Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Tables from the years 1850 - 1899, by date:
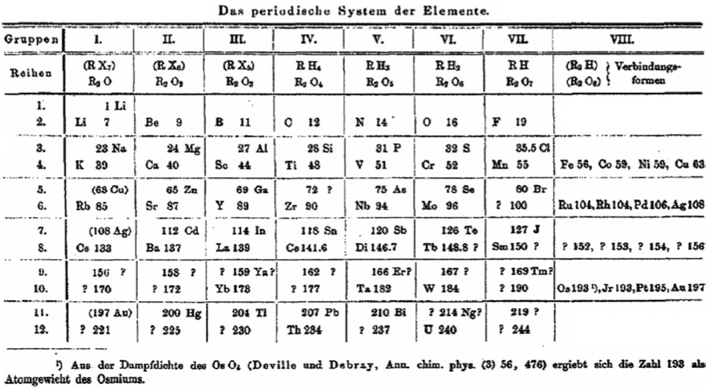
| Year: 1850 | PT id = 474, Type = formulation element weight |
Elements Known in the Year 1850
Elements known in the year 1850, taken from this Wikipedia page:
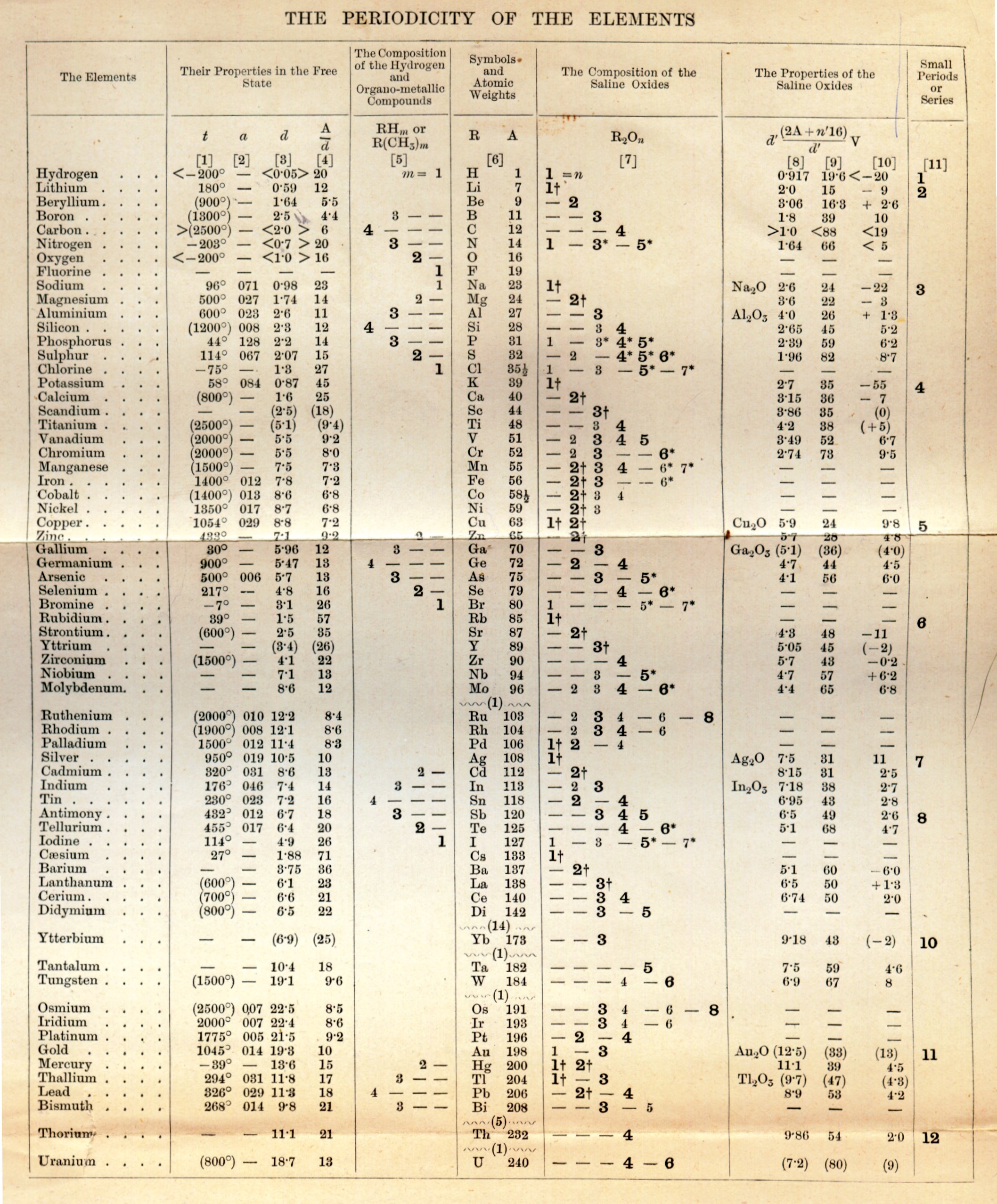
| Year: 1858 | PT id = 1047, Type = formulation review element weight structure |
Cannizzaro's Letter or Sunto
Letter of Professor Stanislao Cannizzaro to Professor S. De Luca: Sunto di un corso di filosofia chimica (Sketch of a Course of Chemical Philosophy) given in the Royal University of Genoa, Il Nuovo Cimento, vol. vii. (1858), pp. 321-366.

Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about, and link to, Cannizzaro's Letter. See a list of other classic chemistry papers.
Read the full letter/paper, in English translation, here. (The Italian version is here.)
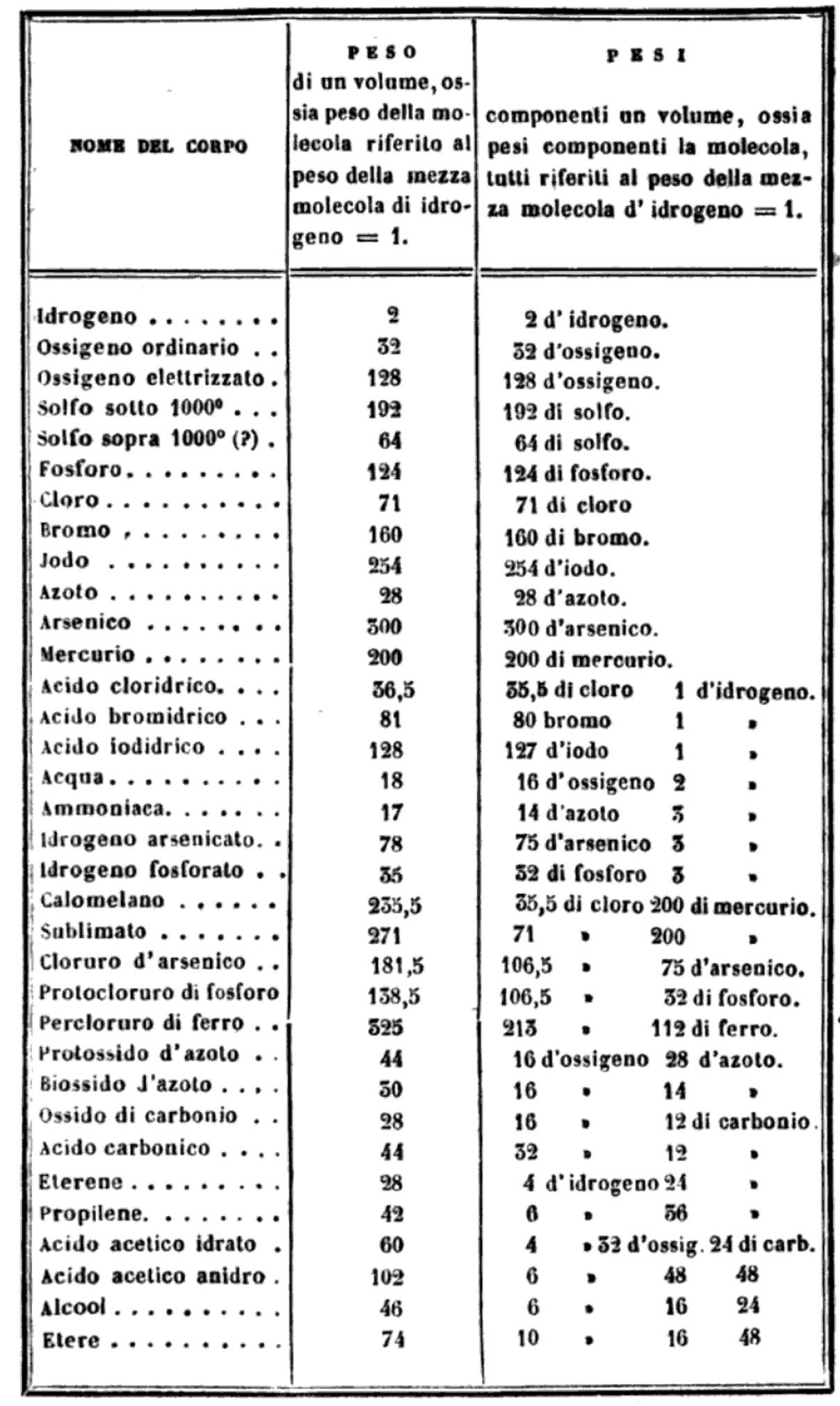
"I believe that the progress of science made in these last years has confirmed the hypothesis of Avogadro, of Ampère, and of Dumas on the similar constitution of substances in the gaseous state; that is, that equal volumes of these substances, whether simple or compound, contain an equal number of molecules: not however an equal number of atoms, since the molecules of the different substances, or those of the same substance in its different states, may contain a different number of atoms, whether of the same or of diverse nature."
From the Science History of Science Institute:
"In 1858 Cannizzaro outlined a course in theoretical chemistry for students at the University of Genoa,where he had to teach without benefit of a laboratory. He used the hypothesis of a fellow Italian, Amedeo Avogadro, who had died just two years earlier, as a pathway out of the confusion rampant among chemists about atomic weights and the fundamental structure of chemical compounds."
Mark Leach writes:
"Before a periodic table of the chemical elements – which orders the elements by atomic weight and then groups them by property – could be developed it was necessary to know the atomic weight values. However, to deduce the atomic weights was a problem as it was necessary to know the ratios of how the elements combined, the stoichiometry.
"Tables of atomic weight data by Dalton (1808), Wollaston (1813), Daubeny (1831) and Kopp & Will (1858) show progress, but the 1858 Cannizzaro letter was the first where the atomic weight data is more or less both complete and accurate, thus removing stiochiometric errors.
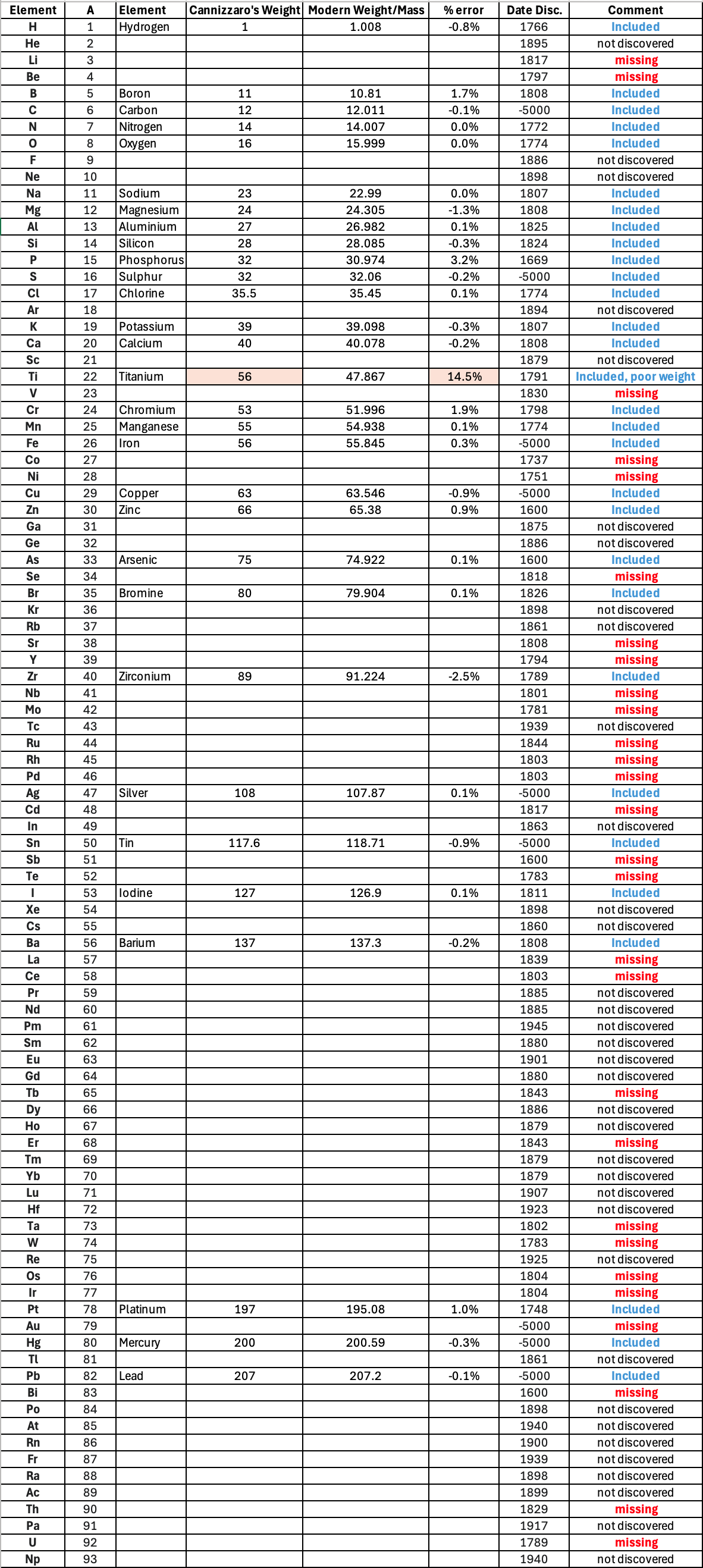
"I have extracted the element atomic weight data from the paper, and given the % error with respect to modern atomic weight/mass data. Only titanium is significantly out! It is clear that Cannizzaron knew that hydrogen, nitrogen, oxygen, chlorine, bromine & iodine existed as diatomic molecules."
| Element | Symbol | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H | 1 | 1.008 | -0.8% |
| Boron | B | 11 | 10.81 | 1.7% |
| Carbon | C | 12 | 12.011 | -0.1% |
| Nitrogen | N | 14 | 14.007 | 0.0% |
| Oxygen | O | 16 | 15.999 | 0.0% |
| Sodium | Na | 23 | 22.99 | 0.0% |
| Magnesium | Mg | 24 | 24.305 | -1.3% |
| Aluminium | Al | 27 | 26.982 | 0.1% |
| Silicon | Si | 28 | 28.085 | -0.3% |
| Sulphur | S | 32 | 32.06 | -0.2% |
| Phosphorus | P | 32 | 30.974 | 3.2% |
| Chlorine | Cl | 35.5 | 35.45 | 0.1% |
| Potassium | K | 39 | 39.098 | -0.3% |
| Calcium | Ca | 40 | 40.078 | -0.2% |
| Chromium | Cr | 53 | 51.996 | 1.9% |
| Manganese | Mn | 55 | 54.938 | 0.1% |
| Iron | Fe | 56 | 55.845 | 0.3% |
| Titanium | Ti | 56 | 47.867 | 14.5% |
| Copper | Cu | 63 | 63.546 | -0.9% |
| Zinc | Zn | 66 | 65.38 | 0.9% |
| Arsenic | As | 75 | 74.922 | 0.1% |
| Bromine | Br | 80 | 79.904 | 0.1% |
| Zirconium | Zr | 89 | 91.224 | -2.5% |
| Silver | Ag | 108 | 107.87 | 0.1% |
| Tin | Sn | 117.6 | 118.71 | -0.9% |
| Iodine | I | 127 | 126.9 | 0.1% |
| Barium | Ba | 137 | 137.3 | -0.2% |
| Platinum | Pt | 197 | 195.08 | 1.0% |
| Mercury | Hg | 200 | 200.59 | -0.3% |
| Lead | Pb | 207 | 207.2 | -0.1% |
| Diatomic Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H2 | 2 | 2.016 | -0.8% |
| Oxygen | O2 | 32 | 31.998 | 0.0% |
| Sulphur | S2 | 64 | 64.12 | -0.2% |
| Chlorine | Cl2 | 71 | 70.9 | 0.1% |
| Bromine | Br2 | 160 | 159.808 | 0.1% |
| Iodine | I2 | 254 | 253.8 | 0.1% |
| Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Water | H2O | 18 | 18.015 | -0.1% |
| Hydrochloric Acid | HCl | 36.5 | 36.458 | 0.1% |
| Methane | CH4 | 16 | 16.043 | -0.3% |
| Hydrogen sulphide | H2S | 34 | 34.076 | -0.2% |
| Diethyl ether | CH3CH2OCH2CH3 | 74 | 74.123 | -0.2% |
| Carbon disulphide | CS2 | 76 | 76.131 | -0.2% |
| Chloroethane | CH3CH2Cl | 64.5 | 64.512 | 0.0% |
Below is a list of the elements showing which ones were included by Cannizzaro and which one were ommitted (because they had not been discovered) or are strangely missing. Odd ommissions (to the modern eye) include: Lithium, Beryllium, Cobalt, Nickel, Palladium, Tungsten and Gold.
| Year: 1858 | PT id = 1348, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1858
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1858 table of data is here.
Mark Leach writes:
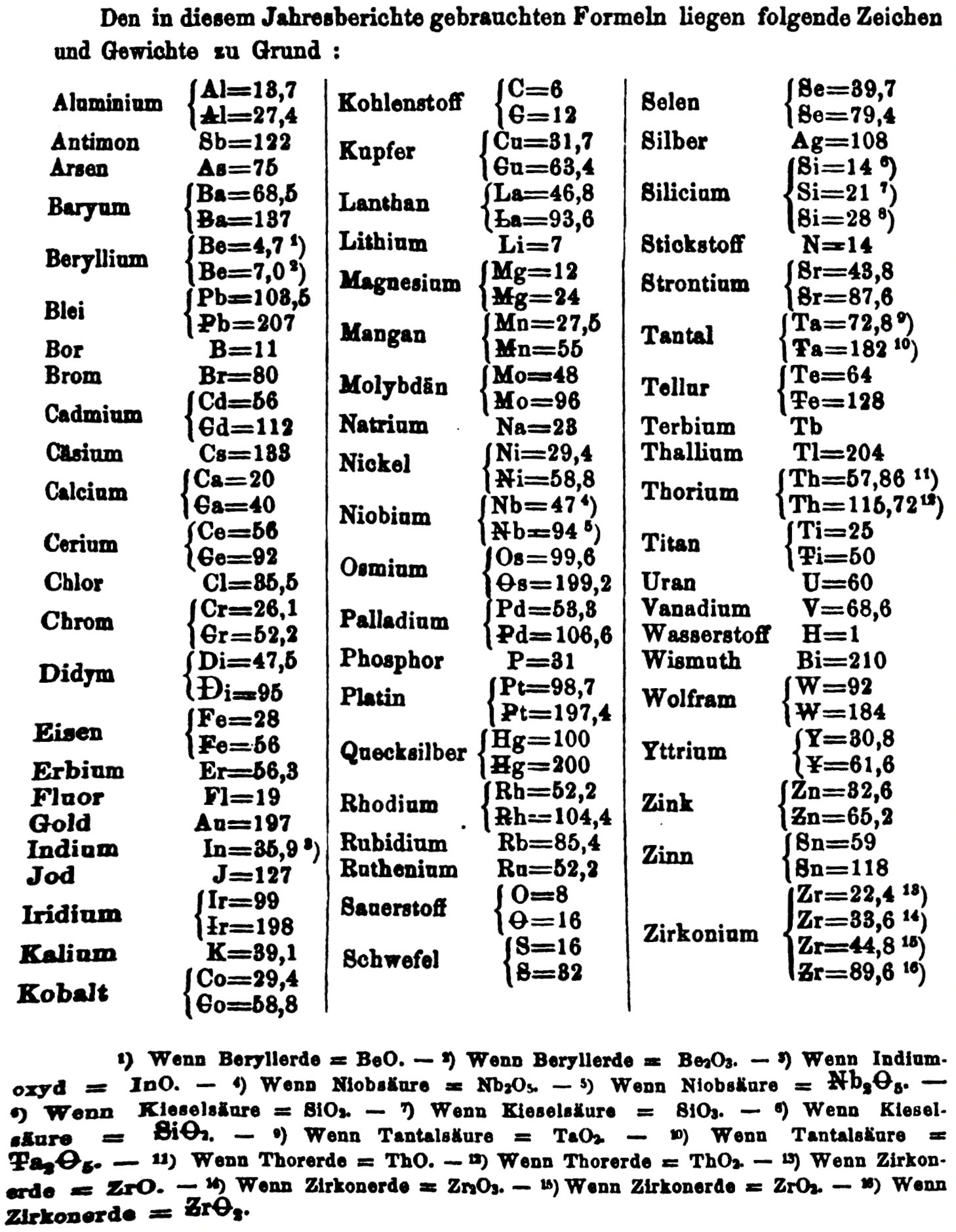
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6
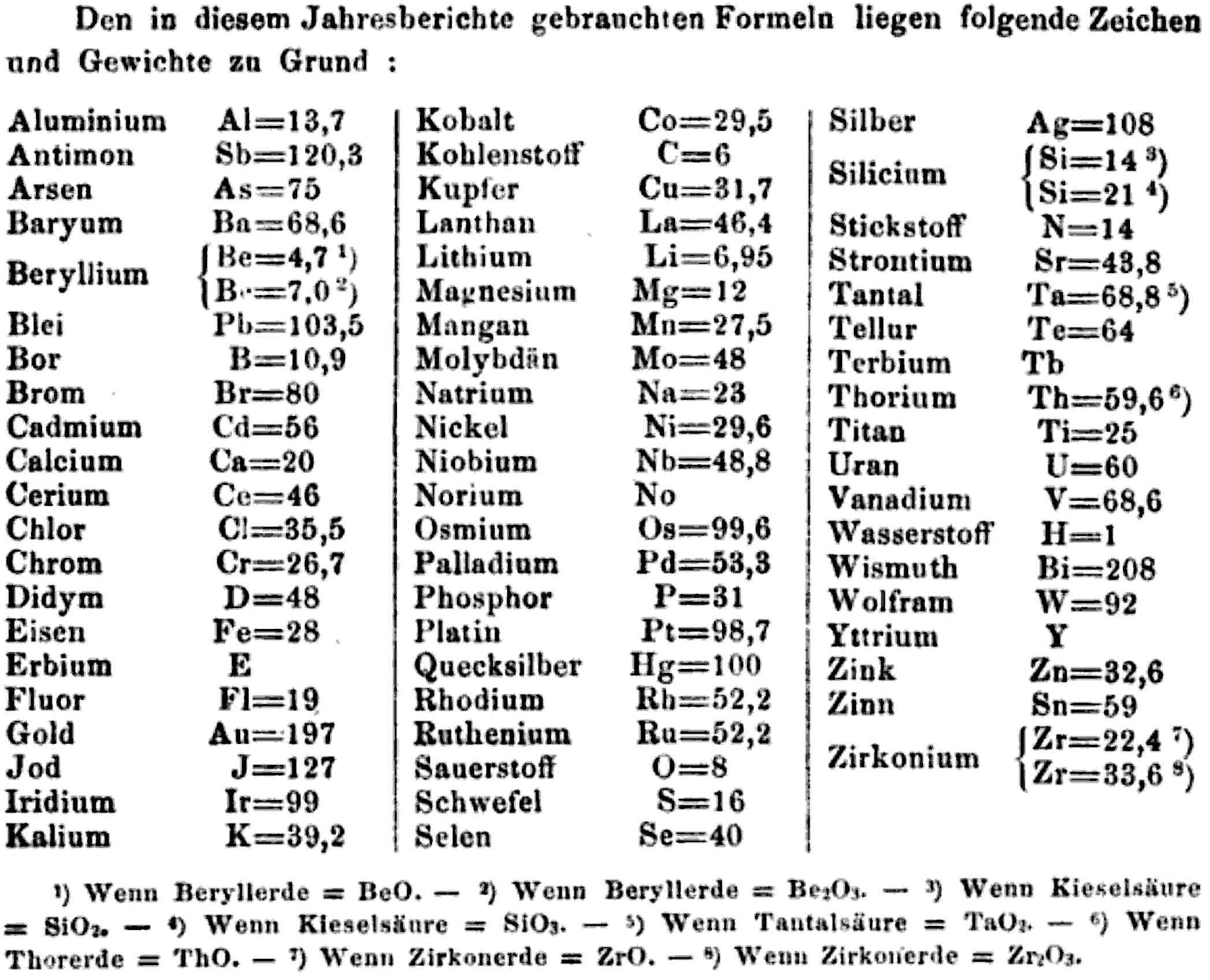
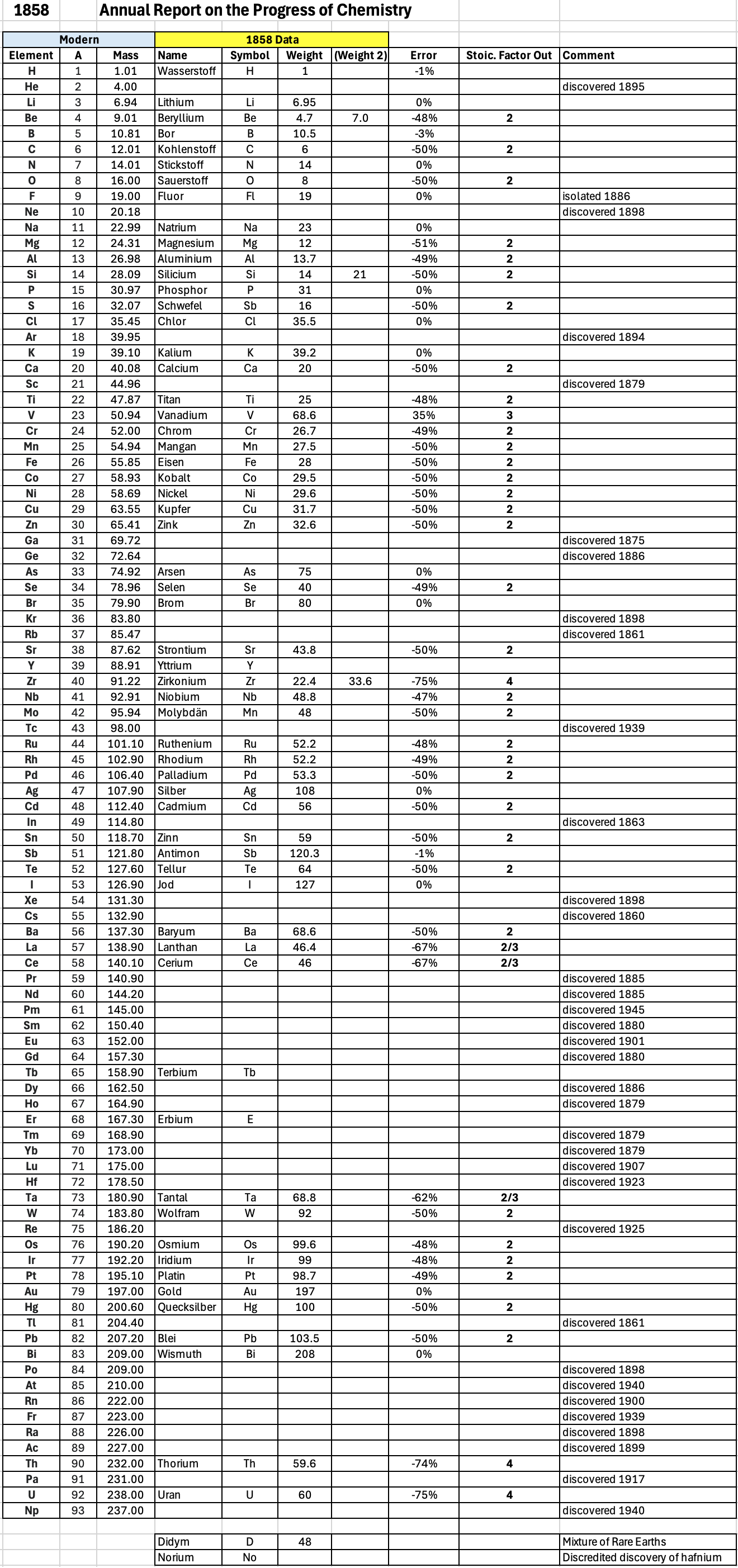
Thanks to René and Mario Rodriguez for the tip!
| Year: 1859 | PT id = 1349, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1859
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1859 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6

