Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Tables from the years before 1800, by date:
| Year: 9000 BCE | PT id = 809, Type = element |
Discovery of Copper
Cu
Copper, atomic number 29, has a mass of 63.546 au.
Copper had its earliest use in about 9000 BCE, and the oldest sample dates from 6000 BCE. It was discovered by Middle East workers and the earliest sample is from Anatolia.
| Year: 7000 BCE | PT id = 862, Type = element |
Discovery of Lead
Pb
Lead, atomic number 82, has a mass of 207.2 au.
Lead had its earliest use in about 7000 BCE, and the oldest sample dates from 3800 BCE. It was discovered by Africa and the earliest sample is from Abydos, Egypt.
| Year: 6000 BCE | PT id = 859, Type = element |
Discovery of Gold
Au
Gold, atomic number 79, has a mass of 196.967 au.
Gold had its earliest use in about 6000 BCE, and the oldest sample dates from 4400 BCE. It was discovered by Bulgaria and the earliest sample is from Varna Necropolis.
| Year: 5000 BCE | PT id = 806, Type = element |
Discovery of Iron
Fe
Iron, atomic number 26, has a mass of 55.845 au.
Iron had its earliest use in about 5000 BCE, and the oldest sample dates from 4000 BCE from Egypt.
| Year: 5000 BCE | PT id = 827, Type = element |
Discovery of Silver
Ag
Silver, atomic number 47, has a mass of 107.868 au.
Silver had its earliest use in about 5000 BCE, and the oldest sample dates from 4000 BCE, and is from Asia Minor.
| Year: 3750 BCE | PT id = 786, Type = element |
Discovery of Carbon
C
Carbon, atomic number 6, has a mass of 12.011 au.
Carbon has many allotropes, including: graphite, diamond, graphene, C60, single wall nanotubes, etc.
Carbon had its earliest use in about 3750 BCE. It was discovered by Egyptians and Sumerians.
| Year: 3500 BCE | PT id = 830, Type = element |
Discovery of Tin
Sn
Tin, atomic number 50, has a mass of 118.71 au.
Tin + copper gives bronze, and so the Bronze Age.
Tin had its earliest use in about 3500 BCE, and the oldest sample dates from 2000 BCE. It is unknown who discovered the element.
| Year: 2000 BCE | PT id = 795, Type = element |
Discovery of Sulfur (Sulphur)
S
Sulfur, atomic number 16, has a mass of 32.068 au.
Sulfur is a pale yellow, odourless, brittle solid.
Sulfur had its earliest use in about 2000 BCE. It was discovered by Chinese/Indians.
| Year: 2000 BCE | PT id = 860, Type = element |
Discovery of Mercury
Hg
Mercury, atomic number 80, has a mass of 200.592 au.
Mercury had its earliest use in about 2000 BCE, and the oldest sample dates from 1500 BCE. It was discovered by Chinese/Indians and the earliest sample is from Egypt.
| Year: 1000 BCE | PT id = 810, Type = element |
Discovery of Zinc
Zn
Zinc, atomic number 30, has a mass of 65.38 au.
Zinc had its earliest use in about 1000 BCE, and the oldest sample dates from 1000 BCE. It was discovered by Indian metallurgists and the earliest sample is from the Indian subcontinent.
| Year: 800 BCE | PT id = 831, Type = element |
Discovery of Antimony
Sb
Antimony, atomic number 51, has a mass of 121.76 au.
Antimony had its earliest use in about 800 BCE.
| Year: 450 BCE | PT id = 229, Type = formulation |
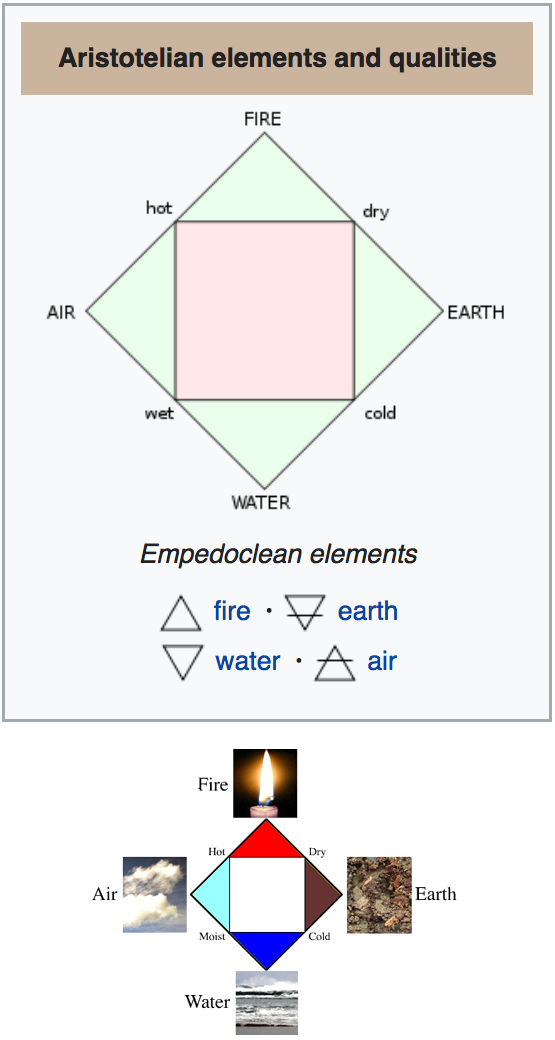
Classical Elements: Earth, Water, Air & Fire
The Greek Classical Elements — Earth, Water, Air, Fire [& Aether] — date from 450 BC or so, and persisted throughout the Middle Ages and into the Renaissance, deeply influencing European thought and culture.
