Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
The 10 most recent entries to the database:
| Year: 1928 | PT id = 1390, Type = formulation spiral |
Another Attempt to Base a Classification of The Elements on Atomic Structure
Simpson, O.J., Another Attempt to Base a Classification of The Elements on Atomic Structure, J. Chem. Educ. 1928, 5, 1, 57 doi.org/10.1021/ed005p57:
| Year: 2023 | PT id = 1389, Type = review formulation |
La Tabla Periódica. El poder de la sistematización. La importancia de la precisión
A video of a presentation (in Spanish) by Manuel Yáñez, a professor at the Autonomous University entitled: La Tabla Periódica. El poder de la sistematización. La importancia de la precisión (The Periodic Table. The power of systematization. The importance of precision.)
| Year: 2020 | PT id = 1388, Type = formulation 3D spiral |
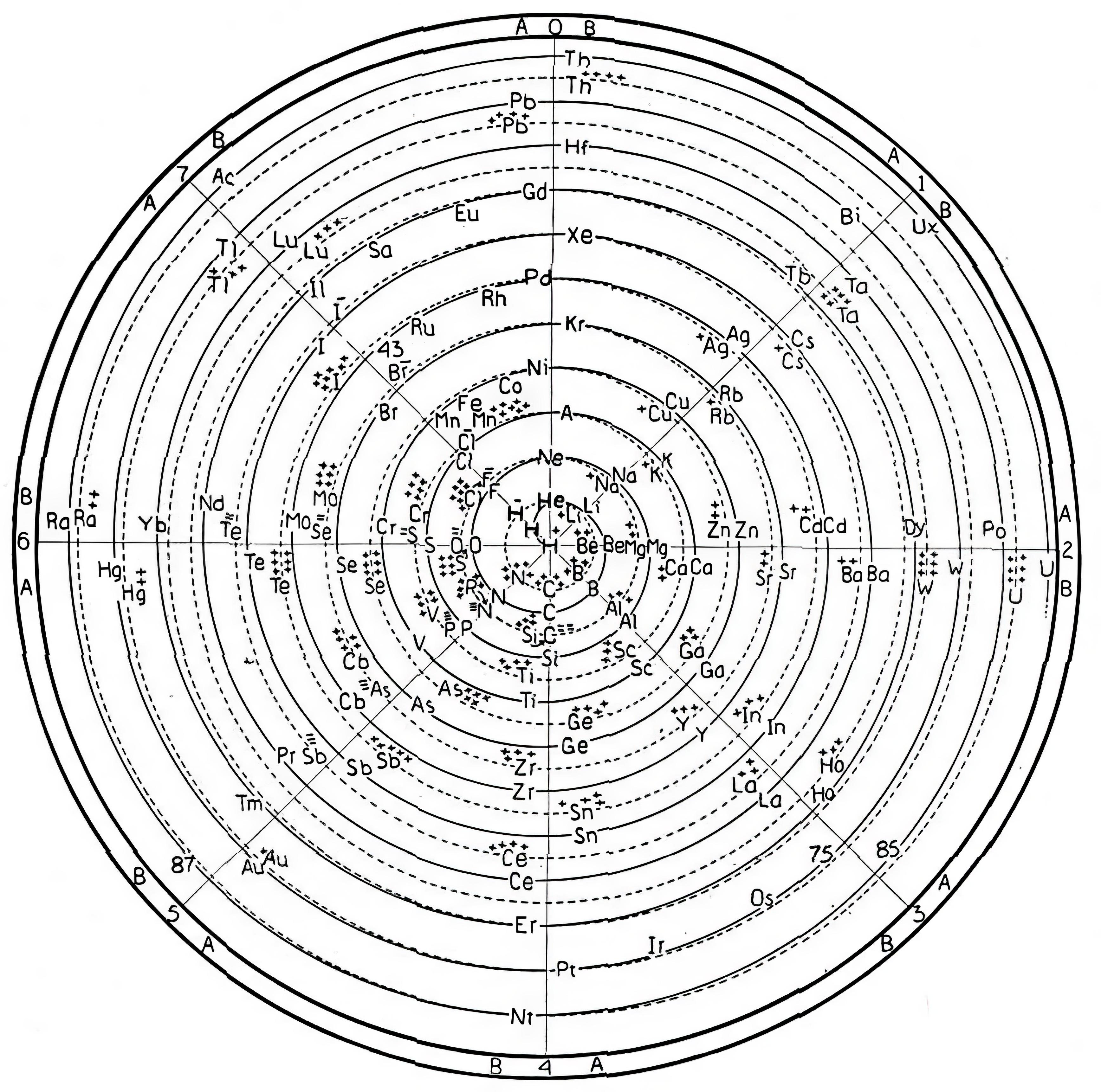
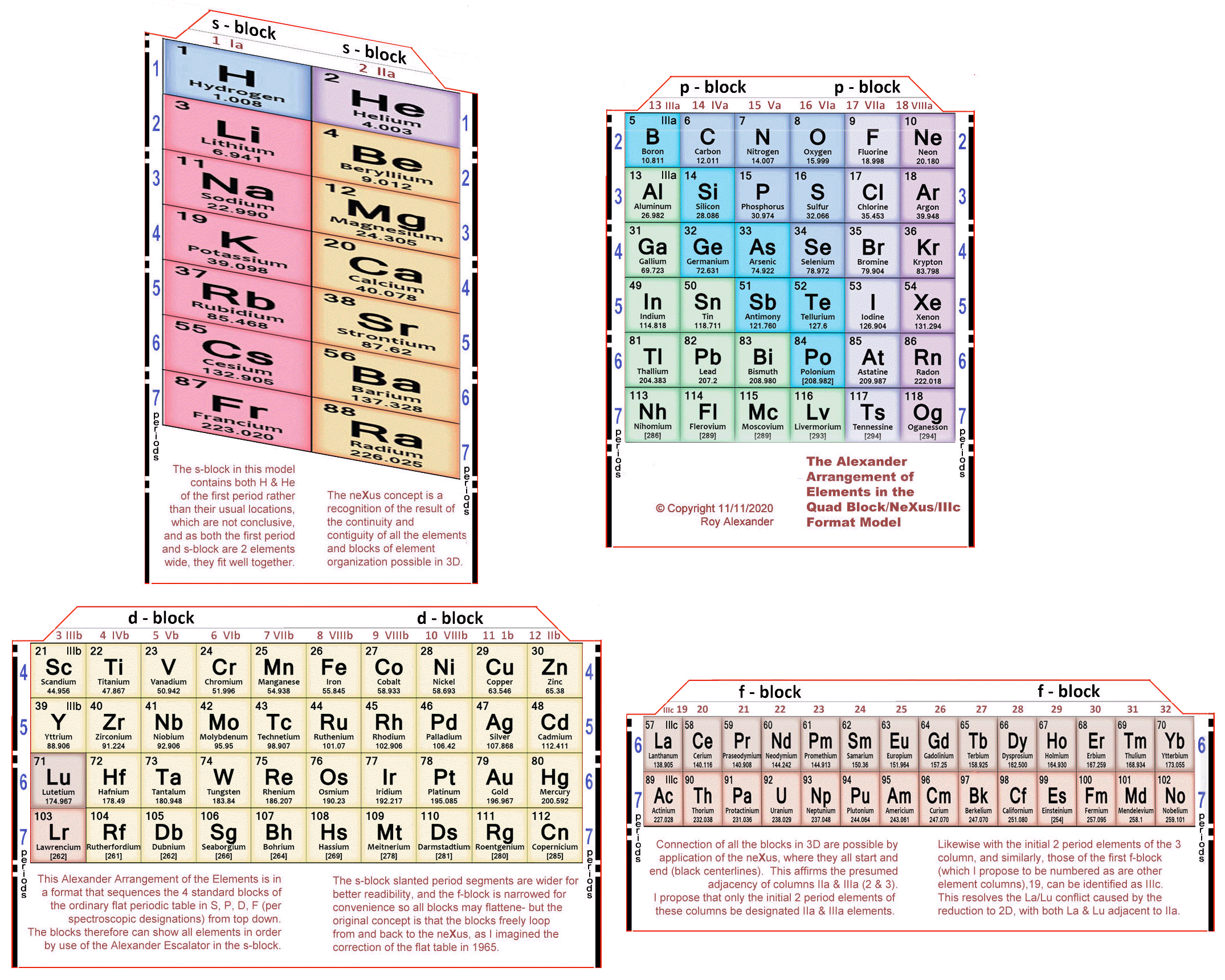
Alexander's Quad Block/neXus/IIIc Model
Roy Alexander's Quad Block/neXus/IIIc Model.

