Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Table database entries referencing the database curator: Mark Leach, by date added
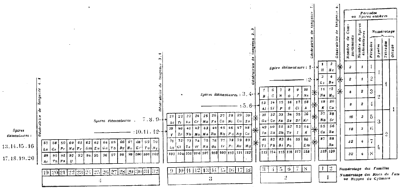
| Year: 1808 | PT id = 5, Type = formulation data element weight structure |
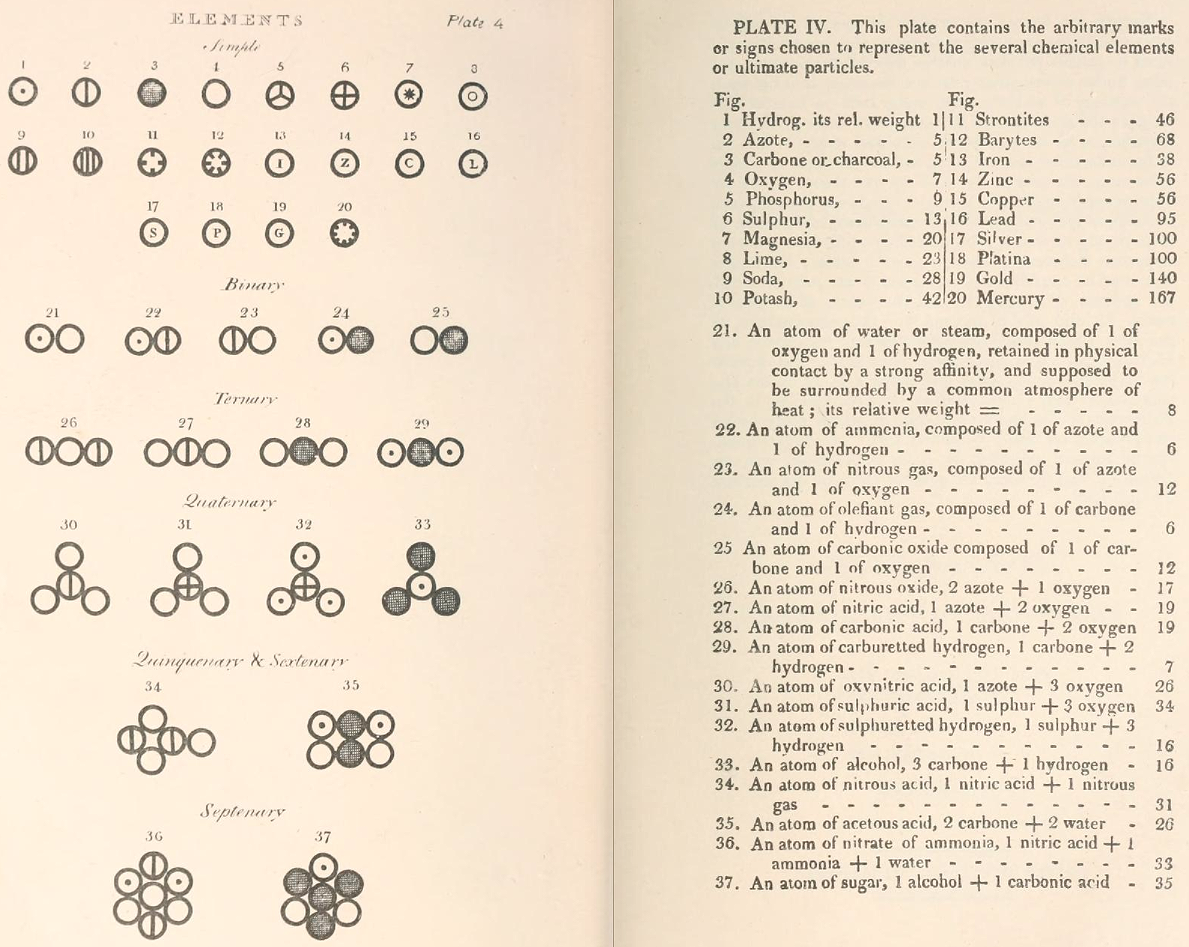
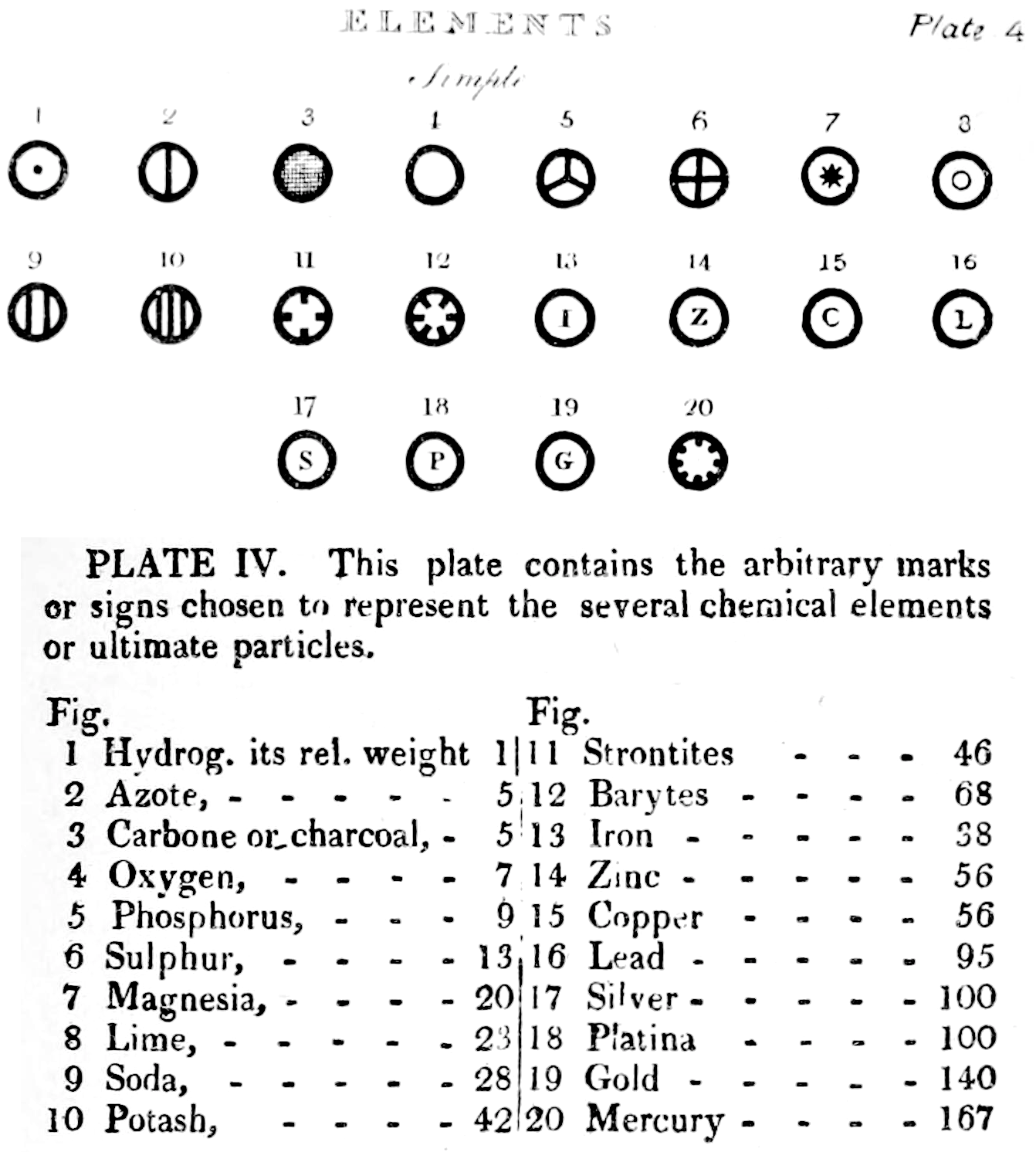
Dalton's Elements
Two pages from John Dalton's A New System of Chemical Philosophy in which he proposed his version of atomic theory based on scientific experimentation (see the scanned book, page 219):
| Name | Modern Symbol | Dalton's Data | Modern Values | % error |
| Hydrog. | H | 1 | 1 | 0% |
| Azote | N | 5 | 14 | -180% |
| Carbone | C | 5 | 12 | -140% |
| Oxygen | O | 7 | 16 | -129% |
| Phosphorus | P | 9 | 31 | -244% |
| Sulphur | S | 13 | 32.1 | -147% |
| Magnesia | Mg | 20 | 24.3 | -22% |
| Lime | Ca | 24 | 40.1 | -67% |
| Soda | Na | 28 | 23 | 18% |
| Potash | K | 42 | 39.1 | 7% |
| Strontites | Sr | 46 | 87.6 | -90% |
| Barytes | Ba | 68 | 137.3 | -102% |
| Iron | Fe | 50 | 55.8 | -12% |
| Zinc | Zn | 56 | 65.4 | -17% |
| Copper | Cu | 56 | 63.5 | -13% |
| Lead | Pb | 90 | 200.6 | -123% |
| Silver | Ag | 190 | 107.9 | 43% |
| Gold | Au | 190 | 197 | -4% |
| Platina | Pt | 190 | 195.1 | -3% |
| Mercury | Hg | 167 | 200.6 | -20% |
- Dalton states that he is considering "chemical elements or ultimate particles"
- Dalton assigns hydrogen as having a relative weight of 1.
- Note the seemingly huge % errors in the atomic weights, compared with modern values.
- These errors occurred because while Dalton had deduced that atoms combine in fixed (stoichiometric) ratios in compounds, he not always know what the ratios were. Thus there were two unknowns: the atomic weights (masses) and the stoichiometric ratios.
By Mark Leach
| Year: 1950 | PT id = 14, Type = formulation |
The modern periodic table is based on quantum numbers and blocks, here.
A periodic table can be constructed by listing the elements by n and l quantum number:
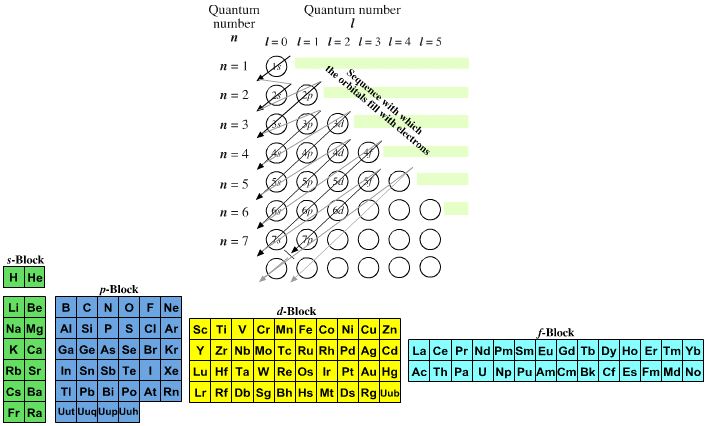
The problem with this mapping is that the generated sequence is not continuous with respect to atomic number atomic number, Z: Check out the sequence Ar to K, 18 to 19.
Named after a French chemist who first published in the formulation in 1929, the Janet or Left-Step Periodic Table uses a slightly different mapping:
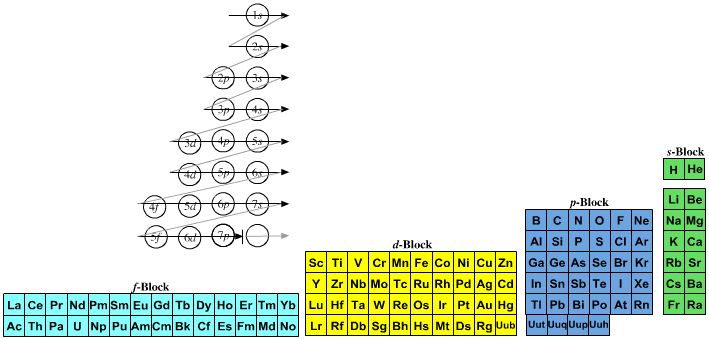
While the Janet periodic table is very logical and clear it does not separate metals from non-metals as well as the Mendeleev version, and helium is a problem chemically.
However, it is a simple mapping to go from the Janet or Left-Step periodic table to a modern formulation of Mendeleev's periodic table:
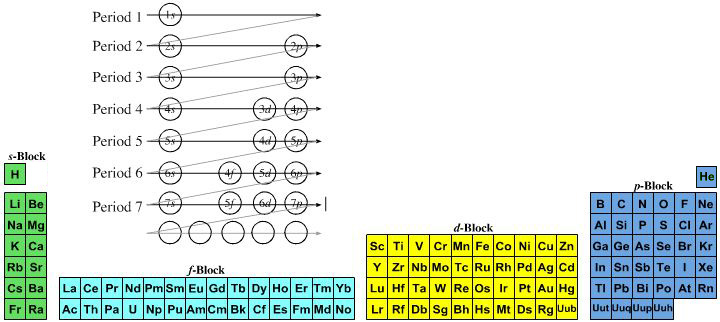
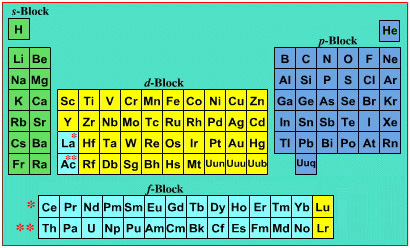
On this page web, "full" f-block included periodic tables are shown wherever possible, as above.
However, the periodic table is usually exhibited in book and on posters in a compressed form with the f-block "rare earths" separated away from the s-block, p-block and d-block elements:
However, the compression used introduces the well known problem known as a "fence post error".
The effect is that:
La and Ac: move from f-block to d-block
Lu and Lr: move from p-block to f-blockChemically, the elements can be fitted in and classified either way. Many thanks to JD for pointing the situation with the periodic table is a fence post error.
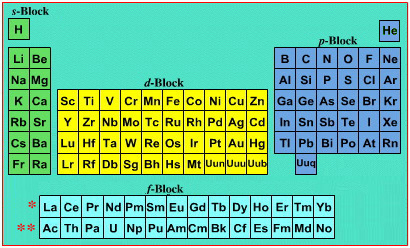
Mark Winter's Web Elements project, here, uses the formulation shown below:
Interestingly, the IUPAC periodic table separates out 15 lanthanides, La-Lu, and 15 actinides, Ac-Lr by leaving gaps in period 3 under Sc & Y:
This corresponds to:
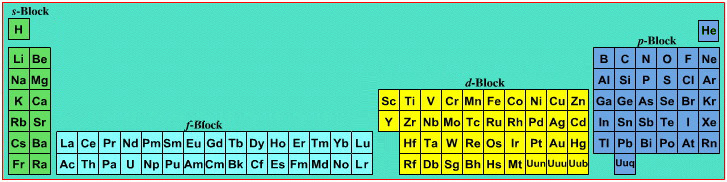
By Mark Leach
| Year: 2006 | PT id = 17, Type = formulation |
Where Should Hydrogen Go?
There are four possible positions for hydrogen:
- A Group 1 element, above Li, because it forms H+ ions.
- A Group 17 element, above F, because it forms H- ions.
- Above and between boron and carbon because it is of intermediate electronegativity.
- In the top middle, because nowhere else is ideal.
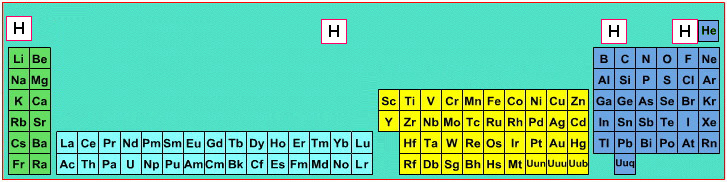
By Mark Leach
| Year: 1993 | PT id = 114, Type = data |
WebElements: The Periodic Table on The Web
Mark Winter's WebElements was started in 1993 when it was one of the first websites on the internet.
Mark Leach's Chemogenesis web book uses the WebElements periodic table as its master data source, and it does not attempt to duplicate it.
|
Abundance of
elements (Earth's crust) |
Electronegativities
|
Oxidation states
in compounds |
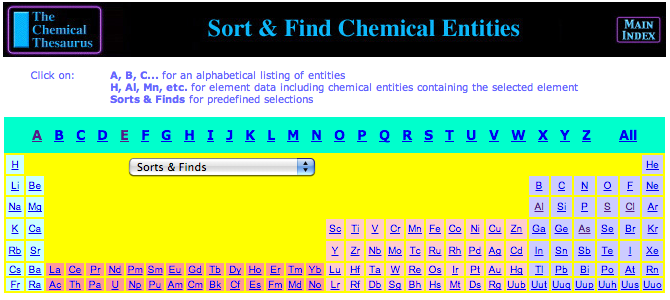
| Year: 2004 | PT id = 115, Type = data |
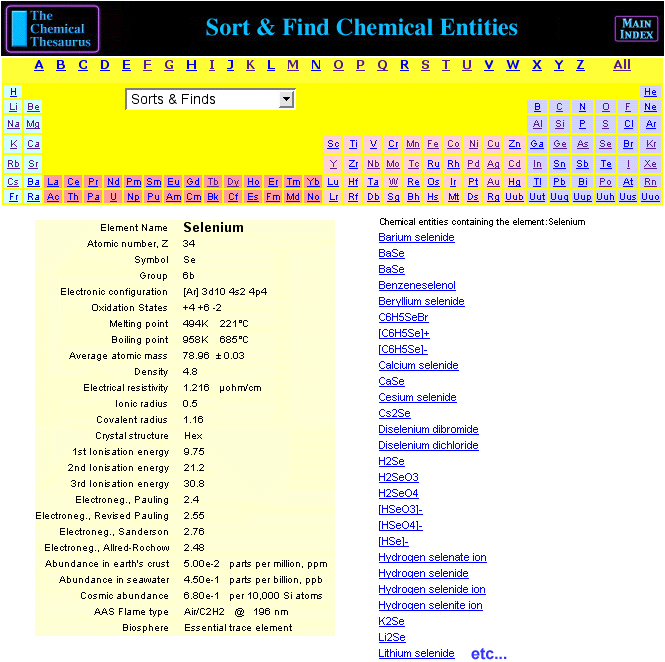
Chemical Thesaurus Periodic Table
Search for chemical reagents, atomic and molecular ions, minerals, isotopes, elemental data, etc., using the periodic table built into The Chemical Thesaurus reaction chemistry database:
By Mark Leach
| Year: 2003 | PT id = 123, Type = data |
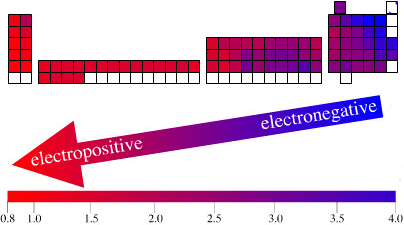
Electronegativity Periodic Table
A periodic table showing electronegativity, "The ability of an atom to attract electron density from a covalent bond" (Linus Pauling). Blue elements are electronegative, red elements are electropositive, and purple elements are intermediate. Notice how hydrogen is intermediate in electronegativity between carbon and boron and is positioned above and between these elements:
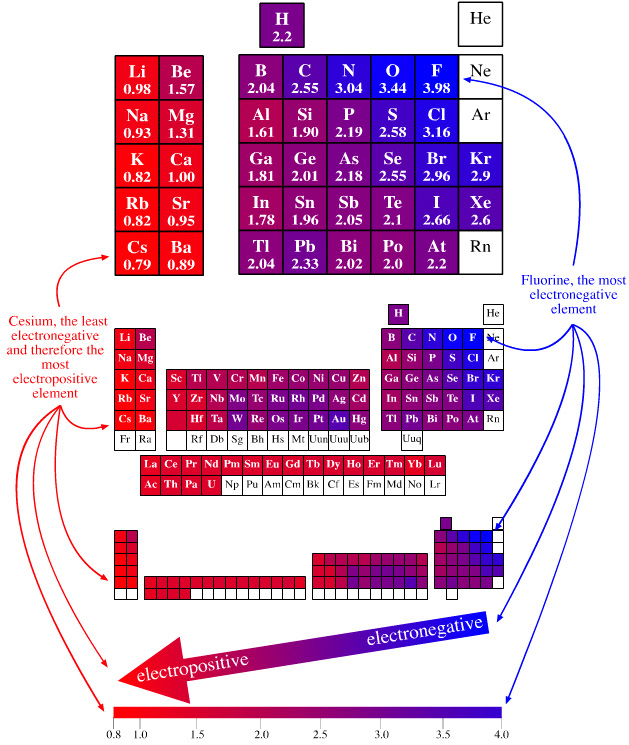
By Mark Leach
| Year: 2004 | PT id = 124, Type = data |
Material Type Periodic Table
All of the the main group elements are common laboratory reagents or chemical in bottles. They appear as metals, metalloid (semi-metals) and non-metals. Most of the non-metals are molecular materials while most of the metalloids have an extended network-covalent structure.
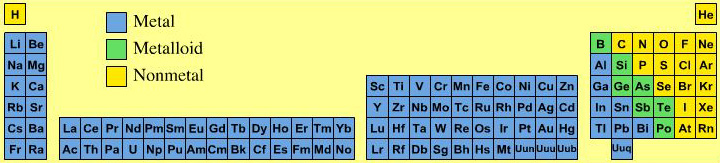
Elsewhere in the chemogenesis web book, material type is discussed in terms of the Laing Tetrahedron, an analysis that classifies binary materials in terms of four extreme types: metallic, ionic, molecular and network. However, none the chemical elements present as ionic materials, only as metals, molecular (van er Waals) and network materials:
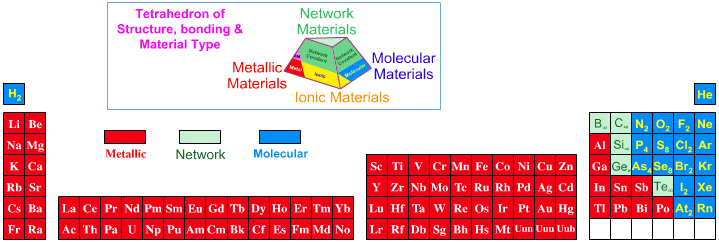
The elements B, C, Si, P, S, Ge, As, Se, Sn, Sb and Te can form allotropes: pure elemental substances that can exist with different crystalline structures from the Wikipedia. Allotropes may be metallic, network or molecular.
By Mark Leach
| Year: 2004 | PT id = 125, Type = data |
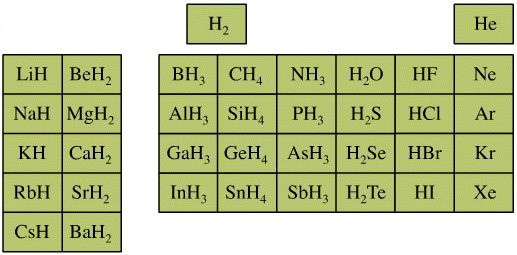
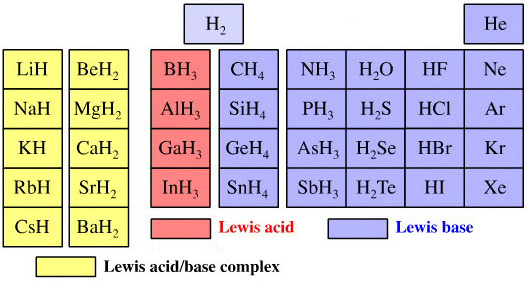
Elemental Hydride Types Periodic Table

- Ionic or Salt-Like Hydrides: Molten LiH conducts electricity and hydrogen gas is liberated at the anode confirming presence of hydride ion H–. The crystal structures show an ionic lattice, and not an LiH molecular lattice.
- Covalent Hydrides are formed by the p-Bolock elements.
- Metallic or Interstitial Hydrides are formed by many d-block and f-block elements when heated with hydrogen under pressure. The hydrides tend to be non-stoichiometric and they may be of variable composition.
- There is a Hydride Gap where elements do not form hydrides. This roughly maps to the Siderophile Elements of the geologist's periodic table (below).
- The Intermediate Hydrides do not fit: beryllium hydride is polymeric, (BeH2)n. Others have properties between metallic and covalent.
The main group elemental hydrides are all well known reagent chemicals. The main group hydrides always give the lowest and most common oxidation state, and all chemicals are molecular in the gas phase. The Group I and II hydrides are ionic materials, but they can be vaporised to give the molecular form.
The chemicals present and behave as Lewis acids, Lewis bases or Lewis acid/base complexes, here:
By Mark Leach
| Year: 2004 | PT id = 126, Type = data |
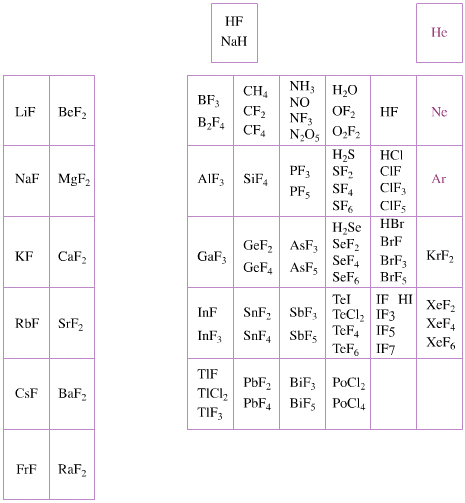
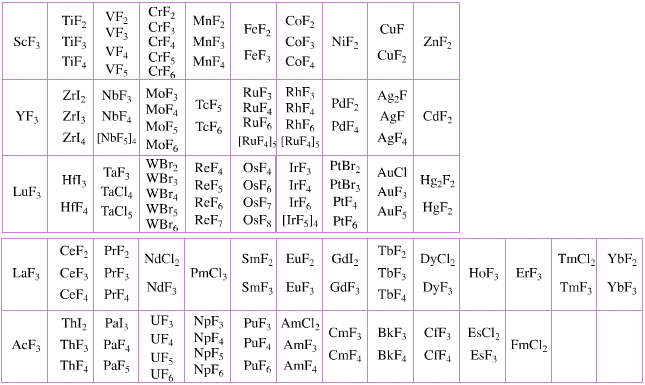
Elemental Oxidation States Periodic Table
The periodic table of fluorides (mainly) shows the range of possible oxidation states. Note that lithium, by way of example, is deemed to have two oxidation states: Li0 (the metal), and Li+ (the lithium ion):
There are a few exceptions and points to note:
- There is a general increase in the number of possible oxidation states towards the lower right hand side of the periodic table.
- Nitrogen(V) fluoride, NF5, is not known, but the nitrogen(V) oxide is: N2O5.
- PtBr2 and PtBr3 are known, but PtF2 and PtF3 are not.
- All elements are known in the zero oxidation state, but apart from: He, Ne & Ar, and these are not shown in the diagram below.
- All data is from WebElements.
By Mark Leach
| Year: 2005 | PT id = 127, Type = data |
Atomic Radii Periodic Table

