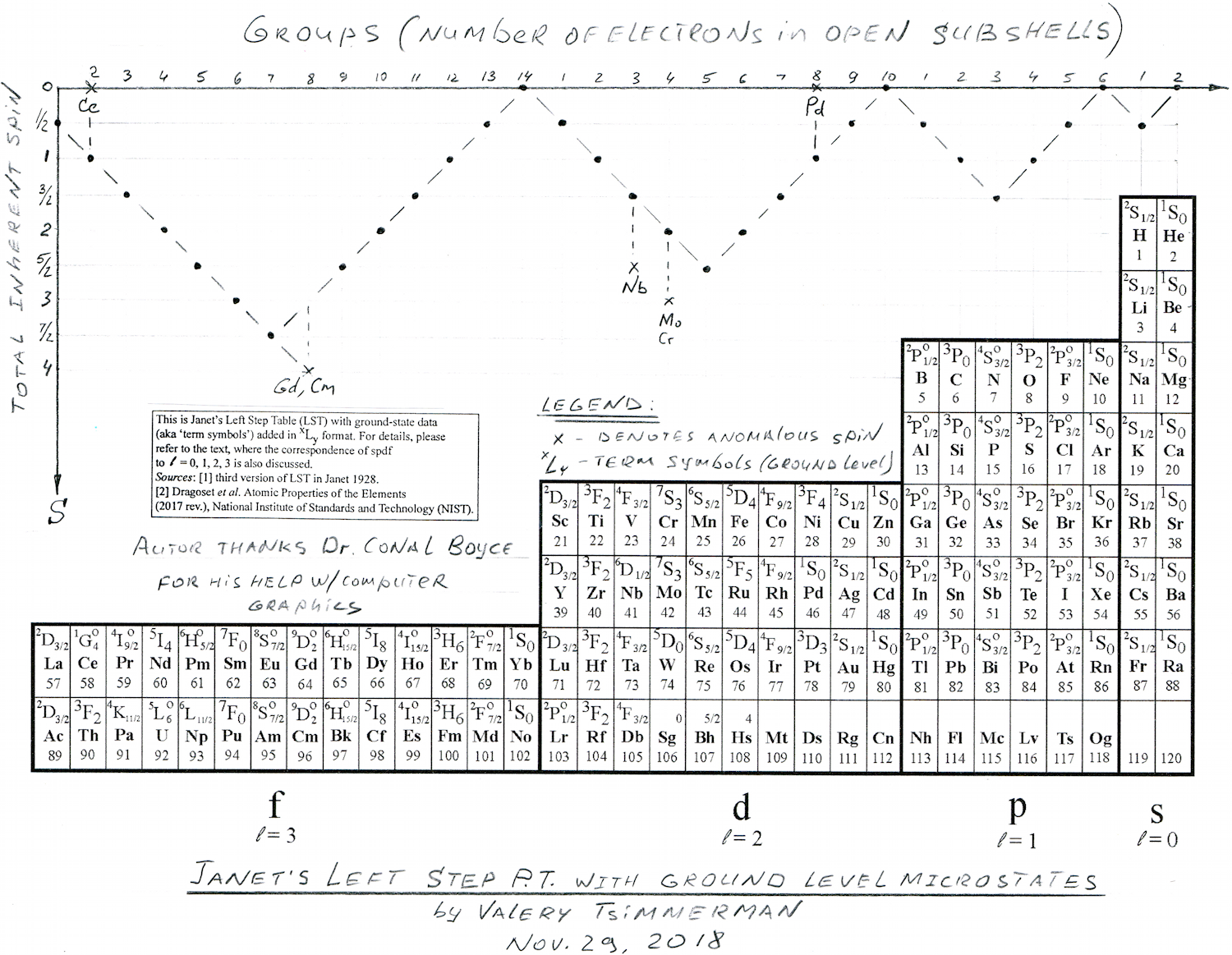
Janet's Left-Step with Ground Level Microstates
By Valery Tsimmerman, who writes:
Janet's LST with ground level microstate information and total spin graph shown for each group of elements. The top line represents number of electrons in open sub-shells (with exception of six anomalous elements). Information shows physical (spectroscopic) basis of the groups.
The zigzag line on top is a graphic representation of Hund's rule showing the total inherent spins of atoms and the total spin of Cu is 1/2, same as for Ag and Au. When it comes to ground level atomic microstates and Hund's rule Cu is not anomalous (2S1/2), despite its anomalous electron configuration.
The diagram represents Hund's Rule that states that "the lowest energy atomic state is the one that maximizes the total spin quantum number for the electrons in the open subshell" (Wikipedia). Y-axis is the total spin and x-axis is number of electrons in open shells (with exception of six anomalous elements).
First, I would like to make couple of general comments. When discussing periodicity, they typically talk about chemical properties and electron configurations/differentiating electrons, etc, but those are not specific enough. For each electron configuration there are multiple microstates. For example, for single electron configuration of carbon there are over 30 microstates and only one of them corresponds to ground level. So, microstates express combined physical/spectroscopic properties of whole atoms and, the most important, combined properties of electrons located in open subshells.
Now, look at ground level term symbols in each group. I see amazing consistency, especially in the main groups. It tells me that groups are not only chemical, but physical!
Looking at periods one can see that all periods in s, p & d blocks begin with elements that have multiplicity M=2 and end with M=1. This is also true for f-block if it starts with La and Ac and ends with Yb and No. This puts Lu and Lr firmly in group 3. Placing La and Ac in group three ruins spectroscopic consistency.
Click here image to enlarge the PT below.
| Year: 2018 | PT id = 1261, Type = formulation |
Kurushkin's 32-Column Periodic Table & Left-step Periodic Table United
Dr Mikhail V. Kurushkin, 32-column Periodic Table & Left-step Periodic Table United: https://bernalinstitute.com/events/bernal-seminar-by-dr-mikhail-v-kurushkin-itmo-universityrussia/
ABSTRACT
The pursuit of optimal representation of the Periodic Table has been a central topic of interest for chemists, physicists, philosophers and historians of science for decades (Leigh, 2009; Scerri, 2009). Should the Periodic Table of Chemical Elements first and foremost serve the needs of chemists as implied by its name? Or should it start from considerations of before quantum mechanics and thus be more appealing to physicists (Scerri, 2010, 2012b)? Is there a representation which overcomes this problem? The Periodic Table is from a fundamental point of view a graphic representation of periodicity as a phenomenon of nature. While the 32-column Periodic Table, popularized by Glenn T. Seaborg, is considered by chemists the most scientifically correct representation (Scerri, 2012a), physicists apparently prefer the Left-step Periodic Table above all (Scerri, 2005; Stewart, 2010). Alternatively, it is suggested that a rigorously fundamental representation of periodicity could only take the form of a spiral as, evidently, the abrupt periods of 2-D Periodic Tables contradict the gradual increase of atomic number, and the spiral representation reconciles this debate (Imyanitov, 2016). An optimal representation is eagerly sought after both for the needs of philosophy of chemistry and chemical education as their never-ending dialogue secures a thorough methodology of chemistry. The aim of the present work is to show that the 32-column Periodic Table and the Left-step Periodic Table can co-exist in mutual tolerance in a form of what Philip Stewart has already called Kurushkin’s Periodic Table (Kurushkin, 2017), Figure 1 below.
René Vernon writes:
"Kurushkin reminds us that the Janet left step table (with Sc-Y-Lu-Lr, and He over Be), and the version of the table with the s-elements on the right (also with Sc-Y-Lu-Lr, and He over Be) are interchangeable.
