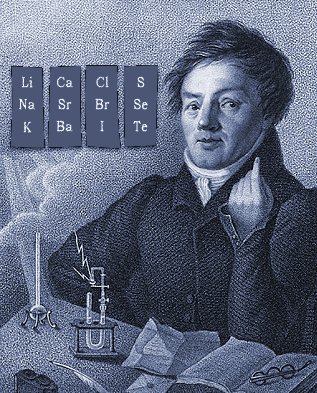Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Elements by Date:
| Year: 9000 BCE | PT id = 809, Type = element |
Discovery of Copper
Cu
Copper, atomic number 29, has a mass of 63.546 au.
Copper had its earliest use in about 9000 BCE, and the oldest sample dates from 6000 BCE. It was discovered by Middle East workers and the earliest sample is from Anatolia.
| Year: 7000 BCE | PT id = 862, Type = element |
Discovery of Lead
Pb
Lead, atomic number 82, has a mass of 207.2 au.
Lead had its earliest use in about 7000 BCE, and the oldest sample dates from 3800 BCE. It was discovered by Africa and the earliest sample is from Abydos, Egypt.
| Year: 6000 BCE | PT id = 859, Type = element |
Discovery of Gold
Au
Gold, atomic number 79, has a mass of 196.967 au.
Gold had its earliest use in about 6000 BCE, and the oldest sample dates from 4400 BCE. It was discovered by Bulgaria and the earliest sample is from Varna Necropolis.
| Year: 5000 BCE | PT id = 806, Type = element |
Discovery of Iron
Fe
Iron, atomic number 26, has a mass of 55.845 au.
Iron had its earliest use in about 5000 BCE, and the oldest sample dates from 4000 BCE from Egypt.
| Year: 5000 BCE | PT id = 827, Type = element |
Discovery of Silver
Ag
Silver, atomic number 47, has a mass of 107.868 au.
Silver had its earliest use in about 5000 BCE, and the oldest sample dates from 4000 BCE, and is from Asia Minor.
| Year: 3750 BCE | PT id = 786, Type = element |
Discovery of Carbon
C
Carbon, atomic number 6, has a mass of 12.011 au.
Carbon has many allotropes, including: graphite, diamond, graphene, C60, single wall nanotubes, etc.
Carbon had its earliest use in about 3750 BCE. It was discovered by Egyptians and Sumerians.
| Year: 3500 BCE | PT id = 830, Type = element |
Discovery of Tin
Sn
Tin, atomic number 50, has a mass of 118.71 au.
Tin + copper gives bronze, and so the Bronze Age.
Tin had its earliest use in about 3500 BCE, and the oldest sample dates from 2000 BCE. It is unknown who discovered the element.
| Year: 2000 BCE | PT id = 795, Type = element |
Discovery of Sulfur (Sulphur)
S
Sulfur, atomic number 16, has a mass of 32.068 au.
Sulfur is a pale yellow, odourless, brittle solid.
Sulfur had its earliest use in about 2000 BCE. It was discovered by Chinese/Indians.
| Year: 2000 BCE | PT id = 860, Type = element |
Discovery of Mercury
Hg
Mercury, atomic number 80, has a mass of 200.592 au.
Mercury had its earliest use in about 2000 BCE, and the oldest sample dates from 1500 BCE. It was discovered by Chinese/Indians and the earliest sample is from Egypt.
| Year: 1000 BCE | PT id = 810, Type = element |
Discovery of Zinc
Zn
Zinc, atomic number 30, has a mass of 65.38 au.
Zinc had its earliest use in about 1000 BCE, and the oldest sample dates from 1000 BCE. It was discovered by Indian metallurgists and the earliest sample is from the Indian subcontinent.
| Year: 800 BCE | PT id = 831, Type = element |
Discovery of Antimony
Sb
Antimony, atomic number 51, has a mass of 121.76 au.
Antimony had its earliest use in about 800 BCE.
| Year: 300 BCE | PT id = 813, Type = element |
Discovery of Arsenic
As
Arsenic, atomic number 33, has a mass of 74.922 au.
Arsenic had its earliest use in about 300 BCE.
| Year: 1000 | PT id = 472, Type = formulation element |
Elements Known in the Year 1000
Elements known in the year 1000, taken from this Wikipedia page:
| Year: 1669 | PT id = 794, Type = element |
Discovery of Phosphorus
P
Phosphorus, atomic number 15, has a mass of 30.974 au.
Phosphorus exists in several allotropic forms including: white, red and black.
Phosphorus was first isolated in 1669 by H. Brand.
| Year: 1700 | PT id = 473, Type = formulation element |
Elements Known in the Year 1700
Elements known in the year 1700, taken from this Wikipedia page:
| Year: 1735 | PT id = 807, Type = element |
Discovery of Cobalt
Co
Cobalt, atomic number 27, has a mass of 58.933 au.
Cobalt was first isolated in 1735 by G. Brandt.
| Year: 1748 | PT id = 858, Type = element |
Discovery of Platinum
Pt
Platinum, atomic number 78, has a mass of 195.084 au.
Platinum was first isolated in 1748 by A. de Ulloa, although it had been used by pre-Colombian Americans.
| Year: 1751 | PT id = 808, Type = element |
Discovery of Nickel
Ni
Nickel, atomic number 28, has a mass of 58.693 au.
Nickel was first isolated in 1751 by F. Cronstedt.
| Year: 1753 | PT id = 863, Type = element |
Discovery of Bismuth
Bi
Bismuth, atomic number 83, has a mass of 208.98 au.
Bismuth was first isolated in 1753 by C.F. Geoffroy.
| Year: 1766 | PT id = 781, Type = element |
Discovery of Hydrogen
H
Hydrogen, atomic number 1, has a mass of 1.008 au.
Hydrogen is the lightest element and by far the most abundant element in the universe: it makes up about about 90% of the universe by weight. Under standard conditions, hydrogen exists as a diatomic molecular gas, H2.
Hydrogen was first isolated and identified as an element in 1766 by H. Cavendish, although it was first made in 1500 by Paracelsus.
| Year: 1771 | PT id = 788, Type = element |
Discovery of Oxygen
O
Oxygen, atomic number 8, has a mass of 15.999 au.
Oxygen exists as a diatomic molecular gas, O2; in this form it makes up about 20% of the atmosphere.
Oxygen was first isolated in 1771 by W. Scheele.
| Year: 1772 | PT id = 787, Type = element |
Discovery of Nitrogen
N
Nitrogen, atomic number 7, has a mass of 14.007 au.
Nitrogen exists as a diatomic molecular gas, N2, and in this form it makes up about 78% of the atmosphere by volume. The element seemed so inert that Lavoisier named it azote, meaning "without life".
Nitrogen was first isolated in 1772 by D. Rutherford.
| Year: 1774 | PT id = 796, Type = element |
Discovery of Chlorine
Cl
Chlorine, atomic number 17, has a mass of 35.452 au.
Chlorine exists as a green diatomic molecular gas, Cl2.
Chlorine was first isolated in 1774 by W. Scheele.
| Year: 1774 | PT id = 805, Type = element |
Discovery of Manganese
Mn
Manganese, atomic number 25, has a mass of 54.938 au.
Manganese was first observed or predicted in 1774 by W. Scheele and first isolated in 1774 by G. Gahn.
| Year: 1781 | PT id = 822, Type = element |
Discovery of Molybdenum
Mo
Molybdenum, atomic number 42, has a mass of 95.95 au.
Molybdenum was first observed or predicted in 1778 by W. Scheele and first isolated in 1781 by J. Hjelm.
| Year: 1782 | PT id = 832, Type = element |
Discovery of Tellurium
Te
Tellurium, atomic number 52, has a mass of 127.6 au.
Tellurium caused great difficulty to the chemists who first tried to develop a periodic table, because it has an atomic weight greater than iodine (126.9). Mendeleev prioritised chemical properties over the anomalous atomic weight data, and correctly classified Te along with O, S, & Se. It was only when nuclear structure and the importance of atomic number was recognised, around 1918, that the issue was explained.
Tellurium was first isolated in 1782 by F.-J.M. von Reichenstein.
| Year: 1783 | PT id = 854, Type = element |
Discovery of Tungsten
W
Tungsten, atomic number 74, has a mass of 183.84 au.
Tungsten was first observed or predicted in 1781 by W. Scheele and first isolated in 1783 by J. and F. Elhuyar.
| Year: 1789 | PT id = 820, Type = element |
Discovery of Zirconium
Zr
Zirconium, atomic number 40, has a mass of 91.224 au.
Zirconium was first observed or predicted in 1789 by H. Klaproth and first isolated in 1824 by J. Berzelius.
| Year: 1789 | PT id = 872, Type = element |
Discovery of Uranium
U ![]()
Uranium, atomic number 92, has a mass of 238.029 au.
Radioactive element with a very long half-life.
Uranium was first observed or predicted in 1789 by H. Klaproth and first isolated in 1841 by E.-M. Péligot.
| Year: 1791 | PT id = 802, Type = element |
Discovery of Titanium
Ti
Titanium, atomic number 22, has a mass of 47.867 au.
Titanium was first observed or predicted in 1791 by W. Gregor and first isolated in 1825 by J. Berzelius.
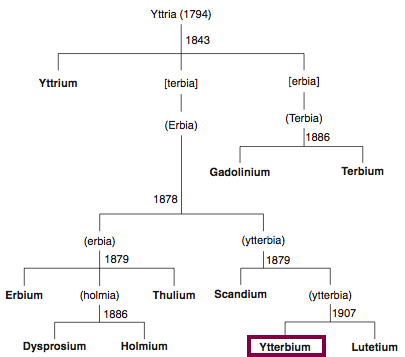
| Year: 1794 | PT id = 819, Type = element |
Discovery of Yttrium
Y
Yttrium, atomic number 39, has a mass of 88.906 au.
Yttrium was first observed or predicted in 1794 by J. Gadolin and first isolated in 1842 by G. Mosander.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
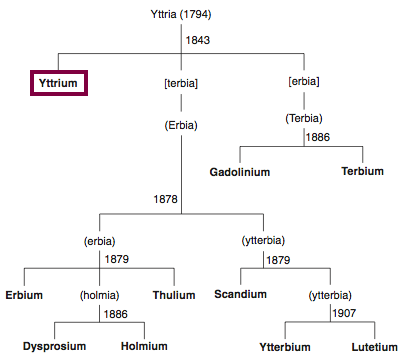
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1798 | PT id = 784, Type = element |
Discovery of Beryllium
Be
Beryllium, atomic number 4, has a mass of 9.012 au.
Beryllium is a metal with a high melting point. At ordinary temperatures it resists oxidation in air. Beryllium compounds are very toxic.
Beryllium was first observed or predicted in 1798 by N. Vauquelin and first isolated in 1828 by F. Wöhler and A. Bussy.
| Year: 1798 | PT id = 804, Type = element |
Discovery of Chromium
Cr
Chromium, atomic number 24, has a mass of 51.996 au.
Chromium was first observed or predicted in 1797 by N. Vauquelin and first isolated in 1798 by N. Vauquelin.
| Year: 1800 | PT id = 235, Type = formulation element |
Elements Known in the Year 1800
Elements known in the year 1800, taken from this Wikipedia page:
| Year: 1801 | PT id = 821, Type = element |
Discovery of Niobium
Nb
Niobium, atomic number 41, has a mass of 92.906 au.
Niobium was first observed or predicted in 1801 by C. Hatchett and first isolated in 1864 by W. Blomstrand.
| Year: 1802 | PT id = 853, Type = element |
Discovery of Tantalum
Ta
Tantalum, atomic number 73, has a mass of 180.948 au.
Tantalum was first isolated in 1802 by G. Ekeberg.
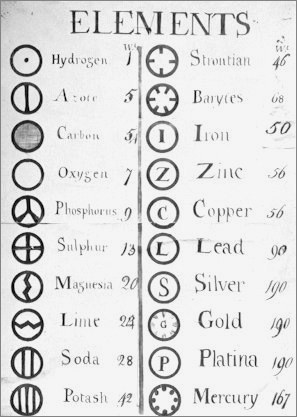
| Year: 1803 | PT id = 4, Type = formulation element weight structure |
Dalton's Postulates About The Elements
Around the year 1803 in Manchester, John Dalton gave a series of lectures in which he presented his postulates:
- Elements are made of tiny particles called atoms.
- The atoms of a given element are different from those of any other element, and the atoms of different elements can be distinguished from one another by their respective relative atomic weigh/mass.
- All atoms of a given element are identical.
- Atoms of one element can combine with atoms of other elements to form chemical compounds, and a given compound always has the same relative numbers of types of atoms.
- Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process, and a chemical reaction simply changes the way atoms are grouped together.
From a very early notebook from around this time:

| Year: 1803 | PT id = 826, Type = element |
Discovery of Palladium
Pd
Palladium, atomic number 46, has a mass of 106.42 au.
Palladium was first isolated in 1803 by H. Wollaston.
| Year: 1803 | PT id = 838, Type = element |
Discovery of Cerium
Ce
Cerium, atomic number 58, has a mass of 140.116 au.
Cerium was first observed or predicted in 1803 by H. Klaproth, J. Berzelius, and W. Hisinger and first isolated in 1838 by G. Mosander.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
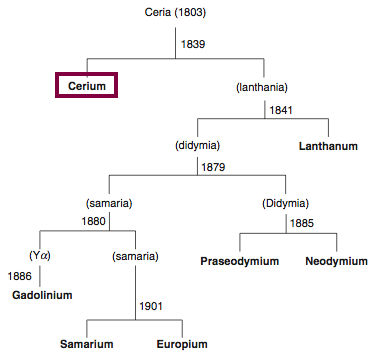
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1803 | PT id = 856, Type = element |
Discovery of Osmium
Os
Osmium, atomic number 76, has a mass of 190.23 au.
Osmium was first isolated in 1803 by S. Tennant.
| Year: 1803 | PT id = 857, Type = element |
Discovery of Iridium
Ir
Iridium, atomic number 77, has a mass of 192.217 au.
Iridium was first isolated in 1803 by S. Tennant.
| Year: 1804 | PT id = 825, Type = element |
Discovery of Rhodium
Rh
Rhodium, atomic number 45, has a mass of 102.906 au.
Rhodium was first isolated in 1804 by H. Wollaston.
| Year: 1807 | PT id = 790, Type = element |
Discovery of Sodium
Na
Sodium, atomic number 11, has a mass of 22.99 au.
Sodium is a Group 1 element, and these are often referred to as the "alkali metals".
Sodium was first isolated in 1807 by H. Davy.
| Year: 1807 | PT id = 799, Type = element |
Discovery of Potassium
K
Potassium, atomic number 19, has a mass of 39.098 au.
Potassium is a Group 1 element, and these are often referred to as the "alkali metals".
Potassium was first isolated in 1807 by H. Davy.
| Year: 1808 | PT id = 5, Type = formulation data element weight structure |
Dalton's Elements
Two pages from John Dalton's A New System of Chemical Philosophy in which he proposed his version of atomic theory based on scientific experimentation (see the scanned book, page 219):
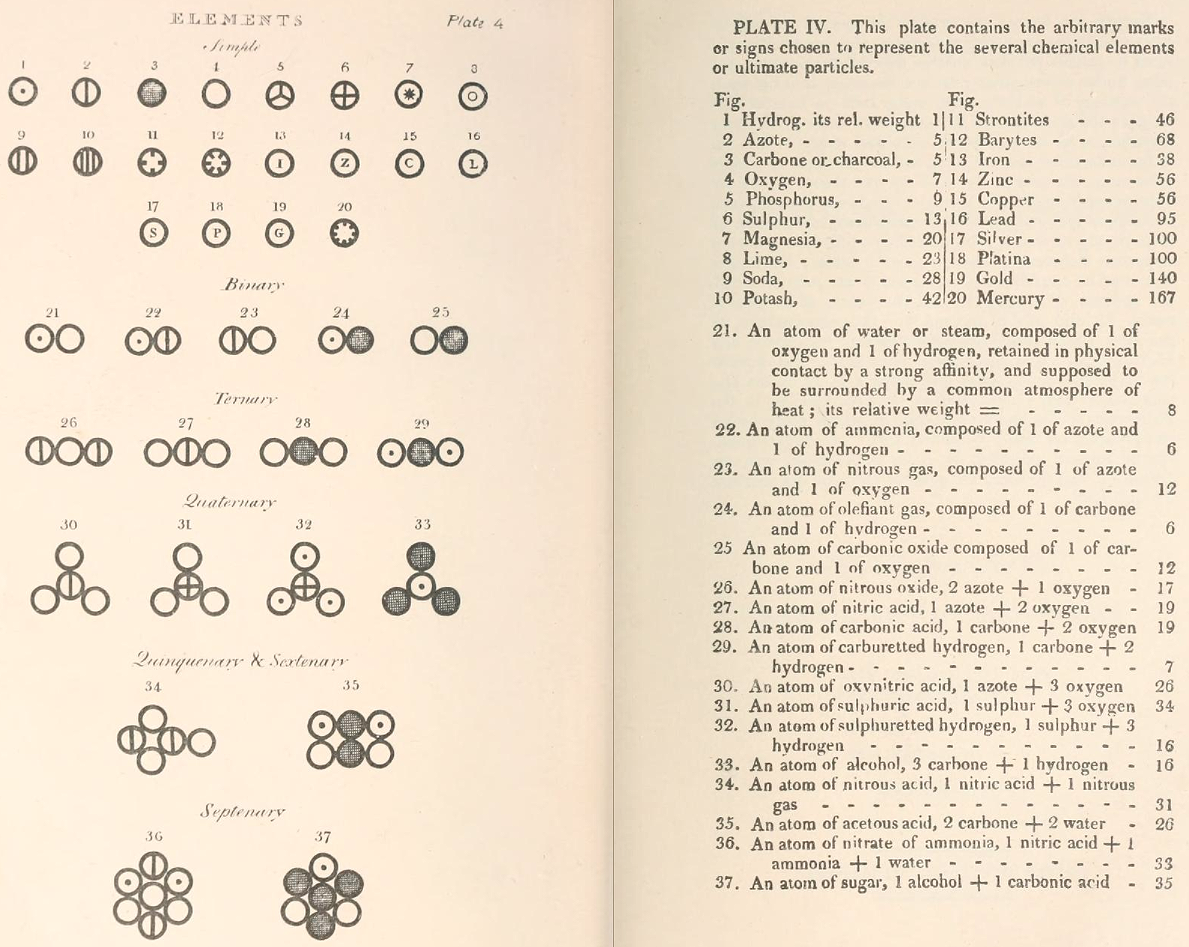
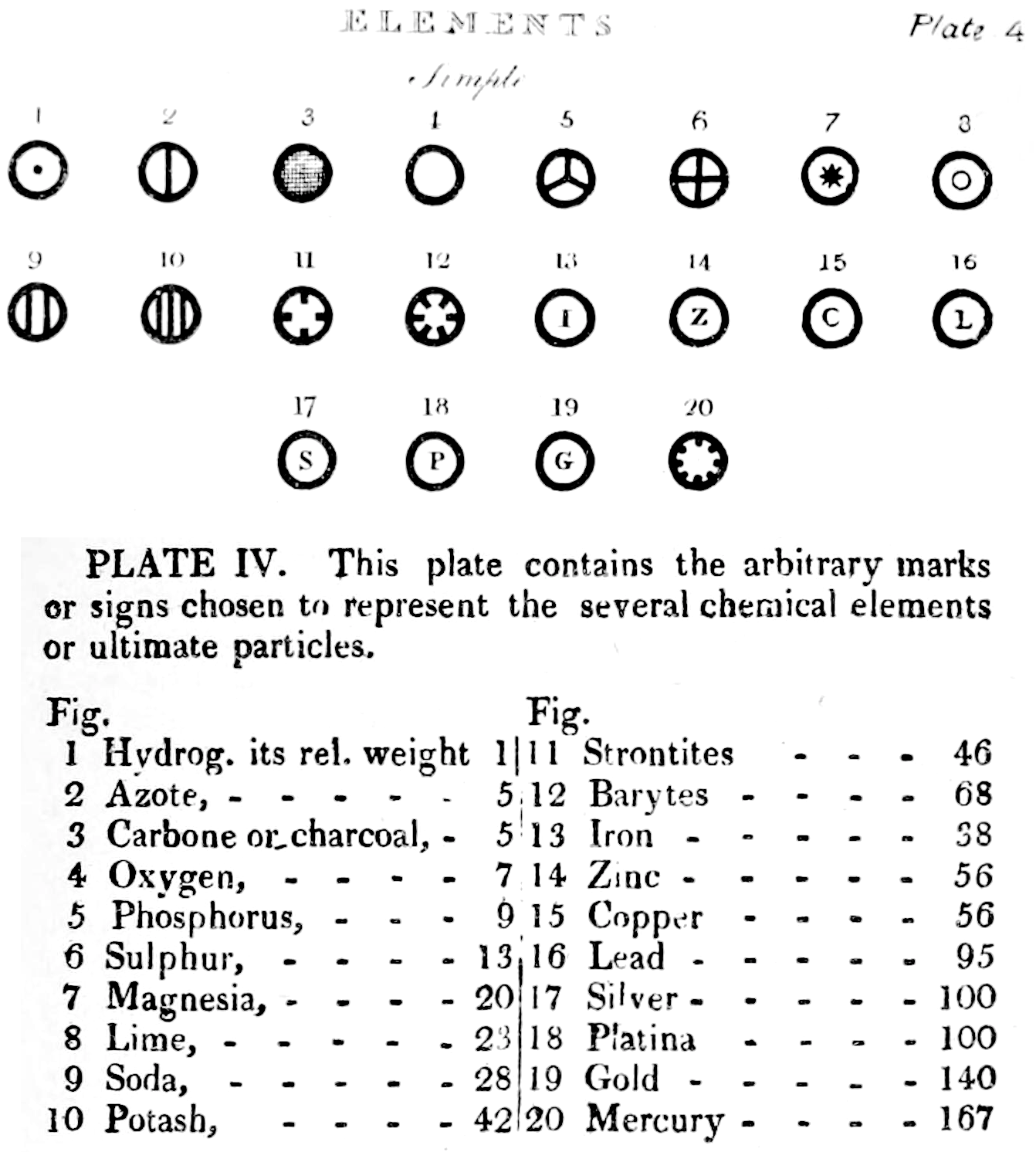
| Name | Modern Symbol | Dalton's Data | Modern Values | % error |
| Hydrog. | H | 1 | 1 | 0% |
| Azote | N | 5 | 14 | -180% |
| Carbone | C | 5 | 12 | -140% |
| Oxygen | O | 7 | 16 | -129% |
| Phosphorus | P | 9 | 31 | -244% |
| Sulphur | S | 13 | 32.1 | -147% |
| Magnesia | Mg | 20 | 24.3 | -22% |
| Lime | Ca | 24 | 40.1 | -67% |
| Soda | Na | 28 | 23 | 18% |
| Potash | K | 42 | 39.1 | 7% |
| Strontites | Sr | 46 | 87.6 | -90% |
| Barytes | Ba | 68 | 137.3 | -102% |
| Iron | Fe | 50 | 55.8 | -12% |
| Zinc | Zn | 56 | 65.4 | -17% |
| Copper | Cu | 56 | 63.5 | -13% |
| Lead | Pb | 90 | 200.6 | -123% |
| Silver | Ag | 190 | 107.9 | 43% |
| Gold | Au | 190 | 197 | -4% |
| Platina | Pt | 190 | 195.1 | -3% |
| Mercury | Hg | 167 | 200.6 | -20% |
- Dalton states that he is considering "chemical elements or ultimate particles"
- Dalton assigns hydrogen as having a relative weight of 1.