By Mark Leach
| Year: 2006 | PT id = 130, Type = data |
Radioactivity Periodic Table
A periodic table showing the elements that have no stable isotopes, so that all samples are radioactive:
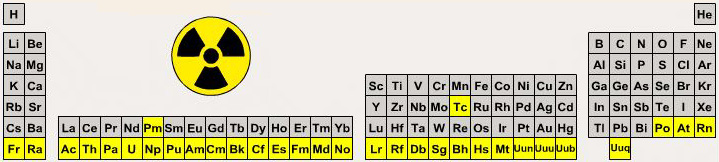
By Mark Leach
| Year: 2006 | PT id = 131, Type = data |
Superconducting Elements
A periodic table showing which elements become superconducting at low temperature.
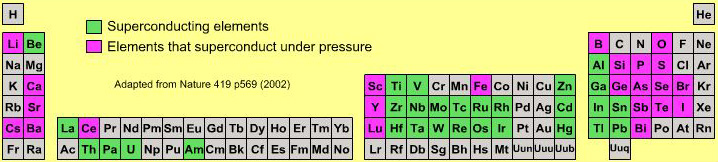
By Mark Leach
| Year: 2004 | PT id = 132, Type = data |
Mass Anomaly Periodic Table
Pairs of atoms where atomic mass does not follow atomic number.
|
Co
|
=
|
58.933 |
Ni
|
=
|
58.69 | ||
|
Ar
|
=
|
39.948 |
K
|
=
|
39.098 | ||
|
Te
|
=
|
127.60 |
I
|
=
|
126.90 |
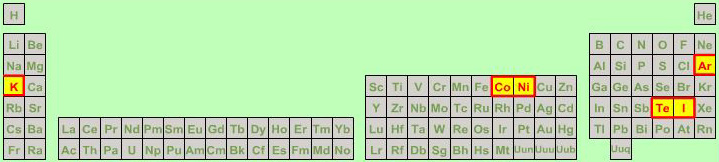
Nature's little quirk – due to the intricacies of nuclear chemistry and isotopic abundance – caused no end of difficulties to the developers of the periodic table in the mid-nineteenth century. Scientists could determine atomic mass, but knew nothing of protons or atomic numbers.
The tellurium-iodine anomaly was a particular problem.
By Mark Leach
| Year: 2012 | PT id = 133, Type = data |
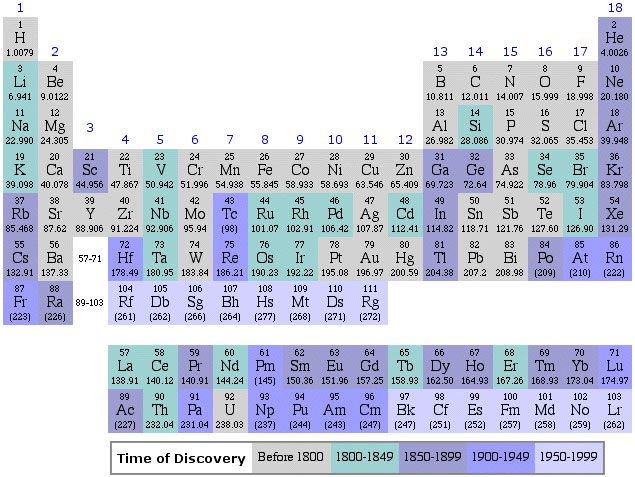
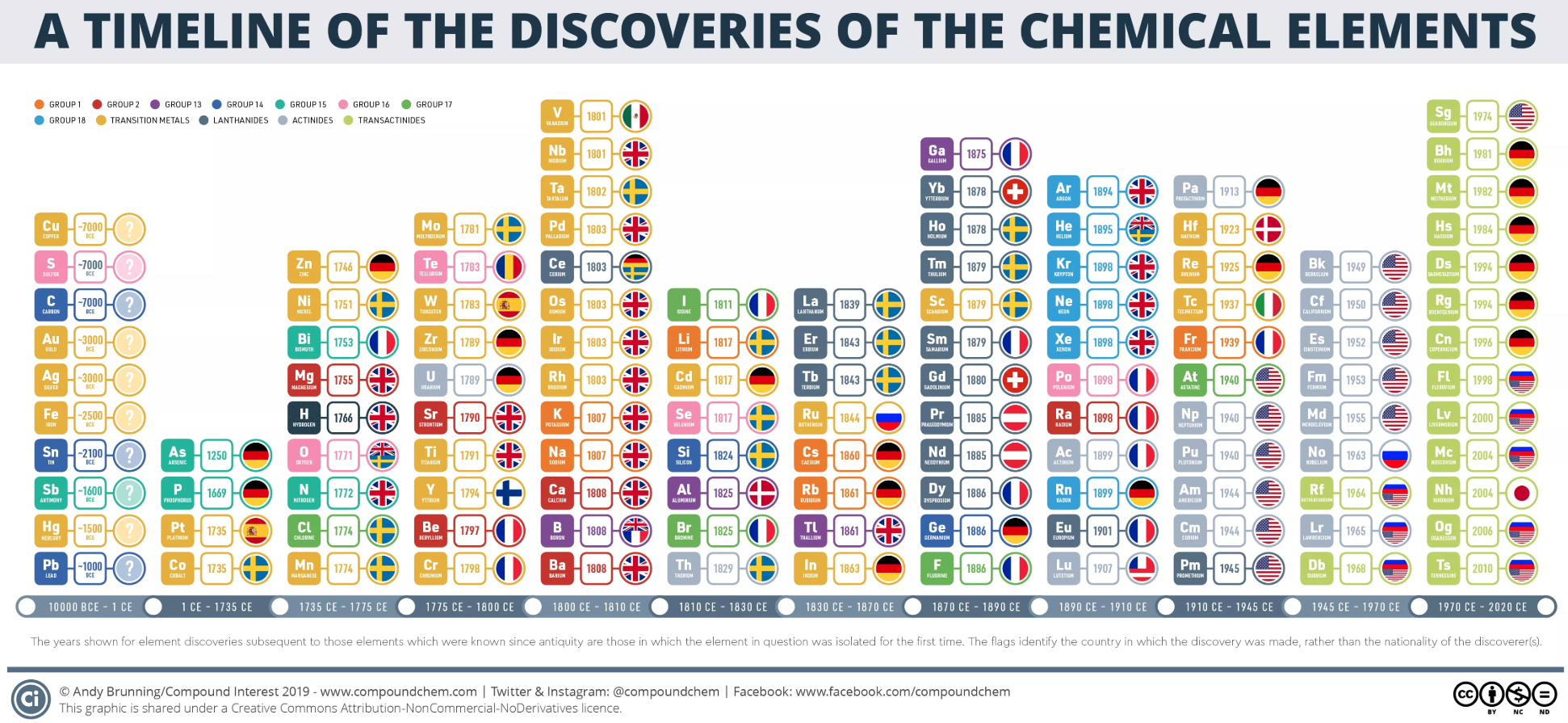
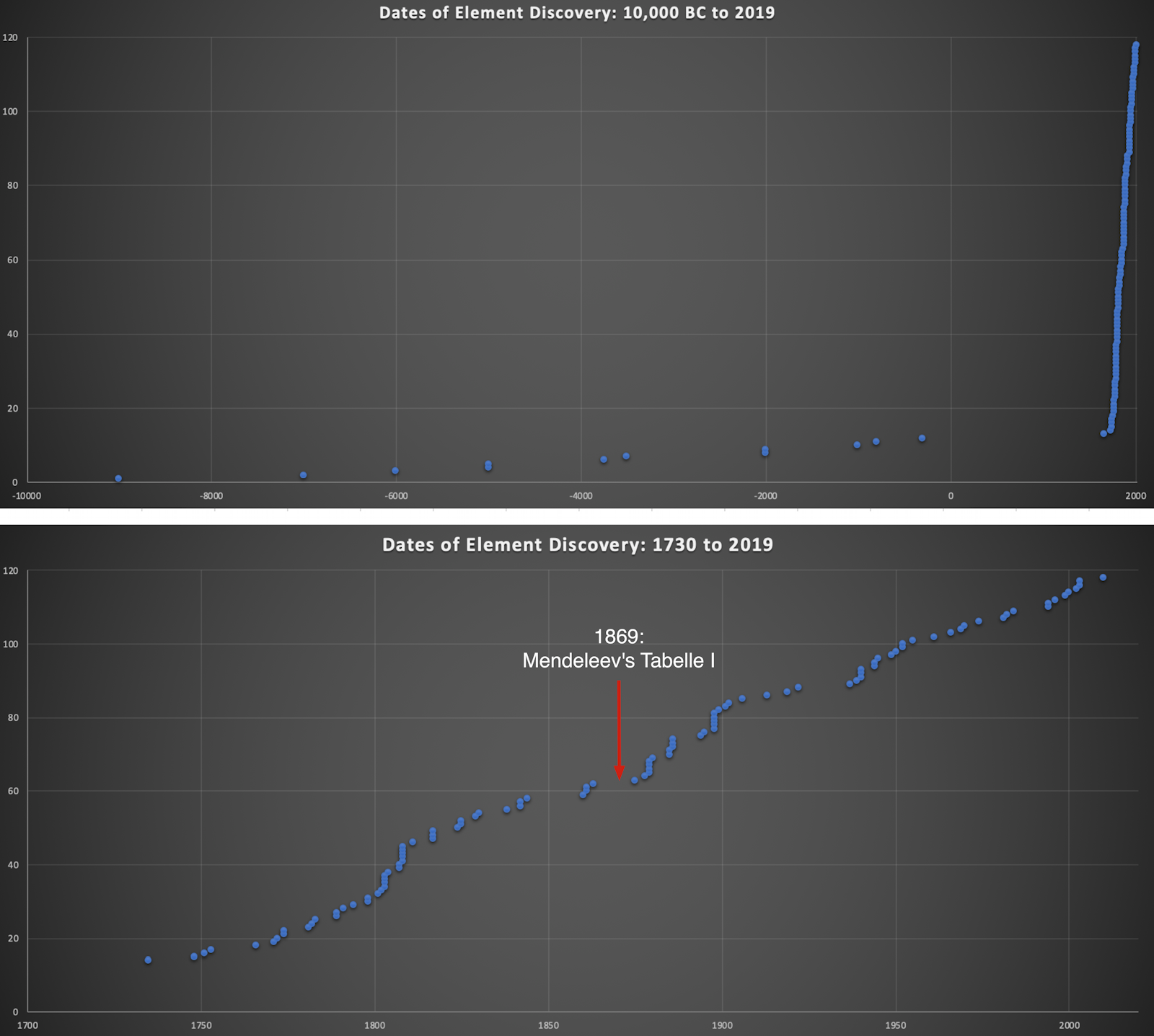
Dates of Discovery of the Elements
The Elements and their dates of discovery, taken from this Wikipedia page:
Two charts showing the dates of discovery of the elements, one from the 'time of the ancients' (10,000 BC) to the present day, and the second from 1700 to the present day.
These show that there were two distinct phases for the discovery of the 118 known elements:
- The first from about 10,000 BC to 1000 AD when 12 elements were discovered/used; one every 900 years or so.
- From 1669 until the present day when the other 106 have been rather steadily (and formally) discovered; one every couple of years.
- The last element to be made/discovered was in 2010.
Data from: this Wikipedia page.
| Discovery of Copper | -9000 |
| Discovery of Lead | -7000 |
| Discovery of Gold | -6000 |
| Discovery of Iron | -5000 |
| Discovery of Silver | -5000 |
| Discovery of Carbon | -3750 |
| Discovery of Tin | -3500 |
| Discovery of Sulfur (Sulphur) | -2000 |
| Discovery of Mercury | -2000 |
| Discovery of Zinc | -1000 |
| Discovery of Antimony | -800 |
| Discovery of Arsenic | -300 |
| Discovery of Phosphorus | 1669 |
| Discovery of Cobalt | 1735 |
| Discovery of Platinum | 1748 |
| Discovery of Nickel | 1751 |
| Discovery of Bismuth | 1753 |
| Discovery of Hydrogen | 1766 |
| Discovery of Oxygen | 1771 |
| Discovery of Nitrogen | 1772 |
| Discovery of Chlorine | 1774 |
| Discovery of Manganese | 1774 |
| Discovery of Molybdenum | 1781 |
| Discovery of Tellurium | 1782 |
| Discovery of Tungsten | 1783 |
| Discovery of Zirconium | 1789 |
| Discovery of Uranium | 1789 |
| Discovery of Titanium | 1791 |
| Discovery of Yttrium | 1794 |
| Discovery of Beryllium | 1798 |
| Discovery of Chromium | 1798 |
| Discovery of Niobium | 1801 |
| Discovery of Tantalum | 1802 |
| Discovery of Palladium | 1803 |
| Discovery of Cerium | 1803 |
| Discovery of Osmium | 1803 |
| Discovery of Iridium | 1803 |
| Discovery of Rhodium | 1804 |
| Discovery of Sodium | 1807 |
| Discovery of Potassium | 1807 |
| Discovery of Boron | 1808 |
| Discovery of Magnesium | 1808 |
| Discovery of Calcium | 1808 |
| Discovery of Strontium | 1808 |
| Discovery of Barium | 1808 |
| Discovery of Iodine | 1811 |
| Discovery of Lithium | 1817 |
| Discovery of Selenium | 1817 |
| Discovery of Cadmium | 1817 |
| Discovery of Silicon | 1824 |
| Discovery of Aluminium (Aluminum) | 1825 |
| Discovery of Bromine | 1825 |
| Discovery of Thorium | 1829 |
| Discovery of Vanadium | 1830 |
| Discovery of Lanthanum | 1838 |
| Discovery of Terbium | 1842 |
| Discovery of Erbium | 1842 |
| Discovery of Ruthenium | 1844 |
| Discovery of Cesium | 1860 |
| Discovery of Rubidium | 1861 |
| Discovery of Thallium | 1861 |
| Discovery of Indium | 1863 |
| Discovery of Gallium | 1875 |
| Discovery of Ytterbium | 1878 |
| Discovery of Scandium | 1879 |
| Discovery of Samarium | 1879 |
| Discovery of Holmium | 1879 |
| Discovery of Thulium | 1879 |
| Discovery of Gadolinium | 1880 |
| Discovery of Praseodymium | 1885 |
| Discovery of Neodymium | 1885 |
| Discovery of Fluorine | 1886 |
| Discovery of Germanium | 1886 |
| Discovery of Dysprosium | 1886 |
| Discovery of Argon | 1894 |
| Discovery of Helium | 1895 |
| Discovery of Neon | 1898 |
| Discovery of Krypton | 1898 |
| Discovery of Xenon | 1898 |
| Discovery of Polonium | 1898 |
| Discovery of Radium | 1898 |
| Discovery of Radon | 1899 |
| Discovery of Europium | 1901 |
| Discovery of Actinium | 1902 |
| Discovery of Lutetium | 1906 |
| Discovery of Protactinium | 1913 |
| Discovery of Rhenium | 1919 |
| Discovery of Hafnium | 1922 |
| Discovery of Technetium | 1937 |
| Discovery of Francium | 1939 |
| Discovery of Astatine | 1940 |
| Discovery of Neptunium | 1940 |
| Discovery of Plutonium | 1940 |
| Discovery of Americium | 1944 |
| Discovery of Curium | 1944 |
| Discovery of Promethium | 1945 |
| Discovery of Berkelium | 1949 |
| Discovery of Californium | 1950 |
| Discovery of Einsteinium | 1952 |
| Discovery of Fermium | 1952 |
| Discovery of Mendelevium | 1955 |
| Discovery of Lawrencium | 1961 |
| Discovery of Nobelium | 1966 |
| Discovery of Rutherfordium | 1969 |
| Discovery of Dubnium | 1970 |
| Discovery of Seaborgium | 1974 |
| Discovery of Bohrium | 1981 |
| Discovery of Meitnerium | 1982 |
| Discovery of Hassium | 1984 |
| Discovery of Darmstadtium | 1994 |
| Discovery of Roentgenium | 1994 |
| Discovery of Copernicium | 1996 |
| Discovery of Flerovium | 1999 |
| Discovery of Livermorium | 2000 |
| Discovery of Oganesson | 2002 |
| Discovery of Nihonium | 2003 |
| Discovery of Moscovium | 2003 |
| Discovery of Tennessine | 2010 |
By Mark Leach
A nice graphic from Compound Interest: (click image to enlarge)
| Year: 2006 | PT id = 134, Type = data misc |
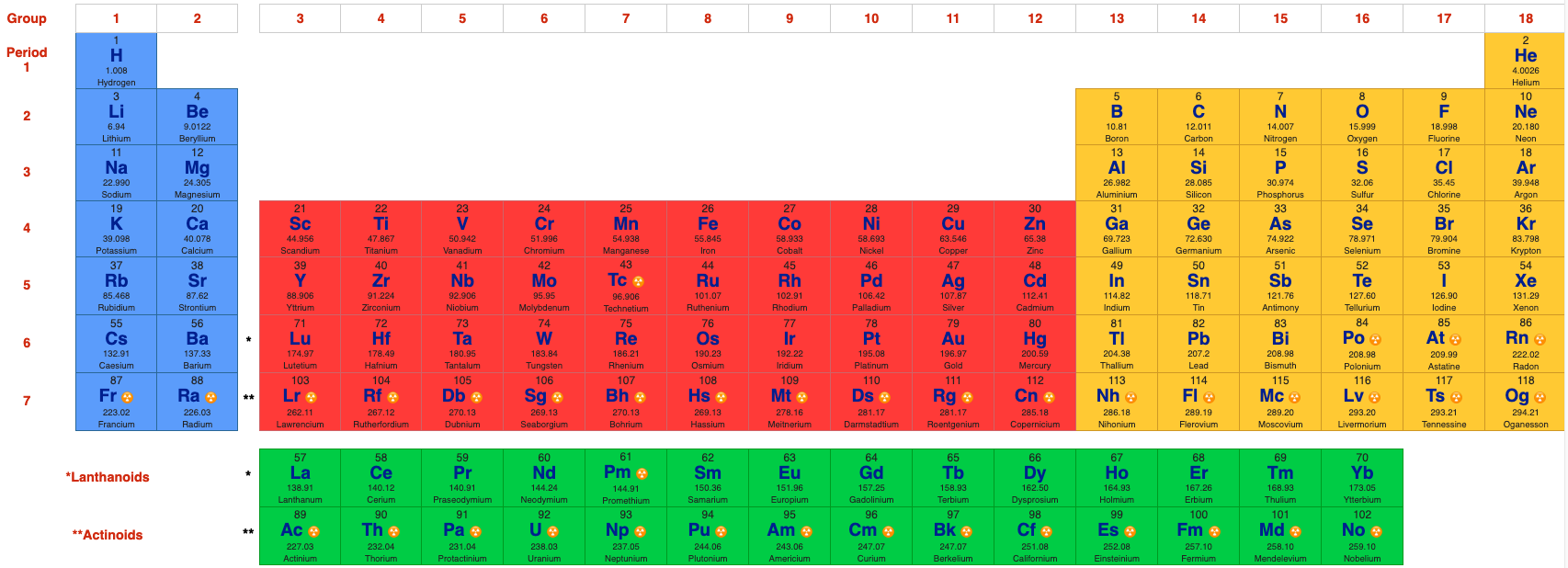
Group Numbering Systems
Phase State: Solid, Liquid, Gas at 20°C & 700°C
By Mark Leach
| Year: 2004 | PT id = 135, Type = data |
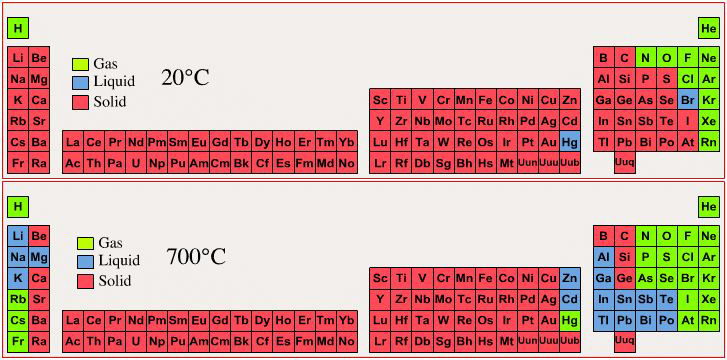
Phase State: Solid, Liquid, Gas at 20°C & 700°C