| Year: 1953 | PT id = 1387, Type = formulation 3D spiral |
Kapustinsky's Pyrimid
Kapustinsky, A. F. (1953). Periodicity in the structure of the electron envelopes and nuclei of atoms Communication 1. Periodic system of the elements and its connection with the theory of numbers and with physicochemical analysis. Bulletin of the Academy of Sciences of the USSR Division of Chemical Science, 2(1), 1–9. Paper as pdf.
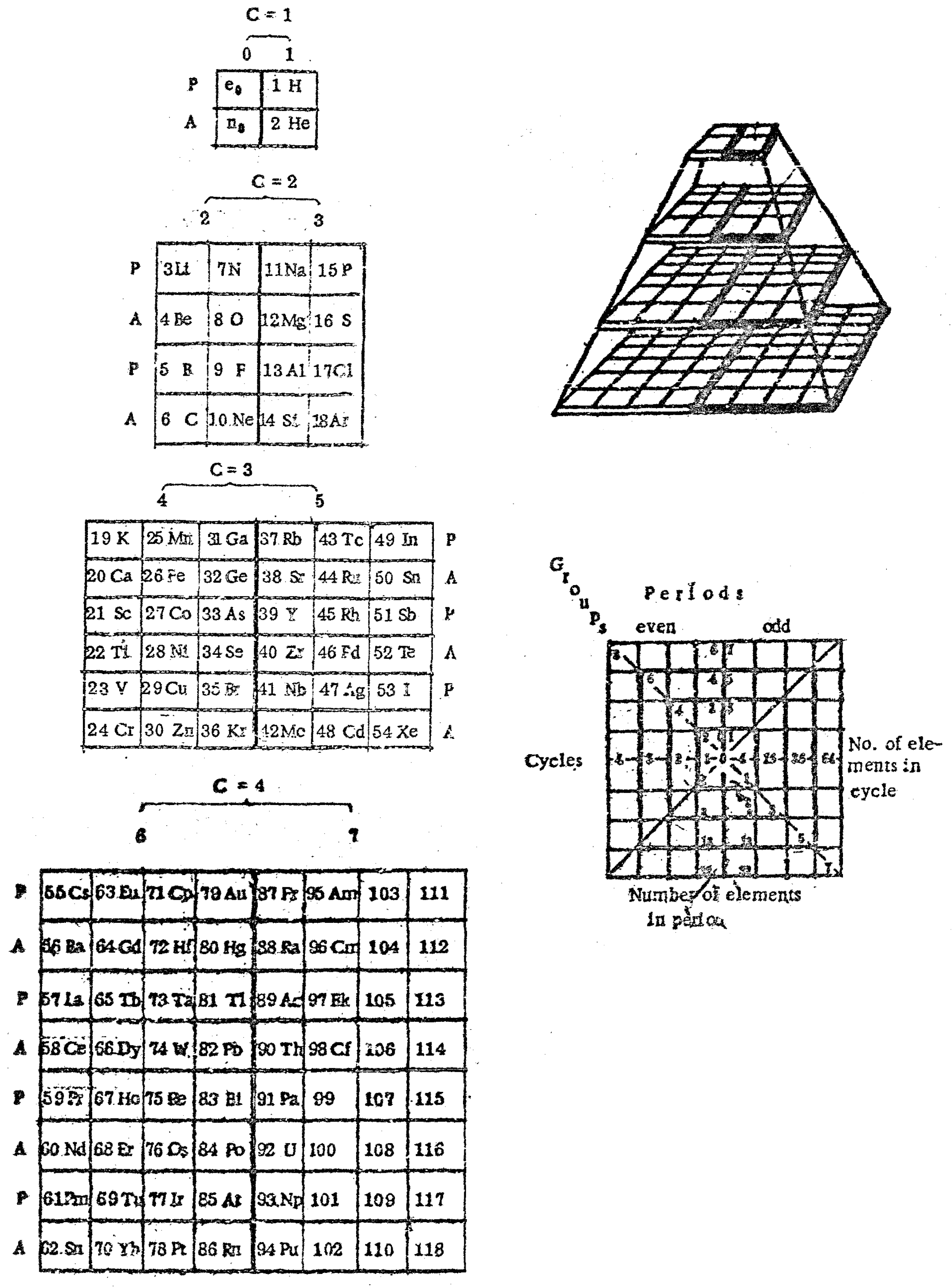
Thanks to René Vernon for the tip!
