Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
The 10 Periodic Tables most recently added to the database:
| Year: 2025 | PT id = 1346, Type = review formulation |
An Algebraic-Quantum Perspective on Atomic Shell Structure
From the ChemRxiv archive by Osman Sualihu:
"An Algebraic-Quantum Perspective on Atomic Shell Structure: The m2 Identity and Chemical Periodicity"


Thanks to Eric Scerri for the tip.
| Year: 2025 | PT id = 1345, Type = element |
Actinium: The Most Annoying Element for the Rare-Earth Industry?
Koen Binnemans writing on Linkedin:
Actinium: The Most Annoying Element for the Rare Earth Industry?
Rare-earth elements (REEs) are often accompanied by radioactive uranium and thorium in their ores. This is particularly problematic for monazite, which can contain more than 15 wt% thorium dioxide. The REE mineral steenstrupine, which occurs in large quantities in Greenland, is so rich in uranium that it can even be mined as a uranium ore. Due to strict safety regulations governing the handling of naturally occurring radioactive materials (NORM), the thorium content of REE ores poses major challenges for REE producers. Thorium is treated as radioactive waste, and its disposal can be very expensive.
It is important to realize that most radioactivity issues are not caused directly by thorium or uranium themselves, since their primary isotopes (thorium-232, uranium-235, and uranium-238) are all very long?lived, but by their radioactive daughter nuclides formed through decay chains.
Most of these radioactive daughter elements have chemical properties sufficiently different from those of the REEs that they can be removed using conventional hydrometallurgical techniques.
However, actinium presents a particular challenge because its chemical properties are similar to those of the REEs, especially lanthanum. The isotope actinium-227, formed by the decay of uranium-235, has a half-life of 22 years. During REE separation, actinium tends to follow lanthanum. In a solvent-extraction circuit, actinium accumulates in the SX battery along with the lanthanum stream.
Although the concentration of actinium is usually very low, lanthanum must be purified as thoroughly as possible, because one important application of lanthanum is in scintillator detectors for ionizing radiation (e.g. (LaBr3:Ce3+ or LaCl3:Ce3+). If lanthanum is contaminated with radioactive actinium, the resulting detector will exhibit significant background noise and therefore poor performance. Another issue is that REE concentrates produced at mining sites may exceed legal radioactivity limits for export to REE refineries due to the presence of actinium.
Therefore, REE processing companies have developed processes to remove actinium from REE concentrates or feed solutions for SX operations. Limited information is available in the scientific literature, but more can be found in patent documents. For instance, read the following patent of CARESTER, https://lnkd.in/evXsXzDB
It should also be noted that actinium has useful applications: actinium?225 is used in radiopharmaceuticals for the precision treatment of tumors. See: PANTERA
SOLVOMET R&I Centre SIM2 KU Leuven

Oak Ridge National Laboratory (Wikipedia) Blue Cerenkov radiation emitted by a sample of actinium-225
| Year: 2025 | PT id = 1344, Type = formulation |
AI Periodic Table: WARNING!