By Mark Leach
| Year: 2005 | PT id = 137, Type = data |
Extraction from Ore to Pure Element
A periodic table showing how pure elements are extracted:
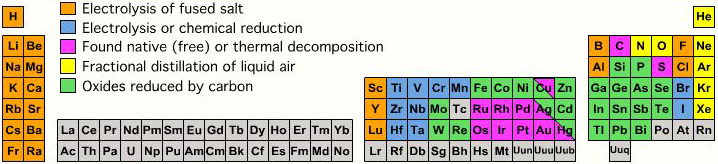
Highly electropositive elements (Na, K) and electronegative elements (Cl2, F2) can only be obtained by electrolysis.
By Mark Leach
| Year: 2004 | PT id = 139, Type = data |
Organic Chemist's Periodic Table
Organic chemistry is dominated by carbon, hydrogen, oxygen and nitrogen. Other elements are commonly encountered in the organic lab, others less commonly and some... almost never at all...
A less than useful formulation (!):
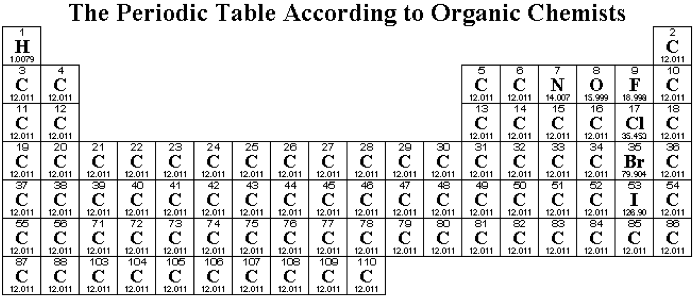
followed by a slightly more useful organic chemist's periodic table:
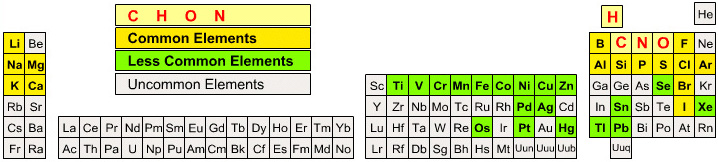
By Mark Leach
| Year: 2004 | PT id = 140, Type = data |
Inorganic Chemist's Periodic Table
Every element has a specialist, somewhere, for whom it is the most important element.
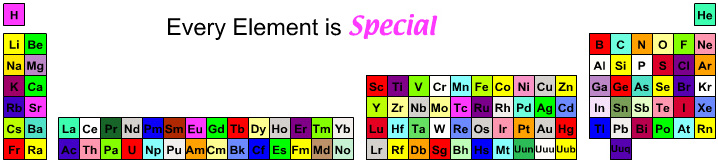
By Mark Leach
| Year: 2005 | PT id = 141, Type = data |
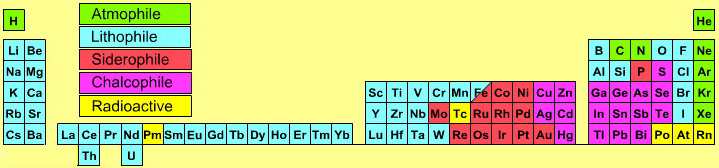
Atmophile Elements - noble gases and covalently bonded gaseous molecules. The atoms and molecules are attracted by weak van der Waals forces and so these elements remain gaseous at room temperature.
Lithophile Elements - Those elements which form ionic bonds generally have filled outer electron shells. They typically bond to oxygen in silicates and oxides.
Siderophile Elements - The metals near iron in the periodic table that exhibit metallic bonding, have a weak affinity for oxygen and sulfur and are readily soluble in molten iron. Examples include iron, nickel, cobalt, platinum, gold, tin, and tantalum. These elements are depleted in the earth crust because they have partitioned into the earth's iron core.
Chalcophile Elements - The elements that bond to S, Se, Te, Sb, and As. These bonds are predominantly covalent in character.
As discussed in more detail here.
By Mark Leach
| Year: 2004 | PT id = 143, Type = data misc |
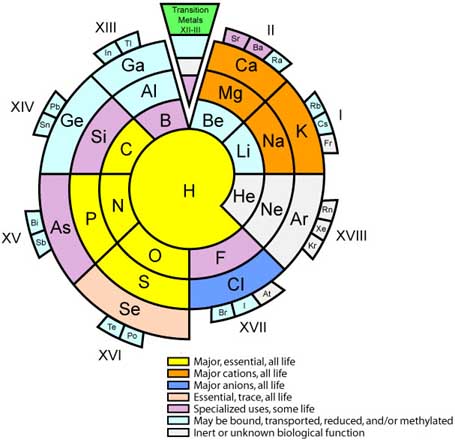
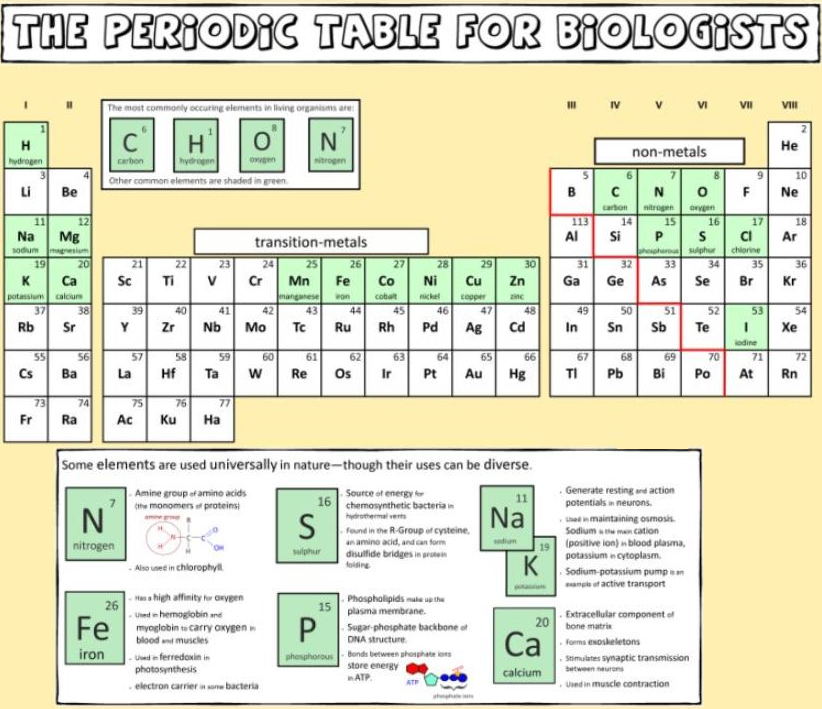
Biologist's Periodic Tables
A periodic table showing where biologically essential (green), essential trace (purple), toxic (red), radioactive (yellow) and of low – but not zero– biological impact (gray) elements are found. Only highly toxic elements are shown in red. Li (as Li+) is biologically active and is used as an antidepressant.
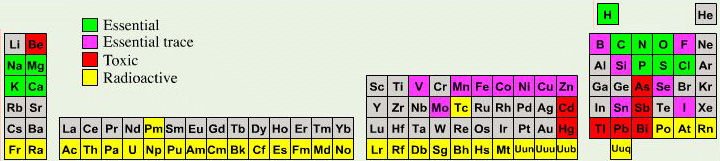
By Mark Leach
or here:
And a periodic table for biologists from Science Videos:
| Year: 2006 | PT id = 144, Type = data |
Astronomer's Periodic Table
Highly amusing for chemists is the astronomer's periodic table because astronomers consider there to be three types of element:
- hydrogen
- helium
- metal
Yup, cosmologists and other professional star gazers consider all elements, atomic number three and up, to be metals.
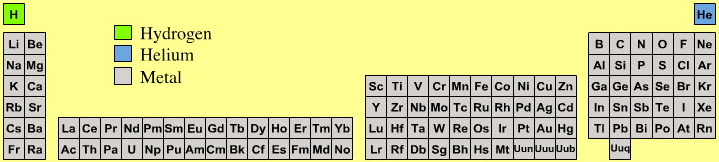
By Mark Leach
| Year: 2005 | PT id = 157, Type = data |
Student's Periodic Table
Students are expected to know that in all equations hydrogen is molecular should [nearly always] be written as H2. Likewise, nitrogen is N2, oxygen O2, fluorine F2, chlorine Cl2, bromine Br2 and iodine I2. But somehow students are expected to know that molecular sulfur, S8, should be written as S and molecular phosphorus, P4, should be written as P.
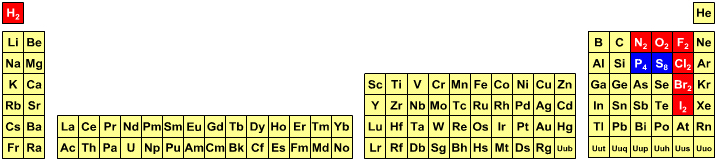
By Mark Leach
| Year: 2003 | PT id = 228, Type = formulation misc |
Elements by Orbital
From elsewhere in Mark Leach's Chemogenesis webbook:
Madelung's Rule tells us that the orbitals fill in the order n + l (lowest first). This gives the sequence:
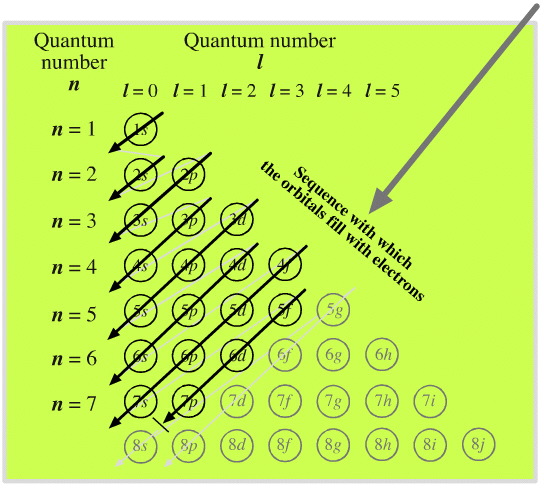
Electronic structure can be illustrated adding electrons to boxes (to represent orbitals). This representation shows the Pauli exclusion principle, the aufbau principle and Hund's rule in action.
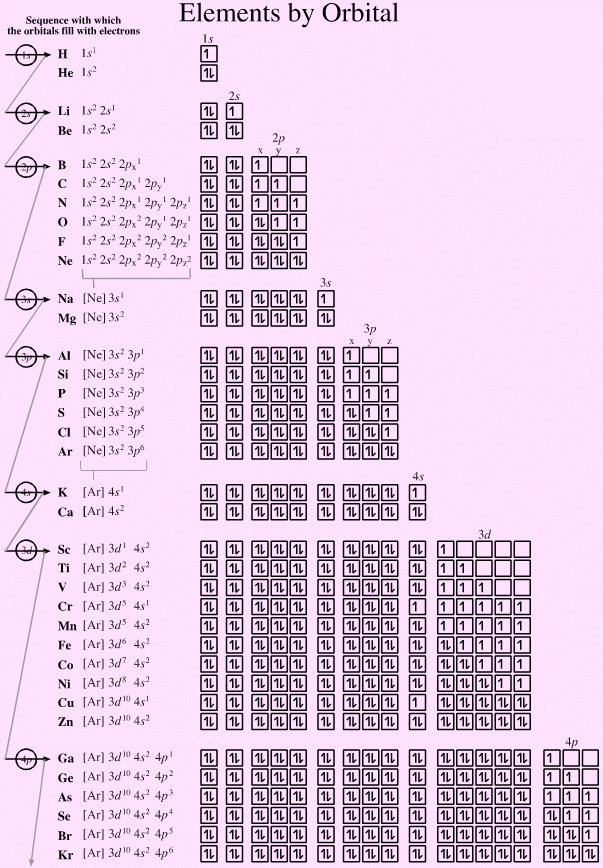
There are some subtle effects with the d block elements chromium, Cr, and copper, Cu. Hund's rule of maximum multiplicity lowers the energy of the 3d orbital below that of the the 4s orbital, due to the stabilisation achieved with a complete and spherically symmetric set of five 3d orbitals containing five or ten electrons. Thus,
- Chromium has the formulation: [Ar] 3d5 4s1 and not: [Ar] 3d4 4s2
- Copper has the formulation: [Ar] 3d10 4s1 and not: [Ar] 3d9 4s2
| Year: 2007 | PT id = 244, Type = data |
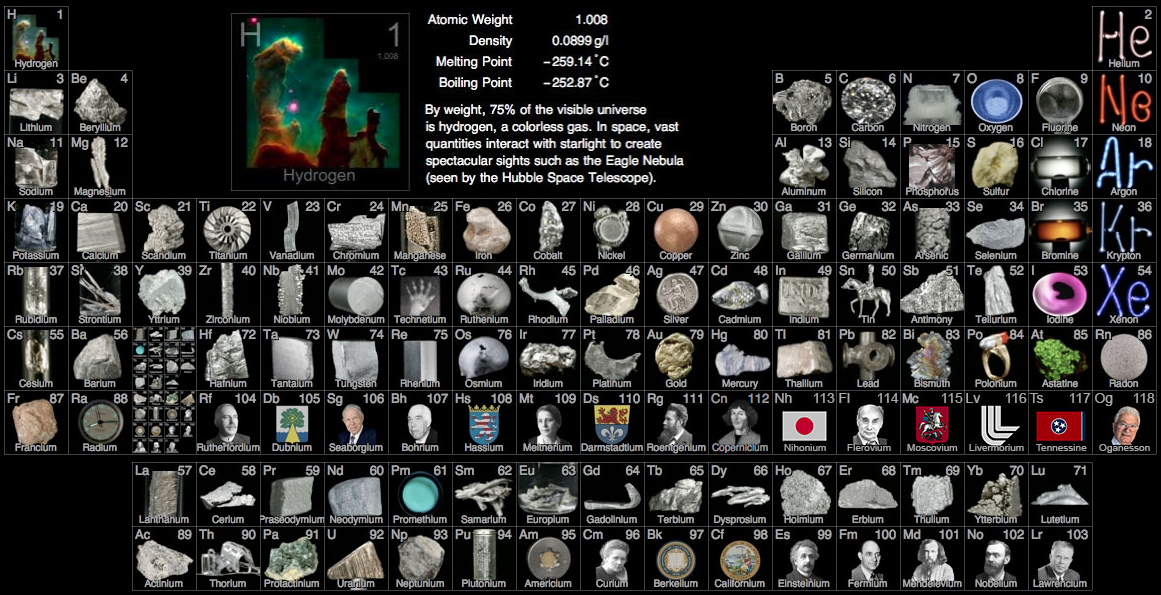
Gray's Photographic Periodic Table
Theodore Gray's Periodic Table.Com is a live version of what is generally regarded as the most beautiful periodic table to be developed so far. It is a treasure trove of pictures, videos and stories. Explore!
Theo is an enthusiast and a collector, and he uses the power of Mathematica (he is a co-founder of Wolfram Research) to drive his astonishing website. It is Theo's aim to be the number one periodic table resource on the web.
Mark Leach, the database curator writes:
"I find Theo's website and approach to be complementary to the more academic WebElements."
| Year: 1843 | PT id = 268, Type = formulation |
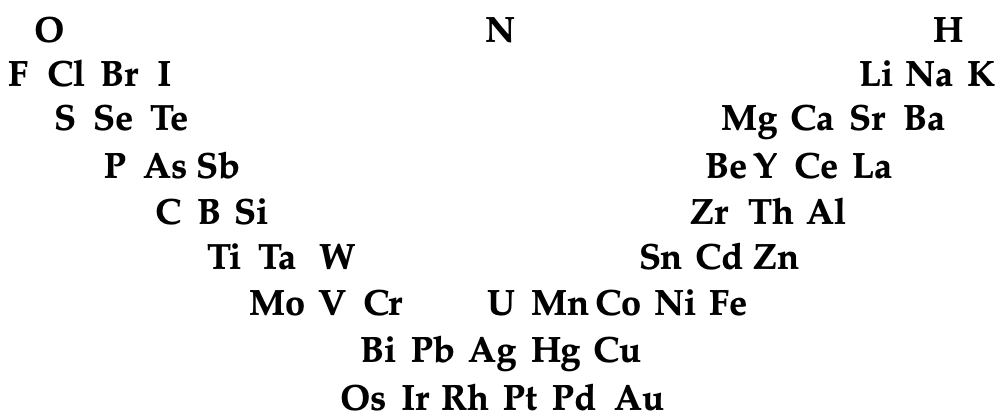
Gmelin's System
L. Gmelin, Handbuch der chemie, 4th ed., Heidelberg, 1843, vol. 1, p. 457 (Many thanks to Carmen Giunta for the ref. update & link.)
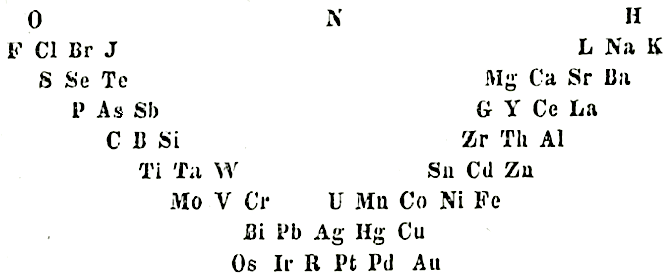
The early and important Gmelin formulation redrawn by Mark Leach with modern element symbols:
- J → I
- G → Be
- L → Li
- R → Rh
| Year: 2006 | PT id = 282, Type = formulation |
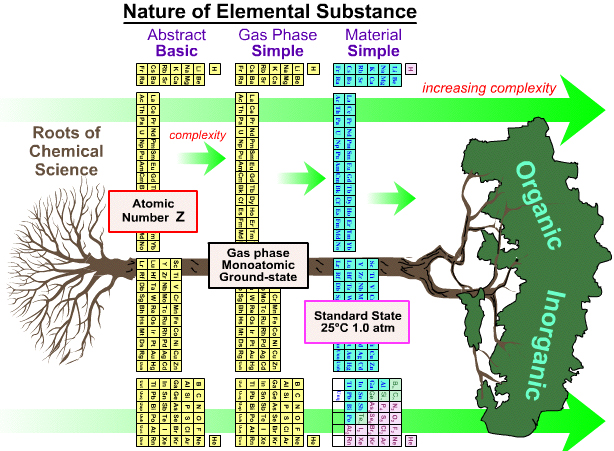
Various Periodic Tables
As discussed on this page of the Chemogenesis webbook, the periodic table is ambiguous as to what it is showing.
Does the PT show the element as the abstract 'basic substance', or gas phase atoms or the material substance?
By Mark Leach
| Year: 2010 | PT id = 335, Type = data misc |
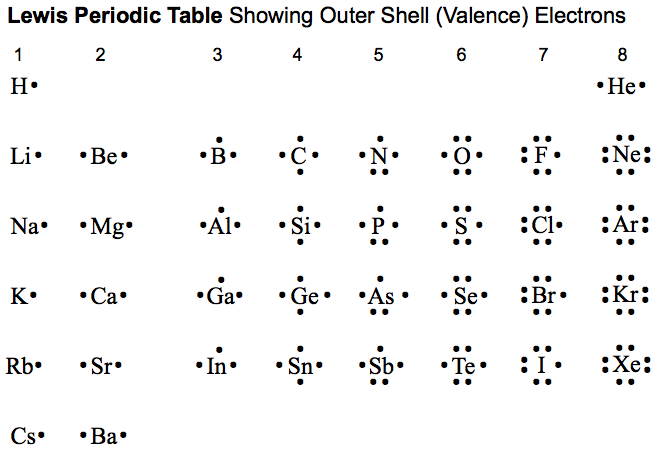
Lewis Octet Periodic Table
A periodic table showing the outer shell of valence electrons associated with Lewis atoms:
By Mark Leach
| Year: 1831 | PT id = 337, Type = formulation element weight |
Daubeny's Teaching Display Board & Wooden Cubes of Atomic Weights
The Museum of the History of Science, Oxford, has a display of Charles Daubeny's teaching materials, including a black painted wooden board with "SYMBOLS OF SIMPLE BODIES": showing symbols, atomic weights and names of elements in two columns, and a small pile of cubes with element symbols.
Charles Daubeny and Chemistry at the Old Ashmolean
Charles Daubeny (1795-1867) was appointed Aldrichian Professor of Chemistry at Oxford in 1822. In 1847 he moved from the original laboratory in this basement [in the museum] to a new one built at his own expense at the Botanic Garden. His apparatus went with him and was preserved there. Daubeny actively campaigned for the teaching of science in Oxford and held several professorships in addition to chemistry. He also conducted research on subjects such as photosynthesis.
From the HSM Database (Inventory no. 17504):
DAUBENY'S LIST OF ATOMIC WEIGHTS Wooden panel, black with white lettering, listing in two columns the symbols and names of twenty elements. This lecture board is identical to the table in the third edition (1831) of E. Turner, 'Elements of Chemistry', apart from the atomic weight for bromine. Daubeny wrote a useful 'Introduction to the Atomic Theory' (published in three versions: 1831, 1840, and 1850), the first edition of which also quotes Turner's table. Probably contemporary with this lecture board are the wooden cubes with the symbols for certain elements.
The period from 1810 to 1860 was crucial in the development of the periodic table. Most of the main group and transition elements had been discovered, but their atomic weights and stoichiometries (combining ratios) had not been fully deduced. Oxygen was assumed to have a weight of 6, and consequently carbon is assumed to have a mass of 6.
Daubeny's element symbols and weights – along with the modern mass data – are tabulated:
| Symbol | Daubeny's Weight | Modern Mass Data | % error | Stoichiometry Error |
| H | 1 | 1 | 0% | |
| C | 6 | 12 | -100% | factor of 2 |
| O | 8 | 16 | -100% | factor of 2 |
| Si | 8 | 28.1 | -251% | factor of 5 (?) |
| Al | 10 | 27 | -170% | factor of 3 |
| Mg | 12 | 24.3 | -103% | factor of 2 |
| N | 14 | 14 | 0% | |
| S | 16 | 32.1 | -101% | factor of 2 |
| P | 16 | 31 | -94% | factor of 2 |
| Fl | 19 | 19 | 0% | |
| Ca | 20 | 40.1 | -101% | factor of 2 |
| Na | 24 | 23 | 4% | |
| Fe | 28 | 55.8 | -99% | factor of 2 |
| Cl | 36 | 35.5 | 1% | |
| K | 40 | 39.1 | 2% | |
| Cu | 64 | 63.5 | 1% | |
| B | 80 | 79.9 | 0% | |
| Pb | 104 | 207 | -99% | factor of 2 |
| I | 124 | 127 | -2% | |
| Hg | 200 | 200.6 | 0% |
While quite a number of weights are close to the modern values, many are way out. However, the error is usually a stiotoimetric factor error.
From the HSM Database (Inventory no. 33732): SET OF WOODEN CUBES ILLUSTRATING ATOMIC WEIGHTS
Forty-two wooden cubes numbered 1-42, painted black with symbols for certain elements, compounds or radicals painted in white on the faces, together with the corresponding atomic, molecular or radical weights. The face markings appear in various combinations:
| H | C | P | Na | Ca° | S | N | K | Fe | K | Na° | Cy | K° |
| 1 | 6 | 16 | 24 | 28 | 16 | 14 | 40 | 28 | 48 | 32 | 26 | 48 |
A typical cube (no. 3) may be represented by the following figure. They present something of an enigma as their faces do not form an obvious pattern. The numbers indicate that there were 42 cubes. In style they are similar to the figures on the panel of atomic weights.
The cubes are listed in Daubeny's 1861 catalogue, p. 11 as: "Wooden cubes for illustrating atomic weight". [See D. R. Oldroyd, The Chemical Lectures at Oxford (1822-1854) of Charles Daubeny, M.D., F.R.S. Notes and Records of the Royal Society, vol. 33 (1979), pp. 217-259.]
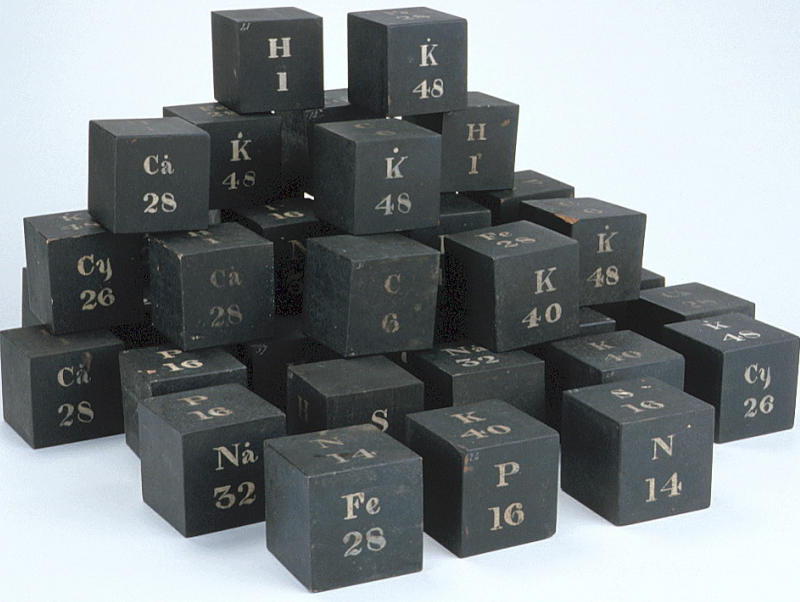
This display was spotted by Eric Scerri who was visiting the museum with Mark Leach in 2010.
There is a virtual tour on the museum, and the above display is in the basement.
| Year: 1904 | PT id = 546, Type = formulation |
Ramsay's Periodic Arrangement of The Elements
From from Scientific American in 1904,, an article by Sir William Ramsay discussing the Periodic Arrangement of The Element:
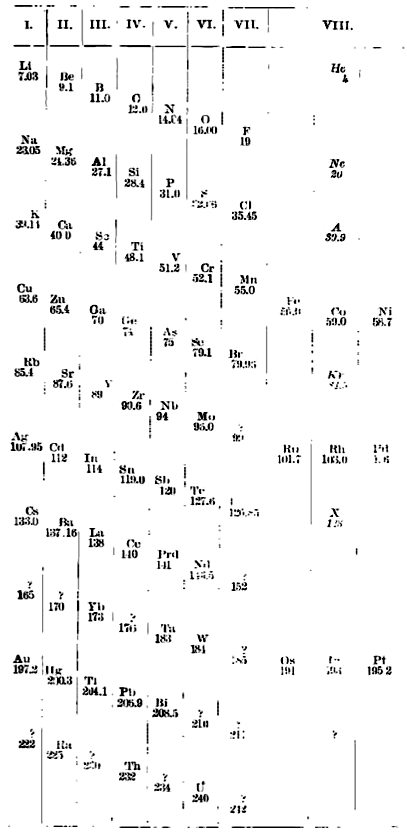
Redrawn by Mark Leach in 2019:
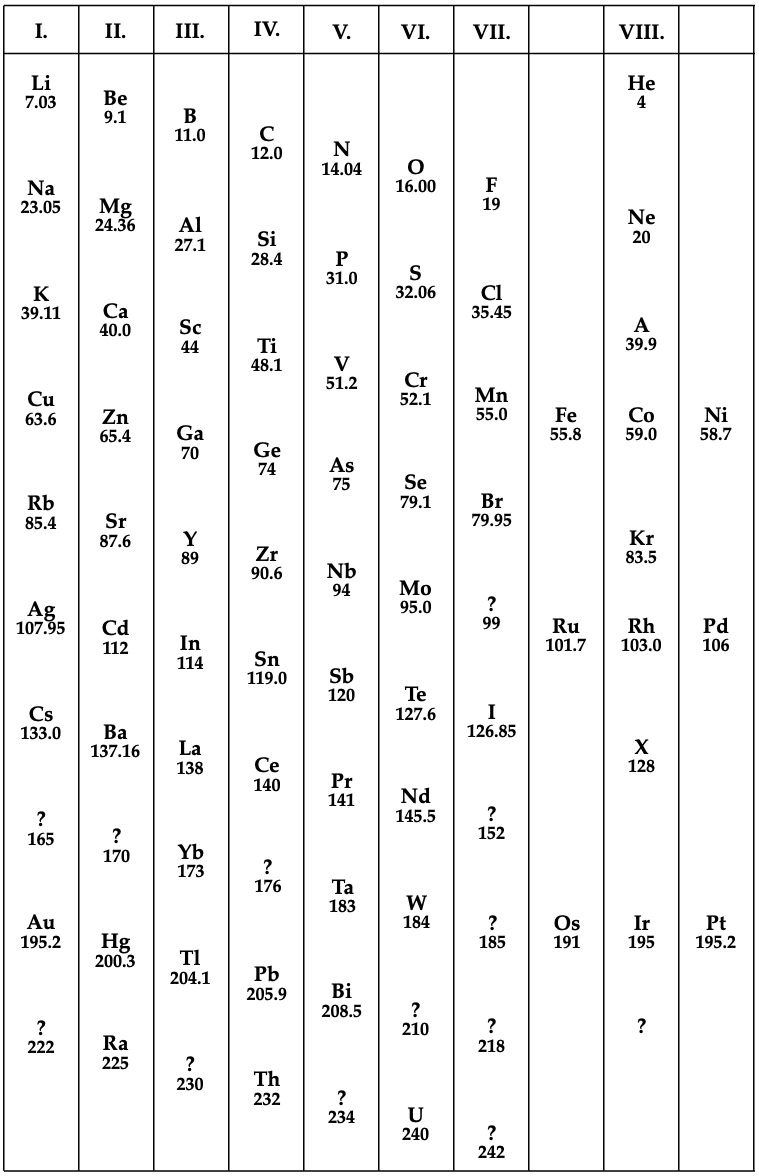
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
| Year: 2013 | PT id = 566, Type = formulation data review |
Electronegativity Chart (Leach)
From Mark R Leach's paper, Concerning electronegativity as a basic elemental property and why the periodic table is usually represented in its medium form, Journal & PDF.
Due to the importance of Pauling's electronegativity scale, as published in The Nature of The Chemical Bond (1960), where electronegativity ranges from Cs 0.7 to F 4.0, all the other electronegativity scales are routinely normalised with respect to Pauling's range.
When the Pauling, Revised Pauling, Mulliken, Sanderson and Allred-Rochow electronegativity scales are plotted together against atomic number, Z, the similarity of the data can be observed. The solid line shows the averaged data:
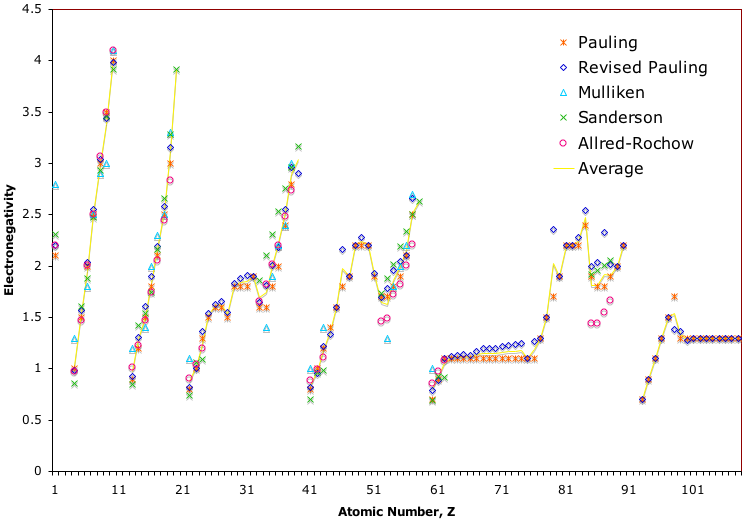
| Year: 2013 | PT id = 610, Type = review |
Top 10 Periodic Tables
There are more than 1000 periodic tables hosted by the Chemogenesis Webbook Periodic Table database, so it can be a little difficult to find the exceptional ones.
Here we present – in our humble opinion – The ten most significant periodic tables in the database.
We present the best:
- Three best data rich periodic tables
- Five formulations which show the development of the modern PT
- One, of many, interesting alternative formulations
- One example of the periodic table being used as an infographic template
Three Excellent, Data Rich Periodic Tables
The first three of our top 10 periodic tables are classic element data repositories.
They all work in the same way: click on the element symbol to get data/information about the selected element. The three are Mark Winter's WebElements, Theo Gray's Photographic Periodic Table & Michael Dayah's Ptable.
- Since 1993 – and with its rather bland interface – WebElements has given access to vast quantities of in depth chemical data & information. This is the professional chemist's periodic table:
- Theo Gray's Photographic Periodic Table is undoubtedly the most attractive PT available in web space, but there is more. Clicking around the website gives access to a host of information, pictures & anecdotes from Theo's extraordinary and extensive collection of chemical elements:
- Ptable has a super-slick, and very fast interface. It is data/information rich and is available in 50 languages:
Five Formulations Showing The History & Development
The next five examples deal with history and development Periodic Table. The first is Dalton's 1808 list of elements, next is Mendeleev's 1869 Tabelle I, then Werner's remarkably modern looking 1905 formulation. This is followed by Janet's Left Step formulation and then a discussion of how and why the commonly used medium form PT formulation, is constructed.
- There are several early listings of chemical substances, including Valentinus' Alchemy Table and Lavoisier's Table of Simple Substances (1789). In 1803 Dalton proposed that matter consists of discrete atoms that combine in fixed ratios, stoichiometry, to form chemical elements. Thus, Dalton's list of chemical elements, plus mass data, must be included in any top ten listing:

- If you examine the periodic tables from Antiquity to 1899, you will see that from about 1830 onwards, proto-periodic tables were coming thick and fast. Significant developments include: Daubeny's Teaching Display Board of Atomic Weights (1831), Chancourtois Telluric Helix (1862) and Newlands octaves (1864).
But, it was Mendeleev's Tabelle I that was first near complete periodic table formulation of the then known elements (no Group 18 rare gasses, note). Crucially, Mendeleev identified gaps and was able to make predictions about the chemical properties of the missing substances. Plus, Mendeleev promoted his ideas with great energy:

- Werner's 1905 Periodic Table is remarkably modern looking. The formulation is a long form that separates transition metals and rare earths, but he guessed wrong on how many existed:

- Janet's Left Step formulation of 1928 is one for the purists as it clearly shows the chemical elements arranged into s, p, d & f-blocks of the recently developed quantum mechanical description of atomic structure:
- The modern (and commonly employed) periodic table is obtained by transforming Janet's Left Step into the modern long form periodic table by rearranging the blocks around. This transformational mapping is discussed in some detail here.
The long form and medium form PTs have electronegativity trending from top-right (electronegative) to bottom left (electropositive), and many aspects of periodicity corollate with electronegativity: atomic radius, first ionisation energy, etc.
Thus, the long form and medium form periodic tables are commonly used in the classroom:
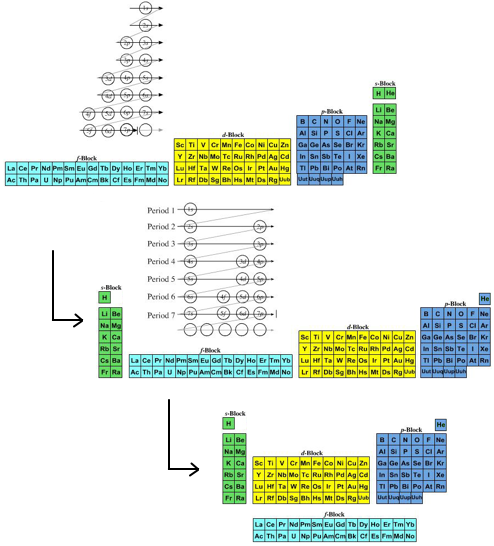
An Alternative Formulation
The internet database contains many, many alternative formulations, and these are often spiral and/or three dimensional. These exemplified by the 1965 Alexander DeskTopper Arrangement. To see the variety of formulations available, check out the Spiral & Helical and 3-Dimensional formulations in the database:

Non-Chemistry PTs
The periodic table as a motif is a useful and commonly used infographic template for arranging many types of object with, from 50 to 150 members.
There are numerous examples in the Non-Chemistry section where dozens of completely random representations can be found:
- Adobe Illustrator Shortcuts
- Adult Positions
- Airline Customer Reviews
- Beer Styles
- And, chosen more or less at random, European Nations:
| Year: 2005 | PT id = 657, Type = data |
Chemical Thesaurus Reaction Chemistry Database Periodic Table
A periodic table front end to the Chemical Thesaurus Reaction Chemistry Database Periodic Table. Clicking on an element gives access to database searches of chemical species and their interactions.
A quote neatly sums up what the ChemThes reaction chemistry database project is trying to achieve:
"The Chemical Thesaurus is a reaction chemistry information system that extends traditional references by providing hyperlinks between related information. The program goes a long way toward meeting its ambitious goal of creating a nonlinear reference for reaction information. With its built-in connections, organizing themes, and multiple ways to sort and view data, The Chemical Thesaurus is much greater than the sum of the data in its database.
"The program does an excellent job of removing the artificial barriers between different subdisciplinary areas of chemistry by presenting a unified vision of inorganic and organic reaction chemistry."
By Mark Leach
| Year: 2018 | PT id = 766, Type = data |
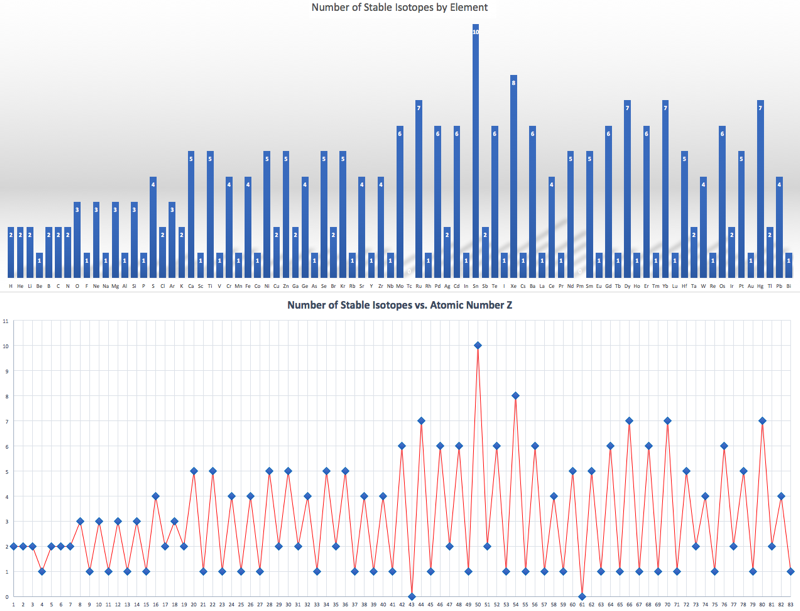
Number of Stable Isotopes by Element
When plotting the number of stable isotopes against element, and against atomic number Z, it is clear that elements with an even atomic number are likely to have more stable isotopes (average 4.9) than elements with an odd atomic number (average 1.3). Click here for the Excel file. There is a Wikipedia page here.
The effect is striking in graphical form:
By Mark Leach
| Year: 2018 | PT id = 774, Type = formulation data |
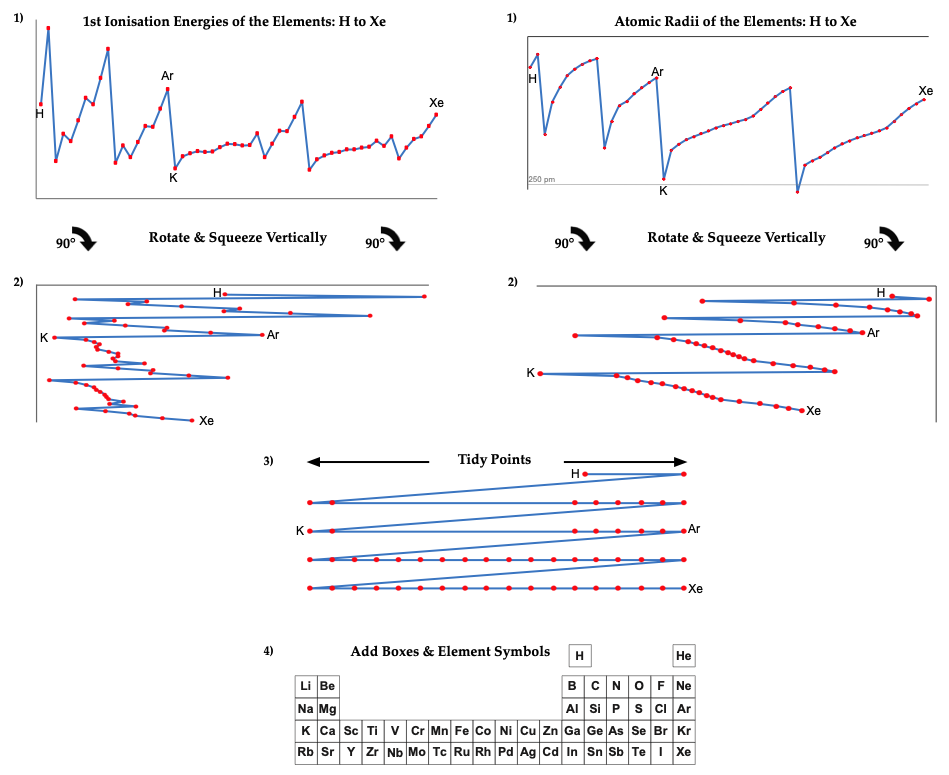
First Ionisation Energy to the Standard Form Periodic Table
There is debate amongst the cognoscenti about the 'best' representation of the periodic table, and how this 'best' formulation can be explained by [rationalized by] quantum mechanics (QM).
Many feel that the Janet PT formulation, the 'Left Step', is the ideal QM PT, but this formulation does not show periodicity very well, and there are issues with the placement of H, He, Be which spill over into questions about their placement in the standard form PT (the periodic table used in classrooms and textbooks around the world).
However, it is possible to get to the conventional standard form PT directly from the first ionisation energy data, where the 1st ionisation energy is the energy required to convert a gas phase atom (M) into its gas phase positive ion plus electron.
M(g) → M+(g) + e–
The process involves:
- taking the 1st ionisation data plot for the elements H to Xe (Z = 1 to 36)
- rotate 90° clockwise and stretch
- move the atoms horizontally into columns
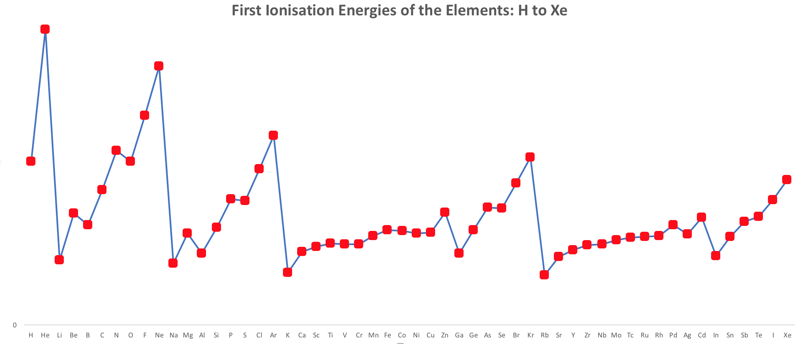
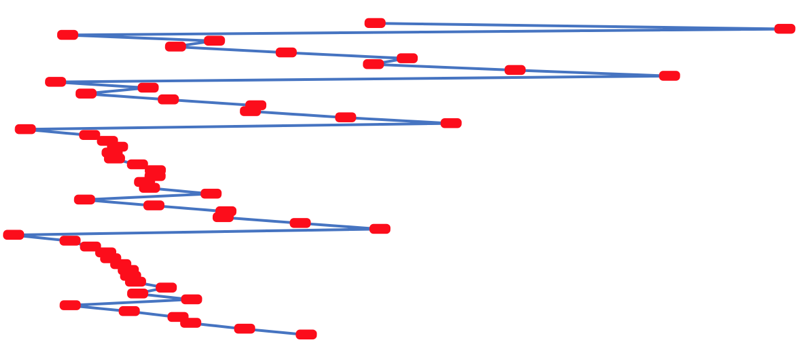
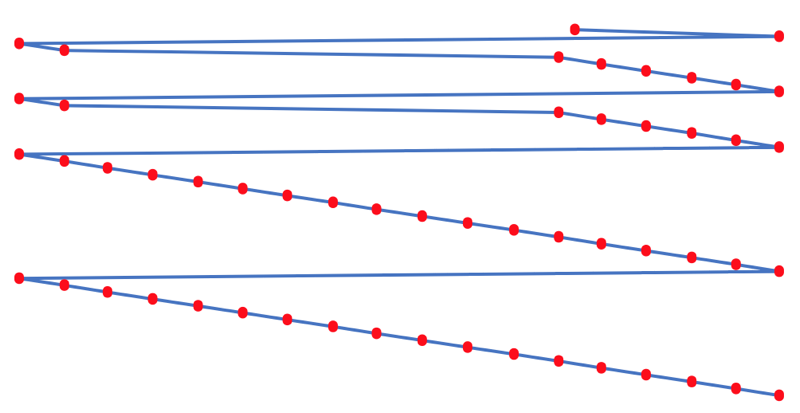
Note that a similar logic can be applied to atomic radius and electronegativity data.
However, there are issues about the measurement of atomic radius, because atoms are 'soft at their edges', and gas phase atomic radius is not precisely defined. And, electronegativity is a derived parameter.
By Mark Leach
| Year: 2012 | PT id = 925, Type = misc data |
Atoms, Orbitals & The Periodic Table
One of several animations and explanations/realisations of quantum physics from Data-Burger, scientific advisor: J. Bobroff, with the support of: Univ. Paris Sud, SFP, Triangle de la Physique, PALM, Sciences à l'Ecole, ICAM-I2CAM.
Mark Leach writes:
"What I particularly like about this video is that it shows the quantum fuzziness of the atoms. This explains/shows how and why induced-dipole/induced-dipole (London force) interactions occur, an important class of van der Waals interaction. At any moment, the electron distribution is not perfectly spherical, which means that there is an instantaneous dipole on the atom. This instantaneous dipole is able to induce a dipole on an adjacent atom, with the effect that the two atoms are attracted when they touch. It is as if atoms are 'sticky' like Velcro.
"This effect explains why the Group 18 noble gas elements are able to form liquids and solids [not He] at low temperatures, and why non-polar molecules, such as P4, S8 and hydrocarbons are able to condense."
| Year: 2019 | PT id = 958, Type = formulation data |
Leach's Empirical Periodic Table
The common/conventional/standard 'medium form' periodic table is based on the 1945 Seaborg formulation, and it is interesting to explore where this formulation – and its 1939 predecessor – come from. (Interestingly, the Werner formulation of 1905 is not cited as a source and there are no other similar formulations in the (this) Periodic Table Database.)
However, it is possible to get to the common/conventional/standard periodic table directly from two readily available data-sets: (1) first ionisation energy of the gas phase atoms, and (2) atomic radius.
The procedure involved plotting the data, rotating 90°, squeezing vertically and smoothing. The points need a little tidying up, and then they can be mapped directly onto the Seaborg formulation periodic table.
The only element which does no obviously 'line-up' with the periodic table is hydrogen, but many modern periodic tables have H floating as it is not obvious if it should be considered to be a Group 1 alkali metal or a Group 17 halogen.
Note:
There are advantages and disadvantages to each data set. The 1st ionisation energy data from NIST is known with up to seven significant figures of precision, but the data jumps about at times due to the presence of the s & p-orbitals, which appears to make the data a little noisy. (Actually, this 'noise' is embedded information about the electronic structure of the atoms.) The atomic radius gives smoother data, but as gas phase atoms do not have hard edges calculated (Clementi 1967) rather than experimental values, must be used.
| Year: 1814 | PT id = 1034, Type = formulation |
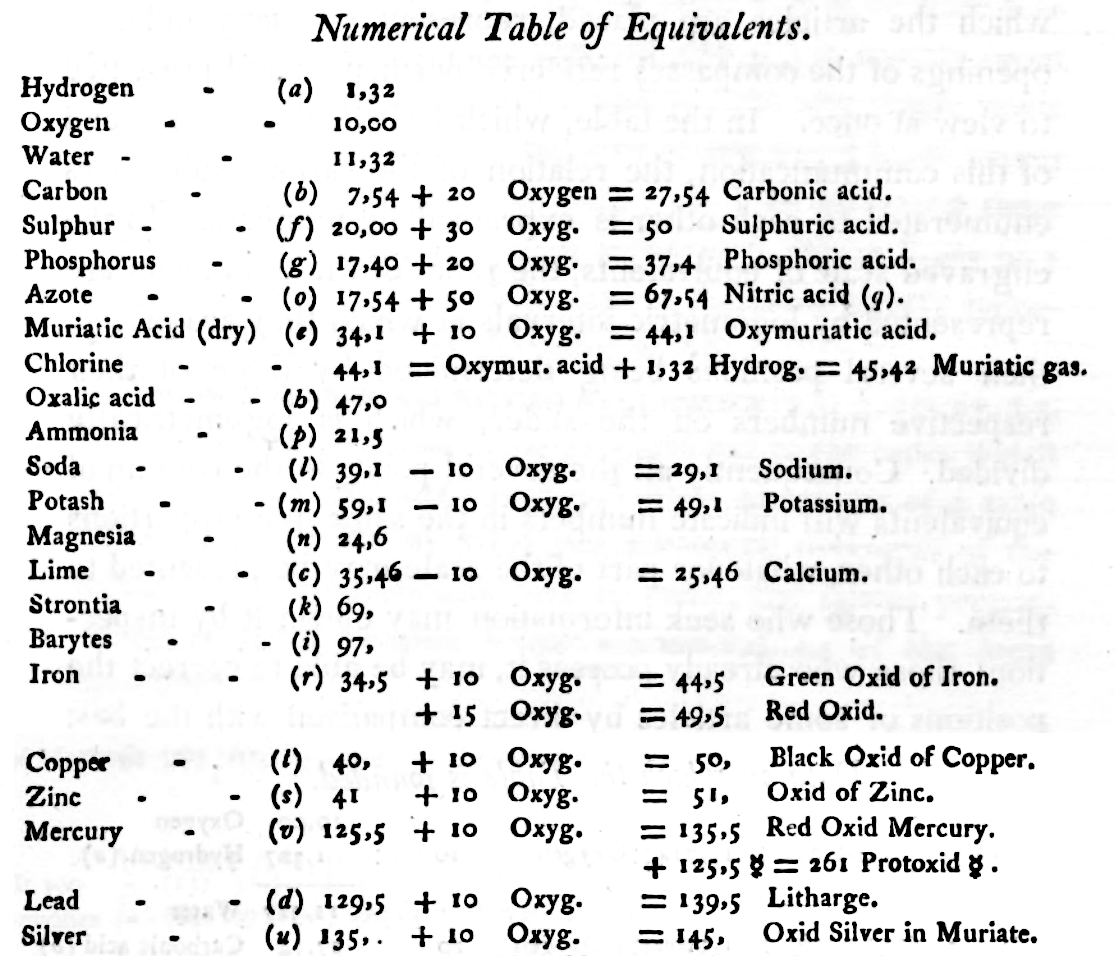
Wollaston's Physical Slide Rule of Chemical Equivalents
From the Science Museum in the UK collection, the Wollaston slide rule of chemical equivalents:
"Three sliding scales of chemical equivalents, all with same manuscripts marks, published by W Cary, devised by W H Wollaston, a leading chemist and natural philosopher during the early 19th century.
"Positioning the slider with the weight of the substance set against it will show you the weights of other substances which will react with it. This fundamental ordering based on measurement paved the way for the periodic table of the elements"
Wollaston uses a decimal scale in which oxygen is defined as having an atomic weight (relative atomic mass) of 10.00 rather than the modern value of 15.999.
Read more here and here, and an entry concerning chemical slide rules:

Mark Leach writes:
"I have edited the image above, setting the scale to zero:"

| Year: 1813 | PT id = 1044, Type = formulation element weight |
Wollaston's Synoptic Scale of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S., or from here.
It is apparent that chemistry the years 1810 to 1850 was largely concerned with discovering the whole number stoichiometric ratios of atoms in chemical compounds.
Wollaston writes in the text above:
"It is impossible in several instances, where only two combinations of the same ingredients are known, to discover which of the compounds is to be regarded as consisting of a pair of single atoms, and since the decision of these questions is purely theoretical, and by no means necessary to the formation of a table adapted to most practical purposes, I have not been desirous of warping my numbers according to an atomic theory, but have endeavored to make practical convenience my sole guide, and have considered the doctrine of simple multiples, on which that of atoms is founded, merely as a valuable assistant in determining, by simple division, the amount of those quantities that are liable to such definite deviations from the original law of Richter."
"Mr. Dalton in his atomic views of chemical combination appears not to have taken much pains to ascertain the actual prevalence of that law of multiple proportions by which the atomic theory is best supported [however] it is in fact to Mr. Dalton that we are indebted for the first correct observation of such an instance of a simple multiple in the union of nitrous gas with oxygen."
"[I have] computed a series of supposed atoms, I [have] assumed oxygen as the decimal unit of my scale [ie. oxygen = 10], in order to facilitate the estimation of those numerous combinations which it forms with other bodies. Though the present table of Equivalents, I have taken care to make oxygen equally prominent on account of the important part it performs in determining the affinities of bodies by the different proportions in which it is united to them.."
Mark Leach writes:
"When Wollaston's equivalent weights are converted from O = 10.00 to the modern value of O = 15.999, the atomic weight values can be seen to be astonishingly accurate.
"However, the language of the article is quite difficult as the meaning of many of the terms is unclear (to me, at least). For example, in modern usage adding 'ia' to a metal implies the oxide: 'magnesia' is magnesium oxide, MgO. I am not clear if this historical usage is consistent. 'Azote' is nitrogen and 'muriatic acid (dry)' is hydrogen chloride gas. I have only analyses/re-calculated the elements and a couple of common/obvious compounds:"
| Wollaston's data | Scaled to O = 15.999 | Modern Values | % error | |
| H (as H2) | 1.32 | 2.112 | 2.016 | 5% |
| O | 10.00 | 15.999 | 15.999 | ref. value |
| H2O | 11.32 | 18.111 | 18.015 | 1% |
| C | 7.74 | 12.383 | 12.011 | 3% |
| S | 20.00 | 31.998 | 32.060 | 0% |
| P | 17.40 | 27.838 | 30.974 | -11% |
| N (as N2) | 17.54 | 28.062 | 28.014 | 0% |
| Cl (as Cl2) | 44.10 | 70.556 | 70.900 | 0% |
| Fe | 34.50 | 55.197 | 55.845 | -1% |
| Cu | 40.00 | 63.996 | 63.546 | 1% |
| Zn | 41.00 | 65.596 | 65.380 | 0% |
| Hg | 125.50 | 200.787 | 200.590 | 0% |
| Pb | 129.50 | 207.187 | 207.980 | 0% |
| Ag | 135.00 | 215.987 | 107.870 | 50% |
- The elements hydrogen, nitrogen (azote) and chlorine have clearly been measured as the diatomic molecules, even if this was unknown to Wollaston in 1813.
- Phosphorus is out by 11%... [fair enough].
- Only silver is clearly wrong, but it is out by 50% so it looks like a simple stoichiometry error: Perhaps the oxide was assumed to be AgO was instead of the correct Ag2O.
Interestingly, Wollaston's analysis is far better than Daubeny's 1831 data seen in Oxford.
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
| Year: 1858 | PT id = 1047, Type = formulation review element weight structure |
Cannizzaro's Letter or Sunto
Letter of Professor Stanislao Cannizzaro to Professor S. De Luca: Sunto di un corso di filosofia chimica (Sketch of a Course of Chemical Philosophy) given in the Royal University of Genoa, Il Nuovo Cimento, vol. vii. (1858), pp. 321-366.