| Year: 1919 | PT id = 1386, Type = formulation |
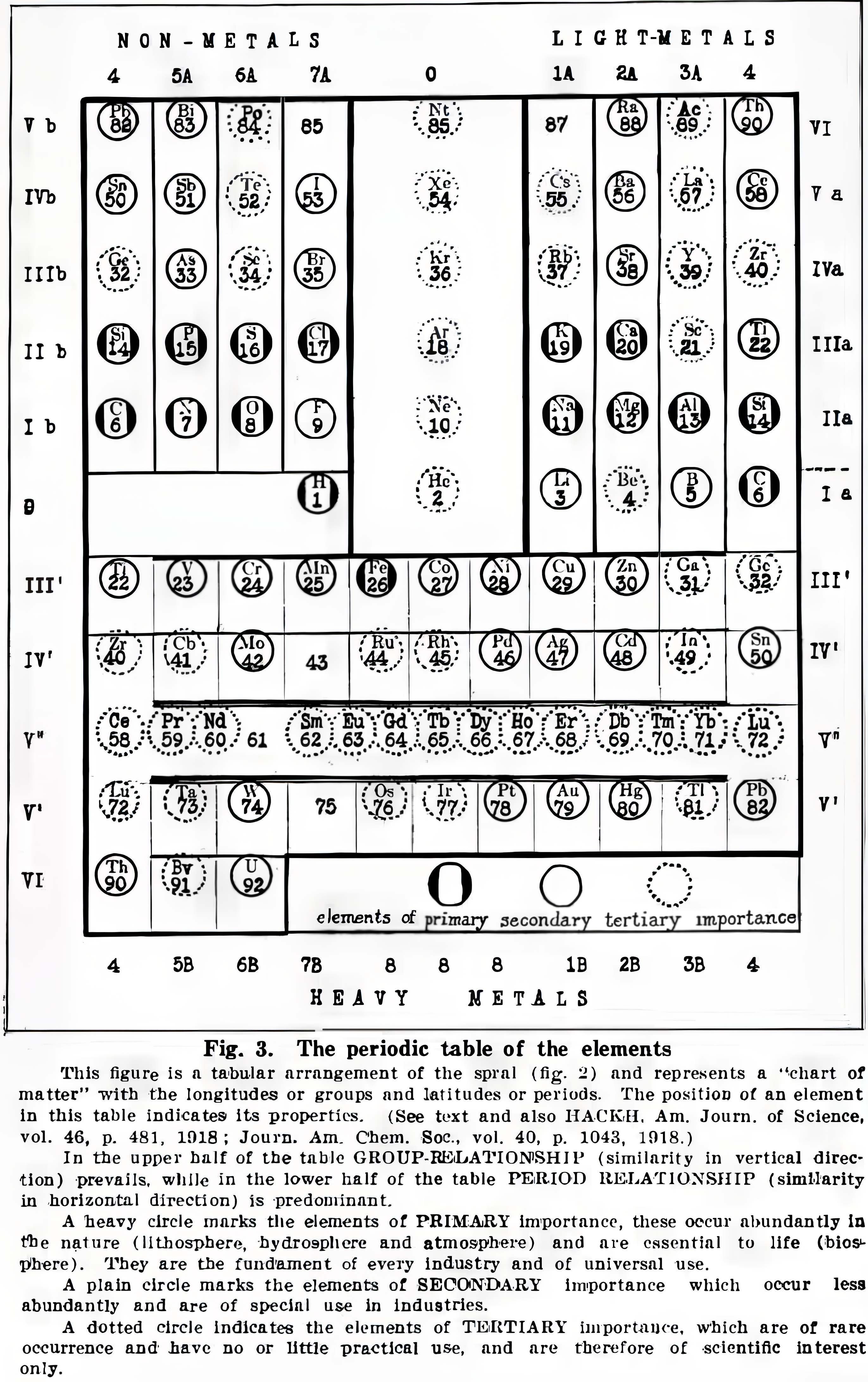
Hackh's Classification of The Chemical Elements
Hackh, I. W. D. (1919). The classification of the chemical elements: The fundament of chemistry, Scientific American, 87 (supp. no. 2253), pp. 146–149 (148). https://zenodo.org/records/2454321
René Vernon writes:
Note that Group 4 (including Lu) appears twice, on the left and right.