From Chemistry World:
"While images of the periodic table generated by a range of different AIs appear plausible at first glance, such as this one by Microsoft’s Copilot, a closer look reveals multiple errors. Calls are being made to outlaw the use of AI for drawing chemical structures in journal publications"
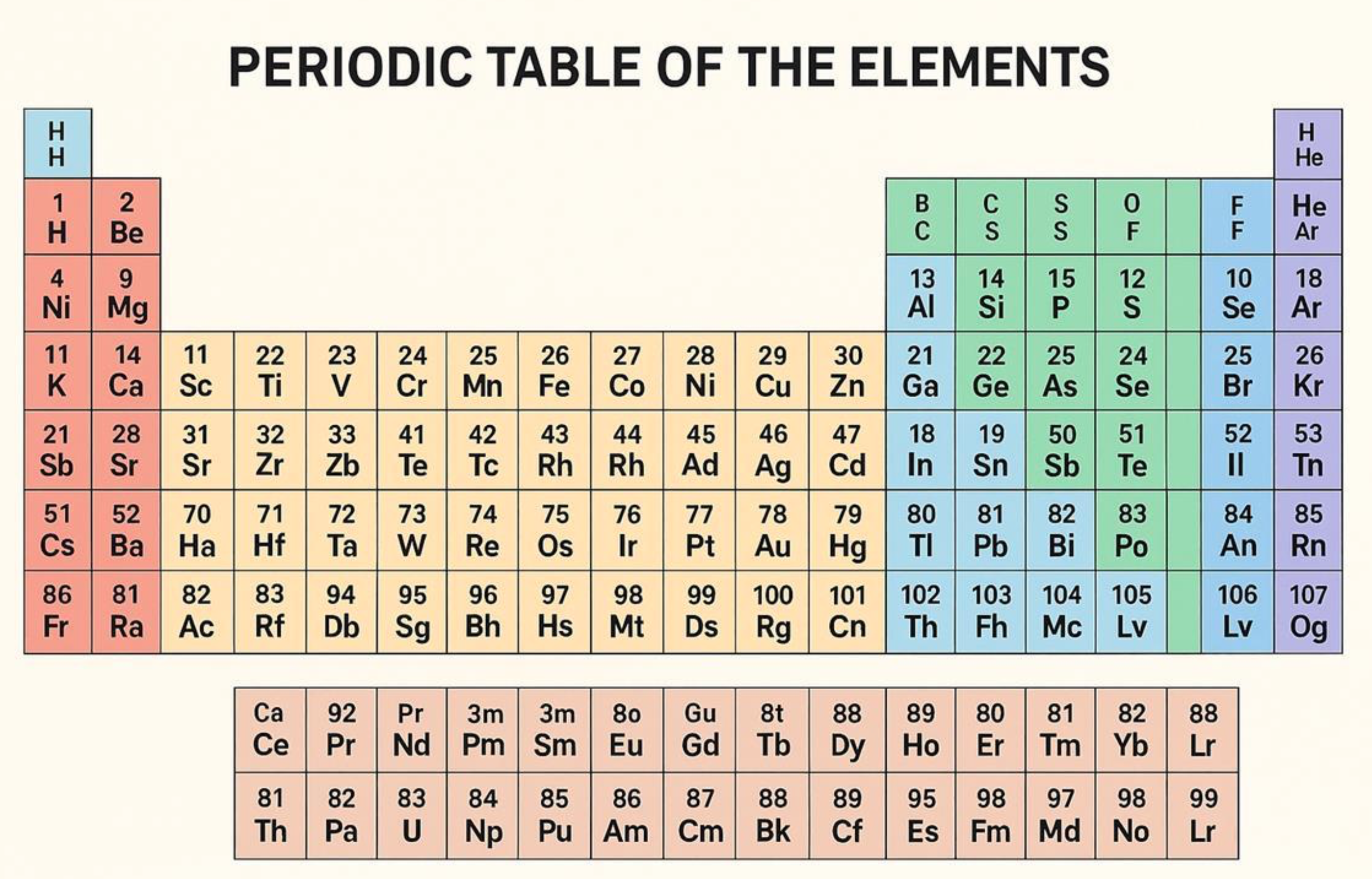
Thanks to Prof. Richard Hull for the tip!
| Year: 2025 | PT id = 1343, Type = non-chem misc |
S Ar Ca Sm: The Elements Required to Deal With Stupidity
S Ar Ca Sm: The Elements Required to Deal With Stupidity

Thanks to Barry for the tip!
