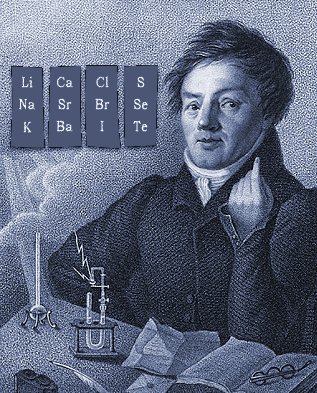Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D. The database holds information on periodic tables, the discovery of the elements, the elucidation of atomic weights and the discovery of atomic structure (and much, much more).
Periodic Tables from the years 1800 - 1849, by date:
| Year: 1800 | PT id = 235, Type = formulation element |
Elements Known in the Year 1800
Elements known in the year 1800, taken from this Wikipedia page:
| Year: 1801 | PT id = 821, Type = element |
Discovery of Niobium
Nb
Niobium, atomic number 41, has a mass of 92.906 au.
Niobium was first observed or predicted in 1801 by C. Hatchett and first isolated in 1864 by W. Blomstrand.
| Year: 1802 | PT id = 853, Type = element |
Discovery of Tantalum
Ta
Tantalum, atomic number 73, has a mass of 180.948 au.
Tantalum was first isolated in 1802 by G. Ekeberg.
| Year: 1803 | PT id = 4, Type = formulation element weight structure |
Dalton's Postulates About The Elements
Around the year 1803 in Manchester, John Dalton gave a series of lectures in which he presented his postulates:
- Elements are made of tiny particles called atoms.
- The atoms of a given element are different from those of any other element, and the atoms of different elements can be distinguished from one another by their respective relative atomic weigh/mass.
- All atoms of a given element are identical.
- Atoms of one element can combine with atoms of other elements to form chemical compounds, and a given compound always has the same relative numbers of types of atoms.
- Atoms cannot be created, divided into smaller particles, nor destroyed in the chemical process, and a chemical reaction simply changes the way atoms are grouped together.
From a very early notebook from around this time:

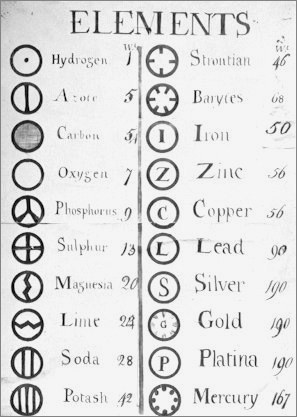
| Year: 1803 | PT id = 826, Type = element |
Discovery of Palladium
Pd
Palladium, atomic number 46, has a mass of 106.42 au.
Palladium was first isolated in 1803 by H. Wollaston.
| Year: 1803 | PT id = 838, Type = element |
Discovery of Cerium
Ce
Cerium, atomic number 58, has a mass of 140.116 au.
Cerium was first observed or predicted in 1803 by H. Klaproth, J. Berzelius, and W. Hisinger and first isolated in 1838 by G. Mosander.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
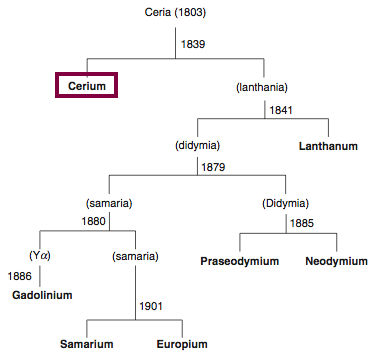
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1803 | PT id = 856, Type = element |
Discovery of Osmium
Os
Osmium, atomic number 76, has a mass of 190.23 au.
Osmium was first isolated in 1803 by S. Tennant.
| Year: 1803 | PT id = 857, Type = element |
Discovery of Iridium
Ir
Iridium, atomic number 77, has a mass of 192.217 au.
Iridium was first isolated in 1803 by S. Tennant.
| Year: 1804 | PT id = 825, Type = element |
Discovery of Rhodium
Rh
Rhodium, atomic number 45, has a mass of 102.906 au.
Rhodium was first isolated in 1804 by H. Wollaston.
| Year: 1807 | PT id = 790, Type = element |
Discovery of Sodium
Na
Sodium, atomic number 11, has a mass of 22.99 au.
Sodium is a Group 1 element, and these are often referred to as the "alkali metals".
Sodium was first isolated in 1807 by H. Davy.
| Year: 1807 | PT id = 799, Type = element |
Discovery of Potassium
K
Potassium, atomic number 19, has a mass of 39.098 au.
Potassium is a Group 1 element, and these are often referred to as the "alkali metals".
Potassium was first isolated in 1807 by H. Davy.