Many thanks to Carmen Giunta, Professor of Chemistry Emeritus, Le Moyne College who provided the information about, and link to, Cannizzaro's Letter. See a list of other classic chemistry papers.
Read the full letter/paper, in English translation, here. (The Italian version is here.)
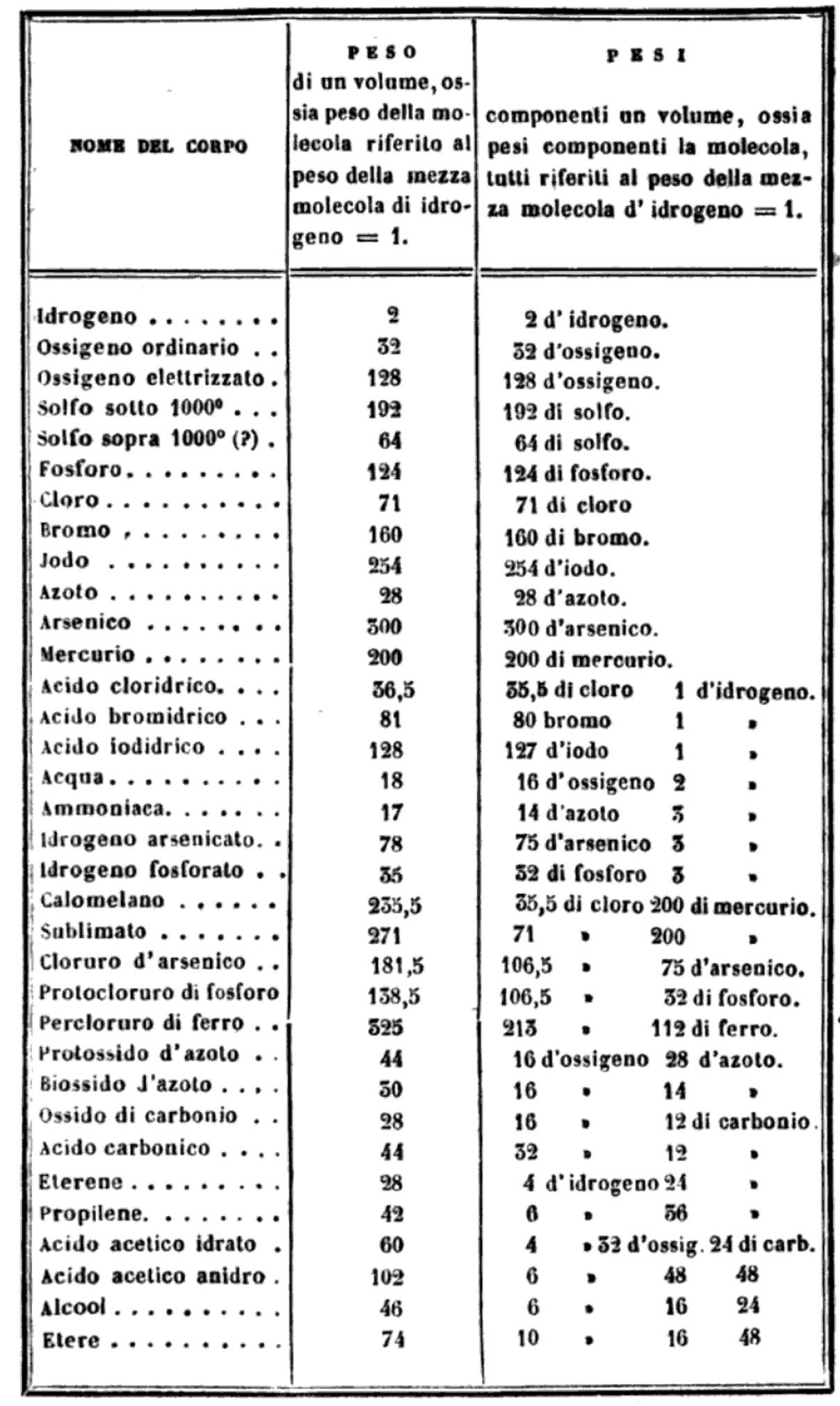
"I believe that the progress of science made in these last years has confirmed the hypothesis of Avogadro, of Ampère, and of Dumas on the similar constitution of substances in the gaseous state; that is, that equal volumes of these substances, whether simple or compound, contain an equal number of molecules: not however an equal number of atoms, since the molecules of the different substances, or those of the same substance in its different states, may contain a different number of atoms, whether of the same or of diverse nature."
From the Science History of Science Institute:
"In 1858 Cannizzaro outlined a course in theoretical chemistry for students at the University of Genoa,where he had to teach without benefit of a laboratory. He used the hypothesis of a fellow Italian, Amedeo Avogadro, who had died just two years earlier, as a pathway out of the confusion rampant among chemists about atomic weights and the fundamental structure of chemical compounds."
Mark Leach writes:
"Before a periodic table of the chemical elements – which orders the elements by atomic weight and then groups them by property – could be developed it was necessary to know the atomic weight values. However, to deduce the atomic weights was a problem as it was necessary to know the ratios of how the elements combined, the stoichiometry.
"Tables of atomic weight data by Dalton (1808), Wollaston (1813), Daubeny (1831) and Kopp & Will (1858) show progress, but the 1858 Cannizzaro letter was the first where the atomic weight data is more or less both complete and accurate, thus removing stiochiometric errors.
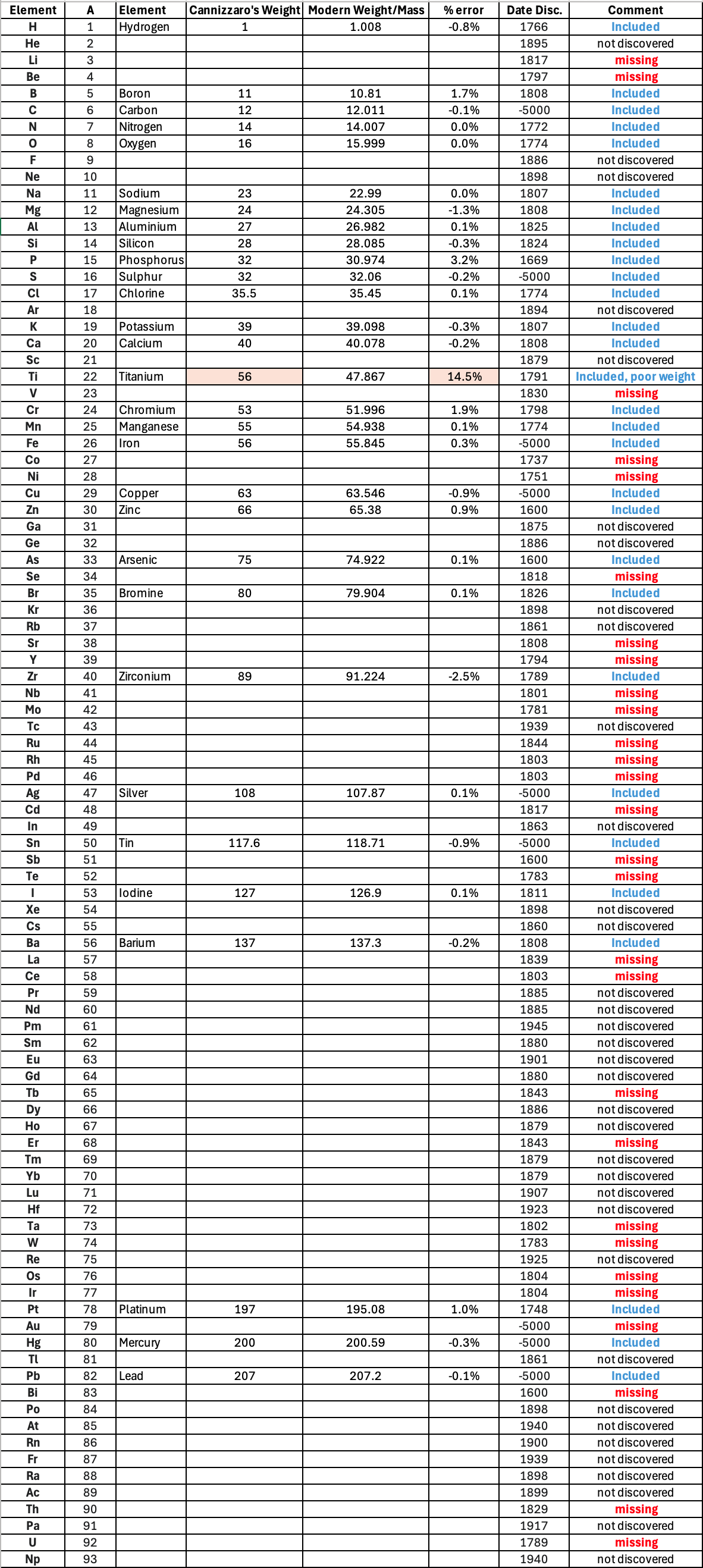
"I have extracted the element atomic weight data from the paper, and given the % error with respect to modern atomic weight/mass data. Only titanium is significantly out! It is clear that Cannizzaron knew that hydrogen, nitrogen, oxygen, chlorine, bromine & iodine existed as diatomic molecules."
| Element | Symbol | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H | 1 | 1.008 | -0.8% |
| Boron | B | 11 | 10.81 | 1.7% |
| Carbon | C | 12 | 12.011 | -0.1% |
| Nitrogen | N | 14 | 14.007 | 0.0% |
| Oxygen | O | 16 | 15.999 | 0.0% |
| Sodium | Na | 23 | 22.99 | 0.0% |
| Magnesium | Mg | 24 | 24.305 | -1.3% |
| Aluminium | Al | 27 | 26.982 | 0.1% |
| Silicon | Si | 28 | 28.085 | -0.3% |
| Sulphur | S | 32 | 32.06 | -0.2% |
| Phosphorus | P | 32 | 30.974 | 3.2% |
| Chlorine | Cl | 35.5 | 35.45 | 0.1% |
| Potassium | K | 39 | 39.098 | -0.3% |
| Calcium | Ca | 40 | 40.078 | -0.2% |
| Chromium | Cr | 53 | 51.996 | 1.9% |
| Manganese | Mn | 55 | 54.938 | 0.1% |
| Iron | Fe | 56 | 55.845 | 0.3% |
| Titanium | Ti | 56 | 47.867 | 14.5% |
| Copper | Cu | 63 | 63.546 | -0.9% |
| Zinc | Zn | 66 | 65.38 | 0.9% |
| Arsenic | As | 75 | 74.922 | 0.1% |
| Bromine | Br | 80 | 79.904 | 0.1% |
| Zirconium | Zr | 89 | 91.224 | -2.5% |
| Silver | Ag | 108 | 107.87 | 0.1% |
| Tin | Sn | 117.6 | 118.71 | -0.9% |
| Iodine | I | 127 | 126.9 | 0.1% |
| Barium | Ba | 137 | 137.3 | -0.2% |
| Platinum | Pt | 197 | 195.08 | 1.0% |
| Mercury | Hg | 200 | 200.59 | -0.3% |
| Lead | Pb | 207 | 207.2 | -0.1% |
| Diatomic Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Hydrogen | H2 | 2 | 2.016 | -0.8% |
| Oxygen | O2 | 32 | 31.998 | 0.0% |
| Sulphur | S2 | 64 | 64.12 | -0.2% |
| Chlorine | Cl2 | 71 | 70.9 | 0.1% |
| Bromine | Br2 | 160 | 159.808 | 0.1% |
| Iodine | I2 | 254 | 253.8 | 0.1% |
| Molecule | Formula | Cannizzaro's Weight | Modern Weight/Mass | % error |
| Water | H2O | 18 | 18.015 | -0.1% |
| Hydrochloric Acid | HCl | 36.5 | 36.458 | 0.1% |
| Methane | CH4 | 16 | 16.043 | -0.3% |
| Hydrogen sulphide | H2S | 34 | 34.076 | -0.2% |
| Diethyl ether | CH3CH2OCH2CH3 | 74 | 74.123 | -0.2% |
| Carbon disulphide | CS2 | 76 | 76.131 | -0.2% |
| Chloroethane | CH3CH2Cl | 64.5 | 64.512 | 0.0% |
Below is a list of the elements showing which ones were included by Cannizzaro and which one were ommitted (because they had not been discovered) or are strangely missing. Odd ommissions (to the modern eye) include: Lithium, Beryllium, Cobalt, Nickel, Palladium, Tungsten and Gold.
| Year: 1939 | PT id = 1056, Type = formulation |
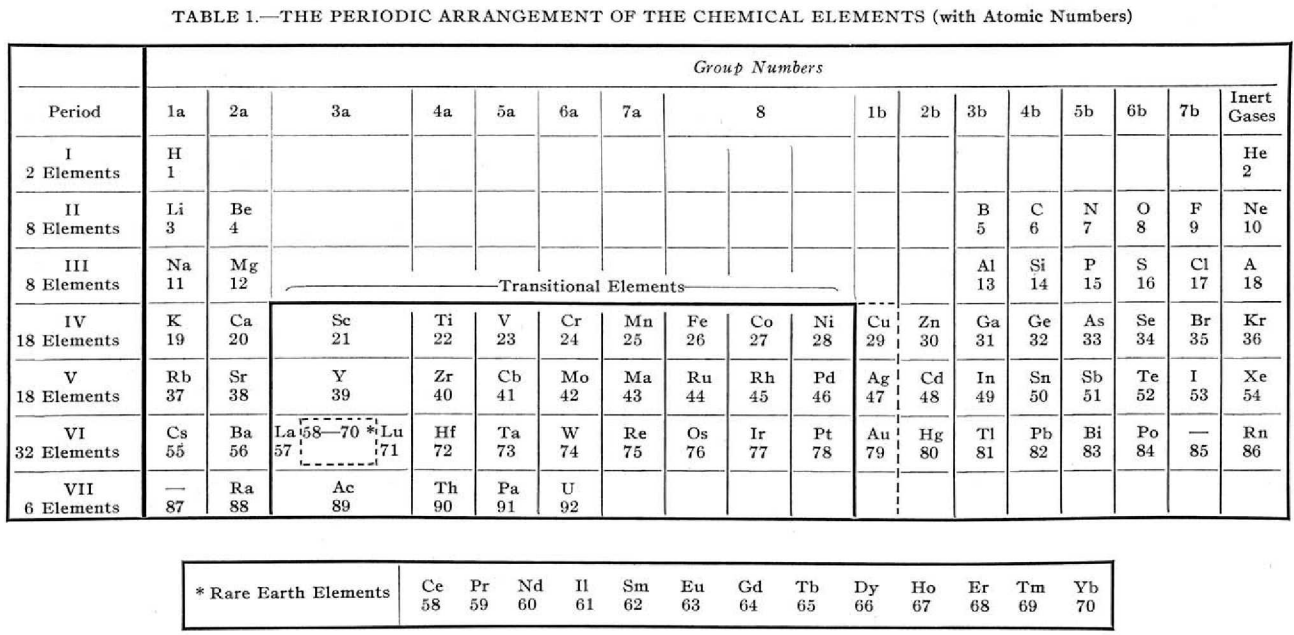
Foster's Periodic Arrangement
L.S. Foster, "Why not modernise the textbooks also? I. The periodic table", Journal of Chemical Education, vol. 16, no. 9, pp. 409–412, https://pubs.acs.org/doi/10.1021/ed016p409
Foster writes:
"The [above] modern periodic table is simply an orderly array of the elements with all unnecessary ornamentation omitted, has been found highly satisfactory for instructional purposes.
"The transitional elements, with two unfilled electron shells, are separated from the non-metallic elements.
"The rare-earth elements, defined as those with three incomplete electron shells, are shown to be those of atomic numbers 58 to 70, while La and Lu, which have only two incomplete electron shells are classified as transitional elements.
"Copper, silver, and gold act as transitional elements except when the state of oxidation is one."
René Vernon writes:
Foster couldn't show the coinage metals – with their full d10 complements – as transitional elements, but by adding a broken line around them he was showing they had the capacity to act as if they were.
I tried to work out how he distinguished La & Lu from Ce to Yb. Foster seems to be saying that La 5d1 6s2, has incomplete 5th and 6th (ie. 6p) shells.
Same for Lu 4f14 5d1 6s2 having incomplete 5th and 6th shells. Whereas, for example, Ce 4f1 5d1 6s2 has incomplete 4th, 5th and 6th shells. Presumably this was in the years before the fact that the 4f shell became full at Yb was widely appreciated. So, strictly speaking, group 3a should have read:
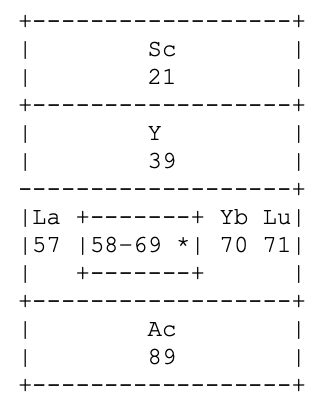
On the other hand, Yb3+ has an f13 configuration, so it does meet his three unfilled shells criterion. Had he known, he probably would've put a broken line around Yb to indicate its full f14 complement but that it normally acted as a rare earth, with an incomplete 4f shell; whereas neither La nor Lu have this capacity.
Good to see Foster put so much thought into organising his table, and his experience with using it for instructional purposes.
Van Spronsen does not mention Forster's table. Mazurs has a reference to Foster's table but lumps it in with the other medium-long tables, not appreciating its subtlety.
Mark Leach writes:
This formulation is very much like the XBL 769-10601, Periodic Table Before World War II used by Seaborg and the Manhattan project and is a precursor to the modern periodic table.
| Year: 2019 | PT id = 1133, Type = formulation data |
Vernon's Oxidation Number Periodic Table
René Vernon's periodic table showing oxidation number trends.
René writes:
- This table is based on two tables by Fernelius (1986), one of which is a portrait version of Bohr's 1922 table, and the second of which is a conventional table highlighting oxidation number trends in groups 4 to 10, and most of the p-block. Ref. Fernelius WC , 'Some reflections on the periodic table and its use', Journal of Chemical Education, vol. 63, no. 3, pp. 263–266 (1986).
- In my table, the transition metals are in groups 4 to 11. As per Mark's Leach's email: "...It's the 'incomplete d-subshell' that gives rise to properties such as: variable oxidation state, catalytic behaviour, d-orbital splitting and [thus] coloured ions/compounds."
- I've included oxidation number details for some elements. I've tried a different way of showing the composition of a bifurcated group 3, which is more in keeping with Bohr's table.
- The horizontal bar of the "T" is for Sc → Ti; the downward bar is for Y → Lu-Lr.
- Thus, Group 3 as Sc-Y-Lu-Lr could be called group 3T.
- La-Ac and Lu-Lr are duplicated in a greyed-out style to make it clearer where the lanthanides and actinides fit into the main body of the table.
- The inner transition metals are clearly delineated. Analogously to the transition metals they're all capable of forming ions with incomplete f sub-shells.
- The early actinides resemble the transition metals.