| Year: 1924 | PT id = 1342, Type = formulation |
Hubbard and His Periodic Table
Henry D. Hubbard in front of his 1924 periodic table from wikimedia:

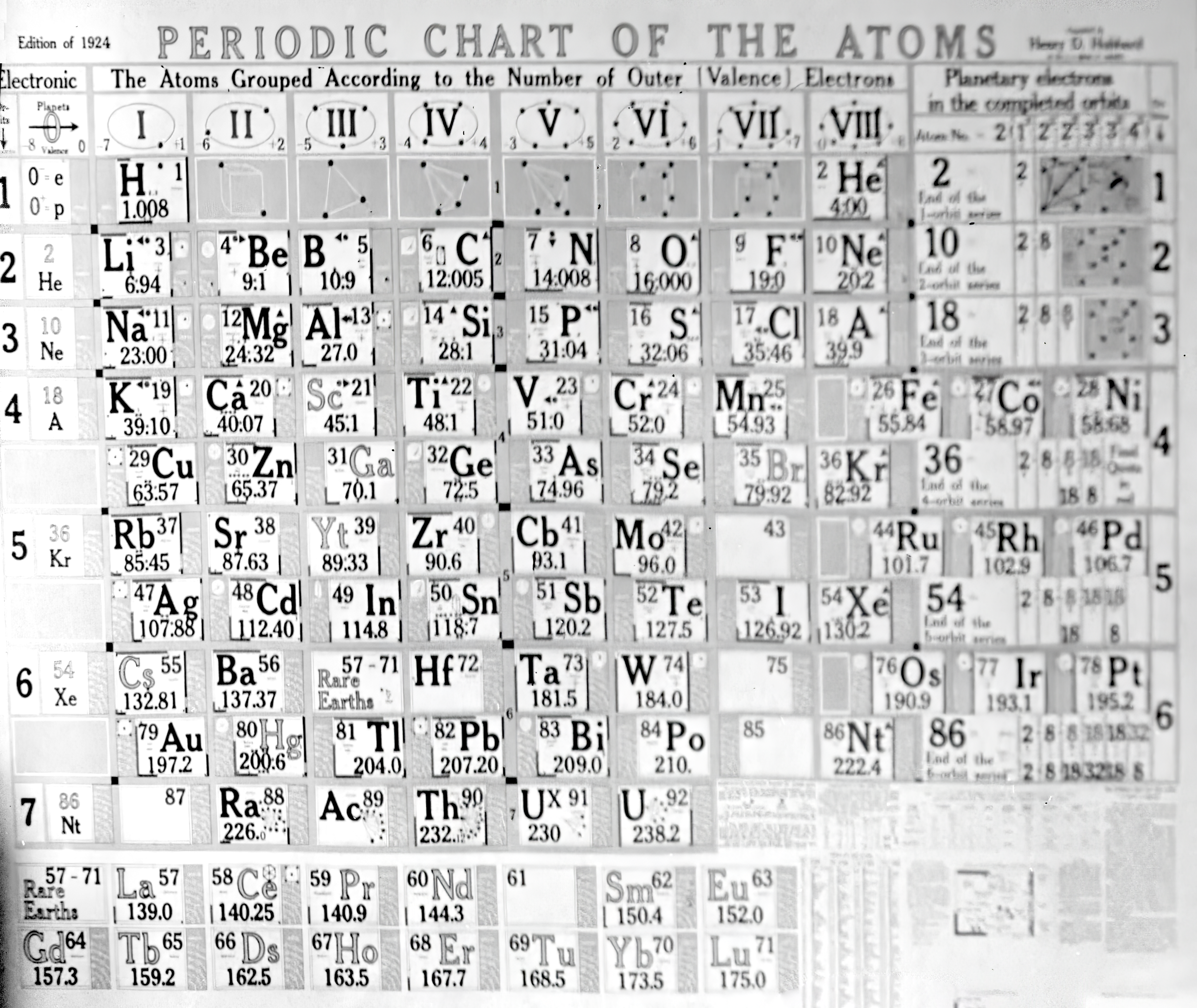
Thanks to Doug Simpson for the tip!