| Year: 1808 | PT id = 5, Type = formulation data element weight structure |
Dalton's Elements
Two pages from John Dalton's A New System of Chemical Philosophy in which he proposed his version of atomic theory based on scientific experimentation (see the scanned book, page 219):
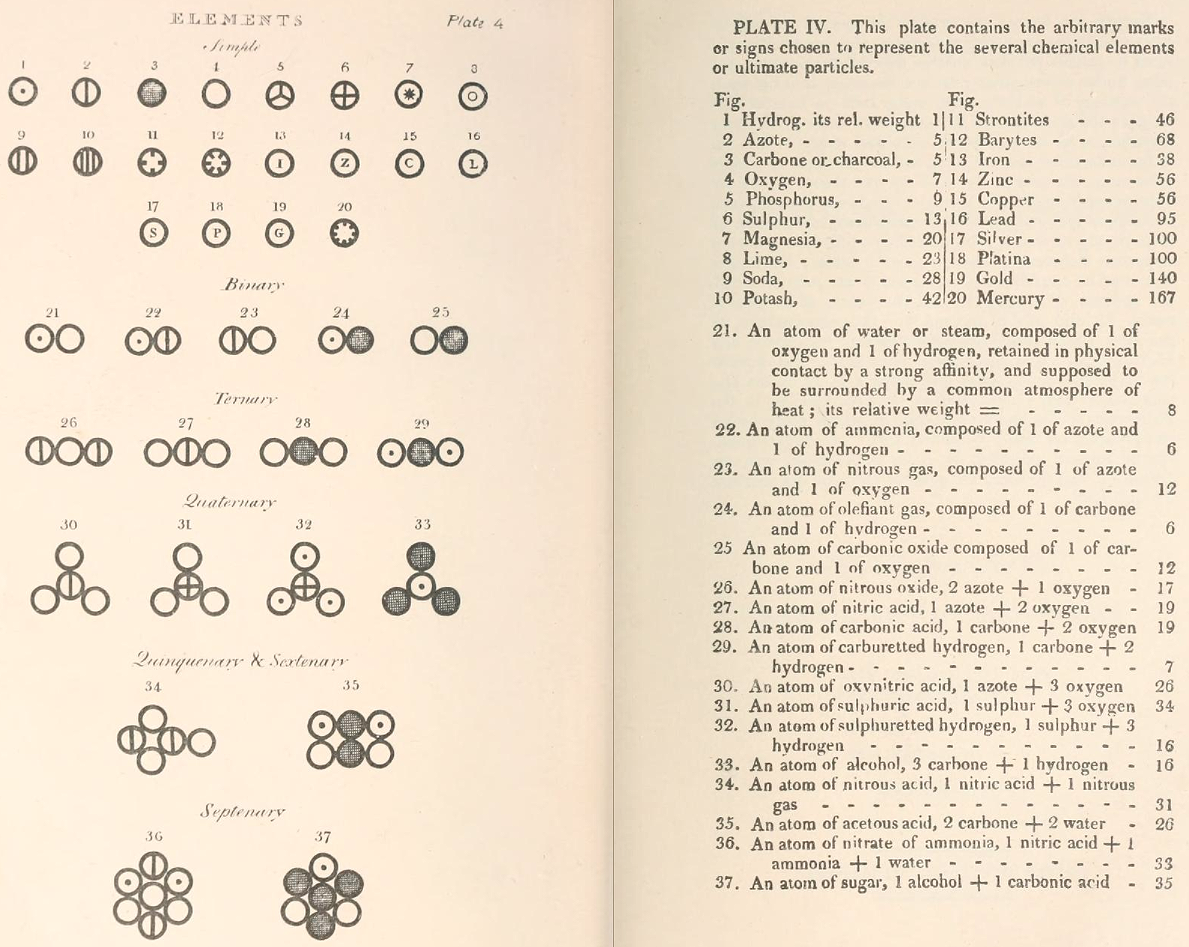
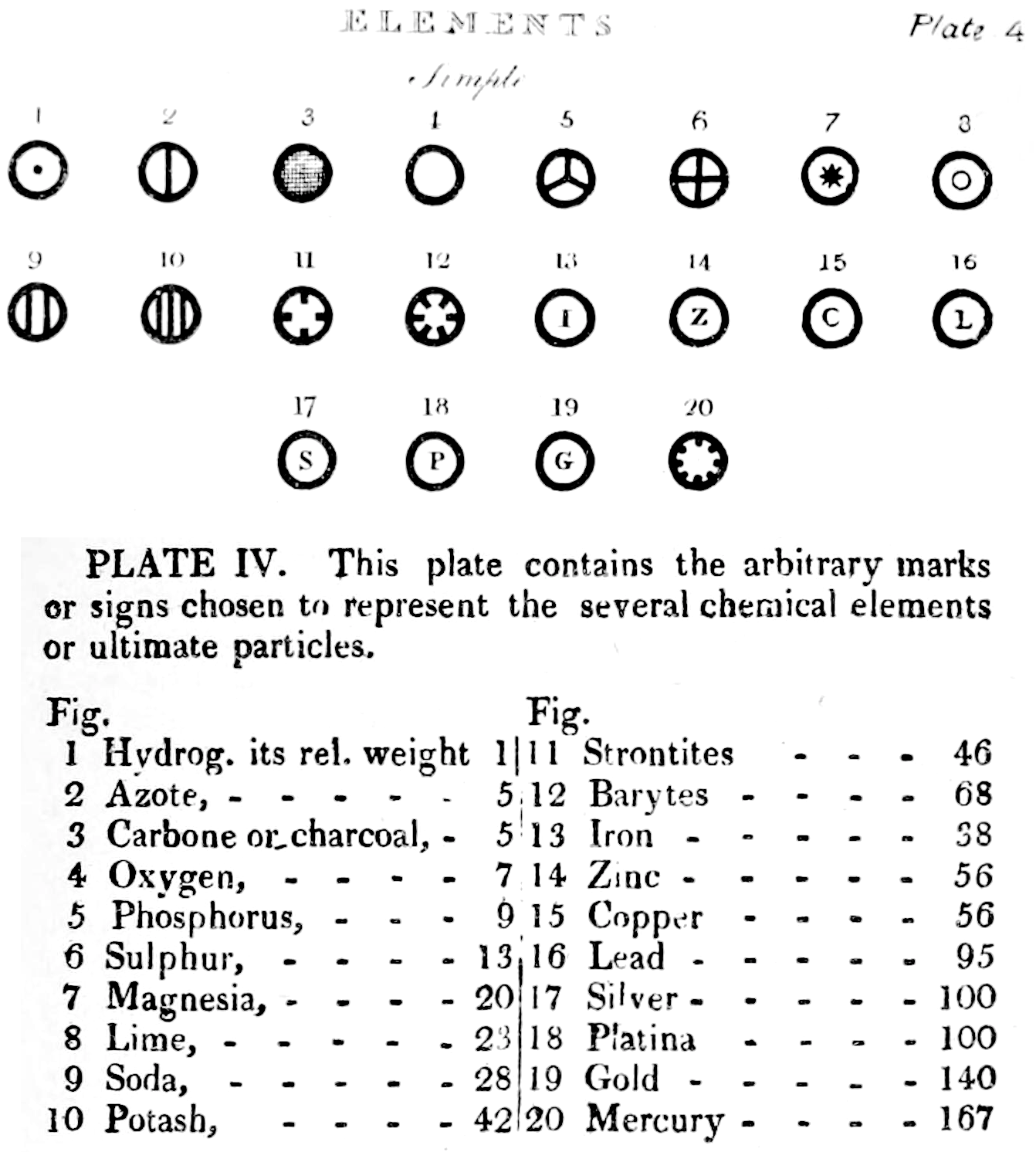
| Name | Modern Symbol | Dalton's Data | Modern Values | % error |
| Hydrog. | H | 1 | 1 | 0% |
| Azote | N | 5 | 14 | -180% |
| Carbone | C | 5 | 12 | -140% |
| Oxygen | O | 7 | 16 | -129% |
| Phosphorus | P | 9 | 31 | -244% |
| Sulphur | S | 13 | 32.1 | -147% |
| Magnesia | Mg | 20 | 24.3 | -22% |
| Lime | Ca | 24 | 40.1 | -67% |
| Soda | Na | 28 | 23 | 18% |
| Potash | K | 42 | 39.1 | 7% |
| Strontites | Sr | 46 | 87.6 | -90% |
| Barytes | Ba | 68 | 137.3 | -102% |
| Iron | Fe | 50 | 55.8 | -12% |
| Zinc | Zn | 56 | 65.4 | -17% |
| Copper | Cu | 56 | 63.5 | -13% |
| Lead | Pb | 90 | 200.6 | -123% |
| Silver | Ag | 190 | 107.9 | 43% |
| Gold | Au | 190 | 197 | -4% |
| Platina | Pt | 190 | 195.1 | -3% |
| Mercury | Hg | 167 | 200.6 | -20% |
- Dalton states that he is considering "chemical elements or ultimate particles"
- Dalton assigns hydrogen as having a relative weight of 1.