- Note the seemingly huge % errors in the atomic weights, compared with modern values.
- These errors occurred because while Dalton had deduced that atoms combine in fixed (stoichiometric) ratios in compounds, he not always know what the ratios were. Thus there were two unknowns: the atomic weights (masses) and the stoichiometric ratios.
By Mark Leach
| Year: 1808 | PT id = 785, Type = element |
Discovery of Boron
B
Boron, atomic number 5, has a mass of 10.814 au.
Boron has properties that are borderline between metal and non-metal (semimetallic).
Boron was first observed or predicted in 1808 by L. Gay-Lussac and L.J. Thénard and first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 791, Type = element |
Discovery of Magnesium
Mg
Magnesium, atomic number 12, has a mass of 24.306 au.
Magnesium is a Group 2 element, and these are called: "alkaline earth metals".
Magnesium was first observed or predicted in 1755 by J. Black and first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 800, Type = element |
Discovery of Calcium
Ca
Calcium, atomic number 20, has a mass of 40.078 au.
Calcium is a Group 2 element, and these are called: "alkaline earth metals".
Calcium was first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 818, Type = element |
Discovery of Strontium
Sr
Strontium, atomic number 38, has a mass of 87.62 au.
Strontium is a Group 2 element, and these are called: "alkaline earth metals".
Strontium was first observed or predicted in 1787 by W. Cruikshank and first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 836, Type = element |
Discovery of Barium
Ba
Barium, atomic number 56, has a mass of 137.327 au.
Barium is a Group 2 element, and these are called: "alkaline earth metals".
Barium was first observed or predicted in 1772 by W. Scheele and first isolated in 1808 by H. Davy.
| Year: 1811 | PT id = 833, Type = element |
Discovery of Iodine
I
Iodine, atomic number 53, has a mass of 126.904 au.
Iodine exists as a black diatomic molecular solid, I2.
Iodine was first isolated in 1811 by B. Courtois.
| Year: 1813 | PT id = 1044, Type = formulation element weight |
Wollaston's Synoptic Scale of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S., or from here.
It is apparent that chemistry the years 1810 to 1850 was largely concerned with discovering the whole number stoichiometric ratios of atoms in chemical compounds.
Wollaston writes in the text above:
"It is impossible in several instances, where only two combinations of the same ingredients are known, to discover which of the compounds is to be regarded as consisting of a pair of single atoms, and since the decision of these questions is purely theoretical, and by no means necessary to the formation of a table adapted to most practical purposes, I have not been desirous of warping my numbers according to an atomic theory, but have endeavored to make practical convenience my sole guide, and have considered the doctrine of simple multiples, on which that of atoms is founded, merely as a valuable assistant in determining, by simple division, the amount of those quantities that are liable to such definite deviations from the original law of Richter."
"Mr. Dalton in his atomic views of chemical combination appears not to have taken much pains to ascertain the actual prevalence of that law of multiple proportions by which the atomic theory is best supported [however] it is in fact to Mr. Dalton that we are indebted for the first correct observation of such an instance of a simple multiple in the union of nitrous gas with oxygen."
"[I have] computed a series of supposed atoms, I [have] assumed oxygen as the decimal unit of my scale [ie. oxygen = 10], in order to facilitate the estimation of those numerous combinations which it forms with other bodies. Though the present table of Equivalents, I have taken care to make oxygen equally prominent on account of the important part it performs in determining the affinities of bodies by the different proportions in which it is united to them.."
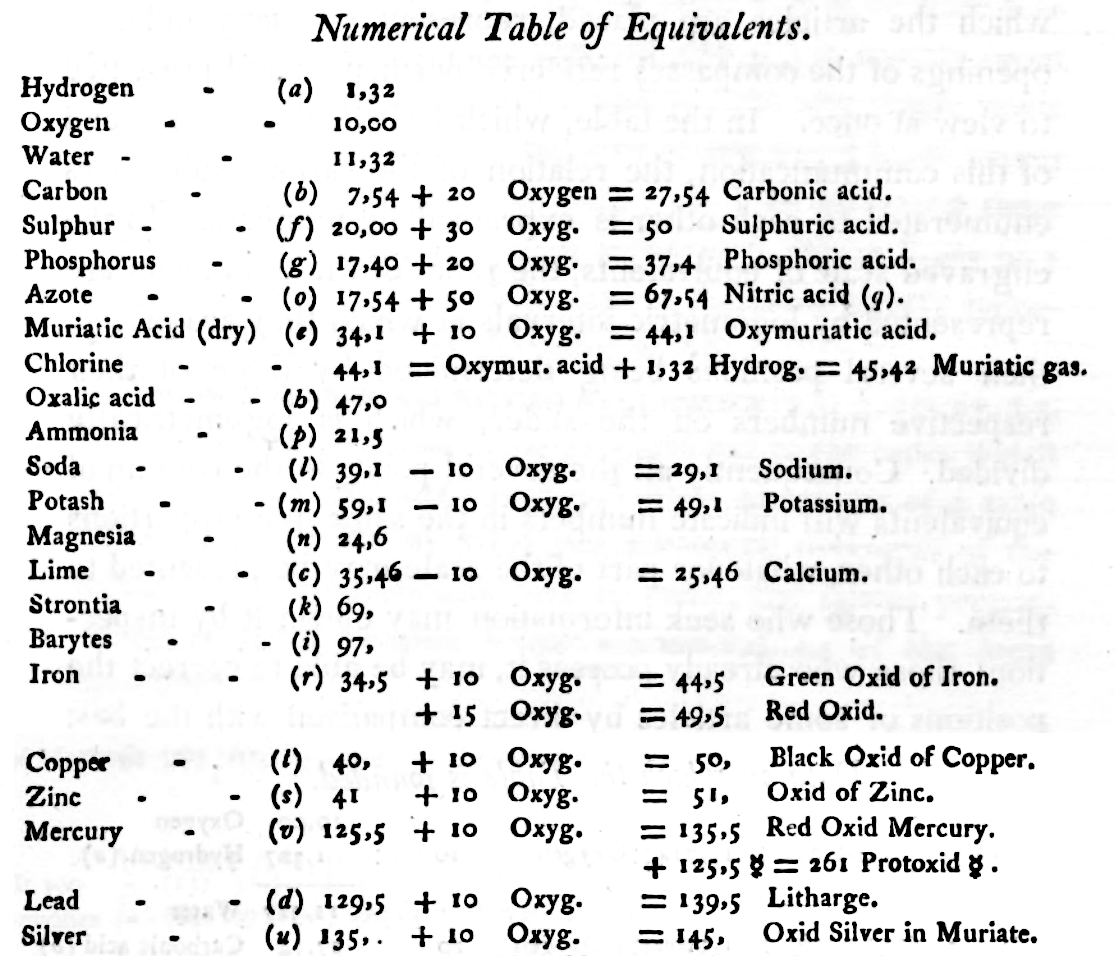
Mark Leach writes:
"When Wollaston's equivalent weights are converted from O = 10.00 to the modern value of O = 15.999, the atomic weight values can be seen to be astonishingly accurate.
"However, the language of the article is quite difficult as the meaning of many of the terms is unclear (to me, at least). For example, in modern usage adding 'ia' to a metal implies the oxide: 'magnesia' is magnesium oxide, MgO. I am not clear if this historical usage is consistent. 'Azote' is nitrogen and 'muriatic acid (dry)' is hydrogen chloride gas. I have only analyses/re-calculated the elements and a couple of common/obvious compounds:"
| Wollaston's data | Scaled to O = 15.999 | Modern Values | % error | |
| H (as H2) | 1.32 | 2.112 | 2.016 | 5% |
| O | 10.00 | 15.999 | 15.999 | ref. value |
| H2O | 11.32 | 18.111 | 18.015 | 1% |
| C | 7.74 | 12.383 | 12.011 | 3% |
| S | 20.00 | 31.998 | 32.060 | 0% |
| P | 17.40 | 27.838 | 30.974 | -11% |
| N (as N2) | 17.54 | 28.062 | 28.014 | 0% |
| Cl (as Cl2) | 44.10 | 70.556 | 70.900 | 0% |
| Fe | 34.50 | 55.197 | 55.845 | -1% |
| Cu | 40.00 | 63.996 | 63.546 | 1% |
| Zn | 41.00 | 65.596 | 65.380 | 0% |
| Hg | 125.50 | 200.787 | 200.590 | 0% |
| Pb | 129.50 | 207.187 | 207.980 | 0% |
| Ag | 135.00 | 215.987 | 107.870 | 50% |
- The elements hydrogen, nitrogen (azote) and chlorine have clearly been measured as the diatomic molecules, even if this was unknown to Wollaston in 1813.
- Phosphorus is out by 11%... [fair enough].
- Only silver is clearly wrong, but it is out by 50% so it looks like a simple stoichiometry error: Perhaps the oxide was assumed to be AgO was instead of the correct Ag2O.
Interestingly, Wollaston's analysis is far better than Daubeny's 1831 data seen in Oxford.
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
| Year: 1817 | PT id = 783, Type = element |
Discovery of Lithium
Li
Lithium, atomic number 3, has a mass of 6.968 au.
Lithium is a reactive metal, of low density: it is the least dense metal.
Lithium was first observed or predicted in 1817 by A. Arfwedson and first isolated in 1821 by W. T. Brande.
| Year: 1817 | PT id = 814, Type = element |
Discovery of Selenium
Se
Selenium, atomic number 34, has a mass of 78.971 au.
Selenium was first isolated in 1817 by J. Berzelius and G. Gahn.
| Year: 1817 | PT id = 828, Type = element |
Discovery of Cadmium
Cd
Cadmium, atomic number 48, has a mass of 112.414 au.
Cadmium was first isolated in 1817 by S. L Hermann, F. Stromeyer and J.C.H. Roloff.
| Year: 1824 | PT id = 793, Type = element |
Discovery of Silicon
Si
Silicon, atomic number 14, has a mass of 28.085 au.
Silicon makes up 25.7% of the earth's crust, and after oxygen is the second most abundant element.
Silicon was first isolated in 1823 by J. Berzelius.
| Year: 1825 | PT id = 792, Type = element |
Discovery of Aluminium (Aluminum)
Al
Aluminium (aluminum), atomic number 13, has a mass of 26.982 au.
Aluminum is a silvery-white metal.
Aluminium was first isolated in 1825 by H.C.Ørsted.
| Year: 1825 | PT id = 815, Type = element |
Discovery of Bromine
Br
Bromine, atomic number 35, has a mass of 79.904 au.
Bromine exists as an orange diatomic molecular liquid, Br2.
Bromine was first isolated in 1825 by J. Balard and C. Löwig.
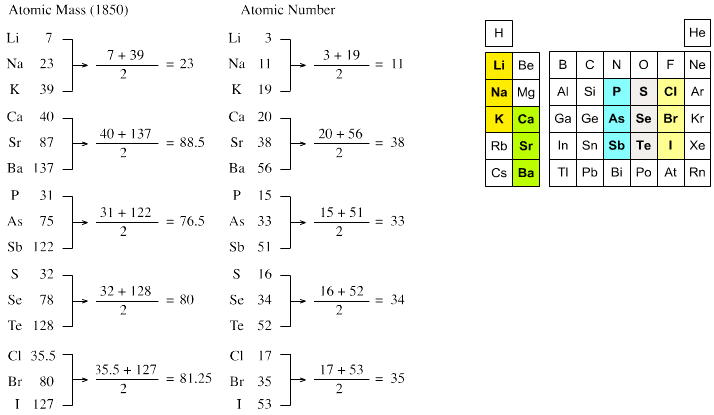
| Year: 1829 | PT id = 6, Type = formulation element |
Döbereiner's Triads
Johann Döbereiner found triads: a sequence of three similar elements, where the middle element has a mass equal to the average of the least and most massive.
A brief biography can be found on the Nature website.
Döbereiner writes in An Attempt to Group Elementary Substances according to Their Analogies (in English)
From Poggendorf's Annalen der Physik und Chemie 15, 301-7 (1829) (in German) [from Henry M. Leicester & Herbert S. Klickstein, eds., A Source Book in Chemistry, 1400-1900 (Cambridge, MA: Harvard, 1952)]:
"The work of Berzelius on the determination of the atomic weights of bromine and iodine has interested me greatly, since it has established the idea, which I expressed earlier in my lectures, that perhaps the atomic weight of bromine might be the arithmetical mean of the atomic weights of chlorine and iodine. This mean is (35.470+126.470)/2 = 80.470. This number is not much greater than that found by Berzelius (78.383); however, it comes so close that it may almost be hoped that the difference will vanish entirely after repeated careful and exact determinations of the atomic weights of these three salt-forming elements. This idea was the motive for an attempt which I made twelve years ago to group substances by their analogies."
[Note: L&K noticed an error in the above math: (35.47 + 126.47)/2 = 80.97 not 80.47. Whoops...]

The diagram below uses mid-nineteenth century atomic mass information rather than modern data. If atomic numbers (Z) are used (a property unknown in 1850), the triads are exact:
| Year: 1829 | PT id = 870, Type = element |
Discovery of Thorium
Th ![]()
Thorium, atomic number 90, has a mass of 232.038 au.
Radioactive element with a very long half-life.
Thorium was first observed or predicted in 1829 by J. Berzelius and first isolated in 1914 by D. Lely, Jr. and L. Hamburger.
| Year: 1830 | PT id = 803, Type = element |
Discovery of Vanadium
V
Vanadium, atomic number 23, has a mass of 50.942 au.
Vanadium was first observed or predicted in 1801 by M. del Río and first isolated in 1830 by N.G.Sefström.
| Year: 1831 | PT id = 337, Type = formulation element weight |
Daubeny's Teaching Display Board & Wooden Cubes of Atomic Weights
The Museum of the History of Science, Oxford, has a display of Charles Daubeny's teaching materials, including a black painted wooden board with "SYMBOLS OF SIMPLE BODIES": showing symbols, atomic weights and names of elements in two columns, and a small pile of cubes with element symbols.
Charles Daubeny and Chemistry at the Old Ashmolean
Charles Daubeny (1795-1867) was appointed Aldrichian Professor of Chemistry at Oxford in 1822. In 1847 he moved from the original laboratory in this basement [in the museum] to a new one built at his own expense at the Botanic Garden. His apparatus went with him and was preserved there. Daubeny actively campaigned for the teaching of science in Oxford and held several professorships in addition to chemistry. He also conducted research on subjects such as photosynthesis.
From the HSM Database (Inventory no. 17504):
DAUBENY'S LIST OF ATOMIC WEIGHTS Wooden panel, black with white lettering, listing in two columns the symbols and names of twenty elements. This lecture board is identical to the table in the third edition (1831) of E. Turner, 'Elements of Chemistry', apart from the atomic weight for bromine. Daubeny wrote a useful 'Introduction to the Atomic Theory' (published in three versions: 1831, 1840, and 1850), the first edition of which also quotes Turner's table. Probably contemporary with this lecture board are the wooden cubes with the symbols for certain elements.
