Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 1886:
| Year: 1886 | PT id = 81, Type = formulation |
Crookes' Periodic Table
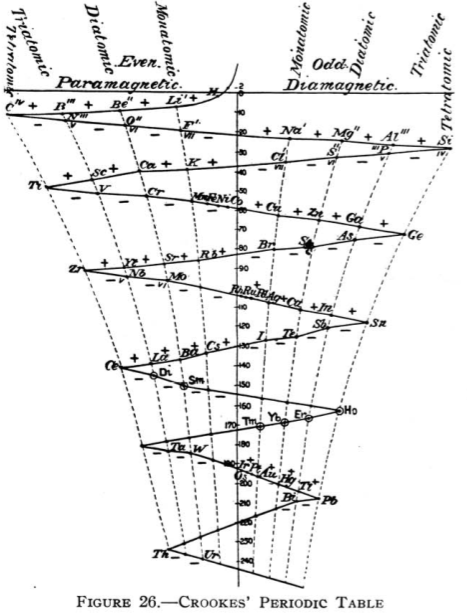
From Quam & Quam's 1934 review paper.pdf
| Year: 1886 | PT id = 798, Type = element |
Discovery of Fluorine
F
Fluorine, atomic number 9, has a mass of 18.998 au.
Fluorine exists as a pale yellow diatomic molecular gas, F2. It is the most electronegative and reactive of all elements: it which reacts with practically all organic and inorganic substances.
Fluorine was first observed or predicted in 1810 by A.-M. Ampére and first isolated in 1886 by H. Moissan.
| Year: 1886 | PT id = 812, Type = element |
Discovery of Germanium
Ge
Germanium, atomic number 32, has a mass of 72.63 au.
Germanium was first isolated in 1886 by C. A. Winkler.
| Year: 1886 | PT id = 846, Type = element |
Discovery of Dysprosium
Dy
Dysprosium, atomic number 66, has a mass of 162.5 au.
Dysprosium was first isolated in 1886 by P.E.L. de Boisbaudran.
Chronology of chemically the splitting of yttria (mixed oxides) into the pure rare-earth metals:
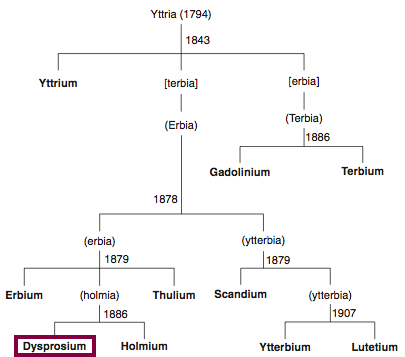
From: CRC Handbook on the Physics and Chemistry of Rare Earths, Chapter 248. Accommodation of the Rare Earths in the Periodic Table: A Historical Analysis
by Pieter Thyssen and Koen Binnemans (ISBN: 978-0-444-53590-0)
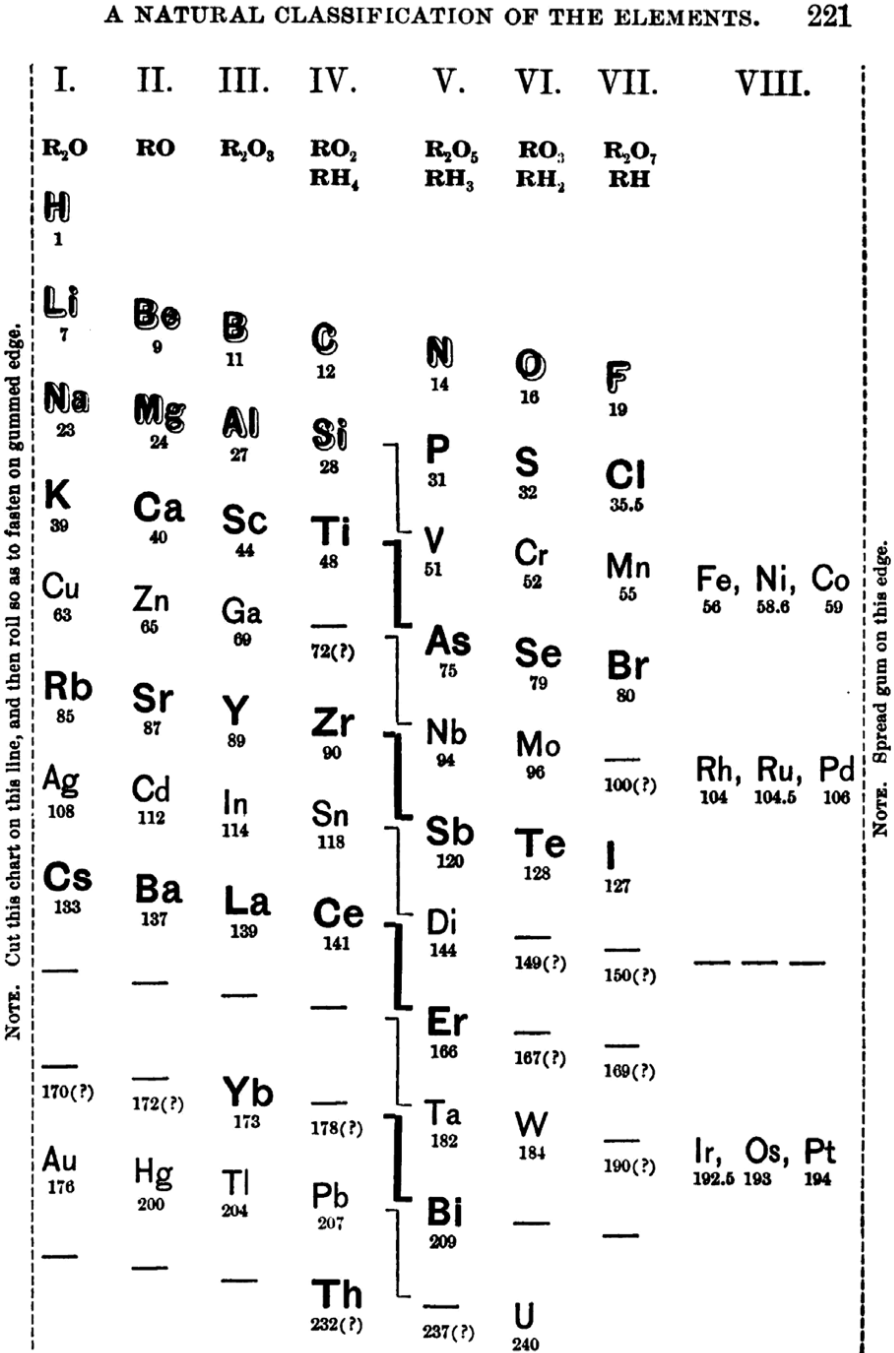
| Year: 1886 | PT id = 1107, Type = formulation spiral 3D |
Shepard's Natural Classification
Shepard's Natural Classification of the Elements, a spiral formulation with instructions for turning it into a three-dimensional table. From: Elements of Inorganic Chemistry, Descriptive and Qualitative (pp221), by J. H. Shepard, (1886), Boston MA, pub. D. C. Heath
René Vernon writes:
Note the instructions along the side, to turn the table into a tube (spiral form) and the 19 spaces from La to eka-Ce. Here, Yb needs to be moved back one column into group II, so as to leave room for Lu under La. Then eka-Ce becomes Hf. This results in La + 15 lanthanoids.