- Note the seemingly huge % errors in the atomic weights, compared with modern values.
- These errors occurred because while Dalton had deduced that atoms combine in fixed (stoichiometric) ratios in compounds, he not always know what the ratios were. Thus there were two unknowns: the atomic weights (masses) and the stoichiometric ratios.
By Mark Leach
| Year: 1808 | PT id = 785, Type = element |
Discovery of Boron
B
Boron, atomic number 5, has a mass of 10.814 au.
Boron has properties that are borderline between metal and non-metal (semimetallic).
Boron was first observed or predicted in 1808 by L. Gay-Lussac and L.J. Thénard and first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 791, Type = element |
Discovery of Magnesium
Mg
Magnesium, atomic number 12, has a mass of 24.306 au.
Magnesium is a Group 2 element, and these are called: "alkaline earth metals".
Magnesium was first observed or predicted in 1755 by J. Black and first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 800, Type = element |
Discovery of Calcium
Ca
Calcium, atomic number 20, has a mass of 40.078 au.
Calcium is a Group 2 element, and these are called: "alkaline earth metals".
Calcium was first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 818, Type = element |
Discovery of Strontium
Sr
Strontium, atomic number 38, has a mass of 87.62 au.
Strontium is a Group 2 element, and these are called: "alkaline earth metals".
Strontium was first observed or predicted in 1787 by W. Cruikshank and first isolated in 1808 by H. Davy.
| Year: 1808 | PT id = 836, Type = element |
Discovery of Barium
Ba
Barium, atomic number 56, has a mass of 137.327 au.
Barium is a Group 2 element, and these are called: "alkaline earth metals".
Barium was first observed or predicted in 1772 by W. Scheele and first isolated in 1808 by H. Davy.
| Year: 1811 | PT id = 833, Type = element |
Discovery of Iodine
I
Iodine, atomic number 53, has a mass of 126.904 au.
Iodine exists as a black diatomic molecular solid, I2.
Iodine was first isolated in 1811 by B. Courtois.
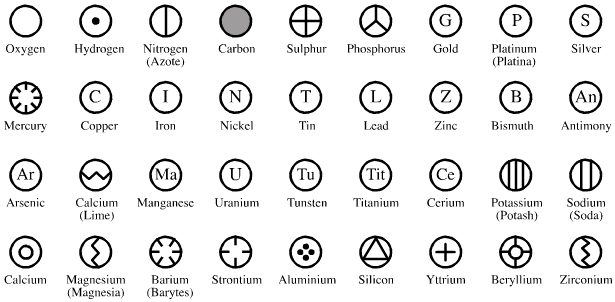
| Year: 1813 | PT id = 1043, Type = formulation |
Wollaston's Slide Rule of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S. – or from here – has a diagram for a slide rule of chemical equivalents:
Wollaston writes:
"In order to shew more clearly the use of this scale, the Plate [diagram of the chemical slide rule] exhibits two different situations of the slider, in one of which oxygen is 10 [oxygen is defined as having an atomic weight/mass of 10.00], and other bodies are in their due proportion to it, so that carbonic acid being 27,54, and lime 35,46, carbonate of lime is placed at 63.
"In the second figure, the slider is represented drawn upwards till 100 corresponds to muriate of soda [sodium chloride, NaCl]; and accordingly the scale then shews how much of each substance contained in the table is equivalent to 100 of common salt. It shews, with regard to the different views of the analysis of this salt, that it contains 46,6 dry muriatic acid [hydrogen chloride], and 53,4 of soda, or 39,8 sodium, and 13,6 oxygen; or if viewed as chlorid of sodium, that it contains 60,2 chlorine, and 39,8 sodium."