The period from 1810 to 1860 was crucial in the development of the periodic table. Most of the main group and transition elements had been discovered, but their atomic weights and stoichiometries (combining ratios) had not been fully deduced. Oxygen was assumed to have a weight of 6, and consequently carbon is assumed to have a mass of 6.
Daubeny's element symbols and weights – along with the modern mass data – are tabulated:
| Symbol | Daubeny's Weight | Modern Mass Data | % error | Stoichiometry Error |
| H | 1 | 1 | 0% | |
| C | 6 | 12 | -100% | factor of 2 |
| O | 8 | 16 | -100% | factor of 2 |
| Si | 8 | 28.1 | -251% | factor of 5 (?) |
| Al | 10 | 27 | -170% | factor of 3 |
| Mg | 12 | 24.3 | -103% | factor of 2 |
| N | 14 | 14 | 0% | |
| S | 16 | 32.1 | -101% | factor of 2 |
| P | 16 | 31 | -94% | factor of 2 |
| Fl | 19 | 19 | 0% | |
| Ca | 20 | 40.1 | -101% | factor of 2 |
| Na | 24 | 23 | 4% | |
| Fe | 28 | 55.8 | -99% | factor of 2 |
| Cl | 36 | 35.5 | 1% | |
| K | 40 | 39.1 | 2% | |
| Cu | 64 | 63.5 | 1% | |
| B | 80 | 79.9 | 0% | |
| Pb | 104 | 207 | -99% | factor of 2 |
| I | 124 | 127 | -2% | |
| Hg | 200 | 200.6 | 0% |
While quite a number of weights are close to the modern values, many are way out. However, the error is usually a stiotoimetric factor error.
From the HSM Database (Inventory no. 33732): SET OF WOODEN CUBES ILLUSTRATING ATOMIC WEIGHTS
Forty-two wooden cubes numbered 1-42, painted black with symbols for certain elements, compounds or radicals painted in white on the faces, together with the corresponding atomic, molecular or radical weights. The face markings appear in various combinations:
| H | C | P | Na | Ca° | S | N | K | Fe | K | Na° | Cy | K° |
| 1 | 6 | 16 | 24 | 28 | 16 | 14 | 40 | 28 | 48 | 32 | 26 | 48 |
A typical cube (no. 3) may be represented by the following figure. They present something of an enigma as their faces do not form an obvious pattern. The numbers indicate that there were 42 cubes. In style they are similar to the figures on the panel of atomic weights.
The cubes are listed in Daubeny's 1861 catalogue, p. 11 as: "Wooden cubes for illustrating atomic weight". [See D. R. Oldroyd, The Chemical Lectures at Oxford (1822-1854) of Charles Daubeny, M.D., F.R.S. Notes and Records of the Royal Society, vol. 33 (1979), pp. 217-259.]
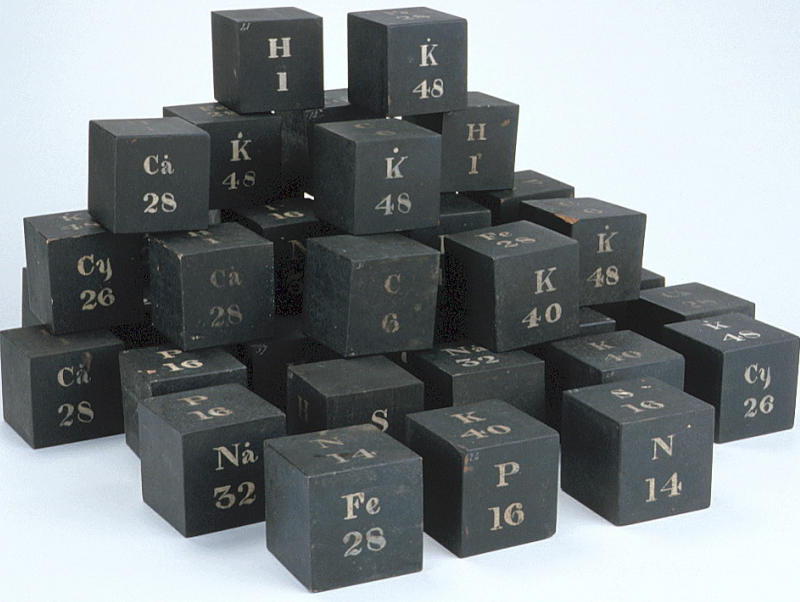
This display was spotted by Eric Scerri who was visiting the museum with Mark Leach in 2010.
There is a virtual tour on the museum, and the above display is in the basement.
| Year: 1838 | PT id = 837, Type = element |
Discovery of Lanthanum
La
Lanthanum, atomic number 57, has a mass of 138.905 au.
Lanthanum was first observed or predicted in 1838 by G. Mosander and first isolated in 1841 by G. Mosander.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
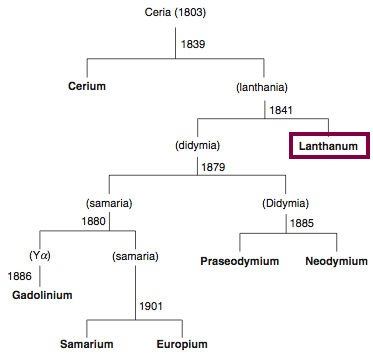
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1842 | PT id = 845, Type = element |
Discovery of Terbium
Tb
Terbium, atomic number 65, has a mass of 158.925 au.
Terbium was first observed or predicted in 1842 by G. Mosander and first isolated in 1886 by J.C.G. de Marignac.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
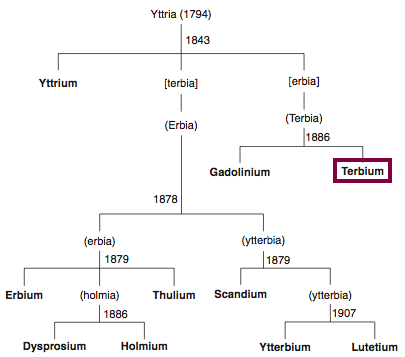
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1842 | PT id = 848, Type = element |
Discovery of Erbium
Er
Erbium, atomic number 68, has a mass of 167.259 au.
Erbium was first observed or predicted in 1842 by G. Mosander and first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
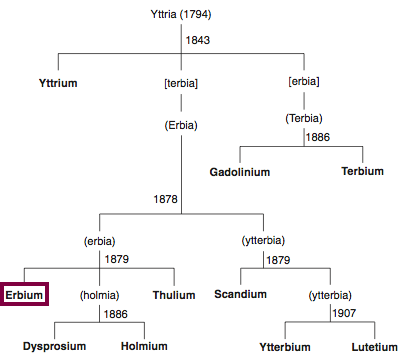
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1844 | PT id = 824, Type = element |
Discovery of Ruthenium
Ru
Ruthenium, atomic number 44, has a mass of 101.07 au.
Ruthenium was first isolated in 1844 by K. Claus.
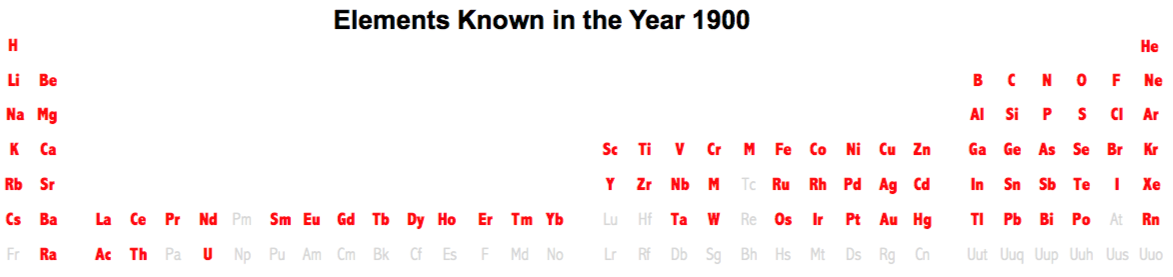
| Year: 1850 | PT id = 474, Type = formulation element weight |
Elements Known in the Year 1850
Elements known in the year 1850, taken from this Wikipedia page:
| Year: 1858 | PT id = 1047, Type = formulation review element weight structure |
Cannizzaro's Letter or Sunto
Letter of Professor Stanislao Cannizzaro to Professor S. De Luca: Sunto di un corso di filosofia chimica (Sketch of a Course of Chemical Philosophy) given in the Royal University of Genoa, Il Nuovo Cimento, vol. vii. (1858), pp. 321-366.

Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about, and link to, Cannizzaro's Letter. See a list of other classic chemistry papers.
Read the full letter/paper, in English translation, here. (The Italian version is here.)
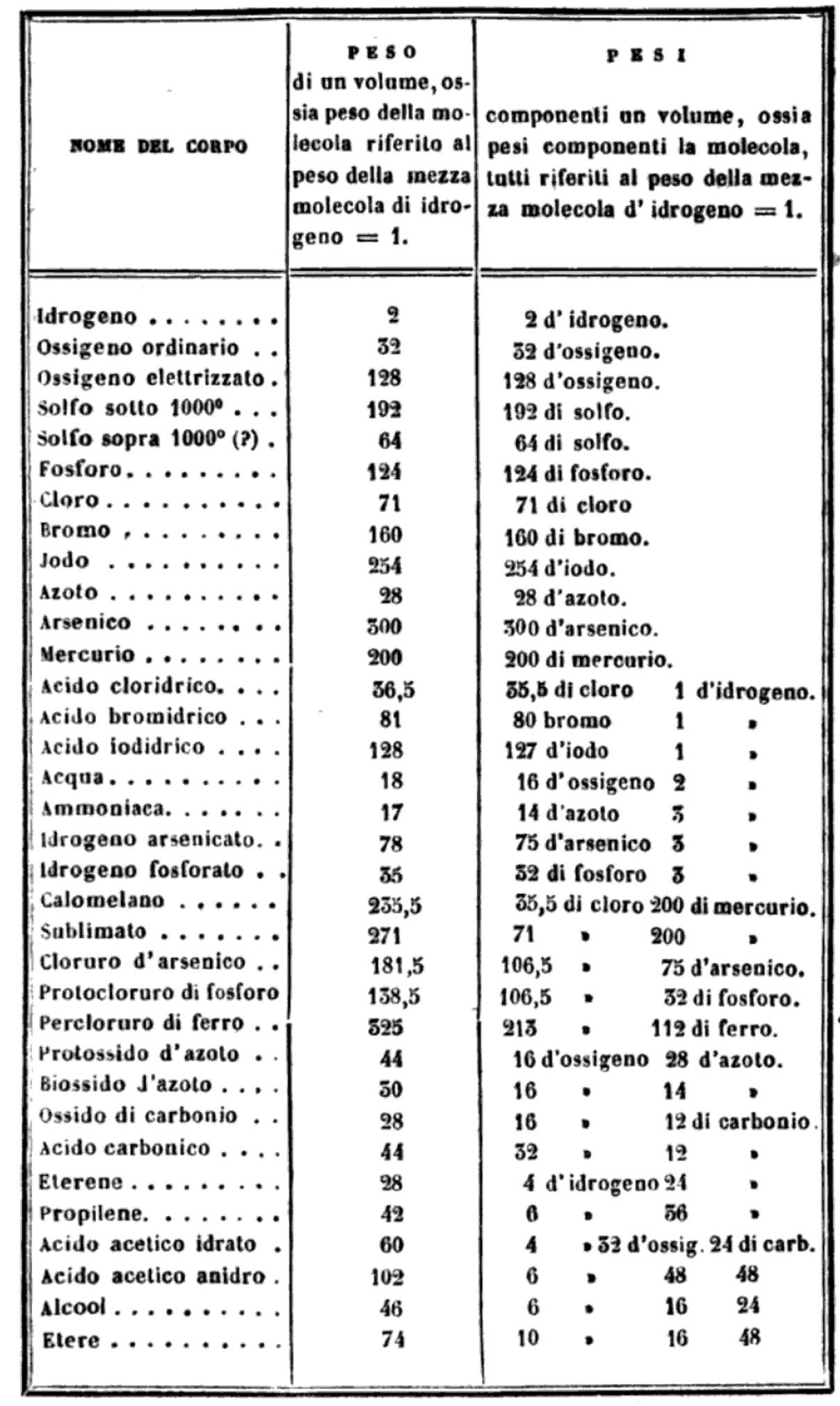
"I believe that the progress of science made in these last years has confirmed the hypothesis of Avogadro, of Ampère, and of Dumas on the similar constitution of substances in the gaseous state; that is, that equal volumes of these substances, whether simple or compound, contain an equal number of molecules: not however an equal number of atoms, since the molecules of the different substances, or those of the same substance in its different states, may contain a different number of atoms, whether of the same or of diverse nature."
From the Science History of Science Institute:
"In 1858 Cannizzaro outlined a course in theoretical chemistry for students at the University of Genoa,where he had to teach without benefit of a laboratory. He used the hypothesis of a fellow Italian, Amedeo Avogadro, who had died just two years earlier, as a pathway out of the confusion rampant among chemists about atomic weights and the fundamental structure of chemical compounds."
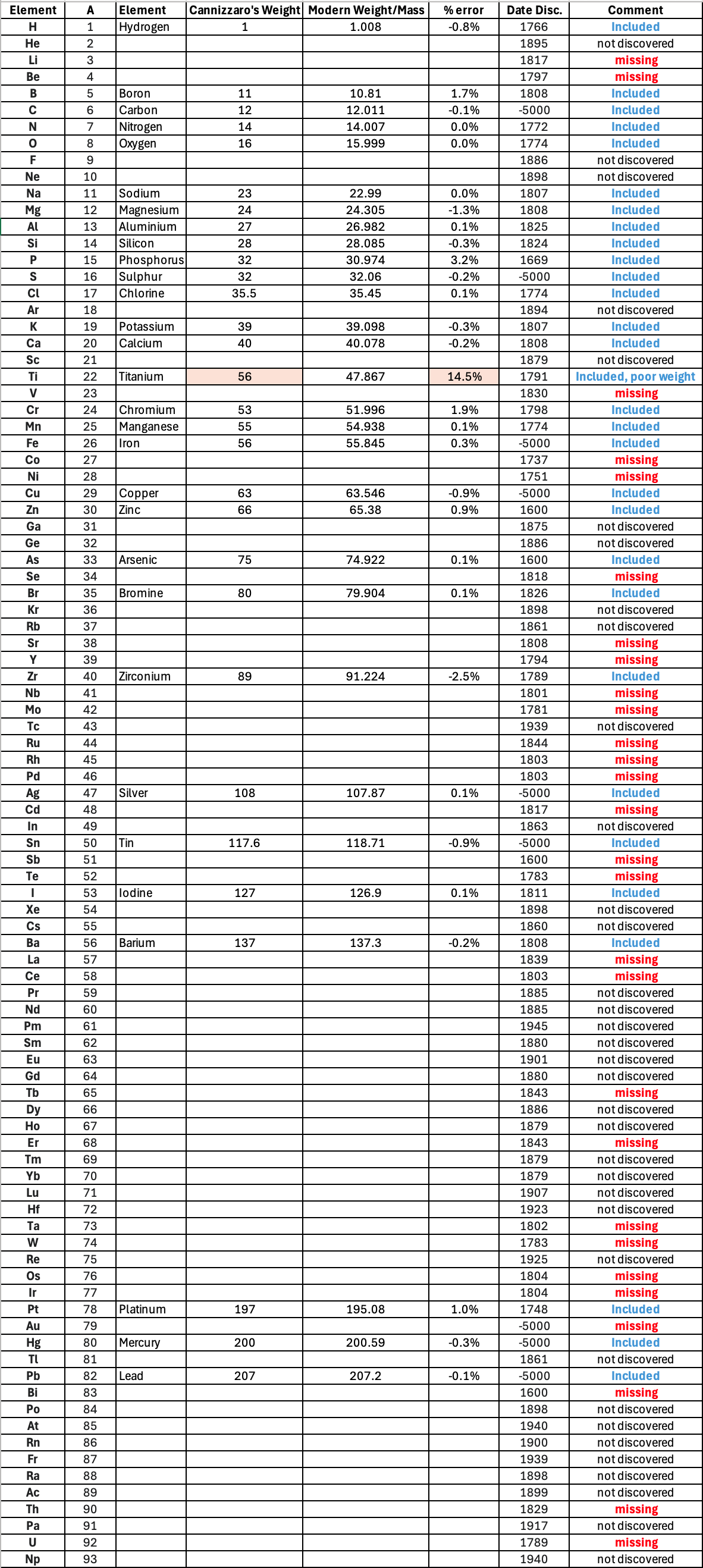
Mark Leach writes:
"Before a periodic table of the chemical elements – which orders the elements by atomic weight and then groups them by property – could be developed it was necessary to know the atomic weight values. However, to deduce the atomic weights was a problem as it was necessary to know the ratios of how the elements combined, the stoichiometry.
"Tables of atomic weight data by Dalton (1808), Wollaston (1813), Daubeny (1831) and Kopp & Will (1858) show progress, but the 1858 Cannizzaro letter was the first where the atomic weight data is more or less both complete and accurate, thus removing stiochiometric errors.