Hackh does not get it quite right when he refers to a vertical similarity prevailing in the upper half of the table and a horizontal similarity in the lower half. A horizontal similarity prevails along the first row of the transition metals; vertical similarities tend to prevail among the second and third row dyads of the transition metals. That said, a horizontal similarity does prevail among the lanthanides.
On the noble gases, Hackh (p. 146) wrote: "...they combined the two extreme ends of a period, they formed the bridge from a non-metallic halogen (electro-negative element) to a metallic alkali (electro-positive element). For this reason we may speak of these elements, the rare or inert gases, as the terminals of the periods, which are either positive nor negative... The first three elements following an inert gas are always strong positive, while the last three before an inert gas are always strong negative and thus a kind of a transition is formed by the fourth element, or the elements of the carbon group."
For chemical properties he wrote: "The chemical characteristics of the elements can equally well be studied, for there are the acid- and base-forming elements on the chart, whose zones gradually infiltrate from strong basic to weak basic to atmospheric to weak acid to strong acid or vice versa."
Read more in the paper.
| Year: 1905 | PT id = 1385, Type = structure |
Einsein's Annus Mirabilis
The annus mirabilis papers (from Latin: annus mirabilis, 'miraculous year') are four papers that Albert Einstein published in the scientific journal Annalen der Physik (Annals of Physics) in 1905. As major contributions to the foundation of modern physics, these scientific publications were the ones for which he gained fame among physicists. They revolutionised science's understanding of the fundamental concepts of space, time, mass, energy, atoms and atomic structure.
- Einstein, Albert (1905) Über einen die Erzeugung und Verwandlung des Lichtes betreffenden heuristischen Gesichtspunkt [On a Heuristic Point of View about the Creation and Conversion of Light] Annalen der Physik (in German). 17 (6): 132–148. doi: https://doi.org/10.1002%2Fandp.19053220607 English translation.
The first paper explained the photoelectric effect, which established the energy of the light quanta, E = hf or E = hν (depending upon context), where h = Planck's constant. This was the only specific discovery mentioned in the citation awarding Einstein the 1921 Nobel Prize in Physics.
- Einstein, Albert (1905) Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen [Investigations on the theory of Brownian Movement] Annalen der Physik (in German). 322 (8): 549–560. doi: https://doi.org/10.1002%2Fandp.19053220806
The second paper explained Brownian motion, D = μkBT which compelled physicists to accept the existence of atoms.
- Einstein, Albert (30 June 1905) Zur Elektrodynamik bewegter Körper [On the Electrodynamics of Moving Bodies]. Annalen der Physik (in German). 17 (10): 891–921. doi: https://onlinelibrary.wiley.com/doi/10.1002/andp.19053221004 English tranlation
The third paper introduced Einstein's special theory of relativity, which proclaims the constancy of the speed of light.
- Einstein, Albert (1905). Ist die Trägheit eines Körpers von seinem Energieinhalt abhängig? [Does the Inertia of a Body Depend Upon Its Energy Content?] Annalen der Physik (in German). 18 (13): 639–641. doi: https://onlinelibrary.wiley.com/doi/10.1002/andp.19053231314 English translation
The fourth, a consequence of special relativity, developed the principle of mass–energy equivalence, expressed in the equation E = mc2, and which led to the discovery and use of nuclear power decades later.
These four papers, together with quantum mechanics and Einstein's later general theory of relativity (1916), are the foundation of modern physics.