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
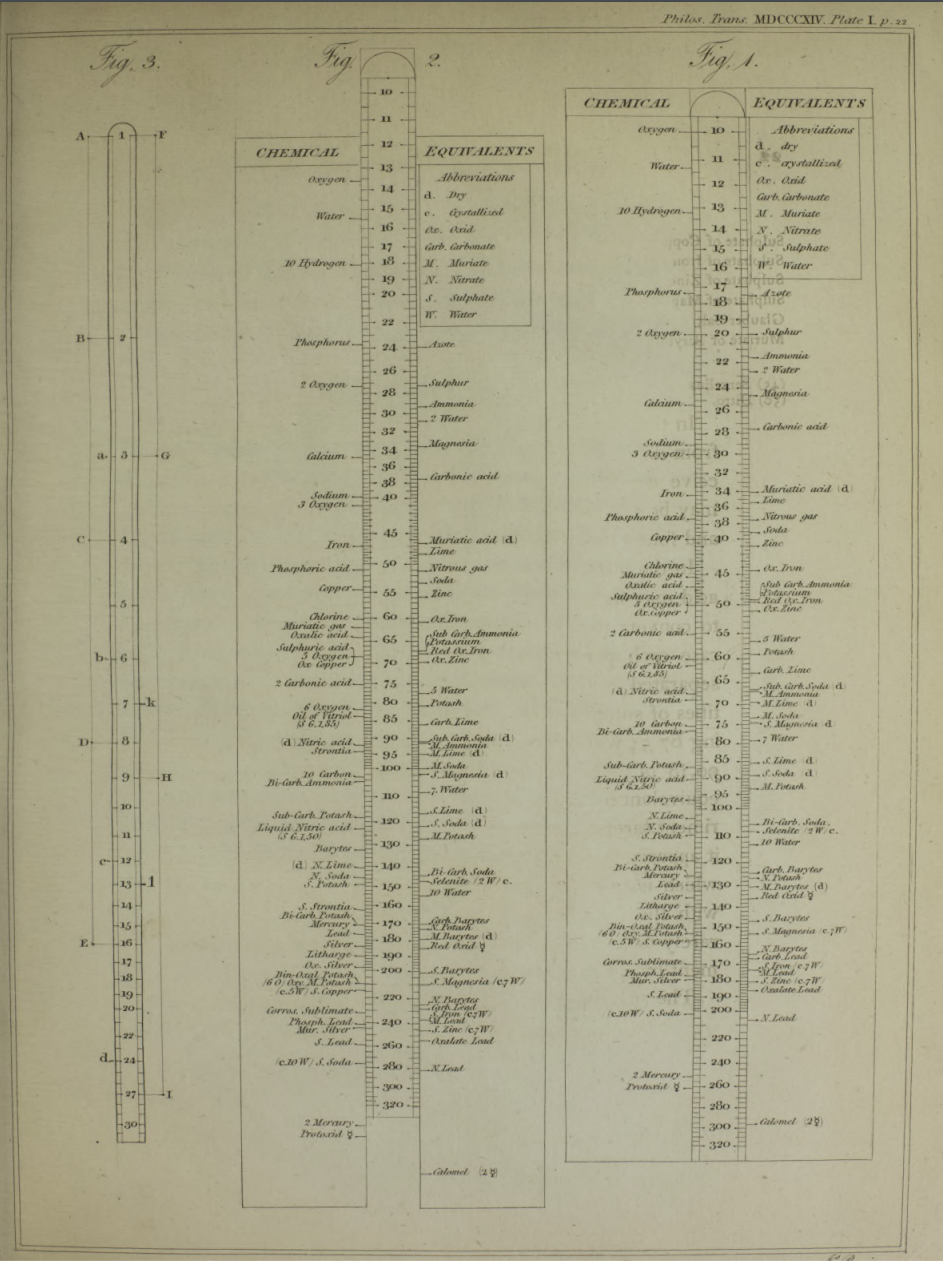
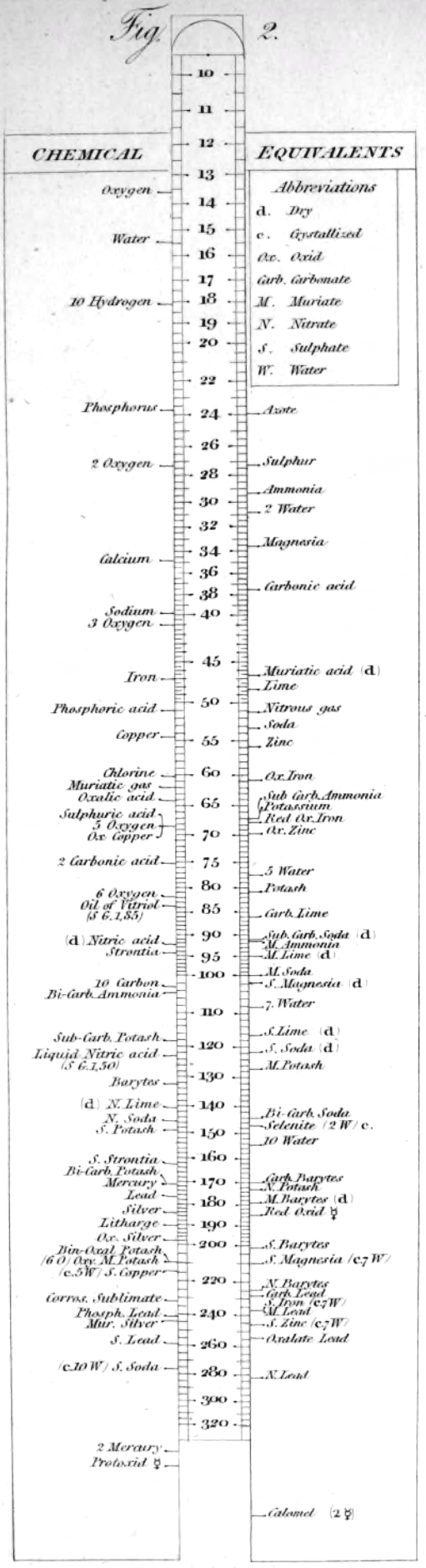
| Year: 1813 | PT id = 1044, Type = formulation element weight |
Wollaston's Synoptic Scale of Chemical Equivalents
Philosophical Transactions: A Synoptic Scale of Chemical Equivalents by William Hyde Wollaston, M.D. Sec. R.S., or from here.
It is apparent that chemistry the years 1810 to 1850 was largely concerned with discovering the whole number stoichiometric ratios of atoms in chemical compounds.