A Greek text Kore Kosmou ("Virgin of the World" - associated with the Egyptian god Thoth - names the four elements fire, water, air, and earth:
And Isis answer made: Of living things, my son, some are made friends with fire, and some with water, some with air, and some with earth, and some with two or three of these, and some with all. And, on the contrary, again some are made enemies of fire, and some of water, some of earth, and some of air, and some of two of them, and some of three, and some of all. For instance, son, the locust and all flies flee fire; the eagle and the hawk and all high-flying birds flee water; fish, air and earth; the snake avoids the open air. Whereas snakes and all creeping things love earth; all swimming things love water; winged things, air, of which they are the citizens; while those that fly still higher love the fire and have the habitat near it. Not that some of the animals as well do not love fire; for instance salamanders, for they even have their homes in it. It is because one or another of the elements doth form their bodies' outer envelope. Each soul, accordingly, while it is in its body is weighted and constricted by these four.
The four elements were used by Hippocrates in describing the human body with an association with the four humours:
- yellow bile (fire)
- black bile (earth)
- blood (air)
- phlegm (water)
Plato characterizes the elements from a list created by the Sicilian philosopher Empedocles called these the four "roots." Plato seems to have been the first to use the term element:
| Year: 300 BCE | PT id = 813, Type = element |
Discovery of Arsenic
As
Arsenic, atomic number 33, has a mass of 74.922 au.
Arsenic had its earliest use in about 300 BCE.
| Year: 1000 | PT id = 472, Type = formulation element |
Elements Known in the Year 1000
Elements known in the year 1000, taken from this Wikipedia page:
| Year: 1520 | PT id = 906, Type = formulation |
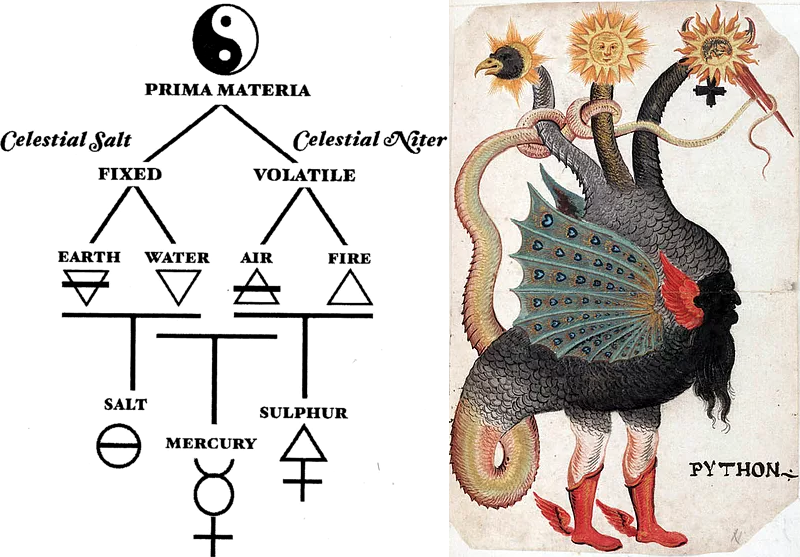
Tria Prima of Alchemy
Paracelsus identified three primes, the tria prima, of alchemy which are related to the Law of the Triangle, in which two components come together to produce the third. Philosophically speaking, Mercury is the Mind; Salt is the Will & Wisdom; and Sulphur is Love.
The three are components or principles of the Philosopher's Stone, and they work potently to transmute any base metal or character into golden perfection. Without these principles, the coveted Stone is ineffectual in its capacity to change vibratory rates.
- Sulfur: The fluid connecting the High and the Low. Sulfur was used to denote the expansive force, evaporation, and dissolution.
- Mercury: The omnipresent spirit of life. Mercury was believed to transcend the liquid and solid states. The belief carried over into other areas, as mercury was thought to transcend life/death and heaven/earth.
- Salt: Base matter. Salt represented the contractive force, condensation, and crystallization.
| Year: 1617 | PT id = 903, Type = formulation |
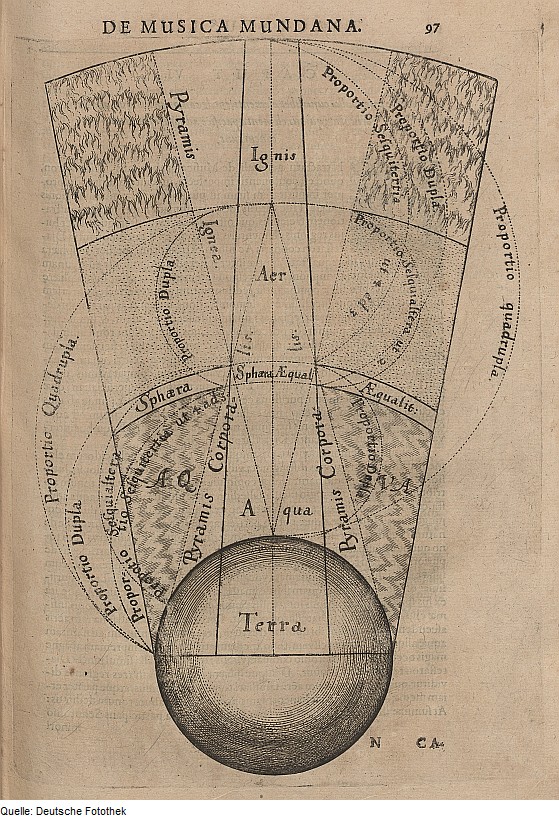
Elemental Spheres of Terra (earth), Aqua (water), Aer (air) & Ignis (fire)
From the German Photo Library Theosophie & Alchemie, a segment of the macrocosm showing the elemental spheres of terra (earth), aqua (water), aer (air), and ignis (fire), by Robert Fludd:
| Year: 1624 | PT id = 965, Type = formulation |
Ripley Scroll
The Ripley Scroll, an illustrated alchemical manuscript, in English and Latin, on vellum, England [perhaps Manchester?] 1624. This item was sold by Christie's in 2017.
There are 23 known versions of the Ripley Scroll (or "Ripley Scrowle").
George Ripley (c. 1415-1490) was one of England's most famous alchemists. His alchemical writings attracted attention not only when they were published in the fifteenth century, but also later in the sixteenth and seventeenth centuries. His writings were studied by noted figures such as the alchemist John Dee, Robert Boyle (who is considered to be the first modern chemist), and even Isaac Newton.