| Year: 1926 | PT id = 1384, Type = structure |
Schrödinger and The Hydrogen Atom
In Parts II and III of Schrödinger's 1926 papers: Annalen der Physik, 79 (1926), pp. 361–376 and Annalen der Physik, 80 (1926), pp. 437–490, the hydrogen atom is addressed.
Here Schrödinger:
- Separates the equation in spherical coordinates
- Solves the radial equation
- Derives hydrogen energy levels:
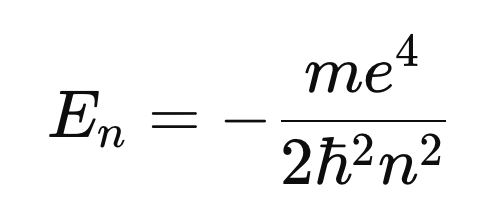
- Shows agreement with the Bohr spectrum
This is the first full wave-mechanical derivation of hydrogen.
There is an on-line English translation of Schrödinger's 1926 papers, published in 1928.

| Year: 1896 | PT id = 1383, Type = structure |
Discovery of Radioactivity
From The Nuclear Wallchart:
In 1896 Henri Becquerel was using naturally fluorescent minerals to study the properties of x-rays, which had been discovered in 1895 by Wilhelm Roentgen. He exposed potassium uranyl sulfate to sunlight and then placed it on photographic plates wrapped in black paper, believing that the uranium absorbed the sun’s energy and then emitted it as x-rays.
This hypothesis was disproved on the 26th-27th of February, when his experiment "failed" because it was overcast in Paris. For some reason, Becquerel decided to develop his photographic plates anyway. To his surprise, the images were strong and clear, proving that the uranium emitted radiation without an external source of energy such as the sun. Becquerel had discovered radioactivity.
Becquerel showed that the radiation he discovered could not be x-rays. X-rays are neutral and cannot be bent in a magnetic field. The new radiation was bent by the magnetic field so that the radiation must be charged and different than x-rays. When different radioactive substances were put in the magnetic field, they deflected in different directions or not at all, showing that there were three classes of radioactivity: negative, positive, and electrically neutral.
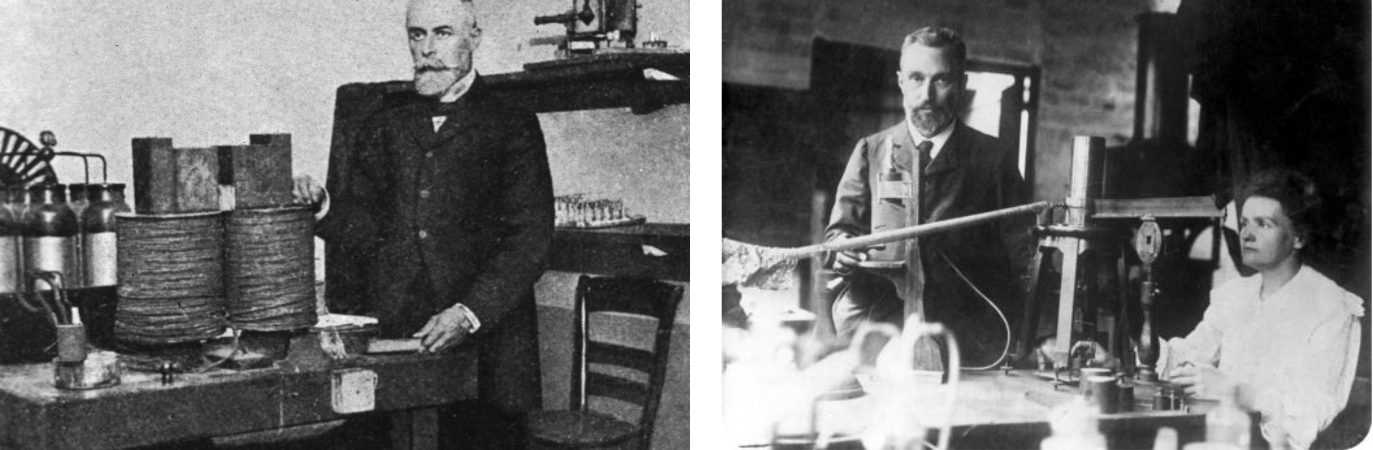
The term radioactivity was actually coined by Marie Curie, who together with her husband Pierre, began investigating the phenomenon recently discovered by Becquerel. The Curies extracted uranium from ore and to their surprise, found that the leftover ore showed more activity than the pure uranium. They concluded that the ore contained other radioactive elements. This led to the discoveries of the elements polonium and radium. It took four more years of processing tons of ore to isolate enough of each element to determine their chemical properties.