"For an earlier paywall version which includes a short video see:
Kurushkin M 2018, Building the periodic table based on the atomic structure, Journal of Chemical Education, vol. 94, no. 7, pp. 976–979, https://pubs.acs.org/doi/10.1021/acs.jchemed.7b00242
"Kurushkin’s interchangeable approach extends to tables with group 3 as either Sc-Y-La-Ac or Sc-Y-Lu-Lr. See Vernon's Yin Yang of The Periodic Table https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=1252"
| Year: 2019 | PT id = 1050, Type = formulation |
Janet Rejuvenated: Stewart-Tsimmerman-Nawa
An updated version of Philip Stewart's Janet Rejuvenated by Valery Tsimmerman redrawn by Nawa.
| Year: 2019 | PT id = 1077, Type = formulation 3D spiral |
Weise's Tetrahedral Periodic Table
A Facebook video by Dmitry Weise showing how the conventional periodic table can be morphed into a tetrahedral formulation via the Janet Left Step:
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2019 | PT id = 984, Type = formulation 3D spiral |
Telluric Remix in Colour
Philip Stewart writes (this is the same text that accompanies the 2018 B/W version):
The Telluric Helix (La Vis Tellurique) was the first graphic representation of the periodic system of the elements, conceived as a spiral wound round a cylinder. It was designed in 1862 by Alexandre-Émile Béguyer de Chancourtois, a French mineralogist. 'Telluric' is from Latin tellus, earth, recalling the 'earths', oxides, in which many elements had been discovered.
My 'Telluric Remix' is a return to the cylinder. It combines ideas from Charles Janet (8, not 7, periods, ending with ns2, defined by a constant sum of the first two quantum numbers, n and l), Edward Mazurs (all members of each electron shell in the same row) and Valery Tsimmerman, (a half square per element).
- The Telluric Remix is topologically the same as my 'Janet Rajeuni' and 'Chemosphere': it maintains the continuous sequence of atomic numbers with the help of arrows, which cascade down, displaying graphically the Janet [Madelung] rule for the order of subshell filling.
- I have placed the s block in the centre to emphasise its pivotal nature and so that there is no question of whether it belongs on the left or on the right. Every shell (Arabic numeral) and every period (Roman numeral) ends with ns2, but the ns electrons combine with f, d or p electrons of elements in the succeeding period to make their valence shells, until ns2+np6, which forms a noble gas. Helium, He, is also noble with a complete n=1 shell and no 1p6.
- Noble gases are marked G. Groups are numbered sequentially within each block, and in general the xth member of the series has x electrons in the subshell. Exceptions are shown by a small d (or two) in the corner, signifying that a d electron replaces an s electron in the d block or an f electron in the f block (note also p in Lr). This makes it easy to determine the electronic structure of each element.
- Click here for a larger version (pdf).
I have not claimed copyright; please copy and share but acknowledge my authorship. stewart.phi@gmail.com
| Year: 2019 | PT id = 1001, Type = review |
Kultovoy's Periodic Table Book
Nicolay Kultovoy, website, as sent me a copy of his Periodic Table book, entitled [Google Translate]: Book 5. Part 11-08. A single quantum mechanical model of the structure of the atomic nucleus and the periodic table of chemical elements of D.I. Mendeleev.
In a mixture of Russian & English, the PDF of the book can be viewed here.
Chapter 1. Triune (electrons, nucleons, chemical elements) quantum mechanical model of Colt. Three
1.1 the Rules of filling of the orbits of electrons.
1.2 Pyramidal lattice.
1.3 models with cubic sieve.
1.4 models with face-centered lattice.
1.5 quantum Mechanical form of the periodic table of chemical elements.
1.6 Stowe-Janet-Scerri Periodic Table.
Chapter 2. A lattice model of the nucleus. Model 62
2.1 Berezovsky G. N.
2.2 I. Boldov
2.4 Konovalov.
2.5 Manturov V.
2.6 Semikov S. A.
2.7 alpha-partial model of the atomic nucleus.
2.8 Burtaev V.
Chapter 3. Various lattice (crystal) model of the nucleus of an atom. One hundred five
3.0 Luis Pauling.
3.1 Valery Tsimmerman. ADOMAH Periodic Table. Model 3-2.
3.2 Klishev B. V. Model 3-1.
3.3 Garai J. Model 3-1.
3.4 Winger E Model 4-2.
3.5 Norman D. Cook. Model 4-1.
3.6 Gamal A. Nasser. Model 4-1.
3.7 D. Asanbaeva Model 4-1.
3.8 Datsuk V. K.
3.9 Bolotov B.
3.10 Djibladze M. I.
3.11 Dyukin S. V.
3.12 A. N. Mishin.
3.13 M. M. Protodyakonov
3.14 Dry I. N.
3.15 Ulf-G. Meißner.
3.16 Foreign works.
Chapter 4. Long-period periodic table. One hundred eighty one
4.1 long-Period representation of the periodic table.
4.2 Artamonov, G. N.
4.3 Galiulin R. V.
4.4 E. K. Spirin
4.5. Khoroshavin L.
4.6 Step form proposed by Thomsen and Bohr.
4.7 Symmetrical shape of the periodic table.
Chapter 5. Construction of a periodic table based on the structure of orbitals. Two hundred twenty one
5.1 construction of the periodic table on the basis of orbitals.
5.2 Short V. M.
5.3 Kulakov, the Novosibirsk table of multiplets.
Chapter 6. Atomic structure. Two hundred forty eight
6.1 Table of isotopes.
6.2 the structure of the orbitals.
| Year: 2020 | PT id = 1162, Type = formulation data review |
Workshop on Teaching 3d-4s Orbitals Presented by Dr. Eric Scerri
Dr. Eric Scerri, UCLA Department of Chemistry & Biochemistry, discusses many of the issues concerning the periodic table: the aufbau principle, Madelung's rule, the electronic and anomalous electronic structures of the transition elements, the Sc2+ ion, the Janet Left Step, Group 3: Sc, Y, Lu, Lr vs. Sc, Y, La, Ac, atomic spectroscopy, etc.
Many of the topics that concern those of us interested in the periodic table are discussed.
Thanks to Eric Scerri – who appears – for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2020 | PT id = 1170, Type = formulation |
Zig-Zag Line, Periodic Table
Periodic Table showing the (regular) zig-zag line by René Vernon who writes:
"It is curious that the full extent of the line has never been properly mapped (to my knowledge).
"Elements on the downside of the line generally display increasing metallic behaviour; elements on the topside generally display increasing nonmetallic behaviour.
"When you see the line you will usually see only about a quarter of it. The line actually runs all the way across the periodic table, as shown, for a total of 44 element box sides.
"Interpretations vary as to where the line runs. None of these is better than any other of them, provided the interpretation is explained to you. The thick black line (at least in the p-block) is the most common version. The metalloids tend to lie to either side of it.
"Polonium and astatine are shown here as post-transition metals although either or both of them are sometimes shown as metalloids (or, in the case of astatine, as a halogen). Polonium conducts electricity like a metal and forms a cation in aqueous solution. In 2013, astatine was predicted to be a centred cubic-metal Condensed Astatine: Monatomic and Metallic This prediction has been cited 35 times, with no dissenters. Astatine also forms a cation in aqueous solution. Oganesson is shown as having (as yet) unknown properties.
"The dashed lines show some alternative paths for the zigzag line.
"The lower one treats the metalloids as nonmetals since metalloid chemistry is predominately nonmetallic. The lower line and the upper line are sometimes shown together used when the metalloids are treated as neither metals nor nonmetals."