Wollaston writes in the text above:
"It is impossible in several instances, where only two combinations of the same ingredients are known, to discover which of the compounds is to be regarded as consisting of a pair of single atoms, and since the decision of these questions is purely theoretical, and by no means necessary to the formation of a table adapted to most practical purposes, I have not been desirous of warping my numbers according to an atomic theory, but have endeavored to make practical convenience my sole guide, and have considered the doctrine of simple multiples, on which that of atoms is founded, merely as a valuable assistant in determining, by simple division, the amount of those quantities that are liable to such definite deviations from the original law of Richter."
"Mr. Dalton in his atomic views of chemical combination appears not to have taken much pains to ascertain the actual prevalence of that law of multiple proportions by which the atomic theory is best supported [however] it is in fact to Mr. Dalton that we are indebted for the first correct observation of such an instance of a simple multiple in the union of nitrous gas with oxygen."
"[I have] computed a series of supposed atoms, I [have] assumed oxygen as the decimal unit of my scale [ie. oxygen = 10], in order to facilitate the estimation of those numerous combinations which it forms with other bodies. Though the present table of Equivalents, I have taken care to make oxygen equally prominent on account of the important part it performs in determining the affinities of bodies by the different proportions in which it is united to them.."
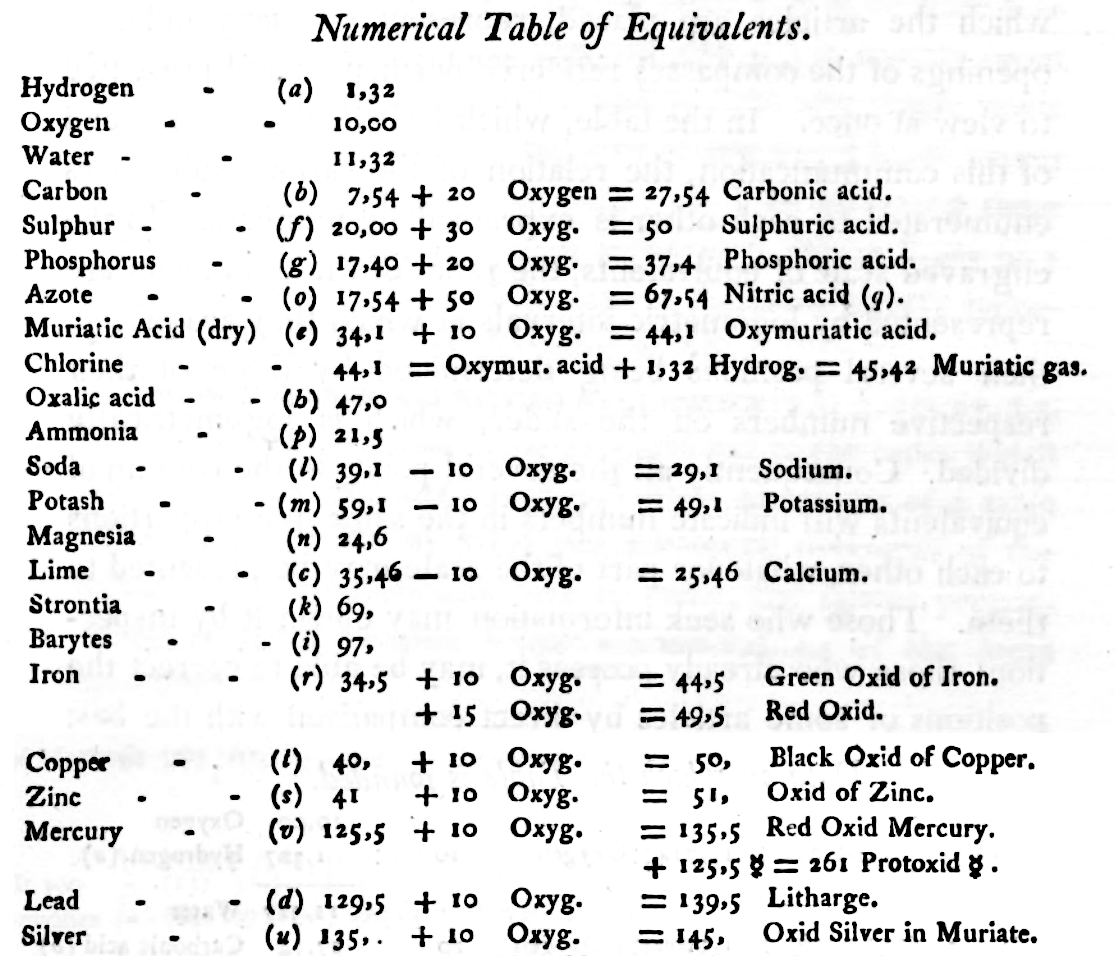
Mark Leach writes:
"When Wollaston's equivalent weights are converted from O = 10.00 to the modern value of O = 15.999, the atomic weight values can be seen to be astonishingly accurate.
"However, the language of the article is quite difficult as the meaning of many of the terms is unclear (to me, at least). For example, in modern usage adding 'ia' to a metal implies the oxide: 'magnesia' is magnesium oxide, MgO. I am not clear if this historical usage is consistent. 'Azote' is nitrogen and 'muriatic acid (dry)' is hydrogen chloride gas. I have only analyses/re-calculated the elements and a couple of common/obvious compounds:"
| Wollaston's data | Scaled to O = 15.999 | Modern Values | % error | |
| H (as H2) | 1.32 | 2.112 | 2.016 | 5% |
| O | 10.00 | 15.999 | 15.999 | ref. value |
| H2O | 11.32 | 18.111 | 18.015 | 1% |
| C | 7.74 | 12.383 | 12.011 | 3% |
| S | 20.00 | 31.998 | 32.060 | 0% |
| P | 17.40 | 27.838 | 30.974 | -11% |
| N (as N2) | 17.54 | 28.062 | 28.014 | 0% |
| Cl (as Cl2) | 44.10 | 70.556 | 70.900 | 0% |
| Fe | 34.50 | 55.197 | 55.845 | -1% |
| Cu | 40.00 | 63.996 | 63.546 | 1% |
| Zn | 41.00 | 65.596 | 65.380 | 0% |
| Hg | 125.50 | 200.787 | 200.590 | 0% |
| Pb | 129.50 | 207.187 | 207.980 | 0% |
| Ag | 135.00 | 215.987 | 107.870 | 50% |
- The elements hydrogen, nitrogen (azote) and chlorine have clearly been measured as the diatomic molecules, even if this was unknown to Wollaston in 1813.
