Periodic Table |
 |
 |
 |
 |
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
The INTERNET Database of Periodic Tables
There are thousands of periodic tables in web space, but this is the only comprehensive database of periodic tables & periodic system formulations. If you know of an interesting periodic table that is missing, please contact the database curator: Mark R. Leach Ph.D.
Use the drop menus below to search & select from the more than 1300 Period Tables in the database:
- SEARCH:
- By Decade
- By Type
-
Pre-Selected
Best Four Periodic Tables for Data All Periodic Tables by Name All Periodic Tables by Date All Periodic Tables by Reverse Date All Periodic Tables, as Added to the Database All Periodic Tables, reverse as Added Elements by Name Elements by Date Discovered Search for: Mendeleev/Mendeléeff Search for: Janet/Left-Step Search for: Eric Scerri Search for: Mark Leach Search for: René Vernon Search for: Electronegativity
-
By Year
2025 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996 1995 1994 1993 1992 1991 1990 1989 1988 1987 1986 1985 1984 1983 1982 1981 1980 1979 1978 1977 1976 1975 1974 1973 1972 1971 1970 1969 1968 1967 1966 1965 1964 1963 1962 1961 1960 1959 1958 1957 1956 1955 1954 1953 1952 1951 1950 1949 1948 1947 1946 1945 1944 1943 1942 1941 1940 1939 1938 1937 1936 1935 1934 1933 1932 1931 1930 1929 1928 1927 1926 1925 1924 1923 1922 1921 1920 1919 1918 1917 