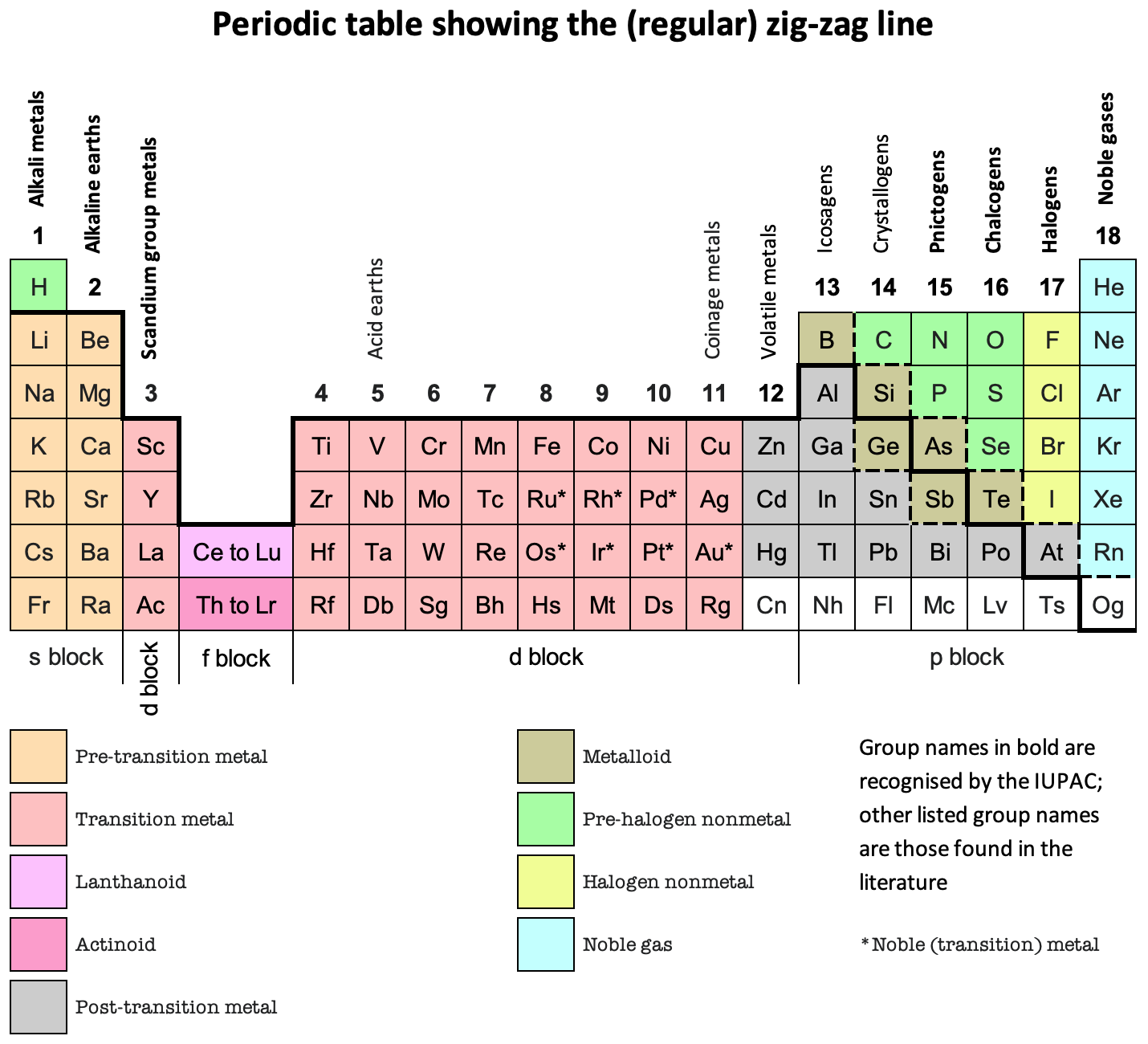
And in Janet Left-Step form:
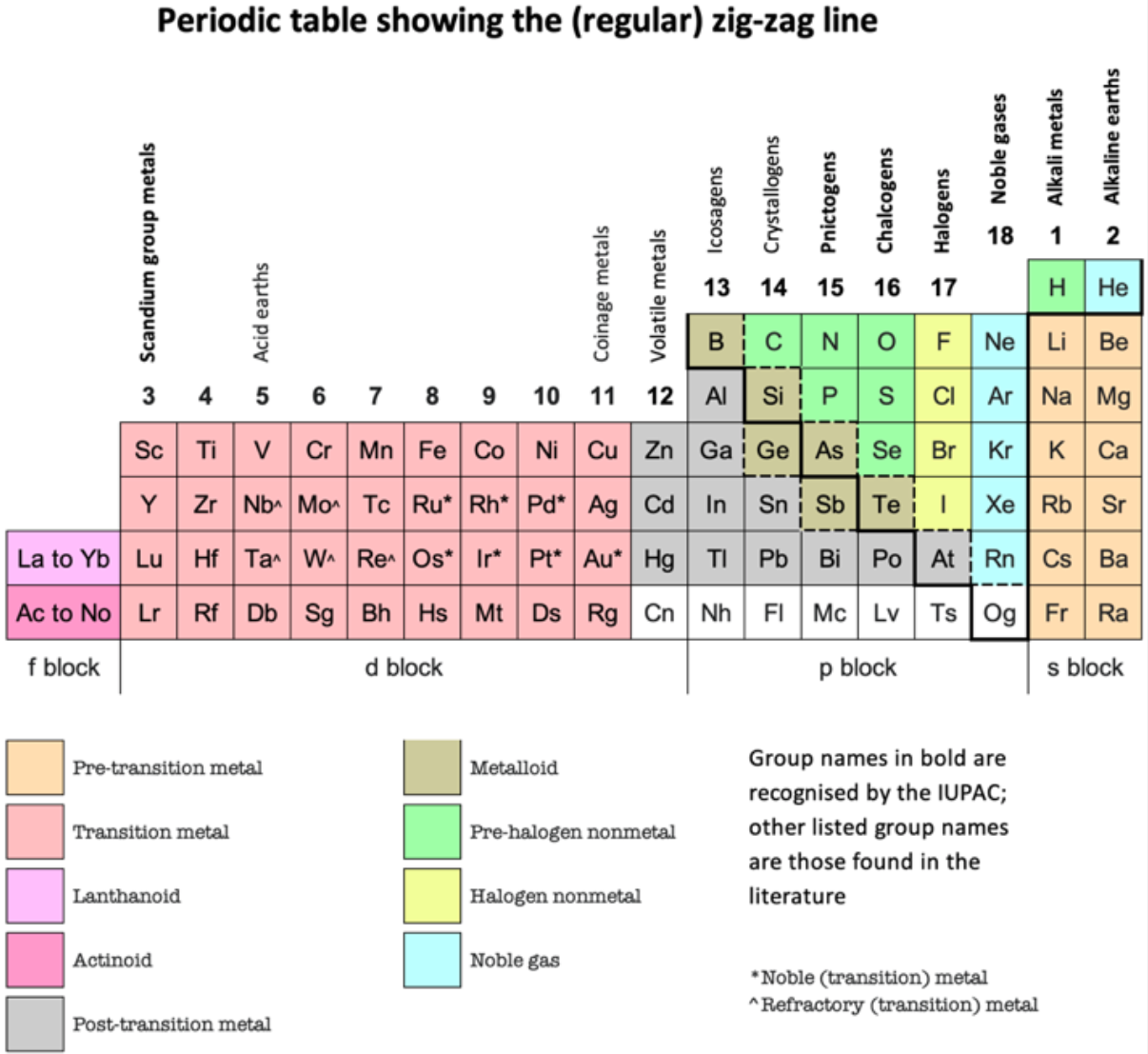
| Year: 2021 | PT id = 1184, Type = formulation |
van Spronsen's Periodic Table: Update
René Vernon writes:
I'd never before realised how clever van Spronsen's 1969 Periodic Table is. It seems to be the ultimate logical electronic version, informed by the actual filling sequence in the gas phase atoms, rather than the idealised sequence.