| Year: 2025 | PT id = 1341, Type = formulation |
Cruciform Periodic Table of the Elements
Don Stenberg, Jr.'s Cruciform Periodic Table of the Elements:
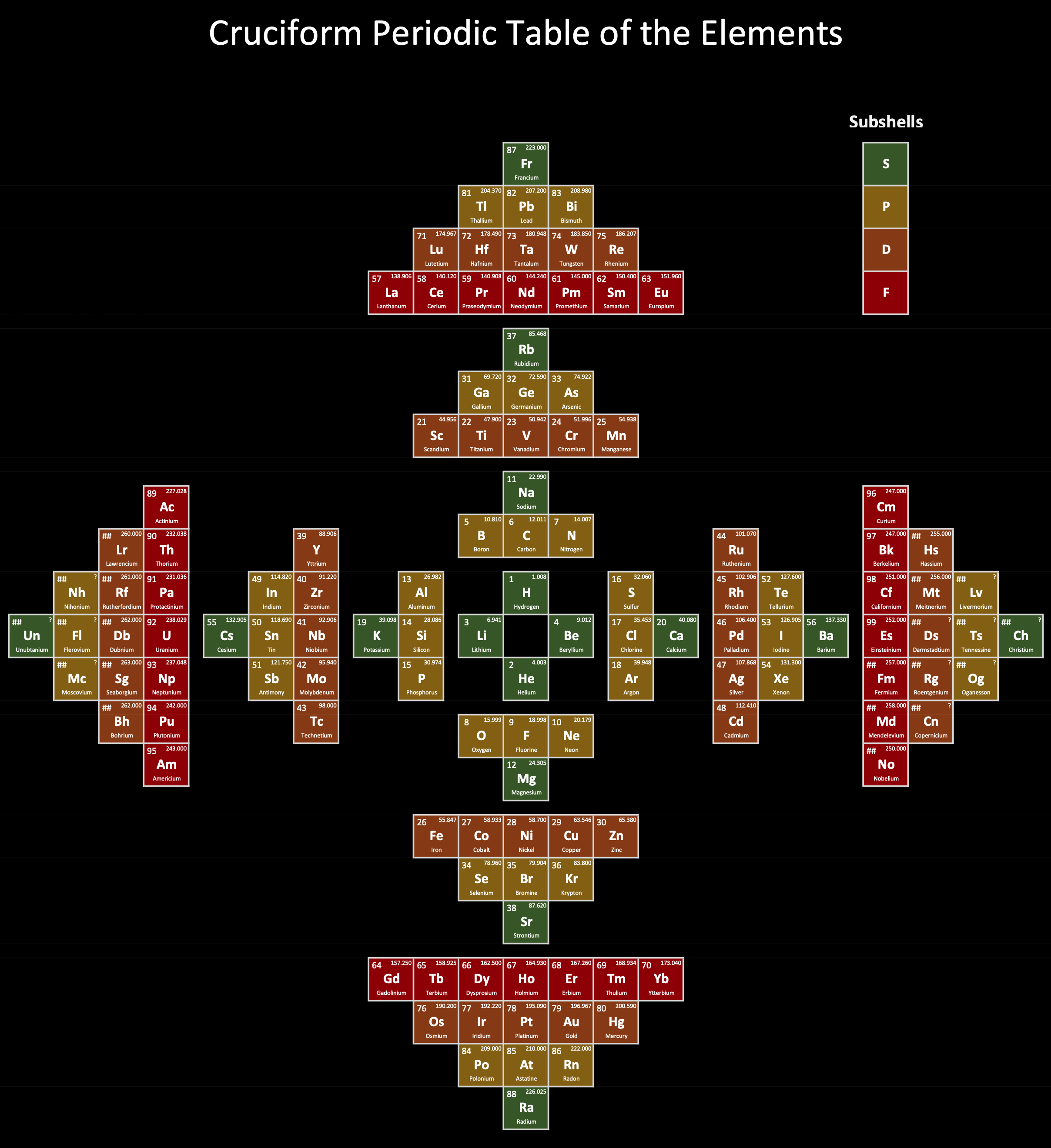
| Year: 2025 | PT id = 1340, Type = formulation |
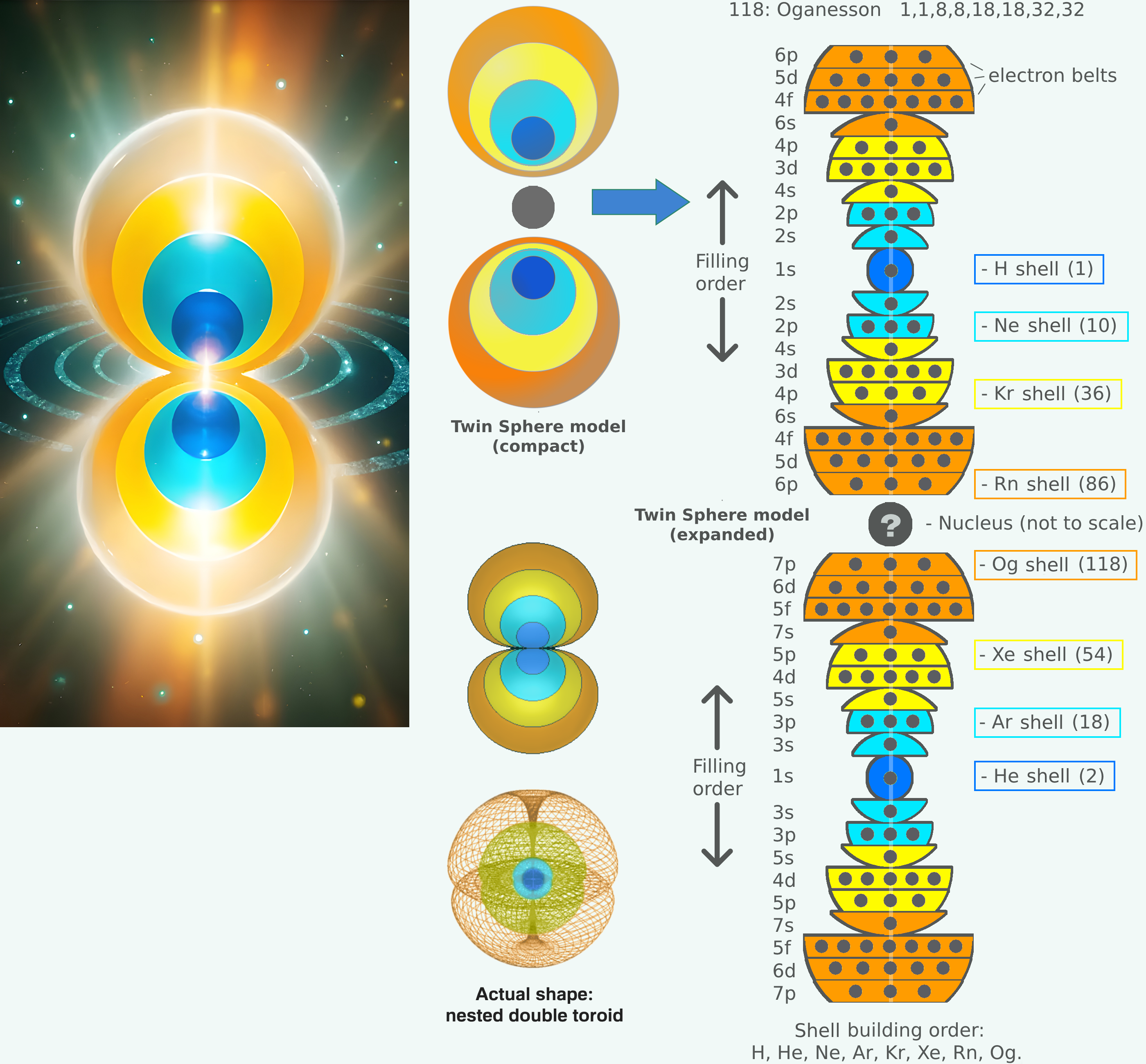
Livengood's Twin Sphere Model
Cody Livengood's Twin Sphere model of the atom. Read the full story: Unlocking the Atom: Introducing the Twin Sphere Model on ResearchGate.
Cody writes:
"Quantum mechanics has long described electrons as probabilistic clouds, an approach that, while mathematically sound, often lacks intuitive clarity about the physical arrangement of electrons in atoms. This abstract portrayal is not only difficult to visualize but makes it challenging to understand why electrons form the distinct patterns observed in the periodic table and other chemical principles or how these patterns influence chemical properties. Could there be a simpler, more deterministic way to describe atomic structure? The Twin Sphere Model offers a compelling alternative. By aligning more closely with the periodic table's structure and other empirical data, it provides a clear, geometric framework that has the potential to transform our understanding of matter, chemistry, and physics overall."