Thanks to René and Mario Rodriguez for the tip!
| Year: 1860 | PT id = 835, Type = element |
Discovery of Cesium
Cs
Cesium (or caesium), atomic number 55, has a mass of 132.905 au.
Cesium is a Group 1 element, and these are often referred to as the "alkali metals".
Cesium was first observed or predicted in 1860 by R. Bunsen and R. Kirchhoff and first isolated in 1882 by C. Setterberg.
| Year: 1860 | PT id = 1048, Type = formulation |
Karlsruhe Congress
The Karlsruhe Congress of 1860 was called so that European chemists could discuss a number of issues, including atomic weights.

From Wikipedia (lightly edited):
"The Karlsruhe meeting ended with no firm agreement on the vexing problem of atomic and molecular weights. However, on the meeting's last day reprints of Stanislao Cannizzaro's 1858 paper on atomic weights were distributed. Cannizzaro's efforts exerted an almost immediate influence on the delegates.
"Lothar Meyer later wrote that on reading Cannizzaro's paper: 'The scales seemed to fall from my eyes.'
"An important long-term result of the Karlsruhe Congress was the adoption of the now-familiar atomic weights. Prior to the Karlsruhe meeting, and going back to Dalton's work in 1803, several systems of atomic weights were in use.
"Following the Karlsruhe meeting, values of about 1 for hydrogen, 12 for carbon, 16 for oxygen, and so forth were adopted. This was based on a recognition that certain common gaseous elements, such as hydrogen, nitrogen, oxygen and chlorine were composed of diatomic molecules and not individual atoms: H2, N2, O2, Cl2, etc."
Once enough elements had been discovered, and their atomic weights correctly deduced, the time was ripe to develop versions of the periodic table systems. These came 'thick & fast' after the Karlsruhe Congress.
Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about the important Karlsruhe Congress.
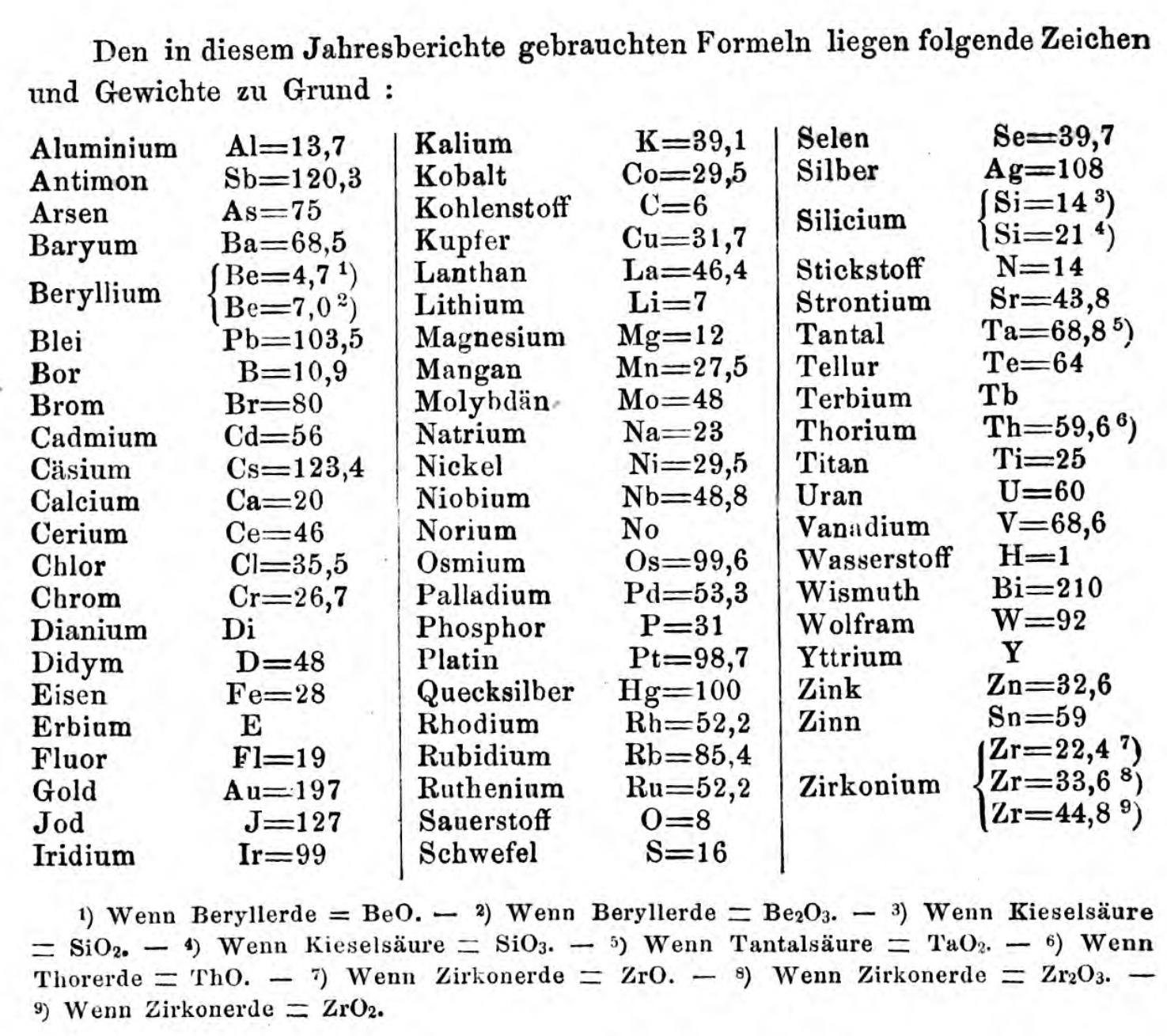
| Year: 1860 | PT id = 1350, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1860
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1860 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si =14 and 21
- Zr = 22.4, 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
| Year: 1861 | PT id = 817, Type = element |
Discovery of Rubidium
Rb
Rubidium, atomic number 37, has a mass of 85.468 au.
Rubidium is a Group 1 element, and these are often referred to as the "alkali metals".
Rubidium was first observed, but not isolated in pure form, in 1861 by R. Bunsen and G. R. Kirchhoff.
| Year: 1861 | PT id = 861, Type = element |
Discovery of Thallium
Tl
Thallium, atomic number 81, has a mass of 204.384 au.
Thallium was first observed or predicted in 1861 by W. Crookes and first isolated in 1862 by C.-A. Lamy.
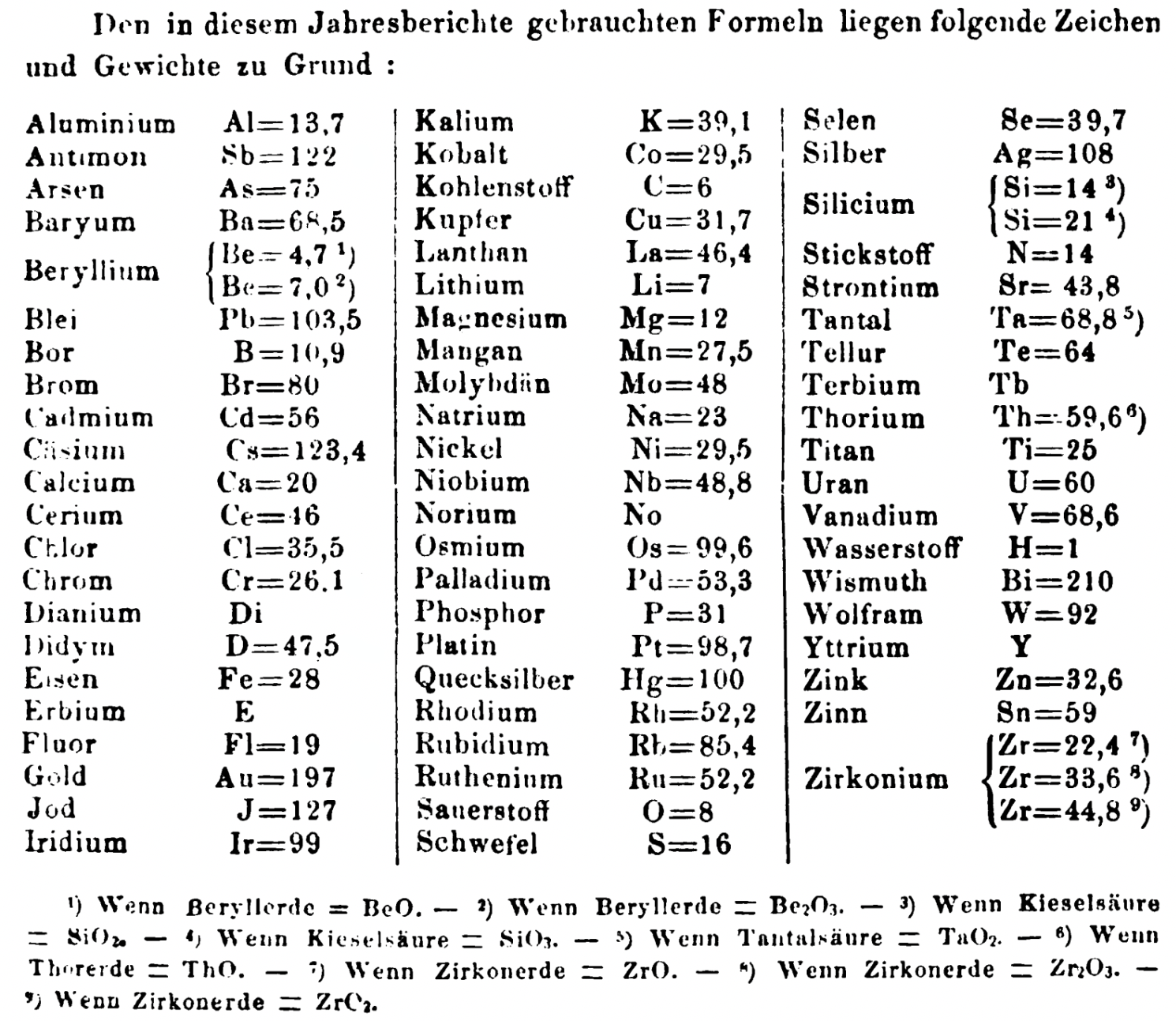
| Year: 1861 | PT id = 1351, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1861
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1861 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
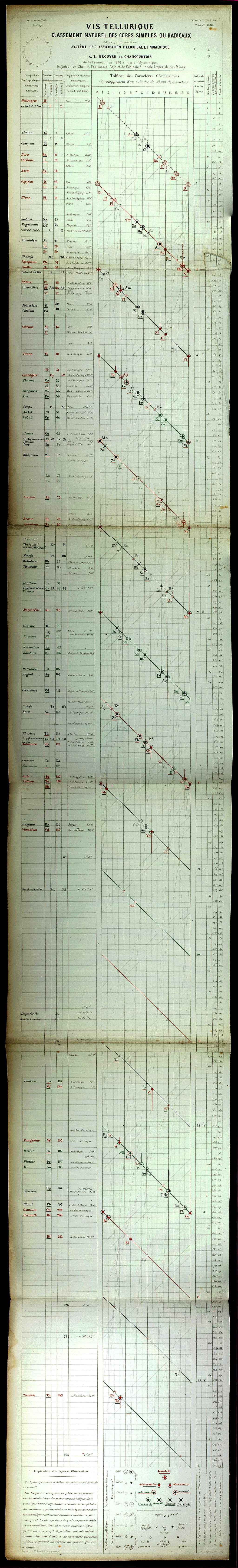
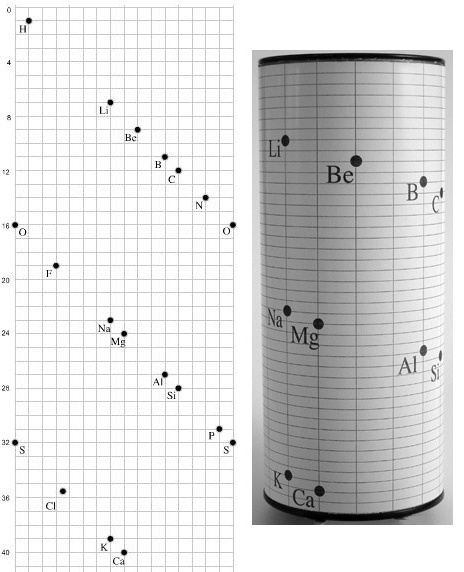
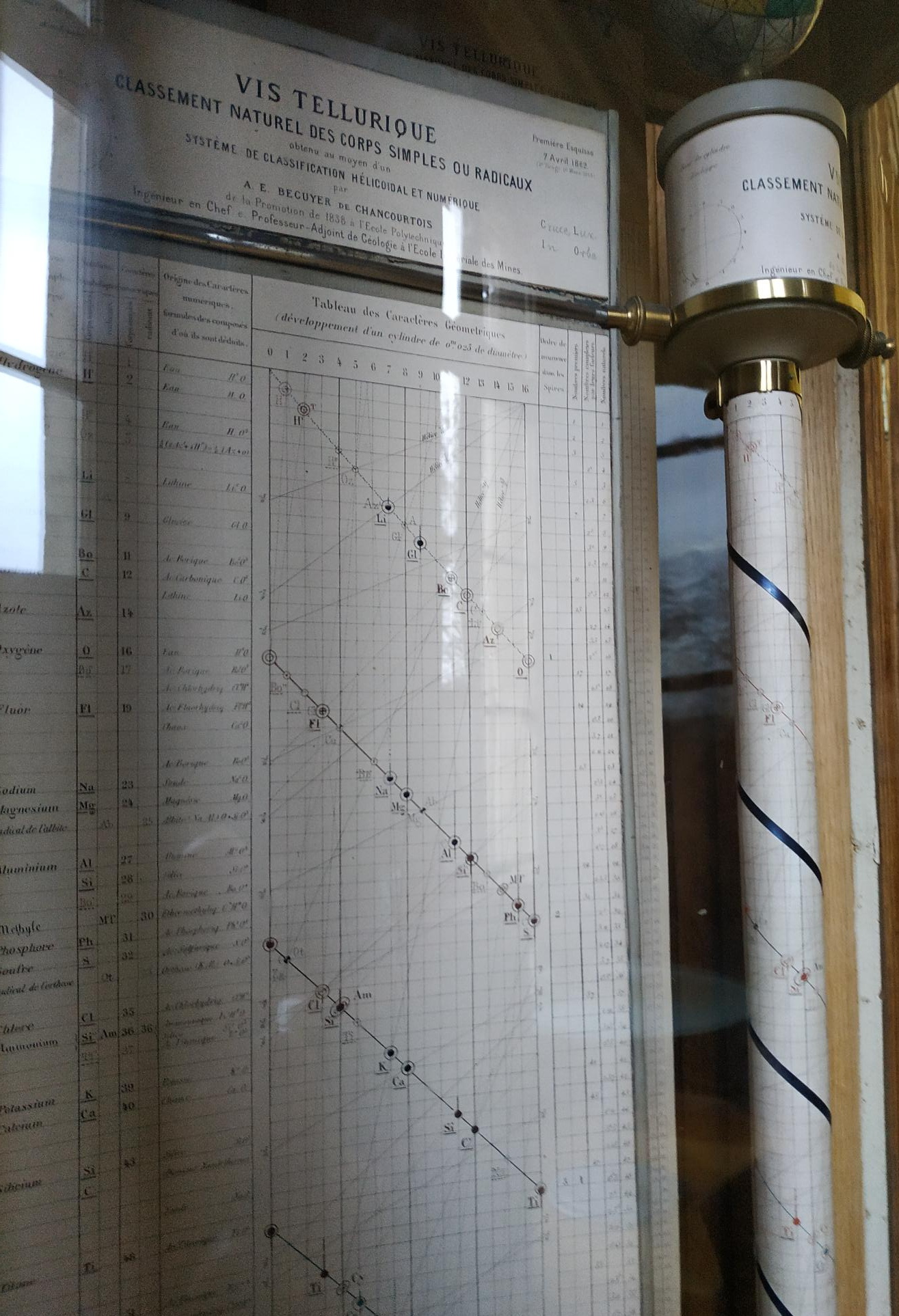
| Year: 1862 | PT id = 7, Type = formulation spiral 3D |
Béguyer de Chancourtois' Vis Tellurique
The French geologist , Alexandre-Émile Béguyer de Chancourtois was the first person to make use of atomic weights to produce a classification of periodicity. He drew the elements as a continuous spiral around a metal cylinder divided into 16 parts. The atomic weight of oxygen was taken as 16 and was used as the standard against which all the other elements were compared. Tellurium was situated at the centre, prompting vis tellurique, or telluric screw.
Many thanks to Peter Wothers – and courtesy of the Master and Fellows of St Catharine's College, Cambridge – comes a high quality image of the original 1862 formulation. Click here, or on the image to enlarge:
Watch Peter Wothers 'unravel' and show Prof. Martyn Poliakoff this first periodic table at 17min 50sec into the YouTube video below:
Some more information:
Chancourtois' original formulation includes elements in their correct places, selected compounds and some elements in more than one place. The helix was an important advance in that it introduced the concept of periodicity, but it was flawed.
It has been suggested that Chancourtois called his formulation a telluric helix because tellurium is found in the middle. However, most elements are found as there their 'earths' – tellus, telluris – or oxides, which for a mineralogist would have been highly significant.
The formulation was rediscovered in the 1889 (P. J. Hartog, "A First Foreshadowing of the Periodic Law" Nature 41, 186-8 (1889)), and since then it has appeared most often in a simplified form that emphasizes the virtues and eliminates its flaws. [Thanks to CG for this info.]
See also:
- Dutch Wikipedia
- ScienceWorld
- Science & Society Picture Library
- Roy Alexander's All Periodic Tables site
A three dimensional models of the telluric helix:
There are representations of the 1862 formulation at the School of Mines at ParisTech:

| Year: 1862 | PT id = 440, Type = formulation |
Meyer's Periodic System
In his book, The Periodic Table: A Very Short Introduction, Eric Scerri writes how Lothar Meyer devised a partial periodic tables consisting of 28 elements arranged in order of increasing atomic weight in which the elements were grouped into vertical columns according to their chemical valences:
| Year: 1862 | PT id = 1352, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1862
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1862 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8