So, H-He are over Li-Be.
Group 3 is Sc-Y-La-Ac since that is where the d-shell starts filling. In the rest of the d-block, there are (4+1) x d5 and (4+2) x d10.
The f-block starts with Ce, as that is where the f-shell starts filling. Notice the high degree of regularity with the 4 x f7 and the 4 x f14, and how Th is treated i.e. as 5f0.
After DIM's 8-column form, I believe the periodic family tree now looks like this:
Three split-blocks
1a. He over Ne; B-Al over Sc-Y-La-Ac = old school form
1b. H-He over F-Ne; ditto = e.g. Soddy 1914?, Kipp 1942?Two split blocks
2a. He over Ne; group 3 as Sc-Y-La-Ac = popular formOne split-block
3a. He over Ne; group 3 as Sc-Y-Lu-Lr = Lu form 3b. He over Be; group 3 as Sc-Y-La-La = forgotten van Spronsen formNo split blocks
4. He over Be = Janet equivalent
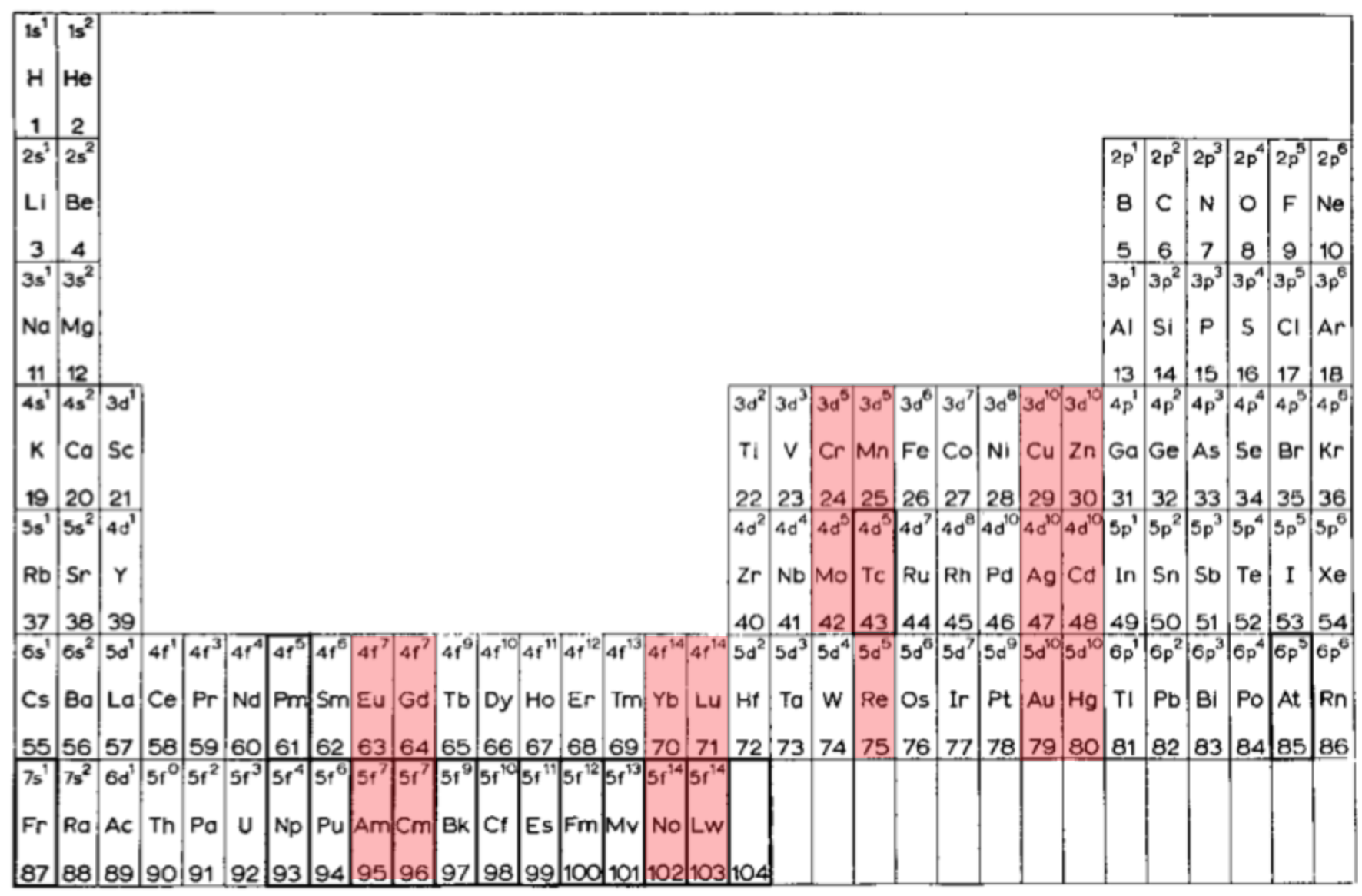
| Year: 2021 | PT id = 1186, Type = formulation |
Helix vs. Screw
Julio Antonio Gutierrez Samanez writes:
Until today, when they write about the work of Chancourtois and his telluric helix wound in a cylinder, still no one alludes to this other telluric helix wound in a cone or screw, the idea is the same: a rope that winds a geometric solid.
The first was devised in 1862, the other in 2008 (146 years later). But, there is a big epistemological difference. In the first, the elementary series presented was: 8, 8, 8, 8, 8 ..., etc., in the second it is: 2, 2, 8, 8, 18, 18, 32, 32. Furthermore, the division of conical radii is regulated by the function 2 (n ^ 2) = 2, 8, 18, 32...
Each binode has two spirals or two periods with the same number of elements, which correspond to the function 4 (n ^ 2). I don't think it is a discovery, it is just the conclusion of the contributions of Rydberg, Janet, and, of course, Chancourtois.