- Phosphorus is out by 11%... [fair enough].
- Only silver is clearly wrong, but it is out by 50% so it looks like a simple stoichiometry error: Perhaps the oxide was assumed to be AgO was instead of the correct Ag2O.
Interestingly, Wollaston's analysis is far better than Daubeny's 1831 data seen in Oxford.
Read more in an entry concerning chemical slide rules.
Thanks to Nawa for the tip!
| Year: 1814 | PT id = 1034, Type = formulation |
Wollaston's Physical Slide Rule of Chemical Equivalents
From the Science Museum in the UK collection, the Wollaston slide rule of chemical equivalents:
"Three sliding scales of chemical equivalents, all with same manuscripts marks, published by W Cary, devised by W H Wollaston, a leading chemist and natural philosopher during the early 19th century.
"Positioning the slider with the weight of the substance set against it will show you the weights of other substances which will react with it. This fundamental ordering based on measurement paved the way for the periodic table of the elements"
Wollaston uses a decimal scale in which oxygen is defined as having an atomic weight (relative atomic mass) of 10.00 rather than the modern value of 15.999.
Read more here and here, and an entry concerning chemical slide rules:

Mark Leach writes:
"I have edited the image above, setting the scale to zero:"

| Year: 1817 | PT id = 783, Type = element |
Discovery of Lithium
Li
Lithium, atomic number 3, has a mass of 6.968 au.
Lithium is a reactive metal, of low density: it is the least dense metal.
Lithium was first observed or predicted in 1817 by A. Arfwedson and first isolated in 1821 by W. T. Brande.
| Year: 1817 | PT id = 814, Type = element |
Discovery of Selenium
Se
Selenium, atomic number 34, has a mass of 78.971 au.
Selenium was first isolated in 1817 by J. Berzelius and G. Gahn.
| Year: 1817 | PT id = 828, Type = element |
Discovery of Cadmium
Cd
Cadmium, atomic number 48, has a mass of 112.414 au.
Cadmium was first isolated in 1817 by S. L Hermann, F. Stromeyer and J.C.H. Roloff.
| Year: 1824 | PT id = 793, Type = element |
Discovery of Silicon
Si
Silicon, atomic number 14, has a mass of 28.085 au.
Silicon makes up 25.7% of the earth's crust, and after oxygen is the second most abundant element.
Silicon was first isolated in 1823 by J. Berzelius.
| Year: 1825 | PT id = 792, Type = element |
Discovery of Aluminium (Aluminum)
Al
Aluminium (aluminum), atomic number 13, has a mass of 26.982 au.
Aluminum is a silvery-white metal.
Aluminium was first isolated in 1825 by H.C.Ørsted.
| Year: 1825 | PT id = 815, Type = element |
Discovery of Bromine
Br
Bromine, atomic number 35, has a mass of 79.904 au.
Bromine exists as an orange diatomic molecular liquid, Br2.
Bromine was first isolated in 1825 by J. Balard and C. Löwig.
| Year: 1829 | PT id = 6, Type = formulation element |
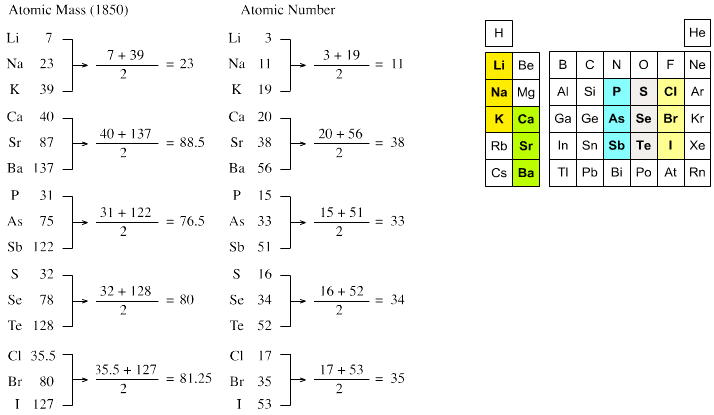
Döbereiner's Triads
Johann Döbereiner found triads: a sequence of three similar elements, where the middle element has a mass equal to the average of the least and most massive.
A brief biography can be found on the Nature website.
Döbereiner writes in An Attempt to Group Elementary Substances according to Their Analogies (in English)
From Poggendorf's Annalen der Physik und Chemie 15, 301-7 (1829) (in German) [from Henry M. Leicester & Herbert S. Klickstein, eds., A Source Book in Chemistry, 1400-1900 (Cambridge, MA: Harvard, 1952)]:
"The work of Berzelius on the determination of the atomic weights of bromine and iodine has interested me greatly, since it has established the idea, which I expressed earlier in my lectures, that perhaps the atomic weight of bromine might be the arithmetical mean of the atomic weights of chlorine and iodine. This mean is (35.470+126.470)/2 = 80.470. This number is not much greater than that found by Berzelius (78.383); however, it comes so close that it may almost be hoped that the difference will vanish entirely after repeated careful and exact determinations of the atomic weights of these three salt-forming elements. This idea was the motive for an attempt which I made twelve years ago to group substances by their analogies."
[Note: L&K noticed an error in the above math: (35.47 + 126.47)/2 = 80.97 not 80.47. Whoops...]

The diagram below uses mid-nineteenth century atomic mass information rather than modern data. If atomic numbers (Z) are used (a property unknown in 1850), the triads are exact:
| Year: 1829 | PT id = 870, Type = element |
Discovery of Thorium
Th ![]()
Thorium, atomic number 90, has a mass of 232.038 au.
