Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 1913:
| 1913 | Moseley's Periodic Law |
| 1913 | Rydberg's Table |
| 1913 | Protactinium, Discovery of |
| 1913 | Rydberg's Periodic Table in style of Spiral with Four Revolutions |
| 1913 | van den Broek's Periodic Table 3 |
| Year: 1913 | PT id = 13, Type = formulation |
Moseley's Periodic Law
Henry Moseley (1887-1915) subjected known elements to x-rays and was able to derive a relationship between x-ray frequency and number of protons.
From Scientific American:
"It was the clever young English physicist, Moseley, who discovered that the atomic number for each element was the number of external electrons in the atom.
"With this discovery came a law concerning the X-ray lines of any element in an X-ray target.
"Moseley's law states that the wavelength of these lines is inversely proportional to the square of the atomic number of the element. Therefore, if we know the atomic number of the element we are looking for, we can predict the wavelength of certain lines in its X-ray spectrum.
"If we set up our X-ray spectrograph so as to catch these lines where we expect them to fall, then, if the element is present in the target which we have chosen to use in our X-ray tube, we should know it. This provides one good way to identify difficult elements, but it is well to have another to use as a check. One of the best of these, and one which is almost as sensitive as the X-ray method, is that of positive ray analysis."
From his paper, The High Frequency Spectra of The Elements, H. G. J. Moseley, M. A. Phil. Mag. (1913), p. 1024, available here:
| Year: 1913 | PT id = 59, Type = formulation |
Rydberg's Table
René Vernon writes:
My source is the 1914 French translation of Rydberg’s 1913 German article.
- Rydberg 1913, Untersuchungen über das System der Grundstoffe, Lunds Univ. Årsskrift, (Acta Univers, Lundensis), vol. 9, no. 18, pp. 1-41
- — 1914, Recherches sur le système des éléments, Journal de Chimie Physique, vol. 12, pp. 585–639, https://doi.org/10.1051/jcp/1914120585

| Year: 1913 | PT id = 871, Type = element |
Discovery of Protactinium
Pa ![]()
Protactinium, atomic number 91, has a mass of 231.036 au.
Radioactive element: Pa is only found in tiny amounts in nature. Most samples are synthetic.
Protactinium was first observed or predicted in 1913 by O. H. Göhring and K. Fajans and first isolated in 1927 by A. von Grosse.
| Year: 1913 | PT id = 973, Type = formulation |
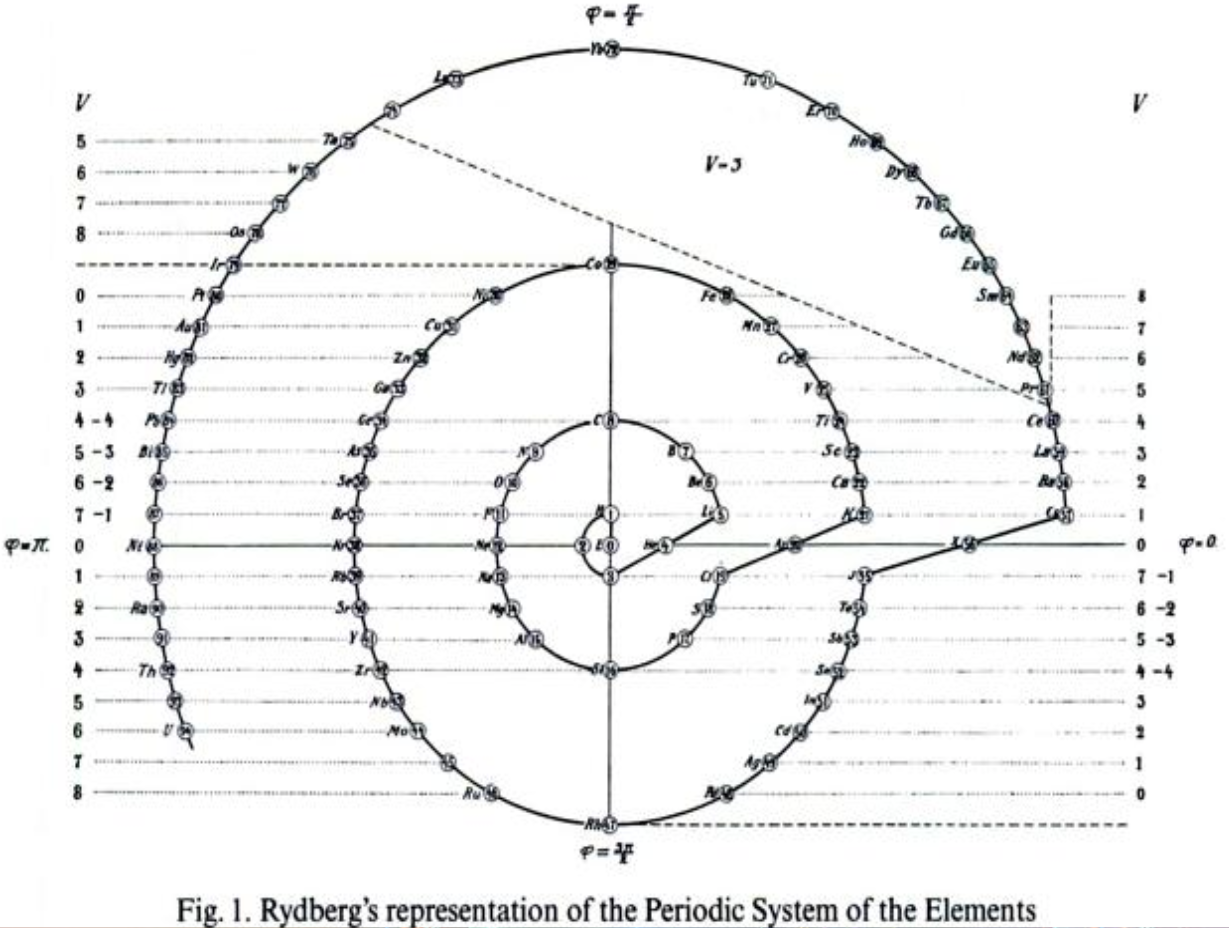
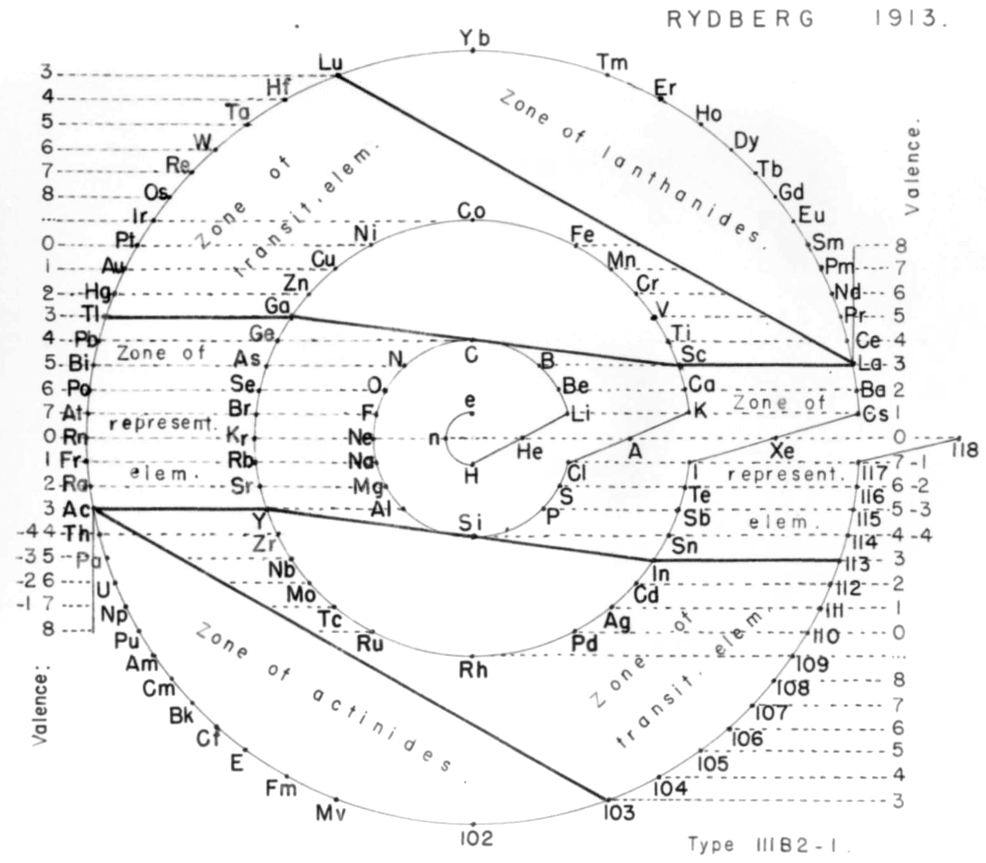
Rydberg's Periodic Table in style of Spiral with Four Revolutions
Periodic table in style of spiral with four revolutions circa 1913 (Original design) and 1957 (Date attributed to slide).