"I have extracted the element atomic weight data from the paper, and given the % error with respect to modern atomic weight/mass data. Only titanium is significantly out! It is clear that Cannizzaron knew that hydrogen, nitrogen, oxygen, chlorine, bromine & iodine existed as diatomic molecules."
| Element | Symbol | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H | 1 | 1.008 | -0.8% |
| Boron | B | 11 | 10.81 | 1.7% |
| Carbon | C | 12 | 12.011 | -0.1% |
| Nitrogen | N | 14 | 14.007 | 0.0% |
| Oxygen | O | 16 | 15.999 | 0.0% |
| Sodium | Na | 23 | 22.99 | 0.0% |
| Magnesium | Mg | 24 | 24.305 | -1.3% |
| Aluminium | Al | 27 | 26.982 | 0.1% |
| Silicon | Si | 28 | 28.085 | -0.3% |
| Sulphur | S | 32 | 32.06 | -0.2% |
| Phosphorus | P | 32 | 30.974 | 3.2% |
| Chlorine | Cl | 35.5 | 35.45 | 0.1% |
| Potassium | K | 39 | 39.098 | -0.3% |
| Calcium | Ca | 40 | 40.078 | -0.2% |
| Chromium | Cr | 53 | 51.996 | 1.9% |
| Manganese | Mn | 55 | 54.938 | 0.1% |
| Iron | Fe | 56 | 55.845 | 0.3% |
| Titanium | Ti | 56 | 47.867 | 14.5% |
| Copper | Cu | 63 | 63.546 | -0.9% |
| Zinc | Zn | 66 | 65.38 | 0.9% |
| Arsenic | As | 75 | 74.922 | 0.1% |
| Bromine | Br | 80 | 79.904 | 0.1% |
| Zirconium | Zr | 89 | 91.224 | -2.5% |
| Silver | Ag | 108 | 107.87 | 0.1% |
| Tin | Sn | 117.6 | 118.71 | -0.9% |
| Iodine | I | 127 | 126.9 | 0.1% |
| Barium | Ba | 137 | 137.3 | -0.2% |
| Platinum | Pt | 197 | 195.08 | 1.0% |
| Mercury | Hg | 200 | 200.59 | -0.3% |
| Lead | Pb | 207 | 207.2 | -0.1% |
| Diatomic Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H2 | 2 | 2.016 | -0.8% |
| Oxygen | O2 | 32 | 31.998 | 0.0% |
| Sulphur | S2 | 64 | 64.12 | -0.2% |
| Chlorine | Cl2 | 71 | 70.9 | 0.1% |
| Bromine | Br2 | 160 | 159.808 | 0.1% |
| Iodine | I2 | 254 | 253.8 | 0.1% |
| Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Water | H2O | 18 | 18.015 | -0.1% |
| Hydrochloric Acid | HCl | 36.5 | 36.458 | 0.1% |
| Methane | CH4 | 16 | 16.043 | -0.3% |
| Hydrogen sulphide | H2S | 34 | 34.076 | -0.2% |
| Diethyl ether | CH3CH2OCH2CH3 | 74 | 74.123 | -0.2% |
| Carbon disulphide | CS2 | 76 | 76.131 | -0.2% |
| Chloroethane | CH3CH2Cl | 64.5 | 64.512 | 0.0% |
Below is a list of the elements showing which ones were included by Cannizzaro and which one were ommitted (because they had not been discovered) or are strangely missing. Odd ommissions (to the modern eye) include: Lithium, Beryllium, Cobalt, Nickel, Palladium, Tungsten and Gold.
| Year: 1858 | PT id = 1348, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1858
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1858 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6
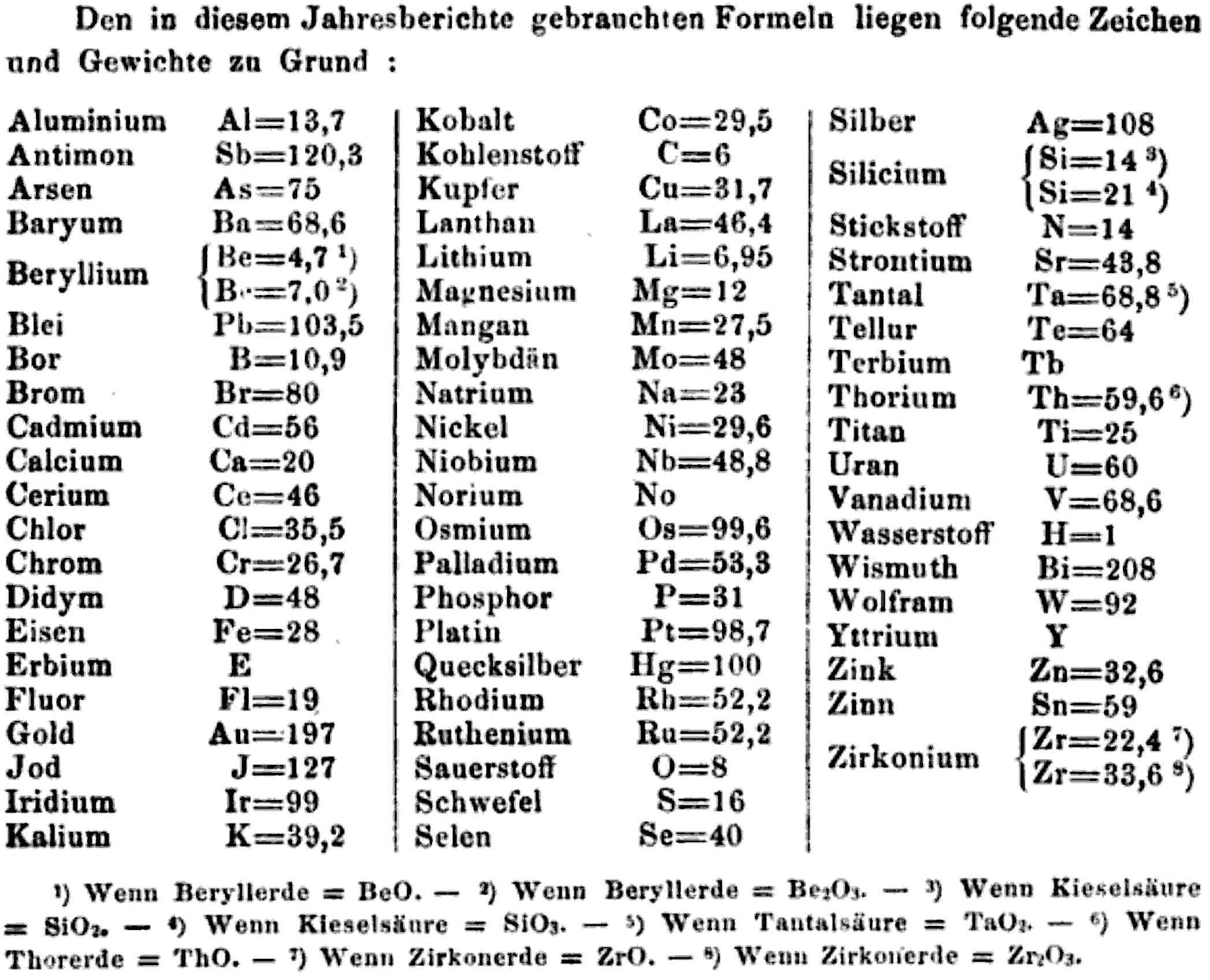
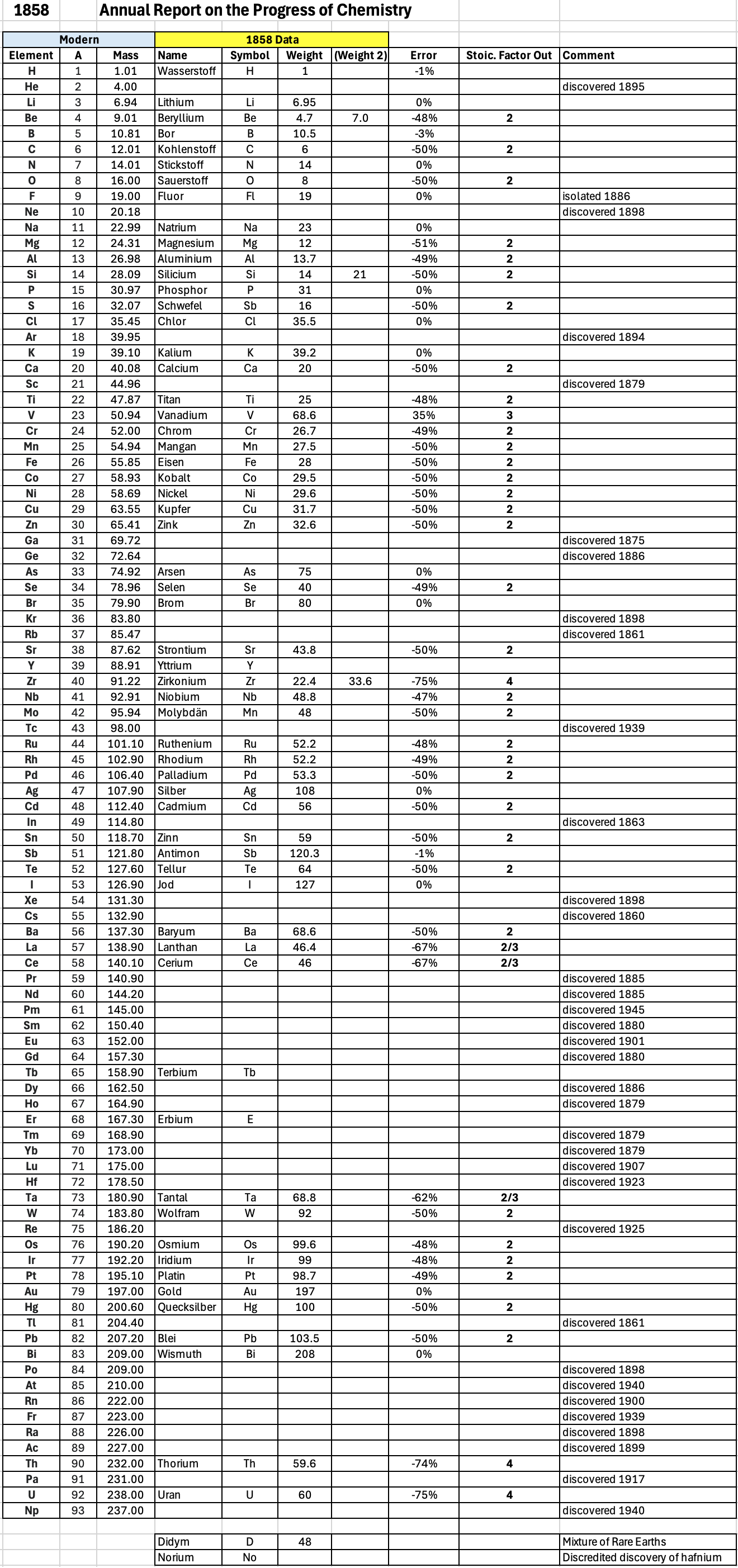
Thanks to René and Mario Rodriguez for the tip!
| Year: 1859 | PT id = 1349, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1859
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1859 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6

Thanks to René and Mario Rodriguez for the tip!
| Year: 1860 | PT id = 835, Type = element |
Discovery of Cesium
Cs
Cesium (or caesium), atomic number 55, has a mass of 132.905 au.
Cesium is a Group 1 element, and these are often referred to as the "alkali metals".
Cesium was first observed or predicted in 1860 by R. Bunsen and R. Kirchhoff and first isolated in 1882 by C. Setterberg.
| Year: 1860 | PT id = 1350, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1860
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1860 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si =14 and 21
- Zr = 22.4, 33.6 and 44.8
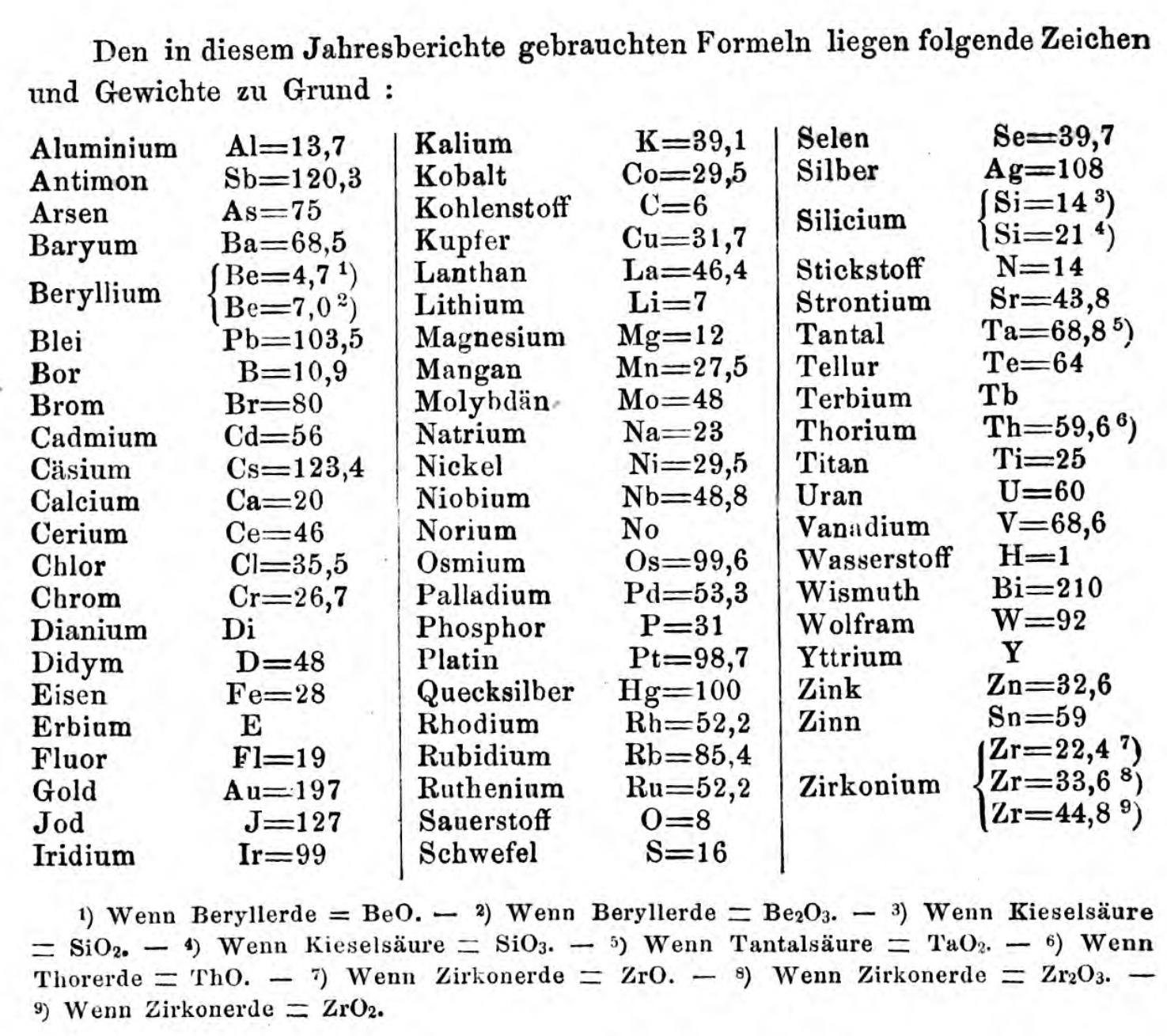
Thanks to René and Mario Rodriguez for the tip!
| Year: 1861 | PT id = 817, Type = element |
Discovery of Rubidium
Rb
Rubidium, atomic number 37, has a mass of 85.468 au.
Rubidium is a Group 1 element, and these are often referred to as the "alkali metals".
Rubidium was first observed, but not isolated in pure form, in 1861 by R. Bunsen and G. R. Kirchhoff.
| Year: 1861 | PT id = 1351, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1861
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1861 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8
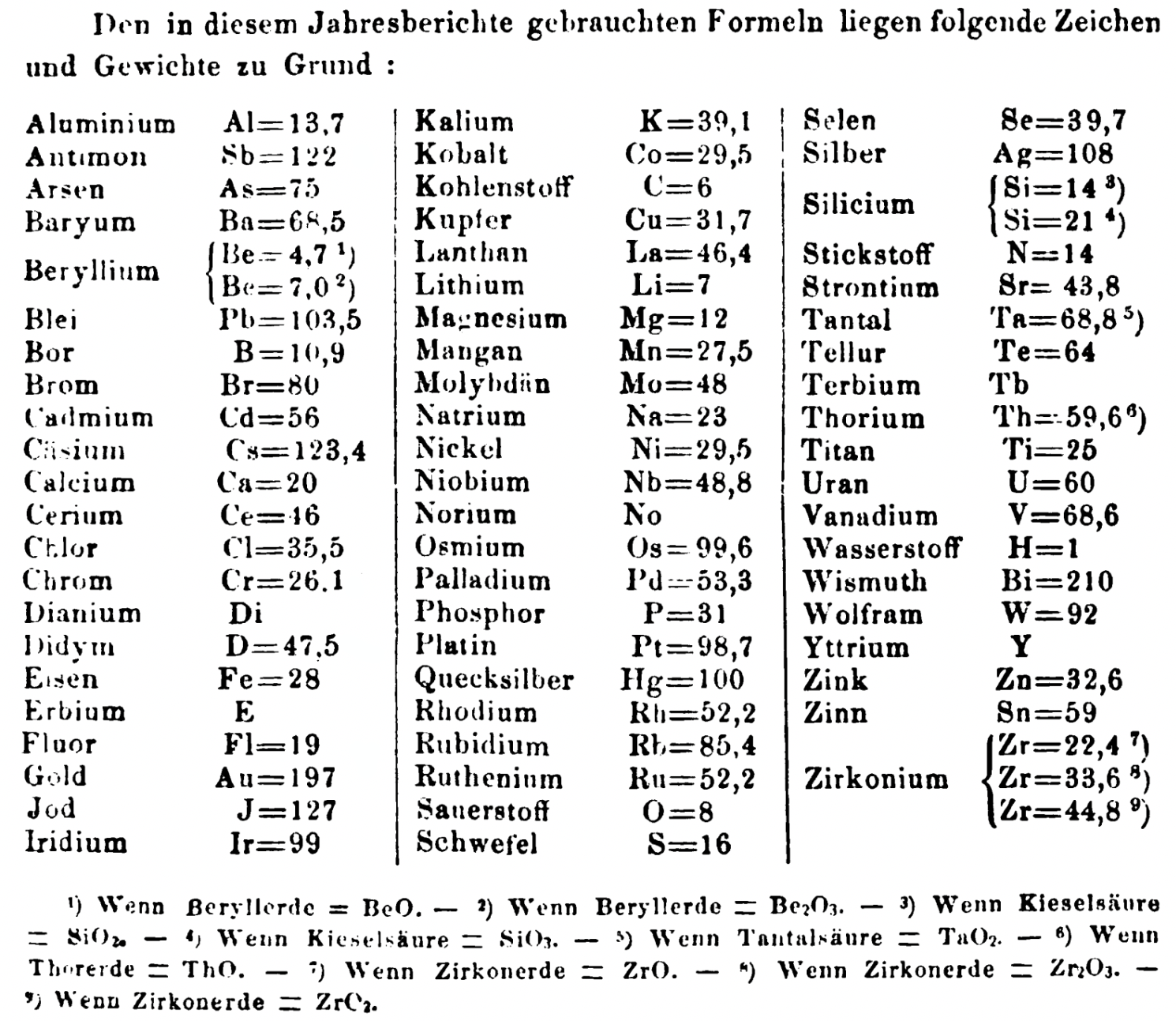
Thanks to René and Mario Rodriguez for the tip!
| Year: 1861 | PT id = 861, Type = element |
Discovery of Thallium
Tl
Thallium, atomic number 81, has a mass of 204.384 au.
Thallium was first observed or predicted in 1861 by W. Crookes and first isolated in 1862 by C.-A. Lamy.
| Year: 1862 | PT id = 1352, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1862
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1862 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8

Thanks to René and Mario Rodriguez for the tip!