Radioactive element with a very long half-life.
Thorium was first observed or predicted in 1829 by J. Berzelius and first isolated in 1914 by D. Lely, Jr. and L. Hamburger.
| Year: 1830 | PT id = 803, Type = element |
Discovery of Vanadium
V
Vanadium, atomic number 23, has a mass of 50.942 au.
Vanadium was first observed or predicted in 1801 by M. del Río and first isolated in 1830 by N.G.Sefström.
| Year: 1831 | PT id = 337, Type = formulation element weight |
Daubeny's Teaching Display Board & Wooden Cubes of Atomic Weights
The Museum of the History of Science, Oxford, has a display of Charles Daubeny's teaching materials, including a black painted wooden board with "SYMBOLS OF SIMPLE BODIES": showing symbols, atomic weights and names of elements in two columns, and a small pile of cubes with element symbols.
Charles Daubeny and Chemistry at the Old Ashmolean
Charles Daubeny (1795-1867) was appointed Aldrichian Professor of Chemistry at Oxford in 1822. In 1847 he moved from the original laboratory in this basement [in the museum] to a new one built at his own expense at the Botanic Garden. His apparatus went with him and was preserved there. Daubeny actively campaigned for the teaching of science in Oxford and held several professorships in addition to chemistry. He also conducted research on subjects such as photosynthesis.
From the HSM Database (Inventory no. 17504):
DAUBENY'S LIST OF ATOMIC WEIGHTS Wooden panel, black with white lettering, listing in two columns the symbols and names of twenty elements. This lecture board is identical to the table in the third edition (1831) of E. Turner, 'Elements of Chemistry', apart from the atomic weight for bromine. Daubeny wrote a useful 'Introduction to the Atomic Theory' (published in three versions: 1831, 1840, and 1850), the first edition of which also quotes Turner's table. Probably contemporary with this lecture board are the wooden cubes with the symbols for certain elements.
The period from 1810 to 1860 was crucial in the development of the periodic table. Most of the main group and transition elements had been discovered, but their atomic weights and stoichiometries (combining ratios) had not been fully deduced. Oxygen was assumed to have a weight of 6, and consequently carbon is assumed to have a mass of 6.
Daubeny's element symbols and weights – along with the modern mass data – are tabulated:
| Symbol | Daubeny's Weight | Modern Mass Data | % error | Stoichiometry Error |
| H | 1 | 1 | 0% | |
| C | 6 | 12 | -100% | factor of 2 |
| O | 8 | 16 | -100% | factor of 2 |
| Si | 8 | 28.1 | -251% | factor of 5 (?) |
| Al | 10 | 27 | -170% | factor of 3 |
| Mg | 12 | 24.3 | -103% | factor of 2 |
| N | 14 | 14 | 0% | |
| S | 16 | 32.1 | -101% | factor of 2 |
| P | 16 | 31 | -94% | factor of 2 |
| Fl | 19 | 19 | 0% | |
| Ca | 20 | 40.1 | -101% | factor of 2 |
| Na | 24 | 23 | 4% | |
| Fe | 28 | 55.8 | -99% | factor of 2 |
| Cl | 36 | 35.5 | 1% | |
| K | 40 | 39.1 | 2% | |
| Cu | 64 | 63.5 | 1% | |
| B | 80 | 79.9 | 0% | |
| Pb | 104 | 207 | -99% | factor of 2 |
| I | 124 | 127 | -2% | |
| Hg | 200 | 200.6 | 0% |
While quite a number of weights are close to the modern values, many are way out. However, the error is usually a stiotoimetric factor error.
From the HSM Database (Inventory no. 33732): SET OF WOODEN CUBES ILLUSTRATING ATOMIC WEIGHTS
Forty-two wooden cubes numbered 1-42, painted black with symbols for certain elements, compounds or radicals painted in white on the faces, together with the corresponding atomic, molecular or radical weights. The face markings appear in various combinations:
| H | C | P | Na | Ca° | S | N | K | Fe | K | Na° | Cy | K° |
| 1 | 6 | 16 | 24 | 28 | 16 | 14 | 40 | 28 | 48 | 32 | 26 | 48 |
A typical cube (no. 3) may be represented by the following figure. They present something of an enigma as their faces do not form an obvious pattern. The numbers indicate that there were 42 cubes. In style they are similar to the figures on the panel of atomic weights.
The cubes are listed in Daubeny's 1861 catalogue, p. 11 as: "Wooden cubes for illustrating atomic weight". [See D. R. Oldroyd, The Chemical Lectures at Oxford (1822-1854) of Charles Daubeny, M.D., F.R.S. Notes and Records of the Royal Society, vol. 33 (1979), pp. 217-259.]
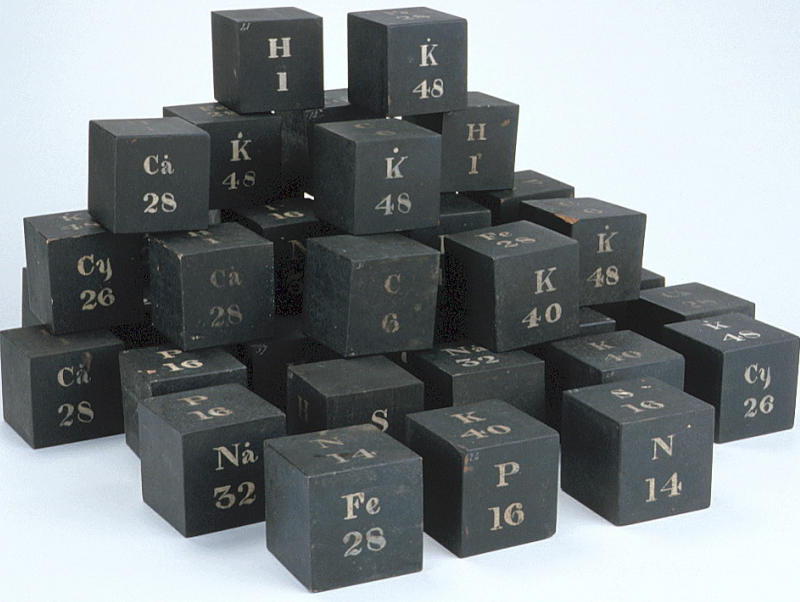
This display was spotted by Eric Scerri who was visiting the museum with Mark Leach in 2010.