Ernest Rutherford, who did many experiments studying the properties of radioactive decay, named these alpha, beta, and gamma (α, β and γ) particles, and classified them by their ability to penetrate matter. Rutherford used an apparatus similar to Becquerel's. When the air from the chamber was removed, the alpha source made a spot on the photographic plate. When air was added, the spot disappeared. Thus, only a few centimeters of air were enough to stop the alpha radiation.
- α-particles carry more electric charge, are more massive, and move slowly compared to β and γ particles, they interact much more easily with matter.
- β-particles are much less massive and move faster, but are still electrically charged. A sheet of aluminum one millimeter thick or several meters of air will stop these electrons [and positrons].
- γ-rays carry no electric charge, they can penetrate large distances through materials before interacting–several centimeters of lead or a meter of concrete is needed to stop most γ-rays.
Henri Becquerel and Marie & Pierre Curie in their labs:
| Year: 2026 | PT id = 1382, Type = formulation spiral |
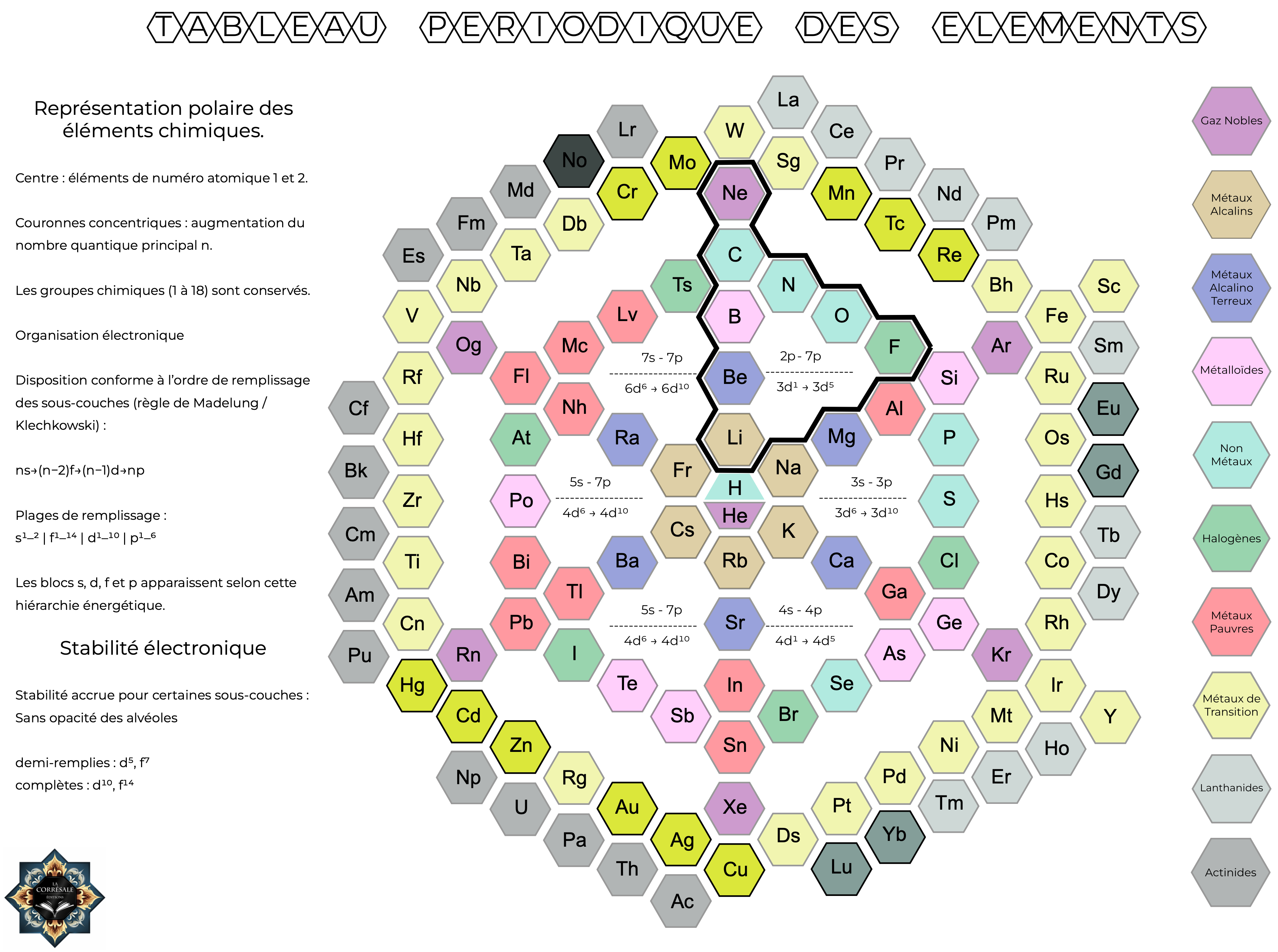
Tableau_Périodique des Elements
La Corrésale writes:
"I have been developing an alternative geometric representation of the periodic table, and I would greatly appreciate your perspective on it.
"The model is a radial (hexagonal) construction with hydrogen and helium positioned at the center, and successive concentric layers corresponding to principal quantum numbers (n = 1-7). The layout follows the Madelung (Klechkowski) filling order, so that the s, p, d, and f blocks arise naturally from the geometric structure.
"Lanthanides and actinides are fully integrated into the continuous sequence rather than separated.
"My intention is not to replace the conventional rectangular table, but to offer a complementary representation that makes the electronic layering and energetic hierarchy more visually explicit. The structure effectively behaves like a “macro-atom,” with shells accumulating outward in a way that mirrors electronic shell construction. Certain periodic trends, such as radial progression and diagonal relationships, appear to become more intuitive in this configuration.