1916 1915 1914 1913 1912 1911 1910 1909 1908 1907 1906 1905 1904 1903 1902 1901 1900 1899 1898 1897 1896 1895 1894 1893 1892 1891 1890 1889 1888 1887 1886 1885 1884 1883 1882 1881 1880 1879 1878 1877 1876 1875 1874 1873 1872 1871 1870 1869 1868 1867 1866 1865 1864 1863 1862 1861 1860 1859 1858 1857 1856 1855 1854 1853 1852 1851 1850 1844 1843 1842 1838 1836 1831 1830 1829 1825 1824 1817 1814 1813 1811 1808 1807 1804 1803 1802 1801 1800 1798 1794 1791 1789 1787 1783 1782 1781 1778 1775 1774 1772 1771 1766 1753 1751 1748 1735 1718 1700 1690 1687 1682 1671 1669 1624 1617 1520 1000 -300 -450 -800 -1000 -2000 -3500 -3750 -5000 -6000 -7000 -9000
Periodic Tables from the year 1930:
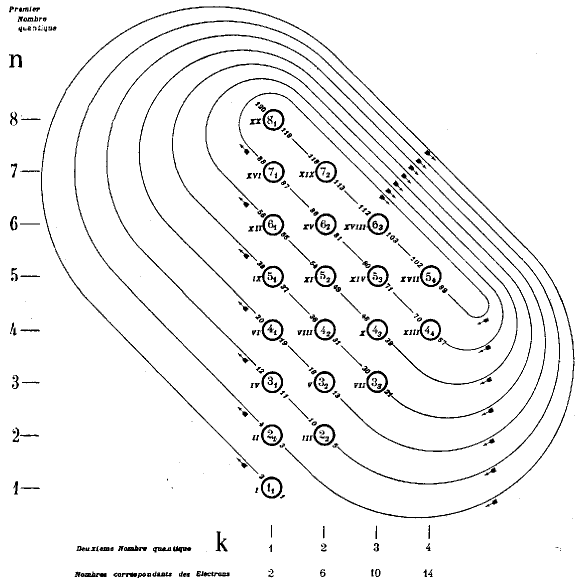
| 1930 | Janet's Shell Filling Diagram |
| 1930 | Gardner & Mazzucchelli's Periodic System Elaborated as Electronic Configuration |
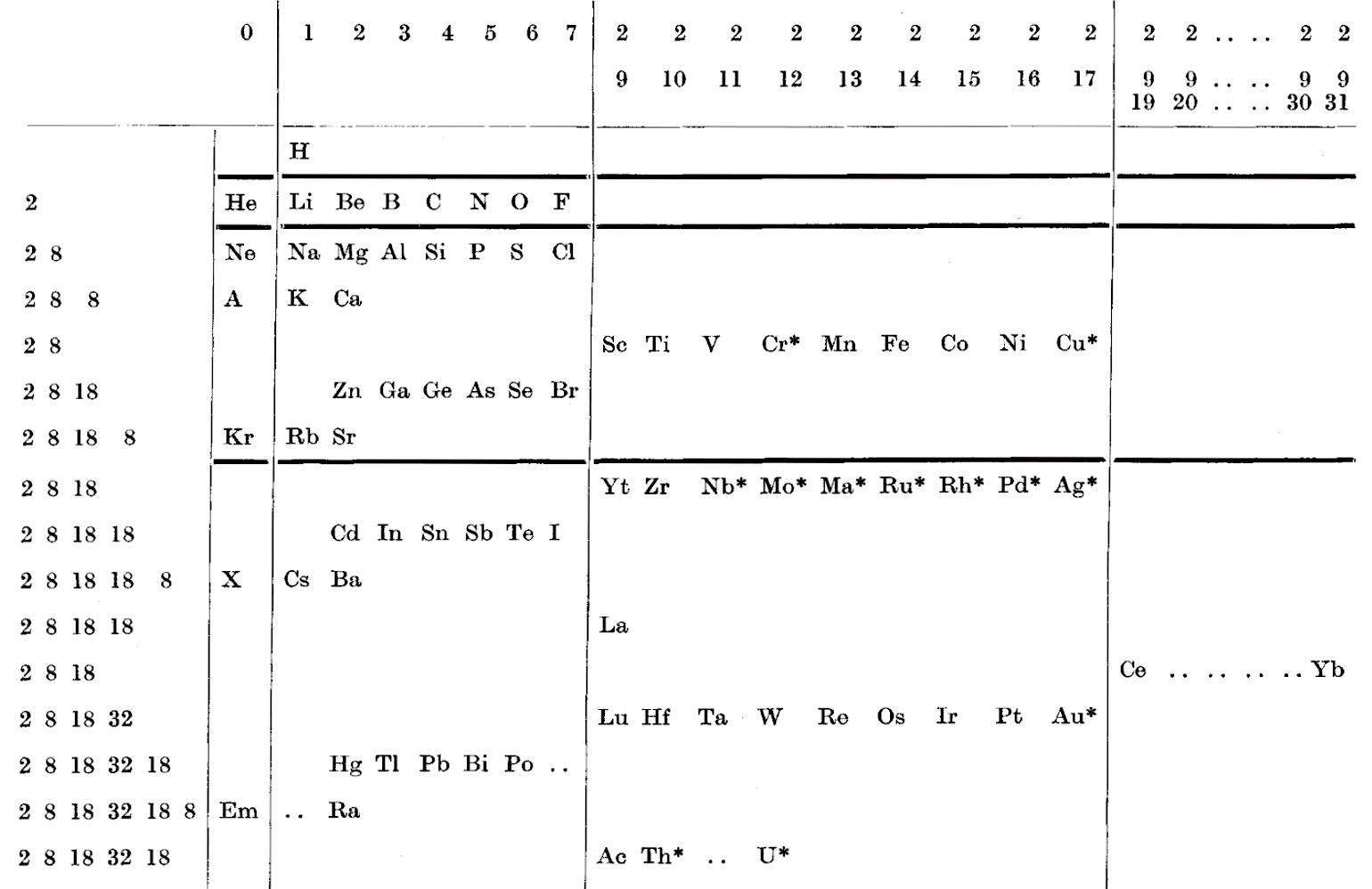
| 1930 | Gardner's Table of Electronic Configurations of the Elements |
| Year: 1930 | PT id = 154, Type = formulation spiral |
Janet's Shell Filling Diagram
Janet produced six papers, in French, which are almost unobtainable as he had them privately printed and didn't distribute them properly. The shell-filling diagram dated from November 1930, six years before Madelung. Note that Janet uses Bohr's radial quantum number, k, which is l+1. In the text he formulates the n+k-1 rule. Information supplied by Philip Stewart.