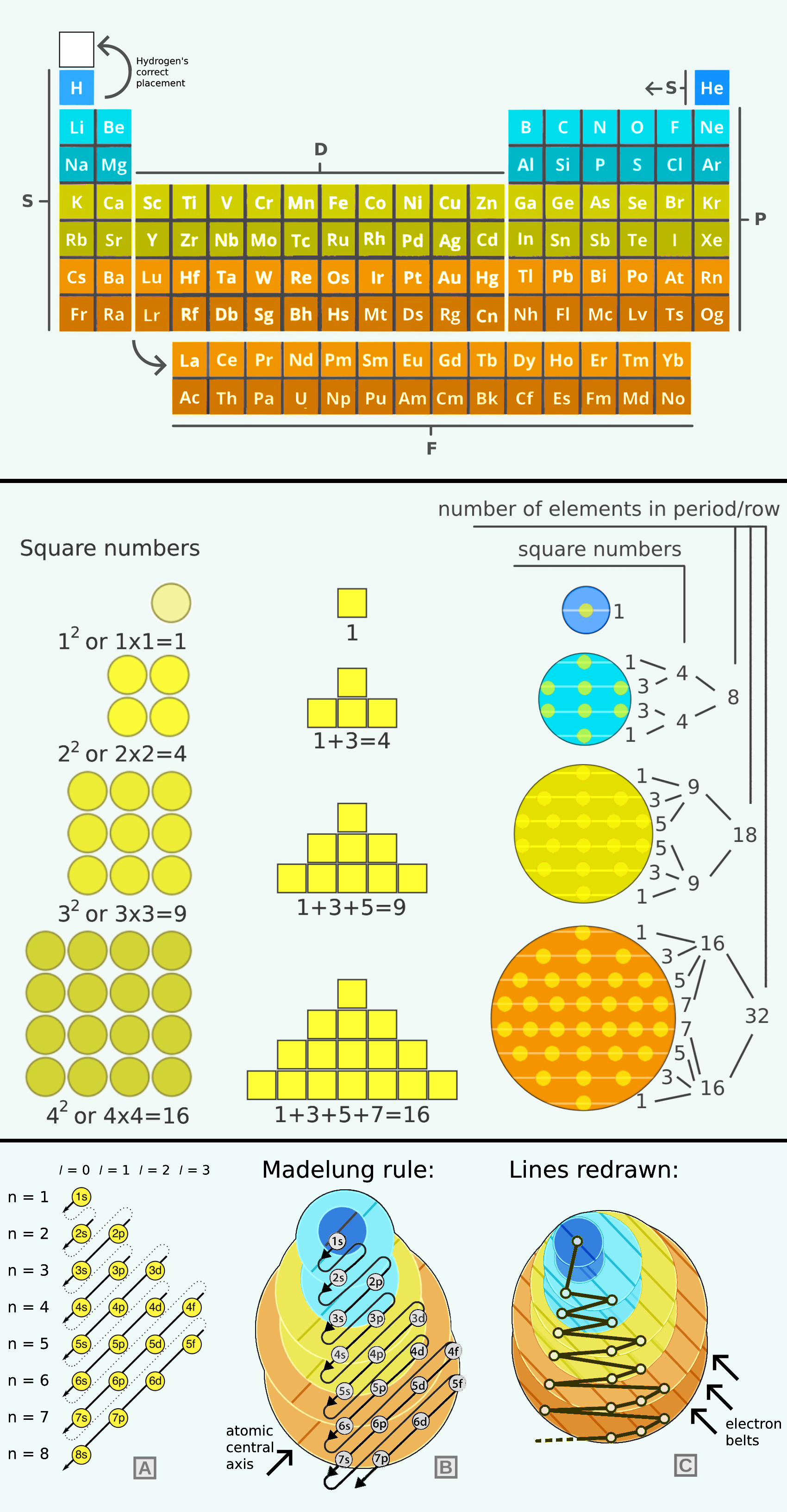
| Year: 2025 | PT id = 1339, Type = non-chem |
Music, Periodic Table of
Even though the author of this image, mycolourmusic.com, do not describe it as a "Periodic Table of Music", we think it fits the bill:
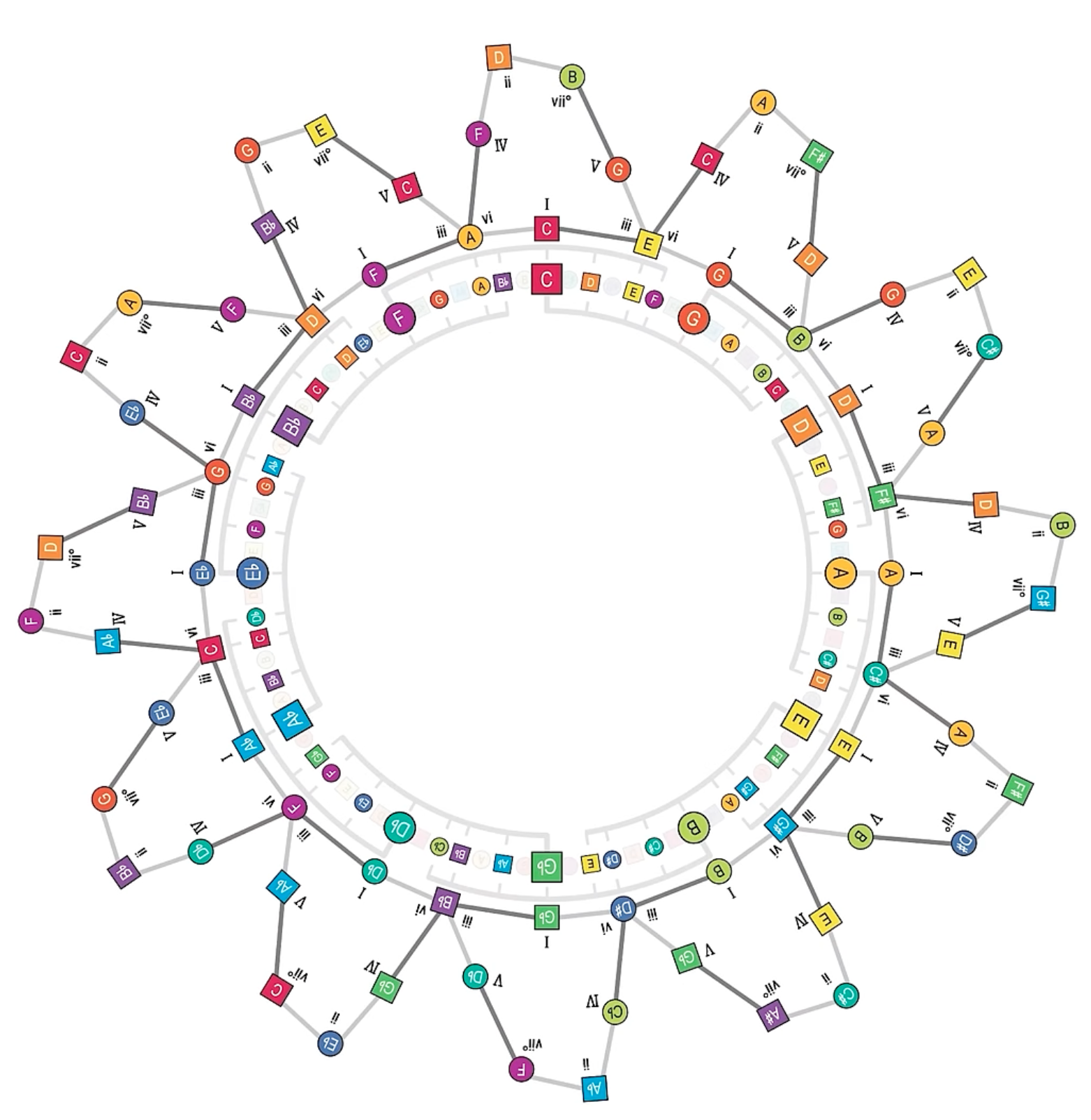
Watch a 60 second video here:
| Year: 2010 | PT id = 1338, Type = misc |
元素 (The Elements song in Japanese)
| Year: 2025 | PT id = 1337, Type = misc |
RIP Tom Lehrer (The Elements Song) - Periodic Table of Videos
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.