Thanks to René and Mario Rodriguez for the tip!
| Year: 1863 | PT id = 829, Type = element |
Discovery of Indium
In
Indium, atomic number 49, has a mass of 114.818 au.
Indium was first observed or predicted in 1863 by F. Reich and T. Richter and first isolated in 1867 by T. Richter.
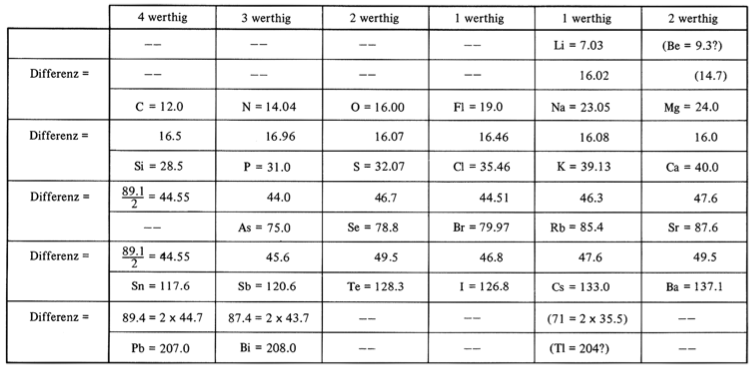
| Year: 1863 | PT id = 1353, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1863
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1863 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- C = 6 and 12
- Hg = 100 and 200
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Zr = 22.4 and 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
| Year: 1864 | PT id = 8, Type = formulation |
Newlands' Octaves
One of the first attempts at a periodic table that arranged the known elements by atomic weight and chemical property, was by John Newlands and is known as "Newlands Octaves".
Newland noticed that if he broke up his list of elements into groups of seven – starting a new row with the eighth element – the first element in each of those groups had similar chemistry.
Note: In the tables below, Newlands Octaves go downwards: H to O, F to S, Cl to Fe, etc.
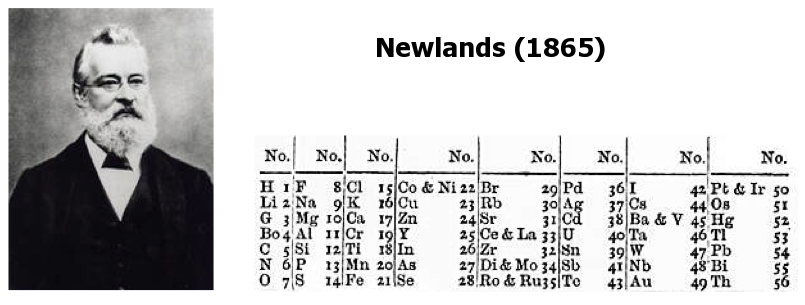
|
H 1
|
F 8
|
Cl 15
|
Co & Ni 22
|
Br 29
|
Pd 36
|
I 42
|
Pt & Ir 50
|
|
Li 2
|
Na 9
|
K 16
|
Cu 23
|
Rb 30
|
Ag 37
|
Cs 44
|
Os 51
|
|
G 3
|
Mg 10
|
Ca 17
|
Zn 24
|
Sr 31
|
Cd 38
|
Ba & V 45
|
Hg 52
|
|
Bo 4
|
Al 11
|
Cr 19
|
Y 25
|
Ce & La 33
|
U 40
|
Ta 46
|
Tl 53
|
|
C 5
|
Si 12
|
Ti 18
|
In 26
|
Zr 32
|
Sn 39
|
W 47
|
Pb 54
|
|
N 6
|
P 13
|
Mn 20
|
As 27
|
Di & Mo 34
|
Sb 41
|
Nb 48
|
Bi 55
|
|
O 7
|
S 14
|
Fe 21
|
Se 28
|
Ro & Ru 35
|
Te 43
|
Au 49
|
Th 56
|
- Seeing the word octave applied to this table may lead one to think that Newlands recognised periods of eight elements with repeating properties, as we do with the modern periodic table, for example: Li Be B C N O F Ne.
- However, each sequence of Newlands' octaves contain only seven elements. Count the elements in the columns! In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not yet been discovered.
- To Newlands, H to F & F to Cl are octaves of eight elements, the eighth element repeating the properties of the first.
There are seven notes in a musical octave: A B C D E F G, after which you start again with A'; similarly for Newlands, seven elements H Li G Bo C N O, then the 8th is F and you start again. [Note that Newlands treated H as a halogen.] More here.
A B C D E F G A
Philip Stewart's musical representation:
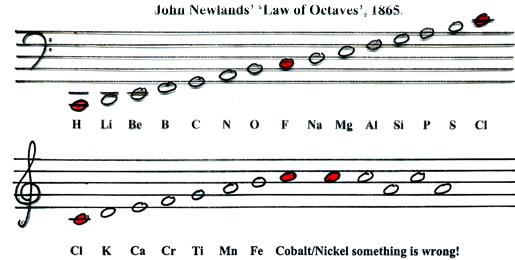
- To Newlands, H to F is an octave of eight elements.
- Today we say Li to Ne & Na to Ar are periods of eight elements, and that that Li and Na are in different periods. Indeed, the Li to Na series consists of nine elements.
- In Newlands' day the group 8 (18) rare gas elements, He, Ne, Ar, Kr & Xe, had not been discovered.
Read more about Newland's Octaves, including a commentary on the original papers in Carmen Giunta's Elements and Atoms: Case Studies in the Development of Chemistry.
| Year: 1864 | PT id = 91, Type = formulation |
Odling's Table of Elements
William Odling's table from: Q. J. Sci., 1864, 1, 642:
| Year: 1864 | PT id = 269, Type = formulation |
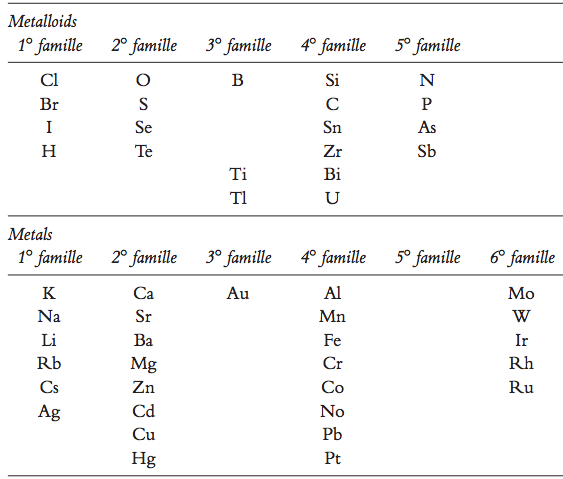
Naquet's Families of Elements
According to Naquet’s 1864 textbook, Principes de Chimie, F. Savy, Paris, (updated by Eric Scerri):
| Year: 1864 | PT id = 1354, Type = formulation element weight |
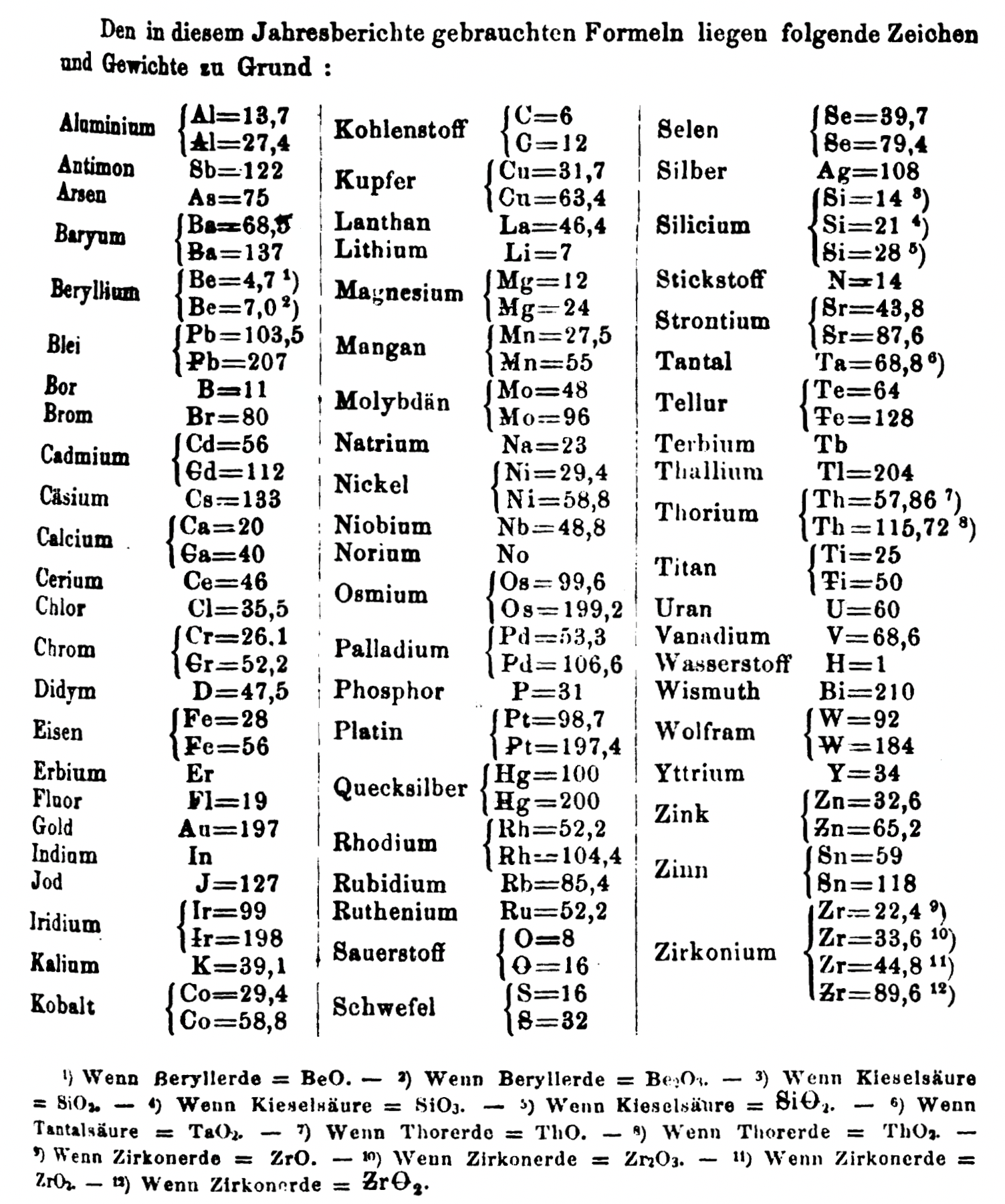
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1864
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1864 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
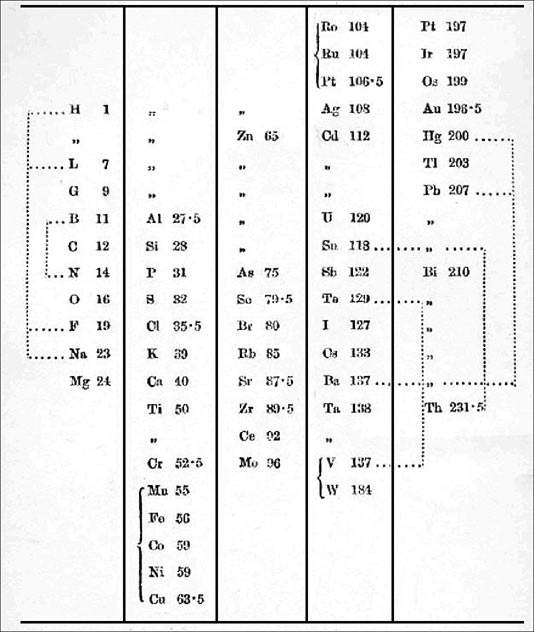
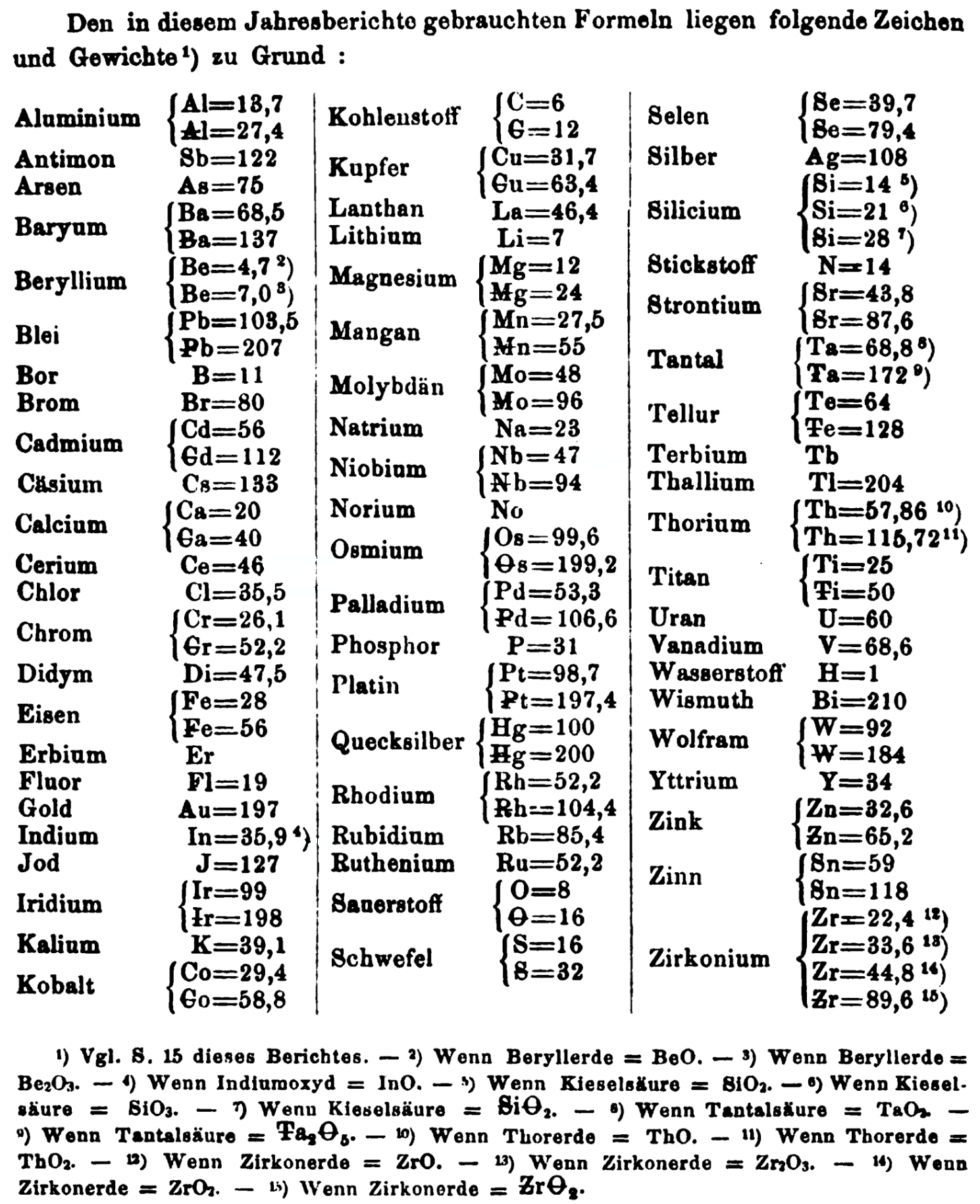
| Year: 1865 | PT id = 1355, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1865
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1865 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
| Year: 1866 | PT id = 545, Type = formulation |
Spectroscope Revelations
From Scientific American in 1866, an article by Henry Draper concerning "The Spectroscope and Its Revelations".
At the time there was no understanding how the spectra were generated but it was recognised that every element produced a unique spectrum. Over 35,000 stars are catalogued/identified by their "HD" [Henry Draper] numbers:
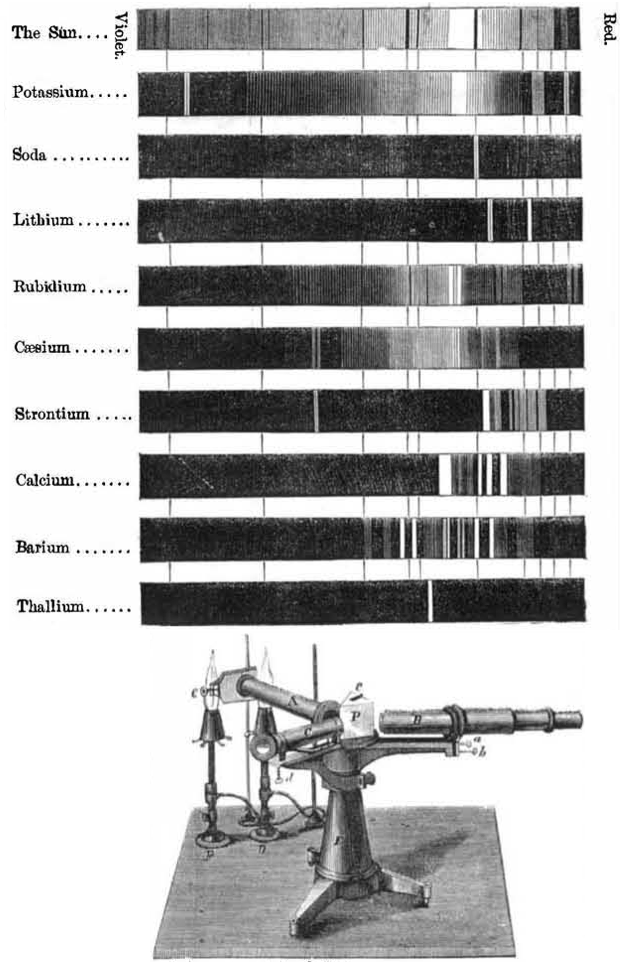
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.Also, thanks to Prof. Emeritus Robert J. Lancashire of The University of the West Indies for corrections & additional information.
| Year: 1866 | PT id = 1356, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1866
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1866 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th = 57.86 and 115.72
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
| Year: 1867 | PT id = 270, Type = formulation spiral |
Hinrichs' Programme of Atomechanics
Gustavus Detlef Hinrichs' spiral "Programme of Atomechanics". Programm der Atomechanik oder die Chemie eine Mechanik de Pantome, Augustus Hageboek, Iowa City, IA (1867).
Hinrichs' system is based on the relationship of what he called: "pantogens, with its atoms called panatoms, which explains the numerical relations of atomic weights and gives a simple classification of the elements."
This classification system culminated in 1867 in his spiral periodic table, which better clarified the groupings of elements. Hinrichs' classification, while distinctly different from the other periodic tables of this period, "seems to capture many of the primary periodicity relationships seen in the modern periodic table... it is not cluttered by attempts to show secondary kinship relationships." (Scerri)
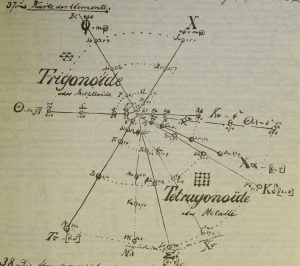
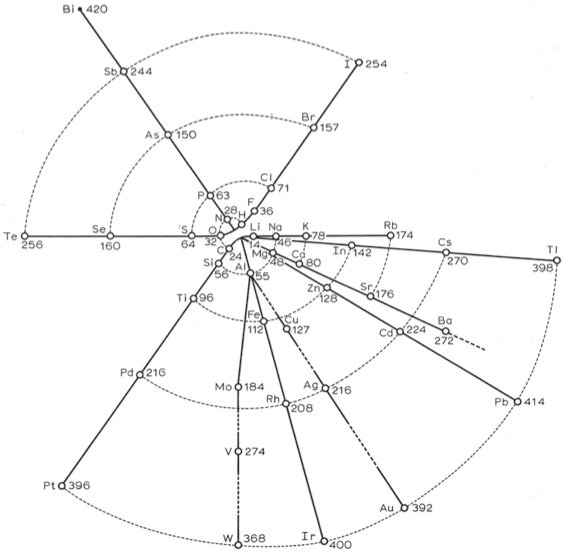
| Year: 1867 | PT id = 1357, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1867
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1867 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th =57.86 and 115.72
- W = 92 and 184
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
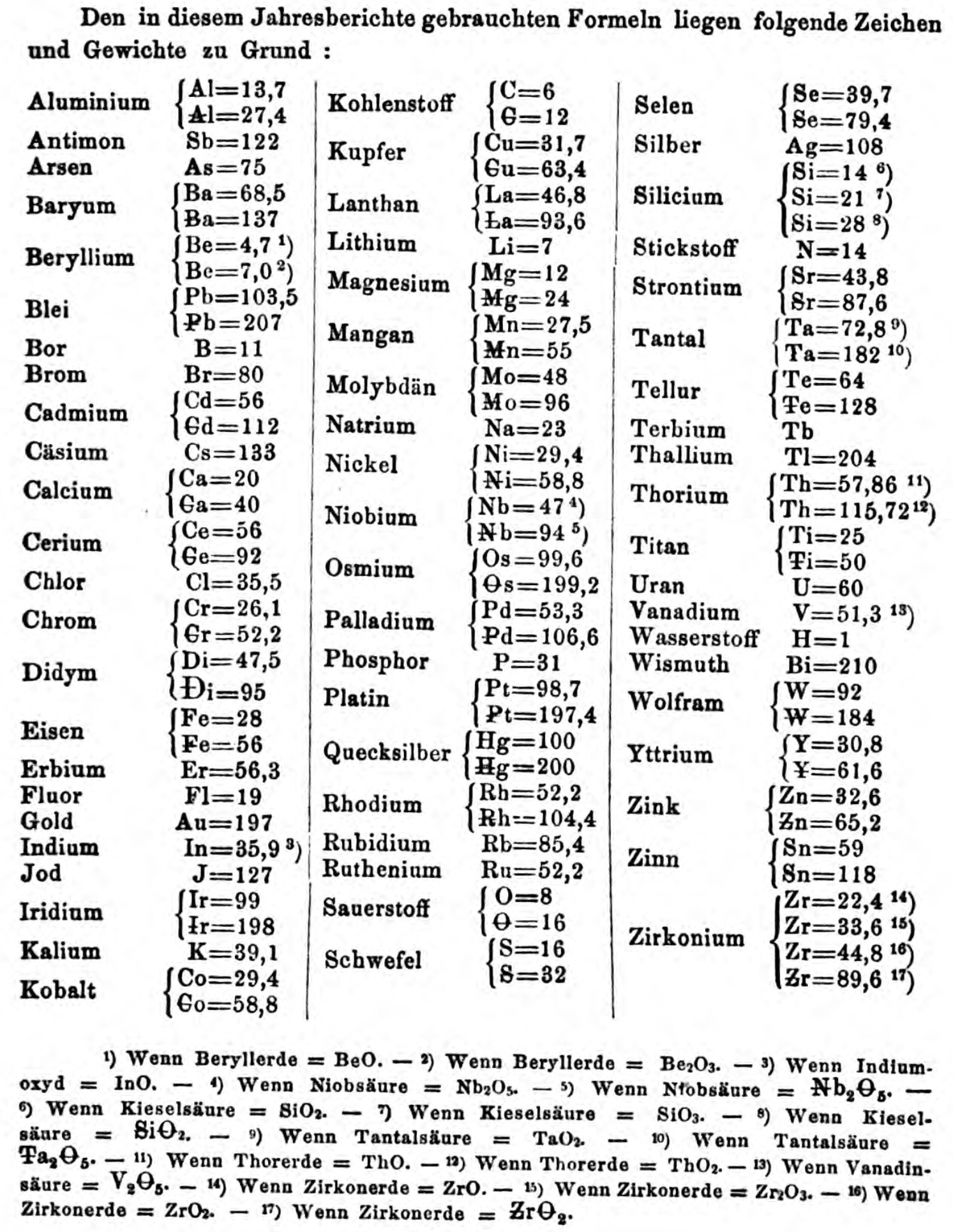
Thanks to René and Mario Rodriguez for the tip!
| Year: 1868 | PT id = 193, Type = formulation |
Handwritten draft of the first version of Mendeleev's Periodic Table
From of Bill Jensen, Curator of the Oesper Collection at the University of Cincinnati:
| Year: 1868 | PT id = 439, Type = formulation |
Meyer's "Lost" Table of 1868
In his book, The Periodic Table: A Very Short Introduction, Eric Scerri writes how Lothar Meyer produced an expanded periodic system for his1868 textbook which contained 53 elements. Unfortunately, the table was misplaced by the publisher and was not appear until after his death in 1895:
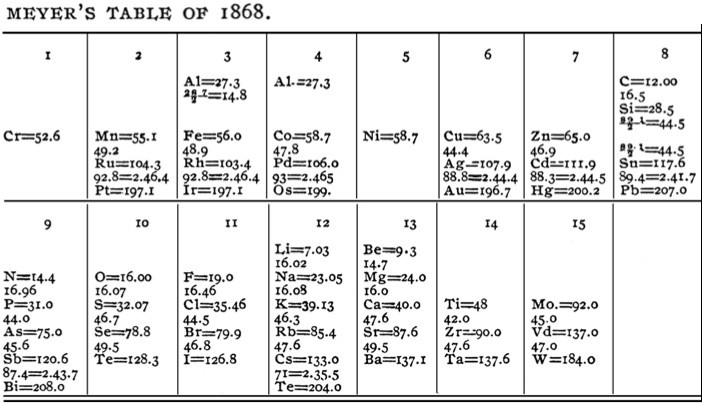
| Year: 1868 | PT id = 1358, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1868
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1868 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53.3 and 106.6
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 44.8 and 89.6
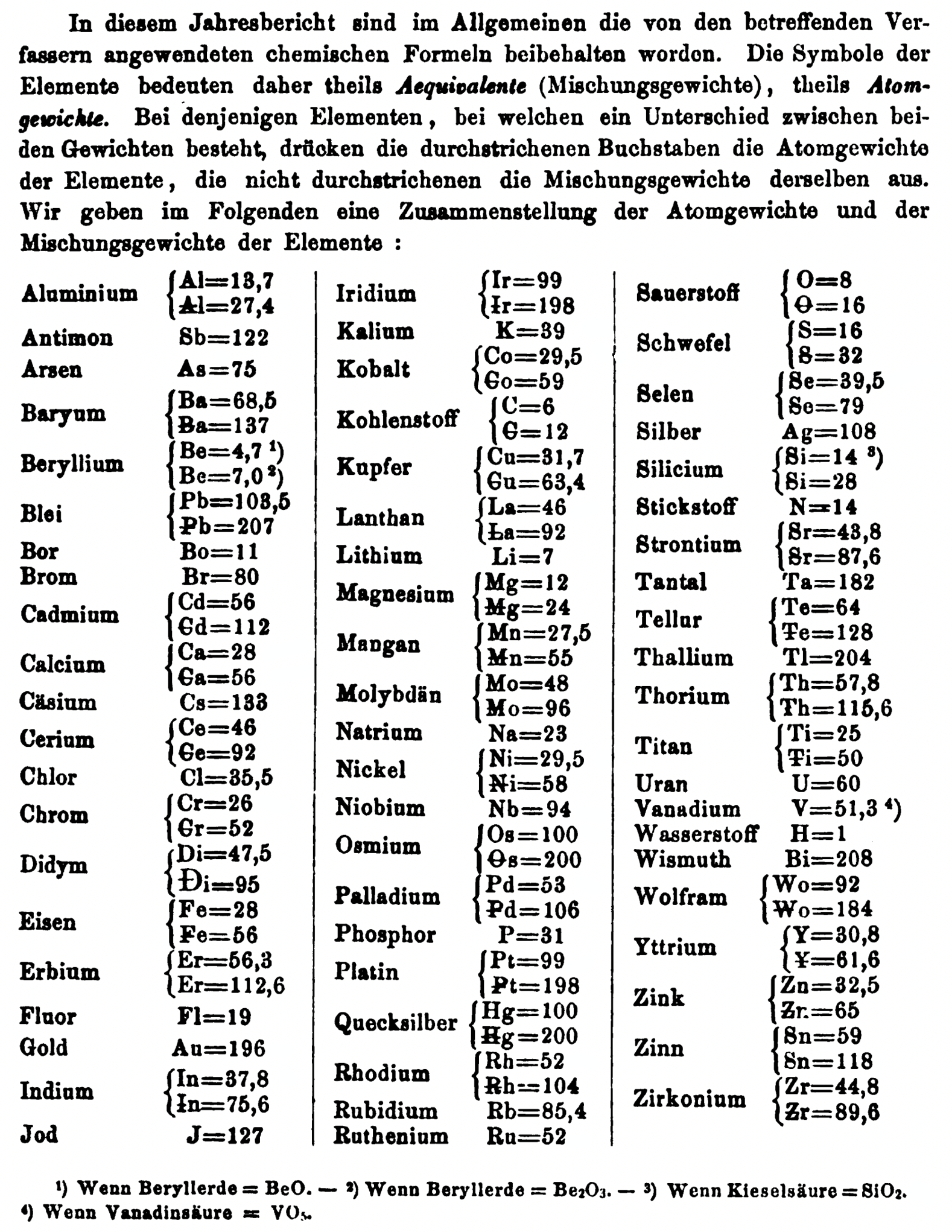
Thanks to René and Mario Rodriguez for the tip!
| Year: 1869 | PT id = 9, Type = formulation |
Mendeleev's Tabelle I
Mendeleev [also spelled Mendeleyev in English] recounted in his diary:
"I saw in a dream a table where all the elements fell into place as required. Awakening, I immediately wrote it down on a piece of paper."