"I am aware that many alternative periodic tables exist, and I have tried to avoid unnecessary aesthetic distortion while preserving structural coherence and regular tiling. I would be very interested to know whether you see any pedagogical or conceptual merit in such a representation, or any structural issues I may have overlooked."
| Year: 1930 | PT id = 1381, Type = structure review |
Quantum Atoms
Dirac, P. A. M. The Principles of Quantum Mechanics. Oxford University Press (1st ed. 1930; 2nd ed. 1935). Wikipeda entry on this work.
von Neumann, J., Mathematische Grundlagen der Quantenmechanik (Mathematical Foundations of Quantum Mechanics), 1932, Springer, Berlin, Germany. Wikepedia entry on this work.
By the 1930s, the mathematics of quantum mechanics was mature, as exemplified by these two text books. Dirac explicitly develops methods for atoms, molecules, radiation, and many-particle systems. Von Neumann formulates a fully general mathematical framework applicable to arbitrarily complex systems (though with few concrete examples).
"The Principles of Quantum Mechanics is an influential monograph written by Paul Dirac and first published by Oxford University Press in 1930. In this book, Dirac presents quantum mechanics in a formal, logically consistent, and axiomatic fashion, making the book the first of its kind. It is based on matrices and operators rather than wave–particle duality. Its 82 sections contain 785 equations with no diagrams. Nor does it have an index, a bibliography, or a list of suggestions for further reading. The first half of the book lays down the foundations of quantum mechanics while the second half focuses on its applications. Dirac did not pursue a historical approach to the subject. Nor did he discuss at length the philosophy of quantum mechanics."
"Von Neumann formalised quantum mechanics using the concept of Hilbert spaces and linear operators. He acknowledged the previous work by Paul Dirac on the mathematical formalisation of quantum mechanics, but was skeptical of Dirac's use of delta functions. He wrote the book in an attempt to be even more mathematically rigorous than Dirac. It was von Neumann's last book in German, afterwards he started publishing in English."
To read Dirac's The Principles of Quantum Mechanics click this link.

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.