Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables referencing the text string "Landau", listed by date:
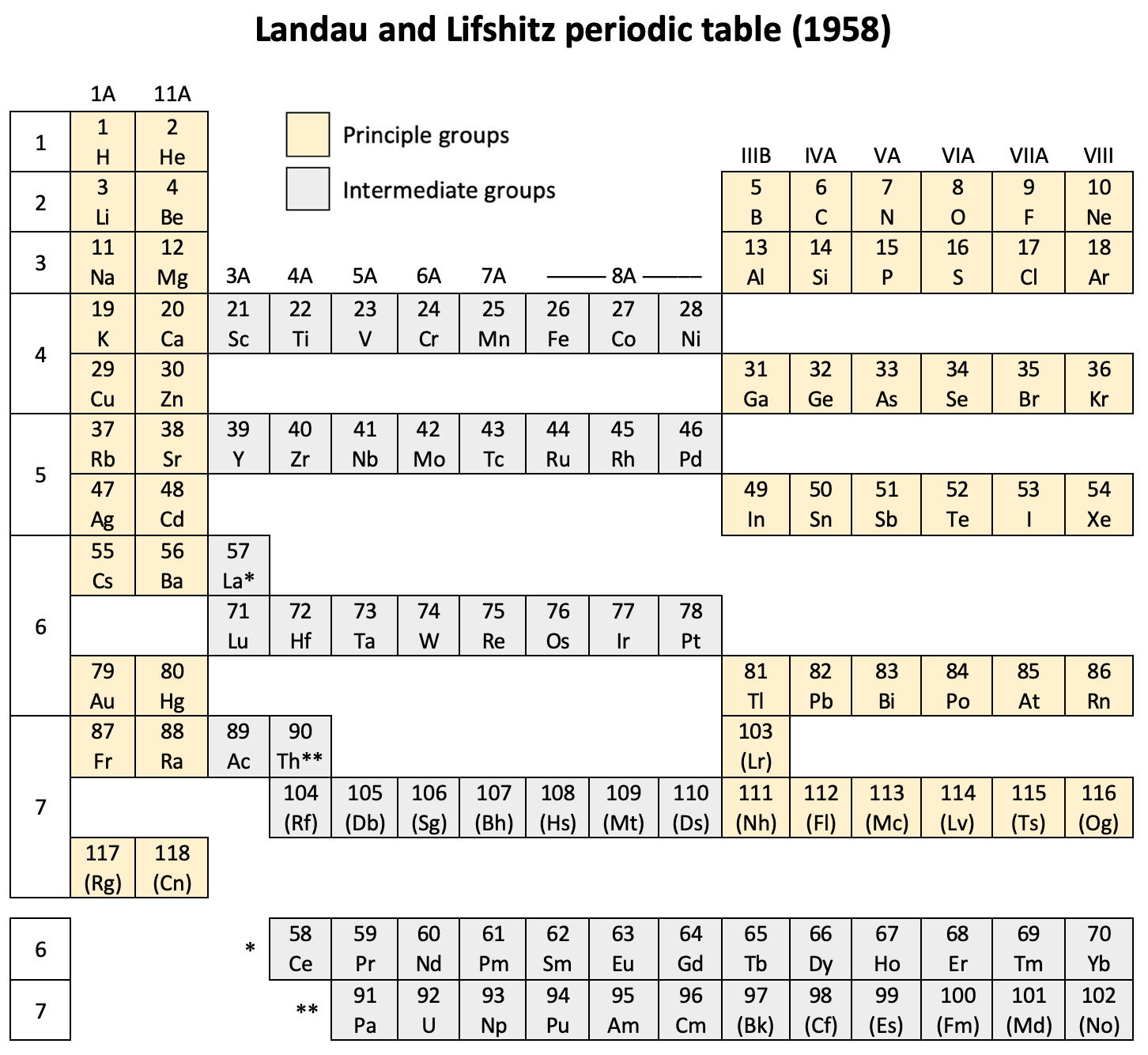
| 1958 | Landau & Lifshitz's Periodic System of Mendeleev |
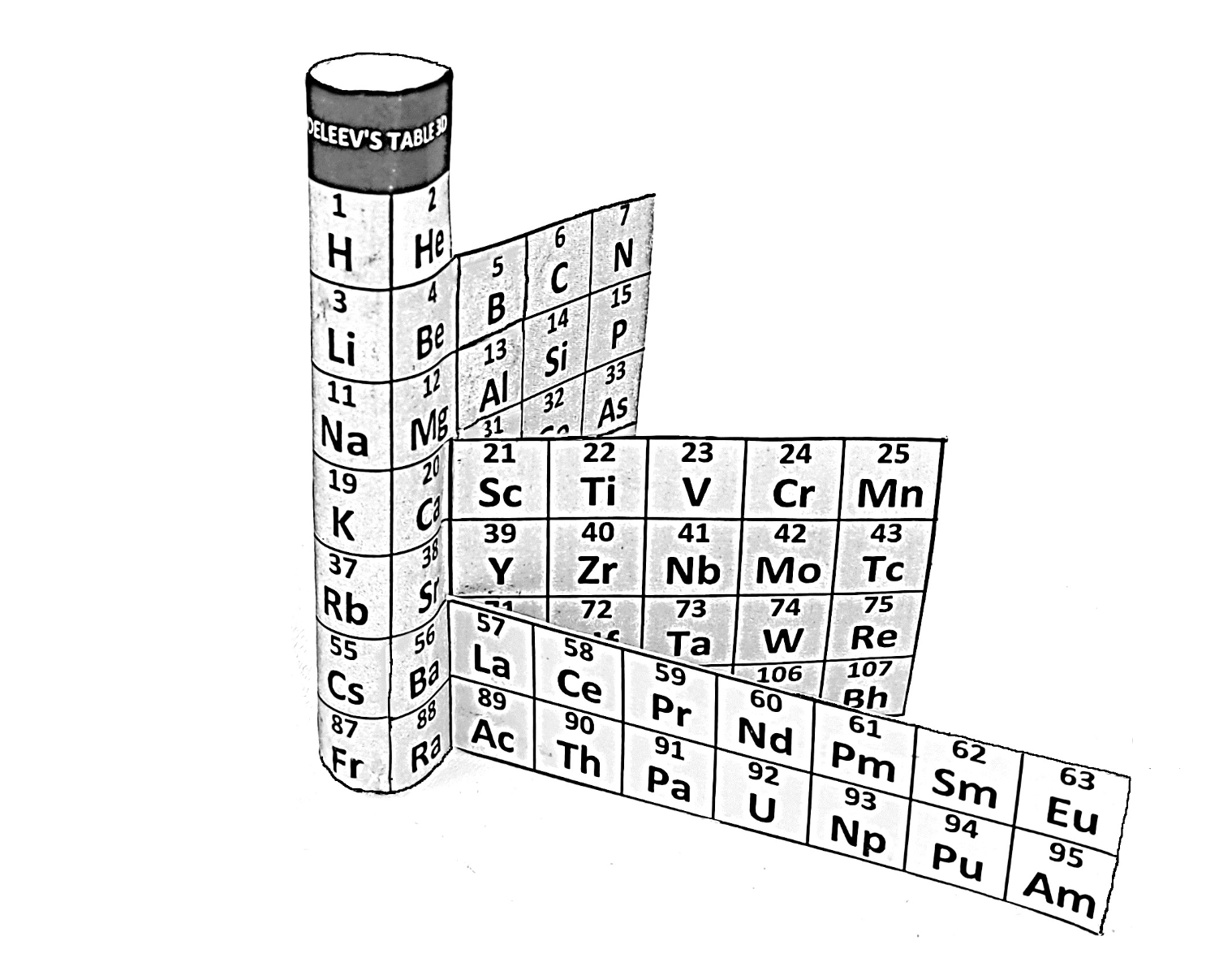
| 2024 | Kudan's 3D Model of The Periodic Table |
| Year: 1958 | PT id = 1148, Type = formulation |
Landau & Lifshitz's Periodic System of Mendeleev
L.D. Landau & E.M. Lifshitz, Quantum Mechanics (Volume 3 of A Course of Theoretical Physics), pages 255-258. (Note: First published in English in 1958, the link is to the 1963 3rd ed. of the English version translated from Russian.)