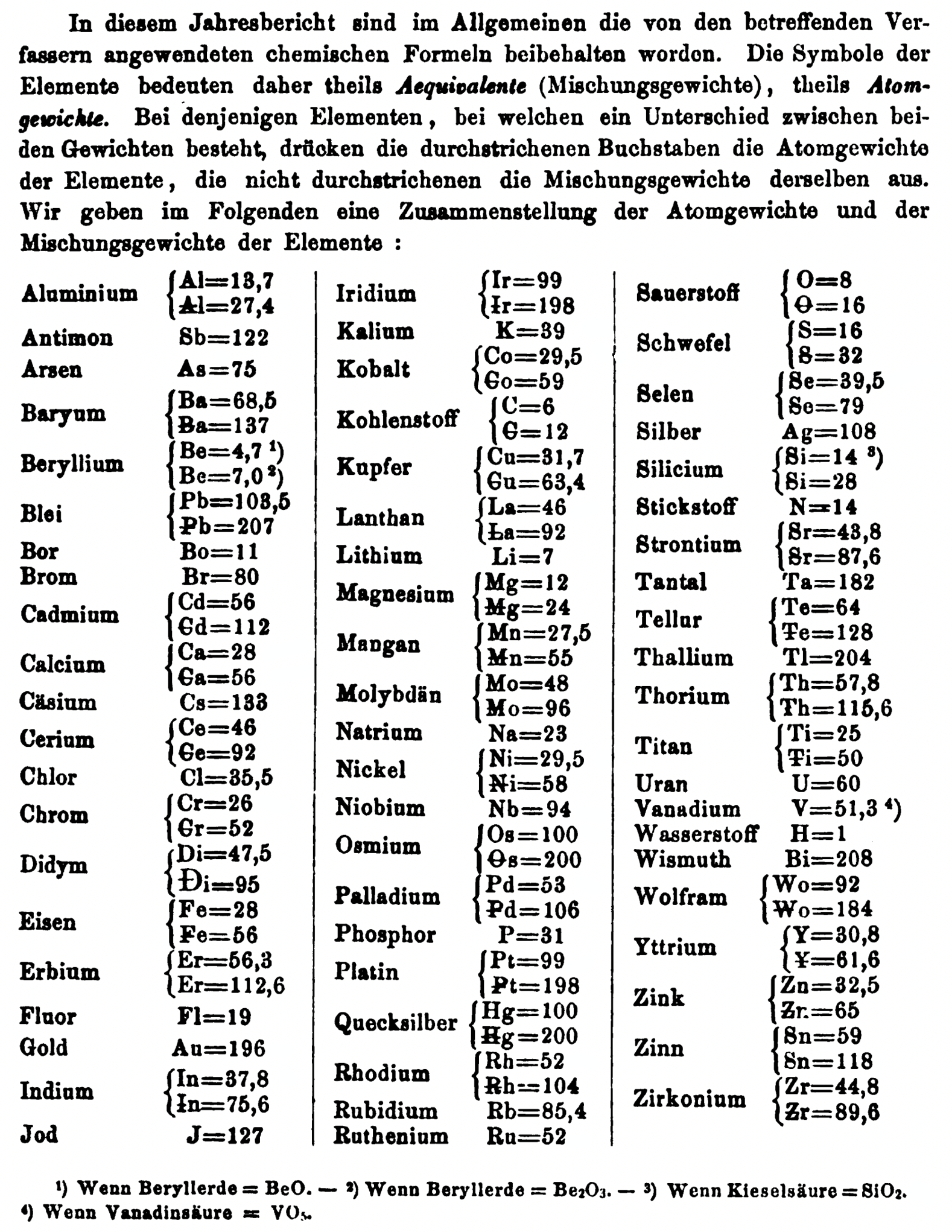
| Year: 1863 | PT id = 829, Type = element |
Discovery of Indium
In
Indium, atomic number 49, has a mass of 114.818 au.
Indium was first observed or predicted in 1863 by F. Reich and T. Richter and first isolated in 1867 by T. Richter.
| Year: 1863 | PT id = 1353, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1863
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1863 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- C = 6 and 12
- Hg = 100 and 200
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Zr = 22.4 and 33.6 and 44.8
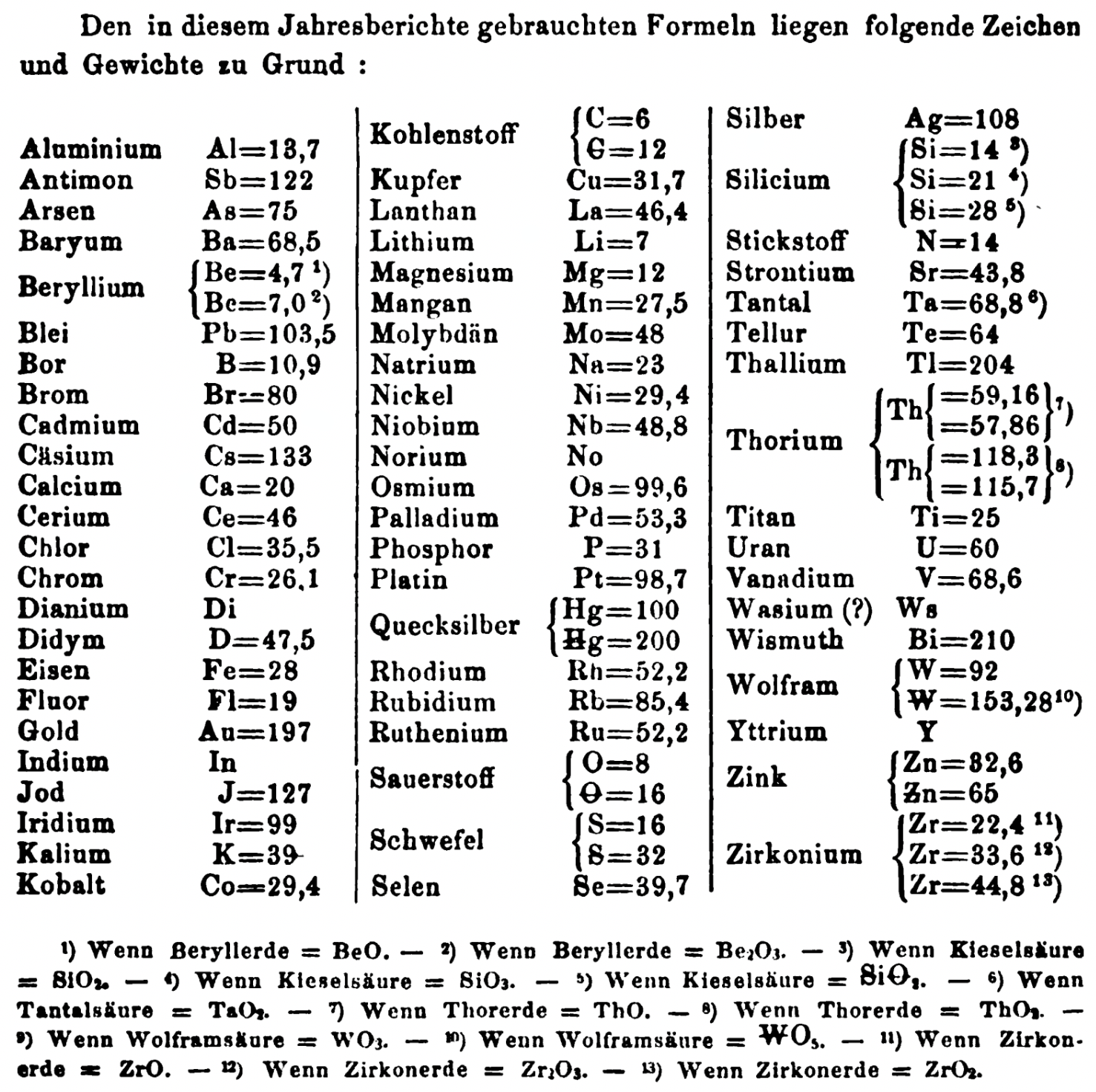
Thanks to René and Mario Rodriguez for the tip!
| Year: 1864 | PT id = 1354, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1864
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1864 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8
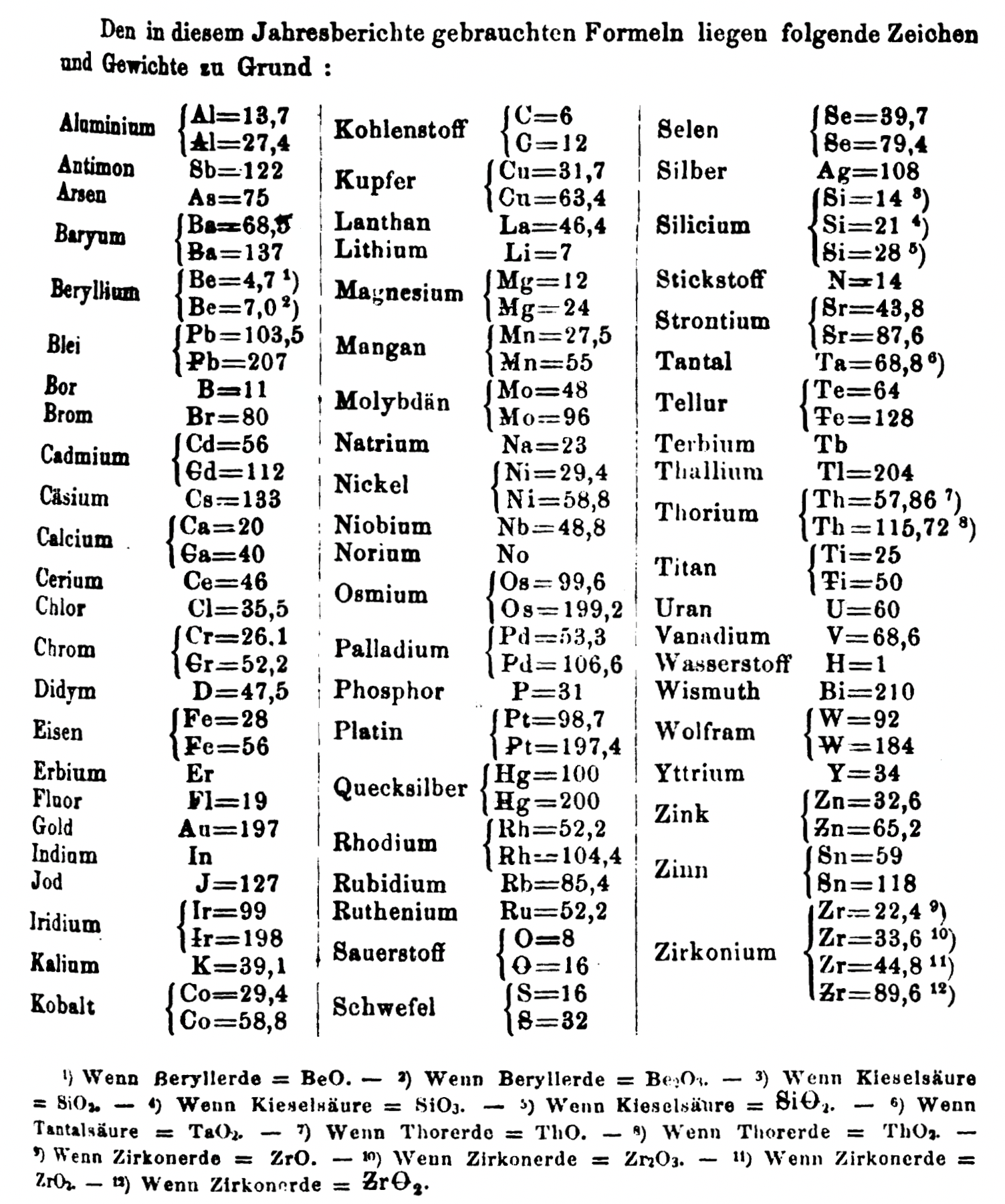
Thanks to René and Mario Rodriguez for the tip!
| Year: 1865 | PT id = 1355, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1865
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1865 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
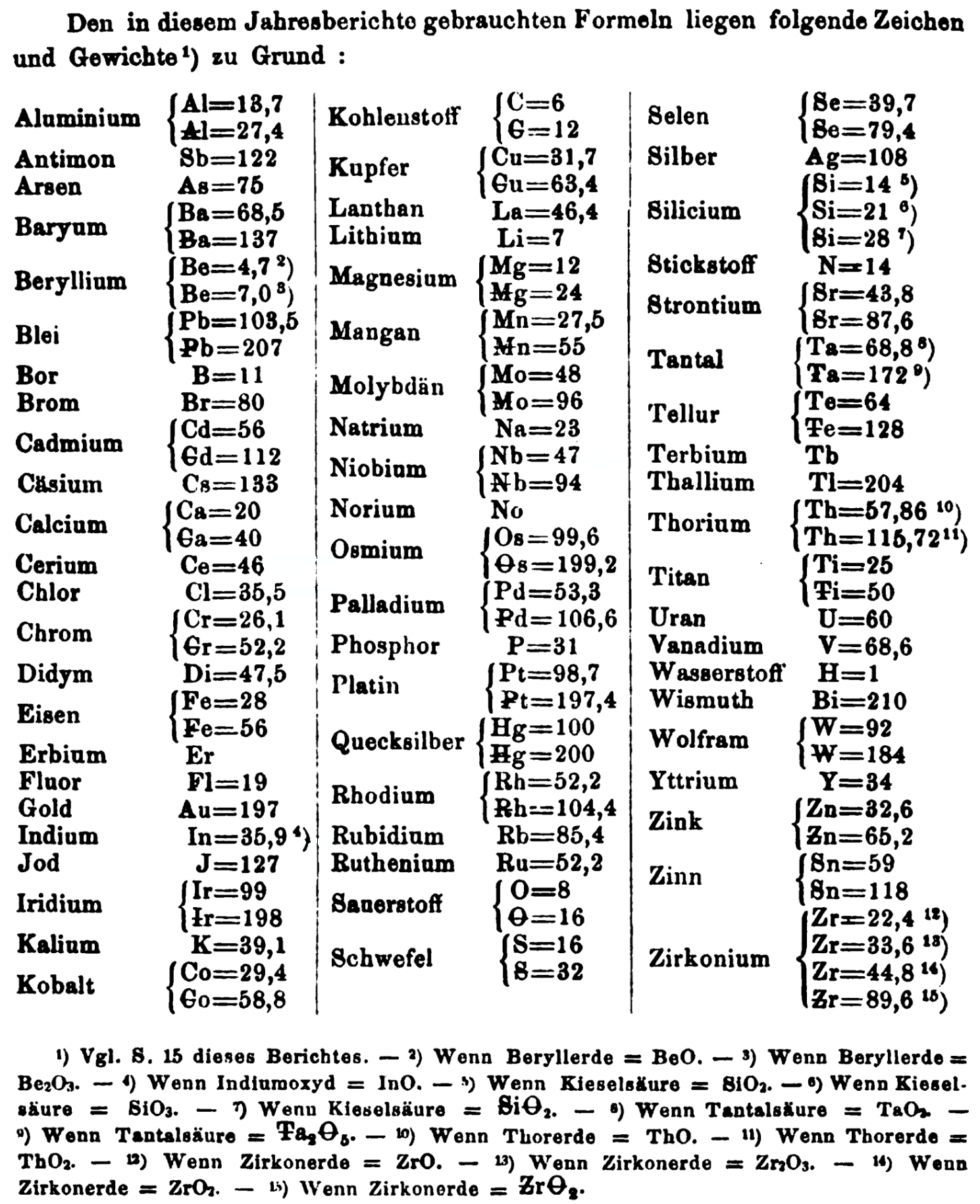
Thanks to René and Mario Rodriguez for the tip!
| Year: 1866 | PT id = 1356, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1866
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1866 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th = 57.86 and 115.72
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
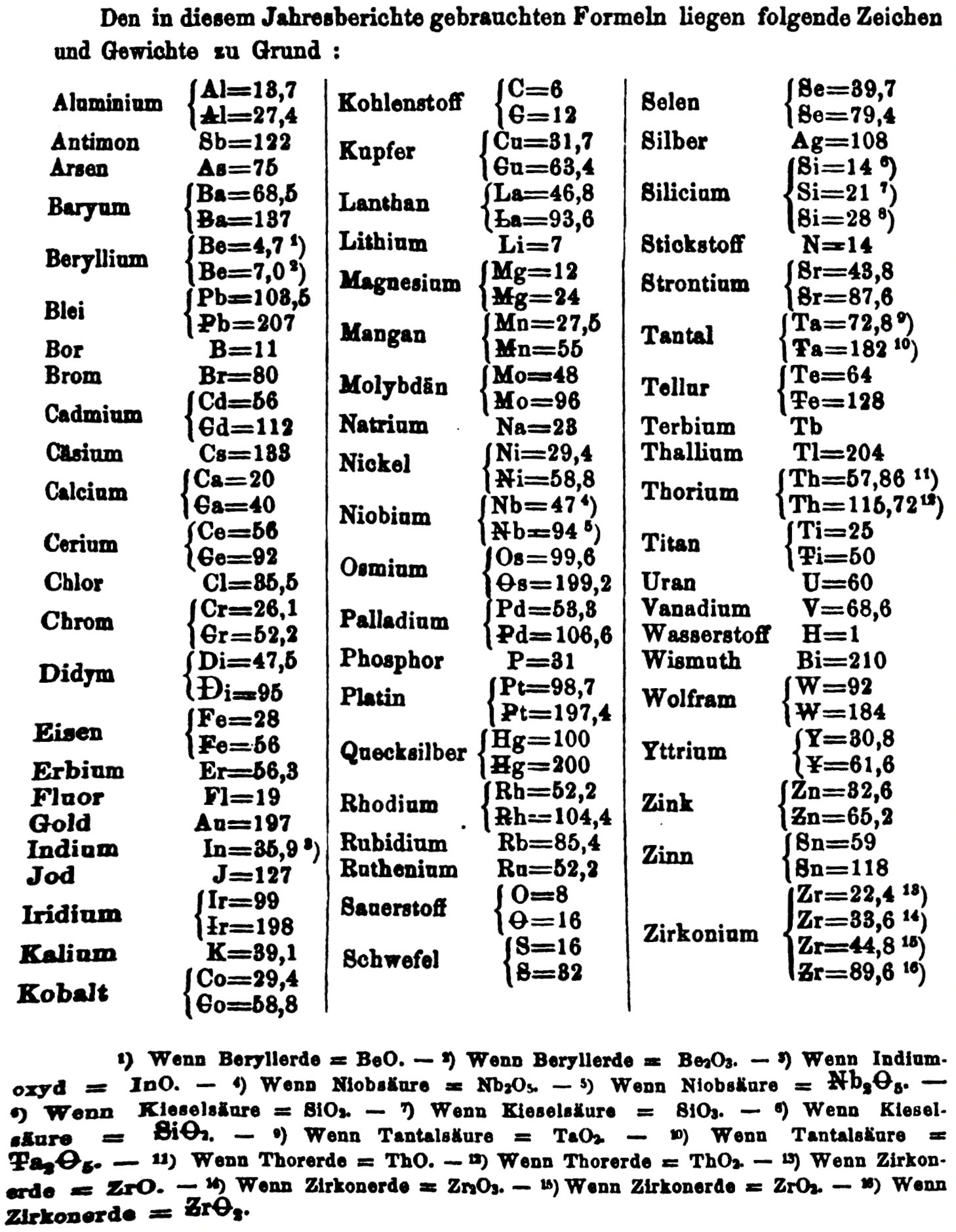
Thanks to René and Mario Rodriguez for the tip!