This table was originated by Swedish physicist Johannes Rydberg (1854-1919) in 1913 and classified by chemist Edward G. Mazurs as Type IIIB2-1 in his seminal work Types of Graphic Representation of the Periodic System of Chemical Elements (1957). The lower version of the table appears as Figure 63 on page 132 of Mazurs' 1957 publication.
| Year: 1913 | PT id = 1000, Type = formulation |
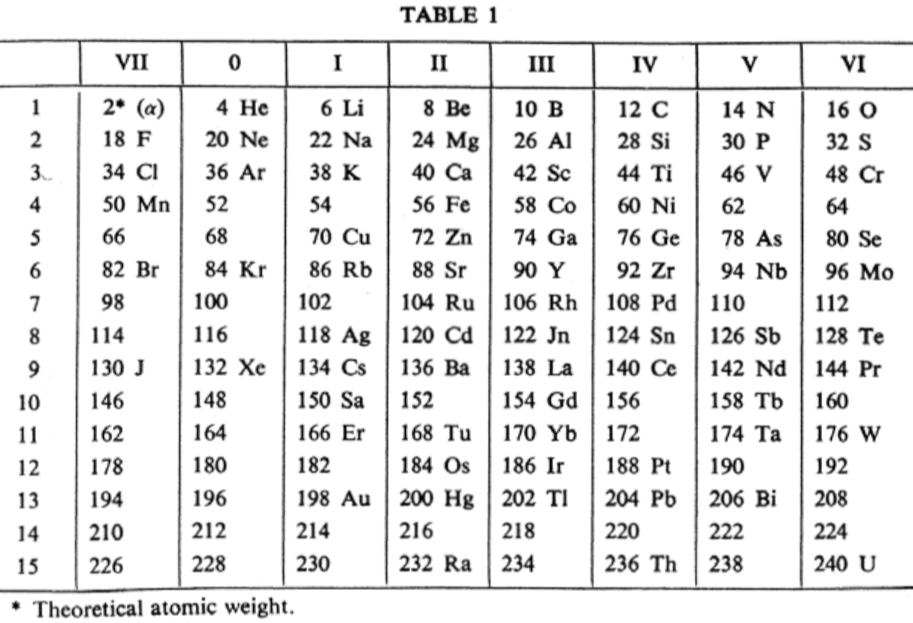
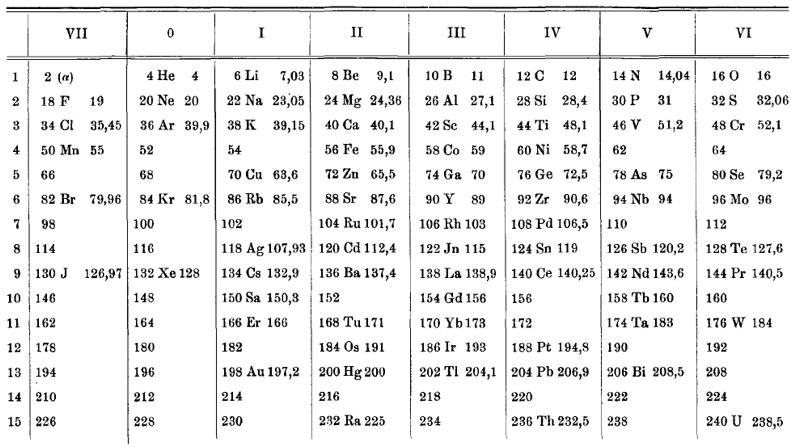
van den Broek's Periodic Table 3
From Wikipedia: Antonius Johannes van den Broek (1870-1926) was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table (now called atomic number) corresponds to the charge of its atomic nucleus. The 1911 inspired the experimental work of Henry Moseley, who found good experimental evidence for it by 1913. van den Broek envisaged the basic building block to be the 'alphon', which weighed twice as much as a hydrogen atom.