Thanks to Marcus Lynch for the tip!
| Year: 1869 | PT id = 1359, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1869
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1869 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 44.8 and 89.6
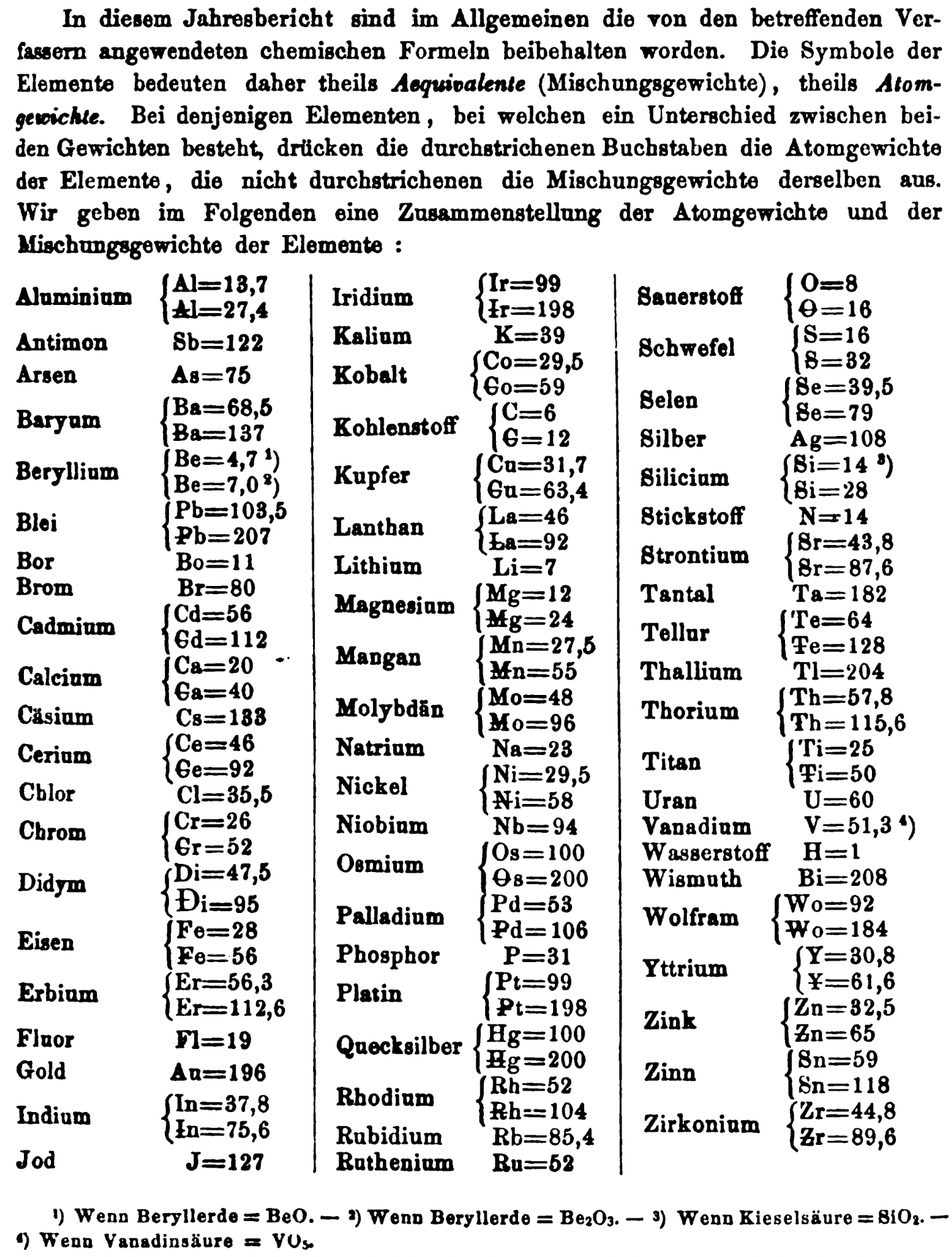
Thanks to René and Mario Rodriguez for the tip!
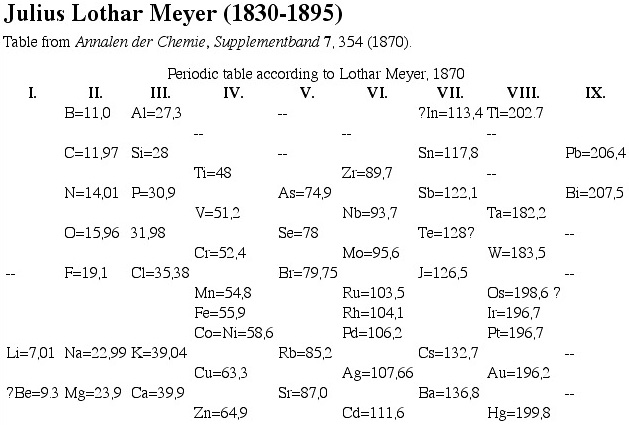
| Year: 1870 | PT id = 12, Type = formulation |
Meyer's Periodic Table. This is rather similar to the Mendeleev attempt at the same time.
| Year: 1870 | PT id = 70, Type = formulation spiral |
Baumhauer's Spiral
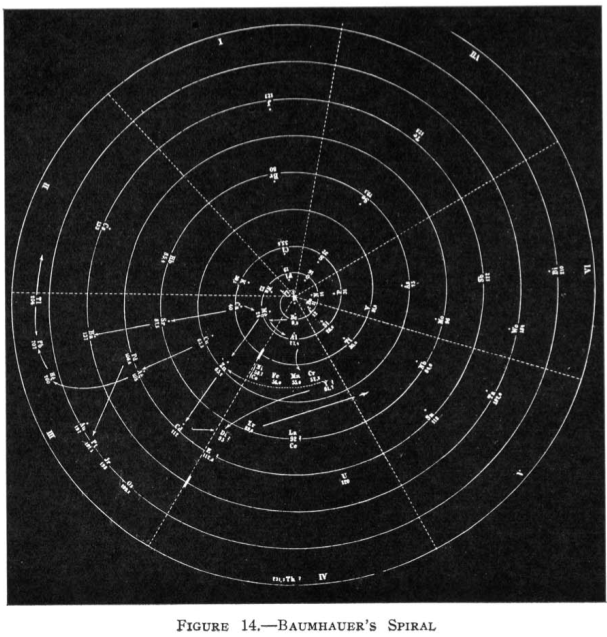
From Quam & Quam's 1934 review paper.pdf
| Year: 1870 | PT id = 454, Type = formulation data |
Baker's Electronegativity Table
Baker's electronegativity table of 1870 differs from Berzelius' listing of 1836 only by the addition of the newly discovered elements. Page 280 and ref. 5 from Bill Jensen's: Electronegativity from Avogadro to Pauling Part II: Late Nineteenth- and Early Twentieth-Century Developments, J. Chem. Educ., 80, 279-287 (2003):

| Year: 1870 | PT id = 1360, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1870
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1870 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
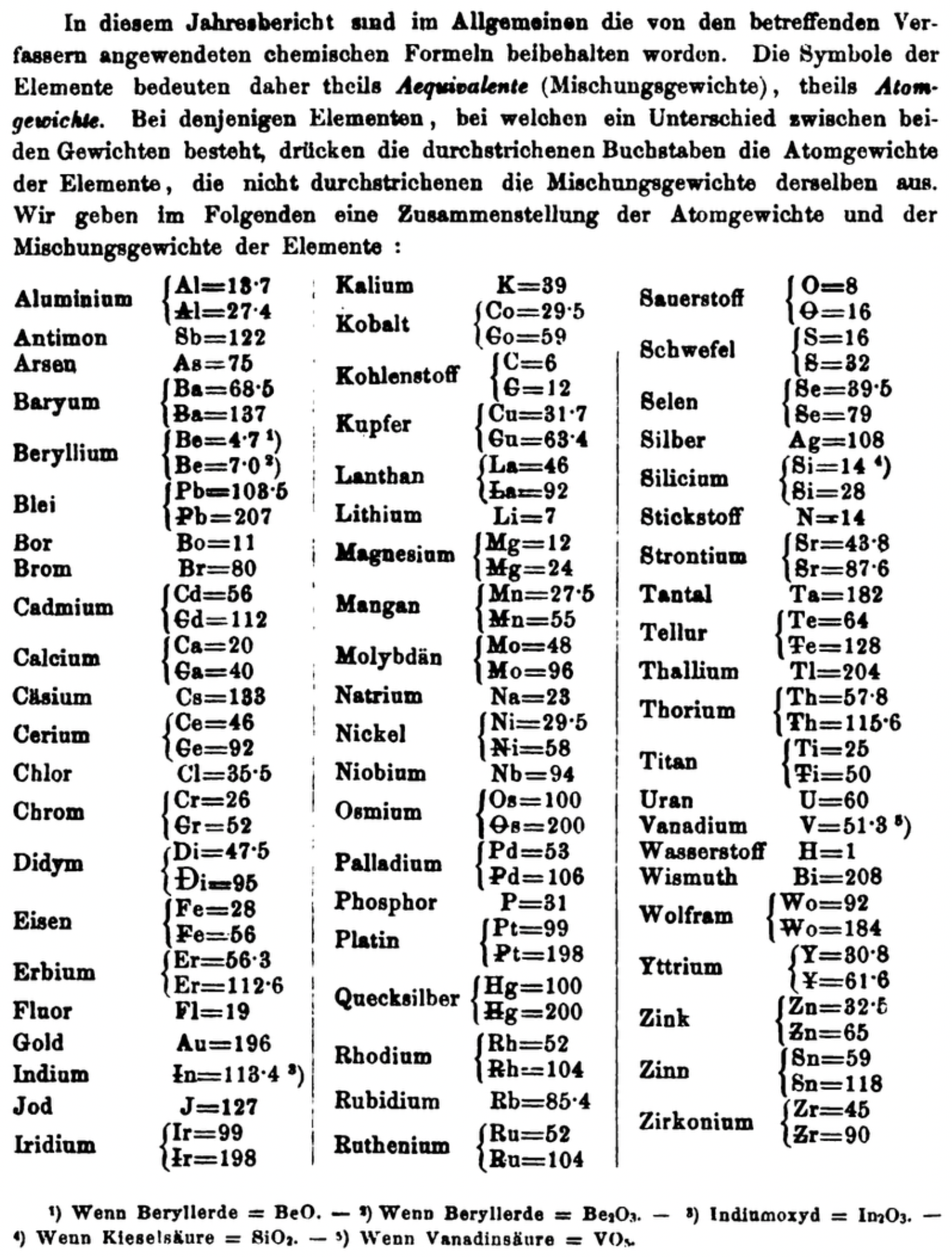
Thanks to René and Mario Rodriguez for the tip!
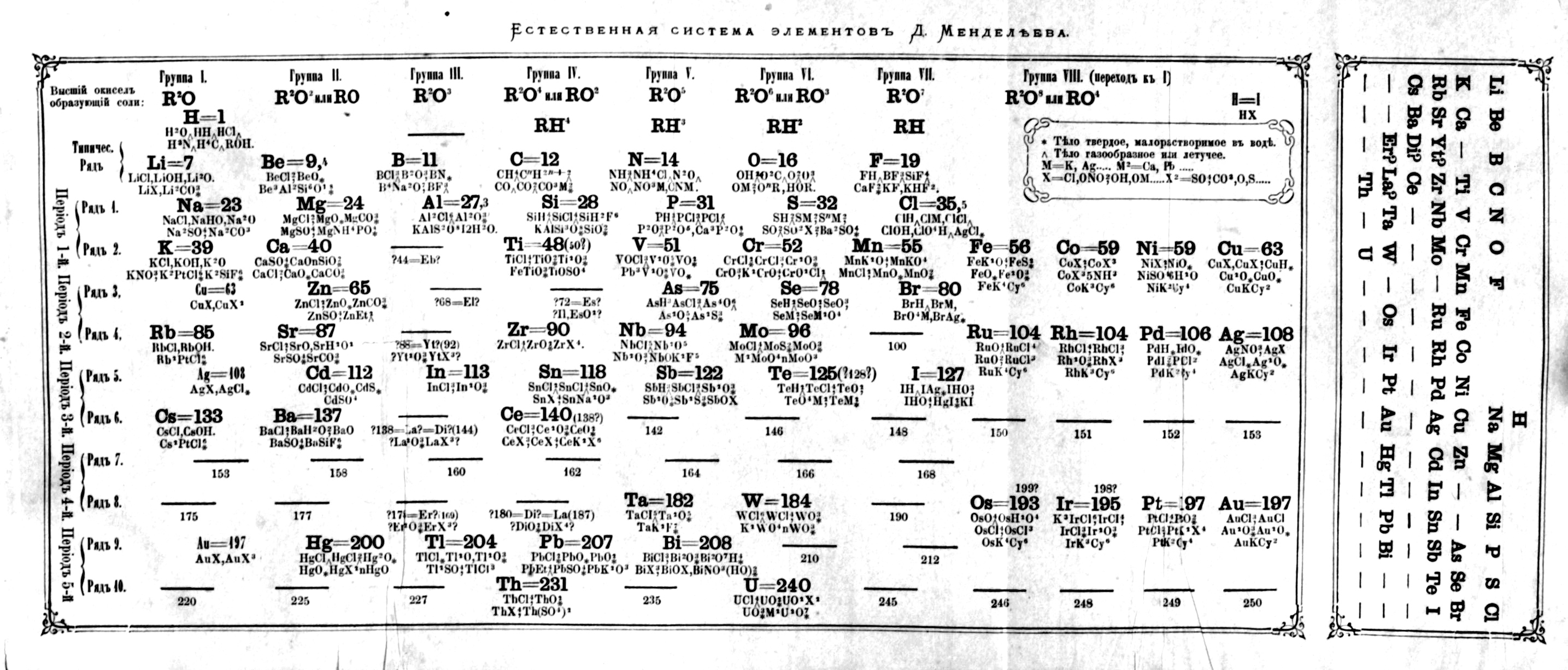
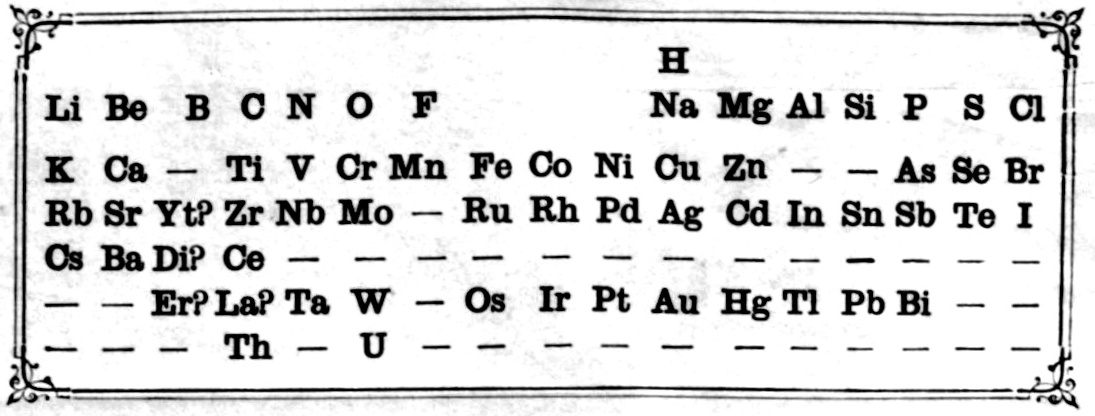
| Year: 1871 | PT id = 10, Type = formulation |
Mendeleev's Tabelle II
Some versions of Mendeleev's Tabelle II of 1871.
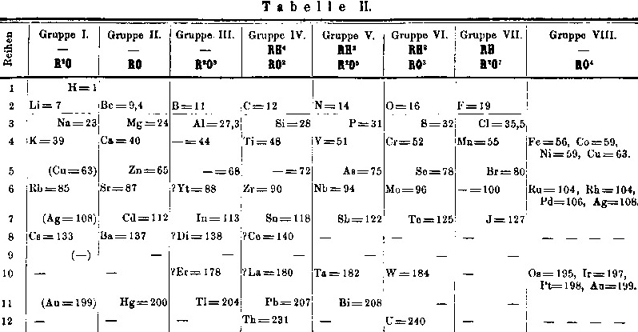
From the second volume of Mendeleev's textbook (click to enlarge):
Notice that on the right hand side, there is an additional formulation:
The two formulations above are discussed by Peter Wothers from the University of Cambridge with Sir Martyn Poliakoff, of the University of Nottingham, at 2:30 into the video below:
Mendeleev's Tabelle II can be shown in semi-modern form with the 'missing' group 18 rare gases and the f-block elements:
An alternative version of Mendeleev's Tabelle II:
| Year: 1871 | PT id = 303, Type = formulation |
Mendeleev's Predicted Elements
In large part, the success of the Mendeleev's analysis can be attributed to the gaps which he predicted would contain undiscovered elements with predictable properties. Mendeleev named these unknown elements using the terms eka, dvi & tri (1, 2 & 3 from the ancient Indian language of Sanskrit).
Mendeleev predictions include:
- Eka-boron (scandium)
- Eka-aluminium (gallium)
- Eka-manganese (technetium)
- Eka-silicon (germanium)
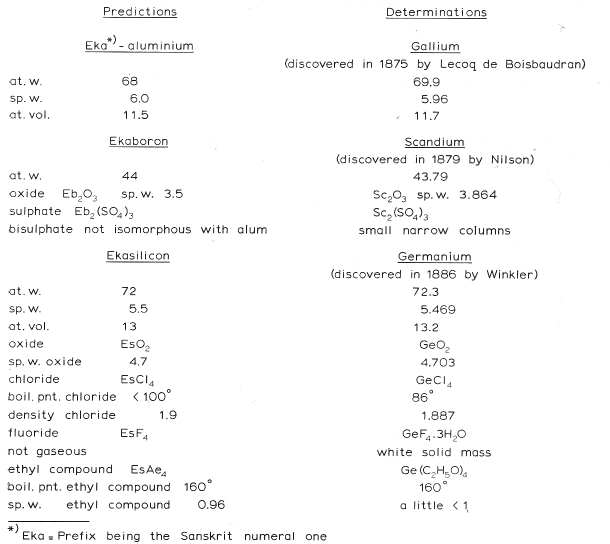
Image from van Spronsen
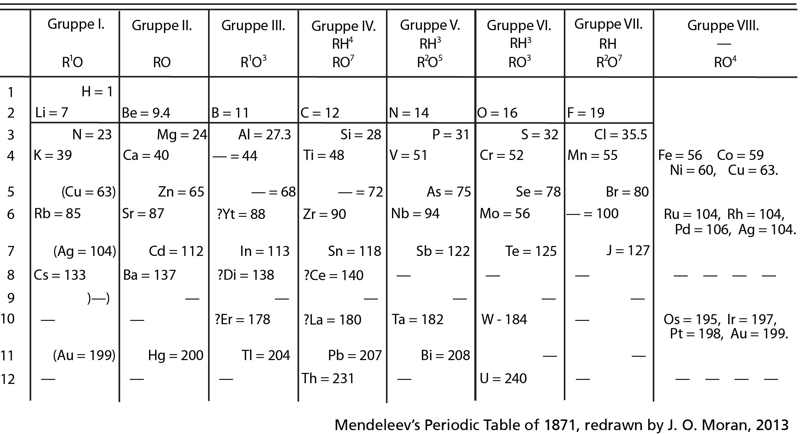
| Year: 1871 | PT id = 621, Type = formulation |
Mendeleev's Periodic Table of 1871, redrawn by J.O. Moran, 2013
Mendeleev's Periodic Table of 1871, redrawn by J.O. Moran, 2013, click here to see full size:
| Year: 1871 | PT id = 1361, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1871
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1871 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
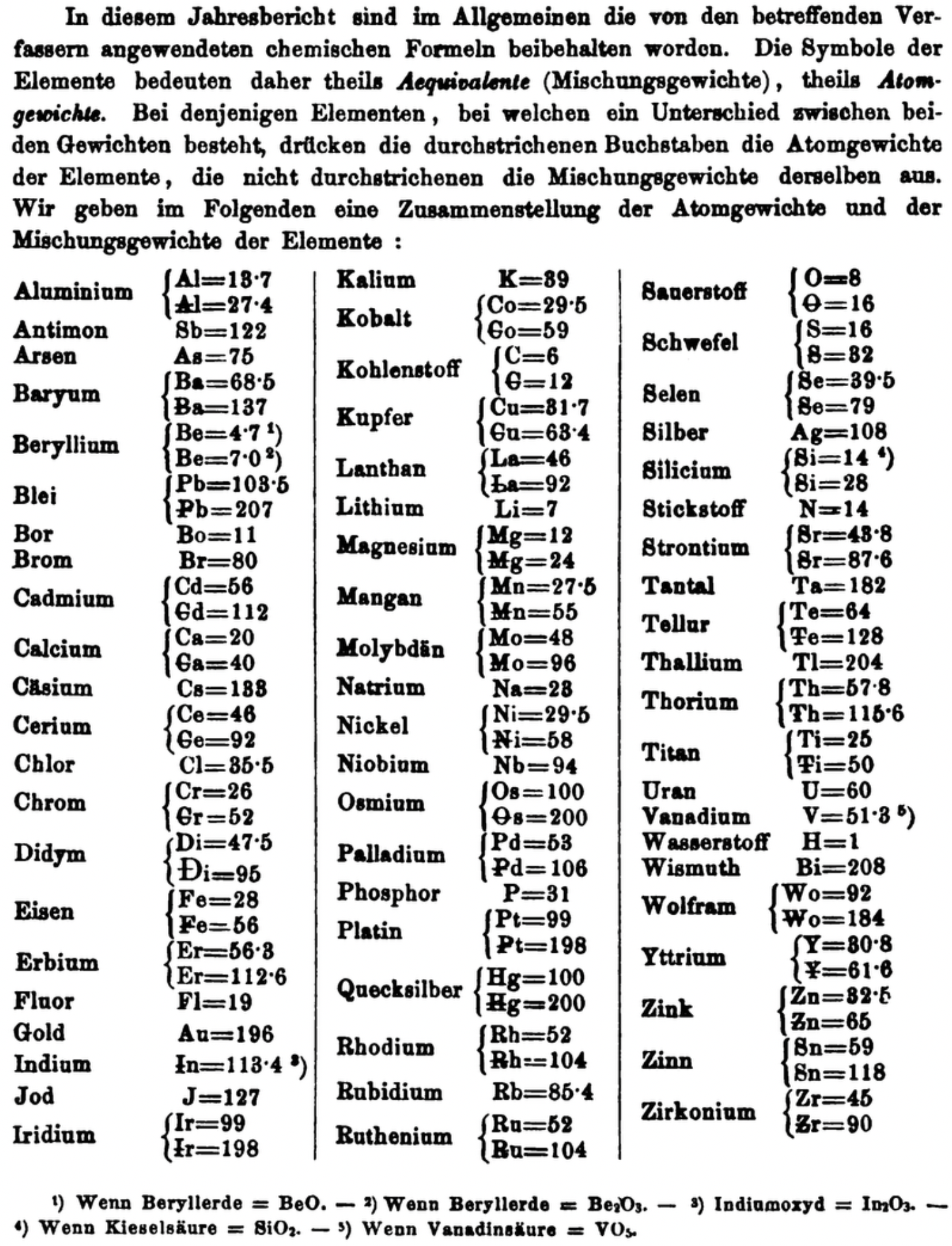
Thanks to René and Mario Rodriguez for the tip!
| Year: 1872 | PT id = 61, Type = formulation |
Mendeléeff's Vertical Table (Q&Q's Spelling)
Quam & Quam's review paper states:
"Mendeléeff's first table was a vertical arrangement in which the elements could be read in order of increasing atomic weight from the top down and in successive series from left to right. The fist column consisted of H and Li; the second, of Be to Na; the third started with Mg.
"An improved form of this table, shown below, appeared in 1872: H occupied the first position separately. The vertical series in order were Li to F, Na to Cl, K to Br, etc., causing the halogens to appear in the same bottom series, while H, Li, Na, Cu, Ag, and Au constituted a midway series, and K, Rb, and Cs formed the topmost series."
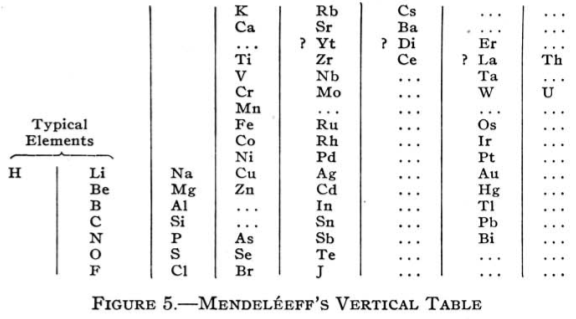
From Quam & Quam's 1934 review paper.pdf
| Year: 1872 | PT id = 284, Type = formulation spiral 3D |
Meyer's Spiral System
Meyer's Spiral System of 1872 (from van Spronsen):