| Year: 1867 | PT id = 1357, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1867
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1867 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th =57.86 and 115.72
- W = 92 and 184
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
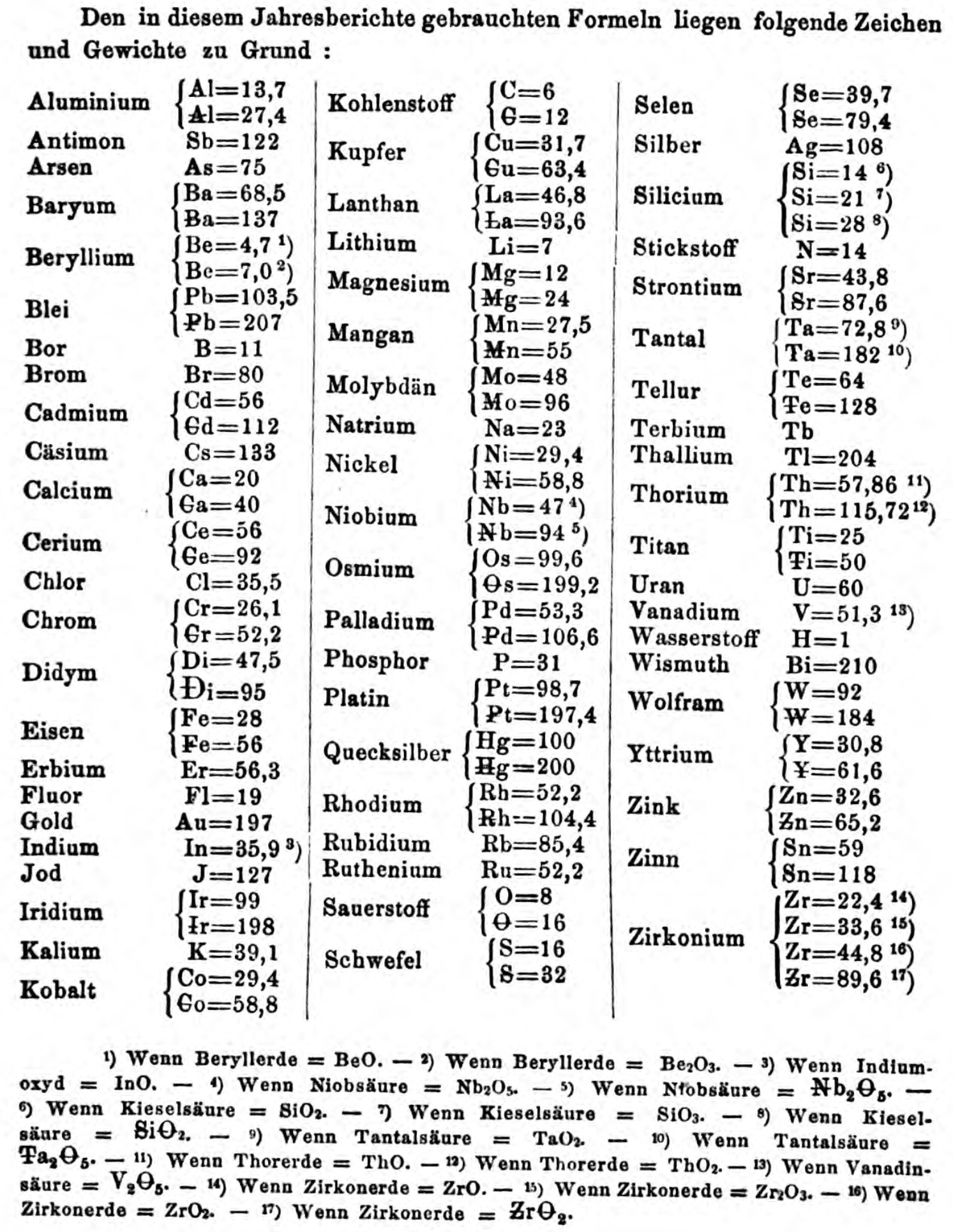
Thanks to René and Mario Rodriguez for the tip!
| Year: 1868 | PT id = 1358, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1868
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1868 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53.3 and 106.6
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
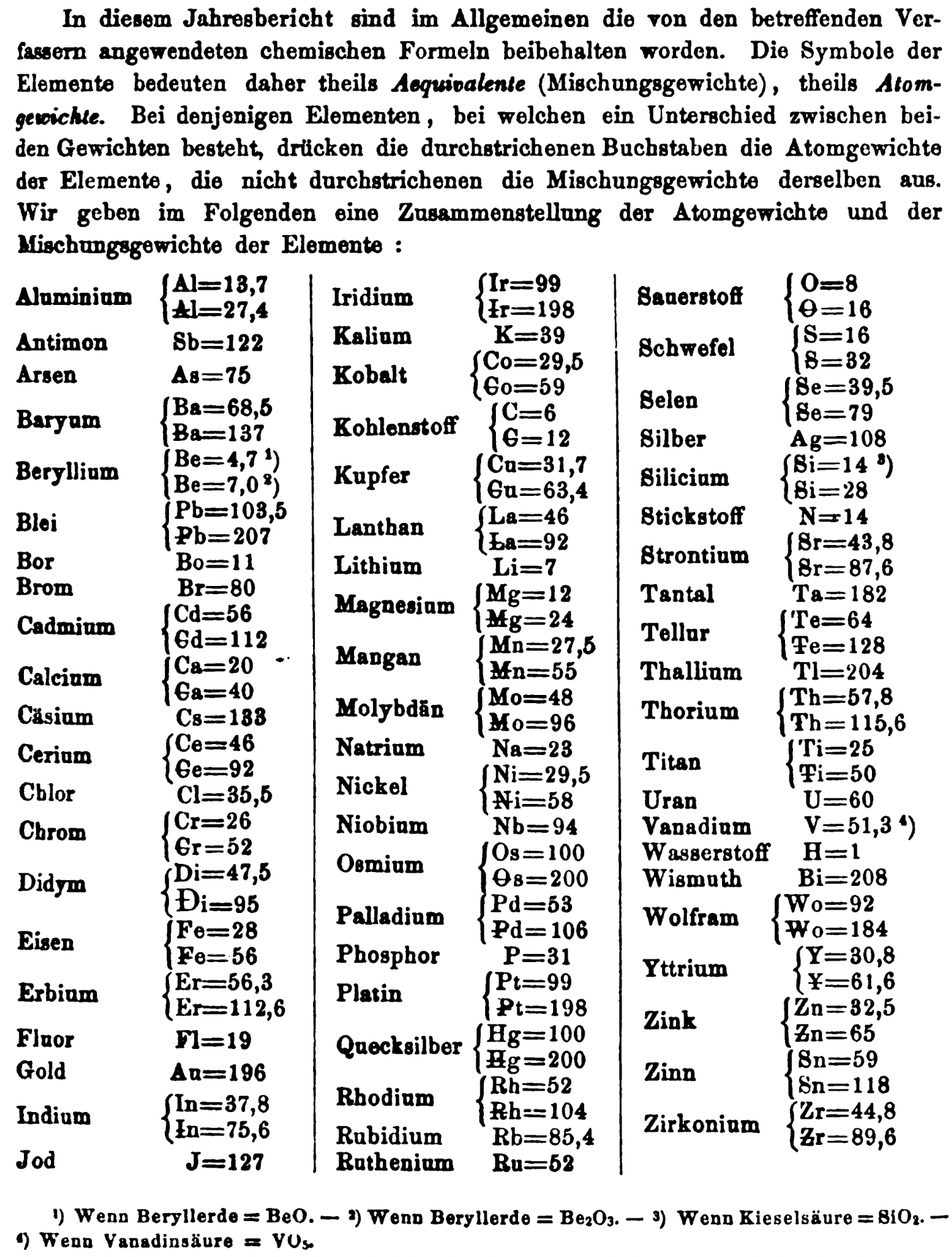
| Year: 1869 | PT id = 1359, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1869
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1869 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 44.8 and 89.6
Thanks to René and Mario Rodriguez for the tip!
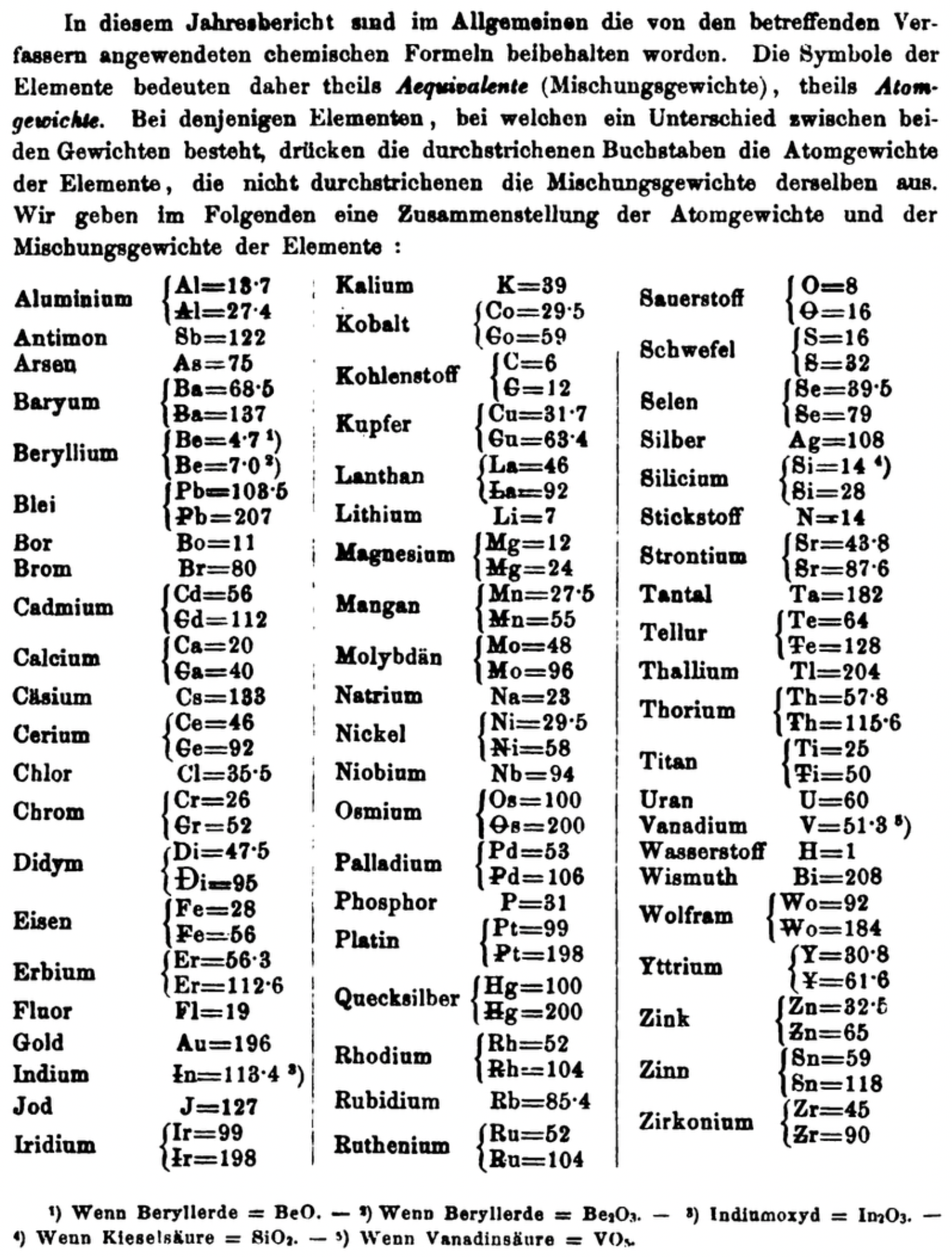
| Year: 1870 | PT id = 1360, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1870
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1870 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
Thanks to René and Mario Rodriguez for the tip!
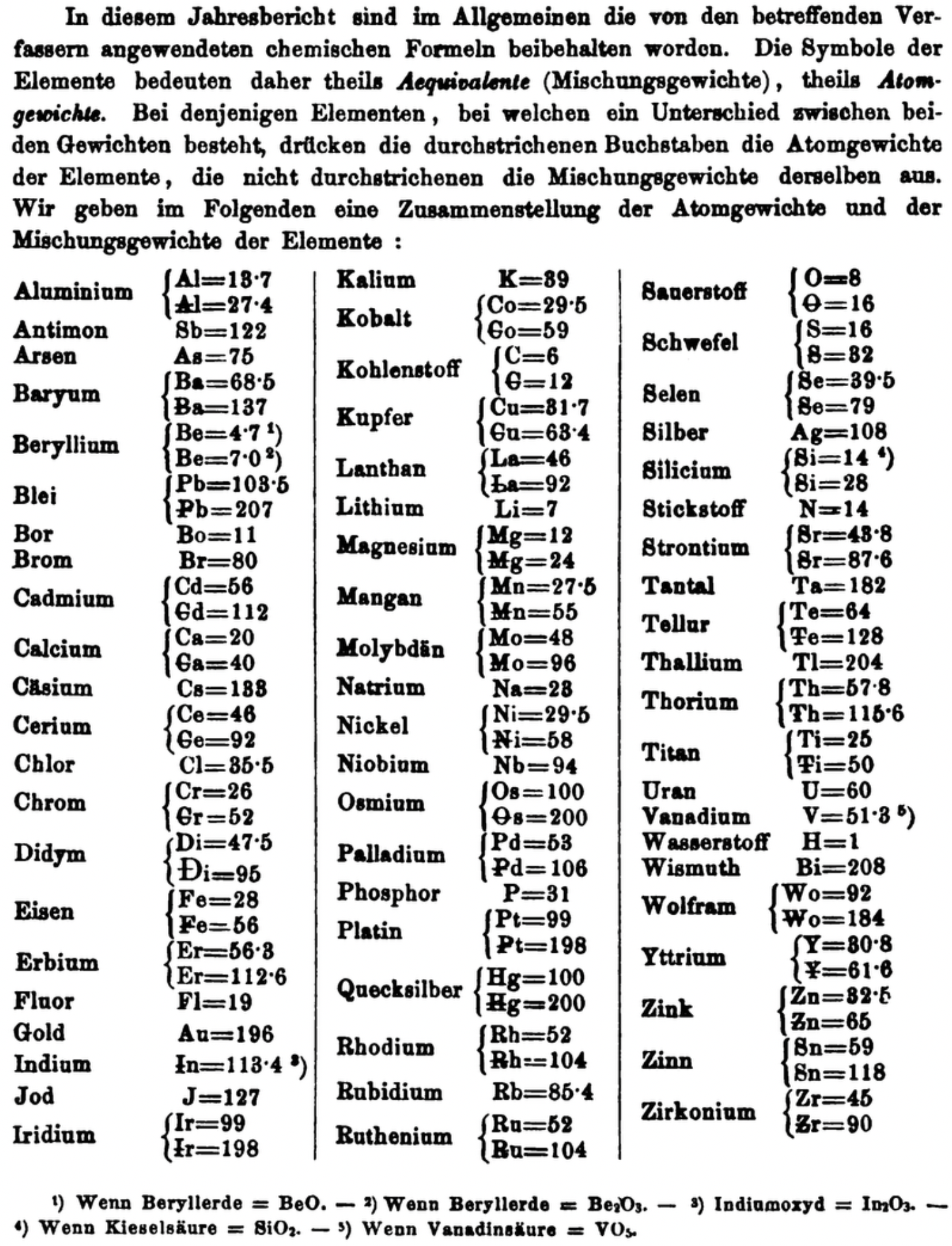
| Year: 1871 | PT id = 1361, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1871
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1871 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
Thanks to René and Mario Rodriguez for the tip!
| Year: 1872 | PT id = 1362, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1872
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1872 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.5 and 59
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.8 and 115.6
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 29.8 and 59.7
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
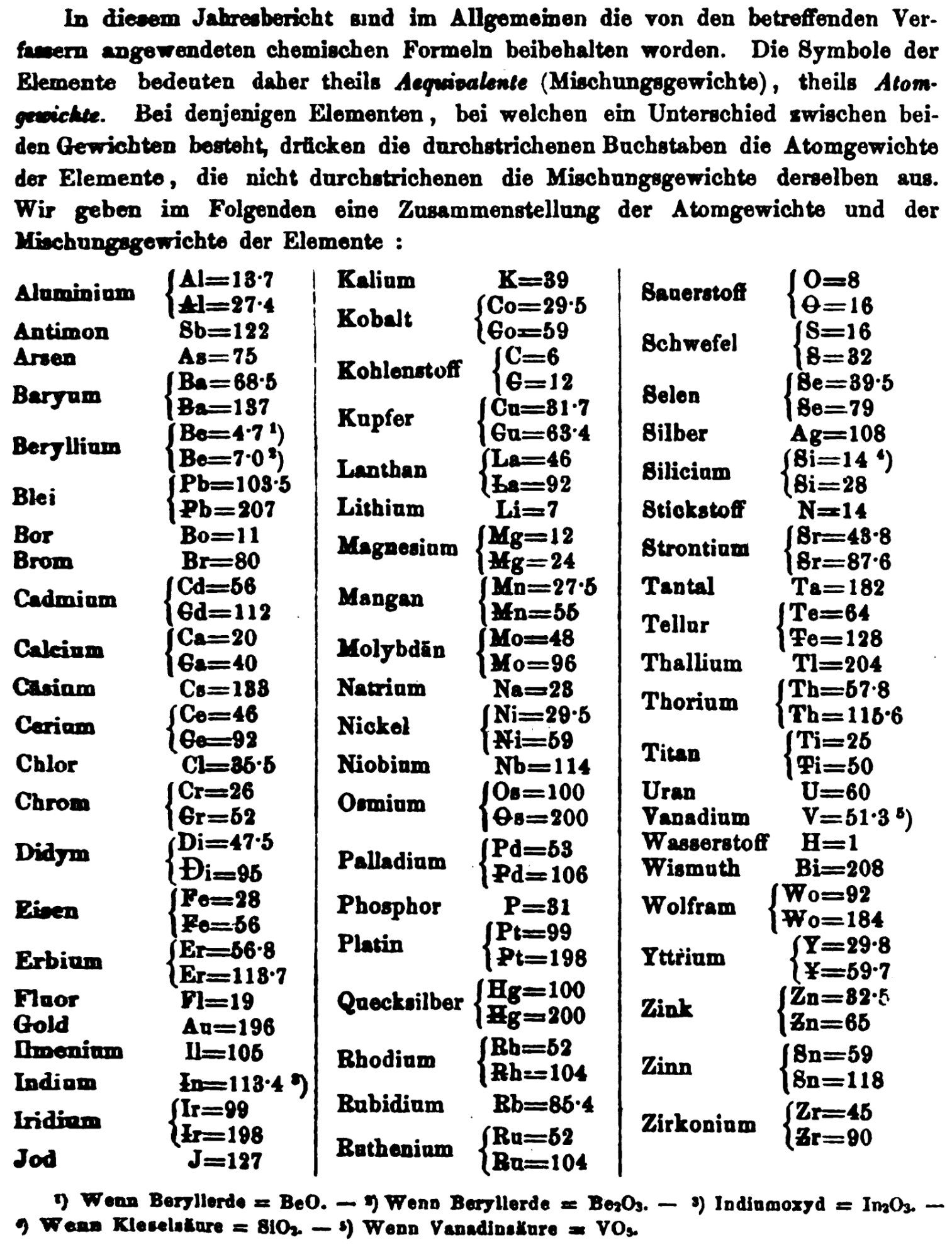
Thanks to René and Mario Rodriguez for the tip!