There is a virtual tour on the museum, and the above display is in the basement.
| Year: 1836 | PT id = 453, Type = formulation data |
Berzelius' Electronegativity Table
Berzelius' electronegativity table of 1836.
The most electronegative element (oxygen or Sauerstoff) is listed at the top left and the least electronegative (potassium or Kalium) lower right. The line between hydrogen (Wasserstoff) and gold seperates the predomently electronegative elements from the electropositive elements. Page 17 and ref. 32 from Bill Jensen's Electronegativity from Avogadro to Pauling Part I: Origins of the Electronegativity Concept, J. Chem. Educ., 73, 11-20 (1996):

| Year: 1838 | PT id = 837, Type = element |
Discovery of Lanthanum
La
Lanthanum, atomic number 57, has a mass of 138.905 au.
Lanthanum was first observed or predicted in 1838 by G. Mosander and first isolated in 1841 by G. Mosander.
Chronology of chemically the splitting of ceria (mixed oxides) into the pure rare-earth metals:
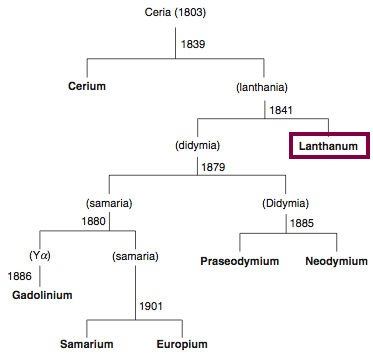
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1842 | PT id = 845, Type = element |
Discovery of Terbium
Tb
Terbium, atomic number 65, has a mass of 158.925 au.
Terbium was first observed or predicted in 1842 by G. Mosander and first isolated in 1886 by J.C.G. de Marignac.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
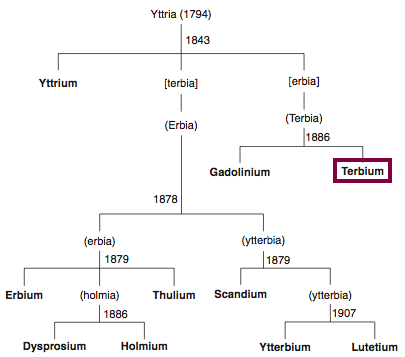
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1842 | PT id = 848, Type = element |
Discovery of Erbium
Er
Erbium, atomic number 68, has a mass of 167.259 au.
Erbium was first observed or predicted in 1842 by G. Mosander and first isolated in 1879 by T. Cleve.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
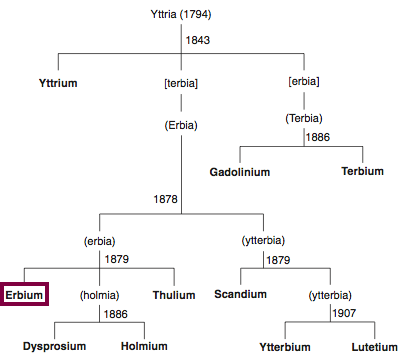
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
| Year: 1843 | PT id = 268, Type = formulation |
Gmelin's System
L. Gmelin, Handbuch der chemie, 4th ed., Heidelberg, 1843, vol. 1, p. 457 (Many thanks to Carmen Giunta for the ref. update & link.)
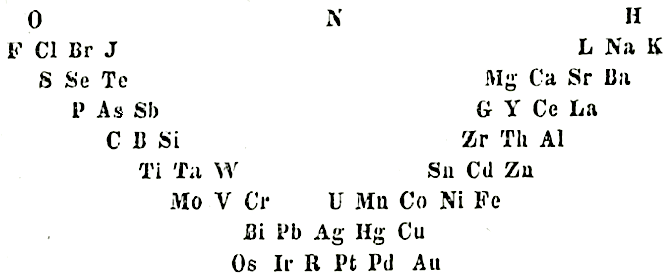
The early and important Gmelin formulation redrawn by Mark Leach with modern element symbols:
- J → I
- G → Be
- L → Li
- R → Rh
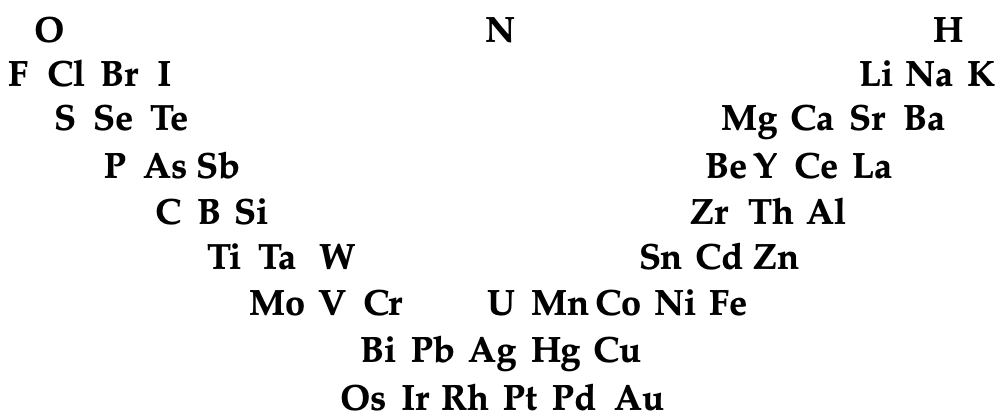
| Year: 1844 | PT id = 824, Type = element |
Discovery of Ruthenium
Ru
Ruthenium, atomic number 44, has a mass of 101.07 au.
Ruthenium was first isolated in 1844 by K. Claus.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.