| Year: 1872 | PT id = 1362, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1872
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1872 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.5 and 59
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.8 and 115.6
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 29.8 and 59.7
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
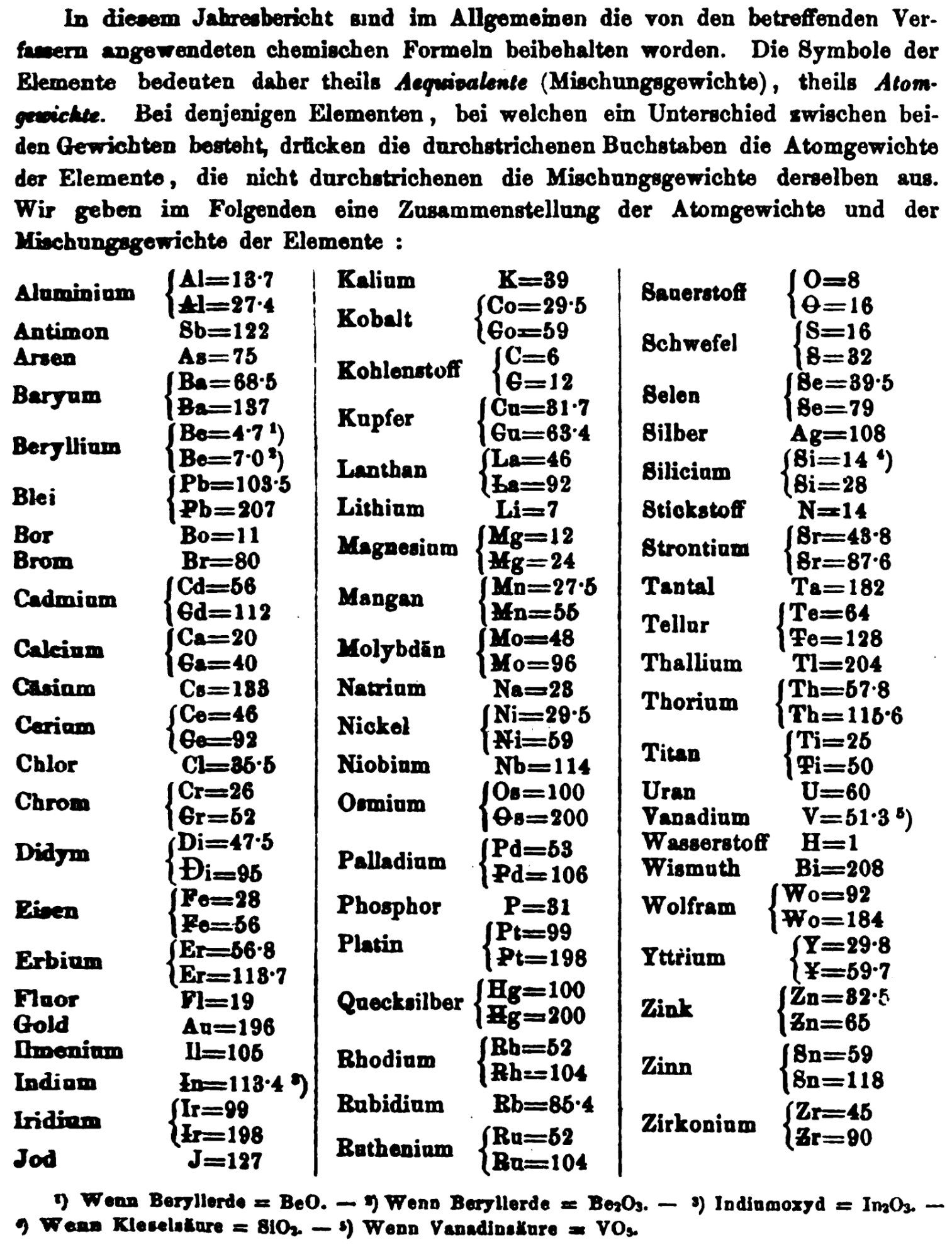
Thanks to René and Mario Rodriguez for the tip!
| Year: 1873 | PT id = 1363, Type = formulation review element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1873
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1873 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systematic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
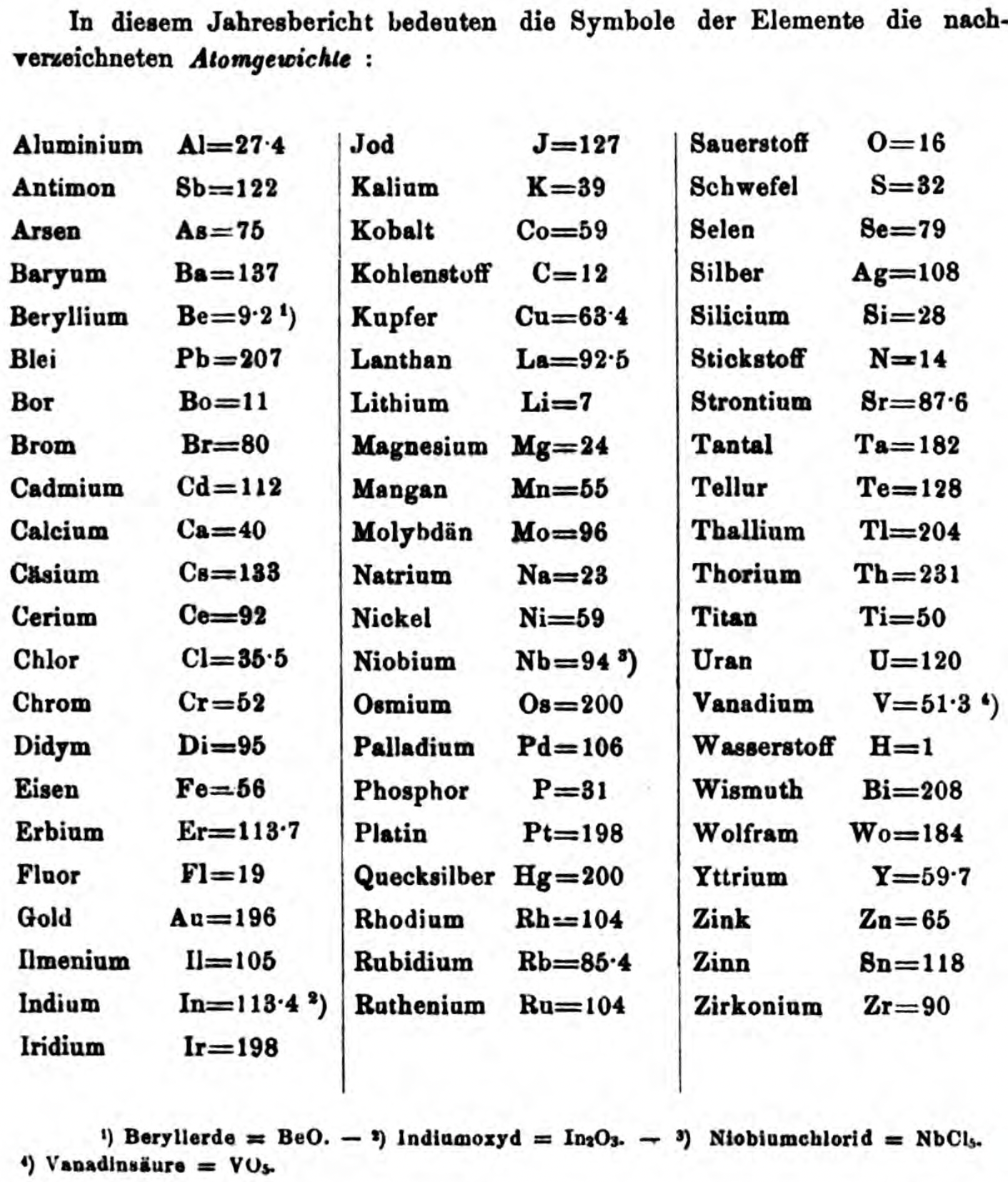
Notes:
- Didym D = 48 was actually a mixture of rare earth elements.
- Ilmenium, Il, was later found to be a mixture of niobium and tantalum.
- Generally, the elements missing had yet to be discovered (dates given below).
- The table below shows the progress from 1858 to 1873.
- By 1873 the only elements with incorrect atomic weights were the (at the time) somewhat obscure strontium, lanthanium, cerium and urananium.
- Previously, many elements were shown with two entries. Clearly, the stoichiometric and mass problems had largely been resolved (and the data agreed upon) by 1873.
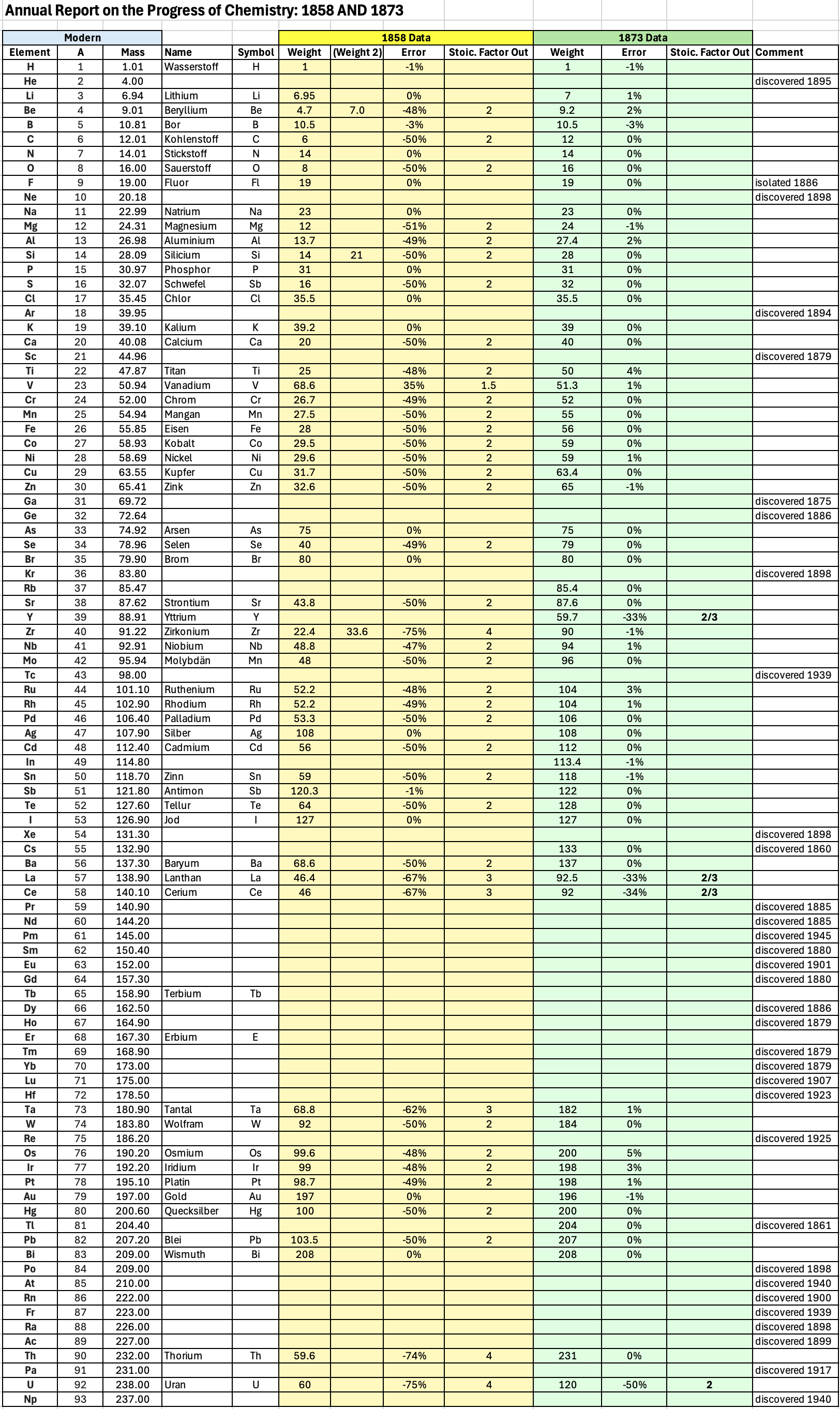
Thanks to René and Mario Rodriguez for the tip!
| Year: 1875 | PT id = 811, Type = element |
Discovery of Gallium
Ga
Gallium, atomic number 31, has a mass of 69.723 au.
Gallium was first isolated in 1875 by P. E. L. de Boisbaudran.
| Year: 1875 | PT id = 1135, Type = formulation |
Gibbes' Synoptical Periodic Table
From page 127 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

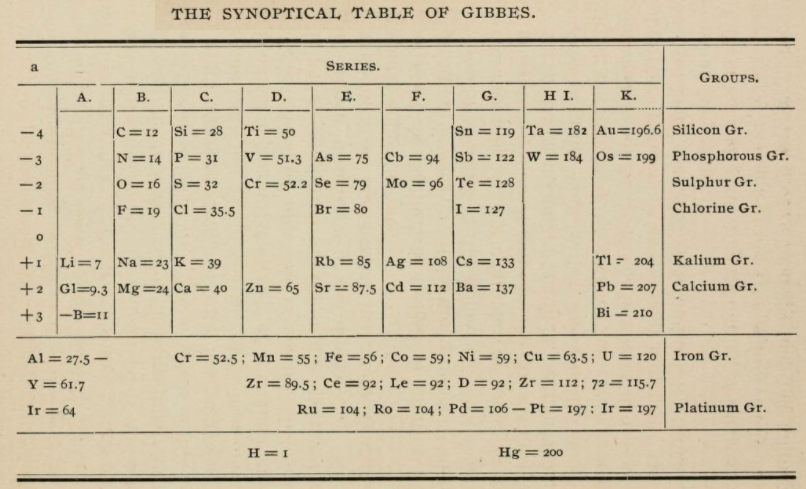
Thanks to René for the tip!
| Year: 1875 | PT id = 1136, Type = formulation spiral |
Concentric Ring Arrangement of Wiik
From page 133 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

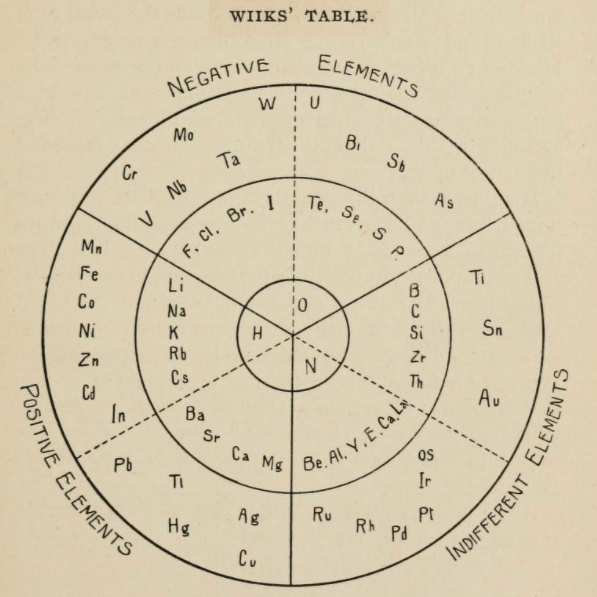
Thanks to René for the tip!
| Year: 1878 | PT id = 850, Type = element |
Discovery of Ytterbium
Yb
Ytterbium, atomic number 70, has a mass of 173.054 au.
Ytterbium was first observed or predicted in 1878 by J.C.G. de Marignac and first isolated in 1906 by C. A. von Welsbach.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
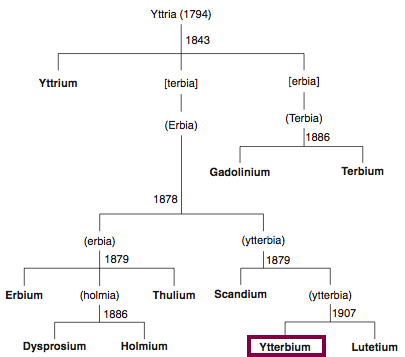
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1878 | PT id = 1137, Type = formulation |
Waechter's Numerical Regularities
From page 136 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:
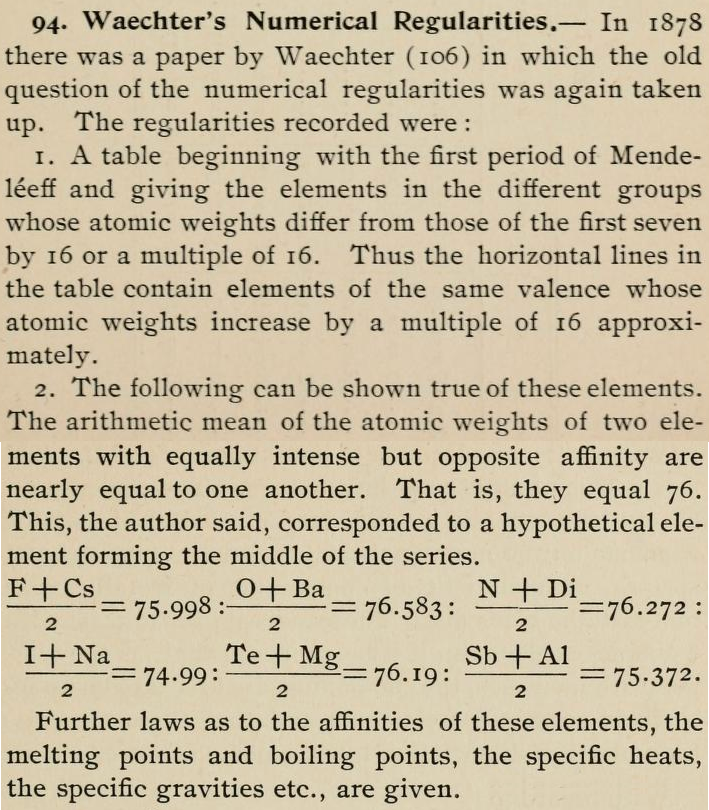
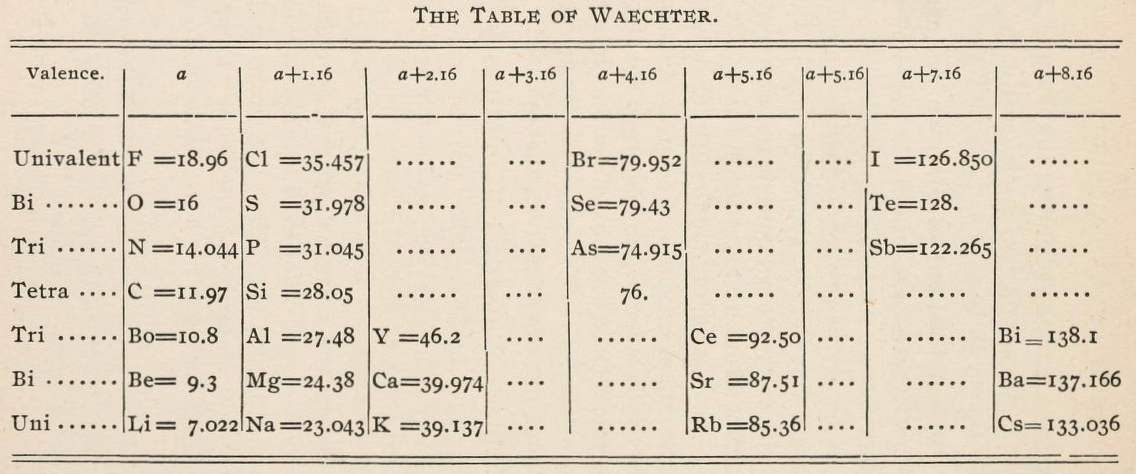
Thanks to René for the tip!
| Year: 1879 | PT id = 801, Type = element |
Discovery of Scandium
Sc
Scandium, atomic number 21, has a mass of 44.956 au.
Scandium was first isolated in 1879 by F. Nilson.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
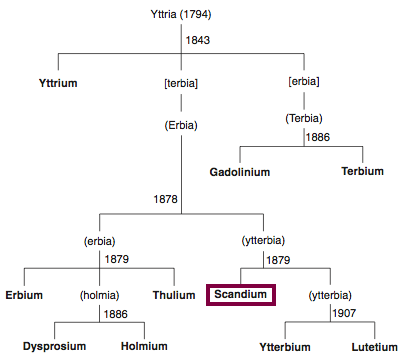
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 842, Type = element |
Discovery of Samarium
Sm
Samarium, atomic number 62, has a mass of 150.36 au.
Samarium was first isolated in 1879 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
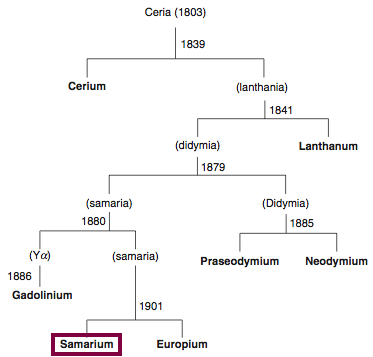
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 847, Type = element |
Discovery of Holmium
Ho
Holmium, atomic number 67, has a mass of 164.93 au.
Holmium was first observed or predicted in 1878 by J.-L. Soret and first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
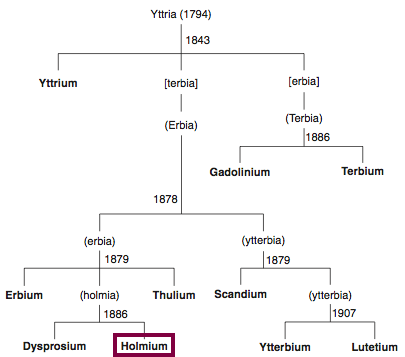
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 849, Type = element |
Discovery of Thulium
Tm
Thulium, atomic number 69, has a mass of 168.934 au.
Thulium was first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
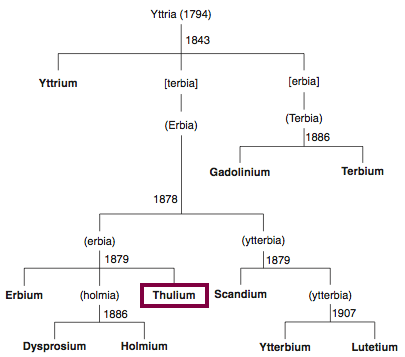
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1880 | PT id = 844, Type = element |
Discovery of Gadolinium
Gd
Gadolinium, atomic number 64, has a mass of 157.25 au.
Gadolinium was first observed or predicted in 1880 by J. C. G. de Marignac and first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
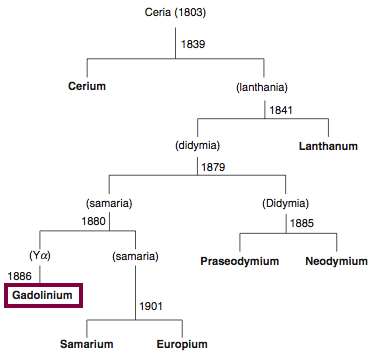
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
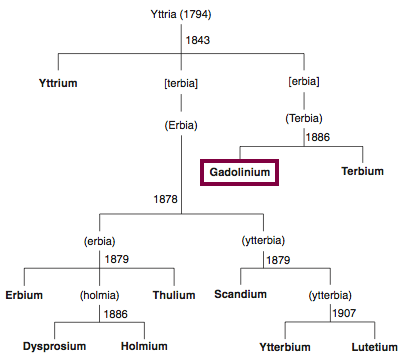
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1880 | PT id = 928, Type = formulation |
Periodische Gesetzmässigkeit der Elemente nach Mendelejeff
A lecture theatre sized periodic table, titled Periodische Gesetzmässigkeit der Elemente nach Mendelejeff, found at St Andrew's University, published and printed in Austria and dating from about from about 1880. Read more about this in The Guardian.