| Year: 1873 | PT id = 1363, Type = formulation review element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1873
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1873 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systematic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
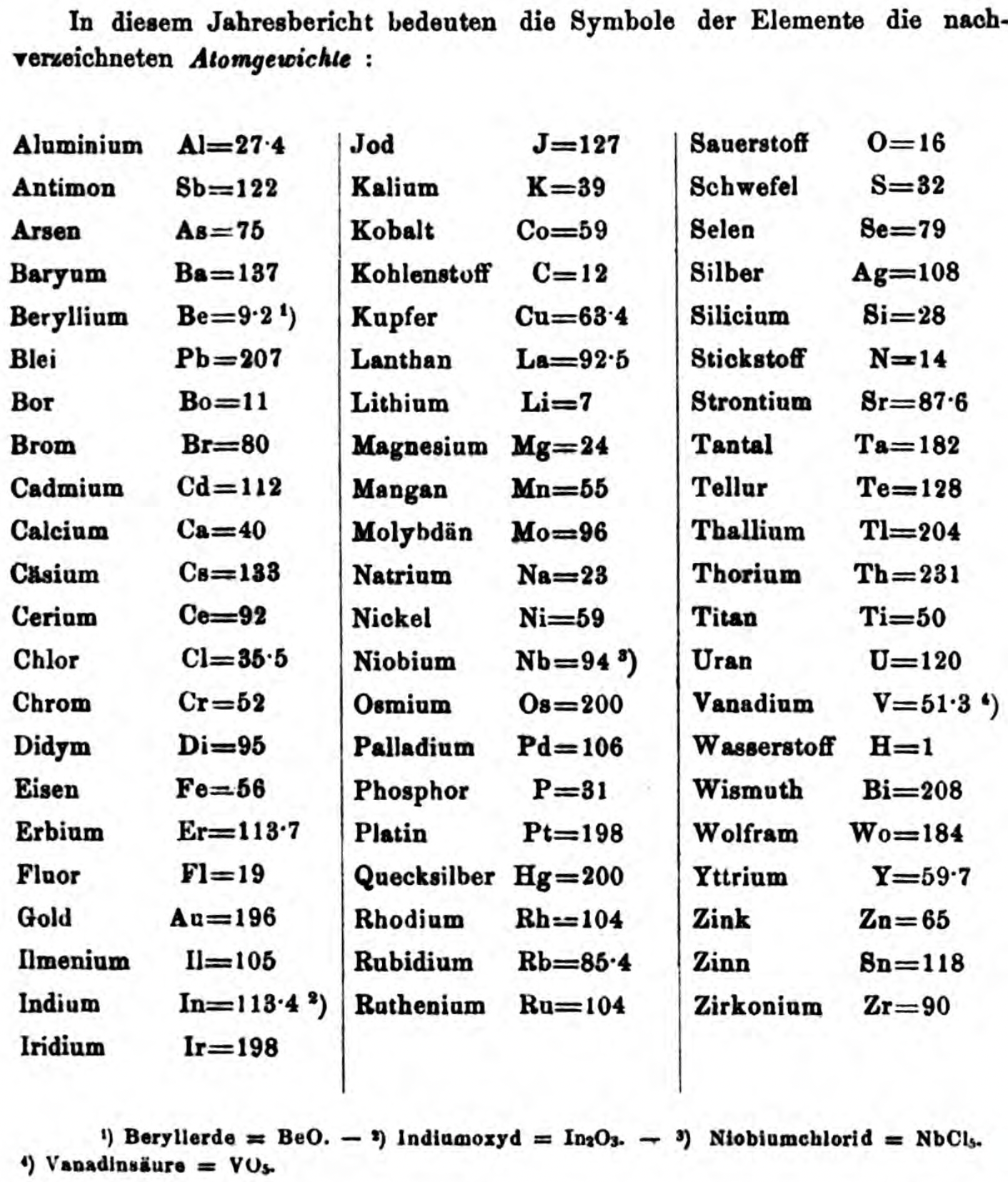
Notes:
- Didym D = 48 was actually a mixture of rare earth elements.
- Ilmenium, Il, was later found to be a mixture of niobium and tantalum.
- Generally, the elements missing had yet to be discovered (dates given below).
- The table below shows the progress from 1858 to 1873.
- By 1873 the only elements with incorrect atomic weights were the (at the time) somewhat obscure strontium, lanthanium, cerium and urananium.
- Previously, many elements were shown with two entries. Clearly, the stoichiometric and mass problems had largely been resolved (and the data agreed upon) by 1873.
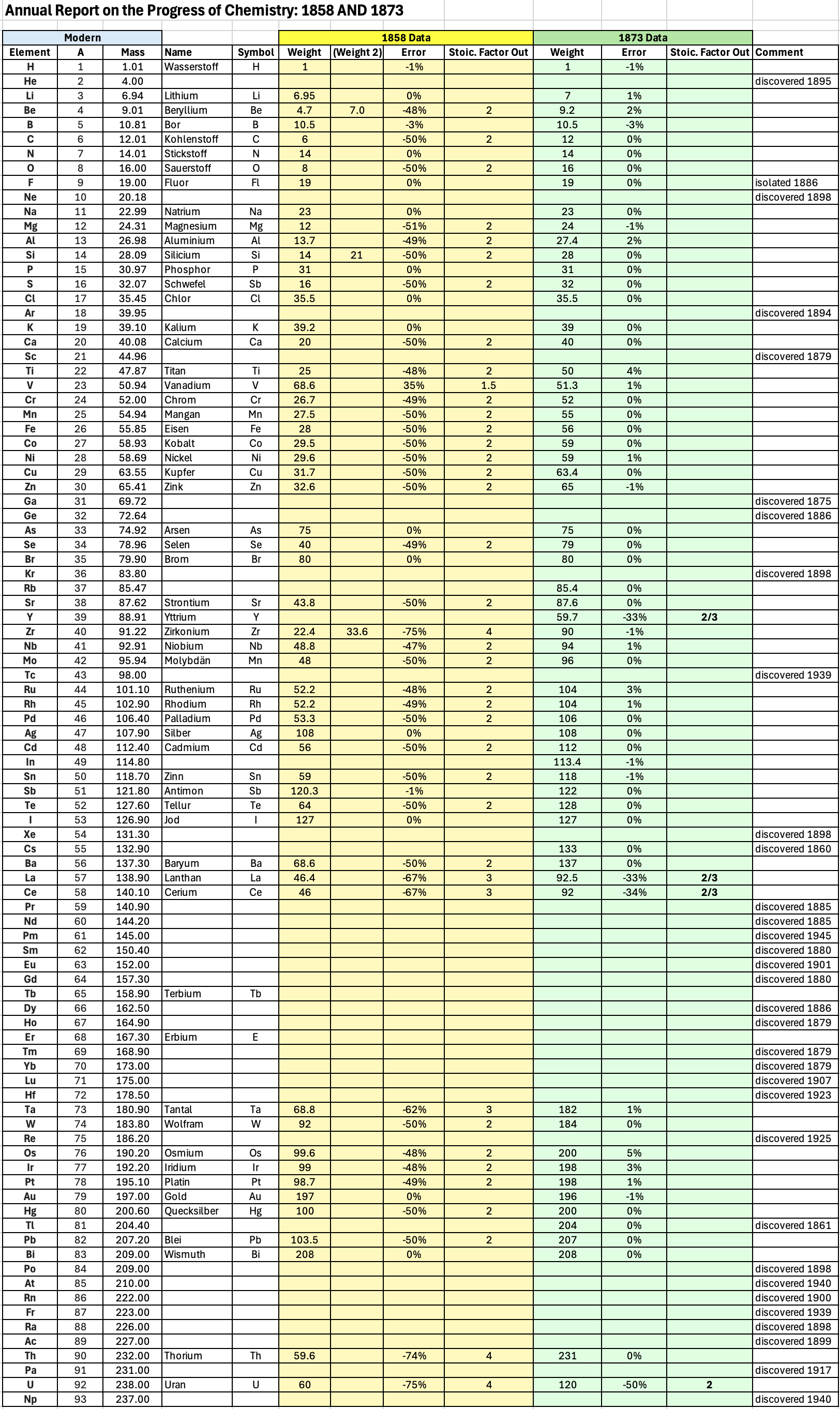
Thanks to René and Mario Rodriguez for the tip!
| Year: 1875 | PT id = 811, Type = element |
Discovery of Gallium
Ga
Gallium, atomic number 31, has a mass of 69.723 au.
Gallium was first isolated in 1875 by P. E. L. de Boisbaudran.
| Year: 1878 | PT id = 850, Type = element |
Discovery of Ytterbium
Yb
Ytterbium, atomic number 70, has a mass of 173.054 au.
Ytterbium was first observed or predicted in 1878 by J.C.G. de Marignac and first isolated in 1906 by C. A. von Welsbach.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 801, Type = element |
Discovery of Scandium
Sc
Scandium, atomic number 21, has a mass of 44.956 au.
Scandium was first isolated in 1879 by F. Nilson.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
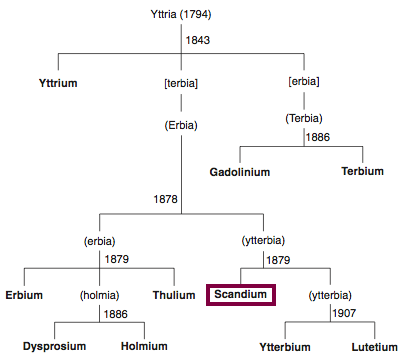
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 842, Type = element |
Discovery of Samarium
Sm
Samarium, atomic number 62, has a mass of 150.36 au.
Samarium was first isolated in 1879 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
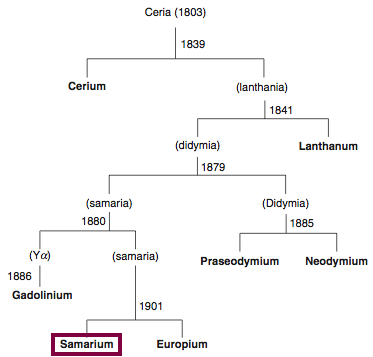
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 847, Type = element |
Discovery of Holmium
Ho
Holmium, atomic number 67, has a mass of 164.93 au.
Holmium was first observed or predicted in 1878 by J.-L. Soret and first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
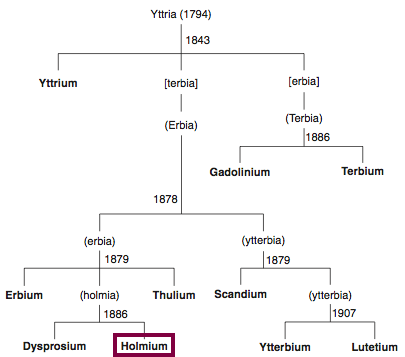
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1879 | PT id = 849, Type = element |
Discovery of Thulium
Tm
Thulium, atomic number 69, has a mass of 168.934 au.
Thulium was first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
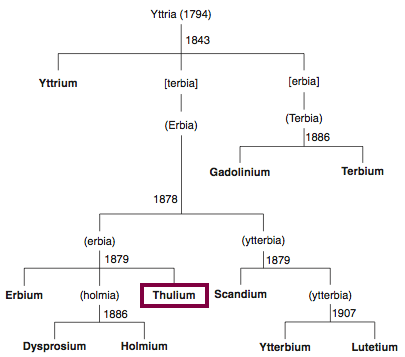
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1880 | PT id = 844, Type = element |
Discovery of Gadolinium
Gd
Gadolinium, atomic number 64, has a mass of 157.25 au.
Gadolinium was first observed or predicted in 1880 by J. C. G. de Marignac and first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
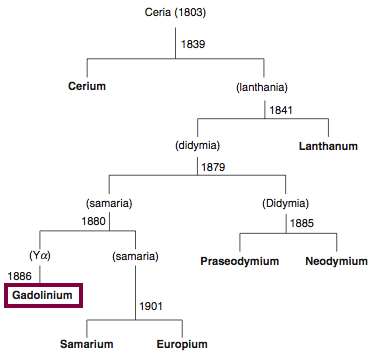
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
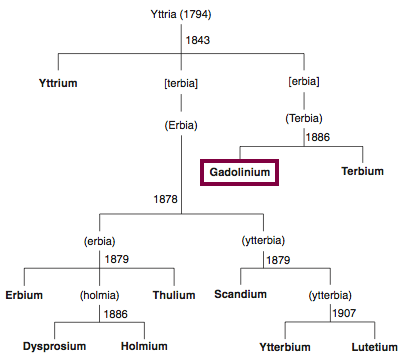
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1885 | PT id = 839, Type = element |
Discovery of Praseodymium
Pr
Praseodymium, atomic number 59, has a mass of 140.908 au.
Praseodymium was first isolated in 1885 by Carl Auer von Welsbach.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
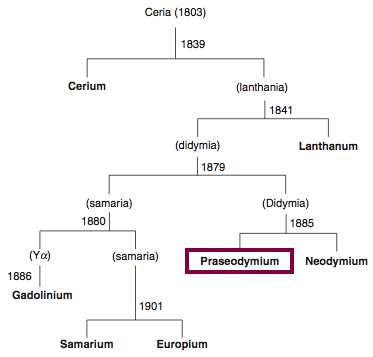
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1885 | PT id = 840, Type = element |
Discovery of Neodymium
Nd
Neodymium, atomic number 60, has a mass of 144.242 au.
Neodymium was first isolated in 1885 by Carl Auer von Welsbach.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
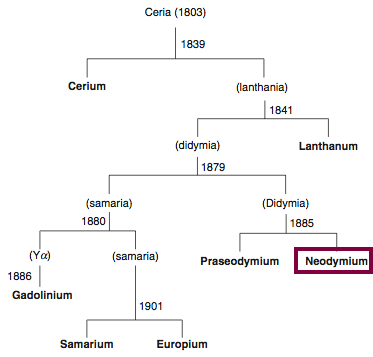
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1886 | PT id = 798, Type = element |
Discovery of Fluorine
F
Fluorine, atomic number 9, has a mass of 18.998 au.
Fluorine exists as a pale yellow diatomic molecular gas, F2. It is the most electronegative and reactive of all elements: it which reacts with practically all organic and inorganic substances.
Fluorine was first observed or predicted in 1810 by A.-M. Ampére and first isolated in 1886 by H. Moissan.
| Year: 1886 | PT id = 812, Type = element |
Discovery of Germanium
Ge
Germanium, atomic number 32, has a mass of 72.63 au.
Germanium was first isolated in 1886 by C. A. Winkler.
| Year: 1886 | PT id = 846, Type = element |
Discovery of Dysprosium
Dy
Dysprosium, atomic number 66, has a mass of 162.5 au.
Dysprosium was first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
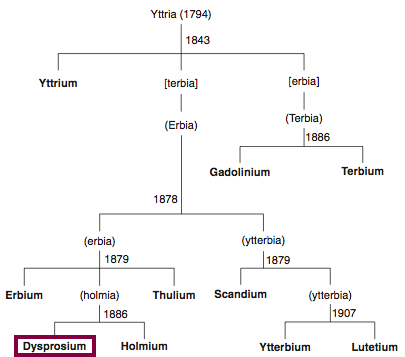
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1894 | PT id = 797, Type = element |
Discovery of Argon
Ar
Argon, atomic number 18, has a mass of 39.948 au.
Argon is a noble gas.
Argon was first isolated in 1894 by Lord Rayleigh and W. Ramsay.
| Year: 1895 | PT id = 782, Type = element |
Discovery of Helium
He
Helium, atomic number 2, has a mass of 4.003 au.
Helium is a noble gas, and is the second most abundant element in the universe after hydrogen.
Helium was first observed or predicted in 1868 by P. Janssen and N. Lockyer from solar spectra, and first isolated in 1895 by W. Ramsay, T. Cleve, and N. Langlet.
| Year: 1898 | PT id = 789, Type = element |
Discovery of Neon
Ne
Neon, atomic number 10, has a mass of 20.18 au.
Neon is a noble gas. It is present in the atmosphere, 1 part in 65000.
Neon was first isolated in 1898 by W. Ramsay and W. Travers.
| Year: 1898 | PT id = 816, Type = element |
Discovery of Krypton
Kr
Krypton, atomic number 36, has a mass of 83.798 au.
Krypton is a noble gas.
Krypton was first isolated in 1898 by W. Ramsay and W. Travers.
| Year: 1898 | PT id = 834, Type = element |
Discovery of Xenon
Xe
Xenon, atomic number 54, has a mass of 131.293 au.
Xenon is a noble gas.
Xenon was first isolated in 1898 by W. Ramsay and W. Travers.
| Year: 1898 | PT id = 864, Type = element |
Discovery of Polonium
Po ![]()
Polonium, atomic number 84, has a mass of 209 au.
Radioactive element.
Polonium was first observed or predicted in 1898 by P. and M. Curie and first isolated in 1902 by W. Marckwald.
| Year: 1898 | PT id = 868, Type = element |
Discovery of Radium
Ra ![]()
Radium, atomic number 88, has a mass of 226 au.
Radioactive element.
Radium was first observed or predicted in 1898 by P. and M. Curie and first isolated in 1902 by M. Curie.
| Year: 1899 | PT id = 866, Type = element |
Discovery of Radon
Rn ![]()
Radon, atomic number 86, has a mass of 222 au.
Radon is a noble gas and it is a radioactive element.
Radon was first observed or predicted in 1899 by E. Rutherford and R. B. Owens and first isolated in 1910 by W. Ramsay and R. Whytlaw-Gray.
| Year: 1900 | PT id = 1284, Type = formulation data element review structure |
History of the Discovery of the Group 18 (erstwhile Group 0) Elements
John Marks has provided a concise history of the discovery of the Group 18 elements and the element name"Nitron/Radon".
Radioactivity was discovered by Becquerel in 1896 and the Curies noted transferred radioactivity rather like the induction of electric or magnetic charge. Radon was discovered in 1900, by Dorn in Halle; Rutherford discovered thoron in 1899; and Debierne discovered actinon in 1903. The time-line is:
- 1868 Lockyer observed the spectrum of helium in the solar corona
- 1894 Ramsay discovers argon
- 1895 Ramsay isolates helium
- 1898 Ramsay discovers krypton, neon & xenon
- 1899 Curie observes an emanation from radium
- 1899 Rutherford observes an emanation from thorium
- 1900 Dorn identifies radon
- 1902 Rutherford & Soddy characterize thoron
- 1903 Rutherford & Soddy isolate radon
- 1903 Debierne observes an emanation from actinium
- 1904 Ramsay names the isotopic emanations exactinio, exradio & exthorio and surmises they are one element, probably an inert gas
- 1908 Professor Sydney Young’s "Stoichiometry" has a periodic table shows niton, Z = 86
- 1909 Ramsay characterizes niton as a group 0 inert gas
- 1910 Cameron's "Radiochemistry" describes the radioactive displacement law
- 1912 The name "niton" accepted by the International Commission for Atomic Weights
- 1913 Soddy expounds theory of isotopes
- 1913 Rydberg's periodic table has Nt (86) for the last inert gas
- 1919 Irving Langmuir's PT has Nt as the last inert gas
- 1922 Niels Bohr’s PT has Nt (86) as the last inert gas
- 1923 GN Lewis’s PT has Nt as the last inert gas
- 1924 CRC’s Handbook of Chemistry and Physics has niton as the last member of Group 0
So niton (from Latin nitens = shining) was noticed by the Curies in 1899 as an emanation from radium. That same year Rutherford noted an identical emanation from thorium, and in 1903 Debierne discovered the same emanation from actinium. All three ('radon', 'thoron' and 'actinon') were identified as an element by Ramsay in 1904 and characterized by him in 1909.
Ramsay named the element niton after its most prominent property viz. that it glowed in the dark.