Read more in Chapter 4, Antonius Van Den Broek, Moseley and the Concept of Atomic Number by Eric Scerri. This chapter can be found in the book: For Science, King & Country: The Life and Legacy of Henry Moseley (Edited by Roy MacLeod, Russell G Egdell and Elizabeth Bruton).
van den Broek's periodic table of 1907: Annalen der Physik, 4 (23), (1907), 199-203
van den Broek's periodic table of 1911: Physikalische Zeitschrift, 12 (1911), 490-497); and also a paper in Nature the same year entitled: The Number of Possible Elements and Mendeléff's "Cubic" Periodic System, Nature volume 87, page 78 (20 July 1911)
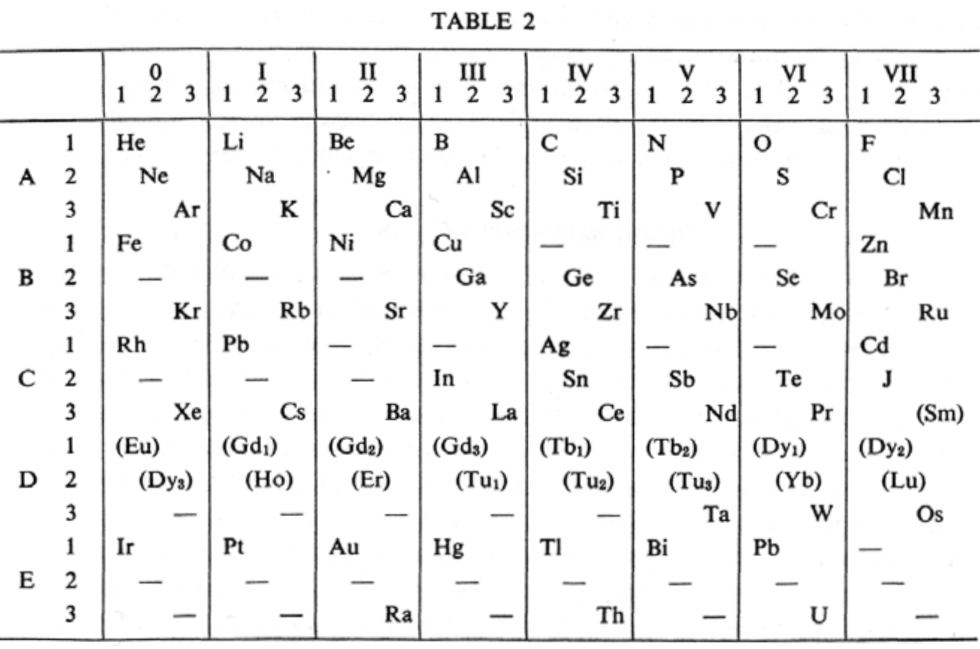
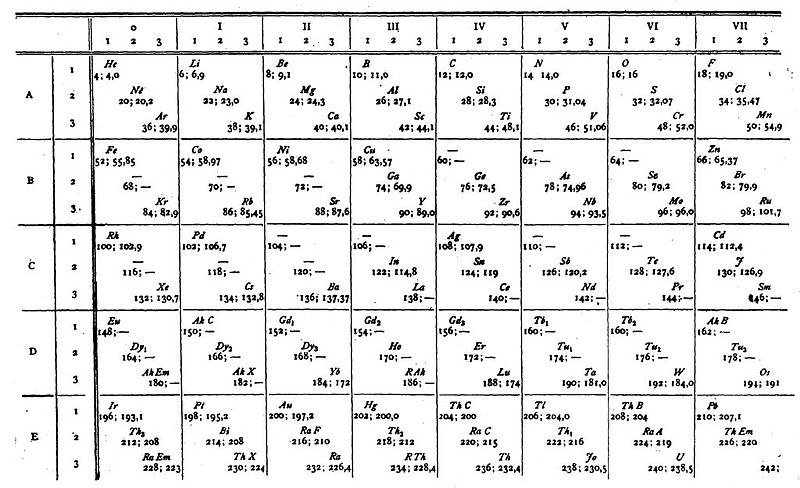
van den Broek's periodic table of 1913: Physikalische Zeitschrift, 14, (1913), 32-41
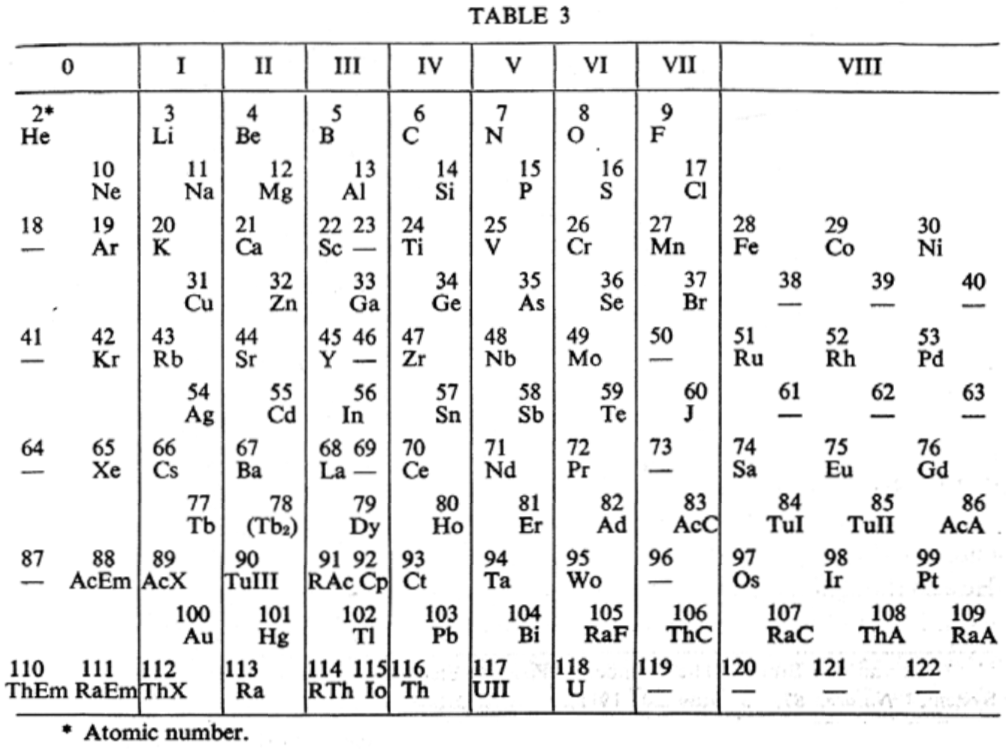
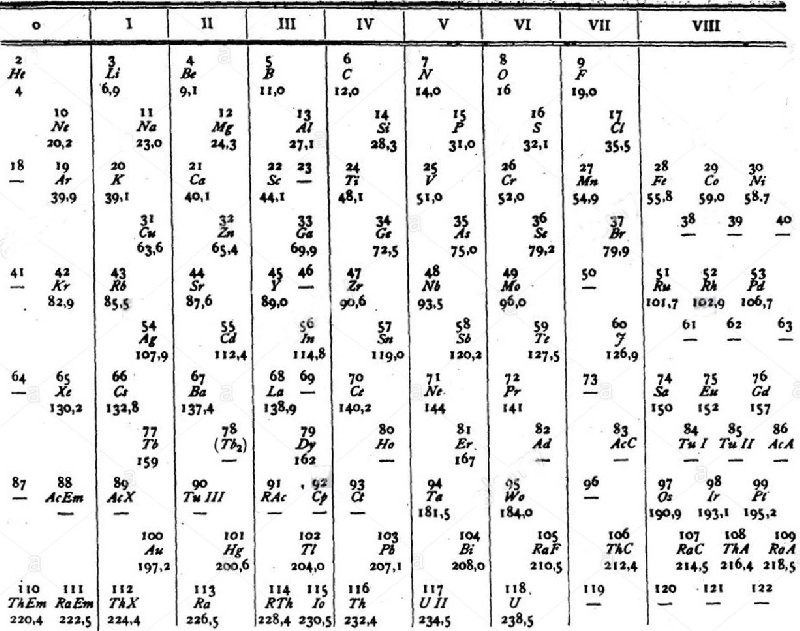
Thanks to Eric Scerri for the tip!
See the website EricScerri.com and Eric's Twitter Feed.
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.