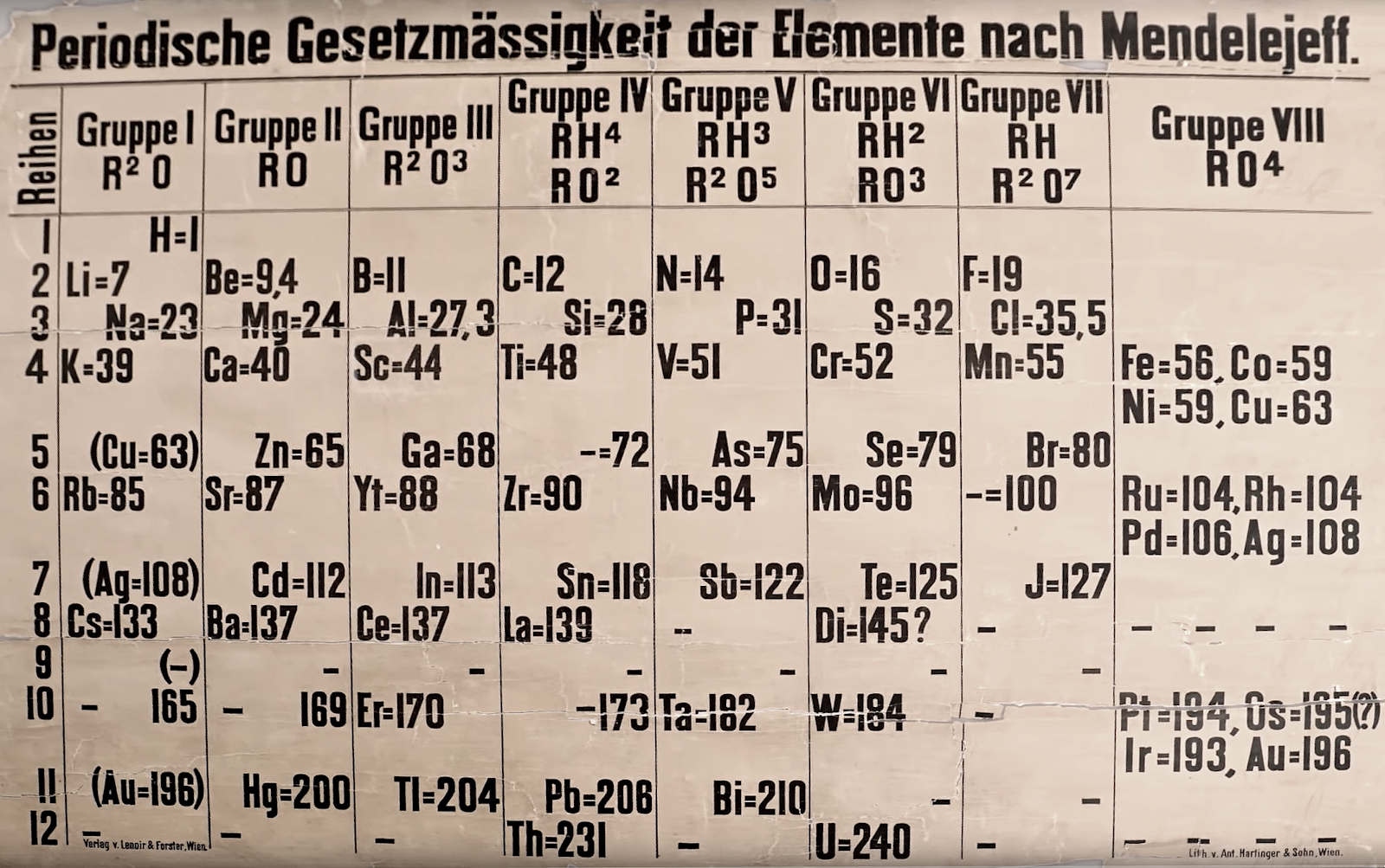
Two YouTube videos about this PT:
| Year: 1881 | PT id = 80, Type = formulation 3D spiral |
Spring's Diagram
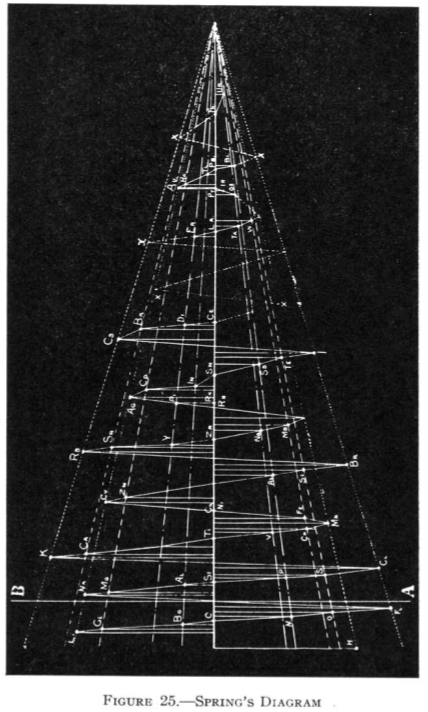
From Quam & Quam's 1934 review paper.pdf
| Year: 1882 | PT id = 66, Type = formulation |
Bayley's Periodic System
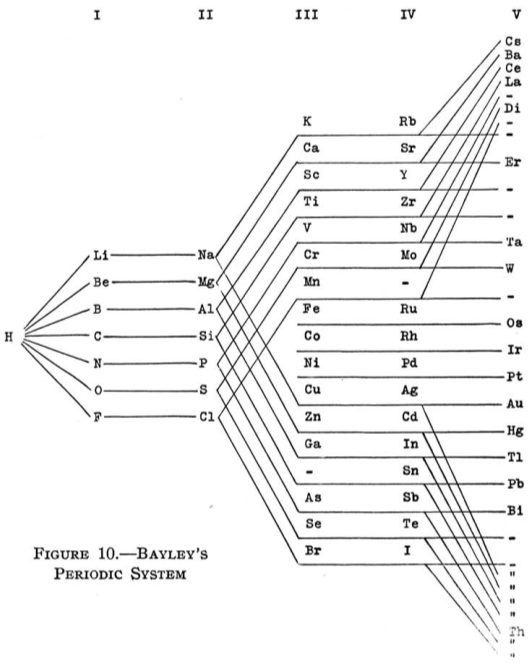
From Quam & Quam's 1934 review paper.pdf
| Year: 1882 | PT id = 363, Type = formulation |
Brauner's Periodic Table
Brauner's periodic table of 1882 with a homologous accommodation of the rare-earth elements, from Chemische Berichte, 15, 1882, p. 15-121:
| Year: 1882 | PT id = 1138, Type = formulation |
Bayley's Attempt
From page 158 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes about Bayley:

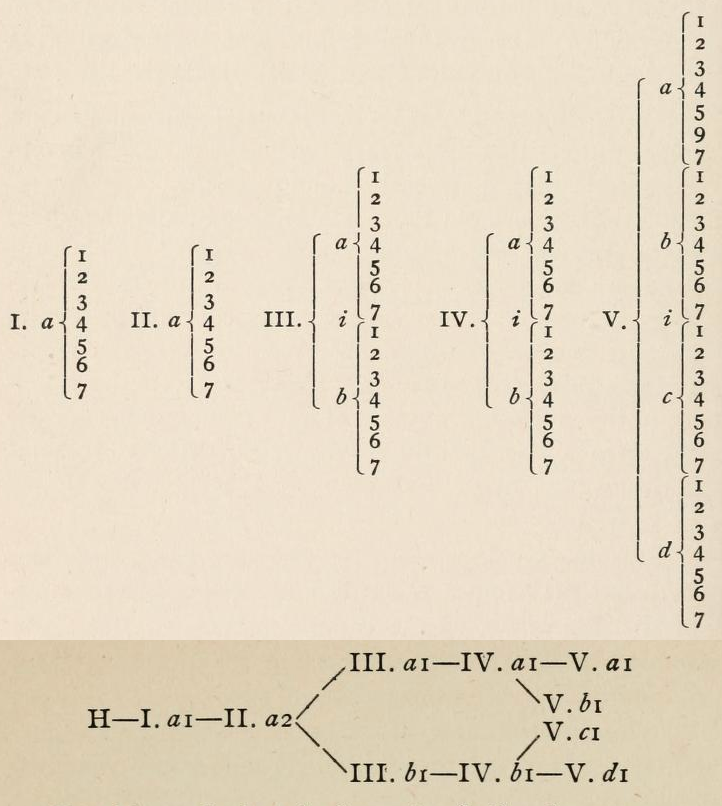
Thanks to René for the tip!
| Year: 1885 | PT id = 839, Type = element |
Discovery of Praseodymium
Pr
Praseodymium, atomic number 59, has a mass of 140.908 au.
Praseodymium was first isolated in 1885 by Carl Auer von Welsbach.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
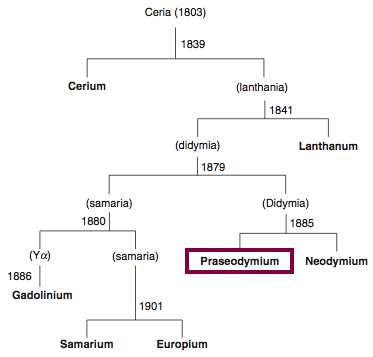
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1885 | PT id = 840, Type = element |
Discovery of Neodymium
Nd
Neodymium, atomic number 60, has a mass of 144.242 au.
Neodymium was first isolated in 1885 by Carl Auer von Welsbach.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
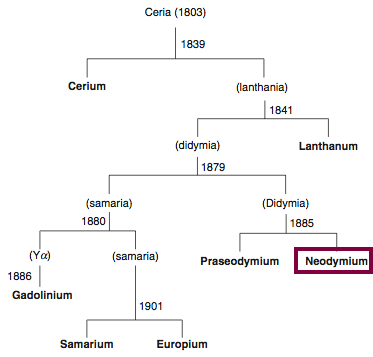
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1885 | PT id = 1139, Type = formulation |
Carnelley & The Periodic Law
From page 172 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:

Thanks to René for the tip!
| Year: 1885 | PT id = 1141, Type = formulation |
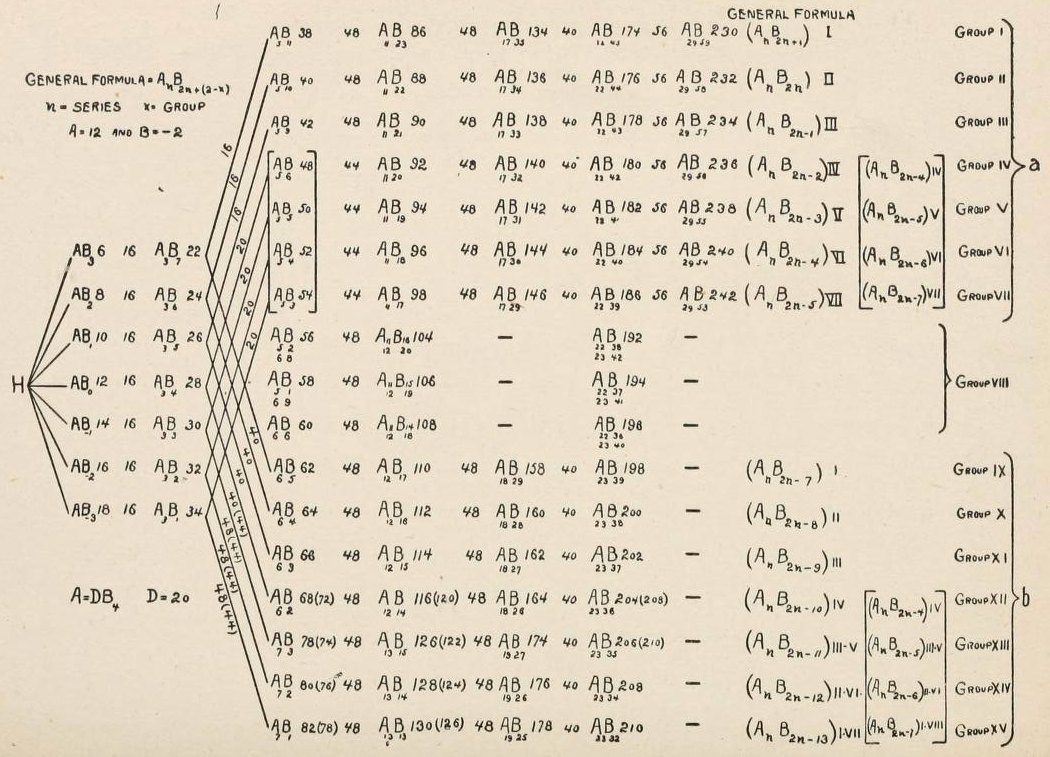
Klieber's Cosmochemical Periodic Table
Klieber's qualitative synthesis of the general composition of celestial objects in the form of a plane periodic system following atomic numbers. His diagram is probably one of the earliest versions of a "cosmochemical periodic table". (The diagram below is clearly redrawn as it has a very modern style.)
I.A. Kleiber, Zh. Russ. Fiziko-Khim. Obshch (St. Petersburg) 1885, 17, 147-171.
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
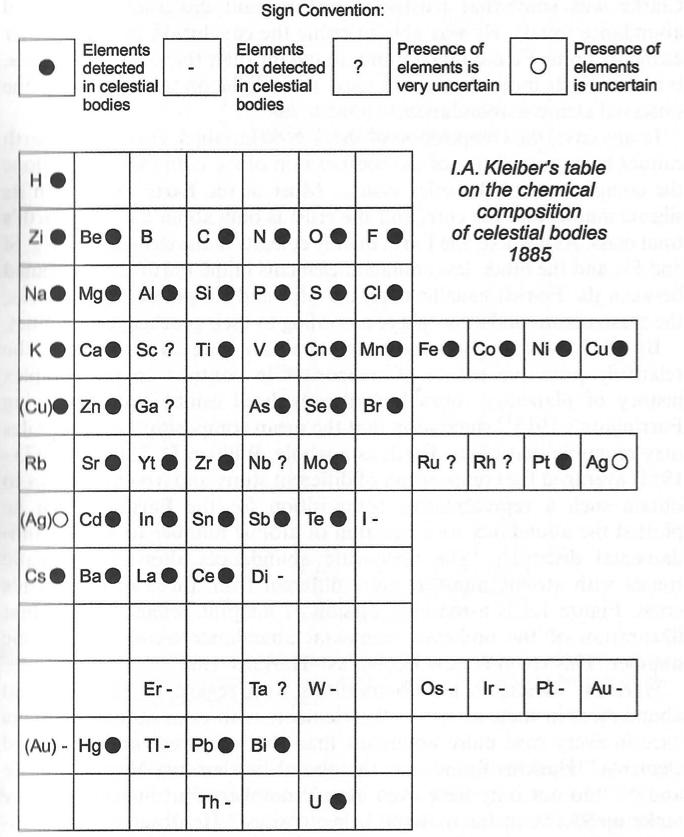
| Year: 1885 | PT id = 1145, Type = formulation review |
von Richter's Periodic System of the Elements
From page 244 of A Text-book of Inorganic Chemistry by Victor von Richter, Published by Blakiston (US ed. in English, 1885). The full text (scanned) is available from archive.org. The first edition was published in 1874 in German. von Richter was was from the Baltic region, in the the Russian empire at the time.
von Richter's work is almost certainly the first chemistry textbook based on the periodic system. Many (indeed most) modern Inorganic Chemistry texts follow this format, but NOT the Chemogenesis web book!
von Richter, writes:

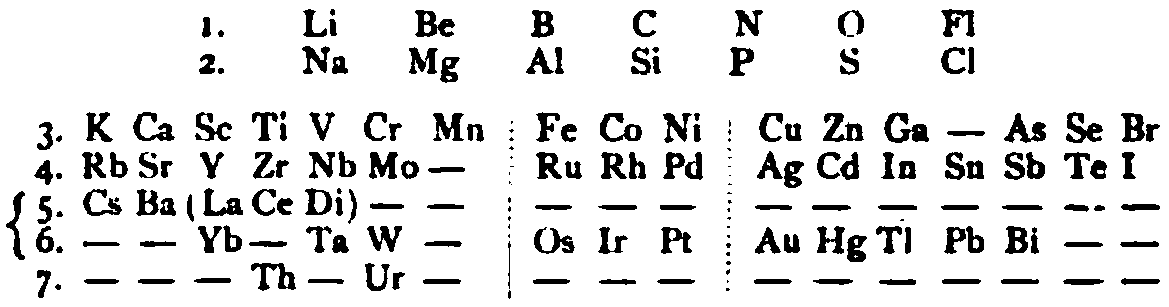

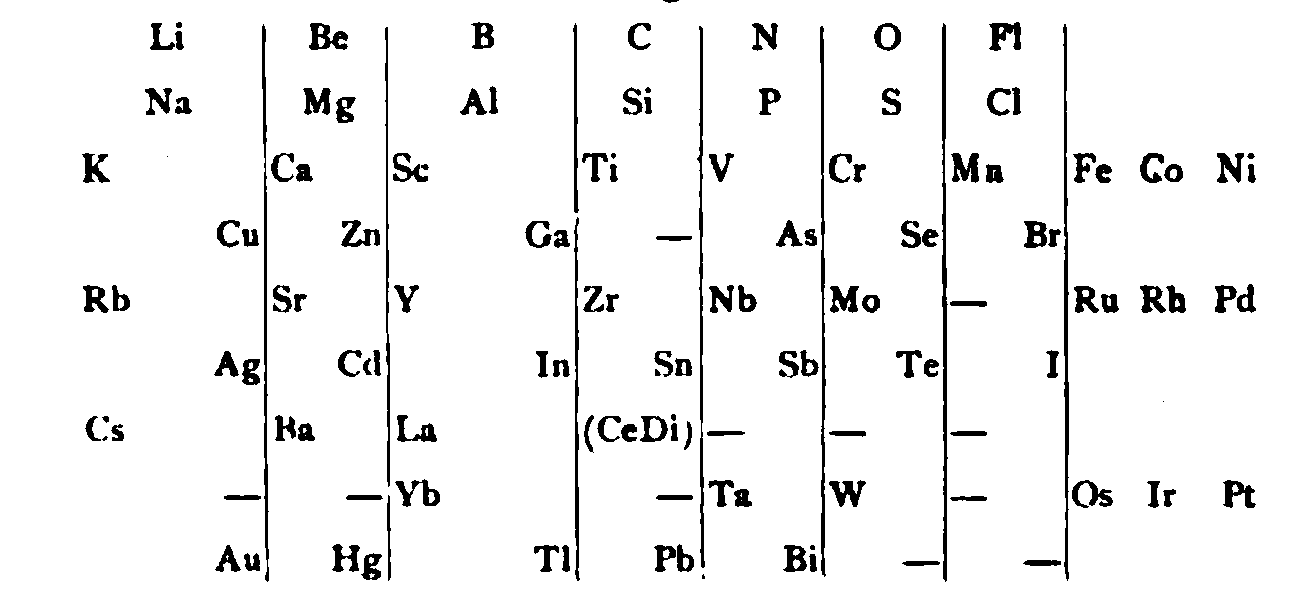
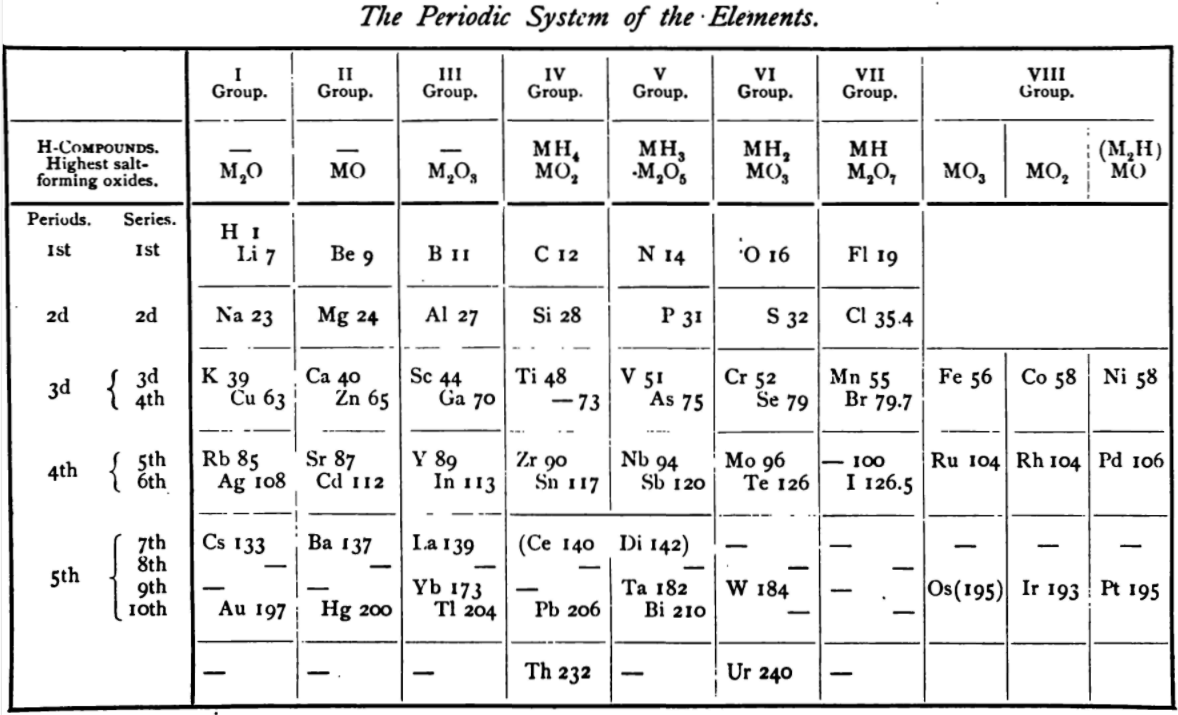
Thanks to René for the tip!
| Year: 1886 | PT id = 81, Type = formulation |
Crookes' Periodic Table
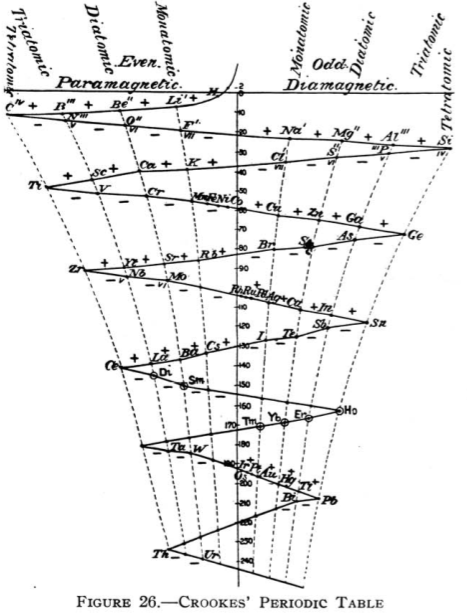
From Quam & Quam's 1934 review paper.pdf
| Year: 1886 | PT id = 798, Type = element |
Discovery of Fluorine
F
Fluorine, atomic number 9, has a mass of 18.998 au.
Fluorine exists as a pale yellow diatomic molecular gas, F2. It is the most electronegative and reactive of all elements: it which reacts with practically all organic and inorganic substances.
Fluorine was first observed or predicted in 1810 by A.-M. Ampére and first isolated in 1886 by H. Moissan.
| Year: 1886 | PT id = 812, Type = element |
Discovery of Germanium
Ge
Germanium, atomic number 32, has a mass of 72.63 au.
Germanium was first isolated in 1886 by C. A. Winkler.
| Year: 1886 | PT id = 846, Type = element |
Discovery of Dysprosium
Dy
Dysprosium, atomic number 66, has a mass of 162.5 au.
Dysprosium was first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
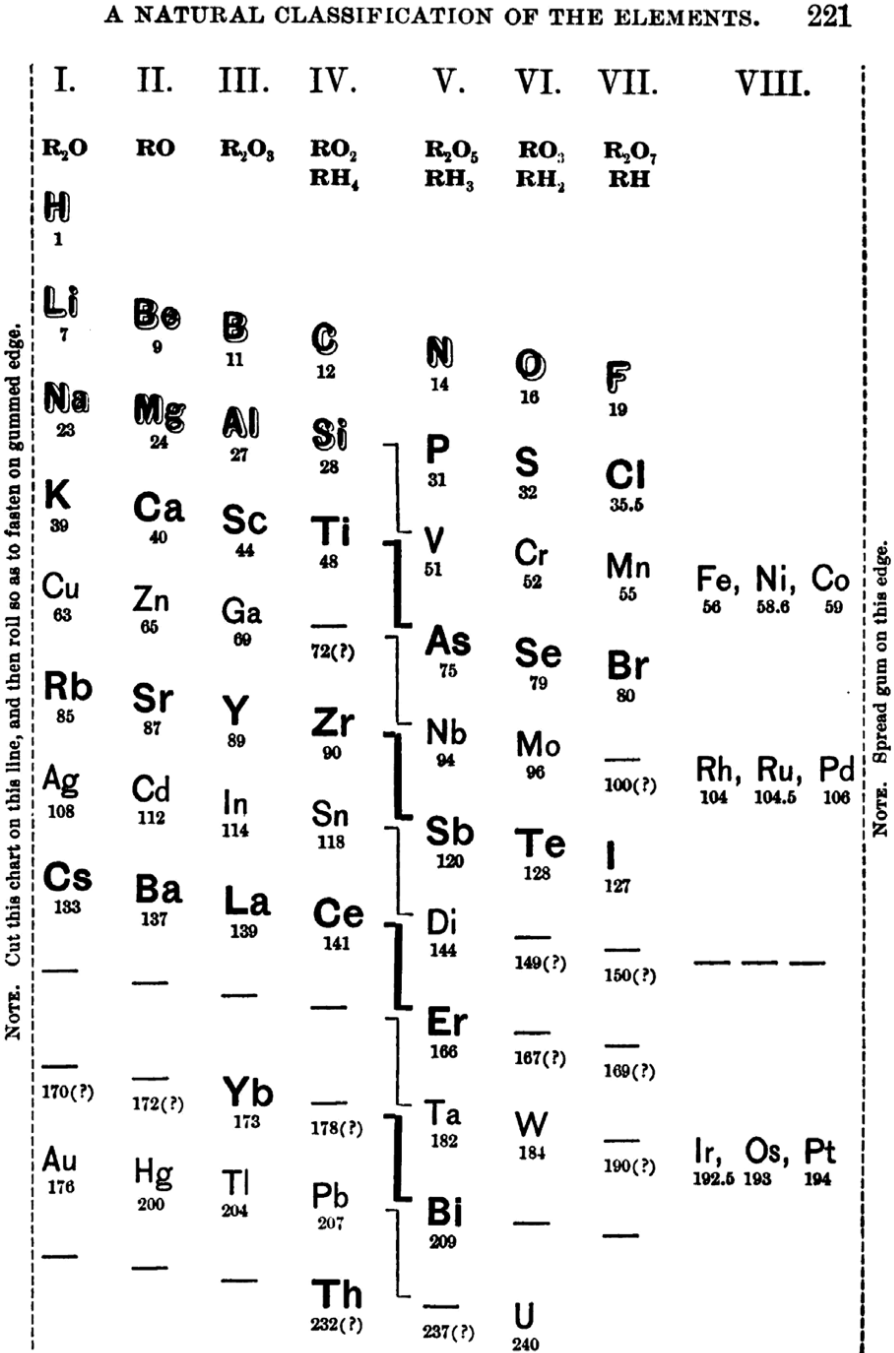
| Year: 1886 | PT id = 1107, Type = formulation spiral 3D |
Shepard's Natural Classification
Shepard's Natural Classification of the Elements, a spiral formulation with instructions for turning it into a three-dimensional table. From: Elements of Inorganic Chemistry, Descriptive and Qualitative (pp221), by J. H. Shepard, (1886), Boston MA, pub. D. C. Heath
René Vernon writes:
Note the instructions along the side, to turn the table into a tube (spiral form) and the 19 spaces from La to eka-Ce. Here, Yb needs to be moved back one column into group II, so as to leave room for Lu under La. Then eka-Ce becomes Hf. This results in La + 15 lanthanoids.
The accompanying text says:
"Elements of most distinct basic character are found towards the left; non-metals predominate in the upper and middle parts of Groups V., VI., and VII. ; while the lower part of the table is marked by the more indifferent elements.
"A double spiral will be traced beyond Si (beginning with P and V respectively) and distinguished by heavy-face and light-face type.
"The harmony of nature here exhibited is most impressive. Is it possible that the so-called elements are really compounds? Did the various 'elements' of the earth and sun once exist as hydrogen, when our solar system was a nebula? And will modern chemists ever revive the famed problem of the alchemists, and seek to turn the base metals into gold? Far more precious than gold is the search for truth; and the more we learn of science, the broader becomes our conception of what we know in part, and the deeper should be our reverence for the infinite thought of the Creator."
| Year: 1886 | PT id = 1108, Type = formulation |
Reynolds' Method of Illustrating the Periodic Law
Reynolds, J. E. (1886). Note on a method of illustrating the periodic law. Chemical News, 54, 1–4
Ann E. Robinson comments:
"Reynolds published a zig-zag version he created, noting it had been 'used in my lecture-room for some years in order to illustrate the periodic character of the relation between the atomic weights and properties of the chemical elements.'
>"Rather than a tabular form, Reynolds's form was a curve, best visualised as a string with seven knots tied in it at regular intervals, providing a 'vibrating system'. Although Reynolds's diagram is presented only as a two-dimensional picture, in a note he remarks that a friend suggested that the installation of 'a vibrating metallic chain, suspended from the ceiling and attached to the floor, would afford a more complete picture.'":
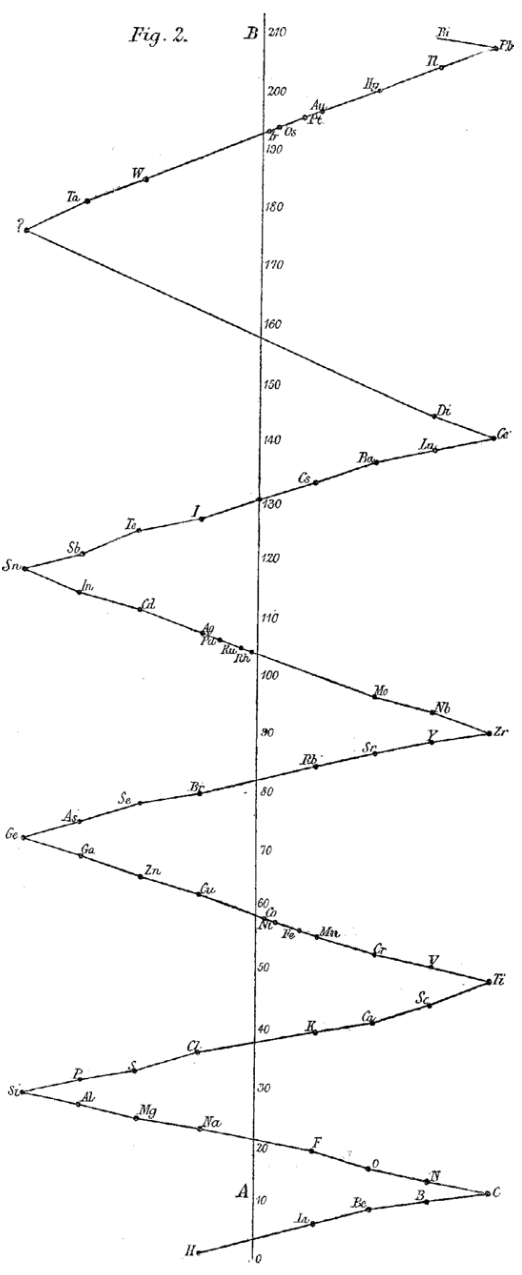
| Year: 1887 | PT id = 82, Type = formulation spiral |
Flavitzky's Arrangement
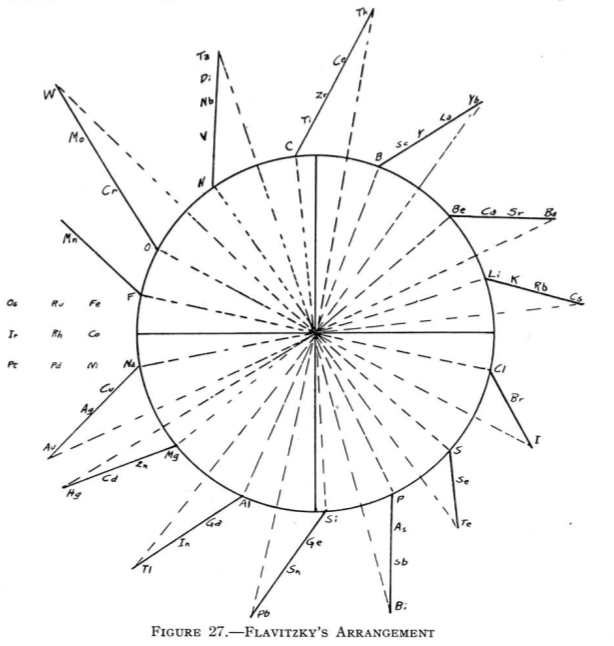
From Quam & Quam's 1934 review paper.pdf
| Year: 1888 | PT id = 997, Type = formulation spiral |
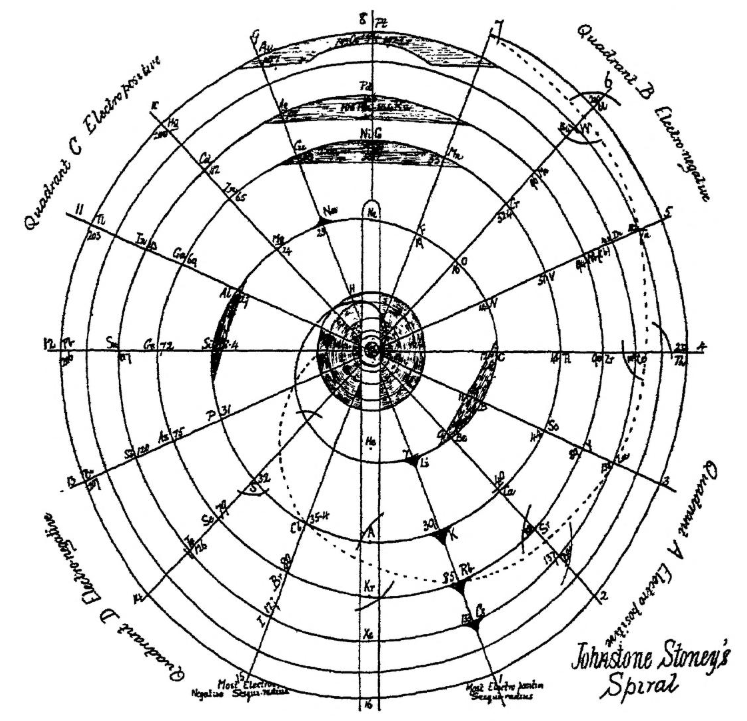
Stoney's Spiral
Johnstone Stoney's Spiral, taken from A. E. Garrett's The Periodic Law (page 167, 1909 pub. D. Appleton And Company). The reference is given – page 167 – is: Phil. Mag. [6], 4, pp 411 et seq.; Proc. Roy. Soc., 1888, p115.
Thanks to Roy Alexander for the tip!
| Year: 1888 | PT id = 1267, Type = formulation spiral |
Stoney's Spiral Periodic Table
In the Proceedings of the Royal Society of London, Series A, Containing Papers of a Mathematical and Physical Character, Volume 85, Issue 580, Aug 1911, p. 472, there is an article On Dr. Johnstone Stoney's Logarithmic Law of Atomic Weights, by Lord Rayleigh (who co-discovered argon in 1894), who writes :
"In the year 1888, Dr. G. Johnstone Stoney communicated to the Society a memoir with title nearly as above, which, however, was not published in full. At the request of the author, who attaches great importance to the memoir, I have recently, by permission of the Council, consulted the original manuscript in the archives of the Society, and I propose to give some extracts, accompanied by a few remarks. The author commenced by plotting the atomic weights of the elements taken as ordinates against a series of natural numbers as abscissæ. But a curve traced through the points thus determined was found to be 'one which has not been studied by mathematicians.
"This sudden transition may have some connection with the fact that no elements have been found on sesqui-radius 16, although the investigation in § 3 shows that the values of m corresponding to the stations on sesqui-radius 16 cannot be dispensed with.
"The vacant places here pointed out are now occupied by the since discovered inert gases. The anticipation is certainly a remarkable one, and it goes far to justify the high claims made for the diagram, as representing in a telling form many of the leading facts of chemistry."
Comment from Mark Leach:
"Notice how the electronegative elements are positioned top right & bottom right and the electropositive elements top left & bottom right."
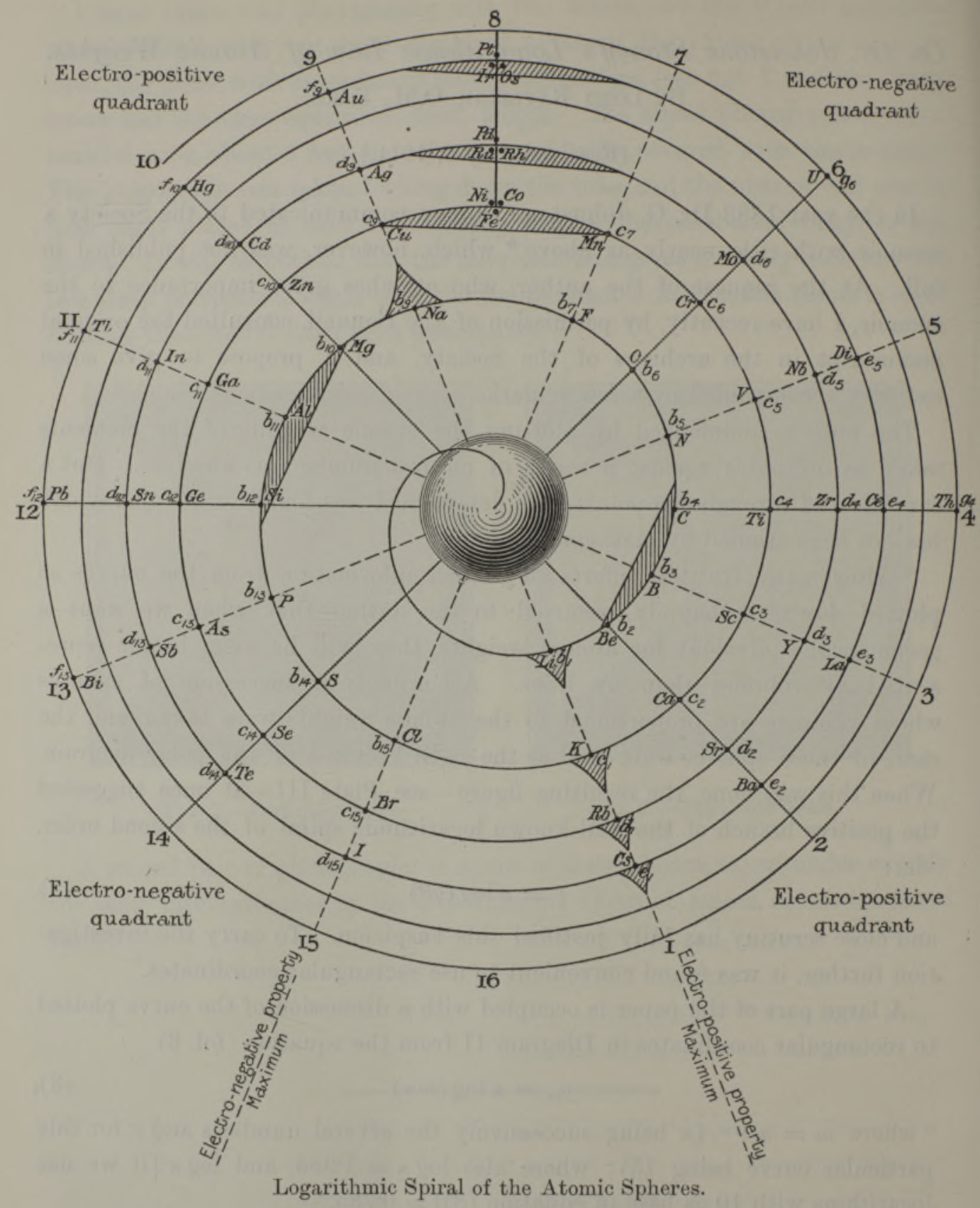
René Vernon writes:
"Stoney has another article in the September 1902 edition of the The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, called Law of Atomic Weights, pp. 411–415. At the back of the journal is an updated fold-out version of Stoney’s table, image attached.
- Ar, Kr and Xe fit on the spiral, and on spoke 16.
- Neon fits on the spiral but is instead on spoke 8.
- Helium is on spoke 18 but is not on the spiral.
- The circle in the middle represents H (p. 414).
"On the page after the updated spiral, there looks to be some printed content, but it is hidden by what looks to be a folded over page."
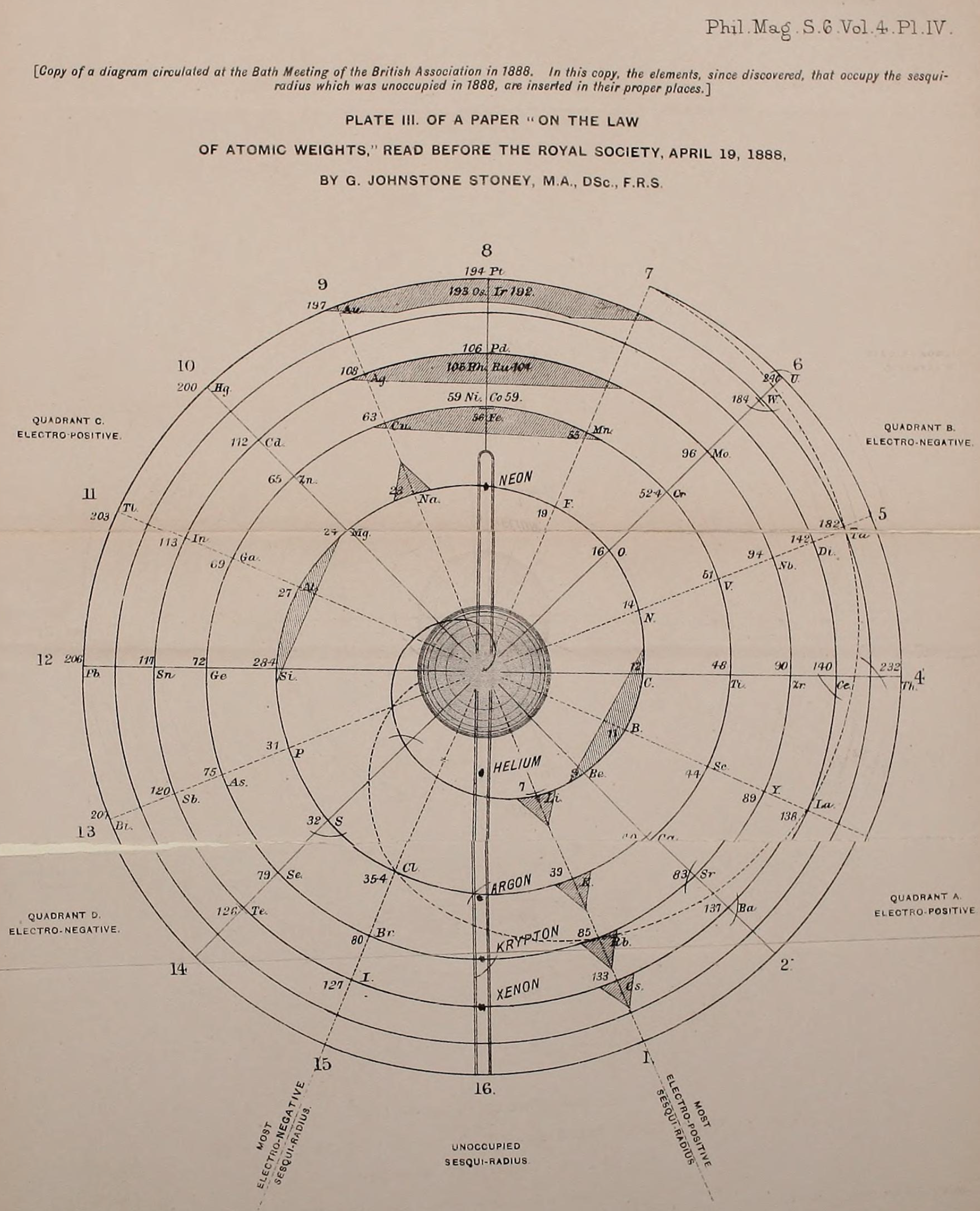
Thanks to René for the tip!
| Year: 1891 | PT id = 954, Type = formulation |
Mendeleev's Table In English
A table, from Wikipedia, showing the periodicity of the properties of many chemical elements, from the first English edition of Dmitrii Mendeleev's Principles of Chemistry (1891, translated from the Russian fifth edition).
It is worth noting that this 1981 formulation shows the presence of gallium and germanium that were not his original table.
| Year: 1891 | PT id = 1140, Type = formulation |
Wendt's Generation-Tree of the Elements
From page 244 of The Development of the Periodic Law by Venable, Francis Preston (1856-1934), Easton, Pa. Chemical Pub. Co (1896). The full text (scanned) is available from archive.org.
Venable writes:
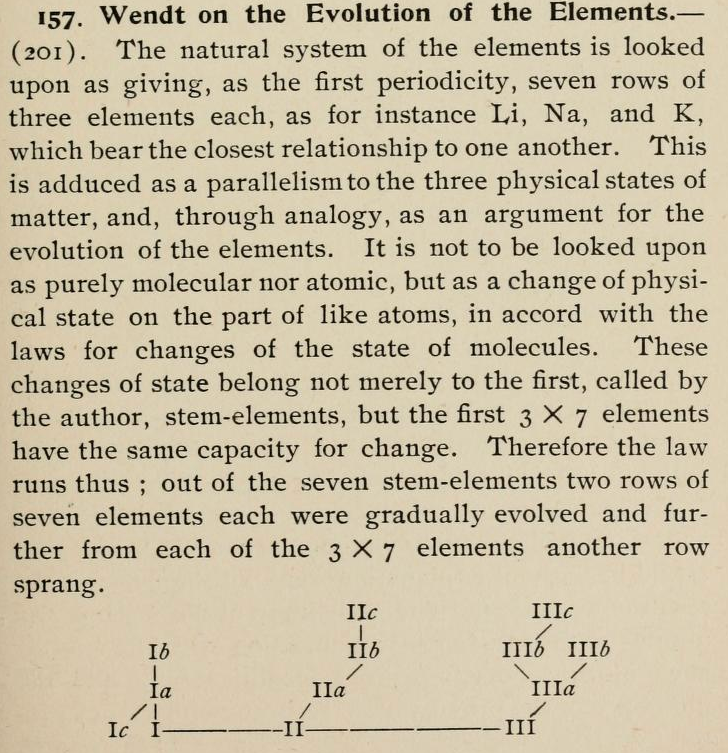
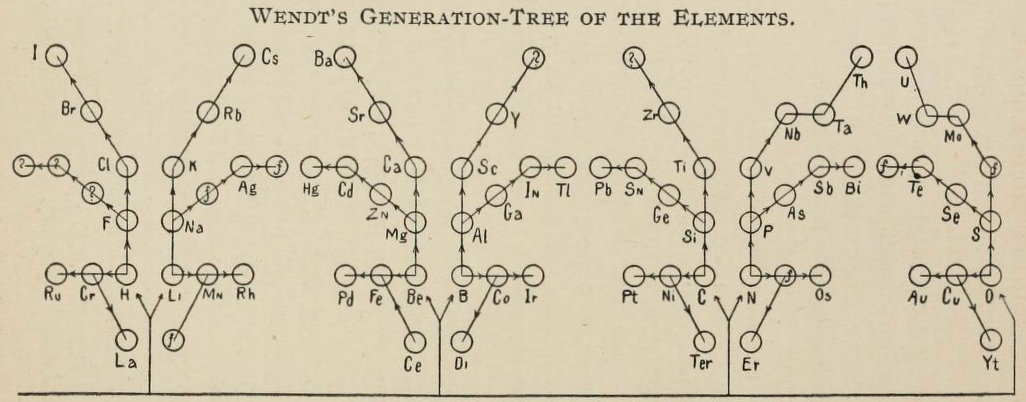
Thanks to René for the tip!
| Year: 1892 | PT id = 62, Type = formulation 3D |
Bassett's Vertical Arrangement
Bassett's Vertical Arrangement is actually designed to be a three dimensional formulation. Quam & Quam's review paper states:
"This table resembles Mendeléeff's vertical arrangement. The Cs period, however, starts far above the horizontal line of K and Rb, thereby giving space to the known and predicted elements of that period. The alkali metals appear in three horizontal lines. Co and Ni are arranged in order of their atomic weights.
"Bassett suggested cutting out the table and rolling it onto a cylinder of such circumference that similar elements would fall in line in Groups. For instance, Li, Na, K, Rb, and Cs would then fall on a line parallel to the axis of the cylinder."
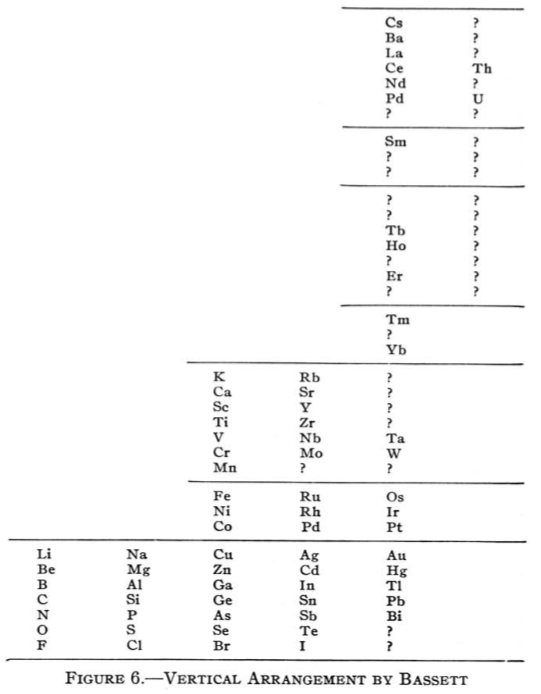
From Quam & Quam's 1934 review paper.pdf
| Year: 1892 | PT id = 247, Type = formulation |
Bassett Dumb-Bell Form
The Basset 'dumb-bell' formulation, ref. H. Basset, Chem. News, 65 (3-4), 19 (1892).
The image is from Concept of Chemical Periodicity: from Mendeleev Table to Molecular Hyper-Periodicity Patterns E. V. Babaev and Ray Hefferlin, here.
| Year: 1893 | PT id = 63, Type = formulation |
Rang's Periodic Arrangement of The Elements
P.J.F. Rang's The Periodic Arrangement of the Elements, Chemical News, vol. 67, p. 178 (1893)
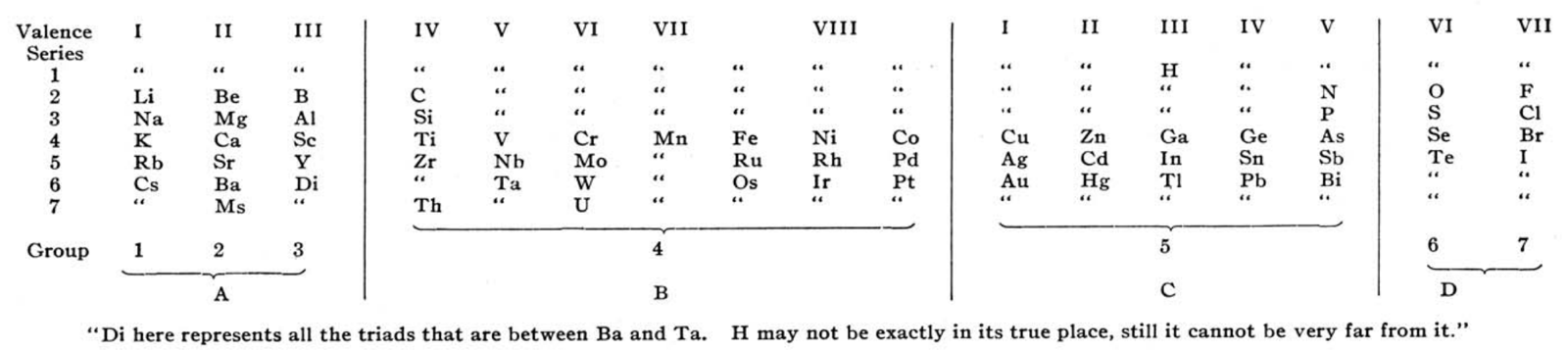
Observing that that Rang's table has four 'groups': A, B, C & D, René Vernon writes:
- Group A contains the strongest positive elements; group D the strongest negative elements. At such an early date, it's odd to see groups 1 to 3 categorised together.
- Group B are the elements with high melting points; "they are all remarkable for their molecular combinations" (presuamably, a reference to multiple oxidation states). At one side of group B are the "anhydro-combinations", probably referring to the simple chemistry of Ti, Zr, [Hf] Nb and Ta being dominated by insoluble oxides. At the other side are the "amin, carbonyl, and cyanogen combination", probably a reference to the group VIII carbonyls, as metal carbonyls had only just been discovered. Ni is shown after Fe, rather than Co.
- Group C includes the "heavy metals that have low melting points"; an early reference to frontier or post-transition metals, as a category.
- Rang says: ...if groups A and D be split up vertically in respectively three and two parts, the table presents seven vertical groups, and horizontally seven more or less complete series. Each group in each of the series 2 and 3 are represent by one element... The octave appears both horizontally and vertically in the table.
- Rang's reference to Di as representing all the triads between Ba and Ta kind of works since Hf would go under Zr, and that would leave 15 Ln or five sets of three. Thus, something like this:

Gd occupies the central position among the Ln. This arrangement won't fit however unless Rang envisaged all 15 Ln occupying the position under Y. - The location of H over | Ga | In | Tl, appears strange... but the electronegativity of H (2.2) is closer to B (2.04) than it is to C (2.55).
From Quam & Quam's 1934 review paper.pdf
| Year: 1893 | PT id = 1151, Type = formulation |
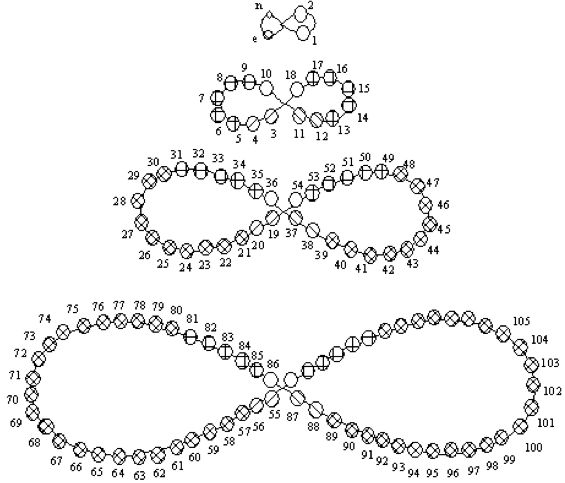
Nechaev's Truncated Cones
René Vernon (who found this formulation) writes:
This weird and wonderful table appears in Teleshov & Teleshova (2019, p. 230). It is attributed by them to Nechaev (1893) and is apparently discussed by Ipatiev (1904):
- The caption accompanying the table is: "Scanning of the projection of rotational bodies in the form of truncated cones as used in Nechaev's spatial construction of the periodic system, 1893."
- Looking at the table it seems to anticipate, after a fashion, the double periodicity noticed by later authors.
- Alternatively, if turned on its side, it would be just five columns wide.
- Between Ce (ignoring Di) and Yb, there are spaces for 12 missing elements, which is one too many.
- Pulling Yb back by one position would have done the trick.
"... We would also like to mention one more version of the periodic table, namely the one offered by V. Ipatiev. Ipatiev's version was one of the first to have been applied in a school textbook, and is also concise and accompanied by a detailed methodological commentary. More specifically, Ipatiev is important in directing our attention to the fact that an essential feature common to all elements should be chosen if the elements are to be systematized. Furthermore, Ipatiev also offered another crucial insight in arguing that this selected feature must satisfy certain conditions, namely: 1) it must be measurable, 2) it must be common to all elements and 3) it must be paramount, i.e. that all the remaining properties of the elements must depend on it [Ipatiev]."
References:
Ipat'ev, V. & Sapozhnikov, A. (1904). Kratkij kurs himii po programme voennyh uchilishh [A concise course in chemistry for military academies]. Sankt-Peterburg: tip. V. Demakova.
Nechaev N. P. (1893). Graficheskoe postroenie periodicheskoj sistemy jelementov Mendeleeva. Sposob Nechaeva [Graphic construction of Mendeleev's periodic system of elements. Nechaev's way]. Moskva: tip. Je. Lissnera i Ju. Romana
Teleshov S, Teleshova E.: The international year of the periodic table: An overview of events before and after the creation of the periodic table. In V Lamanauskas (ed.).: Science and technology Education: Challenges and possible solutions. Proceedings of the 3rd International Baltic Symposium on Science and Technology Education, BalticSTE2019, Šiauliai, 17-20 June, 2019. pp. 227-232, (2019)
| Year: 1894 | PT id = 797, Type = element |
Discovery of Argon
Ar
Argon, atomic number 18, has a mass of 39.948 au.
Argon is a noble gas.
Argon was first isolated in 1894 by Lord Rayleigh and W. Ramsay.
| Year: 1895 | PT id = 364, Type = formulation |
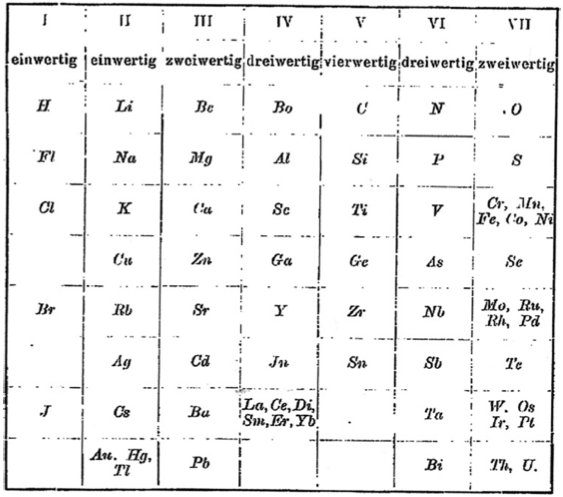
Retger's Periodic Table
Periodic Table of Retgers with an intraperiodic accommodation of the rare earths. Retgers, J.W., 1895. Z. Phys. Chem. 16, 644:
| Year: 1895 | PT id = 368, Type = formulation |
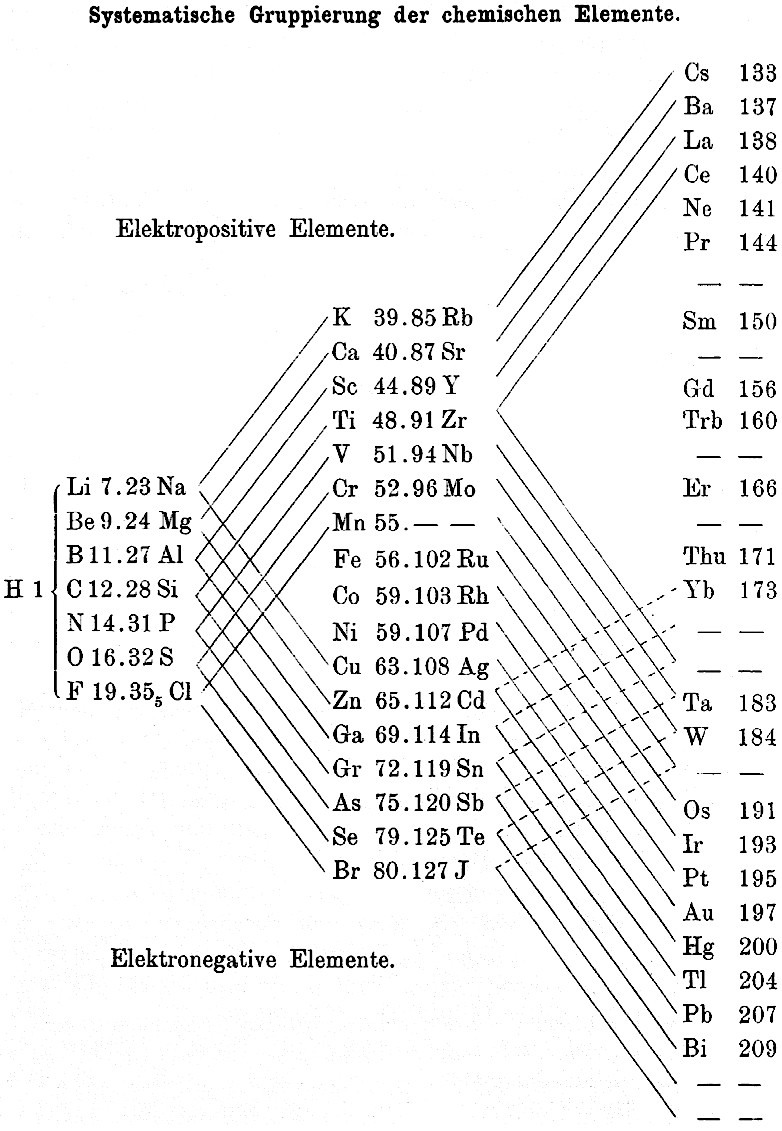
Thomsen's Systematic Arrangement of the Chemical Elements
In 1895 the Danish thermochemist Hans Peter Jørgen Julius Thomsen proposed (Thomsen, J., 1895. Z. Anorg. Chem. 9, 190 & Chemical News, 72, 89–91, p. 90) a pyramidal/ladder representation.
Notice how this formulation identifies the electropositive & electronegative elements with respect to the periodic table, thirty years before Linus Pauling.
| Year: 1895 | PT id = 782, Type = element |
Discovery of Helium
He
Helium, atomic number 2, has a mass of 4.003 au.
Helium is a noble gas, and is the second most abundant element in the universe after hydrogen.
Helium was first observed or predicted in 1868 by P. Janssen and N. Lockyer from solar spectra, and first isolated in 1895 by W. Ramsay, T. Cleve, and N. Langlet.
| Year: 1896 | PT id = 540, Type = formulation |
Richards' Classification of The Elements
This is how the periodic table looked in 1896 in an article by Theodore Richards the pioneer of atomic weight measurement.
Notice all those elements at the bottom that could not be classified, explicitly listed including He and Ar :
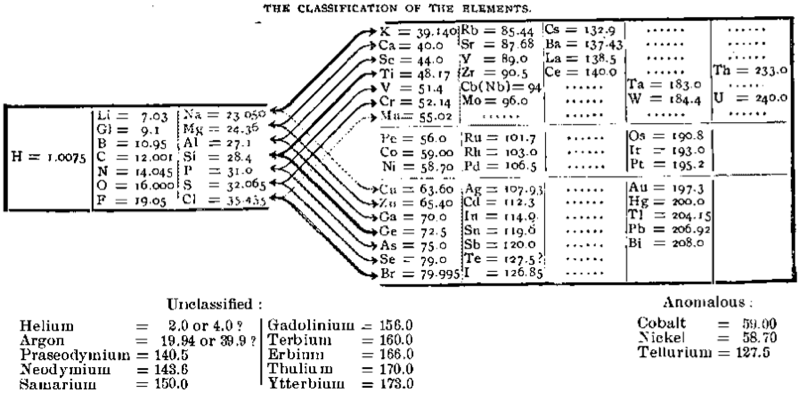
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 1896 | PT id = 1087, Type = formulation |
Ramsay's Elements Arranged in the Periodic System
From The Gases of the Atmosphere, The History of Their Discovery by William Ramsay (and from the Gutenberg Project.)
The author writes pp 220-221:
"In 1863 Mr. John Newlands pointed out in a letter to the Chemical News that if the elements be arranged in the order of their atomic weights in a tabular form, they fall naturally into such groups that elements similar to each other in chemical behaviour occur in the same columns. This idea was elaborated farther in 1869 by Professor Mendeléeff of St. Petersburg and by the late Professor Lothar Meyer, and the table may be made to assume the subjoined form (the atomic weights are given with only approximate accuracy):—"
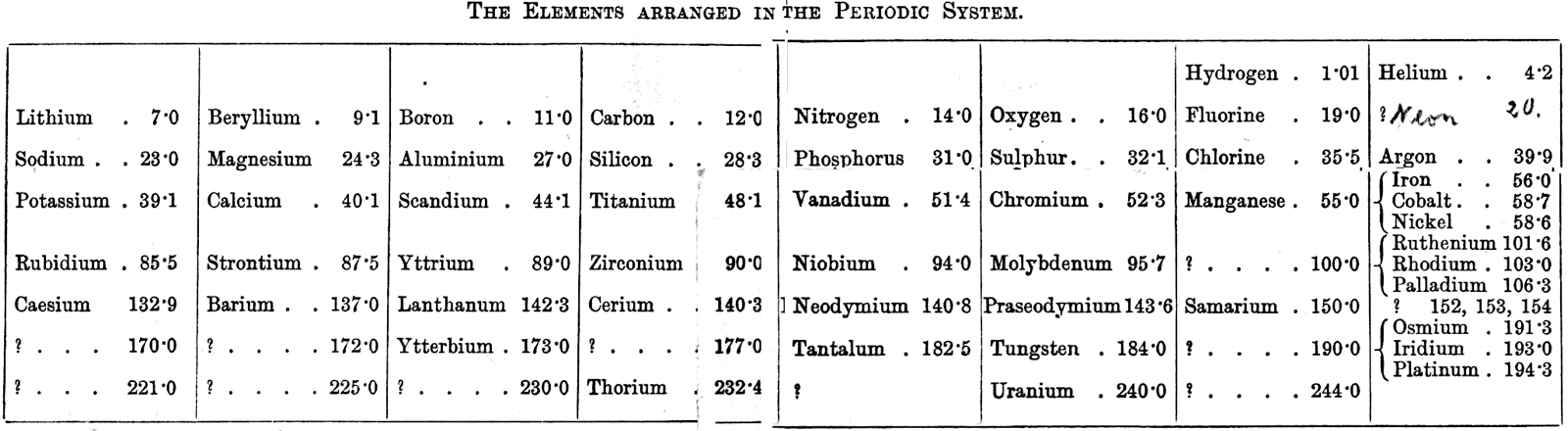
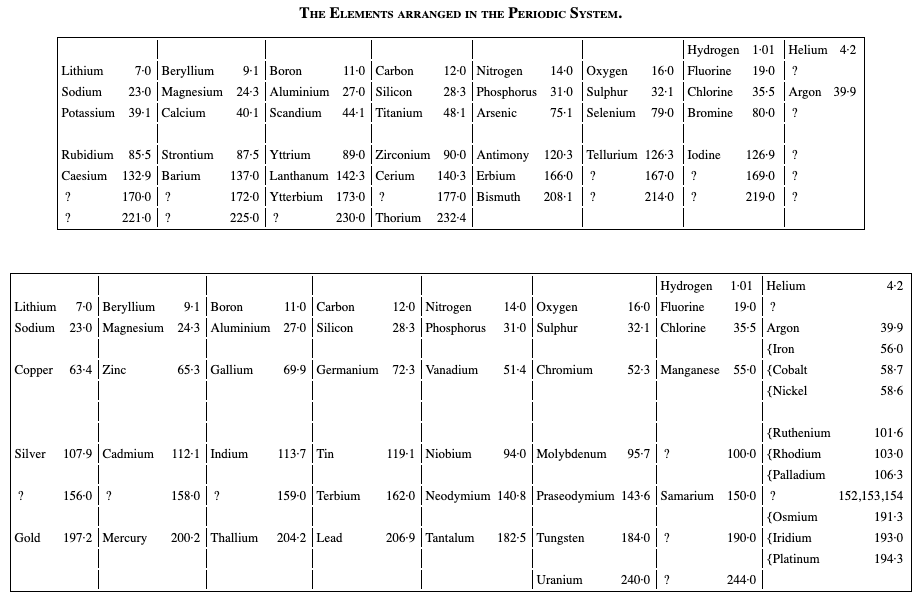
Thanks to René for the tip!
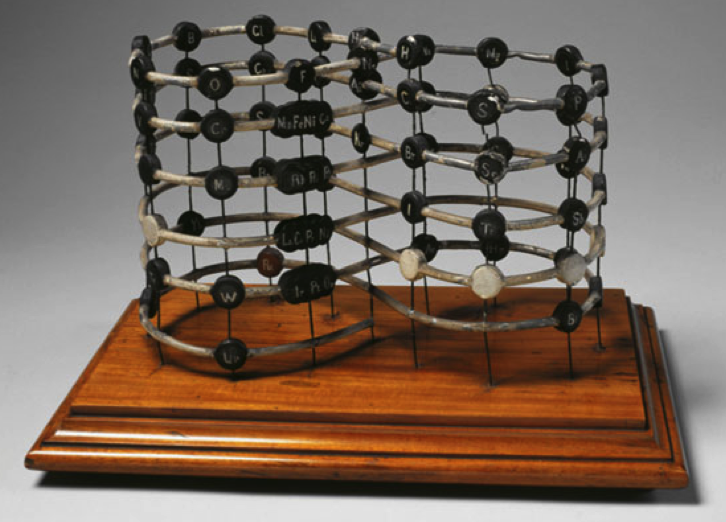
| Year: 1898 | PT id = 75, Type = formulation 3D |
Crookes' vis generatrix
Model of Crookes’ vis generatrix of 1898, built by his assistant, Gardiner. From: Proc. R. Soc. Lond. 63, 408.
The vertical scale represents the atomic weight of the elements from H = 1 to Ur = 239.
Missing elements are represented by a white circle. Similar elements appear underneath each other:
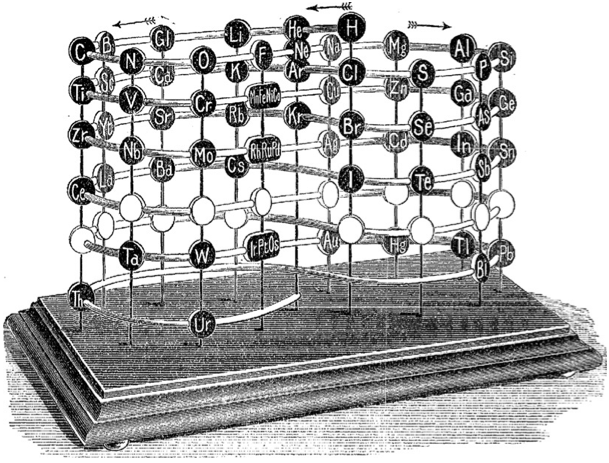
| Year: 1898 | PT id = 789, Type = element |
Discovery of Neon
Ne
Neon, atomic number 10, has a mass of 20.18 au.
Neon is a noble gas. It is present in the atmosphere, 1 part in 65000.
Neon was first isolated in 1898 by W. Ramsay and W. Travers.
| Year: 1898 | PT id = 816, Type = element |
Discovery of Krypton
Kr
Krypton, atomic number 36, has a mass of 83.798 au.
Krypton is a noble gas.
Krypton was first isolated in 1898 by W. Ramsay and W. Travers.
| Year: 1898 | PT id = 834, Type = element |
Discovery of Xenon
Xe
Xenon, atomic number 54, has a mass of 131.293 au.
Xenon is a noble gas.
Xenon was first isolated in 1898 by W. Ramsay and W. Travers.
| Year: 1898 | PT id = 864, Type = element |
Discovery of Polonium
Po ![]()
Polonium, atomic number 84, has a mass of 209 au.
Radioactive element.
Polonium was first observed or predicted in 1898 by P. and M. Curie and first isolated in 1902 by W. Marckwald.
| Year: 1898 | PT id = 868, Type = element |
Discovery of Radium
Ra ![]()
Radium, atomic number 88, has a mass of 226 au.
Radioactive element.
Radium was first observed or predicted in 1898 by P. and M. Curie and first isolated in 1902 by M. Curie.
| Year: 1899 | PT id = 866, Type = element |
Discovery of Radon
Rn ![]()
Radon, atomic number 86, has a mass of 222 au.
Radon is a noble gas and it is a radioactive element.
Radon was first observed or predicted in 1899 by E. Rutherford and R. B. Owens and first isolated in 1910 by W. Ramsay and R. Whytlaw-Gray.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.