With the introduction of Soddy's isotopes, it became clear that: thoron was Nt-220, radon was Nt-222 & actinon was Nt-219.
There are natural traces of other isotopes (e.g. Nt-217, Nt-218) from beta disintegration of astatine. So "radon" was just one isotope of niton.
The foregoing history of niton is uncontroversial and the name niton, Nt, for Z = 86 dates at least from Professor Young´s textbook of stoichiometry in 1908.
In 1912, the name 'niton' was adopted by the International Commission for Atomic weights. Rydberg's PT of 1913 has Nt as the last inert gas, as does Irving Langmuir's PT of 1919, Niels Bohr's PT of 1922, GN Lewis's PT of 1923 and even the CRC's Handbook of Chemistry and Physics in 1924.
John Marks concludes:
"Niton, Nt, for Z = 86, was thus established by its discoverers and accepted by the chemistry (and physics) establishment. Radon, Rn, is an error perpetuated by IUPAC [amongst its many sins].
"Radon is an isotope. We do not refer to hydrogen as 'protium', so why are we referring to niton as 'radon'?"
| Year: 1900 | PT id = 236, Type = formulation element |
Elements Known in the Year 1900
Elements known in the year 1900, taken from this Wikipedia page:
| Year: 1901 | PT id = 843, Type = element |
Discovery of Europium
Eu
Europium, atomic number 63, has a mass of 151.964 au.
Europium was first observed or predicted in 1896 by E.-A. Demarçay and first isolated in 1901 by E.-A. Demarçay.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
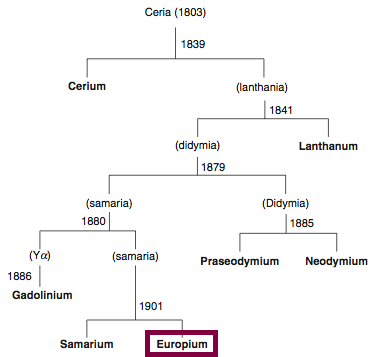
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1902 | PT id = 869, Type = element |
Discovery of Actinium
Ac ![]()
Actinium, atomic number 89, has a mass of 227 au.
Radioactive element.
Actinium was first isolated in 1902 by F. O. Giesel.
| Year: 1906 | PT id = 851, Type = element |
Discovery of Lutetium
Lu
Lutetium, atomic number 71, has a mass of 174.967 au.
Lutetium was first isolated in 1906 by C. A. von Welsbach and G. Urbain.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
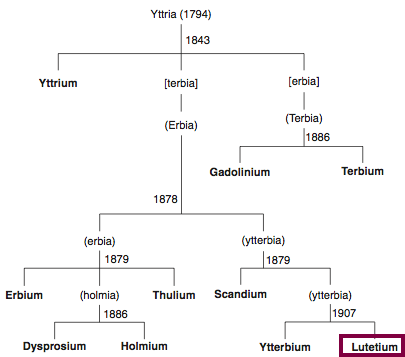
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1907 | PT id = 1332, Type = formulation review element |
Thompson's Electron Rings
After proposing, what became known as the plump-puddding model of the atom in 1904, J.J. Thompson developed the idea in his book The Corpuscular Theory of Matter, Archibale Constable, 1907(available as a scanned document online).
Thompson's Electron Rings are sumarised in this table:

The origional text reads (taken from pages 104-110). Note, for "corpusle" read "electron":



Thanks to Eric Scerri for the tip.
| Year: 1913 | PT id = 13, Type = formulation element structure |
Moseley's Periodic Law and Atomic Number Z
Moseley, H. G. J. The High-Frequency Spectra of the Elements. Philosophical Magazine, 26, 1024–1034 (1913).
"Moseley's law is an empirical law concerning the characteristic X-rays emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913–1914. Until Moseley's work, "atomic number" was merely an element's place in the periodic table and was not known to be associated with any measurable physical quantity.
"In brief, Moseley's law states that the square root of the frequency, ν, of the emitted X-ray is (approximately) proportional to the atomic number":
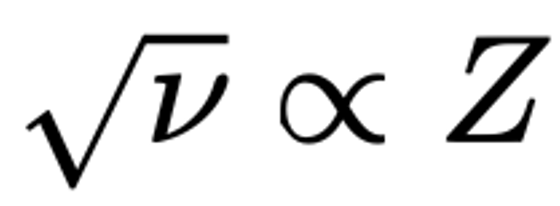
| Year: 1913 | PT id = 871, Type = element |
Discovery of Protactinium
Pa ![]()
Protactinium, atomic number 91, has a mass of 231.036 au.
Radioactive element: Pa is only found in tiny amounts in nature. Most samples are synthetic.
Protactinium was first observed or predicted in 1913 by O. H. Göhring and K. Fajans and first isolated in 1927 by A. von Grosse.
| Year: 1919 | PT id = 855, Type = element |
Discovery of Rhenium
Re
Rhenium, atomic number 75, has a mass of 186.207 au.
Rhenium was first observed or predicted in 1908 by M. Ogawa and first isolated in 1919 by M. Ogawa.
| Year: 1922 | PT id = 852, Type = element |
Discovery of Hafnium
Hf
Hafnium, atomic number 72, has a mass of 178.49 au.
Hafnium was first isolated in 1922 by D. Coster and G. von Hevesy.
| Year: 1937 | PT id = 823, Type = element |
Discovery of Technetium
Tc ![]()
Technetium, atomic number 43, has a mass of 98 au.
Radioactive element: Tc is only found in tiny amounts in nature. Most samples are synthetic.
Technetium was first isolated in 1937 by C. Perrier and E. Segrè. The element had been predicted by Mendeleev in 1871 as eka-manganese.
| Year: 1939 | PT id = 867, Type = element |
Discovery of Francium
Fr ![]()
Francium, atomic number 87, has a mass of 223 au.
Radioactive element.
Francium was first observed in 1939 by M. Perey.
| Year: 1940 | PT id = 865, Type = element |
Discovery of Astatine
At ![]()
Astatine, atomic number 85, has a mass of 210 au.
Radioactive element.
Astatine was first observed or predicted in 1940 by R. Corson, R. MacKenzie and E. Segrè.
| Year: 1940 | PT id = 873, Type = element |
Discovery of Neptunium
Np ![]()
Neptunium, atomic number 93, has a mass of 237 au.
Radioactive element: Np is only found in tiny amounts in nature. Most samples are synthetic.
Neptunium was first observed in 1940 by E.M. McMillan and H. Abelson.
| Year: 1940 | PT id = 874, Type = element |
Discovery of Plutonium
Pu ![]()
Plutonium, atomic number 94, has a mass of 244 au.
Radioactive element: Pu is only found in tiny amounts in nature. Most samples are synthetic.
Plutonium was first observed in 1940 by Glenn T. Seaborg, Arthur C. Wahl, W. Kennedy and E.M. McMillan.
| Year: 1944 | PT id = 875, Type = element |
Discovery of Americium
Am ![]()
Americium, atomic number 95, has a mass of 243 au.
Synthetic radioactive element. It is used in smoke detectors, and so – surprisingly – is present most houses and buildings.
Americium was first observed in 1944 by G. T. Seaborg, R. A. James, O. Morgan and A. Ghiorso.
| Year: 1944 | PT id = 876, Type = element |
Discovery of Curium
Cm ![]()
Curium, atomic number 96, has a mass of 247 au.
Synthetic radioactive element.
Curium was first observed in 1944 by G. T. Seaborg, R. A. James and A. Ghiorso.
| Year: 1945 | PT id = 841, Type = element |
Discovery of Promethium
Pm ![]()
Promethium, atomic number 61, has a mass of 145 au.
Radioactive element: Pm is only found in tiny amounts in nature. Most samples are synthetic.
Promethium was first observed or predicted in 1942 by S. Wu, E.G. Segrè and H. Bethe and first isolated in 1945 by Charles D. Coryell, Jacob A. Marinsky, Lawrence E. Glendenin, and Harold G. Richter.
| Year: 1949 | PT id = 877, Type = element |
Discovery of Berkelium
Bk ![]()
Berkelium, atomic number 97, has a mass of 247 au.
Synthetic radioactive element.
Berkelium was first observed in 1949 by G. Thompson, A. Ghiorso and G. T. Seaborg.
| Year: 1950 | PT id = 878, Type = element |
Discovery of Californium
Cf ![]()
Californium, atomic number 98, has a mass of 251 au.
Synthetic radioactive element.
Californium was first observed in 1950 by S. G. Thompson, K. Street, Jr., A. Ghiorso and G. T. Seaborg.
| Year: 1950 | PT id = 475, Type = formulation element structure |
Elements Known in the Year 1950
Elements known in the year 1950, taken from this Wikipedia page:
| Year: 1952 | PT id = 879, Type = element |
Discovery of Einsteinium
Es ![]()
Einsteinium, atomic number 99, has a mass of 252 au.
Synthetic radioactive element.
Einsteinium was first observed in 1952 by A. Ghiorso et al.
| Year: 1952 | PT id = 880, Type = element |
Discovery of Fermium
Fm ![]()
Fermium, atomic number 100, has a mass of 257 au.
Synthetic radioactive element.
Fermium was first observed in 1952 by A. Ghiorso et al.
| Year: 1955 | PT id = 1086, Type = element misc data |
Element Hunters
A YouTube video, The Element Hunters.
The text accompanying the video says:
"Scientist in Berkeley discover new elements [Californium & Einsteinium] from hydrogen bomb debris in 1951 and then use the 60 inch Cyclotron to create Mendelevium, element 101. The team included Nobel Prize winner Glenn Seaborg and famed element hunter, Albert Ghiorso."
Thanks to Roy Alexander for the tip!
| Year: 1955 | PT id = 881, Type = element |
Discovery of Mendelevium
Md ![]()
Mendelevium, atomic number 101, has a mass of 258 au.
Synthetic radioactive element.
Mendelevium was first observed in 1955 by A. Ghiorso, G. Harvey, R. Choppin, S. G. Thompson and G. T. Seaborg.
| Year: 1961 | PT id = 883, Type = element |
Discovery of Lawrencium
Lr ![]()
Lawrencium, atomic number 103, has a mass of 262 au.
Synthetic radioactive element.
Lawrencium was first observed in 1961 by A. Ghiorso, T. Sikkeland, E. Larsh and M. Latimer.
| Year: 1966 | PT id = 882, Type = element |
Discovery of Nobelium
No ![]()
Nobelium, atomic number 102, has a mass of 259 au.
Synthetic radioactive element.
Nobelium was first observed in 1966 by E. D. Donets, V. A. Shchegolev and V. A. Ermakov.
| Year: 1969 | PT id = 884, Type = element |
Discovery of Rutherfordium
Rf ![]()
Rutherfordium, atomic number 104, has a mass of 267 au.
Synthetic radioactive element.
Rutherfordium was first observed in 1969 by A. Ghiorso et al. and I. Zvara et al.
| Year: 1970 | PT id = 885, Type = element |
Discovery of Dubnium
Db ![]()
Dubnium, atomic number 105, has a mass of 268 au.
Synthetic radioactive element.
Dubnium was first observed in 1970 by A. Ghiorso et al. and V. A. Druin et al.
| Year: 1974 | PT id = 886, Type = element |
Discovery of Seaborgium
Sg ![]()
Seaborgium, atomic number 106, has a mass of 271 au.
Synthetic radioactive element.
Seaborgium was first observed in 1974 by A. Ghiorso et al.
| Year: 1981 | PT id = 887, Type = element |
Discovery of Bohrium
Bh ![]()
Bohrium, atomic number 107, has a mass of 272 au.
Synthetic radioactive element.
Bohrium was first observed in 1981 by G.Münzenberget al.
| Year: 1982 | PT id = 889, Type = element |
Discovery of Meitnerium
Mt ![]()
Meitnerium, atomic number 109, has a mass of 276 au.
Synthetic radioactive element.
Meitnerium was first observed in 1982 by G. Münzenberg, P. Armbrusteret al.
| Year: 1984 | PT id = 888, Type = element |
Discovery of Hassium
Hs ![]()
Hassium, atomic number 108, has a mass of 270 au.
Synthetic radioactive element.
Hassium was first observed in 1984 by G. Münzenberg, P. Armbruster et al.
| Year: 1994 | PT id = 890, Type = element |
Discovery of Darmstadtium
Ds ![]()
Darmstadtium, atomic number 110, has a mass of 281 au.
Synthetic radioactive element.
Darmstadtium was first observed in 1994 by S. Hofmann et al.
| Year: 1994 | PT id = 891, Type = element |
Discovery of Roentgenium
Rg ![]()
Roentgentium, atomic number 111, has a mass of 280 au.
Synthetic radioactive element.
Roentgenium was first observed in 1994 by S. Hofmann et al.
| Year: 1996 | PT id = 892, Type = element |
Discovery of Copernicium
Cn ![]()
Copernicum, atomic number 112, has a mass of 285 au.
Synthetic radioactive element.
Copernicium was first observed in 1996 by S. Hofmann et al.
| Year: 1999 | PT id = 894, Type = element |
Discovery of Flerovium
Fl ![]()
Flerovium, atomic number 114, has a mass of 289 au.
Synthetic radioactive element.
Flerovium was first observed in 1999 by Y. Oganessianet et al.
| Year: 2000 | PT id = 896, Type = element |
Discovery of Livermorium
Lv ![]()
Livermorium, atomic number 116, has a mass of 293 au.
Synthetic radioactive element.
Livermorium was first observed in 2000 by Y. Oganessian et al.
| Year: 2000 | PT id = 476, Type = formulation element |
Elements Known in the Year 2000
Elements known in the year 2000, taken from this Wikipedia page:
| Year: 2002 | PT id = 898, Type = element |
Discovery of Oganesson
Og ![]()
Oganesson, atomic number 118, has a mass of 294 au.
Synthetic radioactive element.
Oganesson was first observed in 2002 by Y. Oganessian et al.
| Year: 2003 | PT id = 893, Type = element |
Discovery of Nihonium
Nh ![]()
Nihonium, atomic number 113, has a mass of 284 au.
Synthetic radioactive element.
Nihonium was first observed in 2003 by Y. Oganessian et al. and K. Morita et al.
| Year: 2003 | PT id = 895, Type = element |
Discovery of Moscovium
Mc ![]()
Moscovium, atomic number 115, has a mass of 288 au.
Synthetic radioactive element.
Moscovium was first observed in 2003 by Y. Oganessian et al.
| Year: 2010 | PT id = 897, Type = element |
Discovery of Tennessine
Ts ![]()
Tennessine, atomic number 117, has a mass of 292 au.
Synthetic radioactive element.
Tennessine was first observed in 2010 by Y. Oganessian et al.
| Year: 2011 | PT id = 477, Type = formulation element |
Elements Known in the Year 2011
Elements known in the year 2011, taken from this Wikipedia page... all the elements to 118 are now known:
| Year: 2019 | PT id = 1246, Type = misc element |
Periodic Table of the Elements Coloring Book
Periodic Table of the Elements Coloring Book
Project managing and chemistry overseen by Yann Brouillette (Faculty, Chemistry, Dawson College). Element representations and cover by Dawson College Illustration & Design students (2nd year)* overseen by Meinert Hansen (Faculty, Illustration & Design, Dawson College).
Thanks to René for the tip!
| Year: 2021 | PT id = 1217, Type = data element misc |
History [of the] Elements and Periodic Table
From the Royal Society of Chemistry (RSC) an interactive Elements and Perioid Table History web page:

Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2022 | PT id = 1236, Type = data misc element |
Which Element is the Best?
The That Chemist YouTube channel asks "Which Element is the Best? Elements 1-20 & Elements 21-40"
That Chemist is a synthetic organic chemist and his bias is in that direction although he gives a variety of examples:
| Year: 2023 | PT id = 1283, Type = data misc non-chem element |
Element Names: The Etymology of The Periodic Table
An excellent video by RobWords about the names of the chemical elements and how they came about:
| Year: 2023 | PT id = 1287, Type = formulation element data misc spiral |
Semicircular Hybrid Chart of the Nuclides
Nawa Nagayasu has produced a new version of the Segrè Chart of the Nuclides.
Nawa writes:
"The chart has the number of neutrons on the [curved] horizontal axis and the number of protons (atomic number) on the vertical axis. I used the IAEA colour coding [scheme]. JAEA's half-life ranks are indicated by simple numbers, not rounded frames.
"In order to fit the whole chart into a semicircle, the axis representing the number of neutrons was made a spiral-like curve. For clarity, the number of neutrons is shown in the middle of each curve."
Yuri Oganessian has commented:
"Nawa Nagayasu is an original and talented designer. After all, it is not easy to work with 118 elements, but now also with isotopes, of which there are more than 3000. The fan design looks attractive and this is very important. This will make people, especially school age, guess the numbers that are written there. So they will gradually delve into the content of the Table, a truly brilliant creation."
| Year: 2025 | PT id = 1345, Type = element |
Actinium: The Most Annoying Element for the Rare-Earth Industry?
Koen Binnemans writing on Linkedin:
Actinium: The Most Annoying Element for the Rare Earth Industry?
Rare-earth elements (REEs) are often accompanied by radioactive uranium and thorium in their ores. This is particularly problematic for monazite, which can contain more than 15 wt% thorium dioxide. The REE mineral steenstrupine, which occurs in large quantities in Greenland, is so rich in uranium that it can even be mined as a uranium ore. Due to strict safety regulations governing the handling of naturally occurring radioactive materials (NORM), the thorium content of REE ores poses major challenges for REE producers. Thorium is treated as radioactive waste, and its disposal can be very expensive.
It is important to realize that most radioactivity issues are not caused directly by thorium or uranium themselves, since their primary isotopes (thorium-232, uranium-235, and uranium-238) are all very long?lived, but by their radioactive daughter nuclides formed through decay chains.
Most of these radioactive daughter elements have chemical properties sufficiently different from those of the REEs that they can be removed using conventional hydrometallurgical techniques.
However, actinium presents a particular challenge because its chemical properties are similar to those of the REEs, especially lanthanum. The isotope actinium-227, formed by the decay of uranium-235, has a half-life of 22 years. During REE separation, actinium tends to follow lanthanum. In a solvent-extraction circuit, actinium accumulates in the SX battery along with the lanthanum stream.
Although the concentration of actinium is usually very low, lanthanum must be purified as thoroughly as possible, because one important application of lanthanum is in scintillator detectors for ionizing radiation (e.g. (LaBr3:Ce3+ or LaCl3:Ce3+). If lanthanum is contaminated with radioactive actinium, the resulting detector will exhibit significant background noise and therefore poor performance. Another issue is that REE concentrates produced at mining sites may exceed legal radioactivity limits for export to REE refineries due to the presence of actinium.
Therefore, REE processing companies have developed processes to remove actinium from REE concentrates or feed solutions for SX operations. Limited information is available in the scientific literature, but more can be found in patent documents. For instance, read the following patent of CARESTER, https://lnkd.in/evXsXzDB
It should also be noted that actinium has useful applications: actinium?225 is used in radiopharmaceuticals for the precision treatment of tumors. See: PANTERA
SOLVOMET R&I Centre SIM2 KU Leuven

Oak Ridge National Laboratory (Wikipedia) Blue Cerenkov radiation emitted by a sample of actinium-225
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.