There is a copy/version of the Ripley Scroll at the British Library.

| Year: 1669 | PT id = 794, Type = element |
Discovery of Phosphorus
P
Phosphorus, atomic number 15, has a mass of 30.974 au.
Phosphorus exists in several allotropic forms including: white, red and black.
Phosphorus was first isolated in 1669 by H. Brand.
| Year: 1671 | PT id = 459, Type = formulation |
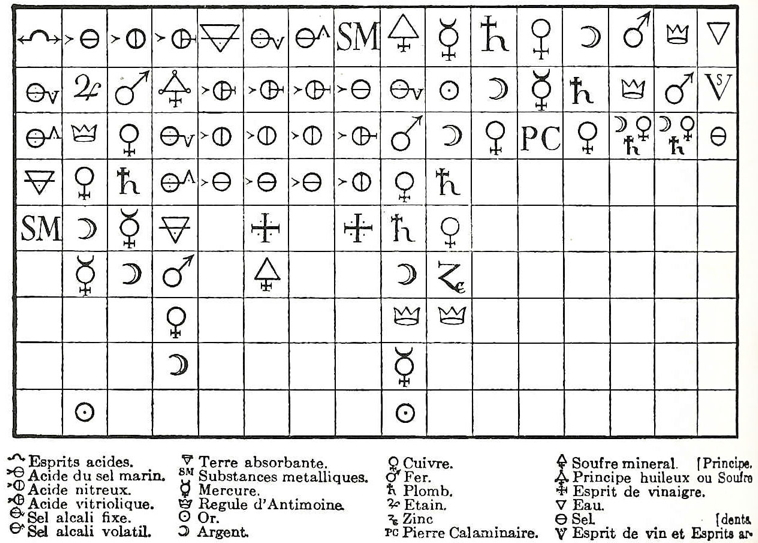
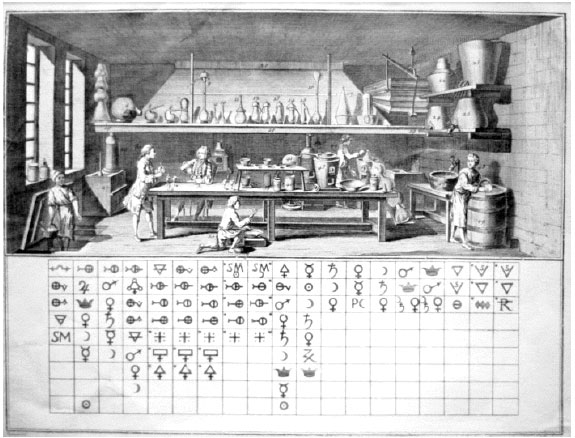
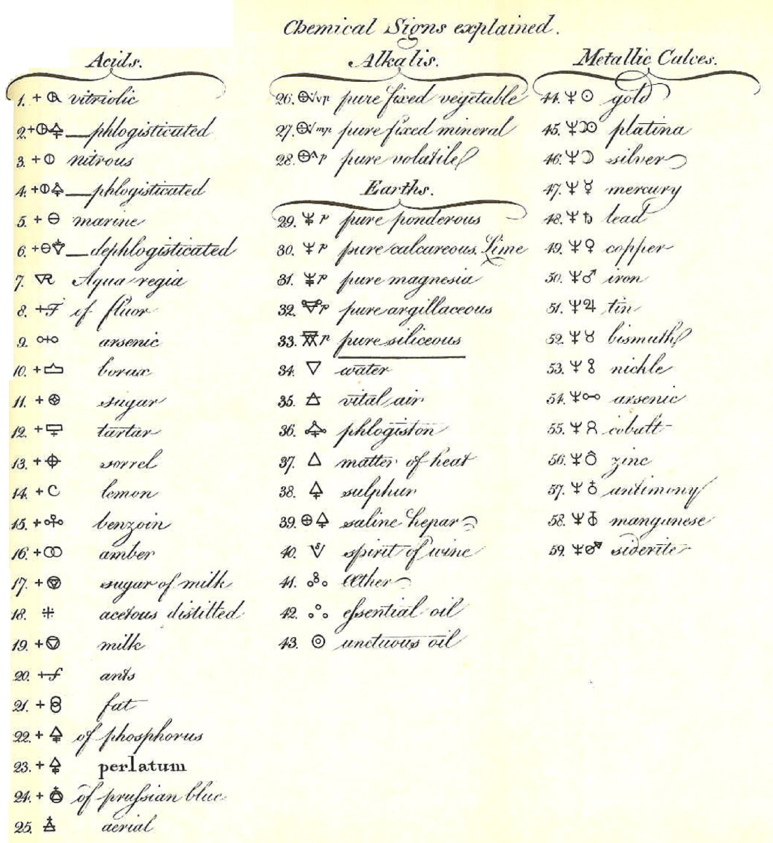
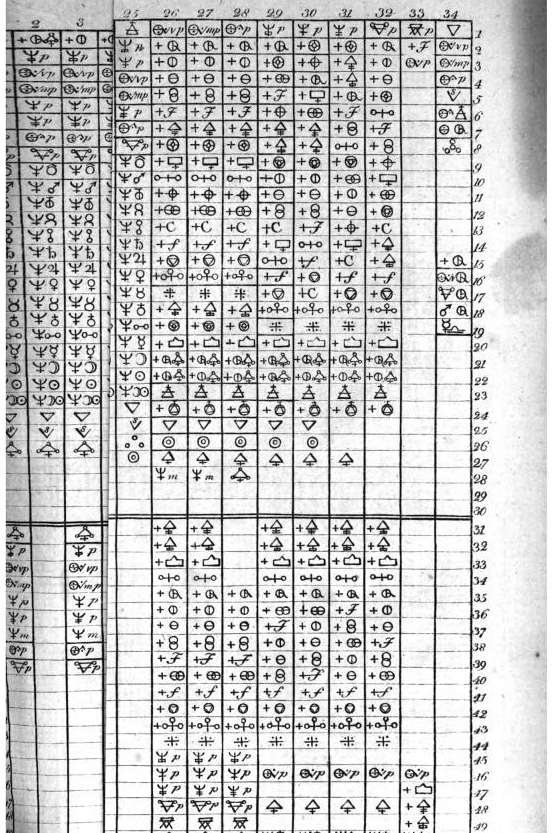
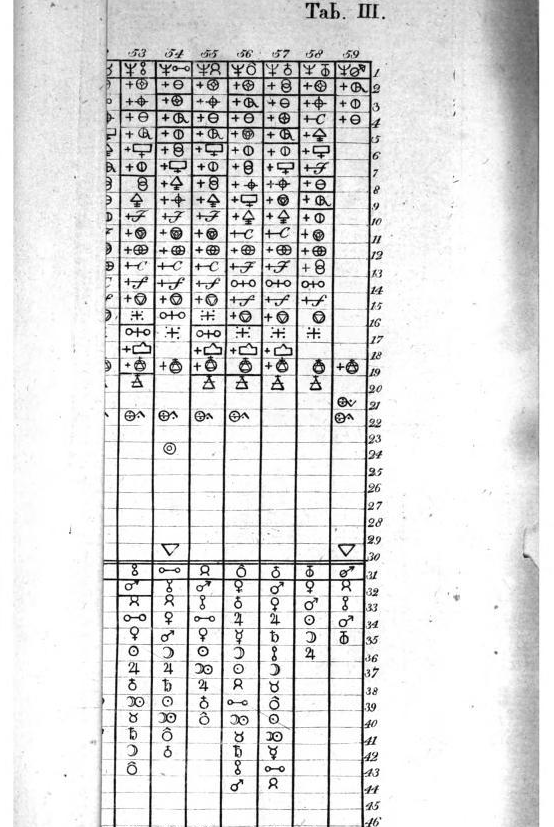
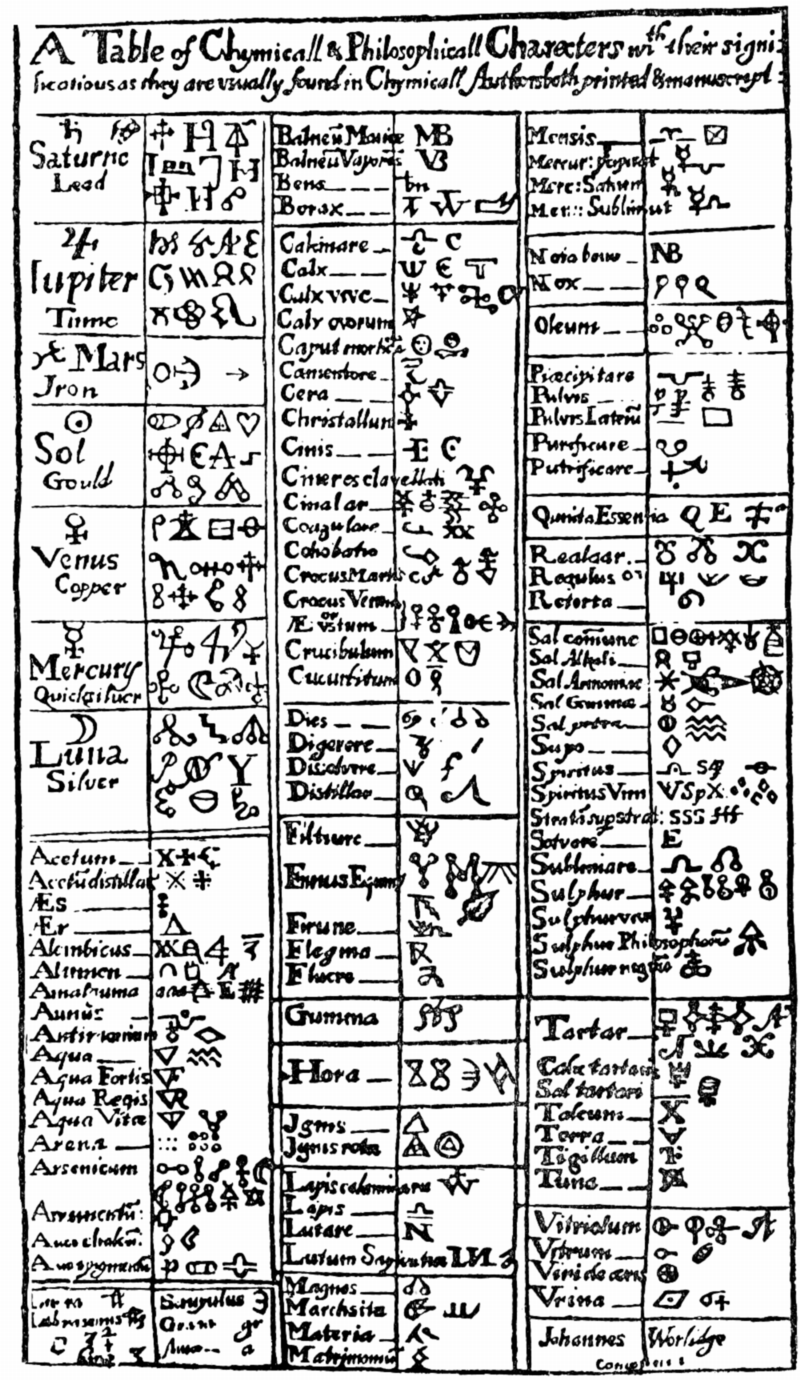
Valentinus' Table of Chymicall & Philosophicall Charecters
A table of alchemical symbols from Basilius Valentinus' (Basil Valentine) The Last Will and Testament:
| Year: 1682 | PT id = 2, Type = formulation early |
Digby's A Choice Collection of Rare Secrets
| Year: 1687 | PT id = 902, Type = formulation |
Alchemical Emblem Showing the Four Classical Elements
From the German Photo Library Theosophie & Alchemie, a seventeenth century alchemical emblem showing the four classical elements (air, fire, earth & water) in the corners of the image, alongside the tria prima on the central triangle:
| Year: 1690 | PT id = 981, Type = formulation |
Newton's Lapis Philosophicus cum suis rotis elementaribus
In 1936 a collection of Newton's papers, amazingly regarded as of "no scientific value" when offered to Cambridge university some fifty years earlier, was purchased at Sotheby's by the respected economist and Newton scholar John Maynard Keynes. Originally left in a stack by Newton when he left his post as the director of the London mint in 1696, these documents had somehow fortuitously escaped the burning of Newton's personal writings arranged after his death, and were discovered two centuries later. Included was a handwritten manuscript entitled Lapis Philosophicus cum suis rotis elementaribus: "The philosophical stone elements with its wheels", Google Translate.