| Year: 1930 | PT id = 696, Type = formulation |
Gardner & Mazzucchelli's Periodic System Elaborated as Electronic Configuration
From Edward G. Mazurs' 1974 (2nd edition) Graphic Representations of the Periodic System During One Hundred Years, University of Alabama Press:
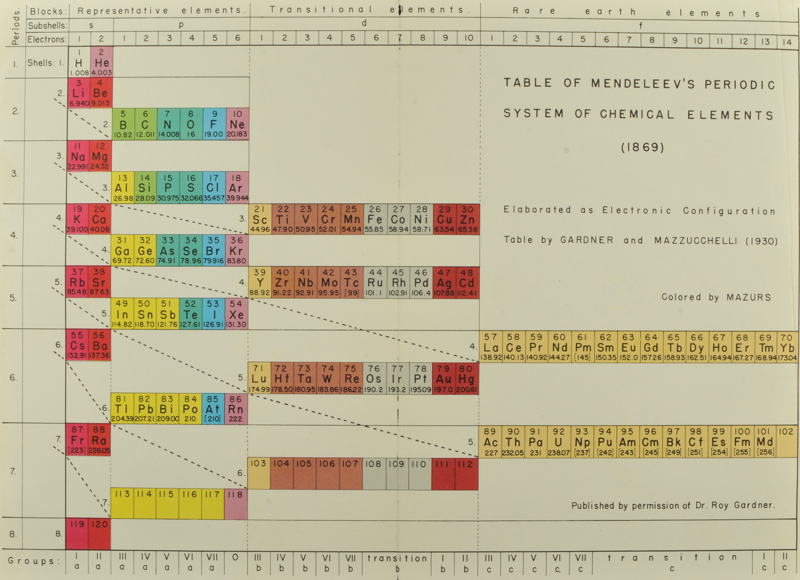
Thanks to Philip Stewart for the tip!
| Year: 1930 | PT id = 1264, Type = formulation |
Gardner's Table of Electronic Configurations of the Elements
A table of electronic configurations of the elements. Nature 125, 146 (1930). https://doi.org/10.1038/125146a0
Abstract:
"MR. ROY GARDNER gave an interesting paper on A Method of Setting out the Classification of the Elements at a recent meeting of the New Zealand Institute. The paper included the accompanying Table, which shows the distribution of electrons into groups corresponding to the principal quantum numbers for all the elements and at the same time preserves the most essential features of the two-dimensional arrangement of Mendeleef. Elements having the same complete groups (that is, all stable groups of 8 or 18) are placed in the same horizontal row, and the vertical columns include elements with the same number of electrons in the incomplete outer groups. The electronic configurations are those given by Sidgwick ("Electronic Theory of Valency", 1927). An asterisk marks elements for which the 'normal' atom is thought to have only one electron in the outermost group, but as practically all these give divalent ions, the point is of minor interest chemically. Distribution of electrons into k-subgroups is unnecessary; these have at present little significance for chemical purposes, and in any case the subgroups are considered to be filled in order to the maxima 2, 6, and 10."
René Vernon writes:
In this table Gardner emphasises the existence of four types of elements:
- those with all "groups" complete
- those with one incomplete group
- those with two incomplete groups (transition elements)
- those with three incomplete groups (rare earth elements)
The upper limits of existence of covalencies of 8, 6, and 4 are marked by heavy horizontal lines.
Note:
- there are nine groups of d-block elements [as we would now call them], and but 13 f-block elements
- La and Lu are treated as d-block elements
- while Yb is counted as an f-block element it was later realised (1937) that the 4f shell is full at Yb, hence it is not clear where Gardner would have placed it (Yb)—seemingly in the 0 column
 |
 |
 |
| What is the Periodic Table Showing? | Periodicity |
© Mark R. Leach Ph.D. 1999 –
Queries, Suggestions, Bugs, Errors, Typos...
If you have any:
Queries
Comments
Suggestions
Suggestions for links
Bug, typo or grammatical error reports about this page,please contact Mark R. Leach, the author, using mark@meta-synthesis.com
This free, open access web book is an ongoing project and your input is appreciated.