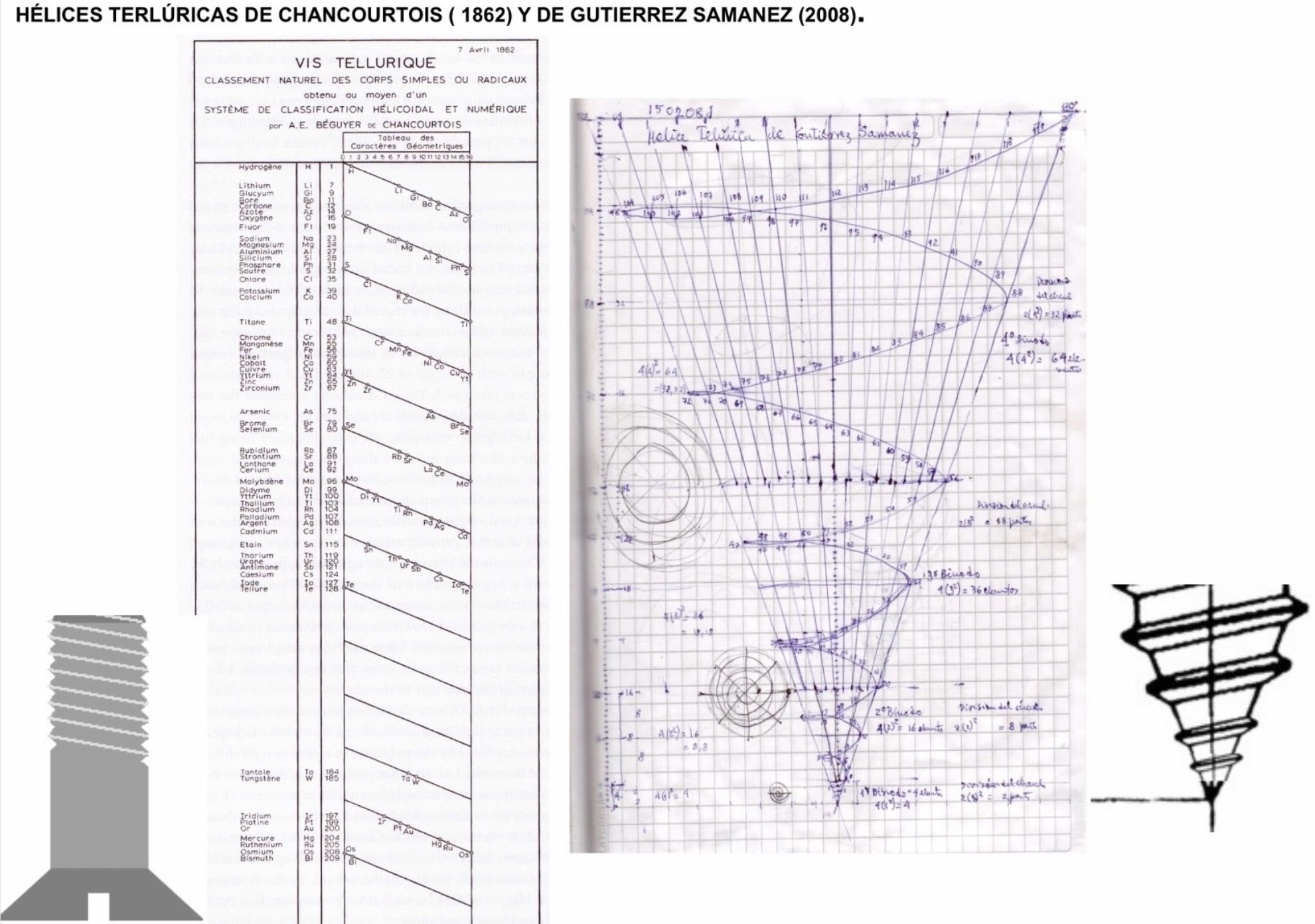
| Year: 2021 | PT id = 1203, Type = formulation |
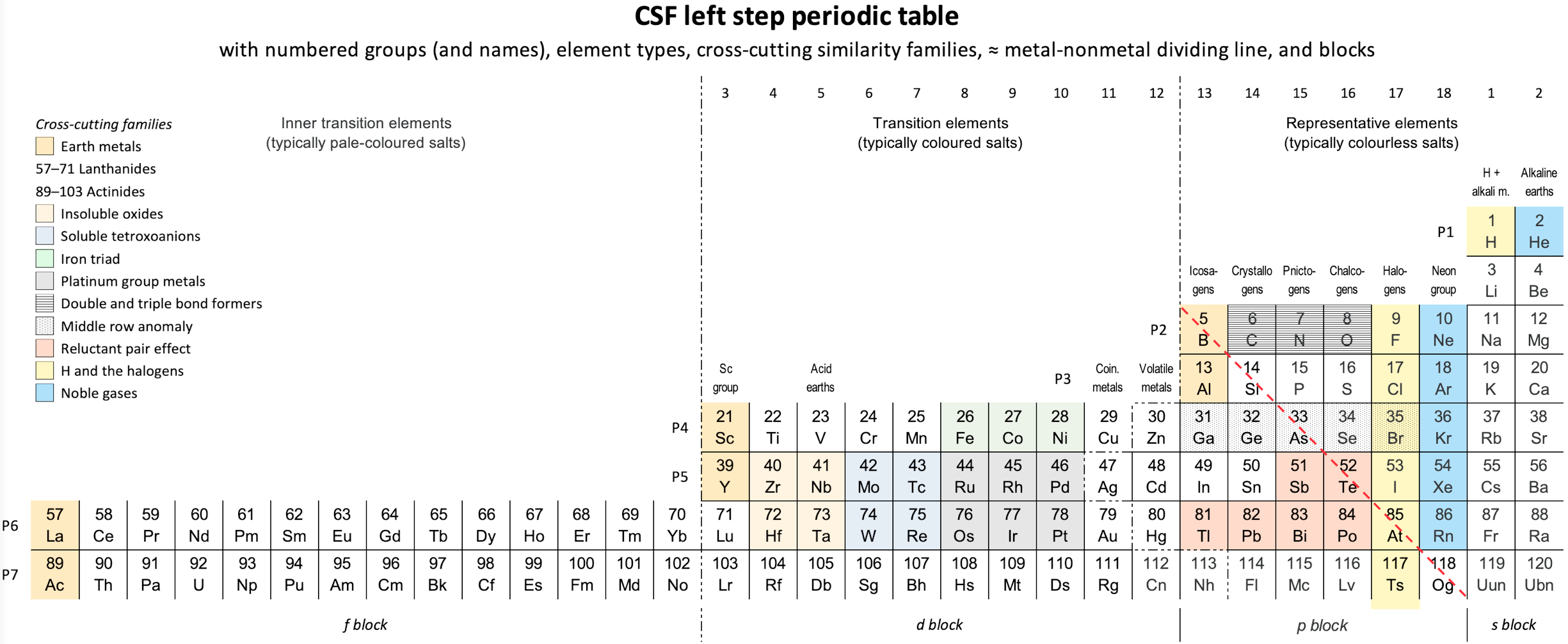
Vernon's CSF Left-Step Periodic Table.
René Vernon's CSF Left-Step Periodic Table.
"I was prompted to switch to He-Be and [to develop a Janet type] left-step periodic table. I suggest it remediates concerns about H and He, and Lu in group 3.
Pros
- There is symmetry in this version.
- The physiochemical relationship of He to Ne is retained.
Cons
- There is a loss of physiochemical regularity in placing He over Be. Even if helium can be enticed to become chemically active, it will still be very much better located in group 18.
- While the d, p, and s blocks start with the appearance of the relevant electron, there is a loss of consistency with La at the start of the f-block. This is confusing to students since there is no such inconsistency in the La form.
- In terms of predominant differentiating electrons in each block, this form is less consistent than an La table.
- There is one less form of "element block-type" symmetry, than in the La form.