Notice how the design below also features in the Ripley Scroll, formulated in the mid-1400s. Newton is known to be influenced by this work:
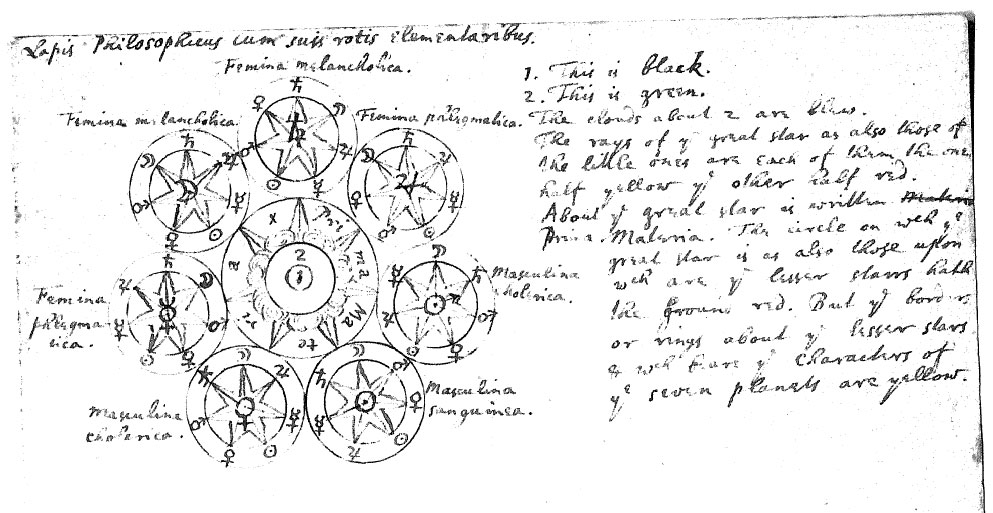
| Year: 1700 | PT id = 473, Type = formulation element |
Elements Known in the Year 1700
Elements known in the year 1700, taken from this Wikipedia page:
| Year: 1718 | PT id = 90, Type = formulation |
Geoffroy's Affinity Table
From Wikipedia, Étienne François Geoffroy's 1718 Affinity Table.
At the head of the column is a substance with which all the substances below can combine.
| Year: 1735 | PT id = 807, Type = element |
Discovery of Cobalt
Co
Cobalt, atomic number 27, has a mass of 58.933 au.
Cobalt was first isolated in 1735 by G. Brandt.
| Year: 1748 | PT id = 858, Type = element |
Discovery of Platinum
Pt
Platinum, atomic number 78, has a mass of 195.084 au.
Platinum was first isolated in 1748 by A. de Ulloa, although it had been used by pre-Colombian Americans.
| Year: 1751 | PT id = 808, Type = element |
Discovery of Nickel
Ni
Nickel, atomic number 28, has a mass of 58.693 au.
Nickel was first isolated in 1751 by F. Cronstedt.
| Year: 1753 | PT id = 863, Type = element |
Discovery of Bismuth
Bi
Bismuth, atomic number 83, has a mass of 208.98 au.
Bismuth was first isolated in 1753 by C.F. Geoffroy.
| Year: 1766 | PT id = 781, Type = element |
Discovery of Hydrogen
H
Hydrogen, atomic number 1, has a mass of 1.008 au.
Hydrogen is the lightest element and by far the most abundant element in the universe: it makes up about about 90% of the universe by weight. Under standard conditions, hydrogen exists as a diatomic molecular gas, H2.
Hydrogen was first isolated and identified as an element in 1766 by H. Cavendish, although it was first made in 1500 by Paracelsus.
| Year: 1771 | PT id = 788, Type = element |
Discovery of Oxygen
O
Oxygen, atomic number 8, has a mass of 15.999 au.
Oxygen exists as a diatomic molecular gas, O2; in this form it makes up about 20% of the atmosphere.
Oxygen was first isolated in 1771 by W. Scheele.
| Year: 1772 | PT id = 787, Type = element |
Discovery of Nitrogen
N
Nitrogen, atomic number 7, has a mass of 14.007 au.
Nitrogen exists as a diatomic molecular gas, N2, and in this form it makes up about 78% of the atmosphere by volume. The element seemed so inert that Lavoisier named it azote, meaning "without life".
Nitrogen was first isolated in 1772 by D. Rutherford.
| Year: 1774 | PT id = 796, Type = element |
Discovery of Chlorine
Cl
Chlorine, atomic number 17, has a mass of 35.452 au.
Chlorine exists as a green diatomic molecular gas, Cl2.
Chlorine was first isolated in 1774 by W. Scheele.
| Year: 1774 | PT id = 805, Type = element |
Discovery of Manganese
Mn
Manganese, atomic number 25, has a mass of 54.938 au.
Manganese was first observed or predicted in 1774 by W. Scheele and first isolated in 1774 by G. Gahn.