| Year: 2021 | PT id = 1179, Type = data review misc |
Chemogenesis In 700 Seconds
The Chemogenesis analysis – by Mark Leach – tells the story of how chemical structure and reactivity emerge from the periodic table of the elements. This video is a rapid dash through the story... which unfolds over many pages of the Chemogenesis Web Book.
| Year: 2021 | PT id = 1232, Type = formulation review |
The Periodic Table: Is it Perfect, is it Fractured or is it Broken?
A video from Mark Leach, who writes:
The periodic table is an icon of science. Indeed, all chemical matter is made from periodic table stuff. The periodic table of the elements is often presented as being:
- With 118 elements the periodic table is now complete
- The periodic table is perfectly described (fully explained) by the application of four quantum numbers with the and some simple rules
- Chemical structure & reactivity can be deduced from the periodicity of the Groups & Periods
However, the chemistry of the chemical elements is actually a little more complicated than this. So, where & why does the predictability 'break'?
| Year: 1888 | PT id = 1267, Type = formulation spiral |
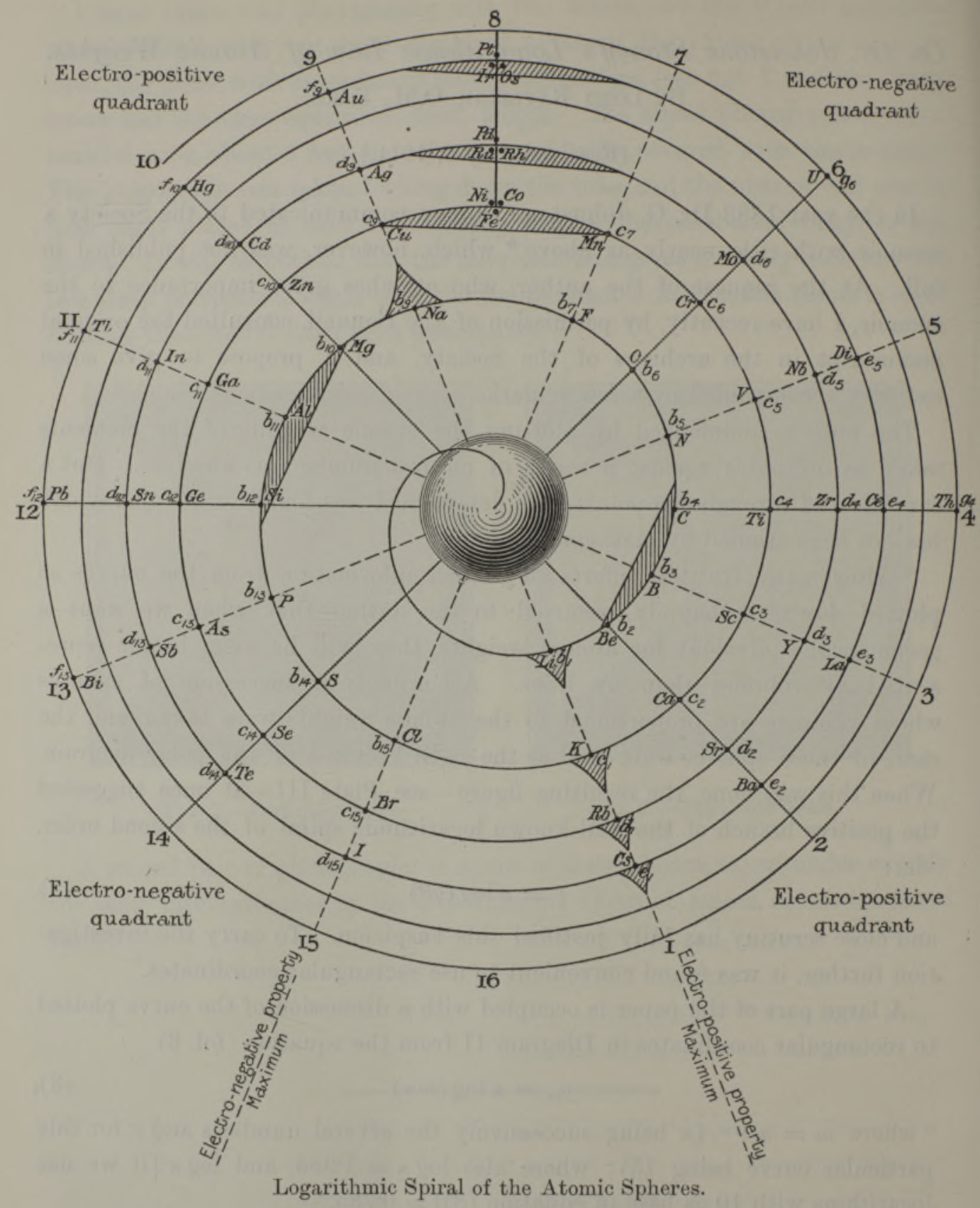
Stoney's Spiral Periodic Table
In the Proceedings of the Royal Society of London, Series A, Containing Papers of a Mathematical and Physical Character, Volume 85, Issue 580, Aug 1911, p. 472, there is an article On Dr. Johnstone Stoney's Logarithmic Law of Atomic Weights, by Lord Rayleigh (who co-discovered argon in 1894), who writes :
"In the year 1888, Dr. G. Johnstone Stoney communicated to the Society a memoir with title nearly as above, which, however, was not published in full. At the request of the author, who attaches great importance to the memoir, I have recently, by permission of the Council, consulted the original manuscript in the archives of the Society, and I propose to give some extracts, accompanied by a few remarks. The author commenced by plotting the atomic weights of the elements taken as ordinates against a series of natural numbers as abscissæ. But a curve traced through the points thus determined was found to be 'one which has not been studied by mathematicians.
"This sudden transition may have some connection with the fact that no elements have been found on sesqui-radius 16, although the investigation in § 3 shows that the values of m corresponding to the stations on sesqui-radius 16 cannot be dispensed with.
"The vacant places here pointed out are now occupied by the since discovered inert gases. The anticipation is certainly a remarkable one, and it goes far to justify the high claims made for the diagram, as representing in a telling form many of the leading facts of chemistry."
Comment from Mark Leach:
"Notice how the electronegative elements are positioned top right & bottom right and the electropositive elements top left & bottom right."
René Vernon writes:
"Stoney has another article in the September 1902 edition of the The London, Edinburgh and Dublin Philosophical Magazine and Journal of Science, called Law of Atomic Weights, pp. 411–415. At the back of the journal is an updated fold-out version of Stoney’s table, image attached.
- Ar, Kr and Xe fit on the spiral, and on spoke 16.
- Neon fits on the spiral but is instead on spoke 8.
- Helium is on spoke 18 but is not on the spiral.
- The circle in the middle represents H (p. 414).
"On the page after the updated spiral, there looks to be some printed content, but it is hidden by what looks to be a folded over page."
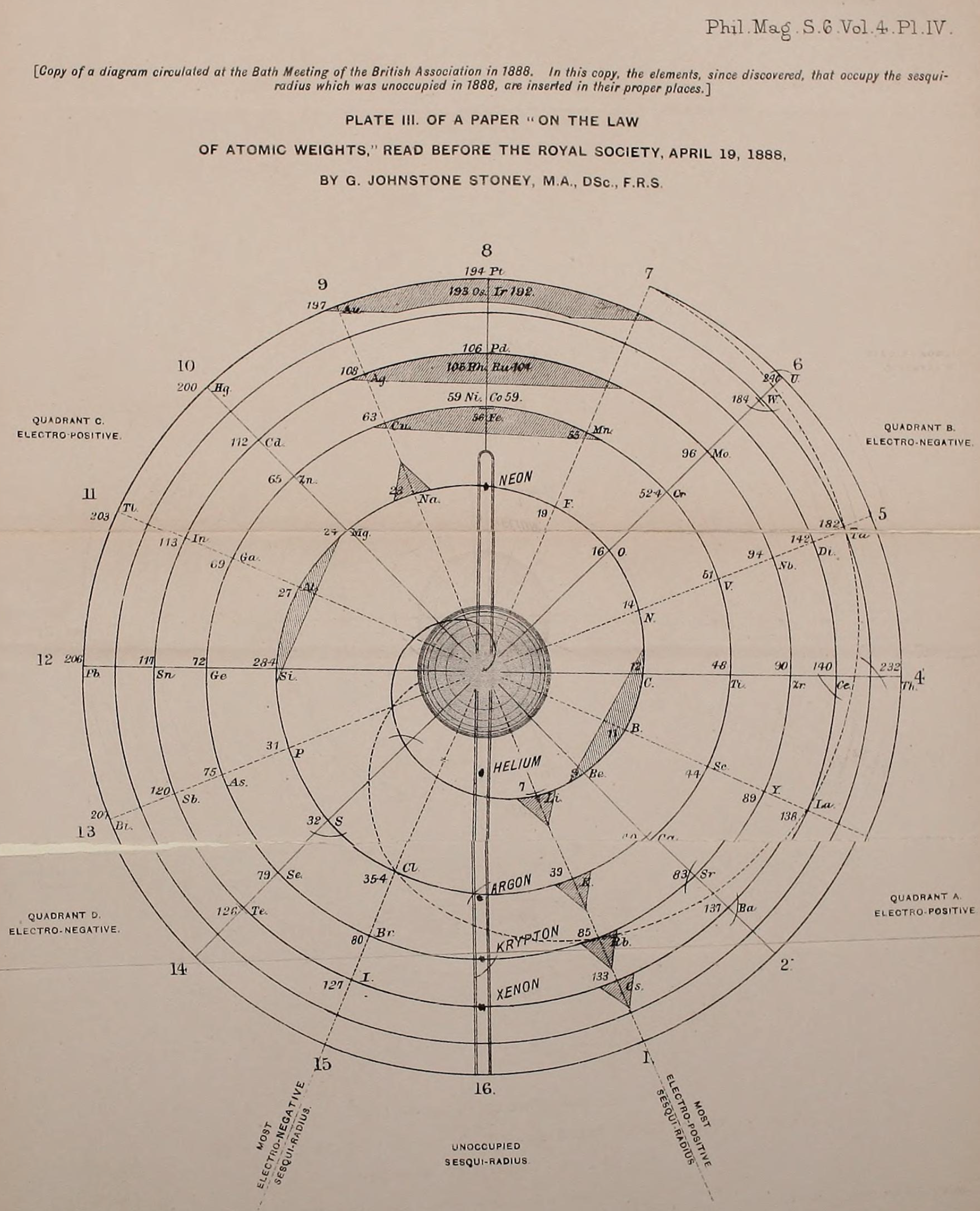
Thanks to René for the tip!
| Year: 1911 | PT id = 1296, Type = formulation |
Emerson's Periodic Table of Atomic Weights
Emerson BK, Helix chemica: A study of the periodic relations of the elements and their graphic representation, American Chemical Journal, vol. 45, pp. 160–210 (1911). The formulation below appears on page 173; a scanned pdf version of the paper can be viewed here.
René Vernon writes:
Emerson includes two elements before hydrogen: "E" (either the luminiferous ether or the electron) and "Coronium". There are also two elements between hydrogen and helium: "Nebulium" and "Protofluorine".
This is the first time I have seen a PT showing four extra elements and where they are supposed to fit.
After La, Emerson incorporates 13 lanthanides (Ce to Lu) as transition elements into his 7th period.
Emerson missed dysprosium, between Tb and Ho.
"A, B and C" at the bottom right are supposed to be 'halogen emanations'.
Mark Leach adds that Emerson's very odd Periodic Table of Atomic Weights does not actually show any atomic weights.
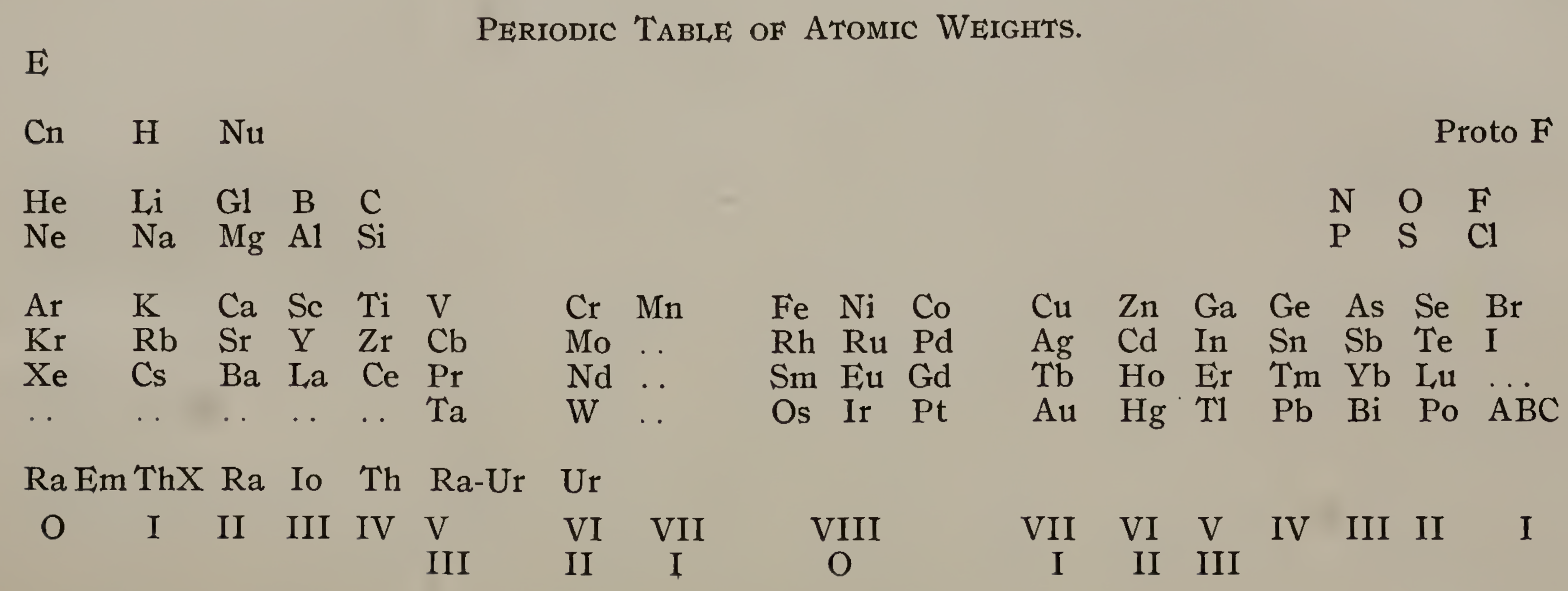
| Year: 2024 | PT id = 1299, Type = misc data |
Can I Lick It? Periodic Table
From Reddit, a "A cool guide to element lickability".
Mark Leach writes:
"I think I would colour calcium yellow as it bubbles hydrogen gas when added to water."
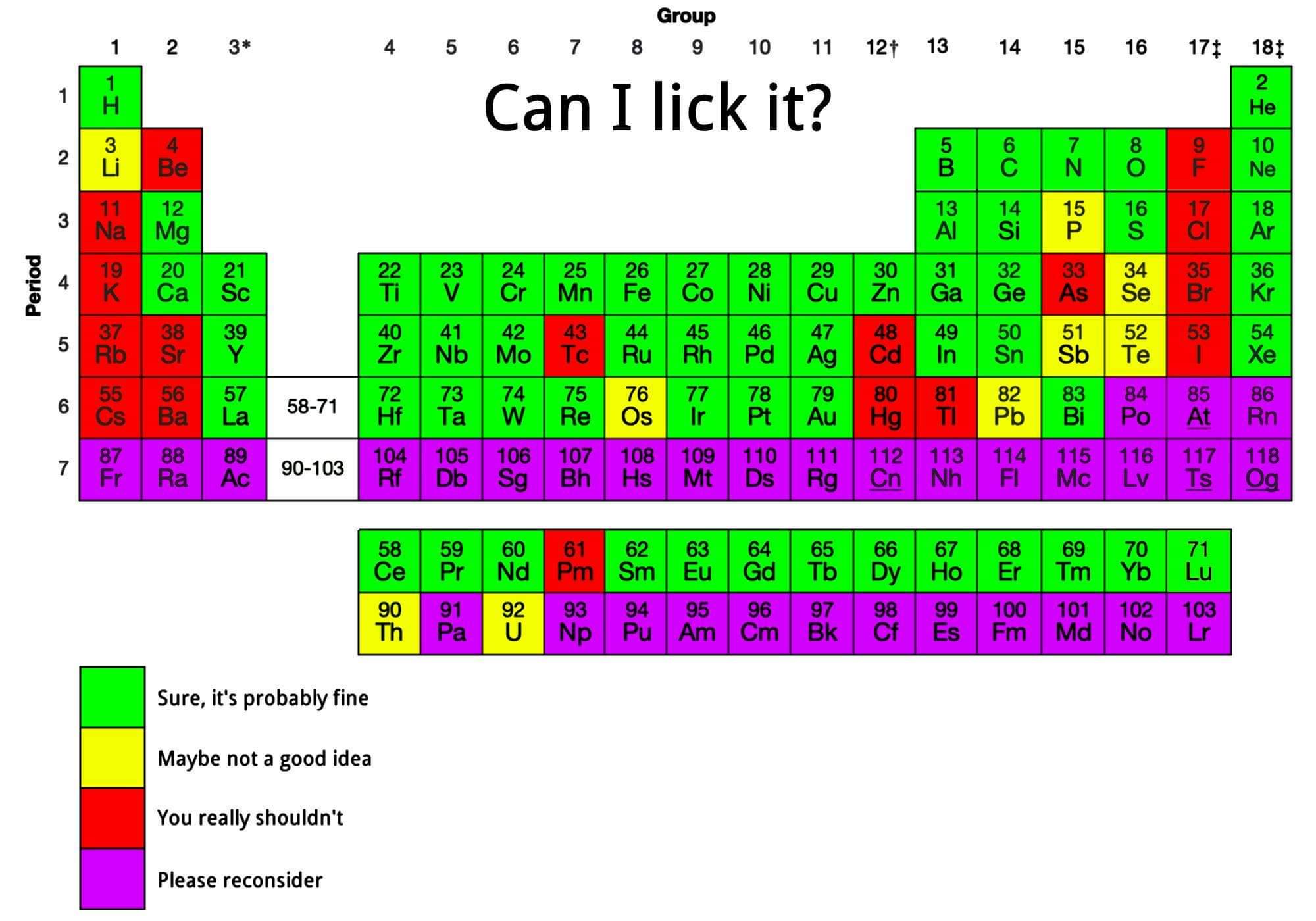
Thanks to René Vernon for the tip!
| Year: 1944 | PT id = 1305, Type = formulation spiral |
Emerson's Spiral Formulation
Emerson EI, 1944, A new spiral form of the periodic table, JChemEd., vol. 21, no. 3, pp. 111–115
René Vernon writes:
Emerson says that the elements in the A groups are called the representative elements because, as Eble states, they "include metals, nonmetals, inert elements, liquids, and gases." Eble RL, 1938, Atomic structure and the periodic table, JChemEd., vol. 15, p. 575
Note the inclusion in Emerson’s table of the neutron as element 0. Astonishingly, Emerson writes: "Element 0, possibly neutron [sic], is considered as a noble gas. Because of its probable chemical inertness and extreme density it might not be detected in a sizeable amount until some future scientist succeeds in sampling the center of the Earth." (p. 111)
(Mark Leach adds: The date is 1944 when the Manhattan Project was in full swing and nothing was being published about nuclear physics and/or neutron interactions. This idea may have come from some type of Popular Science story?)
Other features:
- The A and B groups are diametrically opposed in their positioning. "The electron structure of H either as H+ or H– finds a counterpart in the structure of element 0 or 2." (p. 113)
- The cell spaces for Be and Mg have been stretched on account of uncertainty as to whether they belong to group 2 or group 12. "The break between the periphery of the loops of the spiral along the spaces allotted to Mg and the transition metals of the fourth period serves to indicate that Be and Mg are not to be considered as a kind of prototype of these groups." (p. 111)
- "The C group is shown as a separate segment. If one were not concerned by plane representation the rare earths could be represented as a loop or bulge above the surface of the plane. One might imagine that this group of metals is a sort of hernia of nature that has been excised so as to maintain a flat surface." (p. 112–113)

| Year: 1976 | PT id = 1306, Type = data |
Atomic & Ionic Radii Periodic Table
A periodic table showing atomic and ionic radii from Chem Libre Texts. The text says: Figure 3.7. Source: Ionic radius data from R. D. Shannon, "Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides," Acta Crystallographica 32, no. 5 (1976): 751–767.
Mark Leach writes:
"I came across this periodic table while researching an exam question with a student: Which ion is larger: Na+ or F–?
"Note that N3–, O2–, F–, Ne, Na+, Mg2+ & Al3+ are all isoelectronic in that they all have the same electronic configeration: 1s2, 2s2, 2p6. The only property that changes is the nuclear charge, which increases from + 7 to + 11.
"Thus, N3– will be the largest and Al3+ the smallest in this set of isoelectronic species as the increasing nuclear charge pulls the electrons closer. Likewise from P3– to Sc3+ which are all 1s2, 2s2, 2p6, 3s2, 3p6."

| Year: 2018 | PT id = 1309, Type = review |
Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements
Robinson, Ann E., "Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements" (2018). Doctoral Dissertations. 1385.
https://doi.org/10.7275/12706048 https://scholarworks.umass.edu/dissertations_2/1385. Download and view the PDF.
See Ann E. Robinson's ORCID page.
Mark Leach writes:
"An excellent, comprehensive study that is full of details and references."

| Year: 2024 | PT id = 1312, Type = formulation |
Rectangular Array Model of Chemical Elements
Dr. Laith H . M . Al-ossmi, Rectangular Array Model of Chemical Elements https://www.academia.edu/119516234/Rectangular_Array_Model_of_Chemical_Elements
Abstract: This paper introduces a novel graphical representation of the periodic table, termed the "Rectangular Array Model". This innovative representation adheres to the group numbering recommendations provided by the International Union of Pure and Applied Chemistry (IUPAC). The rectangular array model arranges the known chemical elements (118 elements) according to their similarities in chemical properties, within 7 horizontal periods and 14 vertical groups. Hydrogen has been relocated to join oxygen within the elements of the first period and group 12. The rectangular array model reattached the position of 30 elements of lanthanides and actinidines into the table body. We respectfully encourage educators across various academic institutions to adopt this new periodic table format in their teaching practices.
Mark Leach the PT database administrator & curator writes:
WARNING: This formulation is NOT contiguous with respect to atomic number (surely, a prerequisite for any periodic table formulation?); it does NOT use IUPAC Group Numbers correctly, even though the abstract states that it does; it does not present electron or orbital structure and neither does it show periodicity in any way.

| Year: 1858 | PT id = 1348, Type = formulation element weight |
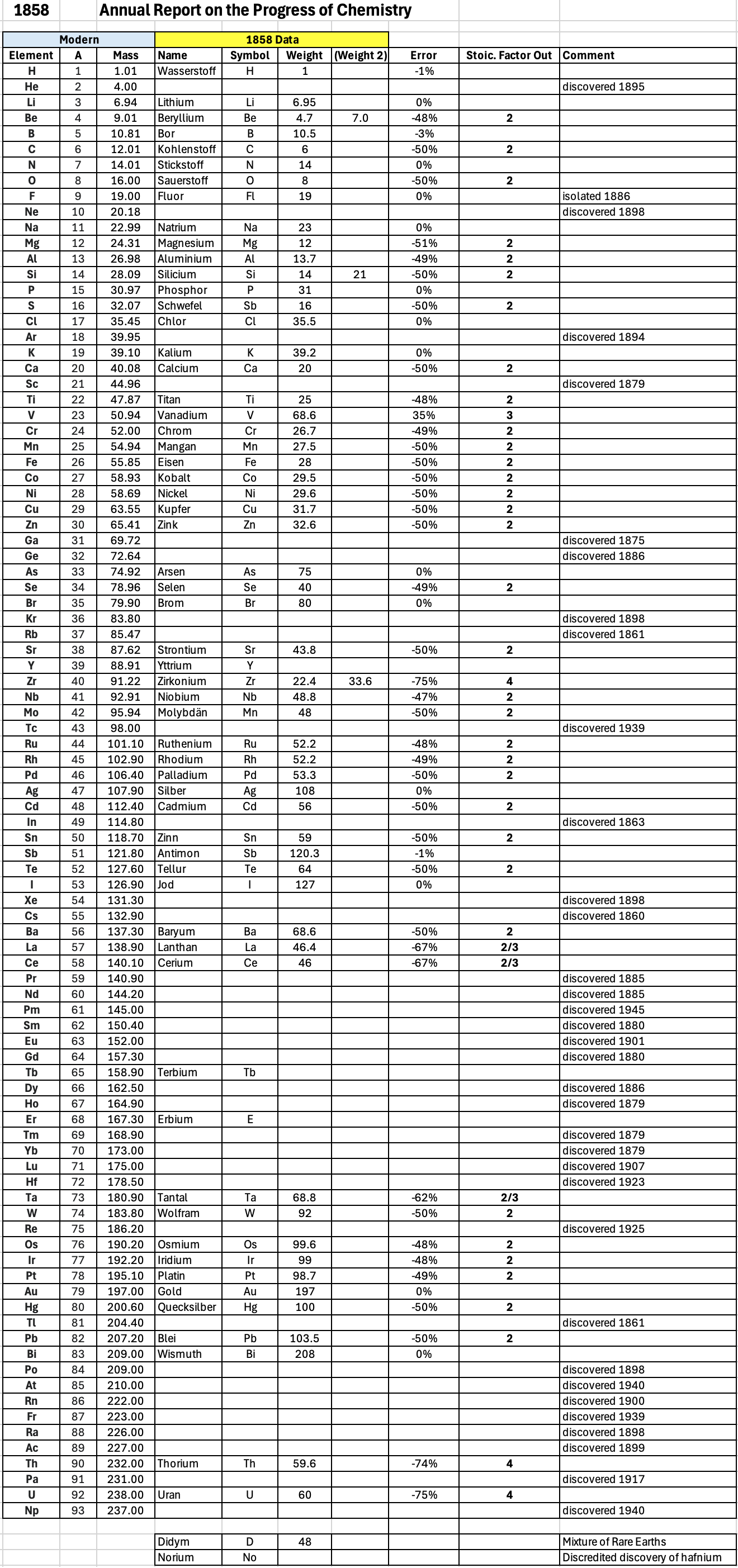
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1858
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1858 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6
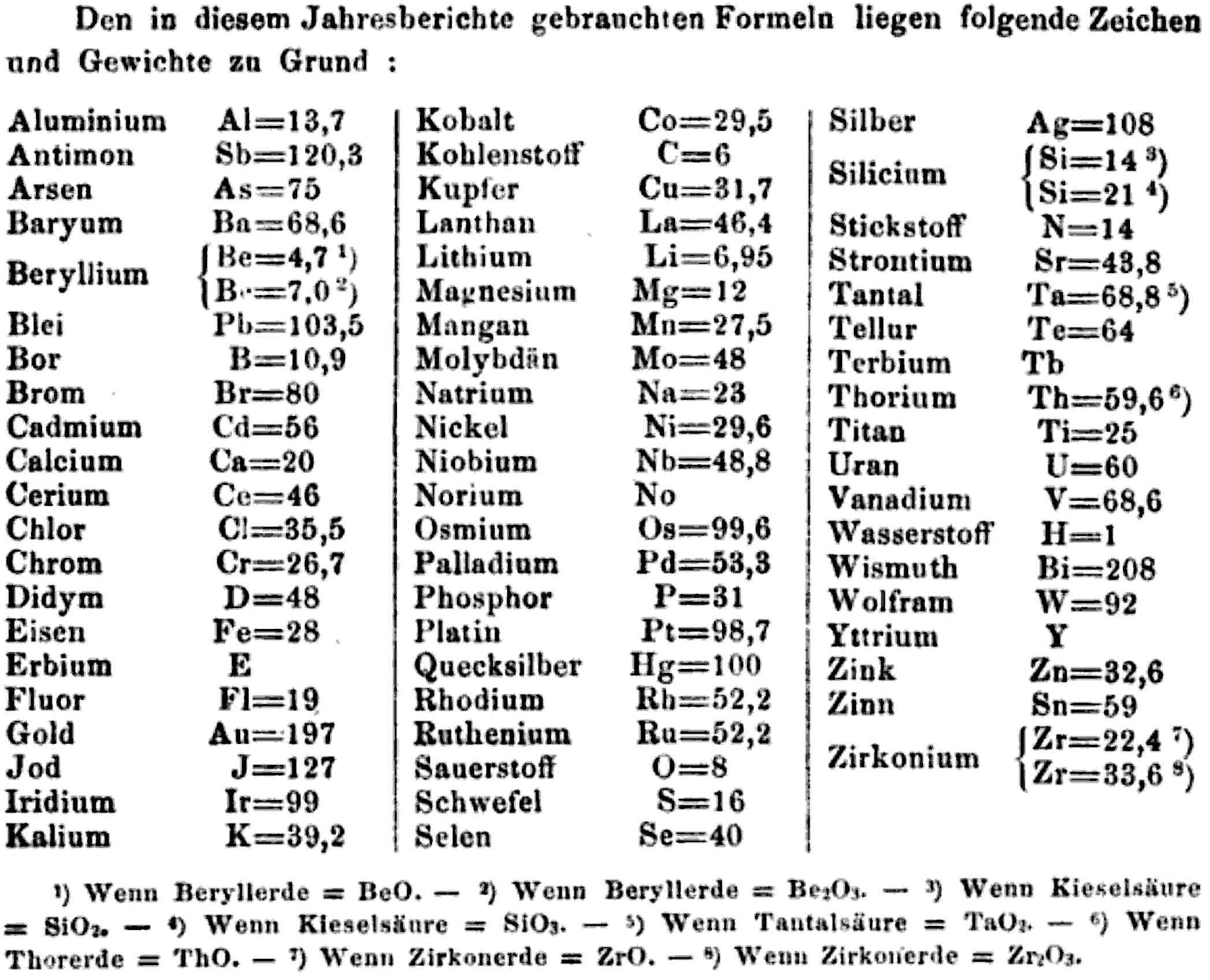
Thanks to René and Mario Rodriguez for the tip!
| Year: 1859 | PT id = 1349, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1859
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1859 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be is shown as 4.7 and 7.0
- Si is shown as 14 and 21
- Zr is shown as 22.4 and 33.6

Thanks to René and Mario Rodriguez for the tip!
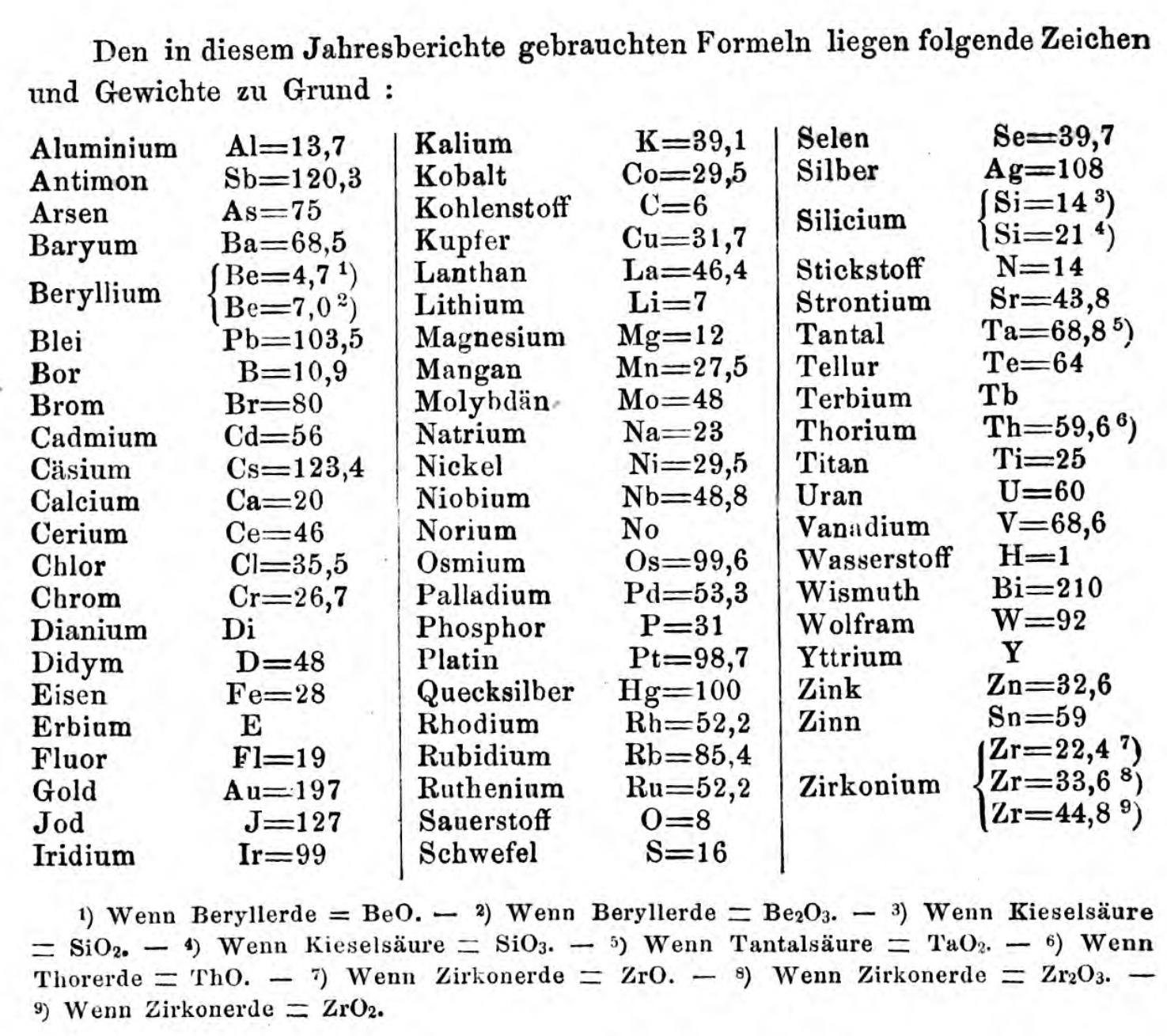
| Year: 1860 | PT id = 1350, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1860
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1860 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si =14 and 21
- Zr = 22.4, 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
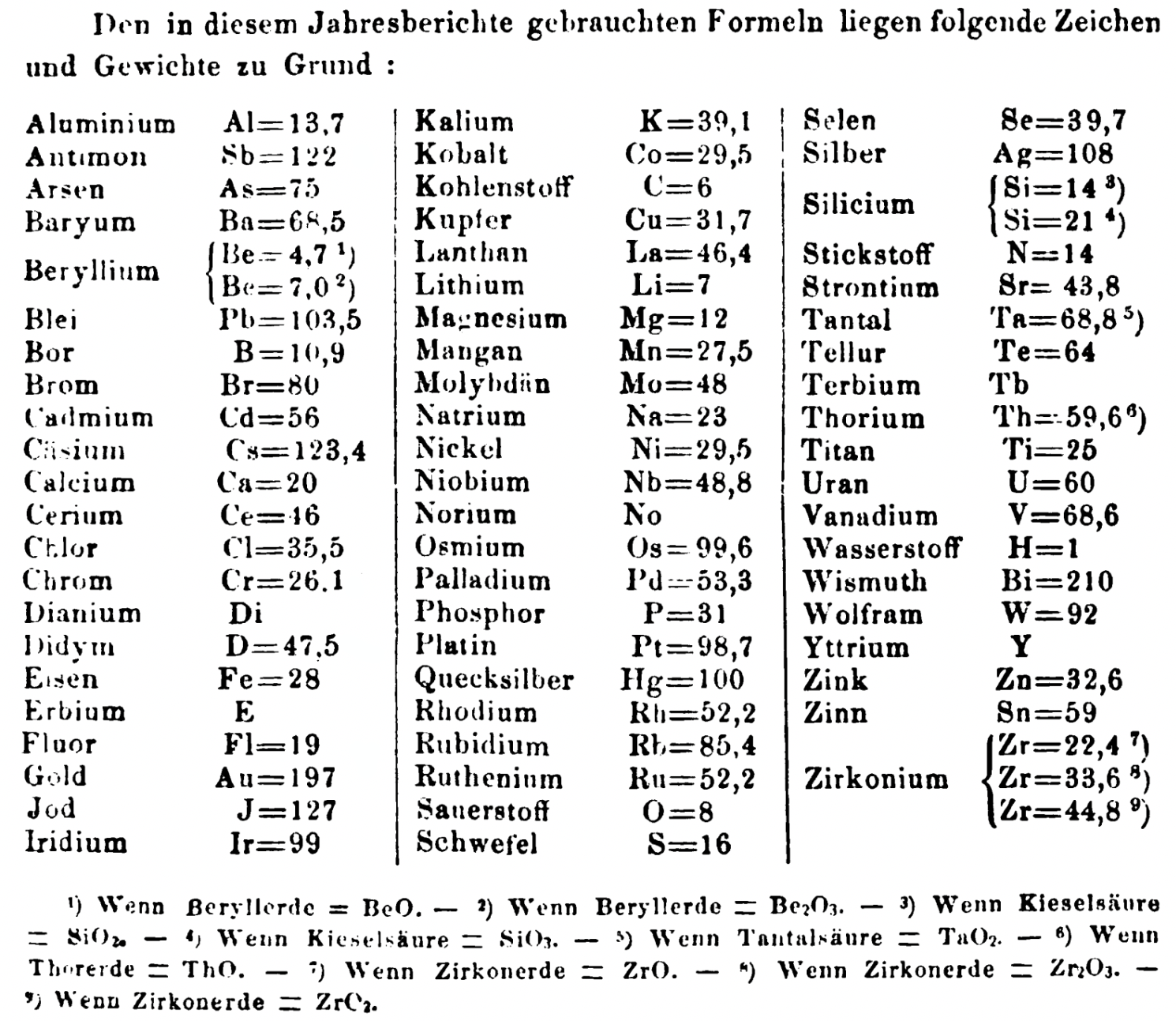
| Year: 1861 | PT id = 1351, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1861
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1861 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
| Year: 1862 | PT id = 1352, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1862
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1862 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- Si = 14 and 21
- Zr = 22.4 and 33.6 and 44.8