René Vernon writes:
The authors discuss aspects of the periodic system of D I Mendeleev. The electron configurations of hydrogen & helium are briefly noted. This is followed by three tables setting out the electron configurations of the s, p, d & f elements.
Some extracts from the text follow:
"The elucidation of the nature of the periodic variation of properties, observed in the series of elements when they are placed in order of increasing atomic number, requires an examination of the peculiarities in the successive completion of the electron shells of atoms." (p. 252)
"Many properties of atoms (including the chemical properties of elements...) depend principally on the outer regions of the electron envelopes." (p. 254)
"The elements containing complete d and f shells (or not containing these shells at all) are called elements of the principal groups; those in which the filling up of these states is actually in progress are called elements of the intermediate groups. These groups of elements are conveniently considered separately." (p. 254)
"We see that the occupation of different states occurs very regularly in the series of elements of the principal groups: first the s states and then the p states are occupied for each principal quantum number n. The electron configurations of the ions of these elements are also regular (until electrons from the d and f shells are removed in the ionisation): each ion has the configuration corresponding to the preceding atom. Thus, the Mg+ ion has the configuration of the sodium atom, and the Mg++ ion that of neon." (p. 255)
"Let us now turn to the elements of the intermediate groups. The filling up of the 3d, 4d, and 5d shells takes place in groups of elements called respectively the iron group, the palladium group and the platinum group. Table 4 gives those electron configurations and terms of the atoms in these groups that are known from experimental spectroscopic data. As is seen from this table, the d shells are filled up with considerably less regularity than the s and p shells in the atoms of elements of the principal groups. Here a characteristic feature is the 'competition' between the s and d states."
"This lack of regularity is observed in the terms of ions also: the electron configurations of the ions do not usually agree with those of the preceding atoms. For instance, the V+ ion has the configuration 3d4 (and not 3d24s2 like titanium) ; the Fe+ ion has 3d64s1 (instead of 3d54s2 as in manganese)."
"A similar situation occurs in the filling up of the 4f shell; this takes place in the series of elements known as the rare earths. † The filling up of the 4f shell also occurs in a slightly irregular manner characterised by the 'competition' between 4f, 5d and 6s states."
"† In books on chemistry, lutetium is also usually placed with the rare-earth elements. This, however, is incorrect, since the 4f shell is complete in lutetium; it must therefore be placed in the platinum group."
"The last group of intermediate elements begins with actinium. In this group the 6d and 5f shells are filled, similarly to what happens in the group of rare-earth elements." (p. 256–257)
The authors exclude lanthanum from the rare earths since the 4f shell has not started filling. Yet actinium and thorium are included by them with what we now call the actinoids even though these two metals have no f electrons. No explanation is provided for this puzzling lack of consistency with their categories.