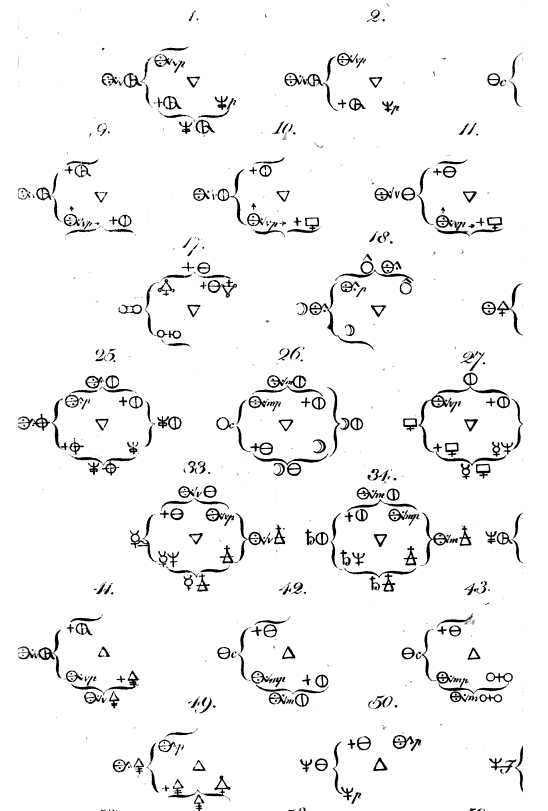
| Year: 1775 | PT id = 515, Type = formulation |
Bergman's Dissertation on Elective Affinities
Alchemical symbols in Torbern Bergman's 1775 Dissertation on Elective Affinities, which was translated from Latin to English in 1783 from Google Books:
| Year: 1778 | PT id = 1, Type = formulation early |
Diderot's Alchemical Chart of Affinities

| Year: 1781 | PT id = 822, Type = element |
Discovery of Molybdenum
Mo
Molybdenum, atomic number 42, has a mass of 95.95 au.
Molybdenum was first observed or predicted in 1778 by W. Scheele and first isolated in 1781 by J. Hjelm.
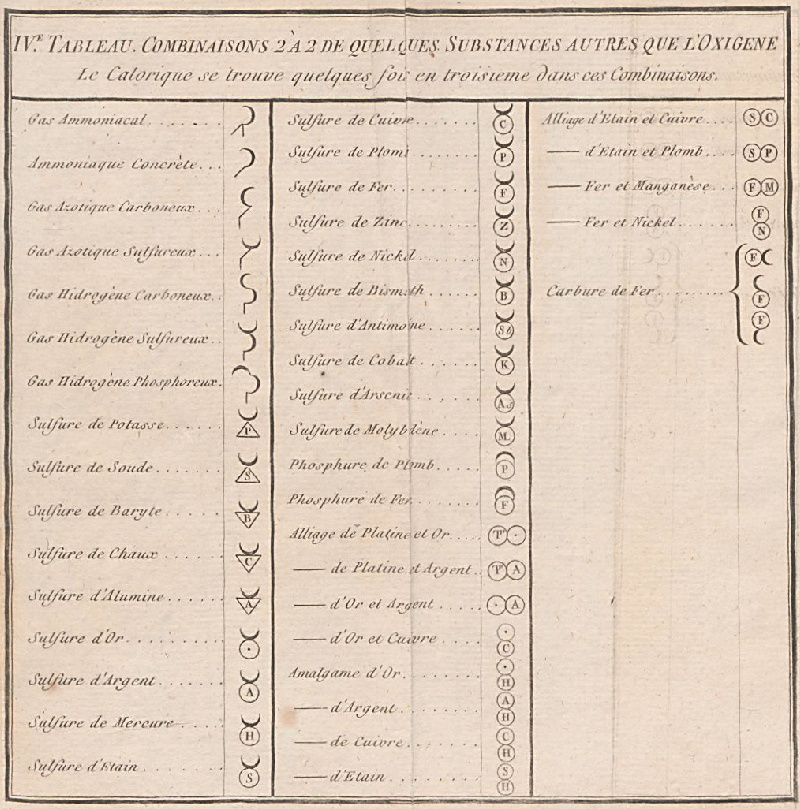
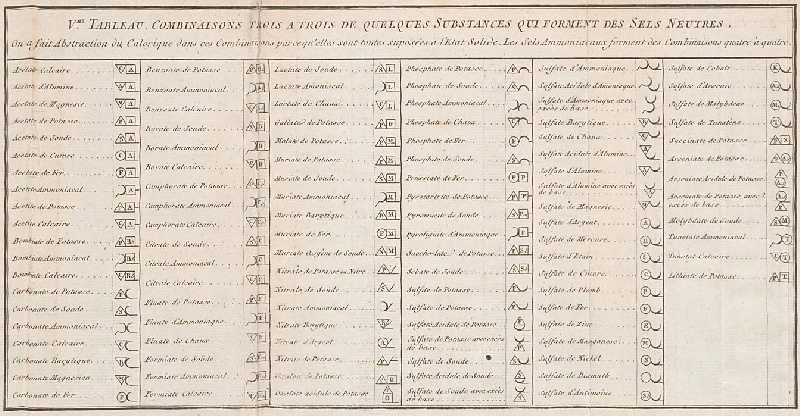
| Year: 1782 | PT id = 297, Type = formulation |
de Morveau's Table of Chemically Simple Substances
de Morveau's table of chemically simple substances (updated with modern representations by Mazurs):
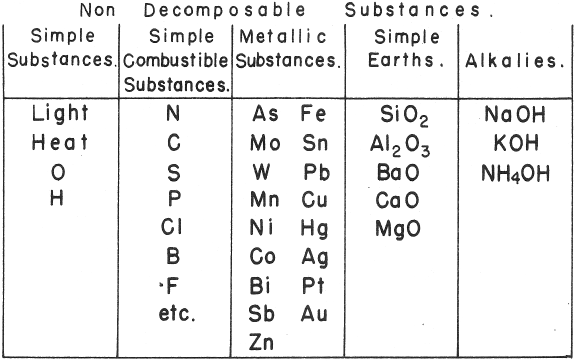
| Year: 1782 | PT id = 832, Type = element |
Discovery of Tellurium
Te
Tellurium, atomic number 52, has a mass of 127.6 au.