The accompanying text says:
"Elements of most distinct basic character are found towards the left; non-metals predominate in the upper and middle parts of Groups V., VI., and VII. ; while the lower part of the table is marked by the more indifferent elements.
"A double spiral will be traced beyond Si (beginning with P and V respectively) and distinguished by heavy-face and light-face type.
"The harmony of nature here exhibited is most impressive. Is it possible that the so-called elements are really compounds? Did the various 'elements' of the earth and sun once exist as hydrogen, when our solar system was a nebula? And will modern chemists ever revive the famed problem of the alchemists, and seek to turn the base metals into gold? Far more precious than gold is the search for truth; and the more we learn of science, the broader becomes our conception of what we know in part, and the deeper should be our reverence for the infinite thought of the Creator."
| Year: 1886 | PT id = 1108, Type = formulation |
Reynolds' Method of Illustrating the Periodic Law
Reynolds, J. E. (1886). Note on a method of illustrating the periodic law. Chemical News, 54, 1–4
Ann E. Robinson comments:
"Reynolds published a zig-zag version he created, noting it had been 'used in my lecture-room for some years in order to illustrate the periodic character of the relation between the atomic weights and properties of the chemical elements.'
>"Rather than a tabular form, Reynolds's form was a curve, best visualised as a string with seven knots tied in it at regular intervals, providing a 'vibrating system'. Although Reynolds's diagram is presented only as a two-dimensional picture, in a note he remarks that a friend suggested that the installation of 'a vibrating metallic chain, suspended from the ceiling and attached to the floor, would afford a more complete picture.'":
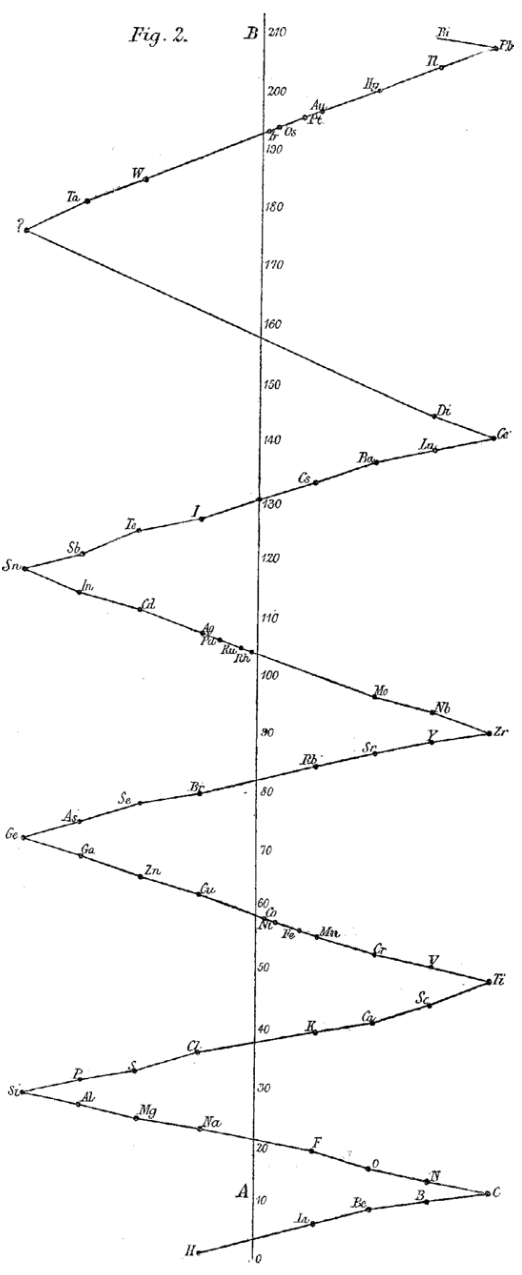
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.