Thanks to René and Mario Rodriguez for the tip!
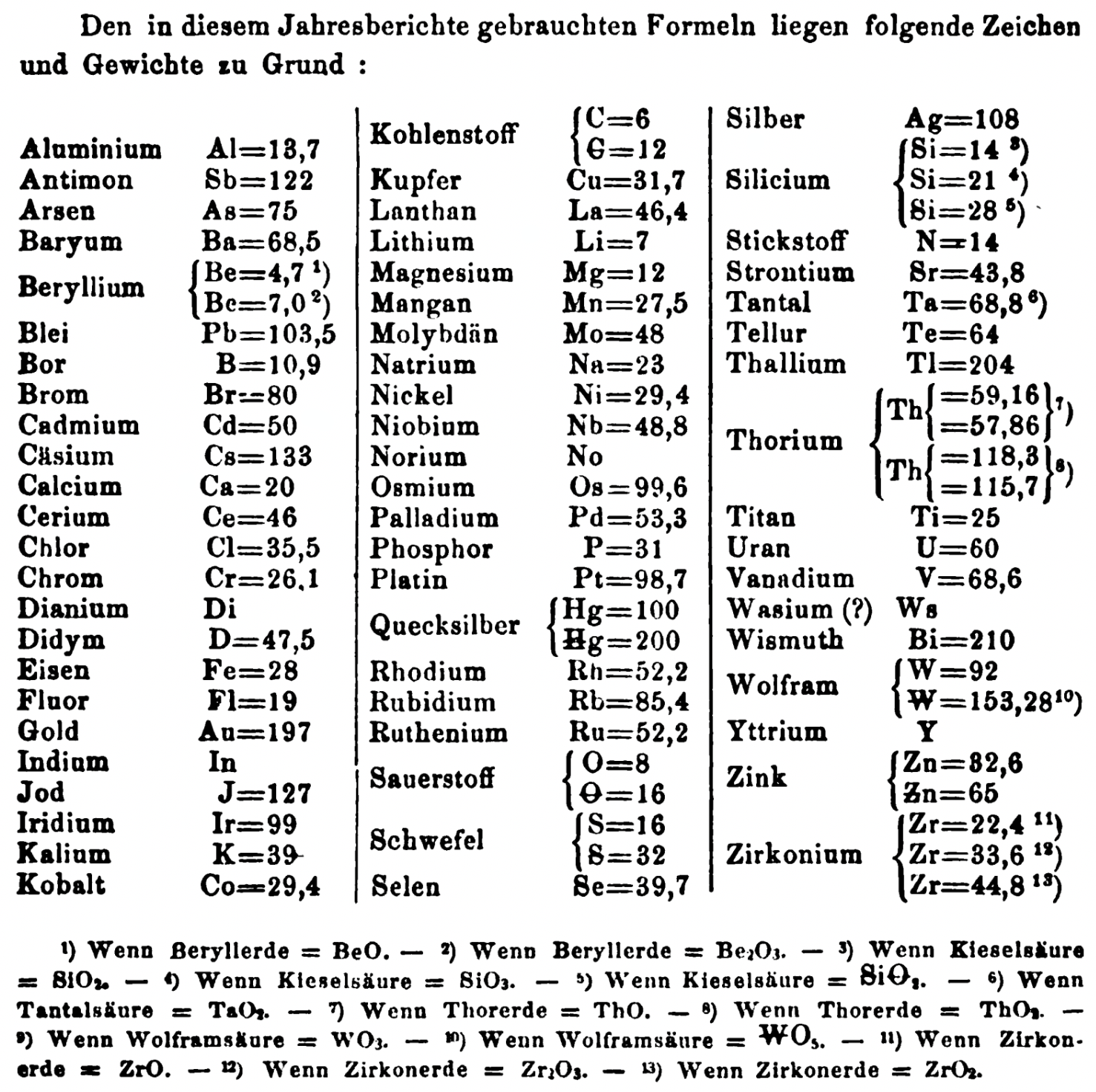
| Year: 1863 | PT id = 1353, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1863
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1863 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Be = 4.7 and 7.0
- C = 6 and 12
- Hg = 100 and 200
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Zr = 22.4 and 33.6 and 44.8
Thanks to René and Mario Rodriguez for the tip!
| Year: 1864 | PT id = 1354, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1864
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1864 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 59.16 and 57.86 and 118.3 and 115.7
- W = 92 and 153.28
- Zn = 32.6 and 65
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8
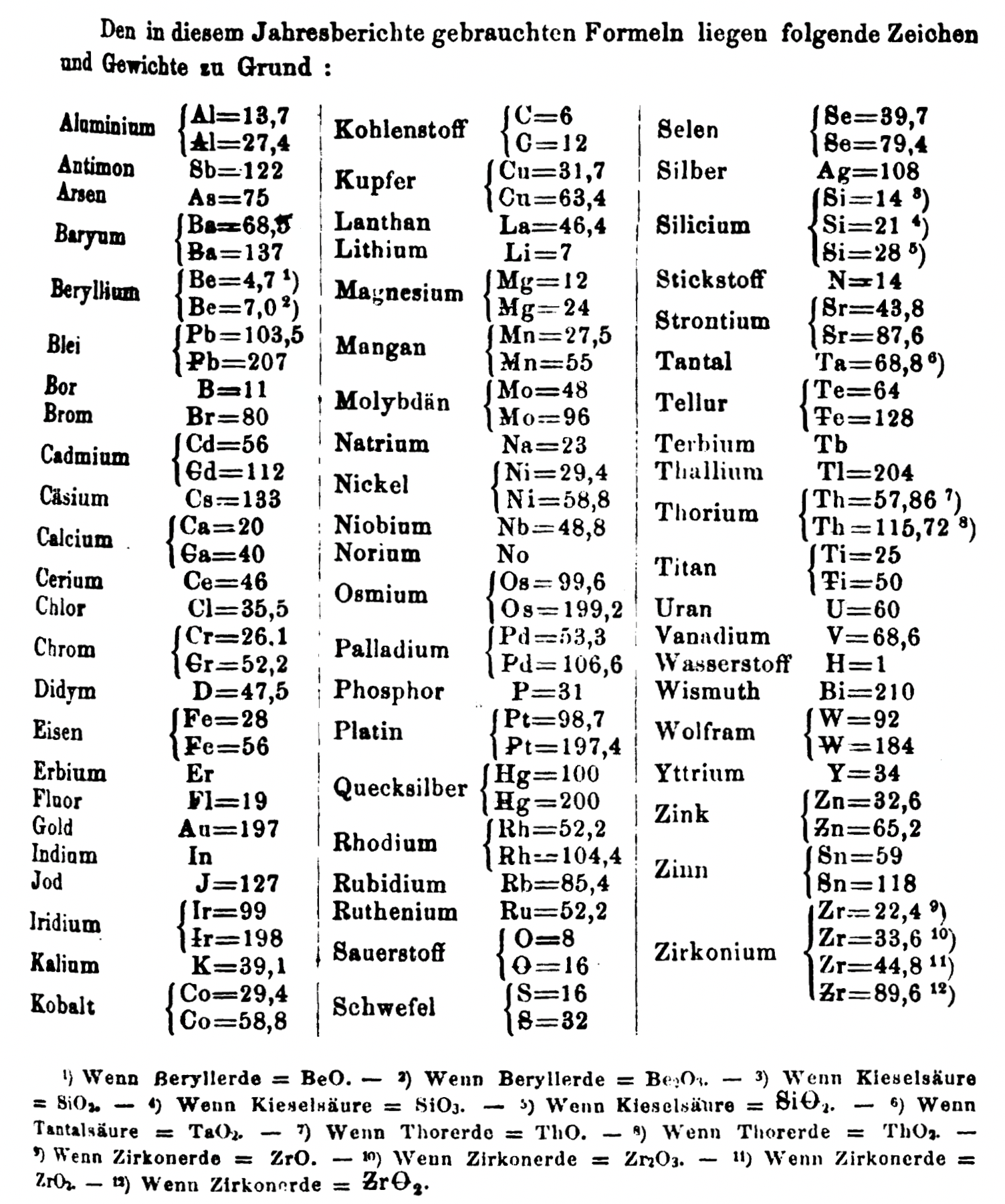
Thanks to René and Mario Rodriguez for the tip!
| Year: 1865 | PT id = 1355, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1865
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1865 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym D = 48 was actually a mixture of rare earth elements.
- Norium No is a discredited claim to be what is now known as hafnium.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
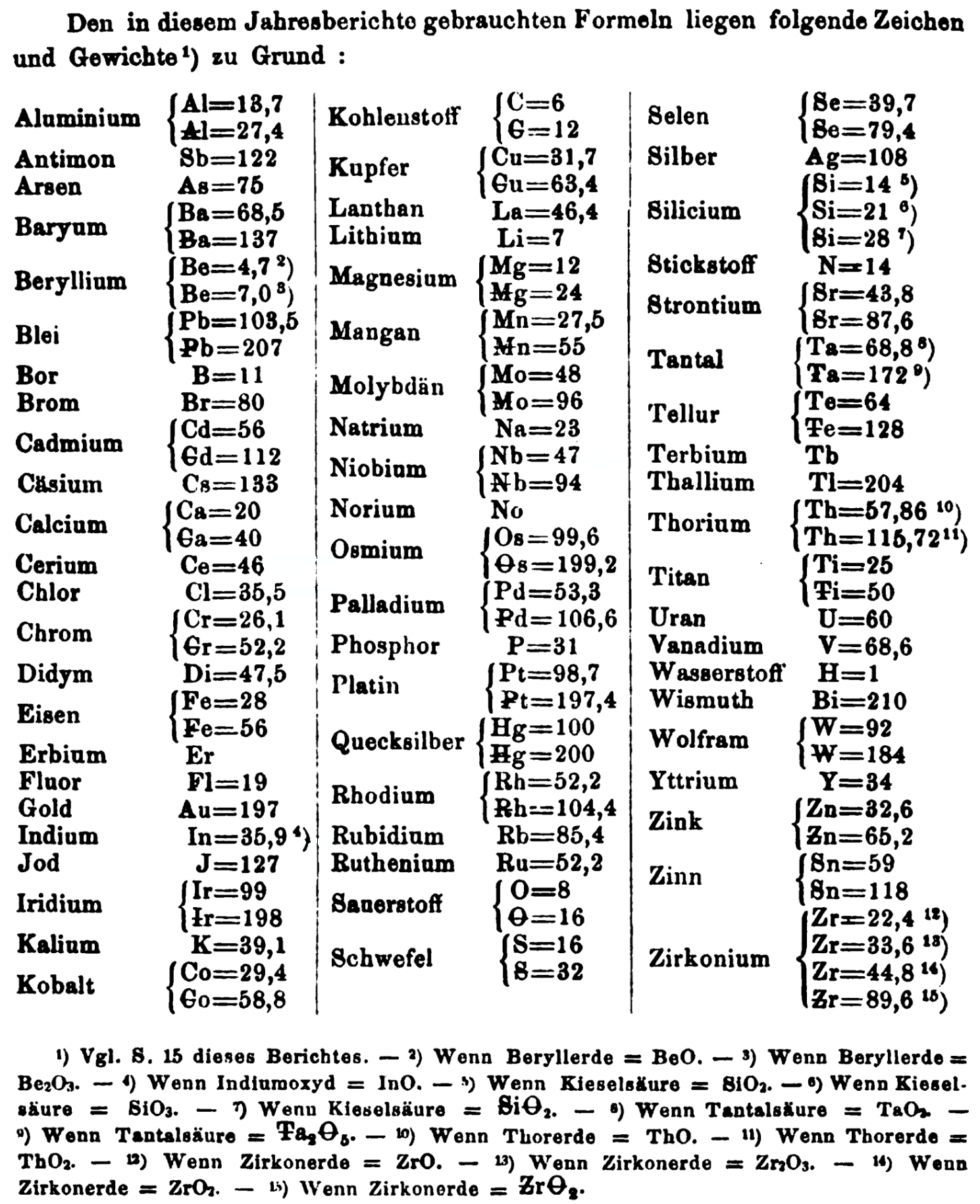
Thanks to René and Mario Rodriguez for the tip!
| Year: 1866 | PT id = 1356, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1866
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1866 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th = 57.86 and 115.72
- W = 92 and 184
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
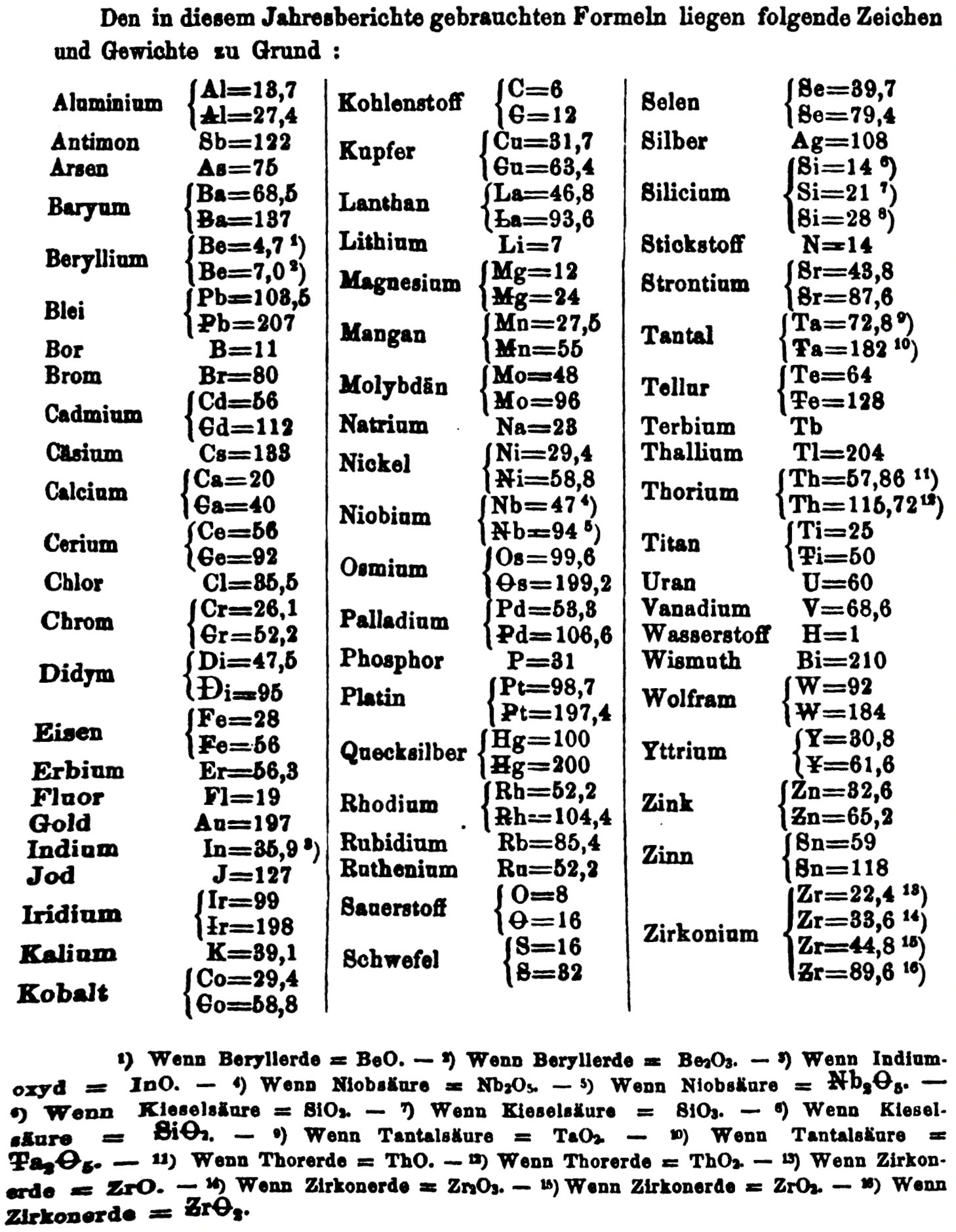
Thanks to René and Mario Rodriguez for the tip!
| Year: 1867 | PT id = 1357, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1867
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1867 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Nb = 47 and 94
- Ni = 29.4 and 58.8
- Os = 99.6 and 199.2
- Pd = 53.3 and 106.6
- Pt = 98.7 and 197.4
- Hg = 100 and 200
- Rh = 52.2 and 104.4
- Si = 14 and 21 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Ta = 68.8 and 172
- Te = 64 and 128
- Ti = 25 and 50
- Th =57.86 and 115.72
- W = 92 and 184
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 22.4 and 33.6 and 44.8 and 89.6
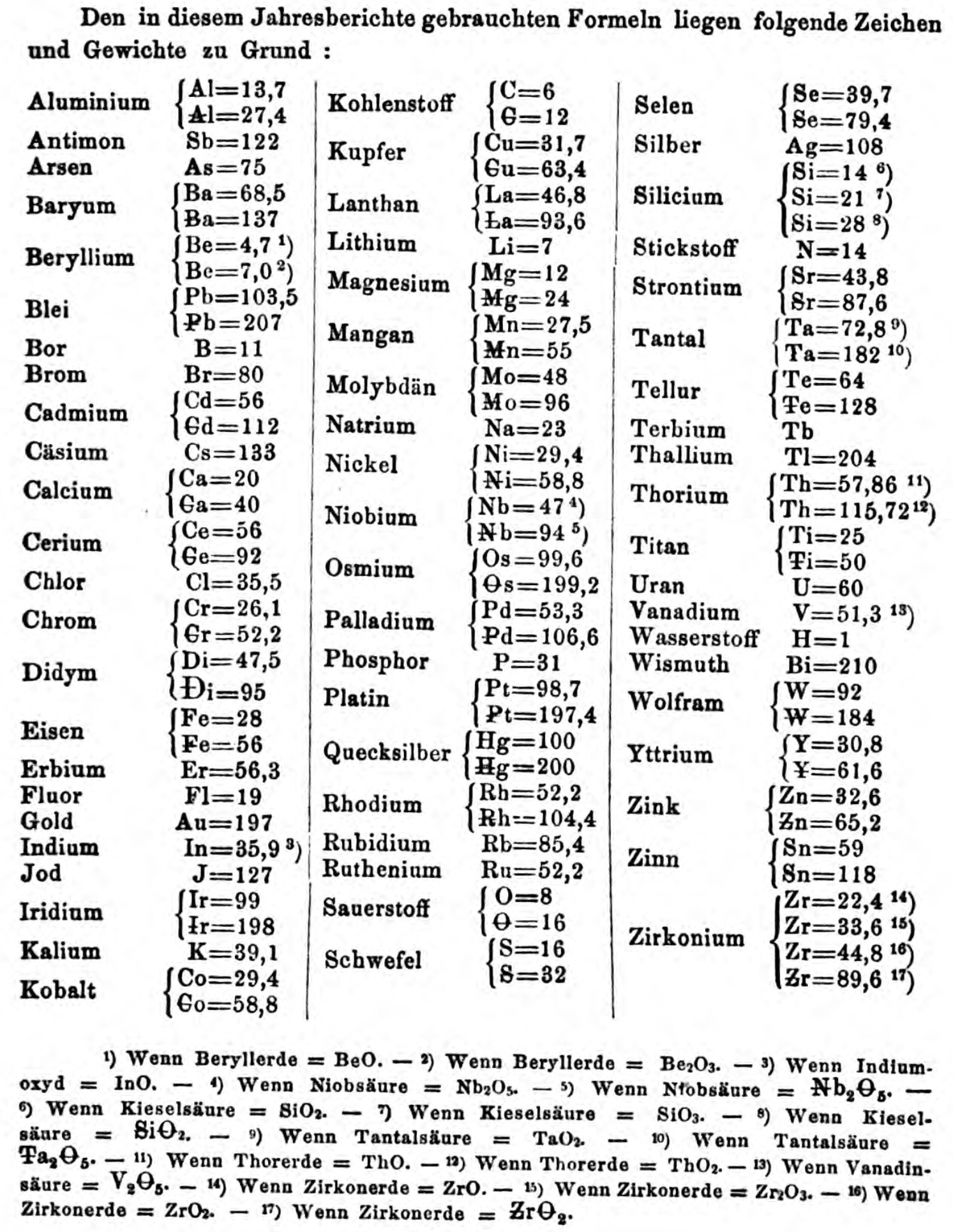
Thanks to René and Mario Rodriguez for the tip!
| Year: 1868 | PT id = 1358, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1868
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1868 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53.3 and 106.6
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.7 and 79.4
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.6 and 65.2
- Sn = 59 and 118
- Zr = 44.8 and 89.6
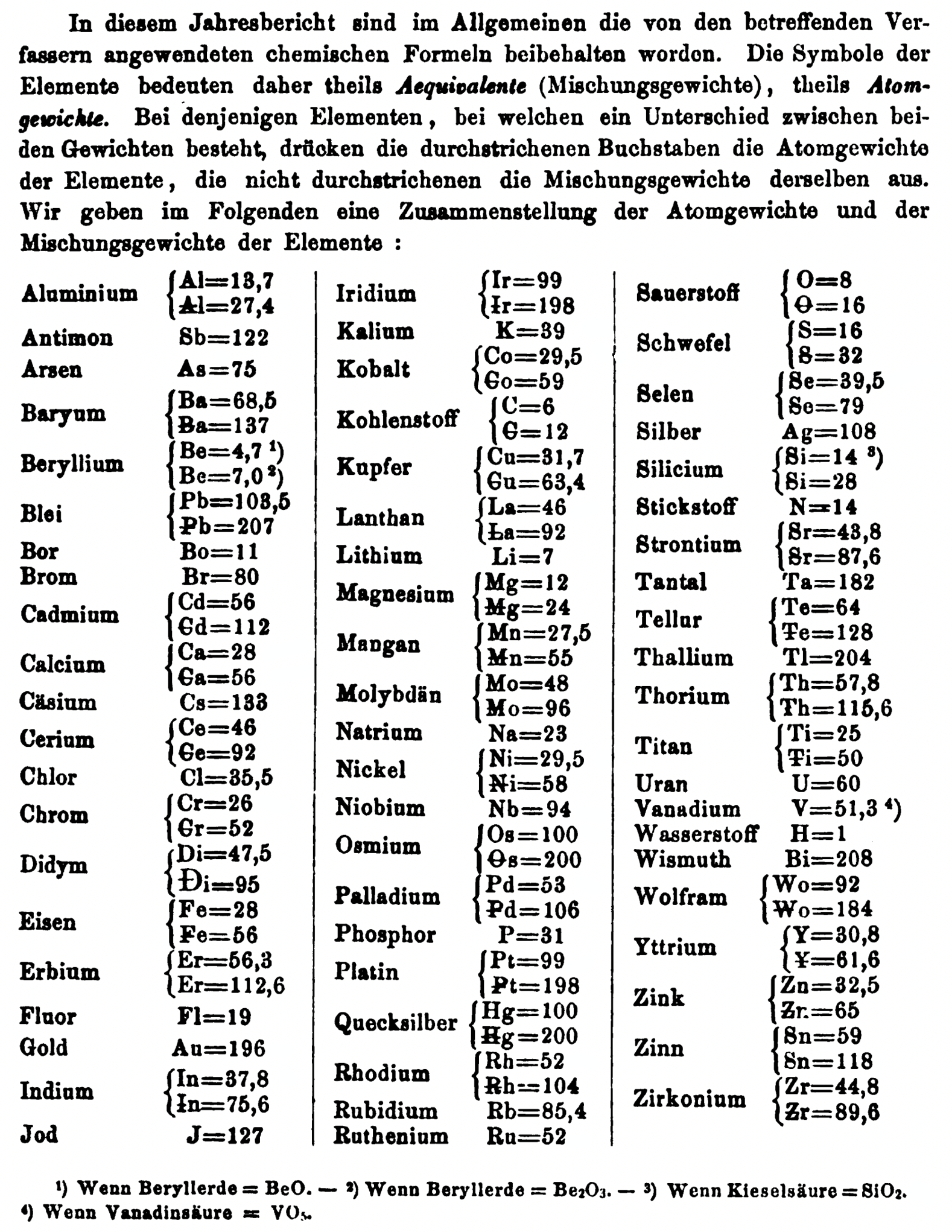
Thanks to René and Mario Rodriguez for the tip!
| Year: 1869 | PT id = 1359, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1869
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1869 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 44.8 and 89.6
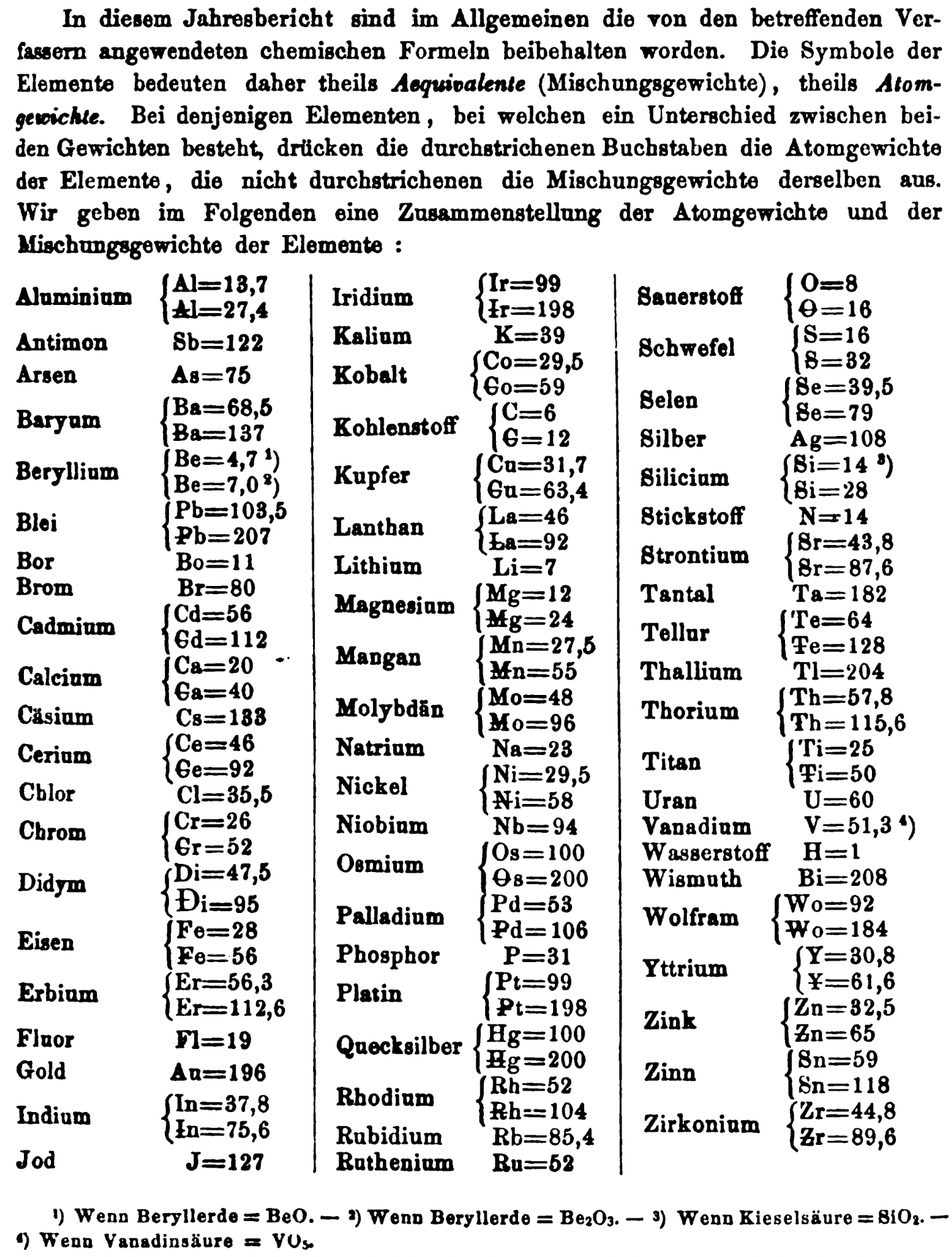
Thanks to René and Mario Rodriguez for the tip!
| Year: 1870 | PT id = 1360, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1870
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1870 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26.1 and 52.2
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.4 and 58.8
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th =57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
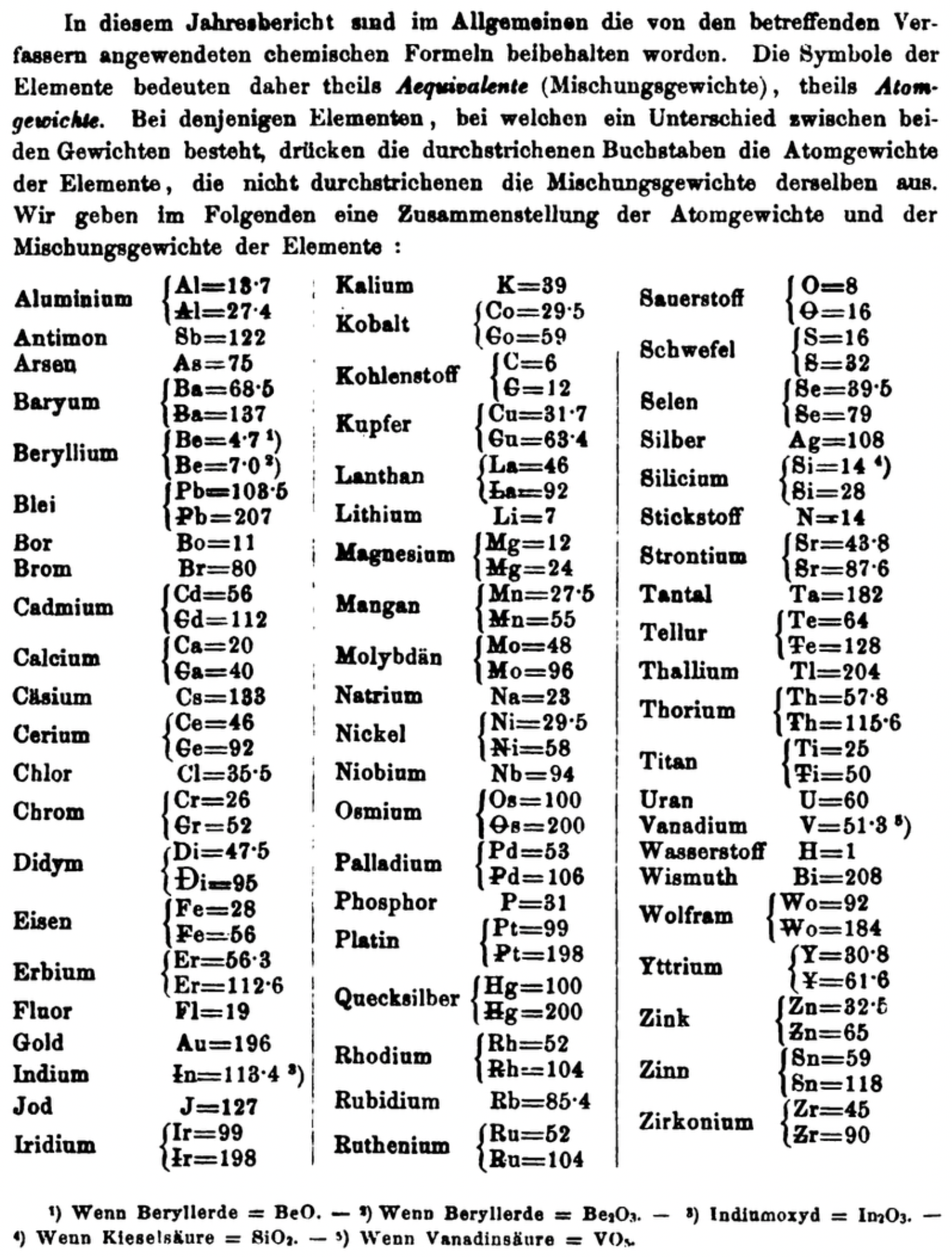
Thanks to René and Mario Rodriguez for the tip!