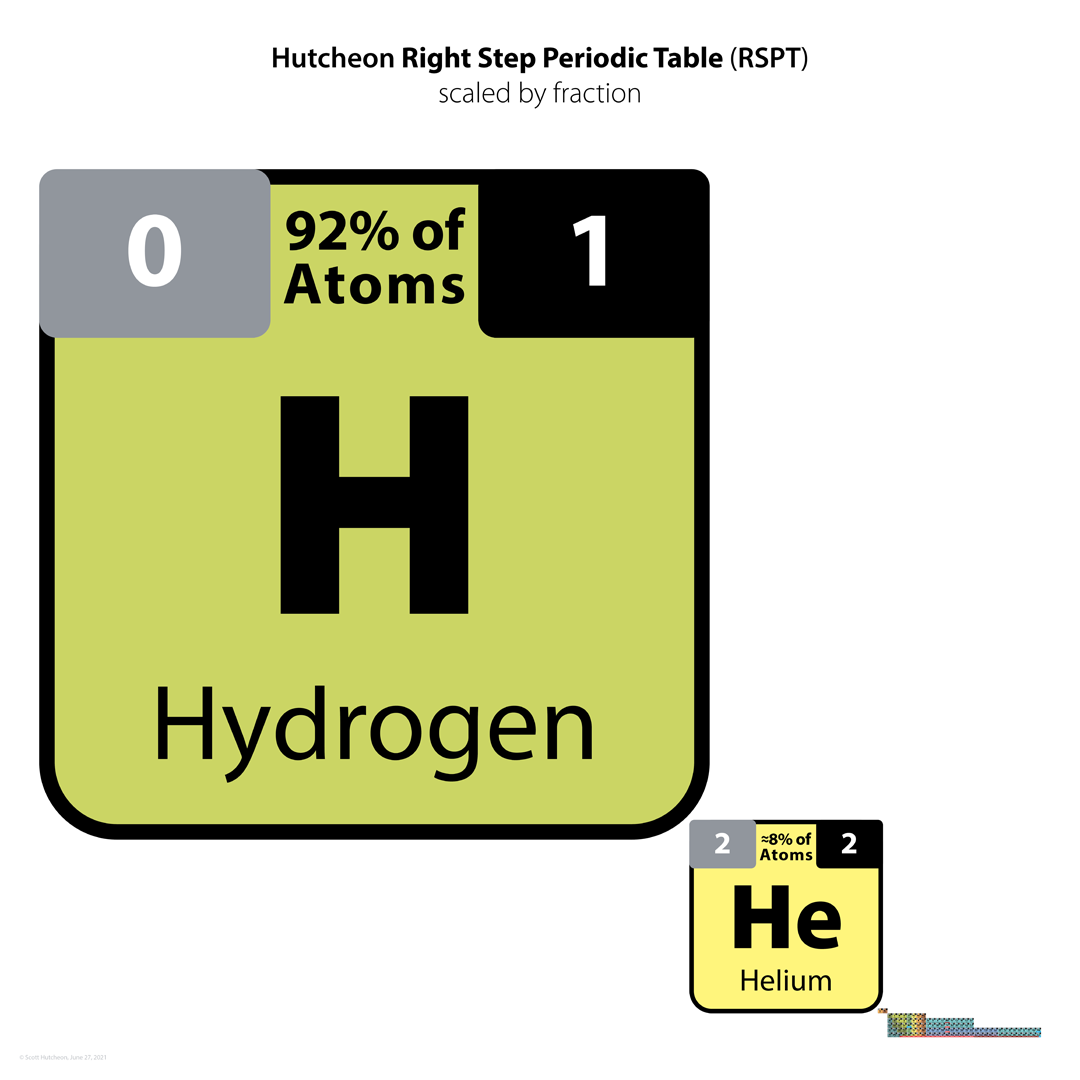
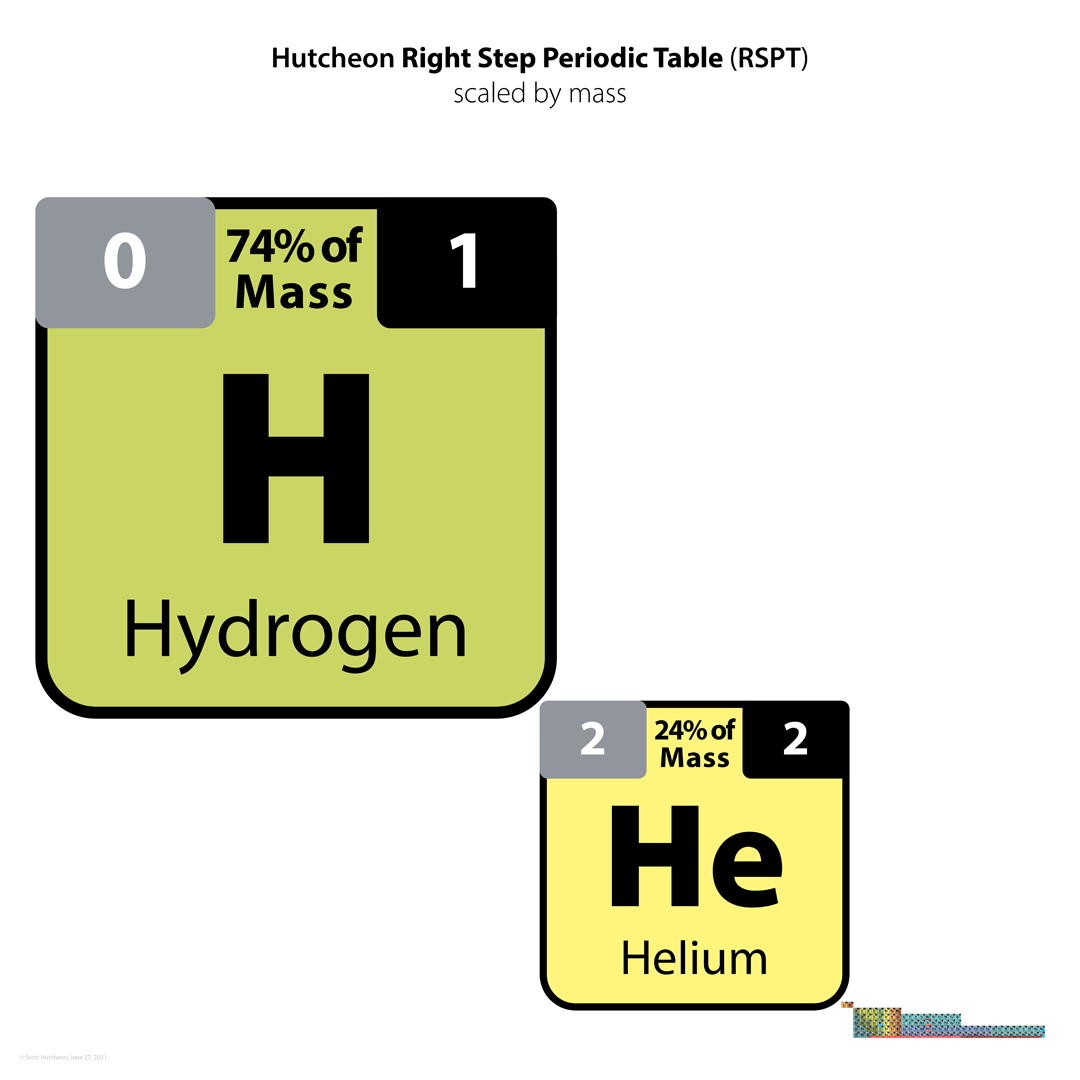
| Year: 2021 | PT id = 1225, Type = formulation |
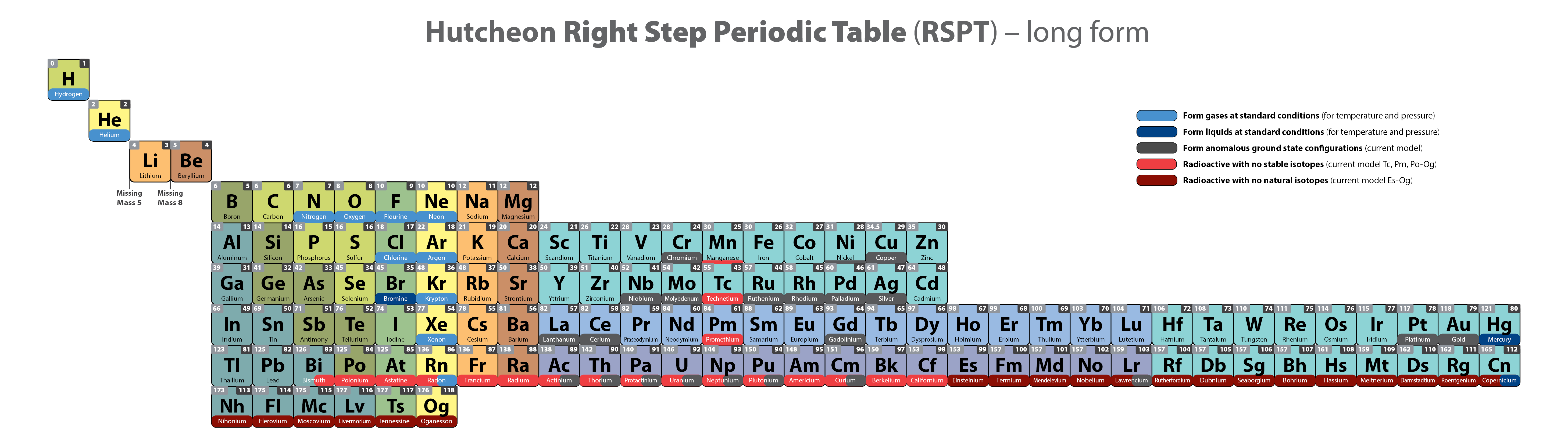
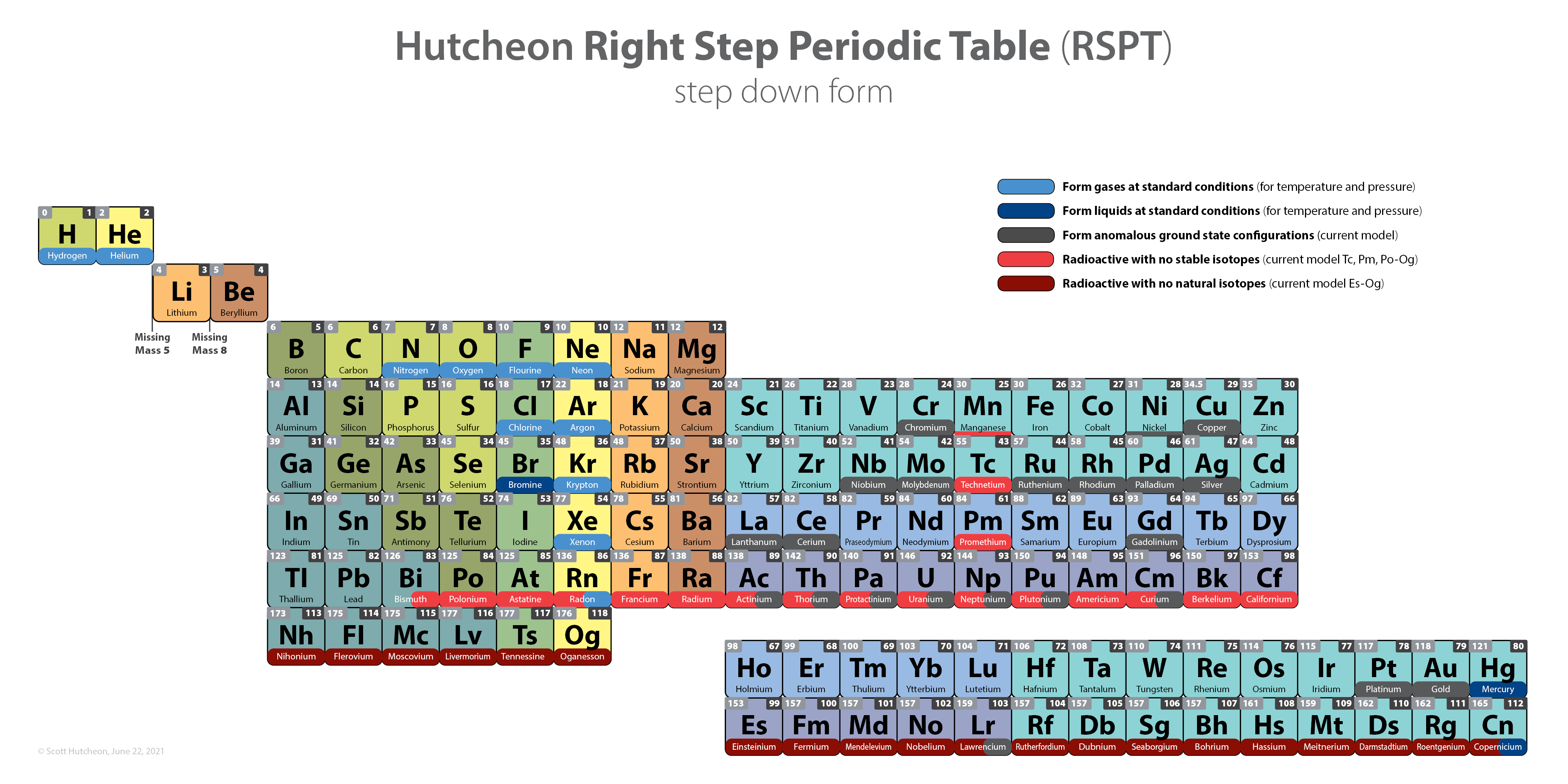
Hutcheon Right Step Periodic Table
From Scott Hutcheon's Linkedin Website:
Abstract:
"Built from first principles, the RSPT is the only Periodic Table (so far) that reflects the periodicity of radioactive elements (natural and artificial) including Tc-Np and Cf/Es, the periodicity of liquids and gases (at standard and other conditions), depicts the 5 and 8 mass roadblocks, and finally clarifies the positions of the initial propagating elements H, He, and Li-Be/B in accordance with their cosmic and stellar evolutionary origins.
"Name inspired by the Janet Left Step Periodic Table (LSPT).
"Bonus Easter Egg: SPOCH BONSe, pronounced Spock Bones, is a new mnemonic device to remember the updated elements considered most essential for human life. Also considered POSCH SeNOB and SNOBS ePOCH."
| Year: 2024 | PT id = 1298, Type = formulation 3D |
Kudan's 3D Model of The Periodic Table
Pavel V. Kudan's 3D model of the Periodic Table via direct download.
Parvel writes:
"The shape of this 3D model allows to show most important thing – H may be aligned over F-Ts and He may be aligned over Ne-Og without classification of H to group F-Ts or He to group Ne-Og. To see that it is needed only that cylinder to be tough (hard) and flat parts to be flexible with ability to change angle. Than is important because according Mendeleev’s principle, maximum valence is main for grouping elements and it is controversial to have element with maximum valence 2 between elements with maximum valence 8.
"Coloring He as gray in the 3D model just reflex the fact that it goes just before the energy gap, as well as coloring Ne-Og in gray show that they too go just before the energy gaps, which makes He and Ne-Og noble. The main is not coloring, but the ability to align and demonstrate.
"You may also remember that the issue of opening the new IUPAC Group 2 project to discuss He group as a continuation of the IUPAC Group 3 project has already been raised in e-mail correspondence with IUPAC some time ago in protection of our reconstruction of Landau’s geometry of the Periodic table.
"I agree with you that double periodicity is important, but also rearrangements of electronic configurations caused by properties of d-orbitals also must be taken in account. For example, Cu has valences 1 or 2, Zn has valence 2 due to special properties of d-orbitals. The 3D model of the Periodic table separates the ability of d-orbitals to steel electrons from s-orbitals and f-orbitals causing of such effects.
"Also when you will have a copy of the 3D model you will see that it unifies both geometry of the Mendeleev’s Periodic table and geometry of the Janet’s Periodic table. Following anticlockwise you may see Mendeleev’s order while following clockwise you may see Janet’s order. It is similar to having the 3D moles of globe as visual aid for better vision of Mendeleev’s Periodic table and Janet’s Periodic table as flat detailed maps."