Tellurium caused great difficulty to the chemists who first tried to develop a periodic table, because it has an atomic weight greater than iodine (126.9). Mendeleev prioritised chemical properties over the anomalous atomic weight data, and correctly classified Te along with O, S, & Se. It was only when nuclear structure and the importance of atomic number was recognised, around 1918, that the issue was explained.
Tellurium was first isolated in 1782 by F.-J.M. von Reichenstein.
| Year: 1783 | PT id = 854, Type = element |
Discovery of Tungsten
W
Tungsten, atomic number 74, has a mass of 183.84 au.
Tungsten was first observed or predicted in 1781 by W. Scheele and first isolated in 1783 by J. and F. Elhuyar.
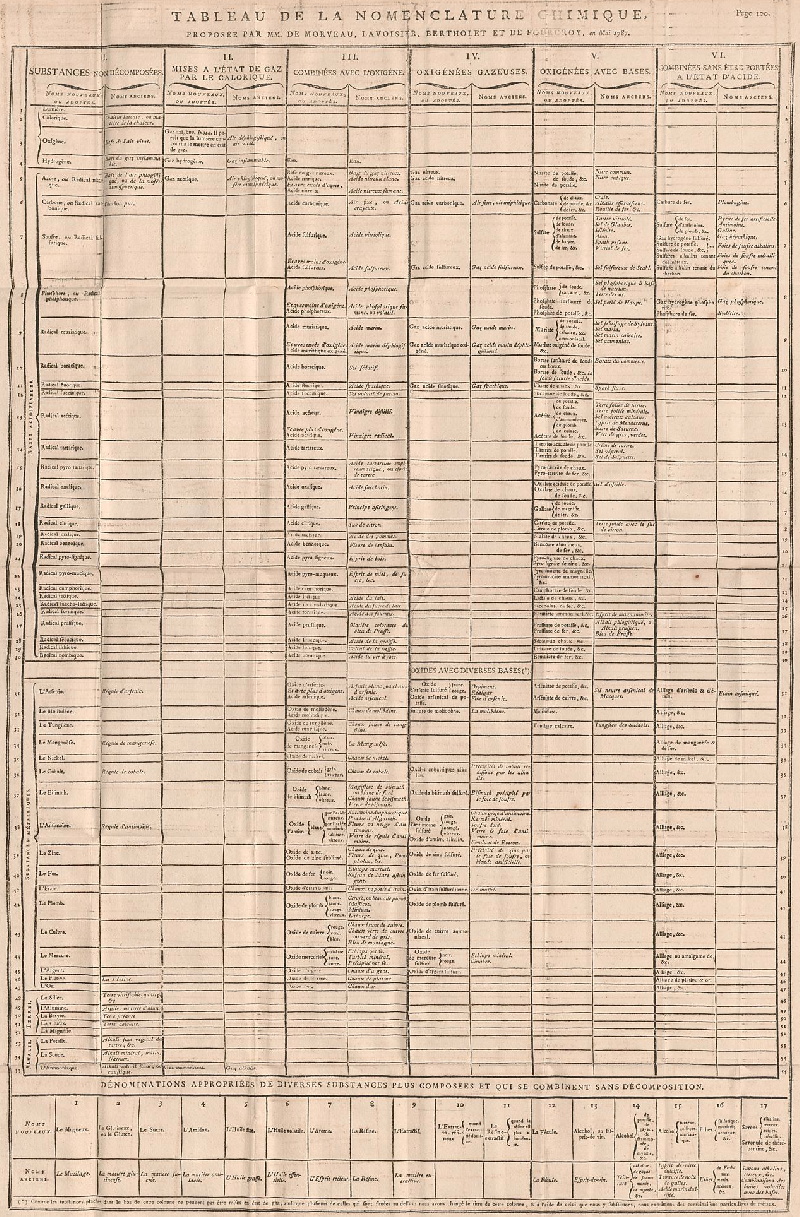
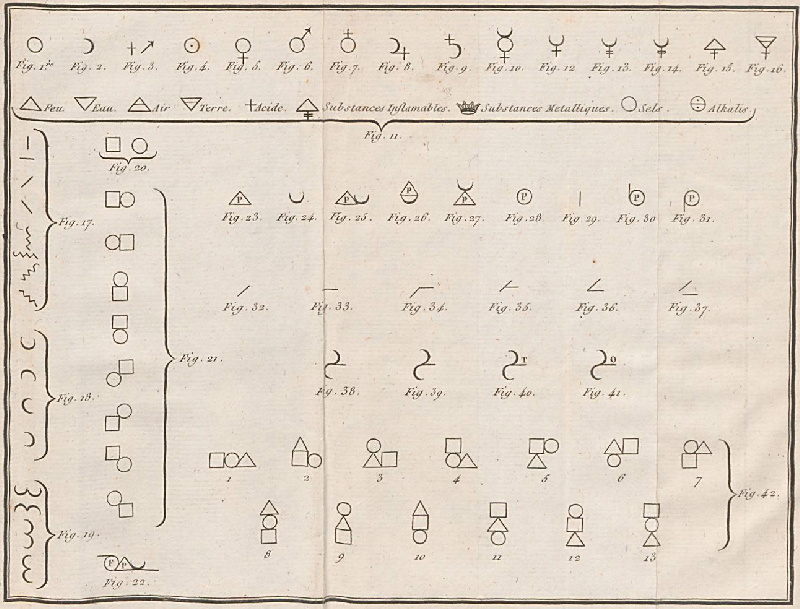
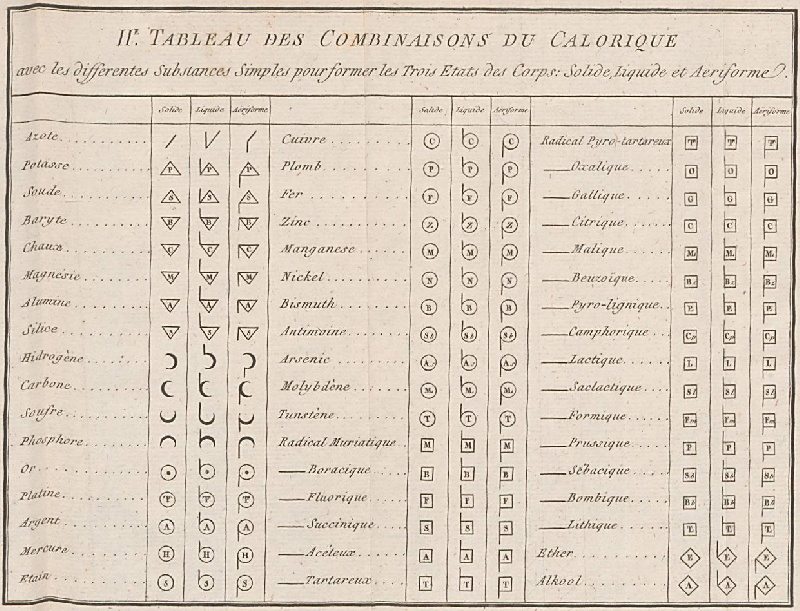
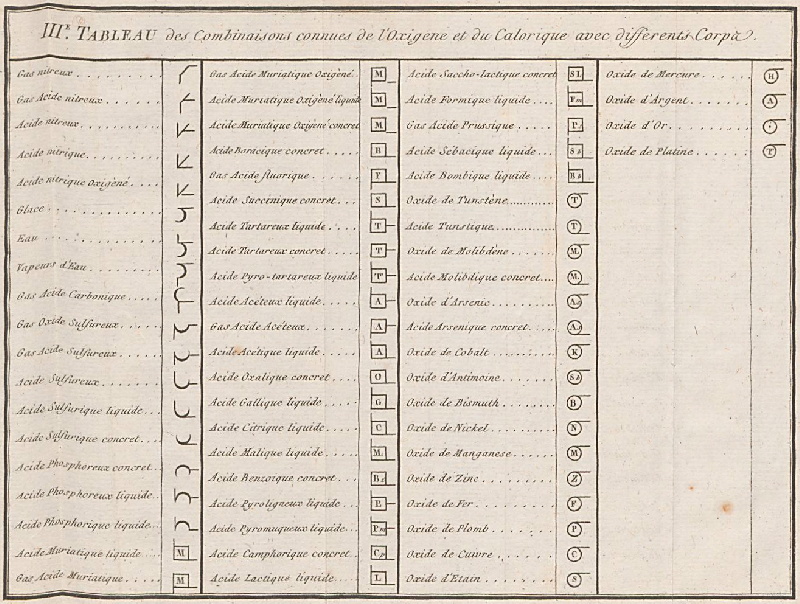
| Year: 1787 | PT id = 964, Type = formulation data |
Méthode de Nomeclature Chimique
By Louis Bernard Guyton de Morveau (1737-1816), Antoine Laurent Lavoisier (1743-1794) , Claude-Louis Berthollet (1748-1822) & Antoine-François de Fourcroy (1755-1809) a book: Méthode de Nomeclature Chimique.
The complete scanned book is available. (Click the 'page view' button, or here.)
The book lists the several hundred chemicals known at the time, including chemical elements, and it discusses the nomenclature (naming). Although not a periodic table as such, the information contained in this book was state of the art for 1787.
Click on an image below to enlarge.
| Year: 1789 | PT id = 3, Type = formulation early |
Antoine Lavoisier
Antoine Lavoisier produced a list chemical substances, that included the 23 known elements. He also refined the concept as before this time, metals - with the exception of mercury - were not considered to be elements. Wikipedia.
A list of 33 simple substances compiled by Lavoisier, from Traité Élémentaire de Chimie, Cuchet, Paris, 1789, p. 192:

From Peter van der Krogt's Elementymology & Elements Multidict web site:
| Lavoisier's Table of Simple Substances (1789) | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Year: 1789 | PT id = 820, Type = element |
Discovery of Zirconium
Zr
Zirconium, atomic number 40, has a mass of 91.224 au.
Zirconium was first observed or predicted in 1789 by H. Klaproth and first isolated in 1824 by J. Berzelius.
| Year: 1789 | PT id = 872, Type = element |
Discovery of Uranium
U ![]()
Uranium, atomic number 92, has a mass of 238.029 au.
Radioactive element with a very long half-life.
Uranium was first observed or predicted in 1789 by H. Klaproth and first isolated in 1841 by E.-M. Péligot.
| Year: 1791 | PT id = 802, Type = element |
Discovery of Titanium
Ti
Titanium, atomic number 22, has a mass of 47.867 au.
Titanium was first observed or predicted in 1791 by W. Gregor and first isolated in 1825 by J. Berzelius.
| Year: 1794 | PT id = 819, Type = element |
Discovery of Yttrium
Y
Yttrium, atomic number 39, has a mass of 88.906 au.
Yttrium was first observed or predicted in 1794 by J. Gadolin and first isolated in 1842 by G. Mosander.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
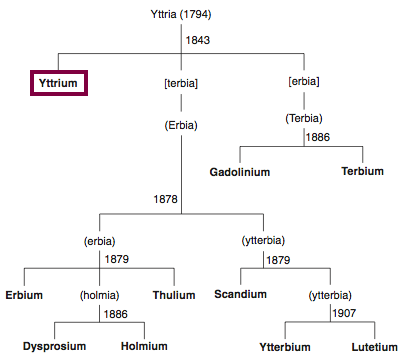
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1798 | PT id = 784, Type = element |
Discovery of Beryllium
Be
Beryllium, atomic number 4, has a mass of 9.012 au.
Beryllium is a metal with a high melting point. At ordinary temperatures it resists oxidation in air. Beryllium compounds are very toxic.
Beryllium was first observed or predicted in 1798 by N. Vauquelin and first isolated in 1828 by F. Wöhler and A. Bussy.
| Year: 1798 | PT id = 804, Type = element |
Discovery of Chromium
Cr
Chromium, atomic number 24, has a mass of 51.996 au.
Chromium was first observed or predicted in 1797 by N. Vauquelin and first isolated in 1798 by N. Vauquelin.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.