| Year: 1871 | PT id = 1361, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1871
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1871 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.4 and 58.8
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.86 and 115.72
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 30.8 and 61.6
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
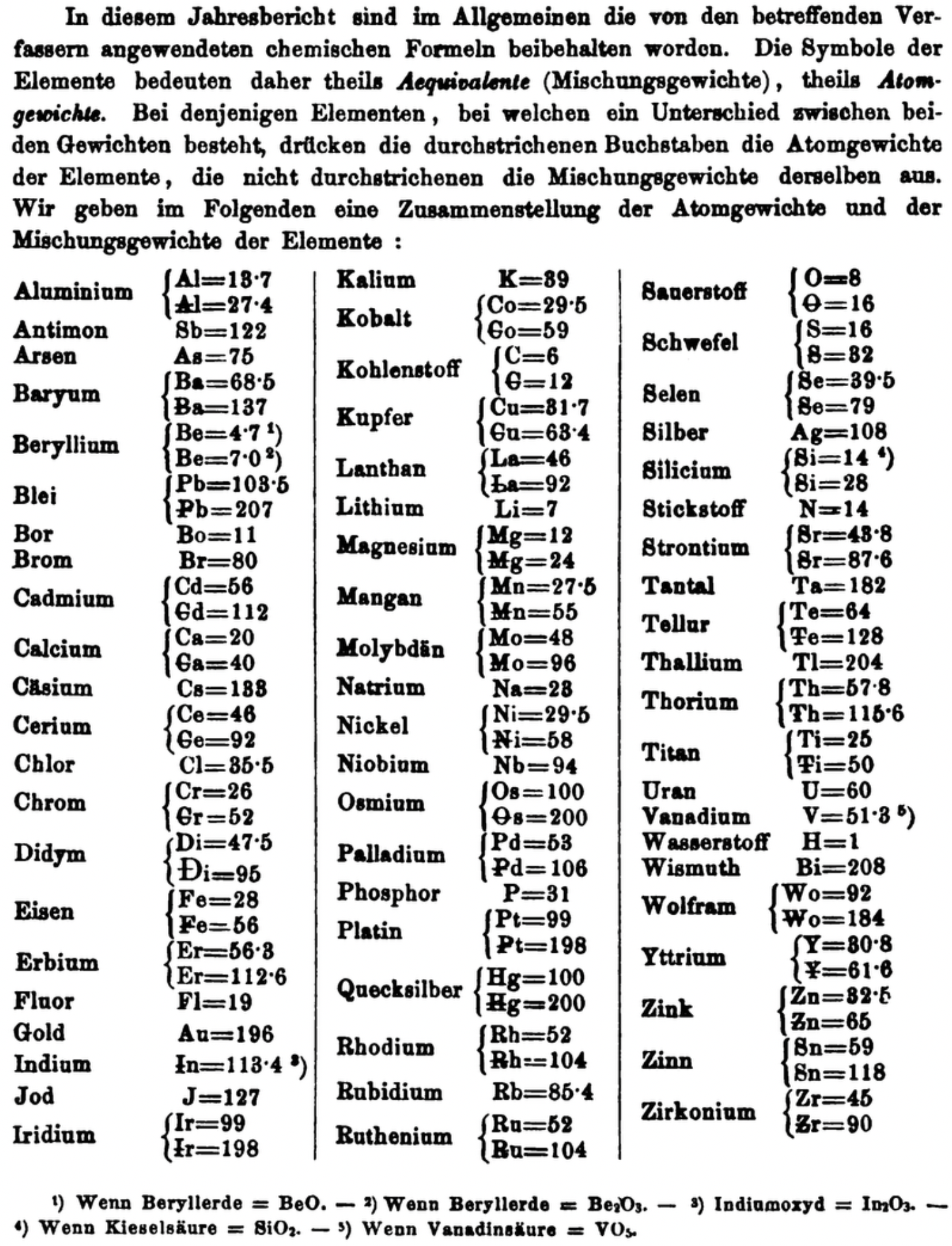
Thanks to René and Mario Rodriguez for the tip!
| Year: 1872 | PT id = 1362, Type = formulation element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1872
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1872 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systermatic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
- Didym Di = 48 and 95 was actually a mixture of rare earth elements.
- The missing elements had yet to be discovered.
- Al = 13.7 and 27.4
- Ba = 68.5 and 137
- Be = 4.7 and 7.0
- Pb = 103.5 and 207
- Cd = 56 and 112
- Ca = 20 and 40
- Ce = 56 and 92
- Cr = 26 and 52
- Fe = 28 and 56
- Er = 56.3 and 112.6
- Ir = 99 and 198
- Co = 29.5 and 59
- C = 6 and 12
- Cu = 31.7 and 63.4
- La = 46.8 and 93.6
- Mg = 12 and 24
- Mn = 27.5 and 55
- Mo = 48 and 96
- Ni = 29.5 and 58
- Os = 100 and 200
- Pd = 53 and 106
- Pt = 99 and 198
- Hg = 100 and 200
- Rh = 52 and 104
- Ru = 52 and 104
- Si = 14 and 28
- O = 8 and 16
- S = 16 and 32
- Se = 39.5 and 79
- Sr = 43.8 and 87.6
- Te = 64 and 128
- Th = 57.8 and 115.6
- Ti = 25 and 50
- Wo = 92 and 184 (note change from W to Wo)
- Y = 29.8 and 59.7
- Zn = 32.5 and 65
- Sn = 59 and 118
- Zr = 45 and 90
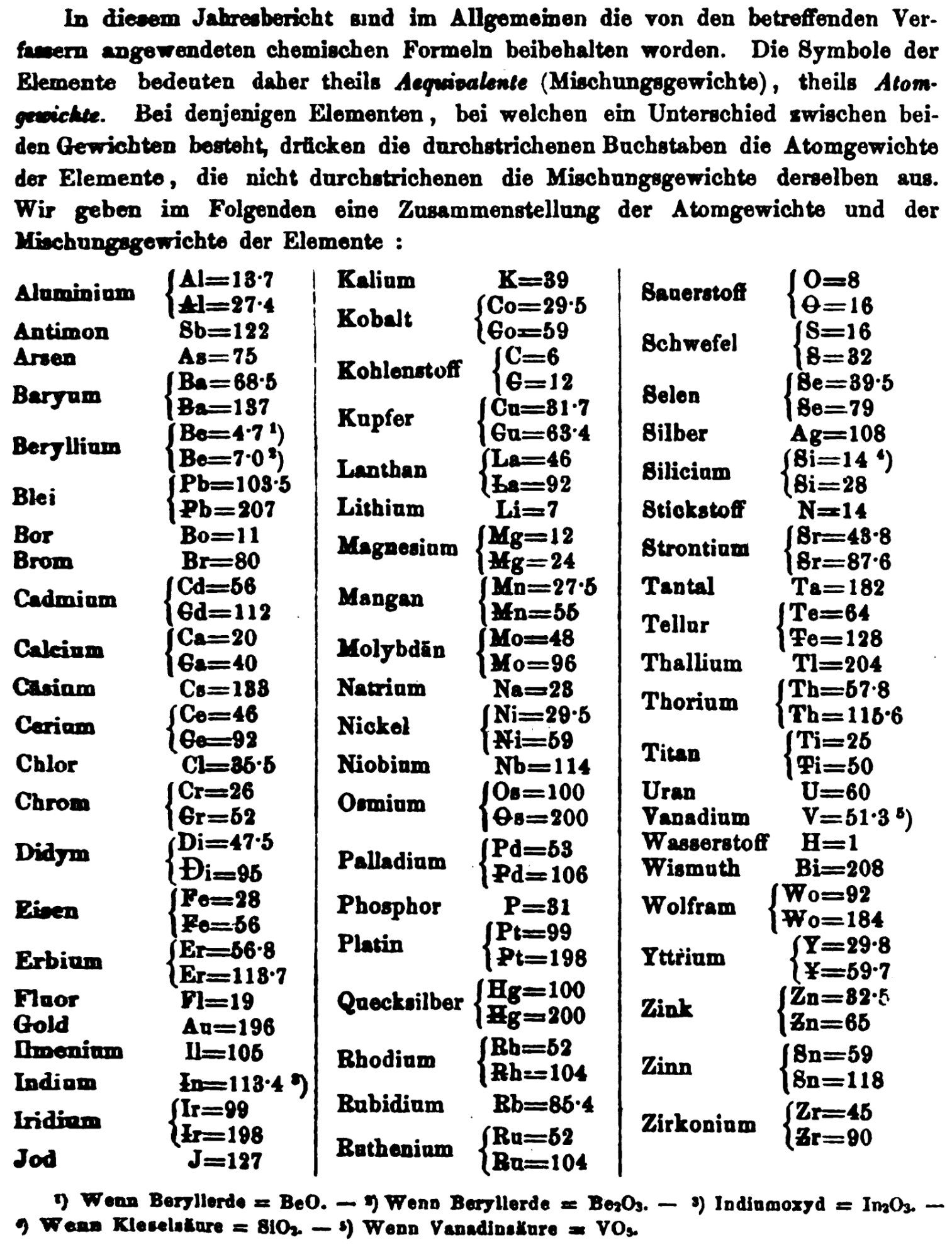
Thanks to René and Mario Rodriguez for the tip!
| Year: 1873 | PT id = 1363, Type = formulation review element weight |
Annual Report on the Progress of Chemistry and Related Areas of Other Sciences 1873
Jahresbericht über die Fortschritte der Chemie und verwandter Theile anderer Wissenschaften. (Annual Report on the progress of chemistry and related areas of other sciences.) HathiTrust Index scanned reports 1847-1910.
The 1873 table of data is here.
Mark Leach writes:
"Every year the annual report started with a list of the known chemical elements and their atomic weights, however, to the modern eye there were many systematic errors. For example, oxygen (Sauerstoff) is given as having a weight of 8 which would have caused – due to the importance of oxides – other atomic weights to be out by a factor of 2 or 3. Once a list of correct atomic weights was known, it would be possible to construct a periodic table of the elements.
"In 1858 the Cannazzario letter gave more correct list of atomic weights and corrected the numerous stoichiometric errors that plagued chemistry at the time. Over the years from 1858 to 1873 the entries in the annual report gradually adopted the Cannazzario logic."
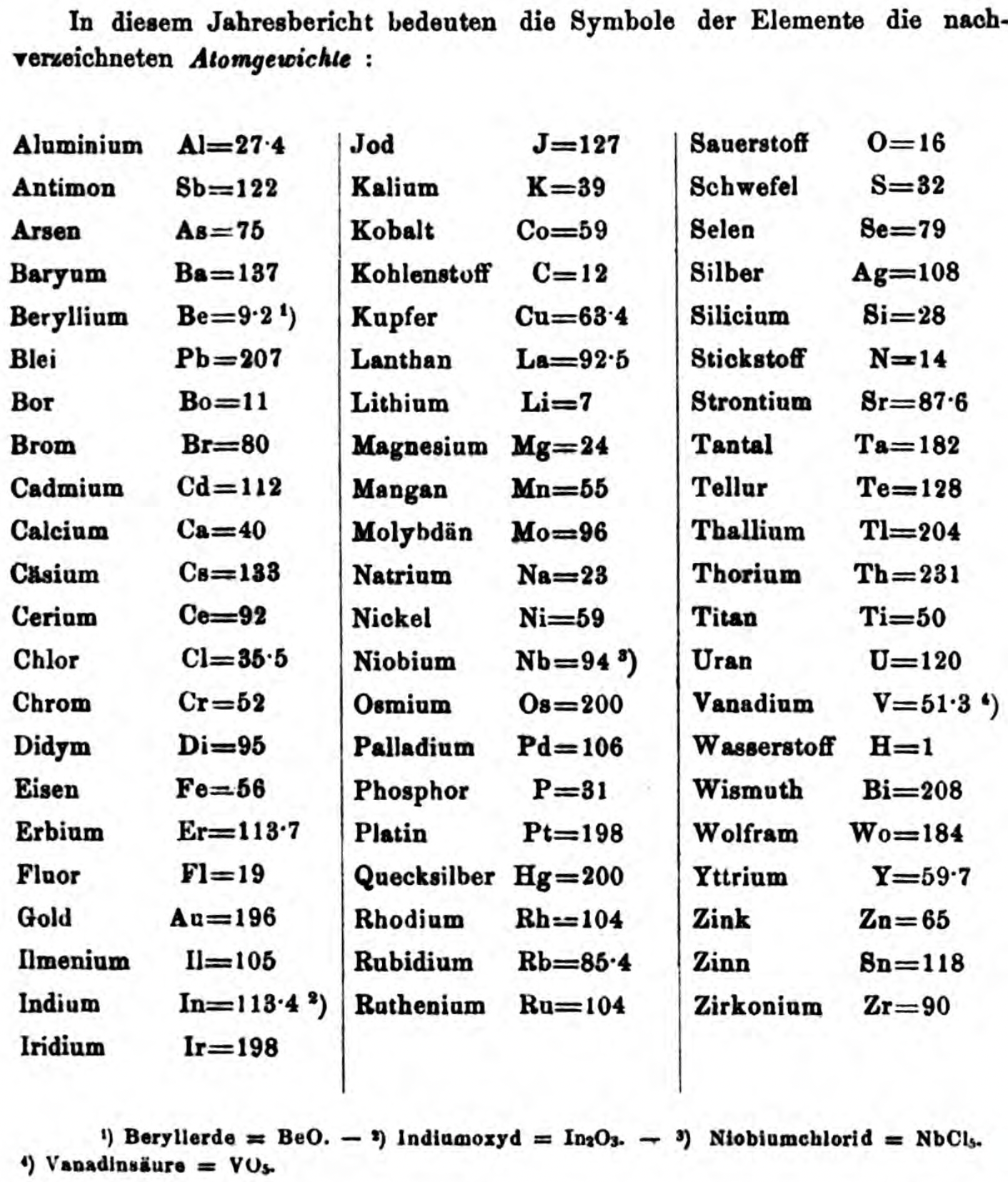
Notes:
- Didym D = 48 was actually a mixture of rare earth elements.
- Ilmenium, Il, was later found to be a mixture of niobium and tantalum.
- Generally, the elements missing had yet to be discovered (dates given below).
- The table below shows the progress from 1858 to 1873.
- By 1873 the only elements with incorrect atomic weights were the (at the time) somewhat obscure strontium, lanthanium, cerium and urananium.
- Previously, many elements were shown with two entries. Clearly, the stoichiometric and mass problems had largely been resolved (and the data agreed upon) by 1873.
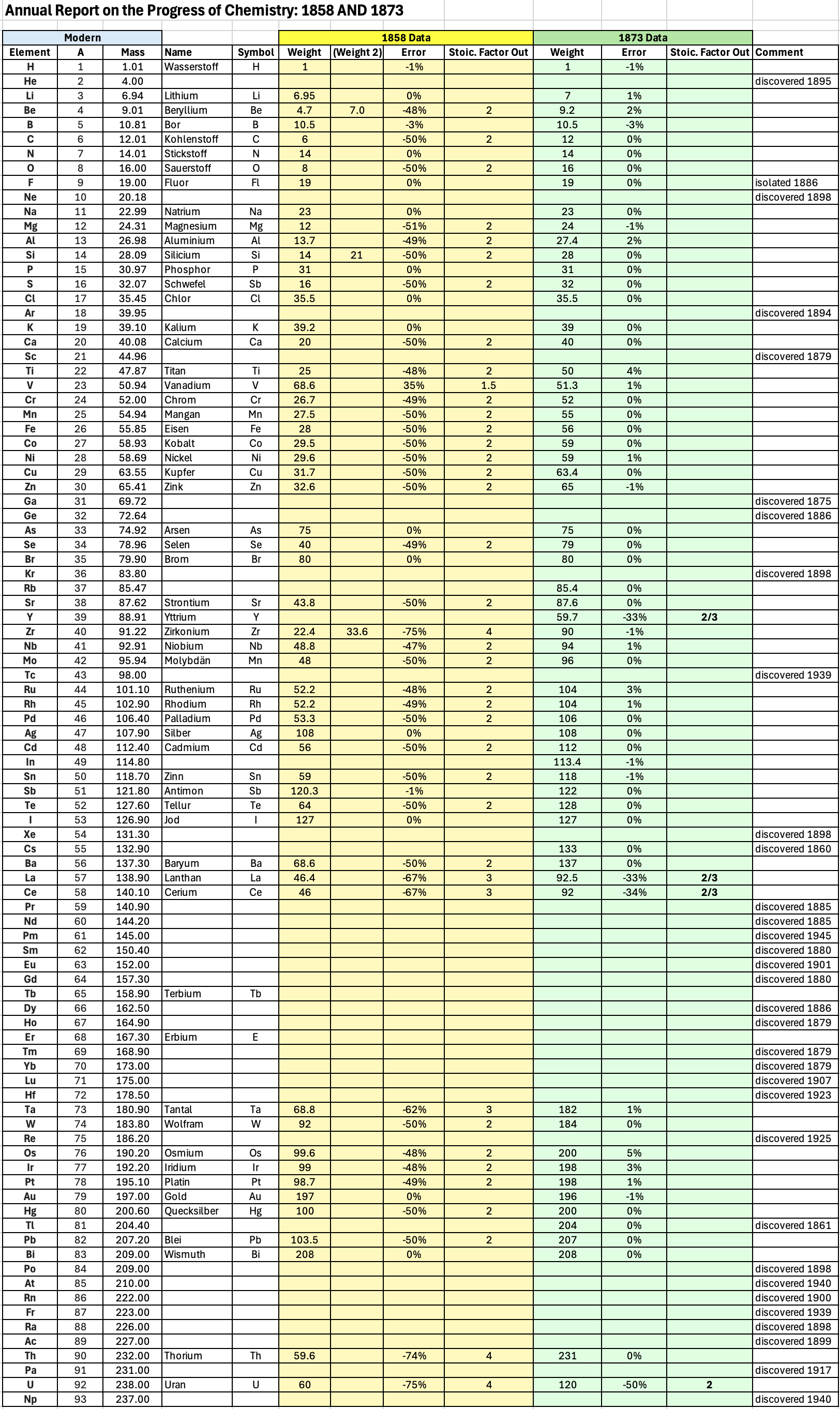
Thanks to René and Mario Rodriguez for the tip!
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.