| Year: 2024 | PT id = 1303, Type = formulation |
Bilateral Symmetry in the Periodic Binodic Table
René Vernon, who developed these ideas, writes:
This table is adapted from the work of Gutiérrez-Samanez (2020), who discusses mathematising the chemical periodic system as a grid, which leads to a quadratic function or “binódica function” formed by pairs of periods or binodos (dyads).
The difference is that whereas Gutiérrez-Samanez showed the first pair of periods as H-He and Li-Be, this table shows the first period as e-n and H-He. Here, e is the electron and n is the neutron. Each pair of periods is shown pancake style rather than in a single row. The formula for the length of each paired period or binode is 2n2 = 2, 8, 16, 32.
The idea of paired periods has a long history; it seems to have originated with Werner in 1905.
According to Jensen:
"The temptation to read more into the shape of the table than is really there is almost overwhelming. Even someone as great as Werner was tempted (1905). Having postulated a missing element between H and He, he decided to perfect the symmetry of his table by guaranteeing that rows of differing length always occurred in pairs. Consequently, he further postulated a row of three missing elements lying above the H-X-He row."
Rydberg (1913, pp. 12–13) used a formula 4n^2 for the number of elements in the paired periods: 4, 16, 32, 64. This formula is also used by Gutiérrez-Samanez.
Paired periods were also used by Janet (1928), Saz (1931), Achimov (1946) and Baca Mendoza (1953).
References
- Achimov EI 1946, Zhur. Obshchei Khim., vol. 16, p. 961; https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=776
- Baca Mendoza O 1953, Leyes Genéticas de los elementos Químicos, Nuevo Sistema Periódico, National University of Cusco, https://www.meta-synthesis.com/webbook/35_pt/Mendoza_PT.pdf, accessed May 12, 2024
- Gutiérrez-Samanez JA 2020, Binódic periodic system: a mathematical approach, Found Chem, vol. 22, pp. 235–266 (255)
- Janet C1928, Essais de classification hélicoïdale des éléments chimiques. Imprimerie Départementale de l’Oise, Beauvais, https://www.meta-synthesis.com/webbook/35_pt/pt_database.php?PT_id=152
- Jensen WB 1986, Classification, symmetry and the periodic table, Computers & Mathematics with Applications, vol. 12B, no. 12, pp. 487–510 (508)
- Rydberg JR 1913, Untersuchungen über das system der grundstoffe, Lunds Univ. Ärsskrift, vol. 9, no. 18. In French: 1914, Recherches sur le système des éléments, Journal de Chimie Physique, vol. 12, p. 585
- Saz E 1931, Iberica, vol. 35, p. 186
- Wemer A 1905, Beitrag zum Aufbau des periodischen Systems, Ber. Deut. Chem. Ges, vol. 38, pp. 914–921, 2022–2027

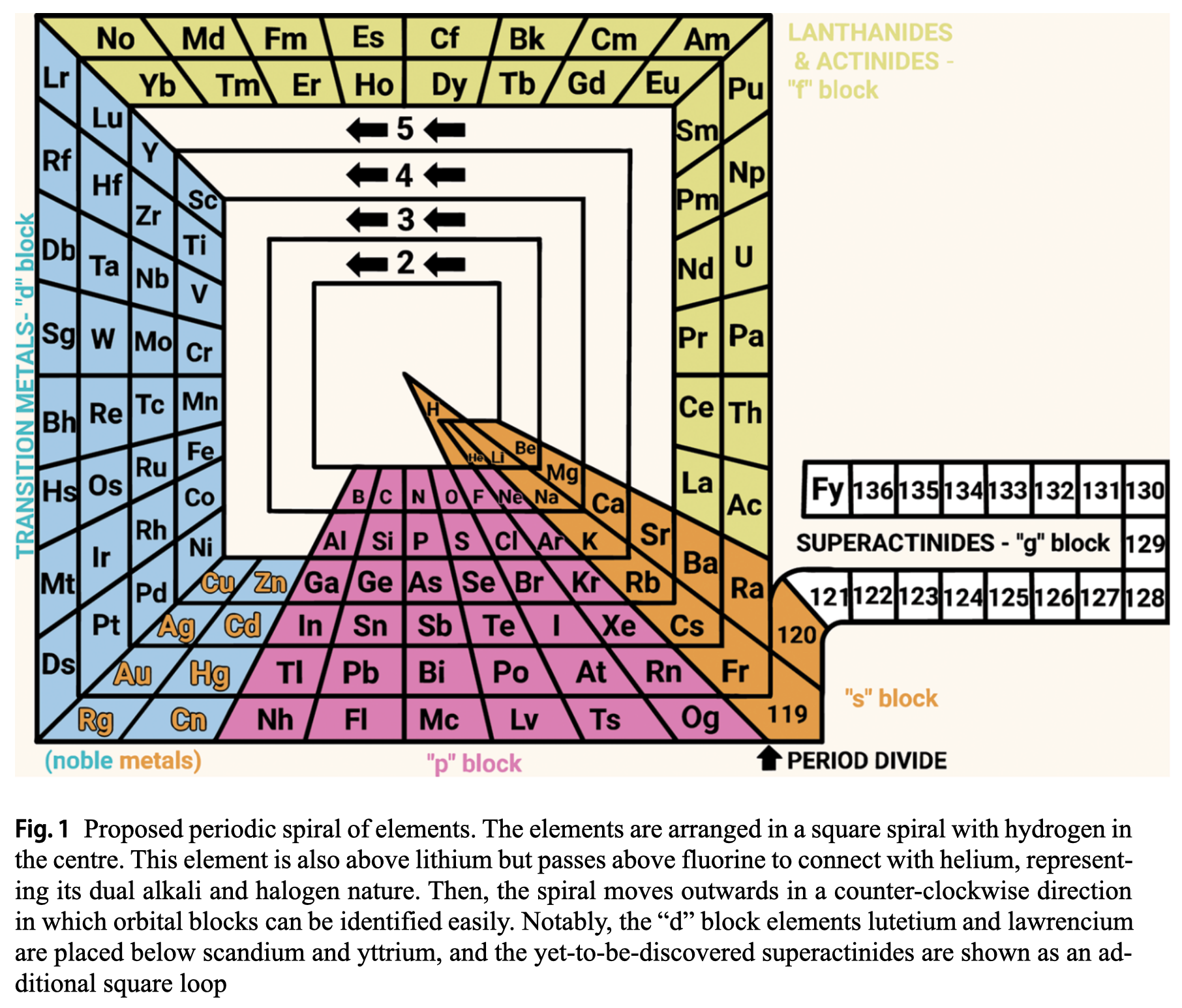
| Year: 2024 | PT id = 1311, Type = formulation spiral |
Rodríguez Peña & García Guerra's Periodic Spiral of The Elements
Rodríguez Peña, M., García Guerra, J.Á. The periodic spiral of elements. Found Chem (2024). https://doi.org/10.1007/s10698-024-09510-4
Abstract There are 2 main problems with the current periodic table: artificial breaks from a given noble gas to the next alkali metal (along with the common protrusion of the "f" block) and hydrogen placed in the alkali group, although this gas also exhibits halogen properties. This paper proposes arranging chemical elements in a square spiral with hydrogen at the centre. This element is also above lithium but passes above fluorine to connect with helium, representing its dual alkali and halogen nature effectively. Then the spiral moves outwards in a counter-clockwise direction, avoiding artificial breaks and following the natural direction of reading for the "s" and "p" blocks elements placed at the bottom of the spiral. Furthermore, this proposed square spiral improves upon previous Janet's and Benfey's representations with a more regular shape to draw, an effective depiction of the dual nature of hydrogen, and easily identifiable orbital blocks without the need for protrusions.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.


 dic System of Chemical Elements
dic System of Chemical Elements