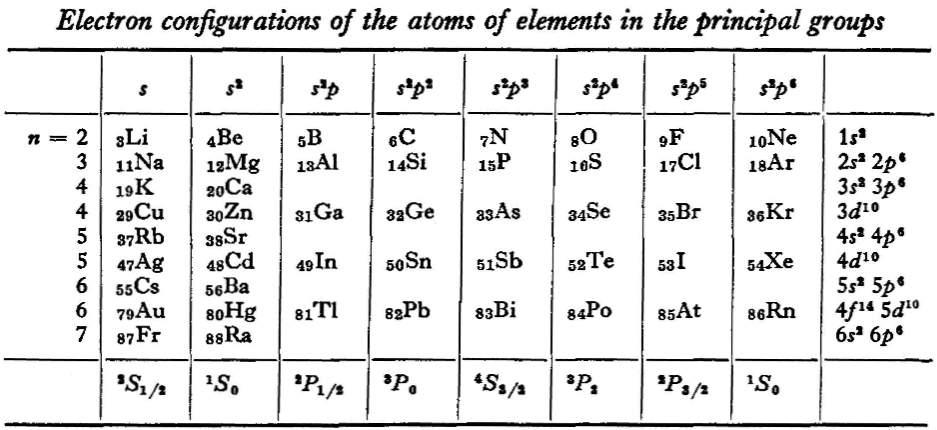
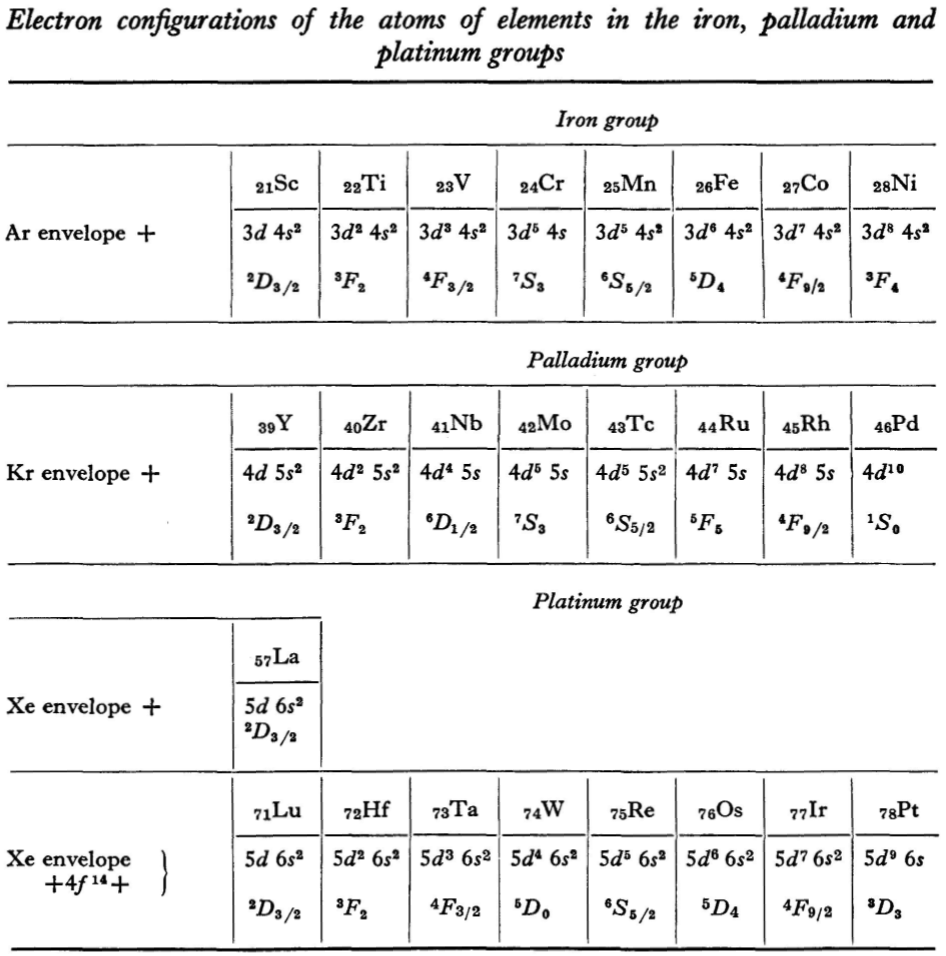
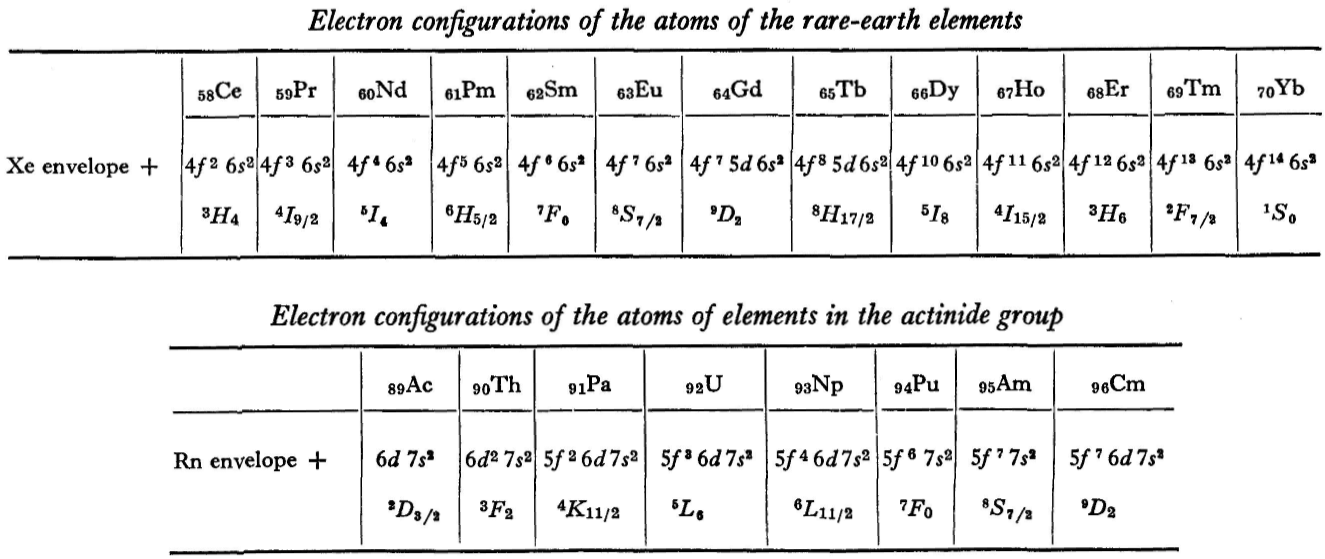
René Vernon writes: I have joined up their one note and three tables. (Curium was the last known element at their time of writing; transcurium elements are shown in parentheses.):
| Year: 2024 | PT id = 1298, Type = formulation 3D |
Kudan's 3D Model of The Periodic Table
Pavel V. Kudan's 3D model of the Periodic Table via direct download.
Parvel writes:
"The shape of this 3D model allows to show most important thing – H may be aligned over F-Ts and He may be aligned over Ne-Og without classification of H to group F-Ts or He to group Ne-Og. To see that it is needed only that cylinder to be tough (hard) and flat parts to be flexible with ability to change angle. Than is important because according Mendeleev’s principle, maximum valence is main for grouping elements and it is controversial to have element with maximum valence 2 between elements with maximum valence 8.
"Coloring He as gray in the 3D model just reflex the fact that it goes just before the energy gap, as well as coloring Ne-Og in gray show that they too go just before the energy gaps, which makes He and Ne-Og noble. The main is not coloring, but the ability to align and demonstrate.
"You may also remember that the issue of opening the new IUPAC Group 2 project to discuss He group as a continuation of the IUPAC Group 3 project has already been raised in e-mail correspondence with IUPAC some time ago in protection of our reconstruction of Landau’s geometry of the Periodic table.
"I agree with you that double periodicity is important, but also rearrangements of electronic configurations caused by properties of d-orbitals also must be taken in account. For example, Cu has valences 1 or 2, Zn has valence 2 due to special properties of d-orbitals. The 3D model of the Periodic table separates the ability of d-orbitals to steel electrons from s-orbitals and f-orbitals causing of such effects.
"Also when you will have a copy of the 3D model you will see that it unifies both geometry of the Mendeleev’s Periodic table and geometry of the Janet’s Periodic table. Following anticlockwise you may see Mendeleev’s order while following clockwise you may see Janet’s order. It is similar to having the 3D moles of globe as visual aid for better vision of Mendeleev’s Periodic table and Janet’s Periodic table as flat detailed maps."

 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.